Abstract
A diverse array of neurometabolic coupling mechanisms exist within the brain to ensure that sufficient metabolite availability is present to meet both acute and chronic energetic demands. Excitatory synaptic activity, which produces the majority of the brain’s energetic demands, triggers a rapid metabolic response including a characteristic shift towards aerobic glycolysis. Herein, astrocytically derived lactate appears to serve as an important metabolite to meet the extensive metabolic needs of activated neurons. Despite a wealth of literature characterizing lactate’s role in mediating these acute metabolic needs, the extent to which lactate supports chronic energetic demands of neurons remains unclear. We hypothesized that synaptic potentiation, a ubiquitous brain phenomenon that can produce chronic alterations in synaptic activity, could necessitate persistent alterations in brain energetics. In freely-behaving rats, we induced long-term potentiation (LTP) of synapses within the dentate gyrus through high-frequency electrical stimulation (HFS) of the medial perforant pathway. Before, during, and after LTP induction, we continuously recorded extracellular lactate concentrations within the dentate gyrus to assess how changes in synaptic strength alter local glycolytic activity. Synaptic potentiation 1) altered the acute response of extracellular lactate to transient neuronal activation as evident by a larger initial dip and subsequent overshoot and 2) chronically increased local lactate availability. Although synapses were potentiated immediately following HFS, observed changes in lactate dynamics were only evident beginning ~24hrs later. Once observed, however, both synaptic potentiation and altered lactate dynamics persisted for the duration of the experiment (~72hrs). Persistent alterations in synaptic strength, therefore, appear to be associated with metabolic plasticity in the form of persistent augmentation of glycolytic activity.
Keywords: long-term potentiation, synaptic plasticity, lactate, glycolysis, hippocampus
1. Introduction
Proper brain function depends upon a precise coupling between neuronal activity and brain metabolism. Macroscopic energetic demands scale linearly as a function of the total number of neurons (Fonseca-Azevedo and Herculano-Houzel, 2012) and brain volume (Karbowski, 2007). Mesoscopically, the small world architecture of the brain, characterized by dense local connectivity and sparse long-range connections (Bassett and Bullmore, 2006), appears ideally suited to maximize signaling complexity while minimizing energetic costs (Tomasi et al., 2013). On the microscopic level, brain metabolism appears to have constrained physiological processes responsible for synaptic activity including the density of functional synapses (Zhou and Liu, 2015), ion fluxes responsible for the action potential (Alle et al., 2009), dynamics of neuronal firing (Hasenstaub et al., 2010), and even the kinetics of postsynaptic glutamate receptors and transporters (Attwell and Gibb, 2005). Collectively, these observations highlight the large extent to which brain structure is constrained by neuroenergetics.
As structure typically begets function, it is not surprising that functional neuronal activity and neuroenergetics are likewise fundamentally intertwined. Energetic demand is 3-fold higher in the synapse-rich gray matter than in white matter (Harris and Attwell, 2012) and brain glucose consumption is coupled to glutamate-glutamine cycling, an index of excitatory neuronal signaling (Sibson et al., 1998). Indeed, a mathematical model of brain energetics predicts that 37% of the entire energy budget of the brain is dedicated to reversing ion flux following activation of postsynaptic receptors (Harris et al., 2012). The high energetic costs of synaptic activity are supported by 1) the subcellular localization of mitochondria, with ~60% of neuropil mitochondria found within the dendrites (Wong-Riley, 1989) and 2) neurometabolic coupling mechanisms that locally increase metabolite supply in response to neuronal activation (Chaigneau et al., 2007; Viswanathan and Freeman, 2007).
During acute neuronal activation, brain metabolism additionally appears to shift from the near complete oxidation of glucose to an increased reliance upon aerobic glycolysis (Fox et al., 1988). A consequent increase in extracellular lactate concentration is typically observed (Hu and Wilson, 1997; Caesar et al., 2008; Li and Freeman, 2015), although the origin and functional consequences of these increases remain contested (Dienel, 2013, 2017; Bak and Walls, 2018; Magistretti and Allaman, 2018; Yellen, 2018). For example, lactate may 1) have important intracellular energetic consequences for the cell in which glycolysis occurs (Lundgaard et al., 2015; Díaz-García et al., 2017; Díaz‐García and Yellen, 2019) and 2) extracellularly, serve primarily as a signaling molecule through the activation of both direct and indirect receptors (Yang et al., 2014; Mosienko et al., 2015; Jourdain et al., 2018) before being extruded across the blood-brain barrier (Dienel, 2017). Alternatively, lactate may primarily serve a metabolic role as outlined in the astrocyte neuron lactate shuttle hypothesis (ANLS; (Pellerin and Magistretti, 1994; Magistretti and Pellerin, 1999; Magistretti and Allaman, 2018). Here, lactate derived from astrocytes is proposed to 1) supply ATP necessary for astrocytic uptake of synaptically-released extracellular glutamate and 2) produce lactate that can be shuttled to neurons as an energetic substrate. This compartmentalization of metabolic processing may additionally regulate neuronal and astrocytic redox states (Kasischke et al., 2004; Aubert and Costalat, 2005; Hirrlinger and Dringen, 2010). Moreover, the ANLS highlights a critical role for lactate in supporting neuronal signaling and provides a compelling mechanism by which astrocytic glycogen (the primary energetic store within the brain) can be rapidly mobilized to support altered metabolic demands (Brown, 2004). Consistent with this idea, glycogen is preferentially situated within astrocytic processes in close proximity to synapses (Calì et al., 2016) and glycogenolysis and subsequent lactate efflux from astrocytes are rapidly triggered by excitatory neuronal activity (Pellerin and Magistretti, 1994; Brown and Ransom, 2007). Synaptic activity, therefore, is a metabolically demanding process that appears to initiate glycolysis and increase extracellular lactate availability. Consistent with these observations, the rate of aerobic glycolysis during development is highest during periods of robust synaptic growth and is correlated in adulthood with the expression of genes responsible for synapse formation and growth (Goyal et al., 2014). Likewise, brain regions that exhibit high functional connectivity have high rates of glucose consumption (Tomasi et al., 2013) and aerobic glycolysis (Vaishnavi et al., 2010).
These associations between synaptic activity and glycolysis raise the distinct possibility that persistent modifications to synaptic strength are accompanied by persistent alterations in lactate dynamics. Lactate appears necessary for the formation of long-term potentiation (Newman et al., 2011; Suzuki et al., 2011) and both the acquisition (Gao et al., 2016; Harris et al., 2019) and reconsolidation (Zhang et al., 2016) of long-term memory. However, the extent to which long-term modifications of synaptic strength are accompanied by persistent modifications in lactate dynamics remains unclear. To address this question, we used fixed potential amperometry to chronically record extracellular lactate concentrations ([lac]) in the dentate gyrus of freely-behaving rats before, during, and up to 72 hours after the induction of long-term potentiation. We observed that synaptic potentiation was associated with persistent alterations in 1) acute lactate dynamics following neuronal activation and 2) chronic lactate availability within the dentate gyrus. Increased energetic demands associated with synaptic potentiation, therefore, may result in metabolic plasticity in the form of persistent augmentation of glycolytic activity.
