Abstract
In this study, transforming growth factor-β1 treatment effectively induced epithelial-mesenchymal transition (EMT) of SMMC-7721 cells, and the expression and function of microRNAs (miRNAs) were determined to understand the processes involved in liver cancer metastasis. Nanoparticle tracking analysis and western blotting were performed to identify exosomes. Transwell and MTS assays were used to assess cell migration and proliferation, respectively. Immunofluorescence microscopy was used to identify the metastasis of exosomes in cells. High-throughput sequencing was used to identify mRNAs and miRNAs in cells and exosomes, respectively. The identified differentially expressed miRNAs (DEmis) were further confirmed using quantitative real-time polymerase chain reaction. An miRNA-target mRNA interaction network was constructed using Cytoscape_V2_8_3. SPSS version 16.0 software with one-way analysis of variance was used for statistical analysis. P < 0.05 was considered statistically significant. The overall size of exosomes in EMT SMMC-7721 cells was smaller than that in normal SMMC-7721 cells. Exosomes of EMT SMMC-7721 cells could promote cell migration and invasion in several cell lines. We identified differentially expressed mRNAs (DEms) and DEmis. Among them, a total of 60 and 78 DEms were upregulated and downregulated, respectively, in EMT SMMC-7721 cells compared with those in SMMC-7721 cells. A total of 709 and 123 DEmis were upregulated and downregulated, respectively, in exosomes in EMT SMMC-7721 cells compared with those in SMMC-7721 cells. hsa-miR-24-3p and hsa-miR-21-5p were further selected for knockdown experiments. Exosomes in cells with hsa-miR-24-3p knockdown could effectively inhibit EMT. hsa-miR-24-3p may be one of the most important molecular markers for EMT in liver cancer, which provides novel clues for the mechanisms involved in liver cancer metastasis.
Keywords: EMT, liver cancer, SMMC-7721, differentially expressed miRNAs, knockdown
Introduction
Primary liver cancer is the fifth most common cancer and is one of the three major malignant tumors that cause tumor-related deaths worldwide. The characteristics of liver cancer include a high recurrence rate, easy metastasis, and an increasing incidence. Currently, effective clinical treatment methods are limited. Therefore, liver cancer remains one of the major diseases threatening human health [1]. The main causes of death in patients with primary liver cancer are invasion and metastasis. The most common site of distant metastases is the lung, which is associated with a high mortality rate and poor prognosis [2]. Although the invasion and metastasis of liver cancer have been previously studied in many clinical and basic studies, the detailed mechanisms of lung metastasis in primary liver cancer remain unclear. Furthermore, there is still a lack of effective prevention and treatment options for the lung metastasis of liver cancer [3]. Therefore, an in-depth study of the specific mechanisms involved in liver metastasis is essential to optimize clinical prevention and improve the survival rate of patients.
Epithelial-mesenchymal transition (EMT) refers to the conversion of polarized epithelial cells to mesenchymal cells under normal physiological and specific pathological conditions [4]. EMT is generally thought to occur at the initial stage of tumor metastasis, allowing tumor cells to migrate and invade other tissues. During EMT, cells lose their typical intercellular connections and cell polarity. At the same time, their morphology changes from the typical cobblestone pattern to a fusiform fibroblast-like shape. Therefore, cell migration and invasion capabilities are enhanced [5]. In the tumor microenvironment, the exchange of information between cells depends on the transmission of the media. The types of media are diverse and include growth factors, cytokines, and chemokines [6]. In addition to the abovementioned media, exosomes have become a major research hotspot in recent years. Exosomes are formed by multivesicular bodies in cells and are released into the extracellular space. Exosomes can carry various substances, including nucleic acids, proteins, and lipids, and participate in the transfer of biological information in the microenvironment [7]. The highest level of mature microRNA (miRNA) has been detected in various exosomes (~41.72%) [8]. Recent studies suggest that mature miRNAs in exosomes play various functional and regulatory roles in recipient cells [9]. miRNAs have been shown to be primarily involved in the post-transcriptional regulation of gene expression [10]. A study has confirmed that miRNAs in exosomes can regulate tumor resistance, metastasis, and immune responses [11]. One ovarian cancer study demonstrated that tumor-associated adipocytes and fibroblasts in the tumor microenvironment produced exosomes containing large amounts of miR-21, which were taken up by tumor cells. miR-21 could inhibit tumor cell apoptosis by targeting apoptosis-related enzyme activators. Thus, miR-21 in exosomes was shown to enhance the resistance of ovarian cancer cells to paclitaxel chemotherapy [12]. In breast cancer, exosomes containing miR-200 produced by highly metastatic tumor cells can enhance the metastatic ability of low-metastatic tumor cells. Additionally, the expression levels of miR-24 were reported to be dysregulated in various types of cancers [13-15]; for example, miR-24 was found to promote breast cancer metastasis [16]. Therefore, identifying the important functions and roles of miRNAs in exosomes in the tumor microenvironment would be helpful to better understand liver metastasis and the associated molecular mechanisms.
In this study, we generated EMT liver cancer cells in vitro. The overall size of exosomes in EMT liver cancer cells was smaller than that in normal liver cancer cells. Following functional analyses and next-generation sequencing, the cell functions and transcript environment were found to differ between EMT SMMC-7721 and normal SMMC-7721 cells. Moreover, we identified differentially expressed miRNAs (DEmis) between the two different groups and studied their functional roles in several cell lines. In particular, hsa-miR-24-3p in exosomes activated fibroblasts to become tumor-associated fibroblasts, promoted epithelialization, and enhanced the tumor growth of liver cancer cells. This evidence is helpful to further understand the process of liver cancer metastasis.
