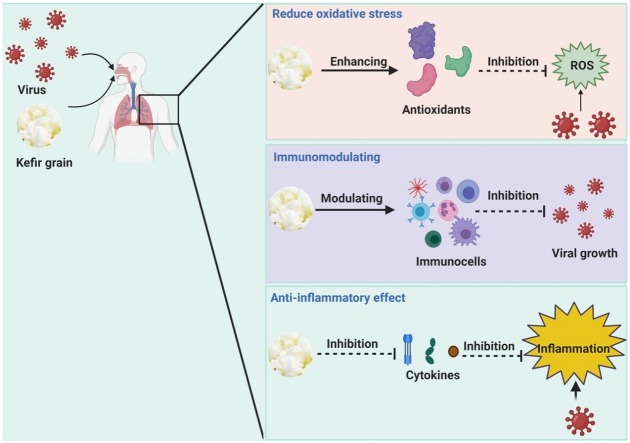
Graphical abstract
Mode of action of kefir supplementary dietary against viral infection.
Abbreviations: μL, Microliter; mg, Milligram; mL, Milliliter; μg, Microgram; mM, Millimolar; h, Hour; IL-2, interleukin-2; IL-12, interleukin-12; IFNγ, interferon gamma
Keywords: Coronaviru, Kefir, Immune system, Antiviral activity, Anti-inflammatory
Highlights
-
•
Kefir and kefir derivatives can suppress viral activity by modulating immune-system responses and/or causing disruption of viral adhesion.
-
•
The antiviral mechanisms of kefir involve enhancement of macrophage production and boosting the activity of proinflammatory cytokines.
-
•
Kefir dietaries have anti-inflammatory activity by inhibiting the activity of proinflammatory cytokines such as IL-1β, TNF-α and IL-6.
-
•
Using kefir (and its byproducts) as an inhibitor of expression of proinflammatory cytokines in COVID-19 patients could be a viable policy.
Abstract
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by a recently discovered coronavirus termed ‘severe acute respiratory syndrome coronavirus 2′ (SARS-CoV-2). Several scholars have tested antiviral drugs and compounds to overcome COVID-19. ‘Kefir’ is a fermented milk drink similar to a thin yogurt that is made from kefir grains. Kefir and its probiotic contents can modulate the immune system to suppress infections from viruses (e.g., Zika, hepatitis C, influenza, rotaviruses). The antiviral mechanisms of kefir involve enhancement of macrophage production, increasing phagocytosis, boosting production of cluster of differentiation-positive (CD4+), CD8+, immunoglobulin (Ig)G+ and IgA+ B cells, T cells, neutrophils, as well as cytokines (e.g., interleukin (IL)-2, IL-12, interferon gamma-γ). Kefir can act as an anti-inflammatory agent by reducing expression of IL-6, IL-1, TNF-α, and interferon-γ. Hence, kefir might be a significant inhibitor of the ‘cytokine storm’ that contributes to COVID-19. Here, we review several studies with a particular emphasis on the effect of kefir consumption and their microbial composition against viral infection, as well as discussing the further development of kefir as a protective supplementary dietary against SARS-CoV-2 infection via modulating the immune response.
1. Introduction
Viral infections can threaten human life [1]. Acute infection of the respiratory tract is associated with viruses, fungi, and bacteria, and cause morbidity and mortality in adults, children, and the immunocompromised [2].
Viruses from the families Paramyxoviridae, Orthomyxoviridae, Adenoviridae, Picornaviridae and Coronaviridae can cause respiratory-tract infections. Viruses from the Coronaviridae family have an envelope and single-stranded RNA. Coronaviruses (CoVs) have the largest RNA genome (30 kB) and infect humans and animals [3,4]. CoVs can infect the lower respiratory tract in older people, those with chronic disease, as well as children [5]. CoV infection can also lead to disorders to gastrointestinal, respiratory, hepatic, and central nervous systems [6,7].
CoVs were the main reason for the outbreak of Middle East respiratory syndrome (MERS) in Saudi Arabia in 2012, and severe acute respiratory syndrome (SARS) in China in 2002–2004 [[8], [9], [10]]. Different CoVs related to SARS have been studied in bats as the reservoir host [7,11,12].
On 12 December 2019, infection by a new CoV that resulted in pneumonia was recognized in Wuhan (Hubei Province, China) [13]. This new CoV was found to have 96 % similarity with CoVs from bats when the whole genome was sequenced [14]. The World Health Organization (WHO) termed this new CoV ‘severe acute respiratory syndrome coronavirus 2′ (SARS-CoV-2) on 11 February 2020. SARS-CoV-2 swept through China initially and has spread worldwide. The WHO named the illness that results from SARS-CoV-2 infection ‘coronavirus disease 2019′ (COVID-19). As of 25 October 2020, COVID-19 has led to 42,624,910 confirmed cases and 1,149,928 deaths in the world [15].
The strategies used to fight viral infections are development of a suitable vaccine or an efficacious antiviral drug to treat infected patients [16]. The mechanism of action of most vaccines is promotion of T-helper type-1 (Th1 or cluster of differentiation (CD)4+) cells to produce different cytokines, such as interferon (IFN)-γ, interleukin (IL)-2, and IL-12. The latter stimulates maturation of killer T cells and enhances the cytotoxicity of natural killer (NK) cells to identify and destroy virus-infected cells [17]. Buisman et al. [18] reported that vaccines induce production of immunoglobulin (Ig)A to protect the body. The mechanism of action of antiviral drugs is based mainly on enhancing the immune system, suppressing virus attachment to target cells, or stopping replication steps [16].
An efficacious drug or vaccine against COVID-19 is not available. However, meticulous hygiene and supporting the immune system are possible prevention strategies. Therefore, investigations to discover appropriate compounds to enhance the immune system and suppress SARS-CoV-2 activity are needed urgently because currently available antiviral medications (e.g., influenza viruses) are not efficacious against SARS-CoV-2 [19,20].
Natural products may enhance the immune system and suppress viral infection. ‘Kefir’ is a fermented milk drink similar to a thin yogurt that is made from kefir grains. Kefir has gained global acceptance as a healthy probiotic (i.e., live microorganism that can provide health benefits when consumed by improving/restoring the gut flora) and has been manufactured on a commercial scale [21]. With respect to human health, kefir has antiviral, antimicrobial, and anti-inflammatory potential. Kefir has been shown to inhibit angiotensin-converting enzyme (ACE) levels, cholesterol metabolism, accelerate wound healing, suppress tumour growth, and cause alterations in the immune system to improve asthma symptoms and allergy [[22], [23], [24], [25]]. Kefir and kefir derivatives (e.g., polysaccharides, protein, peptides) can suppress viral activity by modulating immune-system responses and/or causing disruption of viral adhesion [26,27]. They also act as anti-inflammatory agents by inhibiting the activity of proinflammatory cytokines such as IL-1β, tumour necrosis factor (TNF)-α and IL-6 [27]. Hence, kefir and its byproducts could be employed as protective agents against viral infections.
This review focuses on the antiviral mechanism of kefir and its byproducts. Some suggestions have been formulated regarding the potential of kefir against viruses such as SARS-CoV-2 to help researchers to screen antiviral activity based on this natural product.
2. COVID-19 pathogenesis
COVID-19 pathogenesis is incompletely understood. However, comparison with the mode of action of infection of SARS-CoV and MERS-CoV might offer insights for understanding the infection caused by SARS-CoV-2.
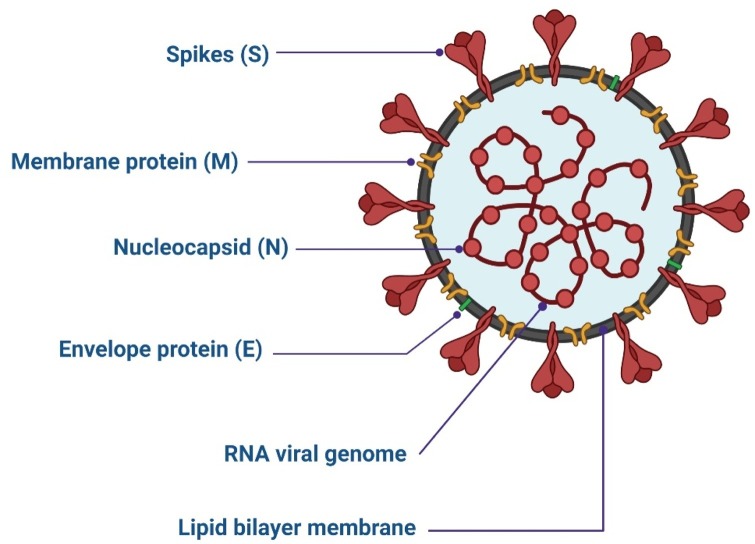
For assembly and infection of CoVs, four structural proteins are employed. The protein responsible for viral–host attachment is a ‘spike’ (S) glycoprotein [28]. The membrane (M) protein is responsible for the shape, curved membrane, and nucleocapsid binding of the virus [29]. Virus release and pathogenesis are the roles of the envelope (E) protein [30,31]. Binding of the RNA genome of the virus is the responsibility of the nucleocapsid (N) protein, but it is also important for virus replication (Fig. 1 ) [32].
Fig. 1.
Structure of SARS-CoV-2.
