Abstract
The acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic has affected millions of individuals, causing major health and economic disruptions worldwide. The pandemic is still raging, with a second and third wave in a few countries, while new infections steadily rise in India. Nutrition and immune status are two critical aspects of fighting the virus successfully. Recently, selenium status was reported to positively correlate with the survival of patients with COVID-19 compared with non-survivors. We analyzed the blood serum levels in 30 apparently healthy individuals and in 30 patients with confirmed COVID-19 infection in the southern part of India. The patients showed significantly lower selenium levels of 69.2 ± 8.7 ng/mL than controls 79.1 ± 10.9 ng/mL. The difference was statistically significant (P = 0.0003). Interestingly, the control group showed a borderline level of selenium, suggesting that the level of this micronutrient is not optimum in the population studied. The results of this exploratory study pave the way for further research in a larger population and suggest that selenium supplementation may be helpful in reducing the effects of the virus.
Keywords: Micronutrient, Immune response, Inflammation, Selenium status, Viral infection, COVID-19
Highlights
-
•
The acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic is a major health concern.
-
•
Nutrition and immune status are two critical aspects of fighting the virus successfully.
-
•
Patients with COVID-19 from southern India showed a significantly lower selenium level in serum compared with controls.
-
•
Controls had borderline levels of selenium, suggesting that the level of this micronutrient is not optimum in the population studied.
-
•
Selenium supplementation may be helpful in reducing the effects of the virus.
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection (COVID-19) pandemic has led to devastating effects on the health and economy worldwide. Age and presence of comorbidities such as obesity, diabetes, cardiovascular diseases, hypertension, and pulmonary diseases are associated with disease severity [1]. More than 80% of the cases are reported to be mild, whereas the rest are associated with severe pulmonary distress, shock, myocardial injury, heart failure, dysfunctional coagulation, and renal failure. The severity of the disease is associated with an overreaction of the immune system, leading to a release of several cytokines and chemokines, also known as cytokine storm [2].
There is no specific therapeutic drug recommended for the treatment of COVID-19. Anti-flu drugs and corticosteroids are being used in clinical settings. Other therapeutic strategies include convalescent sera from recovered individuals, interferon, anti-inflammatory therapies, and ventilator support. The search for vaccines and other pharmacologic agents to prevent viral infection is underway to fight the pandemic.
Selenium is an essential trace element incorporated into 25 selenoproteins having selenocysteine in their active center. Some of these selenoproteins are essential for defense against viral infections, control of thyroid hormone signaling, protection against oxidative stress, protein folding, and mitochondrial health [3]. Glutathione peroxidases and thioredoxin reductase are selenoproteins critical for antiviral defense through their redox signaling and homeostatic activities [3]. Viral infection causes oxidative stress, enhancing the replication and accumulation of mutations in the viral RNA genome, leading to increased virulence and damage to host [4].
Selenium deficiency contributes to mutations in the viral genome to highly virulent forms, and is associated with increased susceptibility and pathogenicity of viral infections, which can be alleviated by adequate levels [4]. Selenoproteins are essential for an effective immune response to infections. Selenium supplementation has been reported to reduce allergic asthma, augment vaccine responses, and reduce the progression of tuberculosis or HIV-1 [5]. India is now reporting increased incidences of COVID-19 infection. To our knowledge, there are very few reports on the average serum selenium levels in Indians. Interestingly, an association was reported between the cure rate from COVID-19 and the basal selenium status in different regions of China [6]. Strengthening this observation, a deficiency of elemental selenium, and its transporter protein levels were reported in patients with COVID-19 in Germany [7]. Based on these reports, in the present exploratory study, we assessed the serum selenium levels in patients with COVID-19 and control individuals to understand the correlation between these levels and viral infection and recovery.
Methods
The study was conducted with 30 COVID-19–positive individuals and 30 apparently healthy controls in the 18 to 45 y age group. Controls did not present with signs or symptoms of viral/bacterial infection, had body temperature between 96°F to 99°F, and had >90% oxygen saturation. SARS-CoV-2 infection in patients was confirmed by the nasopharyngeal swab reverse transcription polymerase chain reaction test. The patients were in stable condition with fever and dyspnea without hypoxemia. Asymptomatic patients, those requiring tube feeding or parenteral nutrition, patients on ventilator support or in unstable condition, and those admitted to the isolation ward for >24 h of confirmed COVID-19–positive test were excluded from the study. Individuals participating in any other study, including any form of dietary supplements/multivitamins or disease-specific oral nutrition supplements, also were excluded from the study. Informed consent was given by all participants. The study was conducted in accordance with the regulatory requirements as per ICMR Guidelines 2017, and the Declaration of Helsinki, Fortaleza, 2013 & the ICH (Step V), Guidance on Good Clinical Practice and applicable regulatory requirements. This clinical trial was registered prospectively with Clinical Trials Registry– India.
