Abstract
Persistent changes in brain stress and glutamatergic function are associated with post-traumatic stress disorder (PTSD). Rodent exposure to the predator odor trimethylthiazoline (TMT) is an innate stressor that produces lasting behavioral consequences relevant to PTSD. As such, the goal of the present study was to assess early (6 hours and 2 days – Experiment 1) and late (4 weeks – Experiment 2) changes to gene expression (RT-PCR) related to stress and excitatory function following TMT exposure in male, Long-Evans rats. During TMT exposure, rats engaged in stress reactive behaviors, including digging and immobility. Further, the TMT group displayed enhanced exploration and mobility in the TMT-paired context one week after exposure, suggesting a lasting contextual reactivity. Gene expression analyses revealed upregulated FKBP5 6 hours post-TMT in the hypothalamus and dorsal hippocampus. Two days after TMT, GRM3 was downregulated in the prelimbic cortex and dorsal hippocampus, but upregulated in the nucleus accumbens. This may reflect an early stress response (FKBP5) that resulted in later glutamatergic adaptation (GRM3). Finally, another experiment four weeks after TMT exposure showed several differentially expressed genes known to mediate excitatory tripartite synaptic function in the prelimbic cortex (GRM5, DLG4 and SLC1A3 upregulated), infralimbic cortex (GRM2 downregulated, Homer1 upregulated), nucleus accumbens (GRM7 and SLC1A3 downregulated), dorsal hippocampus (FKBP5 and NR3C2 upregulated, SHANK3 downregulated) and ventral hippocampus (CNR1, GRM7, GRM5, SHANK3, and Homer1 downregulated). These data demonstrate that TMT exposure induces stress and excitatory molecular adaptations, which could help us understand the persistent glutamatergic dysfunction observed in PTSD.
Keywords: Gene expression, post-traumatic stress disorder, predator odor, TMT, FKBP5, GRM3, GRM5, CNR1, excitatory tripartite synapse, glutamate
Introduction
Post-traumatic stress disorder (PTSD) is a neuropsychiatric disorder that develops in some individuals after experiencing or witnessing trauma1. The American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-5) characterizes PTSD within four symptom clusters including re-experiencing (flashbacks, unwanted memories), avoidance (external reminders), hyperarousal/hypervigilance, and negative mood/thoughts2. In 2013, the lifetime prevalence of PTSD was reported at 6.1%, which was a total of 14.4 million Americans3. Unfortunately, PTSD is a debilitating and enduring disorder with limited treatment options3. Therefore, there exists an urgent need to better understand the biological mechanisms underlying PTSD.
Clinical studies show dysregulated HPA-axis function in patients with PTSD4, 5. For example, some studies report lower baseline cortisol levels in individuals with PTSD compared to trauma-exposed controls without PTSD or controls not reporting a traumatic experience or PTSD6,7,8,9. Additionally, individuals with PTSD show changes in glucocorticoid receptor (GR) expression, binding to glucocorticoids, and associated GR polymorphisms10, 11, 12. Binding of cortisol to the GR downregulates corticotropin releasing factor (CRF) expression in the hypothalamus, acting as a negative feedback of HPA-axis activation13. Interestingly, in PTSD participants, the FK506 binding protein 5 (FKBP5), a chaperone protein that affects GR and mineralocorticoid receptor (MR) binding sensitivity for glucocorticoids and inhibits cellular translocation, is differentially expressed in several RNA sequencing studies compared to control participants both in peripheral blood cells, and post-mortem PFC brain tissue14, 15, 16. Together, these data provide strong support for the hypothesis that PTSD is associated with a persistent dysregulation in the canonical HPA-axis stress system4.
In addition to HPA-axis dysfunction, multiple lines of evidence suggest a role for dysregulated excitatory signaling in PTSD17. For example, studies have reported changes in brain and blood glutamate concentrations in individuals with PTSD compared to control participants18, 19. A positron emission tomography (PET) study showed that individuals with PTSD exhibit enhanced metabotropic glutamate receptor 5 (mGluR5) availability throughout the cortex20, 21. Furthermore, this increased mGluR5 availability positively correlated with avoidance symptom severity in the PTSD group20. Another PET study revealed increased endocannabinoid receptor type 1 (CB1) availability associated with PTSD22, which is interesting because CB1 receptors are inhibitory presynaptic receptors that are functionally linked to post-synaptic mGluR5 signaling23. Finally, preclinical studies show that stress-induced glucocorticoid release modulates multiple aspects of glutamatergic synaptic transmission24, suggesting the possibility that HPA-axis and excitatory dysfunctions associated with PTSD are related.
Animal models of traumatic stress and PTSD have used exposure to the scent of a predator as a stressor that produces lasting behavioral consequences with relevance to traumatic disorders25, 26, 27,28,29,30,31,32,33. For example, exposure to the synthetically derived fox odor 2,5-dihydro-2,4,5-trimethylthiazoline (TMT) produced avoidance and freezing behaviors, indicative of a fear-like response, and increased serum corticosterone, indicative of a stress response26,27. Other studies have shown lasting anxiety-like and hyperarousal behavior31, as well as avoidance of a TMT-paired context or cue32. Additionally, 24 days after a single TMT exposure, re-exposure to the TMT-paired context elicited differential expression of metabotropic glutamate receptor sub-type 5 (GRM5) gene expression in the amygdala and mPFC in a behaviorally-defined “resilient” group compared to “susceptible” and control groups31. Together, these data show that predator odor exposure produces behavioral and molecular changes indicative of a stress response, and a lasting behavioral profile capable of modeling some aspects of PTSD symptomology.
The goal of the present work was to assess early (6 hours and 2 days) and late (4 weeks) gene expression changes throughout the brain following TMT stress exposure. Gene expression analyses focused on stress/HPA-axis function and excitatory neurotransmission-related targets supported by clinical data1, 16, 17, 20. Stress-related genes included NR3C1 (encodes for GR), NR3C2 (encodes for MR), FKBP5, and CRF. Excitatory-related genes included several metabotropic glutamate receptors (GRM2, GRM3, GRM5, GRM7), CNR1 (encodes for CB1), and the SLC1A3 gene (encodes for EAAT-1). The present assessment of glutamate receptors was largely focused on G-protein coupled receptors (GPCRs) primarily because clinical PTSD data support a role for glutamate GPCRs specifically (mGluR5 and CB1). Additionally, changes in GRM5 were followed up by examining genes related to the excitatory post-synaptic density including DLG4 (encodes for PSD-95), Homer1, and SHANK334. Gene expression was examined in the prelimbic cortex, infralimbic cortex, nucleus accumbens, hypothalamus, amygdala, dorsal hippocampus, and ventral hippocampus because analogous brain regions are implicated in PTSD and stress35, 36. Dorsal and ventral hippocampus were separated because these two subregions show considerable differences in circuit inputs/outputs, gene expression, and functional differences in response to stress37,38. Experiment 1 examined the consequences of TMT exposure on gene expression at relatively early time points (6 hrs and 2 days). Next, Experiment 2 investigated context re-exposure reactivity 1 week after TMT exposure and gene expression at a later time point (4 weeks after TMT exposure). Most genes were evaluated in all brain regions at each time point following TMT exposure. Importantly, the TMT exposure protocol for Experiment 2 differed from Experiment 1, precluding direct comparison of gene expression between early and late time points. However, together these data could inform our understanding of the early and late molecular and behavioral changes following an innate stressor.
Methods
Animals
Male Long-Evans rats (n=52; Envigo, Indianapolis, IN) were used for all experiments. Rats arrived at 7 weeks and were handled for at least one min for one week prior to experiments. Rats in Experiment 1 were double-housed upon arrival and single-housed following TMT exposure. Rats in Experiment 2 were single-housed upon arrival and remained single-housed for the duration of the experiment. Rats were housed in ventilated cages with access to food and water ad libitum and maintained in a temperature and humidity controlled vivarium with a 12 hour light/dark cycle. All experiments were conducted during the light cycle. Rats were under continuous care and monitoring by veterinary staff from the Division of Comparative Medicine at UNC-Chapel Hill. All procedures were conducted in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines.
Experiment 1: Assessment of gene expression 6 hours and 2 days after TMT exposure
TMT Exposure
Rats were transported from the vivarium in the home cage to a separate, well-ventilated room that contained the test chambers in which rats were exposed to TMT (45.72 × 17.78 × 21.59 cm; UNC Instrument Shop, Chapel Hill, NC). The length of the back wall of the test chambers was opaque white with two opaque black side walls and a clear, plexiglass front wall to enable video recordings, and a clear sliding lid. A small, metal basket was hung on the right-side wall (17.8 cm above the floor) to hold a piece of filter paper. Rats were placed in the test chamber for 10 min on two consecutive days before the experiment for habituation. On the TMT exposure day, rats were placed in the chambers for 20 mins. During this session, 10 μl of TMT (2,5-dihydro-2,4,5-trimethylthiazoline, 97% purity; SRQ Bio, Sarasota, FL) or water for controls was pipetted onto the filter paper in the metal baskets. The control group was always run before the TMT group to prevent odor contamination. The odor exposure session was video recorded for evaluation of behavior using ANY-maze Video Tracking System (Version 6.12, Stoelting Co. Wood Dale, IL). All animals were transferred from double to single housing after the TMT exposure session. One group of rats was sacrificed 6 hours after the exposure (Control group n=6; TMT group n=12). Another group of rats were sacrificed two days (54 hours) after the exposure (Control group n=6; TMT group n=12). Controls for each time point were pooled for behavioral and gene expression analyses. Groups were staggered such that all rats were sacrificed on the same day.
