This randomized clinical trial compares the ability to prevent intubation in preterm infants by using nasal intermittent positive pressure ventilation for the initial treatment of respiratory distress syndrome or postextubation with either a cannula with long and narrow tubing or short binasal prong and mask interfaces.
Key Points
Question
Is a cannula with long and narrow tubing inferior to short binasal prongs and masks in preterm infants who require nasal intermittent positive pressure ventilation?
Findings
In this noninferiority randomized clinical trial that included 166 preterm infants at 24 weeks’ to 33 weeks and 6 days’ gestation requiring nasal intermittent positive pressure ventilation, intubation within 72 hours occurred in 14% in the group using a cannula with long and narrow tubing and in 18% in the short binasal prongs and masks group (95% CI within the noninferiority margin). Moderate to severe nasal trauma was significantly less common in the group using cannulas with long and narrow tubing.
Meaning
Cannulas with long and narrow tubing were noninferior to short binasal prongs and masks in providing nasal intermittent positive pressure ventilation for preterm infants, while causing significantly less nasal trauma.
Abstract
Importance
Use of cannulas with long and narrow tubing (CLNT) has gained increasing popularity for applying noninvasive respiratory support for newborn infants thanks to ease of use, perceived patient comfort, and reduced nasal trauma. However, there is concern that this interface delivers reduced and suboptimal support.
Objective
To determine whether CLNT is noninferior to short binasal prongs and masks (SPM) when providing nasal intermittent positive pressure ventilation (NIPPV) in preterm infants.
Design, Setting, and Participants
This randomized controlled, unblinded, prospective noninferiority trial was conducted between December 2017 and December 2019 at 2 tertiary neonatal intensive care units. Preterm infants born between 24 weeks’ and 33 weeks and 6 days’ gestation were eligible if presented with respiratory distress syndrome with the need for noninvasive ventilatory support either as initial treatment after birth or after first extubation. Analysis was performed by intention to treat.
Interventions
Randomization to NIPPV with either CLNT or SPM interface.
Main Outcomes and Measures
The primary outcome was the need for intubation within 72 hours after NIPPV treatment began. Noninferiority margin was defined as 15% or less absolute difference.
Results
Overall, 166 infants were included in this analysis, and infant characteristics and clinical condition (including fraction of inspired oxygen, Pco2, and pH level) were comparable at recruitment in the CLNT group (n = 83) and SPM group (n = 83). The mean (SD) gestational age was 29.3 (2.2) weeks vs 29.2 (2.5) weeks, and the mean (SD) birth weight was 1237 (414) g vs 1254 (448) g in the CLNT and SPM groups, respectively. Intubation within 72 hours occurred in 12 of 83 infants (14%) in the CLNT group and in 15 of 83 infants (18%) in the SPM group (risk difference, −3.6%; 95% CI, −14.8 to 7.6 [within the noninferiority margin], χ2 P = .53). Moderate to severe nasal trauma was significantly less common in the CLNT group compared with the SPM group (4 [5%] vs 14 [17%]; P = .01). There were no differences in other adverse events or in the course during hospitalization.
Conclusions and Relevance
In this study, CLNT was noninferior to SPM in providing NIPPV for preterm infants, while causing significantly less nasal trauma.
Trial Registration
ClinicalTrials.gov Identifier: NCT03081611
Introduction
In recent years, in an effort to avoid endotracheal intubation and invasive ventilation, there has been an increased use of noninvasive ventilation in very low-birth-weight preterm infants as the initial respiratory support after birth or postextubation.1,2,3,4,5 Different interfaces are available for applying noninvasive ventilation.6 The most commonly used interfaces to provide nasal intermittent positive pressure ventilation (NIPPV) are the standard short binasal prongs and masks (SPMs). Unfortunately, SPMs are occasionally associated with discomfort and pressure-related nasal injury.7,8 The RAM cannula (Neotech) is made of softer material with a long and narrow tubing (ie, CLNT) for transmitting the pressure to thin-walled prongs. This results in a perceived ease of use, comfort, and less nasal trauma.9 However, there is concern that this long thin interface delivers reduced and suboptimal pressure transmission, tidal volume, and support compared with SPM, especially when leak at the nose is more than minimal.10,11,12,13,14,15,16 Despite its widespread use,17 the clinical efficacy of CLNT was not thoroughly studied. To adopt this method for clinical practice in preterm infants, it has to be proven noninferior to binasal prongs and mask interfaces.
The aim of our study was to compare the ability to prevent intubation in preterm infants by using NIPPV for the initial treatment of respiratory distress syndrome (RDS) or postextubation with either CLNT or SPM interfaces. In this noninferiority trial, we evaluated the statistical null hypothesis that using CLNT would be inferior to SPM in preterm infants who require NIPPV and would result in a higher rate of endotracheal ventilation. Therefore, we hypothesized that using CLNT would be noninferior to SPM.
Methods
Design
This randomized prospective, unblinded, controlled, noninferiority dual-center study was conducted in the tertiary neonatal intensive care units of Rambam and Bnai-Zion Medical Centers in Haifa, Israel, between December 1, 2017, and December 1, 2019. This trial was approved by the local ethics committee that monitored data collection and adverse events. The parents of all participating infants provided written informed consent. If antepartum consent was not sought, parents were approached within 4 hours after NIPPV was initiated. Prior to the study, both interfaces were routinely used in both centers, with preference for CLNT in Rambam and for SPM in Bnai-Zion. The trial protocol and statistical analysis plan are available in Supplement 1.
