Abstract
Background
Prenatal exposure to organophosphate (OP) pesticides associate with impaired neurodevelopment in humans and animal models. However, much uncertainty exists about the brain structural alterations underlying these associations. The objective of this study was to determine whether maternal OP pesticide metabolite concentrations in urine repeatedly measured during gestation are associated with brain morphology and white matter microstructure in 518 preadolescents aged 9–12 years.
Method
Data came from 518 mother—child pairs participating in the Generation R study, a population-based birth cohort from Rotterdam, the Netherlands. Maternal urine concentrations were determined for 6 dialkylphosphates (DAPs)including 3 dimethyl(DM)and 3 diethyl (DE) alkyl phosphate metabolites, collected at early, mid, and late pregnancy. At child’s age 9–12 years, magnetic resonance imaging was performed to obtain T1-weighted images for regional brain volumes and surface-based cortical thickness and surface area, and diffusion tensor images to measure white matter microstructure through fractional anisotropy (FA) and mean diffusivity (MD). Linear regression models were fit for the averaged prenatal exposure across pregnancy.
Results
DE and DM metabolite concentrations were not associated with regional brain volumes, cortical thickness, and cortical surface area. However, a 10-fold increase in averaged DM metabolite concentrations across pregnancy was associated with lower FA (B=−1.00, 95%CI=−1.80, −0.20) and higher MD (B=0.13, 95%CI=0.04, 0.21). Similar associations were observed for DE concentrations.
Conclusions
This study provides the first evidence that OP pesticides may alter normal white matter microstructure in children, which could have consequences for normal neurodevelopment. No associations were observed with structural brain morphology, including regional brain volumes, cortical thickness, or cortical surface area.
Keywords: Organophosphate pesticides, prenatal exposure, MRI, brain structural alterations, neurodevelopment
1. Introduction
Organophosphate (OP) pesticides are chemical agents often used in agriculture to protect crops against insects. At present, five billion pounds of pesticides are being applied worldwide and approximately 33% are OP pesticides (Mahajan et al. 2019). Similarly, between 1998 and 2008 one third of the insecticides used in the Netherlands were OP pesticides (CBS 2020). In the past decade, the use of OP pesticides has been declining in both the Netherlands and European Union (EU) due to stricter legislations. However, several OP pesticides such as malathion are currently approved by the EU and OP pesticide residues are frequently detected on tested vegetables and fruits coming from importation (ChemKap 2017; EU Pesticides database 2020).
Since OP pesticide residues may persist on or in food after crop harvesting (Eaton et al. 2008), there is an increasing concern about their potential harmful health effects. The exposure to OP pesticides generally occurs through the consumption of food (Lu et al. 2008). However, residential exposure can also occur through the use of insecticides in and around the house or by living in close proximity to agricultural lands were OP pesticides are being applied (Fenske et al. 2002; Julien et al. 2007; Lu et al. 2000; Lu et al. 2004; Valcke et al. 2006; Whyatt et al. 2003).
It is well established that the exposure to high concentrations of OP pesticides is neurotoxic to both humans and animals (Costa et al. 2008; Eaton et al. 2008). However, evidence exist that OP pesticide exposure at fairly low-dose levels may also have a negative health effect (Savy et al. 2018; Slotkin et al. 2008). OP pesticides are able to pass the placental and the blood-brain barrier (Bradman et al. 2003) and, during gestation, the development of the human brain is especially susceptible to neurotoxic effects (Rice and Barone 2000). Therefore, pregnancy exposure to low-dose levels of OP pesticides might affect fetal normal brain development.
Although several epidemiological studies have reported associations between pregnancy OP pesticide exposure and offspring’s neuropsychological development (Sapbamrer and Hongsibsong 2019), much uncertainty exists about the brain structural alterations underlying these associations. Magnetic resonance imaging (MRI) is a useful instrument for addressing these knowledge gaps and can help identify the associations between neurotoxic exposures and brain development (Rauh and Margolis 2016).In humans, altered brain morphology and white matter microstructure is associated with impaired cognition, behavior problems, and neurodevelopmental disorders (Dennis and Thompson 2013; Gilmore et al. 2018; Lebel and Deoni 2018; Mizuno et al. 2019). So far, only few animal studies and one small epidemiological study have investigated the relation of OP pesticide exposure on morphological brain measures. Experimental animal studies showed that OP pesticide exposure was associated with smaller brain volume, both thinning and thickening of the cortex, and alterations of white matter microstructure (Mullins et al. 2015; Roy et al. 2004; Roy et al. 2005). In humans, prenatal exposure to the OP pesticide chlorpyrifos measured in cord blood was associated with thinner cortices and alterations in cortical surface area in 40 children at 6–11 years old (Rauh et al. 2012). However, this previous human study only analyzed a specific OP pesticide, was restricted to a small sample size, and was unable to investigate the exposure across the entire pregnancy. Moreover, no previous epidemiological study investigated the association between prenatal OP pesticide exposure and white matter microstructure, which has been observed in a previous animal study (Mullins et al. 2015).
Therefore, the objective of this study was to determine whether maternal OP pesticide metabolite concentrations in urine repeatedly measured during gestation are associated with brain morphology and white matter microstructure in 518 preadolescents aged 9–12 years. Understanding the association between prenatal OP pesticide exposure and brain morphology and white matter microstructure may help explain the association between pregnancy OP pesticide exposure and offspring’s neuropsychological development observed in previous studies. Further, findings of the present study may assist in future policies regarding the regulation of OP pesticide application.
2. Materials and Methods
2.1. Study population and follow-up
This research was embedded in the Generation R Study, a population-based cohort from early fetal life onwards in Rotterdam, the Netherlands, which has been described in detail previously (Kooijman et al. 2016). Figure S1 presents a flowchart of this study. Briefly, all pregnant women who lived in the study area in Rotterdam, the Netherlands and were expected to have a delivery between 2002 and 2006 were eligible. A total of 8879 women were enrolled during pregnancy. A random sample of 800 mother-child pairs were selected for assessment of OP pesticide metabolites among the 1449 that provided three spot urine samples during pregnancy and had child’s neurodevelopmental data at postnatal visits. Of those, 518 children were included in the present study as they had good quality data on MRI measurements at 9–12 years old. Human subjects review for the procedure of this study was carried out and approved by the Medical Ethics Committee of the Erasmus Medical Center, Rotterdam (IRB Registration no.: IRB00001482, MEC-2012–165, MEC-2007–413, MEC, 217.595/2002/202, and MEC 198.782.2001.31). Written informed consent for the children and mothers was provided by the mothers.
