Abstract
Murine coronavirus (CoV) is a beta-CoV that infects mice by binding to carcinoembryonic antigen-related cell adhesion molecule 1. Intraperitoneal infection with the murine CoV strain JHM (JHMV) induces acute mild hepatitis in mice. While both innate and acquired immune responses play a significant role in the protection against murine CoV infection in mice, CD8+ cytotoxic T lymphocytes (CTLs) and interferon-γ are essential for viral clearance in JHMV-induced hepatitis. In addition, CoVs are characterized by high diversity, caused by mutations, recombination, and gene gain/loss. 25V16G is an immune-escape JHMV variant, which lacks a dominant CTL epitope. By evading immune responses, 25V16G establishes persistent infections, leading to granulomatous serositis in interferon-γ-deficient mice. These examples of CoV-associated pathogenesis in mice might provide useful information on other CoV infections, including coronavirus disease 2019 (COVID-19).
Keywords: animal model, coronavirus, cytotoxic T lymphocyte, interferon-γ
Coronaviruses (CoVs) are large, enveloped, positive-stranded RNA viruses that can be divided into four genera: alpha-CoV, beta-CoV, gamma-CoV, and delta-CoV [7]. CoVs are very diverse and are constantly evolving through gene gains and losses, in addition to recombination and accumulation of mutations [1, 10, 30]. Although CoVs are important pathogens in veterinary medicine, they had been overlooked as human pathogens. CoVs are believed to cause 10–30% of common colds, which are usually mild; however, the 2002–2004 severe acute respiratory syndrome (SARS) outbreak and the Middle East respiratory syndrome (MERS) were caused by CoV [37]. In December 2019, another highly pathogenic human CoV, SARS-CoV-2, caused a coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China, which has since spread across the world [25]. SARS-CoV-2 is a beta-CoV [29] that binds to the angiotensin-converting enzyme 2 (ACE2) [40, 47]. Although it has become evident that SARS-CoV-2 primarily causes respiratory disease in humans, the pathogenesis of SARS-CoV-2 infection remains mostly unclear [16]. The severity of COVID-19 varies immensely among individuals, ranging from subclinical to fatal [52]. Epidemiologically, prevalence and severity of the disease increase with age, and are higher in men than in women. Individuals with cardiovascular disease, diabetes, obesity and pulmonary disease have higher mortality. Death is usually the results of anoxia due to pulmonary failure. There is increasing evidence of dysfunction in coagulation and thrombosis in COVID-19 patients [39]. Since asymptomatic infected individuals can shed SARS-CoV-2, controlling person-to-person transmission is extremely challenging [6].
Murine CoV, which belongs to the beta-CoV, infects mice by binding to carcinoembryonic antigen-related cell adhesion molecule 1 [38]. Numerous viral strains have been isolated thus far. Based on their tropism, murine CoV strains can be broadly divided into enterotropic or polytropic [15]. Enterotropic strains include the mouse hepatitis virus (MHV) strains D, Y, RI, and DVIM; they induce enteritis in young weanling mice and are considered responsible for most of the MHV outbreaks in laboratory animal facilities. On the other hand, polytropic strains, such as JHM and A59, primarily cause hepatitis, enteritis, and encephalitis [48], depending on the route of infection [14]. For example, the murine CoV strain JHM (JHMV) is a neurotropic virus; nevertheless, it can cause acute mild hepatitis in different laboratory mouse strains after intraperitoneal infection.
