Abstract
Erythropoiesis‐stimulating agents (ESA) reduce the need for transfusions and improve the quality of life in patients receiving chemotherapy, but several clinical trials have suggested that ESA might have a negative impact on survival. To evaluate the efficacy and safety of ESA, epoetin beta and darbepoetin alfa, including their impact on overall survival and thromboembolic events, we conducted an individual data‐based meta‐analysis of three randomized, placebo‐controlled trials studying Japanese patients with chemotherapy‐induced anemia. All trials were conducted in compliance with Good Clinical Practice. A total of 511 patients with solid tumor or lymphoma (epoetin beta or darbepoetin alfa, n = 273; placebo, n = 238) were included. The ESA significantly reduced the risk of transfusion (relative risk, 0.47; 95% confidence interval, 0.29–0.76). No significant effect of the ESA on overall survival was observed (unadjusted hazard ratio, 1.00; 95% confidence interval, 0.75–1.34). A prespecified subgroup analysis showed no strong interaction between the baseline hemoglobin concentration and the effect of ESA on overall survival. Among the ESA‐treated patients, the highest hemoglobin achieved during the treatment period in each patient had no impact on mortality. No increase in thromboembolic events was observed in the ESA‐treated patients (0.7% vs 1.7% placebo). The ESA reduced the risk of transfusion without a negative impact on the survival of patients with chemotherapy‐induced anemia.
A number of studies have reported that erythropoiesis‐stimulating agents (ESA) are effective in improving anemia and that ESA decrease red blood cell (RBC) transfusion needs and improve the quality of life in cancer patients with chemotherapy‐induced anemia (CIA).1, 2, 3
As an ESA, epoetin alfa was approved in the United States of America (USA) for CIA in 1993 and in Europe, epoetin alfa and epoetin beta were approved in 1994. Subsequently, darbepoetin alfa received European Union (EU) approval in 2001 and USA approval in 2002. Since then, ESA have been commonly used in clinical practice. However, in a clinical trial of epoetin alfa administered to chemotherapy‐treated patients with metastatic breast cancer4 and a clinical trial of radiation‐treated head and neck carcinoma patients,5 reduced survival results of ESA groups compared with placebo groups were reported. After these reports, similar findings from multiple trials were published,6, 7, 8, 9 suggesting negative effects of ESA on cancer patients' prognosis.
In light of these reports, an Oncologic Drugs Advisory Committee met in 2004, 2007 and 2008 in the USA to discuss and investigate the risks of ESA as a CIA treatment. As a result, a boxed warning was added to the product label of each ESA describing the risk of ESA worsening life prognosis and these warnings have remained, with multiple revisions.
Preclinical studies suggested that tumor cells express erythropoietin receptors and that ESA activate these receptors to induce or promote tumor growth.10, 11 However, technical issues (such as anti‐erythropoietin receptor antibody's lack of specificity for erythropoietin receptor) have limited the validity of the findings.12, 13
Several meta‐analyses of randomized controlled trials exploring the risk of worsening the life prognosis for cancer patients on ESA have been reported.14, 15, 16, 17, 18, 19 However, the majority of the clinical trials reviewed in these meta‐analyses were conducted with hemoglobin (Hb) concentrations higher than the standard stated in the current package inserts of ESA in the USA and the EU, that is, the Hb concentrations at the beginning of ESA therapy to be 10 g/dL or lower, and the target Hb concentration of 10–12 g/dL or the minimum level to avoid RBC transfusion. Moreover, some clinical trials were conducted with patients for whom treatments other than ESA were indicated (i.e. patients not receiving chemotherapy).
Erythropoiesis‐stimulating agents have been marketed in Japan with indications for renal anemia, autologous blood collection and anemia of prematurity; however, their use for CIA is not yet approved. The present study analyzed three placebo‐controlled trials conducted from 2006 to 2009 as developmental clinical trials in compliance with Good Clinical Practice (GCP),20, 21, 22 to investigate the possible effects of ESA epoetin beta and darbepoetin alfa on survival using individual patient data provided by Chugai Pharmaceutical Co., Ltd, and Kyowa Hakko Kirin Co., Ltd.