Blood samples and 24-h urine samples were collected from all participants. Selenium status was analyzed by quantitative inductively coupled plasma-mass spectrometry (ICP-MS). All necessary precautions were taken in handling the specimens collected as per standard laboratory guidelines considering COVID-19 complications.
Statistical analysis was performed for all participants with STATA version 15 (StataCorp, College Station, TX, USA). Descriptive statistics are presented for continuous variables, whereas categorical variables are described as their respective percentages. The difference in serum selenium levels between the groups was analyzed using the Welch t test. A statistical significance of P < 0.05 was considered significant.
Results
The demographic data of the participants are presented in Table 1 . The median age was 40.5 y (26–37) in patients with COVID-19 and 33.5 y (37.5–43; P < 0.001) in the control group. The male-to-female ratio was 24:6 and 14:16 in patient and control groups, respectively. Oxygen saturation (SpO2) was significantly lower in patients (91.9 ± 1.15) than in controls (96.9 ± 1.6; P < 0.0001). SpO2 was <95% in all patients, whereas 28 of the 30 control participants had ≥95%. Two control individuals had a saturation of 94%.
Table 1.
Summary statistics of participant demographics*
| Parameter | Controls (n = 30) | COVID-19 patients (n = 30) | P-value |
|---|---|---|---|
| Age (y)* | 33.5 (26–37) | 40.5 (37.5–43) | <0.001 |
| Sex, n (%) | |||
| Male | 14 (46.7) | 24 (80) | <0.001 |
| Female | 16 (53.3) | 6 (20) | |
| BMI (kg/m2) | 25.4 ± 2.6 | 24.5 ± 2.5 | NS |
| SBP (mm Hg) | 121.9 ± 6.4 | 123.3 ± 7.9 | NS |
| DBP (mm Hg) | 77.9 ± 5.2 | 76. 7 ± 7.5 | NS |
| SpO2 (%) | 96.9 ± 1.6 | 91.9 ± 1.1 | <0.0001 |
| Pulse rate (BPM) | 81.4 ± 4.5 | 84.5 ± 4.5 | <0.01 |
BMI, body mass index; BPM, beats per minute; DBP, diastolic blood pressure; NS, non-significant; SBP, systolic blood pressure; SpO2, oxygen saturation
Age represented as median (IQR); sex represented as n (%). All other data represented as mean ± SD.
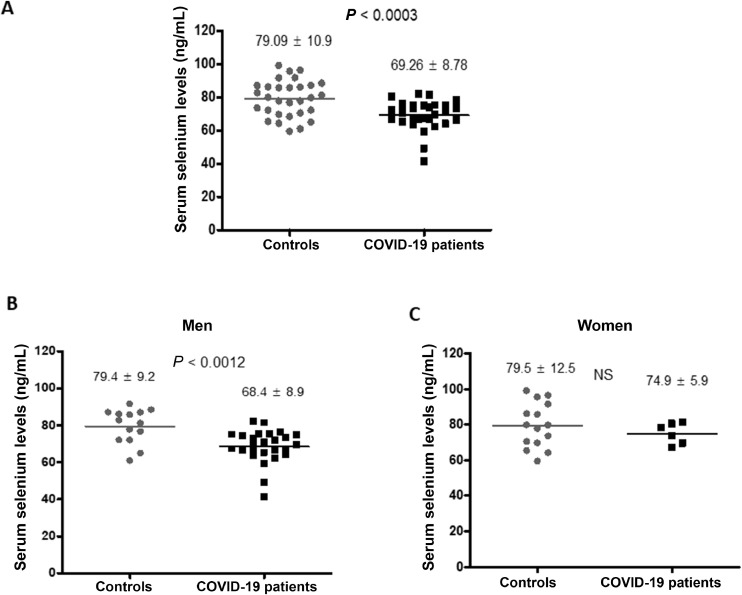
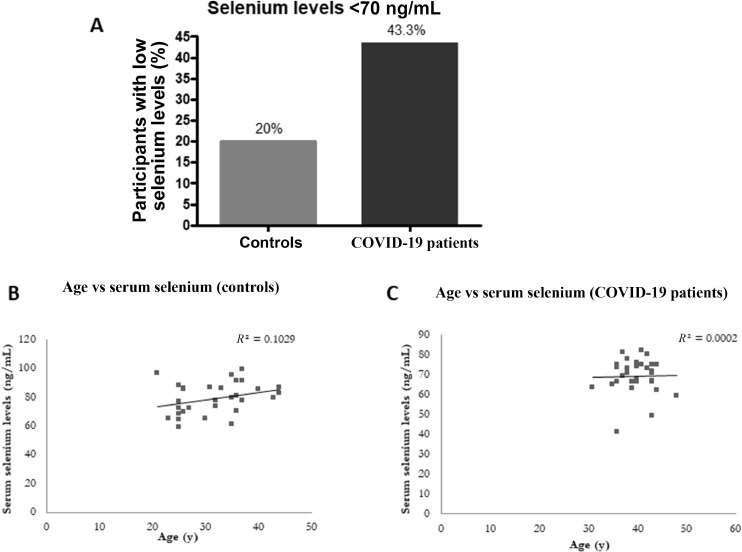
The mean serum selenium levels are represented in Figure 1 . The mean levels were 69.3 ± 8.8 ng/mL in the patients and was 79.1 ± 10.9 ng/mL in the control group. The difference of 9.8 ng/mL was highly significant (P < 0.0003; Fig. 1). The difference in selenium levels showed a similar trend in men (79.4 ± 9.2 versus 68.4 ± 8.2 ng/mL, P < 0.001 in the control and COVID-19 groups, respectively). In women, although the levels were lower in the patient group, the difference was not significant (79.4 ± 12.5 versus 74.9 ± 5.9 ng/mL). We observed that 43.3% of patients with COVID-19 had lower selenium levels compared with 20% of the control group (Fig. 2A). Because the age of controls and patients were not matched in the two groups, we performed a correlation analysis of age and serum selenium levels. The R 2 values were 0.103 for controls and 0.002 for patients (P = not significant for both) suggesting that the difference in age may not have contributed to the difference in serum selenium levels (Fig. 2 B and C).
