Abstract
Background:
Frailty and cognitive impairment are associated with postoperative delirium but are rarely assessed preoperatively. The study was designed to test the hypothesis that preoperative screening for frailty or cognitive impairment identifies patients at risk for postoperative delirium (primary outcome).
Methods:
In this prospective cohort study, we administered frailty and cognitive screening instruments to 229 patients ≥ 70 years old presenting for elective spine surgery. Screening for frailty (5-item FRAIL scale) and cognition (Mini-Cog, Animal Verbal Fluency) were performed at the time of the preoperative evaluation. Demographic data, perioperative variables, and postoperative outcomes were gathered. Delirium was the primary outcome detected by either the Confusion Assessment Method, assessed daily from postoperative day 1 to 3 or until discharge, if patient was discharged sooner, or comprehensive chart review. Secondary outcomes were all other-cause complications, discharge not to home, and hospital length of stay.
Results:
The cohort was 75 [73 to 79 years] years of age, 124/219 (57%) were male. Many scored positive for pre-frailty (117/218; 54%), frailty (53/218; 24%), and cognitive impairment (50 – 82/219; 23–37%). Fifty-five patients (25%) developed delirium postoperatively. On multivariable analysis, frailty (scores 3 to 5) (OR 6.6; 95% CI 1.96 to 21.9; P = 0.002) versus robust (score 0) on the FRAIL scale, lower animal fluency scores (OR 1.08; 95% CI 1.01 to1.51; P = 0.036) for each point decrease in the number of animals named, and more invasive surgical procedures (OR 2.69; 95% CI 1.31 to 5.50; P = 0.007) versus less invasive procedures were associated with postoperative delirium.
Conclusions:
Screening for frailty and cognitive impairment preoperatively using the FRAIL scale and the Animal Verbal Fluency test in older elective spine surgery patients identifies those at high risk for the development of postoperative delirium.
Summary Statement:
Frailty assessed by the FRAIL scale and cognitive impairment using the Animal Verbal Fluency Test are associated with the development of postoperative delirium.
Introduction
Postoperative delirium (POD) is a common complication that afflicts 20–80% of older surgical patients.1 As such, guidelines recommend that older surgical patients undergo preoperative screening for geriatric conditions associated with POD and poor surgical outcomes.2,3 Chief among these are frailty, a geriatric syndrome often described as cumulative impairments in functional reserve, and cognitive impairment. About 10–40% of older community dwelling persons are pre-frail or frail and a similar proportion has cognitive impairment or overt dementia.4 Likewise, both conditions are common in older surgical patients and are associated with a higher risk of developing POD, other in-hospital complications, prolonged hospital length of stay, discharge not to home, hospital readmission, and mortality.5–10 Nonetheless, preoperative screening for frailty or cognitive impairment are not typically performed prior to a surgical procedure.
There are myriad reasons but one is the perception that such screening is unduly burdensome and time-consuming for a surgical setting.8,11–13 This has been addressed to some degree in the case of preoperative cognitive screening, with work by several groups, including us, demonstrating that brief instruments such as the Mini-Cog and Animal Verbal Fluency can identify patients at risk for poor surgical outcomes.10,11,14,15 Similarly, there are numerous validated tools to assess frailty, but little consensus about which are best suited to the preoperative setting. Thus far, investigations of the relationship between frailty and adverse surgical outcomes have largely relied on instruments such as the Frailty Index or the Frailty Phenotype which, are relatively time consuming and labor intensive and therefore unlikely to be widely accepted or adopted in a high throughput preoperative setting. Given the prevalence of frailty and its association with poor surgical outcomes, preoperative assessment of frailty remains an important clinical initiative.
With this in mind, we hypothesized that frailty or cognitive screening using brief tools will identify patients at high risk for POD and other complications. Hence, we designed a prospective study wherein older patients scheduled for elective spine surgery were screened preoperatively for frailty with the FRAIL scale, a validated 5-item questionnaire for predicting decline in health or mortality, and cognition with the Mini-Cog and Animal Verbal Fluency test, which we and others have previously demonstrated can stratify older surgical patients at risk of POD and other adverse outcomes.8,10,11,15–17 Our secondary aims were exploratory in nature and investigate associations between perioperative variables, including frailty and cognitive performance, with all in-hospital complications, discharge to place other than home and hospital length of stay.
