Abstract
It is becoming clearer how neurobiological mechanisms generate ‘liking’ and ‘wanting’ components of food reward. Mesocorticolimbic mechanisms that enhance ‘liking’ include brain hedonic hotspots, which are specialized subregions that are uniquely able to causally amplify the hedonic impact of palatable tastes. Hedonic hotspots are found in nucleus accumbens medial shell, ventral pallidum, orbitofrontal cortex, insula cortex, and brainstem. In turn, a much larger mesocorticolimbic circuitry generates ‘wanting’ or incentive motivation to obtain and consume food rewards. Hedonic and motivational circuitry interact together and with hypothalamic homeostatic circuitry, allowing relevant physiological hunger and satiety states to modulate ‘liking’ and ‘wanting’ for food rewards. In some conditions such as drug addiction, ‘wanting’ is known to dramatically detach from ‘liking’ for the same reward, and this may also occur in over-eating disorders. Via incentive sensitization, ‘wanting’ selectively becomes higher, especially when triggered by reward cues when encountered in vulnerable states of stress, etc. Emerging evidence suggests that some cases of obesity and binge eating disorders may reflect an incentive-sensitization brain signature of cue hyper-reactivity, causing excessive ‘wanting’ to eat. Future findings on the neurobiological bases of ‘liking’ and ‘wanting’ can continue to improve understanding of both normal food reward and causes of clinical eating disorders.
Keywords: Feeding, ‘Liking’, ‘Wanting’, Ventral pallidum, Nucleus accumbens, Prefrontal cortex
1. Introduction
Several decades of neuroscience studies have advanced understanding of how the brain generates behavior related to food reward, motivation, and hunger. A fundamental question that remains is how mesocorticolimbic and hypothalamic circuitry interact to produce reward and the motivation to eat [1–7].
Work in our lab has focused on understanding how mesocorticolimbic systems generate ‘wanting’ and ‘liking’ for food rewards, which have turned out to be somewhat separable. Here we describe how various brain mechanisms produce those two components of food reward. ‘Wanting’ and ‘liking’ usually cohere together, but also can dissociate in particular brain conditions to come apart. Findings have revealed a distributed network of brain hedonic ‘hotspots’ that can amplify hedonic impact or ‘liking’ for food rewards. These ‘liking’ mechanisms differ from larger mesocorticolimbic circuitry that generates incentive salience or ‘wanting’ as motivation to eat. We focus on mechanisms for ‘liking’ and for ‘wanting’, and how these interact with homeostatic hypothalamic circuitry in controlling eating and food reward.
1.1. ‘Liking’ and ‘wanting’ as separate psychological processes
The words liking and wanting are often used interchangeably in ordinary life when talking about rewards. For example, people may want a palatable piece of chocolate because they like the flavor and other sensations of consuming it. In ordinary use, liking means conscious pleasure and wanting means conscious desire, which typically involve cognitive appraisals and declarative goals mediated by cortically-weighted circuitry. But here we use quotations for ‘wanting’ and ‘liking in order to distinguish specific psychological processes from ordinary use [8]. ‘Wanting’ here refers to the particular psychological process of incentive salience, which can occur either consciously or unconsciously, generated by brain mesolimbic circuitry in the form of cue-triggered motivation. When rewards such as palatable foods and their predictive cues are imbued with incentive salience by mesocorticolimbic circuitry, those cues and foods become attractive, and in conscious form able to elicit subjective cravings. Whether conscious or not, incentive salience triggered by cues can also generate behavioral urges to seek and consume their associated rewards [9,10]. In the laboratory, ‘wanting’ is typically measured in humans by subjective craving ratings, and in animals by how much food is pursued, consumed, or preferred over an alternative. ‘Liking’ refers to the hedonic impact of pleasant rewards, which when surfaced into consciousness can result subjective pleasure ratings in adult humans, but which in animals and infant humans can be assessed via objective measures of hedonic orofacial expressions elicited to taste in the affective taste reactivity test [11–15]. ‘Liking’ and ‘wanting’ can become separated in some conditions, as discussed below.
1.2. Measuring hedonic ‘liking’ with the taste reactivity test
The hedonic taste reactivity task measures affective orofacial reactions to tastes of sucrose, quinine, water, etc., and the reactions to any given taste can also be shifted by a variety of relevant physiological, learning, and brain manipulation factors that alter its palatability. Originally pioneered by Steiner for use in human infants [11], the test was adapted for rodents by Grill and Norgren [13]. Orofacial responses to taste are grouped into positive, neutral, and aversive categories. Positive hedonic or ‘liking’ evaluations (Fig. 1a) are reflected in tongue protrusions, paw licks, and lateral tongue protrusions, typically elicited by tastes such as sucrose. By comparison, negative aversive or ‘disgust’ evaluations are reflected by gapes, forelimb, flails, headshakes, paw treading and face washes, and typically elicited by bitter quinine. Many of these orofacial expressions to taste are homologous, or evolutionarily conserved, across mammalian species ranging from human infants to non-human primates, rodents, and horses [14–16]. In our laboratory, rodents are implanted with bilateral oral cannula, which allow taste solutions to be directly infused into their mouths without them having to engage in any appetitive activity to obtain them, and allowing experimenter control of stimulus intensity and duration. Independence from appetitive or instrumental decisions to consume is important in allowing taste reactivity to provide a relatively pure measure of taste-elicited ‘liking’, without being altered by changes in ‘wanting’ that can influence most other behavioral measures of food reward [15,17].
Fig. 1. Brain systems of ‘wanting’ and ‘liking’.
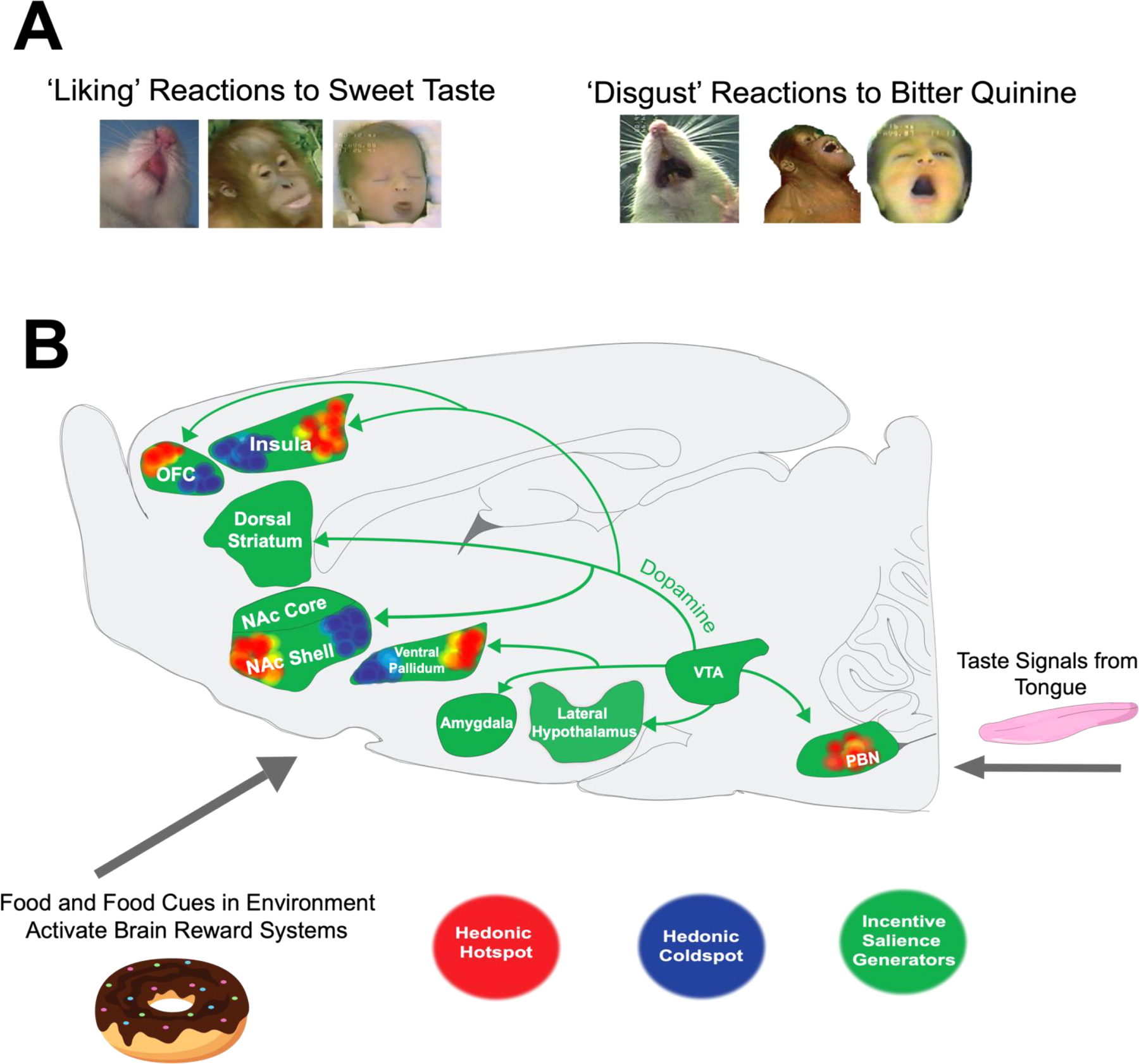
A) Positive hedonic expressions (‘liking’) elicited in response to palatable sucrose solutions (left). Negative aversive orofacial expressions (‘disgust’) in response to bitter quinine solutions (right). Orofacial expressions to palatable and aversive solutions are homologous across various mammalian species that include human infants, nonhuman primates, rodents, and horses. B) Palatable foods and their predictive cues activate mesocorticolimbic reward systems. Sagittal view of a rat brain depicting brain systems of ‘wanting’ and ‘liking’. ‘Wanting’ is generated by mesolimbic dopamine systems originating from the midbrain that project to various limbic structures (pictured in green) to generate incentive salience. ‘Liking’ is mediated by hedonic hotspots (pictured in red) where opioid, orexin, endocannabinoid, and optogenetic manipulations enhance positive orofacial expressions to sucrose taste. By comparison, the same manipulations within the hedonic coldspots (pictured in blue) oppositely suppress ‘liking’ reactions to sucrose solutions.
Tastants with very different sensory properties like sucrose, saccharin, salt, and fats can all evoke similar positive ‘liking’ responses, indicating that hedonic reactions are palatability-specific rather than sensory-specific [14,18–21]. Accordingly, taste reactivity behaviors are not simple inflexible reflexes to a particular sensation, but rather reflect a hedonic evaluation that also depends on the internal state of the organism, including physiological appetite and satiety states, neurobiological states, as well as learned associations carried from previous experiences with the taste. Physiological states like hunger and satiety can shift subjective ratings of palatability for a particular taste in humans, in a phenomenon known as alliesthesia [22–24]. In rodents too, caloric hunger magnifies hedonic ‘liking’ reactions to palatable sweet taste, whereas satiety conversely reduces ‘liking’ [25,26]. Similarly, salt appetite modulates the hedonic impact of the intense saltiness taste of concentrated NaCl. For example, hypertonic concentrations of salt are normally aversive, in the sense that rats mostly display ‘disgust’ reactions when a seawater concentration of NaCl is placed into their mouths. However, when a hormonal state of sodium deficiency or salt depletion is induced, orofacial reactivity to the same intensely salty taste shifts to mostly positive ‘liking’ [20,27–31]. Conversely, modulation by learned associations can be induced by pairing a novel ‘liked’ sweet taste of saccharin as a Pavlovian conditioned stimulus (CS+) with an injection of lithium chloride, which induces malaise, as an unconditioned stimulus (UCS), to produce a conditioned taste aversion (CTA) so that subsequent exposures to saccharin taste instead elicit negative gapes and related ‘disgust’ reactions [32–37].
1.3. Hedonic hotspots: brain mechanisms of ‘liking’
Our laboratory has studied brain generators of taste ‘liking’ by combining central neural manipulations of hedonic circuitry with the taste reactivity measure of ‘liking’ versus ‘disgust’. In brief, pharmacological microinjections, excitotoxin lesions, optogenetic brain stimulation or inhibitions, etc. are used to systematically turn on or turn off particular neural systems in various brain locations during the taste reactivity test. This is coupled with an analysis of local Fos protein expression that allows us to more directly determine the spread of neuronal changes induced by a manipulation that alters ‘liking’, to identify localization of function, and map subregional localization of hedonic mechanisms within a brain structure. These studies have revealed a distributed network of limbic hotspots or small sites within subregions of cortical and subcortical structures in the rat that are capable of amplifying the hedonic impact (Fig. 1b) of sucrose taste [19,38–40]. Brain hedonic hotspots appear to be restricted to particular subregions of limbic structures such as rostrodorsal quadrant of medial shell of nucleus accumbens (NAc), caudolateral half of ventral pallidum (VP), a rostromedial portion orbitofrontal cortex (OFC), a far posterior zone of insula cortex (IC), and the parabrachial nucleus of the brainstem pons (PBN). Brain hedonic hotspots that generate ‘liking’ are embedded within larger mesocorticolimbic circuitry (spanning several entire structures) that is capable of generating incentive salience ‘wanting’, underlying the close interconnection between ‘liking’ and ‘wanting’ functions in reward [38,41–48]. In the following sections we discuss roles of these hedonic hotspots and mesocorticolimbic motivation circuitry in food reward, describe recent findings, and consider their potential roles in normal appetite and in clinical eating disorders and obesity.
2. Hindbrain structures compute early hedonic evaluations
Rudimentary hedonic processing of tastes begins to occur in the brainstem early in pathway of ascending gustatory signals [11,49–52]. For example, brainstem (4th-ventricle) microinjections of a benzodiazepine drug that promotes GABA signaling enhanced positive ‘liking’ reactions to sweet taste, as did microinjections limited to the parabrachial nucleus of the pons, revealing that site as a brainstem hedonic hotspot [53,54]. Brainstem capacity for early hedonic-related processing was also revealed by classic studies of taste reactions in decerebrate rats and in anencephalic infants, both of which lack a functioning forebrain, yet are able to adequately respond to sucrose taste with positive affective reactions, and to quinine with aversive reactions [11,50]. Similarly, decerebrate rats show increases in positive ‘liking’ reactions to intra-oral sucrose after systemic administration of a benzodiazepine drug [55]. For humans and other primates, the causal role of PBN in food hedonics has sometimes been questioned [56,57] on the basis that in primates, gustatory neuroanatomical projections may ascend directly from the hindbrain nucleus of the solitary tract to forebrain thalamus and limbic structures, rather than making an obligatory intermediary relay in PBN as in rodents [58,59]. However, very little data actually exists yet on PBN roles in food reward functions in primates, including humans.
A crucial need for forebrain hierarchical contributions to normal ‘liking’ exists even in rats, evident from observations that many features of normal physiological and associative modulation of ‘liking’ reactions that occur in normal rats are missing in decerebrate rats. For example, decerebrate rats that are transected above the midbrain cannot learn or retain behavioral conditioned taste aversions to a nausea-paired sweet flavor that normally would switch ‘liking’ to ‘disgust’ reactions, suggesting that higher order affective processing involving experience and learning requires forebrain control and cannot be fully mediated by the brainstem on its own [32,35,37,50]. Caloric hunger similarly is reported to fail to enhance positive hedonic reactions to sweet tastes in decerebrate rats [60] unlike in normal rats [25,61], and inducing a hormonal salt appetite state fails to not enhance positive orofacial reactions to the taste of salt [62] again unlike in normal rats [20,27–31]. Those decerebrate failures suggest that the brainstem by itself cannot integrate physiological state or learned associations with tastes to modulate alliesthesia changes in hedonic orofacial reactions, even though some rudimentary processing of such modulating inputs has been reported in brainstem based on electrophysiological measures of neural activity [63–67].
3. The nucleus accumbens medial shell- hotspot for hedonic enhancement
Several decades of research have implicated the nucleus accumbens (NAc) as especially important in food motivation, and the NAc also plays important roles in controlling ‘liking’ reactions. Relevant to ‘wanting’, opioid, dopamine, and GABA/glutamate drug microinjections in the nucleus accumbens, especially in medial shell, can robustly enhance motivation to pursue and eat palatable foods [19,68–82]. Importantly however, the nucleus accumbens is a heterogenous structure with multiple anatomical subregions [83–89] that differentially mediate ‘liking’ and ‘wanting’, at least in response to particular manipulations [19,70,71,75,88]. Beyond the anatomical components of core and shell, there also are important subregional hedonic specializations within the shell, such as the hedonic hotspot within the rostrodorsal quadrant of medial shell. The rostrodorsal quadrant of NAc medial shell was first identified as an important hedonic hotspot (Fig. 1b) for ‘liking’ enhancement by Peciña and Berridge [19]. That hedonic mapping study used microinjections of the mu-opioid receptor agonist (DAMGO) to show that, only in the 1 mm3 rostrodorsal subregion of medial shell did mu opioid stimulation enhance ‘liking’ reactions to sucrose taste, even though opioid stimulation anywhere throughout the entire NAc shell generated robust ‘wanting’ to eat reflected in increased food intake. Opioid stimulations at NAc shell sites other than the rostrodorsal hotspot completely failed to enhance sweetness ‘liking’ reactions at all, even decreasing sucrose ‘liking’ at a hedonic ‘coldspot’ site in caudal shell, despite still increasing ‘wanting’ to eat [19]. That and subsequent mapping studies revealed a clear NAc subregional dissociation between amplification of ‘liking’, which is limited to the rostral medial shell hotspot, versus of ‘wanting’, which can be generated by opioid and some other neurochemical manipulations throughout the entire medial shell as well as NAc core [19,68]. Further illustrating the unique hedonic features of this NAc hotspot, delta opioid and even kappa opioid agonists can enhance sucrose ‘liking’ similarly to mu opioid stimulations when microinjected within the 1 mm3 hotspot in rostrodorsal shell, although kappa opioid stimulation is known to produce negative aversive effects at many other brain sites [70].
Beyond opioid stimulation, orexin and endocannabinoid microinjections within the NAc rostrodorsal shell hotspot also can enhance sucrose ‘liking’ reactions (endocannabinoid enhancements might possibly also extend to caudodorsal shell) [90,91]. Endocannabinoids bind to presynaptic receptors on axonal terminals of NAc neurons, and influence the release of other postsynaptic neurotransmitters [92]. The ability for endocannabinoids in the NAc hotspot to enhance sucrose ‘liking’ appears to require local endogenous opioid mediation [93]. For example, if opioid-blocking naloxone is mixed in the same microinjection into NAc hotspot that contains the endocannabinoid anandamide, the simultaneous opioid blockade prevents the endocannabinoid stimulation from enhancing ‘liking’ reactions to sucrose at all. These findings seem in accordance with research showing that opioid and cannabinoid receptors often co-localize on the same neurons to form heterodimers, and that the two neurochemical signals can functionally interact together to influence motivation for food and drug rewards [94–96].
While opioid, endocannabinoid, orexin, and a few other neurotransmitters act in the NAc hotspot to enhance ‘liking’[38,75,91,93,97–99], mesolimbic dopamine is notably missing from the list of hedonic neurochemical signals. Even in the NAc hotspot of rostrodorsal shell, synaptic dopamine stimulations, such as by amphetamine microinjection or genetic knockdown of the dopamine transporter that boosts dopamine levels in NAc synapses, completely fail to enhance ‘liking’ at all (although potently stimulating cue-triggered ‘wanting’ for sweet reward)[100,101]. Conversely, removing NAc dopamine signals via permanent 6-OHDA lesions or through pharmacological blockade can suppress ‘wanting’ during consuming and instrumental responding tasks [102–113], but fails to impair ‘liking’ reactions [107,114,115].
3.1. Desire versus dread from the nucleus accumbens shell
Another reflection of rostrocaudal differentiation of affective valence functions within the medial shell of NAc is an anatomical gradient of oppositely-valanced appetitive ‘desire’ vs fearful ‘dread’ motivations, revealed by localized microinjections that alter amino acid signaling in inhibitory ways along the anterior to posterior anatomical axis of NAc (Fig. 2a) [88]. For example, these opposite motivations can be produced by microinjections of either the glutamate AMPA antagonist DNQX, which block excitatory glutamate signals, or the GABAa agonist muscimol, which inhibit neuronal activity by opening Cl- ion gates. Microinjections of either drug at sites in rostral shell generate appetitive increases in food intake and can establish conditioned place preference [71,80,116–118]. By comparison, at sites in caudal shell the same pharmacological microinjections can promote active forms of negatively-valenced fearful behaviors such as distress vocalizations or escape attempts and bites when touched, or induce conditioned place avoidance, and elicit spontaneous defensive treading-burying (an antipredator reaction), while often simultaneously reducing appetitive food intake [75,88,119–121]. Intermediate sites between rostral and caudal poles of the NAc shell can produce a mixture of appetitive behavior and fearful behaviors (Fig. 2).
Fig. 2. ‘Liking’, ‘wanting’, desire, and dread in the nucleus accumbens medal shell.
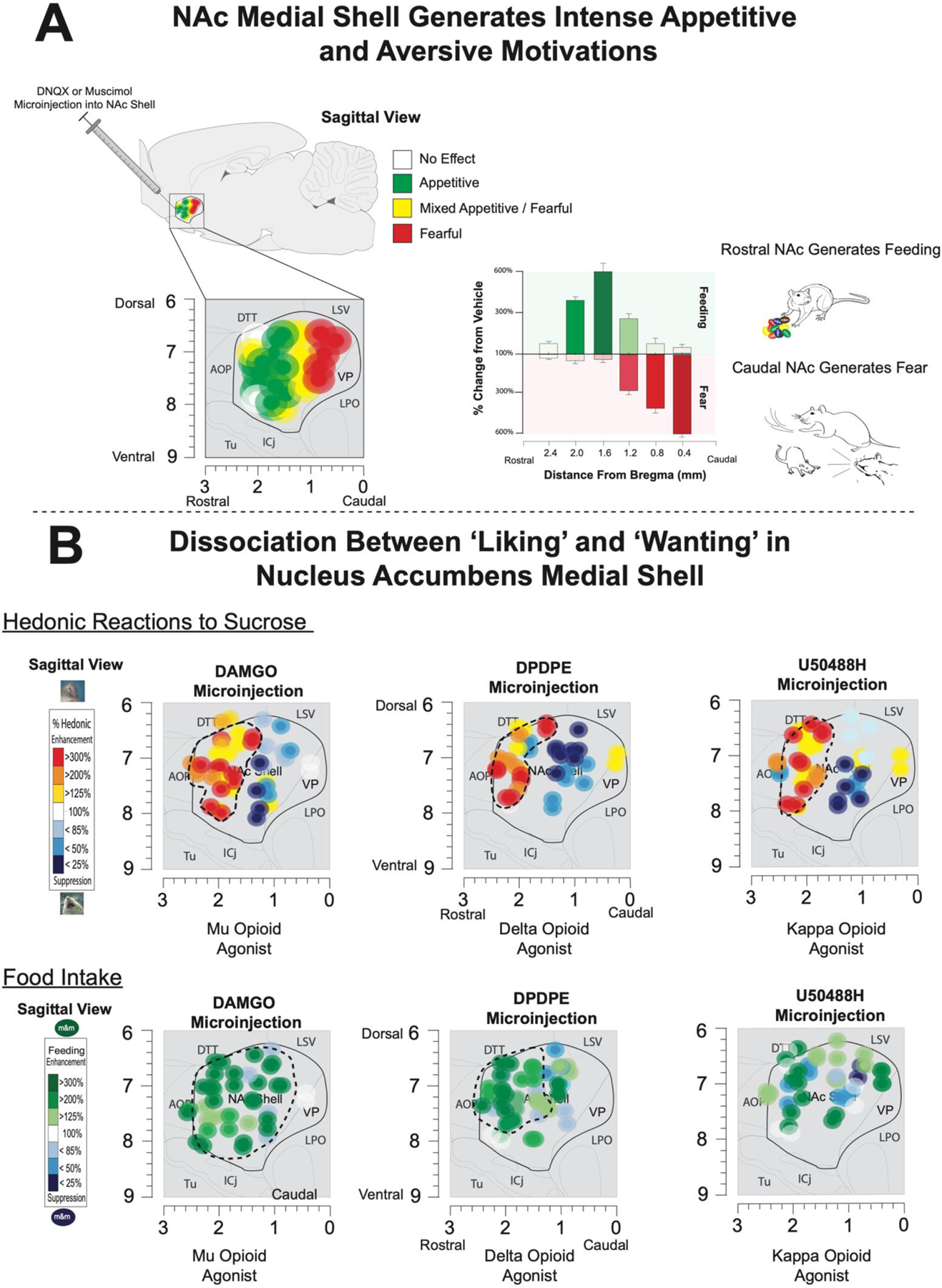
A) Top shows amino acid disruptions (via glutamate AMPA receptor antagonist DNQX or GABAA agonist muscimol) in the medial shell of the nucleus accumbens reveal a rostral to caudal organization of intense motivations. Manipulations into anterior sites produce voracious feeding (shown in green). The same microinjections at posterior sites generate fearful motivations (depicted in red) such as distress calls, bites, escape attempts, and defensive treading. DNQX or muscimol in mid NAc medial shell produce a mix of appetitive and aversive motivations. B) Bottom-top panel shows dissociations between ‘liking’ and ‘wanting’ in the nucleus accumbens medial shell following microinjections of mu-opioid agonists (DAMGO), delta-opioid agonists (DPDPE), and kappa-opioid agonists (U50488H). Similar patterns of hedonic enhancements were found after mu, delta, and kappa opioid agonists. While microinjections into anterior dorsal (in red) sites magnified ‘liking’ expressions to sucrose solutions, posterior manipulations oppositely suppress ‘liking’ expressions (in blue). Bottom panels shows the dissociable effects of mu, delta, and kappa manipulations in the nucleus accumbens medial shell on free-feeding. Mu-opioid agonists generated feeding throughout the entire medial shell. By comparison, delta opioids generate feeding within anterior sites overlapping with the hedonic hotspots. Finally, kappa opioid stimulation did not reliably generate feeding at any site despite generating intense ‘liking’ expressions in the rostrodorsal quadrant. Adapted from Castro & Berridge (2014).