Material and methods
Cell treatments
SMMC-7721, 97L, Hep3B, HepG2, and Huh7 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). All cells were cultured in RPMI 1640 medium containing 10% (v/v) fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA). Cell lines were maintained in a humidified chamber at 5% CO2 and 37°C. SMMC-7721 cells were divided into two subgroups: SMMC-7721 cells treated with transforming growth factor-β1 (TGF-β1; 10 ng/ml) and SMMC-7721 cells without TGF-β1 treatment. pEZ-Lv301 vector was used to generate a knockdown system of hsa-miR-24-3p and hsa-miR-21-5p in SMMC-7721 cells. Endo Fectin-LentiTM and Titer BoostTM reagents (FulenGen, Guangzhou, China) were used to generate lentiviral particles according to the manufacturer’s instructions. Subsequently, the lentiviral transfer vector was added and co-transfected into SMMC-7721 cells with the Lenti-PacTM HIV packaging mix (FulenGen). The supernatant was harvested 48 h after transfection, clarified, and stored at -80°C for further analysis. The sequences of knockdown-treated double small interfering RNAs (siRNAs) are as follows: hsa-miR-24-3p, 5’-CUGUUCCUGCUGAACUGAGCCA-3’ and 5’-UGGCUCAGUUCAGCAGGAACAG-3’; hsa-miR-21-5p, 5’-UCAACAUCAGUCUGAUAAGCUA-3’ and 5’-UAGCUUAUCAGACUGAUGUUGA-3’; and normal control (NC), 5’-CAGUACUUUUGUGUAGUACAA-3’ and 5’-UUGUACUACACAAAAGUACUG-3’. Double-stranded siRNAs were purchased from ReiBo Biotech (China). Cells (5 × 105) were plated in six-well plates containing Dulbecco’s modified Eagle’s medium with 10% FBS and incubated overnight. Lipofectamine 2000 reagent was used to perform transfections (final siRNA concentration, 80 nM; Thermo Fisher Scientific, Waltham, MA, USA).
Exosome extraction
Exosome extraction was performed using the Total Exosome Isolation Reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. Isolated exosomes were stored at 4°C for further analysis. Nanoparticle tracking analysis (NTA) of exosomes was conducted as previously described [17], and the morphology of exosomes was scanned using a scanning electron microscope (SEM).
Animal and tissue collection
Four- to six-week-old male BALB/c nude mice were purchased from Nanjing Biomedical Research Institute of Nanjing University. One injection point was taken from each nude mouse, and 2 × 106 previously treated SMMC-7721 cells (0.5 ml) were inoculated subcutaneously by intraperitoneal injection. After 5 days, clear tumor formation was noted. After 4 weeks, the tumors were collected, and their weight and volume were measured. Mice were euthanized by rapid cervical dislocation after anesthesia by isofluorane inhalation.
Quantitative real-time polymerase chain reaction (qPCR)
Total RNA of exosomes was isolated using a MiniBEST Universal RNA Extraction Kit (Takara, Shiga, Japan). A PrimeScriptTM II Strand cDNA Synthesis Kit (Takara) was used to synthesize cDNA. The primer sequences of five selected miRNAs are as follows: hsa-miR-99b-5p-F, 5’-CACCCGTAGAACCGACCT-3’; hsa-miR-99b-5p-R, 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCGCAAG-3’; hsa-miR-21-5p-F, 5’-GCCGTAGCTTATCAGACTGA-3’; hsa-miR-21-5p-R, 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACA-3’; hsa-miR-23a-3p-F, 5’-AGATCACATTGCCAGGGAT-3’; hsa-miR-23a-3p-R, 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGGAAAT-3’; hsa-miR-24-3p-F, 5’-TGGCTCAGTTCAGCAGGA-3’; hsa-miR-24-3p-R, 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTGTTC-3’; hsa-miR-100-5p-F, 5’-GAACCCGTAGATCCGAACT-3’; and hsa-miR-100-5p-R, 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCACAAG-3’. The 20-µl qPCR reaction mixture contained 10 µl of 2 × SsoAdvancedTM Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA), 1 µl of primers (5 pmol each), 1 µl of DNA template (~20 ng), and 8 µl of double-distilled water. The PCR amplification conditions were as follows: 94°C for 3 min, 40 cycles of 95°C for 15 s, and 60°C for 25 s. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the reference gene [18]. The 2-ΔΔCt method was used to calculate the relative expression of each miRNA.
Western blotting (WB)
Total protein was extracted from exosomes and cells using a homemade cell lysis buffer [phenylmethylsulfonyl fluoride/RIPA cell lysis buffer, 1:120 (v/v)]. The protein was precipitated using 5 × Protein Loading Buffer (ABM, Vancouver, Canada). The mixture was heated at 100°C in a boiling water bath for 5 min and transferred onto a polyvinylidene difluoride membrane (Millipore, Burlington, MA, USA). After blocking for 1 h at room temperature, the membrane was incubated with rabbit polyclonal anti-mouse CD63 (1:1000; Abcam, Cambridge, MA, USA; ab134045), tumor susceptibility gene 101 (TSG101; 1:1000; Abcam; ab125011), HspA8 (1:1000; Cell Signaling Technology, Danvers, MA, USA; 8444), Alix (1:1000; Cell Signaling Technology; 2171), E-cadherin (1:500; Abcam; ab15148), N-cadherin (1:300; Abcam; ab18203), vimentin (1:10000; Abcam; ab24525), β-catenin (1:4000; Abcam; ab6302), and GAPDH (1:2500; Abcam; ab9485) primary antibodies for 15 h. The membranes were then incubated with the appropriate secondary antibodies (1:1500 dilution; Abcam) for 1 h at room temperature. Protein bands were observed using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
Immunofluorescence microscopy
Nuclei were stained with 4’,6-diamidino-2-phenylindole. Each slide was washed with three times with 500 μl of phosphate-buffered saline. Subsequently, exosomes were labeled with PKH67 dye for 24 h (Invitrogen) following the manufacturer’s protocol. Images were obtained using a confocal microscope. Zeiss LCM image browser software was used to produce a composite multicolor image.
Cell migration, invasion, and proliferation
The Transwell Permeable Support Kit (Corning, Corning, NY, USA) was used to assess cell migration. Cells (1 × 106/ml; 100 µl/chamber) were transferred to the upper chamber of each Transwell and were allowed to migrate for 24 h at 37°C. Cells that migrated to the bottom side of the membrane were fixed in methanol and stained with hematoxylin. Cell invasion was analyzed using the Cultrex 24-well BME Cell Invasion Assay kit (Trevigen, Gaithersburg, MD, USA). Briefly, 1 × 103 cells were seeded in the upper wells that were previously coated with a Matrigel basement extract. Growth medium (500 µl) was added to the lower wells. After incubation for 24 h at 37°C, the noninvasive cells on the upper surface were removed, and the cells that migrated to the lower surface were fixed with 500 µl of Cell Dissociation Solution/Calcein-AM (Thermo Fisher Scientific). Cell quantification was performed by fluorimetric analysis with an excitation wavelength of 485 nm and an emission wavelength of 520 nm.
