Abstract
Background:
Perfluoroalkyl substances (PFAS) are suggested to interfere with thyroid hormone during pregnancy and influence fetal neurodevelopment. Epidemiological evidence regarding behavioral difficulties in childhood associated with prenatal PFAS exposure has been inconclusive.
Objective:
We evaluated the association between prenatal PFAS exposure and behavioral difficulties at 7 and 11 years, and investigated the potential mediating role of maternal thyroid hormones.
Methods:
Using pooled samples in the Danish National Birth Cohort established between 1996 and 2002, we estimated the associations between concentrations of six types of PFAS in maternal plasma (median, 8 gestational weeks) and child behavioral assessments from the Strength and Difficulties Questionnaire (SDQ), reported by parents at 7 years (n=2,421), and by parents (n=2,070) and children at 11 years (n=2,071). Behavioral difficulties were defined as having a composite SDQ score above the 90th percentile for total difficulties and externalizing or internalizing behaviors. We used logistic regression to estimate the adjusted Odds Ratio (OR) by doubling increase of prenatal PFAS (ng/ml). The possible mediating effect of maternal thyroid function classified based on thyroid-stimulating hormone (TSH) and free thyroxine (fT4) levels were evaluated.
Results:
Prenatal perfluorononanoic acid (PFNA) was consistently associated with total and externalizing behavioral difficulties in all three SDQ measures reported by parents (OR=1.40, 95% CI: 1.14-1.73 for age 7; OR=1.27, 95% CI: 1.05-1.53 for age 11) or children (OR=1.32, 95% CI: 1.11-1.58) while no consistent associations were observed for other types of PFAS. A small magnitude of natural indirect effects via maternal thyroid dysfunction (ORs ranged from 1.01 to 1.03) of several PFAS were observed for parent-reported total and externalizing behaviors at 7 years only.
Discussion:
Prenatal PFNA exposure was associated with externalizing behavioral difficulties in childhood in repeated SDQ measures at 7 and 11 years. The slight mediating effects of maternal thyroid hormones in early gestation warrant further evaluation.
Keywords: Perfluoroalkyl substances (PFAS), perfluorononanoic acid (PFNA), internalizing behavior, externalizing behavior, hyperactivity/inattentive, emotional problems
INTRODUCTION
Perfluoroalkyl substances (PFAS) are a group of man-made fluorine-containing chemicals that are widespread and persistent in the environment (Houde et al. 2006; Lau et al. 2007). Contaminations from food, drinking water, indoor air and household environments were likely the major PFAS sources for human exposures (D’Eon J and Mabury 2011). In 2002, the manufacturers agreed to gradually phase out the production of perfluorooctane sulfonate (PFOS) and perfluorooctane acid (PFOA) in Europe and in the U.S. due to public concerns about potential health effects (Buck et al. 2011). However, production of other PFAS such as perfluorononanoic acid (PFNA) and perfluorononanoic acid (PFDA) have continued with increasing human exposure until recent years in multiple countries (Bjerregaard-Olesen et al. 2016a; Kato et al. 2011; Nost et al. 2014; Toms et al. 2014).
PFAS have endocrine-disrupting properties (Braun 2017). Population studies have demonstrated that PFAS can interfere with the maternal endocrine system during pregnancy (Ballesteros et al. 2017; Bjerregaard-Olesen et al. 2016b; Inoue et al. 2019; Preston et al. 2018; Wang et al. 2014; Webster et al. 2014). Maternal thyroid hormones are critical for fetal brain development and may affect long-term neuropsychological functions in the offspring (Andersen et al. 2018; Henrichs et al. 2010; Korevaar et al. 2016). Even subtle changes in maternal thyroid function during this critical time window could lead to neurodevelopmental impairs in the child (Andersen et al. 2018; Henrichs et al. 2010; Korevaar et al. 2016). However, the mediating effect of pregnancy thyroid hormones for prenatal PFAS exposure and neurobehavioral outcomes in childhood has never been investigated.