Participants
The study population included preterm infants born between 24 weeks’ and 33 weeks and 6 days’ gestational age as assessed by the obstetrical team. Inclusion criteria were the need for noninvasive ventilatory support for initial treatment or after the first extubation after birth. The need for ventilatory support was assessed by the attending clinician based on clinical signs of RDS (tachypnea, apneic episodes, grunting, and retractions), need for oxygen enrichment for keeping saturation more than 90%, and/or Pco2 level of 60 mm Hg or less.
Excluded were infants with significant morbidity apart from RDS including cardiac disease (not including patent ductus arteriosus), congenital malformation, or if they had cardiovascular or respiratory instability because of sepsis, anemia, or severe intraventricular hemorrhage. Intubation and endotracheal ventilation prior to noninvasive ventilation excluded infants from the initial treatment group. No recruitment for initial therapy did not exclude infants from inclusion for the postextubation arm.
The study population was subdivided into 2 groups. The initial treatment group included infants who needed noninvasive ventilation during the first 7 days of life without prior endotracheal ventilation. The postextubation group included infants who needed noninvasive ventilation during the first 28 days of life after any period of endotracheal ventilation in the neonatal intensive care unit.
Randomization
Prerandomization stratification was done by groups (initial treatment and postextubation) and by birth weight (<1250 g and ≥1250 g) in each study center separately. The randomization sequence was computer generated with block size of 4 and kept in consecutively numbered, opaque envelopes.
Study Intervention
Eligible infants were randomly assigned to NIPPV with either CNLT (RAM [Neotech]) or short NCPAP/NIPPV binasal prongs (INCA Nasal Cannula [CooperSurgical] or EasyFlow prongs [Fritz Stephan GmbH]). The study protocol allowed later use of EasyFlow mask (Fritz Stephan GmbH) or alternating between short prongs and masks in cases of nasal trauma or for prevention of nasal trauma per unit protocol, and both were referred to as SPM for the study purposes. No crossover between CLNT and SPM interfaces was allowed.
CLNT prongs size was selected to fill approximately 80% of nares. The short NCPAP/NIPPV prongs size was selected per the manufacturer’s instructions to fill close to 100% of nares without causing local pressure. If used, the mask size was chosen per the manufacturer’s instructions. Mouth occlusion was allowed with a pacifier or passively. Although debatable,18 we did not use chin straps, according to our routine practice. A nasal barrier dressing was used for protection to all infants.
NIPPV was administered using Leoni (Heinen und Löwenstein) or SLE 5000 (SLE) ventilators on synchronized intermittent mandatory ventilation mode. Initial NIPPV settings were peak inspiratory pressure (PIP) of 14 to 18 cm H2O (according to chest excursion), positive end-expiratory pressure (PEEP) of 6 cm H2O, respiratory rate of 10 to 30 breaths per minute, and inspiratory time of 0.30 to 0.35 seconds. Saturation targets were 90% to 94%. Maximal settings allowed were PIP of 24 cm H2O, PEEP of 8 cm H2O, and respiratory rate of 40 breaths per minute for both interfaces. Weaning from NIPPV to no support or to low flow (ie, ≤2 liters per minute) was considered if there was clinical improvement and the infant was receiving a fraction of inspired oxygen of 0.3 or lower, PIP of 16 cm H2O or less, PEEP of 6 cm H2O or less, and respiratory rate of 20 breaths per minute or less.
Caffeine was given to all infants younger than 32 weeks during the first day of life and to symptomatic infants 32 weeks or older with apnea of prematurity. Exogenous surfactant (200 mg/kg for the first dose and 100 mg/kg for the second dose; 1 to 2 doses as needed), poractant alfa (Chiesi Farmaceutici), was given as rescue therapy. Criteria for surfactant administration on noninvasive ventilation was ongoing fraction of inspired oxygen requirements of 35% or more to 45%. Surfactant administration technique was chosen according to the attending physician discretion.
Outcomes
The primary outcome was treatment failure within 72 hours after initiation of NIPPV, ie, the need for endotracheal ventilation. Infants underwent intubation and mechanical ventilation if they met the following criteria: clinical deterioration (increased respiratory distress) accompanied by at least 1 of the following or worsening of the following: a pH level less than 7.20 and Pco2 level more than 60 mm Hg, oxygen saturation as measured by pulse oximetry less than 90% on fraction of inspired oxygen more than 50%, recurrent significant apneas requiring repeated stimulation or bag-and-mask ventilation despite the use of caffeine and excluding technical problems, and despite allowing the above maximal settings. Moderate to severe nasal trauma19 within 72 hours, resulting in required change of interface, was also considered as failure.
Surfactant administration via the intubation-surfactant-extubation (INSURE) technique, which is done in our units with extubation immediately after surfactant administration, or the minimally invasive surfactant therapy (MIST) technique via a thin catheter, were both not considered a failure of the NIPPV treatment if not immediately followed by endotracheal ventilation.20
Nasal trauma was considered as a secondary outcome. Each infant receiving noninvasive ventilation was assessed routinely for nasal trauma every 4 hours by the nursing team. In case of suspected trauma, the principal investigator on the site graded the nasal injury. The injury was graded, using images, as mild, persistent erythema; moderate, superficial ulceration; and severe, necrosis.19
Other secondary outcomes included treatment failure between 72 hours and 7 days after initiation of NIPPV, reasons for treatment failure, need for surfactant, complications of prematurity, length of invasive and noninvasive ventilation, time to full feeds, and length of hospital stay.