CYTOTOXIC T LYMPHOCYTE (CTL) RESPONSES IN CoV INFECTION
Although both innate and acquired immunity are essential in the protection against murine CoV infections [43, 49], CD8+ cytotoxic T lymphocytes (CTLs) play a pivotal role in viral clearance and inflammatory response in the liver after intraperitoneal JHMV infection in mice [21]. In one study, CD8+ T cell depletion in C57BL/6 (B6) mice using anti-CD8 antibodies significantly increased the viral load in the liver during the acute phase of the infection; a high JHMV titer (>104 PFU/g) was detected in the liver of CD8+ T cell-depleted mice seven days post-infection (p.i.), while no virus was detected in PBS-injected control mice [21]. By contrast, in the liver of CD4+ T cell-depleted mice, the viral titers were only marginally higher than those in control mice [21]. These results suggest that CTLs play a crucial role in virus clearance in JHMV-infected mice. In addition, the adoptive transfer of JHMV-specific CTL clones protects mice from JHMV-induced encephalitis [50, 51]. The importance of CTL-mediated immunity has also been demonstrated in other CoV infections [17, 28, 32, 42]. Taken together, these results suggest that CTL responses are essential for the clearance of CoV infections, similar to other viral infections [2, 33].
PERSISTENT MURINE CoV INFECTION IN IMMUNODEFICIENT HOSTS
Prolonged or severe viral infections are often observed in immunocompromised hosts [31, 35]. For instance, interferon-γ-deficient (GKO) mice are prone to subacute/chronic JHMV infection [23]. Interferon (IFN)-γ is a key antiviral and immunomodulatory cytokine primarily produced by activated natural killer (NK) cells, NKT cells, and T cells during viral infections [27]. In one study, compared to wild-type (WT) B6 mice, the viral titers in the liver of GKO B6 mice at 3 and 5 days p.i. were nearly 10 and 40 times higher, respectively [23]. Although JHMV was cleared from the liver within 7 days p.i. in WT mice, the viral titers remained high at 7 days p.i. and persisted even after 20 days p.i. in GKO B6 mice [23]. GKO BALB/c mice infected with JHMV intraperitoneally showed acute hepatic failure and died within a week, suggesting the genetic background affected the outcome of JHMV-induced hepatitis in GKO mice [22]. Importantly, in other studies with impaired IFN-γ responses, murine CoV clearance was also delayed or suppressed [26, 41]. Therefore, IFN-γ plays a crucial role in viral clearance during murine CoV infection in mice.
In addition to the decrease in viral titers in the liver, hepatitis gradually improves in GKO mice [23]. However, in GKO mice, viral infections are associated with severe granulomatous serositis accompanied by accumulation of a viscous fluid in the abdominal and thoracic cavities, leading to death 16–50 days p.i. [23]. Similar symptoms have been observed in GKO mice naturally infected with murine CoV [12]. Although the involvement of immunological disorders is suspected, the precise mechanisms underlying these conditions remain unknown.
The role of virus-specific antibodies in persistent CoV infections in GKO mice has gained increasing attention. Interestingly, compared to WT mice, GKO mice have 4 times higher titers of virus neutralization antibodies 21 days p.i., likely due to continuous antigenic stimulation in GKO mice [23]. These findings confirm that antibodies are not only ineffective but also possibly aggravate murine CoV-related symptoms. Although there are a lot of studies indicating that antiviral antibodies effectively protect against viral infections [20, 34], it is also known that antibodies against virus enhance viral load and disease severity in some CoV infections [4, 18, 45].
MECHANISMS INVOLVED IN PERSISTENT MURINE CoV INFECTION IN GKO MICE
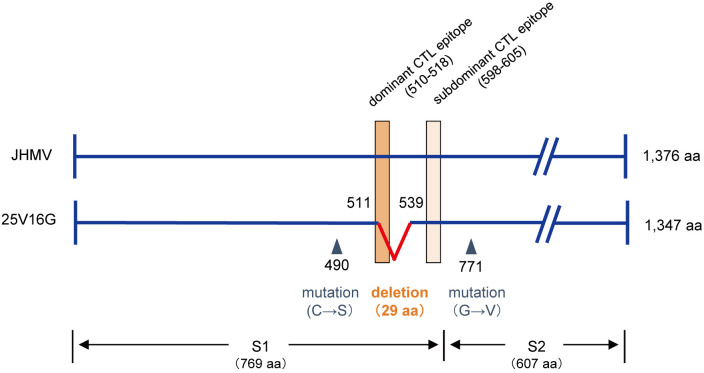
The mechanisms involved in the establishment of persistent CoV infection in GKO mice remain poorly understood. In one study, infectious viruses were isolated from ascites of JHMV-infected GKO mice at 25 days p.i. and cloned virus was named 25V16G after limiting dilution [24]. The clone 25V16G exhibited similar to JHMV replication in various cell lines, including DBT, IC-21, and J774A.1 cells, suggesting a replication potential comparable to that of JHMV [24]. Sequencing has revealed that the S gene of 25V16G has two point mutations [24] and an 87 base deletion in the region encoding for S511–539, which contains a dominant CTL epitope (Fig. 1) [5]. Therefore, it is likely that CTL-escape variants contribute to the establishment of persistent CoV infections in mice.