2. Methods
2.1. Animals and Stereotactic Surgery
Sprague-Dawley rats (3–4 months old, N=9; Charles River, Wilmington, MA) were housed under standard laboratory conditions (12hr light/dark cycle, ad libitum access to food/water). Stereotactic surgery was performed under isoflurane anesthesia (3.5% induction, 2–3% maintenance; V-1 Tabletop, VetEquip, Livermore, CA) to facilitate electrophysiological and electrochemical recordings from the dentate gyrus of freely behaving rats before, during, and after the induction of long-term potentiation (LTP). Preoperative analgesia (Meloxicam; 2mg/kg; MWI, Boise, ID) and an antibiotic treatment (Penicillin; 100,000 units/kg) were administered. The skull was exposed to enable implantation of an indwelling cannula (MD-2250; BASi, West Lafayette, IN) with an affixed local field potential (LFP) recording electrode (Teflon-coated stainless steel wire, 0.008in diameter; A-M systems, Sequim, WA) targeted to the hilus of the dentate gyrus (AP: −3.5mm, ML: −2mm, DV: −3–3.5mm). A stimulating depth electrode (Teflon-coated stainless steel wire, 0.008in diameter) was implanted within the medial perforant path (AP: −8.1mm, ML: −4.2mm, DV: −3.5mm). Two stainless steel screws (Plastics One, Roanoke, VA) were affixed above the cerebellum to serve as references for our recording and stimulating electrodes. Precise final positioning of the stimulating and recording electrodes was achieved by maximizing the size of the electrically evoked response observed in the dentate gyrus following perforant path stimulation (200uS square pulse, 0.1 – 1.5mA). All implants were affixed to the skull with dental acrylic (Lang Dental; Wheeling, IL) and connected, via a flexible cable (Plastics One), to a commutator (Plastics One) that permitted unconstrained movement throughout the rat’s home cage. Postoperative analgesia (Meloxicam) was administered 12–24 hours after the conclusion of surgery. All methods were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Middlebury College’s Institutional Animal Care and Use Committee.
2.2. Amperometric Recordings of Lactate Concentration [Lac]
A minimum of seven days of post-operative recovery passed prior to commencing any experimentation. One day prior to the experimental induction of LTP in vivo, we performed in vitro calibrations of lactate-sensitive amperometric biosensors (Pinnacle Technologies, Lawrence, KS). These sensors consist of an integrated Ag/AgCl reference electrode and a platinum/iridium recording electrode coated with lactate oxidase and a series of selective membranes to increase specificity and sensitivity to lactate. When lactate comes into contact with the biosensor, it is oxidized by lactate oxidase, yielding hydrogen peroxide as a byproduct of the reaction. Continuous application of a 0.6V potential to the recording electrode ensures the rapid oxidation of this hydrogen peroxide and the current generated by this redox reaction can be monitored as a measure of lactate concentration. This entire process is rapid (<1s), highly sensitive and selective for lactate, and generates current that is proportional to the concentration of lactate at the biosensor (Dash et al., 2012; Naylor et al., 2012). Following successful calibration, the biosensor was implanted through the indwelling cannula within the dentate gyrus (directly adjacent to the LFP recording electrode affixed to the outside of the cannula). Biosensor implantation occurred ~20hrs prior to in vivo LTP induction. Once implanted, the biosensor was connected to a headstage amplifier (Pinnacle Technologies) and a data acquisition system (8401 DACS, Pinnacle Technologies) which 1) continuously applied a 0.6V potential to the recording electrode for fixed potential amperometry and 2) recorded redox current (1Hz sampling rate). Amperometric recordings of lactate were continuously performed until the conclusion of the experiment (~72hrs after LTP induction).
2.3. Hippocampal Evoked Responses and Long Term Potentiation Induction
The medial perforant pathway provides monosynaptic input to neurons within the dentate gyrus and serves an ideal model system for monitoring synaptic strength and inducing LTP. Electrical stimulation of the medial perforant pathway reliably produces an evoked response that can be recorded by the LFP implanted within the dentate gyrus. Two primary components of the evoked response can be quantified: 1) the field excitatory postsynaptic potential (fEPSP), a measure of depolarization within the dentate gyrus and 2) the population spike (PS), a measure of subsequent action potential generation. To record evoked responses, LFP electrodes were amplified (100x), filtered with a differential amplifier (low-pass: 10kHz, high-pass: 1Hz; DP-301, Warner Instruments, Hamden, Connecticut), and digitized (10,000Hz; Micro 1401; Signal 4; Cambridge Electronic Design, Cambridge, England). Stimulation electrodes were connected to a battery-powered stimulus isolator unit (World Precision Instruments A365, Sarasota, FL).
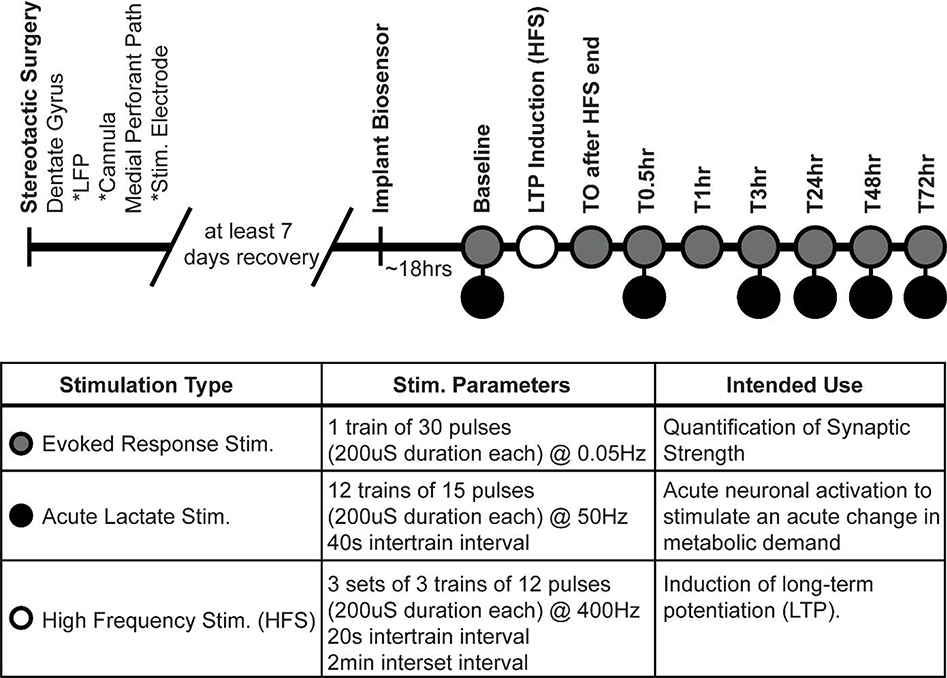
Evoked response stimulations (square pulse, 200μS duration, 0.1–1.5mA) were delivered and a baseline stimulation intensity was determined as that necessary to produce ~40% of the maximal PS that could be elicited. For each rat, baseline stimulation intensity was maintained throughout the experiment. To measure synaptic strength, we quantified the average PS amplitude from 30 baseline stimulations delivered at 0.05Hz (see Figure 1 for complete experimental timeline and summary of all stimulation parameters). To experimentally induce long-term potentiation, high-frequency stimulation (HFS) of the perforant path was delivered to each rat. Herein, each train of stimulations consisted of 12 pulses (200μs square waves, baseline intensity) delivered at 400Hz. 3 sets (2 min interset interval) of 3 trains (20s intertrain interval) comprised our HFS stimulations. In each rat, LTP was induced at 2pm (four hours after light onset).
Figure 1.
Experimental timeline and stimulation parameters. Within the dentate gyrus, an implanted local field potential and lactate-sensitive biosensor were used to record evoked responses and extracellular [lac], respectively. Electrical stimulations of the medial perforant path were used to quantify synaptic strength, produce acute neuronal activation and associated changes in metabolic demand, and produce lasting synaptic potentiation within the dentate gyrus. At time points with two stimulation types, evoked response stimulation always preceded acute lactate stimulations.
2.4. Acute Lactate Stimulations
Acute neuronal activation locally increases metabolic demand and can alter extracellular metabolite availability (Hu and Wilson, 1997; Chaigneau et al., 2007; Viswanathan and Freeman, 2007). While baseline stimulations (200us, square pulses) produced reliable and quantifiable evoked responses within our dentate gyrus LFP, they did not appear to produce sufficient neuronal activation to produce quantifiable changes in extracellular lactate availability (data not shown). Consequently, we developed an acute lactate stimulation (12 trains of 15, 200μs pulses at 50Hz, 40s intertrain interval) that was designed to 1) produce sufficient activation within the dentate gyrus to alter local energetics and 2) not produce lasting alterations in synaptic strength. Acute lactate stimulations were delivered before and after the induction of LTP to assess whether alterations in synaptic strength affect coupling of neuronal activation and extracellular lactate dynamics (see Figure 1 for experimental timeline).