Brain tissue collection and sectioning
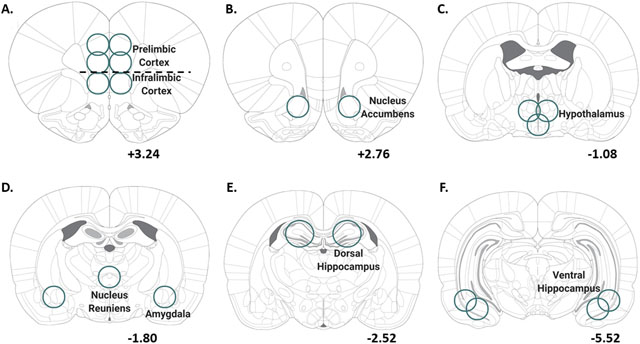
Animals were anesthetized with sodium pentobarbital (Patterson Veterinary, MA; 100 mg/kg, IP) and perfused with ice cold PBS (0.1 M, Fisher, PA) in order to clear peripheral biomolecules from the brain. After perfusion, brains were rapidly extracted and flash frozen with isopentane (Sigma-Aldrich, MI). Brains were stored at −80˚C until brain region sectioning. Brains were sectioned on a cryostat (−20˚C) up to a predetermined bregma for each region of interest (ROI) according to39. Then, a micropunch tool was used to remove tissue specific to each brain region as illustrated in Table 1. Some ROIs were separated by left and right hemispheres, and all qPCR experiments used the right hemisphere when separated. Brain regions that were not bisected by left and right hemisphere used the entire tissue section for qPCR experiments. A complete list of all tissue excision specifications can be found for all ROIs in Table 1. Brain sections were stored at −80˚C until qPCR analysis.
Table 1 –
Details of tissue extraction for each brain region
 | ||
|---|---|---|
| Brain Region | Depth (mm) | Exp 1. Bisected (Y/N) |
| Prelimbic Cortex (A) | 1.0 | Y |
| Infralimbic Cortex (A) | 1.0 | N |
| Nucleus Accumbens (B) | 1.5 | Y |
| Hypothalamus (C) | 1.0 | N |
| Nucleus Reuniens (D) | 1.0 | N |
| Amygdala (D) | 1.0 | N |
| Dorsal Hippo. (E) | 1.5 | Y |
| Ventral Hippo (F) | 1.0 | Y |
Depth: depth of tissue punch; Bisected: Experiment 1 (Y/N): Yes or No – bisected or not bisected only applicable to Experiment 1. The right side of Table 1 shows coronal sections for the start of all brain region tissue punches relative to bregma.
Experiment 2: Assessment of gene expression 4 weeks after TMT exposure
TMT Exposure
Some changes were made to the TMT exposure experimental procedure to improve upon Experiment 1 and capture lasting behavioral and molecular changes in Experiment 2. First, rats were single housed upon arrival, and remained single housed for the duration of the experiment. Second, because the majority of the last 10 min of the 20-min TMT exposure was spent immobile in Experiment 1, the TMT exposure was shortened to 10 min. Third, white bedding (approximately 600 ml) covered the chamber floor for these experiments to examine digging behavior as an additional behavioral measure. Lastly, because a goal of this experiment was to assess reactivity to the context upon re-exposure one week following TMT exposure, animals were not habituated to the chamber environment prior to TMT exposure. Otherwise, the TMT exposure protocol was identical to Experiment 1.
Context Re-exposure
Seven days after TMT exposure, rats were returned to the chambers in which they had been exposed to TMT (including bedding), but without TMT present. This context re-exposure was 5 min in duration and video recorded for behavioral analyses identical to those assessed during TMT exposure.
Brain tissue collection
After the conclusion of the context re-exposure test, rats remained undisturbed in their home cage until sacrifice (4 weeks post-TMT exposure; 3 weeks post-context re-exposure). Rats were deeply anesthetized with 5% isoflurane (Baxter Healthcare, NC) in oxygen, followed by rapid brain extraction and flash freezing in isopentane (Sigma-Aldrich, MI). Brains were stored at −80˚C until brain sectioning. ROI location, and micro-punch width and depth were identical to Experiment 1, but both hemispheres were combined for all Experiment 2 RT-PCR analyses. See Table 1 for tissue excision specifications.
Gene expression analysis using Quantitative Polymerase Chain Reaction (qRT-PCR): Experiments 1 and 2
RNA Extraction-
RNA was extracted from brain tissue using the RNeasy Mini Kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions. RLT lysis buffer containing β-mercaptoethanol (Sigma Aldrich) was used for tissue homogenization. RNA concentration and purity for each sample were determined using a Spectrophotometer (Nanodrop 2000, ThermoScientific).
Reverse Transcription-
RNA was reverse transcribed into cDNA using the QuantiNova Reverse Transcription Kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions. Following reverse transcription, all samples were diluted 1:10 with water (200 uL total), and stored at −20°C before RT-PCR experiments.
RT-PCR-
The StepOnePlus or QuantStudio3 PCR machine (ThermoFisher) was used for all experiments. Importantly, the same machine was used for all experiments conducted on the same brain region and at the same time point. Using a 96-well plate, each sample was run in triplicate using 10 μL total volume per well with the following components: PowerUp Syber green dye (ThermoFisher, containing ROX dye for passive reference), forward and reverse primers (Eton Biosciences Inc., NC, USA), and cDNA template. The PCR was run with an initial activation for 10 mins at 95°C, followed by 40 cycles of the following: denaturation (95°C for 15s), annealing (60°C for 30s), and extension (72°C for 45s). Melt curves were obtained for all experiments to verify synthesis of a single amplicon. All primer sequences are displayed in Table 2.
Table 2 –
Primer sequences used for RT-PCR experiments
| Primer | Forward (5’–3’) | Reverse (5’–3’) |
|---|---|---|
| GRM2 | CCTACAATGTGCTCCTCATCGC | CTGACCTAAAGGGCTGGGAATC |
| GRM3 | ATTCTCAGTCCTCTGCAAGC | AGACCCTGTCACCAATGCTC |
| GRM5 | CTGGCCTTCGTGCCTATCTA | TTT CCG TTG GAGCTTAGGGTT |
| GRM7 | GTGAAAATGTAGACCCAAACAACTG | GGCTGGGTGACAGAATAACTGA |
| SLC1A3 | GGACTGGCTGCTGGATAGAA | ATGGTAAGAATGCAGGGG |
| CRF | CGCCCATCTCTCTGGATCT | ATCAGTTTCCTGTTGCTGTGAG |
| FKBP5 | GAACCCAATGCTGAGCTTATG | ATGTACTTGCCTCCCTTGAAG |
| NR3C1 | GAAAAGCCATCGTCAAAAGGG | TGGAAGCAGTAGGTAAGGAGA |
| NR3C2 | GATTCCAGGTCGTGAAGTGGG | AGAGGAGTTGGCTGTTCGTG |
| CNR1 | TTCAGGTAGCGGGGCATTTT | GCCACAGCTCCGATTCTACA |
| DLG4 | AGTGACAACCAAGAAATACCGCT | CCCTCTGTTCCATTCACCTGC |
| Homerl | GAACAATGCCAAGCTCACCG | CACCGCGTTTGCTTGACTAC |
| SHANK3 | AAGCGTCTTTTCCGCCACTA | AATGGGGGTCTCTGCTTTGG |
| β-actin | CTACAATGAGCTGCGTGTGGC | CAGGTCCAGACGCAGGATGGC |
Data Analyses
Behavioral assessments: TMT exposure and context re-exposure
Using ANY-maze, the length of the rectangular TMT exposure chamber was divided into two compartments for analysis (TMT side and non-TMT side). The basket containing TMT was located on the far end of the TMT side. For Experiment 1, time spent immobile, time spent on the TMT side, distance traveled, and fecal boli during the TMT exposure were assessed. Immobility was operationally defined as the absence of movement other than respiration for longer than 2 seconds. For Experiment 2, time spent digging, time spent immobile, time spent on the TMT side, time spent grooming, distance traveled and fecal boli count were quantified. For Experiment 2, two control and two TMT rats were excluded from the behavioral analyses of TMT exposure due to an error with video recording resulting in 6 control and 6 TMT rats. A two-way RM ANOVA was used to test significant effects of TMT exposure on behavior over time. Sidak’s multiple comparison test was used for all post hoc analyses. In Experiment 2, identical dependent measures were analyzed for the context re-exposure test as those analyzed for the TMT exposure. The context re-exposure was not analyzed across time, but as a total of the 5 min test. Supplementary Table 1 displays Experiment 2 behavioral measures (TMT exposure and context re-exposure) in seconds of the total 5 min test. A student’s t-test was used for all two group comparisons. All data are reported as mean ± SEM. Significance was set at p≤0.05.
