Abstract
Although the fat mass and obesity-associated gene (FTO) correlates with elevated body mass, it is unclear how it contributes to overeating. We tested if individuals with the A allele show greater reward region responsivity to receipt and anticipated receipt of food and money and palatable food images. We also tested if these individuals show greater future weight gain. Initially healthy weight adolescents (Study 1, N = 162; Study 2, N = 135) completed different functional magnetic resonance imaging paradigms and had their body mass measured annually over 3 years. Adolescents with the AA or AT genotypes showed less precuneus and superior parietal lobe response and greater cuneus and prefrontal cortex response to milkshake receipt and less putamen response to anticipated milkshake receipt than those with the TT genotype in separate analyses of each sample. Groups did not differ in response to palatable food images, and receipt and anticipated receipt of money, or in weight gain over 3-year follow-up. Results suggest that initially healthy weight adolescents with vs without the FTO A allele show differential responsivity to receipt and anticipated receipt of food but do not differ in neural response to palatable food images and monetary reward and do not show greater future weight gain.
Keywords: FTO gene, BOLD response, prospective, weight gain, adolescents
Adults with the AA FTO genotype (rs9939609) show higher body mass index (BMI) and report less satiety and stronger preferences for high-calorie food than adults with the TT or AT genotype (Frayling et al., 2007; Cecil et al., 2008; Church et al., 2010). Given that FTO genotypes correlate with dopamine signaling (Sevgi et al., 2015) and consumption of high-calorie foods causes dopamine signaling and activation in reward circuitry (e.g. striatum, amygdala, orbitofrontal cortex [OFC]) (Kringelbach et al., 2003; Ferreira et al., 2012; Stice et al., 2013), as does anticipated palatable food intake and exposure to food images (Stice et al., 2012; van Meer et al., 2015), FTO may alter reward region response to anticipated palatable food receipt and food cues.
FTO is expressed throughout the brain (Frayling et al., 2007), but the neural mechanism by which it increases overeating is unclear. Participants with the AA genotype showed less responsivity in the hypothalamus (r = −0.70), ventral tegmental area (VTA; r = −0.71), posterior insula (r = −0.63), thalamus (r = −0.75) and hippocampus (r = −0.72) to food vs non-food images than participants with the TT genotype when fasted but not when fed (Karra et al., 2013; see Table 1 for a description of the studies). Participants with the AA genotype also showed less activation in the anterior insula (r = −0.87), lateral OFC (r = −0.79) and putamen (r = −0.80) in response to low-calorie vs high-calorie food images than participants with the TT genotype (Karra et al., 2013). Participants with vs without the AA genotype showed less response in the prefrontal cortex (PFC) to high-calorie food images 30 min after ingesting 75 g of glucose (r = −0.90) but no differences when fasted (Heni et al., 2014).
Table 1. Overview of cited research.
| Study | Variants contrasted | Sample | Stimulus | Response |
|---|---|---|---|---|
| Karra et al., 2013 | AA vs TT | Fasted and fed healthy weight adults (N = 24) | Food vs non-food images | ↓ Responsivity in hypothalamus (r = −0.70), VTA (r = −0.71), posterior insula (r = −0.63), thalamus (r = −0.75) and hippocampus (r = −0.72) (present in fasted, not fed) |
| Low- vs high-calorie food images | ↓ Activation in anterior insula (r = −0.87), lateral OFC (r = −0.79) and putamen (r = −0.80) | |||
| Heni et al., 2014 | AA vs TT or AT | Fed and fasted varying weight adults (N = 24) | High-calorie food vs non-food images | ↓ Response in PFC (r = −0.90) (present in fed, not fasted) |
| Kuhn et al., 2016 | AA or AT vs TT | Fed lean adults (N = 77) | Food vs non-food images | ↑ Activation in posterior fusiform gyrus (r = 0.41) |
| Rapuano et al., 2017 | AA and AT vs TT | Fed children (N = 78) | High-calorie food vs non-food commercials | ↑ Responsivity in ventral striatum (r =0 .31), per ROI analyses and medial OFC extending into ventral striatum per whole brain analyses |
| Wiemerslage et al., 2016 | AA vs TT | Fasted healthy weight adults (N = 30) | High- vs low-calorie food images | ↑ Responsivity in posterior cingulate cortex (r = 0.95), cingulate gyrus (r = .97), cuneus (r = .75), precuneus (r = .70) and putamen (r = .85) |
In contrast, participants with the AA or AT genotypes showed greater activation in the posterior fusiform gyrus in response to food vs non-food images than participants with the TT genotype (r = 0.41) (Kuhn et al., 2016). Participants with the AA vs TT genotype showed greater responsivity to high- vs low-calorie food images in the posterior cingulate cortex (r = 0.95), cingulate gyrus (r = 0.97), cuneus (r = 0.75), precuneus (r = 0.70) and putamen (r = 0.85) (Wiemerslage et al., 2016). Participants with the AA or AT genotypes showed greater responsivity to high-calorie food commercials vs non-food commercials in the nucleus accumbens (r = 0.31) and medial OFC vs participants with the TT variant (Rapuano et al., 2017).
Thus, two studies found that individuals with the AA genotype showed less reward region responsivity to food images, whereas three found that these individuals showed greater reward region responsivity. In addition, some studies found differences in a fed, but not a fasted state, though others found the opposite. The mixed findings may have emerged because the studies used small samples, which increased risk for false positive effects (Cremers et al., 2017; Smeets et al., 2019). Further, no studies examined neural response to tastes of high-calorie foods or other rewarding experiences, such as monetary reward, to determine whether aberrant reward region responsivity is specific to food or general.
The primary aim was to test the hypothesis that adolescents with vs without the AA genotype would show greater reward region responsivity to palatable food images, as well as to receipt and anticipated receipt of palatable food and monetary reward, using data from two large samples. We focused on this developmental period because the relation between the FTO genotype and BMI is higher in adolescence than in childhood or adulthood (Hardy et al., 2010; Dwivedi et al., 2012). A secondary aim was to test whether FTO genotypes predict future BMI gain, as past studies have generated inconsistent findings (Jess et al., 2008; Jacobsson et al., 2009; Hardy et al., 2010; Hertel et al., 2011; Hallman et al., 2012; Chuang et al., 2015).