Importantly, the valence tuning of rostral vs caudal sites of medial shell is not static, or determined by anatomical position alone, but instead also can be altered to some extent by shifting the emotional ambience of the testing environment [116,119,120]. For example, rats that receive DNQX microinjections in a calm dark and quiet environment resembling their home cage, which rats prefer over standard laboratory conditions, show enhanced appetitive generation at more widespread sites that extend throughout most of the NAc shell, including caudal portions that otherwise generated fear. Conversely, DNQX microinjections in a more stressfully loud and bright environment shift many NAc shell sites from generating appetitive behavior into instead generating predominantly fearful behaviors [119,121].
Precisely how do DNQX and muscimol actions in NAc shell elicit such intense motivations? A prominent hypothesis of NAc function has been that neuronal inhibitions in NAc medium spiny neurons generate reward motivation [122–131]. By this hypothesis, local NAc neuronal inhibitions suppress axonal release of GABA by output projections of NAc medium spiny neurons onto downstream structures including ventral tegmental area (VTA), lateral hypothalamus (LH), and VP, which consequently disinhibits those target structures into relative excitation [89,132–137]. This NAc inhibition hypothesis is supported by electrophysiological reports that NAc neurons often are phasically inhibited by presentations of reward stimuli, including drugs or palatable foods [124,127,128,138], (although c.f. [41,139–144]). Conversely, aversive bitter tastes and their cues have been reported by some investigators to typically evoke excitatory increases in NAc neuronal firing [128,138]. Similarly, learning a new aversive motivational value for a previously positive reward may shift the electrophysiological response of NAc neurons to tastes from inhibition to excitation. For example, inducing a learned Pavlovian taste aversion to a normally ‘liked’ saccharin solution, by pairing it with nausea, was reported to shift subsequent NAc neuronal responses to that taste from original inhibitions when still rewarding to predominately excitations when ‘disgusting’ [145]. Conversely, appetite states can induce alliesthesia to raise the incentive value of relevant tastes. For example, physiological sodium depletion that shifts affective reactions of intensely hypertonic NaCl tastes from ‘disgust’ to positive ‘liking’, was reported to simultaneously switch the NAc neuronal response to saltiness from excitation to inhibition [146].
By comparison, NAc output targets such as VP or VTA typically encode reward stimuli with electrophysiological excitations, so that as a taste becomes more positively ‘liked’, the greater the neuronal excitation in the posterior VP hotspot [29,147].Therefore, one hypothesis to explain how microinjections of DNQX or muscimol in NAc shell generate intense motivations is that they inhibit the activity of local NAc neurons, shutting off axonal GABA release, and so disinhibit or activate downstream VP, LH and VTA targets [126]. DNQX would merely reduce NAc activity relative to normal levels by blocking excitatory glutamate inputs onto local neurons, whereas muscimol would act on GABA-A receptors to directly open Cl- gates to more powerfully inhibit NAc neurons.
The neural difference in degree of NAc inhibition can create some categorical psychological consequences. Accordingly, DNQX microinjection in rostral shell increases food intake as a form of ‘wanting’ to eat, but does not enhance ‘liking’, whereas muscimol in the rostral shell hotspot increases both ‘wanting’ and ‘liking’ together [99]. Similarly, DNQX in caudal shell only increases motivated ‘fear’ behaviors, whereas muscimol in caudal shell both increases ‘fear’ motivation and induces excessive ‘disgust’ affective reactions to sucrose. Consistent with the idea that NAc inhibition releases projection targets into activation, such NAc drug microinjections increase neuronal activity reflected in Fos expression in downstream structures, including LH, VTA, VP, and paraventricular thalamus (PVT) [116,148,149].
To test whether local neuronal inhibition is actually necessary for DNQX microinjections in NAc shell to cause intense motivations, Hannah Baumgartner, Shannon Cole, and Jeffrey Olney in our laboratory recently tested whether opposing DNQX-induced inhibitions in NAc with optogenetic channelrhodopsin (ChR2) stimulation at the same site would reverse the desire or dread motivations otherwise produced by the DNQX microinjection [116]. They found that the answer was yes: exciting NAc neurons at the same local site as a DNQX microinjection reversed the ability of the microinjected DNQX drug to induce increases in appetitive eating behavior and food intake at rostral shell sites, and similarly reversed the elicitation of defensive or fearful behavior at caudal sites [116]. Further, in support of the hypothesis that NAc neuronal inhibition may be sufficient by itself to generate an intense motivation, Shannon Cole and Jeffrey Olney have also found preliminary evidence that acute inhibition of local neurons in NAc shell, such as by optogenetic inhibitory opsins, may directly elicit increases in motivated behavior [150,151]. For example, rats who received inhibitory viruses targeted at rostral NAc shell sites showed laser-bound increases in eating behavior. These pilot observations support the hypothesis that neuronal inhibitions in NAc shell can be a sufficient cause of increased motivation, as well as being a necessary part of the mechanism by which NAc DNQX microinjections elicit desire or dread [116].
3.2. Neurobiological mechanisms of hedonic hotspots
What neurobiological features of the hedonic hotspots may explain their unique capacities for hedonic enhancement? For example, in rats the NAc hedonic hotspot is a 1 mm3 quadrant of rostrodorsal medial shell, and is the only NAc shell or core site where opioid, endocannabinoid, and orexin stimulations amplify ‘liking reactions to sweet taste [70,75,97,99,152]. Neurobiological evidence suggests that the rostrodorsal subregion of NAc medial shell that contains the hotspot may also have unique neuroanatomical features that differ from other subregions of medial shell [85,86]. For example, one anatomical connectivity tracing study reported that the rostrodorsal subregion of NAc medial shell receives inputs from a distinct subregion of infralimbic cortex in rats, corresponding to Area 25 of the anterior cingulate cortex in humans; those infralimbic inputs to the rostrodorsal hotspot differ from the cortical inputs to other subregions of medial shell [85]. Similarly, the NAc hotspot in rostrodorsal shell sends outputs to distinct subregions of LH and VP that are different from the LH/VP output targets of other NAc shell subregions [85]. Finally, the VP target in turn sends its projections to a particular anterior thalamus subregion that finally projects back to the original infralimbic/A25 cortical subregion, forming a closed-circuit loop that runs through the NAc hotspot. In other words, the NAc hedonic hotspot appears to belong to a distinct cortical-striatal-pallidal-hypothalamic-thalamic-cortical circuit loop that is segregated from other loops running through different subregions of medial shell [85]. Another neuroanatomical study reported that the rostrodorsal hotspot of NAc medial shell has additional distinct features, such as dense projections to subregions of lateral hypothalamus that other NAc subregions may not project to [86]. The rostral hotspot of NAc medial shell also has distinct neurochemical features, such as a higher incidence of parvalbumin neurons than in the caudal coldspot of medial shell [153], and distinct neurochemical responsiveness to mu opioid stimulation [154]. By contrast, the caudal subregion of medial shell, which contains the hedonic coldspot where mu opioid stimulation by DAMGO microinjection (as well as delta or kappa opioid stimulations) oppositely suppresses ‘liking’ (although still increasing ‘wanting’ to eat, at least for mu stimulation), instead has transitional features shared with extended amygdala structures [86]. Which, if any, of these neurobiological features underlie the hotspot’s special ability to enhance ‘liking’ reactions, or rostrocaudal gradients in affective functions of medial shell? The answer to that question is not yet known, but such evidence at least shows that it has a number of unique neuroanatomical and neurochemical features which could eventually be part of that explanation.
4. Ventral pallidum hedonic hotspot
The ventral pallidum receives the densest output projections from nucleus accumbens [132,133,155,156], and ventral pallidum is important in both reward and aversion [29,38,157–174]. The posterior half of the ventral pallidum of rats contains another 0.8 mm3 hedonic hotspot where microinjections of the mu-opioid agonist DAMGO more than doubles hedonic ‘liking’ reactions to sucrose [38,98]. Similar to NAc, though reversed in front to back valence polarity, the VP appears organized along a bivalent anatomical gradient [38]. For example, local opioid stimulation by DAMGO microinjection in the posterior (the same subregion is also lateral and dorsal in VP) half of VP enhanced sucrose ‘liking’ reactions (and increased food intake), whereas the same opioid stimulation in anterior (which is also medial and ventral) VP oppositely suppressed positive ‘liking’ reactions (and suppressed food intake), revealing a rostral VP hedonic coldspot. It may be related that a human neuroimaging study found similar rostrocaudal bivalence, in that anterior VP was reported to activate in response to disgusting images, whereas posterior VP activated to images of palatable foods [170,175]. However, anterior VP still can participate in generating incentive motivation or ‘wanting’ for rewards. A different manipulation of anterior VP, namely local GABA blockade induced via bicuculine antagonist microinjections to disinhibit or excite anterior VP neurons, caused increases in food intake [38]. Similarly, anterior VP has also been shown by others to be important in motivation to pursue drug and foods rewards [166,169].
Within the hedonic hotspot of posterior VP, orexin microinjections also have been found to enhance ‘liking’ reactions to sucrose, just as opioid microinjections do [90]. Furthermore, recent pilot studies using optogenetic stimulation suggest that directly exciting VP neurons via channelrhodopsin in the posterior hotspot similarly enhances positive ‘liking’ expressions, as well as increasing ‘wanting’ to eat [176–178]. By comparison, optogenetic stimulation of LH neurons adjacent to VP, increased only food intake but not hedonic reactions to sucrose, indicating it is possible to increase ‘wanting’ without increasing ‘liking’ [176–179]. Similarly implicating these subregional differences for VP in reward, others have reported that frequency thresholds for electrical self-stimulation in VP are lower in posterior subregions of VP than anterior subregions supporting a special role for caudal ventral pallidum in some reward-related functions [180]. However, as mentioned, anterior VP neurons also contribute to motivation to seek reward, at least in some neurobiological modes and in some situations [38,98,166,169,172]. The functional flexibility and multiple roles of VP subregions is a topic that deserves further investigation.
4.1. Hotspots recruit each other to unanimously enhance ‘liking’ as an integrated hedonic circuit
Some evidence suggests that stimulating one hedonic hotspot (e.g., in either VP, NAc, OFC, or insula) recruits neural activation of other hotspots in different structures, activating the entire array of distributed hotspots as a unitary hedonic circuit to enhance ‘liking’ reactions [1,17,39,98,176,178]. For example, opioid stimulation of the NAc hotspot via NAc DAMGO microinjection recruits distant Fos activation in the VP hotspot when enhancing ‘liking’ reactions to sucrose taste [98], and similarly amplifies electrophysiological firing patterns of neurons in the VP hotspot that encode hedonic ‘liking’ for sucrose [147]. Conversely, local opioid stimulation in the VP hotspot reciprocally recruits Fos activation in the NAc hotspot when enhancing sucrose ‘liking’ [98]. Similarly, in the cortical hedonic hotspots in OFC or insula, DAMGO or orexin microinjections that enhance ‘liking recruit distant Fos increases in subcortical VP and NAc hotspots [39]. Furthermore, evidence suggests that mutual recruitment among hotspots may be causally necessary for any hotspot stimulation to enhance ‘liking’ reactions. Blocking opioid receptors with a naloxone microinjection in one hotspot (either NAc or VP) while simultaneously stimulating the other hotspot, prevents any hedonic enhancement that otherwise would be generated by the DAMGO microinjection in the other hotspot [98]. All in all, these studies suggest that the hedonic hotspots act together as a unified functional circuit for hedonic enhancement, and that disruption of that full circuit recruitment can prevent opioid hotspot stimulation from enhancing affective responses to palatable tastes.
However, while hedonic hotspots recruit each other into action, the exact neuroanatomical connections by which they do so remains as yet unknown. Anatomical tracing evidence suggests that the hotspots do not directly project to each other [85,86]. For example, although NAc and VP as whole structures are heavily interconnected, the NAc subregion of rostrodorsal medial shell that contains the hedonic hotspot primarily projects to the anterior VP that contains the hedonic coldspot and not to the posterior VP hotspot [85,86]. Conversely, the posterior VP hotspot sends reciprocal efferents primarily to the lateral core of the NAc, not to the rostral medial shell that contains the NAc hotspot [85,86,136,155]. In addition, while NAc projects to PBN, which may be a brainstem hedonic hotspot [40], NAc-PBN projections primarily arise from the ventral quadrant of the medial shell, not the rostrodorsal shell quadrant that contains the NAc hedonic hotspot [136]. Similarly, the subregion of prefrontal cortex that projects directly to the NAc shell hotspot is the infralimbic region of ACC (equivalent to Area 25 in humans), and not the anteromedial OFC that contains its cortical hedonic hotspot [85]. A lack of point to point projections among hedonic hotspots indicates that intermediary anatomical relay sites must exist to functionally connect hedonic hotspots together, but the precise identity of these relay sites and connections is not yet known.
4.2. Ventral pallidum hotspot: crucial to normal ‘liking’
Although all hedonic hotspots can produce gains in hedonic ‘liking’ reactions when appropriately stimulated, damage to most hotspots does not produce loss of normal ‘liking’ reactions. The posterior VP hotspot is the only known brain region where excitotoxic or electrolytic neurondestroying lesions can result in loss of normal ‘liking’ reactions and replacement by excessive ‘disgust’ reactions even to sweet taste (Fig. 3). These effects can persist for weeks, underlining the special importance of VP hotspot to normal hedonic function [168,181]. For example, after VP lesions, normally ‘liked’ sucrose taste instead elicits ‘disgust’ reactions such as gapes, headshakes, paw treading, etc., as though the sweet taste had become bitter or otherwise strongly unpalatable [168,181].
Fig. 3. The posterior ventral pallidum is necessary for normal hedonic function.
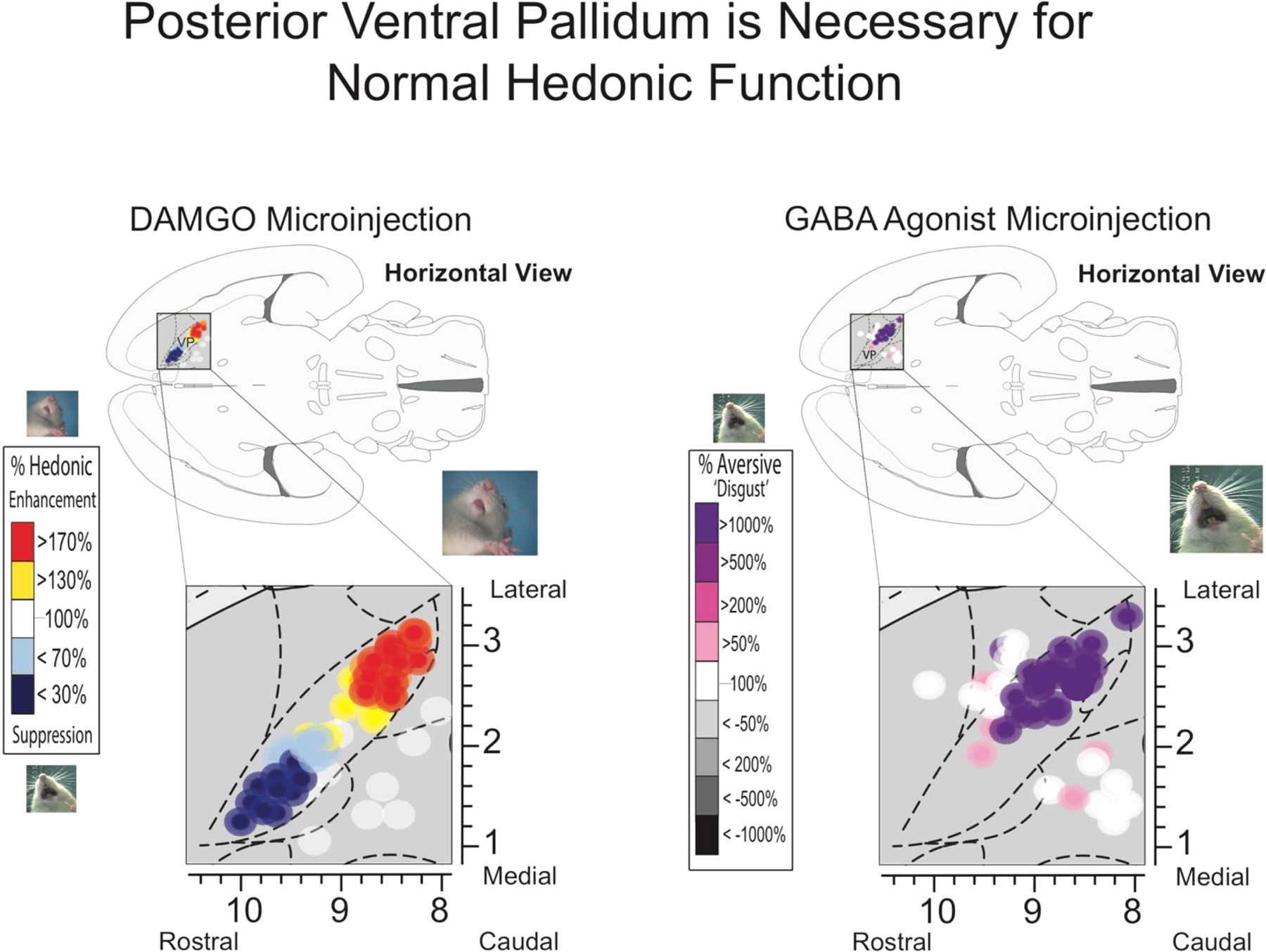
Microinjections of mu-opioid and orexin agonists (left) into the pallidum revealed a rostral to caudal organization of hedonic function. Stimulation of the posterior ventral pallidum ‘hotspot’ causally amplifies sucrose orofacial expressions (‘liking’) while the same manipulations in the caudal hedonic ‘coldspot’ suppress them. Temporary inactivation of posterior VP via GABA agonists generates a reversal of hedonic function so that normally ‘liked’ sucrose solutions elicit aversive ‘disgust’ reactions. Adapted from Smith & Berridge (2005) and Ho & Berridge (2014).
Classic studies in the 1960s using large electrolytic lesions originally attributed lesion-induced ‘disgust’ to damage to the LH [182,183]. However, subsequent more precise mapping using smaller excitotoxin lesions indicated that the crucial ‘disgust-induction’ lesion site was not in LH, but was actually the hedonic hotspot of posterior VP [168]. The large electrolytic lesions to LH of earlier studies typically also damaged posterior VP in addition to the LH, which may account for the negative affective reactions reported by early LH studies [1]. In other words, only damage to the VP hotspot produces dramatic loss of hedonic function. Both LH lesions and VP lesions can cause loss of ‘wanting’ to eat or drink, producing severe adipsia and aphagia, so that lesioned rats require intragastric feeding and hydration to be kept alive. But if they receive that intense nursing for days to weeks, rats slowly begin to independently feed again on soft palatable food, eventually progressing to normal eating and then drinking behavior, although some subtle ingestive functions still remain impaired [183–186].
Beyond ‘disgust’ induction by posterior VP lesions, pharmacological inhibition of posterior VP hotspot neurons, such as by microinjections of GABA agonists, also can induce temporary excessive ‘disgust’ to sweetness that lasts at least for hours [181,187]. Excessive ‘disgust’ induced by pharmacological muscimol/baclofen microinjections in the VP hotspot, as well as by posterior VP lesions, has been interpreted as a ‘release phenomenon’ [181,187], a century-old concept from the early neurologist Hughlings-Jackson for explaining how a neuronal dysfunction produces an active behavioral disorder [188]. That is, the excessive disgust probably results from negative-affect generating circuitry in other brain structures outside the VP, which is released or disinhibited by damage to the positively-valenced hedonic hotspot of posterior VP [181,187].
Our lab is currently testing whether direct optogenetic inhibition of VP neurons can similarly cause loss of hedonic function. Our recent pilot results, using the modified inhibitory channelrhodopsin (SwiChR ++) opsin, which opens negative Cl- ion gates in the neuronal membrane, allowing influx of Cl- ions to make the neuron more negative and less able to fire (similar to an IPSP) [189], suggest that optogenetic inhibition of neurons in the posterior VP hotspot may suppress positive ‘liking’ reactions elicited by sucrose taste, and possibly also increase negative ‘disgust’ reactions to an already aversive quinine solution (Morales & Berridge, 2019 and personal observations). Optogenetic induction of neuronal inhibition may be less intense than that induced by pharmacological GABAergic microinjections, producing weaker behavioral consequences, but results so far suggest that optogenetic inhibition may be enough to suppress positive hedonic valence or increase negative valence under some conditions.
4.3. Potential neurobiological basis of hedonic differences between posterior VP vs anterior VP: ‘liking’ hotspot vs coldspot
What accounts for differences in reward functions between anterior and posterior subregions of VP? One answer may lie in distinct neurobiological features of their neurons, as the ventral pallidum contains multiple types of neurons which can differ in their electrophysiological signatures [191,192], and in their neurochemical identities across anterior-posterior subregions [173,193–196]. For example, electro-physiologically, VP is thought to contain either Type I or Type II cells. The anterior VP contains a mixture of Type I and Type II cells, whereas posterior VP hotspot contains predominantly Type II cells [192]. Type I cells are easily excitable, tonically active, and larger than Type II cells. Type II neurons by contrast exhibit low basal firing rates, require higher thresholds for stimulation, and share some morphological features with NAc MSNs.