Fig. 1.
Selenium levels in controls and COVID-19 patients. (A) Distribution of selenium levels in controls and COVID-19 patients. (B) Selenium levels in men. (C) Selenium levels in women.
Fig. 2.
(A) Percentage of participants with <70 ng/mL of serum selenium levels in control and COVID-19 patient groups. Correlation of age with serum selenium levels in (B) controls and (C) patients with COVID-19.
Discussion
Selenium status varies widely in different parts of the world. Although the normal range in the blood is fixed at 70 to 150 ng/mL, a minimum level of 98.7 ng/mL of serum selenium was required to optimize glutathione peroxidase activity based on 48 European studies and 44 in the Middle East [8,9]. Furthermore, in the National Nutrition Examination Survey, serum selenium levels (1.51 μmol/L or 118.8 ng/mL) was independently associated with anemia in older adults [10]. In the Nutritional Prevention of Cancer (NPI), serum selenium concentrations at 1.34 to 1.54 µmol/l (106–121 ng/mL) showed a protective effect against cancer in clinical studies [11], [12], [13].
Serum selenium concentrations <70 ng/mL are correlated with limiting supplies of the micronutrient as per the Food and Nutrition Board of the Institute of Medicine [14]. We observed that the mean levels in control individuals were only 79.1 ± 10.9 ng/mL, which is much lower than the optimum required levels, suggesting a general deficiency of this micronutrient in the population studied. Urine samples did not show detectable values of selenium.
Nutrition and diet influence the competence of the immune system and influence the risk for and severity of an infection [15]. Micronutrients such as iron, selenium, and zinc provide antioxidant and anti-inflammatory support and are essential for optimal functioning of the immune system [16]. The nutritional status of an individual is associated with the risk for, severity, and outcome of SARS-CoV-2 infection, thus stressing the importance of maintaining macro- and micronutrient status as a preventive measure for COVID-19 [17].
Selenium deficiency is reported to affect 500 million to 1 billion people worldwide, mainly due to inadequate dietary intake. Dietary selenium availability, in turn, is controlled by soil–plant interactions. Loss of selenium-rich soil has reduced the dietary availability of this micronutrient, thus increasing the deficiency of selenium worldwide [18]. India is a vast country with wide geographic soil status. In one study, the northern part of India was reported to have normal levels of soil selenium [19]. Similar data is not available for southern part of the country, where the present study was conducted. One study with 201 adults in Mumbai, reported an average concentration of 100 ng/mL [20]. In contrast, the present results show a borderline low level in the control individuals, suggesting that selenium deficiency may be more widespread than reported.
