Abstract
Regulatory T (Treg) cells expressing the X chromosome-encoded transcription factor Foxp3 represent a specialized immunosuppressive lineage with a well-recognized, essential function in preventing fatal autoimmunity and inflammation. Recent studies revealed that Treg cells can also exert systemic effects on metabolism and partake in tissue repair, suggesting a dual role for these cells in serving and protecting tissues. Here we review multiple means by which Treg cells support tissue function and organismal homeostasis.
eTOC blurb:
Campbell and Rudensky review the roles of regulatory T cells in organismal homeostasis and metabolic health, with a focus on the major immunological and non-immunological mechanisms by which these specialized suppressive cells of the adaptive immune system support the function of various tissues and organs.
Introduction
Cellular differentiation and tissue organization are defining features of metazoans. Unlike unicellular entities, multicellular organisms carry out physiologic functions through the concerted action of specialized cells assembled in discrete units; therefore, safeguarding the function of these units or tissues is of paramount importance to organismal homeostasis. Besides stromal and parenchymal cells, tissues harbor a significant population of recirculating and resident immune cells that include phagocytes, specialized antigen-presenting cells (APC) and innate and adaptive lymphocytes. Regulatory T (Treg) cells, a specialized immunosuppressive lineage of the latter, have been shown to exert systemic effects on metabolism and to partake in tissue repair, suggesting a dual role for adaptive lymphocytes in serving and protecting tissues. This review highlights the main mechanisms by which Treg cells support tissue function.
Immunity related roles of Treg cells in preserving tissue function
First law of adaptive immunity: do no harm
The adaptive immune system of higher vertebrates has a remarkable ability for mounting protective responses against a plethora of pathogens. Somatic rearrangement of gene segments through RAG recombinase activity produces a vast repertoire of antigen receptor specificities, with a theoretical diversity estimated in the order of 1014 unique T cell receptors (TCR) (Murugan et al., 2012). However, being stochastic, the assembly of antigen receptors does not preclude the generation of self-reactive cells, making regulatory mechanisms a necessity by design in order to prevent autoimmunity and consequent impairment in vital tissue functions.
Higher vertebrates employ two major, non-redundant strategies to deal with self-reactivity. These mechanisms, dubbed central and peripheral tolerance, aim to delete or inactivate developing self-reactive lymphocytes and to suppress the activation and potentially destructive activity of escapees, respectively. The presentation of tissue-restricted antigens in the thymus is critical for both processes (Anderson et al., 2002, Aschenbrenner et al., 2007). Thymocytes bearing strongly self-reactive TCRs are largely eliminated in a process known as negative selection (Kisielow et al., 1988, Kappler et al., 1987), while developing CD4+ T cells recognizing self-antigens with moderate to high affinity can be induced to express the transcription factor Foxp3 and differentiate into Treg cells (Lee et al., 2012). Treg cells play an essential role in enforcing peripheral tolerance. Congenital loss-of-function mutations in the Foxp3 gene cause Immunodysregulation, Polyendocrinopathy, Enteropathy, X-linked (IPEX) syndrome, a fatal systemic auto-immune disease targeting endocrine and barrier organs (Bennett et al., 2001). Additionally, ablation of Foxp3-expressing cells in experimental settings leads to widespread immune cell activation, lymphadenopathy and splenomegaly amounting to extensive tissue pathology (Kim et al., 2007, Sugimoto et al., 2017, Kim et al., 2009), indicating a continuous need for Treg cell-mediated suppression throughout the lifespan of animals.
Loss of tissue-specific immune tolerance can have profound effects on organismal physiology. Hashimoto thyroiditis and Grave’s disease are manifestations of autoimmune responses against thyroid antigens including thyroglobulin (Tg), thyroid peroxidase (TPO) and the thyrotropin receptor (TSH-R) (Pearce et al., 2003). Basal metabolic rate, adaptive thermogenesis and energy storage, which are systemically affected by thyroid hormones (Mullur et al., 2014), become severely dysregulated when thyroid function is compromised. Inadequate suppression of auto-reactive lymphocytes is also central to the pathogenesis of type 1 diabetes (T1D), associated with the destruction of pancreatic β-cells by islet-reactive T cells (Pugliese, 2017). T1D and autoimmune thyroid diseases have been linked to polymorphisms in CD25 and CTLA-4, molecules involved in Treg cell differentiation and function (Tomer et al., 2001, Ueda et al., 2003, Chistiakov et al., 2011, Chinen et al., 2016, Wing et al., 2008). Thus, besides being a common feature of IPEX resulting from a severe or complete loss of Treg cell function, autoimmune endocrinopathies may also ensue from milder functional defects in Treg cells.
The critical role of Treg cells in preventing tissue damage arising from immune activation is particularly prominent at organs interfacing with the environment. Barrier sites are continuously exposed to microbial and non-microbial ligands recognized by innate immune sensors and antigen receptors. Treg cell-specific deletion of the anti-inflammatory cytokine IL-10 leads to spontaneous colitis and exacerbated skin and lungs immunoreactivity (Rubtsov et al., 2008), which are reminiscent of the severe enteritis and dermatitis typically observed in IPEX patients. Examination of histopathological sequelae of chronic Treg cell ablation in Specific Pathogen-Free (SPF) or germ-free (GF) animals indicate that, while most organs become equally compromised in the absence of Treg cells, enteric inflammation is remarkably worsened by commensal colonization (Chinen et al., 2010), pointing to a requirement for Treg cells in maintaining diplomatic interactions between host and commensals.
Treg cells and the complexity of the extended “self”
Although the presence of microorganisms at mucosal and epithelial surfaces may seem like an unavoidable consequence of living in a microbial world, the composition of the microbiota is far from being a consequence of a random sampling of the environment. Co-speciation of gut bacteria and hominids indicates that the indigenous microbiota is a product of extensive co-evolution (Moeller et al., 2016). Notably, adaptive lymphocytes were shown to affect the pace and directionality of genetic adaptation in intestinal bacteria (Barroso-Batista et al., 2015), suggesting that these cells may have shaped the evolution of host-commensal interactions in higher vertebrates.
