Abstract
For over three-quarters of a century, organophosphorus (OP) insecticides have been ubiquitously used in agricultural, residential, and commercial settings and in public health programs to mitigate insect borne diseases. Their broad-spectrum insecticidal effectiveness is accounted for by the irreversible inhibition of acetylcholinesterase (AChE), the enzyme that catalyzes acetylcholine (ACh) hydrolysis, in the nervous system of insects. However, because AChE is evolutionarily conserved, OP insecticides are also toxic to mammals, including humans, and acute OP intoxication remains a major public health concern in countries where OP insecticide usage is poorly regulated. Environmental exposures to OP levels that are generally too low to cause marked inhibition of AChE and to trigger acute signs of intoxication, on the other hand, represent an insidious public health issue worldwide. Gestational exposures to OP insecticides are particularly concerning because of the exquisite sensitivity of the developing brain to these insecticides. The present article overviews and discusses: (i) the health effects and therapeutic management of acute OP poisoning during pregnancy, (ii) epidemiological studies examining associations between environmental OP exposures during gestation and health outcomes of offspring, (iii) preclinical evidence that OP insecticides are developmental neurotoxicants, and (iv) potential mechanisms underlying the developmental neurotoxicity of OP insecticides. Understanding how gestational exposures to different levels of OP insecticides affect pregnancy and childhood development is critical to guiding implementation of preventive measures and direct research aimed at identifying effective therapeutic interventions that can limit the negative impact of these exposures on public health.
Keywords: Organophosphorus, acetylcholinesterase, cannabinoid type 1 receptor, developmental neurotoxicity, endocannabinoids
1. Introduction
The discovery in the mid-1930’s of the potent insecticidal properties of organophosphorus (OP) compounds by Gerhard Schrader, a German chemist working in a research program for the discovery of new insecticides, represented a critical breakthrough to overcome the severe food shortage at the time (Costa, 2018; Petroianu, 2009). Being biodegradable, inexpensive, easy to use, and highly effective against a broad spectrum of insects, OP insecticides emerged as a viable alternative to the non-biodegradable organochlorines, whose toxicity and negative impact on the environment were already recognized in the 1940’s (Rosner and Markowitz, 2013). Unfortunately, Gerhard Schrader is best known for his accidental discovery of one of the most toxic man-made chemicals, ethyl N, N'-dimethylphosphoramido cyanidate (also known as tabun), which became the prototype of a class of chemical weapons of mass destruction referred to as nerve agents (Sidell and Borak, 1992).
The insecticidal effectiveness of OP compounds results from the ability of these chemicals or their oxon metabolites to irreversibly inhibit acetylcholinesterase (AChE), the enzyme that hydrolyzes the neurotransmitter acetylcholine (ACh) in the nervous system of insects (Casida and Durkin, 2013). However, because AChE is an evolutionarily conserved enzyme (Wiesner et al., 2007), OP insecticides have undesired effects in mammals. In fact, the lethality and morbidity resulting from occupational, intentional, or accidental poisoning of humans to high doses of these insecticides are well documented (Naughton and Terry, 2018). Equally concerning, though, are the detrimental health effects that have been associated with the more common exposures to environmentally relevant levels of OP insecticides, which are too low to markedly inhibit AChE and trigger clinical signs of acute toxicity.
According to a market estimate published in 2017 by the U.S. Environmental Protection Agency, the use of OP insecticides in the U.S. decreased from nearly 70 million pounds in 2000 to a plateau of 20-23 million pounds per year between 2009 and 2012 (reviewed in Atwood and Paisley-Jones, 2017; Hertz-Picciotto et al., 2018). This decline has been attributed to: (i) the introduction of strict regulations that have either limited or banned the use of some OP insecticides, and (ii) the development of newer generations of insecticides believed to be less toxic (Stone et al., 2009). Yet, according to data from the National Health and Nutrition Examination Survey, the incremental decline in use of OP insecticides between 2001 and 2010 has not been accompanied by a corresponding reduction of population exposure, as assessed by measurements of urine levels of OP metabolites; instead, an initial reduction in exposure between 2001 and 2003 was followed by a subsequent increase between 2003 and 2010 (Gaylord et al., 2020).
Exposures to OP insecticides during pregnancy represent a major public health concern because the developing fetus is highly vulnerable to chemical insults (reviewed in Rock and Patisaul, 2018), and, like most lipophilic chemicals, these insecticides easily cross membrane barriers, including the placenta and the blood brain barrier (Akhtar et al., 2006; Jajoo et al., 2010). A recent study estimated that, between 2001 and 2016, prenatal exposures to environmentally relevant levels of OP insecticides in the U.S. were associated with the cumulative loss of millions of intelligence quotient (IQ) points that cost hundreds of billions of dollars in decreased productivity (Gaylord et al., 2020). This staggering neurodevelopmental disability burden surpassed that associated with environmental exposures to mercury during the same period (Gaylord et al., 2020).
The objectives of the present article are: (i) to discuss the health effects and therapeutic management of acute (intentional, occupational, or accidental) OP exposures during gestation, (ii) to overview epidemiological studies examining associations between prenatal exposures to environmentally relevant levels of OP insecticides and health outcomes of offspring, (iii) to review preclinical evidence of the cause-effect relationship between developmental exposures to low levels of OP insecticides and neurobehavioral deficits and neurochemical alterations later in life, and (iv) to discuss potential mechanisms underlying the impact of environmentally relevant OP exposures on the developing brain.
2. Acute OP Poisoning During Gestation: A Medical Challenge
In the U.S. and other countries, measures that regulate and restrict the use of OP insecticides have markedly reduced the number of cases of acute poisoning throughout the years (Stone et al., 2009). By contrast, in many countries where OP insecticide usage is not as well-regulated, high numbers of cases of acute OP poisoning are still reported to date, many of which are accounted for by the intentional use of OP insecticides in suicides and suicidal attempts, including among pregnant women (Licata et al., 2019; Zangeneh, 2014).
The acute toxicity triggered by acute exposures to high levels of OP compounds results primarily from overstimulation of muscarinic and nicotinic receptors (mAChRs and nAChRs, respectively) by acetylcholine (ACh) that accumulates in the peripheral and central nervous systems as those compounds, or their oxon metabolites, irreversibly inhibit AChE. The cholinergic toxidrome is typically characterized by miosis, profuse secretions, diarrhea, bronchoconstriction, muscle fasciculation, tremors, and seizures (reviewed in Pereira et al., 2014; Taylor, 2017), with clinical presentation varying depending on the OP insecticide (reviewed in Eddleston et al., 2005).
Conventional antidotes used to treat acute OP poisoning include atropine to block mAChR overactivation, oximes (generally pralidoxime, also known as 2-PAM) to reactivate OP-inhibited AChE that is not aged, and a benzodiazepine, as needed, to halt OP-induced convulsions (reviewed in Pereira et al., 2014). However, therapeutic management of acute OP poisoning during gestation is not standardized and presents a serious medical challenge in part because, compared to the adult organism, the developing fetus is more sensitive to the acute toxicity of OP compounds. For instance, the LD50s of the OP insecticides chlorpyrifos, malathion, parathion, and methyl parathion are approximately 5-17 times lower in rats at postnatal days (PND) 1-7, which developmentally correspond to human fetuses in the third trimester of gestation, than in adult rats (Vidair, 2004). Likewise, the LD50s of the OP nerve agents soman and sarin are age dependent in rats (Shih et al., 1990; Wright et al. 2015), with both agents being more toxic at perinatal (PND7) than pubertal (PND28) and adult (PND70) ages (Wright et al., 2015). Of interest, pubertal rats (PND28-30) are more resistant to the toxicity of soman and sarin than adult rats (PND70-240) (Shih et al., 1990; Wright et al. 2015), and, at least for soman, the age dependence of the LD50s between PND30 and PND240 does not correlate with age-related differences in AChE activity in blood or brain (Shih et al., 1990).
The differential sensitivity of the adult and the fetal organisms to the toxic effects of OP compounds may explain, at least in part, the greater lethal potency of these toxicants in pregnant animals compared to adult non-pregnant females. For instance, an earlier study from our laboratory reported that, in adult non-pregnant female guinea pigs, the LD50s (s.c., 24-h lethality) of the OP nerve agents soman and sarin are 27.0 μg/kg (95% confidence interval: 25.2-29.7 μg/kg) and 39.6 μg/kg (95% confidence interval: 34.6-46.5 μg/kg), respectively (Fawcett et al., 2009). By contrast, in pregnant guinea pigs at approximate gestation day 50, the LD50s of soman and sarin have been estimated to be 12.2 μg/kg (95% confidence interval: 11.6-12.9 μg/kg) and 18.0 μg/kg (95% confidence interval: 16.7-19.4 μg/kg), respectively (personal communication). Based on experiments carried out in our laboratory, the oral LD50 of chlorpyrifos in pregnant guinea pigs at approximate 50-day-gestation is estimated to be 300 mg/kg (95% confidence interval: 187.3-480.4 mg/kg, personal communication). It remains to be determined whether, like soman and sarin, chlorpyrifos is more toxic to pregnant than non-pregnant females; the oral LD50 of this insecticide has only been reported for adult male guinea pigs (504 mg/kg with 95% confidence intervals: 299-850 mg/kg; McCollister et al., 1974). Additional factors that can potentially contribute to the greater toxicity of OP compounds in pregnant than non-pregnant females include: (i) a reduced capacity to detoxify these chemicals during gestation (Weitman et al., 1983), and/or (ii) differences in sex hormone levels, given that the estrous cycle and estradiol have been shown to modulate the acute toxicity of the nerve agent sarin and the insecticide parathion, respectively, in rats (Agarwal et al., 1982; Smith et al., 2015).
Although some cases of acute OP poisoning during pregnancy have been successfully managed with conventional antidotes, others have not (Table 1). In addition, limited reports of clinical follow-up of mothers and children who survive an acute OP exposure make it difficult to conclude what impact, if any, conventional antidotes have in preventing neurological complications in children born to mothers intoxicated with OP compounds, insecticides and nerve agents alike, during pregnancy. Complications ranging from recurrent seizures to cognitive deficits and cerebral edema have been observed among children who survived prenatal intoxication with the OP insecticide diazinon (Dahlgren et al., 2004) or the OP nerve agent sarin (Hakeem and Jabri, 2015).
Table 1.
Health outcomes of gestational acute OP poisoning
| OP Compound | # of Cases |
Time of Exposure |
Treatments | Outcomes | References |
|---|---|---|---|---|---|
| Chlorpyrifos | 1 | Not reported | Atropine, 2-PAM | Fetal died before treatment initiation; mother survived | Indu et al., 2016 |
| Chlorpyrifos | 1 | GW 19 | Atropine, 2-PAM | Fetus died 2 h post-exposure, before treatment initiation; mother survived | Sebe et al., 2005 |
| Chlorpyrifos | 1 | GW 29 | Atropine | Maternal and fetal death | Solomon and Moodley, 2007 |
| Diazinon | 1 | GW 21 | None reported | Mother and infant survived. In follow-up that lasted 3 years, child presented seizures and severe cognitive deficits | Dahlgren et al., 2004 |
| Diazinon | 1 | 50 h before delivery | Atropine, 2-PAM | Mother died; infant born with clinical signs of OP poisoning, including bradycardia, continued to be treated with atropine and discharged 12 days later | Jajoo et al., 2010 |
| Diazinon | 1 | GW 26 | Atropine, 2-PAM | Mother recovered in 7 days; infant born 12 weeks later | Kamha et al., 2005 |
| Diazinon | 1 | Close to term | Atropine | Spontaneous labor 28 h after admission; 2.6-kg male infant with normal heart rate, mydriasis and no reaction to light; recovery in 3 days | Shah et al., 1995 |
| Dichlorvos | 2 | GW 39 GW 22 |
Atropine, penehyclidine, 2-PAM Penehyclidine, 2-PAM |
Stillborn child (3.2 kg) 28 h post-admission; mother survived Live premature infant (320 g) 12 h post-admission, died 1 h post-delivery; mother survived |
Sun et al., 2015 |
| Fenthion | 1 | GW 16 | Atropine | Mother recovered; normal delivery | Karalliedde et al., 1988 |
| Methamidophos | 1 | GW 36 | Atropine | Mother recovered; normal delivery | Karalliedde et al., 1988 |
| Oxydemeton-methyl | 1 | GW 4 | Atropine | Infant died at 14 days of age with malformations; mother recovered | Romero et al., 1989 |
| Sarin | 110 | Various | Not reported | Increased miscarriages (45% in exposed vs. 14% in non-exposed), stillbirths (2.7% in exposed vs. 1.2% in the general population), birth defects (6.4% in exposed vs. 3.0% in general population) some leading to perinatal deaths | Hakeem and Jabri, 2015 |
| UnknownA | 21 | GW 8 to 36 | Atropine | 2 mothers and their fetuses died 1 spontaneous abortion 3 patients not followed up 15 patients recovered (2 premature deliveries; 1 mild pre-eclampsia; 2 mild anemia) |
Adhikari et al., 2011 |
| UnknownA,B | 7 | GW 14 to term | Atropine (1) 2-PAM (1) Atropine, 2-PAM (1) No pharmacotreatment (4) |
Intrauterine fetal death Perinatal death Vaginal delivery Intrauterine fetal death (2); full-term (1) and premature (1) deliveries |
Barhoumi et al., 2016 |
| UnknownC | 1 | GW 34-35 | Atropine | A preterm child (1860 g) was delivered by C-section 11 h after admission of the mother. Infant was resuscitated and admitted to the intensity care unit. Mother and infant survived and were discharged after 1-month hospitalization | Weis et al., 1983 |
GW: Gestational weeks. Chlorpyrifos, diazinon, dichlorvos, fenthion, methamidophos, and oxydemeton-methyl are OP insecticides. Sarin is an OP nerve agent.
OP intoxication was confirmed based on: (i) available information of maternal ingestion of an OP insecticide (note, however, that the identity of the OP compound is not provided in these reports), and (ii) typical clinical signs of a cholinergic crisis, including salivation, lacrimation, vomiting, diarrhea, miosis, tachycardia or bradycardia, and/or respiratory failure, presented by the pregnant women.
Inhibition of maternal plasma cholinesterase activity correlated with the severity of intoxication.
OP intoxication was diagnosed based on clinical signs and near full inhibition (that lasted for weeks) of cholinesterase activities in serum and erythrocytes of both mother and infant. Maternal and fetal heart rates at the time of admission were 78 and 140 beats per minute, respectively. While the maternal heart rate was slightly below the normal range of 80 to 90 beats per minute for pregnant women, the fetal heart rate was within the range of 120 to 160 beats per minute considered to be normal (Pildner von Steinburg et al., 2013). Maternal tachycardia has been reported in other cases of gestational OP intoxication (Jajoo et al., 2010; Sun et al., 2015).
The mortality and miscarriages reported in cases of gestational OP poisoning treated with conventional antidotes may be due, at least in part, to the fact that those antidotes do not target mechanisms that are disrupted by the insecticides and are critical for healthy progression of pregnancy. For instance, atropine blocks mAChRs, but, at therapeutically relevant doses, it is unlikely to inhibit nAChRs. Yet, overactivation of both mAChRs and nAChRs during OP poisoning can contribute to: (i) increased myometrium contractility (Luckas et al., 1999; Nas et al., 2007), a well-known cause of premature labor and miscarriages (reviewed in Bygdeman and Swahn, 1990), (ii) an imbalance between a pro- and an anti-inflammatory environment in the placenta that can be detrimental to fetal growth (Paparini et al., 2015; also reviewed in Satyanarayana, 1986), and (iii) fetal neurotoxicity (reviewed in Mactutus, 1989; Slotkin et al., 1997).
Non-selective inhibition of all mAChR subtypes (M1-M5) by atropine may also be detrimental for the treatment of gestational acute OP intoxication, because presynaptic M2 autoreceptors provide an important negative feedback mechanism in which ACh inhibits its own release from cholinergic neurons (Kilbinger and Wessler, 1980; Slutsky et al., 2001). For instance, activation of presynaptic M2 receptors has been shown to suppress vagally induced bronchoconstriction and bronchorrhea (Bowerfind et al., 2002; Lee, 2001). Thus, during acute OP poisoning, the ability of a non-selective mAChR antagonist to suppress the bronchorrhea and bronchoconstriction caused by ACh-induced overactivation of M3 receptors in the airways may be limited by the inhibitory effect of the antagonist on presynaptic M2 receptors. Increased bronchial secretions and bronchoconstriction are main determinants of hypoxia, which, during pregnancy, has been associated with miscarriages, fetal growth retardation, and postnatal neurological complications (reviewed in Hsiao and Patterson, 2012; Hutter et al., 2010).
Cholinolytic drugs that show some degree of selectivity towards mAChRs other than the M2 receptors and inhibit nAChRs are promising candidates for treatment of acute OP intoxication during pregnancy. An example of such drug is (R,S)-trihexyphenidyl, which is approved for treatment of Parkinson’s disease and has been safely used to treat pregnant women suffering from dystonia (Gao et al., 2017; Robottom and Reich, 2011). (R,S)-trihexyphenidyl binds with high, intermediate, and low affinities to M1/M4, M3, and M2/M5 mAChRs, respectively (Dörje et al., 1991). It also inhibits M1 and M3 more selectively than M2 mAChRs (Giachetti et al., 1986; Richards, 1990).
The different muscarinic receptor subtypes are known to signal through different G-protein-coupled mechanisms (reviewed in Moran et al., 2019). Specifically, M1, M3, and M5 receptors couple with Gq to activate phospholipase C, which catalyzes the hydrolysis of phospholipids of membrane into inositol 1,4,5-trisphosphate and 1,2-diacylglycerol – second messengers that increase intracellular Ca2+ signaling. On the other hand, M2 and M4 receptors couple with Gi/o to inhibit adenylyl cyclase and suppress the activity of voltage-gated ion channels. While activation of M1, M3, and M5 receptors increases neuronal excitability, activation of M2 and M4 receptors reduces it (reviewed in Moran et al., 2019). The mean IC50s for (R,S)-trihexyphenidyl to block ACh-induced inositol monophosphate generation in hippocampal slices (M1 responses), ACh-induced contraction of left atrium (M2 responses), and ACh-induced contraction of ileum (M3 responses) are 9.77 nM, 123.03 nM, and 3.55 nM, respectively (Richards, 1990). By comparison, the mean IC50s for atropine to block the same M1-, M2-, and M3-mediated responses are 1.45 nM, 0.77 nM, and 2.95 nM, respectively (Richards, 1990). A more recent study using mice with null mutations of the genes that encode M1, M2, and M4 receptors supports the M2-sparing property of (R,S)-trihexyphenidyl (Joseph and Thomsen, 2017).