Conclusion
This exploratory study demonstrates that selenium status is lower in patients with COVID-19 than in healthy controls, in corroboration with two recently published studies. In the present study, relatively young patients with mild symptoms with slight hypoxia were analyzed, unlike in the German study, wherein patients were older and had severe symptoms. The observational nature of the study precluded any conclusion on the casual relationship between selenium status and COVID-19. Future studies in a larger population would be valuable. Improving the selenium status by nutritional measures or supplementation may be helpful in reducing the devastation caused by this virus in India.
Acknowledgments
The authors acknowledge the clinicians from Apollo Hospital Chennai, and Vagus Hospital Bangalore for providing the COVID-19 patient and control samples. The acknowledge the Neuberg Anand Reference Laboratory, Bangalore, for analyzing the serum selenium levels. Finally, the authors acknowledge the members of ClinWorld team who were involved with the study.
Footnotes
This project was funded by Sami-Sabinsa Group Limited. MM participated in the conceptualization, gathering of resources, and reviewing the manuscript. KN participated in the review and editing of the manuscript MM and SG participated in data curation clinical study supervision, and preparation of the first draft. LM participated in the data validation and writing, reviewing, and editing of the manuscript. All authors have read and agreed to the published version of the manuscript. The authors are affiliated with Sami-Sabinsa Group Limited or Sabinsa Corporation.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song P, Li W, Xie J, Hou Y, You C. Cytokine storm induced by SARS-CoV-2. Clin Chim Acta. 2020;509:280–287. doi: 10.1016/j.cca.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rayman MP. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 4.Guillin OM, Vindry C, Ohlmann T, Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11:2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2012;16:705–743. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am J Clin Nutr. 2020;111:1297–1299. doi: 10.1093/ajcn/nqaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moghaddam A, Heller RA, Sun Q, Seelig J, Cherkezov A, Seibert L. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12:2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoffaneller R, Morse NL. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients. 2015;7:1494–1537. doi: 10.3390/nu7031494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muecke R, Waldschock K, Schomburg L, Micke O, Buentzel J, Kisters K. Whole blood selenium levels and selenium supplementation in patients treated in a family doctor practice in Golßen (State of Brandenburg, Germany): a laboratory study. Integr Cancer Ther. 2018;17:1132–1136. doi: 10.1177/1534735418807971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semba RD, Ricks MO, Ferrucci L, Xue QL, Guralnik JM, Fried LP. Low serum selenium is associated with anemia among older adults in the United States. Eur J Clin Nutr. 2009;63:93–99. doi: 10.1038/sj.ejcn.1602889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinceti M, Crespi CM, Malagoli C, Bottecchi I, Ferrari A, Sieri S. A case-control study of the risk of cutaneous melanoma associated with three selenium exposure indicators. Tumori. 2012;98:287–295. doi: 10.1700/1125.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nomura AM, Lee J, Stemmermann GN, Combs GF., Jr Serum selenium and subsequent risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:883–887. [PubMed] [Google Scholar]
- 13.Duffield-Lillico AJ, Dalkin BL, Reid ME, Turnbull BW, Slate EH, Jacobs ET. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds . National Academies Press; Washington DC: 2000. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. [PubMed] [Google Scholar]
- 15.Im JH, Je YS, Baek J, Chung M-H, Kwon HY, Lee J-S. Nutritional status of patients with coronavirus disease 2019 (COVID-19) Int J Infect Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverio R, Gonçalves DC, Andrade MF, Seelaender M. Coronavirus disease 2019 (COVID-19) and nutritional status: the missing link? [Epub ahead of print] Adv Nutr. 2020 doi: 10.1093/advances/nmaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones GD, Droz B, Greve P, Gottschalk P, Poffet D, McGrath SP. Selenium deficiency risk predicted to increase under future climate change. Proc Natl Acad Sci U S A. 2017;114:2848–2853. doi: 10.1073/pnas.1611576114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadav SK, Singh I, Singh D, Han S-D. Selenium status in soils of northern districts of India. J Environ Manage. 2005;75:129–132. doi: 10.1016/j.jenvman.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Raghunath R, Tripathi RM, Mahapatra S, Sadasivan S. Selenium levels in biological matrices in adult population of Mumbai, India. Sci Total Environ. 2002;285:21–27. doi: 10.1016/s0048-9697(01)00892-0. [DOI] [PubMed] [Google Scholar]