Materials and Methods
The Partners Human Research Committee / Institutional Review Board approved this prospective observational cohort study (# 2016P000012) that was also registered in clinicaltrials.gov (# NCT02922634). This is a primary analysis of data. Between April 17, 2017 and October 9, 2018, study staff members recruited patients ≥ 70 years of age who were scheduled for elective spine surgery at the Brigham and Women’s Hospital and were expected to have an inpatient admission following their procedure. We selected this patient population because spine surgery is the 3rd most common surgical procedure in older persons18 and our prior work showed that nearly 20% of this surgical demographic develops delirium postoperatively.19 This type of surgery is relatively homogeneous and grouped within tiers of invasiveness. Eligible patients were identified by review of the preoperative evaluation schedule in the electronic medical record. Exclusion criteria included planned outpatient surgery; history of overt stroke or brain tumor; uncorrected vision or hearing impairment (unable to see pictures or read or hear instructions); limited use of the dominant hand (limited ability to draw); and/or inability to speak, read or understand English.
We planned to prospectively enroll a total of 229 patients in the study based on a power calculation of the number of patients required for 85% power to detect a 50% difference in POD (primary outcome) at the P = 0.05 level between patients with and without a positive cognitive or frailty screen, assuming a baseline incidence of POD of 15% and approximately a 10% loss to follow up. After obtaining written informed consent, patients were screened using the FRAIL scale to identify frailty and the Mini-Cog and Animal Verbal Fluency tests to evaluate cognitive performance in the Brigham and Women’s Hospital Weiner Center for Preoperative Evaluation on the day of the patient’s scheduled preoperative evaluation which takes place no more than 4 weeks prior to surgery.9,10 The FRAIL scale8,16 is a simple 5 point screen that measures Fatigue, Resistance (ability to climb one flight of stairs), Ambulation (ability to walk one block), Illness (greater than 5 past or current diagnoses) and weight loss (>5%). Each positive response within a domain scores 1 point, yielding a maximum score of 5. Higher scores indicate increased frailty; as described by others, we defined frail as a score of 3 or above and pre-frail as a scores of 1–2. We selected the Mini-Cog and Animal Verbal Fluency tests for cognition because they are brief, have been used previously in older surgical populations, and have been shown to be associated with the development of POD.9–11,15 The Mini-Cog is a simple and validated cognitive screening tool that includes a three-item recall of memory and a clock drawing component that is graded on a 5-point scale, where a score of 2 or less is considered probable cognitive impairment. Animal Verbal Fluency is a similarly simple and brief cognitive screening tool where the subject is asked to name as many animals as possible in 60 seconds and a score of 16 or less has previously been demonstrated to be associated with POD.11,20 For the primary analysis both Mini-Cog and Animal Verbal Fluency scores were analyzed linearly. We categorized the complexity and invasiveness of the surgical procedure according to an established 4-tier rating system: microdiscectomy is a tier 1 procedure; lumbar laminectomy, anterior cervical procedures or minimally invasive fusions are tier 2; lumbar fusion, trauma, or posterior cervical fusion procedures are tier 3; and tumor, infection, deformity, or combined anterior and posterior cervical procedures are tier 4.21 For the analysis, we grouped tiers 1 and 2 (less complex) and 3 and 4 (more complex) together as there were few patients in categories 1 or 4. Other demographic and medical information such as age, sex, body mass index, highest level of education, American Society of Anesthesiologists (ASA) functional status, Metabolic Equivalent of Task (METS), total number of medications, preoperative use of opioids, alcohol consumption, and past medical history of depression and psychiatric comorbidities were obtained from the medical record.
Incidence of POD was the primary outcome. POD was identified both by chart review using published criteria and by direct, independent assessment with the Confusion Assessment Method (CAM).10,22,23 The CAM was administered once per day on postoperative days 1 to 3, or until discharge if the patient was discharged early, by an investigator blinded to chart review information. We used both methods because they are complementary. Delirium typically waxes and wanes so it can be missed if the CAM is administered during the waning period. Conversely, chart review reflects events over an entire day but may miss hypoactive POD, the most common form.1 The secondary outcomes included all in-hospital cardiopulmonary (myocardial infarction, congestive heart failure, cardiac arrest, new onset arrhythmia, pulmonary embolism, reintubation and deep venous thrombosis), infectious (wound infections, pneumonia, sepsis and urinary tract infection), renal (acute renal injury), or cerebrovascular (stroke and transient ischemic accident) complications, discharge to place other than home and hospital length of stay.