Epidemiological evidence in humans has been inconclusive regarding neurobehavioral outcomes in childhood in relation to PFAS exposures (Liew et al. 2018a). Several cross-sectional studies in the U.S. have reported positive associations between children's exposures to some PFAS and impulsivity (Gump et al. 2011), and attention-deficit/hyperactivity disorder (ADHD) (Hoffman et al. 2010; Stein and Savitz 2011). Two studies have also reported that childhood or concurrent exposures to PFAS, including PFOA, PFNA and PFDA, were associated with increasing behavioral problems at 7 years in Faroe Islands (Oulhote et al. 2016) or poorer executive function at 8 years in Cincinnati, Ohio (Vuong et al. 2018). However, findings for prenatal PFAS exposures from longitudinal studies were mixed. Results for diagnoses of ADHD (Liew et al. 2015; Ode et al. 2014; Strom et al. 2014), diagnoses of autism spectrum disorders (Liew et al. 2015; Long et al. 2019; Lyall et al. 2018), parent- or teacher-reported behavioral scores (Fei and Olsen 2011; Lien et al. 2016; Oulhote et al. 2016), or cognitive abilities (Liew et al. 2018b; Vuong et al. 2019) in early childhood were generally null, but weak to moderate associations between child’s behaviors and prenatal PFOA, PFNA and PFDA exposures have also been reported (Hoyer et al. 2018). Variation in exposure concentrations and mixtures or differences in child outcome measures and age of assessment might contribute to the mixed findings (Liew et al. 2018a). Moreover, all these studies were limited by small sample size (usually <1,000 participants except for the one by Hoyer et al. (2018)). A recent investigation of nine European cohorts with a total of 4,826 mother-child pairs found overall no associations between prenatal and early postnatal (up to 24 months of age) PFOS and PFOA exposure and ADHD risk while a potential modifying effect by child sex was suggested for further exploration (Forns et al. 2020).
Within this context, we analyzed the combined prenatal PFAS samples from 2000 mother-child pairs in the Danish National Birth Cohort (DNBC) and evaluated the associations between prenatal PFAS exposures and child’s behavioral difficulties using Strengths and Difficulties Questionnaire (SDQ) reported by parent (7 and 11 years) and the children (11 year). A larger sample with greater statistical power might allow the detection of small to moderate effect size between prenatal PFAS exposures and behavioral outcome measures in childhood. Childhood hyperactivity symptoms might improve with maturational change as age increases (Thapar et al. 2017) but few prior studies have included outcome assessment at multiple time points by different informants. The potential mediation role of maternal thyroid dysfunction in these associations were also evaluated in this study.
MATERIALS AND METHODS
Study Population
The DNBC is a nationwide longitudinal study of pregnant women and their offspring in Denmark; details of the cohort have been described elsewhere (Olsen et al. 2001). Briefly, a total of 100,417 eligible pregnancies were enrolled by the mother’s general practitioners during early gestation (weeks 6–12) from 1996 to 2002. Approximately 50% of all general practitioners in Denmark participated in the study, and 60% of the women invited agreed to participate. Women were ineligible if they did not speak sufficient Danish to complete the telephone interviews or if they intended not to carry their pregnancy to term. A total of four computer-assisted telephone interviews (twice during pregnancy and twice postpartum) were conducted, and two prenatal maternal blood samples were collected and stored (one each in the first and second trimester). Study instrumentation including English versions of the questionnaires are available online at https://www.dnbc.dk/.The source population for this study was defined as 83,389 live-born singleton children enrolled in the DNBC for whom the mothers completed the first prenatal interview and provided a blood sample available for PFAS analyses. Previously, three sub-studies in the DNBC have measured PFAS concentrations using a stored maternal prenatal plasma sample collected in early pregnancy (mean 8.8 weeks) (Fei et al. 2007; Kesmodel et al. 2010; Liew et al. 2014; Liew et al. 2015). Details of the sampling criteria and flowchart of selection for these study samples are presented in figure 1. The present study pooled samples with pre-existing PFAS measures from the three sub-studies. Comparisons of the three study samples and demonstration of the analytical procedures to conduct pooled analyses combining these samples have been shown previously (Ernst et al. 2019; Inoue et al. 2019; Meng et al. 2018). Among the offspring with prenatal PFAS measures, 2,421, 2,070 and 2,071 had complete information on SDQ at 7 years reported by parents, at 11 years reported by parents, and at 11 years reported by children, respectively, for statistical analyses.
