Abstract
Objective:
Evidence links depression and stress to more rapid progression of HIV-1 disease. We conducted a randomized controlled trial to test whether an intervention aimed at improving stress management and emotion regulation, mindfulness-based stress reduction (MBSR), would improve immunological (i.e. CD4+ t-cell counts) and psychological outcomes in persons with HIV-1 infection.
Methods:
We randomly assigned participants with HIV-1 infection and CD4 T-cell counts > 350 cells/μl who were not on antiretroviral therapy in a 1:1 ratio to either an MBSR group (n=89) or an HIV disease self-management skills group (n=88). The study was conducted at the University of California at San Francisco. We assessed immunologic (CD4, c-reactive protein, IL-6, and d-dimer) and psychological measures (Beck Depression Inventory for depression, modified Differential Emotions Scale for positive and negative affect, Perceived stress-scale, and mindfulness) at 3, 6 and 12 months after initiation of the intervention; we used multiple imputation to address missing values.
Results:
We observed statistically significant improvements from baseline to 3-months within the MBSR group in depression, positive and negative affect, perceived stress, and mindfulness; between group differences in change were significantly greater in the MBSR group only for positive affect (per item difference on DES-positive 0.25, 95% CI 0.049, 0.44, p = .015). By 12 months the between group difference in positive affect was not statistically significant, although both groups had trends toward improvements compared to baseline in several psychological outcomes that were maintained at 12-months; these improvements were only statistically significant for depression and negative affect in the MBSR group and perceived stress for the control group. The groups did not differ significantly on rates of antiretroviral therapy initiation (MBSR = 39%, control = 29%, p = .22). After 12 months, the mean decrease in CD4+ T-cell count was 49.6 cells/μl in participants in the MBSR arm, compared to 54.2 cells/μl in the control group, a difference of 4.6 cells favoring the MBSR group (95% CI, −44.6, 53.7, p=.85). The between group differences in other immunologic-related outcomes (c-reactive protein, IL-6, HIV-1 viral load, and d-dimer) were not statistically significant at any time point.
Conclusions:
MBSR improved positive affect more than an active control arm in the 3 months following the start of the intervention. However, this difference was not maintained over the 12-month follow-up and there were no significant differences in immunologic outcomes between intervention groups. These results emphasize the need for further carefully designed research if we are to translate evidence linking psychological states to immunological outcomes into evidence-based clinical practices.
Clinical trial registration:
clinicaltrials.gov registration: NCT00960414
Keywords: HIV-1, Mindfulness, CD4-Positive T-Lymphocytes, Viral Load, Depression, Psychological Stress, CD4 lymphocyte count, emotions, mind-body therapies, immunology, psychoimmunology
Introduction
Despite significant treatment advances, HIV remains a stressful chronic illness for many and is associated with elevated levels of depression.1,2 Stress and depression in HIV are of concern not only because of the deleterious effects on quality of life, but because they are associated with adverse sequelae, including poorer treatment adherence,3,4 increased risk behaviors for HIV transmission,5,6 and potentially more rapid disease progression.7,8 Burack and colleagues found that in a cohort of men with HIV in the pre-highly active antiretroviral era, those with depression had a 38% greater decline in CD4 cells compared with men who were not depressed.7 In a large cohort study of women with HIV, the HIV Epidemiology Research Study (HERS), participants with chronic depressive symptoms had more rapid declines in CD4+ T-cell counts, and were two times more likely to die compared to women with few or no depressive symptoms, after controlling for other prognostic factors.8 Positive psychological states such as positive affect9 and optimism10 are associated with lower risk of mortality among people with HIV, better engagement with care after diagnosis, and greater likelihood of achieving viral suppression when taking antiretroviral therapy.11,12 Although the advent of highly effective antiretroviral therapy has dramatically altered the risk of mortality in HIV infection, engagement in care is critical in obtaining the benefits of treatment, and thus interventions that reduce stress, depression, and negative affect, and increase positive affect likely still provide people living with HIV with multiple benefits.
Mindfulness-Based Stress Reduction (MBSR) is a standardized 8-week program that incorporates several meditation components. It teaches skills to increase awareness and acceptance of moment-to-moment experiences, including difficult emotions and physical discomfort. It is increasing available in many locations in the United States and other countries (including major medical centers), and has well-developed programs to train group leaders, which means that it can be readily disseminated for conditions in which it is shown to be effective. Studies have found MBSR to be an effective component of managing various medical conditions, including chronic pain. Accumulating evidence suggests MBSR is also effective in decreasing depression and perceived stress and increasing positive affect in general populations13 as well as among people coping with significant life stress14 including HIV.15,16 Gayner et al. found that HIV-positive participants randomized to MBSR had significantly lower levels of negative affect and depression and significantly higher levels of positive affect over a 6-month follow-up compared to participants in a usual care control condition.15
The hallmark of HIV-1 disease progression is the depletion of CD4+ T-cells, and this is the key immune measure used to stage disease in HIV-1 clinical management.17 Normal levels are above 500 cells/μl, and serious HIV-1 related opportunistic infections are extremely rare until CD4+ T-cells fall below 200 cells/μl.17 Some evidence suggests MBSR may improve CD4+ T cell counts, which would be an important immunological benefit. Creswell, Myers, Cole, and Irwin demonstrated in a randomized controlled trial that participants with HIV receiving MBSR had a mean increase of 20 CD4+ T-cells/μl compared to a mean decrease of 185 CD4+ T-cells/μl in the control condition (a one-day stress reduction workshop).18 SeyedAlingaghi et al. conducted a randomized trial of MBSR compared to an education control condition in people with HIV.19 Intent-to-treat analysis were not reported but among participants who completed at least 75% of the sessions, participants in the MBSR group showed improvements in physical and psychological symptoms relative to an education control condition. They also reported between-group difference in change in CD4+ T-cell counts with the MBSR group showing significant improvements, although the control condition had significantly higher CD4 count at baseline.