Materials and Methods
Study selection and data collection
Five randomized placebo‐controlled trials investigating epoetin beta or darbepoetin alfa were conducted in compliance with GCP. The trials enrolled Japanese patients who had solid tumors or lymphomas and CIA. Three of the five trials were selected for this meta‐analysis. (Two studies on epoetin beta23, 24 were excluded from the analysis because the survival period was not designated as one of the study end‐points in the protocols prior to the start of the studies.)
Details of the three individual trials are summarized in Table 1. The types of cancer were limited to patients with lung or gynecological tumors receiving platinum‐containing chemotherapy in the Fujisaka trial of epoetin beta22 and the Katakami trial of darbepoetin alfa.20 The dose‐finding study of darbepoetin, that is, the Suzuki trial,21 enrolled patients with any type of solid tumor or malignant lymphoma receiving chemotherapy (not limited to platinum‐containing chemotherapy). In the epoetin beta clinical trial,22 inclusion criteria of the Hb concentration was between 8.0 and 10.0 g/dL, whereas in the two darbepoetin alfa clinical trials,20, 21 the Hb concentration standard at the time of study enrollment was initially 11.0 g/dL or lower. However, the Hb standard was changed to 10.0 g/dL or lower during the Suzuki trial.21 The standard for discontinuation of epoetin beta administration in the Fujisaka trial was a Hb concentration exceeding 12.0 g/dL. In the two darbepoetin alfa trials, the discontinuation standard was initially a Hb concentration exceeding 13.0 g/dL; however, this standard was amended during both trials to a Hb concentration exceeding 12.0 g/dL.
Table 1.
Details of the three clinical trials examined in the meta‐analysis
| Study | n (Total, 511) | Drug | Dosage | Treatment duration | Hemoglobin at enrollment | Hemoglobin ceiling | Cancer | Chemotherapy |
|---|---|---|---|---|---|---|---|---|
| Katakami et al. 200820 | 207 | Darbepoetin alfa | 2.25 mcg/kg qw s.c. | Max. 12 weeks | ≤11.0 g/dL | 13.0 g/dL 12.0 g/dL† | Lung, gynecological | Platinum containing |
| Suzuki et al. 200821 | 123 | Darbepoetin alfa | 4.50 mcg/kg 6.75 mcg/kg q3w s.c. | Max. 12 weeks | ≤11.0 g/dL≤10.0 g/dL† | 13.0 g/dL 12.0 g/dL† | Solid, malignant lymphoma | Any |
| Fujisaka et al. 201122 | 181 | Epoetin beta | 36 000 IU qw s.c. | Max. 12 weeks | 8.0–10.0 g/dL | 12.0 g/dL | Lung, gynecological | Platinum containing |
†After amendment of protocol. qw, every week; q3w, every 3 weeks; s.c., subcutaneous injection.
Individual patient data were provided to the University of Tokyo, Tokyo, Japan, from Chugai Pharmaceutical Co., Ltd, Tokyo, Japan, and Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan in a standardized format. All data were anonymized.
End‐points
The efficacy end‐points of the meta‐analysis were RBC transfusion and transfusion trigger during the period from week 5 of the ESA treatment to the end of the treatment period. The transfusion trigger is defined as either receiving RBC transfusion or a Hb concentration below 8 g/dL. The maximum length of the ESA administration period was 12 weeks. The two end‐points for safety were overall survival starting from the beginning of ESA administration and thromboembolic events (TEE). The TEE include cerebral infarction, cardiac infarction, pulmonary embolism, venous thrombosis, deep‐vein thrombosis, superior vena cava obstruction, arteriosclerosis obliterans and thrombophlebitis.
Statistical analysis
The analytical software sas (version 9; SAS Institute Inc., Cary, NC, USA) was used for all analyses, using a full analysis set. Individual patient data were pooled without stratification for study. For the efficacy end‐points of RBC transfusion and transfusion trigger, Kaplan–Meier estimates of the cumulative incidence of each parameter were calculated in the meta‐analysis and a Z‐test was conducted for comparison between groups using the standard error based on Greenwood's formula. Moreover, for the rate of end‐points that occurred during the trials, the relative risk (RR) and its 95% confidence interval (CI) were determined.
