Abstract
The lung is an organ that is directly exposed to the external environment. Given the large surface area and extensive ventilation of the lung, it is prone to exposure to airborne substances, such as pathogens, allergens, chemicals, and particulate matter. Highly elaborate and effective mechanisms have evolved to protect and maintain homeostasis in the lung. Despite these sophisticated defense mechanisms, the respiratory system remains highly susceptible to environmental challenges. Because of the impact of respiratory exposure on human health and disease, there has been considerable interest in developing reliable and predictive in vitro model systems for respiratory toxicology and basic research. Human air-liquid-interface (ALI) organotypic airway tissue models derived from primary tracheobronchial epithelial cells have in vivo–like structure and functions when they are fully differentiated. The presence of the air-facing surface allows conducting in vitro exposures that mimic human respiratory exposures. Exposures can be conducted using particulates, aerosols, gases, vapors generated from volatile and semi-volatile substances, and respiratory pathogens. Toxicity data have been generated using nanomaterials, cigarette smoke, e-cigarette vapors, environmental airborne chemicals, drugs given by inhalation, and respiratory viruses and bacteria. Although toxicity evaluations using human airway ALI models require further standardization and validation, this approach shows promise in supplementing or replacing in vivo animal models for conducting research on respiratory toxicants and pathogens.
Keywords: Air-liquid-interface (ALI) airway cultures, Exposure system, Inhalation toxicology, Pulmonary drug testing, Pathogen-host interaction
Introduction
The respiratory system is the key interface between the external environment and systemic circulation. It not only allows O2/CO2 gas exchange, but also simultaneously provides a barrier to protect the body from invasion by airborne pathogens and exposure to toxic chemical substances (LeMessurier et al. 2020). The respiratory system is composed of numerous specialized cell types (Fig. 1). It has evolved highly elaborate and effective mechanisms for filtering out, inactivating, and removing foreign materials. These defense mechanisms include mucociliary clearance (MCC) and innate immune capabilities, such as secretion of antimicrobial peptides and cytokines by epithelial cells and resident leukocytes in order to recruit and activate the adaptive immune system (LeMessurier et al. 2020; Sharma et al. 2020). Despite these defense mechanisms, the respiratory system remains highly susceptible to direct effects and/or penetration by potentially toxic pathogens, particles, and gases that are commonly encountered in modern environments. Examples of various sources of potential damage to the respiratory system include air pollution, occupational exposure to chemicals and particles, and tobacco products, as well as chemicals, fumes, particles, and gas exposures from everyday household chemicals, consumer products, and cosmetics. Additionally, respiratory system-specific pathogens (e.g., influenza, tuberculosis, rhinovirus, and coronaviruses) have evolved and adapted to evade the respiratory defense mechanisms (LeMessurier et al. 2020; Sharma et al. 2020). Infection by respiratory bacteria and viruses sickens and kills millions of people worldwide each year as well as inflicts huge economic burdens.
Figure 1.
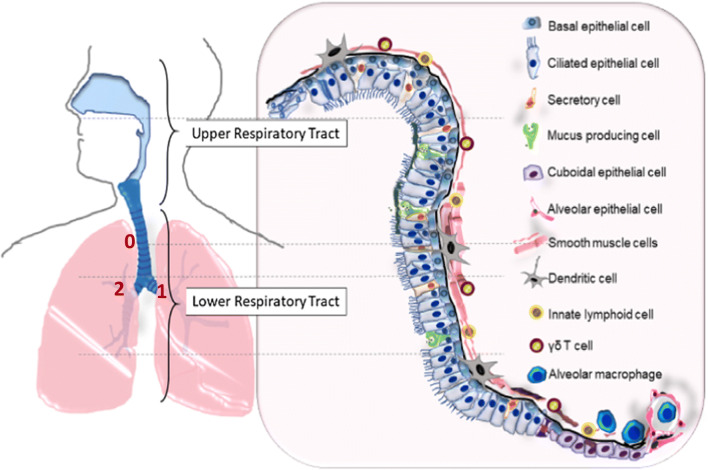
Human respiratory tract is lined with region-specific cell types. Both the upper and lower respiratory tracts are shown in this illustration. The lower respiratory comprises the conducting zone for air passage and respiratory zone for gas exchange between the lung and pulmonary capillaries. The conducting airway consists of the trachea, bronchi, and bronchiole; the respiratory airway consists of the respiratory bronchioles as well as the alveolar ducts and sacs. Each region of the respiratory tract is lined with region-specific cell types as illustrated in this figure. Human airway has 23 generations of dichotomous branching, starting from the trachea as generation 0. The ALI airway tissue models discussed in this review are derived from epithelial cells harvested from generations 0 to 2 (labeled in the graph). Minor modification of the drawing published by LeMessurier et al. (2020) Front Immunol 11: 3. Copyright© 2020 LeMessurier, Tiwary, Morin, and Samarasinghe.
Given the impact of respiratory exposures to human health and disease, development of model systems for respiratory toxicology and basic research has been an area of longstanding interest. Reliable and predictive models of the human respiratory system continue to be a pressing need. Specific applications of respiratory model systems include regulatory safety and hazard assessment of chemicals and nanoparticles (NPs), tobacco research, infectious respiratory disease, and pulmonary drug development (Lacroix et al. 2018).
Animal models, such as mouse, rat, dog, guinea pigs, and non-human primate, have been in widespread use for inhalation toxicology applications for many years. Such approaches have the advantage of identifying systemic effects from inhalation exposure that integrate pharmacokinetic and pharmacodynamic processes in the model animal. However, significant differences in the anatomy, physiology, and breathing patterns between animals and humans have made translation of experimental results obtained in animals to humans problematic. Furthermore, animal models of human respiratory diseases, such as asthma, chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis, are not fully equivalent to the human diseases, leading to high failure rates of clinical trials designed using animal data (Shanks et al. 2009).
The limited success in translating animal data to human outcomes has become increasingly recognized and appreciated in recent years. Ethical concerns regarding the use of animals in toxicological research also have been raised. The National Research Council Report “Toxicity Testing in the 21st Century: A Vision and a Strategy” recommends the replacement of animal tests with relevant in vitro human-based test systems (National Research Council 2007). Transition from animal- to human-based models is ultimately expected to lead to faster and better predictive toxicity assessments and therapeutic development at lower cost. In line with these goals, US federal regulatory agencies consider the development and validation of alternative in vitro methods for acute toxicity testing, including acute inhalation toxicity testing, to be a high priority (Clippinger et al. 2018). Specifically, the US Environmental Protection Agency (EPA) has a stated goal of reducing in vivo acute inhalation testing for pesticide submissions (EPA 2016a; EPA 2016b).
Following the initial development of culture medium capable of supporting in vitro growth of normal human bronchial epithelial cells (Lechner et al. 1981), submerged monolayer cultures derived from human respiratory tissues have been used widely as tools for respiratory research and toxicology studies (Berube et al. 2010). However, these traditional methods, which promote extensive cell proliferation, do not adequately reproduce the differentiated phenotype of in vivo airway epithelial tissues. To circumvent these limitations, culture methods that result in a better representation of three-dimensional, well-differentiated in vivo respiratory tissues have been developed.
Human airway organoids developed from human cell induced pluripotent stem cells, or adult tissue stem cells possess the cell polarity and several of the cell types (e.g., goblet cells, ciliated cells, basal cells, and club cells) found in airway epithelium. Thus, they represent a primary cell–derived in vitro airway epithelial model (van der Vaart and Clevers 2020). Three-dimensional organoids can be maintained successfully under submerged conditions in long-term culture (Sachs et al. 2019). Airway organoids have been used for a variety of applications, including drug screening and as disease models (van der Vaart and Clevers 2020). However, they form as spheres with lumens inside and are absent of an air interface. It is, therefore, not possible to directly expose lung organoids to inhaled substances in their native forms, as occurs in vivo. An alternative airway model that can be exposed directly to gases, vapors, and aerosols involves culturing primary airway cells on microporous membrane scaffolds at the air-liquid interface (ALI) (Adler et al. 1987; Whitcutt et al. 1988).
Compared to submerged monolayer cultures, differentiated, organotypic ALI airway models have a more realistic in vivo–like structure, as well as barrier properties and metabolic functions similar to those found in vivo, and can be dosed in a more human-relevant manner than can submerged or organoid cultures (Wu et al. 1986; Yamaya et al. 1992; Kaartinen et al. 1993). Primary cells that undergo cellular differentiation can reproduce an in vivo–like transcriptional profile similar to that of human airway epithelium (Pezzulo et al. 2011) and replicate in vivo toxicity responses, such as cilia dysfunction (Brekman et al. 2014), squamous metaplasia, and goblet cell hyperplasia (Bolmarcich et al. 2018). Infection by human respiratory pathogens, such as Bordetella pertussis (Soane et al. 2000), influenzae (Chan et al. 2010), and coronavirus (Jia et al. 2005), is also highly dependent on the differentiation state of airway epithelium. The use of human cells in ALI airway culture systems also allows studying the etiology of respiratory diseases, such as asthma, COPD, cystic fibrosis (CF), and idiopathic pulmonary fibrosis, as well as variability in population-based responses.
This review will provide a state-of-the-art update on ALI airway tissue models. A survey of ALI exposure systems and key applications, such as inhalation toxicity testing of NPs and chemicals, tobacco research, pulmonary drug testing, and host-pathogen interactions, will be presented. Finally, the strengths and limitations of the current technology, status of using these in vitro systems as a replacement for in vivo animal models, and recommendations for future directions will also be discussed.
Overview of the Airway ALI System
Organotypic ALI airway tissue models were first successfully developed in the late 1980s by growing primary tracheal epithelial cells from guinea pigs at the air-liquid interface (Alder et al. 1987; Whitcutt et al. 1988). A chamber with a permeable gelatin membrane was employed to separate the culture environment into two compartments that simultaneously exposed cells to air and supplied culture medium through the membrane. Under such conditions, primary airway cells differentiated into heterogeneous cell populations with a polarized mucociliary phenotype resembling the native tissue from which they were derived. With the advances made in stem cell culture techniques, ALI models have been established from human primary cells of the nasal, proximal, and distal airway epithelium (Fuchs et al. 2003; Fulcher et al. 2005; Muller et al. 2013; Huang et al. 2017; Rayner et al. 2019). Models simulating the tracheobronchial epithelium of the lung have been most extensively characterized and employed in respiratory research and, therefore, are the focus of this review.
Functions of Airway Epithelial Cells
The human tracheobronchial epithelial lining consists of a mixed population of secretory cells, ciliated cells, and basal cells organized into a pseudostratified columnar structure, in which all cells reside on basal lamina. Ciliated cells account for 50–70% of the epithelial cell population (Staudt et al. 2014) and each cell has about 200 to 300 cilia of approximately 6 μm in length on its luminal side (Serafini and Michaelson 1977). Cilia are microtubule-based structures. Active ciliary movement depends on both the basal body and the cytoskeleton structures surrounding it and are driven by the energy produced by mitochondria aggregated close to the luminal surface (Harkema et al. 1991).
The mucus-producing goblet cells are the major secretory cells found in tracheobronchial airways. These cells contain mixtures of highly glycosylated electron-lucent mucin granules that can be readily released upon stimulation (Jeffery 1983). Together with beating ciliated cells, they form a dynamic mucociliary escalator, which is one of the major defense mechanisms for trapping and clearing inhaled substances out of the airways without inducing inflammatory responses. Obstruction of the airways as a result of secretory cell hyperplasia and metaplasia as well as compromised MCC is common in subjects with chronic inflammatory airway diseases (Hauber et al. 2006).
Another major type of airway epithelial cell is basal cells. These are undifferentiated epithelial cells and account for approximately 31% of the airway epithelial cell population (Boers et al. 1998). Although a long-held theory is that basal cells are the only progenitor cells that can undergo self-renewal during normal tissue maintenance and terminal differentiation into secretory or ciliated cells in response to tissue injury (reviewed in Berika et al. 2014), increasing evidence demonstrates that other cell types also possess cellular plasticity. For instance, stimulated secretory cells were found to transdifferentiate into ciliated cells (Ayers and Jeffery 1988; Rawlins et al. 2009). Ciliated cells can de-differentiate into squamous cells soon after the injury to preserve tissue integrity and later re-differentiate into secretory cells and ciliated cells to restore an intact layer of airway epithelium (Park et al. 2006). Besides its role as the airway progenitor cells, basal cells have other important structural and regulatory functions. Owing to their central location at the epithelial-mesenchymal trophic unit of the airways, basal cells can interact with various cell types and thus also play a role in regulating inflammatory responses and transepithelial water movement and forming lateral intercellular space along the basement membrane (Evans et al. 2001). A balance between mucus secretion and clearance as well as proportions of different epithelial cell types, therefore, is critical for the health of airway epithelium.
Functions of Airway Epithelium
Epithelial cells are interconnected through a series of junctional complexes, such as tight junctions (TJs), adherens junctions, and desmosomes (Ganesan et al. 2013). Airway epithelium, however, is more than just a physical barrier covering the respiratory tract; it also provides innate immunity crucial for maintaining airway tissue integrity and homeostasis during normal tissue renewal as well as when responding to tissue injuries (Tam et al. 2011). The protective functions of airway epithelium are achieved by its unique cellular structures, such as intercellular junctional complexes and ion channels, and mucociliary escalators, as well as its ability to produce antimicrobial molecules, including cytokines, chemokines, and proteases (Godfrey et al. 1992; Bals 2000; Fahy and Dickey 2010). The epithelial junctional complexes not only modulate the movement of water, ions, and macromolecules, but also function as signaling platforms and regulate proliferation, apoptosis, and differentiation of the epithelial cells (Balda and Matter 2009). Environmental challenges to these junctional complexes could cause membrane leakage and abnormal tissue repair and regeneration, leading to the development of lung diseases, such as asthma, COPD, and lung cancer.
Defects in barrier function have been reported in the lung of subjects with CF and asthma (Rezaee and Georas 2014). Immunohistochemistry staining of asthmatic bronchial epithelial biopsies revealed a reduction in the expression of α-catenin, E-cadherin, ZO-1, and possibly occludin, accompanied by a reduced transepithelial electrical resistance (TEER) and an increased permeability to dextran of less than 20 kDa (de Boer et al. 2008; Xiao et al. 2011). In patients with CF, disorganized TJ strands extend beyond the apical belt (Godfrey et al. 1993). Furthermore, mutations in the cystic fibrosis transmembrane conductance regulator gene (CFTR) result in dysregulation of the depth and barrier functions of TJs (LeSimple et al. 2010). These structural abnormalities impair epithelial functions and significantly increase the susceptibility of CF patients to pathogenic bacteria, such as Pseudomonas aeruginosa (Rezaee and Georas 2014), colonization of which in the airways further disrupts the assembly of TJs (Plotkowski et al. 1999). Thus, in vitro models capable of reproducing the key structural and functional aspects of the airway epithelium will be powerful tools for assessing respiratory toxicity and evaluating the disease potential of airborne substances.
Characteristics of ALI Airway Tissue Models
Primary normal human bronchial epithelial (NHBE) cells isolated from the tracheobronchial region of the airways are de-differentiated cells when they are propagated as monolayers in submerged culture (Wise and Lechner 2002). Although these cells have been used widely for respiratory biology and toxicology studies (Takizawa et al. 1999; Kawasaki et al. 2001; Fields et al. 2005), the presence of a single cell type and the lack of the structural and functional features of the in vivo airway have greatly limited their usefulness as in vitro cell models for toxicity assessment.
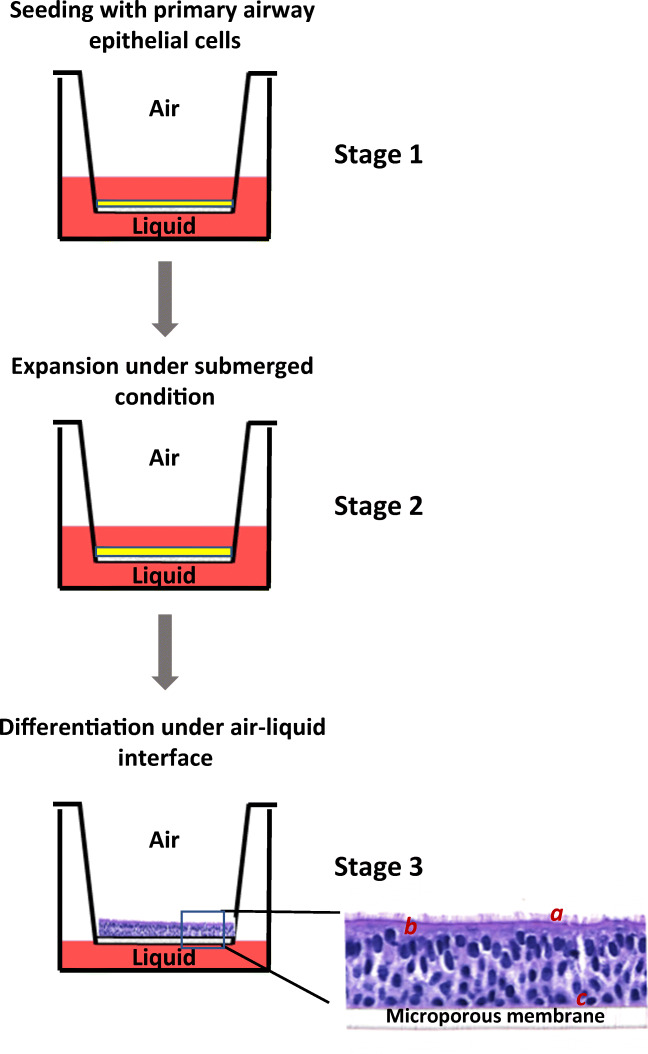
The organotypic ALI airway model is generated in situ by differentiating NHBE cells at an air-liquid interface created by a semi-permeable membrane support (e.g., Corning® Transwell®, Millipore® Millicell®, or Greiner Bio-One ThinCert™ culture insert). The apical surface of the ALI culture is exposed to the surrounding air, which oxygenates the epithelial cells and promotes cellular differentiation (Bebök et al. 2001). Cells are nourished from medium in the basolateral compartment through the microporous membrane. Under these biphasic culture conditions, NHBE cells proliferate and differentiate into a polarized pseudostratified tissue-like structure resembling that of the airway epithelium, with ciliated cells interspersed with goblet cells facing the apical side and basal cells spreading along the basolateral membrane (Fig. 2) (Yamaya et al. 1992; Bals et al. 2004). In addition, extracellular matrix is secreted and deposited onto the basolateral side of the epithelium during differentiation (Cozens et al. 2018).
Figure 2.
A schematic diagram of the human ALI airway tissue model. The procedure of establishing the ALI airway tissue model is schematically illustrated on the left panel. NHBE cells are seeded onto the microporous membrane pre-coated with extracellular matrix proteins, such as collagen (stage 1). Primary cells continue to proliferate under the submerged condition until they reach complete confluence (stage 2). Differentiation is initiated by lifting NHBE cells to the air-liquid interface and feeding with differentiation medium from the basolateral side (stage 3). NHBE cells are differentiated into a pseudostratified phenotype on a microporous membrane around 4 weeks after air-lift. The apical side of the ALI culture is exposed to air; the basolateral side takes up nutrients through microporous membrane. (a) Ciliated cell; (b) mucus-producing goblet cells; (c) basal cells.
Comparison of the transcriptome profiles of the primary cell–based ALI cultures and NHBE cells grown in submerged culture indicates that ALI cultures are much more similar to native epithelial tissues in vivo than are submerged monolayer cultures (Pezzulo et al. 2011). However, subtle differences in gene expression patterns exist (Dvorak et al. 2011). Compared to human airway tissue, ALI cultures express higher levels of basal cell-related genes as well as genes involved in the cell cycle and proliferation. Such discrepancies in gene expression may reflect the difference in the proportion of epithelial cell types between the in vitro and in vivo airway epithelium. The overall similarity in their gene expression also extends to the expression of essential xenobiotic metabolizing enzymes. A broad panel of the phase I and phase II metabolism genes, including cytochrome P450 (CYP) enzymes, glutathione S-transferases (GSTs), and UDP-glucuronosyltransferases (UGTs), is significantly upregulated during maturation of the ALI cultures and their expression remains elevated over a period of 6 months in culture (Boei et al. 2017; Qin et al. 2019). In particular, enzymes expressed exclusively in the lungs, such as CYP2A13, CYP2F1, and CYP4B1 (Carr et al. 2003; Poch et al. 2005; Su et al. 2000), are markedly increased upon differentiation (Newland et al. 2011; Boei et al. 2017; Qin et al. 2019), whereas their expression in undifferentiated NHBE cells is marginal. Enzymatic activity assays using CYP-specific substrates confirm the activity of key xenobiotic metabolism enzymes in ALI cultures.
In contrast to the upregulation observed with many CYP genes, expression of CYP1A1 was found to be significantly lower in differentiated ALI cultures compared to undifferentiated NHBE cells (Qin et al. 2019). This observation, however, is consistent with the report that expression of CYP1A1 was inducible by cigarette smoke (CS) and, therefore, only detected in lung tissues from smokers (MeLemore et al. 1990). Besides CS, exposure of ALI airway cultures to 2,3,7,8-tetrachlorodibenzodioxin (TCDD), aromatic hydrocarbons, and diesel exhaust (DE) also significantly enhanced the expression of CYP1A1 (Boei et al. 2017). Altogether, these observations suggest that well-differentiated ALI cultures possess metabolic capabilities to biotransform xenobiotics that are absent in conventional submerged primary cultures. Conservation of metabolic activities in the ALI cultures is essential for employing such culture systems in toxicity testing and human risk assessment, especially for test articles that require biotransformation. As an example, Qin and colleagues recently demonstrated that the tobacco-specific genotoxicant and carcinogen, 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone (NNK), failed to induce DNA damage in undifferentiated NHBE cells in the absence of an exogenous liver S9 activation system, due to the lack of bioactivation in these cells (Qin et al. 2019).