Subjects and methods
Participants
The studies recruited adolescents between 14 and 17 years of age. We recruited healthy weight adolescents because repeated-measures functional magnetic resonance imaging (fMRI) studies indicate that overeating results in decreased reward region response to food intake and increased reward region response to food images/cues (Stice and Yokum, 2016; Stice et al., 2010a), converging with findings from animal experiments (Davis et al., 2008; Thanos et al., 2008; Johnson and Kenny, 2010). Further, experimentally induced overfeeding increases FTO gene expression (Sirohi et al., 2017). We thought that it is critical to test whether individuals with an AA genotype show different neural response to food stimuli before a history of overeating could induce changes in neural responsivity. Both studies were conducted in accordance with the Declaration of Helsinki (1991; p. 1194) and approved by the Oregon Research Institute’s Institutional Review Board. Participants in Study 1 (N = 162) were recruited from Eugene, Oregon. Participants in Study 2 (N = 135) were recruited from Portland, Oregon. Exclusion criteria were BMI > 25 (however, measured BMI was over 25 for 2 participants in Study 1 and 6 participants in Study 2), binge eating or compensatory behavior or current Axis I psychiatric disorders (assessed via interview using questions from the Schedule for Affective Disorders and Schizophrenia for School Age Children—Epidemiologic Version 5; Orvaschel, 1994), use of psychotropic medications or illicit drugs, any fMRI contra-indicators or dairy allergies. Written informed consent was obtained from parents when they brought their child to the baseline assessment.
In Study 1, one participant did not provide a saliva sample and fMRI data of two participants were collected with acquisition errors. In Study 2, 15 participants did not provide a saliva sample and fMRI data of 3 participants were collected with acquisition errors. Data from these participants were excluded. Findings from the resulting sample of 159 adolescents in Study 1 (79 females; M age = 15.3 ± 1.1; M baseline BMI = 20.8 ± 1.9; 10.1% Hispanic, 2.5% Native Americans, 0.6% Asian, 78.6% European American, 8.2% mixed race/ethnicity) and 117 adolescents in Study 2 (64 females; M age = 15.0 ± 0.9; M baseline BMI = 21.2 ± 2.3; 8.5% Hispanic, 0.9% Native Americans, 5.1% Asian, 6.0% Black or African American, 74.4% European American, 5.1% mixed race/ethnicity) are reported.
FTO rs9939609 genotyping
Participants provided saliva at baseline. Epithelial cells were collected using a commercial product (Oragene, DNA Genotek Inc., Ottawa, Ont). Each 96-well plate included non-templates, DNA standards of known genotype and 10% sample replication for accuracy (100% concordance). Further details are available in Supplementary data. We defined the following FTO groups: AA variant (Study 1, n = 18; Study 2, n = 14), AT variant (Study 1, n = 71; Study 2, n = 55) and TT variant (Study 1, n = 70; Study 2, n = 48). The FTO gene was in Hardy–Weinberg equilibrium (χ2 = 0.06) for the combined sample.
Body mass index
Height was measured to the nearest millimeter by using a stadiometer, and weight was assessed to the nearest 0.1 kg by using digital scales at baseline and at 1-, 2-, and 3-year follow-up. BMI (kg/m2) was calculated. BMI correlates with direct measures of total body fat such as dual energy x-ray absorptiometry (r = 0.80 to 0.90) and with health measures such as atherosclerotic lesions in adolescent samples (Mei et al., 2010). We used raw BMI scores because these are superior to age- and sex-adjusted percentiles or BMI z scores for modeling change over time (Berkey and Colditz, 2007).
Sensory and hedonic measures
Participants were asked to consume their regular meals but to refrain from eating or drinking (except water) for 4 h preceding their scan. Upon arrival to scan sessions, participants rated their hunger on a 20 cm cross-modal visual analog scale (VAS), anchored by −10 (not at all), 0 (neutral) and 10 (never been more hungry). Mean hunger rating was −2.5 in Study 1 and 0.8 in Study 2, suggesting that participants were in a neutral hunger state. Participants tasted the milkshakes and tasteless solution and rated the pleasantness and wanting of the tastes (order counterbalanced). Rating scales ranged from 0 (e.g. most unpleasant sensation ever) to 20 (e.g. most pleasant sensation ever).
Total energy intake and total energy expenditure
In Study 1, we collected data on total energy intake (TEI) and total energy expenditure (TEE) over a 2 week period using Doubly Labeled Water (see Supplementary data for procedures).
fMRI paradigms
In both studies, participants completed similar food reward paradigms. Participants in Study 1 also completed a monetary reward paradigm and participants in Study 2 also completed a food image paradigm.
Study 1 fMRI paradigms
The food receipt paradigm assesses blood oxygen level-dependent (BOLD) response to receipt and anticipated receipt of chocolate milkshake and a tasteless solution. It has activated reward regions (main effects reported in Stice et al., 2012), with activation from this paradigm showing test–retest reliability for weight-stable individuals, sensitivity to the effects of weight change and predictive validity for future weight gain (Stice et al., 2015; (Stice et al., 2008a; Stice et al., 2010b). Stimuli were images of glasses of chocolate milkshake and water that signaled (cued) the delivery of 0.5 cc chocolate milkshake (15 cc total; see Supplementary data for recipe) and tasteless solution, respectively. Tasteless solution consisted of 25 mM KCl and 2.5 mM NaHCO3 in distilled water, the main ionic components of saliva (O’Doherty et al., 2002). On 40% of the milkshake and tasteless solution trials the taste was not delivered following the cue to allow the investigation of the neural response to anticipation of a taste that was not confounded with actual receipt of the taste. In total, there were 30 repeats of both milkshake receipt and tasteless solution receipt and 50 repeats of the glass of milkshake and glass of water. Images were presented for 2 s, followed by a jitter (1–7 s) during which time the screen was blank. Tastes were delivered over 5 s and were followed by a 2 s ‘swallow’ cue. The next image appeared 1–7 s after the ‘swallow’ cue. Order of image presentation was randomized. Tastes were delivered using programmable syringe pumps. Syringes filled with milkshake and tasteless solution were connected via Tygon tubing to a manifold that fit into participants’ mouths and delivered the taste to a consistent tongue segment.
The monetary reward paradigm assesses BOLD response to receipt and anticipated receipt of monetary reward. A coin on the left side of the screen would blink heads (H) and tails (T) 2–4 times for 300 ms per blink before it ‘landed’ on either H or T. After 2 s, a second coin in the middle of the screen blinked 4–6 times before landing on H or T. After 3 s, a third coin blinked 8–10 times on the right side of the screen before landing on H or T. After presentation of the three coins, a 2–3 s message appeared saying ‘You win $3’ or ‘You don’t win’. Stimulus presentations were jittered. Participants won $3 each time three heads or three tails were displayed. Receipt and anticipated receipt of monetary reward in this paradigm activated brain regions implicated in reward (main effects reported in Stice et al., 2012) and differentiated adolescents with vs without family history of obesity (Stice et al., 2011).