Statistical Analysis
Based on our units’ previous data, we estimated that treatment failure (intubation within 72 hours from NIPPV initiation) would be 18% using SPM. We prespecified an absolute noninferiority margin for CLNT of 15% above the failure rate for SPM. This was considered clinically significant and based on previous studies.20,21,22 Using CLNT was considered noninferior to using SPM if the upper limit of the 2-sided 95% CI was less than 15% and the lower limit of the 95% CI was below 0.23
Using a significance level of 5% with 80% power, a sample of 164 infants was required. The calculation was done by power calculator for binary outcome noninferiority trial.24,25
For comparing the binomial proportions in failure rate for noninferiority based on the score test of Farrington and Manning, we used SAS statistical software version 9.4 (SAS Institute) with a noninferiority margin of 0.15. We also compared failure rates using χ2 analysis.
χ2 Test was used to compare dichotomous variables. For continuous variables, we used t test and Mann-Whitney U test for normal and abnormal distribution, respectively. The 95% CI was calculated for secondary outcomes. We used Hodges-Lehmann test for calculating the 95% CIs for medians’ difference. We used Shapiro-Wilks test to assess normal distribution of the results. Analyses were performed using SPSS for Windows version 25 (SPSS Inc). A 2-sided P value of .05 was considered statistically significant. Analysis was performed by intention to treat.
Results
Study Patients
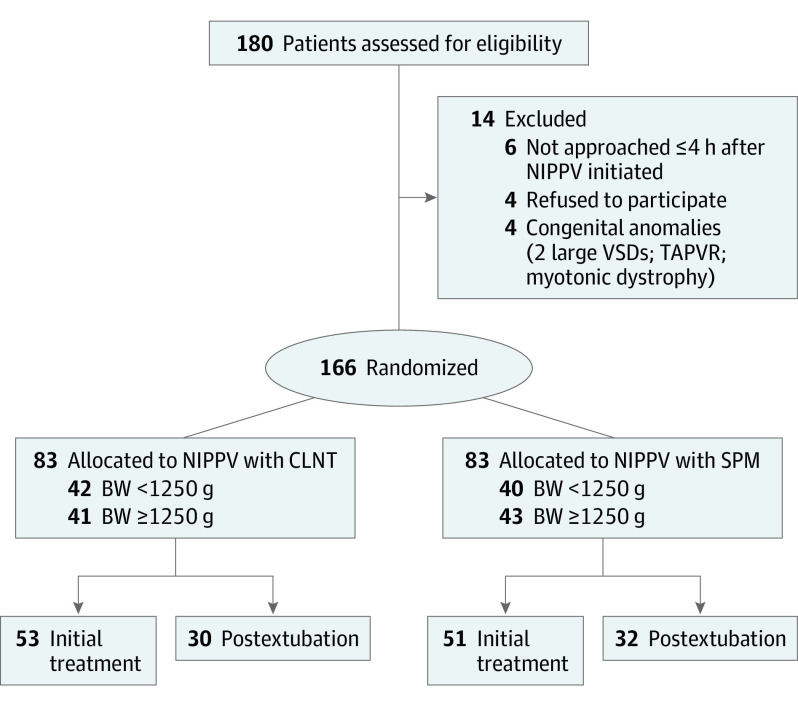
Of 180 infants who needed noninvasive ventilation support during the study period, 14 infants were excluded (Figure 1). In total, 166 infants were randomly assigned to the treatment groups (83 [50%] to CLNT and 83 [50%] to SPM). In the SPM group, infants were started with and spent most of the time with short prongs. There was no crossover between the groups. One infant died in each group, which resulted from late-onset gram-negative sepsis. Baseline demographic and clinical characteristics were comparable between the 2 groups (Table 1).
Figure 1. The CONSORT Flow Diagram.
BW indicates birth weight; CLNT, cannula with long and narrow tubing; NIPPV, nasal intermittent positive pressure ventilation; SPM, short binasal prongs and masks; TAPVR, total anomalous pulmonary venous return; VSD, ventricular septal defect.
Table 1. Characteristics of the Study Groups.
| Characteristic | Long and narrow tubing (n = 83) | Short binasal prong and mask (n = 83) |
|---|---|---|
| Gestational age, mean (SD) [range], wk:d | 29:3 (2:2) [24:2-33:2] | 29:2 (2:5) [24:1-33:6] |
| Birth weight, g | ||
| All, mean (SD) [range] | 1237 (414) [530-2305] | 1254 (448) [500-2490] |
| <1250 | ||
| No. (%) | 42 (51) | 40 (48) |
| Mean (SD) | 918 (220) | 940 (217) |
| ≥1250 | ||
| No. (%) | 41 (49) | 43 (51) |
| Mean (SD) | 1563 (294) | 1539 (390) |
| NIPPV | ||
| As initial treatment | 53 (64) | 51 (61) |
| Start at time from admission, median (range), h | 0 (0-7.5) | 0 (0-4.5) |
| After first extubation | 30 (36) | 32 (39) |
| Start at time from admission, median (range), h | 88 (2-612) | 56 (4-582) |
| Male, No. (%) | 46 (55.4) | 49 (59.0) |
| Prenatal steroids, No. (%) | 68 (82) | 67 (81) |
| Chorioamnionitis (clinical or histological), No. (%) | 3 (4) | 7 (8) |
| Maternal, No. (%) | ||
| Hypertension or preeclampsia | 17 (21) | 22 (267) |
| Diabetes | 7 (8) | 12 (14) |
| Cesarean delivery, No. (%) | 64 (77) | 66 (79) |
| 5-min Apgar score, median (range) | 8 (1-10) | 9 (3-10) |
| Caffeine received in the first 24 h of life, No. (%) | 76 (92) | 75 (90) |
| Before NIPPV initiation, median (range) | ||
| Fio2 | 30 (21-60) | 29 (21-57) |
| co2 | 53 (44-85) | 50 (41-94) |
| pH level | 7.26 (7.00-7.36) | 7.29 (6.92-7.34) |
Abbreviations: Fio2, fraction of inspired oxygen; NIPPV, nasal intermittent positive pressure ventilation.