While these prior studies provide intriguing evidence suggesting benefits of MBSR in HIV, important methodological concerns limit the conclusions that can be made from these trials. None of these prior trials controlled for the amount of attention in a group setting that MBSR provides, making it unclear whether the observed benefits were due to the content delivered to the MBSR group or the benefits of being in a group setting. Secondly, the statistically significant effects of MBSR on CD4+ T-cell counts reported in the Creswell and SeyedAlingaghi trials were based on per protocol analyses rather than intent to treat, and both had high rates of drop-out. In addition, to our knowledge, prior studies have not reported the effects of MBSR on inflammatory biomarkers such as CRP, IL-6, and D-dimer. These inflammatory markers are particularly relevant in HIV-1 infection that is not fully suppressed by effective antiretroviral therapy, as these markers are typically elevated well above levels in a healthy population,20 and are strongly predictive of adverse clinical outcomes, including cardiovascular events and death.21 Links between stress and some of these inflammatory markers22 also support the important of testing whether an intervention aimed at reducing stress improves these measures.
To better assess the effects of MBSR on perceived stress, negative affect, depression, positive affect, rate of disease progression, and inflammatory markers in people living with HIV, we performed a randomized, controlled trial with an attention-matched control condition. We aimed for high rates of participant retention and employed intent-to-treat analyses to address some of the limitations of prior studies. The trial was initiated at a time when antiretroviral therapy was frequently deferred until the CD4+ fell below 350 cells/μl, and we restricted enrollment to persons not on antiviral therapy to assess the effects of the intervention on immunologic outcomes in HIV in the absence of treatment. Participants were followed for 12 months from the start of the intervention to track the durability of intervention effects. We hypothesized that participants in the MBSR condition would show slower rates of CD4 cell decline, decreased depression, negative affect, and perceived stress, and increased positive affect compared to participants in the control condition.
Methods
Design Overview:
This was a single center, randomized controlled parallel trial comparing a standard MBSR course that met weekly for eight weeks to an educational course in HIV that met for the same number of sessions and was designed to control for the group attention in MBSR. Given the need for active involvement in group activities, participants and staff were aware of group assignments. All participants were HIV-1 seropositive. Follow-up for outcome assessment was continued for 12 months from the start of the intervention groups. Enrollment began July 2005 and follow-up was complete in September 2009. The protocol was approved by the University of California, San Francisco Institutional Research Board. All participants gave written, informed consent prior to performing any study procedures.
Participants:
We recruited participants who were 18 years of age or older with HIV-1 infection. The primary study outcome was to assess whether MBSR influenced the rate of decline of CD4+ T-cells, a key measure of disease progression in HIV. We thus aimed to enroll people who were not on antiretroviral therapy and did not have a high likelihood of starting within the next 12 months so that we could assess the effect of MBSR on immune measures independent of antiretroviral therapy, which typically raises CD4+ T-cell counts substantially. When the study began, treatment guidelines recommended that antiretroviral therapy should be initiated before the CD4+ T-cell count decreased below 200 cells/µl, and should be considered in asymptomatic persons with a CD4+ T-cell count below 350 cells/µl.23 To avoid enrolling persons who met clear criteria for initiation of antiretroviral therapy when the study began, we excluded persons with a CD4+ T cell count of ≤ 250 cells/µl. HIV-1 infection was established by history, confirmed by an HIV-1 RNA level of > 100 copies on laboratory testing. Participants could not have used antiretrovirals in the 120 days prior to enrollment to ensure that CD4+ T cell counts had not fallen lower than 250 cells/µl within a short period. Participants were asked not to enroll if they had pre-existing plans to start therapy before the end of follow-up in 12 months, but were informed that once they were enrolled in the study, decisions about initiating antiretroviral therapy were up to them and their doctor, and there would be no consequences in regard to study participation. We used broad recruitment methods including posting flyers, advertisements in local papers and internet sites, and outreach to HIV medical specialists.
Randomization and blinding:
Using a computer-generated randomization list, we randomly assigned participants in a 1:1 ratio using random block sizes of 4–7 to one of the two treatment groups. We used random block sizes to prevent anticipation of treatment assignment and achieve approximately equal group sizes for each wave of the intervention. A database manager generated the randomization sequence with guidance from a study statistician (PB). The database manager, who was otherwise not involved in enrollment, programmed the sequence into a Microsoft Access database. No other study staff had access to the randomization sequence file. Approximately two weeks prior to class start, when participants had completed all enrollment steps, the study Project Director (PM, who was blinded to the block sizes) accessed the allocation sequence using a programmed database that could not be altered once randomized condition was revealed. Due to limitations in staff size, it was not feasible for assessors to be blinded to treatment allocation. However, with the exception of the current medications and brief medical symptoms and conditions interview, all of the psychological measures were done using computer assisted self-interviewing. Personnel performing laboratory assays were masked to group assignment.
Interventions:
The MBSR group used a standard eight-week, manualized course24 that provides systematic training in mindfulness meditation as a self-regulation approach to stress reduction and medical and psychological symptoms 25,26. The course consists of eight weekly classes of 2.5 hours duration (except for the first session, which lasts 3 hours); an 8-hour silent retreat during the sixth week of the program; and assignments for home practice. Content includes body scan meditation, gentle yoga focused on body awareness, sitting meditation, and practices that can be used during daily life to be mindful of stress and emotional state before reacting as well as assignments for 45 minutes per day of meditation and yoga practice 6 days per week for the duration of the course.
The education/control group consisted of 8 weekly group sessions of approximately 1.5 hours each week that covered a variety of educational topics about managing HIV infection. Examples of topics covered included how to work with your doctor effectively, how to interpret common laboratory tests used to follow HIV infection, and how to manage HIV disclosure and other issues in sexual relationships. The groups were based in part on successful seminars performed by an HIV advocacy and information community-based organization in San Francisco, Project Inform, and taught by an experienced group leader who had helped develop these seminars. The goal of the education group was to control for social attention and group interaction time in the MBSR groups, and to make attendance at comparison group sessions attractive. While the education group met for less total time than the MBSR group, MBSR included time for meditation practice in which there was no interaction among the group members, and it was felt that using identical meeting lengths would lead to more group interaction time in the comparison group.
There were eight waves of MBSR and education control groups held during the study. The MBSR groups were taught by five different MBSR leaders; each of the control groups was led by the same leader. The MBSR group leaders had to have had formal teacher training and prior experience in leading MBSR groups. In addition, teachers were observed leading MBSR sessions by our lead MBSR instructor (KB) before selection for this study to insure all study instructors were highly skilled. The control group leader had over five years of experience leading HIV education groups.