Figure 1.
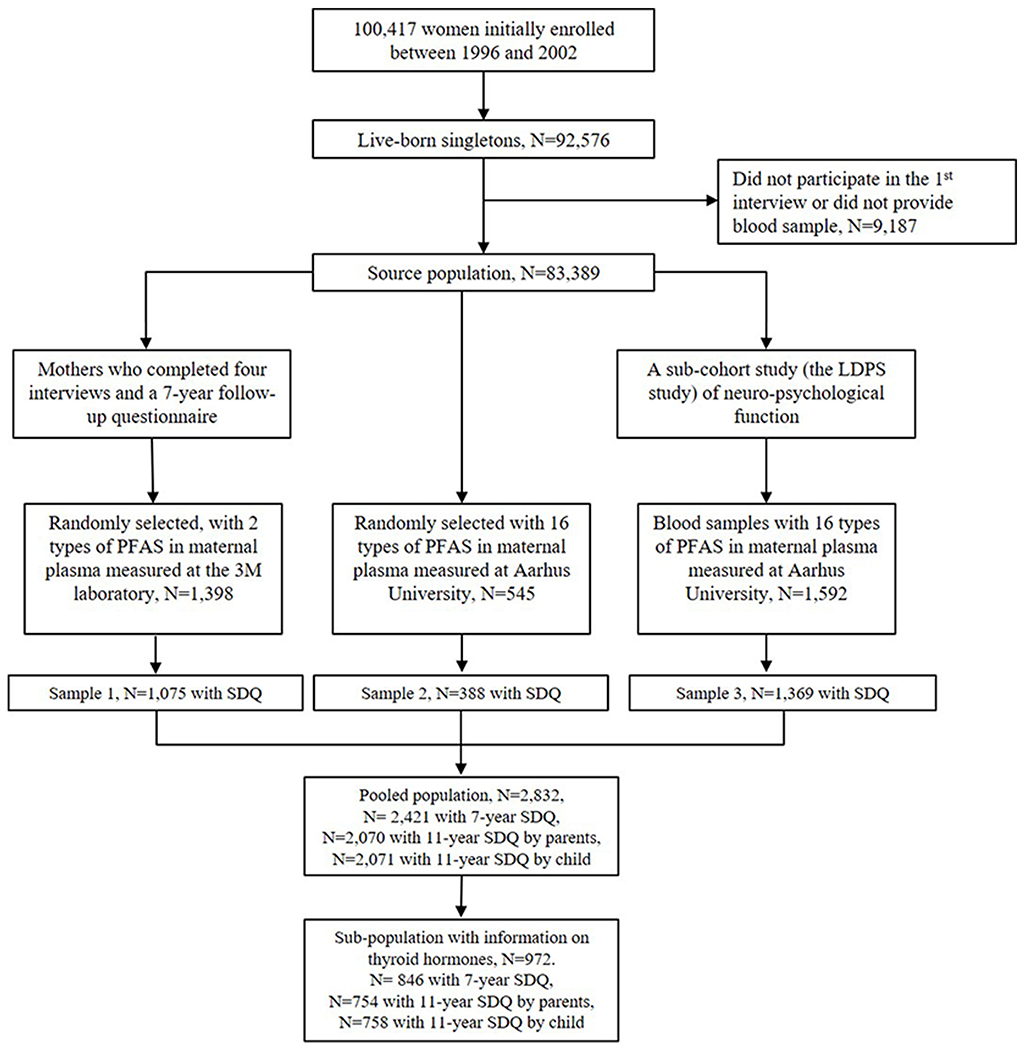
Selection of the DNBC study samples
Written informed consent was obtained from all participants at recruitment. Study procedures have been approved by the Danish data inspectorate (journal number 2016-051-000001, serial number 1297) and the Institutional Review Boards at Yale University (2000024089).
