Abstract
It is unclear how acid-induced lung injury alters the regional lung volume response to mechanical ventilation (MV) and how this impacts protein expression. Using a mouse model, we investigated the separate and combined effects of acid aspiration and MV on regional lung volumes and how these were associated with the proteome. Adult BALB/c mice were divided into four groups: intratracheal saline, intratracheal acid, saline/MV, or acid/MV. Specific tidal volume (sVt) and specific end-expiratory volume (sEEV) were measured at baseline and after 2 h of ventilation using dynamic high-resolution four-dimensional computed tomography (4DCT) images. Lung tissue was dissected into 10 regions corresponding to the image segmentation for label-free quantitative proteomic analysis. Our data showed that acid aspiration significantly reduced sVt and caused further reductions in sVt and sEEV after 2 h of ventilation. Proteomic analysis revealed 42 dysregulated proteins in both Saline/MV and Acid/MV groups, and 37 differentially expressed proteins in the Acid/MV group. Mapping of the overlapping proteins showed significant enrichment of complement/coagulation cascades (CCC). Analysis of 37 unique proteins in the Acid/MV group identified six additional CCC proteins and seven downregulated proteins involved in the mitochondrial respiratory chain (MRC). Regional MRC protein levels were positively correlated with sEEV, while the CCC protein levels were negatively associated with sVt. Therefore, this study showed that tidal volume was associated with the expression of CCC proteins, while low end-expiratory lung volumes were associated with MRC protein expression, suggesting that tidal stretch and lung collapse activate different injury pathways.
NEW & NOTEWORTHY This study provides novel insights into the regional response to mechanical ventilation in the setting of acid-induced lung injury and highlights the complex interaction between tidal stretch and low-end-expiratory lung volumes; both of which caused altered regulation of different injury pathways.
Keywords: acid aspiration, lung imaging, mechanical ventilation, ventilator-induced lung injury proteomics
INTRODUCTION
Treatment of respiratory failure is complicated by the interaction between preexisting lung injury and mechanical ventilation (MV). As such, an individual patient’s outcome is a combination of the lung injury, resulting from the initial insult and the susceptibility of the lung to mechanical stretch. The implementation of protective ventilation strategies with low tidal volume has led to an improvement in outcomes; however, mortality remains unacceptably high (20, 32). Moreover, lower tidal volume ventilation strategies may heighten the risk of atelectasis resulting in further lung injury (17, 29).
The impact of ventilator-induced lung injury (VILI) is further complicated by the heterogeneous response of the lungs to MV, leading to coexistence of contrasting pathological processes (e.g., overdistension and atelectrauma) in different regions of the same lung (6, 7). An understanding of these heterogeneities will help clarify how mechanical forces during MV amplify lung injury and, thus, inform the design of optimized ventilation strategies to minimize such injury. Recently, in healthy mouse lungs, we showed that the magnitude of regional tidal stretch in response to MV was linked to the regional expression of inflammatory genes (37). In line with this, studies in sheep have shown that regional tidal strain in response to MV alters markers of metabolism (34). Intriguingly, in this sheep model and our mouse model, preexisting systemic inflammation modified the relationship between regional tidal strain/volume and inflammation (34, 36), which highlights the importance of considering both regional variation and the effect of prior lung injury when examining the effect of MV on the lung.
Several molecular and cellular mechanisms have been proposed to explain VILI, including the release of proinflammatory cytokines, production of reactive oxygen species, complement activation, and mechanotransduction (20, 25, 31). However, it remains to be determined how these signaling pathways respond to MV in the presence of prior injury and whether they are regulated in a spatial pattern. Recent studies have applied protein profiling to tissue samples to map regional expression of molecular pathways involved in VILI. Studies in healthy mice (8) have shown altered expression of coagulant pathways, while studies in the preterm lamb lung have revealed spatiotemporal patterns in the proteome (21) in response to MV. To our knowledge, however, no studies have examined the regional proteomic response in the adult lung to MV in the setting of prior lung injury.
Acid aspiration is a well-known cause of acute respiratory distress syndrome (ARDS), which leads to respiratory failure and a requirement for MV (24). The purpose of this study was to evaluate the effect of MV in the setting of acid-induced lung injury on the lung volume response, using a four-dimensional computed tomography (4DCT) lung imaging technology (37), and to link this with the regional proteomic response. We hypothesized that preexisting lung injury would alter the lung volume response to MV and that this would result in the dysregulation of multiple molecular pathways in a lung region-specific manner.
MATERIALS AND METHODS
Animal preparation and ventilation.
All animal experiments were approved by the Monash University and University of Tasmania Animal Ethics Committees and conformed to the guidelines of the National Health and Medical Research Council (Australia).
Adult female BALB/c mice were anesthetized, tracheostomized, and mounted upright in a custom-built holder on a rotating stage, as described previously (37). The animals were randomly assigned to four groups (n = 7 per group). The first two groups received intratracheal instillation with either 50 μL of 0.9% saline, or 0.1 M hydrochloric acid with pH 3.0 (Acid), followed by free-breathing for 30 min before euthanasia [Saline/free breathing (FB) and Acid/FB groups]. The second two groups were subjected to 2 h of MV 30 min after intratracheal saline (Saline/MV) or acid (Acid/MV). Ventilated mice were connected via the tracheal cannula to a small animal ventilator (AccuVent 200, Notting Hill Devices, Notting Hill, VIC, Australia) and ventilated at 225 breaths per minute, with a peak inspiratory pressure (PIP) of 12 cmH2O, and a positive end-expiratory pressure (PEEP) of 2 cmH2O. At the end of experiment, mice were euthanized by overdose with pentobarbital sodium (200 mg/kg), and the lung tissue was collected for proteomic analysis (described below).
Lung imaging.