(R,S)-trihexyphenidyl also inhibits, via a use-dependent mechanism, as-of-yet unidentified subtypes of neuronal nAChRs (Gao et al., 1998; Strøm, 2006). This is particularly relevant in the context of acute OP poisoning, because increasing ACh levels, which can favor the use-dependent block of nAChRs by (R,S)-trihexyphenidyl, correlate with increased severity of clinical signs of acute toxicity (Shih and McDonough, 1997). Finally, in contrast to atropine, (R,S)-trihexyphenidyl crosses the blood brain barrier well (Ishizaki et al., 1998).
According to previous studies, (R,S)-trihexyphenidyl is more potent and more effective than atropine in halting seizures induced by OP nerve agents in adult male guinea pigs (McDonough et al., 2000; Shih et al., 2003). For instance, when administered to male guinea pigs 5 min after onset of seizures induced by the nerve agent soman, sarin, or VX, (R,S)-trihexyphenidyl is more potent than atropine in terminating seizure activity (McDonough et al., 2000; Shih et al., 2003). In addition, when delivered 40 min post-seizure onset, (R,S)-trihexyphenidyl, but not atropine, suppresses soman-induced seizures (McDonough et al., 2000). The ability of (R,S)-trihexyphenidyl to not only inhibit M1/M3 receptors more selectively than M2 receptors (Richards, 1999) but also block neuronal nAChRs in addition to N-methyl-D-aspartate type of glutamate receptors (Strøm, 2006) may contribute to its greater potency and effectiveness to treat OP poisoning. It is important to note, however, that the safety and effectiveness of drugs used to treat males are not easily translated to pregnant females, in part because the developing fetus is exquisitely sensitive to the toxic effects of xenobiotics. Thus, research is still needed to optimize therapeutic interventions for treatment of acute OP poisoning during pregnancy.
In summary, as discussed in this section, acute OP poisoning during pregnancy is a major medical problem in part because of the differential sensitivity of the developing and the adult organisms to the acute toxicity of OP compounds. Clinical reports also suggest that treatment of gestational OP intoxication needs to be optimized. Specifically, while there are reports of cases of gestational OP poisoning that have responded to treatment with the non-selective mAChR antagonist atropine and/or the AChE reactivator 2-PAM, there are also reports of cases that have not (see Table 1 and references therein). The discovery of safe and effective medical countermeasures to treat acute OP poisoning during pregnancy will lend support to the initiative launched in 2006 by the World Health Organization to address the issue of acute OP insecticide intoxication, which continues to be very insidious in many low-to-middle income countries (McNab, 2006).
3. Gestational Exposures to Environmentally Relevant Levels of OP Insecticides and Increased Risks of Neurodevelopmental Disorders: A Universal Public Health Issue
Prenatal exposures to environmentally relevant OP insecticide levels, which are too low to cause marked AChE inhibition and trigger overt signs of acute intoxication, have been associated with increased risks of developmental disorders in children. As such, these exposures have become a major public health concern worldwide.
Using different biomarkers of exposure (see Table 2), epidemiological studies have traced significant associations between prenatal exposures to low levels of OP insecticides and: (i) reduced weight and length at birth (Jaacks et al., 2019; Perera et al., 2003; Rauch et al., 2012), (ii) abnormal reflexes in neonates (Engel et al., 2007; Young et al., 2005), (iii) cognitive and motor deficits at 5 months of age (Kongtip et al., 2017), (iv) working memory deficits and reduced full-scale IQ (FSIQ) between the ages of 6 and 9 (Furlong et al., 2006; Horton et al., 2012; Rauh et al., 2011; Rowe et al., 2016), (v) increased risk of attention deficits and hyperactivity between the ages of 2 and 10 (Bouchard et al., 2011; Eskenazi et al., 2007; Rauh et al., 2006; Schmidt et al., 2017; Shelton et al., 2014), and (vi) tremors at the age of 11 (Rauh et al., 2015). Most of the studies that included sex as a factor in the statistical analyses reported that the associations are generally stronger among boys than girls (e.g., Fortenberry et al., 2014; Marks et al., 2010; Rauh et al., 2015).
Table 2.
Epidemiological studies associating developmental exposures to OP insecticides and neurological deficits at different ages after birth.
| Cohort | Subjects Age at Time of Testing |
Biomarker of Exposure | Associated Significant Health Outcome | Reference |
|---|---|---|---|---|
| CHAMACOS cohort (California, U.S.) | Children at 7 years of age | Total DAP levels in urine sampled during pregnancy (~13 and 26 weeks of gestation) and from children at 6 months and 1, 2, 3.5, and 5 years of age |
|
Bouchard et al., 2011 |
| Mount Sinai Children's Environmental Health Cohort, New York | Infants assessed within 5 days of delivery | OP metabolites in maternal urine sampled at ~31 weeks of gestation: DMP, DMTP, DMDTP, DEP, DETP, DEDTP, MDA |
|
Engel et al., 2007 |
| CHAMACOS cohort (California, U.S.) | Children at 6, 12 and 24 months of age | OP metabolites in urine sampled during pregnancy (~13 and 26 weeks of gestation) and from children at time of test: DAPs (DMP, DMTP, DMDP, DEP, DETP, DEDP), MDA, TCPY |
|
Eskenazi et al., 2007 |
| CHAMACOS cohort (California, U.S.) | Children at 6-9 years of age | 6 DAPs in maternal urine sampled once between 25 and 40 weeks of gestation: DMP, DMTP, DMDP DEP, DETP, DEDP |
|
Furlong et al., 2017 |
| Columbia Center for Children•s Environmental Health (New York, U.S.) | Children at 7 years of age | Chlorpyrifos in umbilical cord blood |
|
Horton et al., 2012 |
| Prospective birth cohort from rural Bangladesh | Children at 1 and 2 years of age | OP metabolites in maternal urine sampled at ~11 weeks of gestation: TCPY, 4-nitrophenol, MDA, IMPY |
|
Jaacks et al., 2020 |
| Pregnant women from three hospitals in Thailand | Children at 5 months of age | DAPs in maternal urine sampled at ~28 weeks of gestation: DMP, DEP, DETP, DEDTP (Total DAPs = DMP + DEP + DETP + DEDTP; total DEPs = DEP + DETP + DEDTP) |
|
Kongtip et al., 2017 |
| Columbia Center for Children's Environmental Health (New York, U.S.) | Newborn children | Chlorpyrifos in plasma (maternal blood and umbilical cord blood at delivery) |
|
Perera et al., 2003 |
| MARBLES Cohort (California, U.S.) | Children at 3 years of age | OP metabolites in urine sampled in the 2nd and 3rd trimesters of gestation: DMP, DMTP, DMDP, DEP, DETP, DEDP, TCPY |
|
Philippat et al., 2018 |
| Health Outcomes and Measures of the Environment Cohort (Cincinnati, U.S.) | Newborn children | 6 DAPs in maternal urine sampled at ~16 and 26 weeks of gestation: DMP, DMTP, DMDTP, DEP, DETP, DEDTP |
|
Rauch et al., 2012 |
| Columbia Center for Children's Environmental Health (New York, U.S.) | Children at 1, 2, and 3 years of age | Chlorpyrifos in plasma (maternal blood and umbilical cord blood at delivery) |
|
Rauh et al., 2006 |
| Columbia Center for Children's Environmental Health (New York, U.S.) | Children at 7 years of age | Chlorpyrifos in umbilical cord blood |
|
Rauh et al., 2011 |
| Columbia Center for Children's Environmental Health (New York, U.S.) | Children at 6-11 years of age | Chlorpyrifos in plasma (umbilical cord blood at delivery) |
|
Rauh et al., 2012 |
| Columbia Center for Children's Environmental Health (New York, U.S.) | Children at 11 years of age | Chlorpyrifos in plasma (umbilical cord blood at delivery) |
|
Rauh et al., 2015 |
| CHAMACOS cohort (California, U.S.) | Children at 10 years of age | Residential proximity to commercial OP pesticide usage during pregnancy |
|
Rowe et al., 2016 |
| CHAMACOS cohort (California, U.S.) | Children at 7, 10, and 14 years of age | DAPs in urine sampled during pregnancy (~13 and 26 weeks of gestation): DMP, DMTP, DMDP, DEP, DETP, DEDP |
|
Sagiv et al., 2018 |
| CHAMACOS cohort (California, U.S.) | Adolescents at 15-16 years of age | Residential proximity to commercial use of OP during pregnancy |
|
Sagiv et al., 2019 |
| CHARGE cohort (California, U.S.) | Children at 2-5 years of age | Residential proximity to commercial use of OP during pregnancy and residential use of OP pesticides during pregnancy |
|
Schmidt et al., 2017 |
| CHARGE cohort (California, U.S.) | Children at 2-5 years of age | Residential proximity to commercial use of OP during pregnancy |
|
Shelton et al., 2014 |
| CHAMACOS cohort (California, U.S.) | Infants assessed at >3 days and <2 months of age | 6 DAPs in urine maternal urine sampled at ~13 and 26 weeks gestation and 7 days postpartum: DMP, DMTP, DMDTP, DEP, DETP, DEDTP |
|
Young et al., 2005 |
DAPs, dialkylphosphates; DMP, dimethylphosphate; DMTP, dimethyl thiophosphate; DMDTP, dimethyldithiophosphate; DEP, diethylphosphate; DETP, diethylthiophosphate; DEDTP, diethyldithiophosphate; IMPY, 2-isopropyl-4-methyl-6-hydroxypyrimidine; MDA, malathion diacarboxylic acid; TCPY, 3,5,6- Trichloro-2-pyridinol. MDA and TCPY are malathion- and chlorpyrifos-specific metabolites. IMPY is a specific metabolite of diazinon. 4-nitrophenol is a specific metabolite of parathion and methyl parathion. DAPs are non-specific OP metabolites. For instance, DMPs can be generated from malathion, chlorpyrifos methyl, and phosmet. DEP and DETP can be generated from chlorpyrifos, parathion, diazinon.
Studies of children from a birth cohort living in the agricultural Salinas Valley in California, referred to as the of Center for Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort, have also examined the relationship between prenatal OP exposures and risks of pervasive mental conditions, including autism spectrum disorders (ASD) (Table 2). In 2-year-old children (Eskenazi et al., 2007) and 14-year-old adolescents (Sagiv et al., 2018), maternal urine concentrations of the nonspecific OP metabolites dialkylphosphates (DAPs) during gestation were found to be directly associated with increased odds of ASD-related traits, reported in rating questionnaires completed by parents and/or teachers.
Studies of children from a different cohort – the Childhood Autism Risks from Genetics and Environment (CHARGE) cohort – also revealed that pregnant mothers living near sites of OP insecticide usage were at increased risk of having 2-5-year-old children with clinically confirmed ASD (Table 2) (Schmidt et al., 2017; Shelton et al., 2014). When stratified by timing of exposure, the risk for clinically diagnosed ASD was highest among children born from mothers likely to have been exposed to OP insecticides during the third pregnant trimester and more specifically to chlorpyrifos during the second pregnancy trimester (Shelton et al., 2014). It is noteworthy, however, that Sagiv et al. (2018) found no significant associations between residential proximity of pregnant women to OP use sites and parent-reported ASD-related traits in children from the CHAMACOS cohort. It has been proposed that the discrepancies between the two cohorts could be due to the distinct outcomes assessed (ASD traits reported by parents in standardized scales vs. clinically diagnosed ASD) and/or the distinct characteristics of the populations, with the CHAMACOS cohort having an average higher exposure to OP insecticides than the CHARGE cohort.
Associations between prenatal OP exposures and increased risk for ASD are not unique to the CHAMACOS and CHARGE cohorts. A study of a different cohort, the Markers of Autism Risk in Babies - Learning Early Signs (MARBLES) cohort, also reported significant relationships between levels of OP metabolites measured in maternal urine sampled at multiple times during pregnancy and increased risk of clinically diagnosed ASD among 3-year-old children (Philippat et al., 2018).
An earlier magnetic resonance imaging study was the first to report significant associations between prenatal exposures to an OP insecticide and disruption of the structural integrity of the brain of children (Rauh et al., 2012). Specifically, in a cohort of 6-11-year-old children from low-income urban communities in New York City, umbilical blood cord levels of chlorpyrifos correlated with significant thinning of the frontal and parietal cortices and enlargement of the surface area of brain regions involved in processing attention (e.g., posterior temporal lobe), social recognition (e.g., superior frontal and temporal gyri), and reward (e.g., orbitofrontal areas) (Rauh et al., 2012). According to the same study, the normal direct correlations between FSIQ and the surface area of the different brain regions were either absent or reversed among children, particularly boys, who experienced the higher prenatal chlorpyrifos exposures, represented by umbilical cord blood levels ≥ 4.39 pg/g (Rauh et al., 2012).
More recently, functional near-infrared spectroscopy (fNIRS) was used to examine the relationship between prenatal exposure of adolescents to OP insecticides and activation of specific cerebral cortical regions during tasks of executive function, attention, social cognition, and language comprehension (Sagiv et al., 2019). In this study, the prenatal OP exposure of adolescents was estimated based on the proximity of the residence of their mothers during pregnancy to agricultural sites in California’s Salinas Valley where OP insecticides were used. Prenatal proximity to agricultural sites employing OP insecticides was associated with: (i) reduced activation in the inferior frontal poles of the prefrontal cortex and other regions of the frontal, temporal, and parietal lobes during complex tests of cognitive flexibility and visuospatial working memory, and (ii) increased activation in the left parietal lobule and right temporal/parietal regions during a straightforward test of letter-retrieval working memory (Sagiv et al., 2019). The authors suggested that reduced cortical activation could be due to the prenatal OP exposures leading to development of dysfunctional synaptic connections that are hyporesponsive to the demands of complex cognitive tasks. On the other hand, they interpreted the increased activation of different cortical regions during simple cognitive tasks as a compensatory mechanism to overcome impairments in task-related networks. Such compensatory mechanisms have been detected in young adults overcoming fatigue-induced impairment of cognitive functions (Wang et al., 2016). Sagiv et al. (2019) reported that their findings must be considered with caution because of the small sample size, multiple comparisons, and lack of methods to properly account for innate differences in test performance in a cohort whose performance can be affected by the environmental OP exposures. Nevertheless, based on the patterns of associations between prenatal OP exposures and hyper- or hypoactivation in cerebral cortical regions of adolescents during different cognitive tasks, fNIRS emerges as a cost-effective, non-invasive, and portable tool for continued epidemiological evaluation poised to identify associations between prenatal OP exposures and dysfunctions of specific cortical systems responding to well-defined behavioral tasks.
Despite the ever-growing body of evidence supporting that early life exposures to environmentally relevant levels of OP insecticides are associated with increased risks of neurodevelopmental disorders in children, there are still arguments that these associations could be spurious (Burns et al., 2013; Eaton et al., 2008; Reiss et al., 2012). The skepticism arises in part because epidemiological and clinical studies have not unambiguously established a cause-effect relationship between prenatal OP exposures and increased risk of neurodevelopmental disorders in children. In addition, due to the lack of ideal biomarkers of exposure, these studies have relied on surrogate measures of exposure (Table 2) that are known to have limitations. For instance, residential proximity to OP use sites does not provide information regarding the types of insecticides a resident is exposed to or the timing and duration of the exposure. Umbilical cord levels of OP insecticides provide a snapshot of a recent exposure and are not informative of the total burden of exposure. Likewise, because DAP, diethylphosphate, diethylthiophosphate, and/or dimethylphosphate metabolites of OP insecticides and the parent compounds do not accumulate in the body (Garfitt et al., 2002; Timchalk, 2002), measuring their levels in urine only a few times during pregnancy or at birth can largely underestimate the burden of exposure. In addition, most of those OP metabolites are non-specific, as they do not identify the OP insecticide to which individuals are exposed. Finally, some OP insecticides can be abiotically converted to DAP metabolites, making these metabolites less-than-ideal markers of true exposure to the parent OP compounds (Zhang et al., 2008).
Several lines of evidence, however, make it difficult to ignore the notion that gestational exposures to OP insecticides pose health risks to children. First, based on systematic reviews of the literature (González-Alzaga et al., 2014; Muñoz-Quezada et al., 2013; Sapbamrer and Hongsibsong, 2019) and the works reviewed here, associations between prenatal exposures to OP insecticides and poor health outcomes at birth, childhood, and/or adolescence have been reported by independent research groups working with different population cohorts, biomarkers of exposures, and diagnostic tools. Second, soon after the 2001 government-mandated ban of residential use of chlorpyrifos in the U.S., umbilical cord blood concentrations of this insecticide in a cohort of urban minorities in New York City dropped to levels that no longer associated with reduced weight or length at birth (Whyatt et al., 2005). Third, in that cohort, infants born after the ban had significantly better Mental Development Index and Psychomotor Development Index scores than those born before the ban (Rauh et al., 2006). Fourth, in a cohort study, urine DAP levels were found to be higher among pregnant women with low activity of blood paraoxonase 1 (PON1), an enzyme that detoxifies OP insecticides, than among those with high enzyme activity (Naksen et al., 2015). This and other studies also reported that low maternal PON1 activity strengthens the associations between maternal urine DAP levels and poor neonatal outcomes (Marsillach et al., 2016). Thus, although DAPs are not ideal biomarkers of exposure because they can be generated abiotically in the environment, urinary DAP levels can correctly indicate exposure to OP insecticides.