Primary Outcome
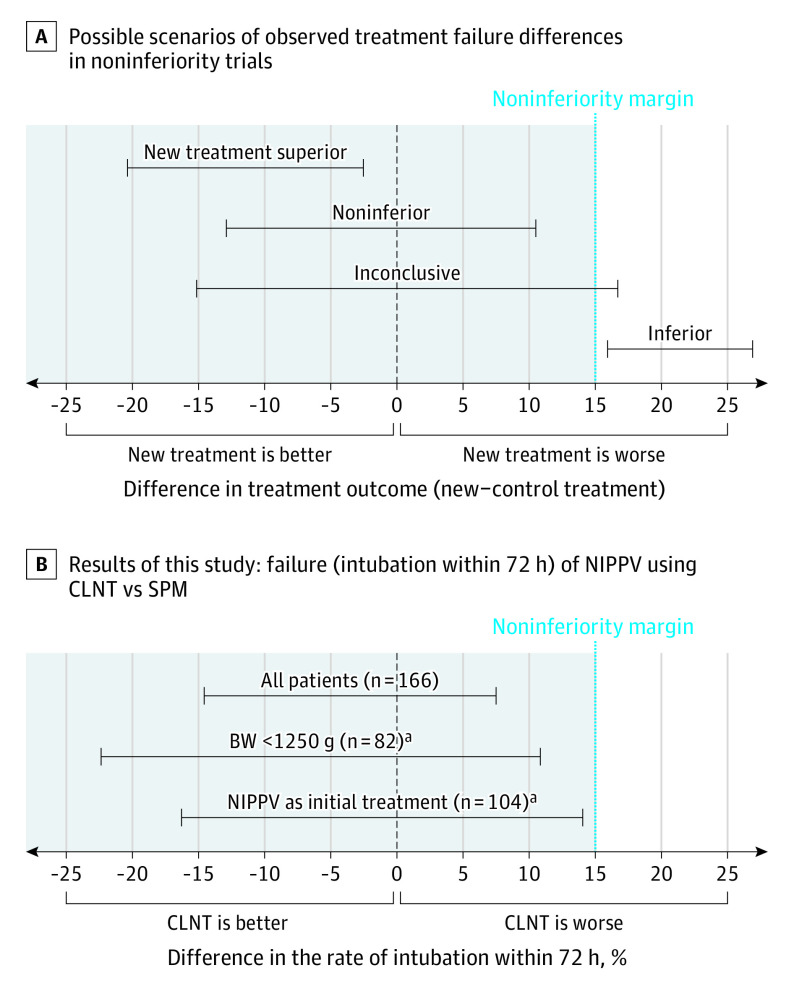
The use of CLNT was noninferior to SPM with regard to the primary outcome. Endotracheal ventilation within 72 hours from randomization occurred in 12 infants (14%) in the group using CLNT and 15 (18%) in the SPM group (risk difference, −3.6%; 95% CI, −14.8 to 7.6; χ2 P = .53) (Table 2). Because the upper limit of the 95% CI was below the noninferiority margin, the noninferiority of CLNT to SPM was confirmed (Figure 2).
Table 2. Primary Outcome: Intubation Within 72 Hours.
| Outcome | Total No. | No./total No. (%) | 95% CI of risk difference | P valuea | |
|---|---|---|---|---|---|
| Long and narrow tubing | Short binasal prong and mask | ||||
| Total study group | 166 | 12/83 (14) | 15/83 (18) | −14.8 to 7.6b | .53 |
| Birth weight, g | |||||
| <1250 | 82 | 7/42 (17) | 9/40 (23) | −22.9 to 11.3b | .51 |
| ≥1250 | 84 | 5/41 (12) | 6/43 (14) | −16.2 to 12.6b | .81 |
| Initial NIPPV group | 104 | 10/53 (19) | 10/51 (20) | −15.9 to 14.4b | .92 |
| Postextubation group | 62 | 2/30 (7) | 5/32 (16) | −24.3 to 6.5b | .43c |
Abbreviation: NIPPV, nasal intermittent positive pressure ventilation.
χ2 For dichotomous parameters and t test and Mann-Whitney test for continuous parameters.
The 95% CI does not include the noninferiority margin (of 15%).
Analysis using Fisher exact test.
Figure 2. Noninferiority Trial Results: Possible Scenarios and Results .
A, Possible scenarios of results for a noninferiority trial according to Piaggio et al.23 B, Results of this study concerning the primary outcome, intubation within 72 hours ( = failure). Error bars indicate 95% CI of the risk difference between using cannulas with long and narrow tubing (CLNT) and short binasal prongs and masks (SPM). BW indicates birth weight; NIPPV, nasal intermittent positive pressure ventilation.
aThe study was underpowered to establish noninferiority in this subgroup.
A subgroup analysis by birth weight (<1250 g or ≥1250 g) and by using NIPPV as initial or after first extubation treatment, although underpowered because of sample size, demonstrated noninferiority of CLNT when compared with SPM (Table 2 and Figure 2). A multivariable regression analysis for the stratified variables revealed that treatment failure with CLNT compared with SPM had an odds ratio of 0.74 (95% CI, 0.32-1.70). There was also no difference in the primary outcome between centers.