For overall survival, we drew a Kaplan–Meier plot for all patients without stratification and for each subgroup based on background characteristics such as the trial, cancer type, Hb concentration category at baseline and Eastern Cooperative Oncology Group performance status. A Cox regression model was used to determine the hazard ratio (HR) of the ESA‐treated group to the placebo‐treated group and for interactions between the effect of ESA and background factors. The tied data were processed using the exact method.25
We also conducted a landmark analysis at 3 months for the ESA‐treated group and analyzed the average Hb concentration and highest Hb concentration achieved during the 3‐month treatment period using a Cox regression model. Patients who responded well to ESA treatments, with high levels of average Hb concentrations during this 3‐month period, might be the patients with better prognoses; therefore, clinically important factors such as baseline age, primary disease or metastatic disease were included in the analyses as adjustment factors.
Results
Description of trials
Our meta‐analysis examined three randomized, placebo‐controlled trials with a total of 511 CIA patients with lung cancer (n = 258), gynecological cancer (n = 138), malignant lymphoma (n = 60) or other types of cancer (n = 55) such as breast cancer, who were administered epoetin beta or darbepoetin alfa (n = 273) or placebo (n = 238). The imbalances in the numbers of patients assigned to the ESA‐treated group and the placebo‐treated group and in the proportions of cancer types between these two groups were caused by the Suzuki21 trial, which included two arms of darbepoetin alfa. Details of the patients included in the meta‐analysis are summarized in Table 2. Except for type of cancer, the baseline characteristics of the patients were generally well balanced between the ESA‐treated group and the placebo‐treated group.
Table 2.
Baseline characteristics of patients with chemotherapy‐induced anemia
| Background factor | ESA (n = 273) | Placebo (n = 238) |
|---|---|---|
| Sex (%) | ||
| Male | 121 (44.3) | 102 (42.9) |
| Female | 152 (55.7) | 136 (57.1) |
| Age (years) (%) | ||
| <65 | 155 (56.8) | 148 (62.2) |
| ≥65 | 118 (43.2) | 90 (37.8) |
| Bodyweighta (kg) | 53.8 ± 9.5 | 53.6 ± 9.4 |
| Type of cancer (%) | ||
| Lung cancer | 130 (47.6) | 128 (53.8) |
| Gynecological cancer | 68 (24.9) | 70 (29.4) |
| Malignant lymphoma | 40 (14.7) | 20 (8.4) |
| Other | 35 (12.8) | 20 (8.4) |
| Tumor diagnosis (%) | ||
| Primary | 184 (67.4) | 173 (72.7) |
| Recurrent | 89 (32.6) | 65 (27.3) |
| ECOG performance status (%) | ||
| 0 | 143 (52.4) | 115 (48.3) |
| 1 | 120 (44.0) | 115 (48.3) |
| 2 | 10 (3.7) | 8 (3.4) |
| Hemoglobin (g/dL) (%) | ||
| ≤9 | 84 (30.8) | 71 (29.8) |
| >9–10 | 101 (37.0) | 98 (41.2) |
| >10 | 88 (32.2) | 69 (29.0) |
| Endogenous EPOa (mU/mL) | 86.4 ± 151.5 | 85.1 ± 83.3 |
Mean ± SD. ECOG, Eastern Cooperative Oncology Group; EPO, erythropoietin; ESA, erythropoiesis‐stimulating agents; SD, standard deviation.
Efficacy
The percentage of patients who received RBC transfusions from week 5 until week 14 were 9% in the ESA‐treated group and 21% in the placebo‐treated group (P < 0.001; Fig. S1) and the risk of transfusion was reduced in the ESA‐treated group by 53% (relative risk [RR], 0.47; 95% CI, 0.29–0.76). The risk of transfusion for major cancer types was RR 0.35 (95% CI, 0.17–0.73) for lung cancer and RR 0.26 (95% CI, 0.08–0.87) for gynecological cancer. The risk of transfusion trigger from week 5 was RR 0.50 (95% CI, 0.38–0.67; Fig. S2).
Overall survival
The median follow‐up period for overall survival of all patients was 13.3 months (min.–max., 1.1–19.4 months). The unadjusted HR for overall survival of all patients was 1.00 (95% CI, 0.75–1.34) and no influence of ESA was identified (Fig. 1). The mortality results for all patients and stratified by trials and baseline characteristics are shown in Figure 2. None of the variables (including cancer type and Hb concentration) influenced mortality. The majority of deaths were cancer‐related in both the ESA‐treated group 95% (94 of 99) and the placebo‐treated group 97% (85 of 88).