Considerable overlap has been observed in the protein composition of apical secretions from ALI airway cultures and normal induced sputum (Kesimer et al. 2009; Baxter et al. 2015). In particular, host defense proteins, such as mucins, proteases, and antimicrobial proteins, are found in both in vitro and in vivo secretions, making up 20–30% of their total mass. Although the secretions have overall similarities, the levels of individual protein components vary (Baxter et al. 2015). For instance, several studies have reported that MUC5B is present in lower abundance in the apical secretions of ALI airway cultures compared to induced sputum (Ali et al. 2011; Baxter et al. 2015). Differences in the collection, processing, and analytical methods may have accounted for the discrepancies. It is also possible, however, that induced sputum could contain molecules secreted by immune cells and other cell types not present in the ALI cultures. Collectively, these differences may confound experimental findings, leading to equivocal conclusions. Methodologies that take potential variables into consideration, therefore, should be developed.
Fully differentiated ALI airway models retain key functions of the in vivo airway epithelium for maintaining homeostasis and responding to environmental challenges. Intercellular junctions, such as TJs and adherens junctions, and drug transporters, such as p-glycoprotein (P-gp), have been identified at the juxta-apical region of ALI airway cultures (Lin et al. 2007; Prytherch et al. 2011; Rayner et al. 2019). The orientation of these apical structures contributes to the polarized morphology of the ALI cultures (Gaillard et al. 2010; Saint-Criq and Gray 2017). Similar to TJs assembled in vivo, the TJ complexes in ALI cultures consist of desmosomes in the subapical regions (Davis et al. 2015). This structural resemblance supports the use of ALI cultures as an in vitro system for investigating the TJ-modulating effects of chemicals, such as interleukin-13 (IL-13), cadmium, and CS (Cao et al. 2015; Schmidt et al. 2019; Tatsuta et al. 2019). Schmidt and colleagues reported that IL-13 altered the expression patterns of claudins, induced the colocalization of TJ proteins with proteasomes, and triggered ubiquitination of TJ proteins, eventually leading to degradation and disruption of the junctional complexes (Schmidt et al. 2019). Functional epithelial ion channels, such as the sodium channel (ENaC) and CFTR, also are present in ALI airway cultures (Enuka et al. 2012; Rayner et al. 2019). These ion channels play essential roles in regulating the height, viscosity, and pH of the airway surface liquid. Conceivably, dysregulation of epithelial ion channels can result in dehydration of the airway surface and impair the function of mucociliary escalators as seen in CF and COPD patients.
Several studies have reported that submerged NHBE cells and differentiated ALI cultures respond differently to environmental challenges. van Wetering and colleagues explored effects of differentiation status on Th2 cytokine-mediated production of eosinophil-attractant chemokines eotaxins in both culture systems (van Wetering et al. 2007). Although gene and protein expression of eotaxins was induced in both NHBE cells and ALI cultures, increases in the level of eotaxin-3 were much higher in ALI cultures. Furthermore, the IL-4-mediated secretion of eotaxin-2 was mitigated by TNFα only in submerged NHBE cells. In another study, Ji and colleagues demonstrated that palladium NPs were taken up at a much lower level in ALI cultures than in submerged NHBE cells (Ji et al. 2017; Wilkinson et al. 2011). Furthermore, the NPs tend to gather at one spot in the ALI cultures, possibly due to the sweeping motion of ciliated cells. Similar differences in response between the two systems also were observed with aldehyde vapors, where significant induction of cytokine release and oxidative stress occurred only in the ALI cultures (Dwivedi et al. 2018).
The differential responses observed in submerged NHBE cells and ALI cultures may result from factors other than their differentiation status. Exposures at the air-liquid interface allow direct exposure of the apical side to airborne substances in their native form, simulating in vivo inhalation exposure. Such exposures prevent potential interactions between the test article and medium components and, therefore, facilitates the uptake of aerosolized particles (Loret et al. 2016). Hypoxia has been proposed as an additional factor underlying the differential responses between the two systems (Ghio et al. 2013). However, this hypothesis needs to be further corroborated using better controlled experimental designs. Nevertheless, these findings suggest that fully differentiated ALI cultures may be more suitable as an in vitro tool for generating toxicity data than traditional submerged cultures. Along with its ability to retain its tissue-specific functions for an extended period, airway ALI tissue models represent a simple and relatively cost-effective and easy-to-use advanced in vitro lung model for acute and sub-acute toxicity evaluations of inhaled substances (Berube et al. 2010; Lacroix et al. 2018).
Unique Endpoints Developed in ALI Airway Models
The structural and functional similarity of ALI airway cultures to in vivo airway epithelium has greatly expanded the possibility of measuring tissue-specific endpoints, such as tissue barrier functions, ion channel physiology, MCC, and tissue morphology, in an in vitro system. Evaluation of tissue responses that are relevant to respiratory diseases is expected to provide valuable information for determining the potential adverse health effects of airborne substances.
Barrier function can be assessed by measuring TEER and paracellular permeability (Strengert and Knaus 2011). TEER measurement is a non-destructive technique that has been widely applied for evaluating epithelial integrity preserved by intact TJs (Srinivasan et al. 2015). Either a Ussing chamber or volt ohm meter (e.g., EVOM2, World Precision Instruments, Sarasota, FL) can be used to measure the flow of electrical current across the cell layer of the ALI cultures. When cultures are well-differentiated, they develop barrier functions that typically have a resistance above 200 Ω cm2. Changes in tissue integrity in response to environmental challenges alter ion flux and can be reflected in electrical resistance or impedance. The tracer flux assay is routinely used for assessing the traverse of aqueous solutes. To conduct this assay, ALI cultures are incubated from the apical side with fluorophore-conjugated probes of different sizes and shapes for a predetermined time. Tracers migrate through the paracellular space and accumulate in medium bathing the basolateral side of the cultures. Concentrations of the tracers in the basolateral medium are measured and an apparent permeability coefficient (Papp) of the epithelium calculated (Strengert and Knaus 2011). Besides these quantitative assays, confocal microscopy also has been used as a visual tool for studying the dynamics of TJs (Buckley et al. 2018). Depending on the purpose of the studies, these methods can be used in combination to comprehensively assess the tissue barrier properties of the ALI cultures under experimental conditions.
Electrophysiological properties of ion transporters, such as ENaC and CFTR, can be evaluated in ALI cultures using a Ussing chamber designed to fit the configuration of the culture inserts (Fulcher et al. 2005; Gianotti et al. 2018). ALI cultures are mounted between 2 hemi-chambers; both are filled with buffer solution (e.g., Kreb’s bicarbonate Ringer’s solution) gassed continuously with a mixture of CO2 and air (e.g., 5% CO2–95% air) for maintaining the physiological pH. Transepithelial electrical resistance, potential difference, and short-circuit current are measured sequentially. By using the Ussing chamber, the role of ion channels in mucosal pathophysiology and effects of test substances on the ion channels can be studied.
The mucociliary escalator is an essential component of the airway innate defense for maintaining the health of the respiratory system (Knowles and Boucher 2002; Fahy and Dickey 2010). It is composed of viscous gel-forming mucins and beating ciliated cells. Airborne substances are first trapped in the mucus and then propelled out of the airways by the coordinated movement of ciliated cells. Compromised MCC results in extended exposure of the airways to inhaled toxicants and contributes to the undesirable health effects associated with diseases, such as CF, COPD, and primary ciliary dyskinesia (PCD) (Rogers 2005; Donaldson et al. 2007; Bush and Hugg 2012). Effects on MCC can be studied indirectly in ALI airway cultures by evaluating responses of the individual components of the mucociliary escalator, i.e., ciliary beat frequency (CBF) and mucus production, or directly by measuring mucociliary transport (MCT) rates.
Commercial software (e.g., Sisson-Ammons Video Analysis System, Clio, MI) is available for quantitatively measuring CBF and active ciliary points (Sisson et al. 2003). Production and secretion of two major gel-forming airway mucins, MUC5AC and MUC5B, can be analyzed semi-quantitatively using commercial or laboratory-developed ELISA assays. The effects of test articles on MCC can be inferred based on the beating of ciliated cells and mucin production. MCT rates also can be measured directly and quantitatively in ALI cultures by tracking the movement of fluorescent microspheres, usually 1 to 2 μm in diameter, over the mucosal surface (Worthington and Tarran 2011). It should be noted that the ALI culture is a closed system where mucus is not continuously removed as occurs in vivo. Conceivably, accumulation of mucus on the apical surface may affect the MCT rate (Sears et al. 2015). For assays assessing barrier functions and MCC, the temperature at which the assays are conducted and the medium formulation may introduce variation into the measurements. Development of standardized protocols, therefore, is critical to ensure the comparability of data from longitudinal studies or between laboratories.
Besides the aforementioned endpoints, tissue morphology has been evaluated using immunohistochemistry staining of formalin-fixed, paraffin-embedded tissue sections (Xiong et al. 2018; Xiong et al. 2019). These measurements provide valuable insight into the responses of individual cell types as well as signs of early morphological changes that may lead to pathogenesis. For instance, goblet cell morphology can be evaluated using Alcian blue/periodic acid-Schiff staining; its ultrastructure can be assessed using electron microscopy. Treatment-associated apoptosis, mitosis, and tissue degeneration can be detected at much lower levels than possible with biochemistry assays. Pathology observations in conjunction with other measurements can generate a comprehensive picture of adverse tissue responses to external stimulation. The ALI airway culture, therefore, is considered a sophisticated in vitro model for evaluating many of the toxicity endpoints used for the in vivo assessment of airborne substances.
Sources of the ALI Cultures
The airway ALI culture is a significant improvement over monolayer NHBE cells. Owing to its structural and functional similarities to in vivo airway epithelium, the ALI model has gained increasing recognition as a physiologically relevant in vitro culture system for respiratory research (Berube et al. 2010). Commercially available culture inserts with semi-permeable membranes effectively support the biphasic culture environment that is required for differentiating the primary cells. In general, three stages are involved in the development of ALI airway cultures: (1) initial expansion of submerged primary cultures on plastic substrata, (2) secondary cell expansion on culture inserts under submerged conditions, and (3) differentiation of primary cells on culture inserts under ALI conditions. Expansion of primary cells in culture dishes not only generates sufficient cells for establishing ALI cultures, but also allows for biobanking cells in limited supply. Culture vessels are usually coated with extracellular matrix proteins, such as collagen, gelatin, or fibronectin, to aid cell attachment and differentiation. To date, a number of culture media and methods have been developed for establishing ALI airway models (Gray et al. 1996; Fulcher et al. 2005; Liu et al. 2007; Prytherch et al. 2011; Cao et al. 2018). Although the medium formulations vary, retinoic acid (RA) is a common and essential ingredient in all recipes for promoting mucociliary differentiation of NHBE cells. The extent of differentiation, proportions of the different cell populations, and the overall morphology depend on the culture methods as well as the passage number of the NHBE cells. Variability in these factors is expected to influence the results of toxicity assessments. Commercial vendors may supply immature cultures as a measure of cost-saving. Under such circumstances, end-users can either request the vendors deliver well-differentiated cultures, which may increase the unit cost of the cultures, or maintain immature cultures in their own laboratories until they are fully differentiated. No matter which option end-users prefer, it should be emphasized to researchers using ALI airway models that only fully differentiated cultures should be employed for experimentation to ensure the validity and reproducibility of the studies. With NHBE cells and expansion and differentiation media becoming commercially available, it is now possible to reproducibly produce ALI cultures in batches of 100 s in-house using easy-to-follow protocols (Cao et al. 2018; Rayner et al. 2019). Generating relatively large numbers of ALI tissue models allows the quantitative measurement of toxicity endpoints using a range of test article concentrations and with sufficient replicates to detect differences between exposure conditions.
Commercial Models
While the methods for production of ALI airway cultures are well established in the literature and cell sources and required materials are readily available, production of well-differentiated ALI airway cultures is a time-consuming endeavor and can also be technically challenging for some laboratories. This situation has led to the establishment of several ready-to-use ALI airway models as commercial products. The first commercially available ALI airway models (EpiAirway™ and EpiAirway-FT™) were introduced by MatTek Corporation (Ashland, MA). Additional commercial ALI models (MucilAir™, SmallAir™, and OncoCilAir™) are available from Epithelix Sarl (Plan-les-Ouates, Switzerland). These products are shipped as viable tissues that are ready-to-use for experimentation following a brief recovery period or additional culturing to allow further differentiation in the end-user laboratory.
“Home-Made” Models
Making airway ALI cultures in-house offers flexibility in designing and carrying out experiments and “home-made” models can be made at lower cost than purchasing commercial models. PneumaCult™ Expansion and Differentiation medium kits developed by STEMCELL Technologies (Vancouver, Canada) are one of the commercial ALI culture methods that are widely used for generating “home-made” ALI airway models. The recently developed PneumaCult™-Ex Plus medium kit supports expansion of primary NHBE cells for up to 6 passages without significantly losing their differentiation potential (Rayner et al. 2019). CnT Airway Proliferation and Differentiation Medium kits developed by CELLnTEC (Bern, Switzerland) and BronchiaLife™ Epithelial Airway Medium Complete Kit coupled with HBTEC Air-Liquid Interface Differentiation Medium (LifeLine Cell Technologies, Frederick, MD) represent other commercial method for establishing “home-made” ALI airway cultures. The components of these media are chemically defined and free of materials of animal or human origins. A range of progenitor cell-targeted growth factors and co-factors as well as trace elements, protective antioxidants, and vitamins are supplemented in the basal proliferation medium to enhance the longevity of the primary large airway epithelial cells. ALI airway cultures generated using these Medium kits have been widely utilized in various areas of respiratory research (Tanaka et al. 2018; Hess et al. 2019; Xia et al. 2020).
To make airway ALI cultures, NHBE cells first are grown in expansion medium on collagen-coated plastic culture dishes until they reach approximately 80% confluence. Cells are gently detached from the culture dish with low concentrations of trypsin-EDTA solution (e.g., 0.025%). Expanded cells are then seeded onto culture inserts at a density of 1.2 × 105 cells/cm2 and allowed to further proliferate on the inserts with expansion medium added to both the apical and basolateral compartments. When they attain complete confluence, the cells are “air-lifted” by removing the medium in the apical compartment and adding differentiation medium to the basolateral compartment. This leaves the apical side of the cultures exposed to air and the basolateral side in contact with the differentiation medium. As discussed above, such culture conditions simulate the native environment and are essential for driving the differentiation of the NHBE cells into the mucociliary phenotypes found in the airway. Achieving fully differentiated ALI cultures takes approximately 4 wk of feeding every 2 or 3 d with fresh differentiation medium. A set of quality standards (e.g., cilia motility, mucus production, and histological examination) is necessary to ensure the quality of the cell cultures and the validity of in vitro findings using these cultures.
Exposure Systems Used with ALI Airway Cultures
Before specialized exposure systems became commercially available, testing of airborne substances often was conducted by applying dissolved test articles directly onto the lung cells. Such exposures not only fail to capture what happens in vivo, but also may introduce irrelevant chemical interactions, limiting the translational value of any findings (Aufderheide 2005; Limbach et al. 2005; Gminski et al. 2010; Raemy et al. 2012; Lenz et al. 2013; Loret et al. 2016; Upadhyay and Palmberg 2018). Dwivedi and colleagues demonstrated that solubilization of chemicals in culture medium blunted the effects of three pulmonary irritants (i.e., acrolein, crotonaldehyde, and hexanal), making direct comparison between the vapor- and aqueous-phase exposures difficult (Dwivedi et al. 2018). Furthermore, interpretation of studies on chemicals known to either react with components of culture medium (Coyle et al. 2018) or partition into the non-bioavailable compartments of the medium, such as lipids and proteins (Gülden and Seibert 2003), must consider the effects of these factors on chemical bioavailability. The latter scenario applies particularly to lipophilic agents, such as aryl hydrocarbon receptor agonists (Lee et al. 2011; Boei et al. 2017). Finally, agglomeration in culture medium is a major problem in testing NPs. Conceivably, these confounding factors could limit the value of in vitro toxicity assessments.
Tarkington and colleagues first described exposing cultures directly to test atmospheres at the air interface to simulate in vivo inhalation exposures (Tarkington et al. 1994). Ideally, design of such exposure systems meets the following criteria: (1) cultivation of lung cell models under ALI conditions, (2) direct contact between airborne test articles and cells, and (3) homogeneous deposition of the test article across the culture surface. Commercial exposure systems have been developed based on these criteria (Aufderheide and Mohr 1999). Direct exposure of the semi-dry apical side of the airway epithelium to test articles in aerosol, vapor, or gas form not only reproduces the proximal airway exposure experienced by humans, but also facilitates more realistic cell-chemical interactions and faster uptake kinetics of the test articles than achieved by treatment of submerged cultures (Lenz et al. 2013; Loret et al. 2016). It is recommended to wash the apical side of the ALI airway cultures with PBS to remove excess mucus secreted by the goblet cells. This is an important practice to ensure homogenous delivery of test articles throughout the culture surface (Jackson et al. 2018). Dosimetry measurement under such conditions can be made by chemical analysis or using quartz crystal microbalances (QCMs) (Lenz et al. 2014; Wang et al. 2019a). However, QCM measurement must be vigorously validated to decouple mass deposition from the influence of viscoelasticity of test articles on vibration of the crystals (Parlak et al. 2013; Wang et al. 2019a).
A variety of exposure systems have been developed for direct exposure of lung cells to test articles in their physiologically relevant forms (Aufderheide and Mohr 1999; Bitterle et al. 2006; Lenz et al. 2013; Thorne and Adamson 2013; Lacroix et al. 2018; Tsoutsoulopoulos et al. 2019; Wang et al. 2019a; Wang et al. 2019b). These exposure systems range from those designed and used by individual research laboratories (Müller et al. 2011; Gualerzi et al. 2012; Amatngalim et al. 2015; Ji et al. 2017; Dwivedi et al. 2018) to widely available systems that are commercially marketed (Aufderheide and Mohr 1999; Okuwa et al. 2010; Li et al. 2012; Tsoutsoulopoulos et al. 2019). Gases, NPs, aerosols, particles, and complex aerosols (e.g., CS and diesel exhaust) have been tested using specialized systems that expose the apical surface of ALI airway cultures (Aufderheide and Mohr 2004; Aufderheide and Gressmann 2007; Okuwa et al. 2010; Lenz et al. 2013; Nara et al. 2013; Lenz et al. 2014; Wang et al. 2019a; Wang et al. 2019b). In the following sections, we will discuss the adaptability and applicability of select commercial exposure systems manufactured by Vitrocell® (Vitrocell® Systems GmbH, Waldkirch, Germany) and CULTEX® (CULTEX Laboratories GmbH, Hannover, Germany), two major equipment manufacturers in the field of in vitro inhalation toxicology.
Vitrocell® Systems
A variety of airborne substances, such as NPs, gases, and complex mixtures (e.g., CS and e-cigarette [e-cig] aerosols) have been tested using exposure systems designed and manufactured by Vitrocell® (Lenz et al. 2014; Neilson et al. 2015; Polk et al. 2016; Ding et al. 2017; Fields et al. 2017; Wang et al. 2019a). These Vitrocell® exposure systems are discussed in this section.
-
a
Liquid Aerosol Exposure Systems
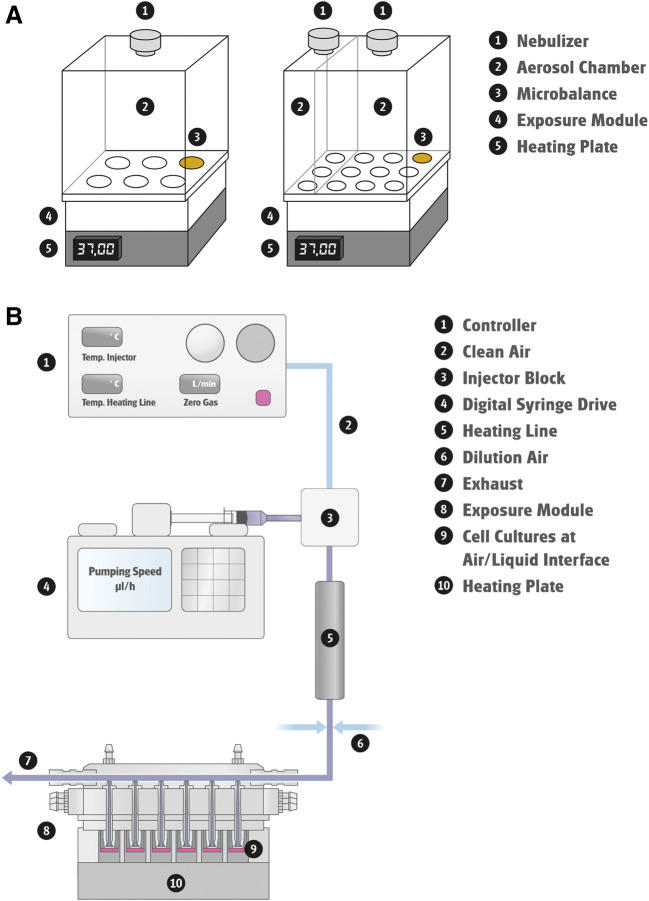
The Cloud System was developed for single exposures of liquid aerosols under ALI conditions. It consists of a nebulizer (Aeroneb® Pro, Aerogen, Galway, Ireland), a removable exposure chamber, a base cultivation module with one position having a QCM for real-time mass deposition measurement, and a heating unit for maintaining a temperature of 37°C during exposure (Fig. 3b). During aerosol exposure, a dense cloud of droplets is produced by aerosolization of chemical solutions/suspensions through the piezoelectrically driven vibrating membrane in the nebulizer. Droplets form a uniform aerosol cloud inside the exposure chamber and uniformly deposit on the apical side of the cells housed in the base module over a period of 5 minutes. The overall deposition efficiency and dose rate of test articles is high, making it amenable for drug testing. The Cloud System has been used for the efficacy testing of pulmonary drugs and the safety evaluation of NPs and airborne particles (Lenz et al. 2014; Chortarea et al. 2017; Röhm et al. 2017; Wang et al. 2019a). Reproducible, spatially uniform deposition of test aerosols in each position of the exposure module was confirmed by fluorometric and chemistry analysis (Lenz et al. 2014; Röhm et al. 2017; Wang et al., 2019a). Furthermore, aerosolized vehicles (e.g., phosphate-buffered saline) by themselves do not affect cell viability, CBF, or IL-8 induction in lung models, indicating that operation of the Cloud System does not induce undesirable stress to the cells (Lenz et al. 2014; Wang et al., 2019a). Altogether, these observations support its application for exposing cells to liquid aerosols generated from non-volatile agents, NPs, and therapeutics. Compared with the more complex aerosol generation systems, the Cloud System is lower in cost, smaller in dimensions, and easier to maintain. However, this simple setup may not be optimal for simulating environmental or occupational exposures where subjects are exposed to airborne toxicants for hours on a daily basis. A continuous aerosol generation system, such as the BioAerosol Nebulizing Generator, equipped with a reservoir for solutions and suspensions, in combination with an in vitro exposure system, such as the 24/48 exposure module, could overcome this limitation. Test articles are continuously delivered to the nebulizer at a predefined speed by a peristaltic pump, allowing precise adjustment of aerosol concentrations without altering the airflow rate. Aerosols can be further diluted to lower concentrations before entering the exposure module. This setup has greatly broadened the range of test substances as well as dosing regimens for toxicity assessment. Manche and colleagues evaluated the genotoxicity of 3 alcohols that are commonly used for prevention of nosocomial infections (Manche et al. 2018). Mucoepidermal lung carcinoma NCI-H292 cells grown under ALI conditions were exposed intermittently to up to 85% (w/w) alcohol aerosols for a total of 60 minutes. Responses in both in vitro micronucleus and comet assays were negative at all concentrations tested.