Study data were managed using Research Electronic Data Capture (REDCap) hosted at Partners Healthcare.24
Statistical Analysis
Data were analyzed by several methods using two-tailed testing. We first evaluated data for normality and outliers, and no action was required. We performed a missing data analysis (numbers reported in the results and tables) and a complete case analysis was performed. For univariate analysis, we used Mann-Whitney U test for non-normal distributions (data reported as median [25th, 75th percentiles]) or the independent samples t test for normally distributed continuous variables (data reported as mean ± standard deviation (SD)), and Chi Square test for categorical variables (data reported as count (%)) to compare differences between POD and no POD groups. For multivariable analysis, all the covariates with P ≤ 0.1 on univariate analysis (body mass index, American Society of Anesthesiologists physical status, Metabolic Equivalent of Task, total number of medications, preoperative use of opioids, animal fluency test score, FRAIL scale score and invasiveness of the surgical procedure) were entered into a backwards stepwise logistic regression model for prediction of the primary outcome, incidence of POD. Age as a continuous variable and the Mini-Cog score were forced into the multivariable model. These variables were selected to account for possible confounding, no variables were analyzed as effect modifiers. The Hosmer-Lemeshow goodness of fit test was performed to evaluate model-fitting of the logistic multivariable model. Variables included in the model were tested for multicollinearity using the Variance Inflation Factor (VFI) and correlation matrix. We performed the same univariate statistical analysis described above for having complications other than POD, discharge to place other than home and hospital length of stay (secondary outcomes) and further used the Spearman’s rank-order correlation test and Kruskal-Wallis rank sum test as appropriate. We performed a post-hoc analysis to investigate the association between the FRAIL scale scores and the ASA physical status (both as ordinal variables) using the Spearman’s rank-order correlation test. A sensitivity model was conducted post-hoc where we forced possible confounders based on theory (to further include alcohol consumption, depression and psychiatric history) into the model with the relevant pre-screening predictors. The significance threshold was set at P < 0.05. All analyses were performed with statistical software IBM SPSS Statistics for Macintosh, Version 25.0 (Armonk, NY: IBM Corp).
Results