As described previously (37), 4DCT images were captured using a liquid-metal-jet X-ray source coupled with a high-speed detector (23). A three-dimensional velocimetry technique (9) was applied to the dynamic images to calculate regional tidal volume (Vt), while Hounsfield Units were used to determine the aeration fraction at end expiration to obtain end-expiratory volume (EEV). The whole lung was segmented into 10 regions (four right lobes and six left regions), and the images were processed to calculate the lung volumes in these regions (37). Total and regional Vt and EEV were normalized by the variation in regional lung size at end expiration, calculated as specific Vt (sVt) = regional Vt/regional lung volume, specific end-expiratory volume (sEEV) = regional EEV/regional lung volume, and strain = sVt/sEEV.
Tissue extraction and peptide sample preparation.
Lung tissue was dissected into 10 regions corresponding to the image segmentation as described above (Supplementary Fig. S1 at https://doi.org/10.6084/m9.figshare.12616757; all supplemental figures may be found at this website). Lung proteins were extracted using T-PER tissue protein extraction reagent (Thermo Scientific, Rockford, IL), and total protein was quantified using a Bradford protein assay kit (Thermo Scientific), according to the manufacturer’s instructions.
Sample volumes corresponding to 50-µg aliquots were cleaned by precipitation in nine volumes of ethanol. Protein samples were then reduced using 10 mM dithiothreitol overnight at 4°C and alkylated using 50 mM iodoacetamide in the dark for 2 h. Proteins were digested with 2 µg mass spectrometry-grade trypsin/Lys-C mix (Promega, Madison, WI) on 500 µg Sera Beads (GE Healthcare, Chicago, IL), as recommended for the SP3 method (15). Peptide samples (1 µg) were analyzed by data-dependent mass spectrometry using an Ultimate 3000 RSLCnano and Q-Exactive HF (Thermo Scientific), as described in Supplementary Methods S1 (https://doi.org/10.6084/m9.figshare.12616763).
Database searching and criteria for protein identification.
Data files were imported into MaxQuant version 1.6.5.0 (https://maxquant.org/) and MS/MS spectra were searched using the Andromeda search engine against the complete Mus musculus UniProt reference proteome. Default settings for protein identification by Orbitrap MS/MS were used, with the match-between-runs function enabled, including a maximum of two missed cleavages, mass error tolerances of 20 ppm, and then 4.5 ppm for initial and main peptide searches, respectively, 0.5 Da tolerance for fragment ions, and carbamidomethyl modification of cysteine and variable methionine oxidation. A false discovery rate (FDR) of 0.01 was used for both peptide-spectrum matches and protein identification. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the data set identifier PXD013485 (available at http://www.ebi.ac.uk/pride/archive/projects/PXD013485).
Determination of relative protein abundance and statistical analysis.
We used MaxLFQ, the MaxQuant algorithm, for peptide intensity determination, and normalization (5), using pairwise comparison of unique and razor peptide intensities and a minimum ratio count of 2. The proteinGroups output files generated by MaxQuant analysis were processed as follows. The normalized label-free quantification (LFQ) intensity values, MS/MS counts and the numbers of razor and unique peptides for each of the identified proteins were imported into Perseus software version 1.5.031 (http://perseus-framework.org/). Protein groups identified either as potential contaminants (prefixed with CON_), identified by modified site only, by reverse database matching, or on the basis of a single matching peptide were removed. LFQ intensity values were then log2–transformed and a filter applied to include only proteins detected in a minimum of 70% of the samples. Missing values were replaced with random intensity values for low-abundance proteins based on a normal distribution of protein abundances using default MaxQuant parameters. Mean LFQ values for each treatment group were compared using a two-sided regularized t-test with a stabilization (s0) factor of 0.1 (30) and proteins with a permutation-based FDR (q value) < 5% were considered to be significant.
Bioinformatics analysis.
Functional category and Gene Ontology annotation were performed on differentially expressed proteins. The KEGG database was used to identify significantly enriched KEGG pathways. The Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database of physical and functional interactions was used to analyze the protein-protein interaction of selected proteins. A Benjamini-corrected P < 0.05 was used as the cut-off for significantly enriched biological processes.
Data analysis.
Differences in regional lung volumes and protein expression between groups and regions were analyzed using two-way ANOVA with main effects, interactions, and repeated measures, as appropriate. Aligned rank transformation was applied for nonparametric data. A Holm-Sidak post hoc test was implemented when ANOVA indicated significance of the main treatment effect or interaction. Total lung volume indices were compared between groups using t tests. SigmaPlot (v. 13, Systat Software, San Jose, CA) was used for statistical analyses. Principal component analysis (PCA) was conducted on proteomic data using SPSS (version 20.0: SPSS, Chicago, IL). Pearson correlation index was calculated to determine the association among different continuous variables. Data are presented as means (SD) or median (range). Differences were considered statistically significant if P < 0.05 (P < 0.1 for interaction terms).
RESULTS
Control lung volumes and proteomics at baseline.
As expected, the spatial distribution of lung volumes was not uniform in the normal ventilated lung (Saline/MV group) (Fig. 1, A and E; Supplemental Fig. S2, A and B). Regional sVt was substantially lower in the accessory lobe R4 than other regions, particularly compared with R3 (P = 0.013), L5 (P = 0.009), and L6 (P = 0.013), whereas sEEV did not show notable regional differences. In terms of the baseline proteomic responses, the heat map showed regional heterogeneity in the protein profile (Supplemental Fig. S3A). We used principal component analysis (PCA) to reduce the complexity of the proteomics data and to examine global trends of protein expression in the lung regions (Supplemental Fig. S3B). In the saline group, most of the variance (63.90%) in protein expression was captured by the first three principal components. The PCA analysis suggested that L3, L6, and L1 were significantly different from other regions in relation to proteomic responses. The significant variation in baseline lung function and protein expression provided further rationale for subsequent regional analysis.