2.5. Data Analyses and Statistics
All electrophysiological and amperometric data were processed and analyzed using custom scripts in Mathworks MATLAB (Natick, MA). Additional inferential statistics were conducted using SPSS (IBM, Armonk, NY). To assess the statistical significance of changes in PS amplitude over time, we employed a repeated measure ANOVA. As technical challenges pairing acute electrical stimulation and amperometric recordings of lactate resulted in several missing data points (four time points across three rats), acute amperometric data were instead analyzed with linear mixed effects models (fixed effect of stimulation time, random effect of subject, maximum likelihood estimation, compound symmetry covariance). Such an approach enabled us to fully incorporate all of our recorded data when assessing the statistical significance of stimulation time point on extracellular lactate dynamics.
To quantify population spike amplitude, we first drew a tangent between the two apices of the evoked response. The amplitude of a vertical line draw between this tangent line and the trough of the PS served as our measure of PS amplitude (see Figure 3A; McNaughton and Barnes, 1977; Korol and Gold, 2008; Dash et al., 2018). PS amplitudes were averaged across the 30 stimulations comprising a baseline stimulation session.
Amperometric recordings of extracellular [lac] were processed as previously described to 1) remove the effects of non-faradaic current generated upon the initial polarization of the recording electrode and 2) account for the slow, progressive decline in faradaic current produced by the biosensor that arises from enzymatic degradation of the applied lactate oxidase enzyme (Dash et al., 2009, 2012, 2013). Herein, we removed the initial 5 hours of recorded lactate data (wherein polarization-induced non-faradaic current may be present), fit a line to the remaining data to model linear decay, and rectified the lactate data to analytically correct for this delay. This corrected amperometric signal was then used for all further analyses.
For analyses of acute changes in [lac] following neuronal activation, individual stimulation time points were extracted from the chronic lactate signal using TTL markers generated at the time of stimulation. [Lac] was normalized as a function of pre-stimulation baseline levels and parameters characterizing activity-induced changes in [lac] were calculated including the magnitude and timing of the initial decrease in [lac] and the magnitude and timing of the ensuring overshoot in [lac]. For analyses of chronic changes in [lac], we binned and averaged [lac] in one-hour increments and expressed [lac] as a function of pre-LTP baseline values.
2.6. Chronic Lactate Controls
In the experiments described above, extracellular [lac] were recorded for up to 72hrs following LTP induction. As LTP was induced in all rats, any observed changes in [lac] over time could be confounded by methodological and/or analytical constraints inherent to our chronic amperometric biosensor recordings. To control for these constraints, we performed similar analyses to those described above in two distinct control populations in which extracellular [lac] were chronically recorded, but LTP was never induced. The first control population (see Dash et al., 2012) was comprised of chronic amperometric recordings of [lac] from frontal cortical regions of freely-behaving Wistar Kyoto rats (N=10). This control population reflects almost entirely spontaneous alterations in extracellular [lac] as either no experimental manipulations were undertaken (N=5) or only a single 3 hour sleep restriction was undertaken across the entire recording duration (N=5). The second control population (N=6) was comprised of rats used to pilot the experimental procedures used in the present manuscript and consequently mirror all experimental approaches except in the specific characteristics of the electrical stimulations they received. Here, individual rats may have received variations in their acute lactate stimulation parameters and/or HFS stimulation; critically, however, we did not observe synaptic potentiation in any of these rats following any stimulation.
3. Results
We sought to investigate how changes in synaptic strength affect glycolytic neuroenergetics. To do so, we chronically recorded extracellular [lac] within the dentate gyrus before and after induction of LTP (see Figure 1A for timeline and a description of stimulation parameters).
3.1. Dynamic changes in extracellular [lac] in response to acute neuronal activation
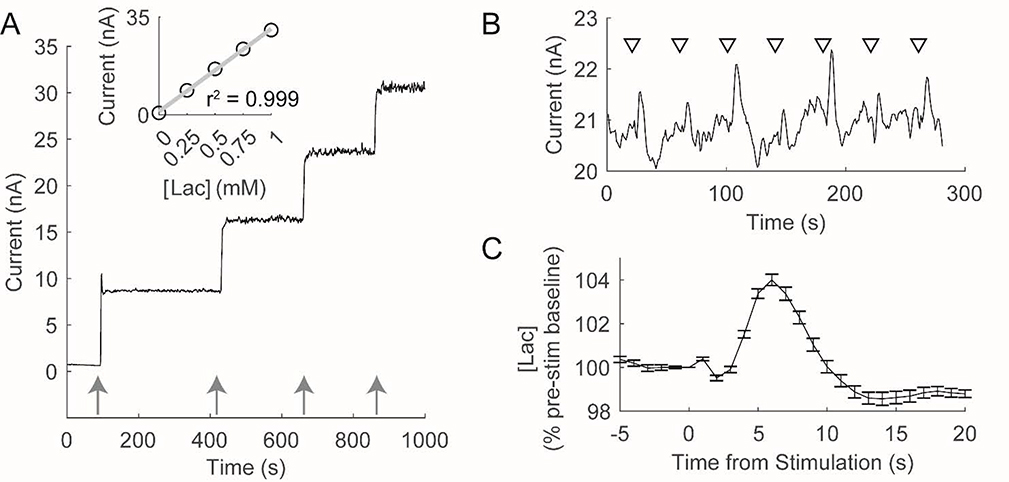
Lactate is an important brain metabolite whose availability rapidly changes in response to physiological demands and/or pathological conditions (De Bruin et al., 1990; Dash et al., 2012; Naylor et al., 2012; Matsui et al., 2017; Newman et al., 2017). Fixed potential amperometry, an electrochemical technique that enables continuous monitoring of an analyte of interest with high temporal resolution, is well-suited to characterize both rapid alterations in extracellular [lac] and prolonged changes in lactate availability across multiple days (Dash et al., 2012; Naylor et al., 2012; Newman et al., 2017). Figure 2A depicts a typical in vitro calibration of a lactate-sensitive amperometric biosensor used in this study to monitor extracellular [lac]. In response to increases in [lac], this biosensor rapidly (~1s) generates an increase in current that is linear across the range of [lac] expected to be observed in vivo. Across all calibrations, biosensors were highly sensitive to changes in [lac] with an average limit of detection of 5.71 ± 0.90μM.
Figure 2.
Amperometric biosensors are sensitive to in vitro and in vivo changes in lactate concentration ([lac]). A) In vitro calibration of a lactate sensitive biosensor. Arrows depict the addition of lactate (0.25mM). Lactate biosensors rapidly (~1s) produce an increase in current in response to changes in [Lac] that is linear across the expected range of [Lac] in vivo (see inset). B) Baseline electrical stimulations of the perforant path in an individual produce reliable changes in extracellular [lac]. Triangles depict each stimulation wherein 15, 200uS pulses were delivered at 50Hz. C) Average change in [Lac] observed during baseline stimulation in an individual rat. Following stimulation, extracellular [Lac] initially decreases followed by an overshoot of pre-stimulation levels.
Calibrated biosensors were implanted into the dentate gyrus of freely behaving rats to record extracellular [lac]. Electrical stimulation of the medial perforant path (12 trains of 15 pulses at 50Hz, 40s intertrain interval), which provides excitatory synaptic input to the dentate gyrus, produced consistent alterations in extracellular [lac] (Figures 2B/C). Following each stimulation train, [lac] initially decline (98.41 ± 0.98% of pre-stim levels; t(8) = −4.57, p = 0.002), reaching a nadir in 2.92 ± 0.47 seconds. [Lac] subsequently increase, reaching a peak (103.18 ± 1.34% of pre-stim levels; t(8) = 6.74, p = 1.47 × 10−4) in 6.93 ± 1.03 seconds. These observed changes in [lac] are 1) consistent with previous reports describing an initial dip and subsequent overshoot in [lac] following acute neuronal activation (Hu and Wilson, 1997; Aubert et al., 2005) and 2) demonstrate the effectiveness of using lactate-sensitive biosensors to monitor changes in [lac] in vivo.