-
b
Vapor Generation and Exposure Systems
The Vitrocell® Spiking System is designed for exposing ALI airway cultures to gases, vapors, and gases generated from volatile and semi-volatile substances. This system consists of two components, a vapor generation system and a cell exposure module. The vapor generation system has a controller for adjusting the flow of clean air and system temperature and a digital syringe drive for injecting test articles at a predefined speed (Fig. 3B). Volatiles or semi-volatiles are vaporized by heating to their boiling points at the injection site. Vapors then are delivered through a heated line to the cell exposure module, where secondary dilution of the test articles takes place by mixing incoming vapors perpendicularly with clean (dilution) air. Concentrations of the test articles can be further adjusted by changing the rate of secondary dilution air flow. The cell exposure module houses the lung models, supplies culture medium to basolateral side of the ALI cultures, and maintains temperature and humidity during the treatment. The test articles are drawn from the dilution system into the exposure module and through a trumpet system by a steady vacuum. Most Vitrocell® exposure modules employ the trumpet technology for active diffusion and deposition of vaporized and aerosolized test articles onto the cells. Performance of the 24/48 exposure module, which exposes up to 48 culture inserts simultaneously, was characterized for CS delivery (Majeed et al. 2014). Results from multiple measurements, including photometry, in-line QCM, and chemistry analysis, demonstrated uniform distribution of CS vapors and particles, with an observed coefficient of variation of 12.2% between inserts.
Recently, Oldham and colleagues conducted an in-depth evaluation of the 24/48 exposure module on the deposition efficiency and uniformity of monodisperse solid fluorescent particles with various mass median aerodynamic diameters (MMAD) and compared the experimental measurements with computational fluid dynamics (CFD)–predicted values (Oldham et al. 2020). Deposition of these particles across the surface of culture inserts was visualized using fluorescence microscopy. In general, deposition efficiency was highly dependent upon MMAD and in good agreement with CFD prediction. However, uniformity of deposition within individual insert exceeded ± 45% of the mean for particles with smaller MMAD. The heterogeneity of particle deposition within the culture inserts revealed by this study stressed the importance of determining the appropriate number of culture replicates based on both aerosol size and the sensitivity of the in vitro endpoints when using this exposure system for particle toxicty testing.
For particles and aerosols, QCMs can be incorporated into the exposure module for real-time monitoring of the delivered doses (Adamson et al. 2013). Although QCMs are not applicable for a system intended for gas exposures, in-line Fourier transform infrared (FTIR) spectroscopy may be used to provide dose measurements in real time. By using the Spiking System, cells can be efficiently and consistently exposed at the ALI to ppm or ppb concentrations of vapors and gases under conditions simulating respiratory exposures in vivo.
The vaporization efficiency of volatile and semi-volatile test substances depends on their chemical and physical properties, such as boiling point, stability, and purity. Theoretically, the Spiking System can vaporize chemicals with boiling points of up to 400°C. Some volatiles and semi-volatiles, however, become degraded or polymerize at temperatures even below their boiling points. When working with these types of chemicals, the highest temperature, at which these undesirable side reactions do not occur, should be used to guide the choice of a heating temperature. However, vaporization efficiency will decrease at lower temperatures, as will the maximal achievable concentration. The range of in vitro test concentrations, therefore, may be limited, which is a major disadvantage for testing thermally unstable chemicals or chemicals with tendency to polymerize at elevated temperatures. The vaporization capacity of the Spiking System also can be limiting. When the injection volume per unit time exceeds the system capacity, droplets will form at the tip of the injection syringe, which will interfere with vapor generation, resulting in large variations in delivered concentrations. Droplet formation is highly likely to occur with chemicals in aqueous solutions, such as formaldehyde.
To ensure consistent generation of vapors and gases, in-line real-time dose monitoring systems, such as gas chromatography-mass spectrometry or FTIR, are highly recommended. Alternatively, test articles can be collected into impingers connected at the end of the vapor generation system and analyzed chemically. However, such methods examine only the average vapor concentrations over a predefined period of time and, therefore, may overlook short-term variations in vapor concentrations. To ensure exposure of cells to consistent concentrations of vapors throughout the study, especially when conducting repeated exposures, it is recommended that the vapor generation system be validated on a daily basis.
Very few studies have been conducted using the Spiking System and most of them have not been published in peer-reviewed journals. However, the Spiking System has been used to evaluate the respiratory toxicity of vapors generated from styrene, formaldehyde, and acrolein (Wang et al. 2019b; Brandwein et al. 2020; Ren et al. 2020). Wang and colleagues repeatedly exposed human ALI cultures to styrene vapors to simulate inhalation exposure in humans. Styrene vapors were collected into impingers containing methanol and the concentration measured by analytical chemistry before conducting each cell exposure. System validation demonstrated a vapor generation efficiency with variations of less than 10% (Wang et al. 2019b). The 3M Strategic Toxicology Laboratory used the Spiking System to expose cells to acrolein vapors at the ALI. Comparison with findings made under the submerged culture conditions revealed that acrolein vapor induced dose-dependent toxicity at much lower concentrations (Brandwein et al. 2020). More testing should be conducted in order to comprehensively evaluate the performance and capability of the Spiking System and develop standard methods for system validation and cell exposure.
-
c
Cigarette Smoking Robots
Early in vitro assessments of CS were conducted by exposing submerged lung cells to cigarette smoke extract (CSE) or condensate (CSC) diluted in physiological buffer or culture medium (Thaikoottathil et al. 2009; Schamberger et al. 2015). Given the complex composition of CS, such exposure methods fail to simulate the exposure experienced by smokers and may influence the outcomes of in vitro studies (St-Laurent et al. 2009). To overcome these limitations, whole smoke exposure systems have been developed for conducting cell exposures. Commercial smoking robots, such as Vitrocell® VC1 and VC10 (Adamson et al. 2017; Keyser et al. 2019) and Borgwaldt RM20S (Phillips et al. 2005; Azzopardi et al. 2015), have been used for exposing cells to freshly generated CS aerosols under ALI conditions and using standard or customized smoke-generating regimens. The Vitrocell® Smoke Exposure Systems allow for consistent and reproducible generation and delivery of CS or e-cig aerosols to ALI cultures (Adamson et al. 2014; Behrsing et al. 2018). In general, these systems consist of two main components, i.e., a smoking machine for generating CS or e-cig aerosols and an exposure chamber for diluting and delivering the aerosols to the cells. VC1 is a manual smoking machine. Its design is based on the VC10 Smoking Robot technology. The scope of application of both VC1 and VC10 ranges from in vitro experiments to chemical analysis (Adamson et al. 2013; Thorne et al. 2013).
Extensive characterization of the Vitrocell® Smoke Exposure System demonstrated consistent particulate deposition, vapor phase marker CO delivery, and biological responses (Thorne et al. 2013). In a recent study, nicotine was validated as a CS dosimetry marker for the VC1 smoking robot (Behrsing et al. 2018). In another study, deposition of CS particles generated by the VC10 smoking robot was measured using QCM and results compared across 6 independent laboratories. Little or no difference in particle deposition between positions within the exposure module was observed, demonstrating reproducible performance of the VC10 smoking robots. To adapt the conventional smoking robots for e-cig research, Vitrocell® VapeStarters are used for activating various e-cig products and, therefore, have greatly expanded the range of tobacco products that can be tested with the systems. Aerosol deposited mass per puff as well as nicotine and carbonyl concentrations are commonly used for e-cig dosimetry measurement (Adamson et al. 2017; Iskandar et al, 2019; Bishop et al. 2019).
Figure 3.
Representative in vitro exposure system from Vitrocell®. (A) Vitrocell® cloud system. Configurations with single or double aerosol chambers and various insert sizes are available. The setup with double chambers (on the right) allows conducting vehicle exposures simultaneously with chemical exposures. (B) Vitrocell® Spiking System.
CULTEX® Systems
First-generation CULTEX® exposure modules exposed cells to test chemicals at the ALI using specially designed inlet nozzles positioned in close proximity to the cells. These exposure modules were used for the in vitro assessment of particles, mineral fibers, wood dust, volatile compounds, CS, and therapeutics under ALI conditions (Ritter et al. 2001; Aufderheide et al. 2003; Aufderheide and Mohr 2004; Aufderheide and Gressmann 2007; Deschl et al. 2011). Characterization of the CULTEX® modules was carried out by validating gas delivery in the exposure module and assessing particulate deposition using fluorescence spectrophotometry (Ritter et al. 2001; Aufderheide et al. 2003; Deschl et al. 2011). Reproducible and dose-dependent particle deposition was demonstrated in these studies.
Unlike the linear design of the Vitrocell® Exposure Modules and first-generation CULTEX systems, newer CULTEX® Exposure Systems have adopted a radial flow system (RFS) (Aufderheide et al. 2011). Radial arrangement of the cell cultures mitigates against formation of a test article concentration gradient. The test atmosphere is introduced into the exposure module through a central inlet, allowing uniform delivery, distribution, and deposition of the test article within the device (Aufderheide et al. 2013). CFD was also used to determine particle number and mass distribution of the test articles in this study. CULTEX® RFS Exposure Systems have been used for toxicity assessment of CS, NPs, and gases (Aufderheide et al. 2011; Aufderheide et al. 2013; Rach et al. 2014; Steinritz et al. 2013; Tsoutsoulopoulos et al. 2019). A modified version of the exposure module was used for analyzing the mutagenic potency of airborne substances in the AMES assay (Aufderheide et al., 2011). In a recent study, the acute toxicity of a set of 20 pre-selected test articles was assessed using the CULTEX® RFS and compared with in vivo reference data (Tsoutsoulopoulos et al. 2019). This study demonstrates a high level of concordance, specificity, and sensitivity as well as good intra/inter-laboratory reproducibility for the in vitro findings. CULTEX® offers a diverse range of exposure systems for in vitro toxicology research, including the CULTEX® RFS, CULTEX® RFS Compact, and CULTEX® LongTermCultivation-Continuous (LTC-C), all based on the radial flow design.
Applications of ALI Cultures
Owing to their physiological similarity to in vivo airway epithelium, primary cell–based ALI tissue cultures have been employed as in vitro models for elucidating the mechanisms underlying respiratory diseases, including acute epithelial injury, fibrosis, COPD, asthma, and cancer, as well as for studying pathogen-host interactions. In recent years, these models have been increasingly adapted for in vitro respiratory toxicity testing. Early studies relied on the direct application of the test article onto the apical side of the cultures to mimic inhalation exposure or addition to the basolateral medium to simulate systemic exposure. Although such exposures are easy to conduct, the physiological relevance of treating the air interface of ALI cultures with liquid solutions has been questioned. The development of aerosol/vapor generation and delivery systems has greatly expanded the range of test articles to include nanomaterials, volatile chemicals, and non-volatile chemicals, as well as CS aerosols and e-cig vapors, with exposures simulating in vivo inhalation exposures under an in vitro setting. Key applications of ALI cultures for respiratory research (summarized in Table 1) will be highlighted in the following sections.
Table 1.
Summary of applications using primary cell–based ALI airway tissue models
| Areas of application | Description | References |
|---|---|---|
| Pulmonary drug testing | Studying deposition and absorption of inhalable drugs | Acosta et al. 2016; Reus et al. 2014 |
| Studying active and passive drug transport | Lin et al. 2007; Madlova et al. 2009 | |
| Evaluate efficacy of antiviral and antibiotic drug candidates | Brockman-Schneider et al. 2014; Mata et al. 2012; Outlaw et al. 2019; Palermo et al. 2009; Tanabe et al. 2011; Triana-Baltzer et al. 2010; Zimmermann et al. 2009 | |
| Pathogen-host interaction | Respiratory viral infection, e.g., influenza virus, rhinovirus, respiratory syncytial virus, and SARS-CoV-2 coronavirus | Matrosovich et al. 2004; Zhang et al. 2005a; Jakiela et al. 2008; Zhang et al. 2002; Dijkman et al. 2009; Kindler et al. 2013; Zhu et al. 2020; Pyrc et al. 2010; Banach et al. 2009 |
| Respiratory bacterial infection, e.g., Bacillus anthracis, Bordetella pertussis, Mycobacterium tuberculosis, and Pseudomonas aeruginosa | Powell et al. 2015; Gasperini et al. 2017; Guevara et al. 2016; Raffel et al. 2013; Schwab et al. 2002; Balder et al. 2009; Prince et al. 2018; Reuschl et al. 2017; Matsuyama et al. 2018; Zhang et al. 2005b; Zulianello et al. 2006; Soong et al. 2011; Verkaik et al. 2014 | |
| Toxicity testing for inhaled chemical agents | Nanoparticles (NPs) | |
| Evaluating biological and toxicological effects of inhaled NPs | Tilly et al. 2020; Kooter et al. 2017; Geiser et al. 2017 | |
| Assessing pathological effects of NPs on normal and diseased airway and relate the data to existing epidemiological studies | Sacks et al. 2011; Kooter et al. 2019; Ji et al. 2019; Geiser et al. 2017; Beyeler et al. 2018 | |
| Complex mixtures | ||
| Testing toxicity and biological effects of inhaled complex mixtures, e.g., e-cig, gasoline/diesel exhaust, and wood-burning stove particulates | Adamson et al. 2013; Adamson et al. 2014; Thorne et al. 2013; Behrsing et al. 2018; Corbett et al. 2019; Moses et al. 2017; Antherieu et al. 2017; Fields et al. 2017; Scheffler et al. 2015; Zarcone et al. 2016; Zarcone et al. 2017; Zarcone et al. 2018; Künzi et al. 2015; Vaughan et al. 2019; Hawley and Volckens, 2013; Shaykhiev et al. 2011; Shaykhiev and Crystal, 2014; Forteza et al. 2012; Iskandar et al. 2015; Ishikawa and Ito, 2017; Aufderheide et al. 2017; Amatngalim et al. 2018; Mertens et al. 2017; Schamberger et al. 2015 | |
| Studying genome-wide association of ontological responses to complex mixture exposure | Corbett et al. 2019; Moses et al. 2017 | |
| Single chemicals | ||
| Testing acute and sub-acute toxicity of inhaled single chemicals | Seagrave et al. 2010; Lantz et al. 2001; Tollstadius et al. 2019; Wang et al. 2019a | |
| Evaluating mechanisms of toxicity induced by single chemical exposure |
Gwinn et al. 2017; Foster et al. 2017; McGraw et al. 2020; Kelly et al. 2014; Foster et al. 2017; McGraw et al. 2020 |
In Vitro Toxicity Testing for Inhaled Agents
-
a
Nanoparticles
Engineered nanomaterials (ENMs) are used in a wide range of applications. As the production and usage of ENMs surge, risks of exposure to NPs also increase, especially considering that aerosolization of NPs is unavoidable during manufacturing processes (Cassee et al. 2011; Lu et al. 2012; Zhu et al. 2017; Evangelista et al. 2019). Respiratory exposure to NPs is anticipated during the production, usage, and disposal of ENMs, which have demonstrated their cytotoxicity, genotoxicity, fibrogenicity, and carcinogenic potential in both in vivo and in vitro models (Shvedova et al. 2008; Li et al. 2010; Wang et al. 2011). However, such risks have not been well characterized due to many challenges, including the impracticality of assessing the toxicities of the vast number of ENMs in animals. With the rapid development in nanotechnology resulting in an expected increase in human respiratory exposure, cost- and time-effective methods are urgently needed to expedite the toxicity evaluation of ENM aerosols (Durantie et al. 2017; Clippinger et al. 2018).
Traditional in vitro approaches using submerged cell cultures have been widely used as a primary choice for toxicity screening of inhaled NPs. However, it is increasingly recognized that the characteristics of NPs under submerged conditions deviate from that of the inhaled “dry” NPs to which humans are exposed in vivo. For example, interaction of NPs with culture medium components could affect their hydrodynamic dimeter, agglomeration, morphology, and surface modification; these factors will affect the settling rate and efficiency of NPs, which directly impact their delivered doses in vitro (DeLoid et al. 2015; Moore et al. 2015; Roszak et al. 2016). Some metal oxide NPs can be solubilized in culture medium; solubilization of NPs, however, is known to artificially alter their biological activities under submerged exposure conditions (Utembe et al. 2015; Kornberg et al. 2017). Exposing NP aerosols at the air interface could circumvent many of these issues and, overall, better represents how pulmonary NP exposures occur in vivo. Several studies have compared cellular responses between submerged and air-interface conditions (Raemy et al. 2012; Tilly et al. 2020). The deposited doses per surface area were first calculated for the respective studies to ensure that the comparison was made at equivalent doses. For submerged cultures, deposited doses can be estimated based on the administered concentrations, particle characteristics, and particokinetics (Deloid et al. 2014). For NP aerosol exposure, surface doses can be directly measured using QCM or atomic absorption spectroscopy (Raemy et al. 2012; Tilly et al. 2020). Differences were noted in dose- and time-response patterns and lowest-observed-adverse-effect levels. Primary cell–based ALI airway cultures have been increasingly used for NP toxicity evaluation (Kooter et al. 2017). The physiological relevance of the primary cell–based ALI cultures, along with their air interface, provides several advantages for nanotoxicology research, including the following: (1) minimal NP agglomeration, (2) avoidance of hydrolytic reactions or surface modifications of NPs by culture medium, (3) controlled and measurable delivered doses, and (4) assessment of airway tissue responses to test NPs. Geiser and colleagues conducted toxicity evaluations of silver (Ag) and carbon (C) NP aerosols in ALI cultures using a portable nano aerosol exposure system (Geiser et al. 2017). A single, short-time exposure to these aerosols induced moderate cytotoxicity and pro-inflammatory responses in a dose-response manner. In another study, Kooter and colleagues compared the toxicity of pristine (nCuO) and carboxylated (nCuOCOOH) NP aerosols in ALI cultures from asthmatic and healthy donors (Kooter et al. 2019). Asthma enhanced the sensitivity of the airway models to these NPs, possibly due to a combination of the hyperreactive respiratory tract and inefficient MCC in these cultures. The effect of donor disease state on tissue vulnerability to external stimuli was further explored in a study on aerosolized carbon nanoparticles (CNPs). ALI cultures mimicking normal and chronic bronchitis (CB) mucosa were exposed to CNP aerosols using the XposeALI® system (Ji et al. 2019). In general, responses involving inflammation, oxidative stress, tissue repair, and cell type–specific marker gene expression were more pronounced in the ALI airway cultures simulating CB. These in vitro findings are consistent with epidemiological studies where an association was established between the susceptibility of CB subjects and exposure to particulate matter (Sacks et al. 2011). The concordance between the in vitro and in vivo observations supports the value of employing ALI airway tissue models for elucidating mechanisms underlying the vulnerability of predisposed individuals to NP exposures. The reliability of this approach was demonstrated in studies that explored the toxicity of multi-walled carbon nanotubes (MWCNTs) (Beyeler et al. 2018). Airway ALI cultures derived from healthy and COPD donors were exposed acutely for 24 h to concentrations of MWCNTs that were set based on the permissible exposure limit of 1 μg/m3 recommended by the National Institute for Occupational Safety and Health (NIOSH). Acute treatment at low doses of MWCNTs caused minimal changes in epithelial integrity, cell death, and inflammatory responses, while the concurrent positive control Dörentruper Quartz, which is known to induce pro-inflammatory cytokine expression, upregulated expression of a range of inflammatory and oxidative stress genes, regardless of the disease status. As MWCNTs pose hazards to workers at manufacturing facilities, long-term in vitro studies using repeated exposure regimens at human-relevant doses will be necessary to appropriately assess their potential risks. Such exposure regimens are only possible in cell models that have relatively long life spans, like ALI airway cultures. Taken together, these studies highlight the applicability and unique advantages of such in vitro approaches for comprehensive assessments of the health hazards posed by NPs.