Fig. 1.
Structure of S protein of murine coronavirus strain JHM (JHMV) and 25V16G, and cytotoxic T lymphocyte (CTL) epitope in C57BL/6 mice. The S protein has a molecular weight of 180 kDa and is composed of S1 and S2 domains [11]. Dominant (510–518) and subdominant (598–605) CTL epitopes within the S protein are from Castro and Perlman [5] and are indicated with rectangles. A deletion and two point mutations were observed in the S protein of 25V16G, indicated with a wedge shape line and triangles, respectively.
WT and GKO B6 mice were inoculated with 25V16G and JHMV to compare the pathogenicity of the two strains. In WT mice, the viral titers of 25V16G were approximately 60 times higher than those of JHMV 5 days p.i., and in contrast to JHMV, 25V16G could still be detected 7 and 14 days p.i. (Takagaki and Kyuwa, unpublished data). Intraperitoneal 25V16G infection in GKO mice induced fatal fulminant hepatitis accompanied by abundant viral replication [24]. By contrast, JHMV infection in GKO mice induces the formation of focal necrotic lesions, accompanied by modest viral replication [24]. These findings support the notion that 25V16G evades CTL-mediated immune responses, having cytopathic effects on murine hepatocytes, although further studies are necessary.
ROLE OF CTL AND IFN-γ IN MURINE CoV INFECTION-ASSOCIATED DISEASE
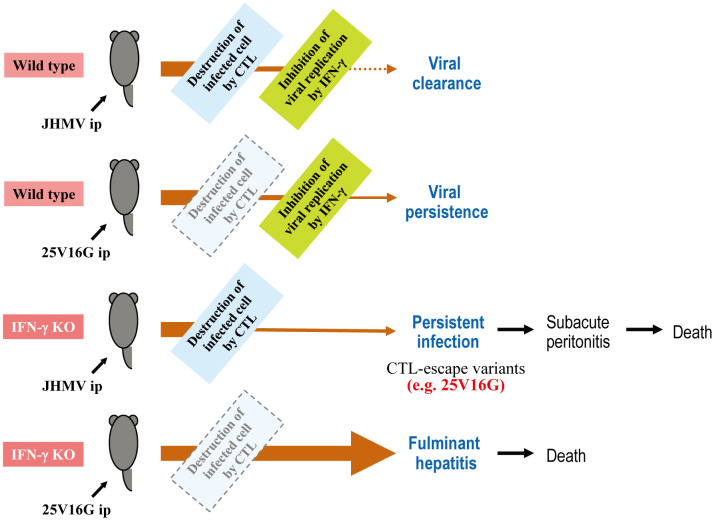
The roles of CTL and IFN-γ in JHMV and 25V16G infections in WT and GKO B6 mice are summarized in Fig. 2. WT B6 mice developed acute hepatitis after intraperitoneal JHMV infection, and the virus was cleared within a week by CTLs, IFN-γ, and other antiviral responses. By contrast, 25V16G was not cleared from the liver of WT B6 mice, probably due to its ability to escape CTL-mediated immune responses [5]. In GKO mice, which produce CTL- but not IFN-γ-mediated immune responses, JHMV infection was not cleared, resulting in subacute infection, highlighting that IFN-γ is essential for viral clearance. Furthermore, these findings suggest that CTL-escape variants could contribute to the establishment of persistent infections. In the absence of both CTL- and IFN-γ-mediated immune responses, mice are highly susceptible to the cytopathic effects of CoV, developing severe fulminant hepatitis.