Figure 1.
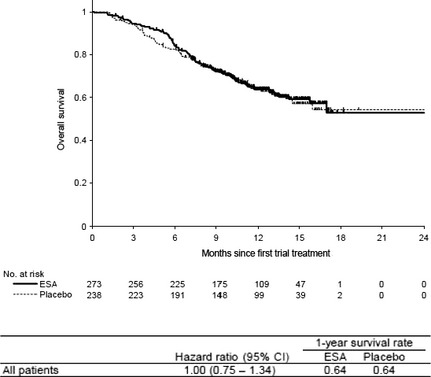
Overall survival. Kaplan–Meier curve for all patients treated with erythropoiesis‐stimulating agents (ESA) or placebo. The median follow‐up period for overall survival of all patients was 13.3 months (min.–max., 1.1–19.4 months). The unadjusted hazard ratio for overall survival of all patients was 1.00 (95% confidence interval [CI], 0.75–1.34) with a 1‐year survival rate of 64% for the ESA‐treated group and placebo‐treated group.
Figure 2.
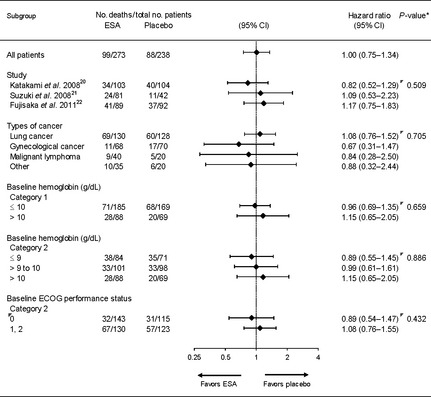
Mortality risk stratified by trials and patient characteristics. Forest plots of the hazard ratio (HR) for overall survival from the three trials for all patients and stratified by trial and baseline characteristics. The position of each diamond indicates the HR estimate. Horizontal lines indicate the 95% confidence interval (CI). None of the variables (including cancer type and hemoglobin concentration) influenced patient mortality. *Test for interaction. ECOG, Eastern Cooperative Oncology Group.
The mortality rate (i.e. number of deaths) during the treatment period (the active study period) was seven patients in the ESA‐treated group and seven patients in the placebo‐treated group.
The landmark analysis at 3 months for the ESA‐treated patients (n = 256) indicated a tendency for patients with a mean Hb concentration of 11–11.5 g/dL during the 3‐month treatment period (n = 39) to have the lowest risk of mortality (adjusted HR, 0.43; 95% CI, 0.17–1.07; reference, mean hemoglobin <10 g/dL). However, the highest Hb concentration achieved during the ESA‐treatment period in each patient (mean, 11.7 g/dL; min.–max., 7.7–15.4 g/dL) had no impact on mortality.
Thromboembolic events
The frequency of TEE was 0.7% in the ESA‐treated patients and 1.7% in the placebo‐treated patients. The TEE reported in the ESA group were one case each of axillary vein thrombosis and pulmonary embolism and in the placebo group, one case each of cerebral infarction, hemorrhagic cerebral infarction, superior vena cava obstruction and arteriosclerosis obliterans. There was no death due to TEE.
Discussion
Our meta‐analysis based on individual patient data with a median follow‐up period of over 1 year did not identify an increased risk of mortality due to the ESA, epoetin beta and darbepoetin alfa. A meta‐analysis published by the Cochrane Collaboration, similarly based on the individual patient data of all 13 933 patients (including those other than CIA) from 53 studies, reported an increased mortality risk of 17% (HR, 1.17; 95% CI, 1.06–1.30) by ESA administration based on overall survival during the active study period.14 However, that meta‐analysis found that when the analysis was limited to the cases of 10 441 chemotherapy‐treated patients from 38 studies, an increase in the mortality risk was 10% (HR, 1.10; 95% CI, 0.98–1.24).14
There are several differences between our analysis and that of the Cochrane Collaboration. First, our analysis included patients with CIA only, which is the approved indication for ESA treatment in the EU and USA. Second, our analysis examined randomized, placebo‐controlled trials conducted in compliance with GCP as developmental clinical trials by pharmaceutical companies between 2006 and 2009, after discussion of the possible contribution of ESA to decreased life prognosis. Therefore, patient selection and ESA administration in the three trials were conducted under close supervision and observation by physicians throughout the study period. The third difference is that all three trials analyzed the measured Hb concentration at least once a week during the study period and when the Hb concentration exceeded the set standard level the ESA administration was discontinued immediately, as indicated by the protocol. Thus, the increased risk associated with ESA was not observed in our analysis of Japanese CIA patients who were appropriately selected, underwent close Hb concentration monitoring and received strict dosing adjustment of ESA.