2.2. Urine collection and analysis of OP pesticide metabolites
A more detailed description of urine specimen collection and measurement of OP pesticide metabolites have been published previously (van den Dries et al. 2018) and can be found in the supplement (Methods S1). Briefly, 6 non-specific urinary dialkylphosphate (DAP) metabolites of OP pesticides were measured from urine samples collected at <18, 18–25, and >25 weeks of gestation by gas chromatography coupled with tandem mass spectrometry (GC—MS/MS). These include 3 diethyl alkyl phosphate (DE) and 3 dimethyl alkyl phosphate (DM) metabolites. Creatinine concentrations were also measured in order to correct for urinary dilution. All urine samples had detectable concentrations of most metabolites. The intraclass correlation for DAP metabolite concentrations was weak for a single concentration (0.22–0.26) and moderate for the average of the 3 concentrations (0.51–0.54)(van den Dries et al. 2019).
2.3. Magnetic Resonance Imaging
Details of the neuroimaging acquisition and processing can be found in the supplemental material (Methods 2). The global brain metrics derived from T1-weighted images included total brain volume, cerebral and cerebellar white and grey matter volume, and subcortical grey matter volume. Additionally, we focused on the corpus callosum and the subcortical regions: amygdala, caudate nucleus, hippocampus, pallidum, putamen, nucleus accumbens and the thalamus (Muetzel et al. 2019). Surface-based thickness and surface area maps were made of the cerebral cortex (Muetzel et al. 2019). Diffusion tensor imaging (DTI) images were used to fit diffusion tensors at each voxel and fractional anisotropy (FA) and mean diffusivity (MD) were computed (Cook et al. 2006). Twelve major white matter tracts were identified via probalistic tractography with the FSL plugin AutoPtx (de Groot et al. 2015; Muetzel et al. 2017). These included the forceps minor and major, and the bilateral tracts of the cingulum bundle, corticospinal tract, the inferior/superior longitudinal fasciculi and the uncinate fasciculus. The mean FA and MD per tract, weighted by connectivity distribution, were then computed. A confirmatory factor analysis was performed to model a single latent FA and MD measures across the 12 tracts, which represented global FA and MD across the brain (Muetzel et al. 2015). Global FA indicates the tendency for preferential water diffusion in white matter tracts. A lower FA score indicates in general that the comprising axons are less densely packed and the directionality of the water diffusion is not uniformly directed as compared with well-organized tracts. Global MD describes the magnitude of average water diffusion in all directions within brain tissue, with higher values generally occurring in white matter tracts that show a less well-organized structure (Alexander et al. 2007).
2.4. Potential confounders
Potential adjustment variables were selected a priori defined as the minimal sufficient adjustment set with a Directed Acyclic Graph (DAG) using the Dagitty software (Textor et al. 2017). The DAG was based on previous studies of prenatal OP pesticide exposure and neurodevelopment and on biologically plausible covariate—exposure and covariate—outcome associations observed in our data (see Figure S2). We further included adjustment variables that are ancestor of the exposure and ancestor of the outcome to increase precision. The adjustment variables were household income (less than 1200 euro/month (i.e., less than the social security level of the Netherlands), 1200 – 2000 euro/month, more than 2000 euro/month), highest achieved level of education (low (less than 3 years of high school), intermediate (3 or more years of secondary education), and high (university degree or higher vocational training)), ethnical background (Dutch, other Western, and non-Western), maternal age at enrolment, marital status (married/ living with partner versus single), parity (0, 1, or 2 or more children), smoking habits during pregnancy (none, only until pregnancy known, or continued after pregnancy known), and gestational alcohol use (none, only until pregnancy known, continued infrequently (<1 glass/week) or continued regularly ((≥1 glass/week), pre-pregnancy body mass index (BMI) (kg/m2), maternal IQ (assessed when the mother-child pairs visited the research center for the 6-year examination and measured by using the computerized Ravens Advanced Progressive Matrices Test, set I (Prieler 2003)), child sex, and the child age at the MRI scan.
2.5. Statistical methods
Total DE (nmol/l) was defined as the sum of diethylphosphate, diethylthiophosphate, and diethyldithiophosphate metabolite concentrations. Total DM (nmol/l) was created by summing dimethylphosphate, dimethylthiophosphate, and dimethyldithiophosphate metabolite concentrations. Total DAP (nmol/l) was created by summing the 6 metabolites. These concentrations were creatinine adjusted (nmol/g creatinine) and transformed using a log transformation (base 10) to improve linearity of the dose-response relation and model fit. Few concentrations were missing because of an inadequate sample or machine error. We therefore imputed missing concentrations (<1%) and missing confounder information 10 times by using the Multivariate Imputation by Chained Equations (MICE) package in R (R core Team 2015; van Buuren and Groothuis-Oudshoorn 2011). We included total brain volume and global FA in the imputation procedure as predictors, but we did not impute brain measures.
As a first step, we applied linear regression to examine the association of averaged DE, DM, and DAP concentrations over pregnancy with brain volumes. To account for multiple testing (14 tests for each exposure), we applied the false discovery rate correction. As a second step, we investigated metabolite concentrations — brain volume associations for each exposure time point (early, mid, and late pregnancy) separately. We performed this second step for the identification of possible periods of susceptibility and to have the ability to compare our findings with studies that only used one urine sample during gestation to measure the exposure to OP pesticides. These analyses were also corrected for multiple testing using the FDR correction. The same multiple linear regressions were applied for the DAP — global white matter tract (FA and MD) associations. Post-hoc analyses were run on the 12 major individual white matter tracts when the analysis yielded a significant association between prenatal DAP metabolite concentrations and global white matter tracts. We explored whole-brain vertex-wise statistics using the QDECR R package (https://qdecr.com) for total DE, DM, and DAP metabolite concentrations in association with local cortical thickness and cortical surface area. Vertex-wise analyses were corrected for multiple testing by the application of Gaussian Monte Carlo null-Z simulations (The cluster-forming threshold defined as p < 0001). Next. these analyses were also corrected by applying a Bonferroni adjustment for the analyses of both hemispheres.