As a complex ensemble of foreign genomes, the gut microbiota represents a unique challenge to the adaptive immune system. Microbial antigens represent a missing page in the syllabus of thymic education, meaning that the deletion of potentially pathogenic commensal-reactive effector T cells and the generation of commensal-specific Treg cells, as a rule, do not take place during T cell differentiation in the thymus. A solution may have emerged during the radiation of placental mammals, when a cis-regulatory element first appeared in the Foxp3 locus of eutherian species, likely due to retrotransposon activity (Samstein et al., 2012). The Conserved Non-Coding Sequence 1 (CNS1) facilitates the differentiation of peripherally-induced or extrathymically generated Treg (pTreg) cells from naïve CD4+ T cells without affecting thymic Treg cell ontogeny (Zheng et al., 2010). Teleologically, inducing Treg cell differentiation in the periphery serves two partially overlapping goals: it diverts commensal (and dietary) antigen-specific T cells away from the effector T cell pool while also bridging a gap in the TCR repertoire of Treg cells (Zhou et al., 2008; Josefowicz et al., 2012; Kim et al., 2016; see(Ai et al., 2014) for review). It was recently demonstrated that the same commensal-specific TCR can support the differentiation of both effector and pTreg cells in a context-dependent manner (Xu et al., 2018b, Wegorzewska et al., 2019), denoting that these two cell fates are indeed related and may be reciprocally regulated by environmental cues. Moreover, in light of the importance of TCR signaling in Treg cell function (Levine et al., 2014, Schmidt et al., 2015, Feng et al., 2015, Levine et al., 2017) and the deleterious effects of reduced Treg cell TCR diversity (Feng et al., 2015; Levine et al., 2017) it seems intuitive that the ability to expand the TCR repertoire of Treg cells via extrathymic generation is likely advantageous.
Considering that the composition of the microbiota may vary across species and even between individuals, it is not surprising that the immune system interprets contextual cues and not specific bacterial antigens as tolerogenic signals. Nevertheless, the nature and mechanism of action of the tolerogenic cues deployed by microbes remain poorly characterized and have become the target of intense research in the past decade. Common products of microbial fermentation such as short-chain fatty acids (SCFA) were shown to facilitate the differentiation of pTreg cells (Smith et al., 2013, Arpaia et al., 2013, Furusawa et al., 2013), providing a molecular basis for the enhanced capacity of bacteria such as Clostridia cluster IV and XIV to promote Treg cell accumulation in the gut (Atarashi et al., 2013, Atarashi et al., 2011). Besides degradation of dietary fiber, the microbiota contributes to other important physiological functions including detoxification of xenobiotics, synthesis of vitamins and resistance to colonization by pathogens (Maurice et al., 2013, Buffie et al., 2015, Magnusdottir et al., 2015). It is tempting to speculate that microbial metabolites involved in these processes would also be interpreted by the host as tolerogenic signals and perhaps regulate other essential aspects of host-microbial interactions. This scenario would suggest that the establishment of immunological tolerance follows a “utilitarian” criterion and that monitoring the metabolic output of the microbiota is a viable strategy to accommodate various configurations of microbial communities with similar functions.
Despite constituting a numerically small subset, pTreg cells are essential for organismal health, possibly due to their superior capacity to control effector T cells sharing the same microbial antigen specificity (Xu et al., 2018b). RORγt-expressing Treg cells, which by-and-large represent pTreg cells induced by the intestinal microbiota (Sefik et al., 2015, Ohnmacht et al., 2015), prevent pathological imprinting of the immune system early in life (Al Nabhani et al., 2019). Moreover, impaired pTreg cell generation associated with loss of the CNS1 enhancer results in age-dependent pathology at mucosal sites and profound perturbations in the intestinal microbiota (Josefowicz et al., 2012, Campbell et al., 2018). Specifically, pTreg cells were shown to promote metabolic diversity within gut bacteria and to significantly shape the luminal metabolome. pTreg cell-deficient animals mount heightened type 2 immune responses during colonization, thus compromising a niche for bacteria closely interacting with the intestinal epithelium. The increased activation of innate lymphoid cells (ILC) in this setting, i.e. genetic pTreg deficiency, suggests that, in addition to restraining commensal-specific T cells, pTreg cells may further support the microbiota by suppressing innate immune cell types during community assembly. Animals with impaired pTreg cell generation show reduced body weight and aberrant gene expression in the intestinal epithelium exclusively in the presence of commensal bacteria, further highlighting the non-redundant role of this Treg cell subset in shaping and abetting the function of this hybrid “superorgan”.
Additional functions of Treg cells in tissue physiology
Two tales of tolerance
With the remarkable exception of commensal microbes, encounters with viruses and bacteria often pose a threat to the health of animals. The host-pathogen interaction strategies can be principally divided into tolerance or resistance. In this setting, tolerance is often stated as a defense strategy that minimizes tissue damage or its consequences to the health status of an organism without reducing pathogen burden, while resistance would entail achieving a higher degree of health by curtailing microbial burden (Ayres and Schneider, 2012, Martins et al., 2019).
Beyond limiting collateral tissue damage due to uncontrolled immune responses, Treg cells can directly contribute to tissue repair during infection through the secretion of growth factors. Selective Treg cell deficiency in an epithelial growth factor family member amphiregulin (Areg) imparts severe lung damage during influenza virus infection with concomitant reduction in blood oxygen saturation (Arpaia et al., 2015). The positive effects of Treg cells were observed in the absence of measureable alterations in antiviral immune responses or pathogen load, which are indicative of host defense through tolerance. Similarly, Treg cells were shown to limit liver pathology during murine cytomegalovirus (MCMV) infection without affecting viral titers (Popovic et al., 2017). Thus, in addition to their classical role enforcing immunological tolerance, Treg cells are also instrumental for tolerance as a host defense strategy during infection.
An essential role of Treg cells in Tissue Regeneration
Another important aspect of Treg cell involvement in tissue maintenance is their contribution to stem cell (SC) niches in various organs in physiologic and pathologic settings. In the bone marrow, Treg cells were found to enforce hematopoietic SC (HSC) quiescence, a state necessary for their longevity (Hirata et al., 2018, Fujisaki et al., 2011). Disrupting Treg cell entry into the bone marrow via CXCR4 deletion increased HSC proliferation, which could be recapitulated by Treg cell-specific ablation of the exonucleotidase CD39. Although degradation of extracellular ATP via CD39/CD73 is a known contributor to Treg cell-mediated suppression (Borsellino et al., 2007), signaling through the adenosine receptor may represent novel mechanism affecting the maintenance of the HSC pool.