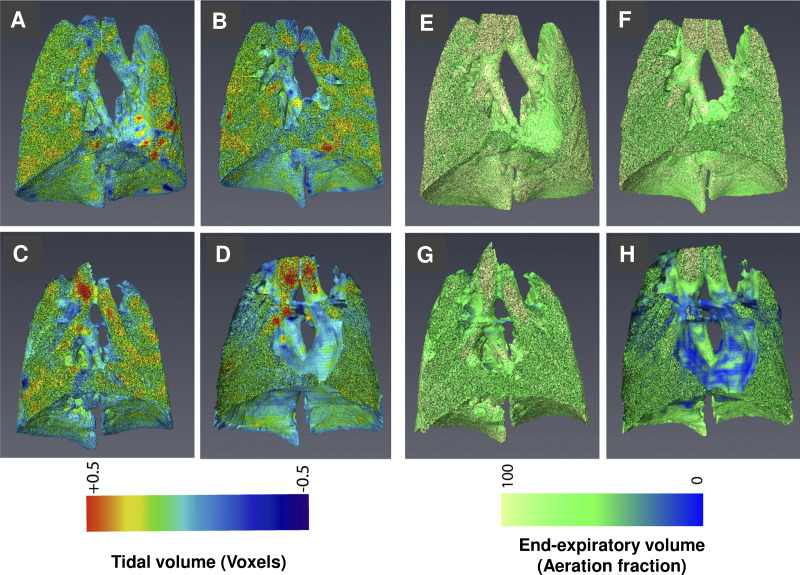
Fig. 1.
Lung imaging in the four experimental groups. Representative three-dimensional images illustrating specific tidal volume (sVt; A–D) and specific end-expiratory volume (sEEV; E–H) for a ventilated mouse at baseline (A, E), a mouse ventilated for 2 h (B, F), an acid-treated mouse at baseline (C, G) and a mouse ventilated for 2 h following acid exposure (D, H).
Effect of mechanical ventilation and acid on lung volumes.
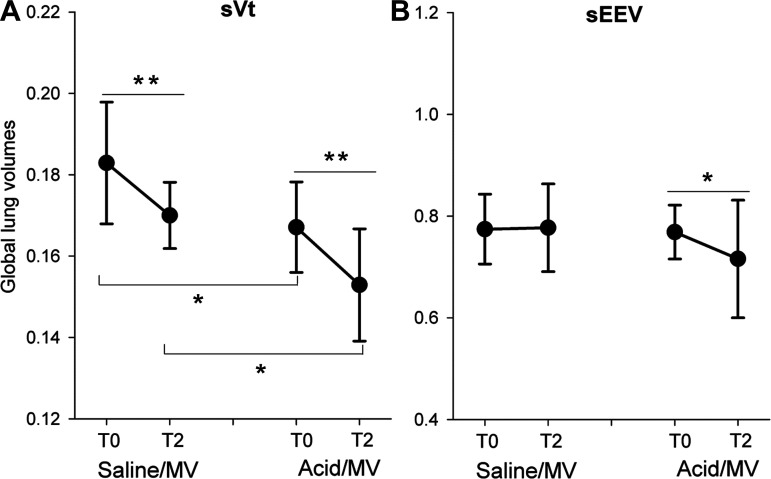
Both acid aspiration (P = 0.012) and 2 h of MV (P = 0.002) caused significant reductions in global sVt (Fig. 2A). However, there was no interaction between acid aspiration and MV on the effect of global sVt (P = 0.84), suggesting that these effects were additive (Fig. 2A).
Fig. 2.
Changes in lung volumes in different experimental conditions. The changes in sVt (A) and sEEV (B) at baseline (T0) and after 2 h of MV (T2) in saline- and acid-exposed mice. Values are mean (SD) with n = 7 per group. *P < 0.05; **P < 0.01. MV, mechanical ventilation; sEEV, specific end-expiratory volume; sVt, specific tidal volume.
There was a significant interaction between acid aspiration and MV and their association with global sEEV (P = 0.10). There was no effect of acid on sEEV at the start of MV (P = 0.97) and no effect of MV on sEEV in the saline group (P = 0.92). There was, however, a significant decrease in sEEV in response to MV in the acid aspiration group (P = 0.035). Acid aspiration (P = 0.54) and MV (P = 0.61) had no effect on global strain (data not shown). When assessed regionally, sVt and sEEV in R4 were lower than the other regions (P < 0.001; Supplemental Fig. S2, C and D); however, such variation was independent of acid exposure (P = 0.895).
Identification of differentially expressed proteins.
Despite the presence of highly abundant proteins such as albumin and hemoglobin, we achieved a comparable proteome depth (Supplemental Figure S4) to similar studies (27). After data filtering, 1,943 proteins (FDR < 1%) were quantified across all samples relative to the control (Saline/FB) group. On this basis, 46, 73, and 114 proteins were classified as differentially expressed in the Acid/FB, Saline/MV, and Acid/MV groups, respectively (Fig. 3, A–C) based on the q < 0.05 cut-off. Further analysis, after controlling for repeated measurements, identified 27 (upregulation: 20; downregulation: 7) in the Acid/FB group, 62 (upregulation: 53; downregulation: 9) in the Saline/MV group and 89 (upregulation: 64; downregulation: 25) in the Acid/MV group (Supplemental Table S1 at https://doi.org/10.6084/m9.figshare.12616766; all supplemental tables may be found at this site). These protein sets were used for further analysis.