Secondary Outcomes and Adverse Events
Infants who failed CLNT and SPM were comparable in characteristics and reasons for failure. The median (range) time from randomization to intubation was similar in both groups (12 [1-54] hours and 5 [1-38] hours, respectively; P = .10). There was no failure related to nasal trauma within 72 hours.
Moderate and severe nasal trauma were significantly less common in the group using CLNT compared with the SPM group (4 [5%] vs 14 [17%]; 95% CI, 0.02-0.22; P = .01). There were only 2 severe nasal injury cases, both in the SPM group.
There were no statistically significant differences in the NIPPV parameters (pressures, rate, and fraction of inspired oxygen) that were applied while using CLNT or SPM. (Table 3). Overall surfactant administration rate was similar in both groups (Table 3). There was also no difference between the 2 groups in surfactant administration using INSURE (7 [8%] vs 7 [8%]; 95% CI, −0.09 to 0.09; P > .99) or by MIST (5 [6%] vs 3 [4%]; 95% CI, −0.05 to 0.10; P = .47), respectively. There was no difference between the groups in any of the parameters of the neonatal course or complications (Table 3).
Table 3. Secondary Outcomes and Adverse Events.
| Outcome | No. (%) | 95% CI of risk difference | |
|---|---|---|---|
| Long and narrow tubing (n = 83) | Short binasal prongs and mask (n = 83) | ||
| Intubation after 72 h to 7 d, No./total No. (%) | 4/83 (5) | 2/83 (2) | −4.2 to 9.5 |
| Nasal traumaa | |||
| All | 13 (16) | 24 (29) | 0.5 to 25.0 |
| Moderate to severe | 4 (5) | 14 (17) | 2.5 to 22.0 |
| Maximal NIPPV settings, median (range) | |||
| PIP | 19 (12-24) | 18 (14-22) | 0 to 2.0 |
| PEEP | 6 (5-8) | 6 (5-7) | 0 |
| Fio2 | 30 (24-100) | 30 (22-100) | 0 to 5.0 |
| Respiratory rate | 30 (10-40) | 25 (8-45) | 0 to 5.0 |
| Surfactant therapy | |||
| Total (including MIST/INSURE) | 39 (47) | 38 (46) | −14.0 to 16.0 |
| 2 Doses | 16 (19) | 12 (14) | −6.0 to 16.0 |
| MIST or INSURE | 12 (14) | 10 (12) | −8.0 to 13.0 |
| Ventilatory support, median (range), d | |||
| High-frequency ventilation | 3 (0-40) | 3 (0-43) | −1.0 to 1.0 |
| Conventional ventilation | 3 (0-61) | 2 (0-56) | 0 to 2.0 |
| Noninvasive ventilation (NIPPV) | 5 (1-101) | 4 (1-57) | 0 to 3.0 |
| Fio2, ≥21% | 5 (1-260) | 8 (1-133) | −5.0 to 3.0 |
| Complications of prematurity | |||
| Pneumothorax | 3 (4) | 2 (2) | −5.0 to 8.0 |
| Late-onset sepsis (culture proven) | 5 (6) | 4 (5) | −6.5 to 9.0 |
| Bronchopulmonary dysplasiab | 14 (17) | 13 (16) | −10.0 to 12.5 |
| Intraventricular hemorrhage grade | |||
| ≥1 | 7 (8) | 8 (10) | −8.0 to 10.5 |
| ≥3 | 1 (1) | 1 (1) | −5.4 to 5.4 |
| Necrotizing enterocolitis (stage ≥2) | 2 (2) | 4 (5) | −4.2 to 9.5 |
| Treated patent ductus arteriosus | 10 (12) | 9 (11) | −8.9 to 11.3 |
| Death | 1 (1) | 1 (1) | −5.4 to 5.4 |
| Time to 140 mL/kg/d enteral feeding, median (range), d | 9 (4-45) | 8 (4-33) | 0 to 2.0 |
| Time to discharge, median (range), d | 59 (14-262) | 64 (16-152) | −10.0 to 7.0 |
Abbreviations: INSURE, intubation-surfactant-extubation; Fio2, fraction of inspired oxygen; MIST, minimally invasive surfactant therapy; NIPPV, nasal intermittent positive pressure ventilation; PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure.
Nasal trauma grades: mild, persistent erythema; moderate, superficial ulceration; and severe, necrosis.
Fio2 ≥21% or the need for continuous positive airway pressure or NIPPV at 36 weeks’ gestational age.
Discussion
Our study found that CLNT was noninferior to SPMs in delivering NIPPV to preterm infants born at 24 weeks’ to 33 weeks and 6 days’ gestation, when defining the primary outcome as the need for endotracheal ventilation within 72 hours of noninvasive respiratory support initiation. CLNT was associated with less nasal trauma.
The most important factor in noninvasive ventilation of premature infants is supporting the functional residual capacity with continuous positive airway pressure (CPAP). This might be the reason for the advantage of NCPAP over high-flow nasal cannula in these infants.21,22 While both NCPAP and NIPPV support the functional residual capacity with NCPAP, different studies showed NIPPV to be either as good as or superior to NCPAP.26,27 Thus, we have assessed in our study the NIPPV as our preferred mode and evaluated 2 interfaces.