SMMC-7721 cells were divided into three groups: one control group and two groups treated with exosomes (10 µg/ml) of either SMMC-7721 or EMT SMMC-7721 cells. After 24 h, the solution was removed, and MTS solution was added (10 μl/chamber). The absorbance values at 490 nm (at 24, 48, and 72 h) were determined using a microplate reader.
Cell sequencing
Total RNA of cells was extracted using Trizol reagent according to the manufacturer’s instructions. The purity and concentration were determined using NanoDrop ND1000 (Thermo Fisher Scientific). To prepare the RNA-seq library, ribosomal RNA was separated from total RNA using the Epicentre Ribo-zeroTM rRNA Removal Kit (Epicentre, Madison, WI, USA). Then, a library was constructed using the RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s instructions. After quantification of the library of each group with the Agilent 2100 Bioanalyzer System (Agilent Technologies, Santa Clara, CA, USA), RNA-seq was performed using the HiSeq 2500 platform (Illumina, San Diego, CA, USA).
Exosome sequencing
Total RNA of exosomes was extracted using the MiniBEST Universal RNA Extraction Kit. NanoDrop ND1000 was used to measure the quality and quantity of RNA. Reverse transcription and library preparation were performed using the QIAseq miRNA Library Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. The final library product was assessed using an Agilent 2100 Bioanalyzer System. An Illumina HiSeq 2500 platform was used to sequence the library with a 150-bp paired-end sequencing strategy.
Bioinformatic analysis
Raw reads in the FASTQ format were first processed using in-house Perl scripts to prepare clean reads by deleting reads containing poly-N and adapters as well as low-quality reads. The expected number of reads per kilobase per million mapped reads of both mRNAs and miRNAs in each sample followed Cuffdiff’s statistical analyses. The mRNA reads were mapped to the hg19 genome using TopHat version 2.1.0 (https://ccb.jhu.edu/software/tophat/index.shtml). For miRNA analysis, low-quality reads were removed, and reads with “adapter only” or unclipped reads were discarded. The remaining clean reads with a length between 15 and 30 nucleotides were mapped to hg19 using SOAP version 2.0. miRNAs were identified and annotated using the National Center for Biotechnology Information (NCBI) Refseq data and NCBI GenBank data (http://www.ncbi.nih.gov). The miRNA candidate target mRNAs were predicted using the miRTarBase database (https://bio.tools/mirtarbase). Genes or transcripts with P < 0.05 and fold change ≥ 2 were defined as mRNAs or miRNAs that were differentially expressed between the two groups.
An unsupervised clustering heatmap and Volcano plot were constructed using the R package (https://cran.r-project.org/web/packages/pheatmap/) to obtain an overview of the expression profiles of mRNAs and miRNAs. Gene Ontology (GO) enrichment analysis was performed using the Gene Ontology Consortium website (http://www.geneontology.org) to identify the genetic regulatory networks of the target mRNAs of DEms and DEmis by forming hierarchical categories according to the molecular function, biological process, and cellular component aspects. Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.jp/kegg/) pathway analysis was performed to explore the significant pathways of the target mRNAs of DEms and DEmis. The miRNA-target mRNA interaction network was constructed using Cytoscape_V2_8_3. SPSS version 16.0 with one-way analysis of variance was used for statistical analysis. P < 0.05 was considered statistically significant.
Statistical analysis
SPSS version 16.0 software (IBM, Armonk, NY, USA) was used in this study. Differences between two or more groups were analyzed using the Student’s t-test. P < 0.05 was considered statistically significant.
Results
EMT model construction
To study EMT in hepatocellular carcinoma cell lines, we treated SMMC-7721 cells with TGF-β1 (10 ng/ml) for 21 days. Cell morphology was observed under an optical microscope. Typical morphological changes (high cell length-to-width ratio and shuttle formation) associated with SMMC-7721 cell in EMT could be observed after TGF-β1 treatment (Figure S1A). To further confirm the effects of EMT, changes in the protein expression of EMT-associated factors (vimentin, β-catenin, E-cadherin, and N-cadherin) were analyzed by WB. The expression of N-cadherin, β-catenin, and vimentin in the TGF-β1-treated group was significantly higher than that in the NC group (Figure S1B). In addition, the expression of E-cadherin in the TGF-β1-treated group was significantly lower than that in the NC group. These results indicated that TGF-β1 treatment for 21 days induced typical EMT of SMMC-7721 cells.
Exosomes in SMMC-7721 and EMT SMMC-7721 cells
To investigate the characteristics of exosomes in SMMC-7721 and EMT SMMC-7721 cells, exosomes were isolated from the culture supernatants (1 ml) of approximately 4 × 106 SMMC-7721 and EMT SMMC-7721 cells using a commercial separation kit. NTA of exosomes in both groups suggested that the size of exosomes mainly ranged from 60 to 80 nm (Figure 1A). The overall size of exosomes in EMT SMMC-7721 cells appeared to be smaller than that in SMMC-7721 cells (Figure 1A). The expression levels of exosome markers (CD63, TSG101, HspA8, and Alix) were validated using WB analysis. The results indicated that these four exosome biomarkers were more abundant in the exosomes of SMMC-7721 and EMT SMMC-7721 cells than in SMMC-7721 and EMT SMMC-7721 cells (Figure 1B). In addition, we labeled exosomes with a PKH67 dye. SMMC-7721 cells were incubated with EMT SMMC-7721 exosomes for 24 h. The results showed that PKH67-labeled exosomes were localized in the cytoplasm of SMMC-7721 cells, which indicated that EMT SMMC-7721 cell-derived exosomes could be internalized by SMMC-7721 cells (Figure S2). In summary, these results demonstrated that the exosomes obtained in this study were reliable.
Figure 1.

Isolation and identification of exosomes of SMMC-7721 cells with or without TGF-β1 treatment. A. Overall size analysis of exosomes in SMMC-7721 cells with or without TGF-β1 treatment (left) and SEM analysis of the morphology of exosomes (right). B. WB analysis of exosome biomarkers (CD63, TSG101, HspA8, and Alix) in SMMC-7721 cells with or without TGF-β1 treatment.