Gene expression assessments
We used the ΔΔCt method to determine fold change relative to controls40. Fold changes were normalized so that average control fold change equaled 1. One-way ANOVAs with post hoc Tukey’s multiple comparisons were used for Experiment 1. Experiment 2 data were analyzed using student’s t-tests. Graphs are displayed as transformed fold changes (log2) of controls, from the TMT group only. Zero represents the average fold change of controls. Samples were removed from analysis in case of experimenter error or if determined to be a statistical outlier (greater than 2 std. dev. from the mean). All gene expression data are reported as mean fold change (Supplementary Table 2–4) or the transformed log2 of fold change (Figures) ± SEM. Significance was set at p≤0.05.
Results
Experiment 1: Assessment of gene expression 6 hours and 2 days after TMT exposure
TMT exposure produces immobility and avoidance behaviors
Figure 1 shows the timeline for Experiment 1 (A), and the behavioral response during TMT exposure (B-D). Analysis of the percent time spent immobile across the 20 min session showed a significant main effect of TMT (F(1, 34)=199.6, p < 0.0001), a significant main effect of time (F(3, 102)=63.7, p < 0.0001), and a significant TMT X time interaction (F(3, 102)=24.5, p<0.0001; Fig. 1B). Post hoc analyses showed increased time spent immobile at minute 10, 15, and 20 in the TMT group relative to controls (p<0.05). Total time spent immobile during the 20 min session was 127.9 ± 15.8 s in controls, and 786.2 ± 27.9 s in the TMT group. Analysis of percent time spent on the TMT side showed a main effect of TMT (F(1, 34)=42.8, p < 0.001; Fig. 1C), with the TMT group spending less time on the TMT side. There was no main effect of time or interaction. Total time spent on the TMT side during the 20 min test was 808.0 ± 55.9 s in controls and 180.9 ± 51.1 s in the TMT group. Analysis of distance traveled during exposure yielded a significant main effect of TMT (F (1, 34)=46.3, p < 0.001, Fig. 1D), a significant main effect of time (F(3, 102)=67.1, p < 0.0001), and a significant TMT X time interaction (F(3, 102)=8.3, p < 0.0001). Post hoc analyses showed decreased distance traveled in the TMT group compared to the control group at all time points (p<0.05) except the first 5 min. Fecal boli production during the TMT exposure did not differ between the control and TMT groups (Control: 5.9 ± 1.4; TMT: 7.6 ± 0.5; not shown). Supplementary Figure 1 shows these behavioral data as individual data points. These data show that TMT exposure elicits a behavioral reactivity characteristic of a fear-like behavior in rats.
Figure 1.
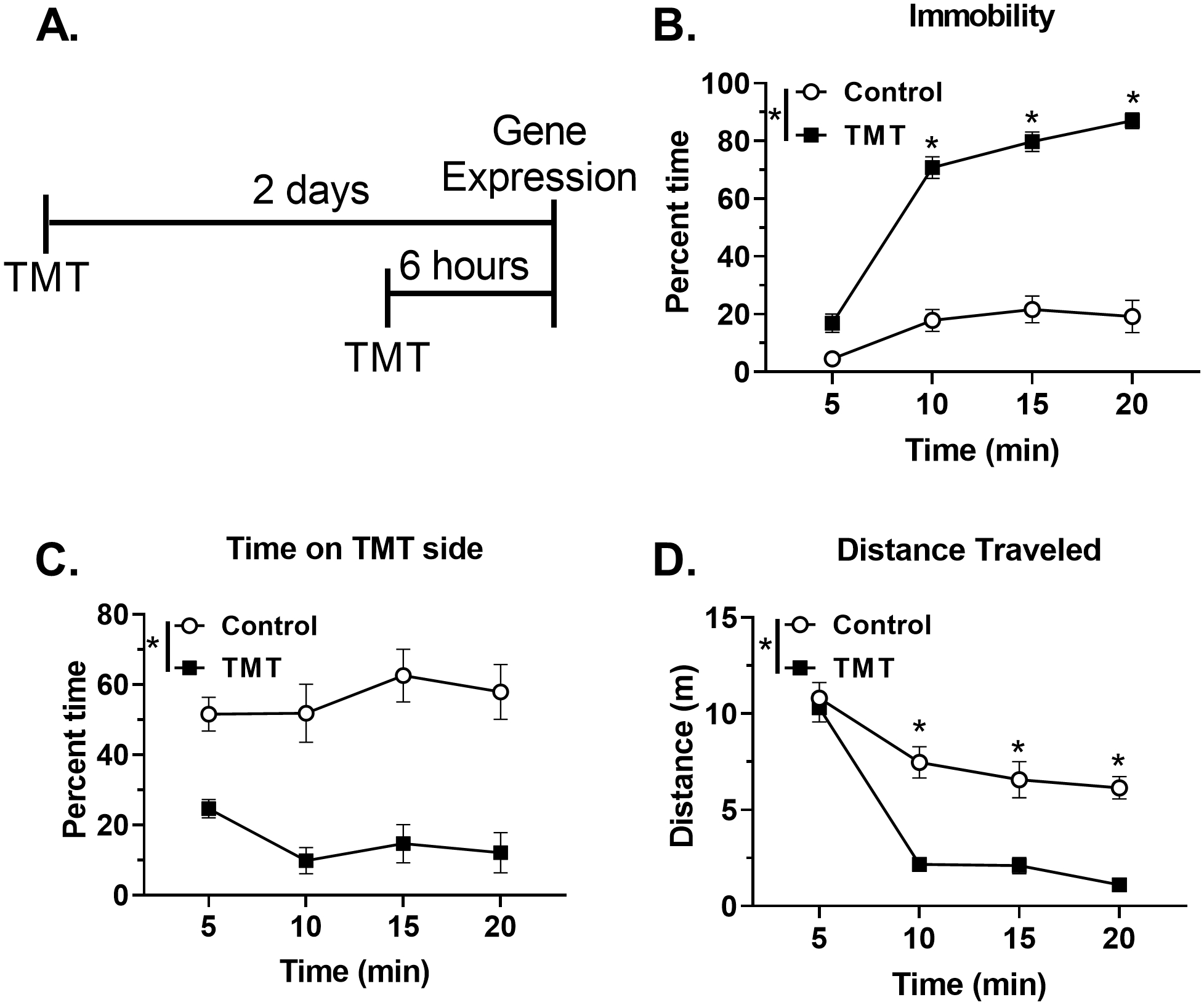
TMT exposure produces immobility and avoidance during exposure (A) Experimental timeline for Experiment 1. During TMT exposure, the TMT group displayed (B) increased percent time immobile, (C) decreased percent time spent on the TMT side, and (D) reduced distance traveled relative to the Control group. n=12 Control; n=24 TMT. * p≤0.05 significantly different from Control.
TMT exposure affects FKBP5 and GRM3 gene expression at early time points
Figure 2 shows gene expression changes 6 hours and 2 days after TMT exposure. In the hypothalamus, FKBP5 was upregulated (F(2, 33)=3.3, p=0.05, Fig. 2A) six hours after exposure (p<0.05), but not 2 days post-exposure. FKBP5 expression in the dorsal hippocampus followed the same pattern (F(2, 32)=5.2, p=0.01, post-hoc p<0.05, Fig. 2A). In contrast, FKBP5 expression was not changed in the ventral hippocampus (Fig. 2A). Additionally, Figure 2B shows significant changes in GRM3 gene expression in the prelimbic cortex, dorsal hippocampus, and nucleus accumbens 2 days following TMT exposure. Specifically, GRM3 was downregulated in the prelimbic cortex (F(2, 30)=5.0, p=0.01) and dorsal hippocampus (F(2, 30)=3.3, p=0.05), but upregulated in the nucleus accumbens (F(2, 31)=3.7, p=0.04) 2 days following TMT exposure (p<0.05). Additionally, we observed an upwards trend for CRF gene expression (p=0.06) 2 days post-TMT (Supplementary Table 3). In contrast, no changes in NR3C1 or NR3C2 expression were observed in either dorsal hippocampus or hypothalamus (Supplementary Table 3). GRM2, GRM5, or GRM7 gene expression were not altered following TMT exposure in any of the brain regions examined at these time points (Supplementary Table 2, 3).
Figure 2.