The findings of our study did not confirm our null hypothesis, which was based mostly on bench studies showing reduced and suboptimal pressure delivered by CLNT.10,12,13,14,15,16 However, we tried to maximize the efficacy of the noninvasive respiratory treatment by using NIPPV with cannula that provides maximal nares occlusion (complete/near complete occlusion or around 80% in SPM and CLNT, respectively) and by allowing ventilatory pressures as described above. Our rates of success were within the reported ranges by other studies.21,28,29 Overall, studies suggest that using CLNT would be potentially more efficacious once lower leak around the cannula and higher ventilatory set pressures are used.6,10,12,13,15,17,30 Gerdes et al12 demonstrated that with 60% to 80% nares occlusion, delivered airway pressures were around 60% of the set CPAP levels. With 100% occlusion, the airway pressure were within 0.5 cm H2O of the set CPAP levels. Furthermore, allowing higher pressure, especially with the high resistance interface of CLNT,16 can improve the noninvasive efficacy. Using CLNT, Claassen et al31 showed that failure of noninvasive ventilation after birth decreased from 45% to 24% as the maximum PEEP used increased from 5.8 to 8.0 cm H2O without differences in rates of pneumothorax. In our study, CLNT was found to be noninferior despite using ventilator pressures, which were not significantly higher compared with the pressures used with SPM. The actual maximal PIP in this study was 22 cm H2O in infants who weighed less than 1250 g. Despite using these pressures and minimizing the nares leak to approximately 20% with CLNT, we did not observe higher rates of air leaks.
While we are not sure that CLNT delivered the intended peak pressures or swing pressures of the NIPPV, it is possible that by the level of occlusion we used and the pressures’ limits we allowed, we achieved functional residual capacity support similar to SPM.12 This could explain the noninferiority of CLNT.32
We have used the synchronized intermittent mandatory ventilation mode for NIPPV. However, we cannot verify that the infants received pressure-synchronized ventilation because the system was open and we used only the pressure sensor of the ventilator. Pressure triggering ventilation is less effective compared with airflow triggering in premature infants receiving ventilation.33 Thus, we used the term NIPPV to describe our mode of nasal support and not synchronized NIPPV. However, this applies to both arms of our study, using different interfaces.14,34
We found only 1 published randomized clinical trial that compared NIPPV using CLNT and short nasal prongs for the initial treatment of RDS.11 Gokce et al11 assessed 126 patients (62 used short prongs and 64 used CLNT) born at 26 weeks’ to 33 weeks and 6 days’ gestational age. In their study, CLNT compared with short prongs was associated with a higher need for invasive ventilation within 72 hours (32.8% vs 9.6%; P = .002). Patients’ characteristics (gestational age and birth weight) were similar, and criteria for intubation were mildly more strict in our study.11 However, Gokce et al11 used CLNT size based on birth weight only and not according to the cannula-to-nares ratio (ie, nares occlusion), hence potentially allowing higher leak and thus less efficacy in delivering pressures. Allowing higher maximal ventilation parameters, potentially more attention to CLNT size-to-nares fit we used, and higher rates of antenatal steroids (82% vs 57%) may explain the difference between the studies, resulting in more efficacious ventilation using CLNT in our study.11 Furthermore, in our study, we used NIPPV for initial treatment of RDS as well as for postextubation and demonstrated noninferiority in both groups as opposed to only initial treatment by Gokce et al.11
Not only did we find a comparable success rate of CLNT and SPM, but we found no differences in the reasons for failure of the 2 interfaces. This was different from the study on a lung model by Mukerji and Belik6 that reported that co2 clearance was lower in CLNT. It is possible that the infants in our randomized clinical trial could compensate and correct the Pco2 and the lung model was not representing the actual clinical condition.
All participating infants had nasal barrier dressing for nasal protection during NIPPV. Moderate and severe nasal trauma12 were significantly less common in the group using CLNT. The incidence of nasal trauma in our study in the SPM group was similar or even lower compared with previous publications.7,8,35,36,37,38,39 Lower incidence of nasal trauma using CLNT is not surprising and was described previously.9,17
We, like others,20 considered surfactant administration with INSURE or MIST, not followed by endotracheal ventilation, as part of the initial care, and not as failure of noninvasive ventilation. This has become part of the acute care of RDS in recent years, lowering the fraction of inspired oxygen threshold for surfactant and allowing infants to enjoy both surfactant and noninvasive ventilation, while assuming more benefit than harm and trying to prevent long-term respiratory and neurologic sequelae. Nevertheless, we used the same criteria and found the same incidence for INSURE and MIST in both groups (approximately 13%). If considering INSURE or MIST a failure of the initial care, then the rate of failure was still comparable in the CLNT and SPM groups (17 of 53 [32%] and 17 of 51 [33%]), respectively).
Limitations and Strengths
Our study had some limitations. It was a dual-center rather than a multicenter randomized clinical trial. Yet, this allowed to decrease the variability and concentrate on centers experienced with noninvasive ventilation. Despite showing no difference, the study was not powered to statistically establish noninferiority in the subgroups, specifically in the groups of infants with birth weight less than 1250 g, which usually needs more respiratory support or as a mode of support for the initial stages of RDS. Yet, 50% of our infants weighed less than 1250 g, and in 62% of the infants, the NIPPV was used as initial support. In both subcategories, there was not even a trend suggesting an inferiority of CLNT. The strengths of our study was its design, no crossover between groups, and 100% participants’ follow-up. Blinding was not possible in this kind of study. We used objective failure criteria and management protocols to reduce the possibility of a bias.
Conclusions
In conclusion, CLNT was noninferior to SPM in providing NIPPV for preterm infants born between 24 weeks’ and 33 weeks and 6 days’ gestation. CLNT resulted in significantly less nasal trauma compared with SPM. Yet, we have to be cautious because of our study limitations. Further larger studies are needed to establish the noninferiority and possible advantage of performing initial NIPPV with CLNT in the group of infants with lower gestational age and more severe RDS.