Cell function
To further investigate the effects of exosomes isolated from SMMC-7721 and EMT SMMC-7721 cells on cell behavior, five cell lines (SMMC-7721, 97L, Hep3B, HepG2, and Huh7) were selected for functional analysis. Transwell chambers were used to assess the in vitro migration ability of these cell lines with different treatments (Figures 2B, S3). The results indicated that exosomes of SMMC-7721 and EMT SMMC-7721 cells could consistently promote cell migration and invasion of the five selected cell lines compared with that in the NC groups. The result of MTS analysis (Figure 2A) in SMMC-7721 cells showed that the proliferation of SMMC-7721 cells increased over time, that exosomes of SMMC-7721 and EMT SMMC-7721 cells consistently promoted cell proliferation in SMMC-7721 cells compared with that in the NC groups (P < 0.01), and that exosomes of EMT SMMC-7721 cells promoted SMMC-7721 cell proliferation compared with that in SMMC-7721 cells at 48 and 72 h (P < 0.0001). In summary, these results suggest that exosomes in both groups contain certain biomaterial that is closely related to cell function, especially in the exosomes of EMT SMMC-7721 cells.
Figure 2.
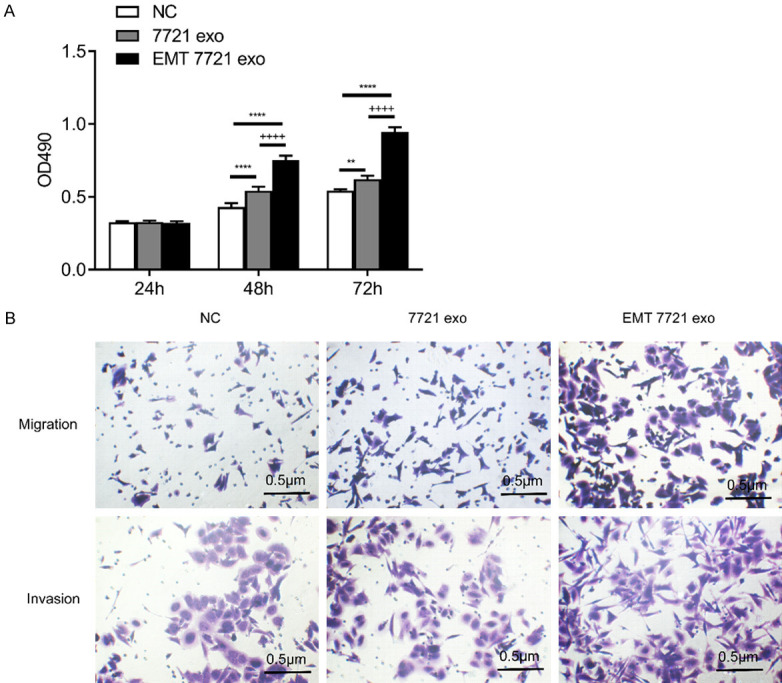
Effects of exosomes isolated from EMT SMMC-7721 and SMMC-7721 cells on SMMC-7721 cell proliferation, migration, and invasion. A. MTS analysis of proliferation. B. Transwell analysis of migration and invasion. All figures are magnified 200 ×. NC represents SMMC-7721 cell, 7721 exo represents exosomes of SMMC-7721 cells, EMT 7721 exo represents exosomes of EMT SMMC-7721 cells, * represents vs. NC, + represents vs. 7721 exo, **; P < 0. 01, ****; P < 0.0001, ++++; P < 0.0001.
DEms in SMMC-7721 and EMT SMMC-7721 cells
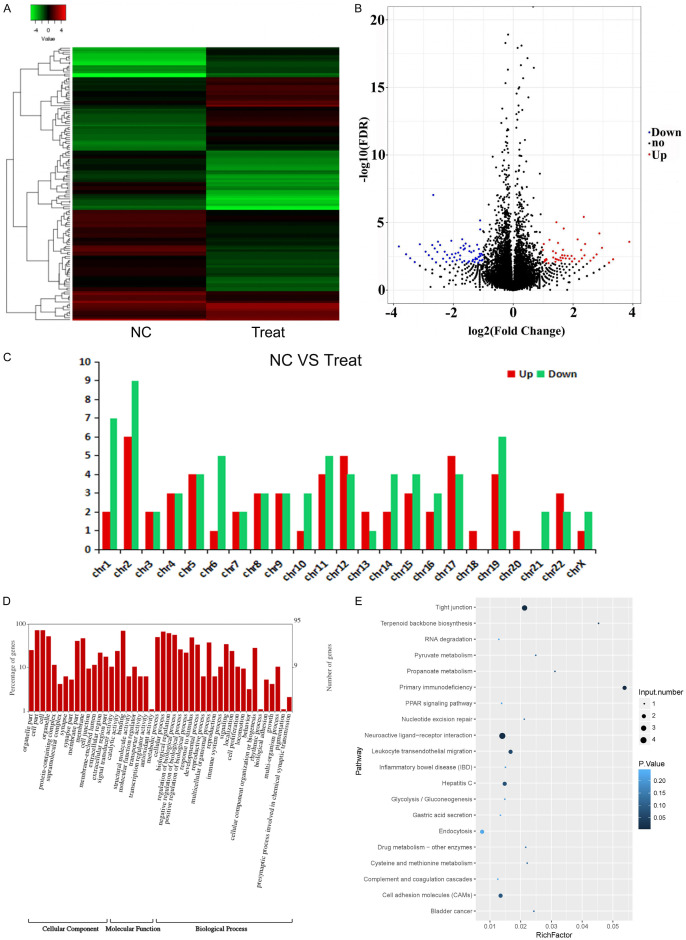
Next-generation sequencing was used to identify DEms between EMT SMMC-7721 and SMMC-7721 cells. A total of 60 DEms were upregulated in EMT SMMC-7721 cells compared with that in SMMC-7721 cells, whereas a total of 78 DEms were downregulated in EMT SMMC-7721 cells compared with that in SMMC-7721 cells (Figure 3A, 3B). The upregulated and downregulated DEms were both enriched in chromosome 2 (Figure 3C). Furthermore, the functions of these DEms were determined from GO analysis (Figure 3D). The three most enriched items of cellular component were cell, cell part, and organelle. The most enriched item of molecular function was binding. The most enriched item of biological process was cellular process. KEGG analysis revealed that the most enriched item was neuroactive ligand-receptor interactions (Figure 3E).
Figure 3.
DEms in SMMC-7721 cells treated with EMT SMMC-7721 (Treat) and SMMC-7721 cells (NC). A. Heatmap of DEms between EMT SMMC-7721 and SMMC-7721 cells. B. Scatter plot of DEms between EMT SMMC-7721 and SMMC-7721 cells. C. Number of DEms in different chromosomes. D. GO analysis of DEms. E. KEGG analysis of DEms.