Neurochemically, approximately ~74% of VP neurons are GABAergic, ~11% are cholinergic, and ~15% are glutamatergic, in mostly separate and non-overlapping populations [173,195,197]. VP glutamate neurons are concentrated in anterior VP [173,197], near the site of the hedonic coldspot [38], whereas posterior VP is more heavily GABAergic. A number of studies have suggested that VP GABAergic neurons contribute primarily to reward-related motivation, whereas VP glutamatergic neurons contribute to aversive motivation, by oppositely modulating the activity of their overlapping downstream targets such as LH, VTA, and lateral habenula (LHb) [162,171,173,197–201].
Are VP GABAergic neurons important in amplifying ‘liking’ and ‘wanting’ for food rewards? To better answer this question, pilot studies in our lab have recently begun to explore this issue via selective optogenetic stimulation of GABA neurons in VP using a Cre-dependent promoter to express ChR2 in the ventral pallidum of GAD-Cre rats [202]. Our preliminary experiments indicate that optogenetic stimulation of VP GABA neurons generates robust feeding, biases and narrows preference for a laser-paired sucrose reward, and promotes self-stimulation [178,190]. Most interestingly, optogenetic stimulation of posterior VP GABA neurons additionally appears to enhance ‘liking’ reactions to sucrose taste, as well as ‘wanting’ to eat [190]. By contrast, inhibiting the same posterior VP GABA neurons with a Cl- ion channel opsin (iC++) may suppress ‘liking’ reactions [190]. Thus, our preliminary results so far support the hypothesis that it is GABAergic neurons in the posterior VP hotspot that are responsible for both gain of function and loss of function changes in hedonic ‘liking’ reactions. Additionally, GABA neurons throughout the entire VP may more generally participate in motivation ‘wanting’ for rewards [38,171,173,181].
5. Cortical hedonic hotspots – insula and orbitofrontal cortex
Beyond subcortical hedonic hotspots, two hotspots in cortex were recently discovered by our lab: one in the anteromedial orbitofrontal cortex, and another in the far-posterior insula cortex of rats. Both of these cortical hedonic hotspots similarly caused hedonic gains of function in sucrose ‘liking’ reactions in response to drug microinjections that deliver mu opioid stimulation or orexin stimulation to local neurons [39]. By contrast, the same opioid/orexin microinjections in other limbic cortex sites outside these hotspots, even in other regions of OFC or insula, fail to enhance sucrose ‘liking’ (and some sites suppress ‘liking’), even if they stimulate ‘wanting’ to eat [39].
The finding that hedonic hotspots exist in the cortex was surprising in one sense, because lesions in cortical areas do not reliably reduce hedonic reactions in either rats or humans [203–208]. That is, damage to the orbitofrontal cortex or insula does not necessarily cause loss of ‘liking’ reactions to foods or other pleasant events. However, gain of hedonic function is different from loss of hedonic function, and in a neural hierarchy a superior structure such as cortex might plausibly cause hedonic gains by activating subcortical hedonic circuitry, without causing hedonic losses when damaged, if the subcortical circuitry is capable on its own of generating basic hedonic reactions. In any case, human neuroimaging data and animal electrophysiological studies have also reported that orbitofrontal cortex and insula at least encode hedonic values of food and other rewards [3,209–215].
In keeping with the hierarchical triggering and cross-hotspot recruitment notions, DAMGO or orexin into the OFC or insula hotspot that enhanced ‘liking’ caused distant increases in neural activation measured by Fos expression in the hedonic hotspots in NAc and VP. This supports the hypothesis that ‘liking’ enhancements caused by neurochemical stimulation of a particular hotspot are mediated by recruiting the entire hedonic circuitry across the brain to activate all hotspots together [1,39,98,147]. The two cortical hedonic hotspots were also shown to bookend a long ‘hedonic coldspot’ strip between them where orexin and DAMGO microinjections oppositely suppressed sucrose hedonic reactions (i.e., stretching from lateral orbitofrontal cortex through insula). Orexin or opioid microinjections in the coldspot strip produced a pattern of Fos changes across the brain quite different from cortical hotspot microinjections, suggesting activation of a separate anti-’liking’ neural circuitry that dampens positive hedonic reactions [39]. It is interesting that an overlapping subregion of posterior insula (posterior to gustatory sensory cortex) also appears crucial to taste aversion learning [216]. Increases in motivational ‘wanting’ to eat, measured as increased consumption of chocolate M&M candies were also produced by all OFC hotspot microinjections and some insula hotspot microinjections, and were also produced by a number of nonhedonic sites in infralimbic cortex, prelimbic cortex, or anterior cingulate cortex (ACC), and even by some sites in the intervening hedonic coldspot strip of posterior-lateral OFC and anterior insula [39].
Current pilot studies in our lab are investigating whether optogenetic ChR2 excitation of neurons in these cortical OFC and insula hedonic hotspots can drive ‘liking’ enhancements, similarly to opioid or orexin neurochemical stimulations of those same OFC or insula subregions. Our preliminary data suggest that optogenetic excitation of neurons in either the anteromedial OFC hotspot or in the far-posterior insula hotspot may indeed double ‘liking’ reactions to sucrose taste [190,217]. However, more mapping may be needed given that a recent report suggested that optogenetic stimulation in anterior insula of mice promotes positive affective reactions whereas posterior insula stimulation evoked ‘disgust’ reactions [218,219]. We also note that some others have reported optogenetic laser self-stimulation of glutamate neurons in insula regions, or of insula-to-amygdala projections [218,220], although others report avoidance of laser-stimulation at some insula sites [218,220,221], suggesting the insula picture in particular may need further clarification.
6. Distributed brain mechanisms of ‘wanting’: nucleus accumbens core, neostriatum, amygdala, lateral hypothalamus and beyond
The mesocorticolimbic brain system that generates incentive salience or ‘wanting’ is anatomically larger than the hedonic hotspot network, including entire structures of NAc, central nucleus of amygdala and parts of neostriatum, etc. Neurochemically it includes dopamine and glutamate, as well as opioid orexin, and endocannabinoid transmitters so that its functionally more robust than the ‘liking’ network (Fig. 1b). [222–231]. This robust network can generate intense incentive motivation and appetite, even without enhancing hedonic ‘liking’.
6.1. Nucleus accumbens core
Incentive motivation to eat can be amplified by manipulations such as opioid-stimulating microinjections throughout the entire nucleus accumbens, including both core and shell. Regarding simple (unconditioned) food intake, some studies have reported that various manipulations in the core as well as shell can enhance free-feeding in rodents, albeit many report less robust effects from the core compared to shell [73,80,81,152,232–236]. However, both core and shell have been shown to potently alter learned instrumental responding for palatable rewards in rats [237–239], which may be due specifically to enhanced cue-triggered ‘wanting’ or incentive salience as shown in an elevated Pavlovian-instrumental transfer (PIT) paradigm after DAMGO or amphetamine microinjections in NAc [239]. Interestingly, lesions to the core, but not shell prevent reallocation of food-related responses in a decision-making task where rats are given the option to lever press for a preferred palatable sucrose reward vs. eating normal laboratory chow that is freely available within the chamber [240].
Overall, the NAc medial shell is especially important for its role in generating intense incentive motivation, whereas the core has been reported to be preferentially activated by reward-predictive cues [1,70,97,146,241–246]. For example, previously drug-associated cues can trigger drug-seeking [247]. Conditioned instrumental responding may be associated with Fos expression in D1 and D2 core medium spiny neurons [143], and specific forms of PIT, which depend upon association of cues with the learned identities of specific foods, are especially reliant on NAc core [248]. Conversely, decreasing dopamine signaling in the core can suppress sign-tracking behavior in rats [249,250].
6.2. The dorsal neostriatum
Parts of the neostriatum, sometimes called dorsal striatum, also participates in generating incentive motivation. Human imaging studies have long shown that food-related cravings are associated with activation of the dorsal striatum [251,252]. This human striatal response to food has been reported to become blunted in those who frequently eat a specific type of food. For example, people who frequently eat ice cream may show suppressed dorsal striatal activation to a milkshake [253]. Similarly, rodent studies have shown that prolonged exposure to a high sugar and fat diet resembling a western diet can alter glutamate, opioid, and dopamine transmission in the dorsal striatum [254]. Lack of dopamine in the dorsal striatum is associated with severe aphagia that ultimately results in death, further implicating neostriatum role in feeding and appetite [255–257].
The dorsomedial part of the neostriatum (DMS) is known for a role in goal-directed learning and motivation [258–265], but it may also play a role in directly generating appetite. For example, microinjection of mu-opioid stimulating DAMGO directly into the dorsomedial neostriatum causes rats to increase food intake [43]. Similarly, levels of the endogenous opioid neurotransmitter enkephalin within dorsomedial neostriatum surge spontaneously when rats begin to eat a palatable food, consistent with an appetite-promoting mechanism [43]. Dorsmedial participation in generating motivation to eat is consistent with evidence that opioid-stimulating microinjections throughout much of the neostriatum can cause increases in food intake [43,68,73,77,266]. However, DAMGO microinjection in the dorsomedial neostriatum that enhances ‘wanting’ to eat sweet food is not accompanied by any enhancement orofacial ‘liking’ reactions to sweetness, suggesting a specific incentive motivation but not hedonic contribution [43]. Finally, selective inhibition in dorsomedial neostriatum of the dopamine D2 ‘stop’ pathway, while preserving the D1 ‘go’ pathway, invigorates motivation to work for a palatable reward during a progressive ratio task [267]. Overall, these results suggest that opioid and dopamine signals in the dorsomedial neostriatum play an important role in modulating incentive motivation to eat.
By comparison, the dorsolateral part of the neostriatum (DLS) has traditionally been described for roles in habit formation [262,268–272], model-free stimulus-response associations [273–277], motor sequences and direct movement control [278–283]. However, DLS also plays a role in motivation for reward. For example, optogenetic stimulation of the direct-path (D1 dopamine receptor expression) and indirect (D2 receptor expressing) neostriatal neurons can promote place-based self-stimulation and avoidance, respectively [284].
The DLS also helps generate incentive salience for learned food cues, visible in an autoshaping or sign-tracking paradigm [42]. Microinjections of DAMGO or of amphetamine into the DLS can enhance attraction to sucrose-related cues. In this situation, rats learn that the insertion of a metal lever into the chamber (Pavlovian CS+) predicts a free sucrose pellet (UCS) [42]. Typically, one group of rats, known as sign-trackers (STs), attribute high incentive salience directly to the predictive lever, and approach and nibble the CS+ lever [285–287]. Another group of rats, known as goal-trackers (GTs), instead are attracted to the sucrose-pellet dish or goal, approaching and nibbling the metal dish. When mu-opioid or dopamine signaling was enhanced in the dorsolateral neostriatum by microinjection of DAMGO or amphetamine, ‘pure’ ST rats that always go to the lever CS+ became even more attracted towards their CS+ lever than before, suggesting intensified incentive salience that is even more narrowly-focused on the CS+ [42]. Similarly, GTs became selectively even more attracted toward their dish, again suggesting intensified and motivation focused on their preferred stimulus. In Pavlovian parlance, the dish is also a type of Pavlovian CS+, but one that is contiguous to sucrose UCS in space and time, whereas the lever is a predictive CS+ whose presentation is correlated with UCS delivery; both CS+ types are traditionally recognized by Pavlovian learning theory.
Further evidence from the same study supported the conclusion that these enhancements of conditioned responding were due to increased motivational attraction to the respective CS+s, rather than to intensified habits. For example, DLS microinjections of DAMGO also increased sign-trackers willingness to learn and work on a new instrumental nosepoke task in order to earn presentations of their lever CS+ (i.e., increased instrumental conditioned reinforcement of an entirely new behavioral response, showing magnified ‘wanting’ for the CS+ as a feature of incentive salience) [42]. Similarly, sign-trackers flexibly followed their lever to a new location in the chamber if it moved after DAMGO microinjections in DLS, rather than repeating the same habitual response of going to the old location.
Amphetamine microinjections that promoted dopamine release in dorsolateral neostriatum made ‘mixed sign-trackers’, which previously mostly went to lever CS+ but sometimes made a ‘goal-tracking’ defection toward the dish CS+, actually switch to become instead purer goal-trackers, again replacing the more habitual response with a different one. That is, DLS dopamine stimulation appeared to enhance motivated attraction to the UCS-proximal dish CS+ at the expense of the predictive lever CS+’s attractiveness for those individuals [42]. Thus, the dorsolateral neostriatum may have important roles in both amplifying incentive motivation and in selecting which competing cues for food reward become most attractive.
A different view of the dorsal neostriatum’s role in eating was recently suggested by Ivan de Araujo, Mark Schatzker, and Dana Small [288], but may possibly be reconciled with our own view expressed above. De Araujo et al. argue that less reliant are the hedonic properties of foods like flavor, taste, and aroma in their ability to generate excessive overeating [288]. They note that vagal sensory projections from the viscera to the hindbrain sensory nucleus of the solitary tract carry signals about caloric content arising from food digestion, and show vagal signals may trigger dopamine release from substantia nigra axons in the dorsal neostriatum [288]. Strikingly, direct optogenetic stimulation of vagal-to-medulla projections supports laser self-stimulation, which they suggest reveals a response-reinforcing signal [289]. Nutrient conditioning of flavor preferences similarly relies on intact dopamine signaling in the dorsal striatum [290,291]. The vagal-neostriatal dopamine reinforcement signal, De Araujo et al. suggest, does not enhance food hedonic palatability but rather strengthens behavior more directly, similar to traditional stimulus-response (S-R) habit stamping-in theories. As de Araujo et al. put it “In other words, reinforcement and habit acquisition can occur seamlessly in the absence of any consciousness-borne flavor appreciation.” (p. 153, [288]).
The hypothesis of de Araujo et al. that vagal nutrient signals act in neostriatum without any “consciousness-borne flavor appreciation” is consistent with our view that neostriatal dopamine fails to enhance ‘liking’. The hypothesis that vagal signals promote learned attraction to foods is also consistent, as de Araujo et al. point out, with many earlier demonstrations by Anthony Sclafani, Kevin Myers and colleagues that intra-gastric calories are able to act as a UCS to establish a conditioned preference for a paired CS flavor in rats, increasing ‘wanting’ to eat that food whether or not it also increases ‘liking’ for the more ‘wanted’ CS flavor [290,292–295]. For example, nutrient conditioning can enhance ‘wanting’ without enhancing ‘liking’ reactions for a bitter/sour CS+ flavor [295], although it can enhance both ‘wanting’ and ‘liking’ together if the CS+ flavor was initially sweet or palatable [294]. Thus, enhanced ‘liking’ is a possible accompaniment but not an obligatory component of nutrient conditioned taste preferences.
Based on all this, we would suggest a possible alternative interpretation to S-R habit reinforcement for the role of vagal-evoked dopamine in neostriatum. That is, given that dopamine in dorsal neostriatum can enhance the incentive salience of specific food cues, as described above [42], vagal-evoked dopamine release in dorsal neostriatum might similarly promote ‘wanting’ to eat evoked by particular food cues associated with vagal stimulation. This would be an incentive motivation mechanism, probably maximally triggered by particular foods that are both caloric and palatable, rather than a behaviorist response stamping-in mechanism, and would not be confined to habits but could promote eating even if food seeking required novel responses or if the food cues moved to new settings.
6.3. The amygdala
The focus of ‘wanting’ onto particular targets is a function in which amygdala also plays an important role. The amygdala is composed of multiple nuclei, including the basolateral nucleus of amygdala (BLA), the medial nucleus of the amygdala (MeA), and the central nucleus of amygdala (CeA) [296–303], and of these, the CeA is particularly important to generating intense incentive salience. The CeA has ‘striatallevel’ status within a cortico-striatal-pallidal macrosystem organization of forebrain structures (in which the BLA has cortical status, and the bed nucleus of stria terminalis (BNST) holds ‘pallidal status’ within the extended amygdala complex [297]). The striatal-level status of the CeA may be relevant to its ability to amplify appetitive motivation. For example, the CeA contains many GABAergic neurons that receive BLA glutamate inputs and mesolimbic dopamine inputs (glutamate-dopamine convergence similar to NAc and neostriatum), and project primarily to BNST as a pallidal-type target [304].
Eating palatable food causes increases in Fos expression in the central amygdala [305,306] and direct manipulations that alter opioid, glutamate, GABA, and several peptides within CeA can potentiate unconditioned food intake [45,307–320]. Conversely, GABAergic inactivation of the CeA or dopamine blockade in CeA suppresses food intake [321,322]. Some recent optogenetic studies have similarly reported that ChR2 activation of various CeA neuronal types amplifies food intake and drinking of palatable sweet solutions [323–326].
The CeA may also play a special role in targeting enhanced ‘wanting’ on to particular learned cues for food rewards. For example, in a sign-tracking/goal-tracking situation, CeA mu-opioid stimulation by DAMGO microinjection selectively enhances the incentive salience of the sucrose-predicting lever CS+ in sign-trackers, but selectively enhances the incentive salience of the sucrose-contiguous dish CS+ in goal-trackers. In both cases it enhances approach towards, and consummatory bites and nibbles to the individual’s preferred metal lever or dish cue [44,45,307]. That suggests the CeA can amplify incentive motivation and focus ‘wanting’ specifically on an already preferred CS + stimulus [44]. Similarly, in a Pavlovian-to-instrumental transfer situation (PIT), CeA opioid stimulation specifically enhances cue-triggered ‘wanting’ by increasing bouts of instrumental lever pressing for sucrose reward when the CS+ is presented, and not in its absence [307]. In addition to its role in food motivation and appetite, CeA signaling has also been shown to be important for cue-induced motivation for drug rewards [327–332]. Conversely, lesion studies suggest that loss of CeA function impairs cue-induced ‘wanting’, suppressing PIT, and other forms of motivation [333–336].
Recently, optogenetic CeA stimulations have been used to amplify and control the direction of ‘wanting’ for a particular target, such as sucrose, cocaine, or even a noxious shock-rod stimulus that delivers electric shocks if touched [46,47,337]. The CeA role is powerful enough to make a rat ‘want what hurts it’ when laser stimulation is paired with voluntary encounters of the noxious shock rod, so that rats paradoxically become compulsively attracted to the shock-rod and subject themselves to shocks again and again [337]. This CeA-driven attraction is mediated in part via recruiting activation of distributed mesocorticolimbic circuitry for incentive motivation [337].
Regarding food in particular, studies by Mike Robinson and Shelley Warlow in our lab showed that pairing such CeA optogenetic stimulation with a sucrose target could make the rat exclusively pursue that laser-paired sucrose target while ignoring an equally good sucrose alternative. CeA stimulation also amplified breakpoint incentive motivation to obtain sucrose in a progressive ratio task [47]. Another study by Robinson and colleagues showed that rats will withstand a painful foot shock in order to gain access to the laser-paired sucrose, and pursue it even when the alternative non-laser paired sucrose reward is 10 times larger [338]. However, Robinson and Warlow found that CeA ChR2 stimulation did not appear to enhance orofacial ‘liking’ reactions for sucrose, despite making rats ‘want’ sucrose more [47]. Pilot results in our lab suggest that pairing optogenetic CeA ChR2 stimulation specifically of CRF neurons in CeA with a particular sucrose target can similarly make that target exclusively preferred over an alternative sucrose option, and so mimic at least some of the CeA ChR2 effects described above [339–341].
Overall, CeA and its control over other mesocorticolimbic circuitry may be involved in sharpening the focus of amplified ‘wanting’ onto cues for a particular incentive target, like a high-caloric palatable food, which could contribute to intense urges to indulge in those foods, leading to overeating.
6.4. Lateral hypothalamus homeostatic interactions with mesocorticolimbic circuitry for ‘liking’ and ‘wanting’
Understanding how ‘liking’, ‘wanting’, and hypothalamic circuitry interact to promote appetite and motivation is an enduring quest. Lateral hypothalamus (LH) may modulate the activity of mesocorticolimbic circuitry, including hedonic hotspots, by integrating homeostatic signals so that relevant hunger/satiety states can enhance or suppress motivated and hedonic behaviors to food rewards at appropriate times [342]. But how might LH help regulate these processes? One obvious potential mechanism is orexin (Fig. 4a), given that it is both a hunger-related hypothalamic signal and an effective enhancer of ‘liking’ reactions in limbic hedonic hotspots [1,39,90,97,148,343,344]. Orexin/hypocretin is a neuropeptide exclusively synthesized in perifornical, lateral, and dorsomedial nuclei of the hypothalamus [345,346], and while implicated generally in arousal throughout the hypothalamus, a subset of orexin neurons in a subregion of lateral hypothalamus are also implicated in reward-related motivation [179,347–352]. LH orexin neurons project widely throughout the brain, including to nucleus accumbens, ventral pallidum, ventral tegmentum, and limbic cortex regions where the hedonic hotspots are located [83,155,349,353–358]. LH orexin is therefore an ideal candidate to help mediate alliesthesia [342,359], the phenomenon in which physiological appetite states enhance hedonic ‘liking’ and palatability ratings of the tastes of relevant foods [23,25,213,360]. Consistent with that hypothesis, direct microinjections of orexin-A into hedonic hotspots in VP, NAc, OFC, and insula amplify ‘liking’ reactions to sucrose as effectively as microinjections of mu opioid DAMGO into those sites [39,90,97].
Fig. 4. Brain Systems for Appetite and Motivation.