Trial protocol and statistical analysis plan
Data sharing statement
References
- 1.Bhandari V. Noninvasive respiratory support in the preterm infant. Clin Perinatol. 2012;39(3):497-511. doi: 10.1016/j.clp.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 2.Kugelman A, Borenstein-Levin L, Jubran H, et al. Less is more: modern neonatology. Rambam Maimonides Med J. 2018;9(3). doi: 10.5041/RMMJ.10344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poets CF, Lorenz L. Prevention of bronchopulmonary dysplasia in extremely low gestational age neonates: current evidence. Arch Dis Child Fetal Neonatal Ed. 2018;103(3):F285-F291. doi: 10.1136/archdischild-2017-314264 [DOI] [PubMed] [Google Scholar]
- 4.Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome: 2019 update. Neonatology. 2019;115(4):432-450. doi: 10.1159/000499361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeVan JM, Brion LP, Wrage LA, et al. ; Eunice Kennedy Shriver NICHD Neonatal Research Network . Change in practice after the surfactant, positive pressure and oxygenation randomised trial. Arch Dis Child Fetal Neonatal Ed. 2014;99(5):F386-F390. doi: 10.1136/archdischild-2014-306057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukerji A, Belik J. Neonatal nasal intermittent positive pressure ventilation efficacy and lung pressure transmission. J Perinatol. 2015;35(9):716-719. doi: 10.1038/jp.2015.61 [DOI] [PubMed] [Google Scholar]
- 7.Imbulana DI, Manley BJ, Dawson JA, Davis PG, Owen LS. Nasal injury in preterm infants receiving non-invasive respiratory support: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2018;103(1):F29-F35. doi: 10.1136/archdischild-2017-313418 [DOI] [PubMed] [Google Scholar]
- 8.Bashir T, Murki S, Kiran S, Reddy VK, Oleti TP. ‘Nasal mask’ in comparison with ‘nasal prongs’ or ‘rotation of nasal mask with nasal prongs’ reduce the incidence of nasal injury in preterm neonates supported on nasal continuous positive airway pressure (nCPAP): a randomized controlled trial. PLoS One. 2019;14(1):e0211476. doi: 10.1371/journal.pone.0211476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nzegwu NI, Mack T, DellaVentura R, et al. Systematic use of the RAM nasal cannula in the Yale-New Haven Children’s Hospital Neonatal Intensive Care Unit: a quality improvement project. J Matern Fetal Neonatal Med. 2015;28(6):718-721. doi: 10.3109/14767058.2014.929659 [DOI] [PubMed] [Google Scholar]
- 10.Bailes SA, Firestone KS, Dunn DK, McNinch NL, Brown MF, Volsko TA. Evaluating the effect of flow and interface type on pressures delivered with bubble CPAP in a simulated model. Respir Care. 2016;61(3):333-339. doi: 10.4187/respcare.04251 [DOI] [PubMed] [Google Scholar]
- 11.Gokce IK, Kaya H, Ozdemir R. A randomized trial comparing the short binasal prong to the RAM cannula for noninvasive ventilation support of preterm infants with respiratory distress syndrome. J Matern Fetal Neonatal Med. 2019;(August):1-7. doi: 10.1080/14767058.2019.1651268 [DOI] [PubMed] [Google Scholar]
- 12.Gerdes JS, Sivieri EM, Abbasi S. Factors influencing delivered mean airway pressure during nasal CPAP with the RAM cannula. Pediatr Pulmonol. 2016;51(1):60-69. doi: 10.1002/ppul.23197 [DOI] [PubMed] [Google Scholar]
- 13.Iyer NP, Chatburn R. Evaluation of a nasal cannula in noninvasive ventilation using a lung simulator. Respir Care. 2015;60(4):508-512. doi: 10.4187/respcare.03560 [DOI] [PubMed] [Google Scholar]
- 14.Matlock DN, Bai S, Weisner MD, et al. Tidal volume transmission during non-synchronized nasal intermittent positive pressure ventilation via RAM cannula. J Perinatol. 2019;39(5):723-729. doi: 10.1038/s41372-019-0333-x [DOI] [PubMed] [Google Scholar]
- 15.Singh N, McNally MJ, Darnall RA. Does the RAM cannula provide continuous positive airway pressure as effectively as the Hudson prongs in preterm neonates? Am J Perinatol. 2019;36(8):849-854. doi: 10.1055/s-0038-1675330 [DOI] [PubMed] [Google Scholar]
- 16.Green EA, Dawson JA, Davis PG, De Paoli AG, Roberts CT. Assessment of resistance of nasal continuous positive airway pressure interfaces. Arch Dis Child Fetal Neonatal Ed. 2019;104:F535-F539. doi: 10.1136/archdischild-2018-315838 [DOI] [PubMed] [Google Scholar]
- 17.Drescher GS, Hughes CW. Comparison of interfaces for the delivery of noninvasive respiratory support to low birthweight infants. Respir Care. 2018;63(10):1197-1206. doi: 10.4187/respcare.05978 [DOI] [PubMed] [Google Scholar]
- 18.MacDonald KD, Davies M, Lam R, et al. Chinstraps are needed for neonatal nasal CPAP: reflections from a non-human primate model. Pediatr Pulmonol. 2020;55(5):1087-1088. doi: 10.1002/ppul.24716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer C, Bertelle V, Hohlfeld J, Forcada-Guex M, Stadelmann-Diaw C, Tolsa JF. Nasal trauma due to continuous positive airway pressure in neonates. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F447-F451. doi: 10.1136/adc.2009.179416 [DOI] [PubMed] [Google Scholar]