DEmis in exosomes
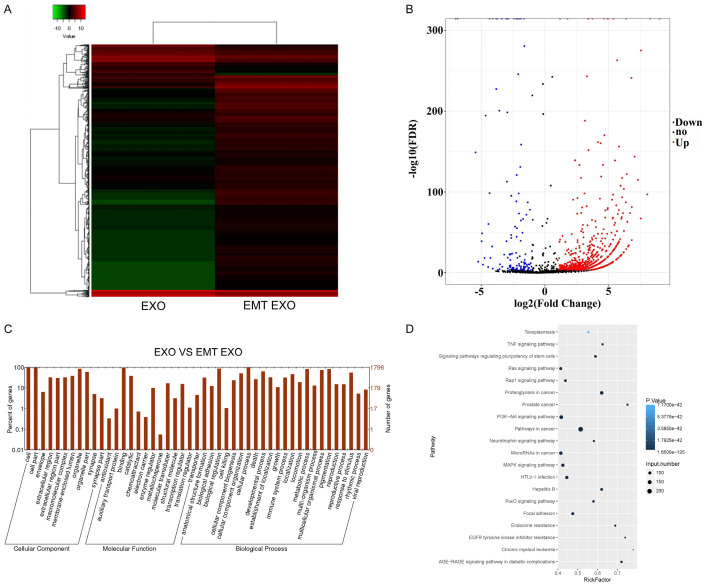
Next-generation sequencing was also used to identify DEmis between exosomes of EMT SMMC-7721 and SMMC-7721 cells. A total of 709 DEmis were upregulated in the exosomes of EMT SMMC-7721 cells compared with that in the exosomes of SMMC-7721 cells, whereas 123 DEmis were downregulated in the exosomes of EMT SMMC-7721 cells compared with that in the exosomes of SMMC-7721 cells (Figure 4A, 4B). Furthermore, the functions of these DEmis were determined using GO analysis (Figure 4C). The three most enriched items of cellular component were cell, cell part, and organelle. The most enriched items of molecular function were binding and catalytic. The most enriched items of biological process were cellular process, biological regulation, metabolic process, and pigmentation. KEGG analysis revealed that the most enriched items were pathways related to cancer and proteoglycans in cancer (Figure 4D).
Figure 4.
DEmis and their functions in the exosomes of EMT SMMC-7721 cells. A. Heatmap of DEmis between the exosomes of EMT SMMC-7721 and SMMC-7721 cells. B. Scatter plot of DEmis between the exosomes of EMT SMMC-7721 and SMMC-7721 cells. C. GO analysis of DEmis. D. KEGG analysis of DEmis. EXO represents exosomes of SMMC-7721 cells. EMT EXO represents exosomes of EMT SMMC-7721 cells.
Moreover, based on the significant differences and upregulated expression determined by sequencing, five DEmis were selected (Table 1) to reconstruct the network of miRNA-mRNA interactions. The five miRNAs were shown to play key roles in this network (Figure 5A). For instance, hsa-miR-21-5p could regulate AKT2 and EGFR, which are closely related to cancer. In addition, these five DEmis were also selected to confirm their expression levels in the exosomes of both groups by qPCR (Figure 5B). As shown in Figure 5B, hsa-miR-100-5p expression was significantly lower in the exosomes of EMT SMMC-7721 cells than in the exosomes of SMMC-7721 cells (P < 0.05). This result was inconsistent with that of next-generation sequencing. We speculated that this might be due to a technical error. The expressions of the other four DEmis were significantly higher in the exosomes of EMT SMMC-7721 cells than in the exosomes of SMMC-7721 cells (P < 0.05), which was consistent with the result of next-generation sequencing.
Table 1.
Expression of five miRNAs in SMMC-7721 and EMT SMMC-7721 exosomes
| geneID | 7721 exosome-Expression | EMT7721 exosome-Expression | 7721 exosome-RPKM | EMT7721 exosome-RPKM | log2 Ratio (EMT7721 exosome/7721 exosome) | Up-Down-Regulation (EMT7721 exosome/7721 exosome) |
|---|---|---|---|---|---|---|
| hsa-miR-99b-5p | 7505 | 52259 | 678.1158769 | 5735.364389 | 3.080281421 | Up |
| hsa-miR-21-5p | 38622 | 228576 | 3489.699054 | 25085.9498 | 2.845705031 | Up |
| hsa-miR-23a-3p | 5330 | 27557 | 481.5932877 | 3024.348657 | 2.650737278 | Up |
| hsa-miR-24-3p | 6044 | 25244 | 546.1069101 | 2770.499601 | 2.342890842 | Up |
| hsa-miR-100-5p | 7153 | 29652 | 646.3108418 | 3254.272467 | 2.332034949 | Up |
Figure 5.
Analysis of selected miRNA-target genes and verification of miRNA expression. A. miRNA-gene network analyses of five miRNAs. Green triangle indicates selected miRNAs. Red ellipse indicates genes that are regulated by miRNAs. B. qPCR analysis of five selected DEmis in the exosomes of SMMC-7721 and EMT SMMC-7721 cells. 7721 exo represents exosomes of SMMC-7721 cells. EMT 7721 exo represents exosomes of EMT SMMC-7721 cells. *; P < 0.05, ***; P < 0.001.
Knockdown analysis of hsa-miR-24-3p and hsa-miR-21-5p in SMMC-7721 cells
The miRNAs with the most number of target genes were hsa-miR-24-3p and hsa-miR-21-5p. To further examine the function of these two miRNAs in exosomes, we categorized SMMC-7721 cells into four groups: SMMC-7721 cells + SMMC-7721 exo group [(miRNA In NC) exo], SMMC-7721 cells + EMT SMMC-7721 exo group [EMT (miRNA In NC) exo], SMMC-7721 cells + EMT SMMC-7721 (hsa-miR-24-3p knockdown) exo group [EMT (miR-24-3p In) exo], and SMMC-7721 cells + EMT SMMC-7721 (hsa-miR-21-5p knockdown) exo group [EMT (miR-21-5p In) exo]. qPCR was used to confirm hsa-miR-24-3p and hsa-miR-21-5p knockdown. The expression of hsa-miR-24-3p and hsa-miR-21-5p in the EMT (miR-24-3p In) exo group and EMT (miR-21-5p In) exo group was significantly downregulated compared with that in the EMT (miRNA In NC) exo group (P < 0.0001; Figure 6A). This result suggested that hsa-miR-24-3p and hsa-miR-21-5p knockdown in EMT SMMC-7721 cells was achieved. The expression of the key members in SMMC-7721 cells was analyzed using WB. Exosomes with hsa-miR-24-3p knockdown treatment inhibited the EMT-related protein expression caused by normal exosomes in EMT SMMC-7721 cells (Figure 6B). However, this phenomenon was not observed in the hsa-miR-21-5p knockdown treatment. In addition, the effects of hsa-miR-24-3p and hsa-miR-21-5p knockdown treatments on cell behavior were determined. Knockdown of hsa-miR-24-3p could inhibit the progressive migration and invasion caused by exosomes in EMT SMMC-7721 cells, whereas knockdown of hsa-miR-21-5p did not affect cell behavior (Figure 6C). The above results suggest that hsa-miR-24-3p in exosomes play a more significant role in the EMT of SMMC-7721 cells.