Child Behavioral Assessment
Child behaviors were assessed using the Strengths and Difficulties Questionnaire (SDQ) when children reached 7 and 11 years old. SDQ is the tool to screen for child behavioral problems (Goodman and Goodman 2009; Goodman 1997; Obel et al. 2004) and the Danish version has been validated in the general population (Niclasen et al. 2012). SDQ contains 5 scales including emotional symptoms, conduct problems, hyperactivity/inattention, peer relationship problems and prosocial behavior. Each scale contains 5 items and each item was scored as 0 for “not true”, 1 for “partly true” and 2 for “very true”. Scores were summed to give a range between 0-10 for each scale. Higher scores for emotional, conduct, hyperactivity and peer relationship subscale indicate increasing behavioral difficulties while higher prosocial scores indicate favorable prosocial behaviors. We calculated the total difficulty score (0 - 40 pts) by summing scores in the emotional, conduct, hyperactivity and peer subscales. According to the SDQ instrumentation (Goodman et al. 2010), we calculated the internalizing difficulty score (0-20 pts) as the sum of the emotional and the peer subscales, and the externalizing difficulty score (0-20 pts) as the sum of the conduct and the hyperactivity subscales. These composite SDQ measures were calculated for parent-reported SDQ at 7 and 11 years, and child-reported SDQ at 11 years.
Exposure Assessment
Prenatal PFAS exposure was measured using stored maternal plasma samples in the DNBC. Blood samples collected were sent by mail to Staten Serum Institute in Copenhagen, Denmark and stored in freezers at −20 °C or −80 °C. Details about the analytic methods for PFAS measures have been described elsewhere (Fei et al. 2007; Liew et al. 2014, 2015). Briefly, a total of 0.1 mL stored maternal plasma was retrieved for each sample identified from the biobank and they were sorted in random order before sending to the laboratories for PFAS analyses. Plasma concentration for PFOS and PFOA in study sample 1 were analyzed in the 3M Toxicology Laboratory, USA (Ehresman et al. 2007) and at the time only these two PFAS compounds could be measured in 2007. Plasma concentrations for sixteen PFAS in the study sample 2 and 3 were analyzed at the Department of Environmental Science at Aarhus University, Denmark (Liew et al. 2014). Both laboratories employed similar analytic methods with a Solid Phase Extraction (SPE) technique for sample extraction and purification, and plasma concentrations for PFAS were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Our statistical analyses focused on 6 PFAS compounds. PFOS and PFOA were quantifiable in all samples measured. For study samples 2 and 3, four other types of PFAS quantified in >90% of samples including perfluorohexan sulfonate (PFHxS) (98%), perfluoroheptane sulfonate (PFHpS) (96%), PFNA (92%), and PFDA (90%) were also included (Liew et al. 2014, 2015). The limits of detection (LOD) were 0.09 for PFOS, 0.07 for PFOA, 0.03 for PFDA, 0.09 for PFNA, 0.03 for PFHxS, and 0.04 for PFHpS. Values below the LOD had been replaced by a single imputation algorithm that included all PFAS and some demographic variables (Liew et al. 2014, 2018).
Comparisons of PFAS measurements in the two laboratories have previously been performed (Liew et al. 2014). Although the absolute PFOS and PFOA values read-out from the 3M laboratory were found to be slightly higher than the Aarhus laboratory (Supplementary Table S1), the correlations of PFOS and PFOA concentrations measured in the same samples (n = 21) produced by the two laboratories were very high (Pearson correlation r = 0.94 for PFOS and r = 0.95 for PFOA) (Meng et al. 2018).
Maternal and Child Covariates
Potential confounders were selected a priori according to directed acyclic graphs (see Figure S1). Information on child’s sex, child’s birth year, maternal age (19-29, 30-34, 35-39), parity (0 or ≥1) was retrieved from the medical register, while other potential confounders including socioeconomic status (SES; low, medium, high), pre-pregnancy body mass index (BMI; <25 or ≥25), gestational week of blood collection, smoking during the 1st trimester (yes/no), alcohol intake during the 1st trimester (≤1/week or >l/week), and parent’s behavioral scores. SES was created based on the highest education and occupation reported for the parents: a higher education (at least four years beyond high school) or an occupation in management position were considered as high, a middle length education and skilled worker positions were considered as medium, and unemployed and unskilled workers were considered as low. Parents also answered 6 questions (possible value of 0, 1, or 2 for each) about their own behavioral problems during childhood, allowing us to generate a parental behavioral problems score (range: 0-12). These covariates were considered as potential confounders in statistical analyses.