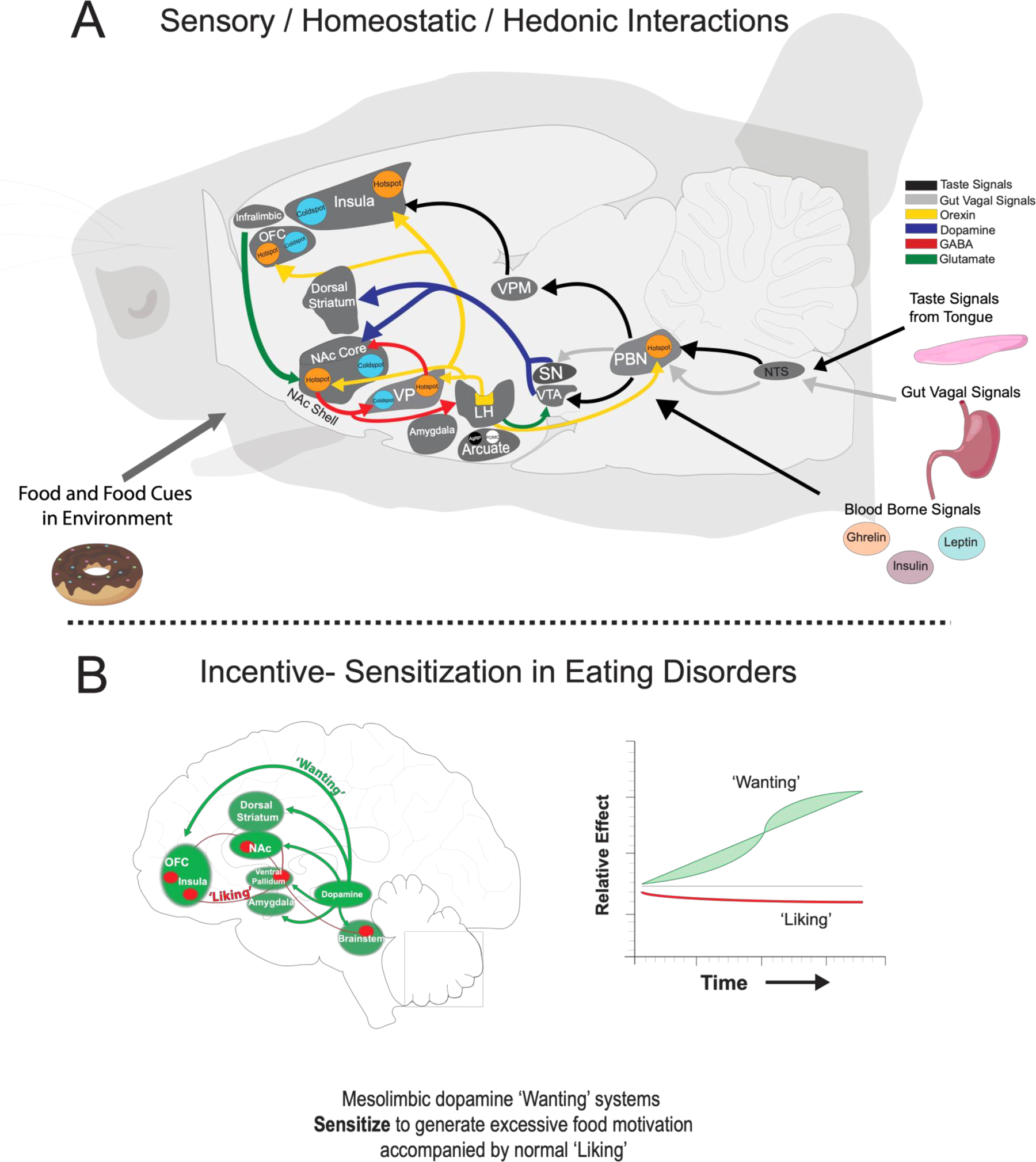
A) Top panel shows a sagittal view of a rat brain with a summary map of connections between hindbrain, hypothalamic, and mesocorticolimbic sites that mediate ‘liking’, ‘wanting’, sensory signals, and appetite. Brain hedonic hotspots (shown in orange) and coldspots (shown in light blue) in parabrachial nucleus, ventral pallidum, nucleus accumbens, orbitofrontal cortex, and insula do not share direct projections. Orexin signals from the lateral hypothalamic modulate mesocorticolimbic activity by integrating circulating signals about hunger/satiety in order to enhance or suppress ‘liking’ and incentive motivation during various physiological states. Additional hypothalamic systems in the arcuate nucleus of the hypothalamus may interact with mesolimbic circuitry so that their activity reflects the incentive value of food and food-related cues in the environment. Colors of arrows denote projection types. Data is based from studies described in text. B) Bottom panel is a sagittal view of mesocorticolimbic systems that mediate ‘liking’ and ‘wanting’ in humans. Individuals with eating disorders may have hyper-reactive mesolimbic dopamine systems that respond to information about food and their related cues in the environment. This enhanced dopamine release may assign excessive incentive salience that results in overconsumption of palatable foods that is independent of how much those foods are actually ‘liked’.
Additional mechanisms for hypothalamic-limbic interactions include AgRP/NPY and POMC neurons in the arcuate nucleus (ARC) and LH [361–364]. ARCAgRP and ARCPOMC neurons send robust projections to LH and their release of AgRP and POMC peptides modulate activity of LH neurons [363,364]. For most of the past 20 years, AgRP neurons have been viewed as simple homeostatic ‘hunger’ neurons, and POMC neurons viewed as ‘satiety’ neurons [365]. However, recent studies indicate that AgRP activity rapidly decreases as soon as palatable food is merely presented, or even when a cue predicting food is encountered, before any actual food has been ingested. Conversely, POMC neuronal activity can rapidly rise when triggered by these encounters or cues, in advance of any physiological satiety [366–368]. One interpretation of these rapid anticipatory changes is that AgRP and POMC neuronal activity reflects an interaction between incentive and hedonic information about available food, implying bidirectional or looping circuitry interactions between mesocorticolimbic-reward and hypothalamic-homeostatic systems [369]. That would be compatible with the increasing recognition that, rather than serving as parallel systems that promote appetite and feeding independently, hedonic and homeostatic systems may be understood as heavily interconnected, which functionally interact to control appetite and eating behavior.
7. Clinical implications of ‘liking’ versus ‘wanting’ dissociation: incentive-sensitization and obesity
The above discussion of brain mechanisms for food ‘wanting’ versus ‘liking’ may carry potential implications for human obesity and eating disorders. In the past decade, a number of obesity investigators have applied the brain-based ‘wanting/liking’ distinction to suggest that in some vulnerable individuals, ‘wanting’ for foods might dissociate and exceed ‘liking’ to cause excessive cue-trigged ‘wants’ to overeat [2,4,5,370–374]. The idea that some cases of extreme over-eating or binge-eating disorders can reflect excessive ‘wanting’, without excessive ‘liking’ invokes the incentive-sensitization theory of addiction, which was originally proposed for drug addiction but recently has been extended to behavioral addictions and to over-eating [375–377]. Incentive-sensitization applied to eating disorders suggests that some individuals may be especially vulnerable to developing neural sensitization of dopamine-related mesocorticolimbic systems of ‘wanting’, and consequently assign the exaggerated incentive salience that results specifically to palatable foods and the act of eating them. The result would be excessive ‘wanting’ to eat (Fig. 4b), typically triggered by palatable food cues or by vivid imagery about such foods, which could become especially exacerbated in moments of stress or emotional arousal that heighten mesolimbic reactivity. Evidence supporting this incentive-sensitization interpretation of overeating comes particularly from neuroimaging studies of obese or binge-eating individuals that have reported a sensitization-type brain activation signature to food cues that is remarkably similar to the signature of people who suffer from drug addiction to drug cues [2,4,370,373].
A potential incentive-sensitization brain explanation for eating disorders is also relevant to debates about the concept of food addiction [370,372,373,378–389]. That is, a legitimate ‘food addiction’ might exist to the degree that some over-eaters truly show incentive-sensitization signatures of brain activation to foods, in the sense that those food-sensitized individuals may experience more intense cue-triggered food cravings than other people do. The ideal brain signature for an eating addiction in the sense of incentive-sensitization would be mesocorticolimbic hyper-reactivity in nucleus accumbens or striatum, ventral tegmentum, amygdala or limbic cortical regions in over-eaters that is triggered by food cues. An incentive-sensitization signature would be hyper-reactive in both of two ways: 1) more intense brain activations triggered by food cues than by money or other reward cues in the same over-eating individual, and 2) more intense brain activations triggered by food cues in sensitized over-eaters than triggered by the same food cues in nonsensitized normal eaters.
Extreme incentive salience attributed to foods is in one sense a natural phenomenon that nearly anyone could experience – at least, under extreme conditions of prolonged starvation, but which most people in the modern world fortunately never experience. For example, during World War 2 a controlled Minnesota study of starvation was carried out using conscientious objectors as volunteers of starvation to better understand starvation consequences and treatments [390]. Gradually the volunteers began to be gripped by intense food cravings as they became extremely underweight: “Some of them (volunteers) obsessively read cookbooks, staring at pictures of food with almost por-nographic interest” [390]. Despite being highly motivated, a number of volunteers could not resist succumbing to temptations to eat, and left the study. Thus, anyone can feel strong urges to eat during extreme physiological starvation that become nearly compulsive. What may be different in sensitized over-eaters is that similarly intense incentive salience is attributed to food cues, due to sensitized hyper-reactivity of mesocorticolimbic ‘wanting’ systems in some vulnerable individuals, even without ever being starved and despite developing obesity.
Some evidence for incentive sensitization in over-eating has come from reports that obesity and binge eating disorder is associated with heightened BOLD signals in ventral striatum, prefrontal cortex, and OFC in response to visual cues of palatable foods compared to individuals without obesity [391–393]. Similarly, individuals with obesity have been reported to have elevated brain responses in striatum, amygdala and orbitofrontal cortex to images of high calorie foods compared to foods low in calories or control images [394–401]. Using PET, one study reported elevation in striatal dopamine release in binge-eating individuals (compared to non-binge eating individuals) when they were given oral methylphenidate, which may pharmacologically prime mesolimbic dopamine reactivity, and their higher dopamine response was positively correlated with binge eating scores [402]. Heightened brain activity to palatable foods also positively correlates with self-reported subjective cravings or ‘wanting’ to eat [403], and individuals with binge eating are reported to have greater EEG reactivity in response to palatable chocolate pictures and increased craving ratings compared to healthy controls [404]. Elevated brain responses to food in individuals with obesity may also be associated with poorer outcomes to behavioral weight loss treatments [405]. Evidence suggests that enhanced brain limbic activity is selective to food rewards in over-eaters, as some studies have not observed increased brain activity to monetary rewards in individuals with binge-eating disorder [403,406]. Overall, these studies suggest that individuals with binge-eating disorder or obesity show incentive sensitization-like features in mesolimbic brain structures to food and food-associated cues, which could produce more intense cue-triggered ‘wanting’ to eat, even if not be matched by more intense ‘liking’ [2,4,5,370–374].
Most telling may be prospective or longitudinal tracking studies that track individuals both before and after they develop obesity. For example, one such study reported that young women showed altered brain responses to learned food cues in ventral pallidum and neostriatum. Women who showed the greatest increase in ventral pallidum BOLD signals and greatest decrease in neostriatal signals were at greatest risk for developing excessive weight gain later in life [407].
Incentive-Sensitization contrasts to Reward Deficiency.
It is worth noting that the incentive-sensitization hypothesis for over-eating contrasts strongly with the reward deficiency hypothesis, which was prominent for several decades in both obesity and drug addiction fields. This reward deficiency idea postulated that obese individuals find foods less rewarding than other individuals, and therefore eat more foods to accumulate rewarding experiences and so make up their reward deficiency. This reward deficiency hypothesis was based on reports that striatal dopamine D2 receptors appear to be down-regulated in some individuals with obesity, at least in the sense that they have reduced labeled-raclopride binding (although reduced binding to vacant receptors may not be able to distinguish between fewer receptors versus higher dopamine release and receptor occupancy). That reduced D2 binding is similar to potential D2 down-regulation in individuals with drug addiction [408–414]; although some studies fail to find D2 binding reductions in people with obesity [415].
Early reward deficiency advocates often drew on the once-popular idea that mesolimbic dopamine mediated ‘liking’ or food pleasure, inferring that lower D2 binding therefore meant a deficiency of pleasure. The reward deficiency hypothesis also assumed that individuals respond to reductions in food pleasure by consuming more food to regain a preferred pleasure level. That assumption views food pleasure reduction as similar to drug dilution, where individuals may consume a greater quantity of a dilute drug (e.g. beer) than of a concentrated drug (e.g. whiskey) to obtain the same alcohol dose. However, sensory incentives such as food obey very different empirical rules. For food rewards, making a food less ‘liked’, typically also makes it less ‘wanted’ less and therefore less consumed [9,416–418]. For example, many parents might be able to attest that putting their children on a diet of unpreferred broccoli, brussels sprouts, or spinach is unlikely to lead to weight gain. Proponents of the reward deficiency hypothesis might object to this example on grounds that reward deficiency is sometimes posited to develop later in life, and only when eating palatable energy-dense foods (e.g., sweet-fatty foods, salty-fatty foods, etc.). However, making a palatable rich food less palatable is still unlikely to make an individual eat more of it. In our view, there is no evidence for the reward-deficiency assumption that individuals eat more as a food becomes less tasty. Rather, people and animals instead typically eat more when the available foods are more ‘liked’ and consequently more ‘wanted’.
Neurobiological problems may also exist for the reward deficiency hypothesis. Evidence from animal experiments where brain dopamine levels are manipulated indicates that increases in food seeking and consumption are more readily produced by increases of dopamine signals in the nucleus accumbens (such as after amphetamine microinjections in nucleus accumbens to promote dopamine release) than by suppression of dopamine signals [239,419–421]. Conversely, suppressing dopamine signals from the nucleus accumbens or neostriatum is most often reported to reduce eating and food seeking in animal studies, rather than cause overeating [422–424]. Similarly in people, inducing suppression of dopamine signaling in ordinary volunteers may actually cause them to eat less rather than to eat more [425]. As a caveat, however, the brain has multiple anatomical dopamine systems, and dopamine and norepinephrine signaling in the paraventricular nucleusof medial hypothalamus can oppositely suppress food intake [426,427]. Appetite-suppressing action of dopamine in the paraventricular nucleus of hypothalamus may explain why amphetaminetype drugs can be dieting aids (i.e., by stimulating hypothalamic dopamine and norepinephrine systems), and conversely why long-term exposure to neuroleptic/anti-psychotic dopamine antagonist drugs can sometimes produce weight gain [428–430].
But if reduction of accumbens/striatal dopamine signals does not cause overeating via reward deficiency, then why are obese individuals often reported to have reductions in striatal D2 receptor binding? An alternative explanation for why D2 dopamine downregulation occurs in many cases of obesity could be that D2 receptor downregulation is a consequence of eating palatable foods and/or weight gain, rather than being its cause. That is, encountering and eating rewarding foods may engage relatively intense mesocorticolimbic signals, possibly involving excessive or repeated dopamine release and related neurobiological over-stimulations, which eventually cause a partially compensatory down-regulation of D2 receptors just as do repeated exposures to addictive drugs.
Further, obesity is a form of extreme satiety that may induce related long-term physiological alliesthesia signals (e.g., high leptin, etc.), as negative-feedback signals that also attempt to dampen future mesocorticolimbic activation to check excessive ‘wanting’ to eat. All these may be viewed as partial compensatory responses tending to oppose the temptation power of palatable food incentives and cues that activate mesocorticolimbic dopamine systems, but which in many individuals fail to fully compensate because they are only partial and because other neuronal components of mesocorticolimbic incentive circuitry remain hyper-reactive to food cues. As a result, those individuals may continue to over-eat even in the face of reduced dopamine D2 receptors in nucleus accumbens or striatum. Evidence for thinking that D2 receptor downregulation is a consequence of continually eating palatable foods and of obesity, rather than a cause of over-eating, is that D2 receptor downregulation in nucleus accumbens and striatum can be induced in normal rats by giving them several weeks of free access to an array of palatably sweet and rich junk foods, on which some of them then become obese [5]. That is, the rats’ D2 downregulation occurs as a consequence of continually eating sweet and fatty junk food and of any consequent obesity that develops over that prolonged period of time. Human evidence that D2 downregulation is primarily a consequence of obesity, and not the cause, is that the suppressed level of D2 dopamine receptors in severely obese humans sometimes rises after they lose weight following bariatric Roux-en-Y surgery, again as a consequence of their change in eating habits and of weight loss induced by the surgery [431,432].
In the end, future prospective human neuroimaging studies may provide the best evidence to help decide between incentive-sensitization and reward deficiency explanations of over-eating and obesity. Tracking changes in brain function in the same individuals both before they become obese and after obesity develops can provide important evidence regarding underlying causal mechanisms. For example, healthy weight adolescents who subsequently gain body fat over 2-or-3 years have been reported to show enhanced brain responses to food cues even before they gained weight [433]. Similarly, healthy weight adolescents who subsequently gain weight have been reported to have higher initial brain responses in taste and reward coding cortical regions like insula and OFC when consuming milkshakes, suggesting limbic hyper-reactivity may be a pre-existing cause of later obesity. However, after they gain weight their brain reactions to the actual taste of milkshakes declines, suggesting that that the reduction may a compensatory consequence of their weight-gain [434].
Partly as a result of many demonstrations of mesocorticolimbic hyper-reactivity to food cues in individuals with obesity, the weight of neuroimaging data may have shifted away from the reward deficiency hypothesis and toward the incentive sensitization hypothesis in the past 10 years [2,4,402,435,436]. For example, a recent meta-analysis of fMRI results concluded that “Extant data provide strong support for the incentive sensitization theory of obesity and… only minimal support for the reward deficit (deficiency) theory” [2]. Similarly, another meta-analysis review of brain imaging studies concluded that “we did not find univocal evidence in favor of a Reward Deficit Hypothesis nor for a systematic deficit of inhibitory cognitive control. We conclude that the available brain activation data (for human obesity)… can be best framed within an Incentive Sensitization Theory” [4]. Such conclusions draw on results such as observation of fMRI hyper-reactivity to food cues in striatum, orbitofrontal cortex and insula cortex in obesity-prone human adolescents even before those individuals went on to gain weight several years later [435]. They are also consistent with reports of higher levels of dopamine release in neostriatum elicited by palatable foods in obese individuals with binge eating disorders than individuals who were not binge eaters, suggesting mesolimbic hyper-reactivity persisted in individuals who binge-eat [402]. Similarly, people who are heavier have been reported to have higher striatal dopamine release than people who were lighter, leading the authors of the study to conclude their results “suggest increase dopamine release with increasing body mass… consistent with… increasing behavioral salience of food being a risk factor for obesity” [436].
8. Conclusion
Mesocorticolimbic structures including the nucleus accumbens, ventral pallidum, orbitofrontal cortex, and insula contain localized hedonic hotspots in specific subregions, where opioid and other specific forms of stimulation can enhance ‘liking’ reactions to palatable foods. The same structures often also contain separable hedonic coldspots where the same neurobiological stimulations suppress ‘liking’. These hotspots and coldspots are nestled within larger mesocorticostriatal ‘wanting’ circuitry where many of the same forms of stimulation plus others (e.g. dopamine) robustly generate intense cue-triggered incentive salience, amplifying motivation to seek and consume palatable food rewards, whether or not ‘liking’ is simultaneously enhanced.
The distinguishable identities of brain systems for ‘liking’ versus ‘wanting’ food rewards has implications for understanding at least some cases of human obesity, binge-eating, and related eating disorders. This particularly appears to apply in the form of incentive-sensitization signatures of hyper-reactivity of brain ‘wanting’ systems in some individuals with obesity or binge eating disorder, which may cause over-eating without necessarily being accompanied by enhanced food ‘liking’. Future research in this area will continue to extend understanding of how mesocorticolimbic systems interact with hypothalamic homeostatic signals to control normal appetite and food reward, and how specific dysregulations in motivation systems contribute to eating disorders and obesity.
Acknowledgements
The research described here was supported by NIH grants MH63649 from NIMH and DA015188 from NIDA to KCB, and IM was supported by training grant DC00011 from NIDCD. We thank Dr. Stephanie Preston for comments on earlier versions of the manuscript. We also thank Hannah Baumgartner, Erin Naffziger, David Nguyen, Shayan Abtahi, and Valerie Trewick for their feedback.
Footnotes
Declaration of Competing Interest
The authors declare no competing financial interests.