Fig. 2.
Role of cytotoxic T lymphocyte (CTL) and interferon (IFN)-γ in murine coronavirus infection in C57BL/6 (B6) mice. The schema illustrates the events and outcomes of murine coronavirus strain JHM (JHMV) and 25V16G infections in wild-type and IFN-γ-deficient (IFN-γ KO) B6 mice. Light blue and yellow-green rectangles indicate CTL- and IFN-γ-mediated immune responses, respectively. The light blue rectangle with a dashed line indicates weak CTL response due to the lack of a dominant CTL epitope. The arrow thickness represents the approximate virus titer.
PERSPECTIVES
The elucidation of the pathogenesis of infectious diseases is imperative to develop vaccines against infectious agents. Animal models play crucial roles in the better understanding of the pathogenesis of infectious diseases [44, 46]. Murine CoV infections might provide useful information on the pathogenesis of human CoV infections including COVID-19, especially in the interaction between viral and host immune responses. In this review, we summarize the role of CTLs and IFN-γ in murine CoV infections in mice. Although different types of vaccines have been developed over the years [9], vaccines that induce virus-specific CTL responses are the most promising for viral infections [3, 19]. Future studies are required to elucidate the dynamics of anti-CoV CTL responses in vivo [13].
In addition, the better understanding of the evolutionary history of the CTL-escape JHMV variant 25V16G would guide the development of effective vaccines for CoV. Because RNA viruses often form quasispecies [8], a 25V16G-like virus might already be present within the original JHMV population. Considering the relevance of intrahost selection pressure in driving viral evolution [36], 25V16G could have emerged during a persistent JHMV infection in GKO mice. It is necessary to analyze SARS-CoV-2 evolution during the episode in each COVID-19 patient. Anyway, the administration of antiviral drugs should be considered during the acute phase of CoV infection to minimize the possibility of the emergence of immune-escape CoV variants.
Acknowledgments
This study was supported by a grant-in-aid for Scientific Research from the Ministry of Education, Culture, Science, Sports, and Technology of Japan.
REFERENCES
- 1.Agostini M. L., Andres E. L., Sims A. C., Graham R. L., Sheahan T. P., Lu X., Smith E. C., Case J. B., Feng J. Y., Jordan R., Ray A. S., Cihlar T., Siegel D., Mackman R. L., Clarke M. O., Baric R. S., Denison M. R.2018. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio 9: E00221–E18. doi: 10.1128/mBio.00221-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auladell M., Jia X., Hensen L., Chua B., Fox A., Nguyen T. H. O., Doherty P. C., Kedzierska K.2019. Recalling the future: Immunological memory toward unpredictable influenza viruses. Front. Immunol. 10: 1400. doi: 10.3389/fimmu.2019.01400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baruah V., Bose S.2020. Immunoinformatics-aided identification of T cell and B cell epitopes in the surface glycoprotein of 2019-nCoV. J. Med. Virol. 92: 495–500. doi: 10.1002/jmv.25698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancel-Tirado S. M., Evans R. B., Yoon K. J.2004. Monoclonal antibody analysis of porcine reproductive and respiratory syndrome virus epitopes associated with antibody-dependent enhancement and neutralization of virus infection. Vet. Immunol. Immunopathol. 102: 249–262. doi: 10.1016/j.vetimm.2004.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro R. F., Perlman S.1995. CD8+ T-cell epitopes within the surface glycoprotein of a neurotropic coronavirus and correlation with pathogenicity. J. Virol. 69: 8127–8131. doi: 10.1128/JVI.69.12.8127-8131.