All models were adjusted for potential confounders described above. Additionally, we adjusted models of subcortical and cerebellar volumes for intracranial volume to ascertain relativity to head size. Models of the other volumes were not adjusted for intracranial volume as they were highly correlated (between r = 0.81 and r = 0.93).
As sensitivity analyses, first, we investigated potential effect modification by sex via interaction terms (P-value for interaction<0.05) to compare our results with previous studies who observed sex specific effects (Rauh et al. 2012; Sagiv et al. 2019). Second, we applied inverse probability weighting to adjust all models for loss to follow-up and to deal with potential selection bias because participants included in this study were older, had higher educational level, and more frequently Dutch as compared to the complete Generation R Study cohort (van den Dries et al. 2018). Third, because diet and the intake of healthy nutrients may confound the association between prenatal OP pesticide exposure (e.g., residues on fruits) (van den Dries et al. 2018) and brain development (e.g., healthy nutrients) (Figure S2), we performed a sensitivity analyses in which we additionally adjusted for maternal fruit and vegetables intake. The consumption of fruit and vegetables was assessed in the first trimester using a modified version of a validated food frequency questionnaire and was adjusted for energy intake (Steenweg-de Graaff et al. 2012).
3. Results
3.1. Descriptive analysis
The median age of the mothers at enrolment was 31.2 years (IQR=5.4) and the median age of the child at MRI assessment was 9.8 years (IQR=0.3) (Table 1). The majority of mothers participating in this study were ethnically Dutch (61.4%), were nulliparous (66.4%), were none smokers (79.1%), had a high educational level (60.2%), and had a high income (73.6%). Total DAP metabolite concentrations comprised mostly DM metabolite concentrations (Table 2). The median nmol/g creatinine concentrations were comparable across the three sampling periods. The median total brain volume was 1209 cm3 (IQR=140 cm3) and median FA was 0.0 (IQR=2.3) (Table S1).
Table 1.
Demographic and lifestyle characteristics of 518 mother-child pairs from the Generation R study population.
| Median (25th, 75th percentile) or % | |
|---|---|
| Maternal characteristics | |
| Age | 31.2 (28.6, 43.0) |
| Missing | - |
| Ethnicity | |
| Dutch | 61.4% |
| Other western | 13.1% |
| Non-western | 25.5% |
| Missing, N | - |
| Educational level | |
| Low | 11.2% |
| Intermediate | 28.6% |
| High | 60.2% |
| Missing, N | 11 |
| Income | |
| <1,200 | 11.6% |
| 1,200–2,00 | 14.8% |
| >2,000 | 73.6% |
| Missing, N | 52 |
| Non-verbal IQ | 100.0 (90.0, 107.0) |
| Missing, N | 7 |
| Body mass index | 23.0 (21.2, 25.9) |
| Missing, N | 2 |
| Parity | |
| 0 | 66.3% |
| 1 | 24.2% |
| >=2 | 9.5% |
| Missing, N | 2 |
| Smoking use during pregnancy | |
| No smoking during pregnancy | 79.1% |
| Until pregnancy recognized | 8.8% |
| Continued during pregnancy | 12.1% |
| missing | 40 |
| Alcohol consumption during pregnancy | |
| No consumption during pregnancy | 33.2% |
| Until pregnancy recognized | 17.7% |
| Continued occasionally | 42.1% |
| Continued frequently | 7.0% |
| Missing, N | 21 |
| Child characteristics | |
| Child age at assessment | 9.8 (9.6, 9.9) |
| Missing, N | - |
| Child sex | |
| Male | 49.4% |
| Female | 50.6% |
| Missing, N | - |
Table 2.
Descriptive statistics of pregnancy DAP metabolite concentrations (N=518)
| nmol/g creatinine | nmol/l | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| min | p25 | p50 | p75 | max | min | p25 | p50 | p75 | max | |
| DM metabolites in nmol/g creatinine * | ||||||||||
| < 18 weeks | 6.6 | 153.6 | 255.3 | 420.4 | 6106.5 | 0.9 | 96.1 | 183.4 | 346.7 | 2627.3 |
| 18 – 25 weeks | 24.8 | 184.2 | 272.1 | 433.6 | 2444.1 | 7.6 | 99.6 | 190.8 | 336.4 | 2396.8 |
| > 25 weeks | 29.2 | 165.8 | 248.9 | 397.6 | 2857.8 | 10.5 | 103.9 | 194.2 | 326.6 | 3300.5 |
| Averaged | 26.3 | 191.3 | 269.3 | 361.8 | 1381.0 | 14.7 | 118.5 | 179.6 | 289.4 | 1105.1 |
| DE metabolites in nmol/g creatinine † | ||||||||||
| < 18 weeks | 0.0 | 25.3 | 46.4 | 86.3 | 3030.5 | 0.0 | 16.0 | 31.3 | 66.2 | 6818.6 |
| 18 – 25 weeks | 3.3 | 25.2 | 43.4 | 79.6 | 624.3 | 0.6 | 13.9 | 30.1 | 58.4 | 1093.4 |
| > 25 weeks | 4.1 | 22.0 | 43.9 | 81.5 | 671.4 | 1.1 | 14.7 | 31.5 | 64.4 | 538.2 |
| Averaged | 2.6 | 29.4 | 44.3 | 68.9 | 601.4 | 3.2 | 19.3 | 30.7 | 49.8 | 407.3 |
| DAP metabolites in nmol/g creatinine ‡ | ||||||||||
| < 18 weeks | 15.4 | 197.3 | 321.4 | 521.7 | 6444.5 | 6.3 | 119.6 | 224.7 | 422.2 | 7798.7 |
| 18 – 25 weeks | 41.0 | 222.2 | 323.0 | 519.8 | 2817.0 | 10.0 | 123.2 | 235.9 | 406.1 | 3056.7 |
| > 25 weeks | 42.1 | 204.1 | 308.0 | 495.7 | 3003.1 | 15.2 | 127.2 | 228.8 | 403.4 | 3332.6 |
| Averaged | 36.7 | 234.6 | 329.9 | 441.2 | 1818.8 | 30.2 | 144.4 | 220.7 | 348.9 | 1259.3 |
Abbreviations: Min=minimum, p25=25th percentile, P50=median (50th percentile), p75=75th percentile, max=maximum, DM= Dimethyl alkyl phosphates, DE= Diethyl alkyl phosphates, DAP= Total dialkyl phosphates.