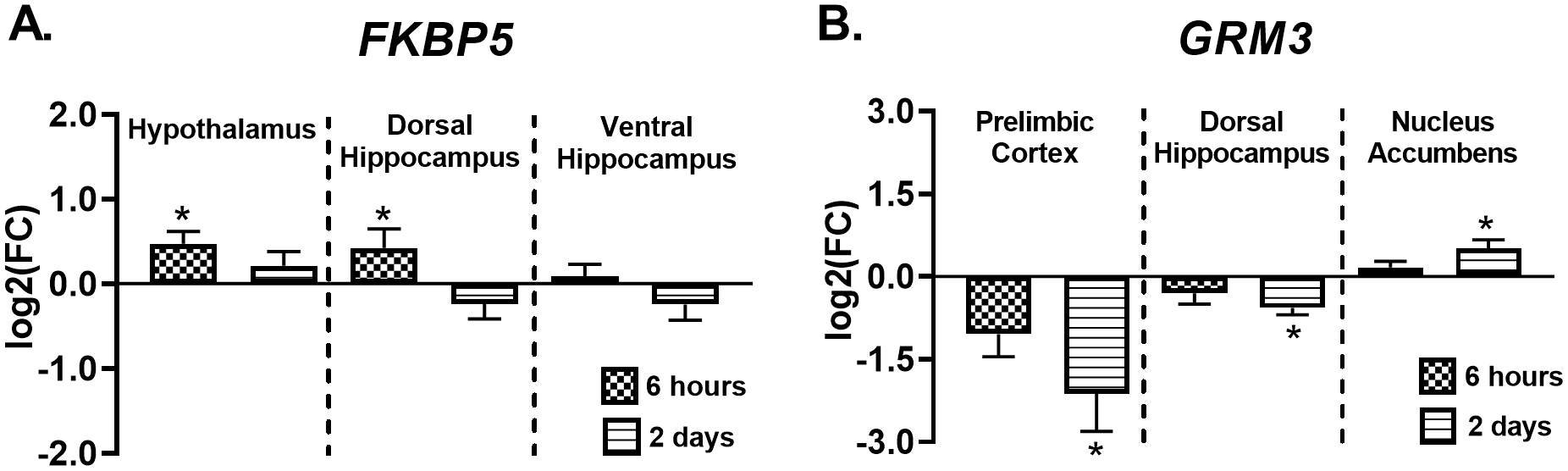
TMT exposure results in differential expression of FKBP5 and GRM3 Six hours after TMT, (A) FKBP5 gene expression was increased in the hypothalamus (n=12 Control; n=12 TMT) and dorsal hippocampus (n=12 Control; n=11 TMT). Two days after TMT, (B) GRM3 gene expression was decreased in the prelimbic cortex (n=11 Control; n=12 TMT) and dorsal hippocampus (n=11 Control; n=11 TMT), but upregulated in the nucleus accumbens (n=12 Control; n=12 TMT). * p≤0.05 significantly different from Control.
Experiment 2: Assessment of gene expression 4 weeks after TMT exposure
TMT exposure produces immobility, avoidance, and digging behaviors
Figure 3 shows the experimental timeline (A) for Experiment 2 and the behavioral response during TMT exposure (B-F). Analysis of percent time digging (Fig. 3B) showed a main effect of TMT (F(1,10)=30.2, p=0.0003), a main effect of time (F(4, 40)=36.4, p<0.0001), and a TMT X time interaction (F(4, 40)=33.5, p<0.0001), with increased digging during the first part of the session (min 2, 4, and 6) in the TMT group compared to the control group (p<0.05). For percent time spent immobile (Fig. 3C), there was a significant main effect of TMT (F(1, 10)=208.6, p<0.0001), a significant main effect of time (F(4, 40)=76.1, p<0.0001), and a significant TMT X time interaction (F(4, 40)=76.4, p<0.0001), with increased immobility during the latter part of the session (min 8 and 10) compared to controls (p<0.05). Analysis of percent time spent on the TMT side showed a significant main effect of TMT (F(1, 10)=10.4, p=0.009, Fig. 3D), with the TMT group spending less time on the TMT side compared to controls, but no main effect of time. Analysis of percent time grooming showed a significant main effect of TMT (F(1, 10)=23.5, p=0.0007, Fig. 3E), but no effect of time or interaction. Overall, TMT-exposed rats groomed less than controls. TMT did not significantly affect overall distance traveled (Fig. 3F). Finally, fecal boli production was increased in the TMT group compared to controls (Control: 0.9 ± 0.5; TMT: 5.4 ± 0.8; t(14)=4.8, p=0.0003). Supplementary Figure 2 shows these behavioral data as individual data points.
Figure 3.
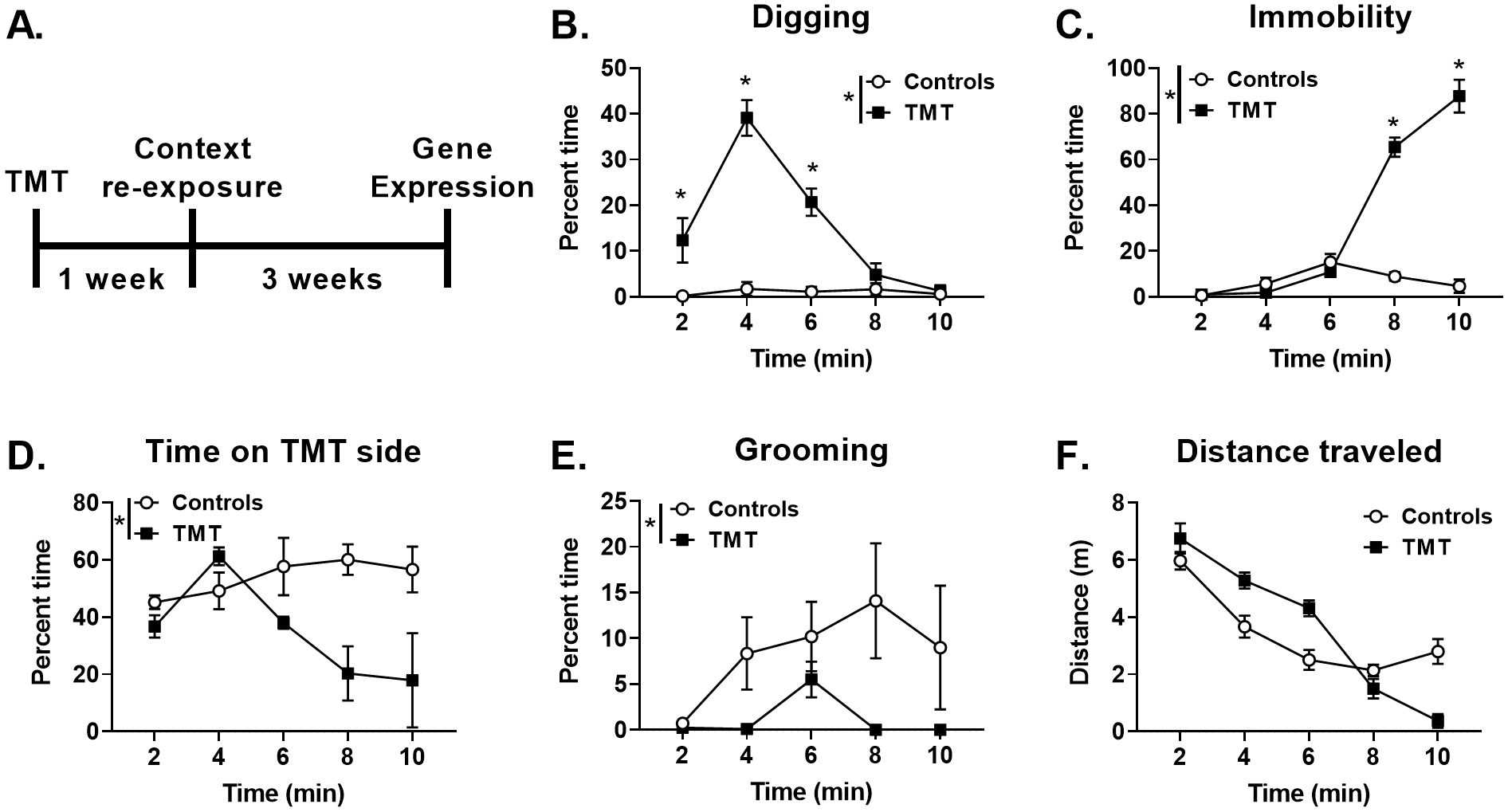
TMT exposure produces digging, immobility, avoidance, and diminished grooming behavior 3A shows the experimental timeline for Experiment 2. During TMT exposure, the TMT group displayed (B) increased percent time digging, (C) increased percent time immobile, (D) decreased percent time spent on the TMT side, (E) decreased percent time spent grooming, and (F) no change in distance traveled. n=6 Control; n=6 TMT. * p≤0.05 significantly different from Control.
Context Re-exposure – enhanced behavioral reactivity to the TMT-paired context
Figure 4 shows the behavioral profile during re-exposure to the previously paired TMT context one week following TMT exposure as individual data points. No group differences were observed for time spent digging during context re-exposure (Fig. 4A). However, the TMT group displayed decreased immobility (t(14)=2.2, p=0.04, Fig. 4B), increased time spent on the TMT side of the chamber (t(14)=3.5, p=0.004, Fig. 4C), and less time grooming (t(14)=3.3, p=0.006, 4D) than controls. TMT exposure did not affect total distance traveled during context re-exposure (Control: 9.0 ± 0.7 m; TMT: 9.4 ± 0.6 m, not shown). Lastly, a trend for increased fecal boli production was observed in the TMT group compared to controls (Control: 0.6 ± 0.3; TMT: 2.6 ± 0.94; t(14)=2.0, p=0.06). Together, these data show a behavioral reactivity to the TMT-paired context.
Figure 4.