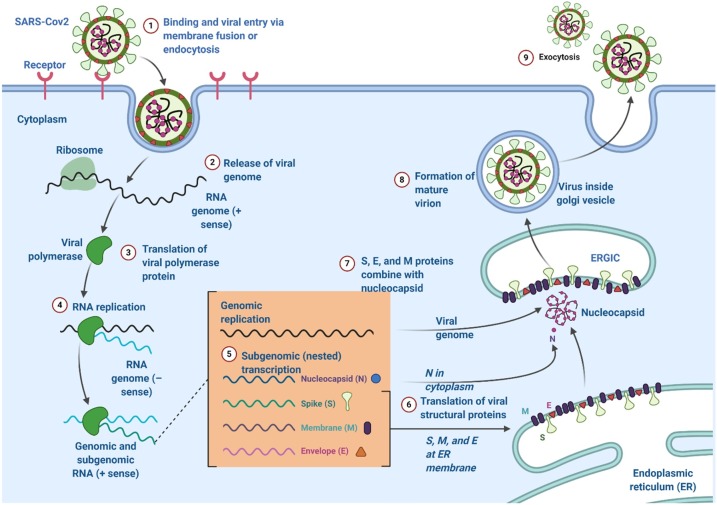
CoVs must reach the cytoplasm to replicate, [33] so they must interact with cell receptors [34,35]. S glycoproteins from SARS-CoV-2 interact with ACE2 receptors on the membranes of lung cells (similar to that seen with SARS-CoV) [14]. Following receptor–virus binding, the virus and cell membrane fuse after breakdown of S glycoproteins by proteolytic enzymes, thereby leading to entry of the viral genome into the cytoplasm [36]. Type-II transmembrane serine proteases are ACE2-associated proteins, and can enhance virus fusion [37,38]. Thereafter, replication of the genomic RNA and mRNA transcription of the virus occur, which involves synthesis of genomic RNA from negative-strand RNA (Fig. 2 ) [39].
Fig. 2.
Lifecycle of coronaviruses.
Consequently, S, E and M proteins are newly formed and incorporated into the endoplasmic reticulum or Golgi body. The nucleocapsid protein and genomic RNA combine for nucleocapsid formation. Then, virus particles develop into endoplasmic reticulum-Golgi intermediate compartment [19]. Thereafter, N proteins capsidate the viral genome for formation of mature viruses [40]. Then, virus particles combine with the plasma membrane for virus release [41].
Symptoms for people infected with SARS-CoV-2 are dry cough, fever, dyspnoea, headache and pneumonia leading to respiratory failure and even death [14]. However, such signs are considered to be mild responses in relation to the symptoms elicited by SARS and MERS [4]. An incubation period of 2–10 days for SARS-CoV-2 ensures a rapid outbreak through person-to-person transmission via contaminated hands and droplets spread through coughs and sneezes [42]. Antiviral medications are not sufficient due to the ability of virus to change its genetic structure [43].
3. Characterisation and potential use of kefir
Kefir was patented first in Eastern Europe, the Balkans, and the Caucasus. It is an acid-alcohol fermented milk with a creamy texture [44,45]. The diameter range of kefir grains is 0.3–3.5 cm [46,47]. Kefir grains comprise probiotic microorganisms that exist in a complex matrix of proteins and polysaccharides [48,49]. Kefir is formed by mixing kefir grains comprising different populations of lactic-acid bacteria (LAB) and yeasts with fresh milk at room temperature. The incubation period offers a great opportunity for the microbial community of kefir grains to develop and spread out, which leads to the addition or loss of bacteria, yeasts and their genes [22,24,25]. Kefir can be formed readily in various ways, including traditional and commercial approaches [50]. Kefir possesses several health benefits [[51], [52], [53]] associated with its microbial community and their metabolic yields, such as organic acids [54,55]. In addition, commercial interest is increasing the use of kefir as a nutritional medium to promote the growth of health-promoting bacteria [[56], [57], [58]]. The principal polysaccharide in kefir grains is the heteropolysaccharide kefiran, which consists of equivalent amounts of galactose and glucose that are mostly formed by Lactobacillus kefiranofaciens [59]. Kefiran increases the viscosity of acidic milk [60]. Kefiran can produce gel materials with good viscosity at low temperatures, and can be used to improve fermented products [61]. Moreover, kefiran has important antitumor, antifungal, antibacterial properties [62,63], as well as immunomodulatory or epithelium-protecting [45], anti-inflammatory [64], healing [53], and antioxidant activity [47].
The most common bacterial genera in kefir grains and milk are Lactococcus, Streptococcus, Lactococcus lactis subspecies lactis, Lactobacillus delbrueckii subspecies bulgaricus, L. helveticus, L. casei subspecies pseudoplantarum, L. skefiri, L. kefir, L. brevis and Streptococcus thermophiles, which account for about 37–90 % of the microbial population (Table 1) [[22], [23], [24], [25]]. For centuries, these bacteria have been known to have important health benefits [65]. Also, other bacterial species, such as Leuconostoc mesenteroides or yeast species, might dominate in some kefir grains [25]. The feature that distinguishes kefir from other fermented dairy products is that kefir grains have many yeast populations [66,67].
The composition of kefir is dependent upon the type of the milk (growth media) used in fermentation. The nutritional importance of kefir is based on its enrichment with various biomolecules (e.g., minerals, sugars, carbohydrates, proteins, peptides, vitamins, fats) as well as secondary metabolites (e.g., catechin, vanillin, ferulic acid, and salicylic acid) [68]. Vitamins B1, B2, B5 and C, elements in high concentrations (e.g., calcium, magnesium, potassium, sodium) and low concentrations (e.g., zinc, copper, iron) as well as essential amino acids (e.g., serine, threonine, alanine, lysine, valine, isoleucine, methionine, phenylalanine, tryptophan) are present in kefir [[69], [70], [71]]. These components have important roles in improving immunomodulation, digestion, metabolism, energy balance, healing, the central nervous system, and homeostasis [[70], [71], [72]]. Nisoli et al. reported that kefiran-containing amino acids extend the healthy lifespan of older people and improve the mental status of patients with severe traumatic brain injury [73].
3.1. Response of the immune system to kefir supplementation
The gut microbiome (GM) is the totality of microorganisms, bacteria, viruses, protozoa, and fungi, and their collective genetic material, present in the gastrointestinal tract (GIT). The GM could be applied for disease treatment/prevention because it can regulate immune responses if applied in suitable amounts [65,74]. The GM has shown efficacy against influenza viruses and Streptococcus pneumoniae in animal models [75].
Different probiotics show different abilities in supporting and regulating the innate and adaptive immune systems [76]. They contain immunostimulatory moieties, including lipoteichoic acid, peptidoglycans, and nucleic acids [77]. Probiotics can also reduce the severity of different types of infections in the GIT [76] and upper respiratory tract [78,79]. Probiotic microorganisms can suppress viral activity and virus entry into host cells by binding with the virus [80].
The potential antiviral mechanism of action for some probiotics could be direct trapping, enhancement of immune compounds, as well as development of bacteriocins, lactic acid and hydrogen peroxide as antiviral agents [81]. The beneficial activities of probiotic microorganisms can operate through a direct effect upon live microbial cells or an indirect effect through microbial secretion of metabolites (biomolecules) [82]. Möller et al. demonstrated that the biomolecules present in fermented dairy milk can enhance production of lymphocytes and Ig molecules [83].
Several scholars have discussed the influence of kefir on the immune system. In a murine model, Yasui et al. investigated the antiviral activity of Bifidobacterium breve upon oral administration against influenza viruses [84]. They showed that B. breve protected the lower respiratory tract from viral infection. Other studies have shown the population of nasal pathogens to be reduced after consumption of fermented milk [85,86].
Vinderola et al. indicated the efficacy of kefir (and other probiotics) as an immune-system enhancer. They showed that kefir can regulate the immune response by increasing the number of IgA+ intestinal and bronchial cells, but also its phagocytic potential for peritoneal and pulmonary macrophages, if applied in mice [87]. Also, they reported that expression of the cytokines IL-4, IL-6 and IL-10 increased in the lamina propria of the small intestine of mice administered kefir. Perdigon et al. reported that mice fed fermented milk showed immune responses such as an increase in the number of IgA+ cells, macrophage activity, and specific antibody responses [[88], [89], [90]]. Can et al. demonstrated that the IgM level increased after giving kefir to Çoruh trout (Salmo coruhensis) [91]. Thoreux and Schmucker studied the immunomodulatory activity of kefir in young rats immunised within the duodenum with cholera toxin (CT) [92]. They found that the level of anti-CT IgA was increased in the serum, Peyer’s patches, mesenteric lymph nodes, spleen, and intestinal lamina propria of rats fed kefir compared with that of rats in the CT group. They suggested that kefir induces an intestinal mucosal immune response against CT in young adult rats, but not in senescent rats. Further investigations showed enhancement of expression of the cytokines IL-1α, IFN-γ, TNF-α, IL-6 and IL-10 after administrating kefiran (produced by the kefir microflora L. kefiranofaciens) to different groups of mice [26]. Similarly, increases in the serum concentration of IFN-γ have been observed in response to smallpox vaccination, suggesting a similar mode of action of kefir to that mentioned above [93]. Indicating the similarity between kefir mechanism and vaccine ability to treat viral infection since IFN-based treatments showed ability in treating hepatitis C virus infecting patient [94,95].
The immunomodulatory effect of kefir also extends to its derivatives. Several studies have demonstrated the potency of the probiotic populations of kefir (e.g., LAB) in modulating specific and nonspecific immune responses [96]. The novel probiotic strain L. kefiranofaciens M1 present in kefir grains has shown immunoregulatory, anti-allergic, anti-asthmatic, and anti-colitis activities in vitro and in vivo in germ-free mice [97]. L. reuteri and L. plantarum have shown positive effects against pneumonia virus-infected mice [98]. A period of 2–12 weeks for consumption of L. plantarum HEAL 9 and L. paracasei 8700 showed a reduction in the risk of acquiring common-cold infections caused by human rhinoviruses via induction of CD4+ and CD8+ cells [99]. Cavicchioli et al. demonstrated that purified bacteriocins of L. lactis subspecies lactis had noticeable inhibitory activity against herpes poliovirus-1 [100]. Moreover, Nanis et al. showed that administration of probiotics such as Lactobacillus acidophilus and Bifidobacteria species enhanced the healing response to treatments of infection by the hepatitis-C virus (HCV) by regulation of IFN-α and ribavirin [95]. Weiss et al. reported that administration of L. acidophilus NCFM resulted in upregulation of the genes linked to murine bone marrow-derived dendritic cells via activation of expression of viral defence genes in a toll-like receptor-2–IFN-β-dependent manner [101].