Study 2 fMRI paradigms
The food receipt paradigm in Study 2 is a block version of the food receipt paradigm in Study 1 and assesses BOLD response to tastes of four chocolate milkshakes varying in sugar and fat content and a tasteless solution (Figure 1A and B): a high-fat/high-sugar milkshake, a high-fat/low-sugar milkshake, a low-fat/high-sugar milkshake and a low-fat/low-sugar milkshake (see Supplementary data for more information on contents). Participants were told that they would receive four different kinds of milkshake but were not informed about the fat and sugar content of the milkshakes. Each milkshake contained the same ice cream base and chocolate syrup. Fat content of each of the milkshakes was manipulated by varying milk type (half-and-half vs 2% milk). Sweetness was manipulated by varying syrup content. No fat substitutes/thickeners or artificial sweeteners were used. The tasteless solution consisted of the same components as mentioned in Study 1. During the task, pictures of glasses of milkshake or water were presented for 1 s to cue the participant that they were about to receive milkshake or tasteless solution. All milkshakes were preceded by the same image of a milkshake. During the taste delivery (0.7 cc) over 5 s (56 cc in total), a fixation cross was shown. Participants were instructed to hold the taste in their mouth until they saw the ‘swallow’ cue on the screen. Delivery of the milkshakes and the tasteless solution occurred in blocks that varied in length (one block presented four, five or seven events in each of the two runs). Only one type of milkshake was delivered per block. After a milkshake block was completed, subjects received a rinse of the tasteless solution followed by a swallow cue (0.5 s) and a jitter (9–11 s). Order of the presentation of blocks (i.e. different milkshakes) was randomized. Two runs (13 min each) were performed. Each run presented three blocks of each of the four milkshake types and the tasteless solution. Tastes of these milkshakes have activated brain regions implicated in reward (striatum), attention (anterior cingulate cortex) and gustatory processing (insula) (Stice et al., 2013).
Fig. 1. Sample timing of (A) the block milkshake receipt paradigm and (B) the food picture paradigm.
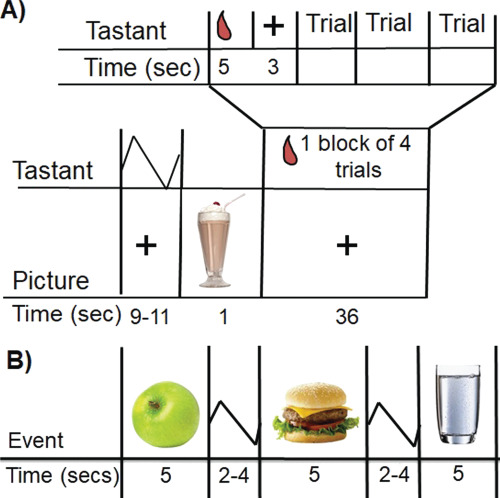
The food image paradigm assesses brain response to images of palatable foods, unpalatable foods and glasses of water. Prior to the scan, participants rated how appetizing they found foods shown in 129 pictures, using a VAS. The images were presented one at a time, in a random order on a computer monitor. A variety of foods were presented, including processed foods, fruits and vegetables. Images were matched for brightness and contrast. Food pictures did not show any logos or product advertisement. During the scan, each participant was exposed to 32 pictures of food they rated the most appetizing and 32 pictures of food they rated the least appetizing, as well as 32 pictures of glasses of water. Exposure to appetizing food pictures in this paradigm activated brain regions implicated in reward (striatum, medial OFC), attention (precuneus, anterior cingulate cortex), motor approach (supplemental motor area) and somatosensory processing (Rolandic operculum) (Stice and Yokum, 2018).
fMRI data analysis
Detailed descriptions of the fMRI data acquisition and data preprocessing are provided in the Supplementary data. Neuroimaging data were preprocessed and analyzed primarily using Statistical Parametric Mapping version 12 (SPM12) (Functional Imaging Laboratory, University College London) in MATLAB (MathWorks). To identify brain regions activated by palatable food receipt in Study 1, we contrasted BOLD signal during receipt of milkshake vs tasteless solution. Activation in response to anticipated food receipt was assessed by contrasting BOLD signal during milkshake cue vs tasteless solution cue. Activation in response to monetary reward was assessed by contrasting BOLD signal at the time a participant ‘won’ (the three coins matched) vs a reward-neutral coin display (the time the first coin stopped blinking). Activation in response to anticipated monetary reward was assessed by contrasting BOLD activation during presentation of the cue signaling, a potential win (i.e. two heads or two tails), vs the reward-neutral coin display. In Study 2, activation in response to the intake of each milkshake was assessed by contrasting BOLD signal during receipt of each of the four milkshakes vs tasteless solution (e.g. high-fat/high-sugar milkshake receipt > tasteless solution receipt). Activation in response to palatable food images was assessed by contrasting BOLD signal during viewing pictures of appetizing food vs unappetizing foods and vs glasses of water. At the individual level, T maps were constructed for comparison of activation within each participant for these contrasts.
The aforementioned individual contrasts were entered into mixed between- and within-subjects second-level 3 (FTO genotype) × 2 (e.g. milkshake receipt > tasteless receipt) analysis of variance (ANOVA) models. Hunger was included as a covariate as this variable modulates neural response to food stimuli (Siep et al., 2009) and ethnicity was included as a covariate to control for population stratification given evidence that this works as well as more complex approaches for this potential confound (Hutchison et al., 2004) (see Supplementary data for results in Caucasians with European ancestry only). Because past studies that used fMRI to examine the functional significance of FTO often controlled for participant BMI (Karra et al., 2013; Rapuano et al., 2017), we likewise used BMI as a covariate. Whole-brain analyses were conducted after the binarized DARTEL-derived sample-specific gray matter mask was applied. We estimated the smoothness of the masked functional data with the three-dimensional FWHM module in AFNI (Version AFNI_17.0.03). This smoothness was then used in 10 000 Monte Carlo simulations of random noise at 3 mm through the gray matter masked data with the 3DClustSim module of AFNI. Simulation results indicated activity surviving a threshold of P < 0.005, with a cluster (k) ≥ 35 as statistically significant correcting for multiple comparisons across whole brain analyses at P < 0.05. We also employed regions of interest (ROI) analyses on neural activity during the food image task in Study 2 to provide a more sensitive test of whether we could replicate peaks reported previously (Karra et al., 2013; Heni et al., 2014; Kuhn et al., 2016; Wiemerslage et al., 2016; Rapuano et al., 2017). We created a bilateral mask that included the regions identified in these studies: PFC Brodmann area 9, hippocampus, hypothalamus, insula, thalamus, VTA, fusiform gyrus, medial OFC, nucleus accumbens, cingulate cortex, cuneus, precuneus and putamen. ROIs were defined using the Wake Forest University Pickatlas toolbox (Maldjian et al., 2003) within SPM.