During the study period, the Weiner Center evaluated 439 patients aged 70 years or older who were scheduled for elective spine surgery (Figure 1). Of these, 33 were ineligible, 41 could not be approached because study personnel were occupied with another enrollment, and 3 declined participation at the front desk. Of the remaining 362 patients, 125 declined and 8 were determined to be ineligible. Of the 229 who gave informed consent, 5 asked to be un-enrolled during the study and were excluded and 5 did not have their procedure. Data from the remaining 219 patients were included in this analysis.
Figure 1:
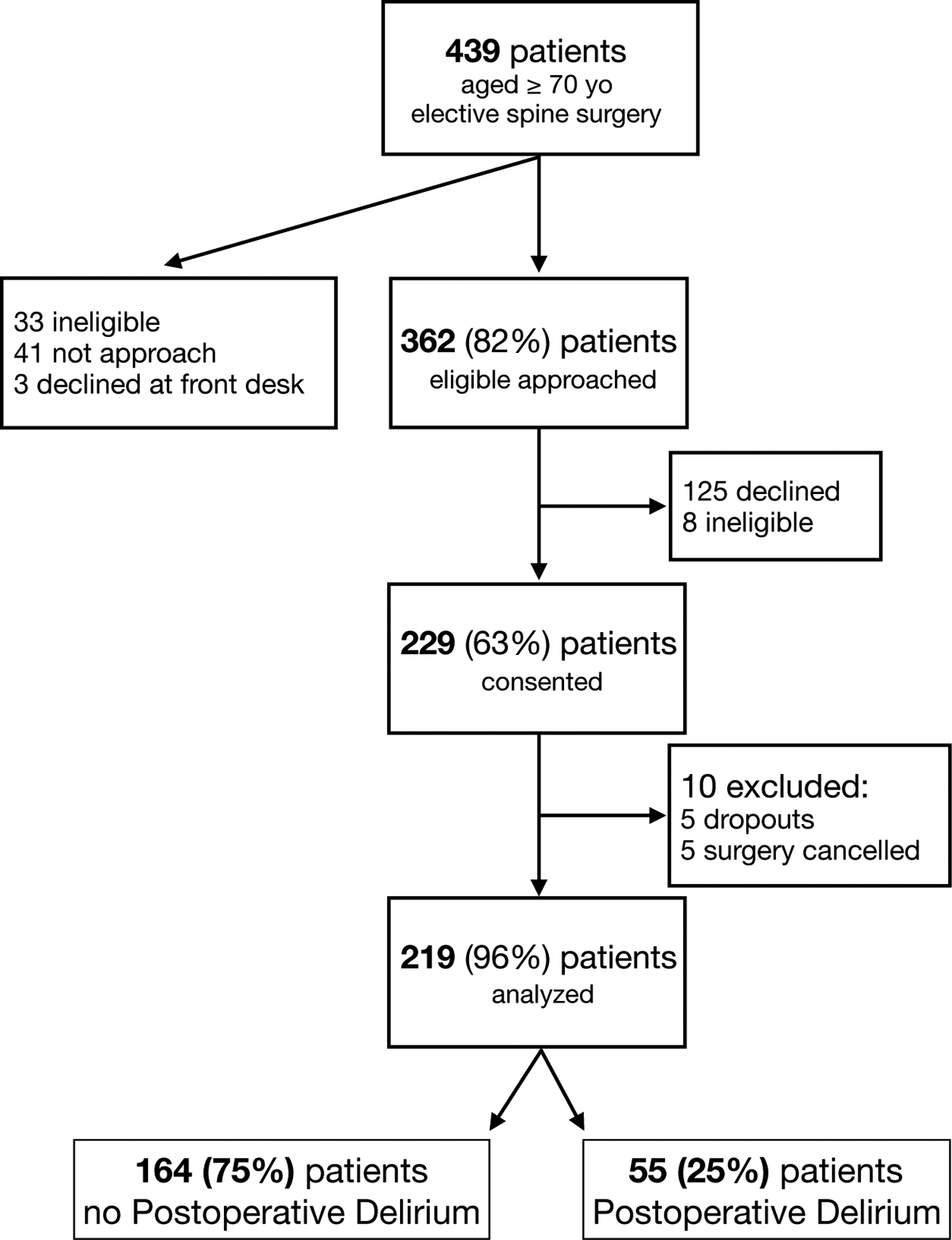
Flow diagram on recruitment, retention and postoperative delirium
As shown in Table 1, of the 219 patients, 2 (0.9%) patients had missing data for body mass index, 7 (3.2%) for highest level of education, 10 (4.6%) for METS, 26 (11.9%) for alcohol consumption, 1 (0.5%) for FRAIL scale, 1 (0.5%) for hospital length of stay and 1 (0.5%) for discharge place.
Table 1.
Baseline characteristics.
| Total = 219 | |
|---|---|
| Age, years, median [25th, 75th percentile] | 75 [73, 79] |
| Sex, n (%) | |
| Male | 124 (57) |
| Female | 95 (43) |
| Body Mass Index, Kg.m2, median [25th, 75th percentile] [N = 217] | 28 [25, 32] |
| College degree or higher, n (%) [N = 212] | 144 (66) |
| ASA physical status ≥ 3, n (%) | 149 (68) |
| METS < 4, n (%) [N = 209] | 70 (32) |
| Total number of medications, median [25th, 75th percentile] | 8 [5, 11] |
| Chronic use of opioids, n (%) | 55 (25) |
| Alcohol consumption, n (%) [N = 193] | 125 (57) |
| Depression, n (%) | 39 (18) |
| Psychiatric history, n (%) | 22 (10) |
| Mini-Cog score, median [25th, 75th percentile] | 4 [3, 5] |
| Animal Fluency Test, mean ± SD | 18 ± 6 |
| FRAIL scale, n (%) [N = 218] | |
| Score 0 (robust) | 48 (22) |
| Scores 1 and 2 (pre-frail) | 117 (54) |
| Scores 3 and 5 (frail) | 53 (24) |
| Surgical invasivenessa, n (%) | |
| Tier 1 + 2 | 111 (51) |
| Tier 3 + 4 | 108 (49) |
SD: standard deviation, ASA: American Society of Anesthesiologists, METS: Metabolic Equivalent of Task.
Surgical invasiveness: tier 1 and 2, microdiscectomy, lumbar laminectomy or anterior cervical procedures, minimally invasive fusions; tier 3 and 4, lumbar fusion, trauma, or posterior cervical fusion procedures, tumor, infection, deformity, or combined anterior and posterior cervical procedures.
The median age of the cohort was 75 years with 57% (N = 124/219) being male (Table 1) and 24% (N = 53/219) scored as frail. Based on the Mini-Cog and verbal fluency scores 23% (N = 50/219) and 37% (N = 82/219) had probable cognitive impairment, respectively. POD developed in 25% (N= 55/219) with (32/219 (58%) detected by chart review only, 2/219 (4%) detected by CAM only, and 21/219 (38%) detected by chart review and CAM). On univariate analysis, higher body mass index, ASA physical score ≥ 3, METS ≤ 4, higher number of medications, chronic use of opioids, fewer animals named on the verbal fluency test, frailty, and more invasive procedures were associated with POD (Table 2).
Table 2.