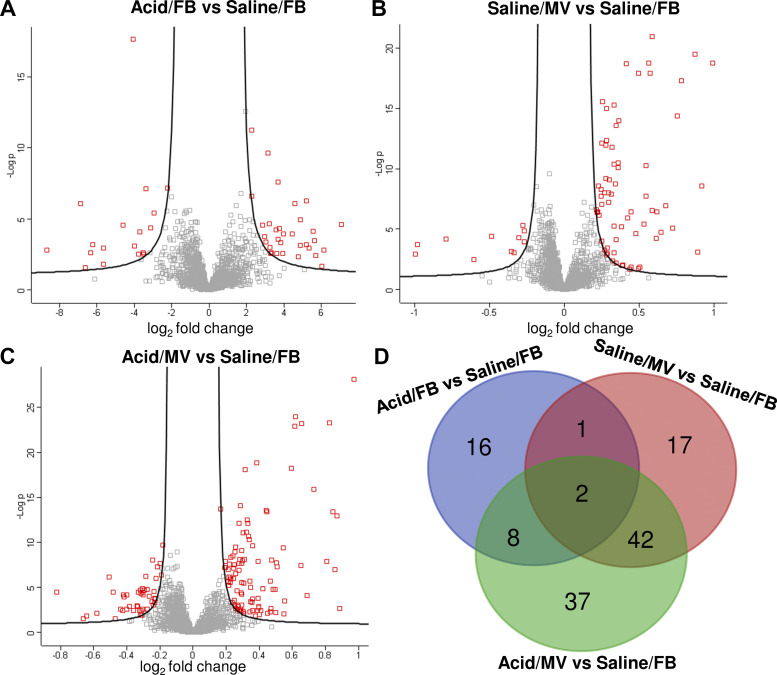
Fig. 3.
Overview of the differentially expressed proteins. Volcano plots for all quantified proteins (n = 1943) from proteomic analyses show −log10 P values (y-axis) versus the log2 fold change (x-axis). The differentially expressed proteins are highlighted in red: Acid/FB vs. saline/FB (A), saline/MV vs. saline/FB (B), Acid/MV vs. Saline/FB (C). The Venn diagram (D) depicts the number of overlapping and uniquely altered proteins among the three groups. FB, free-breathing; MV, mechanical ventilation.
Some of the differentially expressed proteins showed regional heterogeneity in expression level, including five in the Acid/FB group, 43 in Saline/MV, and 61 in Acid/MV with 4 (three upregulated and one downregulated) and eight (all upregulated) proteins showing regional differences that were influenced by treatment (Supplemental Table S2, A–C). Among the differentially expressed proteins, 42 were shared nonredundant proteins in both the Saline/MV and Acid/MV groups, and 37 were uniquely present in Acid/MV group (Fig. 3D).
Functional characterization of differentially expressed proteins.
Functional annotation revealed that 27 of the differentially expressed proteins in the Acid/FB group were involved in transcriptional regulation process, including methylation, acetylation, and RNA-binding (Supplemental Table S2A). In contrast, the 62 dysregulated proteins in the Saline/MV group were involved in platelet aggregation, hemostasis, inflammation, and fibrinolysis (Supplemental Table S2B). Complement and coagulation cascades (CCC), comprising eight proteins (Cfi, Cfd, Fga, Fgb, Fgg, Plg, Serpinc1, and Serpind1) were the most enriched KEGG pathway (Table 1).
Table 1.
Pathway analysis of the differentially expressed proteins in different groups
| KEGG Pathway | Count, % | P | Benjamini (P) | Proteins Involved |
|---|---|---|---|---|
| Proteins Identified from Comparison of Saline/MV and Saline/FB | ||||
| Complement and coagulation cascades | 8 (12.9%) | <0.001 | <0.001 | Cfi, Cfd, Fga, Fgb, Fgg, Plg, Serpinc1, Serpind1 |
| Staphylococcus aureus infection | 6 (9.7%) | <0.001 | <0.001 | Cfi, Cfd, Fgg, H2-Ea-ps, Itgb2, Plg |
| Phagosome | 7 (11.3%) | <0.001 | 0.002 | Coro1a, H2-Ea-ps, Itgb2, Itgb3, Mpo, Thbs1, Tubb1 |
| Platelet activation | 6 (9.7%) | <0.001 | 0.005 | Fermt3, Fga, Fgb, Fgg, Itga2b, Itgb3 |
| Shared Proteins in Saline/MV and Acid/MV Groups | ||||
| Complement and coagulation cascades | 7 (16.7) | <0.001 | <0.001 | Cfi, Fga, Fgb, Fgg, Plg, Serpinc1, Serpind1 |
| Staphylococcus aureus infection | 4 (9.5%) | <0.001 | 0.013 | Cfi, Fgg, Itgb2, Plg |
| Platelet activation | 5 (11.9%) | 0.001 | 0.012 | Fermt3, Fga, Fgb, Fgg, Itgb3 |
| Unique Proteins in Acid/MV Group | ||||
| Complement and coagulation cascades | 6 (16.2%) | <0.001 | <0.001 | C3, C4b, Cfh, Cfb, Kng1, Serpinf2 |
| Parkinson’s disease, Huntington’s disease | 7 (18.9%) | <0.001 | <0.001 | Ndufa9, Cox5a, Cyc1, Uqcrc2, Uqcrc1, Vdac1, Vdac2 |
| Staphylococcus aureus infection | 4 (10.8%) | 0.001 | 0.008 | C3, C4b, Cfh, Cfb |
| Oxidative phosphorylation | 5 (13.5%) | 0.001 | 0.011 | Ndufa9, Cox5a, Cyc1, Uqcrc2, Uqcrc1 |
| Cardiac muscle contraction | 4 (10.8%) | 0.002 | 0.015 | Cox5a, Cyc1, Uqcrc2, Uqcrc1 |
FB, free-breathing; MV, mechanical ventilation.