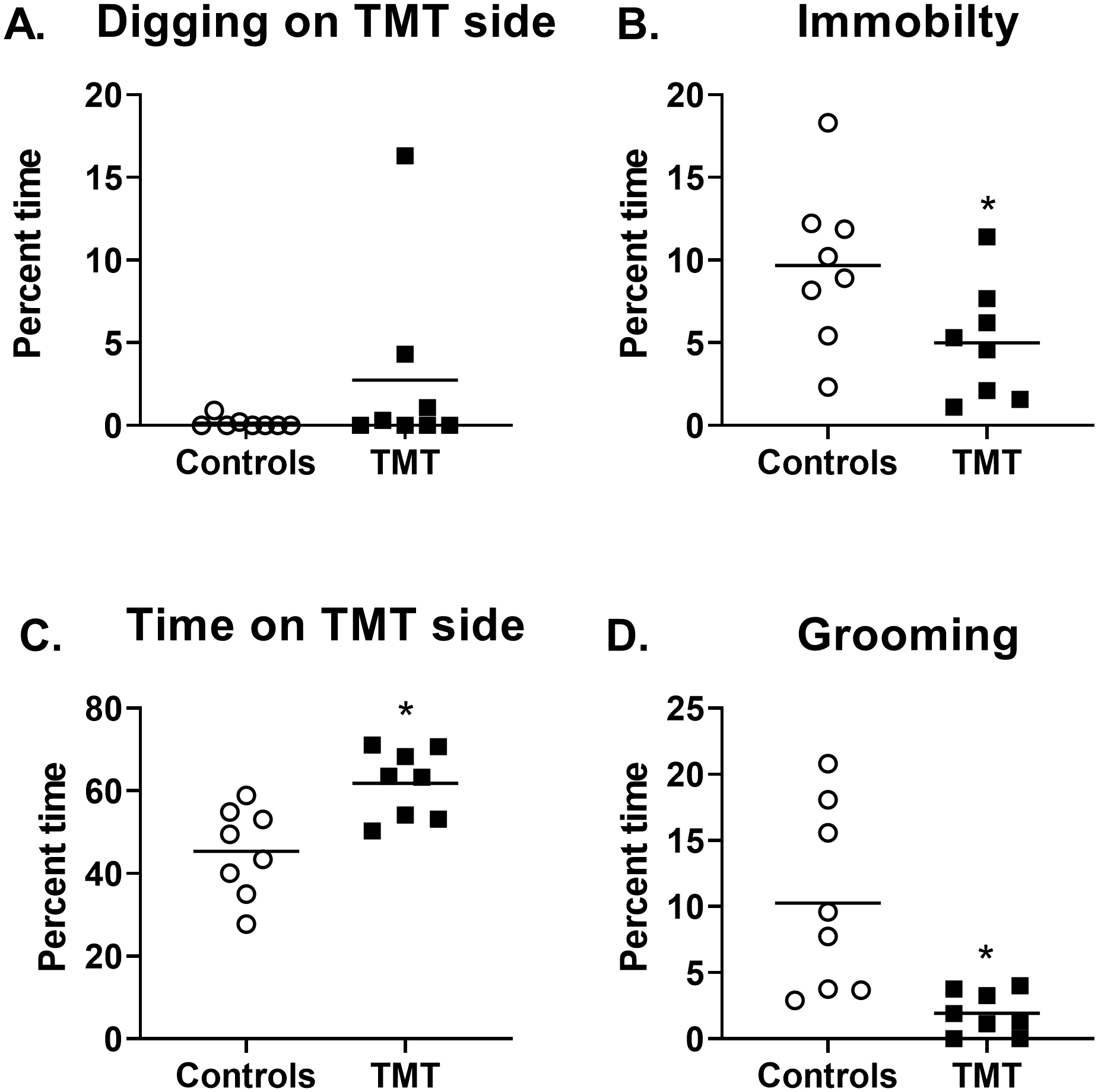
The TMT group displayed context re-exposure behavioral reactivity One week after TMT exposure, (A) no significant difference in digging behavior between the TMT and control group was observed. However, (B) the TMT groups showed less time spent immobile, (C) more time spent on the TMT side of the chamber, and (D) less time grooming relative to controls. n=8 Control; n=8 TMT. * p≤0.05 significantly different from Control.
Gene expression changes four weeks after TMT exposure
Figure 5 shows all significant gene expression changes four weeks after TMT exposure. Figure 5A illustrates upregulation of the stress-related genes, FKBP5 (t(13)=2.2, p=0.04) and NR3C2 (t(13)=2.8, p=0.02), but not NR3C1 in the dorsal hippocampus compared to controls. Figure 5B shows the effects of TMT exposure on the presynaptic, inhibitory receptor gene targets GRM2, GRM7, and CNR1 in the infralimbic cortex, ventral hippocampus and nucleus accumbens. GRM2 was decreased in the infralimbic cortex (t(13)=3.924, p=0.002), but GRM7 and CNR1 were not affected. In the ventral hippocampus, GRM7 (t(13)=2.502, p=0.02) and CNR1 (t(12)=2.930, p=0.01) were both downregulated compared to controls. Similarly, the nucleus accumbens showed decreased GRM7 (t(14)=2.5, p=0.03) and CNR1 (t(13)=3.1, p=0.009) gene expression. Figure 5C shows changes in expression of the synaptic glutamate recycling gene SLC1A3 with an elevation in the prelimbic cortex (t(12)=3.0, p=0.01), and a decrease in the nucleus accumbens (t(13)=3.0, p=0.01). A trend for elevated SLC1A3 expression in the dorsal hippocampus was observed (p=0.06). Figure 5D shows changes in excitatory post-synaptic density and function (GRM5, DLG4, Homer1, and SHANK3) gene expression in prelimbic cortex, infralimbic cortex, ventral hippocampus and dorsal hippocampus. In the prelimbic cortex, a nearly 13-fold increase in GRM5 (t(12)=41.6, p<0.0001) expression was observed in the TMT group compared to controls. This was accompanied by an increase in prelimbic DLG4 (t(13)=2.7, p=0.017), and a trend for an increase in prelimbic Homer1 (t(13)=2.0, p=0.07). The infralimbic cortex did not show changes in GRM5 expression, but did show increased Homer1 (t(14)=2.2, p=0.05) in the TMT group. In contrast to the prelimbic cortex, the ventral hippocampus showed decreased GRM5 expression (t(13)=2.4, p=0.03), as well as decreased SHANK3 (t(13)=4.1, p=0.001) and Homer1 (t(14)=2.6 p=0.02) in the TMT group compared to controls. Similar to the ventral hippocampus, the dorsal hippocampus showed decreased SHANK3 expression (t(13)=4.6, p=0.0005), but GRM5 was not significantly affected.
Figure 5.