Measures
Study assessments were conducted at baseline, post intervention (3 months post baseline), 6 months, and 12 months at the University of California San Francisco. In addition, CD4+ T-cell count and viral load, but not other measures, were obtained at 9 months. Audio Computer-Assisted Self Interview (ACASI) was used to administer the psychological measures as well as to collect detailed demographic and background information including race/ethnicity, age, and gender. Trained research assistants collected health history and medication information using a standardized questionnaire. Study nurses, blind to participant group assignment, completed all blood draws. Blood draws were performed in the morning between 8 am and 11 am. To minimize the effects of diurnal variation on CD4+ T-cell counts, we aimed to schedule participants within plus or minus one hour of the baseline measurement time. To provide better precision of the baseline CD4+ T-cell count measure, we also performed two measures prior to the intervention, about two weeks apart, and averaged them to obtain a baseline value. CD4+ T-cell counts were obtained by standard flow cytometry methods, and calculated by multiplying the proportion of lymphocytes that were CD4+ by the total lymphocyte count measured by a Coulter counter. HIV-1 viral load was measured using polymerase chain reaction (Roche Molecular Diagnostics Amplicor Monitor 1.5). We selected serologic markers related to inflammation to measure based on prior studies that have identified which markers are most strongly associated with increased risk of death in untreated or undertreated HIV-1 infection: IL-6, CRP, and D-dimer.21 were measured at the Penn State University College of Medicine Core Reference Laboratory using enzyme-linked immunosorbent assay (ELISA) kits from the following manufacturers: high sensitivity C-reactive protein (hsCRP), ALPCO; D-dimer, American Diagnostica; and Interleukin 6 (IL-6), R & D systems.
The Beck Depression Inventory (BDI)27, a widely used instrument in depression outcome studies, was used to measure depression symptoms over the past week. Past-week positive and negative affect were measured using a modified version of the Differential Emotions Scale (DES) 28 that assessed nine positive emotions (amused, awe, content, glad, grateful, hopeful, interested,love, and pride) and eight negative emotions (angry, ashamed, contempt, disgust, embarrassed, repentant, sad, and scared). Participants rated how frequently they felt that particular emotion in the past week on a 5-point scale: 0=never to 4=most of the time. We inadvertently omitted the contempt item on the negative affect sub-scale of the mDES from the initial questionnaire, which was not discovered part way through the study; as a result, this item was included for 35% of the participants in the baseline evaluations and 75% of participants at the 12 month follow-up. Our results report an average score of the items obtained. Perceived stress was measured with the Perceived Stress Scale (PSS; Cohen & Nast, 1988). This 10-item measure assesses the degree to which situations in one’s life are appraised as stressful, including how unpredictable, uncontrollable, and overloaded respondents find their lives. We assessed four of the five subscales of the Five Factor Mindfulness Questionnaire29 using an abbreviated version of the measure that included 4 facets: observing, describing, attention/awareness, and nonjudging. The fifth factor of this questionnaire had not been developed when we started the study. We examined the four subscales individually and as part of an overall mindfulness construct (α = .86).
Statistical Methods:
Our primary analysis was intent-to-treat. The pre-specified primary outcome measure was rate of decline in CD4+ T-cells. The psychological measures presented here, HIV-1 viral load, and markers of inflammatory state (hsCRP and d-dimer) were key secondary outcome measures. For sample size estimates, based on prior studies we estimated that the average decline in CD4+ T-cells in the control group would be 64 cells/µl per year, with a SD of 72 cells/µl. Given a sample size of 88 persons per group, we would have 80% power to detect a statistically significant difference between groups if the MBSR group had a 30 cell/µl or greater difference in CD4+ T-cell count decline. To assess outcome measures, we used multiple imputation to replace missing data, based on guidelines for reporting and interpreting results of multiple imputation analyses.30 Missing data were handled using SAS version 9.4 (SAS Institute Inc) procedures PROC MI and MIANALYZE. Imputation models for each outcome variable included treatment arm and values at other timepoints. One hundred data sets were imputed for each outcome using the fully conditional specification method with predictive mean matching. Independent-groups t tests on change scores were done using SAS PROC REG. Because of the profound effects of treatment on CD4+ T-counts, immunological and viral load data were censored following initiation of antiretroviral therapy if this occurred, and multiple imputation was used to address the missing data; psychological outcomes were not censored when antiretroviral therapy was been initiated as we did not expect a significant effect of antiretroviral therapy on these measures.
Results
We randomized 177 participants to either the MBSR (N = 89) or education control (N = 88) group (see Figure 1). Participants were 97% male or male-to-female transgender (n=1) and slightly over half were white (62%) (Table 1). These demographics are similar to those of the HIV epidemic in San Francisco, which were 94% male or transgender, and 61% white at the time the study was performed. Randomization achieved a similar distribution of demographic, laboratory, and psychological measures between the MBSR and education control groups at baseline (Table 1).
Figure 1:

Figure 1 shows the numbers of people screened for, enrolled, retained, and available for particular analyses in the trial. ART = antiretroviral therapy, CD4 = CD4 count, VL = HIV viral load.
Table 1.
Participant characteristics
| Characteristic | MBSR | Control |
|---|---|---|
| n=89 | n=88 | |
| Baseline HIV-1 RNA, median log10 copies/ml (interquartile range) | 4.33 (3.73,4.67) | 4.24 (3.72,4.67) |
| Baseline CD4+ T-cells, median cells/µl (interquartile range) | 437(350,575) | 486(401,590) |
| Male (%) | 85(96%) | 6(98%) |
| Race/Ethnicity (%) | ||
| African-American | 6 (7%) | 8(9%) |
| White | 60(67%) | 49(56%) |
| Other | 23(26%) | 31(35%) |
| Age, median years (range) | 41 (22–63) | 39 (22–66) |
| Prior ART (%) | 21 (23.6%) | 26 (29.6%) |
| BDI (mean, SD) | 9.1 (7.3) | 8.7 (7.0) |
| DES + (mean, SD) | 18.0 (5.8) | 19.3 (5.9) |
| DES − (mean, SD) | 9.0 (5.0) | 9.3 (5.4) |
| PSS (mean, SD) | 18.8 (7.5) | 19.2 (6.5) |
| IL-6 (pg/ml; mean, SD) | 2.03 (4.9) | 10.1 (37.7) |
| hsCRP (mg/L; mean, SD) | 2.0 (4.6) | 1.6 (2.6) |
| D-dimer (ug/L; mean, SD) | 268.6 (309.9) | 249.2 (164.1) |
Note: ART = antiretroviral therapy; BDI = Beck depression inventory; PHQ = Physician Health Questionnaire; DES = Differential Emotions Scale (see methods for modifications); PSS = Perceived Stress Scale; IL-6 = interleukin 6; hsCRP = high sensitivity C-reactive protein
Seventy-three percent of the MBSR and 62% of the education control group completed 6 or more of the sessions. Overall, we retained 82% of the sample for the entire 12 months of the study (Figure 1). Participants who dropped out of the study did not differ significantly in baseline demographic, health, or other characteristics from those who completed the study. The intervention groups did not differ significantly in the proportion that initiated antiretroviral therapy over the course of the follow-up. At 3 months from the start of the intervention period, 5% of the MBSR group participants, and 4% of the control group had started antiretroviral therapy. At 12 months, the proportions who had started antiretroviral therapy were 39% and 29%, respectively (p > .2 for both comparisons).