Univariate associations with postoperative delirium.
| N = 219 | Postoperative Delirium | P - value | |
|---|---|---|---|
| No = 164 (75%) | Yes = 55 (25%) | ||
| Age, years, median [25th, 75th percentile] | 75 [73, 79] | 77 [72, 80] | 0.508a |
| Sex, n (%) | 108 (67) | 36 (72) | 0.720b |
| Male | 94 (57) | 30 (55) | |
| Female | 70 (43) | 25 (46) | |
| Body Mass Index, Kg.m2, median [25th, 75th percentile] [N = 217] | 28 [25, 32] | 30 [26, 34] | 0.025a |
| College degree or higher, n (%) [N = 212] | 108 (67) | 36 (72) | 0.480b |
| ASA physical status ≥ 3, n (%) | 105 (64) | 44 (80) | 0.028b |
| METS < 4, n (%) [N = 209] | 45 (29) | 25 (49) | 0.007b |
| Total number of medications, median [25th, 75th percentile] | 8 [5, 10] | 9 [7,12] | 0.002a |
| Chronic use of opioids, n (%) | 34 (21) | 21 (38) | 0.010b |
| Alcohol consumption, n (%) [N = 193] | 98 (68) | 27 (55) | 0.101b |
| Depression, n (%) | 26 (16) | 13 (24) | 0.192b |
| Psychiatric history, n (%) | 14 (9) | 8 (15) | 0.200b |
| Mini-Cog score, median [25th, 75th percentile] | 4 [3, 5] | 4 [2, 5] | 0.333a |
| Animal Fluency Test, mean ± SD | 19 ± 5 | 17 ± 5 | 0.005c |
| FRAIL scale, n (%) [N = 218] | < 0.001b | ||
| Score 0 (robust) | 43 (26) | 5 (9) | |
| Scores 1 and 2 (pre-frail) | 92 (56) | 25 (46) | |
| Scores 3 and 5 (frail) | 29 (18) | 24 (44) | |
| Surgical invasivenessd, n (%) | 0.002b | ||
| Tier 1 + 2 | 93 (57) | 18 (33) | |
| Tier 3 + 4 | 71 (44) | 37 (67) | |
SD: standard deviation, ASA: American Society of Anesthesiologists, METS: Metabolic Equivalent of Task.
Mann-Whitney U test
Chi square test
Independent samples t test
Surgical invasiveness: tier 1 and 2, microdiscectomy, lumbar laminectomy or anterior cervical procedures, minimally invasive fusions; tier 3 and 4, lumbar fusion, trauma, or posterior cervical fusion procedures, tumor, infection, deformity, or combined anterior and posterior cervical procedures
On the multivariable model, frailty (FRAIL scale score ≥ 3) (odds ratio [OR] 6.6; 95% confidence interval [CI], 1.96 to 21.9; P = 0.002) was a strong independent predictor of POD but this was not the case for pre-frailty (FRAIL scale score 1 or 2) (OR 1.95; 95% CI, 0.60 to 6.3; P = 0.266). Naming fewer animals on the verbal fluency test was associated with increased odds of POD (OR 1.08; 95% CI, 1.01 to 1.51; P = 0.036 for each point decrease in the number of animals named). In contrast, lower scores on the Mini-Cog was not associated with increased risk for POD. Finally, more invasive surgery was associated with greater risk for POD (OR 2.69; 95% CI, 1.31 to 5.50; P = 0.007) (Table 3).
Table 3.
Variables associated with postoperative delirium on multivariable analysis.
| Postoperative Delirium | ||
|---|---|---|
| OR (95% CI) | P - value | |
| Body mass index | 1.06 (0.996; 1.14) | 0.067 |
| Animal Verbal Fluency Test | 1.08* (1.01; 1.51) | 0.036 |
| Scores 3 to 5 (frail) vs Score 0 (robust) | 6.6 (1.96; 21.9) | 0.002 |
| Scores and 2 (pre-frail) vs Score 0 (robust) | 1.95 (0.60; 6.32) | 0.266 |
| Surgical Invasiveness | 2.69 (1.31; 5.50) | 0.007 |
Hosmer and Lemeshow Test Goodness of Fit; P = 0.234
Odds Ratio as per 1 animal decrease
Variables entered in the logistic model: age, body mass index, American Society of Anesthesiologists physical status, Metabolic Equivalent of Task, total number of medications, preoperative use of opioids, Mini-Cog score, animal fluency test score, FRAIL questionnaire score and invasiveness of the surgical procedure.
All in-hospital complications other than POD (N = 219) occurred in 68 patients (31%) and, on univariate analysis, higher body mass index, ASA physical status ≥ 3, METS < 4, total number of medications, chronic use of opioids, alcohol consumption, animal verbal fluency test score frailtyand more invasive procedures were associated with other in hospital complications. (Supplementary Table 1)
Seventy-seven patients (36%) admitted from home (N= 215) were discharged to a place other than home. On univariate analysis, older age, female sex, ASA physical status ≥ 3, METS < 4, total number of medications, psychiatric history, animal verbal fluency test score, FRAIL scale score ≥ 3 and more invasive procedures were associated with discharge to other place than home. (Supplementary Table 2)
On univariate analysis, variables associated with longer hospital length of stay after surgery included higher body mass index, ASA physical status METS < 4, total number of medications, chronic use of opioids, depression, FRAIL scale scoreand more invasive surgical procedures. (Supplementary Table 3)
No in-hospital or 30-day mortality was recorded.