References
- [1].Castro DC, Cole SL, Berridge KC, Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry, Front. Syst. Neurosci 9 (2015) 90, 10.3389/fnsys.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stice E, Yokum S, Neural vulnerability factors that increase risk for future weight gain, Psychol. Bull 142 (2016) 447–471, 10.1037/bul0000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].DiFeliceantonio AG, Coppin G, Rigoux L, Edwin Thanarajah S, Dagher A, Tittgemeyer M, Small DM, Supra-Additive Effects of Combining Fat and Carbohydrate on Food Reward, Cell Metab. 28 (2018) 33–44, 10.1016/j.cmet.2018.05.018 e3. [DOI] [PubMed] [Google Scholar]
- [4].Devoto F, Zapparoli L, Bonandrini R, Berlingeri M, Ferrulli A, Luzi L, Banfi G, Paulesu E, Hungry brains: A meta-analytical review of brain activation imaging studies on food perception and appetite in obese individuals, Neurosci. Biobehav. Rev 94 (2018) 271–285, 10.1016/j.neubiorev.2018.07.017. [DOI] [PubMed] [Google Scholar]
- [5].Robinson MJF, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, Berridge KC, Ferrario CR, Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity, Neuropsychopharmacology 40 (2015) 2113–2123, 10.1038/npp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kringelbach ML, The pleasure of food: underlying brain mechanisms of eating and other pleasures, Flavour 4 (2015) 20, 10.1186/s13411-014-0029-2. [DOI] [Google Scholar]
- [7].Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD, Neural correlates of food addiction, Arch. Gen. Psychiatry 68 (2011) 808–816, 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berridge KC, Kringelbach ML, Pleasure Systems in the Brain, Neuron 86 (2015) 646–664, 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Berridge KC, Evolving concepts of emotion and motivation, Front. Psychol 9 (2018) 1647, 10.3389/fpsyg.2018.01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Winkielman P, Berridge KC, Unconscious emotion, Curr. Dir. Psychol. Sci 13 (2004) 120–123, 10.1111/j.0963-7214.2004.00288.x. [DOI] [Google Scholar]
- [11].Steiner JE, The gustofacial response: observation on normal and anencephalic newborn infants, Symp. Oral Sens. Percept (1973) 254–278. http://www.ncbi.nlm.nih.gov/pubmed/4612820 (accessed December 13, 2017). [PubMed] [Google Scholar]
- [12].Steiner JE, Innate human facial expressions to taste and smell stimulation, Ann. N. Y. Acad. Sci 237 (1974) 229–233, 10.1111/j.1749-6632.1974.tb49858.x. [DOI] [PubMed] [Google Scholar]
- [13].Grill HJ, Norgren R, The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats, Brain Res. 143 (1978) 263–279, 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- [14].Steiner JE, Glaser D, Hawilo ME, Berridge KC, Comparative expression of hedonic impact: Affective reactions to taste by human infants and other primates, Neurosci. Biobehav. Rev 25 (2001) 53–74, 10.1016/S0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- [15].Berridge KC, Measuring hedonic impact in animals and infants: Microstructure of affective taste reactivity patterns, Neurosci. Biobehav. Rev 24 (2000) 173–198, 10.1016/S0149-7634(99)00072-X. [DOI] [PubMed] [Google Scholar]
- [16].Jankunis ES, Whishaw IQ, Sucrose bobs and quinine gapes: Horse (equus caballus) responses to taste support phylogenetic similarity in taste reactivity, Behav. Brain Res 256 (2013) 284–290, 10.1016/j.bbr.2013.08.024. [DOI] [PubMed] [Google Scholar]
- [17].Castro DC, Berridge KC, Advances in the neurobiological bases for food “liking” versus “wanting,”, Physiol. Behav 136 (2014) 22–30, 10.1016/j.physbeh.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Davidson TL, Martin AA, Clark K, Swithers SE, Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation, Q. J. Exp. Psychol 64 (2011) 1430–1441, 10.1080/17470218.2011.552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Peciña S, Berridge KC, Hedonic Hot Spot in Nucleus Accumbens Shell: Where Do -Opioids Cause Increased Hedonic Impact of Sweetness? J. Neurosci 25 (2005) 11777–11786, 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Robinson MJF, Berridge KC, Instant transformation of learned repulsion into motivational “wanting,”, Curr. Biol 23 (2013) 282–289, 10.1016/j.cub.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shin AC, Townsend RL, Patterson LM, Berthoud HR, Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition, Am. J. Physiol. - Regul. Integr. Comp. Physiol 301 (2011) R1267–R1280, 10.1152/ajpregu.00314.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Laeng B, Berridge KC, Butter CM, Pleasantness of a sweet taste during hunger and satiety: Effects of gender and “sweet tooth“, Appetite 21 (1993) 247–254, 10.1006/appe.1993.1043. [DOI] [PubMed] [Google Scholar]
- [23].Cabanac M, Physiological role of pleasure, Science (80-.). 173 (1971) 1103–1107, 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- [24].Cabanac M, Sensory pleasure, Q. Rev. Biol 54 (1979) 1–29, 10.1086/410981. [DOI] [PubMed] [Google Scholar]
- [25].Berridge KC, Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat, Appetite 16 (1991) 103–120, 10.1016/0195-6663(91)90036-R. [DOI] [PubMed] [Google Scholar]
- [26].Cabanac M, Lafrance L, Postingestive alliesthesia: The rat tells the same story, Physiol. Behav 47 (1990) 539–543, 10.1016/0031-9384(90)90123-L. [DOI] [PubMed] [Google Scholar]
- [27].Berridge KC, Grill HJ, Isohedonic tastes support a two-dimensional hypothesis of palatability., Appetite. 5 (1984) 221–31. http://www.ncbi.nlm.nih.gov/pubmed/6524918 (accessed April 15, 2019). [DOI] [PubMed] [Google Scholar]
- [28].Clark JJ, Bernstein IL, Sensitization of salt appetite is associated with increased “wanting” but not “liking” of a salt reward in the sodium-deplete rat, Behav. Neurosci 120 (2006) 206–210, 10.1037/0735-7044.120.1.206. [DOI] [PubMed] [Google Scholar]
- [29].Tindell AJ, Smith KS, Berridge KC, Aldridge JW, Dynamic computation of incentive salience: “wanting” what was never “liked, J. Neurosci 29 (2009) 12220–12228, 10.1523/JNEUROSCI.2499-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tindell AJ, Smith KS, Peciña S, Berridge KC, Aldridge JW, Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J. Neurophysiol 96 (2006) 2399–2409, 10.1152/jn.00727.2006. [DOI] [PubMed] [Google Scholar]
- [31].Berridge KC, Schulkin J, Palability Shift of a Salt-Associated Incentive During Sodium Depletion, Q. J. Exp. Psychol. Sect. B 41 (1989) 121–138, 10.1080/14640748908401188. [DOI] [PubMed] [Google Scholar]
- [32].Berridge KC, Grill HJ, Norgren R, Relation of consummatory responses and preabsoptive insulin release to palatability and learned taste aversions, J. Comp. Physiol. Psychol 95 (1981) 363–382, 10.1037/h0077782. [DOI] [PubMed] [Google Scholar]
- [33].Parker LA, Taste avoidance and taste aversion: Evidence for two different processes, Learn. Behav 31 (2003) 165–172, 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- [34].Spector AC, Breslin P, Grill HJ, Taste Reactivity as a Dependent Measure of the Rapid Formation of Conditioned Taste Aversion: A Tool for the Neural Analysis of Taste-Visceral Associations, Behav. Neurosci 102 (1988) 942–952, 10.1037/0735-7044.102.6.942. [DOI] [PubMed] [Google Scholar]
- [35].Spector AC, Norgren R, Grill HJ, Parabrachial gustatory lesions impair taste aversion learning in rats, Behav. Neurosci 106 (1992) 147–161, 10.1037/0735-7044.106.1.147. [DOI] [PubMed] [Google Scholar]
- [36].Grill HJ, Norgren R, Chronically decerebrate rats demonstrate satiation but not bait shyness, Science 201 (80-) (1978) 267–269, 10.1126/science.663655. [DOI] [PubMed] [Google Scholar]
- [37].Wilkins EE, Bernstein IL, Conditioning method determines patterns of c-fos expression following novel taste-illness pairing, Behav. Brain Res 169 (2006) 93–97, 10.1016/j.bbr.2005.12.006. [DOI] [PubMed] [Google Scholar]
- [38].Smith KS, Berridge KC, The Ventral Pallidum and Hedonic Reward: Neurochemical Maps of Sucrose “Liking” and Food Intake, J. Neurosci 25 (2005) 8637–8649, 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Castro DC, Berridge KC, Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula, Proc. Natl. Acad. Sci 114 (2017) 201705753,, 10.1073/pnas.1705753114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Söderpalm AHVV, Berridge KC, The hedonic impact and intake of food are increased by midazolam microinjection in the parabrachial nucleus, Brain Res. 877 (2000) 288–297, 10.1016/S0006-8993(00)02691-3. [DOI] [PubMed] [Google Scholar]
- [41].Cole SL, Robinson MJF, Berridge KC, Optogenetic self-stimulation in the nucleus accumbens: D1 reward versus D2 ambivalence, PLoS One 13 (2018), 10.1371/journal.pone.0207694 e0207694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].DiFeliceantonio AG, Berridge KC, Dorsolateral neostriatum contribution to incentive salience: Opioid or dopamine stimulation makes one reward cue more motivationally attractive than another, Eur. J. Neurosci 43 (2016) 1203–1218, 10.1111/ejn.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].DiFeliceantonio AG, Mabrouk OS, Kennedy RT, Berridge KC, Enkephalin surges in dorsal neostriatum as a signal to eat, Curr. Biol 22 (2012) 1918–1924, 10.1016/j.cub.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].DiFeliceantonio AG, Berridge KC, Which cue to “want”? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking, Behav. Brain Res 230 (2012) 399–408, 10.1016/j.bbr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mahler SV, Berridge KC, Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J. Neurosci 29 (2009) 6500–6513, 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Warlow SM, Robinson MJF, Berridge KC, Optogenetic central amygdala stimulation intensifies and narrows motivation for cocaine, J. Neurosci 37 (2017), 10.1523/JNEUROSCI.3141-16.2017 3141–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Robinson MJF, Warlow SM, Berridge KC, Optogenetic Excitation of Central Amygdala Amplifies and Narrows Incentive Motivation to Pursue One Reward Above Another, J. Neurosci 34 (2014) 16567–16580, 10.1523/JNEUROSCI.2013-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Peciña S, Schulkin J, Berridge KC, Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 4 (2006) 8, 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Grill HJ, Norgren R, Neurological tests and behavioral deficits in chronic thalamic and chronic decerebrate rats, Brain Res. 143 (1978) 299–312, 10.1016/0006-8993(78)90570-X. [DOI] [PubMed] [Google Scholar]
- [50].Grill HJ, Norgren R, The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats, Brain Res. 143 (1978) 281–297 http://www.ncbi.nlm.nih.gov/pubmed/630410 accessed April 5, 2019. [DOI] [PubMed] [Google Scholar]
- [51].Peciña S, Berridge KC, Brainstem mediates diazepam enhancement of palatability and feeding: Microinjections into fourth ventricle versus lateral ventricle, Brain Res. 727 (1996) 22–30, 10.1016/S0006-8993(96)00325-3. [DOI] [PubMed] [Google Scholar]
- [52].Berridge KC, Liking” and “wanting” food rewards: Brain substrates and roles in eating disorders, Physiol. Behav 97 (2009) 537–550, 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Berridge KC, Peciña S, Benzodiazepines, appetite, and taste palatability, Neurosci. Biobehav. Rev 19 (1995) 121–131, 10.1016/0149-7634(94)00026-W. [DOI] [PubMed] [Google Scholar]
- [54].Soderpalm AH, Berridge KC, Food intake after diazepam, morphine or muscimol: microinjections In the nucleus accumbens shell, Pharmacol. Biochem. Behav 66 (2000) 429–434 S0091–3057(00)00220–3 [pii]. [DOI] [PubMed] [Google Scholar]
- [55].Berridge KC, Brainstem systems mediate the enhancement of palatability by chlordiazepoxide, Brain Res. 447 (1988) 262–268, 10.1016/0006-8993(88)91128-6. [DOI] [PubMed] [Google Scholar]
- [56].Rolls ET, Functions of the anterior insula in taste, autonomic, and related functions, Brain Cogn. 110 (2016) 4–19, 10.1016/j.bandc.2015.07.002. [DOI] [PubMed] [Google Scholar]
- [57].Scott TR, Small DM, The role of the parabrachial nucleus in taste processing and feeding, Ann. N. Y. Acad. Sci Blackwell Publishing Inc, 2009, pp. 372–377, 10.1111/j.1749-6632.2009.03906.x. [DOI] [PubMed] [Google Scholar]
- [58].Norgren R, Leonard CM, Ascending central gustatory pathways, J. Comp. Neurol 150 (1973) 217–237, 10.1002/cne.901500208. [DOI] [PubMed] [Google Scholar]
- [59].Pritchard TC, Hamilton RB, Norgren R, Projections of the parabrachial nucleus in the Old World monkey, Exp. Neurol 165 (2000) 101–117, 10.1006/exnr.2000.7450. [DOI] [PubMed] [Google Scholar]
- [60].Kaplan JM, Roitman M, Grill HJ, Food deprivation does not potentiate glucose taste reactivity responses of chronic decerebrate rats, Brain Res. 870 (2000) 102–108, 10.1016/S0006-8993(00)02406-9. [DOI] [PubMed] [Google Scholar]
- [61].Grill HJ, Roitman MF, Kaplan JM, A new taste reactivity analysis of the integration of taste and physiological state information, Am. J. Physiol. - Regul. Integr. Comp. Physiol (1996) 271, 10.1152/ajpregu.1996.271.3.r677. [DOI] [PubMed] [Google Scholar]
- [62].Grill HJ, Schulkin J, Flynn FW, Sodium Homeostasis in Chronic Decerebrate Rats, Behav. Neurosci 100 (1986) 536–543, 10.1037/0735-7044.100.4.536. [DOI] [PubMed] [Google Scholar]
- [63].Chang FC, Scott TR, Conditioned taste aversions modify neural responses in the rat nucleus tractus solitarius. J. Neurosci 4 (1984) 1850–1862 http://www.ncbi.nlm.nih.gov/pubmed/6737042 accessed April 12, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Giza BK, Scott TR, Blood glucose selectively affects taste-evoked activity in rat nucleus tractus solitarius, Physiol. Behav 31 (1983) 643–650 http://www.ncbi.nlm.nih.gov/pubmed/6665054 accessed December 4, 2019. [PubMed] [Google Scholar]
- [65].Giza BK, Scott TR, Intravenous insulin infusions in rats decrease gustatory-evoked responses to sugars, Am. J. Physiol 252 (1987) R994–1002, 10.1152/ajpregu.1987.252.5.R994. [DOI] [PubMed] [Google Scholar]
- [66].Giza BK, Deems RO, Vanderweele DA, Scott TR, Pancreatic glucagon suppresses gustatory responsiveness to glucose, Am. J. Physiol 265 (1993) R1231–R1237, 10.1152/ajpregu.1993.265.6.R1231. [DOI] [PubMed] [Google Scholar]
- [67].Glenn JF, Erickson RP, Gastric modulation of gustatory afferent activity, Physiol. Behav 16 (1976) 561–568, 10.1016/0031-9384(76)90216-X. [DOI] [PubMed] [Google Scholar]
- [68].Zhang M, Kelley AE, Enhanced intake of high-fat food following striatal mu-opioid stimulation: Microinjection mapping and Fos expression, Neuroscience 99 (2000) 267–277, 10.1016/S0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- [69].Urstadt KR, Kally P, Zaidi SF, Stanley BG, Ipsilateral feeding-specific circuits between the nucleus accumbens shell and the lateral hypothalamus: Regulation by glutamate and GABA receptor subtypes, Neuropharmacology 67 (2013) 176–182, 10.1016/j.neuropharm.2012.10.027. [DOI] [PubMed] [Google Scholar]
- [70].Castro DC, Berridge KC, Opioid Hedonic Hotspot in Nucleus Accumbens Shell: Mu, Delta, and Kappa Maps for Enhancement of Sweetness “Liking” and “Wanting,”, J. Neurosci 34 (2014) 4239–4250, 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Reynolds SM, Berridge KC, Glutamate motivational ensembles in nucleus accumbens: Rostrocaudal shell gradients of fear and feeding, Eur. J. Neurosci 17 (2003) 2187–2200, 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- [72].Stratford TR, Wirtshafter D, NPY mediates the feeding elicited by muscimol injections into the nucleus accumbens shell, Neuroreport 15 (2004) 2673–2676, 10.1097/00001756-200412030-00024. [DOI] [PubMed] [Google Scholar]
- [73].Kelley AE, Swanson CJ, Feeding induced by blockade of AMPA and kainate receptors within the ventral striatum: A microinfusion mapping study, Behav. Brain Res 89 (1997) 107–113, 10.1016/S0166-4328(97)00054-5. [DOI] [PubMed] [Google Scholar]
- [74].Basso AM, Kelley AE, Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference, Behav. Neurosci 113 (1999) 324–336, 10.1037/0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- [75].Reynolds SM, Berridge KC, Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J. Neurosci 22 (2002) 7308–7320 20026734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M, Opioid modulation of taste hedonics within the ventral striatum, Physiol. Behav 76 (2002) 365–377, 10.1016/S0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- [77].Bakshi VP, Kelley AE, Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study, Psychopharmacology (Berl) 111 (1993) 207–214, 10.1007/BF02245525. [DOI] [PubMed] [Google Scholar]
- [78].Bakshi VP, Kelley AE, Sensitization and conditioning of feeding following multiple morphine microinjections into the nucleus accumbens, Brain Res. 648 (1994) 342–346, 10.1016/0006-8993(94)91139-8. [DOI] [PubMed] [Google Scholar]
- [79].Bakshi VP, Kelley AE, Feeding induced by opioid stimulation of the ventral striatum: Role of opiate receptor subtypes, J. Pharmacol. Exp. Ther 265 (1993) 1253–1260. [PubMed] [Google Scholar]
- [80].Maldonado-Irizarry CS, Swanson CJ, Kelley AE, Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus, J. Neurosci 15 (1995) 6779–6788, 10.1523/jneurosci.15-10-06779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Stratford TR, Kelley AE, GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J. Neurosci 17 (1997) 4434–4440 http://www.ncbi.nlm.nih.gov/pubmed/9151760 accessed April 28, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Stratford TR, Swanson CJ, Kelley AE, Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell, Behav. Brain Res 93 (1998) 43–50, 10.1016/S0166-4328(97)00140-X. [DOI] [PubMed] [Google Scholar]
- [83].Groenewegen HJ, Berendse HW, Haber SN, Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents, Neuroscience 57 (1993) 113–142 http://www.ncbi.nlm.nih.gov/pubmed/8278047 accessed April 20, 2019. [DOI] [PubMed] [Google Scholar]
- [84].West EA, Carelli RM, Nucleus accumbens core and shell differentially encode Reward-Associated cues after reinforcer devaluation, J. Neurosci 36 (2016) 1128–1139, 10.1523/JNEUROSCI.2976-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Thompson RH, Swanson LW, Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture, Proc. Natl. Acad. Sci 107 (2010) 15235–15239, 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zahm DS, Parsley KP, Schwartz ZM, Cheng AY, On lateral septum-like characteristics of outputs from the accumbal hedonic “hotspot” of Peciña and Berridge with commentary on the transitional nature of basal forebrain “boundaries,”, J. Comp. Neurol 521 (2013) 50–68, 10.1002/cne.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Li Z, Chen Z, Fan G, Li A, Yuan J, Xu T, Cell-type-specific afferent innervation of the nucleus accumbens core and shell, Front. Neuroanat (2018) 12, 10.3389/fnana.2018.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Reynolds SM, Berridge KC, Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J. Neurosci 21 (2001) 3261–3270 21/9/3261 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Humphries MD, Prescott TJ, The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward, Prog. Neurobiol 90 (2010) 385–417, 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [90].Ho CY, Berridge KC, An orexin hotspot in ventral pallidum amplifies hedonic “liking” for sweetness, Neuropsychopharmacology 38 (2013) 1655–1664, 10.1038/npp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mahler SV, Smith KS, Berridge KC, Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances “liking” of a sweet reward, Neuropsychopharmacology 32 (2007) 2267–2278, 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- [92].Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG, International Union of Pharmacology. XXVII. Classification of cannabinoid receptors, Pharmacol. Rev 54 (2002) 161–202 http://www.ncbi.nlm.nih.gov/pubmed/12037135 accessed April 12, 2019. [DOI] [PubMed] [Google Scholar]
- [93].Mitchell MR, Berridge KC, Mahler SV, Endocannabinoid-Enhanced “Liking” in Nucleus Accumbens Shell Hedonic Hotspot Requires Endogenous Opioid Signals, Cannabis Cannabinoid Res. 3 (2018) 166–170, 10.1089/can.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ferré S, Goldberg SR, Lluis C, Franco R, Looking for the role of cannabinoid receptor heteromers in striatal function, Neuropharmacology 56 (2009) 226–234, 10.1016/j.neuropharm.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Robledo P, Berrendero F, Ozaita A, Maldonado R, Advances in the field of cannabinoid-opioid cross-talk, Addict. Biol 13 (2008) 213–224, 10.1111/j.1369-1600.2008.00107.x. [DOI] [PubMed] [Google Scholar]
- [96].Wenzel JM, Cheer JF, Endocannabinoid Regulation of Reward and Reinforcement through Interaction with Dopamine and Endogenous Opioid Signaling, Neuropsychopharmacology 43 (2018) 103–115, 10.1038/npp.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Castro DC, Terry RA, Berridge KC, Orexin in Rostral Hotspot of Nucleus Accumbens Enhances Sucrose “Liking” and Intake but Scopolamine in Caudal Shell Shifts “Liking” Toward “Disgust” and “Fear”, Neuropsychopharmacology (2016) 1–11, 10.1038/npp.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Smith KS, Berridge KC, Opioid Limbic Circuit for Reward: Interaction between Hedonic Hotspots of Nucleus Accumbens and Ventral Pallidum, J. Neurosci 27 (2007) 1594–1605, 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Faure A, Richard JM, Berridge KC, Desire and dread from the nucleus accumbens: Cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat, PLoS One 5 (2010) e11223, 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wyvell CL, Berridge KC, Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J. Neurosci 20 (2000) 8122–8130 http://www.ncbi.nlm.nih.gov/pubmed/11050134 accessed April 6, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X, Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. J. Neurosci 23 (2003) 9395–9402 http://www.ncbi.nlm.nih.gov/pubmed/14561867 accessed April 6, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Rolls ET, Rolls BJ, Kelly PH, Shaw SG, Wood RJ, Dale R, The relative attenuation of self-stimulation, eating and drinking produced by dopamine-receptor blockade, Psychopharmacologia 38 (1974) 219–230, 10.1007/BF00421374. [DOI] [PubMed] [Google Scholar]
- [103].Zis AP, Fibiger HC, Neuroleptic-induced deficits in food and water regulation: similarities to the lateral hypothalamic syndrome, Psychopharmacologia 43 (1975) 63–68, 10.1007/bf00437616. [DOI] [PubMed] [Google Scholar]
- [104].Wise RA, Raptis L, Effects of naloxone and pimozide on initiation and maintenance measures of free feeding, Brain Res. 368 (1986) 62–68, 10.1016/0006-8993(86)91042-5. [DOI] [PubMed] [Google Scholar]
- [105].Muscat R, Willner P, Effects of dopamine receptor antagonists on sucrose consumption and preference, Psychopharmacology (Berl) 99 (1989) 98–102, 10.1007/bf00634461. [DOI] [PubMed] [Google Scholar]
- [106].Oltmans GA, Harvey JA, Lateral Hypothalamic Syndrome in Rats: A Comparison of the Behavioral and Neurochemical Effects of Lesions Placed in the Lateral Hypothalamus and Nigrostriatal Bundle, J. Comp. Physiol. Psychol 90 (1976) 1051–1062, 10.1037/h0078660. [DOI] [PubMed] [Google Scholar]
- [107].Berridge KC, Venier IL, Robinson TE, Taste reactivity analysis of 6-hydro-xydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function, Behav. Neurosci 103 (1989) 36–45, 10.1037/0735-7044.103.1.36. [DOI] [PubMed] [Google Scholar]
- [108].Schneider LH, Davis JD, Watson CA, Smith GP, Similar effect of raclopride and reduced sucrose concentration on the microstructure of sucrose sham feeding, Eur. J. Pharmacol 186 (1990) 61–70, 10.1016/0014-2999(90)94060-b. [DOI] [PubMed] [Google Scholar]
- [109].Hsiao S, Smith GP, Raclopride reduces sucrose preference in rats, Pharmacol. Biochem. Behav 50 (1995) 121–125, 10.1016/0091-3057(95)00315-N. [DOI] [PubMed] [Google Scholar]
- [110].Smith G, Dopamine and Food Reward, Prog, Psychobiol. Physiol. Psychol 16 (1995) 83–144. [PubMed] [Google Scholar]
- [111].Higgs S, Cooper SJ, The effect of the dopamine D2 receptor antagonist raclopride on the pattern of licking microstructure induced by midazolam in the rat, Eur. J. Pharmacol 409 (2000) 73–80, 10.1016/s0014-2999(00)00802-5. [DOI] [PubMed] [Google Scholar]
- [112].Galistu A, D’Aquila PS, Effect of the dopamine D1-like receptor antagonist SCH 23390 on the microstructure of ingestive behaviour in water-deprived rats licking for water and NaCl solutions, Physiol. Behav 105 (2012) 230–233, 10.1016/j.physbeh.2011.08.006. [DOI] [PubMed] [Google Scholar]
- [113].Cousins MS, Wei W, Salamone JD, Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs, Psychopharmacology (Berl) 116 (1994) 529–537, 10.1007/BF02247489. [DOI] [PubMed] [Google Scholar]
- [114].Treit D, Berridge KC, A comparison of benzodiazepine, serotonin, and dopamine agents in the taste-reactivity paradigm, Pharmacol. Biochem. Behav 37 (1990) 451–456, 10.1016/0091-3057(90)90011-6. [DOI] [PubMed] [Google Scholar]