DM is the sum of dimethylphosphate (DMP), dimethylthiophosphate (DMTP), and dimethyldithiophosphate (DMDTP).
DE is the sum of diethylphosphate (DEP), diethylthiophosphate (DETP), and diethyldithiophosphate (DEDTP).
DAP is the sum of DEDTP, DETP, DEP, DMDTP, DMTP and DMP.
3.2. OP pesticide metabolite concentrations and brain volume
No associations were observed between averaged maternal DE and DM metabolite concentrations and all brain volumes (Table 3). When specific pregnancy periods were analyzed separately, higher DE and DM metabolite concentrations at >25 weeks of gestation were associated with lower thalamus volume, higher DM metabolite concentration at 18–25 weeks of gestation was associated with higher putamen volume, and higher DE metabolite concentrations at >25 weeks of gestation were associated with lower cerebellum cortex volume (Table S2). However, these associations did not remain after correction for multiple testing. The results for the total DAP metabolite concentrations were similar to the results observed for the DE and DM metabolite concentrations (Table S3).
Table 3.
Adjusted* association between averaged log10 transformed maternal concentrations of DM† and DE‡ metabolite concentrations in nmol/g creatinine and brain volumes (N=441) and white matter microstructure (N=474) assessed at child age 10 years.
| Averaged DM metabolite concentrations in nmol/g creatinine | Averaged DE metabolite concentrations in nmol/g creatinine | |||||||
|---|---|---|---|---|---|---|---|---|
| Brain volumes | B | 95%CI | B | 95%CI | ||||
| Total brain | 12.81 | −26.31 | to | 51.92 | 0.22 | −28.98 | to | 29.42 |
| Total gray | 4.69 | −18.75 | to | 28.13 | −3.38 | −20.88 | to | 14.12 |
| Subcortical gray matter | 0.26 | −1.53 | to | 2.04 | −0.69 | −2.03 | to | 0.65 |
| Cerebral white matter | 8.16 | −9.57 | to | 25.90 | 4.27 | −8.98 | to | 17.53 |
| Thalamus§ | −0.36 | −0.74 | to | 0.02 | −0.18 | −0.47 | to | 0.11 |
| Caudate§ | 0.18 | −0.18 | to | 0.55 | −0.11 | −0.38 | to | 0.16 |
| Putamen§ | 0.42 | 0.00 | to | 0.85 | −0.01 | −0.33 | to | 0.31 |
| Pallidum§ | 0.05 | −0.10 | to | 0.20 | −0.05 | −0.16 | to | 0.06 |
| Hippocampus§ | −0.10 | −0.35 | to | 0.16 | 0.02 | −0.17 | to | 0.21 |
| Amygdala§ | 0.05 | −0.08 | to | 0.18 | 0.05 | −0.05 | to | 0.15 |
| Nucleus accumbens§ | 0.00 | −0.07 | to | 0.07 | 0.00 | −0.05 | to | 0.06 |
| Cerebellum cortex§ | −2.68 | −6.51 | to | 1.15 | −2.30 | −5.16 | to | 0.57 |
| Cerebellar white matter§ | −0.08 | −1.08 | to | 0.91 | −0.46 | −1.20 | to | 0.28 |
| Corpus callosum§ | −0.06 | −0.27 | to | 0.16 | −0.01 | −0.17 | to | 0.15 |
| White matter microstructure | B | 95%CI | B | 95%CI | ||||
| Global FA | −1.00 | −1.80 | to | −0.20 | −0.63 | −1.24 | to | −0.02 |
| Global MD | 0.13 | 0.04 | to | 0.21 | 0.06 | 0.00 | to | 0.13 |
Abbreviations: DM= Dimethyl alkyl phosphates, DE= Diethyl alkyl phosphates, FA= fractionalanisotropy, MD= mean diffusivity
Adjusted for age of child during the magnetic resonance imaging assessment, sex of child, and maternal age, ethnicity (Dutch, other-western and non-western), education (low, intermediate and high), income (low, middle and high), marital status, alcohol consumption during pregnancy (no alcohol consumption during pregnancy, alcohol consumption until pregnancy was known, occasionally alcohol consumption during pregnancy and frequently alcohol consumption during pregnancy), body mass index, parity (0, 1, 2+), and smoking use during pregnancy (no smoking during pregnancy, smoked until pregnancy wasknown, smoked during pregnancy).
DM is the sum of dimethylphosphate, dimethylthiophosphate, and dimethyldithiophosphate.
DE is the sum of diethylphosphate, diethylthiophosphate, and diethyldithiophosphate.
Additionally adjusted for intracranial volume
3.3. OP pesticide metabolite concentrations and white matter microstructure
Table 3 presents the association between averaged DM and DE metabolite concentrations and white matter microstructure. We observed an association between a 10-fold increase in averaged DM and DE metabolite concentrations and lower FA (B=−1.00 (95%CI: −1.80, −0.20) and B=−0.63 (95%CI: −1.24, −0.02), respectively). Next, a 10-fold increase in averaged DM and DE metabolite concentrations were associated with higher MD (B=0.13 (95%CI: 0.04, 0.21) and B=0.06 (95%CI: 0.00, 0.13), respectively). Regarding the specific pregnancy periods, we observed similar associations at DM and DE concentrations at <18 weeks and at 18–25 weeks of gestation (Table S3). The associations between maternal DAP metabolite concentrations and white matter microstructure were comparable to the results of DE and DM metabolite concentrations (Table S3).