In study sample 3, a total of 972 mother-child pairs also had maternal thyroid stimulating hormone (TSH) and free thyroxine (fT4) concentrations measured in the same maternal blood samples (gestational week of sampling, median = 8.8) where PFAS levels were obtained (Andersen and Olsen 2017; Laurberg et al. 2016). We used these samples to evaluate the potential mediating roles of maternal thyroid function in the analyses assessing prenatal PFAS exposure and behavioral difficulties in the offspring. TSH and fT4 were measured by a Dimension Vista automated immunoassay (Siemens Healthcare Diagnostics, Eschborn, Germany), as previously described (Laurberg et al. 2016). We classified maternal thyroid dysfunction according to the pregnancy week-specific reference ranges (2.5 and 97.5 percentiles) previously established for the DNBC (Andersen and Olsen 2017; Laurberg et al. 2016). Maternal week-specific TSH or fT4 levels not within the reference range were considered as thyroid dysfunction.
Statistical Analysis
Pearson correlations between different SDQ measures were evaluated. PFAS concentrations (ng/mL) were log2-transformed. Therefore, the effect estimate was reported for a 2-fold increase in each of the PFAS exposure. We used the multivariate logistic regression to estimate the odds ratios (OR) and 95% CI for binary classification of total, internalizing, and externalizing difficulties in the offspring according to 2-fold increase in each of the prenatal PFAS exposure, adjusting for abovementioned potential confounders. An outcome score above the 90th percentile was chosen a priori as the cut-off to indicate behavioral difficulties. The specific cut-off scores can be found in supplementary table S3. A sensitivity analysis was performed for the outcome classification using the 90th percentile ± 2 as the cutoff to define total difficulties, and the score at the 90th percentile ± 1 as the cutoff to define internalizing difficulties and externalizing difficulties. Non-linear relations between prenatal PFAS exposures and outcomes were also evaluated. We used general additive models (GAM) to test nonlinearity using penalized smoothing regression splines with a degree of 3. Departure from linearity (P-value < 0.1 for splines) in GAM was compared to models with PFAS included as a linear term.
Some sex-specific associations for prenatal PFAS exposure and neurological and pubertal endpoints were showed in previous DNBC studies (Ernst et al. 2019; Liew et al. 2014). We therefore also performed sex-stratified analyses here for behavioral outcomes. Tests of heterogeneity were also performed by assessing the p-value of the interaction term for each PFAS and child’s sex in the regression models.
There has been increasing interests of estimating the effect of exposures to PFAS mixture on potential health outcomes in epidemiological research (Braun et al. 2016). We first constructed a co-adjusted pollutants model including all six types of PFAS to estimate the exposure effect for a specific type of PFAS. Moreover, we used weighted quantile sum approach (WQS) to estimate the joint exposure effect of prenatal PFAS mixture on behavioral difficulties in childhood (Carrico et al. 2015). A WQS index was created using all six types of PFAS and each PFAS was assigned a weight that reflects the strength of that PFAS and outcome association, and the collinearity between that PFAS and other type of PFAS in the mixture (Carrico et al. 2015). We constrained the sum of weights to 1.0 to incorporate each mixture components into a single effect estimate, and we also constrained each mixture component effect to be positive based on findings from the individual PFAS model. The WQS index was scaled for an interquartile range (IQR) increase to improve interpretability.
Mediation analysis was performed to assess whether maternal thyroid hormone levels may mediate the potential association between prenatal PFAS exposures and child’s behavioral outcomes using the subset with maternal thyroid hormone information. We estimated the mediating role of maternal thyroid anomalies using the regression-based approach (Valeri and Vanderweele 2013). Within the counterfactual framework, the total effects of PFAS can be decomposed into controlled direct effect and natural indirect effect through maternal thyroid dysfunction. In this study, the controlled direct effect estimates the effect of prenatal PFAS exposures when maternal thyroid function status is fixed at the reference group (defined as both TSH and free T4 levels were within the gestational week specific reference range), while prenatal PFAS exposure changes from a baseline level to an index level. The natural indirect effect estimates how much the outcome would change on average when the PFAS exposure were set at an index level, while the thyroid dysfunction were changed from the level it would take at the reference exposure level to the level it would take at the index exposure level (Valeri and Vanderweele 2013). This approach required a specification of a baseline level and an index level for prenatal PFAS exposure (Valeri and Vanderweele 2013). We set the baseline level and the index level as the 25th and 75th of each PFAS and allowed potential interaction between the exposure and the mediator. The proportion mediated indicates how much total effects were attributed to the specific mediating path evaluated. Mediation analyses were conducted using the logistic regression analyses for binary classification of SDQ endpoints, binary classification of thyroid dysfunction according to gestational week-specific cut-off, and binary PFAS exposures.