In summary, epidemiological studies have provided evidence that prenatal exposures to environmentally relevant levels of OP insecticides, which are generally below the levels needed to cause pronounced AChE inhibition and trigger clinical signs of acute toxicity, are associated with disrupted functional and structural integrity of the brain of children. However, these studies are not poised to establish cause-effect relationships between prenatal OP exposures and neurobehavioral deficits or neuroanatomical alterations later in life. It is in this context that preclinical studies have become essential to demonstrate the developmental neurotoxicity induced by low levels of OP insecticides.
4. Preclinical Evidence of the Developmental Neurotoxicity of OP Insecticides
As extensively discussed in recent reviews (Abreu-Villaça and Levin, 2017; Burke et al., 2017), preclinical studies from independent research groups have provided evidence to support the notion that OP insecticides are developmental neurotoxicants. Specifically, following pre- and/or perinatal exposures to low levels of these insecticides, rodents exhibit neurobehavioral deficits, including learning and memory impairments, that are accompanied by neurochemical alterations in different brain regions. However, as outlined below, effects resulting from developmental exposures to OP insecticides vary with the timing of exposure, are often sexually dimorphic, and differ among compounds (Tables 3 and 4).
Table 3.
Neurobehavioral effects of developmental exposures of rodents to OP insecticides.
| OP Insecticide |
Species/S train |
Dose Regimen | Vehicle | Time of Exposure |
Starting Testing Age |
Behavioral Test | Measured Outcomes (Affected sex) |
Reference |
|---|---|---|---|---|---|---|---|---|
| Chlorpyrifos | SD Rats | 5 mg/kg/day, s.c. | DMSO | GD9-12 | PND4 |
|
|
Icenogle et al., 2004 |
| Chlorpyrifos | ND4 Mice | 1 or 5 mg/kg/day, s.c. | DMSO | GD17- 20 | PND60 |
|
|
Haviland et al., 2010 |
| Chlorpyrifos | SD Rats | 1 mg/kg/day, s.c. | DMSO | GD17-20 | Adolescence, Adulthood | · 16-arm radial maze |
|
Levin et al., 2002 |
| Chlorpyrifos | SD Rats | 1 mg/kg/day, s.c. | DMSO | PND1-4 | PND52 |
|
|
Aldridge et al., 2005 |
| Chlorpyrifos | SD Rats | 1 mg/kg/day, s.c. | DMSO | PND1-4 | PND60 |
|
|
Levin et al., 2001 |
| Chlorpyrifos | Guinea Pigs | 20 mg/kg/day, s.c. | Peanut Oil | GD52-62 | PND40 |
|
|
Mamczarz et al., 2016 |
| Chlorpyrifos | Guinea Pigs | 20 mg/kg/day, s.c. | Peanut Oil | GD52-62 | PND40 |
|
|
Mullins et al., 2015 |
| Chlorpyrifos | C57Bl6/J mice | 2.5 or 5.0 mg/kg/day, oral gavage | Corn Oil | GD12-15 | PND6-12A PND90B |
|
Lan et al., 2017 | |
| Chlorpyrifos | SD Rats | 0.3, 1, or 5 mg/kg/day, oral gavage | Corn Oil | GD6-PND10 | PND22-24 |
|
|
(Maurissen et al., 2000) |
| CD1 Mice | 1 or 3 mg/kg/day, s.c. | DMSO | PND1-4 PND11-14 | PND35 |
|
|
(Ricceri et al., 2003) | |
| CD1 Mice | 3 mg/kg/day, s.c. | Peanut Oil | PND 11-14 | PND1A PND2B PND4C |
|
|
(Venerosi et al., 2008) | |
| CD1 Mice | 1 or 3 mg/kg/day, s.c. | Peanut Oil | PND11-14 | PND35 PND60 PND90 |
|
|
(Ricceri et al., 2006) | |
| SD Rats | 1 mg/kg/day, s.c.A 5 mg/kg/day, s.c.B |
DMSO | PND1-4A PND11-14B |
PND30 |
|
|
(Dam et al., 2000) | |
| Diazinon | SD Rats | 0.5 mg/kg/day, s.c. | DMSO | PND11-14 | PND30 |
|
|
Timofeeva et al., 2008a |
| Diazinon | SD Rats | 0.5 or 2 mg/kg/day, s.c. | DMSO | PND1-14 | PND52 |
|
|
Roegge et al., 2008 |
| Parathion | SD Rats | 0.1 or 0.2 mg/kg/day, s.c. | DMSO | PND1-4 | PND30 |
|
|
Timofeeva et al., 2008a |
| Parathion | SD Rats | 0.1 or 0.2 mg/kg/day, s.c. | DMSO | PND 1-4 | PND50A PND81B PND112C |
|
|
Timofeeva et al., 2008b |
DMSO, dimethyl sulfoxide; GD, gestation day; ND4 mice are outbred mice descended from the Swiss Webster stock rederived by the University of Notre Dame, Indiana; PND, postnatal day; SD, Sprague-Dawley. The letters M and F in parentheses stand for male and female, respectively. The superscripted capital letters in each row are intended to indicate when a behavioral test was conducted at a specific testing age or when the time of exposure resulted in a specific measured outcome.
indicates behavioral phenotypes observed in rodent models of autism-related disorders.
Table 4.
Neurochemical effects of developmental exposures of rats to OP insecticides.
| OP Insecticide |
Dose Regimen | Time of Exposure |
Testing Age | Effects (Sex Affected) | Reference |
|---|---|---|---|---|---|
| Chlorpyrifos | 1 mg/kg/day, s.c. | PND1-4 | PND60 |
|
Aldridge et al., 2004 |
| 5 mg/kg/day, s.c. | GD17-20 | GD21 |
|
Garcia et al., 2005 | |
| 20, 40 mg/kg/day, s.c. | GD17-20 | GD21 |
|
Garcia et al., 2002 | |
| 5, 20, 40 mg/kg/day, s.c. | GD17-20 | GD21 |
|
Garcia et al., 2003 | |
| 5 mg/kg/day, s.c. | GD17-20 | PND30 |
|
||
| 5 mg/kg/day, s.c. | PND11-14 | PND15, PND20 |
|
||
| 5 mg/kg/day, s.c. | PND11-14 | PND30 |
|
Garcia et al., 2002, 2003 | |
| 1 mg/kg/day, s.c. | PND1-4 | PND5 |
|
Slotkin and Seidler, 2007 | |
| 1 mg/kg/day, s.c. | PND1-4 | PND5, PND10, PND30 |
|
Garcia et al., 2002 | |
| Diazinon | 2 mg/kg/day, s.c. | PND1-4 | PND5 |
|
Slotkin and Seidler, 2007 |
| Diazinon | 0.5 mg/kg/day, s.c. | PND1-4 | PND30, PND60, PND100 |
|
Slotkin et al., 2008 |
| Parathion | 0.1, 0.2 mg/kg/day, s.c. | PND1-4 | PND30, PND60 |
|
Slotkin et al., 2009 |
GAP-43, growth associated protein 43; GD, gestation day; GFAP, glial fibrillary acidic protein; MBP, myelin-binding protein; mobp, gene encoding myelin associated oligodendrocyte basic protein; mpz, gene encoding myelin protein zero; myef2, gene encoding myelinexpressionfactor-2, myt, gene encoding myelin transcription factor 1; NF68, neurofilament 68; NF200, neurofilament 200; PND, postnatal day; 5HT1A, serotonin type 1A receptor; 5HTT, serotonin transporter. The letters M and F in parentheses stand for male and female, respectively. All studies were carried out in Sprague-Dawley rats.
Key events underlying brain development, including cell proliferation, migration, and differentiation into neurons and glia, in addition to synaptogenesis, myelination, and integration of neurons and non-neuronal cells into functional networks, are well conserved among different mammalian species; however, their timing is species specific (Reemst et al., 2016; Semple et al., 2013). Researchers have investigated the developmental neurotoxicity resulting from exposures to OP insecticides during three periods of fetal/perinatal brain development that have been identified as uniquely vulnerable to xenobiotics – neurulation, sexual differentiation of the brain, and brain growth spurt (Rice and Barone, 2000).
Neurulation, i.e. the generation of the neural tube, is an early-gestation event in precocial species (e.g. humans, non-human primates, and guinea pigs) and a mid-gestation event in altricial species (e.g. rats and mice). For instance, in guinea pigs (gestation term: 65 days), this event takes place during gestation days (GD) 13.5-16.5 (Monie, 1976). By contrast, in rats and mice (gestation term: 21 days), neurulation occurs during GD9-9.5 and GD10.5-11, respectively (Greene and Copp, 2009).
Sexual differentiation of the fetal brain in precocial species precedes the period of brain growth spurt, which is characterized by a major increase of the brain weight due to the rapid proliferation of astroglial and oligodendroglial cells, neurogenesis, synaptogenesis, and dendritic arborization (Dobbing and Sands, 1973; Guerri, 1998). By contrast, the two periods largely overlap in altricial species, beginning close to the time of birth and extending during the first 2-3 postnatal weeks. For example, in guinea pigs, the initial sexual differentiation of the brain takes place during GD30-37, whereas the brain growth spurt is a prenatal event that begins around GD 45 and ends before birth (Byrnes et al., 2003; Dobbing and Sands, 1970; MacLusky and Naftolin, 1981). On the other hand, in rats, the initial sexual differentiation of the fetal brain takes place during GD18-PND10, thereby overlapping the brain growth spurt period, which starts around GD20, peaks during PND7-10, and ends by the third postnatal week (Dobbing and Sands, 1979).
Preclinical studies have reported that spatial learning and memory deficits are typically observed at adolescence or young adult ages in rodents exposed pre- and/or perinatally to doses of chlorpyrifos that induce no clinical signs of acute toxicity and cause minimal to no inhibition of brain AChE activity (reviewed in Abreu-Villaça and Levin, 2017; Burke et al., 2017). However, the sexual dimorphism of the effects depends on the time of the developmental exposure to chlorpyrifos. For example, following prenatal exposure to chlorpyrifos (5 mg/kg/day, s.c.) during the neurulation period (GD 9 to GD12), adult rats present reference and working memory deficits in the 16-arm radial maze, with males and females being equally affected (Icenogle, 2004). Following prenatal exposure to chlorpyrifos (1 or 5 mg/kg/day, s.c.) during the start of the initial sexual differentiation of the brain (GD17-20), adult mice and rats also present reference and working memory deficits in a foraging maze; however, females are more affected than males (Haviland et al., 2010; Levin et al., 2002). On the other hand, when the exposure to chlorpyrifos takes place during PND1-4, a time of ongoing brain growth spurt and sexual differentiation of the brain, adult male rats present more pronounced working and reference memory deficits than adult female rats (Aldridge et al., 2005; Levin et al., 2001). Likewise, male-biased spatial learning and working memory deficits are apparent in prepubertal guinea pigs prenatally exposed to chlorpyrifos (25 mg/kg/day, s.c.) between GD52 and 62, a time during which the fetal brain growth spurt period is ongoing in this species (Mamczarz et al., 2016; Mullins et al., 2015).
The effects of developmental exposures to chlorpyrifos in mice and rats are not limited to cognitive deficits, and the timing of exposure appears to be an important determinant of the neurobehavioral outcomes. For instance, pregnant C57Bl6/J mice orally exposed to low doses of chlorpyrifos between GD12 and GD15 had offspring that presented delayed development of neonatal reflexes at early postnatal ages and autistic-like behaviors, including repetitive behaviors, reduced social interaction, and limited exploration of novel objects, at young adult ages (Lan et al., 2017). However, rats or mice presented no autistic-like behaviors after being exposed to chlorpyrifos during GD6-PND10, PND1-4, PND11-14, or for 3 days prenatally starting on or after GD13.5 (reviewed in Williams and DeSesso, 2014). The sensitivity of the developmental window of the brain to timed chemical insult is not limited to OP toxicants. For instance, a single dose of valproic acid (400 mg/kg, i.p.) delivered to pregnant mice at GD12.5 induces autistic-like behaviors in the offspring (Arndt et al., 2005; Kataoka et al., 2013). However, this same dose of valproic acid delivered at GD9 or GD14.5 induces neural tube defects (Shafique and Winn, 2020) or no behavioral outcomes (Kataoka et al., 2013), respectively.
The neurobehavioral deficits induced by developmental exposures of rodents to OP insecticides are not always the same for different compounds. For instance, targeting the brain growth spurt in rats with doses of diazinon or parathion that trigger no clinical signs of acute toxicity and cause ≤25% inhibition of brain AChE activity results in neurobehavioral outcomes that differ from those induced by chlorpyrifos. Specifically, young adult rats exposed during PND1-4 to chlorpyrifos (1 mg/kg/day, s.c.) present both working and reference memory deficits in the radial arm maze (Levin et al., 2001). By contrast, young adult rats exposed during PND1-4 to diazinon (0.5 mg/kg/day, s.c.) present only working memory deficits and those exposed during the same neonatal ages to parathion (0.1 or 0.2 mg/kg, s.c.) exhibit neither reference nor working memory impairments (Timofeeva et al., 2008a, 2008b). In addition, while the working memory deficit resulting from the neonatal exposure to chlorpyrifos is sex dependent (Levin et al., 2001), that caused by the neonatal exposure to diazinon is not (Timofeeva et al., 2008a). As it will be discussed later, distinct mechanisms of action may explain the findings that behavioral deficits resulting from developmental exposures to different OP compounds are compound specific.
Neonatal exposures to chlorpyrifos, diazinon, and parathion also affect anxiety- and depression-related behaviors in a compound-specific manner. For example, compared to young adult rats developmentally exposed to vehicle, age-matched rats exposed neonatally to chlorpyrifos (1 mg/kg/day, s.c., PND1-4) present increased anxiety-related behavior in the elevated-plus-maze and anhedonia in a chocolate milk-preference task, with males being more affected than females (Aldridge et al., 2005). By contrast, in the same behavioral tests, both young adult male and female rats neonatally exposed to parathion (0.1 or 0.2 mg/kg/day, s.c., PND1-4) present increased anxiety-related behavior when compared to their control counterparts, but exhibit no anhedonia (Timofeeva et al., 2008b). Finally, young adult rats neonatally exposed to diazinon (0.5 mg/kg/day, s.c., PND1-4) exhibit suppressed anxiety-related behavior, with males being more affected than females, and anhedonia, with males and females being equally affected (Roegge et al., 2008).
The effects of neonatal exposures to chlorpyrifos, diazinon, and parathion on cognitive and non-cognitive behaviors are accompanied by compound-specific neurochemical alterations in the serotoninergic system in various regions of the adult rat brain. These findings are of major significance because serotoninergic pathways play a critical role in spatial navigation, working memory, reversal learning, decision-making, anxiety, and depression (Albert et al., 2019; Bacqué-Cazenave et al., 2020; Clark et al., 2004; Homberg, 2012). Thus, following the early neonatal exposures to chlorpyrifos referred to above, young adult rats present male-biased upregulation of cerebral cortical serotonin type 1A (5HT1A) receptors and female-biased downregulation of cerebral cortical 5HT transporters (5HTT) (Aldridge et al., 2004). By contrast, the early neonatal exposures to parathion induce sex-independent 5HT1A receptor upregulation in the frontal/parietal cortex and downregulation in the hippocampus at PND30 and PND60 (Slotkin et al., 2009), and the early neonatal exposures to diazinon induce male-biased downregulation of cerebral cortical 5HT1A receptors and female-biased upregulation of cerebral cortical 5HTT (Slotkin et al., 2008).
The neurobehavioral deficits and neurochemical alterations observed long after developmental exposures to OP insecticides may result from disruption of such processes as cellular proliferation and differentiation into neurons and glia, myelination, and synaptogenesis during the critical phases of brain development. In this regard, developmental exposures of rats to chlorpyrifos, parathion, and diazinon have been shown to alter the expression of protein markers associated with glia and neuronal differentiation in the brain.
Late gestational exposures of rats to chlorpyrifos (5 mg/kg/d, s.c., GD17-20) have been reported to upregulate the expression of the following proteins in the fetal forebrain and midbrain/brain stem at GD21: (i) myelin-binding protein (MBP, a marker of oligodendrocytes), (ii) neurofilament 68 (NF68, a neuronal marker), and (iii) glial fibrillary acidic protein (GFAP, a marker of astrocytes) (Garcia et al., 2005, 2003). At PND30, however, expression of MBP, NF68, and neurofilament 200 (NF200, a marker of developing axons) is downregulated in the forebrain and midbrain/brain stem of female but not male rats (Garcia et al., 2003). These findings would suggest that the late gestational exposure of rats to low levels of chlorpyrifos causes an immediate increase in differentiation of progenitor cells into astrocytes, oligodendrocytes, and neurons, which is followed by reduced numbers of these cells at adolescent ages. However, in the absence of an analysis of density of different cell types at various ages after the exposure, the possibility that chlorpyrifos dysregulates protein expression but has no effect on cell density cannot be ruled out.
Following a late postnatal (PND11-14) exposure of rats to chlorpyrifos, an initial downregulation of MBP and GFAP expression at PND15 is followed by a more pronounced downregulation of MBP in addition to downregulation of NF68 and NF200 and upregulation of GFAP at PND30, with the effects being observed among males but not females (Garcia et al., 2003). The sex dependence of the effects of the gestational and late neonatal chlorpyrifos exposures on the expression of glial, neuronal, and axonal markers in the brain parallels the sex dependence of the detrimental effects of those same exposures on neurobehavior in adult rats. Thus, dysregulated neuronal and glia differentiation and/or dysregulated expression of neuron- and glia-associated proteins may be related to the neurological deficits that emerge following exposures of the developing brain to chlorpyrifos.
The effects of developmental exposures to chlorpyrifos and diazinon on the expression of glial and neuronal markers are also compound specific. Following an early neonatal exposure chlorpyrifos (1 mg/kg/day, s.c., PND1-4), 5-day-old rats present in different brain regions: (i) no significant change in expression of NF68 or NF200, (ii) downregulation of GFAP expression, (iii) upregulation of the gene that encodes growth associated protein 43 (GAP-43), a protein expressed at high levels in axonal growth cones, and (iv) downregulation of the expression of the oligodendrocyte-specific genes mobp and mpz (Garcia et al., 2003, 2002; Slotkin and Seidler, 2007). By contrast, following neonatal exposure of rats to diazinon (1 or 2 mg/kg/day, s.c., PND1-4), expression of the oligodendrocyte-specific genes myef and myt is strongly upregulated at PND5 (Slotkin and Seidler, 2007).