Declines in CD4+ T-cells were similar in both intervention groups (Table 2, Figure 2). After 12 months, the mean decrease in CD4+ T-cell count among persons who did not initiate anti-retroviral therapy was 55.4 cells/μl in participants in the MBSR arm, compared to 62.5 cells/μl in the control group, a non-significant difference of 7.0 cells/μl favoring the MBSR group (95% CI, −61.1, 47.1, p=.80). Using multiple imputation to estimate values of CD4+ T-cells in all participants resulted in similar findings, with a 4.6 cells/μl difference favoring the MBSR group (p = .85, Table 2). Although HIV-1 viral load increased slightly more in the control group over 12 months compared to the MBSR group in persons who did not start anti-retroviral therapy, with a difference of −0.11 copies/ml log10, this was not statistically significant (95% CI −0.32, 0.10, p = .30); we obtained similar results using multiple imputation for missing data, with a −0.086 copies/ml log10 difference favoring the MBSR group (p = .39, Table 2). We did not observe statistically significant differences in inflammation related measures, including hsCRP, IL-6, and d-dimer between intervention groups (Table 2).
Table 2:
Mean changes in biological and psychological outcomes at 3 and 12 months.
| MBSR (SD) n=89 |
Control (SD) n=88 |
Difference (95% CI) |
P value | |
|---|---|---|---|---|
| Biological Outcomes | ||||
| CD4 T cells (cells/μl) | ||||
| 3 mo | −27.53(132.47) | −5.58 (129.17) | −21.96 (−60.78, 16.86) | .052 |
| 12 mo | −49.65*(160.94) | −54.24*(149.83) | 4.59 (−44.54, 53.71) | .85 |
| HIV-1 viral load (log10 copes/ml) | ||||
| 3 mo | 0.022(0.47) | 0.068 (0.46) | −0.046(−0.18, 0.09) | .50 |
| 12 mo | 0.0070(0.65) | 0.93 (0.64) | −0.086 (−0.29, 0.11) | .39 |
| IL-6 (pg/ml) | ||||
| 3 mo | 0.70(15.55) | 2.29 (18.45) | −1.60 (−6.70, 3.51) | .54 |
| 12 mo | 0.30(12.80) | −2.30 (12.96) | 2.60 (−1.26, 6.45) | .19 |
| hsCRP (mg/L) | ||||
| 3 mo | −0.59 (5.47) | 0.12 (5.62) | −0.71 (−2.37, 0.95) | .40 |
| 12 mo | −0.030 (4.50) | −0.49 (4.49) | 0.46 (−0.86, 1.77) | .49 |
| D-dimer (ug/L) | ||||
| 3 mo | −0.64 (293.54) | 15.06 (299.11) | −15.70(−106.9,75.48) | .73 |
| 12 mo | 71.17 (618.53) | 17.06 (640.87) | 54.11 (−133.34, 241.56) | .57 |
| Psychological outcomes | ||||
| Depression (BDI) | ||||
| 3 mo | −1.90* (6.82) | −0.51 (6.84) | −1.39 (−3.42, 0.65) | .18 |
| 12 mo | −1.98* (7.93) | −1.45 (8.23) | −0.53 (−2.94, 1.88) | .66 |
| Positive Affect (DES) | ||||
| 3 mo | 0.17* (0.66) | −0.073 (0.67) | 0.25(0.049, 0.44) | .015 |
| 12 mo | 0.13 (0.73) | 0.12 (0.76) | 0.011(−0.22, 0.24) | .93 |
| Negative Affect (DES) | ||||
| 3 mo | −0.15 (0.61) | 0.012 (0.60) | −0.16 (−0.34, 0.016) | .074 |
| 12 mo | −0.16* (0.73) | −0.048 (0.74) | −0.11 (−0.033, 0.11) | .32 |
| Perceived Stress (PSS) | ||||
| 3 mo | −1.55* (5.39) | −0.21 (5.48) | −1.34 (−2.96, 0.29) | .11 |
| 12 mo | −0.75 (6.42) | −1.97* (6.69) | 1.22 (−0.75, 3.18) | .22 |
| Mindfulness (overall) | ||||
| 3 mo | 0.13* (0.36) | 0.088* (0.37) | 0.045 (−0.064, 0.15) | .41 |
| 12 mo | 0.14* (0.36) | 0.16* (0.37) | −0.015 (−0.13, 0.10) | .78 |
| Acting with Awareness | ||||
| 3 mo | 0.14* (0.62) | 0.11 (0.62) | 0.038 (−0.14, 0.22) | .68 |
| 12 mo | 0.24* (0.69) | 0.23* (0.69) | 0.016 (−0.19, 0.22) | .88 |
| Non-Judging | ||||
| 3 mo | 0.33* (0.70) | 0.063 (0.70) | 0.26 (0.056, 0.47) | .013 |
| 12 mo | 0.26* (0.73) | 0.14 (0.77) | 0.12 (−0.11, 0.34) | .31 |
| Observing | ||||
| 3 mo | 0.024 (0.56) | 0.056 (0.56) | −0.032 (−0.20, 0.14) | .71 |
| 12 mo | 0.043 (0.54) | 0.17*(0.57) | −0.12 (−0.29, 0.041) | .14 |
| Describing | ||||
| 3 mo | 0.13* (0.55) | 0.16* (0.56) | −0.028 (−0.19, 0.14) | .74 |
| 12 mo | 0.16*(0.57) | 0.23*(0.58) | −0.073 (−0.24, 0.10) | .40 |
= Within group differences with p-values < .05 are designated with an asterisk.