The three study samples were selected according to different sampling strategies and some maternal and child characteristics were oversampled in study sample 2 and 3 due to study purposes. We used inverse probability selection weight technique (IPSW) to adjust for sampling differences and possible bias due to non-participation at 7 and 11 years. Details of the IPSW can be found in our previous publications (Liew et al. 2018b; Meng et al. 2018). About 2.5 % samples had at least one missing value for the covariates except for parent’s behavioral scores (~15%). Missing covariate were replaced in multiple imputations that included all PFAS and the above-mentioned covariates. Ten complete datasets were generated and analyzed (Yuan 2010). The WQS analysis was performed using gWQS package in R version 3.6.1 (R Core Team). All other statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Demographic and other characteristics of the pooled sample (unweighted) and in the subset with information on thyroid hormones are presented in Table 1. The characteristics for each study samples are showed in supplementary table S2. As expected, there were more male infants in sample 2 and more women had alcohol intake during pregnancy in sample 3 due to over-sampling strategy by design. Other study characteristics were similar across study samples. The distribution of parent-reported SDQ scores at 7 and 11 years were comparable, while the child-reported total and internalizing scores were slightly higher than parent-reports at 11 years (Table S3). There were moderate correlations comparing repeated parent-reports of total SDQ scores at 7 and 11 years (r=0.62), as well as the comparisons for parent and child reported SDQ at 11 years (r=0.58) (Table S4).
Table 1.
Distribution of selected characteristics in the pooled sample and the sub-sample with the availability of thyroid hormones measures
| Maternal Characteristics | Pooled sample (N=2,832) | Sub-sample with thyroid hormones (N=972) |
|---|---|---|
| Maternal age (years) | ||
| 19-24 | 208 (7.3) | 84 (8.6) |
| 25-29 | 1090 (38.5) | 340 (35.0) |
| 30-34 | 1072 (37.9) | 392 (40.3) |
| 35+ | 462 (16.3) | 156 (16.1) |
| Child’s sex | ||
| Female | 1272 (44.9) | 473 (48.7) |
| Male | 1560 (55.1) | 499 (51.3) |
| Birth year | ||
| 1998-2000 | 1478 (52.2) | 487 (50.1) |
| 2001-2003 | 1354 (47.8) | 485 (49.9) |
| Parity | ||
| 0 | 1316 (46.5) | 515 (53.0) |
| ≥1 | 1441 (50.9) | 432 (44.4) |
| Missing | 75 (2.7) | 25 (2.6) |
| Maternal social-occupational status | ||
| High | 1964 (69.4) | 708 (72.8) |
| Medium | 787 (27.8) | 239 (24.6) |
| Low | 75 (2.7) | 22 (2.3) |
| Missing | 6 (0.2) | 3 (0.3) |
| Maternal smoking during the 1st trimester | ||
| No | 2075 (73.3) | 661 (68.0) |
| Yes | 757 (26.7) | 311 (32.0) |
| Maternal alcohol intake during the 1st trimester | ||
| None | 582 (20.6) | 122 (12.6) |
| ≤4 glass per week | 793 (28.0) | 125 (12.9) |
| >4 glasses per week | 1457 (51.5) | 725 (74.6) |
| Maternal pre-pregnancy BMI | ||
| <25 | 2031 (71.7) | 710 (73.1) |
| ≥25 | 742 (26.2) | 237 (24.4) |
| Missing | 59 (2.1) | 25 (2.6) |
| Gestational week of blood sampling | ||
| ≤8 weeks | 1241 (43.8) | 447 (46.0) |
| >8 weeks | 1521 (53.7) | 525 (54.0) |
| Missing | 70 (2.5) | 0 |
| Parent’s behavioral score during their childhood | ||
| 1-3 | 1985 (70.1) | 680 (70.0) |
| 4-7 | 368 (13.0) | 128 (13.1) |
| 8-12 | 65 (2.3) | 19 (19.5) |
| Missing | 414 (14.6) | 145 (14.9) |
| Maternal subclinical thyroid dysfunction in early gestationsa | ||
| No | NA | 850 (87.5) |
| Yes | NA | 122 (12.6) |
Maternal subclinical thyroid anomality was based on the week-specific 2.5th and 97.5th percentiles for thyroid stimulating hormone and free thyroxine established for the DNBC.