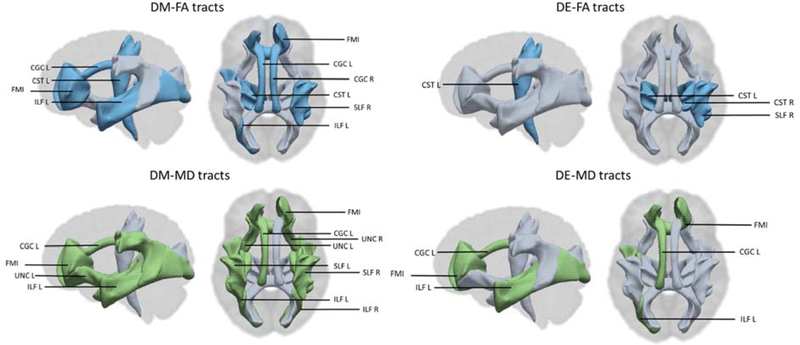
Regarding the individual 12 major white matter tracts, we observed that DM metabolite concentrations averaged across pregnancy were associated with lower FA and higher MD in most of the tracts except for the uncinate fasciculus tract of left hemisphere, the forceps major, and the corticospinal tract of the right hemisphere (Figure 1 and Table S4). We observed that higher averaged DE metabolite concentrations were associated with lower FA in the superior longitudinal fasciculus tract and the corticospinal tracts, and with higher MD in the cingulate gyrus of the cingulum tract of the left hemisphere, the forceps minor tract, and the inferior longitudinal fasciculus tract of the left hemisphere (Figure 1 and Table S5). The results of the total DAP metabolite concentrations were also almost identical to the results observed for the DM and DE metabolite concentrations together (Table S6).
Figure 1. The white matter tracts of global fractional anisotropy (FA) and global mean diffusivity (MD) that are associated (blue= negative, green = positive) with maternal dimethyl (DM) and diethyl (DE) alkyl phosphate metabolite concentrations in nmol/g creatinine.
CGC= Cingulum bundle, CST= corticospinal tract, ILF= inferior longitudinal fasciculus, SLF= superior longitudinal fasciculus, UNC=uncinated fasciculus, FMI= forceps minor, L=left hemisphere, R= right hemisphere
3.4. OP pesticide metabolite concentrations and cortical surface area and cortical thickness
We did not find any evidence of an association between prenatal DM, DE, or DAP metabolite concentrations and cortical thickness and surface area using whole-brain vertex-wise analyses (data not shown).
3.5. Sensitivity analyses
Effect modification by sex was not observed in the association between averaged DAP metabolite concentrations with cortical and subcortical volumes or with white matter microstructure (Table S7). When models were re-run correcting for the potential selection bias using inverse probability weighting, results were similar to the main analyses (Table S8). Of note, a 10-fold increase in averaged DAP metabolite concentrations was significantly associated with a 0.50 (95%CI=−0.89, −0.11) decrease in thalamus volume, but the result did not survive the multiple testing correction. Finally, the results in which we additionally adjusted for maternal diet (see Table S9) were similar to the main results.
4. Discussion
In this population-based study, we observed that OP pesticide metabolites concentrations measured during pregnancy were not associated with brain morphological measures including cortical brain volumes, cortical thickness, and cortical surface area in pre-adolescents aged 9–12 years. However, we showed that higher prenatal exposure to OP pesticides, in particular during early and mid-pregnancy, was associated with lower FA and higher MD, generally considered as indicators for atypical white matter microstructure integrity. When we explored the specific white matter tracts, we observed that OP pesticide exposure was associated with projection, association, limbic system, and callosal fibers.
Although prior studies have noted the importance of the use of neuroimaging tools to address existing research gaps by identifying structural neurotoxic effects of prenatal OP pesticide exposure on the brain (Rauh and Margolis 2016), only one small epidemiological study has investigated this research question (Rauh et al. 2012). This study observed that prenatal exposure to chlorpyrifos, which devolves into the DE metabolites diethylphosphate and diethylthiophosphate (Sudakin and Stone 2011), was predictive of enlargement of the cortical surface in several areas including superior and middle temporal gyrus, post-central gyrus, superior frontal gyrus, cuneus and precuneus, and gyrus rectus (Rauh et al. 2012). Furthermore, increased exposure was associated with lower cortical thickness of frontal, temporal, and parietal regions. In contrast to these findings, no evidence of an association with cortical surface area and cortical thickness was observed in our study. The inconsistency in the results may be explained by the differences in OP pesticide exposure, in exposure assessment methodology, or in study populations. Rauh et al. (2012) measured a single OP pesticide chlorpyrifos in cord blood, while in our study we measured DAP metabolites at early-, mid-, and late-pregnancy in urine as a biomarker of OP pesticide exposure. DAP metabolites provide non-specific data about the total exposure to several OP pesticides instead of the exposure to a single OP pesticide. Mothers in this study were most likely exposed to a combination of different OP pesticides that also produce DE metabolites. Of all the insecticides that were applied in 2004 in the Netherlands, 32% were OP pesticides that produces DM metabolites and only 1% were pesticides that generate DE metabolites (CBS 2020). Of the latter, the OP pesticide chlorpyrifos accounted for 1/3 of the total generated DE metabolites. This suggests that exposure to chlorpyrifos may have been lower in our population. However, between 2004 and 2006 OP pesticide residues of chlorpyrifos coming from importation have been detected on tested vegetables and fruits (ChemKap 2017). Of note, DAP metabolite concentrations in this study are higher compared to most other birth-cohort studies (van den Dries et al. 2018). Other differences between the studies relate to socio-economic status, as the population in the previous study was socially disadvantaged. It is conceivable that in these populations unmeasured background risk factors related to both chlorpyrifos exposure and brain morphology might lead to potential residual confounding.
To our knowledge, this is the first epidemiological study that investigated prenatal exposure to OP pesticides and white matter microstructure. In preadolescents aged 9–12 years, the development of many white matter tracts, such as projection of the prefrontal cortex, is still ongoing (Lebel et al. 2019). Altered maturation of white matter microstructure might therefore result in neurodevelopmental problems with long-term clinical implications. Studies have found that altered white matter microstructure is associated with impaired cognition, behavior problems, and neurodevelopmental disorders (Lebel and Deoni 2018). We observed that increased OP pesticide exposure during pregnancy was associated with lower FA and higher MD of white matter [e.g., a 10-fold increase in averaged DM metabolite concentrations was associated with 1.00 lower FA (95%CI: −1.80, −0.20)], and that the direction of the associations was consistent across most specific tracts. To help interpret these results we calculated the association of child age with white matter microstructure, as age is a robust determinant of the latter. A one-year increase in age was associated with 0.88 (95%CI= 0.35, 1.42) increase in global FA and a 0.10 (95%CI= −0.16, −0.05) decrease in global MD. This implies that, for example, a 10-fold increase in averaged DM concentrations during pregnancy has a similar effect as being 1.1 years younger in terms of white matter microstructure.