- 20.Lavizzari A, Colnaghi M, Ciuffini F, et al. Heated, humidified high-flow nasal cannula vs nasal continuous positive airway pressure for respiratory distress syndrome of prematurity: a randomized clinical noninferiority trial. JAMA Pediatr. Published online August 8, 2016. doi: 10.1001/jamapediatrics.2016.1243 [DOI] [PubMed] [Google Scholar]
- 21.Roberts CT, Owen LS, Manley BJ, et al. ; HIPSTER Trial Investigators . Nasal high-flow therapy for primary respiratory support in preterm infants. N Engl J Med. 2016;375(12):1142-1151. doi: 10.1056/NEJMoa1603694 [DOI] [PubMed] [Google Scholar]
- 22.Manley BJ, Owen LS, Doyle LW, et al. High-flow nasal cannulae in very preterm infants after extubation. N Engl J Med. 2013;369(15):1425-1433. doi: 10.1056/NEJMoa1300071 [DOI] [PubMed] [Google Scholar]
- 23.Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594-2604. doi: 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 24.Sealed Envelope Power (sample size) calculators. Accessed October 24, 2017. https://www.sealedenvelope.com/power/binary-noninferior/
- 25.Blackwelder WC. “Proving the null hypothesis” in clinical trials. Control Clin Trials. 1982;3(4):345-353. doi: 10.1016/0197-2456(82)90024-1 [DOI] [PubMed] [Google Scholar]
- 26.Lemyre B, Laughon M, Bose C, Davis PG. Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants. Cochrane Database Syst Rev. 2016;12:CD005384. doi: 10.1002/14651858.CD005384.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemyre B, Davis PG, De Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst Rev. 2017;2:CD003212. doi: 10.1002/14651858.CD003212.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirpalani H, Millar D, Lemyre B, Yoder BA, Chiu A, Roberts RS; NIPPV Study Group . A trial comparing noninvasive ventilation strategies in preterm infants. N Engl J Med. 2013;369(7):611-620. doi: 10.1056/NEJMoa1214533 [DOI] [PubMed] [Google Scholar]
- 29.Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B, Bader D. Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: a randomized, controlled, prospective study. J Pediatr. 2007;150(5):521-526, 526.e1. doi: 10.1016/j.jpeds.2007.01.032 [DOI] [PubMed] [Google Scholar]
- 30.Claassen CC, Hillman NH, Brown K, Williams HL, Strand ML. Comparison of bubble CPAP devices using RAM cannula for extubation failure in very low birth weight infants: randomized and cohort studies. Neonatology. 2019;115(1):28-35. doi: 10.1159/000493156 [DOI] [PubMed] [Google Scholar]
- 31.Claassen C, Strand M, Williams H, Hillman N Is 8 the new 5 when using a RAM Cannula for Bubble CPAP? 6 years of RAM Cannula experience (board 447). Abstract presented at: Pediatric Academies Societies Meeting; April 28, 2019; Baltimore, Maryland. Publication no. 2852.447. [Google Scholar]
- 32.Gupta A, Rodriguez L, Hallinan S, Szynal A, Keszler M CPAP vs. unsynchronized NIPPV at equal mean airway pressure (MAP) (board 442). Abstract presented at: Pediatric Academies Societies Meeting; April 28, 2019; Baltimore, Maryland. Publication no. 2851.442. [Google Scholar]
- 33.Dimitriou G, Greenough A, Cherian S. Comparison of airway pressure and airflow triggering systems using a single type of neonatal ventilator. Acta Paediatr. 2001;90(4):445-447. doi: 10.1080/080352501750126393 [DOI] [PubMed] [Google Scholar]
- 34.Owen LS, Morley CJ, Dawson JA, Davis PG. Effects of non-synchronised nasal intermittent positive pressure ventilation on spontaneous breathing in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2011;96(6):F422-F428. doi: 10.1136/adc.2010.205195 [DOI] [PubMed] [Google Scholar]
- 35.Sharma D, Kaur A, Farahbakhsh N, Agarwal S. To compare nasal mask with binasal prongs in delivering continuous positive airway pressure for reducing need of invasive ventilation: randomized controlled trial. J Matern Fetal Neonatal Med. 2019;(August):1-7. doi: 10.1080/14767058.2019.1651272 [DOI] [PubMed] [Google Scholar]
- 36.Newnam KM, McGrath JM, Salyer J, Estes T, Jallo N, Bass WT. A comparative effectiveness study of continuous positive airway pressure-related skin breakdown when using different nasal interfaces in the extremely low birth weight neonate. Appl Nurs Res. 2015;28(1):36-41. doi: 10.1016/j.apnr.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 37.Jasani B, Ismail A, Rao S, Patole S. Effectiveness and safety of nasal mask versus binasal prongs for providing continuous positive airway pressure in preterm infants: a systematic review and meta-analysis. Pediatr Pulmonol. 2018;53(7):987-992. doi: 10.1002/ppul.24014 [DOI] [PubMed] [Google Scholar]
- 38.Chandrasekaran A, Thukral A, Jeeva Sankar M, Agarwal R, Paul VK, Deorari AK. Nasal masks or binasal prongs for delivering continuous positive airway pressure in preterm neonates-a randomised trial. Eur J Pediatr. 2017;176(3):379-386. doi: 10.1007/s00431-017-2851-x [DOI] [PubMed] [Google Scholar]
- 39.Fjaeldstad A, Cipliene R, Ramsgaard-Jensen T, Ebbesen F. [Septum necrosis following CPAP treatment of preterm infant]. Ugeskr Laeger. 2014;176(11):V12120735. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
Data sharing statement