Figure 6.
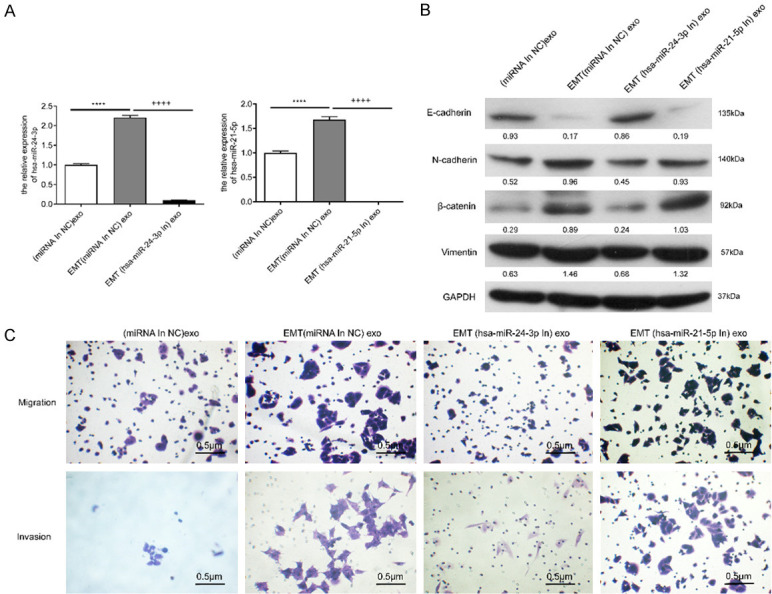
miRNA inhibitor in SMMC-7721 cells interfered with different exosomes to verify the effect of exosomes of SMMC-7721 cells. A. qPCR confirmation of hsa-miR-24-3p and hsa-miR-21-5p knockdown in different exosomes. ****; P < 0.0001 (vs. exosomes of SMMC-7721 cells); ++++; P < 0.0001 (vs. exosomes of EMT SMMC-7721 cells). B. WB analysis of EMT-related proteins in SMMC-7721 cells with different exosome treatments. C. Migration and invasion analysis of SMMC-7721 cells with different exosome treatments. (miRNA In NC) exo, exosomes of SMMC-7721 cells; EMT (miRNA In NC) exo, exosomes of EMT SMMC-7721 cells; EMT (hsa-miR-24-3p In) exo, exosomes of EMT SMMC-7721 cells with hsa-miR-24-3p knockdown treatment; EMT (hsa-miR-21-5p In) exo, exosomes of EMT SMMC-7721 cells with hsa-miR-21-5p knockdown treatment. All figures are magnified 200 ×.
Knockdown analysis of hsa-miR-24-3p in nude mice
To further verify the function of hsa-miR-24-3p in exosomes, SMMC-7721 cells were categorized into four different groups as follows: SMMC-7721 cells (Control), SMMC-7721 cells + SMMC-7721 exo group (EXO), SMMC-7721 cells + EMT SMMC-7721 exo group (EMT EXO), and SMMC-7721 cells + EMT SMMC-7721 (hsa-miR-24-3p knockdown) exo group [EMT EXO (miR-24-3p In)]. The cells were inoculated subcutaneously via intraperitoneal injection. Figure 7A shows the effects of different treatments on the weight of nude mice. The weight of mice in the Control group was apparently higher than that of mice in the EMT EXO (miR-24-3p In) group. In addition, the size and weight of the tumors in the EMT EXO (miR-24-3p In) group were apparently decreased compared with those in the EMT EXO groups (P < 0.05) (Figure 7B, 7C). Using qPCR, we found that the expression of hsa-miR-24-3p in the exosomes of the EMT Exo (miR-24-3p In) group was indeed reduced compared with that in the EMT EXO groups (P < 0.0001) (Figure 7D), which proves that the hsa-miR-24-3p knockdown experiment was indeed successful. The exosomes with the hsa-miR-24-3p knockdown treatment could inhibit the expression of EMT-related proteins caused by exosomes in EMT SMMC-7721 cells (Figure 7E); this result of WB was the same as that processed directly in cells (Figure 6B). Therefore, these results demonstrate that the exosomes of SMMC-7721 cells with hsa-miR-24-3p knockdown could effectively inhibit EMT of SMMC-7721 cells.
Figure 7.