Mapping of the 42 overlapping proteins dysregulated in Saline/MV confirmed that these CCC proteins were also present in the double hit model of acid aspiration and MV. Analysis of 37 unique proteins in the Acid/MV group identified an additional six proteins relating to the enriched CCC pathway (C3, C4b, Cfh, Cfb, Kng1, and Serpinf2). Other pathways identified from the set of unique proteins, consisted of seven downregulated proteins that have been linked to Parkinson’s disease, Huntington’s disease, and cardiac muscle contraction. On further examination, these proteins belong to mitochondrial respiratory chain components (MRC), including Ndufa9 (Complex I), Cyc1, Uqcrc2, Uqcrc1 (Complex III), Cox5a (Complex IV), and Vdac1, and Vdac2 (mitochondrial porin family). The details for the functional annotation of these overlapping and unique proteins can be found in Supplemental Table S2, C and D.
A regulatory network was established using the differentially expressed proteins (89) in the Acid/MV group (Supplemental Fig. S5). Confirming our pathway analysis above, the network comprised two clear clusters: 1) proteins associating with complement, coagulation, and fibrinolysis as hubs and 2) proteins involving the mitochondrial respiratory chain.
Association between identified protein sets and lung volumes.
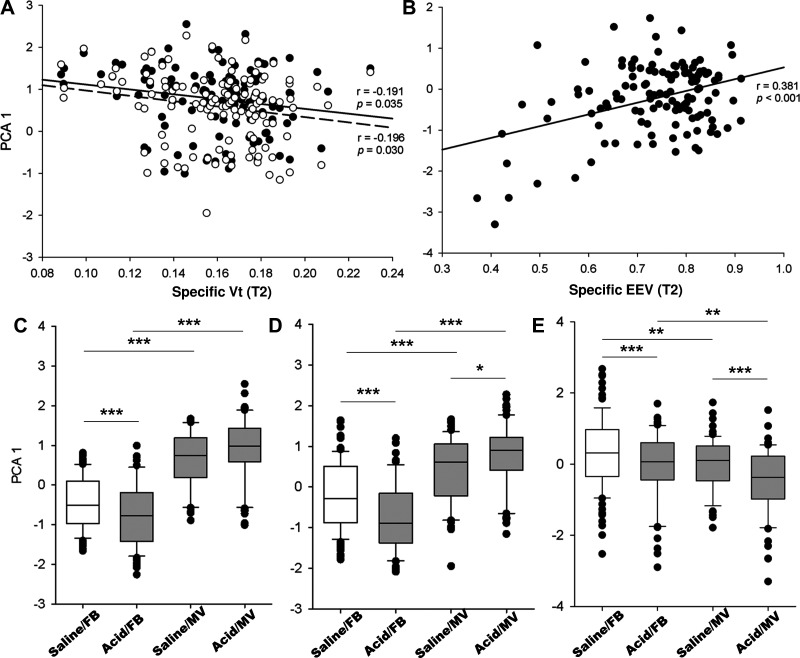
Because of functional relevance and strong correlation of the proteins belonging to the same pathway (Supplemental Fig. S5; P < 0.001 for all associations), we employed principal component analysis (PCA) to group these variables. Using this approach, we identified a principal component (PCA 1) for overlapping CCC proteins accounting for 61.07% of the total variance, as well as a PCA 1 for unique CCC proteins (78.60%), and a PCA 1 for unique MRC proteins (80.16%).
PCA 1 for both shared and unique CCC proteins was negatively associated with regional sVt (P < 0.05) (Fig. 4A), whereas PCA 1 for the MRC proteins was positively correlated with regional sEEV (r = 0.381, P < 0.001) (Fig. 4B). In contrast, regional strain was not associated with the expression of CCC or MRC proteins (P > 0.05).
Fig. 4.
Regional lung volume and protein expression. Principal component analysis (PCA) was applied to group the correlated proteins in the complement and coagulation cascades (CCC), and mitochondrial respiratory chain (MRC), respectively. Scatterplots show correlations between PCA1 for overlapping (solid line and black plot) and unique CCC proteins (dashed line and white plot) and sVt (A), and between PCA1 for unique MRC proteins (B) and sEEV. Also shown are the overall comparisons of PCA 1 for overlapping (C) and unique (D) CCC proteins and unique MRC proteins (E). Saline/FB (n = 99), Acid/FB (n = 66), Saline/MV (n = 63), Acid/MV (n = 60). Values are expressed as medians (25th and 75th centile) for C–E. *P < 0.05, **P < 0.01, ***P < 0.001. MV, mechanical ventilation; FB, free-breathing; sEEV, specific end-expiratory volume; sVt, specific tidal volume.
There was a significant interaction between acid aspiration and MV and their association with the PCA 1 score for both overlapping (P < 0.001) and unique (P < 0.001) CCC proteins (Fig. 4, C and D). For the overlapping CCC proteins, MV increased the PCA 1 score in both the saline (P < 0.001) and acid exposed (P < 0.001) mice. Acid aspiration had no additional effect on PCA 1 score in the ventilated mice (P = 0.07). In contrast, acid aspiration was associated with a decrease in PCA 1 score in the free-breathing mice (P < 0.001). For the unique CCC proteins, MV increased the PCA 1 score in both the saline (P < 0.001) and acid-exposed (P < 0.001) mice. Acid aspiration increased the PCA 1 score in the ventilated mice (P = 0.03). In contrast, acid aspiration was associated with a decrease in PCA 1 score in the free-breathing mice (P < 0.001). Acid aspiration (P < 0.001) and MV (P = 0.002) were associated with significant decreases in PCA 1 score for the unique MRC proteins. There was no interaction between acid aspiration and MV and the association with PCA 1 score for the MRC proteins (P = 0.86) (Fig. 4E).