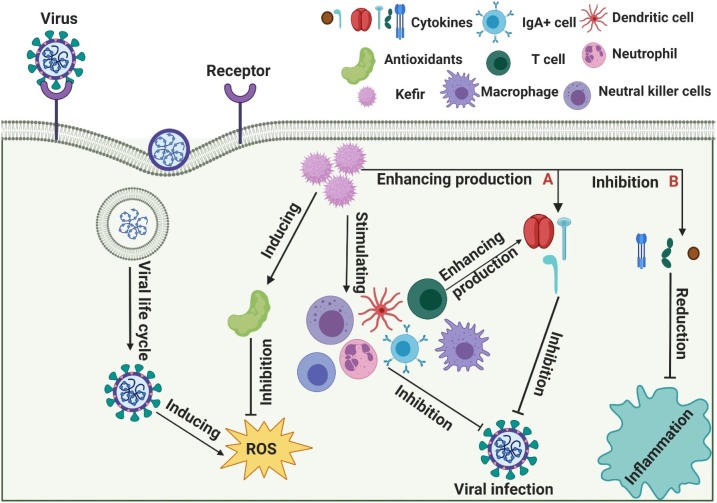
Adiloğlu et al. studied the effect of oral administration of kefir on the human innate immune system [102]. They supplied 18 healthy participants with kefir for 6 weeks, and measured serum levels of TNF-α, IL-1, IL-5, IL-8, transforming growth factor-β, haemoglobin, creatinine and alanine aminotransferase. Results showed increases in immune-reaction polarisation for the Th1 type, reduction in the response of the Th2 type and, consequently, an allergic response. Also, a reduction in IL-8 expression after kefir supplementation might control the inflammatory response by inhibiting neutrophil chemotaxis. In contrast, an increase in IL-5 expression might enhance IgA secretion in the gastrointestinal mucosa, resulting in a more efficient immune response in the intestinal lumen (Fig. 3 ).
Fig. 3.
Potential mechanism of action of kefir against viral infection. (A) Kefir enhances the immune response by stimulating cytokine production, including interferon gamma (IFN-γ), interleukin (IL)-2, and IL-12. (B) Kefir inhibits the inflammatory response by suppressing cytokine production, such as IL-6, IL-1 and tumour necrosis factor (TNF)-α.
3.2. Anti-inflammatory activity of kefir
Viral infection is associated with overexpression of cell cytokines. For example, COVID-19 is associated with overactivation of effector T cells and additional synthesis of inflammatory cytokines (especially IL-6). Such action is termed the ‘cytokine storm’, and has been assigned as a life-threatening complication in patients treated with antibody-based immune therapy. Besides, other cytokines, such as IL-1, TNF-α, and IFN-g, are also formed. All products participate in the pathological events resulting in plasma leakage, vascular permeability, and intravascular coagulation [103,104]. Giavanni et al. suggested a relationship between the cytokine storm and SARS-CoV-2-induced pneumonia symptoms [104]. No increase in SARS-CoV-2-driven pneumonia has been reported in patients with immune disorders and taking cytokine blockers. Scholars have speculated that patients with immune-mediated diseases taking IL-6 inhibitors (or compounds that inhibit immune pathways terminating in IL-6 production or mediation of IL-6 signalling) might be protected against SARS-CoV-2-driven pneumonia.
Along with the microbial community present in kefir, other fermentation products and metabolites have important activities. Many of these byproducts may have extensive outcomes in the host without the presence of a microbial community [48]. Chen et al. studied the anti-inflammatory and antioxidant activities of kefir peptides against particulate material smaller than 4 μm (PM4.0)-induced lung inflammation in transgenic homozygous nuclear factor-kappa B (NF-κB)-luciferase+/+ mice [27]. They demonstrated that kefir peptides had potent anti-inflammatory effects through reduction of expression of the proinflammatory cytokines IL-lβ, IL-4, IL-6 and TNF-α in lung tissue by inhibition of NF-κB signalling. Also, the peptides acted as antioxidant agents that reduced levels of reactive oxygen species through stimulation of activity of total superoxide dismutase in the lung. Rosa et al. reported that 10 weeks of kefir feeding could decrease expression of proinflammatory cytokines (IL-1β), oxidative-stress markers (malondialdehyde, hydroperoxides) in the adipose tissue of hypertensive rats [105]. Andrade et al. showed that kefir administration resulted in a 42 % reduction of TNF-α/IL-10 expression and a 50 % reduction in expression of (proinflammatory) IL-6 expression, concurrent with enhancement of (anti-inflammatory) IL-10 expression [106]. The freeze-dried polysaccharide extract of Tibetan kefir has shown potent inhibitory action of hyaluronidase with minimal activity at 2.08 mg/mL [105]. Seo et al. reported that extracellular vesicles generated from kefir reduced inflammation in intestinal cells by suppressing the synthesis of proinflammatory cytokines [107]. Also, they reported a significant decrease in bodyweight loss and rectal bleeding when inflammatory bowel disease-induced mice were treated with kefir-derived Lactobacillus extracellular vesicles.
An important study by Morsy et al. demonstrated that drinking kefir might benefit patients with chronic HCV infection [108]. Also, they showed considerable amelioration of HCV infection if kefir was taken by patients, thereby suggesting its ability to stimulate the immune system besides its anti-inflammatory and antioxidant effects. Carasi et al. studied the anti-inflammatory activity of L. kefiri CIDCA 8348, which reduced expression of proinflammatory mediators in Peyer’s patches and mesenteric lymph nodes, but enhanced IL-10 production [109]. In addition, in the ileum, IL-10, chemokine (C-X-C motif) ligand-1 and mucin 6 genes were induced whereas, in the colon, mucin-4 expression was upregulated and expression of IFN-g, granulocyte-macrophage colony-stimulating factor, and IL-1b genes was downregulated.
3.3. Antiviral activity of kefir
A considerable increase in the number of viral diseases, especially those due to new emerging viruses (Chikungunya, Dengue, Ebola, Zika, SARS-CoV-2) has wrought havoc on public health worldwide [106,110]. Also, there are not enough drugs being developed to halt the development of resistance to antiviral drugs.
A strong relationship between human nutrition, the immune system and their role in the occurrence, development and suppression of infectious diseases might be a beneficial concept for seeking products that enhance the immune system [111]. One meta-analysis suggested that probiotics and prebiotics can enhance the immunogenicity of influenza vaccines in adults [112].
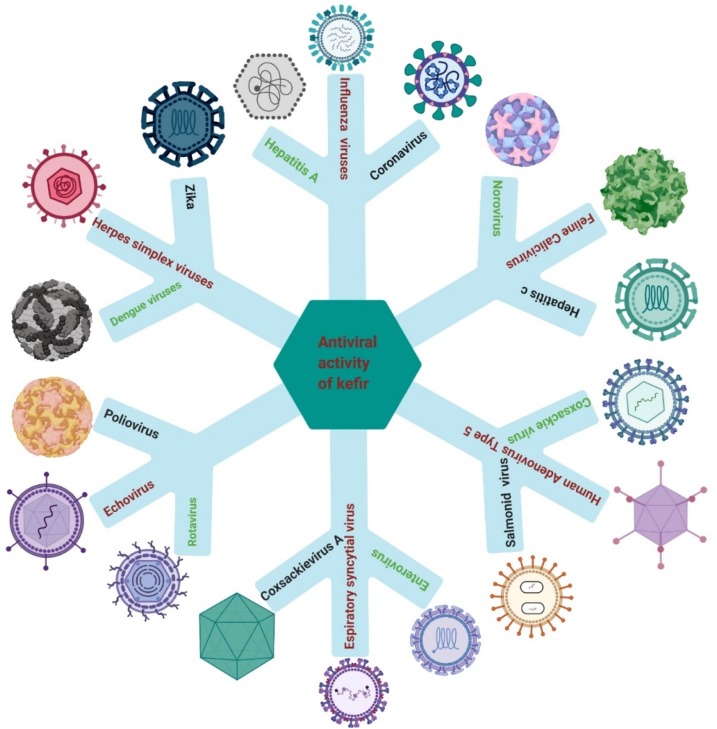
With regard to antiviral drugs with few side effects, several scholars have investigated if probiotic products could be used as remedies alongside antiviral agents [[113], [114], [115]]. de Andrade et al. demonstrated that the ability of kefir (37.5 μg/mL) against the Zika virus occurred via suppression of the effect on epithelial cells, or by antagonism of the impact of the virus on the proliferation of T-lymphocytes [106]. Employment of L. kefiri (100 μg/mL) has been shown to enhance the development of antiviral cytokines and human monocyte-derived dendritic cells so that they might be applied as antiviral and anticancer agents [116]. Yeast, as one of the components of kefir, has been shown to be a site for replication of RNA viruses such as HCV and SARS; this feature may help understanding the mechanism of viral-replication reduction. Approximately 100 non-essential yeast genes influence replication of the RNA viruses of some plants (e.g., tomato bushy stunt virus, Brome mosaic virus), so yeast might also affect other human RNA viruses [117,118]. Parsons and colleagues isolated amantadine hydrochloride from yeast and showed it to have antiviral ability ( Fig. 4 ) [119].
Fig. 4.
Antiviral activities of kefir.
3.3.1. Virus-induced pneumonia
Influenza viruses produce seasonal outbreaks regularly in humans that can be counteracted with antiviral therapy and vaccines. Nevertheless, these therapeutic actions frequently show limited efficacy in immunosuppressed or older persons. Moreover, the high genetic flexibility of viruses hamper the efficacy of active vaccines and antiviral agents [120]. In these situations, circadian administration of probiotic microbes might be helpful against influenza viruses [121].