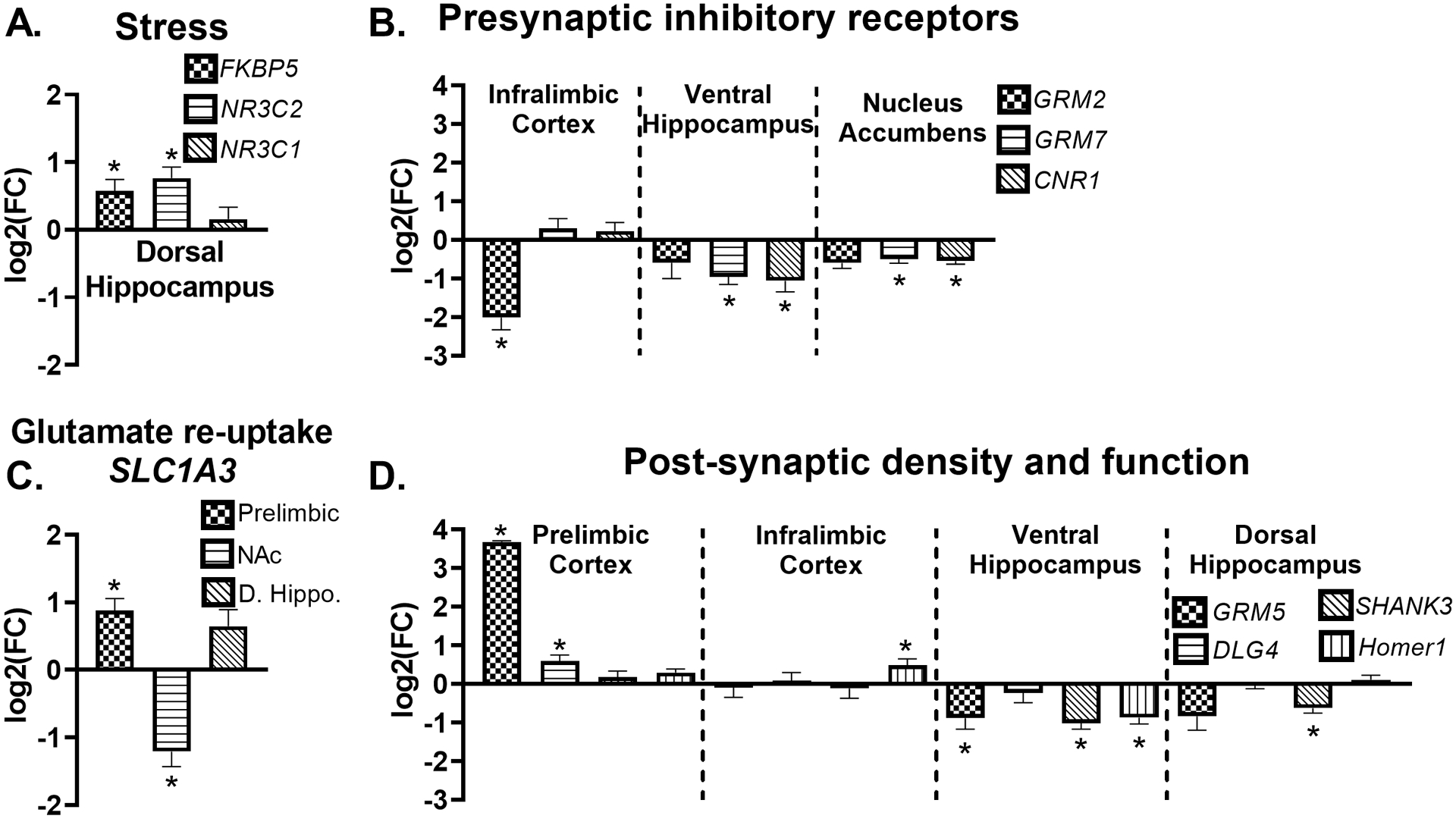
Gene expression changes four weeks after TMT exposure Four weeks after TMT, (A) FBKP5 (n=7 Control; n=8 TMT) and NR3C2 (n=7 Control; n=8 TMT) gene expression were upregulated in the dorsal hippocampus. (B) SLC1A3 was upregulated in the prelimbic cortex (n=6 Control; n=8 TMT), and decreased in the nucleus accumbens (n=7 Control; n=8 TMT). (C) GRM2 was downregulated in the infralimbic cortex (n=7 Control; n=8 TMT), GRM7 (n=8 Control; n=7 TMT) and CNR1 (n=7 Control; n=7 TMT) were decreased in the ventral hippocampus, and GRM7 downregulated in the nucleus accumbens (n=8 Control; n=8 TMT). (D) GRM5 (n=7 Control; n=7 TMT) and DLG-4 (n=7 Control; n=8 TMT) were upregulated in the prelimbic cortex, and Homer1 increased in the infralimbic cortex n=7 Control; n=8 TMT). GRM5 (n=8 Control; n=7 TMT), Homer1 (n=8 Control; n=7 TMT) and SHANK3 (n=8 Control; n=7 TMT) were decreased in the ventral hippocampus, and dorsal hippocampus also showed (D) decreased SHANK3 expression (n=7 Control; n=8 TMT). * p≤0.05 significantly different from Control.
Discussion
These data demonstrate that exposure to the predator odor TMT induces both early (6 hours and 2 days) and late (4 weeks) gene expression changes related to stress and excitatory synaptic function. Among other targets, TMT exposure affected brain gene expression of FKPB5, GRM5, and CNR1, which have all been implicated in PTSD16,20,22. Therefore, these data further validate TMT exposure stress as a model capable of capturing some molecular aspects associated with PTSD.
First, we established that TMT exposure produced a behaviorally-defined stress response. In both Experiments 1 and 2, rats engaged in immobility and avoidance (decreased time spent on the TMT side) behavior. Note that immobility was operationally defined as lack of movement for more than 2 seconds as assessed using ANY-maze software. Therefore, immobility likely captures both inactivity and freezing, which is characteristic of a fear response in rodents41 and observed during TMT exposure42. Importantly, during the last 2 mins of TMT exposure, the TMT group was immobile for approximately 90% of the time, compared to 9% for controls. In Experiment 2, the addition of bedding to the chamber enabled quantification of an additional stress-reactive behavior - digging. Rats engaged in digging behavior during the first 6 mins of exposure, which ultimately resulted in a pile of bedding under the TMT source. Digging behavior appears similar to defensive burying behavior, which rodents display in response to predator odors43. However, because hanging the predator odor from a basket makes burying impossible, digging may reflect a failed attempt at defensive burying behavior. By min 8, the TMT group began to engage in immobility and avoidance behavior similar to Experiment 1. Results from Experiment 1, which used a longer, 20 min TMT exposure, support the observation that once rats begin engaging in immobility and avoidance behaviors, they remain that way until the termination of the experiment. Finally, control rats spent significantly more time grooming than the TMT group, presumably because the TMT group was engaging in digging and immobility behaviors. Increased digging, immobility, avoidance, and decreased grooming during the TMT exposure are consistent with previous findings using TMT stress26,43,30,44. Together, these data reflect a shift from an early, active approach behavior (digging) to a passive immobility and avoidance behavior.
In Experiment 2 one week after TMT, rats were re-exposed to the context in which they were exposed to TMT in order to assess for a lasting behavioral response to the TMT-paired context. Rats in the TMT group showed an altered behavioral response to the TMT-paired context relative to controls. This was displayed by behaviors indicative of exploration and increased activity, such as decreased immobility, increased time spent in the TMT side of the chamber, and decreased time spent grooming. In contrast to the immobility observed during TMT exposure (previously discussed), immobility during the context re-exposure likely reflects inactivity, and not freezing, especially given that the TMT group spent only 5% of the total time immobile (controls: 10%). Therefore, a possible interpretation of decreased immobility (i.e., increased mobility) and grooming in the TMT group during context re-exposure is that control rats showed greater habituation to the context (more inactivity, more grooming), whereas rats that previously experienced the context paired with TMT engaged in more exploratory behavior (less inactivity, less grooming), which may reflect stress-reactivity or hypervigilance to the TMT-associated context. Surprisingly, the TMT group spent slightly more time on the TMT side of the chamber than controls. This could reflect an exploratory behavior of the basket that previously contained TMT or greater exploration of the TMT side in general as this was the side that was previously avoided and thus less explored on the initial exposure. Therefore, while these context re-exposure results do not directly implicate a fear-like response, they may instead reflect enhanced arousal to the TMT-associated context, which is an important behavioral phenotype relevant to PTSD1,33. TMT exposure resulted in both immediate (6 h and 2 days) and late changes (4 weeks) in stress-related genes. FKBP5 gene expression was increased in the hypothalamus and dorsal hippocampus 6 hours following TMT, but returned to control values 2 days after TMT. Additionally, CRF mRNA trended upwards (p=0.06) 2 days after TMT exposure in the hypothalamus. Together, these data suggest engagement of the HPA-axis, and peripheral glucocorticoid release in response to TMT exposure45. Interestingly, FKBP5 was also upregulated 4 weeks after TMT in the dorsal hippocampus, which showed increased NR3C2 (encoding MR), but not NR3C1 (encoding GR), expression as well. Previous experiments demonstrate that stress-induced increases in glucocorticoids bind to the GR and MR, which then translocate into the cell nucleus serving as transcription factors13. Glucocorticoid-activated transcription induces the upregulation of FKBP5, and FKBP5 binds to the GR and MR, blocking their translocation into the nucleus, and therefore serving as an immediate negative feedback loop for stress-induced increases in FKBP5 gene expression13. This could account for the observed increases in FKBP5 expression 6 hours after TMT, but not 2 days after TMT in the dorsal hippocampus and hypothalamus.. Interestingly, PTSD is associated with altered brain FKBP5 transcription and functionally relevant changes in FKBP5-GR complex16,46. These data suggest that the TMT exposure model of stress could be used to investigate the role of FKBP5 in both early and late effects of stress.
Stress-induced increases in glucocorticoid levels affect multiple aspects of glutamate/excitatory brain neurotransmission, indicating a functional relationship between stress and excitatory synaptic transmission24. Therefore, finding of changes in the stress-related genes were followed up by examining genes known to modulate glutamate neurotransmission. At early time points, we identified differential expression of GRM3 across three brain regions. At long-term time points, we showed that genes known to mediate presynaptic neurotransmitter release (i.e., GRM2, GRM7, CNR1), synaptic glutamate recycling (i.e., SLC1A3), and post-synaptic excitatory signaling (i.e., GRM5, DLG4, Homer1, SHANK3) were differentially expressed following TMT exposure.
Two days after TMT exposure, GRM3 (encoding mGluR3) was decreased in the prelimbic cortex and dorsal hippocampus, but increased in the nucleus accumbens, with no significant changes in GRM2, GRM5 and GRM7 expression in any brain region examined at early time points. GRM3 is part of Group II metabotropic glutamate receptors (mGluR2 and 3), which are coupled to Gi/Go to negatively regulate adenylyl cyclase activity47. These receptors are considered largely presynaptic, acting as inhibitory receptors to diminish neurotransmitter release47. However, mGluR3 is also expressed at the post-synaptic membrane and on astrocyte projections at the tripartite synapse47, and displays a post-synaptic site of function in the prelimbic cortex48. Group II mGluRs play an important role in stress, with several studies demonstrating a functional role of these receptors in stress-induced anhedonia/depressive- and anxiety-like behavioral phenotypes47, 48, 49,50,51. Negative allosteric modulation of mGluR3 has antidepressant-like effects in the forced swim and marble burying tests52. Interestingly, mGluR3-mediated long-term depression (LTD) on excitatory prelimbic neurons was abolished following restraint stress48, which is consistent with our findings that TMT stress downregulates GRM3 expression in the prelimbic cortex. Therefore, the observed effects of predator odor stress on GRM3 gene expression may reflect stress-induced changes to synaptic plasticity that could in part underlie the observed changes in subsequent context re-exposure behavioral reactivity and gene expression changes related to the excitatory synapse53.