On the post-hoc analysis, ASA physical status was weakly correlated with the FRAIL scale scores (rs = 0.179, P = 0.008). The sensitivity model consisting of theoretically important confounders yielded consistent results:significant association between verbal fluency test (OR 1.08; 95% CI 1.002 to 1.15; P = 0.043), frailty (FRAIL scale score ≥ 3) (OR 7.1; 95% CI 1.84 to 27.2; P = 0.004) and more invasive surgical procedures (OR 3.05; 95%CI 1.42 to 6.54; P = 0.004).
Discussion
This study demonstrates that frailty is prevalent in older patients undergoing elective spine surgery and, along with cognitive impairment and the invasiveness of the surgical procedure, frailty is associated with POD. Unlike most previous work, we assessed and identified frailty using a simple, brief screening tool, the FRAIL Scale, rather than a lengthy battery and demonstrate that it is sufficient to identify patients at risk for unfavorable surgical outcomes. This is crucial in a preoperative setting, where time is limited and geriatric expertise many not be available. Furthermore, we concurrently screened patients for cognitive impairment, a common feature of frailty and a well-established independent risk factor for development of POD.3 Confirming previous work, we demonstrate that the older surgical population has a high prevalence of cognitive impairment and that poor cognition is associated with a high incidence of POD. Therefore, while both frailty and cognitive impairment are common in older elective surgical patients and predict greater risk for postoperative cognitive and medical complications, frailty appears to be the stronger risk factor of the two.
Frailty is an age-related syndrome featuring multi-organ loss of reserve and resiliency and increased vulnerability to stressors. It is common in the community, with a prevalence between 10–65% depending upon age and the tool used to assess it and over 40% are pre-frail.4,8,24 Frailty is also common in older elective surgical populations; 38–54% of those ≥ age 70 score as pre-frail and 35–41% as frail on comprehensive frailty measures.7 However, longer, detailed frailty instruments have been used in nearly all frailty studies in surgical patients which, due to time constraints, are unlikely to be widely adopted in clinical practice, and the few that used brief measures were conducted retrospectively and/or involved urgent or emergency surgery.8 There is no agreement about the optimal tool for assessing frailty and prevalence estimates vary somewhat with the criteria and instrument used. We chose the FRAIL Scale because it is brief and requires neither measurements (e.g. walking speed, grip strength) nor medically-trained personnel. The Scale has high specificity but low sensitivity, so may underestimate the prevalence of frailty. Nonetheless, it performed well in our setting as judged by the fact that the percentage of our cohort who scored as frail is similar to that reported in surgical populations by others using longer instruments and that the brief screen for frailty verified associations of this geriatric syndrome with POD and all-cause complications. Therefore, the FRAIL Scale appears to be well-suited for a high-throughput environment such as the preoperative evaluation clinic. While there is some correlation between ASA physical status and the FRAIL scale score (our post-hoc analysis suggests a weak yet statistically significant positive correlation), the ASA physical status does not predict perioperative risks, but when used with other factors such as the type of surgery, frailty scores and other markers of deconditioning it can be helpful in predicting perioperative risks.25,26 The FRAIL score measures fatigue, resistance, ambulation, illnesses and loss of weight and is very objective. In contrast, the ASA physical status is a subjective assessment of the fitness of patients before surgery and measures their medical co-morbidities.
Frail persons are often cognitively impaired as well, and poor cognition is a risk factor for development of POD and other unfavorable surgical outcomes. As such, we screened patients separately for baseline cognitive status. We used the Mini-Cog and Animal Verbal
Fluency tests because both tools are brief and easy to administer and have been shown previously to predict risk for POD in older surgical patients.10,11 Our results compare well with prior work in geriatric patients scheduled for various elective surgical procedures, with most reporting that 15–63% of patients, are cognitively impaired before surgery. In fact, the 23% incidence of probable cognitive impairment by Mini-Cog and 37% by Animal Verbal Fluency identified in this study are consistent with the findings of others. Likewise, our data show that poor preoperative cognitive status is associated with a greater prevalence of POD, a relationship identified previously using different tests and surgical populations.11,15 Here, however, contrary to our previous work and the work of others, poor preoperative cognition was associated with POD by Animal Verbal fluency but not Mini-Cog.10 The reasons for this discrepancy are not clear but may be related to several factors. Animal Verbal Fluency is scored on an unlimited-point scale whereas the Mini-Cog is scored on a 5-point scale and this may reduce the statistical power of the latter to detect differences; or perhaps because Animal Verbal Fluency has high sensitivity and low specificity as suggested by a higher incidence of cognitive impairment (37%) than the Mini-Cog (23%).