The bacterial strain lactic acid Enterococcus faecium NCIMB 10,415 can blunt the effects of influenza viruses via direct contact and interactions [20]. Choi et al. studied the antiviral effect of the cell-free supernatants (CFS) of five yogurts fermented below anaerobic incubation with L. acidophilus, L. rhamnosus, L. plantarum, Streptococcus thermophilus, and Bifidobacterium bifidum compared with that of seven RNA viruses (including influenza viruses) (122). They showed that yogurt metabolites fermented with probiotic bacteria could be employed to improve drugs and fermented milk-based foods. Maruo et al. studied the antiviral potential of milk fermented with exopolysaccharide-producing Lactococcus lactis subspecies cremoris in mice [123]. They demonstrated that the mice lungs of the group treated with L. lactis subspecies Cremoris-fermented milk had a significant decrease in the virus titre compared with that of the control group. Goto et al. investigated the antiviral outcomes of non-live and live L. acidophilus in mice infected with influenza virus (H1N1). They suggested that the improvement in NK-cell activity in the lung elicited by several antiviral cytokines and chemokines following oral administration of L. acidophilus might protect against influenza-virus infection [124]. A comparison study between a commercially available drug against influenza virus (H1N1) and isolates of L. plantarum showed that the latter had higher efficacy [125]. Oral intake of L. rhamnosus improved the survival rate of mice by motivating humoral and cellular immune responses, and presented improved resistance in the host against influenza-virus infection [120]. Moreover, Bae and colleagues screened the antiviral properties of L. plantarum and Leuconostoc mesenteroides probiotics on human seasonal influenza viruses and avian influenza viruses [121]. They reported that viral replication in mouse lungs was controlled significantly by these probiotics. Lactobacillus gasseri has several important effects, [121] and shows significant activity against the respiratory syncytial virus (RSV), which is the main causative pathogen of pneumonia and bronchiolitis in children [126]. The RSV titre in mice lungs is reduced considerably following L. gasseri treatment, and a similar pattern is observed for expression of proinflammatory cytokines resulting from RSV infection.
A cellular proteomic study showed SNF2-related CBP activator protein to be a bioactive molecule in the activity of L. gasseri versus the RSV [126]. In addition, the b-glucans of Saccharomyces cerevisiae have shown effects against the swine influenza virus by increasing production of IFN-g and nitric oxide [127]. Frieman et al. designed a specific assay to detect the small molecules responsible for blocking SARS-CoV replication based on their suppression of non-structural protein 3 (nsp3) or papain-like protease (PLP). The rationale for this screening was that enhanced expression of nsp3 in S. cerevisiae caused a remarkably slow growth of the phenotype [128]. PLP is crucial for virus replication. To discover which molecules are responsible for suppression of SARS-CoV replication, a yeast-based assay was designed for PLP activity. A set of molecules was screened to test their inhibitory effect of PLP and maintain growth. NSC158362 blocked SARS-CoV replication exclusively, but no effect on the protease, deubiquitinase, or anti-IFN activities of nsp3 was detected, which suggested an inhibitory mechanism for SARS-CoV replication in which PLP activity was not clearly evident. Instead, direct inhibition via modification of PLP function might be expected. Moreover, the activity of PLP proteases was inhibited in a cell-based assay when treated with the suppressor NSC158011 (Table 1 ).
Table 1.
Probiotic microorganisms in kefir and their antiviral activity.
| Microbial species | Antiviral activity | References |
|---|---|---|
| Lactobacillus casei | Rotavirus | [24,142,143,144] |
| Lactobacillus brevis | Herpes simplex virus type 2 (HSV-2) | [24,25,142,143,145] |
| Lactobacillus plantarum | Echovirus E7 and E19, Influenza virus H1N1, Coxsackie virus, Influenza virus, Seasonal and Avian Influenza viruses | [23,54,142,145] |
| Lactobacillus acidophilus | Hepatitis C, Influenza virus, Rotavirus, Coxsackie | [142,143,145,146] |
| Lactobacillus gasseri | Influenza A virus, Espiratory syncytial virus (RSV) | [142,143] |
| Lactobacillus crispatus | HSV-2 | [142,147] |
| Lactobacillus amylovorus | Echovirus E7 and E19 | [142] |
| L. rhamnosus | Influenza virus, Herpes simplex virus type 1, Coxsackie | [142] |
| L. sakei | Salmonid viruses | [142] |
| L. reuteri | Coxsackievirus A and Enterovirus 71 | [142] |
| Lactococcus lactis subsp. lactis | Feline Calicivirus,norovirus (NV), Herpes simplex virus 1 (HSV-1) and Poliovirus (PV-1) | [22,24,25,54,143,144,147,148,149,150] |
| Lactococcus lactis subsp. cremoris | Influenza virus | [149,150,151] |
| Streptococcus thermophilus | Coxsackie, Influenza virus | [122] |
| Leuconostoc spp. | Human Adenovirus Type 5 | [[143]] |
| Leuconostoc mesenteroides | Salmonid viruses, Seasonal and Avian Influenza viruses | [22,24,142,144,151] |
| Bifidobacterium spp. | Rotavirus | [151] |
| B. longum | Rotavirus | [[146]] |
| Saccharomyces cerevisiae | Swine influenza virus, Coronaviruses | [24,25,128,143,147,152,153,154,155,156] |
| Ganoderma lucidum | Enterovirus 71 | [152] |
| Penicillium sp. Vega 347 | Dengue viruses | [152] |
3.3.2. Rotaviruses
Rotaviruses are the source of diarrhoeal disease in infants and young children. Several studies have demonstrated that Lactobacillus species (e.g., L. casei and L. acidophilus) and Bifidobacterium species (e.g., B. longum) have activity against rotaviruses [114,129,130]. The activity of L. casei and Bifidobacterium species against rotavirus infection has been observed by construction of NSP4 protein and Ca2+ release. The study showed a decrease in the influence of the rotavirus infection by reducing the destruction of the cells ( Table 1 ) [114].
3.3.3. Herpes simplex virus (HSV)
The HSV is the leading cause of herpes infection, which can develop in many parts of the body, but most frequently on the genitals (HSV-2) or mouth (HSV-1) [100,[131], [132], [133]]. L. lactis subspecies lactis, L. rhamnosus and L. brevis, and L. crispatus have activity against HSV-1 and HSV-2, respectively [100]. A recent study revealed that purified bacteriocins of L. lactis subspecies lactis had noticeable inhibitory activity against HSV-1 and could be used as new antiviral agents [100]. Moreover, Khani et al. stated that L. rhamnosus improved macrophage viability for HSV-1 removal [131]. L. brevis has an inhibitory effect on HSV-2 reproduction related to composites with a molecular weight >10 kDa that is possible because of a heat-resistant, non-protein cell-surface bacterial component [132]. Mousavi et al. screened the inhibitory activity of L. crispatus against HSV-2 in mammalian Vero and HeLa cell lines: [133] L. crispatus appeared to ‘catch’ HSV-2 particles. Moreover, realisation of L. crispatus microcolonies on the cell surface might block HSV-2 receptors and avoid viral entrance to cells during early infection ( Table 1 ).
3.3.4. Enteroviruses
Enteroviruses are the source of many infections that are, in general, mild. Nevertheless, enterovirus infection of the central nervous system can cause serious health disorders [134]. Most enteroviruses reproduce in the GIT, so LAB can defend against them in the GIT [134]. Numerous studies have shown the activity of commercially available probiotics as antiviral agents against selected enteroviruses [134,135]. In 2016, two commercially available probiotics were investigated for antiviral activity. Lactobacillus reuteri Protectis and Lactobacillus casei Shirota were examined against enterovirus 71 (EV71), coxsackievirus type A (CA), strain 6 (CA6) and CA16 (the main pathogen responsible for hand, foot and mouth disease) in human colon and skeletal muscle cell lines. L. reuteri Protectis showed substantial activity against EV71 [135]. The authors maintained that the antiviral outcome was reached through a physical interaction between virus particles and bacteria, which stopped virus admission into the mammalian host cell. An antiviral outcome was not documented for L. casei Shirota. Sunmola et al. examined the antiviral activity of the LAB L. plantarum and L. amylovorus, against enteroviruses [134]. They demonstrated the antiviral activity of L. plantarum and L. amylovorus in bacterial cell pellets, CFS, and broth culture against echovirus 7 (E7) and E19 before and after treatment. In addition, inhibitory activity against coxsackieviruses was shown by L. plantarum, L. acidophilus and L. rhamnosus strains and their derivatives (Table 1) [122,136].
3.3.5. Other viruses
Foodborne viruses, such as noroviruses (NVs) and the hepatitis-A virus, are major public-health concerns that necessitate development of new and efficacious methods to stop foodborne viral infections [137]. Aboubakr et al. determined the antiviral activity of probiotic LAB against feline calicivirus (an alternative to human NVs) [137]. They demonstrated that use of L. lactis subspecies lactis resulted in a reduction in virus titres. L. reuteri shows significant activity against CA by direct bacteria–virus interaction that impaired CA entry into host cells [135]. Moreover, investigation of the administration of probiotics such as L. acidophilus and Bifidobacteria species revealed an enhanced healing response to anti−HCV treatments by regulation of IFN-α and ribavirin ( Table 1 ) [95].
4. Conclusions and future aspects
The COVID-19 pandemic is wreaking havoc worldwide, and is a major concern for all scientific communities. A specific vaccine against SARS-CoV-2 is not available, but antiviral agents (ribavirin, acyclovir, ganciclovir, neuraminidase inhibitors) are under investigation [19]. However, any attempt to find suitable treatment to fight COVID-19 must involve the immune system.
Kefir and its components have a crucial regulatory role in the immune response. In this respect, activity has been reported against the Zika virus, HCV, hepatitis-B virus, influenza virus (H1N1), HSV, rhinoviruses and retroviruses.
It has been postulated that some COVID-19 patients die after the massive inflammatory response resulting from a cytokine storm involving 1 L-6, IL-1, TNF-α, and IFN-γ. A proposed initial solution to protect patients from the cytokine storm is blockade of IL-6 function or administration of a compound to suppress inflammation. Kefir can inhibit the activity of proinflammatory cytokines. Using kefir (and its byproducts) as an inhibitor of expression of proinflammatory cytokines in COVID-19 patients could be a viable policy.