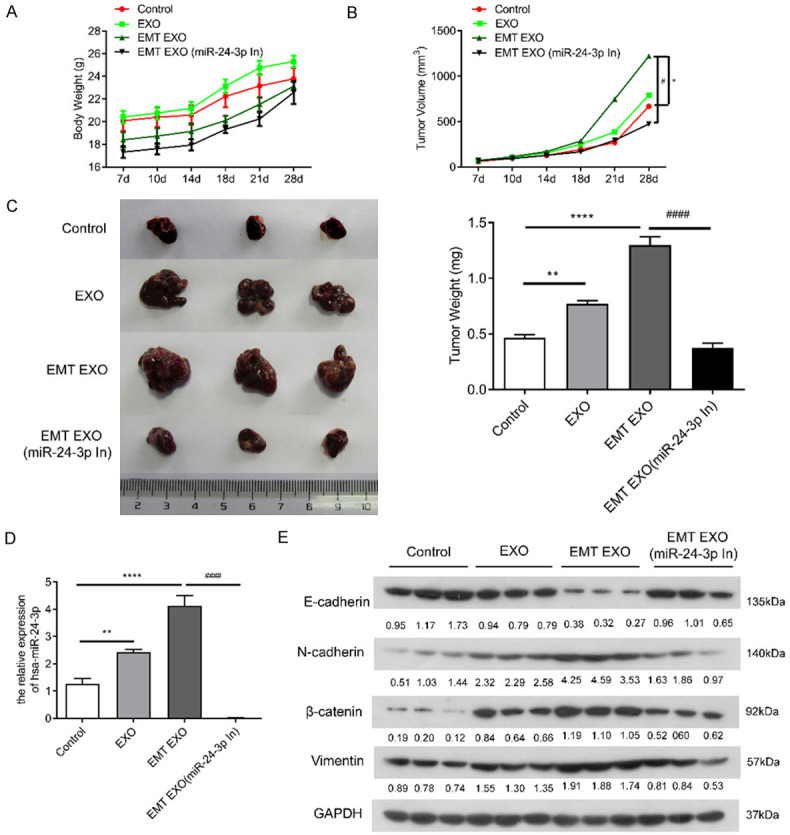
Effect of exosomes isolated from SMMC-7721 cells on tumor formation in nude mice combined with hsa-miR-24-3p knockdown in the exosomes of EMT SMMC-7721 cells. A. Effect of different treatments on the weight of nude mice. B. Effect of different treatments on subcutaneous tumor formation volume. *; P < 0.05 (vs. SMMC-7721 cells); #; P < 0.05 (vs. exosomes of EMT SMMC-7721 cells). C. Phenotype and weight of different treatments on subcutaneous tumors. **; P < 0.01, ****; P < 0.0001 (vs. SMMC-7721 cells), ####; P < 0.0001 (vs. exosomes of EMT SMMC-7721 cells). D. qPCR analysis of hsa-miR-24-3p expression following different treatments in tumors. **; P < 0.01, ****; P < 0.0001 (vs. SMMC-7721 cells), ####; P < 0.0001 (vs. exosomes of EMT SMMC-7721 cells). E. WB analysis of E-cadherin, N-cadherin, vimentin, and β-catenin expression following different treatments in tumors. Control, SMMC-7721 cells; EXO, SMMC-7721 cells treated with exosomes of SMMC-7721 cells; EMT EXO, SMMC-7721 cells treated with exosomes of EMT SMMC-7721 cells; [EMT EXO (miR-24-3p In)], SMMC-7721 cells treated with EMT SMMC-7721 exosomes with hsa-miR-24-3p knockdown.
Discussion
In the tumor microenvironment, tumor-associated fibroblasts are the major components of the stroma. These cells are activated fibroblasts that secrete various cytokines, which play an important role in promoting tumor progression and metastasis [19,20]. For example, a previous study has demonstrated that tumor-associated fibroblasts promote tumor growth and angiogenesis by secreting large amounts of stromal cell-derived factor 1 in invasive mammary glands [21]. Tumor-associated fibroblasts also regulate the inflammatory microenvironment. Studies have confirmed that tumor-associated fibroblasts secrete interleukin-6 and -8, which promote inflammation microcirculation [22,23]. Owing to the heterogeneity of tumor-associated fibroblasts, many specific markers are used, such as α-SMA, fibroblast-specific protein 1, fibroblast activation protein, and platelet-derived growth factor receptor. Among them, α-SMA is one of the most common markers and is widely used for the identification of tumor fibroblasts [24]. Exosomes are membranous vesicles that are produced by multivesicular bodies in the cell and released into the extracellular space. Heat shock protein 70, TSG101, and CD63 are the most widely used biomarkers to identify exosomes [7]. Tumor cell-derived exosomes play an important role in promoting tumor progression. For example, exosomes containing EMT-associated proteins produced by melanoma cells with high metastatic ability can promote bone marrow precursor cells, which could promote the metastatic ability of primary tumors [25]. In the present study, we successfully constructed an EMT model by treating SMMC-7721 cells with TGF-β1. Cell function analysis indicated that exosomes of EMT SMMC-7721 cells significantly promoted cell migration and invasion of SMMC-7721, HepG2, 97L, Hep3B, and Huh7 cells. Using high-throughput sequencing, we analyzed DEms and DEmis between EMT SMMC-7721 and normal SMMC-7721 cells, and the results suggested that the transcript environment was indeed different between these two types of cells.
Various studies have confirmed that miRNAs in exosomes regulate tumor resistance, metastasis, and immune response. Therefore, miRNAs in exosomes play an important role in the development of various tumors. Previous studies suggested that tumor-associated adipocytes and fibroblasts in the tumor microenvironment produced exosomes containing large amounts of miR-21, which were taken up by tumor cells. Mao et al. suggested that miR-21 promotes the proliferation and migration and inhibits the apoptosis of A375 human melanoma cells by inhibiting SPRY1, PDCD4, and PTEN via the extracellular signal-regulated kinase/nuclear factor-kB signaling pathway [26]. Luo et al. demonstrated that miR-21 promoted tumor cell migration and invasion by activating Sox2 and β-catenin signaling pathways in glioma cells [27]. Previous studies have also shown that miR-24 plays an important role in tumorigenesis and progression by regulating target gene expression. For example, Lu et al. demonstrated that miR-24 functioned as an oncogene and was upregulated in breast cancer [15]. Liu et al. demonstrated that miR-24 suppressed osteosarcoma metastasis by targeting Ack1 via the AKT/MMP pathways [28]. Meanwhile, we demonstrated that miRNAs were differentially expressed in exosomes of EMT SMMC-7721 and normal SMMC-7721 cells. EMT SMMC-7721 cells were able to produce a greater variety of DEmis in exosomes compared with that in normal SMMC-7721 cells. The functions of these DEmis were closely related to pathways involved in cancer and proteoglycans in cancer. After the functional studies of two selected DEmis (hsa-miR-24-3p and hsa-miR-21-5p), it was revealed that hsa-miR-24-3p indeed played an important role in regulating cell behavior. Following the analysis of cell functions and nude mice experiments, we showed that exosomes in EMT SMMC-7721 cells could effectively promote liver cell migration and invasion. Importantly, our results suggest that the analysis of hsa-miR-24-3p in exosomes may provide clues to the processes involved in EMT and liver cancer metastasis. miRNAs may be released along with exosomes during the communication between EMT cancer cells and normal cancer cells in vitro.
However, due to the complexity of biomaterials in exosomes, we cannot exclude the possibility that other EMT-driven proteins, mRNAs, or miRNAs in the exosomes could potentially play a role in intercellular communication.