- [115].Peciña S, Berridge KC, Parker LA, Pimozide does not shift palatability: Separation of anhedonia from sensorimotor suppression by taste reactivity, Pharmacol. Biochem. Behav 58 (1997) 801–811, 10.1016/S0091-3057(97)00044-0. [DOI] [PubMed] [Google Scholar]
- [116].Baumgartner HM, Cole SL, Olney JJ, Berridge KC, Desire or Dread from Nucleus Accumbens Inhibitions: Reversed by Same-Site Optogenetic Excitations. J. Neurosci 40 (2020) 2737–2752, 10.1523/JNEUROSCI.2902-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Stratford TR, Kelley AE, Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior, J. Neurosci 19 (1999) 11040–11048 http://www.ncbi.nlm.nih.gov/pubmed/10594084 accessed April 27, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Stratford TR, Wirtshafter D, Evidence that the nucleus accumbens shell, ventral pallidum, and lateral hypothalamus are components of a lateralized feeding circuit, Behav. Brain Res 226 (2012) 548–554, 10.1016/j.bbr.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Reynolds SM, Berridge KC, Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens, Nat. Neurosci 11 (2008) 423–425, 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Richard JM, Berridge KC, Nucleus Accumbens Dopamine/Glutamate Interaction Switches Modes to Generate Desire versus Dread: D1 Alone for Appetitive Eating But D1 and D2 Together for Fear. J. Neurosci 31 (2011) 12866–12879, 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Richard JM, Plawecki AM, Berridge KC, Nucleus accumbens GABAergic inhibition generates intense eating and fear that resists environmental retuning and needs no local dopamine, Eur. J. Neurosci 37 (2013) 1789–1802, 10.1111/ejn.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Carlezon WA, Wise RA, Microinjections of phenycyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward, Psychopharmacology (Berl) 128 (1996) 413–420, 10.1007/s002130050151. [DOI] [PubMed] [Google Scholar]
- [123].Cheer JF, Heien MLAV, Garris PA, Carelli RM, Wightman RM, Simultaneous dopamine and single-unit recordings reveal accumbens GABAergic responses: Implications for intracranial self-stimulation, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 19150–19155, 10.1073/pnas.0509607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Roitman MF, Wheeler RA, Carelli RM, Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output, Neuron 45 (2005) 587–597, 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- [125].Taha SA, Fields HL, Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens, J. Neurosci 25 (2005) 1193–1202, 10.1523/JNEUROSCI.3975-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE, The structural basis for mapping behavior onto the ventral striatum and its subdivisions, Brain Struct. Funct 213 (2008) 17–27, 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Roitman MF, Wheeler RA, Wightman RM, Carelli RM, Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli, Nat. Neurosci 11 (2008) 1376–1377, 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RMM, Behavioral and Electrophysiological Indices of Negative Affect Predict Cocaine Self-Administration, Neuron 57 (2008) 774–785, 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- [129].Carlezon WA, Thomas MJ, Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis, Neuropharmacology 56 (2009) 122–132, 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Krause M, German PW, Taha SA, Fields HL, A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding, J. Neurosci 30 (2010) 4746–4756, 10.1523/JNEUROSCI.0197-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Taha SA, Fields HL, Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior, J. Neurosci 26 (2006) 217–222, 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Mogenson GJ, Swanson LW, Wu M, Neural projections from nucleus accumbens to globus pallidus, substantia innominata, and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J. Neurophysiol 3 (1983) 189–202 http://www.ncbi.nlm.nih.gov/pubmed/6822855 accessed December 4, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Zahm DS, Heimer L, Two transpallidal pathways originating in the rat nucleus accumbens, J. Comp. Neurol 302 (1990) 437–446, 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- [134].Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C, Specificity in the projection patterns of accumbal core and shell in the rat, Neuroscience 41 (1991) 89–125, 10.1016/0306-4522(91)90202-Y. [DOI] [PubMed] [Google Scholar]
- [135].Lu XY, Ghasemzadeh MB, Kalivas PW, Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens, Neuroscience 82 (1998) 767–780 http://www.ncbi.nlm.nih.gov/pubmed/9483534 accessed April 19, 2019. [DOI] [PubMed] [Google Scholar]
- [136].Usuda I, Tanaka K, Chiba T, Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: Biotinylated dextran amine study, Brain Res. 797 (1998) 73–93, 10.1016/S0006-8993(98)00359-X. [DOI] [PubMed] [Google Scholar]
- [137].Zhou L, Furuta T, Kaneko T, Chemical organization of projection neurons in the rat accumbens nucleus and olfactory tubercle, Neuroscience 120 (2003) 783–798, 10.1016/S0306-4522(03)00326-9. [DOI] [PubMed] [Google Scholar]
- [138].Hollander JA, Ijames SG, Roop RG, Carelli RM, An examination of nucleus accumbens cell firing during extinction and reinstatement of water reinforcement behavior in rats, Brain Res. 929 (2002) 226–235, 10.1016/s0006-8993(01)03396-0. [DOI] [PubMed] [Google Scholar]
- [139].Koo JW, Lobo MK, Chaudhury D, Labonté B, Friedman A, Heller E, Peña CJ, Han MH, Nestler EJ, Loss of BDNF signaling in D1R-expressing NAc neurons enhances morphine reward by reducing GABA inhibition, Neuropsychopharmacology 39 (2014) 2646–2653, 10.1038/npp.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun HS, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ, Cell type - Specific loss of BDNF signaling mimics optogenetic control of cocaine reward, Science (80-.). 330 (2010) 385–390, 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Steinberg EE, Boivin JR, Saunders BT, Witten IB, Deisseroth K, Janak PH, Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens, PLoS One 9 (2014) e94771, 10.1371/journal.pone.0094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Natsubori A, Tsutsui-Kimura I, Nishida H, Bouchekioua Y, Sekiya H, Uchigashima M, Watanabe M, De Kerchove D’Exaerde A, Mimura M, Takata N, Tanaka KF, Ventrolateral striatal medium spiny neurons positively regulate food-incentive, goal-directed behavior independently of D1 and D2 selectivity, J. Neurosci 37 (2017) 2723–2733, 10.1523/JNEUROSCI.3377-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, Sousa N, Rodrigues AJ, Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation, Nat. Commun (2016) 7, 10.1038/ncomms11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A, Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens, Neuron 76 (2012) 790–803, 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Roitman MF, Wheeler RA, Tiesinga PHE, Roitman JD, Carelli RM, Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning, Learn. Mem 17 (2010) 539–546, 10.1101/lm.1869710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Loriaux AL, Roitman JD, Roitman MF, Nucleus accumbens shell, but not core, tracks motivational value of salt, J. Neurophysiol 106 (2011) 1537–1544, 10.1152/jn.00153.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Smith KS, Berridge KC, Aldridge JW, Disentangling pleasure from incentive salience and learning signals in brain reward circuitry, Proc. Natl. Acad. Sci 108 (2011) E255–E264, 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE, Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment, Eur. J. Neurosci 19 (2004) 376–386, 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- [149].Stratford TR, Activation of feeding-related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell, Brain Res. 1048 (2005) 241–250, 10.1016/j.brainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- [150].Cole SL, Mechanisms of motivation in the nucleus accumbens, University of Michigan, 2018. [Google Scholar]
- [151].Olney JJ, Berridge KC, Releasing motivation: Direct inhibition of nucleus accumbens shell neurons promotes motivated behaviors, in: Neurosci. Meet. Plan, Society for Neuroscience, San Diego, CA, 2018: p. 600.28 / FFF18. [Google Scholar]
- [152].Peciña S, Berridge KC, Opioid eating site in accumbens shell mediates food intake and hedonic “liking”: map based on microinjection Fos plumes, Brain Res. 863 (2000) 71–86 www.elsevier.com. [DOI] [PubMed] [Google Scholar]
- [153].Trouche S, Koren V, Doig NM, Ellender TJ, El-Gaby M, Lopes-dos-Santos V, Reeve HM, Perestenko PV, Garas FN, Magill PJ, Sharott A, Dupret D, A Hippocampus-Accumbens Tripartite Neuronal Motif Guides Appetitive Memory in Space, Cell 176 (2019) 1393–1406, 10.1016/j.cell.2018.12.037 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Britt JP, McGehee DS, Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens, J. Neurosci 28 (2008) 1672–1681, 10.1523/JNEUROSCI.4275-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Groenewegen HJ, Russchen FT, Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: A tracing and immunohistochemical study in the cat, J. Comp. Neurol 223 (1984) 347–367, 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- [156].Zahm DS, Heimer L, Specificity in the efferent projections of the nucleus accumbens in the rat: Comparison of the rostral pole projection patterns with those of the core and shell, J. Comp. Neurol 327 (1993) 220–232, 10.1002/cne.903270205. [DOI] [PubMed] [Google Scholar]
- [157].Richard JM, Ambroggi F, Janak PH, Fields HL, Ventral Pallidum Neurons Encode Incentive Value and Promote Cue-Elicited Instrumental Actions, Neuron 90 (2016) 1165–1173, 10.1016/j.neuron.2016.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Chang SE, Smedley EB, Stansfield KJ, Stott JJ, Smith KS, Optogenetic inhibition of ventral pallidum neurons impairs context-driven salt seeking, J. Neurosci 37 (2017) 5670–5680, 10.1523/JNEUROSCI.2968-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Chang SE, Todd TP, Smith KS, Paradoxical accentuation of motivation following accumbens-pallidum disconnection, Neurobiol. Learn. Mem 149 (2018) 39–45, 10.1016/j.nlm.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Ahrens AM, Meyer PJ, Ferguson LM, Robinson TE, Wayne Aldridge J, Neural activity in the ventral pallidum encodes variation in the incentive value of a reward cue, J. Neurosci 36 (2016) 7957–7970, 10.1523/JNEUROSCI.0736-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Ahrens AM, Ferguson LM, Robinson TE, Aldridge JW, Dynamic encoding of incentive salience in the ventral pallidum: Dependence on the form of the reward cue, ENeuro (2018) 5, 10.1523/ENEURO.0328-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Knowland D, Lilascharoen V, Pacia CP, Shin S, Wang EHJ, Lim BK, Distinct Ventral Pallidal Neural Populations Mediate Separate Symptoms of Depression, Cell 170 (2017) 284–297, 10.1016/j.cell.2017.06.015 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Tindell AJ, Berridge KC, Aldridge JW, Ventral Pallidal Representation of Pavlovian Cues and Reward: Population and Rate Codes, J. Neurosci 24 (2004) 1058–1069, 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW, Ventral pallidal neurons code incentive motivation: Amplification by mesolimbic sensitization and amphetamine, Eur. J. Neurosci 22 (2005) 2617–2634, 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- [165].Itoga CA, Berridge KC, Aldridge JW, Ventral pallidal coding of a learned taste aversion, Behav. Brain Res 300 (2016) 175–183, 10.1016/j.bbr.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Farrell MR, Ruiz CM, Castillo E, Faget L, Khanbijian C, Liu S, Schoch H, Rojas G, Huerta MY, Hnasko TS, Mahler SV, Ventral pallidum is essential for cocaine relapse after voluntary abstinence in rats, Neuropsychopharmacology 44 (2019) 2174–2185, 10.1038/s41386-019-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Shimura T, Imaoka H, Yamamoto T, Neurochemical modulation of ingestive behavior in the ventral pallidum, Eur. J. Neurosci 23 (2006) 1596–1604, 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- [168].Cromwell HC, Berridge KC, Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 624 (1993) 1–10 http://www.ncbi.nlm.nih.gov/pubmed/8252379 accessed March 12, 2019. [DOI] [PubMed] [Google Scholar]
- [169].Mahler SV, Vazey EM, Beckley JT, Keistler CR, Mcglinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G, Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking, Nat. Neurosci 17 (2014) 577–585, 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Calder AJ, Beaver JD, Davis MH, Van Ditzhuijzen J, Keane J, Lawrence AD, Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods, Eur. J. Neurosci 25 (2007) 3422–3428, 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- [171].Wulff AB, Tooley J, Marconi LJ, Creed MC, Ventral pallidal modulation of aversion processing, Brain Res. (2019) (1713) 62–69, 10.1016/j.brainres.2018.10.010. [DOI] [PubMed] [Google Scholar]
- [172].Ottenheimer D, Richard JM, Janak PH, Ventral pallidum encodes relative reward value earlier and more robustly than nucleus accumbens, Nat. Commun (2018) 9, 10.1038/s41467-018-06849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Faget L, Zell V, Souter E, McPherson A, Ressler R, Gutierrez-Reed N, Yoo JH, Dulcis D, Hnasko TS, Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum, Nat. Commun 9 (2018) 849, 10.1038/s41467-018-03125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Reichard RA, Parsley KP, Subramanian S, Zahm DS, Dissociable effects of dopamine D1 and D2 receptors on compulsive ingestion and pivoting movements elicited by disinhibiting the ventral pallidum, Brain Struct. Funct 224 (2019) 1925–1932, 10.1007/s00429-019-01879-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ, Individual Differences in Reward Drive Predict Neural Responses to Images of Food, J. Neurosci 26 (2006) 5160–5166, 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Castro DC, Berridge KC, Optogenetic enhancement of food ‘liking’ versus ‘wanting’ in the ventral pallidum hotspot and lateral hypothalamus, Neurosci. Meet. Plan. Soc. Neurosci (2013) 867.06/LLL17. [Google Scholar]
- [177].Olney JJ, Berridge KC, Optogenetic excitation of the ventral pallidum promotes motivation towards natural rewards, Neurosci. Meet. Plan. Soc. Neurosci (2017) 244.13 NN17. [Google Scholar]
- [178].Olney JJ, Castro DC, Urstadt K, Kotian A, Berridge KC, Optogenetic excitation of the ventral pallidum and lateral hypothalamus promotes ‘wanting’ but only the posterior ventral pallidum enhances ‘liking,’, Soc. Neurosci. Meet. Plan. Soc. Neurosci (2019) 592.04. T11. [Google Scholar]
- [179].Urstadt KR, Berridge KC, Optogenetic mapping of feeding and self-stimulation within the lateral hypothalamus of the rat, PLoS One 15 (2020) e0224301,, 10.1371/journal.pone.0224301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Panagis G, Miliaressis E, Anagnostakis Y, Spyraki C, Ventral pallidum self-stimulation: a moveable electrode mapping study, Behav. Brain Res 68 (1995) 165–172, 10.1016/0166-4328(94)00169-G. [DOI] [PubMed] [Google Scholar]
- [181].Ho CY, Berridge KC, Excessive disgust caused by brain lesions or temporary inactivations: Mapping hotspots of the nucleus accumbens and ventral pallidum, Eur. J. Neurosci 40 (2014) 3556–3572, 10.1111/ejn.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Schallert T, Whishaw IQ, Two types of aphagia and two types of sensorimotor impairment after lateral hypothalamic lesions: Observations in normal weight, dieted, and fattened rats, J. Comp. Physiol. Psychol 92 (1978) 720–741, 10.1037/h0077504. [DOI] [PubMed] [Google Scholar]
- [183].Teitelbaum P, Epstein AN, The lateral hypothalamic syndrome: Recovery of feeding and drinking after lateral hypothalamic lesions, Psychol. Rev 69 (1962) 74–90, 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- [184].Teitelbaum P, Stellar E, Recovery from the failure to eat produced by hypothalamic lesions, Science (80-.). 120 (1954) 894–895, 10.1126/science.120.3126.894. [DOI] [PubMed] [Google Scholar]
- [185].Rodgers WL, Epstein AN, Teitelbaum P, Lateral hypothalamic aphagia: motor failure or motivational deficit? Am. J. Physiol 208 (1965) 334–342, 10.1152/ajplegacy.1965.208.2.334. [DOI] [PubMed] [Google Scholar]
- [186].Teitelbaum P, Cheng M-F, Rozin P, Stages of recovery and development of lateral hypothalamic control of food and water intake, Ann. N. Y. Acad. Sci 157 (1969) 849–860, 10.1111/j.1749-6632.1969.tb12923.x. [DOI] [PubMed] [Google Scholar]
- [187].Khan HA, Urstadt KR, Mostovoi NA, Berridge KC, Mapping excessive “disgust” in the brain: Ventral pallidum inactivation recruits distributed circuitry to make sweetness “disgusting,”, Cogn. Affect. Behav. Neurosci (2019), 10.3758/s13415-019-00758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Hughlings Jackson J, Selected Writings of John Hughlings Jackson, Staples Press, London, 1958. [Google Scholar]
- [189].Berndt A, Lee SY, Wietek J, Ramakrishnan C, Steinberg EE, Rashid AJ, Kim H, Park S, Santoro A, Frankland PW, Iyer SM, Pak S, Ährlund-Richter S, Delp SL, Malenka RC, Josselyn SA, Carlén M, Hegemann P, Deisseroth K, Structural foundations of optogenetics: Determinants of channelrhodopsin ion selectivity, Proc. Natl. Acad. Sci 113 (2016) 822–829, 10.1073/pnas.1523341113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Morales I, Berridge KC, Cortical Optogentic Stimulation of “Liking” and Ventral Pallidum Inhibition for “Disgust,”, Soc. Neurosci. Meet. Plan. Soc. Neurosci (2019) 592.03. T10. [Google Scholar]
- [191].Bengtson CP, Osborne PB, Electrophysiological properties of cholinergic and noncholinergic neurons in the ventral pallidal region of the nucleus basalis in rat brain slices, J. Neurophysiol 83 (2000) 2649–2660, 10.1152/jn.2000.83.5.2649. [DOI] [PubMed] [Google Scholar]
- [192].Kupchik YM, Kalivas PW, The rostral subcommissural ventral pallidum is a mix of ventral pallidal neurons and neurons from adjacent areas: An electrophysiological study, Brain Struct. Funct 218 (2013) 1487–1500, 10.1007/s00429-012-0471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Geisler S, Derst C, Veh RW, Zahm DS, Glutamatergic afferents of the ventral tegmental area in the rat, J. Neurosci 27 (2007) 5730–5743, 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Geisler S, Zahm DS, Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions, J. Comp. Neurol 490 (2005) 270–294, 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- [195].Gritti I, Mainville L, Jones BE, Codistribution of GABA- with acetylcholine-synthesizing neurons in the basal forebrain of the rat, J. Comp. Neurol 329 (1993) 438–457, 10.1002/cne.903290403. [DOI] [PubMed] [Google Scholar]
- [196].Hur EE, Zaborszky L, Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: A combined retrograde tracing in situ hybridization, J. Comp. Neurol 483 (2005) 351–373, 10.1002/cne.20444. [DOI] [PubMed] [Google Scholar]
- [197].Tooley J, Marconi L, Alipio JB, Matikainen-Ankney B, Georgiou P, Kravitz AV, Creed MC, Glutamatergic Ventral Pallidal Neurons Modulate Activity of the Habenula–Tegmental Circuitry and Constrain Reward Seeking, Biol. Psychiatry 83 (2018) 1012–1023, 10.1016/j.biopsych.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Hjelmstad GO, Xia Y, Margolis EB, Fields HL, Opioid modulation of ventral pallidal afferents to ventral tegmental area neurons, J. Neurosci 33 (2013) 6454–6459, 10.1523/JNEUROSCI.0178-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Levi LA, Inbar K, Nachshon N, Bernat N, Gatterer A, Inbar D, Kupchik YM, Projection-specific potentiation of ventral pallidal glutamatergic outputs after abstinence from cocaine, J. Neurosci (2019), 10.1523/JNEUROSCI.0929-19.2019 0929–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Prasad AA, Xie C, Chaichim C, Nguyen JH, McClusky HE, Killcross S, Power JM, McNally GP, Complementary roles for ventral pallidum cell types and their projections in relapse, J. Neurosci (2019), 10.1523/JNEUROSCI.0262-19.2019 0262–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Stephenson-Jones M, Bravo-Rivera C, Ahrens S, Furlan A, Fernandes-Henriques C, Li B, Opposing contributions of GABAergic and glutamatergic ventral pallidal neurons to motivational behaviours, BioRxiv (2019) 594887,, 10.1101/594887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Sharpe MJ, Marchant NJ, Whitaker LR, Richie CT, Zhang YJ, Campbell EJ, Koivula PP, Necarsulmer JC, Mejias-Aponte C, Morales M, Pickel J, Smith JC, Niv Y, Shaham Y, Harvey BK, Schoenbaum G, Lateral Hypothalamic GABAergic Neurons Encode Reward Predictions that Are Relayed to the Ventral Tegmental Area to Regulate Learning, Curr. Biol 27 (2017) 2089–2100, 10.1016/j.cub.2017.06.024 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT, The regulatory function of self-conscious emotion: Insights from patients with orbitofrontal damage. J. Pers. Soc. Psychol 85 (2003) 594–604, 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- [204].Feinstein JS, Rudrauf D, Khalsa SS, Cassell MD, Bruss J, Grabowski TJ, Tranel D, Bilateral limbic system destruction in man. J. Clin. Exp. Neuropsychol 32 (2010) 88–106, 10.1080/13803390903066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Philippi CL, Feinstein JS, Khalsa SS, Damasio AR, Tranel D, Landini G, Williford K, Rudrauf D, Preserved Self-Awareness following Extensive Bilateral Brain Damage to the Insula, Anterior Cingulate, and Medial Prefrontal Cortices, PLoS One 7 (2012), 10.1371/journal.pone.0038413 e38413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Hashimoto K, Spector AC, Extensive lesions in the gustatory cortex in the rat do not disrupt the retention of a presurgically conditioned taste aversion and do not impair unconditioned concentration-dependent licking of sucrose and quinine, Chem. Senses 39 (2014) 57–71, 10.1093/chemse/bjt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].King CT, Hashimoto K, Blonde GD, Spector AC, Unconditioned oromotor taste reactivity elicited by sucrose and quinine is unaffected by extensive bilateral damage to the gustatory zone of the insular cortex in rats, Brain Res. 1599 (2015) 9–19, 10.1016/j.brainres.2014.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Wirsig CR, Grill HJ, Contribution of the rat’s neocortex to ingestive control: I. Latent learning for the taste of sodium chloride, J. Comp. Physiol. Psychol 96 (1982) 615–627, 10.1037/h0077911. [DOI] [PubMed] [Google Scholar]
- [209].Jezzini A, Mazzucato L, La Camera G, Fontanini A, Processing of hedonic and chemosensory features of taste in medial prefrontal and insular networks, J. Neurosci 33 (2013) 18966–18978, 10.1523/JNEUROSCI.2974-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Small DM, Changes in brain activity related to eating chocolate: From pleasure to aversion, Brain 124 (2001) 1720–1733, 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- [211].de Araujo IE, Kringelbach ML, Rolls ET, McGlone F, Human Cortical Responses to Water in the Mouth, and the Effects of Thirst, J. Neurophysiol 90 (2006) 1865–1876, 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- [212].de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N, Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain, Eur. J. Neurosci 18 (2003) 2059–2068, 10.1046/j.1460-9568.2003.02915.x. [DOI] [PubMed] [Google Scholar]
- [213].Kringelbach ML, O’Doherty J, Rolls ET, Andrews C, Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness, Cereb. Cortex 13 (2003) 1064–1071, 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- [214].Mena JD, Sadeghian K, Baldo BA, Induction of hyperphagia and carbohydrate intake by μ-opioid receptor stimulation in circumscribed regions of frontal cortex. J. Neurosci 31 (2011) 3249–3260, 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Mena JD, Selleck RA, Baldo BA, Mu-Opioid Stimulation in Rat Prefrontal Cortex Engages Hypothalamic Orexin/Hypocretin-Containing Neurons, and Reveals Dissociable Roles of Nucleus Accumbens and Hypothalamus in Cortically Driven Feeding, J. Neurosci 33 (2013) 18540–18552, 10.1523/JNEUROSCI.3323-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Schier LA, Hashimoto K, Bales MB, Blonde GD, Spector AC, High-resolution lesion-mapping strategy links a hot spot in rat insular cortex with impaired expression of taste aversion learning, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 1162–1167, 10.1073/pnas.1315624111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Morales I, Berridge KC, Optogenetic stimulation of the orbitofrontal cortex enhances food “liking” vs “wanting,”, Neurosci. Meet. Plan. Soc. Neurosci (2018) 600.04 / EEE20. [Google Scholar]