SARS CoV-2 replication is dependent upon pH, so elucidating the link between kefir consumption and its ability to change the pH would be worthwhile [44,45]. Studies have reported the pH of kefir to be acidic (pH 4.6) [21,138]. This acidity is related to different populations of acidic bacteria [139,140]. Rea et al. reported that the acidic pH of kefir grains may interfere with pathogenic activities [139]. Because of its ability to produce acidic secondary metabolites and for them to not be degraded, kefir might change the pH in a specific area when it is consumed. Fusion of CoVs occurs in mildly alkaline pH [141], so ascertaining the connection between kefir consumption and pH alterations in a specific body site and viral infection would be worthwhile.
Based on all studies undertaken on kefir and its probiotic microbes, kefir may act as a protective agent against viral infections.
Authors’ contributions
All authors contributed to data analysis, drafting, or revising the article. All authors gave final approval of the published article and are accountable for all aspects of the work.
Consent for publication
Not applicable.
Data availability
The data supporting this article are available in Fig. 1, Fig. 2, Fig. 3, Fig. 4 and Table 1. The data sets analyzed in the present study are available from the corresponding author upon reasonable request.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
References
- 1.Kanauchi O., Andoh A., AbuBakar S., Yamamoto N. Probiotics and paraprobiotics in viral infection: clinical application and effects on the innate and acquired immune systems. Curr. Pharm. Des. 2018;24(6):710–717. doi: 10.2174/1381612824666180116163411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamison D.T., Breman J.G., Measham A.R., Alleyne G., Claeson M., Evans D.B. The World Bank; 2006. Disease Control Priorities in Developing Countries. [PubMed] [Google Scholar]
- 3.Pyrc K., Berkhout B., van der Hoek L. Identification of new human coronaviruses. Expert Rev. Anti. Ther. 2007;5(2):245–253. doi: 10.1586/14787210.5.2.245. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerna G., Campanini G., Rovida F., Percivalle E., Sarasini A., Marchi A. Genetic variability of human coronavirus OC43‐, 229E‐, and NL63‐like strains and their association with lower respiratory tract infections of hospitalized infants and immunocompromised patients. J. Med. Virol. 2006;78(7):938–949. doi: 10.1002/jmv.20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., Guo D. Molecular mechanisms of coronavirus RNA capping and methylation. Virol. Sin. 2016;31(1):3–11. doi: 10.1007/s12250-016-3726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge X.-Y., Li J.-L., Yang X.-L., Chmura A.A., Zhu G., Epstein J.H. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503(7477):535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaki A.M., Van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 9.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan Y., Zhao K., Shi Z.L., Zhou P. Bat coronaviruses in China. Viruses. 2019;11(3) doi: 10.3390/v11030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu B., Zeng L.P., Yang X.L., Ge X.Y., Zhang W., Li B. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13(11) doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L., Wu Z., Ren X., Yang F., He G., Zhang J. Novel SARS-like betacoronaviruses in bats, China, 2011. Emerg Infect Dis. 2013;19(6):989–991. doi: 10.3201/eid1906.121648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Organization WH . 2020. Coronavirus Disease (COVID-19) Pandemic. [Google Scholar]
- 16.Dhama K., Karthik K., Khandia R., Chakraborty S., Munjal A., Latheef S.K. Advances in designing and developing vaccines, drugs, and therapies to counter Ebola virus. Front. Immunol. 2018;9:1803. doi: 10.3389/fimmu.2018.01803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baskar P., Collins G., Dorsey-Cooper B., Pyle R., Nagel J., Dwyer D. Serum antibodies to HIV-1 are produced post-measles virus infection: evidence for cross-reactivity with HLA. Clin. Exp. Immunol. 1998;111(2):251. doi: 10.1046/j.1365-2249.1998.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buisman A.-M., Abbink F., Schepp R.M., Sonsma J.A., Herremans T., Kimman T.G. Preexisting poliovirus-specific IgA in the circulation correlates with protection against virus excretion in the elderly. The Journal of infectious diseases. 2008;197(5):698–706. doi: 10.1086/527487. [DOI] [PubMed] [Google Scholar]
- 19.Li X., Song Y., Wong G., Cui J. Bat origin of a new human coronavirus: there and back again. Science China Life Sciences. 2020;63(3):461–462. doi: 10.1007/s11427-020-1645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z., Chai W., Burwinkel M., Twardziok S., Wrede P., Palissa C. Inhibitory influence of Enterococcus faecium on the propagation of swine influenza A virus in vitro. PloS one. 2013;8(1) doi: 10.1371/journal.pone.0053043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen B., Gurakan G.C., Unlu G. Kefir: a multifaceted fermented dairy product. Probiotics Antimicrob Proteins. 2014;6(3-4):123–135. doi: 10.1007/s12602-014-9168-0. [DOI] [PubMed] [Google Scholar]
- 22.Chen H.C., Wang S.Y., Chen M.J. Microbiological study of lactic acid bacteria in kefir grains by culture-dependent and culture-independent methods. Food Microbiol. 2008;25(3):492–501. doi: 10.1016/j.fm.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 23.MGDCP Miguel, Cardoso P.G., de Assis Lago L., Schwan R.F. Diversity of bacteria present in milk kefir grains using culture-dependent and culture-independent methods. Food ResInt. 2010;43:1523–1528. [Google Scholar]
- 24.Simova E., Beshkova D., Angelov A., Hristozova T., Frengova G., Spasov Z. Lactic acid bacteria and yeasts in kefir grains and kefir made from them. J. Ind. Microbiol. Biotechnol. 2002;28(1):1–6. doi: 10.1038/sj/jim/7000186. [DOI] [PubMed] [Google Scholar]
- 25.Witthuhn R.C., Schoeman T., Britz T.J. Isolation and characterization of the microbial population of different South African kefir grains. Int JDairyTechnol. 2004;57:33–37. [Google Scholar]
- 26.Vinderola G., Perdigon G., Duarte J., Thangavel D., Farnworth E., Matar C. Effects of kefir fractions on innate immunity. Immunobiology. 2006;211(3):149–156. doi: 10.1016/j.imbio.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Chen H.-L., Hung K.-F., Yen C.-C., Laio C.-H., Wang J.-L., Lan Y.-W. Kefir peptides alleviate particulate matter< 4 μm (PM 4.0)-induced pulmonary inflammation by inhibiting the NF-κB pathway using luciferase transgenic mice. Sci. Rep. 2019;9(1):1–13. doi: 10.1038/s41598-019-47872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv. Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011;174(1):11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeDiego M.L., Álvarez E., Almazán F., Rejas M.T., Lamirande E., Roberts A. A severe acute respiratory syndrome coronavirus that lacks the E gene is attenuated in vitro and in vivo. J. Virol. 2007;81(4):1701–1713. doi: 10.1128/JVI.01467-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieto-Torres J.L., DeDiego M.L., Verdia-Baguena C., Jimenez-Guardeno J.M., Regla-Nava J.A., Fernandez-Delgado R. Severe acute respiratory syndrome coronavirus envelope protein ion channel activity promotes virus fitness and pathogenesis. PLoS Pathog. 2014;10(5) doi: 10.1371/journal.ppat.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui L., Wang H., Ji Y., Yang J., Xu S., Huang X. The nucleocapsid protein of coronaviruses acts as a viral suppressor of RNA silencing in mammalian cells. J. Virol. 2015;89(17):9029–9043. doi: 10.1128/JVI.01331-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7(6):439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enjuanes L., Almazán F., Sola I., Zuñiga S. Biochemical aspects of coronavirus replication and virus-host interaction. Annu. Rev. Microbiol. 2006;60:211–230. doi: 10.1146/annurev.micro.60.080805.142157. [DOI] [PubMed] [Google Scholar]
- 36.Fehr A.R., Perlman S. Springer; 2015. Coronaviruses: an Overview of Their Replication and Pathogenesis. Coronaviruses; pp. 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85(2):873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertram S., Glowacka I., Müller M.A., Lavender H., Gnirss K., Nehlmeier I. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J. Virol. 2011;85(24):13363–13372. doi: 10.1128/JVI.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Haan C.A., Rottier P.J. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 2005;64:165–230. doi: 10.1016/S0065-3527(05)64006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zolnikova O., Komkova I., Potskherashvili N., Trukhmanov A., Ivashkin V. Application of probiotics for acute respiratory tract infections. Ital. J. Med. 2018;12(1):32–38. [Google Scholar]
- 44.Fontán M.C.G., Martínez S., Franco I., Carballo J. Microbiological and chemical changes during the manufacture of Kefir made from cows’ milk, using a commercial starter culture. Int. Dairy J. 2006;16(7):762–767. [Google Scholar]
- 45.Serafini F., Turroni F., Ruas-Madiedo P., Lugli G.A., Milani C., Duranti S. Kefir fermented milk and kefiran promote growth of Bifidobacterium bifidum PRL2010 and modulate its gene expression. Int. J. Food Microbiol. 2014;178:50–59. doi: 10.1016/j.ijfoodmicro.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 46.Garrote G.L., Abraham A.G., De Antoni G.L. Biotechnology of Lactic Acid Bacteria: Novel Applications. 2010. Microbial interactions in Kefir: a natural probiotic drink; p. 327. [Google Scholar]