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng V. C., Wong S. C., Chuang V. W., So S. Y., Chen J. H., Sridhar S., To K. K., Chan J. F., Hung I. F., Ho P. L., Yuen K. Y.2020. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J. Infect. 81: 107–114. doi: 10.1016/j.jinf.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cong Y., Verlhac P., Reggiori F.2017. The interaction between Nidovirales and autophagy components. Viruses 9: E182. doi: 10.3390/v9070182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolan P. T., Whitfield Z. J., Andino R.2018. Mechanisms and concepts in RNA virus population dynamics and evolution. Annu. Rev. Virol. 5: 69–92. doi: 10.1146/annurev-virology-101416-041718 [DOI] [PubMed] [Google Scholar]
- 9.Enjuanes L., Zuñiga S., Castaño-Rodriguez C., Gutierrez-Alvarez J., Canton J., Sola I.2016. Molecular basis of coronavirus virulence and vaccine development. Adv. Virus Res. 96: 245–286. doi: 10.1016/bs.aivir.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forni D., Cagliani R., Clerici M., Sironi M.2017. Molecular evolution of human coronavirus genomes. Trends Microbiol. 25: 35–48. doi: 10.1016/j.tim.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frana M. F., Behnke J. N., Sturman L. S., Holmes K. V.1985. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J. Virol. 56: 912–920. doi: 10.1128/JVI.56.3.912-920.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.France M. P., Smith A. L., Stevenson R., Barthold S. W.1999. Granulomatous peritonitis and pleuritis in interferon-gamma gene knockout mice naturally infected with mouse hepatitis virus. Aust. Vet. J. 77: 600–604. doi: 10.1111/j.1751-0813.1999.tb13199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halle S., Halle O., Förster R.2017. Mechanisms and dynamics of T cell-mediated cytotoxicity in vivo. Trends Immunol. 38: 432–443. doi: 10.1016/j.it.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 14.Hirano N., Murakami T., Taguchi F., Fujiwara K., Matumoto M.1981. Comparison of mouse hepatitis virus strains for pathogenicity in weanling mice infected by various routes. Arch. Virol. 70: 69–73. doi: 10.1007/BF01320795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homberger F. R., Zhang L., Barthold S. W.1998. Prevalence of enterotropic and polytropic mouse hepatitis virus in enzootically infected mouse colonies. Lab. Anim. Sci. 48: 50–54. [PubMed] [Google Scholar]
- 16.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B.2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janice Oh H. L., Ken-En Gan S., Bertoletti A., Tan Y. J.2012. Understanding the T cell immune response in SARS coronavirus infection. Emerg. Microbes Infect. 1: e23. doi: 10.1038/emi.2012.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaume M., Yip M. S., Cheung C. Y., Leung H. L., Li P. H., Kien F., Dutry I., Callendret B., Escriou N., Altmeyer R., Nal B., Daëron M., Bruzzone R., Peiris J. S.2011. Anti-severe acute respiratory syndrome coronavirus spike antibodies trigger infection of human immune cells via a pH- and cysteine protease-independent FcγR pathway. J. Virol. 85: 10582–10597. doi: 10.1128/JVI.00671-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalita P., Padhi A. K., Zhang K. Y. J., Tripathi T.2020. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb. Pathog. 145: 104236. doi: 10.1016/j.micpath.2020.104236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krammer F., Palese P.2015. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov. 14: 167–182. doi: 10.1038/nrd4529 [DOI] [PubMed] [Google Scholar]
- 21.Kyuwa S., Machii K., Shibata S.1996. Role of CD4+ and CD8+ T cells in mouse hepatitis virus infection in mice. Exp. Anim. 45: 81–83. doi: 10.1538/expanim.45.81 [DOI] [PubMed] [Google Scholar]