In summary, data reviewed in this section support the notion that developmental exposures to low levels of different OP insecticides are causally related to neurobehavioral deficits and neurochemical alterations in the brain later in life. The findings that neurodevelopmental effects of chlorpyrifos, diazinon, and parathion are compound specific strongly suggest that, as developmental neurotoxicants, OP insecticides cannot be grouped in a single class of chemicals that act via a shared mechanism of action. The next section provides an overview of AChE-unrelated mechanisms that may contribute to the developmental neurotoxicity of OP insecticides.
5. Potential Molecular Mechanisms Underlying the Developmental Neurotoxicity of OP Insecticides
In vitro studies have been instrumental in providing direct evidence demonstrating that AChE-unrelated mechanisms are important determinants of the neurotoxic effects of OP insecticides. For instance, 24-h exposure of primary cultures from the cerebral cortex of fetal mice (GD15-16) to 10 μM and 30-100 μM chlorpyrifos inhibits AChE activity by ~50% and 80-90%, respectively, and comparable exposure to 1-100 μM chlorpyrifos-oxon inhibits AChE activity by nearly 100% (Rush et al., 2010). However, 24-h exposure of the cultures to 100 μM chlorpyrifos reduces cell viability by 50%, and similar exposure to 10-100 μM chlorpyrifos-oxon reduces cell viability by no more than 10%. Similar results were obtained with diazinon and diazoxon. In addition, the mAChR antagonist atropine and the nAChR antagonist mecamylamine had no effect on the cytotoxic effects of chlorpyrifos and diazinon. Altogether these results revealed that AChE inhibition and cholinergic overactivation do not underlie the cytotoxic effects of these OP insecticides. In fact, numerous findings suggested that a common mechanism of action cannot account for the cytotoxic effects of diazinon and chlorpyrifos in the primary cortical cultures.
Chlorpyrifos-induced cytotoxicity in the primary cortical cultures was characterized by the typical non-specific DNA breakdown of necrotic cell death, accompanied by a robust increase in extracellular glutamate concentrations, suppressed by ionotropic glutamate receptor antagonists, and exacerbated by the caspase inhibitor Z-ValAla-Asp-fluoromethylketone (ZVAD) (Rush et al., 2010). On the other hand, diazinon-induced cytotoxicity was characterized by the typical DNA fragmentation of apoptosis, was not accompanied by significant changes in glutamate concentrations, was unaffected by ionotropic glutamate receptor antagonists, and was suppressed by the caspase inhibitor ZVAD (Rush et al., 2010). Thus, while in this system chlorpyrifos-induced cytotoxicity could be accounted primarily for by glutamate-mediated excitotoxicity, diazinon-induced cytotoxicity was likely a result of apoptosis (Rush et al., 2010). An earlier study also reported that exposure of PC12 cells to chlorpyrifos induces a more robust and longer-lasting increase in ionotropic glutamate receptor gene expression than does exposure to diazinon (Slotkin and Seidler, 2009). It is, however, unknown whether increased cell death is part of the pathway of the developmental neurotoxicity triggered by environmentally relevant levels of these insecticides.
At concentrations that induce no change in cell viability and have no significant effect on the catalytic activity of AChE, diazinon and its oxon metabolite can inhibit neurite outgrowth in primary hippocampal cultures (Pizzurro et al., 2014) and in cultures of neuroblastoma N2a cells (Flaskos et al., 2007). In N2a cell cultures, diazoxon-induced inhibition of neurite outgrowth is accompanied by decreased expression (or integrity) of GAP-43 and reduced phosphorylation of the neurofilament heavy chain (Sidiropoulou et al., 2009). It has been hypothesized that oxidative stress contributes to diazinon- and diazoxon-induced inhibition of neurite extension because: (i) inhibition of neurite extension could be prevented by antioxidants, and (ii) both diazinon and diazoxon had been shown to increase the production of reactive oxidative species (Giordano et al., 2007; Pizzurro et al., 2014).
Similar to diazinon and diazoxon, chlorpyrifos and chlorpyrifos-oxon inhibit neurite outgrowth in N2a cell cultures (Sachana et al., 2005) and in PC12 cell cultures (Das and Barone, 1999). In N2a cell cultures, suppression of neurite extension by chlorpyrifos and its oxon metabolite is also accompanied by reduced expression (or integrity) of GAP-43 and α-tubulin (Sachana et al., 2005). In contrast to diazoxon, however, chlorpyrifos-oxon does not affect phosphorylation of the neurofilament heavy chain in N2a cell cultures (Flaskos et al., 2011), a finding that suggests distinct mechanisms are likely to underlie the effects of diazoxon and chlorpyrifos-oxon on neurite outgrowth.
It has been reported that, axonal growth in primary cultures of superior cervical ganglion neurons is significantly inhibited by low nanomolar concentrations of chlorpyrifos or chlorpyrifos-oxon, which are devoid of significant effect on the catalytic activity of AChE (Howard et al., 2005). Experiments carried out in primary cultures of rat dorsal ganglion neurons from mice with a null mutation in the AChE-encoding gene indicated that the morphogenic, but not the catalytic property of AChE contributes to the inhibitory effects of chlorpyrifos on axonal growth (Yang et al., 2008). In addition, there is mounting evidence that chlorpyrifos and its oxon metabolite can interact with and disrupt the integrity of structural proteins that are essential not only for neurite outgrowth but also axonal transport.
Axonal transport, which is required for both establishment of specialized structures during neuronal development and maintenance of functional synaptic connections in the mature brain (Bury and Sabo, 2016), is reduced by some, but not all OP insecticides. For instance, fast anterograde axonal transport in a rat optic nerve preparation is reportedly insensitive to parathion (Reichert and Abou-Donia, 1980). By contrast, repeated exposure of adult rats to low doses of chlorpyrifos causes significant impairment of both fast anterograde and retrograde axonal transport of vesicles in sciatic nerves, with the effects lasting at least 14 days after the last dose (Terry et al., 2007, 2003). In primary cultures of the fetal rat cerebral cortex, axonal transport of membrane-bound organelles is also inhibited by concentrations of chlorpyrifos and chlorpyrifos-oxon that are devoid of effects on cell viability or AChE catalytic activity (Gao et al., 2017; Middlemore-Risher et al., 2011).
It has been proposed that inhibition of axonal transport by chlorpyrifos and its oxon metabolite, an effect found to be insensitive to mAChR or nAChR antagonists (Gao et al., 2017), can be due, at least in part, to the ability of these compounds to directly interact with and inhibit tubulin polymerization (Prendergast et al., 2007). The impaired anterograde axonal transport induced by chlorpyrifos and chlorpyrifos-oxon may also be a result of their direct interactions with kinesin (Gearhart et al., 2007). It remains to be determined whether inhibition of neurite outgrowth and disruption of axonal transport takes place in the developing brain exposed to environmentally relevant levels of these insecticides and contributes to the neurologic deficits that emerge later in life.
Earlier studies have reported that some OP insecticides and their oxon metabolites can modulate the activity of the endocannabinoid (eCB) system, which plays a critical role in several aspects of neural development, from neuroprogenitor cell proliferation to synaptogenesis. For instance, chlorpyrifos-oxon, paraoxon, and diazoxon reportedly displace binding of the cannabinoid type 1 receptor (CB1R)-selective ligand [3H]CP 55,940 from mouse brain membranes with IC50s of 14 nM, 1.2 μM, and 2 μM, respectively. Likewise, chlorpyrifos, parathion, and diazinon displace [3H]CP 55,940 binding with IC50s of 35 μM, 43 μM, and >100 μM, respectively (Quistad et al., 2002). In vitro and in vivo studies further revealed that chlorpyrifos, parathion, malaoxon, and diazinon inhibit fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), the enzymes that hydrolyze and inactivate the eCBs anandamide (AEA) and 2-arachidonylglycerol (2-AG), respectively (Alexander et al., 2016; Quistad et al., 2006, 2002, 2001).
Exposure of the developing mammalian brain to doses of chlorpyrifos that are too low to inhibit AChE results in significant inhibition of FAAH activity. Specifically, FAAH activity was found to be approximately 20% lower in the brain of 17-day-old rats orally exposed to chlorpyrifos (0.5 or 0.75 mg/kg/day) during PND10-16 than in the brain of vehicle-exposed, age-matched rats (Buntyn et al., 2017; Carr et al., 2014, 2020). By contrast, neither MAGL nor AChE activity in the brain was affected by the chlorpyrifos exposure (Buntyn et al., 2017; Carr et al., 2020, 2014). This is of major significance in the context of the developmental neurotoxicity of chlorpyrifos, and, possibly other OP insecticides, because activation of CB1Rs and CB2Rs is known to promote neuroprogenitor cell proliferation and affect neural differentiation (Aguado et al., 2005; Berghuis et al., 2007; Trazzi et al., 2010). While long-term exposures to CB1R agonists favor the differentiation of neuroprogenitor cells into mature neurons (Compagnucci et al., 2013; Jiang, 2005; Soltys et al., 2010), short-term exposures drive neuroprogenitor cell differentiation into glial cells (Soltys et al., 2010). At late stages of fetal brain development, axonal growth cones also express high levels of CB1Rs whose activation by eCBs restricts axonal elongation and guides postsynaptic target selection (Berghuis et al., 2007). Thus, by altering eCB signaling during development, OP insecticides can disrupt processes that shape the nervous system structurally and functionally from embryogenesis through adulthood.
A recent study from our laboratory tested the hypothesis that, at concentrations below the threshold for AChE inhibition, chlorpyrifos, acting via CB1R signaling, disrupts neurodifferentiation. This hypothesis was tested using the SHSY-5Y cell line, which is a subclone of the SK-SN-SH line originally isolated from a bone marrow biopsy of a 4-year-old female neuroblastoma patient and has the capacity to differentiate into mature neuron populations (Adem et al., 1987; Shipley et al., 2016; Tosetti et al., 1998). SHSY-5Y cells have been used as an in vitro model of OP-induced neurotoxicity (e.g., Raszewski et al., 2015), because they express: (i) cholinergic proteins, including AChE, nicotinic receptor subunits, muscarinic receptors, and the vesicular acetylcholine transporter (Kovalevich and Langford, 2013), and (ii) non-cholinergic targets of OP insecticides, including CB1Rs, FAAH, and MAGL (Marini et al., 2009; Pasquariello et al., 2009).
In SHSY-5Y cell extracts, chlorpyrifos concentration dependently inhibited the catalytic activity of AChE (Todd et al., 2018), with the lowest effective concentration (LoEC) and IC50 being 30 μM and 181 μM, respectively. Following 24-h exposure of SHSY-5Y cultures to chlorpyrifos (1 μM-1 mM dissolved in DMSO; final concentration of DMSO in medium containing 1% fetal bovine serum was 0.01%), there was also a concentration-dependent reduction of cell viability, which was assessed by means of the MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) assay. The LoEC and the IC50 for chlorpyrifos to reduce cell viability were 30 μM and 135 μM, respectively. Using the same assays, an earlier study reported that the IC50 for chlorpyrifos to suppress cell viability in SHSY-5Y cultures was 313 μM (Raszewski et al., 2015). The differences in the IC50s can be accounted for by the fact that in our study, cultures were incubated with chlorpyrifos in medium containing 1% serum to reduce non-specific binding of chlorpyrifos to proteins in serum, whereas, in the earlier study, chlorpyrifos was added to medium containing 15% serum (Raszewski et al., 2015).
To examine whether neurodifferentiation is altered by chlorpyrifos concentrations that affect neither cell viability nor AChE activity, SHSY-5Y cultures were incubated for 7 days with chlorpyrifos (0.3-3μM)-containing medium, and, on the 8th day, cells were: (i) harvested for Western blot analysis of expression of nestin, a marker of neuroprogenitor cells, and NeuN, a marker of post-mitotic neurons that is not expressed in actively proliferating cells (Gilyarov, 2008; Gusel’nikova and Korzhevskiy, 2015; Lucassen et al., 2010), or (ii) processed immunocytochemically for analysis of number of nestin- and NeuN-immunopositive cells.
Following the 7-day exposure of SHSY-5Y cells to 0.3 μM chlorpyrifos, there was a significant increase in nestin expression that was not accompanied by changes in NeuN expression. As the concentration of chlorpyrifos increased to 3 μM, expression of nestin was significantly downregulated, while expression of NeuN was significantly upregulated (Todd et al., 2018). While a similar 7-day exposure of SHSY-5Y cultures to the neutral CB1R antagonist AM4113 alone had no significant effect on NeuN or nestin expression, chlorpyrifos (3 μM) in the presence of AM4113 was devoid of effect on the expression of NeuN or nestin (Todd et al., 2018). Exposure of the cultures to chlorpyrifos (3 μM) also resulted in increased phosphorylation of p38 and extracellular signal-regulated kinases 1/2 (Todd et al., 2018), which are downstream signaling pathways known to be activated by CB1R agonists (reviewed in Howlett et al., 2010). These results suggest that: (i) chlorpyrifos does not act as a CB1R antagonist, and (ii) chlorpyrifos-induced effects on NeuN and nestin expression are, at least in part, mediated by CB1R signaling.
The immunocytochemical analysis suggested that the effects of chlorpyrifos on nestin and NeuN expression were due to changes in cellular differentiation. In SHSY-5Y cultures, four cellular phenotypes were identified as Nestin+NeuN−, Nestin−NeuN+, Nestin−NeuN+, and Nestin+NeuN+. Based on literature reports (Lucassen et al., 2010), Nestin+NeuN− and Nestin−NeuN+ were interpreted as neuroprogenitor cells and post-mitotic neurons, respectively. Although continuous exposure of the SHSY-5Y cultures to 3 μM chlorpyrifos did not alter total number of cells, it significantly reduced the % of Nestin+NeuN− cells and increased the % of Nestin−NeuN+ cells (Todd et al., 2018).
The findings discussed above lend support to the hypothesis that, in differentiating systems with a low degree of tonic eCB activity, chlorpyrifos may directly (via receptor binding and activation) or indirectly (via FAAH inhibition) increase CB1R activity in neuroprogenitor cells and, thereby, accelerate their differentiation into mature neurons (Figure 1). On the other hand, in differentiating systems with a high degree of tonic eCB activity maintained by 2-AG, OP insecticides that act as partial CB1R agonists or inhibit FAAH, and, thereby, increase endogenous levels of AEA, an eCB that acts as a partial CB1R agonist, may reduce CB1R signaling in neuroprogenitor cells and decelerate their differentiation into mature neurons. It remains to be demonstrated whether, by disrupting eCB signaling, chlorpyrifos and other OP insecticides can indeed accelerate or decelerate the normal process of neuronal differentiation in the developing brain and lead to the generation of dysfunctional synaptic connections, which have been proposed to contribute to the pathophysiology of neurodevelopmental disorders (Zoghbi and Bear, 2012).
Figure 1. Diagrammatic representation of a hypothetical mechanism underlying the developmental neurotoxicity of chlorpyrifos (CPF).
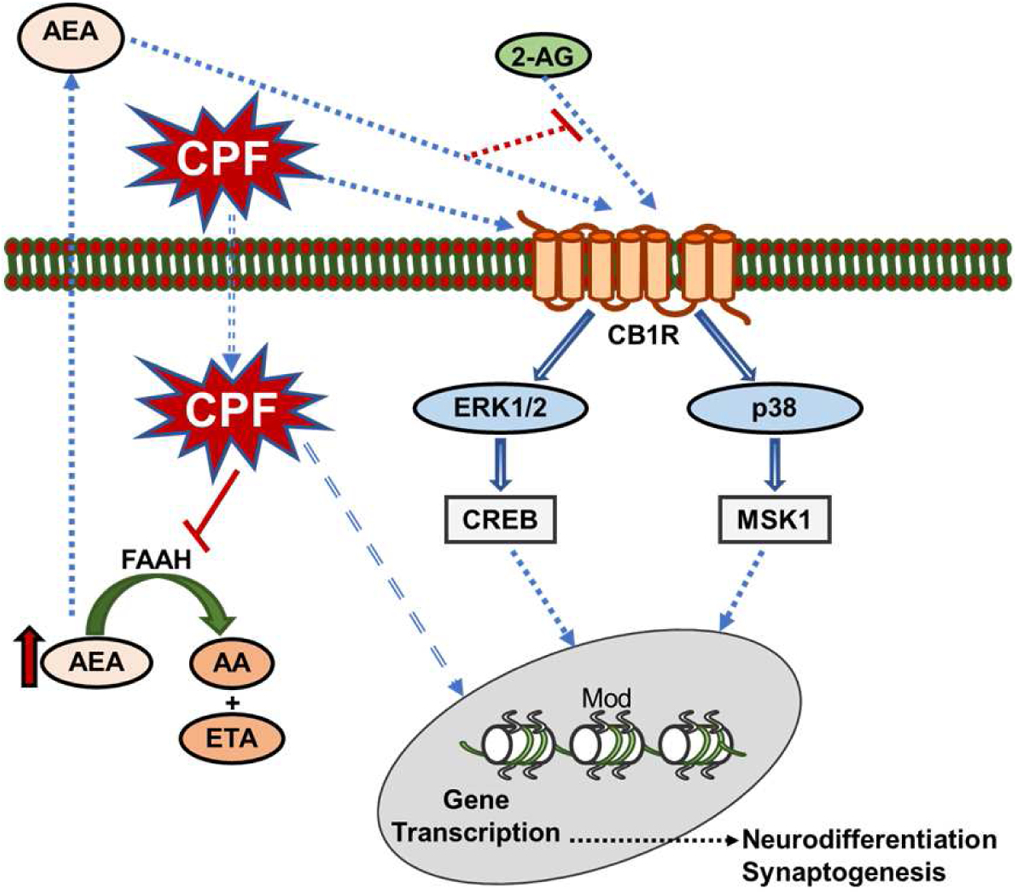
This diagram illustrates the hypothesis that, in systems with a low degree of tonic eCB signaling, CPF may directly (via receptor binding and activation) or indirectly (via FAAH inhibition) increase CB1R signaling in neuroprogenitor cells and, thereby, accelerate their differentiation into mature neurons. On the other hand, in systems with a high degree of tonic eCB activity maintained by 2-AG, chlorpyrifos, acting as a partial CB1R agonist or inhibiting FAAH, and, thereby, increasing endogenous levels of AEA, may reduce CB1R signaling in neuroprogenitor cells and decelerate their differentiation into mature neurons. Parts of images from Motifolio drawing toolkits (www.motifolio.com) were used in the figure preparation. (2-AG, 2-arachidonylglycerol; AEA, anandamide; AA, arachidonic acid; CB1R, cannabinoid type 1 receptor; CREB, cAMP response element-binding protein; ERK1/2, extracellular signal-regulated kinases 1/2; ETA, ethanolamine; FAAH, fatty acid amide hydrolase; MSK1, mitogen- and stress-activated protein kinase-1; mod, histone modifications).