BMI change analyses
BMI data from baseline and 1, 2 and 3 year follow-ups were used in random intercept, mixed effects growth curve analyses (SAS Inc. version 9.3) to model BMI change, which use maximum likelihood estimation to accommodate missing data (Singer et al., 1996). We examined empirical growth plots, fit an unconditional means model, fit an unconditional linear growth model and fit unconditional nonlinear models. Linear growth models consistently showed a better fit than higher order polynomials.
Results
Table 2 presents the descriptive statistics of the genotype groups per study. There were no significant relations between genotype status and ethnicity, sex and socioeconomic status, suggesting that these variables were not potential confounds. ANOVA models examined group differences in sensory and hedonic ratings, TEI and TEE (Table 3). Bonferroni corrections were used (Study 1: P ≤ 0.002; Study 2: P ≤ 0.001). In Study 1, there were significant genotype group differences on milkshake pleasantness (F2,156 = 6.34, P = 0.002). AA genotypes reported lower milkshake pleasantness ratings (M pleasantness = 12.3 ± 4.1) than TT genotypes (M pleasantness = 14.6 ± 2.1). There were no significant group differences on milkshake wanting, TEI or TEE. In Study 2, there were no significant differences between genotype groups on any the outcomes.
Table 2. Descriptive statistics for the genotype groups.
| Genotype | N | Ethnicity |
|---|---|---|
| Study 1 (n = 159) | ||
| FTO | ||
| AA | 18 (55.6% Female) | 16.7% Hispanic, 77.8% Caucasian, 22.2% mixed races |
| AT | 71 (46.5% Female) | 4.2% Hispanic, 93% Caucasian, 1.4% American Indian/Alaska Native, 5.6% mixed races |
| TT | 77 (51.4% Female) | 17.1% Hispanic, 78.6% Caucasian, 2.9% American Indian/Alaska Native, 1.4 Asian, 17.1% mixed races |
| Study 2 (n = 117) | ||
| FTO | ||
| AA | 14 (50.0% Female) | 0% Hispanic, 100% Caucasian |
| AT | 55 (52.7% Female) | 14.5% Hispanic, 65.5% Caucasian, 1.8% American Indian/Alaska Native, 1.8% Asian, 7.3 American African, 20.0% mixed races |
| TT | 48 (58.3% Female) | 6.3% Hispanic, 75% Caucasian, 10.4% Asian, 6.3% African American, 8.3% mixed races |
Table 3. Behavioral measures.
| FTO AA | FTO AT | FTO TT | Test statistics | |
|---|---|---|---|---|
| Study 1 | n = 18 | n = 71 | n = 70 | |
| BMI at baseline | 20.7 ± 1.9 | 20.7 ± 1.9 | 20.9 ± 1.9 | F 2,156 = 0.30, P = 0.74 |
| Age | 14.9 ± 1.1 | 15.6 ± 1.0 | 15.1 ± 1.0 | F 2,156 = 5.99, P = 0.003 |
| Parental education | 4.2 ± 1.0 | 4.4 ± 1.0 | 4.0 ± 1.1 | F 2,156 = 2.07, P = 0.13 |
| Hunger | 4.8 ± 4.1 | 7.6 ± 4.3 | 8.2 ± 4.1 | F 2,156 = 4.56, P = 0.01 |
| Milkshake pleasantness | 12.3 ± 4.1 | 14.4 ± 2.3 | 14.6 ± 2.1 | F 2,156 = 6.34, P = 0.002 |
| Milkshake wanting | 13.2 ± 2.8 | 13.7 ± 2.5 | 13.6 ± 2.2 | F 2,156 = 0.34, P = 0.71 |
| Tasteless solution pleasantness | 8.0 ± 1.7 | 7.8 ± 2.7 | 8.1 ± 3.0 | F 2,156 = 0.22, P = 0.80 |
| Tasteless solution wanting | 6.4 ± 3.5 | 5.9 ± 3.9 | 6.0 ± 4.0 | F 2,156 = 0.09, P = 0.91 |
| Total energy intake | 2505 ± 891 | 2636 ± 787 | 2593 ± 886 | F 2,151 = 0.18, P = 0.83 |
| Total energy expenditure | 2587 ± 508 | 2641 ± 588 | 2529 ± 539 | F 2,151 = 0.68, P = 0.51 |
| Study 2 | n = 14 | n = 55 | n = 48 | |
| BMI | 21.6 ± 2.2 | 20.8 ± 2.2 | 21.5 ± 2.5 | F 2,114 = 1.41, P = 0.25 |
| Age | 14.9 ± 0.9 | 15.1 ± 0.8 | 15.0 ± 0.8 | F 2,114 = 0.43, P = 0.65 |
| Parental education | 4.7 ± 0.8 | 4.3 ± 1.1 | 4.2 ± 0.9 | F 2,114 = 1.26, P = 0.29 |
| Hunger | 11.3 ± 5.1 | 11.1 ± 4.2 | 10.5 ± 4.2 | F 2,114 = 0.31, P = 0.73 |
| High-fat/high-sugar milkshake pleasantness | 15.3 ± 2.2 | 14.6 ± 3.3 | 14.5 ± 3.5 | F 2,114 = 0.30, P = 0.74 |
| High-fat/high-sugar milkshake wanting | 14.2 ± 2.6 | 13.8 ± 3.5 | 14.0 ± 3.5 | F 2,114 = 0.10, P = 0.93 |
| High-fat/low-sugar milkshake pleasantness | 13.1 ± 3.2 | 11.9 ± 3.7 | 11.2 ± 4.1 | F 2,114 = 1.46, P = 0.24 |
| High-fat/low-sugar milkshake wanting | 13.3 ± 2.8 | 11.7 ± 3.8 | 10.9 ± 4.9 | F 2,114 = 1.73, P = 0.18 |
| Low-fat/high-sugar milkshake pleasantness | 12.5 ± 3.5 | 13.5 ± 4.0 | 12.2 ± 5.1 | F 2,114 = 1.15, P = 0.32 |
| Low-fat/high-sugar milkshake wanting | 12.1 ± 3.3 | 12.6 ± 4.2 | 11.6 ± 5.3 | F 2,114 = 0.31, P = 0.73 |