The analysis for each background factor showed that no variables (including baseline Hb concentration) influenced mortality. Thus, we conducted a landmark analysis starting from the 3‐month time point for the relationship between Hb concentration after ESA administration and mortality (the subjects for the analysis were the patients with a follow up of 3 months or more). This analysis revealed that the highest Hb levels achieved during the ESA‐treatment period for each patient were within the range of 7.7–15.4 g/dL and the Hb level did not influence mortality. This finding indicates that a temporary rise in Hb concentration to a high level does not negatively affect mortality.
Moreover, as an exploratory investigation, we used a model for predicting the patients whose Hb concentrations respond well to ESA administration using the patients' background factors. We defined good responders as those with a >0.5 g/dL increase in Hb concentration when the average first‐month Hb was compared with the baseline. With this model, we extracted the patients who were expected to be good responders from the placebo‐treated group and matched them with ESA‐treated patients with good responses. This matching was implemented with randomly sorted ESA‐treated patients and the HR was estimated after one‐to‐one matching. For the sensitivity analysis, the above analysis was iterated 10 times and the range of estimated HR was 0.54–0.83 (standard error [SE], 0.30–0.32) for the good responders and 0.82–1.10 (SE, 0.19–0.20) for the remainder (the non‐good responders). If the response to ESA affects mortality and overall survival is improved in the good responders, the HR would be <1; conversely, if the ESA have a negative influence on the non‐good responders, the HR would be over 1. However, we found no significant difference between these two subgroups and therefore the results suggest that the level of Hb concentration response to ESA does not change the degree of influence of ESA on mortality.
In the present study, no increase in TEE was observed in the ESA‐treated patients (0.7% vs 1.7% placebo). Due to the low incidence of TEE and the lack of a structured approach for identifying TEE we could not conduct a detailed analysis, but in general the incidence of TEE in Japanese patients is considered to be low.26 Previous studies of ESA (not included in the present study) showed that few Japanese CIA patients treated with ESA or placebo experienced TEE.23, 24, 27, 28
As in previous reports,17, 18 the present analysis further confirms that ESA reduce the need for RBC transfusions (RR, 0.47). However, none of the trials in the present study showed any significant difference in quality of life between the ESA‐treated and placebo‐treated groups. Although a higher percentage of patients received RBC transfusions in the placebo‐treated group than in the ESA‐treated group, the mean changes in the Functional Assessment of Cancer Therapy‐Anemia total fatigue subscale score were better in the ESA‐treated group.
In conclusion, treatment with the ESA epoetin beta and darbepoetin alfa reduced the risk of transfusion without a negative impact on overall survival in Japanese patients with CIA where appropriate patient selection, monitoring of Hb concentration and strict dosing adjustment were conducted. Further investigations regarding Hb concentrations after ESA administration are needed, as is a cohort study on the relationship between the response to ESA administration and mortality.
Disclosure Statement
Y.O. has received honoraria from and has had a consultant or advisory relationship with Chugai Pharmaceutical Co., Ltd and Kyowa Hakko Kirin Co., Ltd. In addition, Y.O. is a Chairman of the Board in Statcom Co., Ltd and has owned stocks of Statcom Co., Ltd. T.S. received honoraria and research funding from Chugai Pharmaceutical Co., Ltd. N.K. received honoraria from Chugai Pharmaceutical Co., Ltd and Kyowa Hakko Kirin Co., Ltd. N.S. received honoraria from Chugai Pharmaceutical Co., Ltd and Kyowa Hakko Kirin Co., Ltd. In addition, N.S. received research funding from Chugai Pharmaceutical Co., Ltd. T.H. had an advisory relationship with Chugai Pharmaceutical Co., Ltd and Kyowa Hakko Kirin Co., Ltd (the advisory role was defined in the delegated research contract between National Hospital Organization, Nagoya Medical Center and each company). In addition, T.H. received research funding from Chugai Pharmaceutical Co., Ltd. All remaining authors have no conflict of interest.