Notes (Table 2): Changes are calculated as the Baseline value minus the follow-up time point. The difference represents the MBSR group minus the Control group. Multiple imputation was used to estimate missing values. P-values are derived from independent sample t tests. mo = months; BDI = Beck depression inventory; DES = Differential Emotions Scale (see methods for modifications); hsCRP = high sensitivity C-reactive protein. Acting with awareness, non-judging observing, and describing represent sub-scales of the mindfulness measure. When this study was initiated, the fifth facet on the current Five Facet Mindfulness Scale, nonjudging, had not been included in the measure. 29
Figure 2:
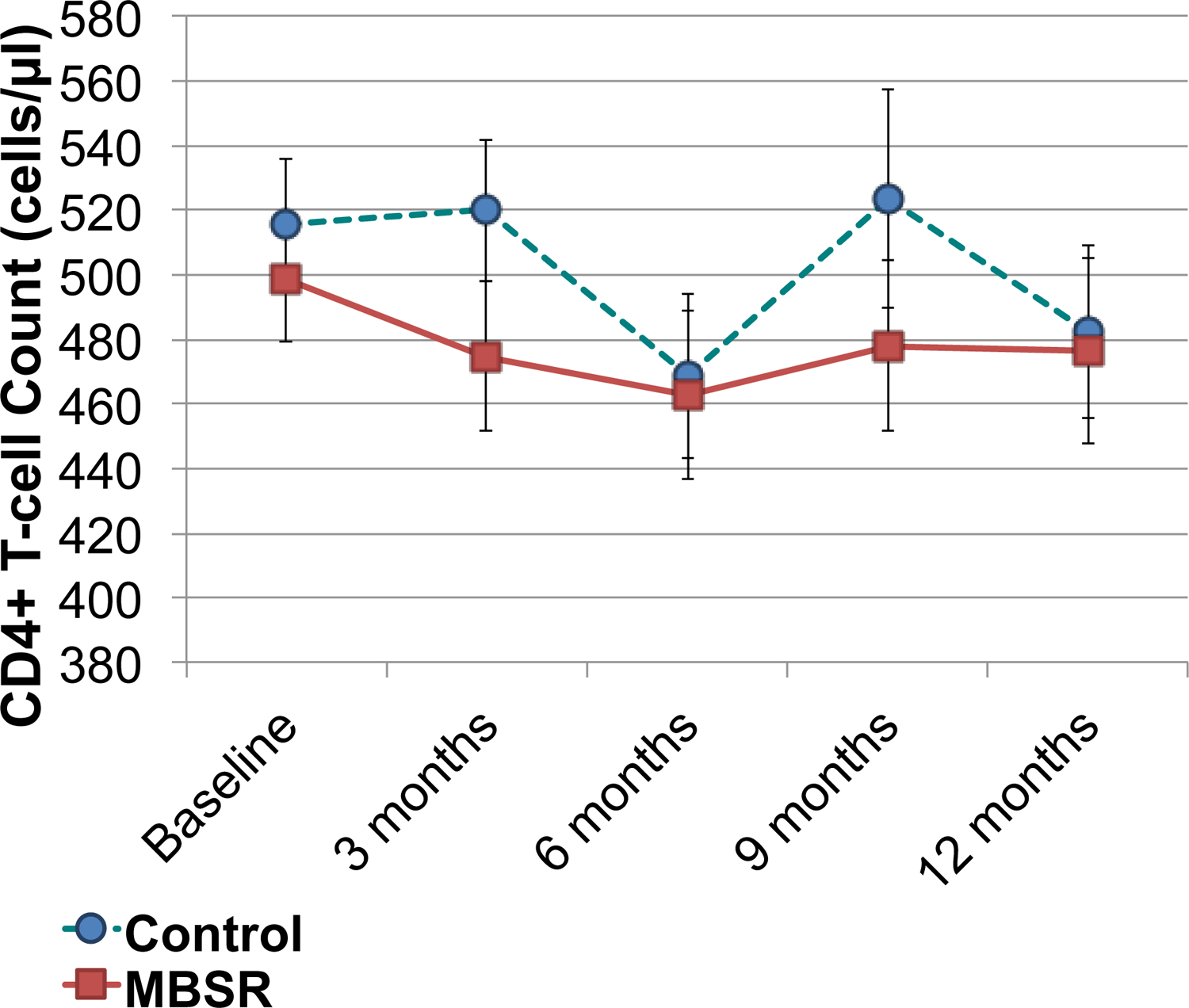
CD4 T-cell count during follow-up by group. Baseline represents pre-intervention levels. Other time periods represent months from the beginning of the MBSR or control group seminars. The dashed line with circles represents the control group. The solid line with squares represents the MBSR group. No differences achieved a p-value < .05.
To address the question of whether participants who were experiencing greater stress or depression at the start of the intervention might have greater benefit from MBSR, we conducted additional analyses of outcomes presented in Table 2 on three subgroups: (1) those with a score of ≥ 14 on the Beck Depression Inventory (consistent with mild or greater depression),31 (2) those with a score of ≥ 14 on the Perceived Stress Scale (representing a score above the mean in a typical US population),32 and (3) Perceived Stress Scale ≥ 27, which has been suggested as a cut-off for high perceived stress. None of these analyses suggested particular benefits for MBSR on CD4 count or HIV viral load in the defined sub-groups. For example, in the 69 MBSR participants and 67 control participants with Perceived Stress Scale score ≥ 14, there was a 16 cell/μl greater drop in CD4 T cell count in the MBSR group at 3 months (95% CI 61 cell greater decrease, 30 cell increase), and a 13 cell/μl lesser drop at 12 months (95% CI 43 cell greater decrease, 69 cell increase). P-values for all comparisons in change in CD4+ T cell count and HIV viral load between MBSR and control groups for each sub-group we assessed were > .4 at 3 and 12 months.