- 22.Kyuwa S., Shibata S., Tagawa Y., Iwakura Y., Machii K., Urano T.2002. Acute hepatic failure in IFN-gamma-deficient BALB/c mice after murine coronavirus infection. Virus Res. 83: 169–177. doi: 10.1016/S0168-1702(01)00432-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyuwa S., Tagawa Y., Shibata S., Doi K., Machii K., Iwakura Y.1998. Murine coronavirus-induced subacute fatal peritonitis in C57BL/6 mice deficient in gamma interferon. J. Virol. 72: 9286–9290. doi: 10.1128/JVI.72.11.9286-9290.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyuwa S., Takagaki S., Matsuyama S., Taguchi F., Saegusa J., Iwakura Y., Tagawa Y., Yoshikawa Y.2010. Characterization of a variant virus from ascitic fluid of subacute granulomatous serositis in interferon-gamma-deficient C57BL/6 mice persistently infected with murine coronavirus strain JHM. Viral Immunol. 23: 437–442. doi: 10.1089/vim.2010.0008 [DOI] [PubMed] [Google Scholar]
- 25.Lai C. C., Shih T. P., Ko W. C., Tang H. J., Hsueh P. R.2020. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 55: 105924. doi: 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane T. E., Paoletti A. D., Buchmeier M. J.1997. Disassociation between the in vitro and in vivo effects of nitric oxide on a neurotropic murine coronavirus. J. Virol. 71: 2202–2210. doi: 10.1128/JVI.71.3.2202-2210.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee A. J., Ashkar A. A.2018. The dual nature of type I and type II interferons. Front. Immunol. 9: 2061. doi: 10.3389/fimmu.2018.02061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu G., Wang Q., Liu N., Xiao Y., Tong T., Liu S., Wu D.2012. Infectious bronchitis virus nucleoprotein specific CTL response is generated prior to serum IgG. Vet. Immunol. Immunopathol. 148: 353–358. doi: 10.1016/j.vetimm.2012.06.028 [DOI] [PubMed] [Google Scholar]
- 29.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W. J., Wang D., Xu W., Holmes E. C., Gao G. F., Wu G., Chen W., Shi W., Tan W.2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395: 565–574. doi: 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makino S., Keck J. G., Stohlman S. A., Lai M. M.1986. High-frequency RNA recombination of murine coronaviruses. J. Virol. 57: 729–737. doi: 10.1128/JVI.57.3.729-737.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKay S. L., Guo A., Pergam S. A., Dooling K.2019. Herpes zoster risk in immunocompromised adults in the United States: A systematic review. Clin. Infect. Dis https://doi:10.1093/cid/ciz1090. [DOI] [PMC free article] [PubMed]
- 32.Meng F., Ren Y., Suo S., Sun X., Li X., Li P., Yang W., Li G., Li L., Schwegmann-Wessels C., Herrler G., Ren X.2013. Evaluation on the efficacy and immunogenicity of recombinant DNA plasmids expressing spike genes from porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. PLoS One 8: e57468. doi: 10.1371/journal.pone.0057468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migueles S. A., Laborico A. C., Shupert W. L., Sabbaghian M. S., Rabin R., Hallahan C. W., Van Baarle D., Kostense S., Miedema F., McLaughlin M., Ehler L., Metcalf J., Liu S., Connors M.2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3: 1061–1068. doi: 10.1038/ni845 [DOI] [PubMed] [Google Scholar]
- 34.Nakanaga K., Yamanouchi K., Fujiwara K.1986. Protective effect of monoclonal antibodies on lethal mouse hepatitis virus infection in mice. J. Virol. 59: 168–171. doi: 10.1128/JVI.59.1.168-171.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogimi C., Englund J. A., Bradford M. C., Qin X., Boeckh M., Waghmare A.2019. Characteristics and outcomes of coronavirus infection in children: The role of viral factors and an immunocompromised state. J. Pediatric Infect. Dis. Soc. 8: 21–28. doi: 10.1093/jpids/pix093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parameswaran P., Wang C., Trivedi S. B., Eswarappa M., Montoya M., Balmaseda A., Harris E.2017. Intrahost selection pressures drive rapid dengue virus microevolution in acute human infections. Cell Host Microbe 22: 400–410.e5. doi: 10.1016/j.chom.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paules C. I., Marston H. D., Fauci A. S.2020. Coronavirus infections-more than just the common cold. JAMA 323: 707–708. doi: 10.1001/jama.2020.0757 [DOI] [PubMed] [Google Scholar]