A role for Treg cells in supporting the niche of intestinal stem cells (ISC) was also recently suggested (Biton et al., 2018). The differentiation of ISCs into specialized subsets such as Paneth cells or goblet cells allows for tailored responses to various challenges via alterations in the cellular makeup of the tissue, enabling changes in organ physiology in response to functional demands. Treg cell ablation leads to the depletion of the Lgr5+ISC pool and aberrant enterocyte differentiation as a result of both direct and indirect effects of Treg cells on the ISC niche. While the regulatory cytokine IL-10 was shown to directly boost the frequency of ISC in organoid cultures, effector T cell cytokines including IFNγ and IL-13 induced precursor cell pool skewing towards committed lineages with a concomitant reduction in the ISCs. Being the predominant source of IL-10 in the gut, Treg cells are capable of controlling epithelial differentiation by simultaneously restraining effector T cells and promoting ISC self-renewal. Hence, regulation of tissue composition represents a mechanism by which Treg cells affect intestinal physiology and such a mechanism may be at play in other tissues with high turnover rates.
Treg cells also associate with SC in the skin, another major barrier organ. Body hair not only reinforces the physical resistance of the skin but also contributes to thermoregulation indispensable for homeotherms. Skin-resident Treg cells preferentially localize to hair follicles (HF) and display population dynamics highly correlated with hair growth cycles (Ali et al., 2017). Remarkably, HFs failed to transition into anagen in animals subjected to Treg cell depletion. Expression of the Notch ligand Jagged-1 on Treg cells was suggested to facilitate SC proliferation in HFs, indicating that, in addition to soluble factors, Treg cells may also support the SC niche via direct cell-cell communication. Besides their role in supporting hair growth, Treg cells have been shown to promote the barrier function of the skin by partaking in wound healing (Nosbaum et al., 2016). In the event of a breach, Treg cells are recruited from secondary lymphoid organs (SLO) and acquire an activated phenotype characterized by high expression of CD25, CTLA-4 and ICOS. Treg cell ablation early after injury increased the presence of pro-inflammatory immune cell types - prominently of myeloid origin – and delayed wound closure. In addition to epidermal SC, HF SC also contribute to skin regeneration by giving rise to epithelial cell precursors. Increased neutrophil influx upon Treg cell ablation reduced the migration of HF SC into sites of injury and impaired their differentiation towards keratinocytes (Mathur et al., 2019). Thus, regulation of local inflammatory responses by Treg cells is an important contributor to the maintenance of epidermal barrier function.
The skeletal muscle has a remarkable capacity to grow in response to hypertrophic stimuli and to repair itself after injury. Immune cells are recruited to injured muscle fibers and undergo a switch from an early pro-inflammatory phenotype, to a late, anti-inflammatory infiltrate where Treg cells can make up to 50% of lymphocytes (Burzyn et al., 2013). These Treg cells exhibit expanded TCR clonotypes and distinct transcriptional signatures when compared to their splenic counterparts, including higher expression of IL-10, Areg and the ligand binding subunit of the IL-33 receptor, ST2. Depletion of Treg cells at the onset of tissue damage augmented inflammation and compromised muscle fiber regeneration, leading to a reduction in the myogenic capacity of satellite cells and increased fibrosis. The accruement of Treg cells in injured muscle was shown to be dependent on their influx from SLO and expression of ST2. Of note, the number of IL-33+ PDGFRα+ cells increased quickly after injury, suggesting that stromal cells may be directly involved in driving local expansion or supporting survival and accumulation of Treg cells (Kuswanto et al., 2016). These findings are consistent with a model where sensing of tissue damage through ST2 promotes the accumulation and activity of Treg cells at sites of injury to facilitate tissue repair.
Treg cells can also contribute to the repair of tissues with limited regenerative potential, such as in the central nervous system. Ablation of Treg cells early after brain ischemia increases microglial activation and immune cell infiltration, which promote the enlargement of the infarcted area (Liesz et al., 2009). The protective effects of Treg cells during brain ischemia were dependent on IL-10 expression, furthering the notion that immunoregulatory mechanisms are essential to limit secondary tissue damage driven by inflammation. Brain Treg cells are recruited from the periphery and display evidence of clonal expansion (Ito et al., 2019), which is reminiscent of their features observed during repair processes in other tissues. Additionally, IL-33 production by stromal cells and ST2 expression on Treg cells were also required for Treg cell accumulation after neuronal damage, further supporting the IL-33/ST2 axis as a mechanism promoting an increase in Treg cell numbers in injured tissues. Notably, brain Treg cells were also found to express the serotonin receptor HT7 and respond to this neurotransmitter with increased proliferation and upregulation of suppressive molecules. Pharmacologically enhancing serotonin levels increased Treg cells in the damaged brain, leading to improved neurological function. Mechanistically, Treg cells prevented astrogliosis and the ensuing neuronal apoptosis via Areg secretion. Although astrocytes usually play a beneficial role by providing trophic support to neurons, ischemia or injury can turn these helpful “accessory cells” into neurotoxic agents (Sofroniew, 2014, Okabe and Medzhitov, 2016). The effect of Areg in averting this phenotypic switch expands the known functions of this molecule and suggests an additional role for Treg cells during tissue repair via modulation of accessory cells. In line with this notion, Treg cells were found to promote neuronal remyelination via secretion of CCN3, a factor that induces oligodendrocyte differentiation (Dombrowski et al., 2017). It will be of relevance to investigate whether Treg cells support differentiation and function of other accessory cells at the steady state and in diverse pathological settings such as cancer, where this could potentially be exploited for therapeutic purposes.
The distinct, non-immune function of Treg cells in tissue regeneration appears to be evolutionarily conserved. In agreement with observations in experimental mouse models, Foxp3+ cells were shown to be recruited to sites of injury in zebra fish and to support organ regeneration through the production of tissue-specific repair factors rather than via secretion of the immunomodulatory cytokine IL-10. Acute ablation of Foxp3-expressing cells in zebra fish led to impaired retinal, cardiac and spinal cord regeneration after injury-induced issue damage (Sugimoto et al., 2017; Hui et al., 2017;), supporting an ancestral role for Treg cells in promoting tissue homeostasis.