This work has a number of weaknesses. This was a single-center study of older surgical spine patients, so caution is warranted in generalizing these results to other geriatric surgical patients. In addition, nearly 35% of the patients who were eligible for the study declined participation and it is not known whether the results would be different if they had enrolled (selection bias). Our data may also underestimate the risk of developing POD after this type of surgery as more than 65% of the subjects in this study had at least a college education; this is relevant because educational attainment is associated with a lower risk of cognitive impairment and, hence, delirium. Similarly, because we administered the CAM just once per day during the first 3 postoperative days, we may have missed cases of POD. However, we complemented this evaluation with chart review, which complements the CAM and reflects the waxing and waning course of delirium throughout the day. There were other sources of potential bias during this study, such as interviewer bias or bias from misclassification of exposure and/or outcome that were acknowledged and addressed: we used a standardized protocol for data collection; investigators were trained to score the Mini-Cog and CAM prior to study enrollment; the Mini-Cog clock drawing test was scored by a second blind investigator and, in case of conflicting scores, by a third blind investigator; and CAM and chart review for POD were checked by an independent investigator. We performed a multivariable regression analysis to account for possible confounders, but the role of other unidentified variables cannot be ruled out.
In conclusion, as suggested by The American College of Surgeons and the American Geriatrics Society guidelines, we found that older patients who screen positively for preoperative frailty or cognitive impairment using brief screening tools are at increased risk of developing POD and all-cause morbidity.
Supplementary Material
Funding Statement:
National Institutes of Health AG048522 and AG055833 (DJC) and the Department of Anesthesiology, Perioperative, and Pain Medicine, Brigham and Women’s Hospital, Boston, MA
Footnotes
A subset of these data were presented at Porto Anaesthesiology International Congress – Norte da Anestesia, November 18, 2017, Porto, Portugal
Conflicts of Interest:
MJS: None
RHG: None
BR: None
JDK: None
TRS: None
YL: None
MWG: None
JHC: None
FG: None
DJC: Director of the American Board of Anesthesiology, Member ABMS Committee on Continuous Certification, ACGME – RRC ex-officio member, Executive Editor Anesthesiology, ASA committee member; Grant funding: NIA, NIGMS.
References
- 1.Inouye SK: Delirium in older persons. N Engl J Med 2006; 354: 1157–65 [DOI] [PubMed] [Google Scholar]
- 2.Chow WB, Rosenthal RA, Merkow RP, Ko CY, Esnaola NF, American College of Surgeons National Surgical Quality Improvement P, American Geriatrics S: Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg 2012; 215: 453–66 [DOI] [PubMed] [Google Scholar]
- 3.Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, Cherubini A, Jones C, Kehlet H, MacLullich A, Radtke F, Riese F, Slooter AJ, Veyckemans F, Kramer S, Neuner B, Weiss B, Spies CD: European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol 2017; 34: 192–214 [DOI] [PubMed] [Google Scholar]
- 4.Woo J, Yu R, Wong M, Yeung F, Wong M, Lum C: Frailty Screening in the Community Using the FRAIL Scale. J Am Med Dir Assoc 2015; 16: 412–9 [DOI] [PubMed] [Google Scholar]
- 5.Gleason LJ, Schmitt EM, Kosar CM, Tabloski P, Saczynski JS, Robinson T, Cooper Z, Rogers SO Jr., Jones RN, Marcantonio ER, Inouye SK: Effect of Delirium and Other Major Complications on Outcomes After Elective Surgery in Older Adults. JAMA Surg 2015; 150: 1134–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makhani SS, Kim FY, Liu Y, Ye Z, Li JL, Revenig LM, Vaughan CP, Johnson TM 2nd, Garcia PS, Ogan K, Master VA: Cognitive Impairment and Overall Survival in Frail Surgical Patients. J Am Coll Surg 2017; 225: 590–600 e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper Z, Rogers SO, Jr., Ngo L, Guess J, Schmitt E, Jones RN, Ayres DK, Walston JD, Gill TM, Gleason LJ, Inouye SK, Marcantonio ER: Comparison of Frailty Measures as Predictors of Outcomes After Orthopedic Surgery. J Am Geriatr Soc 2016; 64: 2464–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gleason LJ, Benton EA, Alvarez-Nebreda ML, Weaver MJ, Harris MB, Javedan H: FRAIL Questionnaire Screening Tool and Short-Term Outcomes in Geriatric Fracture Patients. J Am Med Dir