3.2. High Frequency Stimulation Produces Lasting Synaptic Potentiation
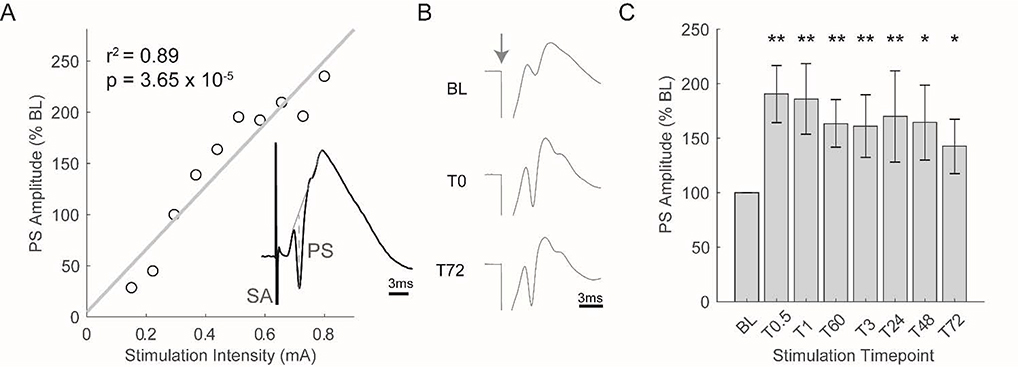
Acute electrical stimulation (single pulse, 200uS duration) of the medial perforant pathway can also be used to elicit a hippocampal evoked response. To determine the baseline stimulation intensity for each rat, we recorded evoked responses across different stimulation intensities (Figure 3A). As stimulation intensity increased (i.e. greater activation of medial perforant pathway), we observed that the amplitude of the PS likewise increased. The stimulation intensity that produced a PS equal to ~40% of the maximal PS elicited was chosen as the baseline stimulation intensity.
Figure 3.
High frequency stimulation (HFS) significantly potentiates the population spike of the evoked response for at least 72hrs. A) Typical input/output curve used to determine the baseline (BL) intensity of perforant path stimulation. BL intensity was chosen to produce an evoked response that contained a population spike equal to ~40% of the maximal population spike that could be elicited. Inset depicts a typical evoked response (SA: stimulation artifact, PS: population spike). B) Average evoked response observed during three separate stimulation periods in an individual rat (stimulation artifacts have been truncated to better visualize the evoked response). Grey arrow depicts time of stimulation. HFS was administered between BL and T0. C) Average population spike observed during each stimulation period (N = 9 rats; **p<0.01, *p<0.05 as compared to BL).
Like stimulation intensity, changes in synaptic strength also affect evoked response magnitude. To quantify changes in synaptic strength, therefore, we assessed population spike amplitude in response to acute stimulations at baseline intensity before and after induction of LTP via high-frequency stimulation (HFS). HFS produced robust synaptic potentiation as evident by a marked increase in population spike amplitude (see Figure 3B for a typical example). Across all rats, HFS stimulation induced significant potentiation of the population spike that was evident immediately after HFS stimulation and persisted for at least 72hrs (F(7,56) = 5.20, p = 1.32 × 10−4; Figure 3C). Thus, we were able to reliably induce long-term potentiation within the dentate gyrus.
3.3. Synaptic Potentiation Induces Changes in the Acute [Lac] Response to Neuronal Activation
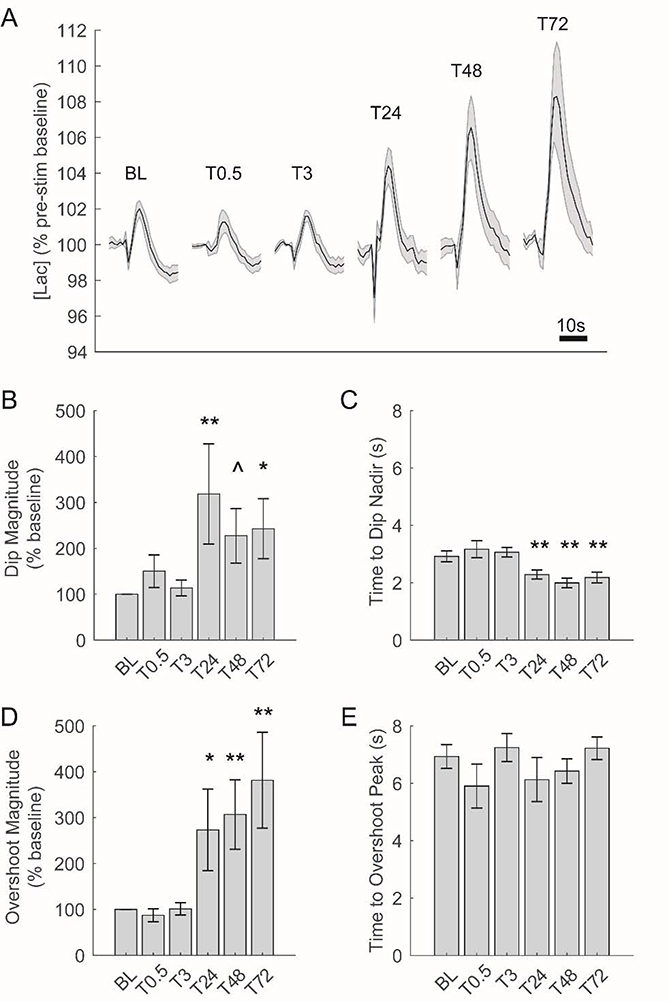
To assess the effects of synaptic potentiation on extracellular [lac] dynamics, we compared changes in [lac] in response to acute neuronal activation observed before and after LTP induction. Across all stimulation time points, we observed a canonical initial dip and subsequent overshoot of baseline [lac] in response to acute neuronal activation (Figure 4A and see Figure 2B/C). The timing and magnitude of these responses, however, were not static across all stimulation time points; instead, we observed pronounced changes in both response timing and magnitude that were initially evident 24hrs after LTP induction and persist for the remainder of the recording period (Figure 4A).
Figure 4.
Changes in [lac] in response to acute electrical stimulation are augmented beginning 24hrs after induction of LTP. A) Average change in [lac] in response to acute electrical stimulation across all rats at each stimulation time point. Each tracing depicts 25s of [lac] (including 5s prior to stimulation). BCDE) Changes in [lac] following acute electrical stimulation before and after LTP induction. 24hrs after LTP induction, the initial decrease in [lac] following stimulation is both significantly larger (B) and faster (C) while the magnitude of ensuing [lac] overshoot is also significantly increased (D). **p<0.01, *p<0.05, and ^p<0.10 as compared to baseline (BL).
To test the statistical significance of these observed changes in dip and overshot characteristics, we used linear mixed models with stimulation time point as a fixed effect and individual subjects as a random effect (see methods). Herein, we observed a significant effect of stimulation time point on the magnitude of the initial dip in [lac] (F(5, 41.86) = 3.14, p =0.02),with a significant increase in dip magnitude evident 24–72hrs after LTP induction (Figure 4B). Likewise, the time to reach the dip’s nadir was significantly affected by stimulation time point (F(5, 41.21) = 9.14, p = 6.55 × 10−6), with a significant decrease in the time to dip nadir present 24–72hrs after LTP induction (Figure 4C). The magnitude of [lac] overshoot was also significantly affected by stimulation time point (F(5, 42.19) = 6.40, p = 1.66 × 10−4), with a significant increase in overshoot magnitude observed 24–72hrs post-LTP (Figure 4D). By contrast, stimulation time point did not significantly affect the time to reach the overshoot peak (F(5, 40.29) = 1.11, p = 0.37; Figure 4E). Thus, beginning 24hrs after the induction of LTP, we observed significant changes in the response of [lac] to acute neuronal stimulation including increased magnitude of both the initial dip and subsequent overshoot of [lac] and a significantly quicker initial dip.