- [218].Peng Y, Gillis-Smith S, Jin H, Tränkner D, Ryba NJP, Zuker CS, Sweet and bitter taste in the brain of awake behaving animals, Nature 527 (2015) 512–515, 10.1038/nature15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Dolensek N, Gehrlach DA, Klein AS, Gogolla N, Facial expressions of emotion states and their neuronal correlates in mice, Science (80-.). 368 (2020) 89–94, 10.1126/science.aaz9468. [DOI] [PubMed] [Google Scholar]
- [220].Wang L, Gillis-Smith S, Peng Y, Zhang J, Chen X, Salzman CD, Ryba NJP, Zuker CS, The coding of valence and identity in the mammalian taste system, Nature 558 (2018) 127–131, 10.1038/s41586-018-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Gehrlach DA, Dolensek N, Klein AS, Roy Chowdhury R, Matthys A, Junghänel M, Gaitanos TN, Podgornik A, Black TD, Reddy Vaka N, Conzelmann KK, Gogolla N, Aversive state processing in the posterior insular cortex, Nat. Neurosci 22 (2019) 1424–1437, 10.1038/s41593-019-0469-1. [DOI] [PubMed] [Google Scholar]
- [222].Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG, The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders, Brain Res. 1350 (2010) 43–64, 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Ferrario CR, Labouèbe G, Liu S, Nieh EH, Routh VH, Xu S, O’Connor EC, Homeostasis meets motivation in the battle to control food intake, J. Neurosci. Soc. Neurosci (2016) 11469–11481, 10.1523/JNEUROSCI.2338-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Olney JJ, Warlow SM, Naffziger EE, Berridge KC, Current perspectives on incentive salience and applications to clinical disorders, Curr. Opin. Behav. Sci 22 (2018) 59–69, 10.1016/j.cobeha.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Kuhn BN, Campus P, Flagel SB, The Neurobiological Mechanisms Underlying Sign-Tracking Behavior, in: Tomie A, Morrow J (Eds.), Sign-Tracking Drug Addict., Michigan Publishing, Ann Arbor, MI, n.d. [Google Scholar]
- [226].Haight JL, Flagel SB, A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses, Front. Behav. Neurosci 8 (2014) 79, 10.3389/fnbeh.2014.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Haight JL, Fuller ZL, Fraser KM, Flagel SB, A food-predictive cue attributed with incentive salience engages subcortical afferents and efferents of the paraventricular nucleus of the thalamus, Neuroscience 340 (2017) 135–152, 10.1016/j.neuroscience.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Yager LM, Pitchers KK, Flagel SB, Robinson TE, Individual Variation in the Motivational and Neurobiological Effects of an Opioid Cue, Neuropsychopharmacology 40 (2015) 1269–1277, 10.1038/npp.2014.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Campus P, Covelo IR, Kim Y, Parsegian A, Kuhn BN, Lopez SA, Neumaier JF, Ferguson SM, Solberg Woods LC, Sarter M, Flagel SB, The paraventricular thalamus is a critical mediator of top-down control of cuemotivated behavior in rats, Elife (2019) 8, 10.7554/eLife.49041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE, A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions, Neuroscience 196 (2011) 80–96, 10.1016/j.neuroscience.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Flagel SB, Watson SJ, Robinson TE, Akil H, Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats, Psychopharmacology (Berl) 191 (2007) 599–607, 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- [232].Maldonado-Irizarry CS, Kelley AE, Differential behavioral effects following microinjection of an NMDA antagonist into nucleus accumbens subregions, Psychopharmacology (Berl) 116 (1994) 65–72, 10.1007/BF02244872. [DOI] [PubMed] [Google Scholar]
- [233].Stratford TR, Holahan MR, Kelley AE, Injections of nociceptin into nucleus accumbens shell or ventromedial hypothalamic nucleus increase food intake, Neuroreport 8 (1997) 423–426, 10.1097/00001756-199701200-00009. [DOI] [PubMed] [Google Scholar]
- [234].Swanson CJ, Heath S, Stratford TR, Kelley AE, Differential behavioral responses to dopaminergic stimulation of nucleus accumbens subregions in the rat, Pharmacol. Biochem. Behav 58 (1997) 933–945, 10.1016/S0091-3057(97)00043-9. [DOI] [PubMed] [Google Scholar]
- [235].Hajnal A, Székely M, Gálosi R, Lénárd L, Accumbens cholinergic interneurons play a role in the regulation of body weight and metabolism, Physiol. Behav 70 (2000) 95–103, 10.1016/S0031-9384(00)00236-5. [DOI] [PubMed] [Google Scholar]
- [236].Pratt WE, Kelley AE, Striatal muscarinic receptor antagonism reduces 24-h food intake in association with decreased preproenkephalin gene expression, Eur. J. Neurosci 22 (2005) 3229–3240, 10.1111/j.1460-9568.2005.04489.x. [DOI] [PubMed] [Google Scholar]
- [237].Pratt WE, Kelley AE, Nucleus accumbens acetylcholine regulates appetitive learning and motivation for food via activation of muscarinic receptors, Behav. Neurosci 118 (2004) 730–739, 10.1037/0735-7044.118.4.730. [DOI] [PubMed] [Google Scholar]
- [238].Kelley AE, Smith-Roe SL, Holahan MR, Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 12174–12179, 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Peciña S, Berridge KC, Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered “wanting” for reward: Entire core and medial shell mapped as substrates for PIT enhancement, Eur. J. Neurosci 37 (2013) 1529–1540, 10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Sokolowski JD, Salamone JD, The role of accumbens dopamine in lever pressing and response allocation: Effects of 6-OHDA injected into core and dorsomedial shell, Pharmacol. Biochem. Behav 59 (1998) 557–566, 10.1016/S0091-3057(97)00544-3. [DOI] [PubMed] [Google Scholar]
- [241].Badrinarayan A, Wescott SA, Weele CMV, Saunders BT, Couturier BE, Maren S, Aragona BJ, Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell, J. Neurosci 32 (2012) 15779–15790, 10.1523/JNEUROSCI.3557-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Mark Wightman R, Carelli RM, Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats, Eur. J. Neurosci 30 (2009) 1889–1899, 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM, Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events, J. Neurosci 28 (2008) 8821–8831, 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Wanat MJ, Kuhnen CM, Phillips PEM, Delays conferred by escalating costs modulate dopamine release to rewards but not their predictors, J. Neurosci 30 (2010) 12020–12027, 10.1523/JNEUROSCI.2691-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H, A selective role for dopamine in stimulus-reward learning, Nature 469 (2011) 53–59, 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Day JJ, Roitman MF, Wightman RM, Carelli RM, Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens, Nat. Neurosci 10 (2007) 1020–1028, 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- [247].Stefanik MT, Kupchik YM, Brown RM, Kalivas PW, Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking, J. Neurosci 33 (2013) 13654–13662, 10.1523/JNEUROSCI.1570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Corbit LH, Balleine BW, The general and outcome-specific forms of pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell, J. Neurosci 31 (2011) 11786–11794, 10.1523/JNEUROSCI.2711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Saunders BT, Robinson TE, The role of dopamine in the accumbens core in the expression of pavlovian-conditioned responses, Eur. J. Neurosci 36 (2012) 2521–2532, 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Fraser KM, Janak PH, Long-lasting contribution of dopamine in the nucleus accumbens core, but not dorsal lateral striatum, to sign-tracking, Eur. J. Neurosci 46 (2017) 2047–2055, 10.1111/ejn.13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Small DM, Jones-Gotman M, Dagher A, Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers, Neuroimage 19 (2003) 1709–1715, 10.1016/S1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- [252].Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Wong C, Gatley SJ, Gifford AN, Ding YS, Pappas N, Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect, Synapse 44 (2002) 175–180, 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- [253].Burger KS, Stice E, Frequent ice cream consumption is associated with reduced striatal response to receipt of an ice cream-based milkshake, Am. J. Clin. Nutr 95 (2012) 810–817, 10.3945/ajcn.111.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Fritz BM, Muñoz B, Yin F, Bauchle C, Atwood BK, A High-fat, High-sugar ‘Western’ Diet Alters Dorsal Striatal Glutamate, Opioid, and Dopamine Transmission in Mice, Neuroscience 372 (2018) 1–15, 10.1016/j.neuroscience.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Zhou QY, Palmiter RD, Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic, Cell 83 (1995) 1197–1209, 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- [256].Hnasko TS, Perez FA, Scouras AD, Stoll EA, Gale SD, Luquet S, Phillips PEM, Kremer EJ, Palmiter RD, Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 8858–8863, 10.1073/pnas.0603081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, Palmiter RD, Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice, Neuron 30 (2001) 819–828, 10.1016/S0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- [258].Furlong TM, Supit ASA, Corbit LH, Killcross S, Balleine BW, Pulling habits out of rats: adenosine 2A receptor antagonism in dorsomedial striatum rescues meth-amphetamine-induced deficits in goal-directed action, Addict. Biol 22 (2017) 172–183, 10.1111/adb.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Shan Q, Ge M, Christie MJ, Balleine BW, The acquisition of goal-directed actions generates opposing plasticity in direct and indirect pathways in dorsomedial striatum, J. Neurosci 34 (2014) 9196–9201, 10.1523/JNEUROSCI.0313-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Hart G, Leung BK, Balleine BW, Dorsal and ventral streams: The distinct role of striatal subregions in the acquisition and performance of goal-directed actions, Neurobiol. Learn. Mem 108 (2014) 104–118, 10.1016/j.nlm.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Balleine BW, Neural bases of food-seeking: Affect, arousal and reward in corticostriatolimbic circuits, Physiol. Behav, Elsevier Inc, 2005, pp. 717–730, 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- [262].Balleine BW, Delgado MR, Hikosaka O, The role of the dorsal striatum in reward and decision-making, J. Neurosci 27 (2007) 8161–8165, 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Balleine BW, Liljeholm M, Ostlund SB, The integrative function of the basal ganglia in instrumental conditioning, Behav. Brain Res 199 (2009) 43–52, 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- [264].Yin HH, Knowlton BJ, Balleine BW, Blockade of NMDA receptors in the dorsomedial striatum prevents action-outcome learning in instrumental conditioning, Eur. J. Neurosci 22 (2005) 505–512, 10.1111/j.1460-9568.2005.04219.x. [DOI] [PubMed] [Google Scholar]
- [265].Yin HH, Ostlund SB, Knowlton BJ, Balleine BW, The role of the dorsomedial striatum in instrumental conditioning, Eur. J. Neurosci 22 (2005) 513–523, 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- [266].Perry ML, Pratt WE, Baldo BA, Overlapping striatal sites mediate scopolamine-induced feeding suppression and mu-opioid-mediated hyperphagia in the rat, Psychopharmacology (Berl) 231 (2014) 919–928, 10.1007/s00213-013-3317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Poyraz FC, Holzner E, Bailey MR, Meszaros J, Kenney L, Kheirbek MA, Balsam PD, Kellendonk C, Decreasing striatopallidal pathway function enhances motivation by energizing the initiation of goal-directed action, J. Neurosci 36 (2016) 5988–6001, 10.1523/JNEUROSCI.0444-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Graybiel AM, Habits, Rituals, and the Evaluative Brain, Annu. Rev. Neurosci 31 (2008) 359–387, 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- [269].Lipton DM, Gonzales BJ, Citri A, Dorsal striatal circuits for habits, compulsions and addictions, Front. Syst. Neurosci (2019) 13, 10.3389/fnsys.2019.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Malvaez M, Wassum KM, Regulation of habit formation in the dorsal striatum, Curr. Opin. Behav. Sci 20 (2018) 67–74, 10.1016/j.cobeha.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Burton AC, Nakamura K, Roesch MR, From ventral-medial to dorsal-lateral striatum: Neural correlates of reward-guided decision-making, Neurobiol. Learn. Mem 117 (2015) 51–59, 10.1016/j.nlm.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Smith KS, Graybiel AM, A dual operator view of habitual behavior reflecting cortical and striatal dynamics, Neuron 79 (2013) 361–374, 10.1016/j.neuron.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Schultz W, Reward prediction error, Curr. Biol 27 (2017) R369–R371, 10.1016/j.cub.2017.02.064. [DOI] [PubMed] [Google Scholar]
- [274].Schultz W, Dickinson A, Neuronal Coding of Prediction Errors, Annu. Rev. Neurosci 23 (2000) 473–500, 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- [275].Schultz W, Predictive reward signal of dopamine neurons. J. Neurophysiol 80 (1998) 1–27 http://www.ncbi.nlm.nih.gov/pubmed/9658025 accessed May 25, 2017. [DOI] [PubMed] [Google Scholar]
- [276].Schultz W, Dopamine signals for reward value and risk: basic and recent data, Behav. Brain Funct 6 (2010) 24, 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Schultz W, Getting formal with dopamine and reward, Neuron 36 (2002) 241–263, 10.1016/S0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- [278].Hawes SL, Evans RC, Unruh BA, Benkert EE, Gillani F, Dumas TC, Blackwell KT, Multimodal plasticity in dorsal striatum while learning a lateralized navigation task, J. Neurosci 35 (2015) 10535–10549, 10.1523/JNEUROSCI.4415-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM, Concurrent activation of striatal direct and indirect pathways during action initiation, Nature 494 (2013) 238–242, 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Yttri EA, Dudman JT, Opponent and bidirectional control of movement velocity in the basal ganglia, Nature 533 (2016) 402–406, 10.1038/nature17639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Kreitzer AC, Malenka RC, Striatal Plasticity and Basal Ganglia Circuit Function, Neuron 60 (2008) 543–554, 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Barbera G, Liang B, Zhang L, Gerfen CR, Culurciello E, Chen R, Li Y, Lin DT, Spatially Compact Neural Clusters in the Dorsal Striatum Encode Locomotion Relevant Information, Neuron 92 (2016) 202–213, 10.1016/j.neuron.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].V Kalueff A, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC, Neurobiology of rodent self-grooming and its value for translational neuroscience, Nat. Rev. Neurosci 17 (2016) 45–59, 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Kravitz AV, Tye LD, Kreitzer AC, Distinct roles for direct and indirect pathway striatal neurons in reinforcement, Nat. Neurosci 15 (2012) 816–818, 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE, Quantifying individual variation in the propensity to attribute incentive salience to reward cues, PLoS One (2012) 7, 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Flagel SB, Akil H, Robinson TE, Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction, Neuropharmacology 56 (2009) 139–148, 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Robinson TE, Flagel SB, Dissociating the Predictive and Incentive Motivational Properties of Reward-Related Cues Through the Study of Individual Differences, Biol. Psychiatry 65 (2009) 869–873, 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].de Araujo IE, Schatzker M, Small DM, Rethinking Food Reward, Annu. Rev. Psychol 71 (2020) 139–164, 10.1146/annurev-psych-122216-011643. [DOI] [PubMed] [Google Scholar]
- [289].Han W, Tellez LA, Perkins MH, Perez IO, Qu T, Ferreira J, Ferreira TL, Quinn D, Liu ZW, Gao XB, Kaelberer MM, Bohórquez DV, Shammah-Lagnado SJ, de Lartigue G, de Araujo IE, A Neural Circuit for Gut-Induced Reward, Cell 175 (2018) 665–678, 10.1016/j.cell.2018.08.049 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Tellez LA, Han W, Zhang X, Ferreira TL, Perez IO, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE, Separate circuitries encode the hedonic and nutritional values of sugar, Nat. Neurosci 19 (2016) 465–470, 10.1038/nn.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Zhang L, Han W, Lin C, Li F, de Araujo IE, Sugar metabolism regulates flavor preferences and portal glucose sensing, Front. Integr. Neurosci (2018) 12, 10.3389/fnint.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Sclafani A, Post-ingestive positive controls of ingestive behavior, Appetite 36 (2001) 79–83, 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- [293].Sclafani A, From appetite setpoint to appetition: 50 years of ingestive behavior research, Physiol. Behav 192 (2018) 210–217, 10.1016/j.physbeh.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Myers KP, Sclafani A, Conditioned enhancement of flavor evaluation reinforced by intragastric glucose: II. Taste reactivity analysis, Physiol. Behav 74 (2001) 495–505, 10.1016/S0031-9384(01)00596-0. [DOI] [PubMed] [Google Scholar]
- [295].Myers KP, Sclafani A, Conditioned acceptance and preference but not altered taste reactivity responses to bitter and sour flavors paired with intragastric glucose infusion, Physiol. Behav 78 (2003) 173–183, 10.1016/S0031-9384(02)00890-9. [DOI] [PubMed] [Google Scholar]
- [296].Swanson LW, Petrovich GD, What is the amygdala? Trends Neurosci. 21 (1998) 323–331 http://www.ncbi.nlm.nih.gov/pubmed/9720596 accessed March 14, 2019. [DOI] [PubMed] [Google Scholar]
- [297].Swanson LW, The amygdala and its place in the cerebral hemisphere, Ann. N. Y. Acad. Sci 985 (2003) 174–184 http://www.ncbi.nlm.nih.gov/pubmed/12724158 accessed March 16, 2019. [DOI] [PubMed] [Google Scholar]
- [298].Alheid GF, Heimer L, New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata, Neuroscience 27 (1988) 1–39, 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- [299].De Olmos JS, Heimer L, The concepts of the ventral striatopallidal system and extended amygdala, Ann. N. Y. Acad. Sci (1999) 1–32, 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- [300].Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S, Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors, Neuron 93 (2017) 1464–1479, 10.1016/J.NEURON.2017.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Baxter MG, Murray EA, The amygdala and reward, Nat. Rev. Neurosci 3 (2002) 563–573, 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- [302].Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S, Antagonistic negative and positive neurons of the basolateral amygdala, Nat. Neurosci 19 (2016) 1636–1646, 10.1038/nn.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].Janak PH, Tye KM, From circuits to behaviour in the amygdala, Nature 517 (2015) 284–292, 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].McDonald AJ, Cytoarchitecture of the central amygdaloid nucleus of the rat, J. Comp. Neurol 208 (1982) 401–418, 10.1002/cne.902080409. [DOI] [PubMed] [Google Scholar]
- [305].Wu Q, Lemus MB, Stark R, Bayliss JA, Reichenbach A, Lockie SH, Andrews ZB, The Temporal Pattern of cfos Activation in Hypothalamic, Cortical, and Brainstem Nuclei in Response to Fasting and Refeeding in Male Mice, Endocrinology 155 (2014) 840–853, 10.1210/en.2013-1831. [DOI] [PubMed] [Google Scholar]
- [306].Valdivia S, Patrone A, Reynaldo M, Perello M, Acute high fat diet consumption activates the mesolimbic circuit and requires orexin signaling in a mouse model, PLoS One (2014) 9, 10.1371/journal.pone.0087478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Mahler SV, Berridge KC, What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex, Psychopharmacology (Berl) 221 (2012) 407–426, 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Fekete ÉM, Bagi ÉE, Tóth K, Lénárd L, Neuromedin C microinjected into the amygdala inhibits feeding, Brain Res. Bull 71 (2007) 386–392, 10.1016/j.brainresbull.2006.10.007. [DOI] [PubMed] [Google Scholar]
- [309].Corwin RL, Robinson JK, Crawley JN, Galanin Antagonists Block Galanin-induced Feeding in the Hypothalamus and Amygdala of the Rat, Eur. J. Neurosci 5 (1993) 1528–1533, 10.1111/j.1460-9568.1993.tb00221.x. [DOI] [PubMed] [Google Scholar]
- [310].Will MJ, Franzblau EB, Kelley AE, The amygdala is critical for opioid-mediated binge eating of fat, Neuroreport 15 (2004) 1857–1860, 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- [311].Baldo BA, Alsene KM, Negron A, Kelley AE, Hyperphagia induced by GABAA receptor-mediated inhibition of the nucleus accumbens shell: Dependence on intact neural output from the central amygdaloid region, Behav. Neurosci 119 (2005) 1195–1206, 10.1037/0735-7044.119.5.1195. [DOI] [PubMed] [Google Scholar]
- [312].Andrezjewski ME, Sadeghian K, Kelley AE, Central amygdalar and dorsal striatal NMDA receptor involvement in instrumental learning and spontaneous behavior, Behav. Neurosci 118 (2004) 715–729, 10.1037/0735-7044.118.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Giraudo SQ, Kotz CM, Billington CJ, Levine AS, Association between the amygdala and nucleus of the solitary tract in μ-opioid induced feeding in the rat, Brain Res. 802 (1998) 184–188, 10.1016/S0006-8993(98)00602-7. [DOI] [PubMed] [Google Scholar]
- [314].Gosnell BA, Involvement of μ opioid receptors in the amygdala in the control of feeding, Neuropharmacology 27 (1988) 319–326, 10.1016/0028-3908(88)90050-0. [DOI] [PubMed] [Google Scholar]
- [315].Giraudo SQ, Billington CJ, Levine AS, Effects of the opioid antagonist nal-trexone on feeding induced by DAMGO in the central nucleus of the amygdala and in the paraventricular nucleus in the rat, Brain Res. 782 (1998) 18–23, 10.1016/S0006-8993(97)01140-2. [DOI] [PubMed] [Google Scholar]
- [316].Kim EM, Quinn JG, Levine AS, O’Hare E, A bi-directional μ-opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat, Brain Res. 1029 (2004) 135–139, 10.1016/j.brainres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- [317].Levine AS, Olszewski PK, Mullett MA, Pomonis JD, Grace MK, Kotz CM, Billington CJ, Intra-amygdalar injection of DAMGO: Effects on c-Fos levels in brain sites associated with feeding behavior, Brain Res. 1015 (2004) 9–14, 10.1016/j.brainres.2004.04.039. [DOI] [PubMed] [Google Scholar]
- [318].Vígh J, Lénárd L, Fekete É, Hernádi I, Bombesin injection into the central amygdala influences feeding behavior in the rat, Peptides 20 (1999) 437–444, 10.1016/S0196-9781(99)00023-6. [DOI] [PubMed] [Google Scholar]
- [319].Kask A, Schiöth HB, Tonic inhibition of food intake during inactive phase is reversed by the injection of the melanocortin receptor antagonist into the paraventricular nucleus of the hypothalamus and central amygdala of the rat, Brain Res. 887 (2000) 460–464, 10.1016/S0006-8993(00)03034-1. [DOI] [PubMed] [Google Scholar]
- [320].Pang YY, Chen XY, Xue Y, Han XH, Chen L, Effects of secretin on neuronal activity and feeding behavior in central amygdala of rats, Peptides 66 (2015) 1–8, 10.1016/j.peptides.2015.01.012. [DOI] [PubMed] [Google Scholar]
- [321].Anderberg RH, Anefors C, Bergquist F, Nissbrandt H, Skibicka KP, Dopamine signaling in the amygdala, increased by food ingestion and GLP-1, regulates feeding behavior, Physiol. Behav 136 (2014) 135–144, 10.1016/j.physbeh.2014.02.026. [DOI] [PubMed] [Google Scholar]
- [322].Miñano FJ, Meneres Sancho MS, Sancibrián M, Salinas P, Myers RD, GABAA receptors in the amygdala: role in feeding in fasted and satiated rats, Brain Res. 586 (1992) 104–110, 10.1016/0006-8993(92)91377-Q. [DOI] [PubMed] [Google Scholar]
- [323].Torruella-Suárez ML, Vandenberg JR, Cogan ES, Tipton GJ, Teklezghi A, Dange K, Patel GK, McHenry JA, Hardaway JA, Kantak PA, Crowley NA, DiBerto JF, Faccidomo SP, Hodge CW, Stuber GD, McElligott ZA, Manipulations of Central Amygdala Neurotensin Neurons Alter the Consumption of Ethanol and Sweet Fluids in Mice, J. Neurosci 40 (2020) 632–647, 10.1523/JNEUROSCI.1466-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Hardaway JA, Halladay LR, Mazzone CM, Pati D, Bloodgood DW, Kim M, Jensen J, DiBerto JF, Boyt KM, Shiddapur A, Erfani A, Hon OJ, Neira S, Stanhope CM, Sugam JA, Saddoris MP, Tipton G, McElligott Z, Jhou TC, Stuber GD, Bruchas MR, Bulik CM, Holmes A, Kash TL, Central Amygdala Prepronociceptin-Expressing Neurons Mediate Palatable Food Consumption and Reward, Neuron (2019), 10.1016/j.neuron.2019.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Douglass AM, Kucukdereli H, Ponserre M, Markovic M, Gründemann J, Strobel C, Alcala Morales PL, Conzelmann K-K, Lüthi A, Klein R, Central amygdala circuits modulate food consumption through a positive-valence mechanism, Nat. Neurosci 20 (2017) 1384–1394, 10.1038/nn.4623. [DOI] [PubMed] [Google Scholar]