Figure 2 presents the associations between prenatal PFAS exposure and behavioral difficulties in childhood. Several PFAS appear to be associated with total behavioral difficulties at 7 years reported by parents. But the associations for most PFAS were attenuated towards the null in parent and child reports at 11 years, except for PFNA (OR=1.23, 95% CI: 1.01-1.50 at 11 years in parent reports, OR=1.30, 95% CI: 1.08-1.57 at 11 years in child report). The consistent associations of PFNA were mainly driven by externalizing difficulties (OR=1.40, 95% CI: 1.14-1.73 for parental report at 7 years and OR=1.27, 95% CI: 1.05-1.53 at 11 years, OR=1.32, 95% CI: 1.11-1.58 for child report at 11 years). Suggestive associations were also observed for PFDA, PFNA and PFHpS with internalizing difficulties reported by children at 11 years (ORs were 1.20 (95% CI: 0.99-1.44), 1.22 (95% CI: 1.01-1.47) and 1.19 (95% CI: 0.99-1.42), respectively), while an inverse association was also found for PFHxS and internalizing behaviors reported by parents (OR=0.77, 95% CI: 0.66-0.88) and children (OR=0.81, 95% CI: 0.70-0.94) at 11 years.
Figure 2.
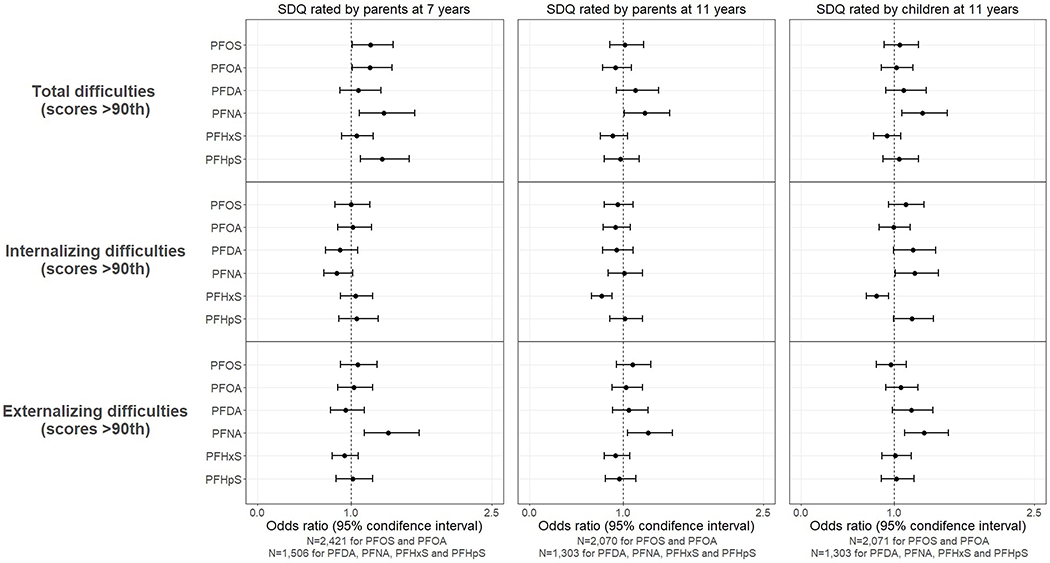
Odds ratio (OR) and 95% confidence interval (CI) for behavioral difficulties in children according to per doubling increase in prenatal PFAS level (ng/ml) in logistic regression models. Models were adjusted for sex, birth year, maternal age, parity, maternal social-occupational status, maternal pre-pregnancy BMI, gestational week of blood draw, smoking during the 1st trimester, alcohol intake during the 1st trimester, and parent’s behavioral scores.