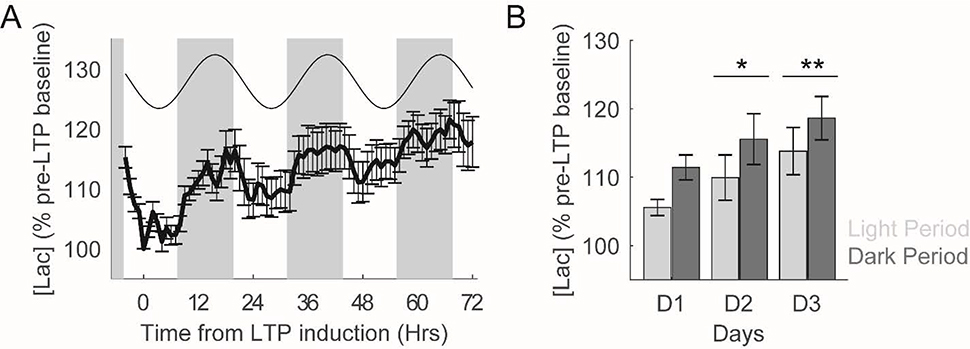
3.4. Synaptic Potentiation Induces Changes in Chronic [Lac] Availability
In addition to the acute changes in [lac] following evoked neuronal activation described above, LTP could additionally alter spontaneous lactate availability across longer timescales (i.e. hours to days). To test this possibility, we first averaged [lac] observed within each rat across consecutive one hour blocks throughout the recording period and then created a composite average across all rats. Similar to previous observations (Dash et al., 2012), a clear circadian rhythm in extracellular [lac] was visually apparent (Figure 5A). We therefore modelled this circadian variation in [lac] as a best-fitting cosine function (least squares fit). This model explained a significant amount of variance in [lac] (r(75) = 0.62, p =1.83 × 10−9), and moreover revealed a rhythm with a 24hr periodicity, peak-to-trough amplitude of 9.00% of pre-LTP baseline values, and an acrophase at 7pm (i.e. nine hours after dark onset; see Figure 5A).
Figure 5.
LTP induction produces persistent increases in extracellular [lac]. A) Extracellular [lac] across 72hrs post-LTP induction. In addition to exhibiting a clear circadian rhythmicity, [lac] are consistently increased across the days following LTP induction. Grey boxes depict lights off. Best fitting cosine function to model circadian rhythmicity is presented above [lac] tracing. B) Average extracellular [lac] during the light and dark periods during the three days of data depicted in A. [Lac] are significantly higher during the dark period as compared to the light period. Moreover, relative to the day of LTP induction (D1), [lac] are significantly higher during each of the next two days. **p<0.01 and *p<0.05.
In addition to circadian modulation of extracellular [lac], the availability of this important metabolite also appeared to be modulated by LTP as evident by a sustained increase in extracellular [lac] in the days following LTP induction (see Figures 5A/B). To quantify this effect, we averaged [lac] within the light and dark periods for each day of our extracellular lactate recordings. A linear mixed model with individual rats as random effects and 1) light/dark period and 2) recording day as fixed effects was generated to quantify the effect of these variables on extracellular lactate availability (Figure 5B). Consistent with our circadian analyses above, we observed a significant effect of light/dark period (F (1, 37.63) = 6.66, p = 0.01), with [lac] during the dark period 5.28 ± 0.61% higher than those observed during the light period. Strikingly, we also observed a significant effect of recording day (F (2, 37.73) = 4.09, p =0.03), with post-hoc analyses indicating that [lac] were significantly higher in the two days following LTP induction (Figure 5B). The interaction between light/dark period and recording day was not observed to be statistically significant (F (2, 37.64) = 0.07, p =0.93). Thus, following synaptic potentiation, we observed persistent increases in extracellular lactate availability.
To assess whether these chronic alterations in extracellular [lac] arise as a consequence of synaptic potentiation (as opposed to arising from unintended consequences of methodological approaches associated with chronic amperometric recordings), we performed similar analyses to those described above on two distinct data sets in which extracellular [lac] were recorded for up to 72hrs but synaptic potentiation was not induced. We first analyzed data in which extracellular [lac] were continuously recorded from frontal cortical areas in male Wistar Kyoto rats across the sleep/wake cycle (see Dash et al., 2012 for complete methodological details). Consistent with results in the present study, we observed a significant effect of the light/dark period on extracellular [lac] (F (1, 39.96) = 5.41, p=0.03). However, no significant effects of recording day (F (2, 41.47) = 0.20, p=0.82) or interaction between recording day and light/dark period (F (2, 39.96) = 0.14, p=0.87) were observed. Thus, absent experimental LTP induction, extracellular [lac] were not observed to increase as a function of experiment duration. We observe similar findings when analyzing a subset of pilot recordings of extracellular [lac] within the dentate gyrus that were used to develop the final stimulation parameters used in the present study, but did not result in LTP induction (see methods for details). Again, [lac] were significantly affected by the light/dark period (F (1, 28.67) = 11.85, p=0.002), but not recording day (F (2, 28.64) = 0.58, p=0.57) or interaction between light/dark period and recording day (F (2, 28.64) = 0.16, p=0.85). Together, these results support a direct role of synaptic potentiation in producing chronic increases in extracellular [lac] characterized in the present study.
4. Discussion
Through simultaneous recordings of extracellular [lac] and electrically-evoked field potentials within the dentate gyrus of freely behaving rats, we assessed how synaptic potentiation affects extracellular lactate dynamics. HFS of the medial perforant pathway reliably produced significant potentiation of synapses within the dentate gyrus that persisted for the duration of our recordings (~72hrs post-LTP induction). Synaptic potentiation was associated with alterations in both chronic extracellular lactate availability and in the acute response of lactate to transient neuronal activation. Strikingly, the expression of this metabolic plasticity was delayed relative to LTP induction; despite pronounced potentiation of synapses occurring immediately following LTP induction, statistically significant alterations in both acute and chronic lactate measures were not observed until ~24hrs later. Thus, synaptic plasticity appears to be associated with metabolic plasticity in the form of persistent alterations in glycolytic activity.
The brain is equipped with a diverse array of interdependent neurometabolic coupling mechanisms to ensure that sufficient metabolic supply is directed to meet the extensive energetic demands associated with acute and chronic alterations in neuronal activity. Herein, local changes in cerebral blood flow (Chaigneau et al., 2007; Viswanathan and Freeman, 2007), efficacy of nutrient transport across the blood-brain barrier (Leybaert, 2005), and changes in oxidative and/or glycolytic metabolic processes (Fox et al., 1988) have all been reported to offset energetic demands associated with excitatory neuronal activation. To this end, extracellular [lac] have been consistently reported to increase following acute neuronal activity (Hu and Wilson, 1997; Caesar et al., 2008; Li and Freeman, 2015), although the source of this lactate and its functional consequences remain contested (Dienel, 2013, 2017; Bak and Walls, 2018; Magistretti and Allaman, 2018; Yellen, 2018). Some researchers have argued that acute increases in extracellular [lac] arise following neuronal glycolysis or from a combination of neuronal/astrocytic glycolysis (Lundgaard et al., 2015; Díaz-García et al., 2017; Díaz‐García and Yellen, 2019). Here, energetic benefits are proposed to occur at the site of glycolysis prior to lactate efflux to the extracellular space. Within the extracellular space, lactate may then serve important signaling functions (Yang et al., 2014; Mosienko et al., 2015; Jourdain et al., 2018; Magistretti and Allaman, 2018), before ultimately being extruded from the brain (Dienel, 2017). Other researchers, within the broad framework of the astrocyte neuron lactate shuttle hypothesis (ANLS), argue that extracellular lactate is largely derived from astrocytic glycolysis and goes on to serve as a critical energy substrate within neurons (Pellerin and Magistretti, 1994; Magistretti and Pellerin, 1999; Magistretti and Allaman, 2018). Following synaptic activation, glutamate reuptake within astrocytes triggers rapid glycogenolysis and lactate efflux to the extracellular space where it can be taken up by neurons and ultimately converted to ATP (Pellerin and Magistretti, 1994). Excitatory synaptic activity is ultimately dependent upon these mechanisms as their disruption produces a pronounced reduction in EPSC amplitude (Nagase et al., 2014).