-
b
CS and e-Cig Aerosols
CS is a complex mixture of combustion products in the form of particulate matter, vapors, and aerosols (Rodgman and Perfetti, 2013), many of which are known carcinogens (Hecht 2012) and pulmonary irritants (Thorne et al. 2015; Gonzalez-Suarez et al. 2016). COPD, one of the respiratory diseases that are highly associated with CS exposure, is characterized in the airway epithelium by basal cell hyperplasia, squamous cell formation, loss of barrier function, decreased cilia biogenesis and function, and goblet cell hyperplasia (Shaykhiev and Crystal, 2014). These adverse tissue responses, in addition to key early molecular initiating events, have been reproduced using primary cell–based ALI airway cultures. Acute exposure to CS caused ontological, cytological, and genome-wide changes reflective of the in vivo pathogenesis associated with CS (Shaykhiev et al. 2011; Forteza et al. 2012). For example, CS-induced changes in the expression of xenobiotic metabolism gene in co-cultures of differentiated ALI cultures with fibroblasts are well-correlated with those observed in bronchial scrapings from smokers (Iskandar et al. 2015).
The in vitro endpoints measured in ALI cultures under different exposure protocols can be compared to the pathology observed in human airway epithelium to associate the impact of exposure metrics on CS toxicity. While several smoking regimens are accepted as the standards, the Canadian Intense (CI) regimen, which often generates higher levels of reactive aldehydes and nicotine (Pazo et al. 2016), caused a more rapid induction in cytotoxicity compared to the International Organization for Standardization (ISO) 3308 regimen, although the maximal responses produced by the two protocols were similar (Bishop et al. 2019). Aufderheide and colleagues examined the effects of repeated CS exposures on metaplasia in ALI airway mono- and co-cultures with lung fibroblasts (Aufderheide et al. 2017). A total of 13 CS exposures induced the formation of non-hyperplastic cytokeratin 13–positive areas, accompanied by reduced mucus secretion, in both mono- and co-cultures. Studies examining the effects of repeated CS exposures on differentiation of the ALI cultures have also be conducted. Findings from these studies support the use of these cultures to model bronchial re-epithelialization during tissue repair as well as normal epithelial maintenance (Ishikawa and Ito, 2017). Differences in the toxicity of CS generated from cigarettes with different product designs can be characterized using the ALI systems. Although acute exposures are often used for these studies, the relatively longer life span of ALI airway cultures makes them amenable to repeated, sub-acute treatments (i.e., treatments that last up to several months), which can provide useful information on CS toxicity.
Apart from assessing the toxicity of CS by itself, studies have been conducted to understand the association between the host-pathogen interaction and pulmonary injuries in smokers. CS suppresses host defenses in the lungs of smokers (Herr et al. 2009). Sub-acute exposures of ALI cultures to CS decrease the expression of respiratory host defense proteins as a result of aberrant epithelial differentiation (Ishikawa and Ito, 2017), leading to a deficiency in the transepithelial transport of IgA (Amatngalim et al. 2018). Mertens and colleagues co-exposed ALI airway cultures to CS and IL-13 and investigated the effects of CS on pathogenesis of asthma (Mertens et al. 2017). The gene expression signature of IL-13 was irreversibly modulated by CS, demonstrating the importance of the interaction between the Th2 genes and CS in the development of asthma. These studies provided mechanistic evidence on the vulnerability of smokers to respiratory pathogens.
Electronic cigarettes (e-cigs) have been promoted as a safer alternative to conventional cigarettes; however, e-cig aerosols contain chemicals associated with pulmonary injury, including reactive aldehydes, particulate matter, and volatile organic compounds (Kosmider et al. 2014). Despite rapid growth in the use of e-cigs, their long-term health effects remain largely unknown. The recent outbreak of acute respiratory injury among e-cig users in the USA highlights the urgency of developing methodologies for assessing the potential risks of e-cigs and other novel tobacco products and has stimulated research on the toxicodynamics of e-cig aerosols (Perrine et al. 2019). Genome-wide association studies have correlated ontological responses in e-cig-exposed ALI cultures to those in bronchial scrapings from e-cig users (Moses et al. 2017; Corbett et al. 2019). The concordance between the in vitro and in vivo data suggests the suitability of employing ALI airway cultures for evaluating the pathological changes induced by inhaled substances, including CS and e-cig aerosols.
With regard to e-cig dosimetry, Shen and colleagues demonstrated that nicotine content was a leading predictor of e-cig aerosol cytotoxicity, as aerosols from nicotine-containing e-cig products were more potent than their nicotine-free analogues in ALI cultures (Shen et al. 2016). Relatively easy to quantitate, nicotine has been suggested as a dosimetry marker for in vitro e-cig aerosol exposures (Adamson et al. 2017; Bishop et al. 2019).
To date, most investigations on e-cig aerosol exposures have employed acute exposures (Scheffler et al. 2015; Moses et al. 2017; Antherieu et al. 2017; Fields et al. 2017). With the experience gained from repeated CS exposures, it should be possible to use airway ALI cultures to conduct sub-acute exposure studies with e-cig aerosols. Although the in vitro toxicity assessment of e-cig aerosols is still in its infancy, the primary cell–based ALI airway tissue model system has proven to be a relevant platform for screening alternative tobacco products as well as elucidating the mechanisms underpinning e-cig aerosol toxicity to human bronchial epithelium.
-
c
Chemicals
Primary cell–based ALI culture systems have been used increasingly for chemical toxicity testing, particularly for testing irritants and oxidizers. Testing, however, has been impeded due to the difficulty in developing standardized methods for generating and delivering chemical aerosols and vapors, which is complicated by the vast differences in the physiochemical characteristics of test articles with potential health concerns. Gases and vapors readily reach pulmonary tissues by respiration. There are many examples where ALI cultures have been exposed to volatile agents using relatively crude exposure method that involve ad hoc approaches devised in-house, such as the vapor cup (Seagrave et al. 2010; Gwinn et al. 2017). A limitation of this approach, however, is that it relies on the theoretical estimation of a nominal vapor exposure concentration based on a head space calculation using the chemical partition coefficient and other factors (Kelly et al. 2014). True control of the exposure concentrations, ideally by performing real-time monitoring, can be technically and logistically challenging. Nevertheless, the simplicity of the vapor cup exposure method has a major advantage of eliminating the need for the complex vapor generation, monitoring, and exposure systems necessary for a continuous flow-through in vitro inhalation exposure system. A simple procedure of direct application of chemical solutions or suspensions has also been shown as a quick and useful screening method for acute respiratory toxicants and irritants. After pipetting the suspension or solution of chemical test agent directly onto the apical tissue surface, the tissue insert is capped to prevent evaporation of vehicle or volatile components. Tissue viability is determined following a 3-h incubation period. This method correlates well with Global Harmonized System (GSH) and EPA acute inhalation toxicity categories determined by in vivo rat inhalation exposures studies and allows for relatively fast screening of large numbers of chemicals (Jackson et al. 2018).
Human ALI airway models have been extensively used to evaluate potential mechanisms of airway toxicity and fibrosis (obliterative bronchiolitis) induced by occupational exposure to artificial butter flavoring chemicals, such as 2,3-butanedione (diacetyl [DA]), including DA-induced basal cell effects (Gwinn et al. 2017; Foster et al. 2017; McGraw et al. 2020) and involvement of EGFR signaling pathway (Kelly et al. 2014; Foster et al. 2017; McGraw et al. 2020). Exposure of ALI airway mono- or co-cultures with donor-matched fibroblasts to DA vapors using vapor cups caused airway epithelial injury with histopathological evidence of basal/suprabasal cell spongiosis (Gwinn et al. 2017) as well as hyperphosphorylation and cross-linking of basal cell–specific keratins (Foster et al. 2017). In another study, McGraw and colleagues demonstrated increased ubiquitination of keratin 5 and decreased expression of p63, both of which are basal cell markers, and proteasome activity was shown to be involved in basal cell repair of injury induced by DA vapors (McGraw et al. 2020). Collectively, these findings suggest that basal cells are a major target of DA toxicity. Analyses of the secreted proteasome in ALI airway cultures exposed to DA vapors revealed secretion of pro-inflammatory/pro-fibrotic cytokines and chemokines, including the EGFR ligands, TGFα, and amphiregulin (AREG), as well as IL-1α, IL-6, and IL-8 (which is EGFR-dependent) (Kelly et al. 2014; Brass et al. 2017; Gwinn et al. 2017; Kelly et al. 2019). These observations suggest an important role for EGFR signaling in DA toxicity.
Exposure to non-volatile agents also presents difficulties, considering the technical feasibility of agent delivery, dose quantitation, reactivity/partition of test agents within the delivery system, and the physiological relevance of the exposure modality. A balance between practicality and relevance must be struck to produce responses relevant to humans and with minimal artifacts. The development of aerosol exposure modules, such as the Vitrocell® Cloud System and similar setups, has significantly eased toxicodynamic studies of aerosolized non-volatiles (Lantz et al. 2001; Tollstadius et al. 2019), particularly for reproducibly generating aerosols over extended periods of time. Wang and colleagues repeatedly exposed ALI airway cultures to dihydroxyacetone (DHA), an active constituent of the sunless tanning products, once a week for up to 5 weeks (Wang et al. 2019a). While DHA induced dose-dependent reduction in mucin secretion and CBF, these effects were reversed upon cessation of the treatment, suggesting that DHA has only transient effects on pulmonary clearance and host defense mechanisms.
The adverse effects of gasoline and DE emissions also have been investigated in ALI airway tissue models (Künzi et al. 2015; Zarcone et al. 2018). DE was found to be cytotoxic in ALI cultures from healthy donors and increase intercellular permeability as well as expression of markers for inflammation and oxidative stress (Zarcone et al. 2016). A follow-up study using ALI airway cultures having underlying co-morbidities, i.e., cultures made from donors with COPD and cultures infected with Haemophilus influenzae, revealed that COPD and Haemophilus infection did not modulate the tissue responses to DE emissions (Zarcone et al. 2017). These results are not surprising as pulmonary changes observed in vivo rely on endogenous cell types (Zhao et al. 2009) that are not present in differentiated in vitro ALI airway cultures.
With the increasing use of biodiesel to offset fossil fuel consumption, the toxicity of biodiesel blends has become a health concern. Vaughan and colleagues assessed the cytotoxicity and inflammatory responses in ALI airway cultures to emissions from biodiesel blended with and without the fuel additive triacetin (Vaughan et al. 2019). Although combustion of biodiesel blends generated less particulate matter than diesel alone, their combustion products caused significantly greater cytotoxicity and secretion of IL-8, particularly with blends containing 10% triacetin. These findings are congruent with an in vivo study that reported increased inflammation and oxidative stress responses in mice exposed to biodiesel emissions (Yanamala et al. 2013).
In another study, the cytotoxicity and inflammatory responses induced by gasoline engine emissions were examined in ALI cultures using the CULTEX® exposure system equipped with an electrostatic precipitator (Vaughan et al. 2019). This study showed that gasoline engine emissions produced a dose-dependent induction of cytotoxicity, oxidative stress, and inflammatory responses. A customized electrostatic aerosol exposure system (Volckens et al. 2009) has been used to characterize exposures to indoor wood smoke in a manner simulating smoke exposure during cooking (Hawley and Volckens 2013). Increased generation of particulate matter 10 (PM10: inhaled particles with diameters of 10 μm or less) was generally correlated with modulation of markers for inflammation and oxidative stress, suggesting that traditional wood-burning stoves may lead to chronic diseases, a result that is in accordance with epidemiological findings (Po et al. 2011). Taken together, studies conducted in ALI airway cultures have generated valuable in vitro toxicity profiles of vehicle exhaust and environmental emissions that could be valuable for making in vitro-in vivo comparisons. While some studies have included ALI airway models made from sensitive populations, e.g., COPD, more research is required to examine the accuracy of these models to recapitulate toxicity responses among high-risk populations.
Pulmonary Drug Testing
The large surface area of human lungs is characterized by a unique air-epithelium interface that is well-suited for drug absorption and uptake. Drug administration by inhalation is a non-invasive route that can bypass the first-pass metabolism by the liver, overcome poor absorption of drugs by the gastrointestinal tract, and reduce systemic side effects (Patton and Byron 2007). Pulmonary drug delivery also provides an excellent solution for delivering locally acting drugs to treat respiratory diseases, such as asthma, cystic fibrosis, COPD, and CB (Laube 2014). Novel aerosol delivery devices have enabled the local deposition of accurately controlled concentrations of aerosolized medications in the lung (Chandel et al. 2019). So far, there are more than 20 active pharmacological ingredients (APIs) that have been formulated as inhaler products (Labiris and Dolovich 2003; Stein and Thiel 2017). The clinical efficacy of inhaled drugs depends on the effectiveness of aerosol generation as well as factors affecting their bioavailability, such as their regional deposition, absorption, and clearance (Olsson et al. 2011).
After deposition on the luminal surface of the respiratory epithelium, aerosolized drugs first need to overcome removal by mucociliary escalators (Haghi et al. 2014). Residual drugs then are transported into the cell via either passive or active mechanisms, depending on their molecular size, lipid solubility, and site of absorption (Olsson et al. 2011). Ninety percent of small molecular weight (150–200 Da) hydrophobic drugs are rapidly absorbed by passive diffusion (Patton et al. 2004). In vitro ALI airway models have been employed for assessing the deposition and absorption of inhalable drugs across the epithelium as well as for distinguishing between passive diffusion and active transport (Reus et al. 2014; Acosta et al. 2016). Lin and colleagues reported that the transepithelial permeability of a panel of anti-allergy drugs, including fexofenadine-HCl, albuterol, albuterol sulfate, dexamethasone, budesonide, and triamcinolone acetonide, was linearly correlated with their lipophilicity (Lin et al. 2007). Among these drugs, fexofenadine-HCl was transported symmetrically between the apical and basolateral sides of the ALI cultures. This observation is consistent with findings in human airways (Lechapt-Zalcman et al. 1997; Thiebaut et al. 1987) and may reflect either a relatively low expression of P-gp transporters in the ALI cultures or saturation of this drug transporter by high concentrations of fexofenadine-HCl.
Because of the importance of P-gp transporters for drug disposition, the effect of selective inhibitors, such as GF120918A, was evaluated using digoxin as a model substrate (Madlova et al. 2009). In this study, the Papp and efflux values were calculated for digoxin transport in the ALI cultures. GF120918A significantly increased the efflux ratio of digoxin transport only in ALI cultures that had been cultured for 21 days or longer. These results suggest that P-gp activity may depend on the differentiation status of the cultures, emphasizing the importance of using fully differentiated primary cell–based ALI cultures for drug transport research. In another study, Hoffmann and colleagues evaluated the absorption of over 30 chemicals and established threshold values for their permeability coefficient in ALI airway cultures (Hoffmann et al. 2018). Results from this work demonstrated that these cultures are an effective barrier model for studying the absorption kinetics of different classes of chemicals and generating useful information for toxicokinetic modeling and chemical risk assessment.
Human ALI airway cultures have been increasingly used for testing antiviral and antibiotic drug candidates (Zimmermann et al. 2009; Triana-Baltzer et al. 2010; Mata et al. 2012). Antimicrobial agents of different classes, such as macrolides, quinolones, and β-lactams, have been used widely in treating respiratory tract infections. Macrolides (e.g., azithromycin [AZM] and clarithromycin) and fluoroquinolones (e.g., maxifloxacin [MXF]) exert anti-inflammatory effects by attenuating cytokine release both in vitro and in vivo (Rubin and Henke 2004; Shalit et al. 2006; Tauber and Nau 2008). Zimmermann and colleagues further examined whether or not such immunomodulatory effects could be reproduced in airway epithelial cells (Zimmermann et al. 2009). ALI cultures were treated with cefuroxime (CXM), AZM, levofloxacin (LVX), and MXF at therapeutic concentrations, followed by treatment with TNF-α to simulate pro-inflammatory conditions. AZM and MXF were more effective than CXM and LVX in inhibiting the release of TNF-α-induced cytokines, such as GM-CSF and IL-8. The MXF finding is consistent with previous observations, suggesting that it may attenuate inflammation in bronchial epithelial cells.
In addition to their anti-microbial and anti-inflammatory activities, macrolides also mitigate mucus hypersecretion, a common feature seen in many respiratory diseases, such as asthma and COPD (Rubin et al. 1997; Kaneko et al. 2003; López-Boado and Rubin 2008; Ou et al. 2008). For instance, clarithromycin was found to inhibit IL-13-induced mucus hypersecretion and goblet cell hyperplasia in ALI cultures by inactivating the NF-κB signaling pathway (Tanabe et al. 2011). IL-13 is a pro-fibrotic cytokine secreted by T helper type 2 (Th2) cells that is associated with allergic responses in humans (Gour and Wills-Karp 2015). These findings indicate that the ALI airway model may also be a useful tool for screening drugs to treat secretory hyperresponsive diseases, such as asthma.
Pathogen-Host Interaction
Animal models are important tools for studying infectious diseases of the respiratory system. However, the animal model should be selected carefully due to differences in anatomy, physiology, respiration rates, and inhalation pharmacokinetics between species (Davies and Morris 1993; Harkema et al. 2006; Corley et al. 2012; Swearengen 2018). Furthermore, elucidating the mechanisms underlying microbial infections using animal models has always been challenging. In addition, the high cost and ethical concerns about conducting animal research contribute to the increasing popularity of using in vitro pulmonary cell models for conducting studies on respiratory pathogens. Among the existing in vitro lung models, physiologically relevant human ALI airway cultures provide a natural infection target for a variety of human respiratory pathogens, and thus are a valuable culture system for mechanistic studies on pathogen-host interactions.
a. Virus-Host Interaction
The interplay between host cells and viral factors in the pathogenesis of several common human respiratory viruses, including influenza virus (Matrosovich et al. 2004; Zhang et al. 2005a), rhinovirus (Jakiela et al. 2008), and respiratory syncytial virus (Zhang et al. 2002), has been investigated in ALI airway cultures. Utilization of ALI cultures derived from individuals with respiratory diseases, such as asthma (Hackett et al. 2011; Bai et al. 2015) or COPD (Osei et al. 2016; Osei and Hackett 2020), also allows the investigation of the role of pathogens in disease exacerbation. Human ALI airway cultures also have been employed for identifying novel antiviral drug candidates (Palermo et al. 2009; Mata et al. 2012; Brockman-Schneider et al. 2014; Outlaw et al. 2019). Triana-Baltzer and colleagues initially evaluated the pharmacodynamics and antiviral activity of DAS181 in ALI cultures (Triana-Baltzer et al. 2010). DAS181 is a sialidase fusion protein that effectively inhibits the entry of influenza virus by cleaving sialic acid receptors from airway epithelium. Approximately 80% of sialic acid receptors could be removed by DAS181 within 15 minutes, with the effects persisting for at least 2 days. Findings from this study also provided useful information on the treatment regimen and dose selection for DAS181, which is currently under clinical trials as Fludase® (Davidson 2018).
ALI airway cultures have been used to study the propagation kinetics and cytopathic effects of novel human viruses targeting the respiratory system; these are measurements that are difficult to make using traditional approaches (Banach et al. 2009; Dijkman et al. 2009; Pyrc et al. 2010; Kindler et al. 2013; Zhu et al. 2020). Recently, Zhu and colleagues used ALI airway cultures to characterize the cytopathic effects of a novel betacoronavirus associated with the COVID-19 outbreak (Zhu et al. 2020). Bronchoalveolar lavage fluid from patients with pneumonia was directly inoculated onto the apical surface of the ALI cultures and genome sequencing of isolated virus was conducted. Functional assays also revealed significant reduction in cilia beating and morphological changes were observed as early as 4 days after inoculation.
b. Bacteria-Host Interaction
Bacterial infections of the respiratory system normally begin with the adherence and colonization of pathogens on the apical surface of the airway epithelium, followed by internalization into host cells or bacterial invasion to reach the subepithelial space (Hasan et al. 2018). Polarized ALI airway cultures are a powerful tool for studying the infection of a range of human respiratory pathogens, such as Bacillus anthracis (Powell et al. 2015), Bordetella pertussis (Gasperini et al. 2017), Haemophilus influenzae (Raffel et al. 2013), Burkholderia cepacia (Schwab et al. 2002), Moraxella catarrhalis (Balder et al. 2009), Mycoplasma pneumoniae (Prince et al. 2018), Mycobacterium tuberculosis (Reuschl et al. 2017), Mycobacteroides abscessus (Matsuyama et al. 2018), Pseudomonas aeruginosa (Zulianello et al. 2006), Staphylococcus aureus (Soong et al. 2011), and Streptococcus pneumoniae (Verkaik et al. 2014).
Opportunistic bacteria, such as P. aeruginosa, invade airway epithelium with compromised TJs (Plotkowski et al. 1999). Zulianello and colleagues exposed ALI cultures to strains of P. aeruginosa expressing different virulence factors (Zulianello et al. 2006). The strains that secrete rhamnolipids most efficiently compromised barrier integrity and increased the paracellular permeability of the ALI airway cultures. Work by Zhang and colleagues identified Toll-like receptor 5 (TLR5) on the apical surface of the ALI cultures and defined its key role in mediating epithelial responses to P. aeruginosa (Zhang et al. 2005b). Considering the importance of TLRs in recognizing pathogens and activating the innate immunity of airway epithelium (Hopkins and Sriskandan 2005), findings from this study provide a better understanding of the mechanisms underlying bacterial invasion and the responses provoked in airway epithelial cells.