DISCUSSION
The aim of our study was to assess the lung volume response to mechanical ventilation (MV) with and without prior acid-induced lung injury and to link this to the regional proteomic response. We found that combined acid exposure and MV reduced tidal volume (Vt) and end-expiratory volume (EEV), while MV alone (Saline/MV group) had minimal effect on these parameters. Proteomic analysis revealed a number of differentially expressed proteins, some of which were unique to exposure to acid, MV, and the combination of both. Mapping of the overlapping proteins showed significant enrichment of complement and coagulation cascades (CCC), while analysis of the unique proteins in the Acid/MV group identified additional proteins related to CCC and the mitochondrial respiratory chain (MRC). Importantly, regional MRC protein levels were positively correlated with sEEV, while the CCC protein levels were negatively associated with regional sVt. This study suggests that preexisting acid injury aggravates the deleterious response of the lung to MV by decreasing lung volumes. The regional loss of EEV is linked to downregulation of mitochondrial respiration-associated proteins, while the regional decrease in Vt is linked to activation of complement and coagulation pathways.
Although acid aspiration is a common cause of direct lung injury that can lead to respiratory failure and a requirement for MV, data are scarce on the interactions between acid and MV, particularly the fundamental molecular mechanisms of the potential synergistic effects of these respiratory insults. In analyzing the separate effects of acid and MV, our data showed that acid aspiration caused a decrease in Vt. The deleterious effects of acid aspiration on lung function are well described and include decreasing lung volume (4), which is consistent with decreased lung compliance as a result of pulmonary edema (13). The rapid respiratory response to acid, as indicated by the difference in Vt at baseline within 30 min of acid instillation, is also consistent with the previous observations, whereby the greatest impairment in pulmonary gas exchange occurs 20 min after acid aspiration (26). This is probably linked to the rapid development of acid-induced pulmonary edema before subsequent recruitment of inflammatory cells, including neutrophils (14). We found that the low tidal volume ventilation strategy induced minimal changes in lung volume, which is consistent with previous studies (25, 35) and the clinical observation that low tidal volume plus moderate PEEP, minimizes lung injury in the acute respiratory distress syndrome (10). However, when combined with acid-induced lung injury, there was evidence of a pronounced reduction in lung volumes without any change in lung strain after 2 h of MV, suggesting that even so-called “protective” mechanical ventilation has the capacity to exacerbate acid-induced lung injury. It is interesting to note that this is in clear contrast to our previous study (9) using a model of endotoxemia-induced lung injury, in which we observed an increase in sEEV in response to lung injury; likely due to gas trapping at end-expiration. This discrepancy may be related to the difference in the nature of the injury that was induced before MV in the two studies and highlights the importance of understanding the contribution of preexisting injury to the deleterious effects of MV.
In addition to the effects on lung mechanics outlined above, both MV and acid exposure altered the proteome. Exposure to acid alone altered the expression of 27 proteins that are mainly associated with transcriptional regulation processes, consistent with a rapid host response to injury. On the other hand, MV enriched the complement and coagulation cascades (CCC) with additional proteins in this pathway identified in the Acid/MV group. CCC are characteristic of acute lung injury (1, 33) and other inflammatory conditions in the lung including pneumonia, and sepsis (1) and have been shown to be linked to ventilator induced lung injury (VILI) (22, 28), and multiorgan failure (1). In VILI, as a result of tidal stretch, both local and systemic complement systems are activated, resulting in increased vascular permeability and pulmonary edema (18, 22), which can subsequently limit lung expansion, and reduce lung compliance. As such, the CCC proteins are regarded as theoretical therapeutic targets to prevent VILI (22, 28, 33). Consistent with this, we found a negative correlation between the regional expression of CCC and regional Vt after 2 h of ventilation and no association between CCC proteins and regional strain, indicating that the regions with the lowest compliance (i.e., the lowest tidal volume) had the highest expression of CCC.
Interestingly, analysis of 37 differentially expressed proteins in the Acid/MV group led to the identification of seven downregulated proteins involved in mitochondrial respiratory chain (MRC). Reduction of MRC proteins may decrease ATP generation and energy supply, and lead to excessive reactive oxygen species, which can stimulate pathological processes, including inflammation, cellular damage, mutations, and apoptosis (19). Endothelial mitochondrial depolarization has been proposed as an important mechanism of global loss of endothelial barrier function and pulmonary edema in acid-induced lung injury (14). In addition, evidence linking mitochondrial dysfunction with MV also exists. For example, oxidative mitochondrial DNA (mtDNA) damage occurs during ventilation with high peak inflation pressures (11), and repair of mtDNA damage attenuates oxidative stress and protects against lung injury in moderately severe VILI (12). Recently, Pereira-Fantini et al. (21) reported region-specific mitochondrial alterations in the preterm lamb lung subjected to MV, including an increase in the expression of respiratory chain complex IV (cytochrome-c oxidase) proteins in the nondependent lung and decreased expression of respiratory chain complex III and V proteins in the gravity-dependent lung. In our study, we showed that downregulation of MRC proteins was associated with low sEEV, suggesting that regions exposed to low lung volumes are susceptible to mitochondrial dysfunction. This is consistent with the concept of atelectasis-induced lung injury and the impact of inadequate lung volumes on clinical progression of patients with respiratory failure (17).
One of the limitations of the current study is that we were unable to determine the effect of acid alone at the 2 h time point due to animal welfare concerns. Hence, whether downregulation of MRC proteins is a result of acid exposure or the interaction between acid exposure and mechanical ventilation is unclear, although the association between MRC expression and lung volumes would suggest the latter. Furthermore, we used acid with a pH of 3.0, which is higher than the pH typically used in these models (16). However, most ICU patients have gastric juice pH in the range of 3.0 to 4.0 (3), and pH 4.0 has been reported to cause lung injury (2), suggesting our model is clinically relevant. It is also important to note that we used pressure-driven MV in our study with no adjustment of tidal volume over the course of ventilation. This differs from the clinical situation which typically involves volume-driven MV, which may have impacted the pathways that were stimulated. Nonetheless, we have shown that low lung volumes (both EEV and Vt) are associated with alterations in protein expression within pathways that are likely to promote lung injury.