In the present study, we observed that acute neuronal activation caused extracellular [lac] to 1) initially decrease, 2) subsequently increase above pre-stimulation baseline levels (i.e. overshoot), and 3) ultimately return to baseline levels (see Figures 2B/C and 4). As these data were recorded in freely-behaving animals, in response to physiologically-relevant activation patterns (i.e. 15 pulses @ 50Hz, 200uS pulse durations), they represent a novel extension of canonical observations characterizing similar lactate dynamics in the dentate gyrus of anesthetized rats following 5s bouts of perforant pathway stimulation (Hu and Wilson, 1997). Similar dynamics have been modeled (Aubert et al., 2005; Jolivet et al., 2015) and have recently been observed to also occur directly within astrocytes, but not neurons (Zuend et al., 2020). Collectively, these data may reflect activity-dependent 1) rapid neuronal uptake of lactate through monocarboxylate transporter 2 (MCT2). which is highly expressed within neurons, 2) acute glycogenolysis and subsequent lactate efflux through MCT1/MCT4 isoforms highly expressed within astrocytes, and 3) cessation of the acute metabolic response (Hu and Wilson, 1997; Aubert et al., 2005; Pellerin et al., 2005). Of note, while our lactate-sensitive biosensor was centered adjacent to the dentate gyrus LFP, the active sensing cavity of the biosensor extends 1mm. Consequently, the magnitude of our observed acute lactate response may underestimate changes in extracellular [lac] following neuronal activation as perforant pathway stimulation is unlikely to have fully activated neurons situated along the entire length of the biosensor. Nevertheless, these results provide further evidence of the association between synaptic activity and acute lactate dynamics.
Given the high energetic demands associated with synaptic activity, it is perhaps not surprising that synaptic plasticity is both supported and constrained by neuroenergetic processes. ATP consumption to sustain and restore ion gradients, remodel cytostructural elements, and support kinase activity is critical for LTP induction (Mattson and Liu, 2002) and insufficient ATP prevents the expression of late-phase LTP through the downstream effects of AMP-activated protein kinase (Potter et al., 2010). Lactate, derived from astrocytic glycogen stores, appears to play a prominent role in supporting the energetic demands of synaptic potentiation; LTP expression is blocked by inhibition of glycogenolysis (Suzuki et al., 2011) or disruption of the astrocytic syncytium through diminished gap junctions (Murphy-Royal et al., 2020), effects that are rescued by lactate infusion. Behavioral experiments, in which long-term memory formation is blocked through glycogenolysis inhibition and/or antagonism of monocarboxylate transporters (Newman et al., 2011; Suzuki et al., 2011), provide further support for the role of lactate in synaptic plasticity.
In the present study, we build upon these previous observations and demonstrate that in addition to its role during LTP-induction, lactate dynamics may also be altered to provide persistent support to meet the needs of potentiated synapses. Although synaptic potentiation occurred immediately after HFS, an altered acute response of lactate to neuronal activation (characterized by a significantly larger initial decrease and a significantly larger subsequent overshoot) was only observed beginning 24hrs after HFS. Thus, these altered acute lactate dynamics are unlikely to arise simply in response to greater activation of potentiated synapses. Instead, metabolic plasticity in the form of altered gene/protein expression and/or increased glycogen availability may provide an explanation for these results. Tadi et al., 2015 previously reported that inhibitory avoidance training results in upregulated mRNA expression of monocarboxylate transporters (MCT1 and MCT4), glycolytic enzymes (lactate dehydrogenase A/B), and genes associated with glycogen metabolism (glycogen branching enzyme 1 and glycogen synthase). Similar to the time course observed in our study, many of these changes were first observed 24hrs following training (or increased their expression at this time point) and were still elevated 72hrs post-training. Increased lactate production (i.e. glycogen metabolism and glycolytic enzyme activity) and efflux from astrocytes (MCT1/MCT4) may therefore underlie the increased overshoot observed in the present study. As novel object recognition learning has recently been shown to increase the size and density of astrocytic glycogen granules (Vezzoli et al., 2020), these genetic changes may be complemented by increased glycogen stores. Unlike the astrocytic MCT1/MCT4, MCT2 mRNA expression levels were unaffected by inhibitory avoidance training (Tadi et al., 2015). It has previously been reported, however, that activity-dependent regulation of MCT2 expression can occur via post-transcriptional modifications without altering mRNA expression levels (Pierre et al., 2003). The larger initial decrease in extracellular [lac] observed in our study, therefore, may yet arise from a larger neuronal influx supported by increased MCT2 expression.
It is unclear whether the above changes in gene/protein expression would be initiated directly following HFS stimulation (similar to those that support the expression of LTP itself (Nguyen et al., 1994; Chen et al., 2017)) or alternatively, whether they may arise as a consequence of prolonged and enhanced synaptic activity of potentiated synapses. In vitro, synaptic activity extensively modulates astrocytic gene expression; increased synaptic activity significantly upregulates genes involved in the enzymatic production and transport of lactate thereby increasing glycolytic capacity of astrocytes (Hasel et al., 2017). Similarly, synaptic activity has been shown to produce alterations in neuronal gene expression that also support a shift in energetic balance towards aerobic glycolysis (Bas-Orth et al., 2017). Consistent with these observations, 1) across development the highest rates of aerobic glycolysis are associated with peak synaptic development and 2) in the adult brain, regions that exhibit high levels of aerobic glycolysis likewise exhibit increased gene expression associated with synapse formation and growth (Goyal et al., 2014). Synaptic plasticity (be it developmental or in the adult brain), therefore appears to be associated with widespread metabolic plasticity to ensure sufficient lactate availability is present to support the energetic demands of synaptic activity.
A multitude of factors have been shown to alter extracellular lactate availability within the hippocampus including behavioral state (Dash et al., 2012; Naylor et al., 2012), stress (De Bruin et al., 1990), exercise (Matsui et al., 2017), and learning (Newman et al., 2017). Throughout our amperometric recordings, we observed fluctuations in extracellular lactate concentrations across diverse temporal scales (from seconds to hours to days) that likely reflect the contributions of these various regulators. To wit, a prominent circadian rhythm in extracellular [lac] was readily apparent in our recordings (see Figure 5), as previously documented (Dash et al., 2012). Despite the multitude of effectors that could influence lactate availability, we nonetheless also observed that [lac] were significantly higher across the two days following LTP-induction than the day of induction. This observation raises the possibility that synaptic potentiation produces persistent increases in baseline lactate availability. We were unable to record past ~72hrs post-LTP induction because of enzymatic degradation of the amperometric biosensor (Dash et al., 2009, 2012). Therefore, we do not know whether increased lactate availability ultimately returns to pre-LTP baseline levels or alternatively, persists for the duration that synaptic potentiation is expressed.