| Low-fat/low-sugar milkshake pleasantness | 11.1 ± 3.1 | 9.7 ± 3.6 | 9.5 ± 4.4 | F 2,114 = 0.98, P = 0.38 |
| Low-fat/low-sugar milkshake wanting | 10.4 ± 3.4 | 9.4 ± 3.5 | 9.2 ± 4.9 | F 2,114 = 0.47, P = 0.62 |
| Tasteless solution pleasantness | 9.4 ± 2.5 | 10.5 ± 3.1 | 11.1 ± 3.4 | F 2,114 = 1.60, P = 0.21 |
| Tasteless solution wanting | 11.8 ± 3.9 | 10.9 ± 3.3 | 11.6 ± 3.6 | F 2,114 = 0.56, P = 0.57 |
| FTO AA (n = 32) | FTO AT (n = 126) | FTO TT (n = 118) | Test statistics | |
| BMI at baseline | 20.8 ± 2.1 | 21.2 ± 2.1 | 20.8 ± 2.0 | F 2,272 = 0.85, P = 0.43 |
| Change in BMI | 0.3 ± 0.8 | 0.4 ± 0.5 | 0.4 ± 0.3 | F 2,271 = 1.14, P = 0.32 |
Study 1: response to receipt and anticipated receipt of food and monetary reward
Whole brain analyses revealed differences in right putamen response to anticipated milkshake receipt and left cuneus response to milkshake receipt (Figure 2; Table 4). AA (M contrasting BOLD signal during milkshake receipt vs tasteless solution receipt [M BOLD contrast] = −0.0 ± 0.2; effect size r = −0.35) and AT carriers (M BOLD contrast = 0.01 ± 0.2; r = −0.30) showed less putamen response to anticipated milkshake receipt than TT carriers (M BOLD contrast = 0.16 ± 0.3; Figure 2A). AA (M BOLD contrast = 0.56 ± 0.8; r = 0.27) and AT carriers (M BOLD contrast = 0.56 ± 0.7; r = 0.28) showed greater cuneus response to milkshake receipt than TT carriers (M BOLD contrast = 0.16 ± 0.6; Figure 2B). Both peaks remained significant when including milkshake pleasantness as a covariate in the models. There were no differences between AA and AT carriers in responsivity to receipt and anticipated milkshake receipt. Exploratory analyses tested whether BOLD response in the putamen and cuneus peaks correlated with milkshake pleasantness and wanting ratings. Subject-level parameter estimates for these peak coordinates were extracted from SPM and exported to SPSS. There were no significant correlations between the parameter estimates in these peak coordinates and the hedonic ratings. There were no significant group differences in BOLD response to monetary reward and anticipated monetary reward.
Fig. 2. Significant group-by-cue interactions in BOLD signal in (A) right putamen (Montreal Neurological Institute, MNI coordinates: 24, 21, −3; Z = 3.90; k = 61) in response to the contrast milkshake cue > tasteless solution cue and (B) cuneus (MNI coordinates: −6, −78, 12; Z = 3.10; k = 35) in response to the contrast milkshake receipt > tasteless solution receipt in Study 1.
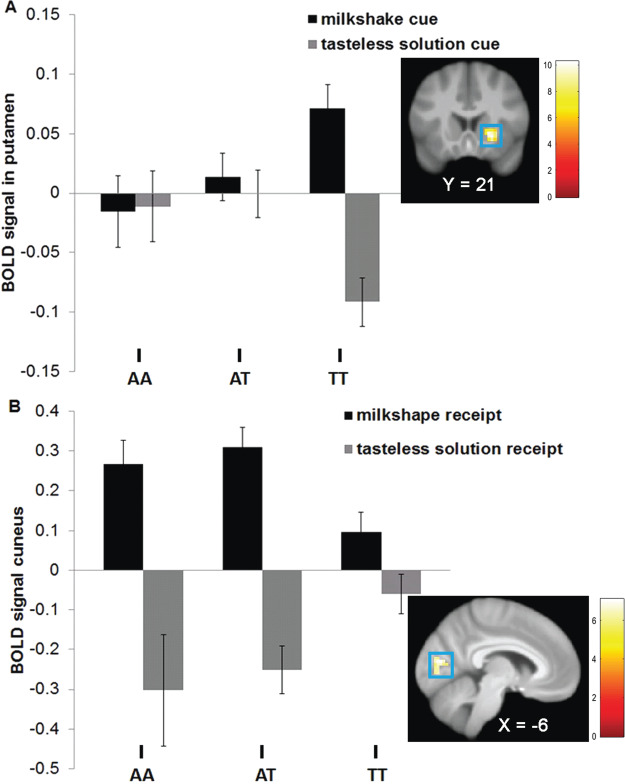
Table 4. Significant group-by-cue interactions in BOLD activity between FTO genotypes AA, AT and TT in Study 1 and Study 2 based on whole-brain analyses.
| Contrast and region | K | Z value | MNI coordinates | r (Z/√N) | 95% CI |
|---|---|---|---|---|---|
| Study 1 | |||||
| Milkshake cue > tasteless solution cue | |||||
| Putamen | 61 | 3.91 | 24, 21,-3 | 0.31 | 0.04–0.11 |
| Milkshake receipt > tasteless solution receipt | |||||
| Cuneus | 35 | 3.09 | −6, −78, 12 | 0.25 | 0.27–0.49 |
| Study 2 | |||||
| Low-fat/low-sugar milkshake receipt > tasteless solution receipt | |||||
| Precuneus | 44 | 3.34 | 27, −66, 33 | 0.31 | −0.16-0.04 |
Notes. For all contrasts, activated regions, number of contiguous voxels (k), Z values and coordinates within the MNI coordinate system are displayed. Effect sizes (r) were derived from the Z values (Z/√N). CI = confidence interval.
To examine the dominant influence of the A allele, we also compared AA and AT vs TT carriers. Paralleling the 3 × 2 ANOVA results, compared to the TT group, the AA/AT group showed less putamen response (Montreal Neurological Institute, MNI coordinates: 24, 21, −3; Z = 3.72; k = 61) to anticipated milkshake receipt (AA/AT M BOLD activity in response to contrast = 0.01 ± 0.2; TT M BOLD contrast = 0.13 ± 0.2; r = −0.32) and greater cuneus response (MNI coordinates: −6, −78, 12; Z = 3.20; k = 60) to milkshake receipt (AA/AT M BOLD contrast = 0.45 ± 0.6; TT M BOLD contrast = 0.13 ± 0.5, r = 0.28). No differences emerged in response to monetary reward and anticipated monetary reward.