In conclusion, our data show that low tidal volume ventilation with moderate PEEP does not exacerbate lung inhomogeneity but predisposes the lung to elevated expression of CCC proteins. The detrimental effects of MV on the lung were magnified by prior exposure to acid, leading to the loss of lung volume. Importantly, we were also able to identify a potential impact of acid exposure, in combination with MV, on mitochondrial function, which was linked to regional end-expiratory volume, suggesting a novel effect of atelectasis in promoting cell injury. Overall, this study provides novel insights into regulatory mechanisms of VILI following acid aspiration and highlights the fact that both tidal stretch and end-expiratory volume may contribute to further lung injury.
GRANTS
This work was supported by the NHMRC APP1077905 (G.R.Z., A.F., and P.A.D.), NHMRC APP1160774 (to G.R.Z. and S.D.), Royal Hobart Hospital Research Foundation no. 19-206 (to G.R.Z. and P.A.D.), the Multi-modal Australian Sciences Imaging and Visualization Environment (eu92, to S.D.), and Australian Research Council Discovery Early Career Researcher Award (DE180101133, to S.D.).
DISCLOSURES
Stephen Dubsky and Andreas Fouras have financial interests in the commercialization of an imaging technology related to this publication. All other authors declare no conflicts of interest, financial or otherwise.
AUTHOR CONTRIBUTIONS
P.A.D. and G.R.Z. conceived and designed research; S.Y., M. Preissner, E.B., R.W., M. Pavez, S.D., A.F., and G.R.Z. performed experiments; Y.S. analyzed data; Y.S. and G.R.Z. interpreted results of experiments; Y.S. and M. Preissner prepared figures; Y.S. and G.R.Z. drafted manuscript; S.Y., Y.S., M. Preissner, R.W., M. Pavez, S.D., P.A.D., and G.R.Z. edited and revised manuscript; S.Y., Y.S., M. Preissner, R.W., M. Pavez, S.D., P.A.D., A.F., and G.R.Z. approved final version of manuscript.
REFERENCES
- 1.Abraham E. Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol 22: 401–404, 2000. doi: 10.1165/ajrcmb.22.4.f184. [DOI] [PubMed] [Google Scholar]
- 2.Allen GB, Leclair TR, von Reyn J, Larrabee YC, Cloutier ME, Irvin CG, Bates JH. Acid aspiration-induced airways hyperresponsiveness in mice. J Appl Physiol (1985) 107: 1763–1770, 2009. doi: 10.1152/japplphysiol.00572.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonten MJ, Gaillard CA, van der Geest S, van Tiel FH, Beysens AJ, Smeets HG, Stobberingh EE. The role of intragastric acidity and stress ulcus prophylaxis on colonization and infection in mechanically ventilated ICU patients. A stratified, randomized, double-blind study of sucralfate versus antacids. Am J Respir Crit Care Med 152: 1825–1834, 1995. doi: 10.1164/ajrccm.152.6.8520743. [DOI] [PubMed] [Google Scholar]
- 4.Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med 160: 109–116, 1999. doi: 10.1164/ajrccm.160.1.9803046. [DOI] [PubMed] [Google Scholar]
- 5.Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics 13: 2513–2526, 2014. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cressoni M, Chiurazzi C, Gotti M, Amini M, Brioni M, Algieri I, Cammaroto A, Rovati C, Massari D, di Castiglione CB, Nikolla K, Montaruli C, Lazzerini M, Dondossola D, Colombo A, Gatti S, Valerio V, Gagliano N, Carlesso E, Gattinoni L. Lung inhomogeneities and time course of ventilator-induced mechanical injuries. Anesthesiology 123: 618–627, 2015. doi: 10.1097/ALN.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 7.Dargaville PA, Rimensberger PC, Frerichs I. Regional tidal ventilation and compliance during a stepwise vital capacity manoeuvre. Intensive Care Med 36: 1953–1961, 2010. doi: 10.1007/s00134-010-1995-1. [DOI] [PubMed] [Google Scholar]
- 8.Ding H, Wang Y, Dong W, Ren R, Mao Y, Deng X. Proteomic lung analysis of mice with ventilator-induced lung injury (VILI) using iTRAQ-based quantitative proteomics. Chem Pharm Bull (Tokyo) 66: 691–700, 2018. doi: 10.1248/cpb.c17-00844. [DOI] [PubMed] [Google Scholar]
- 9.Dubsky S, Hooper SB, Siu KK, Fouras A. Synchrotron-based dynamic computed tomography of tissue motion for regional lung function measurement. J R Soc Interface 9: 2213–2224, 2012. doi: 10.1098/rsif.2012.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths MJD, McAuley DF, Perkins GD, Barrett N, Blackwood B, Boyle A, Chee N, Connolly B, Dark P, Finney S, Salam A, Silversides J, Tarmey N, Wise MP, Baudouin SV. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res 6: e000420, 2019. doi: 10.1136/bmjresp-2019-000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Potter BJ, Wilson GL, Gillespie MN, Parker JC. Mitochondrial-targeted DNA repair enzyme 8-oxoguanine DNA glycosylase 1 protects against ventilator-induced lung injury in intact mice. Am J Physiol Lung Cell Mol Physiol 304: L287–L297, 2013. doi: 10.1152/ajplung.00071.