It is interesting to speculate as to the functional consequences of both the acute and chronic alterations in extracellular lactate dynamics that we observed following synaptic potentiation. Lactate is necessary during LTP induction or memory formation to support de novo transcription and translation required for long-term, but not short-term, plasticity and memory (Suzuki et al., 2011; Descalzi et al., 2019), an effect that may be mediated by lactate potentiating NMDA signaling within neurons and altering neuronal redox state (Yang et al., 2014). Transiently reducing extracellular [lac] does not impair memory retrieval (Harris et al., 2019), yet lactate is necessary to support memory reconsolidation (Zhang et al., 2016). The persistent increase in lactate availability observed ~24hrs following LTP induction in the present study, may therefore reflect a metaplastic effect that could regulate consolidation and/or reconsolidation processes that are likewise dependent on protein synthesis (Abraham and Williams, 2008). Alternatively, persistent increases in extracellular [lac] may 1) serve to support the increased energetic demands associated with potentiated synapses and/or 2) play a neuroprotective role. As postsynaptic receptor activation has been modelled to account for 37% of the brain’s energy budget (Harris et al., 2012), and LTP is defined by increased postsynaptic activity, increased [lac] may counteract elevated energetic needs be serving as a precursor to ATP formation and/or a regulator of redox state (Kasischke et al., 2004; Hirrlinger and Dringen, 2010; Magistretti and Allaman, 2018). If lactate is primarily serving a metabolic role, it would be important to determine if newly potentiated neurons experience an energy deficit until metabolic plasticity is able to supply sufficient lactate. Lastly, persistent increases in lactate may serve a neuroprotective role; high lactate concentrations have been shown to reduce NMDAR-dependent excitotoxic cell death (Jourdain et al., 2018).
Ultimately, further study of the duration of these glycolytic effects is warranted; are [lac] persistently elevated or are there mechanisms (e.g. homeostatic plasticity (Turrigiano, 2012)) that lead to a return to baseline levels? Although persistent increases in extracellular lactate may have beneficial effects as outlined above, increased dependence upon aerobic glycolysis may have negative long-term consequences. Elevated [lac] and glycolytic activity are also characteristic of the aged brain (Ross et al., 2010) and neurodegenerative disorders including Parkinson’s and Alzheimer’s disease (Yao et al., 2009, 2011; Liguori et al., 2015). Indeed, a significant spatial correlation has been observed between brain regions that exhibit high levels of aerobic glycolysis in healthy young adults and brain regions that exhibit significant amyloid-β plaque deposition in older individuals who manifest clinical signs of Alzheimer’s (Vlassenko et al., 2010). While it remains unclear whether elevated glycolysis contributes to the development of these pathologies, or whether it may serve a neuroprotective role (Newington et al., 2013; Tang, 2020), it is critical that the long-term consequences of synaptic plasticity and consequent metabolic plasticity for brain health continue to be characterized.
Supplementary Material
Highlights.
Characterized how long term potentiation affects extracellular lactate dynamics
Potentiation causes enhanced acute lactate response to neuronal activity
Potentiation causes chronic increases in lactate availability
Acute and chronic changes in lactate manifest ~24hrs after synaptic potentiation
5.
Funding
This work was supported by Middlebury College start-up funds and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103449. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham WC, Williams JM (2008) LTP maintenance and its protein synthesis-dependence. Neurobiology of Learning and Memory 89:260–268. [DOI] [PubMed] [Google Scholar]
- Alle H, Roth A, Geiger JR (2009) Energy-efficient action potentials in hippocampal mossy fibers. Science 325:1405–1408. [DOI] [PubMed] [Google Scholar]
- Attwell D, Gibb A (2005) Neuroenergetics and the kinetic design of excitatory synapses. Nat Rev Neurosci 6:841–849. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R (2005) Interaction between Astrocytes and Neurons Studied using a Mathematical Model of Compartmentalized Energy Metabolism: Journal of Cerebral Blood Flow & Metabolism 25:1476–1490. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R, Magistretti PJ, Pellerin L (2005) Brain lactate kinetics: Modeling evidence for neuronal lactate uptake upon activation. PNAS 102:16448–16453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Walls AB (2018) CrossTalk opposing view: lack of evidence supporting an astrocyte-to-neuron lactate shuttle coupling neuronal activity to glucose utilisation in the brain. J Physiol 596:351–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas-Orth C, Tan Y-W, Lau D, Bading H (2017) Synaptic activity drives a genomic program that promotes a neuronal Warburg effect. J Biol Chem:jbc.M116.761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E (2006) Small-world brain networks. Neuroscientist 12:512–523. [DOI] [PubMed] [Google Scholar]
- Brown AM (2004) Brain glycogen re-awakened. J Neurochem 89:537–552. [DOI] [PubMed] [Google Scholar]
- Brown AM, Ransom BR (2007) Astrocyte glycogen and brain energy metabolism. Glia 55:1263–1271. [DOI] [PubMed] [Google Scholar]
- Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M (2008) Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo: Glutamate receptor-dependent lactate production. The Journal of Physiology 586:1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì C, Baghabra J, Boges DJ, Holst GR, Kreshuk A, Hamprecht FA, Srinivasan M, Lehväslaiho H, Magistretti PJ (2016) Three-dimensional immersive virtual reality for studying cellular compartments in 3D models from EM preparations of neural tissues. Journal of Comparative Neurology 524:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaigneau E, Tiret P, Lecoq J, Ducros M, Knöpfel T, Charpak S (2007) The Relationship between Blood Flow and Neuronal Activity in the Rodent Olfactory Bulb. J Neurosci 27:6452–6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PB, Kawaguchi R, Blum C, Achiro JM, Coppola G, O’Dell TJ, Martin KC (2017) Mapping Gene Expression in Excitatory Neurons during Hippocampal Late-Phase Long-Term Potentiation. Front Mol Neurosci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash MB, Ajayi S, Folsom L, Gold PE, Korol DL (2018) Spontaneous Infraslow Fluctuations Modulate Hippocampal EPSP-PS Coupling. eNeuro 5:403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash MB, Bellesi M, Tononi G, Cirelli C (2013) Sleep/wake dependent changes in cortical glucose concentrations. JNeurochem 124:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash MB, Douglas CL, Vyazovskiy VV, Cirelli C, Tononi G (2009) Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J Neurosci 29:620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash MB, Tononi G, Cirelli C (2012) Extracellular levels of lactate, but not oxygen, reflect sleep homeostasis in the rat cerebral cortex. Sleep 35:909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin LA, Schasfoort EM, Steffens AB, Korf J (1990) Effects of stress and exercise on rat hippocampus and striatum extracellular lactate. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 259:R773–R779. [DOI] [PubMed] [Google Scholar]
- Descalzi G, Gao V, Steinman MQ, Suzuki A, Alberini CM (2019) Lactate from astrocytes fuels learning-induced mRNA translation in excitatory and inhibitory neurons. Commun Biol 2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-García CM, Mongeon R, Lahmann C, Koveal D, Zucker H, Yellen G (2017) Neuronal Stimulation Triggers Neuronal Glycolysis and Not Lactate Uptake. Cell Metabolism 26:361–374.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-García CM, Yellen G (2019) Neurons rely on glucose rather than astrocytic lactate during stimulation. Journal of Neuroscience Research 97:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA (2013) Astrocytic energetics during excitatory neurotransmission: What are contributions of glutamate oxidation and glycolysis? Neurochemistry International 63:244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA (2017) Lack of appropriate stoichiometry: Strong evidence against an energetically important astrocyte–neuron lactate shuttle in brain. Journal of Neuroscience Research 95:2103–2125. [DOI] [PubMed] [Google Scholar]
- Fonseca-Azevedo K, Herculano-Houzel S (2012) Metabolic constraint imposes tradeoff between body size and number of brain neurons in human evolution. PNAS 109:18571–18576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C (1988) Nonoxidative glucose consumption during focal physiologic neural activity. Science 241:462–464. [DOI] [PubMed] [Google Scholar]
- Gao V, Suzuki A, Magistretti PJ, Lengacher S, Pollonini G, Steinman MQ, Alberini CM (2016) Astrocytic β2-adrenergic receptors mediate hippocampal long-term memory consolidation. PNAS 113:8526–8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME (2014) Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell metabolism 19:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Attwell D (2012) The Energetics of CNS White Matter. J Neurosci 32:356–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D (2012) Synaptic Energy Use and Supply. Neuron 75:762–777. [DOI] [PubMed] [Google Scholar]