Treg cells in metabolic homeostasis
The term “homeostasis” is commonly used to refer to the maintenance of physiological variables around a predefined set point (Kotas and Medzhitov, 2015). Within these definitions, the adipose tissue is a central hub for metabolic homeostasis, regulating insulin-dependent glucose uptake and long-term regulation of body weight via leptin secretion. At the steady state, Treg cells support adipose tissue function by keeping local and systemic inflammation in check. Circulating levels of TNFα, a cytokine known to antagonize insulin signaling in adipocytes (Hotamisligil et al., 1994), begin to rise as early as 24 hours after experimental Treg cell depletion (Nystrom et al., 2014). Impaired insulin receptor activity in the epididymal white adipose tissue (eWAT) manifests within days of Treg cell ablation, with local production of inflammatory mediators and compensatory increases in insulin secretion following suit (Feuerer et al., 2009). These observations indicate that the systemic immunomodulatory function of Treg cells is essential to maintain insulin sensitivity and metabolic homeostasis even in the absence of environmental stressors.
Although operational in a wide range of conditions, the adipose tissue becomes dysfunctional during prolonged overnutrition, leading to metabolic diseases. Foxp3+ cells are enriched in the eWAT of lean, but not obese mice, and show distinct a TCR repertoire and transcriptome compared to Treg cells from SLO (Feuerer et al., 2009, Kolodin et al., 2015). Fat Treg cells express high levels of the transcriptional factor PPARγ, the master regulator of adipocyte differentiation. In addition to TCR specificity, PPARγ along with BATF and IRF-4, are major drivers for Treg cell accumulation in the eWAT (Cipolletta et al., 2012, Li et al., 2018). eWAT-resident Treg cells can expand robustly in the presence IL-33, consistent with their abundant expression of ST2 (Kolodin et al., 2015). Notably, the basally high levels of IL-33 in the eWAT undergo further increase during diet-induced obesity (DIO). Adipose progenitor stem cells (APSC) and mesenchymal stromal cells (mSC) are the predominant sources of IL-33 in this adipose depot, with the latter being significantly expanded during nutritional overload (Mahlakoiv et al., 2019, Spallanzani et al., 2019). Thus, the decrease in fat Treg cells is likely not caused by paucity of IL-33 but rather due to other factors that may counteract IL-33 activity. Indeed, elevated levels of IFNγ in the adipose tissue block IL-33-dependent Treg cell accumulation (Molofsky et al., 2015), posing a possibility that supraphysiological levels of this alarmin are required to overcome its inhibition. Abrogating IFNγ production or signaling alleviates metabolic dysfunction in DIO (Stolarczyk et al., 2013), further suggesting a detrimental role for type 1 immunity in this context. Interestingly, while boosting the number of fat-resident Treg cells improves metabolic parameters in obesity, the natural increase in Foxp3+ cells associated with aging was shown to contribute to the development of age-dependent insulin resistance (Bapat et al., 2015). These findings indicate that pathologies in the diabetic spectrum may arise from distinct perturbations in the adipose tissue that are directly or indirectly related to the local abundance and functionality of Treg cells, and highlight the importance of an adequate immunological tone in promoting metabolic homeostasis.
Treg cells also affect organismal metabolism by supporting hepatic tissue function. Similar to their eWAT counterparts, liver Treg cells express high levels of ST2 and expand vigorously in response to IL-33 (Xu et al., 2018a). Depletion of Treg cells early in life resulted in autoimmune hepatitis and metabolic disorders, denoting that the establishment of immunological tolerance is essential to preserve liver function (Li et al., 2019). Moreover, ablation of Treg cells altered hepatic metabolism and aggravated hyperlipidemia and atherosclerosis in genetically susceptible animals (Klingenberg et al., 2013). These changes were associated with increases in hepatic IFNγ and TNFα levels, reinforcing the notion that regulation of type 1 inflammation by Treg cells is required for tissue homeostasis and optimal metabolic tenor.
Conclusions
The ability to adjust tissue function in response to environmental cues is essential to the fitness of metazoans. Treg cells constitute an additional regulatory layer embedded within tissue-intrinsic homeostatic mechanisms, expanding the range of environmental inputs that can be detected and integrated into physiological responses. Besides contributing to the preservation of tissue function and to the overall organismal integrity of vertebrates upon encounter with invading pathogens, these cells enabled the co-evolution of complex microbial communities that provide essential services, which include, but are not limited to, nutrient utilization, synthesis of essential metabolites and detoxification. The restricted TCR repertoire of Foxp3-expressing cells in various organs implies that signaling through their antigen receptor is not only important for Treg cell ontogeny but may also determine their ability to localize to tissues and support their homeostatic functions. Bony fish have functional FOXP3 orthologs and rely on Foxp3-expressing lymphocytes to suppress autoimmunity and to support tissue repair upon injury (Hui et al., 2017, Sugimoto et al., 2017). Like mammalian Treg cells, zebrafish Treg cells are quickly recruited to injured sites where they produce organ-specific growth factors, suggesting that their tissue repair “program” is flexible and may be instructed by the environment. While current studies of mammalian Treg cells support the existence of invariant features of their tissue repair program, the extent to which this program can be further shaped by environmental cues remains undetermined. Investigating Treg cells in different modalities of tissue injury within the same organ will be instrumental to determine which context-specific modules can be appended or whether additional functions must be gained by sub-specialization.
Figure 1: Roles of regulatory T (Treg) cells in tissue pathophysiology and metabolism in vertebrates.