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashizume M, Mouner M, Chouteau JM, Gorodnya OM, Ruchko MV, Wilson GL, Gillespie MN, Parker JC. Mitochondrial targeted endonuclease III DNA repair enzyme protects against ventilator induced lung injury in mice. Pharmaceuticals (Basel) 7: 894–912, 2014. doi: 10.3390/ph7080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heuer JF, Sauter P, Pelosi P, Herrmann P, Brück W, Perske C, Schöndube F, Crozier TA, Bleckmann A, Beißbarth T, Quintel M. Effects of pulmonary acid aspiration on the lungs and extra-pulmonary organs: a randomized study in pigs. Crit Care 16: R35, 2012. doi: 10.1186/cc11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hough RF, Islam MN, Gusarova GA, Jin G, Das S, Bhattacharya J. Endothelial mitochondria determine rapid barrier failure in chemical lung injury. JCI Insight 4: e124329, 2019. doi: 10.1172/jci.insight.124329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes CS, Moggridge S, Müller T, Sorensen PH, Morin GB, Krijgsveld J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat Protoc 14: 68–85, 2019. doi: 10.1038/s41596-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy TP, Johnson KJ, Kunkel RG, Ward PA, Knight PR, Finch JS. Acute acid aspiration lung injury in the rat: biphasic pathogenesis. Anesth Analg 69: 87–92, 1989. doi: 10.1213/00000539-198907000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Kilpatrick B, Slinger P. Lung protective strategies in anaesthesia. Br J Anaesth 105, Suppl 1: i108–i116, 2010. doi: 10.1093/bja/aeq299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu K, Mao YF, Zheng J, Peng ZY, Liu WW, Liu Y, Xu WG, Sun XJ, Jiang CL, Jiang L. SC5b-9-induced pulmonary microvascular endothelial hyperpermeability participates in ventilator-induced lung injury. Cell Biochem Biophys 67: 1421–1431, 2013. doi: 10.1007/s12013-013-9675-8. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Chen Z. The pathophysiological role of mitochondrial oxidative stress in lung diseases. J Transl Med 15: 207, 2017. doi: 10.1186/s12967-017-1306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oeckler RA, Hubmayr RD. Ventilator-associated lung injury: a search for better therapeutic targets. Eur Respir J 30: 1216–1226, 2007. doi: 10.1183/09031936.00104907. [DOI] [PubMed] [Google Scholar]
- 21.Pereira-Fantini PM, Pang B, Byars SG, Oakley RB, Perkins EJ, Dargaville PA, Davis PG, Nie S, Williamson NA, Ignjatovic V, Tingay DG. Preterm lung exhibits distinct spatiotemporal proteome expression at initiation of lung injury. Am J Respir Cell Mol Biol 61: 631–642, 2019. doi: 10.1165/rcmb.2019-0084OC. [DOI] [PubMed] [Google Scholar]
- 22.Petersen B, Busch T, Gaertner J, Haitsma JJ, Krabbendam S, Ebsen M, Lachmann B, Kaisers UX. Complement activation contributes to ventilator-induced lung injury in rats. J Physiol Pharmacol 67: 911–918, 2016. [PubMed] [Google Scholar]
- 23.Preissner M, Murrie RP, Pinar I, Werdiger F, Carnibella RP, Zosky GR, Fouras A, Dubsky S. High resolution propagation-based imaging system for in vivo dynamic computed tomography of lungs in small animals. Phys Med Biol 63: 08NT03, 2018. doi: 10.1088/1361-6560/aab8d2. [DOI] [PubMed] [Google Scholar]
- 24.Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med 39: 818–826, 2011. doi: 10.1097/CCM.0b013e31820a856b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Eur Respir J Suppl 42: 2s–9s, 2003. doi: 10.1183/09031936.03.00420103. [DOI] [PubMed] [Google Scholar]
- 26.Richter T, Bergmann R, Musch G, Pietzsch J, Koch T. Reduced pulmonary blood flow in regions of injury 2 hours after acid aspiration in rats. BMC Anesthesiol 15: 36, 2015. doi: 10.1186/s12871-015-0013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosa RL, Berger M, Santi L, Driemeier D, Barros Terraciano P, Campos AR, Guimarães JA, Vainstein MH, Yates JR III, Beys-da-Silva WO. Proteomics of rat lungs infected by Cryptococcus gattii reveals a potential Warburg-like effect. J Proteome Res 18: 3885–3895, 2019. doi: 10.1021/acs.jproteome.9b00326. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K, Saha D, Shattino I, Pavlov VI, Stahl GL, Finnegan P, Melo MF. Complement 3 is involved with ventilator-induced lung injury. Int Immunopharmacol 11: 2138–2143, 2011. doi: 10.1016/j.intimp.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 377: 1904–1905, 2017. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 30.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uhlig S. Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am J Physiol Lung Cell Mol Physiol 282: L892–L896, 2002. doi: 10.1152/ajplung.00124.2001. [DOI] [PubMed] [Google Scholar]
- 32.Villar J, Blanco J, Kacmarek RM. Current incidence and outcome of the acute respiratory distress syndrome. Curr Opin Crit Care 22: 1–6, 2016. doi: 10.1097/MCC.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 33.Ware LB, Camerer E, Welty-Wolf K, Schultz MJ, Matthay MA. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol 291: L307–L311, 2006. doi: 10.1152/ajplung.00157.2006. [DOI] [PubMed] [Google Scholar]
- 34.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Zheng H, Venegas JG, Vidal Melo MF. Effect of local tidal lung strain on inflammation in normal and lipopolysaccharide-exposed sheep. Crit Care Med 42: e491–e500, 2014. doi: 10.1097/CCM.0000000000000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ, Juffermans NP, Schultz MJ. Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care 13: R1, 2009. doi: 10.1186/cc7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yen S, Preissner M, Bennett E, Dubsky S, Carnibella R, Murrie R, Fouras A, Dargaville PA, Zosky GR. Interaction between regional lung volumes and ventilator-induced lung injury in the normal and endotoxemic lung. Am J Physiol Lung Cell Mol Physiol 318: L494–L499, 2020. doi: 10.1152/ajplung.00492.2019. [DOI] [PubMed] [Google Scholar]
- 37.Yen S, Preissner M, Bennett E, Dubsky S, Carnibella R, O’Toole R, Roddam L, Jones H, Dargaville PA, Fouras A, Zosky GR. The link between regional tidal stretch and lung injury during mechanical ventilation. Am J Respir Cell Mol Biol 60: 569–577, 2019. doi: 10.1165/rcmb.2018-0143OC. [DOI] [PubMed] [Google Scholar]