- Harris RA, Lone A, Lim H, Martinez F, Frame AK, Scholl TJ, Cumming RC (2019) Aerobic Glycolysis Is Required for Spatial Memory Acquisition But Not Memory Retrieval in Mice. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasel P et al. (2017) Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nature Communications 8:15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Otte S, Callaway E, Sejnowski TJ (2010) Metabolic cost as a unifying principle governing neuronal biophysics. PNAS 107:12329–12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirrlinger J, Dringen R (2010) The cytosolic redox state of astrocytes: Maintenance, regulation and functional implications for metabolite trafficking. Brain Research Reviews 63:177–188. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wilson GS (1997) A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem 69:1484–1490. [DOI] [PubMed] [Google Scholar]
- Jolivet R, Coggan JS, Allaman I, Magistretti PJ (2015) Multi-timescale Modeling of Activity-Dependent Metabolic Coupling in the Neuron-Glia-Vasculature Ensemble. PLOS Computational Biology 11:e1004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Rothenfusser K, Ben-Adiba C, Allaman I, Marquet P, Magistretti PJ (2018) Dual action of L-Lactate on the activity of NR2B-containing NMDA receptors: from potentiation to neuroprotection. Sci Rep 8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski J (2007) Global and regional brain metabolic scaling and its functional consequences. BMC Biol 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW (2004) Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 305:99–103. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE (2008) Epinephrine converts long-term potentiation from transient to durable form in awake rats. Hippocampus 18:81–91. [DOI] [PubMed] [Google Scholar]
- Leybaert L (2005) Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cereb Blood Flow Metab 25:2–16. [DOI] [PubMed] [Google Scholar]
- Li B, Freeman RD (2015) Neurometabolic coupling between neural activity, glucose, and lactate in activated visual cortex. Journal of Neurochemistry 135:742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C, Stefani A, Sancesario G, Sancesario GM, Marciani MG, Pierantozzi M (2015) CSF lactate levels, τ proteins, cognitive decline: a dynamic relationship in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 86:655–659. [DOI] [PubMed] [Google Scholar]
- Lundgaard I, Li B, Xie L, Kang H, Sanggaard S, Haswell JDR, Sun W, Goldman S, Blekot S, Nielsen M, Takano T, Deane R, Nedergaard M (2015) Direct neuronal glucose uptake heralds activity-dependent increases in cerebral metabolism. Nature Communications 6:6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I (2018) Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci 19:235–249. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L (1999) Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci 354:1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Omuro H, Liu Y-F, Soya M, Shima T, McEwen BS, Soya H (2017) Astrocytic glycogen-derived lactate fuels the brain during exhaustive exercise to maintain endurance capacity. PNAS 114:6358–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Liu D (2002) Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromol Med 2:215–231. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA (1977) Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. Journal of Comparative Neurology 175:439–453. [DOI] [PubMed] [Google Scholar]
- Mosienko V, Teschemacher AG, Kasparov S (2015) Is L-Lactate a Novel Signaling Molecule in the Brain? Journal of Cerebral Blood Flow & Metabolism 35:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Royal C, Johnston AD, Boyce AKJ, Diaz-Castro B, Institoris A, Peringod G, Zhang O, Stout RF, Spray DC, Thompson RJ, Khakh BS, Bains JS, Gordon GR (2020) Stress gates an astrocytic energy reservoir to impair synaptic plasticity. Nature Communications 11:2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase M, Takahashi Y, Watabe AM, Kubo Y, Kato F (2014) On-Site Energy Supply at Synapses through Monocarboxylate Transporters Maintains Excitatory Synaptic Transmission. J Neurosci 34:2605–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor E, Aillon DV, Barrett BS, Wilson GS, Johnson DA, Johnson DA, Harmon HP, Gabbert S, Petillo PA (2012) Lactate as a Biomarker for Sleep. Sleep 35:1209–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newington JT, Harris RA, Cumming RC (2013) Reevaluating Metabolism in Alzheimer’s Disease from the Perspective of the Astrocyte-Neuron Lactate Shuttle Model. Journal of Neurodegenerative Diseases 2013:e234572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE (2011) Lactate Produced by Glycogenolysis in Astrocytes Regulates Memory Processing. PLOS ONE 6:e28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Scavuzzo CJ, Gold PE, Korol DL (2017) Training-induced elevations in extracellular lactate in hippocampus and striatum: Dissociations by cognitive strategy and type of reward. Neurobiology of Learning and Memory 137:142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER (1994) Requirement of a critical period of transcription for induction of a late phase of LTP. Science 265:1104–1107. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bergersen LH, Halestrap AP, Pierre K (2005) Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J Neurosci Res 79:55–64. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. PNAS 91:10625–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K, Debernardi R, Magistretti PJ, Pellerin L (2003) Noradrenaline enhances monocarboxylate transporter 2 expression in cultured mouse cortical neurons via a translational regulation. Journal of Neurochemistry 86:1468–1476. [DOI] [PubMed] [Google Scholar]
- Potter WB, O’Riordan KJ, Barnett D, Osting SMK, Wagoner M, Burger C, Roopra A (2010) Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS ONE 5:e8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JM, Öberg J, Brené S, Coppotelli G, Terzioglu M, Pernold K, Goiny M, Sitnikov R, Kehr J, Trifunovic A, others (2010) High brain lactate is a hallmark of aging and caused by a shift in the lactate dehydrogenase A/B ratio. PNAS 107:20087–20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG (1998) Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA 95:316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM (2011) Astrocyte-Neuron Lactate Transport Is Required for Long-Term Memory Formation. Cell 144:810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadi M, Allaman I, Lengacher S, Grenningloh G, Magistretti PJ (2015) Learning-Induced Gene Expression in the Hippocampus Reveals a Role of Neuron -Astrocyte Metabolic Coupling in Long Term Memory. PLOS ONE 10:e0141568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL (2020) Glucose, glycolysis, and neurodegenerative diseases. J Cell Physiol. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Volkow ND (2013) Energetic cost of brain functional connectivity. PNAS 110:13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G (2012) Homeostatic Synaptic Plasticity: Local and Global Mechanisms for Stabilizing Neuronal Function. Cold Spring Harbor Perspectives in Biology 4:a005736–a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME (2010) Regional aerobic glycolysis in the human brain. PNAS 107:17757–17762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzoli E, Calì C, De Roo M, Ponzoni L, Sogne E, Gagnon N, Francolini M, Braida D, Sala M, Muller D, Falqui A, Magistretti PJ (2020) Ultrastructural Evidence for a Role of Astrocytes and Glycogen-Derived Lactate in Learning-Dependent Synaptic Stabilization. Cereb Cortex 30:2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD (2007) Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci 10:1308–1312. [DOI] [PubMed] [Google Scholar]
- Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA (2010) Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta ) deposition. PNAS 107:17763–17767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MTT (1989) Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends in Neurosciences 12:94–101. [DOI] [PubMed] [Google Scholar]
- Yang J, Ruchti E, Petit J-M, Jourdain P, Grenningloh G, Allaman I, Magistretti PJ (2014) Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. PNAS 111:12228–12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD (2009) Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. PNAS 106:14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Gandhi S, Burchell VS, Plun-Favreau H, Wood NW, Abramov AY (2011) Cell metabolism affects selective vulnerability in PINK1-associated Parkinson’s disease. JCellSci 124:4194–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G (2018) Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J Cell Biol 217:2235–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xue Y, Meng S, Luo Y, Liang J, Li J, Ai S, Sun C, Shen H, Zhu W, Wu P, Lu L, Shi J (2016) Inhibition of Lactate Transport Erases Drug Memory and Prevents Drug Relapse. Biological Psychiatry 79:928–939. [DOI] [PubMed] [Google Scholar]
- Zhou H, Liu G (2015) Regulation of density of functional presynaptic terminals by local energy supply. Molecular brain 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuend M, Saab AS, Wyss MT, Ferrari KD, Hösli L, Looser ZJ, Stobart JL, Duran J, Guinovart JJ, Barros LF, Weber B (2020) Arousal-induced cortical activity triggers lactate release from astrocytes. Nature Metabolism 2:179–191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.