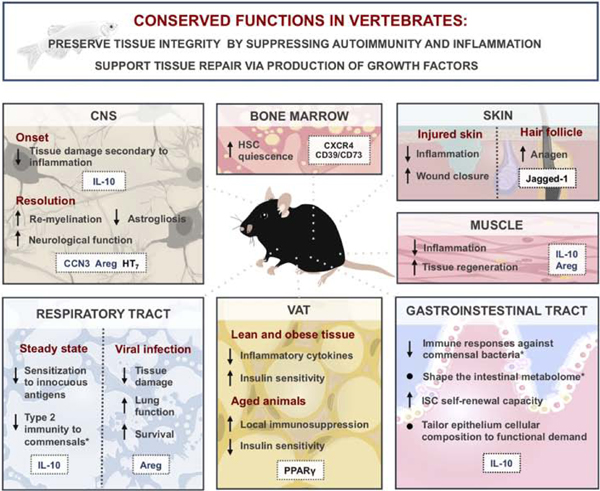
Conserved functions of Treg cells across vertebrate species (top blue box). Specific functions of Treg cells in various tissues are summarized in bottom panels with molecules implicated in corresponding Treg cell functions highlighted in white boxes. Secreted molecules depicted in blue; surface or intracellular molecules depicted in black. Asterisks (*) denote functions ascribed to extrathymically generated Treg cells. From top left to right: Treg cells in the central nervous system (CNS) limit secondary tissue damage and contribute to tissue repair during ischemia and spinal cord injury. Treg cell production of adenosine via ectonucleotidases CD39 and CD73 has been suggested to support the quiescence of hematopoietic stem cells (HSC) in the bone marrow. Skin Treg cells expressing a Notch receptor ligand Jagged-1 promote wound closure through a contact-dependent Notch signaling in stem cells to promote anagen. Treg cells infiltrate the muscle upon injury and suppress inflammation, facilitating tissue repair and regeneration. Intestinal Treg cells shape the composition and function of the microbiota and affect the make-up of the gut epithelium via direct and indirect effects on intestinal stem cells (ISC). Dual role of Treg cells in visceral adipose tissue (VAT) function: in young animals, Treg cells promote insulin sensitivity in both lean and obese tissue, while a buildup of fat Treg cells in older animals contributes to insulin resistance. In the lung, Treg cells support tissue homeostasis by preventing exacerbated immune responses to innocuous environmental antigens at the steady state and by partaking in tissue repair during injury.
Acknowledgments:
This work was supported by NIH/NCI Cancer Center Support Grant (CCSG) P30CA008748, NIH grant R37AI034206, the Ludwig Center at Memorial Sloan Kettering, and the Hilton-Ludwig Cancer Prevention Initiative (Conrad N. Hilton Foundation and Ludwig Cancer Research). A.Y.R. is an investigator with the Howard Hughes Medical Institute. We thank Miguel F. de Jesus (MSKCC) for help with digital art. We apologize to many colleagues working in the field for not being able to cite all their publications in this mini-review due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- AI TL, SOLOMON BD & HSIEH CS 2014. T-cell selection and intestinal homeostasis. Immunol Rev, 259, 60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL NABHANI Z, DULAUROY S, MARQUES R, COUSU C, AL BOUNNY S, DEJARDIN F, SPARWASSER T, BERARD M, CERF-BENSUSSAN N & EBERL G. 2019. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity, 50, 1276–1288.e5. [DOI] [PubMed] [Google Scholar]
- ALI N, ZIRAK B, RODRIGUEZ RS, PAULI ML, TRUONG HA, LAI K, AHN R, CORBIN K, LOWE MM, SCHARSCHMIDT TC, TARAVATI K, TAN MR, RICARDO-GONZALEZ RR, NOSBAUM A, BERTOLINI M, LIAO W, NESTLE FO, PAUS R, COTSARELIS G, ABBAS AK & ROSENBLUM MD 2017. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell, 169, 1119–1129 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON MS, VENANZI ES, KLEIN L, CHEN Z, BERZINS SP, TURLEY SJ, VON BOEHMER H, BRONSON R, DIERICH A, BENOIST C & MATHIS D. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science, 298, 1395–401. [DOI] [PubMed] [Google Scholar]
- ARPAIA N, CAMPBELL C, FAN X, DIKIY S, VAN DER VEEKEN J, DEROOS P, LIU H, CROSS JR, PFEFFER K, COFFER PJ & RUDENSKY AY 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature, 504, 451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARPAIA N, GREEN JA, MOLTEDO B, ARVEY A, HEMMERS S, YUAN S, TREUTING PM & RUDENSKY AY 2015. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell, 162, 1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASCHENBRENNER K, D’CRUZ LM, VOLLMANN EH, HINTERBERGER M, EMMERICH J, SWEE LK, ROLINK A & KLEIN L. 2007. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol, 8, 351–8. [DOI] [PubMed] [Google Scholar]
- ATARASHI K, TANOUE T, OSHIMA K, SUDA W, NAGANO Y, NISHIKAWA H, FUKUDA S, SAITO T, NARUSHIMA S, HASE K, KIM S, FRITZ JV, WILMES P, UEHA S, MATSUSHIMA K, OHNO H, OLLE B, SAKAGUCHI S, TANIGUCHI T, MORITA H, HATTORI M & HONDA K. 2013. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature, 500, 232–6. [DOI] [PubMed] [Google Scholar]
- ATARASHI K, TANOUE T, SHIMA T, IMAOKA A, KUWAHARA T, MOMOSE Y, CHENG G, YAMASAKI S, SAITO T, OHBA Y, TANIGUCHI T, TAKEDA K, HORI S, IVANOV II, UMESAKI Y, ITOH K & HONDA K. 2011. Induction of colonic regulatory T cells by indigenous Clostridium species. Science, 331, 337–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AYRES JS & SCHNEIDER DS 2012. Tolerance of infections. Annu Rev Immunol, 30, 271–94. [DOI] [PubMed] [Google Scholar]
- BAPAT SP, MYOUNG SUH J, FANG S, LIU S, ZHANG Y, CHENG A, ZHOU C, LIANG Y, LEBLANC M, LIDDLE C, ATKINS AR, YU RT, DOWNES M, EVANS RM & ZHENG Y. 2015. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature, 528, 137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARROSO-BATISTA J, DEMENGEOT J & GORDO I. 2015. Adaptive immunity increases the pace and predictability of evolutionary change in commensal gut bacteria. Nat Commun, 6, 8945.26615893 [Google Scholar]
- BENNETT CL, CHRISTIE J, RAMSDELL F, BRUNKOW ME, FERGUSON PJ, WHITESELL L, KELLY TE, SAULSBURY FT, CHANCE PF & OCHS HD 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet, 27, 20–1. [DOI] [PubMed] [Google Scholar]