Supporting information
Fig. S1. Kaplan–Meier curve of the time to first red blood cell transfusion during the period from week 5 of treatment to the end of the treatment period for all patients treated with erythropoiesis‐stimulating agents or placebo.
Fig. S2. Kaplan–Meier curve of the time to transfusion trigger during the period from week 5 of treatment to the end of the treatment period for all patients treated with erythropoiesis‐stimulating agents or placebo.
Acknowledgments
This study was supported by Chugai Pharmaceutical Co., Ltd, Tokyo, Japan and Kyowa Hakko Kirin Co., Ltd, Tokyo, Japan.
(Cancer Sci 2013; 104: 481–485)
References
- 1. Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B, Epoetin Alfa Study Group . Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double‐blind, placebo‐controlled trial. J Clin Oncol 2001; 19: 2865–74. [DOI] [PubMed] [Google Scholar]
- 2. Osterborg A, Brandberg Y, Molostova V et al Randomized, double‐blind, placebo‐controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. J Clin Oncol 2002; 20: 2486–94. [DOI] [PubMed] [Google Scholar]
- 3. Vansteenkiste J, Pirker R, Massuti B et al Double‐blind, placebo‐controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst 2002; 94: 1211–20. [DOI] [PubMed] [Google Scholar]
- 4. Leyland‐Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol 2003; 4: 459–60. [DOI] [PubMed] [Google Scholar]
- 5. Henke M, Laszig R, Rübe C et al Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double‐blind, placebo‐controlled trial. Lancet 2003; 362: 1255–60. [DOI] [PubMed] [Google Scholar]
- 6. Hedenus M, Adriansson M, San Miguel J et al Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double‐blind, placebo‐controlled study. Br J Haematol 2003; 122: 394–403. [DOI] [PubMed] [Google Scholar]
- 7. Wright JR, Ung YC, Julian JA et al Randomized, double‐blind, placebo‐controlled trial of erythropoietin in non‐small‐cell lung cancer with disease‐related anemia. J Clin Oncol 2007; 25: 1027–32. [DOI] [PubMed] [Google Scholar]
- 8. Smith RE Jr, Aapro MS, Ludwig H et al Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double‐blind, placebo‐controlled study. J Clin Oncol 2008; 26: 1040–50. [DOI] [PubMed] [Google Scholar]
- 9. Thomas G, Ali S, Hoebers FJ et al Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol 2008; 108: 317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeong JY, Feldman L, Solar P, Szenajch J, Sytkowski AJ. Characterization of erythropoietin receptor and erythropoietin expression and function in human ovarian cancer cells. Int J Cancer 2008; 122: 274–80. [DOI] [PubMed] [Google Scholar]
- 11. Jeong JY, Hoxhaj G, Socha AL, Sytkowski AJ, Feldman L. An erythropoietin autocrine/paracrine axis modulates the growth and survival of human prostate cancer cells. Mol Cancer Res 2009; 7: 1150–7. [DOI] [PubMed] [Google Scholar]
- 12. Jelkmann W, Bohlius J, Hallek M, Sytkowski AJ. The erythropoietin receptor in normal and cancer tissues. Crit Rev Oncol Hematol 2008; 67: 39–61. [DOI] [PubMed] [Google Scholar]
- 13. Aapro M, Jelkmann W, Constantinescu SN, Leyland‐Jones B. Effects of erythropoietin receptors and erythropoiesis‐stimulating agents on disease progression in cancer. Br J Cancer 2012; 106: 1249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bohlius J, Schmidlin K, Brillant C et al Recombinant human erythropoiesis‐stimulating agents and mortality in patients with cancer: a meta‐analysis of randomised trials. Lancet 2009; 373: 1532–42. [DOI] [PubMed] [Google Scholar]
- 15. Glaspy J, Crawford J, Vansteenkiste J et al Erythropoiesis‐stimulating agents in oncology: a study‐level meta‐analysis of survival and other safety outcomes. Br J Cancer 2010; 102: 301–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bennett CL, Silver SM, Djulbegovic B et al Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer‐associated anemia. JAMA 2008; 299: 914–24. [DOI] [PubMed] [Google Scholar]