Four weeks after TMT exposure, several changes in genes encoding Gi/o-coupled presynaptic glutamate receptors were observed. GRM2 expression was decreased in the infralimbic cortex (Il). As previously stated, mGluR2 receptors are presynaptic, inhibitory receptors47 that have been implicated in stress susceptibility54. Additionally, GRM7 (encoding mGluR7) and CNR1 (encoding CB1) were decreased in the ventral hippocampus and the nucleus accumbens. mGluR7 is part of Group III mGluRs, which are Gi/o coupled GPCRs, reducing cyclic AMP formation similar to that of Group II mGluRs, but show different affinity for glutamate compared to Group II receptors55. mGluR7 displays low affinity for glutamate, and activation reduces glutamate release under conditions of high extracellular glutamate concentrations55. Additionally, the CB1 receptor is a presynaptic receptor that when activated by endocannabinoids inhibits neurotransmitter release, and has been implicated in stress and PTSD22,56. Together, these results demonstrate that downregulation of presynaptic, inhibitory receptor gene expression in infralimbic cortex, ventral hippocampus, and nucleus accumbens are persistent consequences of TMT stress, suggesting possible late effects on extracellular glutamate concentrations.
To follow-up on these results, we examined expression of SLC1A3 (encodes the EAAT-1 receptor) as a more direct indicator of changes in extracellular glutamate concentrations. SLC1A3 gene expression was increased in the prelimbic cortex and decreased in the nucleus accumbens. EAAT-1 is expressed on astrocyte projections at the tripartite synapse, and serves to recycle extracellular glutamate from the synapse57. Interestingly, PTSD is associated with high extracellular glutamate, and changes in other neurotransmitter concentrations18. These data provide further support for the hypothesis that TMT exposure induced late changes in excitatory molecular composition related to synaptic glutamate levels.
Next, we investigated whether these changes in presynaptic genes and extracellular glutamate markers were accompanied by changes in the post-synaptic glutamate receptor, GRM5 (encoding mGluR5). GRM5 was differentially expressed in both the prelimbic cortex (increased) and ventral hippocampus (decreased). mGluR5 receptors are part of Group I mGluRs that are Gq/s coupled, increasing cAMP activity on the postsynaptic cell, and affecting NMDAR-mediated excitability58. Additionally, preclinical studies have demonstrated a functional role for mGluR5 signaling in stress-related behaviors31,59,60. Interestingly, in a clinical study in individuals with PTSD, cortical mGluR5 availability was increased relative to healthy controls, and positively correlated with avoidance symptom severity20. Therefore, the nearly 13-fold increase in GRM5 expression in the prelimbic cortex observed in Experiment 2 suggests that increased stress-related cortical mGluR5 might be transcriptionally regulated, and may be a conserved, long-term adaptation that persists following a severe stressor. In contrast to the prelimbic cortex, the ventral hippocampus showed decreased expression of GRM5, suggesting the possibility that GRM5 plays a distinct stress-related role depending on the brain region. Group I mGluR agonism induces robust electrophysiological readouts particularly in the ventral hippocampus, which may play a role in fear memory or extinction mechanisms61,62. In addition to the post-synaptic GPCR changes observed, future work will assess the expression of post-synaptic ionotropic glutamate receptors at these time points following TMT stress exposure.
To follow-up on the changes in GRM5, we examined gene expression of intracellular post-synaptic targets that complex with glutamate membrane receptors (DLG4, SHANK3, and Homer1) in brain regions where GRM5 was changed (prelimbic cortex and ventral hippocampus)34. Further, we examined the dorsal hippocampus and infralimbic cortex to determine if changes were specific to the ventral hippocampus and prelimbic cortex. Interestingly, at least one of these post-synaptic density genes (DLG4, SHANK3, Homer1) were changed in regions where GRM5 was also affected. Further, these gene expression changes were in the same directionality of expression as GRM5. Specifically, DLG4 was increased in the prelimbic cortex, Homer1 increased in the infralimbic cortex, SHANK3 and Homer1 decreased in the ventral hippocampus, and SHANK3 decreased in the dorsal hippocampus. It is interesting to note that many more gene expression changes were observed in the ventral compared to dorsal hippocampus, which is in agreement with previous data demonstrating that stress impacts the ventral more so than the dorsal hippocampus37. Together, these results suggest that TMT exposure produced late changes in gene expression related to excitatory post-synaptic signaling.
As previously mentioned, predator odor stress models have been used to behaviorally -define stress-“susceptible” and “resilient” groups in an effort to improve the face validity of predator odor models25,29,32. However, the goal of the present experiments was to provide a comprehensive and dynamic assessment of glutamate- and stress-related genes throughout the brain following a single exposure to the predator odor TMT. To this end, the sample sizes used were not sufficient to define sub-populations, but it will be important for future work to define how the genes identified here play a role in resilience and vulnerability to develop maladaptive behavioral changes. Context re-exposure assessments one week after TMT exposure showed that 2 out of 8 TMT-exposed rats engaged in digging behavior, while most rats in the TMT group did not engage in digging, and resembled the control group. Therefore, it stands to reason that a larger sample size of TMT-exposed animals may produce two distinct sub-groups based on a context reactivity measure – i.e. “diggers” and “non-diggers”, which could be used to assess individual differences in contextual stress reactivity. Another consideration is that these studies were conducted in male rats only. Sex differences in response to predator odor are documented63 and women suffer from PTSD at three times the rate of men3,16. Therefore, future work should examine sex differences in response to TMT stress.
While conclusions about function cannot be determined based on gene expression data alone, the magnitude of gene expression changes related to a similar function (i.e., excitatory/glutamate signaling and the stress response) suggests the strong possibility that some adaptation to stress mechanisms and excitatory signaling occurred following TMT stress. These data show how TMT stress affects early and late glutamate- and stress-related gene expression throughout the brain relevant to targets identified in the clinical literature. It is interesting to note that more gene expression changes were observed at late compared to early time points; however, the methodological differences between the experiments may have affected the differences observed. Nevertheless, the presence of glutamate- and stress- related gene expression changes one month after stressor exposure builds upon our understanding of the late molecular changes following predator odor stress, which could inform our understanding of traumatic stress disorders.
Supplementary Material
Acknowledgements:
This work was supported in part by the National Institute of Health AA026537 (JB) and by the Bowles Center for Alcohol Studies. RET was supported by NS007431. The authors thank Abigail Garcia-Baza for her help with behavioral analyses.
Footnotes
Conflicts of interest: none.
Data Availability Statement: Data available on request from the authors.