The sexual dimorphism of the eCB system in the developing brain could contribute to the sex-biased effects of chlorpyrifos on neurodevelopment because the eCB system is sexually dimorphic not only in the mature but also in the developing brain (Fattore and Fratta, 2010). For instance, CB1R density in the forebrain is higher in 2-day-old female than male rats (de Fonseca et al., 1993), and FAAH expression in the amygdala is higher in female than male rats at 4 days of age (Krebs-Kraft et al., 2010). In part via stimulating microglia-induced phagocytosis of newborn astrocytes in neonatal rats, CB1R activation has been shown to shape the sexual differentiation of the developing amygdala and juvenile play behavior (Argue et al., 2017; VanRyzin et al., 2019).
Increasing evidence indicates that disruption of the epigenome by early-life insults, including environmental toxicants, can also affect the development of the nervous system and increase the risk for neurological disorders later in life (Balmer et al., 2014). This is relevant to the developmental neurotoxicity of OP insecticides because in vitro and in vivo studies have reported that some OP insecticides have significant effects on epigenetic markers, including covalent histone modifications and DNA methylation. For example, in one study, exposure of human neuronal progenitor cells to chlorpyrifos (57 μM, 12 h) increased levels of trimethylated histone 3 lysine 4 (H3K4) (Kim et al., 2016), which maintains the expression of pluripotency-associated genes during the neural differentiation of embryonic stem cells (Roidl and Hacker, 2014). In our recent study, the increased neurodifferentiation observed following a 7-day exposure of SHSY-5Y cells to 3 μM chlorpyrifos was accompanied by a p38-dependent increase in levels of dimethylated H3K4 (Todd et al., 2018). In another study, exposure of pregnant mice to chlorpyrifos-methyl (4, 20, 100 mg/kg/day, p.o.; GD7–12) resulted in hypomethylation of the h19 gene, which has been associated with intrauterine and postnatal growth retardation (Murphy et al., 2012), in different fetal organs (Shin et al., 2015). Finally, exposure of human myelogenous leukemia K562 cells to a low concentration of diazinon (0.1 μM, 12 h) induced hypermethylation of several genes, including that which encodes histone deacetylase 3 (HDAC3) (Zhang et al., 2013). In the developing brain, HDAC3 has been proposed to maintain the neural stem cell state, in part by retaining the nuclear localization of a transcriptional repressor known to prevent the differentiation of progenitor cells into neurons – the silencing mediator of retinoid and thyroid hormone receptors (Soriano and Hardingham, 2011; Yu et al., 2005).
Although it is unclear how OP insecticides alter histone modifications and DNA methylation, it is worth mentioning that these epigenetic mechanisms are also affected by exposure of the developing brain to cannabinoid ligands. For instance, increased levels of dimethylated histone 3 lysine 9 (H3K9) and decreased levels of trimethylated H3K4, which typically suppress gene expression, have been observed in the nucleus accumbens of 2-day-old rats exposed to tetrahydrocannabinol during GD5-PND2 (DiNieri et al., 2011). These changes in the epigenome were accompanied by reduced expression of the dopamine receptor D2 in the nucleus accumbens, which was proposed to contribute to increased addiction vulnerability later in life (DiNieri et al., 2011). Studies are needed to test the hypothesis that, via eCB-dependent and/or eCB-independent mechanisms, disruption of the epigenome in the developing brain contributes to the neurobehavioral deficits that develop later in life following developmental exposures to OP insecticides.
Post-translational histone modifications are also recognized as important mechanisms underlying sexual differentiation of the brain and the sex-dependent effects of environmental insults (Singh et al., 2019). In the brain, the sexual dimorphism of some covalent histone modifications, including acetylated H3K9, is established by the hormonal-dependent masculinization of the brain (Tsai et al., 2009). However, sex differences for other covalent histone modifications, including methylated H3K4, can be defined by sex chromosome-associated genes that encode the enzymes needed to catalyze these modifications (Singh et al., 2019). Of interest, developmental exposures of mice to lead acetate induce sex- and brain region-specific alterations in a number of covalent histone modifications, including acetylated H3K9 and methylated H3K4 and H3K9, some of which persist through adulthood (Schneider et al., 2016; Varma et al., 2017). Thus, sex-dependent effects of some OP insecticides on neurodevelopment could also be related to sex-biased alterations of histone modifications.
In summary, acting via AChE-unrelated mechanisms, different OP insecticides alter the neurochemistry and the structural integrity of neurons and glial cells. In this respect, it is important to note that some OP insecticides, including chlorpyrifos, diazinon, and malathion, have been shown to modify the activity of components of the eCB system, including CB1 receptors and FAAH, and to alter histone modifications. Research is needed to establish the role of these mechanisms on the developmental neurotoxicity of different OP insecticides.
6. Conclusions
Intentional, occupational, and accidental exposures to high doses of OP insecticides represent a major public health issue in countries where these insecticides are poorly regulated. Poisoning with OP insecticides is conventionally treated with the non-selective mAChR antagonist atropine to suppress the hypercholinergic tone resulting from OP-induced irreversible inhibition of AChE, in addition to an oxime to reactivate AChE and a benzodiazepine, as needed, to halt OP-induced seizures (reviewed in Pereira et al., 2014; Taylor, 2017). However, as discussed in this review, research is still needed to optimize the treatment of OP poisoning during pregnancy because clinical reports indicate that some cases of gestational acute OP intoxication respond to the conventional antidotes, while others do not (see Table 1 and references therein).
More concerning, however, are the ubiquitous exposures to environmentally relevant levels of OP insecticides, which are too low to trigger clinical signs of acute toxicity and are particularly detrimental to the normal development of the brain. Epidemiological studies continue to provide evidence that prenatal exposures to low levels of OP insecticides are associated with increased risks of intellectual disabilities and pervasive developmental disorders (see Table 2 and references therein). In addition, preclinical studies have demonstrated that prepubertal and adult rodents present neurobehavioral deficits and neurochemical alterations in the brain following exposures to different OP insecticides during well-defined periods of prenatal/perinatal development (see Tables 3 and 4 and references therein).
The finding that the developmental neurotoxicity of OP insecticides is compound specific has important implications. First, it can explain, at least in part, why the strength of associations between prenatal OP exposures and specific neurological deficits in children varies among epidemiological studies, given that, in most of these studies, exposures are assessed based on measurements of non-specific OP metabolites in urine or residential proximity to sites that use various OP insecticides. Second, it suggests that, as developmental neurotoxicants, different OP insecticides are likely to act via distinct mechanisms.
Several lines of evidence discussed here strongly support the concept that mechanisms other than the catalytic activity of AChE contribute to the developmental neurotoxicity of OP insecticides. Different OP insecticides have been shown to interact with and/or modify the activity of: (i) the M2 mAChR subtype (Howard and Pope, 2002; Huff et al., 1994), (ii) structural proteins such as tubulin and kinesin (Gearhart et al., 2007; Prendergast et al., 2007), and (iii) components of the eCB system, including CB1Rs, FAAH, and MAGL (Alexander et al., 2016; Quistad et al., 2006, 2002, 2001). Results from experiments carried out with the neuroblastoma cell line SHSY-5Y support a mechanistic hypothesis linking eCB signaling and developmental neurotoxicity induced by some OP insecticides (Todd et al., 2018). Prenatal/perinatal exposures to chlorpyrifos and other OP insecticides capable of disrupting eCB signaling may be accounted for by CB1R -dependent acceleration or deceleration of neurodifferentiation contributing to the development of dysfunctional synapses (Figure 1). It is also plausible that eCB-dependent and/or -independent disruption of the epigenome in the developing brain by different OP insecticides can disrupt the expression of genes associated with normal neurodevelopment and, thereby, have enduring detrimental effects on brain structure and function (Figure 1).
The ability of a given molecular target to contribute to the developmental neurotoxicity of different OP insecticides will depend on not only the expression and function of that target in the developing nervous system during the time of exposure, but also the concentration of the insecticides at the target and the potency with which they act at that target. The high lipophilicity of many OP insecticides, particularly diethyl phosphoryl insecticides such as chlorpyrifos, diazinon, parathion, and fenitrothion, can favor their distribution and accumulation in fat-enriched tissue, including the brain. For instance, the brain:blood partition coefficient for chlorpyrifos in rats ranges from 4 to 33, as estimated by the octanol:water partitioning of this insecticide and the brain lipid content, and is proportional to the blood lipid content; the higher the blood lipid levels are, the lower the portioning of chlorpyrifos between fat-enriched tissue and blood is (Ellison et al., 2011; Lowe et al., 2009; Timchalk, 2002). However, the degree to which chlorpyrifos and other OP insecticides accumulate in the brain and other fat-enriched tissues of fetuses remains largely unknown, making it difficult to validate physiologically based pharmacokinetic and pharmacodynamic (PB-PK/PD) models that have been developed to integrate target tissue concentrations and dynamic responses in subjects exposed to those insecticides.
It is also noteworthy that red blood cell AChE inhibition is the measure of effect used in the PB-PK/PD models generated to refine risk assessment of intoxication by OP insecticides at different ages and during pregnancy (Lowe et al., 2009; Poet et al., 2004; Timchalk, 2002; Smith et al., 2014). Yet, as demonstrated in the various works reviewed here, AChE inhibition does not appear to be a major determinant of the developmental neurotoxicity triggered by low levels of OP insecticides.
Research is critically needed to identify the molecular mechanisms by which OP insecticides trigger developmental neurotoxicity. However, as discussed in various reviews, this is a challenging task that will require the use a multidisciplinary in vitro-in vivo strategy (Crofton et al., 2011; Hollander et al., 2020; Smirnova et al., 2014), as in vitro testing can overcome the shortcomings of the excessively resource-intensive in vivo evaluation of developmental neurotoxicity. An in-vitro approach suitable to address the developmental neurotoxicity of OP insecticides will need to rely on: (i) the use of a model system that maintains relevance to the sex-biased effects of OP insecticides in the developing brain, and (ii) translation of the neurobehavioral deficits that emerge long after early life exposures to OP insecticides into cellular endpoints that can be quantified in vitro. Relevant in vitro model systems may include primary cultures of fetal brain tissue from rodents, cultures of human induced-pluripotent stem cells, as well as 3-D organoid cultures, each of which can be sex specific. In addition, cellular endpoints relevant to neurodevelopment may include proliferation and differentiation of neural stem cells and/or neuroprogenitor cells, neurite outgrowth, and synaptogenesis. Integrating structural, functional, and transcriptome analyses will be essential to identify molecular “signatures” underlying the developmental neurotoxicity of different OP insecticides. Such a mechanistic framework can, then, be used to design hypothesis-driven in vivo studies aimed at identifying effective interventions for disorders associated with prenatal exposures to low levels of OP insecticides.
Characterizing the mechanisms by which different OP insecticides disrupt normal brain development can also lead to identification of biomarkers of effect that are more specific and sensitive than AChE inhibition for assessment of the health risks posed by low-level exposures of pregnant women and children to these insecticides. These risk assessments are critically needed to support the implementation of risk management plans that seek to protect the most vulnerable sectors of the population against the detrimental health effects of OP insecticides and to encourage research and development of alternative pest-management strategies that are safe but also meet the increasing demands for food security and control of vector-borne diseases worldwide (Bonner and Alavanja, 2017; Hertz-Picciotto et al., 2018; Popp et al., 2013).
Highlights.
Irreversible AChE inhibition underlies the cholinergic crisis in acute OP poisoning
Treatment of acute OP insecticide poisoning during pregnancy is not optimized
Acute toxicity and AChE inhibition are not required for developmental OP neurotoxicity
Cannabinoid signaling can contribute to the developmental OP neurotoxicity
Acknowledgements
The authors are indebted to Ms. Mabel A. Zelle for her technical assistance. This work was funded in part by the National Institutes of Health through the National Institute of Environmental Health Sciences (Grant R01ES027822).