- 47.Chen Z., Shi J., Yang X., Nan B., Liu Y., Wang Z. Chemical and physical characteristics and antioxidant activities of the exopolysaccharide produced by Tibetan kefir grains during milk fermentation. Int. Dairy J. 2015;43:15–21. [Google Scholar]
- 48.Bourrie B.C., Willing B.P., Cotter P.D. The microbiota and health promoting characteristics of the fermented beverage kefir. Front. Microbiol. 2016;7:647. doi: 10.3389/fmicb.2016.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Radhouani H., Goncalves C., Maia F.R., Oliveira J.M., Reis R.L. Biological performance of a promising Kefiran-biopolymer with potential in regenerative medicine applications: a comparative study with hyaluronic acid. J. Mater. Sci. Mater. Med. 2018;29(8):124. doi: 10.1007/s10856-018-6132-7. [DOI] [PubMed] [Google Scholar]
- 50.Bensmira M., Nsabimana C., Jiang B. Effects of fermentation conditions and homogenization pressure on the rheological properties of Kefir. LWT-Food Science and Technology. 2010;43(8):1180–1184. [Google Scholar]
- 51.Vasiljević D.Z., Mansouri A., Anzi L., Sordan R., Stojanović G.M. Performance analysis of flexible ink-jet printed humidity sensors based on graphene oxide. IEEE Sens. J. 2018;18(11):4378–4383. [Google Scholar]
- 52.McCue P.P., Shetty K. A model for the involvement of lignin degradation enzymes in phenolic antioxidant mobilization from whole soybean during solid-state bioprocessing by Lentinus edodes. Process. Biochem. 2005;40(3–4):1143–1150. [Google Scholar]
- 53.Rodrigues K.L., Caputo L.R.G., Carvalho J.C.T., Evangelista J., Schneedorf J.M. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents. 2005;25(5):404–408. doi: 10.1016/j.ijantimicag.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 54.Garrote G.L., Abraham A.G., De Antoni G.L. Chemical and microbiological characterisation of kefir grains. J. Dairy Res. 2001;68(4):639–652. doi: 10.1017/s0022029901005210. [DOI] [PubMed] [Google Scholar]
- 55.Ismaiel A.A., Ghaly M.F., El-Naggar A.K. Milk kefir: ultrastructure, antimicrobial activity and efficacy on aflatoxin B1 production by Aspergillus flavus. Curr. Microbiol. 2011;62(5):1602–1609. doi: 10.1007/s00284-011-9901-9. [DOI] [PubMed] [Google Scholar]
- 56.Vinderola G., Perdigón G., Duarte J., Farnworth E., Matar C. Effects of the oral administration of the products derived from milk fermentation by kefir microflora on immune stimulation. J. Dairy Res. 2006;73(4):472–479. doi: 10.1017/S002202990600197X. [DOI] [PubMed] [Google Scholar]
- 57.Medrano M., Pérez P.F., Abraham A.G. Kefiran antagonizes cytopathic effects of Bacillus cereus extracellular factors. Int. J. Food Microbiol. 2008;122(1–2):1–7. doi: 10.1016/j.ijfoodmicro.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 58.AMdO Leite, Miguel M.A.L., Peixoto R.S., Rosado A.S., Silva J.T., Paschoalin V.M.F. Microbiological, technological and therapeutic properties of kefir: a natural probiotic beverage. Braz. J. Microbiol. 2013;44(2):341–349. doi: 10.1590/S1517-83822013000200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.ZAJŠEK K., Kolar M., GORŠEK A. Characterisation of the exopolysaccharide kefiran produced by lactic acid bacteria entrapped within natural kefir grains. Int. J. Dairy Technol. 2011;64(4):544–548. [Google Scholar]
- 60.Rimada P.S., Abraham A.G. Kefiran improves rheological properties of glucono-δ-lactone induced skim milk gels. Int. Dairy J. 2006;16(1):33–39. [Google Scholar]
- 61.Zajšek K., Goršek A., Kolar M. Cultivating conditions effects on kefiran production by the mixed culture of lactic acid bacteria imbedded within kefir grains. Food Chem. 2013;139(1–4):970–977. doi: 10.1016/j.foodchem.2012.11.142. [DOI] [PubMed] [Google Scholar]
- 62.Cevikbas A., Yemni E., Ezzedenn F.W., Yardimici T., Cevikbas U., Stohs S. Antitumoural antibacterial and antifungal activities of kefir and kefir grain. Phytother. Res. 1994;8(2):78–82. [Google Scholar]
- 63.Wang Y., Ahmed Z., Feng W., Li C., Song S. Physicochemical properties of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir. Int. J. Biol. Macromol. 2008;43(3):283–288. doi: 10.1016/j.ijbiomac.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 64.Rodrigues K., Carvalho J., Schneedorf J. Anti-inflammatory properties of kefir and its polysaccharide extract. Inflammopharmacology. 2005;13(5-6):485–492. doi: 10.1163/156856005774649395. [DOI] [PubMed] [Google Scholar]
- 65.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 66.Tamang J.P., Watanabe K., Holzapfel W.H. Review: diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 2016;7:377. doi: 10.3389/fmicb.2016.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamime A.Y. Fermented milks: a historical food with modern applications--a review. Eur. J. Clin. Nutr. 2002;56(Suppl 4):S2–S15. doi: 10.1038/sj.ejcn.1601657. [DOI] [PubMed] [Google Scholar]
- 68.Bensmira M., Jiang B. Total phenolic compounds and antioxidant activity of a novel peanut based kefir. Food Sci. Biotechnol. 2015;24(3):1055–1060. [Google Scholar]
- 69.Sarkar S. Potential of kefir as a dietetic beverage–a review. Br. Food J. 2007 [Google Scholar]
- 70.Simova E., Simov Z., Beshkova D., Frengova G., Dimitrov Z., Spasov Z. Amino acid profiles of lactic acid bacteria, isolated from kefir grains and kefir starter made from them. Int. J. Food Microbiol. 2006;107(2):112–123. doi: 10.1016/j.ijfoodmicro.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 71.Bakircioglu D., Topraksever N., Yurtsever S., Kizildere M., Kurtulus Y.B. Investigation of macro, micro and toxic element concentrations of milk and fermented milks products by using an inductively coupled plasma optical emission spectrometer, to improve food safety in Turkey. Microchem. J. 2018;136:133–138. [Google Scholar]
- 72.Grohmann U., Bronte V. Control of immune response by amino acid metabolism. Immunol. Rev. 2010;236(1):243–264. doi: 10.1111/j.1600-065X.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- 73.Bifari F., Nisoli E. Branched‐chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. Br. J. Pharmacol. 2017;174(11):1366–1377. doi: 10.1111/bph.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heineman J., Bubenik S., McClave S., Martindale R. Fighting fire with fire: is it time to use probiotics to manage pathogenic bacterial diseases? Curr. Gastroenterol. Rep. 2012;14(4):343–348. doi: 10.1007/s11894-012-0274-4. [DOI] [PubMed] [Google Scholar]
- 75.Ichinohe T., Pang I.K., Kumamoto Y., Peaper D.R., Ho J.H., Murray T.S. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guandalini S. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2011;45(Suppl):S149–53. doi: 10.1097/MCG.0b013e3182257e98. [DOI] [PubMed] [Google Scholar]
- 77.Pimentel-Nunes P., Soares J.B., Roncon-Albuquerque R., Jr., Dinis-Ribeiro M., Leite-Moreira A.F. Toll-like receptors as therapeutic targets in gastrointestinal diseases. Expert Opin. Ther. Targets. 2010;14(4):347–368. doi: 10.1517/14728221003642027. [DOI] [PubMed] [Google Scholar]
- 78.Lehtoranta L., Pitkaranta A., Korpela R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33(8):1289–1302. doi: 10.1007/s10096-014-2086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park M.K., Ngo V., Kwon Y.M., Lee Y.T., Yoo S., Cho Y.H. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0075368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salminen S., Nybom S., Meriluoto J., Collado M.C., Vesterlund S., El-Nezami H. Interaction of probiotics and pathogens—benefits to human health? Curr. Opin. Biotechnol. 2010;21(2):157–167. doi: 10.1016/j.copbio.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 81.Al Kassaa I., Hober D., Hamze M., Chihib N.E., Drider D. Antiviral potential of lactic acid bacteria and their bacteriocins. Probiotics Antimicrob. Proteins. 2014;6(3–4):177–185. doi: 10.1007/s12602-014-9162-6. [DOI] [PubMed] [Google Scholar]
- 82.Takano T. Anti-hypertensive activity of fermented dairy products containing biogenic peptides. Antonie Van Leeuwenhoek. 2002;82(1–4):333–340. [PubMed] [Google Scholar]
- 83.Möller N.P., Scholz-Ahrens K.E., Roos N., Schrezenmeir J. Bioactive peptides and proteins from foods: indication for health effects. Eur. J. Nutr. 2008;47(4):171–182. doi: 10.1007/s00394-008-0710-2. [DOI] [PubMed] [Google Scholar]
- 84.Yasui H., Kiyoshima J., Hori T., Shida K. Protection against influenza virus infection of mice fed Bifidobacterium breve YIT4064. Clin. Diagn. Lab. Immunol. 1999;6(2):186–192. doi: 10.1128/cdli.6.2.186-192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Gutierrez R.C., Santos V., Nader-Macías M.E. Protective effect of intranasally inoculated Lactobacillus fermentum against Streptococcus pneumoniae challenge on the mouse respiratory tract. FEMS Immunol. Med. Microbiol. 2001;31(3):187–195. doi: 10.1111/j.1574-695X.2001.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 86.Aldinucci C., Bellussi L., Monciatti G., Passali G., Salerni L., Passali D. Effects of dietary yoghurt on immunological and clinical parameters of rhinopathic patients. Eur. J. Clin. Nutr. 2002;56(12):1155–1161. doi: 10.1038/sj.ejcn.1601465. [DOI] [PubMed] [Google Scholar]