The multiple cell types that make up the ALI cultures have been used to study the cell type–specific pathogenic effects of respiratory pathogens like Haemophilus influenzae and Bordetella pertussis, which primarily adhere to non-ciliated cells (Ketterer et al. 1999) and ciliated cells (Tuomanen and Hendley 1983; Wilson et al. 1991; Soane et al. 2000), respectively. B. pertussis is the major pathogen responsible for whooping cough or pertussis in humans (Mallory and Hornor 1912). It is known to cause ciliostasis and disrupt MCC in airway epithelium. However, the molecular mechanism underlying its adherence and colonization has not been thoroughly investigated due to the lack of suitable in vitro models. Guevara and colleagues developed a quantitative adherence assay in ALI airway cultures and identified multiple mutations in the fimbrial adhesin subunits that may contribute to B. pertussis adherence, confirming the essential role of FimD adhesion in this process (Guevara et al. 2016).
Conclusions
Efforts are currently underway to develop alternatives for in vivo inhalation toxicity testing by the development of in vitro airway/lung approaches (e.g., ALI airway models) consistent with the 3Rs principles of replacement, reduction, and refinement (Russell and Burch 1959). Conducting in vivo inhalation toxicity studies using whole-body or nose-only exposure systems is expensive and time-consuming and typically requires a large number of animals. The goal of using alternative methods, like human in vitro ALI airway cultures, ultimately is to replace inhalation toxicity testing in animals with in vitro approaches.
Before in vitro approaches can ever replace in vivo inhalation studies, however, ALI culture models must be fully validated to optimally reproduce the airway/lung biology of native tissue. Validation also should include assessing the reproducibility of the endpoints that can be measured with ALI cultures across different batches of both commercial and “home-made” models as well as the transferability of results between independent testing laboratories. One important element for validating any new assay for making regulatory decisions is determining its performance relative to an accepted standard (see, for instance, OECD, 2005). One problem with validating performance of ALI airway assays is that these models and endpoints are mainly developed using human tissue, while most reference data from an accepted validated test have been generated with rodents (e.g., studies conforming with OECD Test Guidelines 412 and 413). Although it is clearly the case that a human-based system will be more valuable for assessing human health risk than a rodent system, in this case, it may be necessary to develop rodent ALI airway data as an approach to bridge data between rodent and human. Ultimately, validated in vitro assays must be accepted by both regulatory agencies (e.g., US EPA, US FDA, Health Canada, NIEHS) and the industry as an alternative or replacement to in vivo testing. Acceptance often involves consensus-forming mediated by groups such as the Organisation for Economic Cooperation and Development (OECD) and The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).
Although ALI airway cultures have not yet been incorporated into regulatory guidelines, international workshops have been organized to standardize approaches for assessing airborne substances in these cultures (Clippinger et al. 2018; Lacroix et al. 2018). Efforts are ongoing to develop a set of criteria for standardizing every aspect of the studies utilizing ALI airway cultures, including culture preparation, assay performance, and exposure procedures. For instance, Institute for In Vitro Sciences (Bethesda, MD) has coordinated a multi-institutional validation study to develop standards for CBF measurement in ALI airway cultures. At this time, ALI airway culture models are not ready to fully replace inhalation studies in animals but can be effectively used to complement in vivo studies as a tool (1) to perform initial screening for chemical- or drug-induced toxicity in order to prioritize compounds for further (and more extensive) testing in vivo and/or (2) for mechanistic and follow-up evaluations. Such applications of ALI airway models can potentially refine how in vivo testing is conducted and reduce both the number of animals used for inhalation toxicity testing and the time for conducting the studies.
The use of standard in vivo models to predict adverse chemical- or drug-induced airway effects in humans can be complicated by species-specific differences in the respiratory tract between rodents and humans. Although these species differences produce challenges for validating in vitro airway models, viewed differently, a major advantage of ALI cultures is that the tissues can be of human origin and thus are reflective of human airway/lung biology. ALI cultures are composed of the critical functioning cell types present within the airway epithelium and, therefore, can be used effectively for evaluating human-relevant toxic effects and mechanisms involved in airway responses to chemical or drug exposures. In addition, ALI cultures can be used in conjunction with physiologically relevant exposure systems for vapors/gases, aerosols, or particles, although further optimization of high-throughput approaches is needed for efficient screening of large numbers of compounds.
Resident cells within ALI airway epithelial models can secrete pro- and anti-inflammatory cytokines; however, these cultures lack mesenchymal cells as well immune cells, such as neutrophils, monocytes/macrophages, and lymphocytes that are recruited from the circulation. These cells perform a critical role in regulating inflammation and the immune responses induced within the airways by external stimuli. Improved ALI culture models, therefore, are needed to incorporate these additional cell types as well as endothelial and mesenchymal cells to more realistically reproduce inflammatory responses. Full-thickness (FT) ALI models have been developed to include a thin basement membrane-like lining with a subepithelial collagen-rich matrix that contains donor-matched fibroblasts. Mechanical forces exerted within the airway/lung can also be engineered into ALI cultures. Microphysiological systems, such as lung-on-a-chip, have integrated flow and/or mechanical forces (e.g., stretching to reflect the breathing motion) into in vitro airway models. Additional work is needed to determine how well these in vitro airway models can be created to best recapitulate airway fibrosis, remodeling, and repair from repeated injury with relevance to chemical/drug-induced sub-acute/chronic toxicity. It should be noted that one of the features of the basic ALI human airway model is that they remain structurally and functionally useful for months in culture, which lends them to conducting sub-acute dosing protocols, similar to those conducted in animal models and that occur with human exposures. Making a more complex model, however, may affect the stability of airway models, reducing their useable life span in culture.
Most testing with human ALI cultures has been conducted with culture models generated from single donors, with little consideration given to the age, sex, race, and health condition of the donor. There are data indicating that pre-existing diseases of the donors, such as asthma (Hackett et al. 2011; Bai et al. 2015) and COPD (Osei et al. 2016; Osei and Hackett 2020), can affect the biology of airway models in a way that can be used to assess the effects of inhalation exposures on susceptible populations. However, effects of lifestyle factors, including use of tobacco or electronic cigarettes and occupational or environmental chemical exposures, on airway epithelium may possibly be reversible when airway cells are removed from the context of the in vivo lung environment and subjected to long-term in vitro culturing in the absence of the initiating stimulus. For instance, while some gene expression changes caused by tobacco smoke are reported to be irreversible, many other changes are reversible following smoking cessation (Beane et al. 2007). Nevertheless, certain aspects of in vivo disease states can be recapitulated in vitro by supplementing the missing factors in cell culture. One example is to recapitulate goblet cell hyperplasia in in vitro bronchial models by exposing the cultures to cigarette smoke or by adding T cell–derived cytokines, such as IL-13, or other exogenous stimuli (Bolmarcich et al. 2018).
Thus, in order to use data from ALI airway cultures in a scientifically rigorous manner and understand variability among results, the effects of the inherent demographic differences among healthy, non-smoking donors on the responses measured in ALI cultures must be understood. One potential solution for minimizing variability among donors is developing models derived from a single induced pluripotent stem cell line that could be used for standardized testing. On the other hand, the study of cultures derived from a variety of healthy and diseased individuals (Hackett et al. 2011, Bai et al. 2015) will enable a more comprehensive understanding of the responses of both healthy and diseased human populations to environmental agents and therapeutics.
Conducting inhalation toxicology studies in vitro, under conditions simulating human inhalation exposures, is technically challenging. Although improvement and standardization are still needed, the combination of ALI airway tissue models and progress in developing exposure systems offer unique opportunities to advance in vitro respiratory toxicology and the human risk assessment for a broad range of airborne substances as well as chemicals that indirectly impact lung health.
Acknowledgments
Baiping Ren is an Oak Ridge Institute for Science and Education (ORSIE) fellow and supported under an Interagency Agreement between FDA and NIEHS (FDA IAG no. 224-17-0502 and NIH IAG no. AES12013).
Abbreviations
- ALI
Air-liquid-interface
- AZM
Azithromycin
- CB
Chronic bronchitis
- CBF
Cilia beating frequency
- CF
Cystic fibrosis
- CFD
Computational fluid dynamics
- CI
Canadian Intense
- COPD
Chronic obstructive pulmonary disease
- CS
Cigarette smoke
- CSC
Cigarette smoke condensate
- CSE
Cigarette smoke extract
- CXM
Cefuroxime
- DA
Diacetyl
- DE
Diesel exhaust
- ENM
Engineered nanomaterial
- GHS
Global Harmonization System
- ICH
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- IL-13
Interleukin-13
- ISO
International Organization for Standardization
- LVX
Levofloxacin
- MCC
Mucociliary clearance
- MCT
Mucociliary transport
- MMAD
Mass median aerodynamic diameter
- MWCNT
Multi-walled carbon nanotube
- MXF
Moxifloxacin
- NHBE
Normal human bronchial epithelial cells
- NIOSH
National Institute for Occupational Safety and Health
- NP
Nanoparticle
- OECD
Organisation for Economic Co-operation and Development
- PCD
Primary ciliary dyskinesia
- P-gp
p-Glycoprotein
- QCM
Quartz crystal microbalance
- RA
Retinoic acid
- TCDD
2,3,7,8-Tetrachlorodibenzodioxin
- TEER
Transepithelial electrical resistance
- TJ
Tight junction
Funding
This research was supported [in part] by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Compliance with Ethical Standards
Disclaimer
The opinions expressed in this review are those of the authors and do not necessarily represent the official positions of the Food and Drug Administration, the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, or the National Toxicology Program positions or policies. Mention of any company or product does not constitute endorsement by the FDA, NIOSH/CDC, or NTP.
References
- Acosta MF, Muralidharan P, Meenach SA, Hayes D, M-Black S, Mansour HM. In vitro pulmonary cell culture in pharmaceutical inhalation aerosol delivery: 2-D, 3-D, and in situ bioimpactor models. Curr Pharm Des. 2016;22:2522–2531. doi: 10.2174/1381612822666160202142104. [DOI] [PubMed] [Google Scholar]
- Adamson J, Li X, Cui H, Thorne D, Xie F, Gaca MD. Nicotine quantification in vitro: a consistent dosimetry marker for e-cigarette aerosol and cigarette smoke generation. Appl In Vitro Toxicol. 2017;3:14–27. doi: 10.1089/aivt.2016.0025. [DOI] [Google Scholar]
- Adamson J, Thorne D, Dalrymple A, Dillon D, Meredith C. Assessment of cigarette smoke particle deposition within the Vitrocell® exposure module using quartz crystal microbalances. Chem Cent J. 2013;7:50–50. doi: 10.1186/1752-153X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson J, Thorne D, Errington G, Fields W, Li X, Payne R, Krebs T, Dalrymple A, Fowler K, Dillon D, Xie F, Meredith C. An inter-machine comparison of tobacco smoke particle deposition in vitro from six independent smoke exposure systems. Toxicol in Vitro. 2014;28:1320–1328. doi: 10.1016/j.tiv.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Adler KB, Schwarz JE, Whitcutt MJ, Wu R. A new chamber system for maintaining differentiated Guinea pig respiratory epithelial cells between air and liquid phases. Biotechniques. 1987;5:462–465. [Google Scholar]
- Ali M, Lillehoj EP, Park Y, Kyo Y, Kim KC. Analysis of the proteome of human airway epithelial secretions. Proteome Sci. 2011;9:4. doi: 10.1186/1477-5956-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatngalim GD, Schrumpf JA, Dishchekenian F, Mertens TCJ, Ninaber DK, van der Linden AC, Pilette C, Taube C, Hiemstra PS, van der Does AM (2018) Aberrant epithelial differentiation by cigarette smoke dysregulates respiratory host defence. Eur Respir J 51: pii 1701009. [DOI] [PubMed]
- Amatngalim GD, van Wijck Y, de Mooij-Eijk Y, Verhoosel RM, Harder J, Lekkerkerker AN, Janssen RAJ, Hiemstra PS. Basal cells contribute to innate immunity of the airway epithelium through production of the antimicrobial protein RNase 7. J Immunol. 2015;194:3340–3350. doi: 10.4049/jimmunol.1402169. [DOI] [PubMed] [Google Scholar]
- Antherieu S, Garat A, Beauval N, Soyez M, Allorge D, Garcon G, Lo-Guidice JM. Comparison of cellular and transcriptomic effects between electronic cigarette vapor and cigarette smoke in human bronchial epithelial cells. Toxicol in Vitro. 2017;45:417–425. doi: 10.1016/j.tiv.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Aufderheide M. Direct exposure methods for testing native atmospheres. Exp Toxicol Pathol. 2005;57:213–226. doi: 10.1016/j.etp.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Aufderheide M, Gressmann H. A modified Ames assay reveals the mutagenicity of native cigarette mainstream smoke and its gas vapour phase. Exp Toxicol Pathol. 2007;58:383–392. doi: 10.1016/j.etp.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Aufderheide M, Halter B, Möhle N, Hochrainer D. The CULTEX RFS: a comprehensive technical approach for the in vitro exposure of airway epithelial cells to the particulate matter at the air-liquid interface. Biomed Res Int. 2013;2013:734137. doi: 10.1155/2013/734137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufderheide M, Ito S, Ishikawa S, Emura M. Metaplastic phenotype in human primary bronchiolar epithelial cells after repeated exposure to native mainstream smoke at the air-liquid interface. Exp Toxicol Pathol. 2017;69:307–315. doi: 10.1016/j.etp.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Aufderheide M, Knebel JW, Ritter D. An improved in vitro model for testing the pulmonary toxicity of complex mixtures such as cigarette smoke. Exp Toxicol Pathol. 2003;55:51–57. doi: 10.1078/0940-2993-00298. [DOI] [PubMed] [Google Scholar]
- Aufderheide M, Mohr U. CULTEX — a new system and technique for the cultivation and exposure of cells at the air/liquid interface. Exp Toxicol Pathol. 1999;51:489–490. doi: 10.1016/S0940-2993(99)80121-3. [DOI] [PubMed] [Google Scholar]
- Aufderheide M, Mohr U. A modified CULTEX® system for the direct exposure of bacteria to inhalable substances. Exp Toxicol Pathol. 2004;55:451–454. doi: 10.1078/0940-2993-00348. [DOI] [PubMed] [Google Scholar]
- Aufderheide M, Scheffler S, Möhle N, Halter B, Hochrainer D. Analytical in vitro approach for studying cyto- and genotoxic effects of particulate airborne material. Anal Bioanal Chem. 2011;401:3213–3220. doi: 10.1007/s00216-011-5163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers MM, Jeffery PK. Proliferation and differentiation in mammalian airway epithelium. Eur Respir J. 1988;1:58–80. [PubMed] [Google Scholar]
- Azzopardi D, Haswell LE, Foss-Smith G, Hewitt K, Asquith N, Corke S, et al. Evaluation of an air-liquid interface cell culture model for studies on the inflammatory and cytotoxic responses to tobacco smoke aerosols. Toxicol in Vitro. 2015;29:1720–1728. doi: 10.1016/j.tiv.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Bai J, Smock SL, Jackson GR, Jr, MacIsaac KD, Huang Y, Mankus C, Oldach J, Roberts B, Ma YL, Klappenbach JA, Crackower MA, Alves SE, Hayden PJ. Phenotypic responses of differentiated asthmatic human airway epithelial cultures to rhinovirus. PLoS One. 2015;10:e0118286. doi: 10.1371/journal.pone.0118286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788:761–767. doi: 10.1016/j.bbamem.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Balder R, Krunkosky TM, Nguyen CQ, Feezel L, Lafontaine ER. Hag mediates adherence of Moraxella catarrhalis to ciliated human airway cells. Infect Immun. 2009;77:4597–4608. doi: 10.1128/IAI.00212-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res. 2000;1:141–150. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bals R, Beisswenger C, Blouquit S, Chinet T. Isolation and air-liquid interface culture of human large airway and bronchiolar epithelial cells. J Cyst Fibros. 2004;2(Suppl):49–51. doi: 10.1016/j.jcf.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Banach B, Orenstein JM, Fox LM, Randell SH, Rowley AH, Baker SC. Human airway epithelial cell culture to identify new respiratory viruses: coronavirus NL63 as a model. J Virol Methods. 2009;156:19–26. doi: 10.1016/j.jviromet.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter A, Thain S, Banerjee A, et al. Targeted omics analyses, and metabolic enzyme activity assays demonstrate maintenance of key mucociliary characteristics in long term cultures of reconstituted human airway epithelia. Toxicol in Vitro. 2015;29:864–875. doi: 10.1016/j.tiv.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Beane J, Sebastiani P, Liu G, Brody JS, Lenburg ME, Spira A. Reversible and permanent effects of tobacco smoke exposure on airway epithelial gene expression. Genome Biol. 2007;8:R201. doi: 10.1186/gb-2007-8-9-r201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebök Z, Tousson A, Schwiebert LM, Venglarik CJ. Improved oxygenation promotes CFTR maturation and trafficking in MDCK monolayers. Am J Physiol Cell Physiol. 2001;280:C135–C145. doi: 10.1152/ajpcell.2001.280.1.C135. [DOI] [PubMed] [Google Scholar]
- Behrsing H, Aragon M, Adamson J, Sheehan D, Gaca M, Curren R, Hill E. Characterization of a Vitrocell VC1 using nicotine dosimetry: an essential component toward standardized in vitro aerosol exposure of tobacco and next generation nicotine delivery products. Appl In Vitro Toxicol. 2018;4:159–166. doi: 10.1089/aivt.2018.0001. [DOI] [Google Scholar]
- Berika M, Elgayyar MW, El-Hashash HK. Asymmetric cell division of stem cells in the lung and other systems. Front Cell Dev Biol. 2014;2:33. doi: 10.3389/fcell.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube K, Prytherch Z, Job C, Hughes T. Human primary bronchial lung cell constructs: the new respiratory models. Toxicology. 2010;278:311–318. doi: 10.1016/j.tox.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Beyeler S, Chortarea S, Rothen-Rutishauser B, Petri-Fink A, Wick P, Tschanz SA, von Garnier C, Blank F. Acute effects of multi-walled carbon nanotubes on primary bronchial epithelial cells from COPD patients. Nanotoxicology. 2018;12:699–711. doi: 10.1080/17435390.2018.1472310. [DOI] [PubMed] [Google Scholar]
- Bishop E, Haswell L, Adamson J, Costigan S, Thorne D, Gaca M. An approach to testing undiluted e-cigarette aerosol in vitro using 3D reconstituted human airway epithelium. Toxicol in Vitro. 2019;54:391–401. doi: 10.1016/j.tiv.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Bitterle E, Karg E, Schroeppel A, Kreyling WG, Tippe A, Ferron GA, Schmid O, Heyder J, Maier KL, Hofer T. Dose-controlled exposure of A549 epithelial cells at the air–liquid interface to airborne ultrafine carbonaceous particles. Chemosphere. 2006;65:1784–1790. doi: 10.1016/j.chemosphere.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Boei JJ, Vermeulen S, Klein B, et al. Xenobiotic metabolism in differentiated human bronchial epithelial cells. Arch Toxicol. 2017;91:2093–2015. doi: 10.1007/s00204-016-1868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 1998;157:2000–2006. doi: 10.1164/ajrccm.157.6.9707011. [DOI] [PubMed] [Google Scholar]
- Bolmarcich J, Wilbert S, Jackson GR, Oldach J, Bachelor M, Kenney T, Wright CD, Hayden PJ. In vitro human airway models for study of goblet cell hyperplasia and mucus production: effects of Th2 cytokines, double -stranded RNA, and tobacco smoke. Appl In Vitro Toxicol. 2018;4:332–346. doi: 10.1089/aivt.2017.0001. [DOI] [Google Scholar]
- Brandwein DH, Bettmann FA, DeLorme MP, Eveland AT, Milchak LM (2020) Comparison of vapor and liquid phase acrolein exposures to air liquid interface (ALI) cell cultures. 2020 Annual Meeting Abstract Supplement, Society of Toxicology.