Abbreviations
- 2-AG
2-arachidonylglycerol
- ACh
acetylcholine
- AChE
acetylcholinesterase
- AEA
anandamide
- ASD
autism spectrum disorders
- CB1R
cannabinoid type 1 receptor
- CHAMACOS
Center for Health Assessment of Mothers and Children of Salinas
- CHARGE
Childhood Autism Risks from Genetics and Environment
- CPF
chlorpyrifos
- CREB
cAMP response element-binding protein
- DAP
dialkylphosphate
- eCB
endocannabinoid
- ERK1/2
extracellular signal-regulated kinases ½
- ETA
ethanolamine
- FAAH
fatty acid amide hydrolase
- fNIRS
functional near-infrared spectroscopy
- GAP-43
growth associated protein 43
- GD
gestation day
- GFAP
glial fibrillary acidic protein
- HDAC3
histone deacetylase 3
- H3K4
histone 3 lysine 4
- H3K9
histone 3 lysine 9
- IC50
half-maximal inhibitory concentration
- IQ
intelligence quotient
- LD50
median lethal dose
- LoEC
lowest effective concentration
- mAChR
muscarinic receptor
- MAGL
monoacylglycerol lipase
- MARBLES
Markers of Autism Risk in Babies - Learning Early Signs
- MBP
myelin-binding protein
- mobp
gene encoding myelin associated oligodendrocyte basic protein
- mpz
gene encoding myelin protein zero
- MSK1
mitogen- and stress-activated protein kinase-1
- MTT
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide
- myef2
gene encoding myelinexpressionfactor-2
- myt
gene encoding myelin transcription factor 1
- nAChR
nicotinic receptor
- NF200
neurofilament 200
- NF68
neurofilament 68
- OP
organophosphorus
- PB-PK/PD
physiologically based pharmacokinetic and pharmacodynamic
- PND
postnatal day
- PON1
paraoxonase 1
- 5HT
serotonin
- 5HT1A
serotonin type 1A receptor
- 5HTT
serotonin transporter
- ZVAD
Z-ValAla-Asp-fluoromethylketone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Rahman A, Blumenthal G, Abou-Donia S, Ali F, A. A-M, Abou-Donia M, 2002. Pharmacokinetic profile and placental transfer of a single intravenous injection of [14C]chlorpyrifos in pregnant rats. Arch. Toxicol 76, 452–459. 10.1007/s00204-002-0366-2 [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Levin ED, 2017. Developmental neurotoxicity of succeeding generations of insecticides. Environ. Int 99, 55–77. 10.1016/j.envint.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adem A, Mattsson MEK, Nordberg A, Påhlman S, 1987. Muscarinic receptors in human SH-SY5Y neuroblastoma cell line: regulation by phorbol ester and retinoic acid-induced differentiation. Dev. Brain Res 33, 235–242. 10.1016/0165-3806(87)90156-8 [DOI] [PubMed] [Google Scholar]
- Adhikari K, Ghosh A, Alauddin MD, Moitra A, Datta AK, 2011. Organophosphate poisoning in pregnancy. J Obstet Gynaecol 31, 290–292. 10.3109/01443615.2010.545901 [DOI] [PubMed] [Google Scholar]
- Agarwal DK, Misra D, Agarwal S, Seth PK, Kohli JD, 1982. Influence of sex hormones on parathion toxicity in rats: Antiacetylcholinesterase activity of parathion and paraoxon in plasma, erythrocytes, and brain. J. Toxicol. Environ. Health 9, 451–459. 10.1080/15287398209530177 [DOI] [PubMed] [Google Scholar]
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzmán M, Galve-Roperh I, 2005. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 19, 1704–1706. 10.1096/fj.05-3995fje [DOI] [PubMed] [Google Scholar]
- Akhtar N, Srivastava MK, Raizada RB, 2006. Transplacental disposition and teratogenic effects of chlorpyrifos in rats. J. Toxicol. Sci 31, 521–527. 10.2131/jts.31.521 [DOI] [PubMed] [Google Scholar]
- Albert PR, Le François B, Vahid-Ansari F, 2019. Genetic, epigenetic and posttranscriptional mechanisms for treatment of major depression: the 5-HT1A receptor gene as a paradigm. J. Psychiatry Neurosci 44, 164–176. 10.1503/jpn.180209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA, 2005. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ. Health Perspect 113, 527–531. 10.1289/ehp.7867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA, 2004. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ. Health Perspect 112, 148–155. 10.1289/ehp.6713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S, Carter W, Clark J, Lo T, Garle M, 2006. A novel fluorescence-based assay for FAAH activity, in: 16th Annual Symposium on the Cannabinoids, Burlington, Vermont, International Cannabinoid Research Society; p. 121 https://icrs.co/SYMPOSIUM.2006/2006.ICRS.Program.and.Abstracts.pdf [Google Scholar]
- Argue KJ, VanRyzin JW, Falvo DJ, Whitaker AR, Yu SJ, McCarthy MM, 2017. Activation of both CB1 and CB2 endocannabinoid receptors is critical for masculinization of the developing medial amygdala and juvenile social play behavior. eneuro 4, ENEURO.0344-16.2017. 10.1523/ENEURO.0344-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt TL, Stodgell CJ, Rodier PM, 2005. The teratology of autism. Int. J. Dev. Neurosci 23, 189–199. 10.1016/j.ijdevneu.2004.11.001 [DOI] [PubMed] [Google Scholar]
- Atwood D, Paisley-Jones C, 2017. Pesticides industry sales and usage. U.S. Environmental Protection Agency; https://www.epa.gov/sites/production/files/2017-01/documents/pesticides-industry-sales-usage-2016_0.pdf [Google Scholar]
- Bacquá-Cazenave J, Bharatiya R, Barrière G, Delbecque J-P, Bouguiyoud N, Di Giovanni G, Cattaert D, De Deurwaerdère P, 2020. Serotonin in animal cognition and behavior. Int. J. Mol. Sci 21, 1649 10.3390/ijms21051649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer NV, Klima S, Rempel E, Ivanova VN, Kolde R, Weng MK, Meganathan K, Henry M, Sachinidis A, Berthold MR, Hengstler JG, Rahnenführer J, Waldmann T, Leist M, 2014. From transient transcriptome responses to disturbed neurodevelopment: role of histone acetylation and methylation as epigenetic switch between reversible and irreversible drug effects. Arch. Toxicol 88, 1451–1468. 10.1007/s00204-014-1279-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhoumi MH, Bannour B, Barhoumi T, Jouini R, Marwene N, Fatnassi MR, 2016. Intoxications aigues aux organophosphores chez la femme enceinte. Pan Afr. Med. J 25 10.11604/pamj.2016.25.227.11041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T, 2007. Hardwiring the brain: Endocannabinoids shape neuronal connectivity. Science 316, 1212–1216. 10.1126/science.1137406 [DOI] [PubMed] [Google Scholar]
- Bonner MR, Alavanja MCR, 2017. Pesticides, human health, and food security. Food Energy Secur. 6, 89–93. 10.1002/fes3.112 [DOI] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B, 2011. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ. Health Perspect 119, 1189–1195. 10.1289/ehp.1003185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowerfind WML, Fryer AD, Jacoby DB, 2002. Double-stranded RNA causes airway hyperreactivity and neuronal M2 muscarinic receptor dysfunction. J. Appl. Physiol 92, 1417–1422. 10.1152/japplphysiol.00934.2001 [DOI] [PubMed] [Google Scholar]
- Buntyn RW, Alugubelly N, Hybart RL, Mohammed AN, Nail CA, Parker GC, Ross MK, Carr RL, 2017. Inhibition of endocannabinoid-metabolizing enzymes in peripheral tissues following developmental chlorpyrifos Exposure in Rats. Int. J. Toxicol 36, 395–402. 10.1177/1091581817725272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RD, Todd SW, Lumsden E, Mullins RJ, Mamczarz J, Fawcett WP, Gullapalli RP, Randall WR, Pereira EFR, Albuquerque EX, 2017. Developmental neurotoxicity of the organophosphorus insecticide chlorpyrifos: from clinical findings to preclinical models and potential mechanisms. J. Neurochem 142, 162–177. 10.1111/jnc.14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CJ, McIntosh LJ, Mink PJ, Jurek AM, Li AA, 2013. Pesticide exposure and neurodevelopmental outcomes: Review of the epidemiologic and animal studies. J. Toxicol. Environ. Health Part B 16, 127–283. 10.1080/10937404.2013.783383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury LAD, Sabo SL, 2016. Building a Terminal: Mechanisms of Presynaptic Development in the CNS. The Neuroscientist 22, 372–391. 10.1177/1073858415596131 [DOI] [PubMed] [Google Scholar]
- Bygdeman M, Swahn ML, 1990. Uterine contractility during pregnancy and the effect of abortifacient drugs. Baillières Clin. Obstet. Gynaecol 4, 249–261. 10.1016/S0950-3552(05)80225-1 [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Reynolds JN, Brien JF, 2003. Brain growth spurt-prenatal ethanol exposure and the guinea pig hippocampal glutamate signaling system. Neurotoxicol. Teratol 25, 303–310. 10.1016/S0892-0362(02)00354-9 [DOI] [PubMed] [Google Scholar]
- Carr RL, Alugubelly N, de Leon K, Loyant L, Mohammed AN, Patterson ME, Ross MK, Rowbotham NE, 2020. Inhibition of fatty acid amide hydrolase by chlorpyrifos in juvenile rats results in altered exploratory and social behavior as adolescents. NeuroToxicology 77, 127–136. 10.1016/j.neuro.2020.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Graves CA, Mangum LC, Nail CA, Ross MK, 2014. Low level chlorpyrifos exposure increases anandamide accumulation in juvenile rat brain in the absence of brain cholinesterase inhibition. NeuroToxicology 43, 82–89. 10.1016/j.neuro.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Durkin KA, 2013. Anticholinesterase insecticide retrospective. Chem. Biol. Interact 203, 221–225. 10.1016/j.cbi.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Cools R, Robbins TW, 2004. The neuropsychology of ventral prefrontal cortex: Decision-making and reversal learning. Brain Cogn. 55, 41–53. 10.1016/S0278-2626(03)00284-7 [DOI] [PubMed] [Google Scholar]
- Compagnucci C, Di Siena S, Bustamante MB, Di Giacomo D, Di Tommaso M, Maccarrone M, Grimaldi P, Sette C, 2013. Type-1 (CB1) cannabinoid receptor promotes neuronal differentiation and maturation of neural stem cells. PLoS ONE 8, e54271 10.1371/journal.pone.0054271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, 2018. Organophosphorus compounds at 80: Some old and new issues. Toxicol. Sci 162, 24–35. 10.1093/toxsci/kfx266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton KM, Mundy WR, Lein PJ, Bal-Price A, Coecke S, Seiler AEM, Knaut H, Buzanska L, Goldberg A, 2011. Developmental neurotoxicity testing: recommendations for developing alternative methods for the screening and prioritization of chemicals. ALTEX 28, 9–15. 10.14573/altex.2011.1.009 [DOI] [PubMed] [Google Scholar]
- Dahlgren JG, Takhar HS, Ruffalo CA, Zwass M, 2004. Health effects of diazinon on a family. J. Toxicol. Clin. Toxicol 42, 579–591. 10.1081/CLT-200026979 [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA, 2000. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Dev. Brain Res 121, 179–187. 10.1016/S0165-3806(00)00044-4 [DOI] [PubMed] [Google Scholar]
- Das KP, Barone S, 1999. Neuronal differentiation in PC12 cells is inhibited by chlorpyrifos and its metabolites: Is acetylcholinesterase inhibition the site of action? Toxicol. Appl. Pharmacol 160, 217–230. 10.1006/taap.1999.8767 [DOI] [PubMed] [Google Scholar]
- de Fonseca FR, Ramos JA, Bonnin A, Fernández-Ruiz JJ, 1993. Presence of cannabinoid binding sites in the brain from early postnatal ages: NeuroReport 4, 135–138. 10.1097/00001756-199302000-00005 [DOI] [PubMed] [Google Scholar]
- DiNieri JA, Wang X, Szutorisz H, Spano SM, Kaur J, Casaccia P, Dow-Edwards D, Hurd YL, 2011. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry 70, 763–769. 10.1016/j.biopsych.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J, 1979. Comparative aspects of the brain growth spurt. Early Hum. Dev 3, 79–83. 10.1016/0378-3782(79)90022-7 [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J, 1973. Quantitative growth and development of human brain. Arch. Dis. Child 48, 757–767. 10.1136/adc.48.10.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J, 1970. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 17, 115–123. 10.1016/0006-8993(70)90311-2 [DOI] [PubMed] [Google Scholar]
- Dörje F, Wess J, Lambrecht G, Tacke R, Mutschler E, Brann MR, 1991. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J. Pharmacol. Exp. Ther 256, 727–733. PMID: 1994002 [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte-Hermann R, Spencer PS, 2008. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol 38, 1–125. 10.1080/10408440802272158 [DOI] [PubMed] [Google Scholar]
- Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, Juszczak E, Hittarage A, Azhar S, Dissanayake W, Sheriff MR, Szinicz L, Dawson AH, Buckley NA, 2005. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. The Lancet 366, 1452–1459. 10.1016/S0140-6736(05)67598-8 [DOI] [PubMed] [Google Scholar]
- Ellison CA, Smith JN, Lein PJ, Olson JR, 2011. Pharmacokinetics and pharmacodynamics of chlorpyrifos in adult male Long-Evans rats following repeated subcutaneous exposure to chlorpyrifos. Toxicology 287, 137–144. 10.1016/j.tox.2011.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JG, Wolff MS, 2007. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am. J. Epidemiol 165, 1397–1404. 10.1093/aje/kwm029 [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP, 2007. Organophosphate pesticide exposure and neurodevelopment in Young Mexican-American children. Environ. Health Perspect 115, 792–798. 10.1289/ehp.9828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattore L, Fratta W, 2010. How important are sex differences in cannabinoid action?: Sex differences in cannabinoid action. Br. J. Pharmacol 160, 544–548. 10.1111/j.1476-5381.2010.00776.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett WP, Aracava Y, Adler M, Pereira EFR, Albuquerque EX, 2009. Acute toxicity of organophosphorus compounds in guinea pigs is sex- and age-dependent and cannot be solely accounted for by acetylcholinesterase inhibition. J. Pharmacol. Exp. Ther 328, 516–524. 10.1124/jpet.108.146639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaskos J, Harris W, Sachana M, Muñoz D, Tack J, Hargreaves AJ, 2007. The effects of diazinon and cypermethrin on the differentiation of neuronal and glial cell lines. Toxicol. Appl. Pharmacol 219, 172–180. 10.1016/j.taap.2006.10.033 [DOI] [PubMed] [Google Scholar]
- Flaskos J, Nikolaidis E, Harris W, Sachana M, Hargreaves AJ, 2011. Effects of sub-lethal neurite outgrowth inhibitory concentrations of chlorpyrifos oxon on cytoskeletal proteins and acetylcholinesterase in differentiating N2a cells. Toxicol. Appl. Pharmacol 256, 330–336. 10.1016/j.taap.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Fortenberry GZ, Meeker JD, Sánchez BN, Barr DB, Panuwet P, Bellinger D, Schnaas L, Solano-González M, Ettinger AS, Hernandez-Avila M, Hu H, Tellez-Rojo MM, 2014. Urinary 3,5,6-trichloro-2-pyridinol (TCPY) in pregnant women from Mexico City: Distribution, temporal variability, and relationship with child attention and hyperactivity. Int. J. Hyg. Environ. Health 217, 405–412. 10.1016/j.ijheh.2013.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong CE, Holland N, Richter RJ, Bradman A, Ho A, Eskenazi B, 2006. PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenet. Genomics 16, 183–190. 10.1097/01.fpc.0000189796.21770.d3 [DOI] [PubMed] [Google Scholar]
- Gao J, Naughton SX, Beck WD, Hernandez CM, Wu G, Wei Z, Yang X, Bartlett MG, Terry AV, 2017. Chlorpyrifos and chlorpyrifos oxon impair the transport of membrane bound organelles in rat cortical axons. NeuroToxicology 62, 111–123. 10.1016/j.neuro.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Liu BY, Cui WY, Li LJ, Fan QH, Liu CG, 1998. Pharmacology: Anti-nicotinic properties of anticholinergic antiparkinson drugs. J. Pharm. Pharmacol 50, 1299–1305. 10.1111/j.2042-7158.1998.tb03349.x [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Qiao D, Slotkin TA, 2002. Chlorpyrifos targets developing glia: effects on glial fibrillary acidic protein. Dev. Brain Res 133, 151–161. 10.1016/S0165-3806(02)00283-3 [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Slotkin TA, 2005. Developmental neurotoxicity of chlorpyrifos: targeting glial cells. Environ. Toxicol. Pharmacol 19, 455–461. 10.1016/j.etap.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Slotkin TA, 2003. Developmental neurotoxicity elicited by prenatal or postnatal chlorpyrifos exposure: effects on neurospecific proteins indicate changing vulnerabilities. Environ. Health Perspect 111, 297–303. 10.1289/ehp.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfitt SJ, Jones K, Mason HJ, Cocker J, 2002. Exposure to the organophosphate diazinon: data from a human volunteer study with oral and dermal doses. Toxicol. Lett 134, 105–113. 10.1016/S0378-4274(02)00178-9 [DOI] [PubMed] [Google Scholar]
- Gaylord A, Osborne G, Ghassabian A, Malits J, Attina T, Trasande L, 2020. Trends in neurodevelopmental disability burden due to early life chemical exposure in the USA from 2001 to 2016: A population-based disease burden and cost analysis. Mol. Cell. Endocrinol 502, 110666 10.1016/j.mce.2019.110666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart D, Sickles D, Buccafusco J, Prendergast M, Terryjr A, 2007. Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol. Appl. Pharmacol 218, 20–29. 10.1016/j.taap.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Giachetti A, Giraldo E, Ladinsky H, Montagna E, 1986. Binding and functional profiles of the selective M1 muscarinic receptor antagonists trihexyphenidyl and dicyclomine. Br. J. Pharmacol 89, 83–90. 10.1111/j.1476-5381.1986.tb11123.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilyarov AV, 2008. Nestin in central nervous system cells. Neurosci. Behav. Physiol 38, 165–169. 10.1007/s11055-008-0025-z [DOI] [PubMed] [Google Scholar]
- Giordano G, Afsharinejad Z, Guizzetti M, Vitalone A, Kavanagh TJ, Costa LG, 2007. Organophosphorus insecticides chlorpyrifos and diazinon and oxidative stress in neuronal cells in a genetic model of glutathione deficiency. Toxicol. Appl. Pharmacol 219, 181–189. 10.1016/j.taap.2006.09.016 [DOI] [PubMed] [Google Scholar]
- González-Alzaga B, Lacasaña M, Aguilar-Garduño C, Rodriguez-Barranco M, Ballester F, Rebagliato M, Hernández AF, 2014. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol. Lett 230, 104–121. 10.1016/j.toxlet.2013.11.019 [DOI] [PubMed] [Google Scholar]
- Greene NDE, Copp AJ, 2009. Development of the vertebrate central nervous system: formation of the neural tube. Prenat. Diagn 29, 303–311. 10.1002/pd.2206 [DOI] [PubMed] [Google Scholar]
- Guerri C, 1998. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol. Clin. Exp. Res 22, 304–312. 10.1111/j.1530-0277.1998.tb03653.x [DOI] [PubMed] [Google Scholar]
- Gusel’nikova VV, Korzhevskiy DE, 2015. NeuN as a neuronal nuclear antigen and neuron differentiation marker. Acta Naturae 7, 42–47. [PMC free article] [PubMed] [Google Scholar]
- Hakeem O, Jabri S, 2015. Adverse birth outcomes in women exposed to Syrian chemical attack. Lancet Glob. Health 3, e196 10.1016/S2214-109X(15)70077-X [DOI] [PubMed] [Google Scholar]