Study 2: response to food receipt and food images
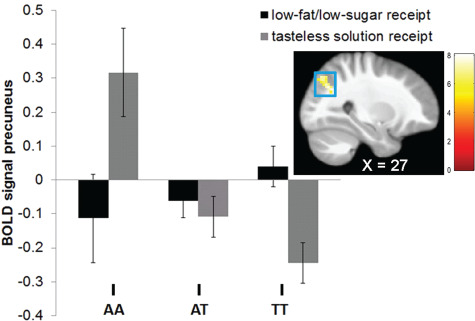
Whole brain analyses revealed a difference in right precuneus response to low-fat/low-sugar milkshake receipt (Table 4). AA (M contrasting BOLD signal during low-fat/low-sugar milkshake receipt vs tasteless solution receipt [M BOLD contrast] = −0.43 ± 0.6; r = −0.53) and AT carriers (M BOLD contrast = 0.0 ± 0.5; r = −0.24) showed less right precuneus response to low-fat/low-sugar milkshake receipt > tasteless solution receipt (Figure 3) than TT carriers (M BOLD contrast = 0.29 ± 0.5). The AA carriers also showed less precuneus response to this contrast than AT carriers (r = −0.39); however, this effect was partly driven by elevated BOLD response to the tasteless solution receipt in A homozygotes (Figure 3). There were no differences in response to high-fat/high-sugar milkshake receipt, high-fat/low-sugar milkshake receipt and low-fat/high-sugar milkshake receipt or in response to palatable food images. Exploratory analyses tested whether precuneus activity in response to the low-fat/low-sugar milkshake receipt correlated with milkshake pleasantness and wanting ratings. We found that parameter estimates in this peak coordinate correlated significantly with pleasantness (r = 0.23, P = 0.01) and wanting (r = 0.19, P = 0.04) of the low-fat/low-sugar milkshake.
Fig. 3. Significant group-by-cue interactions in BOLD signal in the precuneus (MNI coordinates: 27, −66, 33; Z = 3.34; k = 44) in response to the contrast low-fat/low-sugar milkshake receipt > tasteless solution receipt in Study 2.
We also examined the dominant influence of the A allele with a 2 (AA/AT vs TT) × 2 (e.g. high-fat/high sugar milkshake receipt > tasteless receipt) ANOVA. Compared to TT carriers, the AA/AT carriers showed elevated PFC (MNI coordinates: 27, 30, 45; Z = 3.61; k = 50) response to low-fat/high-sugar milkshake receipt (AA/AT M BOLD contrast = −0.09 ± 0.4; TT M BOLD contrast = −0.44 ± 0.5, r = 0.36) and less superior parietal lobe (MNI coordinates: −24, −63, 38; Z = 3.27; k = 39) response to the low-fat/low-sugar milkshake receipt (AA/AT M BOLD contrast = −0.14 ± 0.6; TT M BOLD contrast = 0.34 ± 0.7, r = −0.35).
We also conducted ROI analyses on neural activity during the food picture task using peaks identified in previous fMRI studies reviewed above; no cluster reached significance.
Relations between FTO and BMI gain over 3 year follow-up
We tested for genotype group differences in BMI gain over 3 year follow-up. We combined the two samples (N = 276; AA = 32, AT = 126; TT = 118) to maximize sensitivity. On average BMI increased 0.48 units per year over follow-up, reflecting moderate weight gain. There were no differences among the three genotype groups on BMI gain [F2,271 = 1.14, P = 0.32, partial eta squared = 0.01; Table 2], controlling for baseline BMI. There were also no group differences in baseline BMI [F2,272 = 0.85, P = 0.43, partial eta squared = 0.00; Table 2].
Discussion
This is the first study to test whether individuals with different FTO variants showed differential neural response to tastes and anticipated tastes of high-calorie milkshakes. Individuals with the AA and AT variants vs the TT variant showed greater cuneus response to milkshake receipt in Study 1 and less precuneus response to milkshake receipt in Study 2. Further, individuals with either the AA or AT variant vs the TT variant showed greater PFC response and less superior parietal lobe response to milkshake taste in Study 2. Although the PFC and precuneus had been reported in previous FTO papers (Heni et al., 2014; Wiemerslage et al., 2016), the results were in the opposite directions. Both cuneus and precuneus have been implicated in visual processing (Cavanna and Trimble, 2006) and respond to palatable food pictures and tastes (van der Laan et al., 2011; Stice et al., 2012). Further, the precuneus is functionally connected with reward-related regions, such as the striatum, through communication of the salience of visual stimuli (Engelmann et al., 2012). Precuneus activation correlated with pleasantness rating of and wanting for the low-fat/low-sugar milkshake. The fact that these effects appear to be contradictory does not inspire confidence in the conclusion that healthy weight adolescents with the AA variant show reliably weaker or stronger differences in neural response to tastes of palatable beverages. Further, the fact that most of the contrasts involving tastes of the four milkshakes that varied in fat and sugar content did not yield consistent differences likewise does not inspire confidence in this conclusion.
We also found that adolescents with the AA and AT variants showed weaker putamen response to anticipated milkshake taste, than those with the TT variant. The putamen has been found to respond to images of high-calorie foods and anticipated palatable food tastes (Stice et al., 2012; Tang et al., 2012; Stice et al., 2013). Thus, our findings may suggest that AA and AT carriers show less reward region response to anticipated food reward. Karra and associates (2013) found an interaction between nutritional state (fed vs fasted) and AA vs TT genotype status, wherein AA carriers showed less responsivity to both high-calorie and low-calorie food images in a region that contained the putamen compared to TT carriers when fed, but not when fasted. Although this finding was in response to food images, rather than anticipated milkshake receipt, both results suggest that individuals with the AA variant show less recruitment of a region implicated in reward to food stimuli than those with the TT variant. It should be noted that the milkshake receipt and anticipated receipt paradigm reliably differentiates lean vs overweight individuals (Stice et al., 2008b) and has predicted future weight gain in three samples, including those from Study 1 and Study 2 (Stice et al., 2008a; Stice et al., 2015; Stice and Yokum, 2018), providing evidence of discriminative and predictive validity.
This is also the first study to test whether the FTO variant groups showed differential neural response to receipt and anticipated receipt of monetary reward. However, we did not observe any differences in response to monetary reward, providing little support for the hypothesis that the FTO genotypes differ in general reward responsivity.