- 87.Vinderola C.G., Duarte J., Thangavel D., Perdigón G., Farnworth E., Matar C. Immunomodulating capacity of kefir. J. Dairy Res. 2005;72(2):195–202. doi: 10.1017/s0022029905000828. [DOI] [PubMed] [Google Scholar]
- 88.Perdigon G., de Macias M.E.N., Alvarez S., Oliver G., De Ruiz Holgado A.A.P. Prevention of gastrointestinal infection using immunobiological methods with milk fermented with Lactobacillus casei and Lactobacillus acidophilus. J. Dairy Res. 1990;57(2):255–264. doi: 10.1017/s002202990002687x. [DOI] [PubMed] [Google Scholar]
- 89.Perdigon G., De Macias M., Alvarez S., Oliver G., de Ruiz Holgado A.P. Systemic augmentation of the immune response in mice by feeding fermented milks with Lactobacillus casei and Lactobacillus acidophilus. Immunology. 1988;63(1):17. [PMC free article] [PubMed] [Google Scholar]
- 90.Cano P.G., Perdigón G. Probiotics induce resistance to enteropathogens in a re-nourished mouse model. J. Dairy Res. 2003;70(4):433–440. doi: 10.1017/s0022029903006472. [DOI] [PubMed] [Google Scholar]
- 91.Can E., Kutluyer F., Sonay F.D., Köse Ö. The use of kefir as potential probiotic in Çoruh trout (Salmo coruhensis): effects on growth performance and immunoglobulin (IgM) levels. Afr. J. Biotechnol. 2012;11(30):7775–7780. [Google Scholar]
- 92.Thoreux K., Schmucker D.L. Kefir milk enhances intestinal immunity in young but not old rats. J. Nutr. 2001;131(3):807–812. doi: 10.1093/jn/131.3.807. [DOI] [PubMed] [Google Scholar]
- 93.Umlauf B.J., Ovsyannikova I.G., Haralambieva I.H., Kennedy R.B., Vierkant R.A., Pankratz V.S. Correlations between vaccinia-specific immune responses within a cohort of armed forces members. Viral Immunol. 2011;24(5):415–420. doi: 10.1089/vim.2011.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Braciale T.J., Hahn Y.S. Immunity to viruses. Immunol. Rev. 2013;255(1) doi: 10.1111/imr.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Allam Nanis G., Salem Mohamed L., Elbatae Hassan, MM Nabieh. Lactobacillus acidophilus and Bifidobacteria spp having antibacterial and antiviral effects on chronic HCV infection. Afr. J. Microbiol. Res. 2018;13(5) [Google Scholar]
- 96.Isolauri E., Sütas Y., Kankaanpää P., Arvilommi H., Salminen S. Probiotics: effects on immunity. Am. J. Clin. Nutr. 2001;73(2):444s–450s. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 97.Chen Y.-P., Chen Y.-P. Effects of Lactobacillus kefiranofaciens M1 isolated from kefir grains on germ-free mice. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0078789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gabryszewski S.J., Bachar O., Dyer K.D., Percopo C.M., Killoran K.E., Domachowske J.B. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J. Immunol. 2011;186(2):1151–1161. doi: 10.4049/jimmunol.1001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Berggren A., Ahrén I.L., Larsson N., Önning G. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. Eur. J. Nutr. 2011;50(3):203–210. doi: 10.1007/s00394-010-0127-6. [DOI] [PubMed] [Google Scholar]
- 100.Cavicchioli V.Q., Carvalho O.V., Paiva J.C., Todorov S.D., Silva Junior A., Nero L.A. Inhibition of herpes simplex virus 1 (HSV-1) and poliovirus (PV-1) by bacteriocins from lactococcus lactis subsp. Lactis and enterococcus durans strains isolated from goat milk. Int. J. Antimicrob. Agents. 2018;51(1):33–37. doi: 10.1016/j.ijantimicag.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 101.Weiss G., Rasmussen S., Zeuthen L.H., Nielsen B.N., Jarmer H., Jespersen L. Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll‐like receptor‐2‐dependent mechanism. Immunology. 2010;131(2):268–281. doi: 10.1111/j.1365-2567.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adiloğlu A., Gönülateş N., Işler M., Senol A. The effect of kefir consumption on human immune system: a cytokine study. Mikrobiyol. Bul. 2013;47(2):273–281. doi: 10.5578/mb.4709. [DOI] [PubMed] [Google Scholar]
- 103.Chen C., Zhang X., Ju Z., He W. Advances in the research of cytokine storm mechanism induced by Corona Virus Disease 2019 and the corresponding immunotherapies. Zhonghua shao shang za zhi= Zhonghua shaoshang zazhi= Chinese journal of burns. 2020;36:E005. doi: 10.3760/cma.j.cn501120-20200224-00088. [DOI] [PubMed] [Google Scholar]
- 104.Monteleone G., Sarzi-Puttini P.C., Ardizzone S. The Lancet Rheumatology. 2020. Preventing COVID-19-induced pneumonia with anticytokine therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosa D.D., Grześkowiak ŁM., Ferreira C.L., Fonseca A.C.M., Reis S.A., Dias M.M. Kefir reduces insulin resistance and inflammatory cytokine expression in an animal model of metabolic syndrome. Food Funct. 2016;7(8):3390–3401. doi: 10.1039/c6fo00339g. [DOI] [PubMed] [Google Scholar]
- 106.Ribeiro de Andrade Gabrielle, Neves Irys Viana, Marques Vaniky Duarte, Borges Monamaris Marques, Broring Tiago Antonio Martinhago, Anjos Marina Tavaresdos. Influence of a kefir-derived antimicrobial fraction on zika virus cytopathic effects and lymphocyte proliferation. JOJ Immuno Virology. 2017;2(2) [Google Scholar]
- 107.Seo M., Park E., Ko S., Choi E., Kim S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2, 4, 6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J. Dairy Sci. 2018;101(10):8662–8671. doi: 10.3168/jds.2018-15014. [DOI] [PubMed] [Google Scholar]
- 108.Morsy B.M., Mahmoud A.M., Zanaty M.I., Abdel-Moneim A., Abo-Seif M.A. Beneficial effects of milk kefir in patients with chronic hepatitis C virus infection. Int. J. Bioassays. 2014;3(6):3086–3091. [Google Scholar]
- 109.Carasi P., Racedo S.M., Jacquot C., Romanin D.E., Serradell M., Urdaci M. Impact of kefir derived Lactobacillus kefiri on the mucosal immune response and gut microbiota. J. Immunol. Res. 2015;2015 doi: 10.1155/2015/361604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spiteri G., Fielding J., Diercke M., Campese C., Enouf V., Gaymard A. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25(9) doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Villena J., Oliveira M.L.S., Ferreira P.C., Salva S., Alvarez S. Lactic acid bacteria in the prevention of pneumococcal respiratory infection: future opportunities and challenges. Int. Immunopharmacol. 2011;11(11):1633–1645. doi: 10.1016/j.intimp.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 112.Lei W.-T., Shih P.-C., Liu S.-J., Lin C.-Y., Yeh T.-L. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017;9(11):1175. doi: 10.3390/nu9111175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fernandez-Duarte K.P., Olaya-Galan N.N., Salas-Cardenas S.P., Lopez-Rozo J., Gutierrez-Fernandez M.F. Bifidobacterium adolescentis (DSM 20083) and Lactobacillus casei (Lafti L26-DSL): probiotics able to block the in vitro adherence of rotavirus in MA104 cells. Probiotics Antimicrob. Proteins. 2018;10(1):56–63. doi: 10.1007/s12602-017-9277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Olaya Galan N.N., Ulloa Rubiano J.C., Velez Reyes F.A., Fernandez Duarte K.P., Salas Cardenas S.P., Gutierrez Fernandez M.F. In vitro antiviral activity of Lactobacillus casei and Bifidobacterium adolescentis against rotavirus infection monitored by NSP4 protein production. J. Appl. Microbiol. 2016;120(4):1041–1051. doi: 10.1111/jam.13069. [DOI] [PubMed] [Google Scholar]
- 115.Starosila D., Rybalko S., Varbanetz L., Ivanskaya N., Sorokulova I. Anti-influenza activity of a Bacillus subtilis probiotic strain. Antimicrob. Agents Chemother. 2017;61(7) doi: 10.1128/AAC.00539-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghoneum M., Felo N., Agrawal S., Agrawal A. A novel kefir product (PFT) activates dendritic cells to induce CD4+ T and CD8+ T cell responses in vitro. Int. J. Immunopathol. Pharmacol. 2015;28(4):488–496. doi: 10.1177/0394632015599447. [DOI] [PubMed] [Google Scholar]
- 117.Panavas T., Serviene E., Brasher J., Nagy P.D. Yeast genome-wide screen reveals dissimilar sets of host genes affecting replication of RNA viruses. Proc. Natl. Acad. Sci. 2005;102(20):7326–7331. doi: 10.1073/pnas.0502604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kushner D.B., Lindenbach B.D., Grdzelishvili V.Z., Noueiry A.O., Paul S.M., Ahlquist P. Systematic, genome-wide identification of host genes affecting replication of a positive-strand RNA virus. Proc. Natl. Acad. Sci. 2003;100(26):15764–15769. doi: 10.1073/pnas.2536857100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Parsons A.B., Lopez A., Givoni I.E., Williams D.E., Gray C.A., Porter J. Exploring the mode-of-action of bioactive compounds by chemical-genetic profiling in yeast. Cell. 2006;126(3):611–625. doi: 10.1016/j.cell.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 120.Song J.A., Kim H.J., Hong S.K., Lee D.H., Lee S.W., Song C.S. Oral intake of Lactobacillus rhamnosus M21 enhances the survival rate of mice lethally infected with influenza virus. J. Microbiol. Immunol. Infect. 2016;49(1):16–23. doi: 10.1016/j.jmii.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 121.Bae J.Y., Kim J.I., Park S., Yoo K., Kim J.I.H., Joo W. Effects of Lactobacillus plantarum and Leuconostoc mesenteroides probiotics on human seasonal and avian influenza viruses. J. Microbiol. Biotechnol. 2018;28(6):893–901. doi: 10.4014/jmb.1804.04001. [DOI] [PubMed] [Google Scholar]