- Haviland JA, Butz DE, Porter WP, 2010. Long-term sex selective hormonal and behavior alterations in mice exposed to low doses of chlorpyrifos in utero. Reprod. Toxicol 29, 74–79. 10.1016/j.reprotox.2009.10.008 [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Sass JB, Engel S, Bennett DH, Bradman A, Eskenazi B, Lanphear B, Whyatt R, 2018. Organophosphate exposures during pregnancy and child neurodevelopment: Recommendations for essential policy reforms. PLOS Med. 15, e1002671 10.1371/journal.pmed.1002671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Cory-Slechta DA, Jacka FN, Szabo ST, Guilarte TR, Bilbo SD, Mattingly CJ, Moy SS, Haroon E, Hornig M, Levin ED, Pletnikov MV, Zehr JL, McAllister KA, Dzierlenga AL, Garton AE, Lawler CP, Ladd-Acosta C, 2020. Beyond the looking glass: recent advances in understanding the impact of environmental exposures on neuropsychiatric disease. Neuropsychopharmacol. 45, 1086–1096. 10.1038/s41386-020-0648-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, 2012. Serotonergic modulation of conditioned fear. Scientifica 2012, 1–16. 10.6064/2012/821549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A, Bucelli R, Jett D, Bruun D, Yang D, Lein P, 2005. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol. Appl. Pharmacol 207, 112–124. 10.1016/j.taap.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Howard MD, Pope CN, 2002. In vitro effects of chlorpyrifos, parathion, methyl parathion and their oxons on cardiac muscarinic receptor binding in neonatal and adult rats. Toxicology 170, 1–10. 10.1016/S0300-483X(01)00498-X [DOI] [PubMed] [Google Scholar]
- Horton MK, Kahn LG, Perera F, Barr DB, Rauh V, 2012. Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory? Neurotoxicol. Teratol 34, 534–541. 10.1016/j.ntt.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Blume LC, Dalton GD, 2010. CB(1) cannabinoid receptors and their associated proteins. Curr. Med. Chem 17, 1382–1393. 10.2174/092986710790980023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH, 2012. Placental regulation of maternal-fetal interactions and brain development. Dev. Neurobiol 72, 1317–1326. 10.1002/dneu.22045 [DOI] [PubMed] [Google Scholar]
- Huff RA, Corcoran JJ, Anderson JK, Abou-Donia MB, 1994. Chlorpyrifos oxon binds directly to muscarinic receptors and inhibits cAMP accumulation in rat striatum. J. Pharmacol. Exp. Ther 269, 329–335. PMID: 7513360 [PubMed] [Google Scholar]
- Hutter D, Kingdom J, Jaeggi E, 2010. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: A review. Int. J. Pediatr 2010, 1–9. 10.1155/2010/401323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icenogle L, 2004. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicol. Teratol 26, 95–101. 10.1016/j.ntt.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Indu TH, Roopa BS, Ponnusankar S, 2016. Intentional chlorpyrifos poisoning in pregnant woman and subsequent fetal death. Int. J. Health Allied Sci 5, 39–41. 10.4103/2278-344X.173887 [DOI] [Google Scholar]
- Ishizaki J, Yokogawa K, Nakashima E, Ohkuma S, Ichimura F, 1998. Characteristic subcellular distribution, in brain, heart and lung, of biperiden, trihexyphenidyl, and (−)-quinuclidinyl benzylate in rats. Biol. Pharm. Bull 21, 67–71. 10.1248/bpb.21.67 [DOI] [PubMed] [Google Scholar]
- Jaacks LM, Diao N, Calafat AM, Ospina M, Mazumdar M, Ibne Hasan MOS, Wright R, Quamruzzaman Q, Christiani DC, 2019. Association of prenatal pesticide exposures with adverse pregnancy outcomes and stunting in rural Bangladesh. Environ. Int 133, 105243 10.1016/j.envint.2019.105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajoo M, Saxena S, Pandey M, 2010. Transplacentally acquired organophosphorus poisoning in a newborn: case report. Ann. Trop. Paediatr 30, 137–139. 10.1179/146532810X12703902516202 [DOI] [PubMed] [Google Scholar]
- Jiang W, 2005. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Invest 115, 3104–3116. 10.1172/JCI25509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph L, Thomsen M, 2017. Effects of muscarinic receptor antagonists on cocaine discrimination in wild-type mice and in muscarinic receptor M 1 , M 2 , and M 4 receptor knockout mice. Behav. Brain Res 329, 75–83. 10.1016/j.bbr.2017.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamha AA, Al Omary IYM, Zalabany HA, Hanssens Y, Adheir FS, 2005. Organophosphate poisoning in pregnancy: A case report. Basic Clin. Pharmacol. Toxicol 96, 397–398. 10.1111/j.1742-7843.2005.pto_09.x [DOI] [PubMed] [Google Scholar]
- Karalliedde L, Senanayake N, Ariaratnam A, 1988. Acute organophosphorus insecticide poisoning during pregnancy. Hum. Toxicol 7, 363–364. 10.1177/096032718800700412 [DOI] [PubMed] [Google Scholar]
- Kataoka S, Takuma K, Hara Y, Maeda Y, Ago Y, Matsuda T, 2013. Autism-like behaviours with transient histone hyperacetylation in mice treated prenatally with valproic acid. Int. J. Neuropsychopharmacol 16, 91–103. 10.1017/S1461145711001714 [DOI] [PubMed] [Google Scholar]
- Kilbinger H, Wessler I, 1980. Inhibition by acetylcholine of the stimulation-evoked release of [3H]acetylcholine from the guinea-pig myenteric plexus. Neuroscience 5, 1331–1340. 10.1016/0306-4522(80)90205-5 [DOI] [PubMed] [Google Scholar]
- Kim HY, Wegner SH, Van Ness KP, Park JJ, Pacheco SE, Workman T, Hong S, Griffith W, Faustman EM, 2016. Differential epigenetic effects of chlorpyrifos and arsenic in proliferating and differentiating human neural progenitor cells. Reprod. Toxicol 65, 212–223. 10.1016/j.reprotox.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongtip P, Techasaensiri B, Nankongnab N, Adams J, Phamonphon A, Surach A, Sangprasert S, Thongsuksai A, Srikumpol P, Woskie S, 2017. The impact of prenatal organophosphate pesticide exposures on Thai infant neurodevelopment. Int. J. Environ. Res. Public. Health 14, 570 10.3390/ijerph14060570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalevich J, Langford D, 2013. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology, in: Amini S, White MK (Eds.), Neuronal Cell Culture, Methods in Molecular Biology. Humana Press, Totowa, NJ, pp. 9–21. 10.1007/978-1-62703-640-5_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM, 2010. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc. Natl. Acad. Sci 107, 20535–20540. 10.1073/pnas.1005003107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan A, Kalimian M, Amram B, Kofman O, 2017. Prenatal chlorpyrifos leads to autism-like deficits in C57B16/J mice. Environ. Health 16, 43 10.1186/s12940-017-0251-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, 2001. Selective muscarinic receptor antagonists for airway diseases. Curr. Opin. Pharmacol 1, 223–229. 10.1016/S1471-4892(01)00040-6 [DOI] [PubMed] [Google Scholar]
- Levario-Carrillo M, Amato D, Ostrosky-Wegman P, González-Horta C, Corona Y, Sanin LH, 2004. Relation between pesticide exposure and intrauterine growth retardation. Chemosphere 55, 1421–1427. 10.1016/j.chemosphere.2003.11.027 [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA, 2002. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol. Teratol 24, 733–741. 10.1016/S0892-0362(02)00272-6 [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA, 2001. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev. Brain Res 130, 83–89. 10.1016/S0165-3806(01)00215-2 [DOI] [PubMed] [Google Scholar]
- Licata C, Liu L, Mole D, Thorp J, Chand R, Chaulagain S, 2019. Social and cultural factors leading to suicide attempt via organophosphate poisoning in Nepal. Case Rep. Psychiatry 2019, 1–3. 10.1155/2019/7681309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe ER, Poet TS, Rick DL, Marty MS, Mattsson JL, Timchalk C, Bartels MJ, 2009. The Effect of Plasma Lipids on the Pharmacokinetics of Chlorpyrifos and the Impact on Interpretation of Blood Biomonitoring Data. Toxicol. Sci 108, 258–272. 10.1093/toxsci/kfp034 [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czéh B, 2010. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur. Neuropsychopharmacol 20, 1–17. 10.1016/j.euroneuro.2009.08.003 [DOI] [PubMed] [Google Scholar]
- Luckas MJM, Taggart MJ, Wray S, 1999. Intracellular calcium stores and agonist-induced contractions in isolated human myometrium. Am. J. Obstet. Gynecol 181, 468–476. 10.1016/S0002-9378(99)70580-6 [DOI] [PubMed] [Google Scholar]
- MacLusky N, Naftolin F, 1981. Sexual differentiation of the central nervous system. Science 211, 1294–1302. 10.1126/science.6163211 [DOI] [PubMed] [Google Scholar]
- Mactutus CF, 1989. Developmental neurotoxicity of nicotine, carbon monoxide, and other tobacco smoke constituents. Ann. N. Y. Acad. Sci 562, 105–122. 10.1111/j.1749-6632.1989.tb21010.x [DOI] [PubMed] [Google Scholar]
- Mamczarz J, Pescrille JD, Gavrushenko L, Burke RD, Fawcett WP, DeTolla LJ, Chen H, Pereira EFR, Albuquerque EX, 2016. Spatial learning impairment in prepubertal guinea pigs prenatally exposed to the organophosphorus pesticide chlorpyrifos: Toxicological implications. NeuroToxicology 56, 17–28. 10.1016/j.neuro.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini P, Moriello AS, Cristino L, Palmery M, De Petrocellis L, Di Marzo V, 2009. Cannabinoid CB1 receptor elevation of intracellular calcium in neuroblastoma SH-SY5Y cells: Interactions with muscarinic and δ-opioid receptors. Biochim. Biophys. Acta BBA - Mol. Cell Res 1793, 1289–1303. 10.1016/j.bbamcr.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B, 2010. Organophosphate pesticide exposure and attention in young Mexican-American children: The CHAMACOS study. Environ. Health Perspect 118, 1768–1774. 10.1289/ehp.1002056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsillach J, Costa LG, Furlong CE, 2016. Paraoxonase-1 and early-life environmental exposures. Ann. Glob. Health 82, 100 10.1016/j.aogh.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurissen JPJ, Shankar MR, Mattsson JL, 2000. Chlorpyrifos. Neurotoxicol. Teratol 22, 237–246. 10.1016/S0892-0362(99)00062-8 [DOI] [PubMed] [Google Scholar]
- McCollister SB, Kociba RJ, Humiston CG, McCollister DD, Gehring PJ, 1974. Studies of the acute and long-term oral toxicity of chlorpyrifos (O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate). Food Cosmet. Toxicol 12, 45–61. 10.1016/0015-6264(74)90321-6 [DOI] [PubMed] [Google Scholar]
- McDonough JH, Zoeffel LD, McMonagle J, Copeland TL, Smith CD, Shih TM, 2000. Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Res. 38, 1–14. 10.1016/s0920-1211(99)00060-1 [DOI] [PubMed] [Google Scholar]
- McNab C, 2006. Pesticides are a leading suicide method. World Health Organization; https://www.who.int/mediacentre/news/notes/2006/np24/en/ [Google Scholar]
- Middlemore-Risher M-L, Adam B-L, Lambert NA, Terry AV, 2011. Effects of chlorpyrifos and chlorpyrifos-oxon on the dynamics and movement of mitochondria in rat cortical neurons. J. Pharmacol. Exp. Ther 339, 341–349. 10.1124/jpet.111.184762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monie IW, 1976. Comparative development of the nervous, respiratory, and cardiovascular systems. Environ. Health Perspect 18, 55–60. 10.1289/ehp.761855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran SP, Maksymetz J, Conn PJ, 2019. Targeting muscarinic acetylcholine receptors for the treatment of psychiatric and neurological disorders. Trends Pharmacol. Sci 40, 1006–1020. 10.1016/j.tips.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RJ, Xu S, Pereira EFR, Pescrille JD, Todd SW, Mamczarz J, Albuquerque EX, Gullapalli RP, 2015. Prenatal exposure of guinea pigs to the organophosphorus pesticide chlorpyrifos disrupts the structural and functional integrity of the brain. NeuroToxicology 48, 9–20. 10.1016/j.neuro.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Quezada MT, Lucero BA, Barr DB, Steenland K, Levy K, Ryan PB, Iglesias V, Alvarado S, Concha C, Rojas E, Vega C, 2013. Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: A systematic review. NeuroToxicology 39, 158–168. 10.1016/j.neuro.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R, Ibáñez L, Hattersley A, Tost J, 2012. IGF2/H19 hypomethylation in a patient with very low birthweight, precocious pubarche and insulin resistance. BMC Med. Genet 13, 42 10.1186/1471-2350-13-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naksen W, Prapamontol T, Mangklabruks A, Chantara S, Thavornyutikarn P, Srinual N, Panuwet P, Barry Ryan P, Riederer AM, Barr DB, 2015. Associations of maternal organophosphate pesticide exposure and PON1 activity with birth outcomes in SAWASDEE birth cohort, Thailand. Environ. Res 142, 288–296. 10.1016/j.envres.2015.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nas T, Barun S, Öztürk GS, Vural IM, Ercan ZS, Sarioglu Y, 2007. Nicotine potentiates the electrical field stimulation-evoked contraction of non-pregnant rabbit myometrium. Tohoku J. Exp. Med 211, 187–193. 10.1620/tjem.211.187 [DOI] [PubMed] [Google Scholar]
- Naughton SX, Terry AV, 2018. Neurotoxicity in acute and repeated organophosphate exposure. Toxicology 408, 101–112. 10.1016/j.tox.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrea EM, Morales V, Ngoumgna E, Prescilla R, Tan E, Hernandez E, Ramirez GB, Cifra HL, Manlapaz ML, 2002. Prevalence of Fetal Exposure to Environmental Toxins as Determined by Meconium Analysis. NeuroToxicology 23, 329–339. 10.1016/S0161-813X(02)00077-3 [DOI] [PubMed] [Google Scholar]
- Paparini D, Gori S, Grasso E, Scordo W, Calo G, Pérez Leirós C, Ramhorst R, Salamone G, 2015. Acetylcholine contributes to control the physiological inflammatory response during the peri-implantation period. Acta Physiol. 214, 237–247. 10.1111/apha.12494 [DOI] [PubMed] [Google Scholar]
- Pasquariello N, Catanzaro G, Marzano V, Amadio D, Barcaroli D, Oddi S, Federici G, Urbani A, Finazzi Agrò A, Maccarrone M, 2009. Characterization of the endocannabinoid system in human neuronal cells and proteomic analysis of anandamide-induced apoptosis. J. Biol. Chem 284, 29413–29426. 10.1074/jbc.M109.044412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira EFR, Aracava Y, DeTolla LJ, Beecham EJ, Basinger GW, Wakayama EJ, Albuquerque EX, 2014. Animal models that best reproduce the clinical manifestations of human intoxication with organophosphorus compounds. J. Pharmacol. Exp. Ther 350, 313–321. 10.1124/jpet.114.214932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Tsai W-Y, Kinney P, Camann D, Barr D, Bernert T, Garfinkel R, Tu Y-H, Diaz D, Dietrich J, Whyatt RM, 2003. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ. Health Perspect 111, 201–205. 10.1289/ehp.5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroianu GA, 2009. The synthesis of phosphor ethers: who was Franz Anton Voegeli? Pharm. 64, 269–275. PMID: 19435147 [PubMed] [Google Scholar]
- Philippat C, Barkoski J, Tancredi DJ, Elms B, Barr DB, Ozonoff S, Bennett DH, Hertz-Picciotto I, 2018. Prenatal exposure to organophosphate pesticides and risk of autism spectrum disorders and other non-typical development at 3 years in a high-risk cohort. Int. J. Hyg. Environ. Health 221, 548–555. 10.1016/j.ijheh.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pildner von Steinburg S, Boulesteix A-L, Lederer C, Grunow S, Schiermeier S, Hatzmann W, Schneider K-TM, Daumer M, 2013. What is the “normal” fetal heart rate? PeerJ 1, e82 10.7717/peerj.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzurro DM, Dao K, Costa LG, 2014. Astrocytes protect against diazinon- and diazoxon-induced inhibition of neurite outgrowth by regulating neuronal glutathione. Toxicology 318, 59–68. 10.1016/j.tox.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poet TS, Kousba AA, Dennison SL, Timchalk C, 2004. Physiologically Based Pharmacokinetic/Pharmacodynamic Model for the Organophosphorus Pesticide Diazinon. NeuroToxicology 25, 1013–1030. 10.1016/j.neuro.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Popp J, Pető K, Nagy J, 2013. Pesticide productivity and food security. A review. Agron. Sustain. Dev 33, 243–255. 10.1007/s13593-012-0105-x [DOI] [Google Scholar]
- Prendergast MA, Self RL, Smith KJ, Ghayoumi L, Mullins MM, Butler TR, Buccafusco JJ, Gearhart DA, Terry AV, 2007. Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience 146, 330–339. 10.1016/j.neuroscience.2007.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Caboni P, Liang SN, Casida JE, 2006. Monoacylglycerol lipase inhibition by organophosphorus compounds leads to elevation of brain 2-arachidonoylglycerol and the associated hypomotility in mice. Toxicol. Appl. Pharmacol 211, 78–83. 10.1016/j.taap.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Quistad GB, Nomura DK, Sparks SE, Segall Y, Casida JE, 2002. Cannabinoid CB1 receptor as a target for chlorpyrifos oxon and other organophosphorus pesticides. Toxicol. Lett 135, 89–93. 10.1016/S0378-4274(02)00251-5 [DOI] [PubMed] [Google Scholar]
- Quistad GB, Sparks SE, Casida JE, 2001. Fatty acid amide hydrolase inhibition by neurotoxic organophosphorus pesticides. Toxicol. Appl. Pharmacol 173, 48–55. 10.1006/taap.2001.9175 [DOI] [PubMed] [Google Scholar]
- Raszewski G, Lemieszek MK, Łukawski K, Juszczak M, Rzeski W, 2015. Chlorpyrifos and cypermethrin induce apoptosis in human neuroblastoma cell line SH-SY5Y. Basic Clin. Pharmacol. Toxicol 116, 158–167. 10.1111/bcpt.12285 [DOI] [PubMed] [Google Scholar]
- Rauch SA, Braun JM, Barr DB, Calafat AM, Khoury J, Montesano MA, Yolton K, Lanphear BP, 2012. Associations of prenatal exposure to organophosphate pesticide metabolites with gestational age and birth weight. Environ. Health Perspect 120, 1055–1060. 10.1289/ehp.1104615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R, 2011. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ. Health Perspect 119, 1196–1201. 10.1289/ehp.1003160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garcia WE, Whyatt RM, Horton MK, Barr DB, Louis ED, 2015. Prenatal exposure to the organophosphate pesticide chlorpyrifos and childhood tremor. NeuroToxicology 51, 80–86. 10.1016/j.neuro.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW, 2006. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 118, e1845–e1859. 10.1542/peds.2006-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, Peterson BS, 2012. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc. Natl. Acad. Sci 109, 7871–7876. 10.1073/pnas.1203396109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reemst K, Noctor SC, Lucassen PJ, Hol EM, 2016. The indispensable roles of microglia and astrocytes during brain development. Front. Hum. Neurosci 10 10.3389/fnhum.2016.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert BL, Abou-Donia MB, 1980. Inhibition of fast axoplasmic transport by delayed neurotoxic organophosphorus esters: a possible mode of action. Mol. Pharmacol 17, 56–60. PMID: 6155603 [PubMed] [Google Scholar]