Global FA and MD are indicators of white matter microstructure (Alexander et al. 2007). FA describes the propensity for enhanced water diffusion in the white matter tracts whereas MD expresses the scale of average water diffusion in every direction within brain tissue (Alexander et al. 2007). A lower FA and higher MD can be a result of several reasons including lower packing of axons, higher membrane permeability, disturbance of internal axonal structure, and decreased myelination (Lebel et al. 2019). Animal studies also observed similar associations in white matter microstructure in relationship with exposure to OP pesticides. Prenatally chlorpyrifos exposed guinea pigs had lower FA and higher MD within the corpus callosum and the amygdala and rats postnatal (day 1 until day 6) exposed to chlorpyrifos had a decreased expression of the myelin-associated glycoprotein in the brain which is crucial for the preservation of the mature myelinated unit (Betancourt et al. 2006; Mullins et al. 2015). Moreover, chlorpyrifos exposure reduces the polymerization of tubulin (Grigoryan and Lockridge 2009). Tubulin is an protein which plays an important role in the creation of microtubules which are needed for the preservation of the structural and functional integrity of axons (Grigoryan and Lockridge 2009; Terry 2012). In our study we further found an association between OP pesticide exposure and white matter microstructure specific tracts present in projection, association, limbic system, and callosal fibers. Further work is required to confirm our observed association between prenatal OP pesticide exposure and altered white matter microstructure in children.
We observed that the first and second trimester (<18 weeks, and 18–25 weeks) OP pesticide exposure was driving the associated with lower FA and higher MD. White matter growth starts in early gestation and myelination begins in the second trimester (Dubois et al. 2014; Huang et al. 2009; Rice and Barone 2000). OP pesticide exposure has been shown to disrupt the expression of genes and proteins important for the myelination (Slotkin and Seidler 2007). During the second trimester, the development of white matter is especially dependable on signaling pathways such as extracellular ligands, secreted molecules, and transcriptional regulations (Emery 2010). Thus, OP pesticide exposure may alter the courses of later brain development by influencing axonal growth adhering and group formation and white matter myelination via gene expression alteration.
This study has several limitations. First, urinary DAP metabolite concentrations provide information regarding the joint exposure to multiple OP pesticides instead of providing specific information regarding the exact OP pesticide exposure (Duggan et al. 2003; Margariti et al. 2007; Wessels et al. 2003). It is therefore unknown to which specific OP parent pesticide(s) our study population was exposed. However, the use of DAP metabolites as biomarkers of OP pesticides is also a strength because it allows for the identification and comparison OP pesticide exposure levels within and between study populations (Bravo et al. 2004). Second, DAP metabolites are characterized by a short half-life and are excreted in urine within one or two days. This implies that the biomarker concentrations may differ from day-to-day within each subject as a consequence of variable contact with exposure sources (e.g., variable diet patterns) and result in (within-subject) temporal variability (Lu et al. 2008; Needham 2005). Although we included 3 urine spot samples which is more frequent that most other studies exploring the association between prenatal OP pesticide exposure and neurodevelopment, it would be preferable to collect more urine specimens during pregnancy to reduce the measurement error and attenuation bias caused by the within-subject variably (Perrier et al. 2016). Third, this study was restricted by the nonappearance of information on possible residential pesticide use by the participant, another household member, or a professional exterminator. Participants in this study might have been exposed through the use of residential products which may contain OP pesticides such as insecticides for the lawn and garden (e.g., emulsifiable concentrate), insecticides for house plants, residential pest products (e.g., fly control insecticides and moth killer cassettes), and flea products for pets. Although the use of products that contain OP pesticides is unlikely to confound the association between prenatal OP pesticide exposure and brain morphology, such information would be helpful in determining the exact sources of exposure. The Generation R Study is representative of an urban population of which the exposure to OP pesticides most likely occurs through diet (van den Dries et al. 2018). The results of this study may therefore not be fully generalizable to semi-urban and rural areas in the Netherlands where the source of OP pesticides exposure could be different Finally, this study was limited by the absence of information on exposure to other types of pesticides. While we included many possible confounders in our analyses, we cannot eliminate the existence of potential residual confounding in this study as a consequence of unidentified background risk factors that are predictive of OP pesticide exposure and brain development.
The present study has a number of strengths. This study has a large sample size and the availability of potential confounders such as the IQ of the mother and socioeconomic factors. Further, the scanning procedure in which all brains were scanned using the same MRI scanner and software to reduce potential measurement error is another strength.
In conclusion, prenatal OP pesticide exposure was not associated with cortical brain volume, cortical thickness, and cortical surface area in preadolescents aged 9–12 years. However, we found that prenatal OP pesticide exposure was associated with lower FA and higher MD of white matter, and that early and mid-pregnancy were particularly sensitive windows of vulnerability of OP pesticide exposure. These findings suggest that prenatal exposure to worldwide commonly applied OP pesticides may alter normal white matter microstructure development in children, which could have consequences for normal neurodevelopment. Besides structural brain changes, functional brain alteration may also provide opportunities to deepen the understanding of the effects of prenatal exposure to OP pesticides on the brain, as a recent study has done (Sagiv et al. 2019). Future longitudinal studies on brain imaging are warranted to reproduce our findings, as well as to investigate the mediating role of the structural and functional brain alterations in the association between prenatal exposure to OP pesticides and neuropsychological development. If the findings of his study are confirmed, public health policies that aim towards stricter regulation and control of OP pesticides application should be implemented both in Europe and worldwide.
Supplementary Material
Highlights.
We explored whether prenatal exposure to OP pesticides is associated brain morphology and white matter microstructure in 518 preadolescents
Maternal urinary concentrations of 6 DAP metabolites were measured during early, mid, and late pregnancy
MRI was performed to obtain brain volumes, cortical thickness, surface area, and DTI images to measure white matter microstructure
Higher prenatal exposure to OP pesticides was associated with lower FA and higher MD, indicating atypical white matter microstructure integrity
Acknowledgement
We would like to acknowledge all the partners involved in the Generation R Study which includes the Erasmus Medical Center, Rotterdam, the Faculty of Social Sciences of the Erasmus University Rotterdam, Rotterdam Homecare Foundation and Stichting Trombosedienst & Artsenlaboratorium Rijnmond. Further, the authors sincerely appreciate Dr. Frank Pierik effort for arranging the basis for the NIEHS-TNO-Erasmus University partnership.