We did not observe any differences in neural response across genotype groups to appetizing food images. This is in contrast to the five published studies that examined this question, though two found that AA carriers showed less responsivity of various brain regions to palatable food images, whereas the other three found that AA carriers showed greater responsivity of other brain regions to palatable food images. Our null findings are noteworthy because our samples were larger than those used in past studies and because the effect sizes reported in the earlier studies were large (ranging from r’s = −.90 to.97). Results from these six studies collectively suggest that individuals with vs without the AA genotype do not show reliably weaker or stronger neural responsivity of regions implicated in reward or related functions to palatable food images.
A secondary aim was to test whether FTO genotypes predicted future increases in BMI. Although studies have reported that AA carriers showed greater weight gain over time (Jess et al., 2008; Hardy et al., 2010; Hallman et al., 2012; Chuang et al., 2015), we were unable to replicate this predictive effect, consistent with null findings from other studies (Jacobsson et al., 2009; Hertel et al., 2011). We had a power of 0.80 or greater to detect a small effect of r = .17 or greater in the prediction of future BMI change, suggesting sufficient sensitivity. We found no group differences in objectively measured energy intake and energy expenditure, in contrast with the findings of another study that used Doubly Labeled Water to measure energy expenditure in children (Cecil et al., 2008).
It is important to consider the study limitations. First, the number of AA carriers was relatively small, reducing sensitivity in analyses comparing these individuals to those with other FTO variants. Second, the fact that our samples included only healthy weight adolescents might have reduced sensitivity to detecting a relation between FTO variants and BMI change. Nonetheless, our sample is reasonably representative, in that 78% of adolescents are not yet overweight or obese by the age of 15 years, and BMI values ranged from 16.2 to 26.3, suggesting an adequate range. Third, differences in the paradigms used across studies might have contributed to the inconsistent findings regarding neural response to food images. Finally, the evidence that obesity is a polygenic disorder implies that future studies that use fMRI to investigate the functional significance genetic risk should examine a broader range of genes.
In conclusion, we used fMRI to examine the functional significance of FTO genotypes using five validated paradigms and larger samples than previous studies that address this important question. The present study extends this literature by finding that healthy weight AA carriers showed differential activation of regions implicated in reward and attention in response to receipt and anticipated receipt of chocolate milkshake, though the findings did not appear to be consistent with regard to the valence of the effects. Another novel feature of this study is that we tested for differences in neural response to receipt and anticipated receipt of monetary reward, though we did not observe significant effects. Further, we found no evidence that AA carriers showed differential neural responsivity to palatable food images and as such were unable to replicate results reported by five previous studies that used fMRI to examine the functional significance of FTO genotypes, which also had not produced any findings that replicated. It will thus be critical for future studies to examine the mechanism through which FTO genotypes increase risk or excessive weight gain.
Funding
This work was supported by National Institutes of Health (DK-080760) and (DK-092468).
Author contributions
The authors contributed in the following ways: E.S. and S.Y. designed and conducted the research; S.Y. performed statistical analyses; E.S. and S.Y. wrote the paper; E.S. had primary responsibility for final content. All authors read and approved the final manuscript. The authors report no conflict of interest with respect to the content of this paper.
Acknowledgments
The authors thank the Lewis Center for Neuroimaging at the University of Oregon for their assistance in data collection for these projects.
Contributor Information
Eric Stice, Stanford University, Stanford, CA 94305, USA.
Sonja Yokum, Oregon Research Institute, Eugene, OR, 97403, USA.
Pascale Voelker, University of Oregon, Eugene, OR, 97403, USA.
References
- Berkey C.S., Colditz G.A. (2007). Adiposity in adolescents: change in actual BMI works better than change in BMI z score for longitudinal studies. Annals of Epidemiology, 17(1), 44–50. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3), 564–83. [DOI] [PubMed] [Google Scholar]
- Cecil J.E., Tavendale R., Watt P., Hetherington M.M., Palmer C.N. (2008). An obesity-associated FTO gene variant and increased energy intake in children. The New England Journal of Medicine, 359(24), 2558–66. [DOI] [PubMed] [Google Scholar]
- Chuang Y.F., Tanaka T., Beason-Held L.L., An Y., Terracciano A., Sutin A.R., Thambisetty M. (2015). FTO genotype and aging: pleiotropic longitudinal effects on adiposity, brain function, impulsivity and diet. Molecular Psychiatry, 20(1), 133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church C., Moir L., McMurray F., Girard C., Banks G.T., Teboul L., Cox R.D. (2010). Overexpression of Fto leads to increased food intake and results in obesity. Nature Genetics, 42(12), 1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers H.R., Wager T.D., Yarkoni T. (2017). The relation between statistical power and inference in fMRI. PLoS One, 12(11), e0184923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.F., Tracy A.L., Schurdak J.D., Tschop M.H., Lipton J.W., Clegg D.J., Benoit S.C. (2008). Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behavioral Neuroscience, 122, 1257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi O.P., Tabassum R., Chauhan G., Ghosh S., Marwaha R.K., Tandon N., et al. (2012). Common variants of FTO are associated with childhood obesity in a cross-sectional study of 3,126 urban Indian children. PLoS One, 7, e47772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann J.M., Versace F., Robinson J.D., Minnix J.A., Lam C.Y., Cui Y., Cinciripini P.M. (2012). Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage, 60(1), 252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira J.G., Tellez L.A., Ren X., Yeckel C.W., de Araujo I.E. (2012). Regulation of fat intake in the absence of flavour signalling. The Journal of Physiology, 590(4), 953–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M., McCarthy M.I. (2007). A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science, 316(5826), 889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallman D.M., Friedel V.C., Eissa M.A., Boerwinkle E., Huber J.C. Jr., Harrist R.B., Berenson G.S. (2012). The association of variants in the FTO gene with longitudinal body mass index profiles in non-Hispanic white children and adolescents. International Journal of Obesity, 36(1), 61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R., Wills A.K., Wong A., Elks C.E., Wareham N.J., Loos R.J., Ong K.K. (2010). Life course variations in the associations between FTO and MC4R gene variants and body size. Human Molecular Genetics, 19(3), 545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heni M., Kullmann S., Veit R., Ketterer C., Frank S., Machicao F., Fritsche A. (2014). Variation in the obesity risk gene FTO determines the postprandial cerebral processing of food stimuli in the prefrontal cortex. Molecular Metabolism, 3(2), 109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel J.K., Johansson S., Sonestedt E., Jonsson A., Lie R.T., Platou C.G., Njolstad P.R. (2011). FTO, type 2 diabetes, and weight gain throughout adult life: a meta-analysis of 41,504 subjects from the Scandinavian HUNT, MDC, and MPP studies. Diabetes, 60(5), 1637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison K., Stallings M., McGeary J., Bryan A. (2004). Population stratification in the candidate gene study: fatal threat or red herring? Psychological Bulletin, 130, 66–79. [DOI] [PubMed] [Google Scholar]