- Reiss R, Neal B, Lamb JC, Juberg DR, 2012. Acetylcholinesterase inhibition dose–response modeling for chlorpyrifos and chlorpyrifos-oxon. Regul. Toxicol. Pharmacol 63, 124–131. 10.1016/j.yrtph.2012.03.008 [DOI] [PubMed] [Google Scholar]
- Ricceri L, Markina N, Valanzano A, Fortuna S, Cometa MF, Meneguz A, Calamandrei G, 2003. Developmental exposure to chlorpyrifos alters reactivity to environmental and social cues in adolescent mice. Toxicol. Appl. Pharmacol 191, 189–201. 10.1016/S0041-008X(03)00229-1 [DOI] [PubMed] [Google Scholar]
- Ricceri L, Venerosi A, Capone F, Cometa MF, Lorenzini P, Fortuna S, Calamandrei G, 2006. Developmental neurotoxicity of organophosphorous pesticides: Fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicol. Sci 93, 105–113. 10.1093/toxsci/kfl032 [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S, 2000. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect 108, 511–533. 10.1289/ehp.00108s3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MH, 1990. Rat hippocampal muscarinic autoreceptors are similar to the M2 (cardiac) subtype: comparison with hippocampal M1, atrial M2 and ileal M3 receptors. Br. J. Pharmacol 99, 753–761. 10.1111/j.1476-5381.1990.tb13002.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robottom BJ, Reich SG, 2011. Exposure to high dosage trihexyphenidyl during pregnancy for treatment of generalized dystonia: Case report and literature review. Neurologist 17, 340–341. 10.1097/NRL.0b013e31822b54d2 [DOI] [PubMed] [Google Scholar]
- Rock KD, Patisaul HB, 2018. Environmental mechanisms of neurodevelopmental toxicity. Curr. Environ. Health Rep 5, 145–157. 10.1007/s40572-018-0185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED, 2008. Developmental diazinon neurotoxicity in rats: Later effects on emotional response. Brain Res. Bull 75, 166–172. 10.1016/j.brainresbull.2007.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roidl D, Hacker C, 2014. Histone methylation during neural development. Cell Tissue Res. 356, 539–552. 10.1007/s00441-014-1842-8 [DOI] [PubMed] [Google Scholar]
- Romero P, Barnett PG, Midtling JE, 1989. Congenital anomalies associated with maternal exposure to oxydemeton-methyl. Environ. Res 50, 256–261. 10.1016/S0013-9351(89)80006-4 [DOI] [PubMed] [Google Scholar]
- Rosner D, Markowitz G, 2013. Persistent pollutants: A brief history of the discovery of the widespread toxicity of chlorinated hydrocarbons. Environ. Res 120, 126–133. 10.1016/j.envres.2012.08.011 [DOI] [PubMed] [Google Scholar]
- Rowe C, Gunier R, Bradman A, Harley KG, Kogut K, Parra K, Eskenazi B, 2016. Residential proximity to organophosphate and carbamate pesticide use during pregnancy, poverty during childhood, and cognitive functioning in 10-year-old children. Environ. Res 150, 128–137. 10.1016/j.envres.2016.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush T, Liu XQ, Hjelmhaug J, Lobner D, 2010. Mechanisms of chlorpyrifos and diazinon induced neurotoxicity in cortical culture. Neuroscience 166, 899–906. 10.1016/j.neuroscience.2010.01.025 [DOI] [PubMed] [Google Scholar]
- Sachana M, Flaskos J, Hargreaves AJ, 2005. Effects of chlorpyrifos and chlorpyrifos-methyl on the outgrowth of axon-like processes, tubulin, and GAP-43 in N2a Cells. Toxicol. Mech. Methods 15, 405–410. 10.1080/15376520500194767 [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Bruno JL, Baker JM, Palzes V, Kogut K, Rauch S, Gunier R, Mora AM, Reiss AL, Eskenazi B, 2019. Prenatal exposure to organophosphate pesticides and functional neuroimaging in adolescents living in proximity to pesticide application. Proc. Natl. Acad. Sci 116, 18347–18356. 10.1073/pnas.1903940116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Harris MH, Gunier RB, Kogut KR, Harley KG, Deardorff J, Bradman A, Holland N, Eskenazi B, 2018. Prenatal organophosphate pesticide exposure and traits related to autism spectrum disorders in a population living in proximity to agriculture. Environ. Health Perspect 126, 047012 10.1289/EHP2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapbamrer R, Hongsibsong S, 2019. Effects of prenatal and postnatal exposure to organophosphate pesticides on child neurodevelopment in different age groups: a systematic review. Environ. Sci. Pollut. Res 26, 18267–18290. 10.1007/s11356-019-05126-w [DOI] [PubMed] [Google Scholar]
- Satyanarayana M, 1986. A correlative review of acetylcholine synthesis in relation to histopathology of the human syncytiotrophoblast. Acta Obstet. Gynecol. Scand 65, 567–572. 10.3109/00016348609158388 [DOI] [PubMed] [Google Scholar]
- Schmidt RJ, Kogan V, Shelton JF, Delwiche L, Hansen RL, Ozonoff S, Ma CC, McCanlies EC, Bennett DH, Hertz-Picciotto I, Tancredi DJ, Volk HE, 2017. Combined prenatal pesticide exposure and folic acid intake in relation to autism spectrum disorder. Environ. Health Perspect 125, 097007 10.1289/EHP604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Anderson DW, Kidd SK, Sobolewski M, Cory-Slechta DA, 2016. Sex-dependent effects of lead and prenatal stress on post-translational histone modifications in frontal cortex and hippocampus in the early postnatal brain. NeuroToxicology 54, 65–71. 10.1016/j.neuro.2016.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebe A, Satar S, Alpay R, Kozaci N, Hilal A, 2005. Organophosphate poisoning associated with fetal death: a case study. Mt. Sinai J. Med. N. Y 72, 354–356. PMID: 16184302 [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ, 2013. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol 106–107, 1–16. 10.1016/j.pneurobio.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafique S, Winn LM, 2020. Gestational exposure to valproic acid upregulates total Stat3 protein expression while downregulating phosphorylated Stat3 in CD-1 mouse embryos with neural tube defects. Birth Defects Res. bdr2.1666. 10.1002/bdr2.1666 [DOI] [PubMed] [Google Scholar]
- Shah AM, Chattopadhyay A, Khambadkone SM, Dixit KM, Irani SF, 1995. Neonatal mydriasis due to effects of atropine used for maternal Tik-20 poisoning. J. Postgrad. Med 41, 21–22. PMID: 10740698 [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, Hansen RL, Hertz-Picciotto I, 2014. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The CHARGE study. Environ. Health Perspect 122, 1103–1109. 10.1289/ehp.1307044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TM, Duniho SM, McDonough JH, 2003. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol. Appl. Pharmacol 188, 69–80. 10.1016/s0041-008x(03)100019-x. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH, 1997. Neurochemical mechanisms in soman-induced seizures. J. Appl. Toxicol. JAT 17, 255–264. [DOI] [PubMed] [Google Scholar]
- Shin H-S, Seo J-H, Jeong S-H, Park S-W, Park Y-I, Son S-W, Kang H-G, Kim JS, 2015. Effect on the H19 gene methylation of sperm and organs of offspring after chlorpyrifos-methyl exposure during organogenesis period: Effect on the h19 gene methylation of sperm and organs. Environ. Toxicol 30, 1355–1363. 10.1002/tox.21923 [DOI] [PubMed] [Google Scholar]
- Shipley MM, Mangold CA, Szpara ML, 2016. Differentiation of the SH-SY5Y human neuroblastoma cell line. J. Vis. Exp 53193 10.3791/53193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidell FR, Borak J, 1992. Chemical warfare agents: II. nerve agents. Ann. Emerg. Med 21, 865–871. 10.1016/S0196-0644(05)81036-4 [DOI] [PubMed] [Google Scholar]
- Sidiropoulou E, Sachana M, Flaskos J, Harris W, Hargreaves AJ, Woldehiwet Z, 2009. Diazinon oxon affects the differentiation of mouse N2a neuroblastoma cells. Arch. Toxicol 83, 373–380. 10.1007/s00204-008-0339-1 [DOI] [PubMed] [Google Scholar]
- Singh G, Singh V, Schneider JS, 2019. Post-translational histone modifications and their interaction with sex influence normal brain development and elaboration of neuropsychiatric disorders. Biochim. Biophys. Acta BBA - Mol. Basis Dis 1865, 1968–1981. 10.1016/j.bbadis.2018.10.016 [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ, 2009. Developmental neurotoxicity of parathion: Progressive effects on serotonergic systems in adolescence and adulthood. Neurotoxicol. Teratol 31, 11–17. 10.1016/j.ntt.2008.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, McCook EC, Seidler FJ, 1997. Cryptic brain cell injury caused by fetal nicotine exposure is associated with persistent elevations of c-fos protooncogene expression. Brain Res. 750, 180–188. 10.1016/S0006-8993(96)01345-5 [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Levin ED, Seidler FJ, 2008. Developmental neurotoxicity of low dose diazinon exposure of neonatal rats: Effects on serotonin systems in adolescence and adulthood. Brain Res. Bull 75, 640–647. 10.1016/j.brainresbull.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, 2009. Protein kinase C is a target for diverse developmental neurotoxicants: Transcriptional responses to chlorpyrifos, diazinon, dieldrin and divalent nickel in PC12 cells. Brain Res. 1263, 23–32. 10.1016/j.brainres.2009.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, 2008. Developmental neurotoxicants target neurodifferentiation into the serotonin phenotype: Chlorpyrifos, diazinon, dieldrin and divalent nickel. Toxicol. Appl. Pharmacol 233, 211–219. 10.1016/j.taap.2008.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, 2007. Comparative developmental neurotoxicity of organophosphates in vivo: Transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res. Bull 72, 232–274. 10.1016/j.brainresbull.2007.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky I, Silman I, Parnas I, Parnas H, 2001. Presynaptic M2 muscarinic receptors are involved in controlling the kinetics of ACh release at the frog neuromuscular junction. J. Physiol 536, 717–725. 10.1111/j.1469-7793.2001.00717.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JN, Hinderliter PM, Timchalk C, Bartels MJ, Poet TS, 2014. A human life-stage physiologically based pharmacokinetic and pharmacodynamic model for chlorpyrifos: Development and validation. Regul. Toxicol. Pharmacol 69, 580–597. 10.1016/j.yrtph.2013.10.005 [DOI] [PubMed] [Google Scholar]
- Smith CD, Wright LKM, Garcia GE, Lee RB, Lumley LA, 2015. Hormone-dependence of sarin lethality in rats: Sex differences and stage of the estrous cycle. Toxicol. Appl. Pharmacol 287, 253–257. 10.1016/j.taap.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova L, Hogberg HT, Leist M, Hartung T, 2014. Developmental neurotoxicity - challenges in the 21st century and in vitro opportunities. ALTEX 31, 129–156. 10.14573/altex.1403271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon GM, Moodley J, 2007. Acute chlorpyrifos poisoning in pregnancy: A case report. Clin. Toxicol 45, 416–419. 10.1080/15563650601117988 [DOI] [PubMed] [Google Scholar]
- Soltys J, Yushak M, Mao-Draayer Y, 2010. Regulation of neural progenitor cell fate by anandamide. Biochem. Biophys. Res. Commun 400, 21–26. 10.1016/j.bbrc.2010.07.129 [DOI] [PubMed] [Google Scholar]
- Soriano FX, Hardingham GE, 2011. In cortical neurons HDAC3 activity suppresses RD4-dependent SMRT export. PLoS ONE 6, e21056 10.1371/journal.pone.0021056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DL, Sudakin DL, Jenkins JJ, 2009. Longitudinal trends in organophosphate incidents reported to the National Pesticide Information Center, 1995–2007. Environ. Health 8, 18 10.1186/1476-069X-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøm B, 2006. Block of neuronal nicotinic receptors by NMDA receptor antagonists Screening inhibitors with fluorescence probes (Thesis in Pharmacology). University of Oslo; https://www.duo.uio.no/bitstream/handle/10852/12114/Bjorns_masterthesis_final.pdf?sequence=1&isAllowed=y [Google Scholar]
- Sun L, Li Guo-qiang, Yan P, Liu Y, Li Guo-feng, Wei L-Q, 2015. Clinical management of organophosphate poisoning in pregnancy. Am. J. Emerg. Med 33, 305.e1–305.e3. 10.1016/j.ajem.2014.05.057 [DOI] [PubMed] [Google Scholar]
- Taylor P, 2017. Anticholinesterase Agents, Chapter 10, 13th ed, Goodman and Gilman’s the Pharmacological Basis of Therapeutics. McGraw Hill Companies, New York, NY. [Google Scholar]
- Terry AV, Gearhart DA, Beck WD, Truan JN, Middlemore M-L, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ, 2007. Chronic, intermittent exposure to chlorpyrifos in rats: Protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J. Pharmacol. Exp. Ther 322, 1117–1128. 10.1124/jpet.107.125625 [DOI] [PubMed] [Google Scholar]
- Terry AV, Stone JD, Buccafusco JJ, Sickles DW, Sood A, Prendergast MA, 2003. Repeated exposures to subthreshold doses of chlorpyrifos in rats: Hippocampal damage, impaired axonal transport, and deficits in spatial learning. J. Pharmacol. Exp. Ther 305, 375–384. 10.1124/jpet.102.041897 [DOI] [PubMed] [Google Scholar]
- Timchalk C, 2002. A physiologically based pharmacokinetic and pharmacodynamics (PBPK/PD) model for the organophosphate insecticide chlorpyrifos in rats and humans. Toxicol. Sci 66, 34–53. 10.1093/toxsci/66.1.34 [DOI] [PubMed] [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED, 2008a. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol. Teratol 30, 38–45. 10.1016/j.ntt.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeeva OA, Sanders D, Seemann K, Yang L, Hermanson D, Regenbogen S, Agoos S, Kallepalli A, Rastogi A, Braddy D, Wells C, Perraut C, Seidler FJ, Slotkin TA, Levin ED, 2008b. Persistent behavioral alterations in rats neonatally exposed to low doses of the organophosphate pesticide, parathion. Brain Res. Bull 77, 404–411. 10.1016/j.brainresbull.2008.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd SW, Lumsden E, Randall WR, Albuquerque EX, Pereira EFR, 2018. The organophosphorus pesticide chlorpyrifos induces neuronal differentiation of SH-SY5Y neuroblastoma cells. The Toxicologist 162, 309 https://www.toxicology.org/pubs/docs/Tox/2018Tox.pdf [Google Scholar]
- Tosetti P, Taglietti V, Toselli M, 1998. Functional changes in potassium conductances of the human neuroblastoma cell line SH-SY5Y during in vitro differentiation. J. Neurophysiol 79, 648–658. 10.1152/jn.1998.79.2.648 [DOI] [PubMed] [Google Scholar]
- Trazzi S, Steger M, Mitrugno VM, Bartesaghi R, Ciani E, 2010. CB1 cannabinoid receptors increase neuronal precursor proliferation through AKT/glycogen synthase kinase-3β/β-catenin signaling. J. Biol. Chem 285, 10098–10109. 10.1074/jbc.M109.043711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H-W, Grant PA, Rissman EF, 2009. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics 4, 47–53. 10.4161/epi.4.1.7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin JW, Marquardt AE, Argue KJ, Vecchiarelli HA, Ashton SE, Arambula SE, Hill MN, McCarthy MM, 2019. Microglial phagocytosis of newborn cells is induced by endocannabinoids and sculpts sex differences in juvenile rat social play. Neuron 102, 435–449.e6. 10.1016/j.neuron.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma G, Sobolewski M, Cory-Slechta DA, Schneider JS, 2017. Sex- and brain region-specific effects of prenatal stress and lead exposure on permissive and repressive post-translational histone modifications from embryonic development through adulthood. NeuroToxicology 62, 207–217. 10.1016/j.neuro.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venerosi A, Cutuli D, Colonnello V, Cardona D, Ricceri L, Calamandrei G, 2008. Neonatal exposure to chlorpyrifos affects maternal responses and maternal aggression of female mice in adulthood. Neurotoxicol. Teratol 30, 468–474. 10.1016/j.ntt.2008.07.002 [DOI] [PubMed] [Google Scholar]
- Vidair CA, 2004. Age dependence of organophosphate and carbamate neurotoxicity in the postnatal rat: extrapolation to the human. Toxicol. Appl. Pharmacol 196, 287–302. 10.1016/j.taap.2003.12.016 [DOI] [PubMed] [Google Scholar]
- Wang C, Trongnetrpunya A, Samuel IBH, Ding M, Kluger BM, 2016. Compensatory neural activity in response to cognitive fatigue. J. Neurosci 36, 3919–3924. 10.1523/jneurosci.3652-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis OF, Müller FO, Lyell H, Badenhorst CH, van Niekerk P, 1983. Materno-fetal cholinesterase inhibitor poisoning. Anesth. Analg 62, 233–235. PMID: 6829926 [PubMed] [Google Scholar]
- Weitman SD, Vodicnik MJ, Lech JJ, 1983. Influence of pregnancy on parathion toxicity and disposition. Toxicol. Appl. Pharmacol 71, 215–224. 10.1016/0041-008x(83)90338-1 [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Camann D, Perera FP, Rauh VA, Tang D, Kinney PL, Garfinkel R, Andrews H, Hoepner L, Barr DB, 2005. Biomarkers in assessing residential insecticide exposures during pregnancy and effects on fetal growth. Toxicol. Appl. Pharmacol 206, 246–254. 10.1016/j.taap.2004.11.027 [DOI] [PubMed] [Google Scholar]
- Wiesner J, Kříž Z, Kuča K, Jun D, Koča J, 2007. Acetylcholinesterases – the structural similarities and differences. J. Enzyme Inhib. Med. Chem 22, 417–424. 10.1080/14756360701421294 [DOI] [PubMed] [Google Scholar]
- Williams AL, DeSesso JM, 2014. Gestational/perinatal chlorpyrifos exposure is not associated with autistic-like behaviors in rodents. Crit. Rev. Toxicol 44, 523–534. 10.3109/10408444.2014.907772 [DOI] [PubMed] [Google Scholar]
- Yang D, Howard A, Bruun D, Aiua-Alemani M, Pickart C, Lein PJ, 2008. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol. Appl. Pharmacol 228, 32–41. 10.1016/j.taap.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, Barr DB, Furlong CE, Holland NT, 2005. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. NeuroToxicology 26, 199–209. 10.1016/j.neuro.2004.10.004 [DOI] [PubMed] [Google Scholar]
- Yu C, Markan K, Temple KA, Deplewski D, Brady MJ, Cohen RN, 2005. The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor γ transcriptional activity and repress 3T3-L1 adipogenesis. J. Biol. Chem 280, 13600–13605. 10.1074/jbc.M409468200 [DOI] [PubMed] [Google Scholar]
- Zangeneh M, 2014. Frequency of attempted suicide methods and the fetal outcomes in pregnant women in Kermanshah. J. Womens Health Care 03 10.4172/2167-0420.1000164 [DOI] [Google Scholar]
- Zhang X, Driver JH, Li Y, Ross JH, Krieger RI, 2008. Dialkylphosphates (DAPs) in fruits and vegetables may confound biomonitoring in organophosphorus insecticide exposure and risk assessment. J. Agric. Food Chem 56, 10638–10645. 10.1021/jf8018084 [DOI] [PubMed] [Google Scholar]
- Zhang X-H, Liu S-S, Yi F, Zhuo M, Li B-M, 2013. Delay-dependent impairment of spatial working memory with inhibition of NR2B-containing NMDA receptors in hippocampal CA1 region of rats. Mol. Brain 6, 13 10.1186/1756-6606-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY, Bear MF, 2012. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol 4, a009886–a009886. 10.1101/cshperspect.a009886 [DOI] [PMC free article] [PubMed] [Google Scholar]