Funding: This research received financial support from the intramural research program of the National Institute of Environmental Health Sciences, National Institutes of Health (Grant# HHSN273201500003C). The general design of the Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam; the Erasmus University Rotterdam; Netherlands Organization for Health Research and Development (ZonMw); the Netherlands Organization for Scientific Research (NWO); and the Ministry of Health, Welfare and Sport. Neuroimaging was supported by the ZonMw TOP project no. 91211021, and super computing computations for imaging processing were supported by the NWO Physical Sciences Division (Exacte Wetenschappen) and SURFsara (Cartesius compute cluster, https://www.surf.nl). Henning Tiemeier was supported by a grant of the Netherlands Organization for Scientific Research (NWO grant No. 024.001.003, Consortium on Individual Development, and NWO/ZonMW grant 016.VICI.170.200). Mònica Guxens is funded by a Miguel Servet fellowship (MS13/00054, CPII18/00018) awarded by the Spanish Institute of Health Carlos III. Monica Guxens acknowledges support from the Spanish Ministry of Science and Innovation and State Research Agency through the “Centro de Excelencia Severo Ochoa 2019–2023” Program (CEX2018-000806-S), and support from the Generalitat de Catalunya through the CERCA Programme. Dr. Hanan El Marroun was supported by Stichting Volksbond Rotterdam, the Dutch Brain Foundation (De Hersenstichting, project number GH2016.2.01) and the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 733206 LifeCycle). Funders for this research had no participation in the design and conduct of the study.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and consent to participate: Human subjects review for the procedure of this study was carried out and approved by the Medical Ethics Committee of the Erasmus Medical Center, Rotterdam (IRB Registration no.: IRB00001482, MEC-2012-165, MEC-2007-413, MEC, 217.595/2002/202, and MEC 198.782.2001.31). Written informed consent for the children and mothers was provided by the mothers.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander AL, Lee JE, Lazar M, Field AS. 2007. Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt AM, Burgess SC, Carr RL. 2006. Effect of developmental exposure to chlorpyrifos on the expression of neurotrophin growth factors and cell-specific markers in neonatal rat brain. Toxicological Sciences 92:500–506. [DOI] [PubMed] [Google Scholar]
- Bradman A, Barr DB, Claus Henn BG, Drumheller T, Curry C, Eskenazi B. 2003. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: A validation study. Environmental Health Perspectives 111:1779–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Caltabiano LM, Weerasekera G, Whitehead RD, Fernandez C, Needham LL, et al. 2004. Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification. 14:249. [DOI] [PubMed] [Google Scholar]
- CBS. 2020(Accessed 20 March 2020).
- ChemKap. 2017. Agricultural products quality programme chemkap.
- Cook PA, Bai Y, Nedjati-Gilani S, Seunarine KK, Hall MG, Parker GJ, et al. Camino: Open-source diffusion-mri reconstruction and processing. In: Proceedings of the 14th scientific meeting of the international society for magnetic resonance in medicine, 2006, Vol. 2759Seattle WA, USA, 2759. [Google Scholar]
- Costa LG, Giordano G, Guizzetti M, Vitalone A. 2008. Neurotoxicity of pesticides: A brief review. Front Biosci 13:1240–1249. [DOI] [PubMed] [Google Scholar]
- de Groot M, Ikram MA, Akoudad S, Krestin GP, Hofman A, van der Lugt A, et al. 2015. Tract-specific white matter degeneration in aging: The rotterdam study. Alzheimer's & Dementia 11:321–330. [DOI] [PubMed] [Google Scholar]
- Dennis EL, Thompson PM. 2013. Typical and atypical brain development: A review of neuroimaging studies. Dialogues Clin Neurosci 15:359–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Höppi PS, Hertz-Pannier L. 2014. The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. neuroscience 276:48–71. [DOI] [PubMed] [Google Scholar]
- Duggan A, Charnley G, Chen W, Chukwudebe A, Hawk R, Krieger RI, et al. 2003. Di-alkyl phosphate biomonitoring data: Assessing cumulative exposure to organophosphate pesticides. Regulatory Toxicology and Pharmacology 37:382–395. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, et al. 2008. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Critical Reviews in Toxicology 38:1–125. [DOI] [PubMed] [Google Scholar]
- Emery B. 2010. Regulation of oligodendrocyte differentiation and myelination. Science 330:779–782. [DOI] [PubMed] [Google Scholar]
- EU Pesticides database. 2020. https://eceuropaeu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN.