- Brass DM, Gwinn WM, Valente AM, Kelly FL, Brinkley CD, Nagler AE, Moseley MA, Morgan DL, Palmer SM, Foster MW. The diacetyl-exposed human airway epithelial secretome: new insights into flavoring-induced airway disease. Am J Respir Cell Mol Biol. 2017;56:748–795. doi: 10.1165/rcmb.2016-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekman A, Walters MS, Tilley AE, Crystal RG. FOXJ1 prevents cilia growth inhibition by cigarette smoke in human airway epithelium in vitro. Am J Respir Cell Mol Biol. 2014;51:688–700. doi: 10.1165/rcmb.2013-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman-Schneider RA, Pickles RJ, Gern JE. Effects of vitamin D on airway epithelial cell morphology and rhinovirus replication. PLoS One. 2014;9:e86755. doi: 10.1371/journal.pone.0086755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley AG, Looi K, Iosifidis T, et al. Visualisation of multiple tight junctional complexes in human airway epithelial cells. Biol Proced Online. 2018;20:3. doi: 10.1186/s12575-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush A, Hogg C. Primary ciliary dyskinesia: recent advances in epidemiology, diagnosis, management and relationship with the expanding spectrum of ciliopathy. Expert Rev Respir Med. 2012;6:663–682. doi: 10.1586/ers.12.60. [DOI] [PubMed] [Google Scholar]
- Cao X, Lin H, Muskhelishvili L, Latendresse J, Richter P, Heflich RH. Tight junction disruption by cadmium in an in vitro human airway tissue model. Respir Res. 2015;16:30. doi: 10.1186/s12931-015-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Wang Y, Xiong R, Muskhelishvili L, Davis K, Richter PA, Heflich RH. Cigarette whole smoke solutions disturb mucin homeostasis in a human in vitro airway tissue model. Toxicology. 2018;409:119–128. doi: 10.1016/j.tox.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Carr BA, Wan J, Hines RN, Yost GS. Characterization of the human lung CYP2F1 gene and identification of a novel lung-specific binding motif. J Biol Chem. 2003;278:15473–15483. doi: 10.1074/jbc.M300319200. [DOI] [PubMed] [Google Scholar]
- Cassee FR, van Balen EC, Singh C, Green D, Muijser H, Weinstein J, Dreher K. Exposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additive. Crit Rev Toxicol. 2011;41:213–229. doi: 10.3109/10408444.2010.529105. [DOI] [PubMed] [Google Scholar]
- Chan RW, Yuen KM, Yu WC, Ho CC, Nicholls JM, Peiris JS, Chan MC. Influenza H5N1 and H1N1 virus replication and innate immune responses in bronchial epithelial cells are influenced by the state of differentiation. PLoS One. 2010;5:e8713. doi: 10.1371/journal.pone.0008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandel A, Goyal AK, Ghosh G, Rath G. Recent advances in aerosolised drug delivery. Biomed Pharmacother. 2019;112:108601. doi: 10.1016/j.biopha.2019.108601. [DOI] [PubMed] [Google Scholar]
- Chortarea S, Barosova H, Clift MJD, Wick P, Petri-Fink A, Rothen-Rutishauser B. Human asthmatic bronchial cells are more susceptible to subchronic repeated exposures of aerosolized carbon nanotubes at occupationally relevant doses than healthy cells. ACS Nano. 2017;11:7615–7625. doi: 10.1021/acsnano.7b01992. [DOI] [PubMed] [Google Scholar]
- Clippinger AJ, Allen D, Jarabek AM, Corvaro M, Gaca M, Gehen S, Hotchkiss JA, Patlewicz G, Melbourne J, Hinderliter P, Yoon M, Huh D, Lowit A, Buckley B, Bartels M, BeruBe K, Wilson DM, Indans I, Vinken M. Alternative approaches for acute inhalation toxicity testing to address global regulatory and non-regulatory data requirements: an international workshop report. Toxicol in Vitro. 2018;48:53–70. doi: 10.1016/j.tiv.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett SE, Nitzberg M, Moses E, Kleerup E, Wang T, Perdomo C, Perdomo C, Liu G, Xiao X, Liu H, Elashoff DA, Brooks DR, O'Connor GT, Dubinett SM, Spira A, Lenburg ME. Gene expression alterations in the bronchial epithelium of e-cigarette users. Chest. 2019;156:764–773. doi: 10.1016/j.chest.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corley RA, Kabilan S, Kuprat AP, Carson JP, Minard KR, Jacob RE, Timchalk C, Glenny R, Pipavath S, Cox T, Wallis CD, Larson RF, Fanucchi MV, Postlethwait EM, Einstein DR. Comparative computational modeling of airflows and vapor dosimetry in the respiratory tracts of rat, monkey, and human. Toxicol Sci. 2012;128:500–516. doi: 10.1093/toxsci/kfs168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JP, Rinaldi RJ, Johnson GT, Bourgeois MM, McCluskey J, Harbison RD. Acrolein measurement and degradation in Dulbecco’s Modified Eagle Medium: an examination of in-vitro exposure metrics. Toxicol Mech Methods. 2018;28:115–121. doi: 10.1080/15376516.2017.1370755. [DOI] [PubMed] [Google Scholar]
- Cozens D, Sutherland E, Marchesi F, Taylor G, Berry CC, Davies RL. Temporal differentiation of bovine airway epithelial cells grown at an air-liquid interface. Sci Rep. 2018;8:14893. doi: 10.1038/s41598-018-33180-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer WI, Sharma HS, Baelemans SM, et al. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol. 2008;86:105–112. doi: 10.1139/Y08-004. [DOI] [PubMed] [Google Scholar]
- Davidson S. Treating influenza infection, from now and into the future. Front Immunol. 2018;9:1946. doi: 10.3389/fimmu.2018.01946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–1095. doi: 10.1023/A:1018943613122. [DOI] [PubMed] [Google Scholar]
- Davis AS, Chertow DS, Moyer JE, et al. Validation of normal human bronchial epithelial cells as a model for influenza A infections in human distal trachea. J Histochem Cytochem. 2015;63:312–328. doi: 10.1369/0022155415570968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloid GM, Cohen JM, Darrah T, Derk R, Rojanasskul L, Pyrgiotakes G, Wohleben W, Demokritou P. Estimating the effective density of engineered nanomaterials for in vitro dosimetry. Nat Commun. 2014;5:3514. doi: 10.1038/ncomms4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLoid GM, Cohen JM, Pyrgiotakis G, Pirela SV, Pal A, Liu J, Srebric J, Demokritou P. Advanced computational modeling for in vitro nanomaterial dosimetry. Part Fibre Toxicol. 2015;12:32. doi: 10.1186/s12989-015-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschl U, Vogel J, Aufderheide M. Development of an in vitro exposure model for investigating the biological effects of therapeutic aerosols on human cells from the respiratory tract. Exp Toxicol Pathol. 2011;63:593–598. doi: 10.1016/j.etp.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Dijkman R, Koekkoek SM, Molenkamp R, Schildgen O, van der Hoek L. Human bocavirus can be cultured in differentiated human airway epithelial cells. J Virol. 2009;83:7739–7748. doi: 10.1128/JVI.00614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Weindl P, Wimmer C, Mayer P, Krebs T, Schmid O (2017) Characterization of the air-liquid interface cell exposure (ALICE-CLOUD) system for in-vitro toxicological studies of engineered nanomaterials (ENMs). European Aerosol Conference.
- Donaldson SH, Corcoran TE, Laube BL, Bennett WD. Mucociliary clearance as an outcome measure for cystic fibrosis clinical research. Proc Am Thorac Soc. 2007;4:399–405. doi: 10.1513/pats.200703-042BR. [DOI] [PubMed] [Google Scholar]
- Durantie E, Vanhecke D, Rodriguez-Lorenzo L, Delhaes F, Balog S, Septiadi D, Bourquin J, Petri-Fink A, Rothen-Rutishauser B. Biodistribution of single and aggregated gold nanoparticles exposed to the human lung epithelial tissue barrier at the air-liquid interface. Part Fibre Toxicol. 2017;14:49. doi: 10.1186/s12989-017-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak A, Tilley AE, Shaykhiev R, Wang R, Crystal RG. Do airway epithelium air-liquid cultures represent the in vivo airway epithelium transcriptome? Am J Respir Cell Mol Biol. 2011;44:465–473. doi: 10.1165/rcmb.2009-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi AM, Upadhyay S, Johanson G, et al. Inflammatory effects of acrolein, crotonaldehyde and hexanal vapors on human primary bronchial epithelial cells cultured at air-liquid interface. Toxicol in Vitro. 2018;46:219–228. doi: 10.1016/j.tiv.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Enuka Y, Hanukoglu I, Edelheit O, Vaknine H, Hanukoglu A. Epithelial sodium channels (ENaC) are uniformly distributed on motile cilia in the oviduct and the respiratory airways. Histochem Cell Biol. 2012;137:339–353. doi: 10.1007/s00418-011-0904-1. [DOI] [PubMed] [Google Scholar]
- EPA (Environmental Protection Agency) (2016a) Process for evaluating and implementing alternative approaches to traditional in vivo acute toxicity for FIFRA regulatory use. Available via: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/process-establishing-implementing-alternative.
- EPA (Environmental Protection Agency) (2016b) Letter to stakeholders on EPA office of pesticide programs’ goal to reduce animal testing. Available via: https://www.regulations.gov/document?D=EPA-HQ-OPP-2016-0093-0003.
- Evangelista ACJ, de Morais JF, Tam V, Soomro M, Torres Di Gregorio L, Haddad AN. Evaluation of carbon nanotube incorporation in cementitious composite materials. Materials (Basel) 2019;12:1504. doi: 10.3390/ma12091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res. 2001;27:401–415. doi: 10.1080/019021401300317125. [DOI] [PubMed] [Google Scholar]
- Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields WR, Leonard RM, Odom PS, et al. Gene expression in normal human bronchial epithelial (NHBE) cells following in vitro exposure to cigarette smoke condensate. Tox Sci. 2005;86:84–91. doi: 10.1093/toxsci/kfi179. [DOI] [PubMed] [Google Scholar]
- Fields WR, Maione A, Keyser B, Bombick B. Characterization and application of the VITROCELL VC1 smoke exposure system and 3D EpiAirway models for toxicological and e-cigarette evaluations. Applied In Vitro Toxicology. 2017;3:68–83. doi: 10.1089/aivt.2016.0035. [DOI] [Google Scholar]
- Forteza RM, Casalino-Matsuda SM, Falcon NS, Valencia Gattas M, Monzon ME. Hyaluronan and layilin mediate loss of airway epithelial barrier function induced by cigarette smoke by decreasing E-cadherin. J Biol Chem. 2012;287:42288–42298. doi: 10.1074/jbc.M112.387795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MW, Gwinn WM, Kelly FL, Brass DM, Valente AM, Moseley MA, Thompson JW, Morgan DL, Palmer SM. Proteomic analysis of primary human airway epithelial cells exposed to the respiratory toxicant diacetyl. J Proteome Res. 2017;16:538–549. doi: 10.1021/acs.jproteome.6b00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Hollins AJ, Laue M, Schaefer UF, Roemer K, Gumbleton M, Lehr CM. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res. 2003;311:31–45. doi: 10.1007/s00441-002-0653-5. [DOI] [PubMed] [Google Scholar]
- Fulcher L, Gabriel S, Burns KA, Yankaskas JR, Randell SH (2005) Well-differentiated human airway epithelial cell cultures. In: Picot J (ed) Methods in molecular medicine 2nd Edition volume 107. Humana Press, Totowa New Jersey, pp183–206. [DOI] [PubMed]
- Gaillard EA, Kota P, Gentzsch M, et al. Regulation of the epithelial Na+ channel and airway surface liquid volume by serine proteases. Pflugers Arch. 2010;460:1–17. doi: 10.1007/s00424-010-0827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S, Comstock AT, Sajjan US. Barrier function of airway tract epithelium. Tissue Barriers. 2013;1:e24997. doi: 10.4161/tisb.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasperini G, Arato V, Pizza M, Aricò B, Leuzzi R. Physiopathological roles of spontaneously released outer membrane vesicles of Bordetella pertussis. Future Microbiol. 2017;12:1247–1259. doi: 10.2217/fmb-2017-0064. [DOI] [PubMed] [Google Scholar]
- Geiser M, Jeannet N, Fierz M, Burtscher H (2017) Evaluating adverse effects of inhaled nanoparticles by realistic in vitro technology. Nanomaterials (Basel) 7: pii E49. [DOI] [PMC free article] [PubMed]
- Ghio AJ, Dailey LA, Soukup JM, et al. Growth of human bronchial epithelial cells at an air-liquid interface alters the response to particle exposure. Part Fibre Toxicol. 2013;10:25. doi: 10.1186/1743-8977-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti A, Delpiano L, Caci E. In vitro methods for the development and analysis of human primary airway epithelia. Front Pharmacol. 2018;9:1176. doi: 10.3389/fphar.2018.01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gminski R, Tang T, Mersch-Sundermann V. Cytotoxicity and genotoxicity in human lung epithelial A549 cells caused by airborne volatile organic compounds emitted from pine wood and oriented strand boards. Toxicol Lett. 2010;196:33–41. doi: 10.1016/j.toxlet.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Godfrey RW, Severs NJ, Jeffery PK. Freeze-fracture morphology and quantification of human bronchial epithelial tight junctions. Am J Respir Cell Mol Biol. 1992;6:453–458. doi: 10.1165/ajrcmb/6.4.453. [DOI] [PubMed] [Google Scholar]
- Godfrey RW, Severs NJ, Jeffery PK. Structural alterations of airway epithelial tight junctions in cystic fibrosis: comparison of transplant and postmortem tissue. Am J Respir Cell Mol Biol. 1993;9:148–156. doi: 10.1165/ajrcmb/9.2.148. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Suarez I, Martin F, Marescotti D, Guedj E, Acali S, Johne S, Dulize R, Baumer K, Peric D, Goedertier D, Frentzel S, Ivanov NV, Mathis C, Hoeng J, Peitsch MC. In vitro systems toxicology assessment of a candidate Mmdified risk tobacco product shows reduced toxicity compared to that of a conventional cigarette. Chem Res Toxicol. 2016;29:3–18. doi: 10.1021/acs.chemrestox.5b00321. [DOI] [PubMed] [Google Scholar]
- Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine. 2015;75:68–78. doi: 10.1016/j.cyto.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1996;14:104–112. doi: 10.1165/ajrcmb.14.1.8534481. [DOI] [PubMed] [Google Scholar]
- Gualerzi A, Sciarabba M, Tartaglia G, Sforza C, Donetti E. Acute effects of cigarette smoke on three-dimensional cultures of normal human oral mucosa. Inhal Toxicol. 2012;24:382–389. doi: 10.3109/08958378.2012.679367. [DOI] [PubMed] [Google Scholar]
- Guevara C, Zhang C, Gaddy JA, Iqbal J, Guerra J, Greenberg DP, Decker MD, Carbonetti N, Starner TD, McCray PB, Jr, Mooi FR, Gómez-Duarte OG. Highly differentiated human airway epithelial cells: a model to study host cell–parasite interactions in pertussis. Infect Dis. 2016;48:177–188. doi: 10.3109/23744235.2015.1100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülden M, Seibert H. In vitro-in vivo extrapolation: estimation of human serum concentrations of chemicals equivalent to cytotoxic concentrations in vitro. Toxicology. 2003;189:211–222. doi: 10.1016/S0300-483X(03)00146-X. [DOI] [PubMed] [Google Scholar]
- Gwinn WM, Flake GP, Bousquet RW, Taylor GJ, Morgan DL. Airway injury in an in vitro human epithelium-fibroblast model of diacetyl vapor exposure: diacetyl-induced basal/suprabasal spongiosis. Inhal Toxicol. 2017;29:310–321. doi: 10.1080/08958378.2017.1369604. [DOI] [PubMed] [Google Scholar]
- Hackett TL, Singhera GK, Shaheen F, Hayden P, Jackson GR, Hegele RG, Van Eeden S, Bai TR, Dorscheid DR, Knight DA. Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to respiratory syncytial virus and air pollution. Am J Respir Cell Mol Biol. 2011;45:1090–1100. doi: 10.1165/rcmb.2011-0031OC. [DOI] [PubMed] [Google Scholar]
- Haghi M, Ong HX, Traini D, Young P. Across the pulmonary epithelial barrier: integration of physicochemical properties and human cell models to study pulmonary drug formulations. Pharmacol Ther. 2014;144:235–252. doi: 10.1016/j.pharmthera.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol. 2006;34:252–269. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- Harkema JR, Mariassy SA, George J, Hyde DM, Plopper CG. Lung biology in health and disease volume 55. New York: Dekker; 1991. Epithelial cells of the conducting airways: a species comparison; pp. 3–39. [Google Scholar]
- Hasan S, Sebo P, Osicka R. A guide to polarized airway epithelial models for studies of host-pathogen interactions. FEBS J. 2018;285:4343–4358. doi: 10.1111/febs.14582. [DOI] [PubMed] [Google Scholar]
- Hauber HP, Foley SC, Hamid Q. Mucin overproduction in chronic inflammatory lung disease. Can Respir J. 2006;13:327–335. doi: 10.1155/2006/901417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley B, Volckens J. Proinflammatory effects of cookstove emissions on human bronchial epithelial cells. Indoor Air. 2013;23:4–13. doi: 10.1111/j.1600-0668.2012.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht SS. Lung carcinogenesis by tobacco smoke. Int J Cancer. 2012;131:2724–2732. doi: 10.1002/ijc.27816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr C, Beisswenger C, Hess C, Kandler K, Suttorp N, Welte T, Schroeder JM, Vogelmeier C. Suppression of pulmonary innate host defence in smokers. Thorax. 2009;64:144–149. doi: 10.1136/thx.2008.102681. [DOI] [PubMed] [Google Scholar]
- Hess BM, Thomas DG, Weber TJ, Hutchison JR, Straub TM, Bruckner-Lea CJ, Powell JD, Kabilan S, Corley RA. An integrated experimental-computational approach for predicting virulence in New Zealand white rabbits and humans following inhalation exposure to Bacillus anthracis spores. PLoS One. 2019;14:e0219160. doi: 10.1371/journal.pone.0219160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W, Gradinaru J, Farcal L, Caul-Futy M, Huang S, Wiszniewski L, Parissis N, Morath S, Fortaner S, Cole T, Reginato E, Carrupt PA, Constant S, Coecke S. Establishment of a human 3D tissue-based assay for upper respiratory tract absorption. Appl In Vitro Toxicol. 2018;4:139–148. doi: 10.1089/aivt.2017.0035. [DOI] [Google Scholar]
- Hopkins PA, Sriskandan S. Mammalian Toll-like receptors: to immunity and beyond. Clin Exp Immunol. 2005;140:395–407. doi: 10.1111/j.1365-2249.2005.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Boda B, Vernaz J, Ferreira E, Wiszniewski L, Constant S. Establishment and characterization of an in vitro human small airway model (SmallAir™) Eur J Pharm Biopharm. 2017;118:68–72. doi: 10.1016/j.ejpb.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Ito S. Repeated whole cigarette smoke exposure alters cell differentiation and augments secretion of inflammatory mediators in air-liquid interface three-dimensional co-culture model of human bronchial tissue. Toxicol in Vitro. 2017;38:170–178. doi: 10.1016/j.tiv.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Iskandar AR, Xiang Y, Frentzel S, Talikka M, Leroy P, Kuehn D, Guedj E, Martin F, Mathis C, Ivanov NV, Peitsch MC, Hoeng J. Impact assessment of cigarette smoke exposure on organotypic bronchial epithelial tissue cultures: a comparison of mono-culture and coculture model containing fibroblasts. Toxicol Sci. 2015;147:207–221. doi: 10.1093/toxsci/kfv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskandar AR, Zanetti F, Marescotti D, Titz B, Sewer A, Kondylis A, et al. Application of a multi-layer systems toxicology framework for in vitro assessment of the biological effects of classic tobacco e-liquid and its corresponding aerosol using an e-cigarette device with MESH technology. Arch Toxicol. 2019;93:3229–3247. doi: 10.1007/s00204-019-02565-9. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Jr, Maione AG, Klausner M, Hayden PJ. Prevalidation of an acute inhalation toxicity test using the EpiAirway in vitro human airway model. Appl In Vitro Toxicol. 2018;4:149–158. doi: 10.1089/aivt.2018.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery PK. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis. 1983;128:S14–S20. doi: 10.1164/arrd.1983.128.2P2.S14. [DOI] [PubMed] [Google Scholar]
- Ji J, Ganguly K, Mihai X, Sun J, Malmlof M, Gerde P, Upadhyay S, Palmberg L. Exposure of normal and chronic bronchitis-like mucosa models to aerosolized carbon nanoparticles: comparison of pro-inflammatory oxidative stress and tissue injury/repair responses. Nanotoxicology. 2019;13:1362–1379. doi: 10.1080/17435390.2019.1655600. [DOI] [PubMed] [Google Scholar]
- Ji J, Hedelin A, Malmlof M, et al. Development of combining of human bronchial mucosa models with XposeALI® for exposure of air pollution nanoparticles. PLoS One. 2017;12:e0170428. doi: 10.1371/journal.pone.0170428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, Farzan M, Wohlford-Lenane C, Perlman S, McCray PB., Jr ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia. J Virol. 2005;79:14614–14621. doi: 10.1128/JVI.79.23.14614-14621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen L, Nettesheim P, Adler KB, Randell SH. Rat tracheal epithelial cell differentiation in vitro. In Vitro Cell Dev Biol Anim. 1993;296:481–492. doi: 10.1007/BF02639383. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Yanagihara K, Seki M, Kuroki M, Miyazaki Y, Hirakata Y, Mukae H, Tomono K, Kadota J, Kohno S. Clarithromycin inhibits overproduction of muc5ac core protein in murine model of diffuse panbronchiolitis. Am J Physiol Lung Cell Mol Physiol. 2003;85:L847–L853. doi: 10.1152/ajplung.00216.2002. [DOI] [PubMed] [Google Scholar]
- Kawasaki S, Talizawa H, Takami K, et al. Benzene-extracted components are important for the major activity of diesel exhaust particles: effect on interleukin-8 gene expression in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2001;24:419–426. doi: 10.1165/ajrcmb.24.4.4085. [DOI] [PubMed] [Google Scholar]