- BITON M, HABER AL, ROGEL N, BURGIN G, BEYAZ S, SCHNELL A, ASHENBERG O, SU CW, SMILLIE C, SHEKHAR K, CHEN Z, WU C, ORDOVAS-MONTANES J, ALVAREZ D, HERBST RH, ZHANG M, TIROSH I, DIONNE D, NGUYEN LT, XIFARAS ME, SHALEK AK, VON ANDRIAN UH, GRAHAM DB, ROZENBLATT-ROSEN O, SHI HN, KUCHROO V, YILMAZ OH, REGEV A & XAVIER RJ 2018. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell, 175, 1307–1320 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORSELLINO G, KLEINEWIETFELD M, DI MITRI D, STERNJAK A, DIAMANTINI A, GIOMETTO R, HOPNER S, CENTONZE D, BERNARDI G, DELL’ACQUA ML, ROSSINI PM, BATTISTINI L, ROTZSCHKE O & FALK K. 2007. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood, 110, 1225–32. [DOI] [PubMed] [Google Scholar]
- BUFFIE CG, BUCCI V, STEIN RR, MCKENNEY PT, LING L, GOBOURNE A, NO D, LIU H, KINNEBREW M, VIALE A, LITTMANN E, VAN DEN BRINK MR, JENQ RR, TAUR Y, SANDER C, CROSS JR, TOUSSAINT NC, XAVIER JB & PAMER EG 2015. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature, 517, 205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURZYN D, KUSWANTO W, KOLODIN D, SHADRACH JL, CERLETTI M, JANG Y, SEFIK E, TAN TG, WAGERS AJ, BENOIST C & MATHIS D. 2013. A special population of regulatory T cells potentiates muscle repair. Cell, 155, 1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL C, DIKIY S, BHATTARAI SK, CHINEN T, MATHEIS F, CALAFIORE M, HOYOS B, HANASH A, MUCIDA D, BUCCI V & RUDENSKY AY 2018. Extrathymically Generated Regulatory T Cells Establish a Niche for Intestinal Border-Dwelling Bacteria and Affect Physiologic Metabolite Balance. Immunity, 48, 1245–1257 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHINEN T, KANNAN AK, LEVINE AG, FAN X, KLEIN U, ZHENG Y, GASTEIGER G, FENG Y, FONTENOT JD & RUDENSKY AY 2016. An essential role for the IL-2 receptor in Treg cell function. Nat Immunol, 17, 1322–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHINEN T, VOLCHKOV PY, CHERVONSKY AV & RUDENSKY AY 2010. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J Exp Med, 207, 2323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHISTIAKOV DA, CHISTIAKOVA EI, VORONOVA NV, TURAKULOV RI & SAVOST’ANOV KV 2011. A variant of the Il2ra / Cd25 gene predisposing to graves’ disease is associated with increased levels of soluble interleukin-2 receptor. Scand J Immunol, 74, 496–501. [DOI] [PubMed] [Google Scholar]
- CIPOLLETTA D, FEUERER M, LI A, KAMEI N, LEE J, SHOELSON SE, BENOIST C & MATHIS D. 2012. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature, 486, 549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOMBROWSKI Y, O’HAGAN T, DITTMER M, PENALVA R, MAYORAL SR, BANKHEAD P, FLEVILLE S, ELEFTHERIADIS G, ZHAO C, NAUGHTON M, HASSAN R, MOFFAT J, FALCONER J, BOYD A, HAMILTON P, ALLEN IV, KISSENPFENNIG A, MOYNAGH PN, EVERGREN E, PERBAL B, WILLIAMS AC, INGRAM RJ, CHAN JR, FRANKLIN RJM & FITZGERALD DC 2017. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci, 20, 674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENG Y, VAN DER VEEKEN J, SHUGAY M, PUTINTSEVA EV, OSMANBEYOGLU HU, DIKIY S, HOYOS BE, MOLTEDO B, HEMMERS S, TREUTING P, LESLIE CS, CHUDAKOV DM & RUDENSKY AY 2015. A mechanism for expansion of regulatory T-cell repertoire and its role in self-tolerance. Nature, 528, 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEUERER M, HERRERO L, CIPOLLETTA D, NAAZ A, WONG J, NAYER A, LEE J, GOLDFINE AB, BENOIST C, SHOELSON S & MATHIS D. 2009. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med, 15, 930–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJISAKI J, WU J, CARLSON AL, SILBERSTEIN L, PUTHETI P, LAROCCA R, GAO W, SAITO TI, LO CELSO C, TSUYUZAKI H, SATO T, COTE D, SYKES M, STROM TB, SCADDEN DT & LIN CP 2011. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature, 474, 216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUSAWA Y, OBATA Y, FUKUDA S, ENDO TA, NAKATO G, TAKAHASHI D, NAKANISHI Y, UETAKE C, KATO K, KATO T, TAKAHASHI M, FUKUDA NN, MURAKAMI S, MIYAUCHI E, HINO S, ATARASHI K, ONAWA S, FUJIMURA Y, LOCKETT T, CLARKE JM, TOPPING DL, TOMITA M, HORI S, OHARA O, MORITA T, KOSEKI H, KIKUCHI J, HONDA K, HASE K & OHNO H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature, 504, 446–50. [DOI] [PubMed] [Google Scholar]
- HIRATA Y, FURUHASHI K, ISHII H, LI HW, PINHO S, DING L, ROBSON SC, FRENETTE PS & FUJISAKI J. 2018. CD150(high) Bone Marrow Tregs Maintain Hematopoietic Stem Cell Quiescence and Immune Privilege via Adenosine. Cell Stem Cell, 22, 445–453 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTAMISLIGIL GS, MURRAY DL, CHOY LN & SPIEGELMAN BM 1994. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A, 91, 4854–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUI SP, SHENG DZ, SUGIMOTO K, GONZALEZ-RAJAL A, NAKAGAWA S, HESSELSON D & KIKUCHI K. 2017. Zebrafish Regulatory T Cells Mediate Organ-Specific Regenerative Programs. Dev Cell, 43, 659–672 e5. [DOI] [PubMed] [Google Scholar]
- ITO M, KOMAI K, MISE-OMATA S, IIZUKA-KOGA M, NOGUCHI Y, KONDO T, SAKAI R, MATSUO K, NAKAYAMA T, YOSHIE O, NAKATSUKASA H, CHIKUMA S, SHICHITA T & YOSHIMURA A. 2019. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature, 565, 246–250. [DOI] [PubMed] [Google Scholar]
- JOSEFOWICZ SZ, NIEC RE, KIM HY, TREUTING P, CHINEN T, ZHENG Y, UMETSU DT & RUDENSKY AY 2012. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature, 482, 395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPPLER JW, ROEHM N & MARRACK P. 1987. T cell tolerance by clonal elimination in the thymus. Cell, 49, 273–80. [DOI] [PubMed] [Google Scholar]