- Fenske RA, Lu C, Barr D, Needham L. 2002. Children's exposure to chlorpyrifos and parathion in an agricultural community in central washington state. Environmental health perspectives 110:549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Knickmeyer RC, Gao W. 2018. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci 19:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan H, Lockridge O. 2009. Nanoimages show disruption of tubulin polymerization by chlorpyrifos oxon: Implications for neurotoxicity. Toxicology and Applied Pharmacology 240:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, et al. 2009. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. Journal of Neuroscience 29:4263–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien R, Adamkiewicz G, Levy JI, Bennett D, Nishioka M, Spengler JD. 2007. Pesticide loadings of select organophosphate and pyrethroid pesticides in urban public housing. 18:167. [DOI] [PubMed] [Google Scholar]
- Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van Ijzendoorn MH, et al. 2016. The generation r study: Design and cohort update 2017. European Journal of Epidemiology 31:1243–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Deoni S. 2018. The development of brain white matter microstructure. NeuroImage 182:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Treit S, Beaulieu C. 2019. A review of diffusion mri of typical white matter development from early childhood to young adulthood. NMR in Biomedicine 32:e3778. [DOI] [PubMed] [Google Scholar]
- Lu C, Fenske RA, Simcox NJ, Kalman D. 2000. Pesticide exposure of children in an agricultural community: Evidence of household proximity to farmland and take home exposure pathways. Environmental research 84:290–302. [DOI] [PubMed] [Google Scholar]
- Lu C, Kedan G, Fisker-Andersen J, Kissel JC, Fenske RA. 2004. Multipathway organophosphorus pesticide exposures of preschool children living in agricultural and nonagricultural communities. Environmental Research 96:283–289. [DOI] [PubMed] [Google Scholar]
- Lu C, Barr DB, Pearson MA, Waller LA. 2008. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environmental Health Perspectives 116:537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan R, Chandel S, Chatterjee S. 2019. Environmental fate of organophosphate residues from agricultural soils to fresh farm produce: Microbial interventions for sustainable bioremediation strategies. In: Microbes and enzymes in soil health and bioremediation:Springer, 211–224. [Google Scholar]
- Margariti MG, Tsakalof AK, Tsatsakis AM. 2007. Analytical of biological monitoring for exposure to pesticides: Recent update. Ther Drug Monit 29:150–163. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Kagitani-Shimono K, Jung M, Makita K, Takiguchi S, Fujisawa TX, et al. 2019. Structural brain abnormalities in children and adolescents with comorbid autism spectrum disorder and attention-deficit/hyperactivity disorder. Translational Psychiatry 9:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muetzel RL, Mous SE, van der Ende J, Blanken LME, van der Lugt A, Jaddoe VWV, et al. 2015. White matter integrity and cognitive performance in school-age children: A population-based neuroimaging study. Neuroimage 119:119–128. [DOI] [PubMed] [Google Scholar]
- Muetzel RL, Blanken LME, van der Ende J, El Marroun H, Shaw P, Sudre G, et al. 2017. Tracking brain development and dimensional psychiatric symptoms in children: A longitudinal population-based neuroimaging study. American Journal of Psychiatry 175:54–62. [DOI] [PubMed] [Google Scholar]
- Muetzel RL, Mulder RH, Lamballais S, Cortes Hidalgo AP, Jansen P, Güroglu B, et al. 2019. Frequent bullying involvement and brain morphology in children. Frontiers in Psychiatry 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RJ, Xu S, Pereira EFR, Pescrille JD, Todd SW, Mamczarz J, et al. 2015. Prenatal exposure of guinea pigs to the organophosphorus pesticide chlorpyrifos disrupts the structural and functional integrity of the brain. Neurotoxicology 48:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham LL. 2005. Assessing exposure to organophosphorus pesticides by biomonitoring in epidemiologic studies of birth outcomes. Environmental Health Perspectives 113:494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C. 2016. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology (Cambridge, Mass) 27:378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieler J. 2003. Raven's advanced progressive matrices. Schufried, Mödling, Austria. [Google Scholar]
- R core Team. 2015. A language and environment for statistical computing. Vienna, austria: R Foundation for Statistical Computing. [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, et al. 2012. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proceedings of the National Academy of Sciences of the United States of America 109:7871–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Margolis AE. 2016. Research review: Environmental exposures, neurodevelopment, and child mental health — new paradigms for the study of brain and behavioral effects. Journal of Child Psychology and Psychiatry 57:775–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S. 2000. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environmental Health Perspectives 108:511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy TS, Seidler FJ, Slotkin TA. 2004. Morphologic effects of subtoxic neonatal chlorpyrifos exposure in developing rat brain: Regionally selective alterations in neurons and glia. Developmental brain research 148:197–206. [DOI] [PubMed] [Google Scholar]
- Roy TS, Sharma V, Seidler FJ, Slotkin TA. 2005. Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats. Developmental brain research 155:71–80. [DOI] [PubMed] [Google Scholar]
- Sagiv SK, Bruno JL, Baker JM, Palzes V, Kogut K, Rauch S, et al. 2019. Prenatal exposure to organophosphate pesticides and functional neuroimaging in adolescents living in proximity to pesticide application. Proceedings of the National Academy of Sciences 116:18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapbamrer R, Hongsibsong S. 2019. Effects of prenatal and postnatal exposure to organophosphate pesticides on child neurodevelopment in different age groups: A systematic review. Environmental Science and Pollution Research 26:18267–18290. [DOI] [PubMed] [Google Scholar]
- Savy CY, Fitchett AE, Blain PG, Morris CM, Judge SJ. 2018. Gene expression analysis reveals chronic low level exposure to the pesticide diazinon affects psychological disorders gene sets in the adult rat. Toxicology 393:90–101. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. 2007. Comparative developmental neurotoxicity of organophosphates in vivo: Transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain research bulletin 72:232–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. 2008. Neonatal exposure to low doses of diazinon: Long-term effects on neural cell development and acetylcholine systems. Environmental Health Perspectives 116:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenweg-de Graaff J, Roza SJ, Steegers EA, Hofman A, Verhulst FC, Jaddoe VW, et al. 2012. Maternal folate status in early pregnancy and child emotional and behavioral problems: The generation r study. Am J Clin Nutr 95:1413–1421. [DOI] [PubMed] [Google Scholar]
- Sudakin DL, Stone DL. 2011. Dialkyl phosphates as biomarkers of organophosphates: The current divide between epidemiology and clinical toxicology. Clinical Toxicology 49:771–781. [DOI] [PubMed] [Google Scholar]
- Terry AV. 2012. Functional consequences of repeated organophosphate exposure: Potential non-cholinergic mechanisms. Pharmacology & therapeutics 134:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GTH. 2017. Robust causal inference using directed acyclic graphs: The r package ‘dagitty’. International Journal of Epidemiology 45:1887–1894. [DOI] [PubMed] [Google Scholar]
- Valcke M, Samuel O, Bouchard M, Dumas P, Belleville D, Tremblay C. 2006. Biological monitoring of exposure to organophosphate pesticides in children living in peri-urban areas of the province of quebec, canada. Int Arch Occup Environ Health 79:568–577. [DOI] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn C. 2011. Mice: Multivariate imputation by chained equations in r.
- van den Dries MA, Pronk A, Guxens M, Spaan S, Voortman T, Jaddoe VW, et al. 2018. Determinants of organophosphate pesticide exposure in pregnant women: A population-based cohort study in the netherlands. International Journal of Hygiene and Environmental Health 221:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Dries MA, Guxens M, Pronk A, Spaan S, El Marroun H, Jusko TA, et al. 2019. Organophosphate pesticide metabolite concentrations in urine during pregnancy and offspring attention-deficit hyperactivity disorder and autistic traits. Environment International 131:105002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels D, Barr DB, Mendola P. 2003. Use of biomarkers to indicate exposure of children to organophosphate pesticides: Implications for a longitudinal study of children's environmental health. Environmental health perspectives 111:1939–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, et al. 2003. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environmental Health Perspectives 111:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.