- Kelly FL, Sun J, Fischer BM, Voynow JA, Kummarapurugu AB, Zhang HL, Nugent JL, Beasley RF, Martinu T, Gwinn WM, Morgan DL, Palmer SM. Diacetyl induces amphiregulin shedding in pulmonary epithelial cells and in experimental bronchiolitis obliterans. Am J Respir Cell Mol Biol. 2014;51:568–574. doi: 10.1165/rcmb.2013-0339OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly FL, Weinberg KE, Nagler AE, Nixon AB, Star MD, Todd JL, Brass DM, Palmer SM. EGFR-dependent IL8 production by airway epithelial cells after exposure to the food flavoring chemical 2,3-butanedione. Toxicol Sci. 2019;169:534–542. doi: 10.1093/toxsci/kfz066. [DOI] [PubMed] [Google Scholar]
- Kesimer M, Kirkham S, Pickles RJ, et al. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol. 2009;296:L92–L100. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketterer MR, Shao JQ, Hornick DB, Buscher B, Bandi VK, Apicella MA. Infection of primary human bronchial epithelial cells by Haemophilus influenzae: macropinocytosis as a mechanism of airway epithelial cell entry. Infect Immun. 1999;67:4161–4170. doi: 10.1128/IAI.67.8.4161-4170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser BM, Leverette R, Hollings M, Seymour A, Reeve L, Fields W. Investigation of multiple whole smoke dosimetry techniques using a Vitrocell® VC10® smoke exposure system. Toxicol Rep. 2019;6:1281–1288. doi: 10.1016/j.toxrep.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler E, Jónsdóttir HR, Muth D, Hamming OJ, Hartmann R, Rodriguez R, Geffers R, Fouchier RA, Drosten C, Müller MA, Dijkman R, Thiel V. Efficient replication of the novel human betacoronavirus EMC on primary human epithelium highlights its zoonotic potential. mBio. 2013;4:e00611–e00612. doi: 10.1128/mBio.00611-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI0215217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooter IM, Grollers-Mulderij M, Duistermaat E, Kuper F, Schoen ED. Factors of concern in a human 3D cellular airway model exposed to aerosols of nanoparticles. Toxicol in Vitro. 2017;44:339–348. doi: 10.1016/j.tiv.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Kooter IM, Ilves M, Grollers-Mulderij M, Duistermaat E, Tromp PC, Kuper F, Kinaret P, Savolainen K, Greco D, Karisola P, Ndika J, Alenius H. Molecular signature of asthma-enhanced sensitivity to CuO nanoparticle aerosols from 3D cell model. ACS Nano. 2019;13:6932–6946. doi: 10.1021/acsnano.9b01823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg TG, Stueckle TA, Antonini JA, Rojanasakul Y, Castranova V, Yang Y, Wang L (2017) Potential toxicity and underlying mechanisms associated with pulmonary exposure to iron oxide nanoparticles: conflicting literature and unclear risk. Nanomaterials (Basel) 7: pii E307. [DOI] [PMC free article] [PubMed]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16:1319–1326. doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzi L, Krapf M, Daher N, Dommen J, Jeannet N, Schneider S, Platt S, Slowik JG, Baumlin N, Salathe M, Prevot AS, Kalberer M, Strahl C, Dumbgen L, Sioutas C, Baltensperger U, Geiser M. Toxicity of aged gasoline exhaust particles to normal and diseased airway epithelia. Sci Rep. 2015;5:11801. doi: 10.1038/srep11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix G, Koch W, Ritter D, Gutleb AC, Larsen ST, Loret T, Zanetti F, Constant S, Chortarea S, Rothen-Rutishauser B, Hiemstra PS, Frejafon E, Hubert P, Gribaldo L, Kearns P, Aublant J-M, Diabaté S, Weiss C, de Groot A, Kooter I. Air–liquid interface in vitro models for respiratory toxicology research: consensus workshop and recommendations. Appl In Vitro Toxicol. 2018;4:91–106. doi: 10.1089/aivt.2017.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbach LK, Li Y, Grass RN, Brunner TJ, Hintermann MA, Muller M, Gunther D, Stark WJ. Oxide nanoparticle uptake in human lung fibroblasts: effects of particle size, agglomeration, and diffusion at low concentrations. Environ Sci Technol. 2005;39:9370–9376. doi: 10.1021/es051043o. [DOI] [PubMed] [Google Scholar]
- Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56:588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz RC, Lemus R, Lange RW, Karol MH. Rapid reduction of intracellular glutathione in human bronchial epithelial cells exposed to occupational levels of toluene diisocyanate. Toxicol Sci. 2001;60:348–355. doi: 10.1093/toxsci/60.2.348. [DOI] [PubMed] [Google Scholar]
- Laube BL. The expanding role of aerosols in systemic drug delivery, gene therapy and vaccination: an update. Transl Respir Med. 2014;2:3. doi: 10.1186/2213-0802-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechapt-Zalcman E, Hurbain I, Lacave R, Commo F, Urban T, Antoine M, Milleron B, Bernaudin JF. MDR1-Pgp 170 expression in human bronchus. Eur Respir J. 1997;10:1837–1843. doi: 10.1183/09031936.97.10081837. [DOI] [PubMed] [Google Scholar]
- Lechner JF, Haugen A, Autrup H, McClendon IA, Trump BF, Harris CC. Clonal growth of epithelial cells from normal adult human bronchus. Cancer Res. 1981;41:2294–2304. [PubMed] [Google Scholar]
- Lee YC, Oslund KL, Thai P, Velichko S, Fujisawa T, Duong T, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced MUC5AC expression: aryl hydrocarbon receptor-independent/EGFR/ERK/p38-dependent SP1-based transcription. Am J Respir Cell Mol Biol. 2011;45:270–276. doi: 10.1165/rcmb.2010-0313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMessurier KS, Tiwary M, Morin NP, Samarasinghe AE. Respiratory barrier as a safeguard and regulator of defense against influenza A virus and Streptococcus pneumoniae. Front Immunol. 2020;11:3. doi: 10.3389/fimmu.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz A-G, Karg E, Brendel E, Hinze-Heyn H, Maier KL, Eickelberg O, Stoeger T, Schmid O. Inflammatory and oxidative stress responses of an alveolar epithelial cell line to airborne zinc oxide nanoparticles at the air-liquid interface: a comparison with conventional, submerged cell-culture conditions. Biomed Res Int. 2013;2013:652632–652632. doi: 10.1155/2013/652632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz AG, Stoeger T, Cei D, Schmidmeir M, Semren N, Burgstaller G, Lentner B, Eickelberg O, Meiners S, Schmid O. Efficient bioactive delivery of aerosolized drugs to human pulmonary epithelial cells cultured in air–liquid interface conditions. Am J Resp Cell Mol. 2014;51:526–535. doi: 10.1165/rcmb.2013-0479OC. [DOI] [PubMed] [Google Scholar]
- LeSimple P, Liao J, Robert R, Gruenert DC, Hanrahan JW. Cystic fibrosis transmembrane conductance regulator trafficking modulates the barrier function of airway epithelial cell monolayers. J Physiol. 2010;588:1195–1209. doi: 10.1113/jphysiol.2009.182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Muralikrishnan S, Ng CT, Yung LY, Bay BH. Nanoparticle-induced pulmonary toxicity. Exp Biol Med. 2010;235:1025–1033. doi: 10.1258/ebm.2010.010021. [DOI] [PubMed] [Google Scholar]
- Li X, Shang P, Peng B, Nie C, Zhao L, Liu H, Xie J. Effects of smoking regimens and test material format on the cytotoxicity of mainstream cigarette smoke. Food Chem Toxicol. 2012;50:545–551. doi: 10.1016/j.fct.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Lin H, Li H, Cho HJ, Bian S, Roh HJ, Lee MK, Kim JS, Chung SJ, Shim CK, Kim DD. Air-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studies. J Pharm Sci. 2007;96:341–350. doi: 10.1002/jps.20803. [DOI] [PubMed] [Google Scholar]
- Liu X, Luo M, Zhang L, Ding W, Yan Z, Engelhardt JF. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Respir Cell Mol Biol. 2007;36:313–323. doi: 10.1165/rcmb.2006-0286OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Boado YS, Rubin BK. Macrolides as immunomodulatory medications for the therapy of chronic lung diseases. Curr Opin Pharmacol. 2008;8:286–291. doi: 10.1016/j.coph.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Loret T, Peyret E, Dubreuil M, et al. Air-liquid interface exposure to aerosols of poorly soluble nanomaterials induces different biological activation levels compared to exposure to suspensions. Part Fibre Toxicol. 2016;13:58. doi: 10.1186/s12989-016-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Zu M, Byun JH, Kim BS, Chou TW. State of the art of carbon nanotube fibers: opportunities and challenges. Adv Mater. 2012;24:1805–1833. doi: 10.1002/adma.201104672. [DOI] [PubMed] [Google Scholar]
- Madlova M, Bosquillon C, Asker D, Dolezal P, Forbes B. In-vitro respiratory drug absorption models possess nominal functional P-glycoprotein activity. J Pharm Pharmacol. 2009;61:293–301. doi: 10.1211/jpp.61.03.0003. [DOI] [PubMed] [Google Scholar]
- Majeed S, Frentzel S, Wagner S, Kuehn D, Leroy P, Guy PA, Knorr A, Hoeng J, Peitsch MC. Characterization of the Vitrocell® 24/48 in vitro aerosol exposure system using mainstream cigarette smoke. Chem Cent J. 2014;8:62. doi: 10.1186/s13065-014-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory FB, Hornor AA. Pertussis: the histological lesion in the respiratory tract. J Med Res. 1912;27:115–124. [PMC free article] [PubMed] [Google Scholar]
- Manche M, Nesslany F, Krebs T (2018) A setup to test toxicity of alcohol inhalation from consumer products. Vitrocell® User Group Meeting. Available via: https://www.vitrocell.com/news/a-setup-to-test-toxicity-of-alcohol-inhalation-from-consumer-products.
- Mata M, Sarrion I, Armengot M, Carda C, Martinez I, Melero JA, Cortijo J. Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: effectiveness of N-acetylcysteine. PLoS One. 2012;7:e48037. doi: 10.1371/journal.pone.0048037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc Natl Acad Sci U S A. 2004;101:4620–4624. doi: 10.1073/pnas.0308001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama M, Martins AJ, Shallom S, Kamenyeva O, Kashyap A, Sampaio EP, Kabat J, Olivier KN, Zelazny AM, Tsang JS, Holland SM. Transcriptional response of respiratory epithelium to nontuberculous mycobacteria. Am J Respir Cell Mol Biol. 2018;58:241–252. doi: 10.1165/rcmb.2017-0218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw MD, Kim SY, Reed C, Hernady E, Rahman I, Mariani TJ, Finkelstein JN. Airway basal cell injury after acute diacetyl (2,3-butanedione) vapor exposure. Toxicol Lett. 2020;325:25–33. doi: 10.1016/j.toxlet.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MeLemore TL, Adelberg S, Liu MC, et al. Expression of CYP1A1 gene in patients with lung cancer: evidence for cigarette smoke-induced gene expression in normal lung tissue and for altered gene regulation in primary pulmonary carcinomas. J Natl Cancer Inst. 1990;82:1333–1339. doi: 10.1093/jnci/82.16.1333. [DOI] [PubMed] [Google Scholar]
- Mertens TCJ, van der Does AM, Kistemaker LE, Ninaber DK, Taube C, Hiemstra PS (2017) Cigarette smoke differentially affects IL-13-induced gene expression in human airway epithelial cells. Physiol Rep 5: pii e13347. [DOI] [PMC free article] [PubMed]
- Moore TL, Rodriguez-Lorenzo L, Hirsch V, et al. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem Soc Rev. 2015;44:6287–6305. doi: 10.1039/C4CS00487F. [DOI] [PubMed] [Google Scholar]
- Moses E, Wang T, Corbett S, Jackson GR, Drizik E, Perdomo C, Perdomo C, Kleerup E, Brooks D, O'Connor G, Dubinett S, Hayden P, Lenburg ME, Spira A. Molecular impact of electronic cigarette aerosol exposure in human bronchial epithelium. Toxicol Sci. 2017;155:248–257. doi: 10.1093/toxsci/kfw198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller L, Brighton LE, Carson JL, Fischer WA, II, Jaspers I. Culturing of human nasal epithelial cells at the air liquid interface. J Vis Exp. 2013;80:e50646. doi: 10.3791/50646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller L, Gasser M, Raemy D, Herzog F, Brandenberger C, Schmid O, Gehr P, Rothen-Rutishauser B, Clift M. Realistic exposure methods for investigating the interaction of nanoparticles with the lung at the air-liquid interface in vitro. Insciences J. 2011;1:30–64. doi: 10.5640/insc.010130. [DOI] [Google Scholar]
- Nara H, Fukano Y, Nishino T, Aufderheide M. Detection of the cytotoxicity of water-insoluble fraction of cigarette smoke by direct exposure to cultured cells at an air–liquid interface. Exp Toxicol Pathol. 2013;65:683–688. doi: 10.1016/j.etp.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Neilson L, Mankus C, Thorne D, Jackson G, DeBay J, Meredith C. Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicol in Vitro. 2015;29:1952–1962. doi: 10.1016/j.tiv.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Newland N, Baxter A, Hewitt K, Minet E. CYP1A1/1B1 and CYP2A6/2A13 activity is conserved in cultures of differentiated primary human tracheobronchial epithelial cells. Toxicol in Vitro. 2011;25:922–929. doi: 10.1016/j.tiv.2011.02.014. [DOI] [PubMed] [Google Scholar]
- National Research Council . Toxicity testing in the 21st century: a vision and a strategy. Washington, DC: National Academy Press; 2007. [Google Scholar]
- OECD (Organisation for Economic Cooperation and Development) (2005) Guidance document on the validation and international acceptance of new or updated test methods for hazard assessment. Series on Testing and assessment, No. 34. Available via: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2005)14&doclanguage=en.
- Okuwa K, Tanaka M, Fukano Y, Nara H, Nishijima Y, Nishino T. In vitro micronucleus assay for cigarette smoke using a whole smoke exposure system: a comparison of smoking regimens. Exp Toxicol Pathol. 2010;62:433–440. doi: 10.1016/j.etp.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Oldham MJ, Castro N, Zhang J, Rostami A, Lucci F, Pithawalla Y, Kuczaj AK, Gilman IG, Kosachevsky P, Hoeng J, Lee KM. Deposition efficiency and uniformity of monodisperse solid particle deposition in the Vitrocell® 24/48 air–liquid-interface in vitro exposure system. Aerosol Sci Technol. 2020;54:52–65. doi: 10.1080/02786826.2019.1676877. [DOI] [PubMed] [Google Scholar]
- Olsson B, Bondesson E, Borgström L, Edsbäcker S, Eirefelt S, Ekelund K, Gustavsson L, Hegelund-Myrbäck T. Pulmonary drug metabolism, clearance, and absorption. In: Smyth H, Hickey A, editors. Controlled pulmonary drug delivery. New York: Advances in Delivery Science and Technology. Springer; 2011. pp. 21–50. [Google Scholar]
- Osei ET, Hackett TL. Epithelial-mesenchymal crosstalk in COPD: an update from in vitro model studies. Int J Biochem Cell Biol. 2020;125:105775. doi: 10.1016/j.biocel.2020.105775. [DOI] [PubMed] [Google Scholar]
- Osei ET, Noordhoek JA, Hackett TL, Spanjer AI, Postma DS, Timens W, Brandsma CA, Heijink IH. Interleukin-1α drives the dysfunctional cross-talk of the airway epithelium and lung fibroblasts in COPD. Eur Respir J. 2016;48:359–369. doi: 10.1183/13993003.01911-2015. [DOI] [PubMed] [Google Scholar]
- Ou XM, Feng YL, Wen FQ, Wang K, Yang J, Deng ZP, Liu DS, Li YP. Macrolides attenuate mucus hypersecretion in rat airways through inactivation of NF-kappaB. Respirology. 2008;13:63–72. doi: 10.1111/j.1440-1843.2007.01213.x. [DOI] [PubMed] [Google Scholar]
- Outlaw VK, Bottom-Tanzer S, Kreitler DF, Gellman SH, Porotto M, Moscona A. Dual inhibition of human parainfluenza type 3 and respiratory syncytial virus infectivity with a single agent. J Am Chem Soc. 2019;141:12648–12656. doi: 10.1021/jacs.9b04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo LM, Porotto M, Yokoyama CC, Palmer SG, Mungall BA, Greengard O, Niewiesk S, Moscona A. Human parainfluenza virus infection of the airway epithelium: viral hemagglutinin-neuraminidase regulates fusion protein activation and modulates infectivity. J Virol. 2009;83:6900–6908. doi: 10.1128/JVI.00475-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Wells JM, Zorn AM, et al. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol. 2006;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlak Z, Biet C, Zauscher S. Decoupling mass adsorption from fluid viscosity and density in quartz crystal microbalance measurements using normalized conductance modeling. Measure Sci Tech. 2013;24:085301. doi: 10.1088/0957-0233/24/8/085301. [DOI] [Google Scholar]
- Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- Patton JS, Fishburn CS, Weers JG. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc. 2004;1:338–344. doi: 10.1513/pats.200409-049TA. [DOI] [PubMed] [Google Scholar]
- Pazo DY, Moliere F, Sampson MM, Reese CM, Agnew-Heard KA, Walters MJ, Holman MR, Blount BC, Watson CH, Chambers DM. Mainstream smoke levels of volatile organic compounds in 50 U.S. domestic cigarette brands smoked with the ISO and Canadian Intense Protocols. Nicotine Tob Res. 2016;18:1886–1894. doi: 10.1093/ntr/ntw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine CG, Pickens CM, Boehmer TK, King BA, Jones CM, DeSisto CL, Duca LM, Lekiachvili A, Kenemer B, Shamout M, Landen MG, Lynfield R, Ghinai I, Heinzerling A, Lewis N, Pray IW, Tanz LJ, Patel A, Briss PA. Characteristics of a multistate outbreak of lung injury associated with E-cigarette use, or vaping - United States, 2019. MMWR Morb Mortal Wkly Rep. 2019;68:860–864. doi: 10.15585/mmwr.mm6839e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo AA, Starner TD, Scheetz TE, et al. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2011;300:L25–L31. doi: 10.1152/ajplung.00256.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J, Kluss B, Richter A, Massey ED. Exposure of bronchial epithelial cells to whole cigarette smoke: assessment of cellular responses. Altern Lab Anim. 2005;33:239–248. doi: 10.1177/026119290503300310. [DOI] [PubMed] [Google Scholar]
- Plotkowski MC, de Bentzmann S, Pereira SH, Zahm JM, Bajolet-Laudinat O, Roger P, Puchelle E. Pseudomonas aeruginosa internalization by human epithelial respiratory cells depends on cell differentiation, polarity, and junctional complex integrity. Am J Respir Cell Mol Biol. 1999;20:880–890. doi: 10.1165/ajrcmb.20.5.3408. [DOI] [PubMed] [Google Scholar]
- Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66:232–239. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- Poch MT, Cutler NS, Yost GS, Hines RN. Molecular mechanisms regulating human BYP4B1 lung-selective expression. Drug Metab Dispos. 2005;33:1174–1184. doi: 10.1124/dmd.105.004523. [DOI] [PubMed] [Google Scholar]
- Polk WW, Sharma M, Sayes CM, Hotchkiss JA, Clippinger AJ. Aerosol generation and characterization of multi-walled carbon nanotubes exposed to cells cultured at the air-liquid interface. Part Fibre Toxicol. 2016;13:20. doi: 10.1186/s12989-016-0131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JD, Hutchison JR, Hess BM, Straub TM. Bacillus anthracis spores germinate extracellularly at air-liquid interface in an in vitro lung model under serum-free conditions. J Appl Microbiol. 2015;119:711–723. doi: 10.1111/jam.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince OA, Krunkosky TM, Sheppard ES, Krause DC (2018) Modelling persistent mycoplasma pneumoniae infection of human airway epithelium. Cell Microbiol 20. 10.1111/cmi.12810 [DOI] [PMC free article] [PubMed]
- Prytherch Z, Job C, Marshall H, Oreffo V, Foster M, BeruBe K. Tissue-specific stem cell differentiation in an in vitro airway model. Macromol Biosci. 2011;11:1467–1477. doi: 10.1002/mabi.201100181. [DOI] [PubMed] [Google Scholar]
- Pyrc K, Sims AC, Dijkman R, Jebbink M, Long C, Deming D, Donaldson E, Vabret A, Baric R, van der Hoek L, Pickles R. Culturing the unculturable: human coronavirus HKU1 infects, replicates, and produces progeny virions in human ciliated airway epithelial cell cultures. J Virol. 2010;84:11255–11263. doi: 10.1128/JVI.00947-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q, Wu Q, Wang Y, et al. Effects of cellular differentiation in human primary bronchial epithelial cells: metabolism of 4-(methylnitrosamine)-1-(3-pyridyl)-1-butanone. Toxicol in Vitro. 2019;55:185–194. doi: 10.1016/j.tiv.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rach J, Budde J, Möhle N, Aufderheide M (2014) Direct exposure at the air–liquid interface: evaluation of an in vitro approach for simulating inhalation of airborne substances. J. App Toxicol 34: 506–515. [DOI] [PubMed]
- Raemy DO, Grass RN, Stark WJ, Schumacher CM, Clift MJ, Gehr P, Rothen-Rutishauser B. Effects of flame made zinc oxide particles in human lung cells - a comparison of aerosol and suspension exposures. Part Fibre Toxicol. 2012;9:33. doi: 10.1186/1743-8977-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffel FK, Szelestey BR, Beatty WL, Mason KM. The Haemophilus influenzae sap transporter mediates bacterium-epithelial cell homeostasis. Infect Immun. 2013;81:43–54. doi: 10.1128/IAI.00942-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BLM. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner RE, Makena P, Prasad GL, Cormet-Boyaka E. Optimization of normal human bronchial epithelial (NHBE) cell 3D cultures for in vitro lung model studies. Sci Rep. 2019;9:500. doi: 10.1038/s41598-018-36735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Wu Q, Bryant M, Wang Y, Cao X (2020) Evaluating respiratory toxicity of formaldehyde in an in vitro human airway epithelial tissue model. 2020 Annual Meeting of Society of Toxicology Abstract Supplement.