We also assessed changes in psychological measures from baseline between groups, both at 3 months following the intensive intervention period, and at 12 months to assess longer-term effects. Within the MBSR group, depressive symptoms, positive affect, and perceived stress all improved significantly from baseline to 3 months (see Table 2). While many of these measures also tended to improve in the control group, none of these measures had statistically significant changes from baseline within the control group. The increase in positive affect was significantly greater in the MBSR group compared to the control from baseline to 3 months (Table 2).
Within the MBSR group, the overall mindfulness measure increased between the study baseline and month 3, and the mindfulness subscales of acting with attention/awareness, nonjudging of inner experience, and describing all improved significantly at 3 months (Table 2). These changes were maintained at 12 months of follow-up. Compared to the control condition, only nonjudging of inner experience increased significantly more in the MBSR group, however.
We assessed practice of both formal (sitting meditation) and informal (use of mindfulness during daily life) practice during and after the main intervention period within the group that received the MBSR intervention (Table 3). We found that there was a decrease in both formal and informal practice between 3 months (shortly after the MBSR program ended) and 6 months, but that both formal and informal practice remained stable between 6 and 12 months. At 12 months, 44% of MBSR group participants reported continued formal meditation practice, with at least one sitting meditation in the past week, and 69% reported use of informal mindfulness practices in the past week.
Table 3. Practice adherence in the MBSR group.
| Practice characteristic | 3 months | 6 months | 12 months |
|---|---|---|---|
| n=80 | n=79 | n=77 | |
| Using formal meditation practice | 57.5% | 40.5% | 44.1% |
| Average minutes/week formal practice* (SD) | 103.5 (69.9) | 100.4 (105.3) | 106.0 (92.7) |
| Using informal meditation practice | 86.3% | 70.1% | 68.8% |
| Average minutes/week informal practice* (SD) | 111.5 (98.7) | 87.5 (88.2) | 94.5 (103.2) |
Average practice minutes per week is only for the participants reporting use of the practice. N represents number of people who responded to the practice questions at the time point. Formal practice represents sitting meditation.
Discussion
We compared the effect of MBSR, a meditation-based program aimed at improved management of stress and emotion, to an HIV self-management group that controlled for the effects of being in a group program, on immunologic and psychological outcomes in people with HIV infection. We performed the trial at a time when recommendations for antiretroviral therapy considered delaying treatment initiation until CD4 T-cell counts fell below 350 cells/μl an acceptable treatment option; we enrolled participants with CD4 T-cell counts above this threshold who were not on antiretroviral therapy. We hypothesized that the MBSR group would show slower declines in CD4 T-cell counts, based on prior data showing an association of more rapid HIV disease progression with depression and stress. As the most recent recommendations for initiating HIV treatment now call for considering treatment as soon as HIV is diagnosed, the question of whether stress reduction interventions can delay the need for antiretroviral therapy no longer has the same treatment implications. Understanding the potential immune effects of mindfulness-based interventions, however, has important implications for other conditions, such as whether such an intervention may be useful in strengthening immune defenses to other viral illnesses.
Contrary to our hypothesis, we found that over a 12-month period, there was no evidence of lower loss of CD4 T-cells in the MBSR group. We assessed differences in CD4 T-cell counts at 3 month from study initiation to assess intervention effects on CD4+ T-cells immediately after the 2-month MBSR course. As CD4 T-cell count declines tend to be slow in HIV, we also hypothesized that a longer duration of follow-up out to 12-months might reveal trends in CD4+ T-cell loss that could take longer to become apparent. However, we found no time point that clearly favored the MBSR group. Our results contrast with those of two prior studies of MBSR in people living with HIV. Creswell and colleagues reported that in a randomized, controlled trial of MBSR compared to a 1-day control seminar, there was a significant time x treatment interaction on CD4 T-cell counts favoring the MBSR group at the end of the 8-week MBSR program in the 48 persons who attended groups in either arm.18 SeyedAlinaghi conducted a 173 person randomized controlled trial and found significant improvements in CD4 T-cell counts in the MBSR group at 3, 6, and 9 months of follow-up, though by 12 months CD4 counts were almost identical to baseline in both groups.19
Differences in control group design may account for some of the differences in results. We compared MBSR to a control group that met for the same number of sessions (8) to control for the effect of attending a group with other persons with HIV. The Creswell et al. study used a control group consisting of a day-long seminar with information, instruction, and introduction to the same mindfulness practices as in the 8-week program, but had no further meetings and participants were not encouraged to engage in any further practice. In the SeyedAlinaghi et al. study, control participants met twice in small groups for a total of 2 hours to receive educational information. Our control group may have been more closely matched to the MBSR group, and perhaps it even exceeded the effects of MBSR groups in providing interaction with other people with HIV. Other studies have suggested that group interaction may provide significant psychological and even health benefits for people with other medical conditions, such as breast cancer 33. Similar group support effects may have been responsible for some of the positive effects of the MBSR program in other studies.
Several other issues suggest the need for caution in interpreting CD4 T-cell count results from these earlier trials, however. In the Creswell et al. study, the difference in CD4 T-cell counts between groups was primarily driven by a drop of 185 cells/μl over 8 weeks in the comparison group.18 This CD4 T-cell count drop is much larger than would be expected over a two-month period based on other studies. For example, in the START trial, which randomized persons with early asymptomatic HIV to immediate or deferred antiretroviral treatment, CD4 T-cells in the deferred treatment group (n=2359) declined on average less than 100 cells/μl over a 12-month period, or approximately half the decline over a 6 times longer follow-up period than that observed in the Creswell study. Given the well-known variability in CD4 T-cells count measurements34,35 and the small sample size in the Creswell study, this suggests that at least part of the difference observed may have been due to chance variation in CD4 count measurements. This is further supported by the fact that the statistically significant difference in CD4 counts was only found in an analysis in which 11 of the 26 persons randomized to the control group were excluded due to non-participation in the 1-day workshop. In intent to treat analysis in which all randomized persons were included, differences in CD4 counts were no longer statistically significant. In the SeyedAlinaghi et al. study, randomization did not achieve well-matched baseline CD4 counts between groups.19 CD4 counts in the MBSR group at baseline were 100 cells/μl lower in the control group (p < .001). Although CD4 counts increased in the MBSR group, mean counts in the MBSR group were lower than the control group throughout the trial. The baseline difference was almost certainly due to chance rather than some error in the randomization process, as the authors acknowledged, but the magnitude of this imbalance makes it more difficult to interpret the observed differences in CD4 counts following MBSR. Because of the known variability of CD4 count measures, we used two baseline measurements performed on different days and averaged them. We also used a protocol in which blood was obtained within a similar two-hour period in the morning each time to limit diurnal variation. These steps may have resulted in a more precise estimate of baseline CD4 counts than in prior studies.