- 38.Peng G., Sun D., Rajashankar K. R., Qian Z., Holmes K. V., Li F.2011. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc. Natl. Acad. Sci. USA 108: 10696–10701. doi: 10.1073/pnas.1104306108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pirofski L. A., Casadevall A.2020. Pathogenesis of COVID-19 from the perspective of the damage-response framework. MBio 11: e01175–e20. doi: 10.1128/mBio.01175-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F.2020. Structural basis of receptor recognition by SARS-CoV-2. Nature 581: 221–224. doi: 10.1038/s41586-020-2179-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schijns V. E., Wierda C. M., van Hoeij M., Horzinek M. C.1996. Exacerbated viral hepatitis in IFN-γ receptor-deficient mice is not suppressed by IL-12. J. Immunol. 157: 815–821. [PubMed] [Google Scholar]
- 42.Seo S. H., Collisson E. W.1997. Specific cytotoxic T lymphocytes are involved in in vivo clearance of infectious bronchitis virus. J. Virol. 71: 5173–5177. doi: 10.1128/JVI.71.7.5173-5177.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skinner D., Marro B. S., Lane T. E.2019. Chemokine CXCL10 and coronavirus-induced neurologic disease. Viral Immunol. 32: 25–37. doi: 10.1089/vim.2018.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutton T. C., Subbarao K.2015. Development of animal models against emerging coronaviruses: From SARS to MERS coronavirus. Virology 479–480: 247–258. doi: 10.1016/j.virol.2015.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takano T., Yamada S., Doki T., Hohdatsu T.2019. Pathogenesis of oral type I feline infectious peritonitis virus (FIPV) infection: Antibody-dependent enhancement infection of cats with type I FIPV via the oral route. J. Vet. Med. Sci. 81: 911–915. doi: 10.1292/jvms.18-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villano J. S., Ogden B. E.2017. Special issue: Infectious disease research: animal models and risk management. Comp. Med. 67: 189–191. [PMC free article] [PubMed] [Google Scholar]
- 47.Wan Y., Shang J., Graham R., Baric R. S., Li F.2020. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 94: E00127–E20. doi: 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss S. R., Leibowitz J. L.2011. Coronavirus pathogenesis. Adv. Virus Res. 81: 85–164. doi: 10.1016/B978-0-12-385885-6.00009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wijburg O. L., Heemskerk M. H., Boog C. J., Van Rooijen N.1997. Role of spleen macrophages in innate and acquired immune responses against mouse hepatitis virus strain A59. Immunology 92: 252–258. doi: 10.1046/j.1365-2567.1997.00340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi K., Goto N., Kyuwa S., Hayami M., Toyoda Y.1991. Protection of mice from a lethal coronavirus infection in the central nervous system by adoptive transfer of virus-specific T cell clones. J. Neuroimmunol. 32: 1–9. doi: 10.1016/0165-5728(91)90065-F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi K., Kyuwa S., Nakanaga K., Hayami M.1988. Establishment of cytotoxic T-cell clones specific for cells infected with mouse hepatitis virus. J. Virol. 62: 2505–2507. doi: 10.1128/JVI.62.7.2505-2507.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou X., Li Y., Li T., Zhang W.2020. Follow-up of asymptomatic patients with SARS-CoV-2 infection. Clin. Microbiol. Infect. 26: 957–959. doi: 10.1016/j.cmi.2020.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]