- KIM J, LAHL K, HORI S, LODDENKEMPER C, CHAUDHRY A, DEROOS P, RUDENSKY A & SPARWASSER T. 2009. Cutting edge: depletion of Foxp3+ cells leads to induction of autoimmunity by specific ablation of regulatory T cells in genetically targeted mice. J Immunol, 183, 7631–4. [DOI] [PubMed] [Google Scholar]
- KIM JM, RASMUSSEN JP & RUDENSKY AY 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol, 8, 191–7. [DOI] [PubMed] [Google Scholar]
- KISIELOW P, BLUTHMANN H, STAERZ UD, STEINMETZ M & VON BOEHMER H. 1988. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature, 333, 742–6. [DOI] [PubMed] [Google Scholar]
- KLINGENBERG R, GERDES N, BADEAU RM, GISTERA A, STRODTHOFF D, KETELHUTH DF, LUNDBERG AM, RUDLING M, NILSSON SK, OLIVECRONA G, ZOLLER S, LOHMANN C, LUSCHER TF, JAUHIAINEN M, SPARWASSER T & HANSSON GK 2013. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest, 123, 1323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLODIN D, VAN PANHUYS N, LI C, MAGNUSON AM, CIPOLLETTA D, MILLER CM, WAGERS A, GERMAIN RN, BENOIST C & MATHIS D. 2015. Antigen-and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab, 21, 543–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOTAS ME & MEDZHITOV R. 2015. Homeostasis, inflammation, and disease susceptibility. Cell, 160, 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUSWANTO W, BURZYN D, PANDURO M, WANG KK, JANG YC, WAGERS AJ, BENOIST C & MATHIS D. 2016. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity, 44, 355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE HM, BAUTISTA JL, SCOTT-BROWNE J, MOHAN JF & HSIEH CS 2012. A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self. Immunity, 37, 475–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE AG, ARVEY A, JIN W & RUDENSKY AY 2014. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol, 15, 1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE AG, HEMMERS S, BAPTISTA AP, SCHIZAS M, FAIRE MB, MOLTEDO B, KONOPACKI C, SCHMIDT-SUPPRIAN M, GERMAIN RN, TREUTING PM & RUDENSKY AY 2017. Suppression of lethal autoimmunity by regulatory T cells with a single TCR specificity. J Exp Med, 214, 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI C, DISPIRITO JR, ZEMMOUR D, SPALLANZANI RG, KUSWANTO W, BENOIST C & MATHIS D. 2018. TCR Transgenic Mice Reveal Stepwise, Multi-site Acquisition of the Distinctive Fat-Treg Phenotype. Cell, 174, 285–299 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI M, ZHAO W, WANG Y, JIN L, JIN G, SUN X, WANG W, WANG K, XU X, HAO J, JIN R, FU W, SUN Y, CHANG Y, HUANG X, ZHOU X, WU H, ZHANG K & GE Q. 2019. A wave of Foxp3(+) regulatory T cell accumulation in the neonatal liver plays unique roles in maintaining self-tolerance. Cell Mol Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIESZ A, SURI-PAYER E, VELTKAMP C, DOERR H, SOMMER C, RIVEST S, GIESE T & VELTKAMP R. 2009. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med, 15, 192–9. [DOI] [PubMed] [Google Scholar]
- MAGNUSDOTTIR S, RAVCHEEV D, DE CRECY-LAGARD V & THIELE I. 2015. Systematic genome assessment of B-vitamin biosynthesis suggests cooperation among gut microbes. Front Genet, 6, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHLAKOIV T, FLAMAR AL, JOHNSTON LK, MORIYAMA S, PUTZEL GG, BRYCE PJ & ARTIS D. 2019. Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33. Sci Immunol, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINS R, CARLOS AR, BRAZA F, THOMPSON JA, BASTOS-AMADOR P, RAMOS S & SOARES MP 2019. Disease Tolerance as an Inherent Component of Immunity. Annu Rev Immunol. [DOI] [PubMed] [Google Scholar]
- MATHUR AN, ZIRAK B, BOOTHBY IC, TAN M, COHEN JN, MAURO TM, MEHTA P, LOWE MM, ABBAS AK, ALI N & ROSENBLUM MD 2019. Treg-Cell Control of a CXCL5-IL-17 Inflammatory Axis Promotes Hair-Follicle-Stem-Cell Differentiation During Skin-Barrier Repair. Immunity, 50, 655–667.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAURICE CF, HAISER HJ & TURNBAUGH PJ 2013. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell, 152, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOELLER AH, CARO-QUINTERO A, MJUNGU D, GEORGIEV AV, LONSDORF EV, MULLER MN, PUSEY AE, PEETERS M, HAHN BH & OCHMAN H. 2016. Cospeciation of gut microbiota with hominids. Science, 353, 380–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLOFSKY AB, VAN GOOL F, LIANG HE, VAN DYKEN SJ, NUSSBAUM JC, LEE J, BLUESTONE JA & LOCKSLEY RM 2015. Interleukin-33 and Interferon-gamma Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity, 43, 161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLUR R, LIU YY & BRENT GA 2014. Thyroid hormone regulation of metabolism. Physiol Rev, 94, 355–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURUGAN A, MORA T, WALCZAK AM & CALLAN CG JR. 2012. Statistical inference of the generation probability of T-cell receptors from sequence repertoires. Proc Natl Acad Sci U S A, 109, 16161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOSBAUM A, PREVEL N, TRUONG HA, MEHTA P, ETTINGER M, SCHARSCHMIDT TC, ALI NH, PAULI ML, ABBAS AK & ROSENBLUM MD 2016. Cutting Edge: Regulatory T Cells Facilitate Cutaneous Wound Healing. J Immunol, 196, 2010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTROM SN, BOURGES D, GARRY S, ROSS EM, VAN DRIEL IR & GLEESON PA 2014. Transient Treg-cell depletion in adult mice results in persistent self-reactive CD4(+) T-cell responses. Eur J Immunol, 44, 3621–31. [DOI] [PubMed] [Google Scholar]