References
- 1.Yehuda R, Hoge CW, McFarlane AC, et al. Post-traumatic stress disorder. Nat Rev Dis Primers. 2015;1:15057. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2013;5. [Google Scholar]
- 3.Goldstein RB, Smith SM, Chou SP, et al. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc Psychiatry Psychiatr Epidemiol. 2016;51(8):1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yehuda R, Boisoneau D, Mason JW, Giller EL. Glucocorticoid receptor number and cortisol excretion in mood, anxiety, and psychotic disorders. Biol Psychiatry. 1993;34(1–2):18–25. [DOI] [PubMed] [Google Scholar]
- 5.De Bellis MD, Chrousos GP, Dorn LD, et al. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. J Clin Endocrinol Metab. 1994;78(2):249–255. [DOI] [PubMed] [Google Scholar]
- 6.Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL Jr., Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nerv Ment Dis. 1990;178(6):366–369. [DOI] [PubMed] [Google Scholar]
- 7.Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary free-cortisol levels in posttraumatic stress disorder patients. J Nerv Ment Dis. 1986;174(3):145–149. [DOI] [PubMed] [Google Scholar]
- 8.Thaller V, Vrkljan M, Hotujac L, Thakore J. The potential role of hypocortisolism in the pathophysiology of PTSD and psoriasis. Coll Antropol. 1999;23(2):611–619. [PubMed] [Google Scholar]
- 9.Glover DA, Poland RE. Urinary cortisol and catecholamines in mothers of child cancer survivors with and without PTSD. Psychoneuroendocrinology. 2002;27(7):805–819. [DOI] [PubMed] [Google Scholar]
- 10.Szeszko PR, Lehrner A, Yehuda R. Glucocorticoids and Hippocampal Structure and Function in PTSD. Harv Rev Psychiatry. 2018;26(3):142–157. [DOI] [PubMed] [Google Scholar]
- 11.Yehuda R, Boisoneau D, Lowy MT, Giller EL Jr., Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry. 1995;52(7):583–593. [DOI] [PubMed] [Google Scholar]
- 12.Castro-Vale I, van Rossum EF, Machado JC, Mota-Cardoso R, Carvalho D. Genetics of glucocorticoid regulation and posttraumatic stress disorder--What do we know? Neurosci Biobehav Rev. 2016;63:143–157. [DOI] [PubMed] [Google Scholar]
- 13.Gjerstad JK, Lightman SL, Spiga F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress. 2018;21(5):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuan PF, Waszczuk MA, Kotov R, et al. Gene expression associated with PTSD in World Trade Center responders: An RNA sequencing study. Transl Psychiatry. 2017;7(12):1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuan P-F, Yang X, Clouston S, et al. Cell type-specific gene expression patterns associated with posttraumatic stress disorder in World Trade Center responders. Translational Psychiatry. 2019;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girgenti MJ, Wang J, Ji D, et al. Transcriptomic Organization of Human Posttraumatic Stress Disorder. bioRxiv. 2020:2020.2001.2027.921403. [Google Scholar]
- 17.Averill LA, Purohit P, Averill CL, Boesl MA, Krystal JH, Abdallah CG. Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neurosci Lett. 2017;649:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishi D, Hashimoto K, Noguchi H, Hamazaki K, Hamazaki T, Matsuoka Y. Glutamatergic system abnormalities in posttraumatic stress disorder. Psychopharmacology (Berl). 2015;232(23):4261–4268. [DOI] [PubMed] [Google Scholar]
- 19.Meyerhoff DJ, Mon A, Metzler T, Neylan TC. Cortical gamma-aminobutyric acid and glutamate in posttraumatic stress disorder and their relationships to self-reported sleep quality. Sleep. 2014;37(5):893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmes SE, Girgenti MJ, Davis MT, et al. Altered metabotropic glutamate receptor 5 markers in PTSD: In vivo and postmortem evidence. Proc Natl Acad Sci U S A. 2017;114(31):8390–8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis MT, Hillmer A, Holmes SE, et al. In vivo evidence for dysregulation of mGluR5 as a biomarker of suicidal ideation. Proceedings of the National Academy of Sciences. 2019;116(23):11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neumeister A, Normandin MD, Pietrzak RH, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry. 2013;18(9):1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olmo IG, Ferreira-Vieira TH, Ribeiro FM. Dissecting the Signaling Pathways Involved in the Crosstalk between Metabotropic Glutamate 5 and Cannabinoid Type 1 Receptors. Mol Pharmacol. 2016;90(5):609–619. [DOI] [PubMed] [Google Scholar]
- 24.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci. 2011;13(1):22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen H, Matar MA, Richter-Levin G, Zohar J. The contribution of an animal model toward uncovering biological risk factors for PTSD. Ann N Y Acad Sci. 2006;1071:335–350. [DOI] [PubMed] [Google Scholar]
- 26.Endres T, Fendt M. Aversion- vs fear-inducing properties of 2,4,5-trimethyl-3-thiazoline, a component of fox odor, in comparison with those of butyric acid. J Exp Biol. 2009;212(Pt 15):2324–2327. [DOI] [PubMed] [Google Scholar]
- 27.Rosen JB, Asok A, Chakraborty T. The smell of fear: innate threat of 2,5-dihydro-2,4,5-trimethylthiazoline, a single molecule component of a predator odor. Front Neurosci. 2015;9:292–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deslauriers J, Toth M, Der-Avakian A, Risbrough VB. Current Status of Animal Models of Posttraumatic Stress Disorder: Behavioral and Biological Phenotypes, and Future Challenges in Improving Translation. Biol Psychiatry. 2018;83(10):895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albrechet-Souza L, Gilpin NW. The predator odor avoidance model of post-traumatic stress disorder in rats. Behav Pharmacol. 2019;30(2 and 3-Spec Issue):105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25(7–8):597–609. [DOI] [PubMed] [Google Scholar]
- 31.Schwendt M, Shallcross J, Hadad NA, et al. A novel rat model of comorbid PTSD and addiction reveals intersections between stress susceptibility and enhanced cocaine seeking with a role for mGlu5 receptors. Transl Psychiatry. 2018;8(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brodnik ZD, Black EM, Clark MJ, Kornsey KN, Snyder NW, España RA. Susceptibility to traumatic stress sensitizes the dopaminergic response to cocaine and increases motivation for cocaine. Neuropharmacology. 2017;125:295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitaker AM, Gilpin NW, Edwards S. Animal models of post-traumatic stress disorder and recent neurobiological insights. Behav Pharmacol. 2014;25(5–6):398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tu JC, Xiao B, Naisbitt S, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23(3):583–592. [DOI] [PubMed] [Google Scholar]
- 35.Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8(4):445–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fenster RJ, Lebois LAM, Ressler KJ, Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci. 2018;19(9):535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ergang P, Vodička M, Soták M, et al. Differential impact of stress on hypothalamic-pituitary-adrenal axis: gene expression changes in Lewis and Fisher rats. Psychoneuroendocrinology. 2015;53:49–59. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates Elsevier/Academic; 2009. [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 41.Lojowska M, Gladwin TE, Hermans EJ, Roelofs K. Freezing promotes perception of coarse visual features. J Exp Psychol Gen. 2015;144(6):1080–1088. [DOI] [PubMed] [Google Scholar]
- 42.Ayers LW, Asok A, Heyward FD, Rosen JB. Freezing to the predator odor 2,4,5 dihydro 2,5 trimethylthiazoline (TMT) is disrupted by olfactory bulb removal but not trigeminal deafferentation. Behav Brain Res. 2013;253:54–59. [DOI] [PubMed] [Google Scholar]
- 43.Hwa LS, Neira S, Pina MM, Pati D, Calloway R, Kash TL. Predator odor increases avoidance and glutamatergic synaptic transmission in the prelimbic cortex via corticotropin-releasing factor receptor 1 signaling. Neuropsychopharmacology. 2019;44(4):766–775. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Wallace KJ, Rosen JB. Predator odor as an unconditioned fear stimulus in rats: elicitation of freezing by trimethylthiazoline, a component of fox feces. Behav Neurosci. 2000;114(5):912–922. [DOI] [PubMed] [Google Scholar]
- 45.Thomas RM, Urban JH, Peterson DA. Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus. Exp Neurol. 2006;201(2):308–315. [DOI] [PubMed] [Google Scholar]
- 46.Li H, Su P, Lai TK, et al. The glucocorticoid receptor-FKBP51 complex contributes to fear conditioning and posttraumatic stress disorder. J Clin Invest. 2020;130(2):877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaki S mGlu2/3 Receptor Antagonists as Novel Antidepressants. Trends Pharmacol Sci. 2017;38(6):569–580. [DOI] [PubMed] [Google Scholar]
- 48.Joffe ME, Santiago CI, Engers JL, Lindsley CW, Conn PJ. Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function. Mol Psychiatry. 2019;24(6):916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshimizu T, Shimazaki T, Ito A, Chaki S. An mGluR2/3 antagonist, MGS0039, exerts antidepressant and anxiolytic effects in behavioral models in rats. Psychopharmacology (Berl). 2006;186(4):587–593. [DOI] [PubMed] [Google Scholar]
- 50.Ago Y, Yano K, Araki R, et al. Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology. 2013;65:29–38. [DOI] [PubMed] [Google Scholar]
- 51.Feyissa AM, Woolverton WL, Miguel-Hidalgo JJ, et al. Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(2):279–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engers JL, Rodriguez AL, Konkol LC, et al. Discovery of a Selective and CNS Penetrant Negative Allosteric Modulator of Metabotropic Glutamate Receptor Subtype 3 with Antidepressant and Anxiolytic Activity in Rodents. J Med Chem. 2015;58(18):7485–7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Menna L, Joffe ME, Iacovelli L, et al. Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology. 2018;128:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasca C, Bigio B, Zelli D, Nicoletti F, McEwen BS. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry. 2015;20(6):755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palazzo E, Marabese I, de Novellis V, Rossi F, Maione S. Metabotropic Glutamate Receptor 7: From Synaptic Function to Therapeutic Implications. Curr Neuropharmacol. 2016;14(5):504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hillard CJ. Stress regulates endocannabinoid-CB1 receptor signaling. Semin Immunol. 2014;26(5):380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rothstein JD, Martin L, Levey AI, et al. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13(3):713–725. [DOI] [PubMed] [Google Scholar]
- 58.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4(2):131–144. [DOI] [PubMed] [Google Scholar]
- 60.Shallcross J, Hámor P, Bechard AR, Romano M, Knackstedt L, Schwendt M. The Divergent Effects of CDPPB and Cannabidiol on Fear Extinction and Anxiety in a Predator Scent Stress Model of PTSD in Rats. Front Behav Neurosci. 2019;13:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J Neurosci. 2009;29(12):3676–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tidball P, Burn HV, Teh KL, Volianskis A, Collingridge GL, Fitzjohn SM. Differential ability of the dorsal and ventral rat hippocampus to exhibit group I metabotropic glutamate receptor-dependent synaptic and intrinsic plasticity. Brain Neurosci Adv. 2017;1(1):2398212816689792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albrechet-Souza L, Schratz CL, Gilpin NW. Sex differences in traumatic stress reactivity of rats with a history of alcohol drinking. bioRxiv. 2019:869990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