Given the sample size and the methodological rigor of our study, which included high retention rates and a control group that was carefully matched for instructor attention and social interaction, we believe our results provide fairly strong evidence against the suggestion from earlier studies that MBSR can significantly improve CD4 counts in HIV, at least in comparison to an attention-matched control group. We also did not find evidence of significant benefits of MBSR for other immunologically related outcomes, including HIV-1 viral load, IL-6, hsCRP, or d-dimer levels. While these results do not apply directly to other conditions, they suggest caution in interpreting some of the potential benefits of mindfulness-based interventions for improving immune function, and underline the need for high-quality trials to evaluate these potential benefits before they can be translated into clinical practice.
In contrast to the immunological outcomes, we found stronger evidence of benefits of MBSR in psychological outcomes. At three months from the initiation of MBSR groups (about 1 month after completion of the 8-week course), we observed statistically significant improvements from baseline within the MBSR group in depression (as measured by the BDI), positive affect, perceived stress, and mindfulness. The control group, however, also experienced improvements in many of these measures as well, suggesting that some of the benefit may have been due to the effects of meeting in a group with other people with the same health condition. When the MBSR group was compared to the active control group, we observed statistically significant improvements in changes in positive affect in the MBSR group at 3 months. This improvement has potential implications for overall health,36–38 as well as for improved psychological health. A recent meta-analysis found insufficient evidence of effects of meditation on positive affect to determine whether it is of benefit in this regard. 39 Our results suggest that MBSR does, in fact, improve positive affect. Given growing evidence that positive affect has unique beneficial psychological and physical health benefits,40 independent of negative affect, research regarding MBSR effects on psychological well-being is worth pursuing.
While MBSR programs are thought to have benefits beyond the end of the intervention, the durability of effects has not been assessed in many studies. This has been raised as an important limitation of earlier research on MBSR41 and was one of the reasons we performed 12-month follow-up in our study. At 12 months after the beginning of the study intervention period, some of the psychological benefits observed in the MBSR group began waning. Of note, the improvement in depression, as measured by the BDI, remained stable and statistically significant compared with baseline within the MBSR group. In comparisons between the MBSR and control groups at 12 months, however, none of the improvements in psychological outcome measures were statistically significant, in part due to improvements in psychological outcome measures in the control group. We found some decrease in the amount of both formal and informal meditation practices between our assessment one month after the MBSR course (3 month time point) and 3 months later (the 6 month time point), but stable practice over the next six months. The decrease in practice after the initial intervention might account for some decreases in psychological benefits, but we found that nearly 70% of participants reported on-going use of informal practices and 44% reported sitting meditation at 12 months, indicating that the initial training resulted in a substantial frequency of on-going practice throughout the study period. In addition, we did not find any statistically significant differences in outcomes when comparing the MBSR participants who practiced formal meditation at 12 months versus those that did not. While the durability of the effect on depression is encouraging, we believe that the overall waning of effects in the MBSR group suggests that further research may be needed to optimize MBSR-based intervention programs if the goal is long-term maintenance of psychological benefits, such as testing of maintenance strategies, longer-duration MBSR, or perhaps identifying which elements of MBSR led to longer term benefit, and augmenting them in the program.
While we believe this study is a more definitive assessment of the effects of MBSR in HIV infection than prior studies, there are several limitations. Our sample size was not large enough to exclude a modest benefit in CD4+ T-cell counts, so our evidence of lack of immunologic benefit from MBSR must be interpreted with some caution. We used a very active control group, which may have provided an even greater opportunity for social interaction than in the MBSR groups. For many participants, this was their first experience of openly discussing their HIV status with other people living with HIV. Anecdotally, many comparison group participants found the groups very beneficial, and our data suggest that there were significant psychological benefits, as evidenced by statistically significant within-group improvements in depression and perceived stress at 12-months, compared to baseline. The benefits of group participation may be important to consider when the alternative to an MBSR group is no group, as is true in most clinical practice settings. In this context, the comparison with an active control group is likely to underestimate the overall psychological benefits of MBSR participation for people with HIV when compared with usual care.
In conclusion, we did not find evidence of immunological benefits of MBSR in people with HIV-1 who were not on anti-retroviral therapy, when compared with an active control group. We did find evidence of psychological benefits of MBSR at 3 months from intervention initiation, but some benefits tended to wane by 12 months. Overall, these results emphasize the need for further carefully designed research if we are to translate evidence linking psychological states to immunological outcomes into evidence-based clinical practices. Our results support some of the psychological benefits of mindfulness-based interventions, but suggest that maintenance of effects may be an important challenge to address in future research.
Figure 3:

HIV viral load levels during follow-up by group. Baseline represents pre-intervention levels. Other time periods represent months from the beginning of the MBSR or control group seminars. The dashed line with circles represents the control group. The solid line with squares represents the MBSR group. No differences achieved a p-value < .05.
Highlights.
MBSR improved positive affect in HIV, compared to an active control
Psychological benefits did not translate into improved immunological outcomes.
Some psychological benefits waned within 10 months after MBSR was completed.