- OHNMACHT C, PARK JH, CORDING S, WING JB, ATARASHI K, OBATA Y, GABORIAU-ROUTHIAU V, MARQUES R, DULAUROY S, FEDOSEEVA M, BUSSLINGER M, CERF-BENSUSSAN N, BONECA IG, VOEHRINGER D, HASE K, HONDA K, SAKAGUCHI S & EBERL G. 2015. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science, 349, 989–93. [DOI] [PubMed] [Google Scholar]
- OKABE Y & MEDZHITOV R. 2016. Tissue biology perspective on macrophages. Nat Immunol, 17, 9–17. [DOI] [PubMed] [Google Scholar]
- PEARCE EN, FARWELL AP & BRAVERMAN LE 2003. Thyroiditis. N Engl J Med, 348, 2646–55. [DOI] [PubMed] [Google Scholar]
- POPOVIC B, GOLEMAC M, PODLECH J, ZELEZNJAK J, BILIC-ZULLE L, LUKIC ML, CICIN-SAIN L, REDDEHASE MJ, SPARWASSER T, KRMPOTIC A & JONJIC S. 2017. IL-33/ST2 pathway drives regulatory T cell dependent suppression of liver damage upon cytomegalovirus infection. PLoS Pathog, 13, e1006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUGLIESE A. 2017. Autoreactive T cells in type 1 diabetes. J Clin Invest, 127, 2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBTSOV YP, RASMUSSEN JP, CHI EY, FONTENOT J, CASTELLI L, YE X, TREUTING P, SIEWE L, ROERS A, HENDERSON WR JR., MULLER W & RUDENSKY AY 2008. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity, 28, 546–58. [DOI] [PubMed] [Google Scholar]
- SAMSTEIN RM, JOSEFOWICZ SZ, ARVEY A, TREUTING PM & RUDENSKY AY 2012. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell, 150, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT AM, LU W, SINDHAVA VJ, HUANG Y, BURKHARDT JK, YANG E, RIESE MJ, MALTZMAN JS, JORDAN MS & KAMBAYASHI T. 2015. Regulatory T cells require TCR signaling for their suppressive function. J Immunol, 194, 4362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEFIK E, GEVA-ZATORSKY N, OH S, KONNIKOVA L, ZEMMOUR D, MCGUIRE AM, BURZYN D, ORTIZ-LOPEZ A, LOBERA M, YANG J, GHOSH S, EARL A, SNAPPER SB, JUPP R, KASPER D, MATHIS D & BENOIST C. 2015. MUCOSAL IMMUNOLOGY. Individual intestinal symbionts induce a distinct population of RORgamma(+) regulatory T cells. Science, 349, 993–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH PM, HOWITT MR, PANIKOV N, MICHAUD M, GALLINI CA, BOHLOOLY YM, GLICKMAN JN & GARRETT WS 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science, 341, 569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOFRONIEW MV 2014. Astrogliosis. Cold Spring Harb Perspect Biol, 7, a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPALLANZANI RG, ZEMMOUR D, XIAO T, JAYEWICKREME T, LI C, BRYCE PJ, BENOIST C & MATHIS D. 2019. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Sci Immunol, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLARCZYK E, VONG CT, PERUCHA E, JACKSON I, CAWTHORNE MA, WARGENT ET, POWELL N, CANAVAN JB, LORD GM & HOWARD JK 2013. Improved insulin sensitivity despite increased visceral adiposity in mice deficient for the immune cell transcription factor T-bet. Cell Metab, 17, 520–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGIMOTO K, HUI SP, SHENG DZ, NAKAYAMA M & KIKUCHI K. 2017. Zebrafish FOXP3 is required for the maintenance of immune tolerance. Dev Comp Immunol, 73, 156–162. [DOI] [PubMed] [Google Scholar]
- TOMER Y, GREENBERG DA, BARBESINO G, CONCEPCION E & DAVIES TF 2001. CTLA-4 and not CD28 is a susceptibility gene for thyroid autoantibody production. J Clin Endocrinol Metab, 86, 1687–93. [DOI] [PubMed] [Google Scholar]
- UEDA H, HOWSON JM, ESPOSITO L, HEWARD J, SNOOK H, CHAMBERLAIN G, RAINBOW DB, HUNTER KM, SMITH AN, DI GENOVA G, HERR MH, DAHLMAN I, PAYNE F, SMYTH D, LOWE C, TWELLS RC, HOWLETT S, HEALY B, NUTLAND S, RANCE HE, EVERETT V, SMINK LJ, LAM AC, CORDELL HJ, WALKER NM, BORDIN C, HULME J, MOTZO C, CUCCA F, HESS JF, METZKER ML, ROGERS J, GREGORY S, ALLAHABADIA A, NITHIYANANTHAN R, TUOMILEHTO-WOLF E, TUOMILEHTO J, BINGLEY P, GILLESPIE KM, UNDLIEN DE, RONNINGEN KS, GUJA C, IONESCU-TIRGOVISTE C, SAVAGE DA, MAXWELL AP, CARSON DJ, PATTERSON CC, FRANKLYN JA, CLAYTON DG, PETERSON LB, WICKER LS, TODD JA & GOUGH SC 2003. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature, 423, 506–11. [DOI] [PubMed] [Google Scholar]
- WEGORZEWSKA MM, GLOWACKI RWP, HSIEH SA, DONERMEYER DL, HICKEY CA, HORVATH SC, MARTENS EC, STAPPENBECK TS & ALLEN PM 2019. Diet modulates colonic T cell responses by regulating the expression of a Bacteroides thetaiotaomicron antigen. Sci Immunol, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WING K, ONISHI Y, PRIETO-MARTIN P, YAMAGUCHI T, MIYARA M, FEHERVARI Z, NOMURA T & SAKAGUCHI S. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science, 322, 271–5. [DOI] [PubMed] [Google Scholar]
- XU L, LI W, WANG X, ZHANG L, QI Q, DONG L, WEI C, PU Y, LI Y, ZHU J, ZHOU S, LIU F, CHEN X & SU C. 2018a. The IL-33-ST2-MyD88 axis promotes regulatory T cell proliferation in the murine liver. Eur J Immunol, 48, 1302–1307. [DOI] [PubMed] [Google Scholar]
- XU M, POKROVSKII M, DING Y, YI R, AU C, HARRISON OJ, GALAN C, BELKAID Y, BONNEAU R & LITTMAN DR 2018b. c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature, 554, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG Y, JOSEFOWICZ S, CHAUDHRY A, PENG XP, FORBUSH K & RUDENSKY AY 2010. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature, 463, 808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