- [326].Han W, Tellez LA, Rangel MJ, Motta SC, Zhang X, Perez IO, Canteras NS, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE, Integrated Control of Predatory Hunting by the Central Nucleus of the Amygdala, Cell 168 (2017) 311–324, 10.1016/j.cell.2016.12.027. e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Lu L, Hope BT, Dempsey J, Liu SY, Bessert JM, Shaham Y, Central amygdala ERK signaling pathway is critical to incubation of cocaine craving, Nat. Neurosci 8 (2005) 212–219, 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- [328].Funk D, Coen K, Tamadon S, Hope BT, Shaham Y, Lê AD, Role of central amygdala neuronal ensembles in incubation of nicotine craving, J, Neurosci. 36 (2016) 8612–8623, 10.1523/JNEUROSCI.1505-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y, The Central Amygdala Nucleus is Critical for Incubation of Methamphetamine Craving, Neuropsychopharmacology 40 (2015) 1297–1306, 10.1038/npp.2014.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, Cifani C, Marchant NJ, Yizhar O, Bossert JM, Chiamulera C, Morales M, Shaham Y, The Anterior Insular Cortex→Central Amygdala Glutamatergic Pathway Is Critical to Relapse after Contingency Management, Neuron 96 (2017) 414–427, 10.1016/j.neuron.2017.09.024. e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Venniro M, Russell TI, Ramsey LA, Richie CT, Lesscher HMB, Giovanetti SM, Messing RO, Shaham Y, Abstinence-dependent dissociable central amygdala microcircuits control drug craving, Proc. Natl. Acad. Sci (2020), 10.1073/pnas.2001615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, Shaham Y, Lu L, Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving, J. Neurosci 28 (2008) 13248–13257, 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Corbit LH, Balleine BW, Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of pavlovian-instrumental transfer, J. Neurosci 25 (2005) 962–970, 10.1523/JNEUROSCI.4507-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ, Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating pavlovian influences on instrumental behaviour, Eur. J. Neurosci 13 (2001) 1984–1992, 10.1046/j.0953-816X.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- [335].Holland PC, Gallagher M, Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer, Eur. J. Neurosci 17 (2003) 1680–1694, 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- [336].Gallagher M, Graham PW, Holland PC, The amygdala central nucleus and appetitive pavlovian conditioning: Lesions impair one class of conditioned behavior, J. Neurosci 10 (1990) 1906–1911, 10.1523/jneurosci.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Warlow SM, Naffziger EE, Berridge KC, The central amygdala recruits mesocorticolimbic circuitry for pursuit of reward or pain, Nat. Commun 11 (2020) 1–15, 10.1038/s41467-020-16407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Tom RL, Ahuja A, Maniates H, Freeland CM, Robinson MJF, Optogenetic Activation of the Central Amygdala Generates Addiction-like Preference for Reward, Eur. J. Neurosci (2018), 10.1111/ejn.13967. [DOI] [PubMed] [Google Scholar]
- [339].Baumgartner HM, Schulkin J, Berridge KC, Optogenetic excitation of limbic corticotropin releasing factor neurons modulates motivation, Soc. Neurosci. Meet. Plan (2018) 600.08. [Google Scholar]
- [340].Baumgartner HM, Olney JJ, Warlow SM, Schulkin J, Berridge KC, Investigating corticotropin releasing factor in mediating appetitive behavior, Soc. Neurosci. Meet. Plan (2017) 244.14. [Google Scholar]
- [341].Baumgartner HM, Huerta-Sanchez LL, Schulkin J, Berridge KC, Excitation and inhibition of limbic corticotropin releasing factor neurons modulates motivation, Soc. Neurosci. Meet. Plan (2019) 592.01. [Google Scholar]
- [342].Berthoud HR, Metabolic and hedonic drives in the neural control of appetite: Who is the boss? Curr. Opin. Neurobiol 21 (2011) 888–896, 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Brown JA, Woodworth HL, Leinninger GM, To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance, Front. Syst. Neurosci (2015) 9, 10.3389/fnsys.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Barson JR, Morganstern I, Leibowitz SF, Similarities in hypothalamic and mesocorticolimbic circuits regulating the overconsumption of food and alcohol, Physiol. Behav 104 (2011) 128–137, 10.1016/j.physbeh.2011.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [345].De Lecea L, Kilduff TS, Peyron C, Gao XB, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FS, Frankel WN, Van Den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity, Proc. Natl. Acad. Sci (1998) 322–327, 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior, Cell 92 (1998) 573–585, 10.1016/S0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- [347].Nevárez N, De Lecea L, Recent advances in understanding the roles of hypocretin/orexin in arousal, affect, and motivation [version 1; referees: 3 approved], F1000Res. (2018) 7, 10.12688/f1000research.15097.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Harris GC, Aston-Jones G, Arousal and reward: a dichotomy in orexin function, Trends Neurosci. 29 (2006) 571–577, 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- [349].Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA, Lateral hypothalamc orexin/hypocretin neurons: a role in reward-seeking and addiction, Brain Res. 1314 (2010) 74–90, 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [350].Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G, Motivational activation: A unifying hypothesis of orexin/hypocretin function, Nat. Neurosci 17 (2014) 1298–1303, 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Berridge CW, España RA, Vittoz NM, Hypocretin/orexin in arousal and stress, Brain Res. 1314 (2010) 91–102, 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, Neve R, Wildes CP, Tye KM, Decoding neural circuits that control compulsive sucrose seeking, Cell 160 (2015) 528–541, 10.1016/j.cell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Baldo BA, Daniel RA, Berridge CW, Kelley AE, Overlapping distributions of orexin/hypocretin- and dopamine-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress, J. Comp. Neurol 464 (2003) 220–237, 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- [354].Yoshida K, McCormack S, España RA, Crocker A, Scammell TE, Afferents to the orexin neurons of the rat brain, J. Comp. Neurol 494 (2006) 845–861, 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [355].Peyron C, Tighe DK, van den Pol AN, De Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci 18 (1998) 9996–10015 10.1.1.335.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [356].Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA, Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its co-transmitter dynorphin in ventral tegmental area, Proc. Natl. Acad. Sci (2014) 111, 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].O’Connor EC, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C, Lüscher C, Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding, Neuron 88 (2015) 553–564, 10.1016/j.neuron.2015.09.038. [DOI] [PubMed] [Google Scholar]
- [358].Tyree SM, De Lecea L, Lateral hypothalamic control of the ventral tegmental area: Reward evaluation and the driving of motivated behavior, Front. Syst. Neurosci (2017) 11, 10.3389/fnsys.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [359].Kelley AE, Baldo BA, Pratt WE, A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward, J. Comp. Neurol (2005) 72–85, 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- [360].Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M, Changes in brain activity related to eating chocolate, Brain 124 (2001) 1720–1733, 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- [361].Chen Y, Essner RA, Kosar S, Miller OH, Lin Y-C, Mesgarzadeh S, Knight ZA, Sustained NPY signaling enables AgRP neurons to drive feeding, Elife (2019) 8, 10.7554/eLife.46348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Elmquist JK, Elias CF, Saper CB, From lesions to leptin: Hypothalamic control of food intake and body weight, Neuron 22 (1999) 221–232, 10.1016/S0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- [363].Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbæk C, Flier JS, Saper CB, Elmquist JK, Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area, Neuron 23 (1999) 775–786, 10.1016/S0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- [364].Chen Y, Lin Y-C, Zimmerman CA, Essner RA, Knight ZA, Hunger neurons drive feeding through a sustained, positive reinforcement signal, Elife (2016) 5, 10.7554/elife.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [365].Williams KW, Elmquist JK, From neuroanatomy to behavior: Central integration of peripheral signals regulating feeding behavior, Nat. Neurosci 15 (2012) 1350–1355, 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Chen Y, Lin YC, Kuo TW, Knight ZA, Sensory Detection of Food Rapidly Modulates Arcuate Feeding Circuits, Cell 160 (2015) 829–841, 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [367].Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, Andermann ML, Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales, Elife (2015) 4, 10.7554/elife.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Betley JN, Xu S, Cao ZFH, Gong R, Magnus CJ, Yu Y, Sternson SM, Neurons for hunger and thirst transmit a negative-valence teaching signal, Nature 521 (2015) 180–185, 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [369].Seeley RJ, Berridge KC, The hunger Games, Cell 160 (2015) 805–806, 10.1016/j.cell.2015.02.028. [DOI] [PubMed] [Google Scholar]
- [370].Ahmed SH, Avena NM, Berridge KC, Gearhardt AN, Guillem K, Food Addiction, Pfaff DW, Volkow ND (Eds.), Eds, Springer, New York, NY, 2016, pp. 3771–3796. [Google Scholar]
- [371].Berthoud HR, Lenard NR, Shin AC, Food reward, hyperphagia, and obesity, Am. J. Physiol. - Regul. Integr. Comp. Physiol 300 (2011) 1266–1277, 10.1152/ajpregu.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Ferrario CR, Food Addiction and Obesity, Neuropsychopharmacology 42 (2017) 361–362, 10.1038/npp.2016.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Gearhardt AN, Boswell RG, White MA, The association of “food addiction” with disordered eating and body mass index, Eat. Behav 15 (2014) 427–433, 10.1016/j.eatbeh.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [374].Robinson MJF, Fischer AM, Ahuja A, Lesser EN, Maniates H, Roles of “wanting” and “liking” in motivating behavior: gamblng, food addition, and drug additions, in: Simpson EH, Balsam PD (Eds.), Behav. Neurosci. Motiv, Eds., Berlin: Springer, 2016, pp. 105–136. [DOI] [PubMed] [Google Scholar]
- [375].Berridge KC, Robinson TE, wanting Liking, and the incentive-sensitization theory of addiction, Am. Psychol 71 (2016) 670–679, 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [376].Robinson TE, Berridge KC, The neural basis of drug craving: An incentive-sensitization theory of addiction, Brain Res. Rev 18 (1993) 247–291, 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- [377].Robinson TE, Berridge KC, Addiction, Annu. Rev. Psychol 54 (2003) 25–53, 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- [378].Westwater ML, Fletcher PC, Ziauddeen H, Sugar addiction: the state of the science, Eur. J. Nutr 55 (2016) 55–69, 10.1007/s00394-016-1229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [379].Wiss DA, Avena NM, Rada P, Sugar addiction: From evolution to revolution, Front. Psychiatry (2018) 9, 10.3389/fpsyt.2018.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [380].Schulte EM, Joyner MA, Potenza MN, Grilo CM, Gearhardt AN, Current Considerations Regarding Food Addiction, Curr. Psychiatry Rep (2015) 17, 10.1007/s11920-015-0563-3. [DOI] [PubMed] [Google Scholar]
- [381].Avena NM, Hoebel BG, Amphetamine-sensitized rats show sugar-induced hyperactivity (cross-sensitization) and sugar hyperphagia, Pharmacol. Biochem. Behav 74 (2003) 635–639, 10.1016/S0091-3057(02)01050-X. [DOI] [PubMed] [Google Scholar]
- [382].Avena NM, Hoebel BG, A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine, Neuroscience 122 (2003) 17–20, 10.1016/S0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- [383].Cameron JD, Chaput J-P, Sjödin AM, Goldfield GS, Brain on Fire: Incentive Salience, Hedonic Hot Spots, Dopamine, Obesity, and Other Hunger Games, Annu. Rev. Nutr 37 (2017) 183–205, 10.1146/annurev-nutr-071816-064855. [DOI] [PubMed] [Google Scholar]
- [384].Carlier N, Marshe VS, Cmorejova J, Davis C, Müller DJ, Genetic Similarities between Compulsive Overeating and Addiction Phenotypes: A Case for “Food Addiction”? Curr. Psychiatry Rep (2015) 17, 10.1007/s11920-015-0634-5. [DOI] [PubMed] [Google Scholar]
- [385].Carter A, Hendrikse J, Lee N, Yücel M, Verdejo-Garcia A, Andrews Z, Hall W, The Neurobiology of “Food Addiction” and Its Implications for Obesity Treatment and Policy, Annu. Rev. Nutr 36 (2016) 105–128, 10.1146/annurev-nutr-071715-050909. [DOI] [PubMed] [Google Scholar]
- [386].Davis C, Loxton NJ, A psycho-genetic study of hedonic responsiveness in relation to “food addiction, Nutrients 6 (2014) 4338–4353, 10.3390/nu6104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [387].Fletcher PC, Kenny PJ, Food addiction: a valid concept? Neuropsychopharmacology 43 (2018) 2506–2513, 10.1038/s41386-018-0203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Rogers PJ, Food and drug addictions: Similarities and differences, Pharmacol. Biochem. Behav 153 (2017) 182–190, 10.1016/j.pbb.2017.01.001. [DOI] [PubMed] [Google Scholar]
- [389].Volkow ND, Wise RA, Baler R, The dopamine motive system: Implications for drug and food addiction, Nat. Rev. Neurosci 18 (2017) 741–752, 10.1038/nrn.2017.130. [DOI] [PubMed] [Google Scholar]
- [390].Keys A, Brozek J, Henschel A, Mickelsen O, Longstreet Taylor H, Wells SM, The Biology of Human Starvation, University of Minnesota University Press, Minneapolis, 1950. [Google Scholar]
- [391].Geliebter A, Ladell T, Logan M, Schweider T, Sharafi M, Hirsch J, Responsivity to food stimuli in obese and lean binge eaters using functional MRI, Appetite 46 (2006) 31–35, 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [392].Schienle A, Schäfer A, Hermann A, Vaitl D, Binge-Eating Disorder, Reward Sensitivity and Brain Activation to Images of Food, Biol. Psychiatry 65 (2009) 654–661, 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- [393].Karhunen LJ, Vanninen EJ, Kuikka JT, Lappalainen RI, Tiihonen J, Uusitupa MIJ, Regional cerebral blood flow during exposure to food in obese binge eating women, Psychiatry Res. - Neuroimaging 99 (2000) 29–42, 10.1016/S0925-4927(00)00053-6. [DOI] [PubMed] [Google Scholar]
- [394].Stice E, Yokum S, Bohon C, Marti N, Smolen A, Reward circuitry responsivity to food predicts future increases in body mass: Moderating effects of DRD2 and DRD4, Neuroimage 50 (2010) 1618–1625, 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [395].Frankort A, Roefs A, Siep N, Roebroeck A, Havermans R, Jansen A, Reward activity in satiated overweight women is decreased during unbiased viewing but increased when imagining taste: An event-related fMRI study, Int. J. Obes 36 (2012) 627–637, 10.1038/ijo.2011.213. [DOI] [PubMed] [Google Scholar]
- [396].Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE, Widespread reward-system activation in obese women in response to pictures of high-calorie foods, Neuroimage 41 (2008) 636–647, 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- [397].Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF, Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals, Neuroimage 37 (2007) 410–421, 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- [398].Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Butler MG, Savage CR, Neural mechanisms associated with food motivation in obese and healthy weight adults, Obesity 18 (2010) 254–260, 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- [399].Holsen LM, Savage CR, Martin LE, Bruce AS, Lepping RJ, Ko E, Brooks WM, Butler MG, Zarcone JR, Goldstein JM, Importance of reward and prefrontal circuitry in hunger and satiety: Prader-Willi syndrome vs simple obesity, Int. J. Obes 36 (2012) 638–647, 10.1038/ijo.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [400].Dimitropoulos A, Tkach J, Ho A, Kennedy J, Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults, Appetite 58 (2012) 303–312, 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [401].Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR, Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control, Int. J. Obes 34 (2010) 1494–1500, 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [402].Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, Galanti K, Selig PA, Han H, Zhu W, Wong CT, Fowler JS, Enhanced striatal dopamine release during food stimulation in binge eating disorder, Obesity 19 (2011) 1601–1608, 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [403].Simon J, Skunde M, Walther S, Bendszus M, Herzog W, Friederich C, Neural signature of food reward processing in bulemic-type eating disorders, Soc. Cogn. Affect. Neurosci 11 (2016) 1393–1401, 10.1093/scan/nsw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [404].Wolz I, Sauvaget A, Granero R, Mestre-Bach G, Baño M, Martín-Romera V, Veciana De Las Heras M, Jiménez-Murcia S, Jansen A, Roefs A, Fernández-Aranda F, Subjective craving and event-related brain response to olfactory and visual chocolate cues in binge-eating and healthy individuals, Sci. Rep 7 (2017) 1–10, 10.1038/srep41736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [405].Murdaugh DL, Cox JE, Cook EW, Weller RE, FMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program, Neuroimage 59 (2012) 2709–2721, 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [406].Balodis IM, Kober H, Worhunsky PD, White MA, Stevens MC, Pearlson GD, Sinha R, Grilo CM, Potenza MN, Monetary reward processing in obese individuals with and without binge eating disorder, Biol. Psychiatry 73 (2013) 877–886, 10.1016/j.biopsych.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [407].Burger KS, Stice E, Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain, Neuroimage 99 (2014) 122–128, 10.1016/j.neuroimage.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [408].Blum K, Liu Y, Shriner R, Gold MS, Reward Circuitry Dopaminergic Activation Regulates Food and Drug Craving Behavior, Curr. Pharm. Des 17 (2012) 1158–1167, 10.2174/138161211795656819. [DOI] [PubMed] [Google Scholar]
- [409].Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE, The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J. R. Soc. Med 89 (1996) 396–400 http://www.ncbi.nlm.nih.gov/pubmed/8774539 accessed December 22, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [410].Comings DE, Blum K, Reward deficiency syndrome: Genetic aspects of behavioral disorders, Prog. Brain Res, Elsevier, 2000, pp. 325–341, 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- [411].Johnson PM, Kenny PJ, Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats, Nat. Neurosci 13 (2010) 635–641, 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [412].Volkow ND, Wise RA, How can drug addiction help us understand obesity? Nat. Neurosci 8 (2005) 555–560, 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- [413].Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusll N, Fowler JS, Brain dopamine and obesity, Lancet 357 (2001) 354–357, 10.1016/S0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- [414].Gardner EL, The neurobiology and genetics of addiction: impliccations of the “reward deficiency syndrome” for therapeutic strategies in chemical dependency, in: Elster J (Ed.), Addict. Entries Exits, Ed, Russell Sage Foundation, 1999, pp. 57–229. [Google Scholar]
- [415].Guo J, Simmons WK, Herscovitch P, Martin A, Hall KD, Striatal dopamine D2-like receptor correlation patterns with human obesity and opportunistic eating behavior, Mol. Psychiatry 19 (2014) 1078–1084, 10.1038/mp.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [416].Bindra D, How adaptive behavior is produced: A perceptual-motivational alternative to response reinforcements, Behav. Brain Sci 1 (1978) 41–52, 10.1017/S0140525X00059380. [DOI] [Google Scholar]
- [417].Bolles RC, Reinforcement, expectancy, and learning, Psychol. Rev 79 (1972) 394–409, 10.1037/h0033120. [DOI] [Google Scholar]
- [418].Toates F, Motivational Systems, Cambridge University Press, Cambridge, 1986. [Google Scholar]
- [419].Evans KR, Vaccarino FJ, Intra-nucleus accumbens amphetamine: Dose-dependent effects on food intake, Pharmacol. Biochem. Behav 25 (1986) 1149–1151, 10.1016/0091-3057(86)90102-4. [DOI] [PubMed] [Google Scholar]
- [420].Evans KR, Vaccarino FJ, Amphetamine- and morphine-induced feeding: evidence for involvement of reward mechanisms, Neurosci. Biobehav. Rev 14 (1990) 9–22, 10.1016/s0149-7634(05)80156-3. [DOI] [PubMed] [Google Scholar]
- [421].Wise RA, Fotuhi M, Colle LM, Facilitation of feeding by nucleus accumbens amphetamine injections: Latency and speed measures, Pharmacol. Biochem. Behav 32 (1989) 769–772, 10.1016/0091-3057(89)90031-2. [DOI] [PubMed] [Google Scholar]
- [422].Salamone JD, Zigmond MJ, Stricker EM, Characterization of the impaired feeding behavior in rats given haloperidol or dopamine-depleting brain lesions, Neuroscience 39 (1990) 17–24, 10.1016/0306-4522(90)90218-s. [DOI] [PubMed] [Google Scholar]
- [423].Sotak BN, Hnasko TS, Robinson S, Kremer EJ, Palmiter RD, Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding, Brain Res. 1061 (2005) 88–96, 10.1016/j.brainres.2005.08.053. [DOI] [PubMed] [Google Scholar]
- [424].Bernal SY, Dostova I, Kest A, Abayev Y, Kandova E, Touzani K, Sclafani A, Bodnar RJ, Role of dopamine D1 and D2 receptors in the nucleus accumbens shell on the acquisition and expression of fructose-conditioned flavor-flavor preferences in rats, Behav. Brain Res 190 (2008) 59–66, 10.1016/j.bbr.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [425].Hardman CA, Herbert VMB, Brunstrom JM, Munafò MR, Rogers PJ, Dopamine and food reward: Effects of acute tyrosine/phenylalanine depletion on appetite, Physiol. Behav 105 (2012) 1202–1207, 10.1016/j.physbeh.2011.12.022. [DOI] [PubMed] [Google Scholar]
- [426].Adan RAH, Vanderschuren LJMJ, la Fleur SE, Anti-obesity drugs and neural circuits of feeding, Trends Pharmacol. Sci 29 (2008) 208–217, 10.1016/j.tips.2008.01.008. [DOI] [PubMed] [Google Scholar]
- [427].Wellman PJ, Davies BT, Morien A, McMahon L, Modulation of feeding by hypothalamic paraventricular nucleus alpha 1- and alpha 2-adrenergic receptors, Life Sci. 53 (1993) 669–679, 10.1016/0024-3205(93)90243-v. [DOI] [PubMed] [Google Scholar]
- [428].Cope MB, Nagy TR, Fernández JR, Geary N, Casey DE, Allison DB, Antipsychotic drug-induced weight gain: Development of an animal model, Int. J. Obes 29 (2005) 607–614, 10.1038/sj.ijo.0802928. [DOI] [PubMed] [Google Scholar]
- [429].Matsui-Sakata A, Ohtani H, Sawada Y, Receptor Occupancy-based Analysis of the Contributions of Various Receptors to Antipsychotics-induced Weight Gain and Diabetes Mellitus, Drug Metab. Pharmacokinet 20 (2005) 368–378, 10.2133/dmpk.20.368. [DOI] [PubMed] [Google Scholar]
- [430].Stefanidis A, Verty ANA, Allen AM, Owens NC, Cowley MA, Oldfield BJ, The role of thermogenesis in antipsychotic drug-induced weight gain, Obesity 17 (2009) 16–24, 10.1038/oby.2008.468. [DOI] [PubMed] [Google Scholar]
- [431].Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, Kumar A, Brasic J, Wong DF, Alterations of centraldopamine receptors before and after gastric bypass surgery, Obes. Surg 20 (2010) 369–374, 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- [432].van der Zwaal EM, de Weijer BA, van de Giessen EM, Janssen I, Berends FJ, van de Laar A, Ackermans MT, Fliers E, la Fleur SE, Booij J, Serlie MJ, Striatal dopamine D2/3 receptor availability increases after long-term bariatric surgery-induced weight loss, Eur. Neuropsychopharmacol 26 (2016) 1190–1200, 10.1016/j.euroneuro.2016.04.009. [DOI] [PubMed] [Google Scholar]
- [433].Stice E, Yokum S, Gain in body fat is associated with increased striatal response to palatable food cues, whereas body fat stability is associated with decreased striatal response, J. Neurosci 36 (2016) 6949–6956, 10.1523/JNEUROSCI.4365-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [434].Yokum S, Stice E, Weight gain is associated with changes in neural response to palatable food tastes varying in sugar and fat and palatable food images: a repeated-measures fMRI study, Am. J. Clin. Nutr 110 (2019) 1275–1286, 10.1093/ajcn/nqz204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [435].Stice E, Yokum S, Burger KS, Epstein LH, Small DM, Youth at risk for obesity show greater activation of striatal and somatosensory regions to food, J. Neurosci 31 (2011) 4360–4366, 10.1523/JNEUROSCI.6604-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [436].Kessler RM, Zald DH, Ansari MS, Li R, Cowan RL, Changes in dopamine release and dopamine D2/3 receptor levels with the development of mild obesity, Synapse (2014), 10.1002/syn.21738. n/a–n/a [DOI] [PubMed] [Google Scholar]