- 122.Choi Hwa-Jung, Song Jae-Hyoung, Ahn Young-Joon, Baek Seung-Hwa, Kwon D.-H. Antiviral activities of cell-free supernatants of yogurts metabolites against some RNA viruses. European Food Research and Technology. 2009;228:945–950. [Google Scholar]
- 123.Maruo T., Gotoh Y., Nishimura H., Ohashi S., Toda T., Takahashi K. Oral administration of milk fermented with Lactococcus lactis subsp. Cremoris FC protects mice against influenza virus infection. Lett. Appl. Microbiol. 2012;55(2):135–140. doi: 10.1111/j.1472-765X.2012.03270.x. [DOI] [PubMed] [Google Scholar]
- 124.Goto H., Sagitani A., Ashida N., Kato S., Hirota T., Shinoda T. Anti-influenza virus effects of both live and non-live Lactobacillus acidophilus L-92 accompanied by the activation of innate immunity. Br. J. Nutr. 2013;110(10) doi: 10.1017/S0007114513001104. [DOI] [PubMed] [Google Scholar]
- 125.Rather Irfan A., Choi Kwang-Ho, Bajpai Vivek K., Park Y.-H. Antiviral mode of action of Lactobacillus plantarum YML009 on Influenza virus H1N1. A Journal of the Bangladesh Pharmacological Society (BDPS). 2015;10:475–482. [Google Scholar]
- 126.Eguchi K., Fujitani N., Nakagawa H., Miyazaki T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019;9(1):4812. doi: 10.1038/s41598-019-39602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jung K., Ha Y., Ha S.K., Han D.U., Kim D.W., Moon W.K. Antiviral effect of Saccharomyces cerevisiae beta-glucan to swine influenza virus by increased production of interferon-gamma and nitric oxide. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2004;51(2):72–76. doi: 10.1111/j.1439-0450.2004.00732.x. [DOI] [PubMed] [Google Scholar]
- 128.Frieman M., Basu D., Matthews K., Taylor J., Jones G., Pickles R. Yeast based small molecule screen for inhibitors of SARS-CoV. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kang J.Y., Lee D.K., Ha N.J., Shin H.S. Antiviral effects of Lactobacillus ruminis SPM0211 and Bifidobacterium longum SPM1205 and SPM1206 on rotavirus-infected Caco-2 cells and a neonatal mouse model. J. Microbiol. 2015;53(11):796–803. doi: 10.1007/s12275-015-5302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee D.K., Park J.E., Kim M.J., Seo J.G., Lee J.H., Ha N.J. Probiotic bacteria, B. Longum and L. Acidophilus inhibit infection by rotavirus in vitro and decrease the duration of diarrhea in pediatric patients. Clin. Res. Hepatol. Gastroenterol. 2015;39(2):237–244. doi: 10.1016/j.clinre.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 131.Khani S., Motamedifar M., Golmoghaddam H., Hosseini H.M., Hashemizadeh Z. In vitro study of the effect of a probiotic bacterium Lactobacillus rhamnosus against herpes simplex virus type 1. Braz. J. Infect. Dis. 2012;16(2):129–135. doi: 10.1016/S1413-8670(12)70293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mastromarino P., Cacciotti F., Masci A., Mosca L. Antiviral activity of Lactobacillus brevis towards herpes simplex virus type 2: role of cell wall associated components. Anaerobe. 2011;17(6):334–336. doi: 10.1016/j.anaerobe.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 133.Mousavi E., Makvandi M., Teimoori A., Ataei A., Ghafari S., Samarbaf-Zadeh A. Antiviral effects of Lactobacillus crispatus against HSV-2 in mammalian cell lines. J. Chin. Med. Assoc. 2018;81(3):262–267. doi: 10.1016/j.jcma.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 134.Sunmola A.A., Ogbole O.O., Faleye T.O.C., Adetoye A., Adeniji J.A., Ayeni F.A. Antiviral potentials of Lactobacillus plantarum, Lactobacillus amylovorus, and Enterococcus hirae against selected Enterovirus. Folia Microbiol (Praha). 2019;64(2):257–264. doi: 10.1007/s12223-018-0648-6. [DOI] [PubMed] [Google Scholar]
- 135.Kuang H., Yang P., Yang L., Aguilar Z.P., Xu H. Size dependent effect of ZnO nanoparticles on endoplasmic reticulum stress signaling pathway in murine liver. J. Hazard. Mater. 2016;317:119–126. doi: 10.1016/j.jhazmat.2016.05.063. [DOI] [PubMed] [Google Scholar]
- 136.Arena M.P., Elmastour F., Sane F., Drider D., Fiocco D., Spano G. Inhibition of coxsackievirus B4 by Lactobacillus plantarum. Microbiol. Res. (Pavia) 2018;210:59–64. doi: 10.1016/j.micres.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 137.Aboubakr H.A., El-Banna A.A., Youssef M.M., Al-Sohaimy S.A., Goyal S.M. Antiviral effects of Lactococcus lactis on feline calicivirus, a human norovirus surrogate. Food Environ. Virol. 2014;6(4):282–289. doi: 10.1007/s12560-014-9164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Karaçalı R., Özdemİr N., Çon A.H. Aromatic and functional aspects of kefir produced using soya milk and Bifidobacterium species. Int. J. Dairy Technol. 2018;71(4):921–933. [Google Scholar]
- 139.Rea M., Lennartsson T., Dillon P., Drinan F., Reville W., Heapes M. Irish kefir‐like grains: their structure, microbial composition and fermentation kinetics. J. Appl. Bacteriol. 1996;81(1):83–94. [Google Scholar]
- 140.Talib N., Mohamad N.E., Yeap S.K., Hussin Y., Aziz M.N.M., Masarudin M.J. Isolation and characterization of Lactobacillus spp. From kefir samples in Malaysia. Molecules. 2019;24(14):2606. doi: 10.3390/molecules24142606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sturman L.S., Ricard C., Holmes K. Conformational change of the coronavirus peplomer glycoprotein at pH 8.0 and 37 degrees C correlates with virus aggregation and virus-induced cell fusion. J. Virol. 1990;64(6):3042–3050. doi: 10.1128/jvi.64.6.3042-3050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nalbantoglu U., Cakar A., Dogan H., Abaci N., Ustek D., Sayood K. Metagenomic analysis of the microbial community in kefir grains. Food Microbiol. 2014;41:42–51. doi: 10.1016/j.fm.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 143.Angulo L., Lopez E., Lema C. Microflora present in kefir grains of the Galician region (North-West of Spain) J. Dairy Res. 1993;60(2):263–267. doi: 10.1017/s002202990002759x. [DOI] [PubMed] [Google Scholar]
- 144.Zanirati D.F., Abatemarco Jr M., de Cicco Sandes S.H., Nicoli J.R., Nunes ÁC., Neumann E. Selection of lactic acid bacteria from Brazilian kefir grains for potential use as starter or probiotic cultures. Anaerobe. 2015;32:70–76. doi: 10.1016/j.anaerobe.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 145.Santos A., San Mauro M., Sanchez A., Torres J., Marquina D. The antimicrobial properties of different strains of Lactobacillus spp. Isolated from kefir. Syst. Appl. Microbiol. 2003;26(3):434–437. doi: 10.1078/072320203322497464. [DOI] [PubMed] [Google Scholar]
- 146.Dobson A., O’Sullivan O., Cotter P.D., Ross P., Hill C. High-throughput sequence-based analysis of the bacterial composition of kefir and an associated kefir grain. FEMS Microbiol. Lett. 2011;320(1):56–62. doi: 10.1111/j.1574-6968.2011.02290.x. [DOI] [PubMed] [Google Scholar]
- 147.Garofalo C., Osimani A., Milanović V., Aquilanti L., De Filippis F., Stellato G. Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiol. 2015;49:123–133. doi: 10.1016/j.fm.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 148.Pintado M.E., Da Silva J.L., Fernandes P.B., Malcata F.X., Hogg T.A. Microbiological and rheological studies on Portuguese kefir grains. Int. J. Food Sci. Technol. 1996;31(1):15–26. [Google Scholar]
- 149.Mainville I., Robert N., Lee B., Farnworth E.R. Polyphasic characterization of the lactic acid bacteria in kefir. Syst. Appl. Microbiol. 2006;29(1):59–68. doi: 10.1016/j.syapm.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 150.Yüksekdağ Z., Beyatli Y., Aslim B. Determination of some characteristics coccoid forms of lactic acid bacteria isolated from Turkish kefirs with natural probiotic. LWT Food Sci. Technol. 2004;37(6):663–667. [Google Scholar]
- 151.Korsak N., Taminiau B., Leclercq M., Nezer C., Crevecoeur S., Ferauche C. Evaluation of the microbiota of kefir samples using metagenetic analysis targeting the 16S and 26S ribosomal DNA fragments. J. Dairy Sci. 2015;98(6):3684–3689. doi: 10.3168/jds.2014-9065. [DOI] [PubMed] [Google Scholar]
- 152.Marsh A.J., O’Sullivan O., Hill C., Ross R.P., Cotter P.D. Sequencing-based analysis of the bacterial and fungal composition of kefir grains and milks from multiple sources. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Latorre-García L., del Castillo-Agudo L., Polaina J. Taxonomical classification of yeasts isolated from kefir based on the sequence of their ribosomal RNA genes. World J. Microbiol. Biotechnol. 2007;23(6):785–791. [Google Scholar]
- 154.Marquina D., Santos A., Corpas I., Munoz J., Zazo J., Peinado J. Dietary influence of kefir on microbial activities in the mouse bowel. Lett. Appl. Microbiol. 2002;35(2):136–140. doi: 10.1046/j.1472-765x.2002.01155.x. [DOI] [PubMed] [Google Scholar]
- 155.Vardjan T., Lorbeg P.M., Rogelj I., Majhenič A.Č. Characterization and stability of lactobacilli and yeast microbiota in kefir grains. J. Dairy Sci. 2013;96(5):2729–2736. doi: 10.3168/jds.2012-5829. [DOI] [PubMed] [Google Scholar]
- 156.Diosma G., Romanin D.E., Rey-Burusco M.F., Londero A., Garrote G.L. Yeasts from kefir grains: isolation, identification, and probiotic characterization. World J. Microbiol. Biotechnol. 2014;30(1):43–53. doi: 10.1007/s11274-013-1419-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this article are available in Fig. 1, Fig. 2, Fig. 3, Fig. 4 and Table 1. The data sets analyzed in the present study are available from the corresponding author upon reasonable request.