- Jacobsson J.A., Riserus U., Axelsson T., Lannfelt L., Schioth H.B., Fredriksson R. (2009). The common FTO variant rs9939609 is not associated with BMI in a longitudinal study on a cohort of Swedish men born 1920-1924. BMC Medical Genetics, 10, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jess T., Zimmermann E., Kring S.I., Berentzen T., Holst C., Toubro S., Sorensen T.I. (2008). Impact on weight dynamics and general growth of the common FTO rs9939609: a longitudinal Danish cohort study. International Journal of Obesity, 32(9), 1388–94. [DOI] [PubMed] [Google Scholar]
- Johnson P.M., Kenny P.J. (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nature Neuroscience, 13(5), 635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karra E., O'Daly O.G., Choudhury A.I., Yousseif A., Millership S., Neary M.T., Batterham R.L. (2013). A link between FTO, ghrelin, and impaired brain food-cue responsivity. The Journal of Clinical Investigation, 123(8), 3539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L., O'Doherty J., Rolls E.T., Andrews C. (2003). Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex, 13(10), 1064–71. [DOI] [PubMed] [Google Scholar]
- Kuhn A.B., Feis D.L., Schilbach L., Kracht L., Hess M.E., Mauer J., Tittgemeyer M. (2016). FTO gene variant modulates the neural correlates of visual food perception. NeuroImage, 128, 21–31. [DOI] [PubMed] [Google Scholar]
- van der Laan L.N., de Ridder D.T., Viergever M.A., Smeets P.A. (2011). The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. NeuroImage, 55(1), 296–303. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–9. [DOI] [PubMed] [Google Scholar]
- van Meer F., van der Laan L.N., Adan R.A., Viergever M.A., Smeets P.A. (2015). What you see is what you eat: an ALE meta-analysis of the neural correlates of food viewing in children and adolescents. NeuroImage, 104, 35–43. [DOI] [PubMed] [Google Scholar]
- Mei H., Chen W., Srinivasan S.R., Jiang F., Schork N., Murray S., Berenson G.S. (2010). FTO influences on longitudinal BMI over childhood and adulthood and modulation on relationship between birth weight and longitudinal BMI. Human Genetics, 128(6), 589–96. [DOI] [PubMed] [Google Scholar]
- O'Doherty J.P., Deichmann R., Critchley H.D., Dolan R.J. (2002). Neural responses during anticipation of a primary taste reward. Neuron, 33(5), 815–26. [DOI] [PubMed] [Google Scholar]
- Orvaschel H. (1994). Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SADS-E) 5, Florida: Nova Southeastern University, Center for Psychological Studies. [Google Scholar]
- Rapuano K.M., Zieselman A.L., Kelley W.M., Sargent J.D., Heatherton T.F., Gilbert-Diamond D. (2017). Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proceedings of the National Academy of Sciences of the United States of America, 114(1), 160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevgi M., Rigoux L., Kuhn A.B., Mauer J., Schilbach L., Hess M.E., Tittgemeyer M. (2015). An obesity-predisposing variant of the FTO gene regulates D2R-dependent reward learning. The Journal of Neuroscience, 35(36), 12584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siep N., Roefs A., Roebroeck A., Havermans R., Bonte M.L., Jansen A. (2009). Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behavioural Brain Research, 198(1), 149–58. [DOI] [PubMed] [Google Scholar]
- Singer J.D., Manning B.M., Formosa T. (1996). Coordinating DNA replication to produce one copy of the genome requires genes that act in ubiquitin metabolism. Molecular and Cellular Biology, 16(4), 1356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S., Van Cleef A., Davis J.F. (2017). Patterned feeding induces neuroendocrine, behavioral and genetic changes that promote palatable food intake. International Journal of Obesity, 41(3), 412–9. [DOI] [PubMed] [Google Scholar]
- Smeets P., Dagher A., Hare T., et al. (2019). Good practice in food-related neuroimaging. The American Journal of Clinical Nutrition, 109(3), 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Yokum S. (2016). Gain in body fat is associated with increased striatal response to palatable food cues, whereas body fat stability is associated with decreased striatal response. The Journal of Neuroscience, 36(26), 6949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Yokum S. (2018). Relation of neural response to palatable food tastes and images to future weight gain: using bootstrap sampling to examine replicability of neuroimaging findings. NeuroImage, 183, 522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Spoor S., Bohon C., Small D.M. (2008a). Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science, 322(5900), 449–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Spoor S., Bohon C., Veldhuizen M.G., Small D.M. (2008b). Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. Journal of Abnormal Psychology, 117(4), 924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Yokum S., Blum K., Bohon C. (2010a). Weight gain is associated with reduced striatal response to palatable food. The Journal of Neuroscience, 30(39), 13105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Yokum S., Bohon C., Marti N., Smolen A. (2010b). Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage, 50(4), 1618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Yokum S., Burger K.S., Epstein L.H., Small D.M. (2011). Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. The Journal of Neuroscience, 31(12), 4360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Yokum S., Burger K., Epstein L., Smolen A. (2012). Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. The Journal of Neuroscience, 32(29), 10093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Burger K.S., Yokum S. (2013). Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. The American Journal of Clinical Nutrition, 98(6), 1377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E., Burger K.S., Yokum S. (2015). Reward region responsivity predicts future weight gain and moderating effects of the TaqIA allele. The Journal of Neuroscience, 35(28), 10316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D.W., Fellows L.K., Small D.M., Dagher A. (2012). Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiology & Behavior, 106(3), 317–24. [DOI] [PubMed] [Google Scholar]
- Thanos P.K., Michaelides M., Piyis Y.K., Wang G.J., Volkow N.D. (2008). Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse, 62(1), 50–61. [DOI] [PubMed] [Google Scholar]
- Wiemerslage L., Nilsson E.K., Solstrand Dahlberg L., Ence-Eriksson F., Castillo S., Larsen A.L., Schioth H.B. (2016). An obesity-associated risk allele within the FTO gene affects human brain activity for areas important for emotion, impulse control and reward in response to food images. The European Journal of Neuroscience, 43(9), 1173–80. [DOI] [PubMed] [Google Scholar]