In summary, miRNAs carried by exosomes of EMT liver cancer cells can activate normal liver cancer cells to promote cell fibrosis. hsa-miR-24-3p may be one of the most important molecular markers of EMT in liver cancer. Our findings provide new insights into the pathological mechanisms of liver cancer metastasis.
Acknowledgements
Authors would like to thank the National Natural Science Foundation of China (81873303), Natural Science Foundation of Guangdong Province, China (2019A1515011013), Key Projects of Educational Commission of Guangdong Province, China (2019KZDXM045), and Medical innovation project of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (2019IIT18) for financial support.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414–420. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Chen L. Tumor microenviroment and hepatocellular carcinoma metastasis. J Gastroenterol Hepatol. 2013;28(Suppl 1):43–48. doi: 10.1111/jgh.12091. [DOI] [PubMed] [Google Scholar]
- 4.Min H, Sun X, Yang X, Zhu H, Liu J, Wang Y, Chen G, Sun X. Exosomes derived from irradiated esophageal carcinoma-infiltrating T cells promote metastasis by inducing the epithelial-mesenchymal transition in esophageal cancer cells. Pathol Oncol Res. 2018;24:11–18. doi: 10.1007/s12253-016-0185-z. [DOI] [PubMed] [Google Scholar]
- 5.Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol. 2016;43:7–13. doi: 10.1016/j.ceb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. J Control Release. 2015;219:278–294. doi: 10.1016/j.jconrel.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, Wang D, See W, Costello BA, Quevedo F, Tan W, Nandy D, Bevan GH, Longenbach S, Sun Z, Lu Y, Wang T, Thibodeau SN, Boardman L, Kohli M, Wang L. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67:33–41. doi: 10.1016/j.eururo.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madar S, Brosh R, Buganim Y, Ezra O, Goldstein I, Solomon H, Kogan I, Goldfinger N, Klocker H, Rotter V. Modulated expression of WFDC1 during carcinogenesis and cellular senescence. Carcinogenesis. 2009;30:20–27. doi: 10.1093/carcin/bgn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Z, Shi K, Yang S, Liu J, Zhou Q, Wang G, Song J, Li Z, Zhang Z, Yuan W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol Cancer. 2018;17:147. doi: 10.1186/s12943-018-0897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawinkels LJ, Paauwe M, Verspaget HW, Wiercinska E, van der Zon JM, van der Ploeg K, Koelink PJ, Lindeman JH, Mesker W, ten Dijke P, Sier CF. Interaction with colon cancer cells hyperactivates TGF-beta signaling in cancer-associated fibroblasts. Oncogene. 2014;33:97–107. doi: 10.1038/onc.2012.536. [DOI] [PubMed] [Google Scholar]
- 13.Lin SC, Liu CJ, Lin JA, Chiang WF, Hung PS, Chang KW. -24 up-regulation in oral carcinoma: positive association from clinical and in vitro analysis. Oral Oncology. 2010;46:204–208. doi: 10.1016/j.oraloncology.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Zhang A, Li Y, Zhang K, Han L, Du W, Yan W, Li R, Wang Y, Wang K. MiR-24 regulates the proliferation and invasion of glioma by ST7L via β-catenin/Tcf-4 signaling. Cancer Letters. 2013;329:174–180. doi: 10.1016/j.canlet.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Lu K, Wang J, Song Y, Zhao S, Liu H, Tang D, Pan B, Zhao H, Zhang Q. miRNA-24-3p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting p27Kip1. Oncol Rep. 2015;34:995. doi: 10.3892/or.2015.4025. [DOI] [PubMed] [Google Scholar]
- 16.Wang XM, Yu DM, McCaughan GW, Gorrell MD. Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cell line. Hepatology. 2005;42:935–945. doi: 10.1002/hep.20853. [DOI] [PubMed] [Google Scholar]
- 17.Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, Giebel B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces. 2011;87:146–150. doi: 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Yao Z, Luo J, Hu K, Lin J, Huang H, Wang Q, Zhang P, Xiong Z, He C, Huang Z, Liu B, Yang Y. ZKSCAN1 gene and its related circular RNA (circZKSCAN1) both inhibit hepatocellular carcinoma cell growth, migration, and invasion but through different signaling pathways. Mol Oncol. 2017;11:422–437. doi: 10.1002/1878-0261.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 21.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Sharon Y, Raz Y, Cohen N, Ben-Shmuel A, Schwartz H, Geiger T, Erez N. Tumor-derived osteopontin reprograms normal mammary fibroblasts to promote inflammation and tumor growth in breast cancer. Cancer Res. 2015;75:963–973. doi: 10.1158/0008-5472.CAN-14-1990. [DOI] [PubMed] [Google Scholar]
- 23.Dror S, Sander L, Schwartz H, Sheinboim D, Barzilai A, Dishon Y, Apcher S, Golan T, Greenberger S, Barshack I, Malcov H, Zilberberg A, Levin L, Nessling M, Friedmann Y, Igras V, Barzilay O, Vaknine H, Brenner R, Zinger A, Schroeder A, Gonen P, Khaled M, Erez N, Hoheisel JD, Levy C. Melanoma miRNA trafficking controls tumour primary niche formation. Nat Cell Biol. 2016;18:1006–1017. doi: 10.1038/ncb3399. [DOI] [PubMed] [Google Scholar]
- 24.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 25.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao XH, Chen M, Wang Y, Cui PG, Liu SB, Xu ZY. MicroRNA-21 regulates the ERK/NF-kappaB signaling pathway to affect the proliferation, migration, and apoptosis of human melanoma A375 cells by targeting SPRY1, PDCD4, and PTEN. Mol Carcinog. 2017;56:886–894. doi: 10.1002/mc.22542. [DOI] [PubMed] [Google Scholar]
- 27.Luo G, Luo W, Sun X, Lin J, Wang M, Zhang Y, Luo W, Zhang Y. MicroRNA21 promotes migration and invasion of glioma cells via activation of Sox2 and betacatenin signaling. Mol Med Rep. 2017;15:187–193. doi: 10.3892/mmr.2016.5971. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Liu Z, Liu Z, Zhang Y, Li Y, Liu B, Zhang K. miR-24 represses metastasis of human osteosarcoma cells by targeting Ack1 via AKT/MMPs pathway. Biochem Biophys Res Commun. 2017;486:211–217. doi: 10.1016/j.bbrc.2017.02.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.