Acknowledgments
Financial support: National Institutes of Health, National Center for Complementary and Integrative Health PO1 AT002024, K24 AT007827, and T32AT003997 (FMH), K01 AT005270 (LGD), UCSF-CTSI grant UL1 RR024131, and K24 MH 093225 (JTM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep 2008;5:163–71. [DOI] [PubMed] [Google Scholar]
- 2.Do AN, Rosenberg ES, Sullivan PS, et al. Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS One 2014;9:e92842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horberg MA, Silverberg MJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. Journal of acquired immune deficiency syndromes 2008;47:384–90. [DOI] [PubMed] [Google Scholar]
- 4.Kong MC, Nahata MC, Lacombe VA, Seiber EE, Balkrishnan R. Association between race, depression, and antiretroviral therapy adherence in a low-income population with HIV infection. J Gen Intern Med 2012;27:1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly JA, Murphy DA, Bahr GR, et al. Factors associated with severity of depression and high-risk sexual behavior among persons diagnosed with human immunodeficiency virus (HIV) infection. Health Psychology 1993;12:215–9. [DOI] [PubMed] [Google Scholar]
- 6.Perdue T, Hagan H, Thiede H, Valleroy L. Depression and HIV risk behavior among Seattle-area injection drug users and young men who have sex with men. AIDS Educ Prev 2003;15:81–92. [DOI] [PubMed] [Google Scholar]
- 7.Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. Jama 1993;270:2568–73. [PubMed] [Google Scholar]
- 8.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. Jama 2001;285:1466–74. [DOI] [PubMed] [Google Scholar]
- 9.Moskowitz JT. Positive affect predicts lower risk of AIDS mortality. Psychosomatic Medicine 2003;65:620–6. [DOI] [PubMed] [Google Scholar]
- 10.Ironson G, Hayward H. Do positive psychosocial factors predict disease progression in HIV-1? A review of the evidence. Psychosom Med 2008;70:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson TE, Weedon J, Cohen MH, et al. Positive Affect and Its Association With Viral Control Among Women With HIV Infection. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 2016. [DOI] [PMC free article] [PubMed]
- 12.Carrico AW, Moskowitz JT. Positive affect promotes engagement in care after HIV diagnosis. Health Psychol 2014;33:686–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyklicek I, Kuijpers KF. Effects of mindfulness-based stress reduction intervention on psychological well-being and quality of life: is increased mindfulness indeed the mechanism? Annals of behavioral medicine : a publication of the Society of Behavioral Medicine 2008;35:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ledesma D, Kumano H. Mindfulness-based stress reduction and cancer: a meta-analysis. Psychooncology 2009;18:571–9. [DOI] [PubMed] [Google Scholar]
- 15.Gayner B, Esplen MJ, DeRoche P, et al. A randomized controlled trial of mindfulness-based stress reduction to manage affective symptoms and improve quality of life in gay men living with HIV. J Behav Med 2012;35:272–85. [DOI] [PubMed] [Google Scholar]
- 16.Duncan LG, Moskowitz JT, Neilands TB, Dilworth SE, Hecht FM, Johnson MO. Mindfulness-based stress reduction for HIV treatment side effects: a randomized, wait-list controlled trial. J Pain Symptom Manage 2012;43:161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner BJ, Hecht FM, Ismail RB. CD4+ T-lymphocyte measures in the treatment of individuals infected with human immunodeficiency virus type 1. A review for clinical practitioners. Arch Intern Med 1994;154:1561–73. [PubMed] [Google Scholar]
- 18.Creswell JD, Myers HF, Cole SW, Irwin MR. Mindfulness meditation training effects on CD4+ T lymphocytes in HIV-1 infected adults: a small randomized controlled trial. Brain, behavior, and immunity 2009;23:184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SeyedAlinaghi S, Jam S, Foroughi M, et al. Randomized controlled trial of mindfulness-based stress reduction delivered to human immunodeficiency virus-positive patients in Iran: effects on CD4(+) T lymphocyte count and medical and psychological symptoms. Psychosom Med 2012;74:620–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neuhaus J, Jacobs DR Jr., Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010;201:1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 2007;21:901–12. [DOI] [PubMed] [Google Scholar]
- 23.Hammerfald K, Eberle C, Grau M, et al. Persistent effects of cognitive-behavioral stress management on cortisol responses to acute stress in healthy subjects--a randomized controlled trial. Psychoneuroendocrinology 2006;31:333–9. [DOI] [PubMed] [Google Scholar]
- 24.Kabat-Zinn J, University of Massachusetts Medical Center/Worcester Stress Reduction Clinic. Full catastrophe living : using the wisdom of your body and mind to face stress, pain, and illness. Delta trade pbk. reissue ed. New York, N.Y.: Delta Trade Paperbacks; 2005. [Google Scholar]
- 25.Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med 1985;8:163–90. [DOI] [PubMed] [Google Scholar]
- 26.Kabat-Zinn J, Massion AO, Kristeller J, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. Am J Psychiatry 1992;149:936–43. [DOI] [PubMed] [Google Scholar]
- 27.Beck A, Ward C, Mendelsen M, al e. An inventory for measuring depression. Arch General Phychiarty 1961;4:561–71. [DOI] [PubMed] [Google Scholar]
- 28.Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crises? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychology 2003;84:365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment 2006;13:27–45. [DOI] [PubMed] [Google Scholar]
- 30.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 32.Cohen S, Janicki-Deverts D. Who’s Stressed? Distributions of Psychological Stress in the United States in Probability Samples from 1983, 2006, and 2009. J Appl Soc Psychol 2012;42:1320–34. [Google Scholar]
- 33.Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet 1989;2:888–91. [DOI] [PubMed] [Google Scholar]
- 34.Hoover DR, Graham NM, Chen B, et al. Effect of CD4+ cell count measurement variability on staging HIV-1 infection. Journal of acquired immune deficiency syndromes 1992;5:794–802. [PubMed] [Google Scholar]
- 35.Raboud JM, Montaner JS, Conway B, et al. Variation in plasma RNA levels, CD4 cell counts, and p24 antigen levels in clinically stable men with human immunodeficiency virus infection. J Infect Dis 1996;174:191–4. [DOI] [PubMed] [Google Scholar]
- 36.Moskowitz JT. Positive affect predicts lower risk of AIDS mortality. Psychosom Med 2003;65:620–6. [DOI] [PubMed] [Google Scholar]
- 37.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull 2005;131:925–71. [DOI] [PubMed] [Google Scholar]
- 38.Sirois BC, Burg MM. Negative emotion and coronary heart disease. A review. Behav Modif 2003;27:83–102. [DOI] [PubMed] [Google Scholar]
- 39.Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA internal medicine 2014;174:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steptoe A, Dockray S, Wardle J. Positive affect and psychobiological processes relevant to health. Journal of personality 2009;77:1747–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bishop SR. What do we really know about mindfulness-based stress reduction? Psychosom Med 2002;64:71–83. [DOI] [PubMed] [Google Scholar]