- Reus AA, Maas WJ, Jansen HT, Constant S, Staal YC, van Triel JJ, Kuper CF. Feasibility of a 3D human airway epithelial model to study respiratory absorption. Toxicol in Vitro. 2014;28:258–264. doi: 10.1016/j.tiv.2013.10.025. [DOI] [PubMed] [Google Scholar]
- Reuschl AK, Edwards MR, Parker R, Connell DW, Hoang L, Halliday A, Jarvis H, Siddiqui N, Wright C, Bremang S, Newton SM, Beverley P, Shattock RJ, Kon OM, Lalvani A. Innate activation of human primary epithelial cells broadens the host response to Mycobacterium tuberculosis in the airways. PLoS Pathog. 2017;13:e1006577. doi: 10.1371/journal.ppat.1006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaee F, Georas SN. Breaking barriers. New insights into airway epithelial barrier function in health and disease. Am J Respir Cell Mol Biol. 2014;50:857–869. doi: 10.1165/rcmb.2013-0541RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter D, Knebel JW, Aufderheide M. In vitro exposure of isolated cells to native gaseous compounds development and validation of an optimized system for human lung cells. Exp Toxicol Pathol. 2001;53:373–386. doi: 10.1078/0940-2993-00204. [DOI] [PubMed] [Google Scholar]
- Rodgman A, Perfetti TA. The chemical components of tobacco and tobacco smoke. 2. Boca Raton Florida: CRC Press; 2013. [Google Scholar]
- Rogers DF. Mucociliary dysfunction in COPD: effect of current pharmacotherapeutic options. Pulm Pharmacol Ther. 2005;18:1–8. doi: 10.1016/j.pupt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Röhm M, Carle S, Maigler F, Flamm J, Kramer V, Mavoungou C, Schmid O, Schindowski K. A comprehensive screening platform for aerosolizable protein formulations for intranasal and pulmonary drug delivery. Int J Pharm. 2017;532:537–546. doi: 10.1016/j.ijpharm.2017.09.027. [DOI] [PubMed] [Google Scholar]
- Roszak J, Catalan J, Jarventaus H, Lindberg HK, Suhonen S, Vippola M, Stepnik M, Norppa H. Effect of particle size and dispersion status on cytotoxicity and genotoxicity of zinc oxide in human bronchial epithelial cells. Mutat Res Genet Toxicol Environ Mutagen. 2016;805:7–18. doi: 10.1016/j.mrgentox.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Rubin BK, Druce H, Ramirez OE, Palmer R. Effect of clarithromycin on nasal mucus properties in healthy subjects and in patients with purulent rhinitis. Am J Respir Crit Care Med. 1997;155:2018–2023. doi: 10.1164/ajrccm.155.6.9196110. [DOI] [PubMed] [Google Scholar]
- Rubin BK, Henke MO. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest. 2004;125(2 Suppl):70S–78S. doi: 10.1378/chest.125.2_suppl.70S. [DOI] [PubMed] [Google Scholar]
- Russell WMS, Burch RL. The principles of humane experimental technique. London: Metheuen; 1959. [Google Scholar]
- Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, Heo I, Böttinger L, Klay D, Weeber F, Huelsz-Prince G, Iakobachvili N, Amatngalim GD, de Ligt J, van Hoeck A, Proost N, Viveen MC, Lyubimova A, Teeven L, Derakhshan S, Korving J, Begthel H, Dekkers JF, Kumawat K, Ramos E, van Oosterhout MFM, Offerhaus GJ, Wiener DJ, Olimpio EP, Dijkstra KK, Smit EF, van der Linden M, Jaksani S, van de Ven M, Jonkers J, Rios AC, Voest EE, van Moorsel CHM, van der Ent CK, Edwin C, van Oudenaarden A, Coenjaerts FE, Linde M, Bont LJ, Peters PJ, Tans SJ, van Zon JS, Boj SF, Vries RG, Beekman JM, Clevers H. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019;38:e100300. doi: 10.15252/embj.2018100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, Ross M. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect. 2011;119:446–454. doi: 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint-Criq V, Gray MA. Role of CFTR in epithelial physiology. Cell Mol Life Sci. 2017;74:93–115. doi: 10.1007/s00018-016-2391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamberger AC, Staab-Weijnitz CA, Mise-Racek N, Eickelberg O. Cigarette smoke alters primary human bronchial epithelial cell differentiation at the air-liquid interface. Sci Rep. 2015;5:8163. doi: 10.1038/srep08163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler S, Dieken H, Krischenowski O, Aufderheide M. Cytotoxic evaluation of e-liquid aerosol using different lung-derived cell models. Int J Environ Res Public Health. 2015;12:12466–12474. doi: 10.3390/ijerph121012466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Braubach P, Schilpp C, et al. IL-13 impairs tight junctions in airway epithelial. Int J Mol Sci. 2019;20:3222. doi: 10.3390/ijms20133222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab U, Leigh M, Ribeiro C, Yankaskas J, Burns K, Gilligan P, Sokol P, Boucher R. Patterns of epithelial cell invasion by different species of the Burkholderia cepacia complex in well-differentiated human airway epithelia. Infect Immun. 2002;70:4547–4555. doi: 10.1128/IAI.70.8.4547-4555.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagrave J, Weber WM, Grotendorst GR. Sulfur mustard vapor effects on differentiated human lung cells. Inhal Toxicol. 2010;22:896–902. doi: 10.3109/08958378.2010.493901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears PR, Ying WN, Ostrowski LE. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2015;309:L99–L108. doi: 10.1152/ajplung.00024.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini SM, Michaelson ED. Length and distribution of cilia in human and canine airways. Bull Eur Physiopathol Respir. 1977;13:551–559. [PubMed] [Google Scholar]
- Shalit I, Halperin D, Haite D, Levitov A, Romano J, Osherov N, Fabian I. Anti-inflammatory effects of moxifloxacin on IL-8, IL-1beta and TNF-alpha secretion and NFkappaB and MAP-kinase activation in human monocytes stimulated with Aspergillus fumigatus. J Antimicrob Chemother. 2006;57:230–235. doi: 10.1093/jac/dki441. [DOI] [PubMed] [Google Scholar]
- Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philos Ethics Humanit Med. 2009;4:1–20. doi: 10.1186/1747-5341-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma L, Feng J, Britto CJ, Dela Cruz CS. Mechanisms of epithelial immunity evasion by respiratory bacterial pathogens. Front Immunol. 2020;11:91. doi: 10.3389/fimmu.2020.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaykhiev R, Crystal RG. Basal cell origins of smoking-induced airway epithelial disorders. Cell Cycle. 2014;13:341–342. doi: 10.4161/cc.27510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaykhiev R, Otaki F, Bonsu P, Dang DT, Teater M, Strulovici-Barel Y, Salit J, Harvey BG, Crystal RG. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell Mol Life Sci. 2011;68:877–892. doi: 10.1007/s00018-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Wolkowicz MJ, Kotova T, Fan L, Timko MP. Transcriptome sequencing reveals e-cigarette vapor and mainstream-smoke from tobacco cigarettes activate different gene expression profiles in human bronchial epithelial cells. Sci Rep. 2016;6:23984. doi: 10.1038/srep23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, Hubbs AF, Mercer RR, Keohavong P, Sussman N, Jin J, Yin J, Stone S, Chen BT, Deye G, Maynard A, Castranova V, Baron PA, Kagan VE. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress, and mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L552–L565. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc. 2003;211:103–111. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- Soane MC, Jackson A, Maskell D, Allen A, Keig P, Dewar A, Dougan G, Wilson R. Interaction of Bordetella pertussis with human respiratory mucosa in vitro. Respir Med. 2000;94:791–799. doi: 10.1053/rmed.2000.0823. [DOI] [PubMed] [Google Scholar]
- Soong G, Martin FJ, Chun J, Cohen TS, Ahn DS, Prince A. Staphylococcus aureus protein A mediates invasion across airway epithelial cells through activation of RhoA GTPase signaling and proteolytic activity. J Biol Chem. 2011;286:35891–35898. doi: 10.1074/jbc.M111.295386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan B, Kolli AR, Esch MB, et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015;20:107–126. doi: 10.1177/2211068214561025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt MR, Rogalski A, Tilley AE, et al. Smoking is associated with a loss of ciliated cells throughout the airways. Am J Resp Crit Care Med. 2014;189:A4097. [Google Scholar]
- St-Laurent J, Proulx LI, Boulet LP, Bissonnette E. Comparison of two in vitro models of cigarette smoke exposure. Inhal Toxicol. 2009;21:1148–1153. doi: 10.3109/08958370902926692. [DOI] [PubMed] [Google Scholar]
- Stein SW, Thiel CG. The history of therapeutic aerosols: a chronological review. J Aerosol Med Pulm Drug Deliv. 2017;30:20–41. doi: 10.1089/jamp.2016.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinritz D, Möhle N, Pohl C, Papritz M, Stenger B, Schmidt A, Kirkpatrick CJ, Thiermann H, Vogel R, Hoffmann S, Aufderheide M. Use of the CULTEX® radial flow system as an in vitro exposure method to assess acute pulmonary toxicity of fine dusts and nanoparticles with special focus on the intra- and inter-laboratory reproducibility. Chem-Bio Int. 2013;206:479–490. doi: 10.1016/j.cbi.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Strengert M, Knaus UG. Analysis of epithelial barrier integrity in polarized lung epithelial cells. Methods Mol Biol. 2011;763:195–206. doi: 10.1007/978-1-61779-191-8_13. [DOI] [PubMed] [Google Scholar]
- Su T, Bao Z, Zhang QY, Smith TJ, Hong JY, Ding X. Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 2000;60:5074–5079. [PubMed] [Google Scholar]
- Swearengen JR. Choosing the right animal model for infectious disease research. Animal Model Exp Med. 2018;1:100–108. doi: 10.1002/ame2.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa H, Ohtoshi T, Kawasaki S, et al. Diesel exhaust particles induce NF-ƙB activation in human bronchial epithelial cells in vitro: importance in cytokine transcription. J Immunol. 1999;162:4705–4711. [PubMed] [Google Scholar]
- Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis. 2011;5:255–273. doi: 10.1177/1753465810396539. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Kanoh S, Tsushima K, Yamazaki Y, Kubo K, Rubin BK. Clarithromycin inhibits interleukin-13–induced goblet cell hyperplasia in human airway cells. Am J Respir Cell Mol Biol. 2011;5:1075–1083. doi: 10.1165/rcmb.2010-0327OC. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Yamaguchi M, Hirai S, Sumi T, Tada M, et al. Characterization of distal airway stem-like cells expressing N-terminally truncated p63 and thyroid transcription factor-1 in the human lung. Exp Cell Res. 2018;372:141–149. doi: 10.1016/j.yexcr.2018.09.020. [DOI] [PubMed] [Google Scholar]
- Tarkington BK, Reen W, Wei-Min S, Nikula KJ, Wilson DW, Last JA. In vitro exposure of tracheobronchial epithelial cells and of tracheal explants to ozone. Toxicol. 1994;88:51–68. doi: 10.1016/0300-483X(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Tatsuta M, Kan-O K, Ishii Y, et al. Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: protective role of cathelicidin LL-37. Respir Res. 2019;20:251. doi: 10.1186/s12931-019-1226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber SC, Nau R. Immunomodulatory properties of antibiotics. Curr Mol Pharmacol. 2008;1:68–79. doi: 10.2174/1874467210801010068. [DOI] [PubMed] [Google Scholar]
- Thaikoottathil JV, Martin RJ, Zdunek J, Weinberger A, Rino JG, Chu HW. Cigarette smoke extract reduces VEGF in primary human airway epithelial cells. Eur Resp J. 2009;33:835–843. doi: 10.1183/09031936.00080708. [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne D, Adamson J. A review of in vitro cigarette smoke exposure systems. Exp Toxicol Pathol. 2013;65:1183–1193. doi: 10.1016/j.etp.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Thorne D, Dalrymple A, Dillon D, Duke M, Meredith C. A comparative assessment of cigarette smoke aerosols using an in vitro air-liquid interface cytotoxicity test. Inhal Toxicol. 2015;27:629–640. doi: 10.3109/08958378.2015.1080773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne D, Kilford J, Payne R, Adamson J, Scott K, Dalrymple A, Meredith C, Dillon D. Characterisation of a Vitrocell® VC 10 in vitro smoke exposure system using dose tools and biological analysis. Chem Cent J. 2013;7:146–146. doi: 10.1186/1752-153X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly TB, Nelson MT, Chakravarthy KB, Shira EA, Debrose MC, Grabinski CM, Salisbury RL, Mattie DR, Hussain SM (2020) In vitro aerosol exposure to nanomaterials: from laboratory to environmental field toxicity testing. Chem Res Toxicol. 10.1021/acs.chemrestox.9b00237 [DOI] [PubMed]
- Tollstadius BF, Silva A, Pedralli BCO, Valadares MC. Carbendazim induces death in alveolar epithelial cells: a comparison between submerged and at the air-liquid interface cell culture. Toxicol in Vitro. 2019;58:78–85. doi: 10.1016/j.tiv.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Triana-Baltzer GB, Babizki M, Chan MC, Wong AC, Aschenbrenner LM, Campbell ER, Li QX, Chan RW, Peiris JS, Nicholls JM, Fang F. DAS181, a sialidase fusion protein, protects human airway epithelium against influenza virus infection: an in vitro pharmacodynamic analysis. J Antimicrob Chemother. 2010;65:275–284. doi: 10.1093/jac/dkp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoutsoulopoulos A, Gohlsch K, Möhle N, Breit A, Hoffmann S, Krischenowski O, Mückter H, Gudermann T, Thiermann H, Aufderheide M, Steinritz D. Validation of the CULTEX® radial flow system for the assessment of the acute inhalation toxicity of airborne particles. Toxicol in Vitro. 2019;58:245–255. doi: 10.1016/j.tiv.2019.03.020. [DOI] [PubMed] [Google Scholar]
- Tuomanen EI, Hendley JO. Adherence of Bordetella pertussis to human respiratory epithelial cells. J Infect Dis. 1983;148:125–130. doi: 10.1093/infdis/148.1.125. [DOI] [PubMed] [Google Scholar]
- Upadhyay S, Palmberg L. Air-liquid interface: relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol Sci. 2018;164:21–30. doi: 10.1093/toxsci/kfy053. [DOI] [PubMed] [Google Scholar]
- Utembe W, Potgieter K, Stefaniak AB, Gulumian M. Dissolution and biodurability: important parameters needed for risk assessment of nanomaterials. Part Fibre Toxicol. 2015;12:11. doi: 10.1186/s12989-015-0088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart J, Clevers H (2020) Airway organoids as models of human disease. J Intern Med. [published online ahead of print, 2020 Apr 29]. [DOI] [PubMed]
- van Wetering S, Zuyderduyn S, Ninaber DK, et al. Epithelial differentiation is a determinant in the production of Eotaxin-2 and -3 by bronchial epithelial cells in response to IL-4 and IL-13. Mol Immunol. 2007;44:803–811. doi: 10.1016/j.molimm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Vaughan A, Stevanovic S, Jafari M, Bowman RV, Fong KM, Ristovski ZD, Yang IA. Primary human bronchial epithelial cell responses to diesel and biodiesel emissions at an air-liquid interface. Toxicol in Vitro. 2019;57:67–75. doi: 10.1016/j.tiv.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Verkaik NJ, Nguyen DT, de Vogel CP, Moll HA, Verbrugh HA, Jaddoe VW, Hofman A, van Wamel WJ, van den Hoogen BG, Buijs-Offerman RM, Ludlow M, de Witte L, Osterhaus AD, van Belkum A, de Swart RL. Streptococcus pneumoniae exposure is associated with human metapneumovirus seroconversion and increased susceptibility to in vitro HMPV infection. Clin Microbiol Infect. 2014;17:1840–1844. doi: 10.1111/j.1469-0691.2011.03480.x. [DOI] [PubMed] [Google Scholar]
- Volckens J, Dailey L, Walters G, Devlin RB. Direct particle-to-cell deposition of coarse ambient particulate matter increases the production of inflammatory mediators from cultured human airway epithelial cells. Environ Sci Technol. 2009;43:4595–4599. doi: 10.1021/es900698a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Luanpitpong S, Castranova V, Tse W, Lu Y, Pongrakhananon V, Rojanasakul Y. Carbon nanotubes induce malignant transformation and tumorigenesis of human lung epithelial cells. Nano Lett. 2011;11:2796–2803. doi: 10.1021/nl2011214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu Q, Muskhelishvili L, Davis K, Bryant M, Cao X. Assessing the respiratory toxicity of dihydroxyacetone using an in vitro human airway epithelial tissue model. Toxicol in Vitro. 2019;59:78–86. doi: 10.1016/j.tiv.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou J, Wu Q, Wynne RA, Bryant M, Cao X (2019b) Integrating respiratory toxicity and genotoxicity endpoints in an in vitro model of human origin for assessing hazard to styrene exposure. EMGS Annual Meeting.
- Whitcutt MJ, Adlet KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol. 1988;24:420–428. doi: 10.1007/BF02628493. [DOI] [PubMed] [Google Scholar]
- Wilkinson KE, Palmberg L, Witasp E, et al. Solution-engineered palladium nanoparticles: model for health effect studies of automotive particulate pollution. ACS Nano. 2011;5:5312–5324. doi: 10.1021/nn1032664. [DOI] [PubMed] [Google Scholar]
- Wilson R, Read R, Thomas M, Rutman A, Harrison K, Lund V, Cookson B, Goldman W, Lambert H, Cole P. Effects of Bordetella pertussis infection on human respiratory epithelium in vivo and in vitro. Infect Immun. 1991;59:337–345. doi: 10.1128/IAI.59.1.337-345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J, Lechner JF. Normal human bronchial epithelial cell culture. In: Freshney RI, Freshney MG, editors. Culture of Epithelial Cells 2nd Edition. New York: Wiley-Liss; 2002. pp. 257–276. [Google Scholar]
- Worthington EN, Tarran R. Methods for ASL measurements and mucus transport rates in cell cultures. Methods Mol Biol. 2011;72:77–92. doi: 10.1007/978-1-61779-120-8_5. [DOI] [PubMed] [Google Scholar]
- Wu R, Sato GH, Whitcutt MJ. Developing differentiated epithelial cell cultures: airway epithelial cells. Fundam Appl Toxicol. 1986;6:580–590. doi: 10.1016/0272-0590(86)90170-3. [DOI] [PubMed] [Google Scholar]
- Xia S, Liu J, Yang Y, Wu M, Ye L, et al. Coupled CRC 2D and ALI 3D cultures express receptors of emerging viruses and are more suitable for the study of viral infections compared to conventional cell lines. Sem Cells Int. 2020;2020:2421689. doi: 10.1155/2020/2421689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Puddicombe SM, Field S, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–556. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- Xiong R, Wu Q, Muskhelishvili L, Davis K, Shemansky JM, Bryant M, Rosenfeldt H, Healy SM, Cao X. Evaluating mode of action of acrolein toxicity in an in vitro human airway tissue model. Toxicol Sci. 2018;166:451–464. doi: 10.1093/toxsci/kfy226. [DOI] [PubMed] [Google Scholar]
- Xiong R, Wu Q, Trbojevich R, Muskhelishvili L, Davis K, Bryant M, Richter R, Cao X. Disease-related responses induced by cadmium in an in vitro human airway tissue model. Toxicol Lett. 2019;303:16–27. doi: 10.1016/j.toxlet.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol Lung Cell Mol Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- Yanamala N, Hatfield MK, Farcas MT, Schwegler-Berry D, Hummer JA, Shurin MR, Birch ME, Gutkin DW, Kisin E, Kagan VE, Bugarski AD, Shvedova AA. Biodiesel versus diesel exposure: enhanced pulmonary inflammation, oxidative stress, and differential morphological changes in the mouse lung. Toxicol Appl Pharmacol. 2013;272:373–383. doi: 10.1016/j.taap.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarcone MC, Duistermaat E, Alblas MJ, van Schadewijk A, Ninaber DK, Clarijs V, Moerman MM, Vaessen D, Hiemstra PS, Kooter IM. Effect of diesel exhaust generated by a city bus engine on stress responses and innate immunity in primary bronchial epithelial cell cultures. Toxicol in Vitro. 2018;48:221–231. doi: 10.1016/j.tiv.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Zarcone MC, Duistermaat E, van Schadewijk A, Jedynska A, Hiemstra PS, Kooter IM. Cellular response of mucociliary differentiated primary bronchial epithelial cells to diesel exhaust. Am J Physiol Lung Cell Mol Physiol. 2016;311:L111–L123. doi: 10.1152/ajplung.00064.2016. [DOI] [PubMed] [Google Scholar]
- Zarcone MC, van Schadewijk A, Duistermaat E, Hiemstra PS, Kooter IM. Diesel exhaust alters the response of cultured primary bronchial epithelial cells from patients with chronic obstructive pulmonary disease (COPD) to non-typeable Haemophilus influenzae. Respir Res. 2017;18:27. doi: 10.1186/s12931-017-0510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bukreyev A, Thompson CI, Watson B, Peeples ME, Collins PL, Pickles RJ. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J Virol. 2005;79:1113–1124. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76:5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by toll-like receptor 5. Infect Immun. 2005;73:7151–7160. doi: 10.1128/IAI.73.11.7151-7160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ma JK, Barger MW, Mercer RR, Millecchia L, Schwegler-Berry D, Castranova V, Ma JY. Reactive oxygen species- and nitric oxide-mediated lung inflammation and mitochondrial dysfunction in wild-type and iNOS-deficient mice exposed to diesel exhaust particles. J Toxicol Environ Health A. 2009;72:560–570. doi: 10.1080/15287390802706330. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhou Z, Mao H, Yang L. Magnetic nanoparticles for precision oncology: theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine. 2017;12:73–87. doi: 10.2217/nnm-2016-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733. [DOI] [PMC free article] [PubMed]
- Zimmermann GS, Neurohr C, Villena-Hermoza H, Hatz R, Behr J. Anti-inflammatory effects of antibacterials on human bronchial epithelial cells. Respir Res. 2009;10:89. doi: 10.1186/1465-9921-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulianello L, Canard C, Köhler T, Caille D, Lacroix JS, Meda P. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect Immun. 2006;74:3134–3147. doi: 10.1128/IAI.01772-05. [DOI] [PMC free article] [PubMed] [Google Scholar]