- 17. Tonelli M, Hemmelgarn B, Reiman T et al Benefits and harms of erythropoiesis‐stimulating agents for anemia related to cancer: a meta‐analysis. CMAJ 2009; 180: E62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ross SD, Allen IE, Henry DH, Seaman C, Sercus B, Goodnough LT. Clinical benefits and risks associated with epoetin and darbepoetin in patients with chemotherapy‐induced anemia: a systematic review of the literature. Clin Ther 2006; 28: 801–31. [DOI] [PubMed] [Google Scholar]
- 19. Seidenfeld J, Piper M, Bohlius J et al Comparative effectiveness of epoetin and darbepoetin for managing anemia in patients undergoing cancer treatment. Agency for Healthcare Research and Quality Comparative Effectiveness Reviews No.3; 2006. May. [PubMed]
- 20. Katakami N, Nishiwaki Y, Fujiwara Y et al Randomized, double‐blind, placebo‐controlled phase III study of weekly administration of darbepoetin alfa (DA) in anemic patients with lung or gynecologic cancer receiving platinum‐containing chemotherapy. Ann Oncol 2008; 19 (Suppl. 8): 277–8. [Google Scholar]
- 21. Suzuki Y, Tokuda Y, Okamoto R et al Randomized, placebo‐controlled phase II study of darbepoetin alfa (DA) administered every three weeks (Q3W) in patients with chemotherapy‐induced anemia (CIA). Ann Oncol 2008; 19 (Suppl. 8): 277. [Google Scholar]
- 22. Fujisaka Y, Sugiyama T, Saito H et al Randomized, phase III trial of epoetin beta to treat chemotherapy‐induced anemia according to the EU Regulation. Br J Cancer 2011; 105: 1267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kunikane H, Watanabe K, Fukuoka M et al Double‐blind randomized control trial of the effect of recombinant human erythropoietin on chemotherapy‐induced anemia in patients with non‐small cell lung cancer. Int J Clin Oncol 2001; 6: 296–301. [DOI] [PubMed] [Google Scholar]
- 24. Tsuboi M, Ezaki K, Tobinai K, Ohashi Y, Saijo N. Weekly administration of epoetin beta for chemotherapy‐induced anemia in cancer patients: results of a multicenter, phase III, randomized, double‐blind, placebo‐controlled study. Jpn J Clin Oncol 2009; 39: 163–8. [DOI] [PubMed] [Google Scholar]
- 25. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley & Sons, 1980. [Google Scholar]
- 26. Aihara T, Takatsuka Y, Ohsumi S et al Phase III randomized adjuvant study of tamoxifen alone versus sequential tamoxifen and anastrozole in Japanese postmenopausal women with hormone‐responsive breast cancer: N‐SAS BC03 study. Breast Cancer Res Treat 2010; 121: 379–87. [DOI] [PubMed] [Google Scholar]
- 27. Morishima Y, Ogura M, Yoneda S et al Once‐weekly epoetin‐beta improves hemoglobin levels in cancer patients with chemotherapy‐induced anemia: a randomized, double‐blind, dose‐finding study. Jpn J Cin Oncol 2006; 36: 655–61. [DOI] [PubMed] [Google Scholar]
- 28. Suzuki Y, Tokuda Y, Fujiwara Y, Minami H, Ohashi Y, Saijo N. Weekly epoetin beta maintains hemoglobin levels and improves quality of life in patients with non‐myeloid malignancies receiving chemotherapy. Jpn J Cin Oncol 2008; 38: 214–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Kaplan–Meier curve of the time to first red blood cell transfusion during the period from week 5 of treatment to the end of the treatment period for all patients treated with erythropoiesis‐stimulating agents or placebo.
Fig. S2. Kaplan–Meier curve of the time to transfusion trigger during the period from week 5 of treatment to the end of the treatment period for all patients treated with erythropoiesis‐stimulating agents or placebo.