Assoc 2017; 18: 1082–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culley DJ, Flaherty D, Reddy S, Fahey MC, Rudolph J, Huang CC, Liu X, Xie Z, Bader AM, Hyman BT, Blacker D, Crosby G: Preoperative Cognitive Stratification of Older Elective Surgical Patients: A Cross-Sectional Study. Anesth Analg 2016; 123: 186–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culley DJ, Flaherty D, Fahey MC, Rudolph JL, Javedan H, Huang CC, Wright J, Bader AM, Hyman BT, Blacker D, Crosby G: Poor Performance on a Preoperative Cognitive Screening Test Predicts Postoperative Complications in Older Orthopedic Surgical Patients. Anesthesiology 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long LS, Wolpaw JT, Leung JM: Sensitivity and specificity of the animal fluency test for predicting postoperative delirium. Can J Anaesth 2015; 62: 603–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flexman AM, Charest-Morin R, Stobart L, Street J, Ryerson CJ: Frailty and postoperative outcomes in patients undergoing surgery for degenerative spine disease. Spine J 2016; 16: 1315–1323 [DOI] [PubMed] [Google Scholar]
- 13.Shoultz TH, Moore M, Reed MJ, Kaplan SJ, Bentov I, Hough C, Taitsman LA, Mitchell SH, So GE, Arbabi S, Phelan H, Pham T: Trauma Providers’ Perceptions of Frailty Assessment: A Mixed-Methods Analysis of Knowledge, Attitudes, and Beliefs. South Med J 2019; 112: 159–163 [DOI] [PubMed] [Google Scholar]
- 14.Robinson TN, Wu DS, Pointer LF, Dunn CL, Moss M: Preoperative cognitive dysfunction is related to adverse postoperative outcomes in the elderly. J Am Coll Surg 2012; 215: 12–7; discussion 17–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dworkin A, Lee DS, An AR, Goodlin SJ: A Simple Tool to Predict Development of Delirium After Elective Surgery. J Am Geriatr Soc 2016; 64: e149–e153 [DOI] [PubMed] [Google Scholar]
- 16.Morley JE, Malmstrom TK, Miller DK: A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012; 16: 601–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdatta L, Perletti G, Maggiulli F, Tamborini F, Pellegatta I, Cherubino M: FRAIL scale as a predictor of complications and mortality in older patients undergoing reconstructive surgery for non-melanoma skin cancer. Oncol Lett 2019; 17: 263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deiner S, Westlake B, Dutton RP: Patterns of surgical care and complications in elderly adults. J Am Geriatr Soc 2014; 62: 829–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Susano MJ, Scheetz SD, Grasfield RH, Cheung D, Xu X, Kang JD, Smith TR, Lu Y, Groff MW, Chi JH, Crosby G, Culley DJ: Retrospective Analysis of Perioperative Variables Associated With Postoperative Delirium and Other Adverse Outcomes in Older Patients After Spine Surgery. J Neurosurg Anesthesiol 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fong TG, Hshieh TT, Wong B, Tommet D, Jones RN, Schmitt EM, Puelle MR, Saczynski JS, Marcantonio ER, Inouye SK: Neuropsychological profiles of an elderly cohort undergoing elective surgery and the relationship between cognitive performance and delirium. J Am Geriatr Soc 2015; 63: 977–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armaghani SJ, Lee DS, Bible JE, Archer KR, Shau DN, Kay H, Zhang C, McGirt MJ, Devin CJ: Preoperative opioid use and its association with perioperative opioid demand and postoperative opioid independence in patients undergoing spine surgery. Spine (Phila Pa 1976) 2014; 39: E1524–30 [DOI] [PubMed] [Google Scholar]
- 22.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI: Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941–8 [DOI] [PubMed] [Google Scholar]
- 23.Kuhn E, Du X, McGrath K, Coveney S, O’Regan N, Richardson S, Teodorczuk A, Allan L, Wilson D, Inouye SK, MacLullich AM, Meagher D, Brayne C, Timmons S, Davis D: Validation of a consensus method for identifying delirium from hospital records. PLoS One 2014; 9: e111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aprahamian I, Cezar NOC, Izbicki R, Lin SM, Paulo DLV, Fattori A, Biella MM, Jacob Filho W, Yassuda MS: Screening for Frailty With the FRAIL Scale: A Comparison With the Phenotype Criteria. J Am Med Dir Assoc 2017; 18: 592–596 [DOI] [PubMed] [Google Scholar]
- 25.Fitz-Henry J: The ASA classification and peri-operative risk. Ann R Coll Surg Engl 2011; 93: 185–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mayhew D, Mendonca V, Murthy BVS: A review of ASA physical status - historical perspectives and modern developments. Anaesthesia 2019; 74: 373–379 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