In sensitivity analysis varying the cutoffs to define childhood behavioral difficulties, the association between PFNA and externalizing difficulties in all three measures remain robust with little changes (Table S5). Effect estimates for other PFAS and outcome measures remain similar or slightly fluctuated when a higher or lower cut-off was used but the overall findings did not change.
There was no evidence to suggest non-linearity (p-value >0.10) when continuous PFAS values were analyzed using GAM in these analyses (results not shown). There were also no sex-specific differences found (p for interaction >0.10 for all SDQ outcome measures), though the effect estimates between PFOS, PFOA, PFNA, PFHpS and the total or externalizing behavioral difficulties at 7 years appeared to be stronger for girls (Supplementary Figure S2).
In the multi-PFAS adjustment model (supplementary figure S3), the positive associations were strengthened between PFNA and externalizing difficulties in all three SDQ measures (OR=2.52, 95% CI: 1.77-3.58 at 7 years and OR=1.77, 95% CI: 1.30-2.40 at 11 years in parent report; OR=1.47, 95% CI: 1.13-1.93 at 11 years in child report) and PFHpS with child-report internalizing difficulties (OR=1.64, 95% CI: 1.07-2.51). The negative association between PFHxS and child-report internalizing difficulties remained unchanged (OR=0.65, 95% CI: 0.54-0.79). In PFAS mixture analyses (Table 2), we found that for an IQR increment of the WQS index was associated with total difficulties in parental report at 7 years (OR=1.44, 95% CI: 1.10-1.98) but the effect sizes decreased at 11-year measures. Elevated ORs (ranges from 1.21 to 1.28) were also observed for externalizing difficulties in parental and child reports at 7 and 11 years according to increasing WQS index of PFAS mixture but the CIs included the null. The estimated OR for the WQS index of PFAS mixture and internalizing difficulties were elevated in child-reported measure only (OR=1.25, 95% CI: 0.95-1.63). The weights for each PFAS compound in the WQS index can be found in supplementary table S5. PFNA contributed the largest weights (0.35 to 0.54) for the total and externalizing difficulties in all outcome measures, consistent with findings in the single pollutant model.
Table 2.
Odds ratio (OR) and 95% confidence interval (CI) for behavioral difficulties in children according to per inter quartile range increase in the weighted quantile sum (WQS) index of prenatal PFAS mixtures .
| Behavioral difficulties | OR (95% CI)a |
||
|---|---|---|---|
| SDQ by parents at 7 years (N=1,506) | SDQ by parents at 11 years (N=1,303) | SDQ by children at 11 years (N=1,303) | |
| Total difficulties | 1.44 (1.10-1.89) | 1.19 (0.89-1.61) | 1.28 (0.96-1.70) |
| Internalizing difficulties | 1.14 (0.86-1.52) | 0.95 (0.72-1.24) | 1.25 (0.95-1.63) |
| Externalizing difficulties | 1.19 (0.91-1.56) | 1.21 (0.92-1.59) | 1.21 (0.93-1.57) |
OR: odds ratio; CI: confidence interval
A WQS index was created using all six types of PFAS to reflect the mixture exposure level.
Models were adjusted for sex, birth year, maternal age, parity, maternal social-occupational status, maternal pre-pregnancy BMI, gestational week of blood draw, smoking during the 1st trimester, and alcohol intake during the 1st trimester, and parent’s behavioral scores.
In mediation analyses using a subset of sample with information on thyroid hormones (Table 3), we found that the estimated total effects in this subset were consistent with the main results using all samples presented in figure 2. The estimated total effects were predominately driven by the estimated controlled direct effects e.g. the exposure effect not mediating through maternal thyroid dysfunction (see supplementary table S6). A small magnitude of estimated natural indirect effect of PFOS, PFOA, PFNA, and PFHpS on maternal thyroid function was found for total and externalizing difficulties at 7 years reported by parents, but there was no evidence for mediating effects of maternal thyroid function for any outcome measures at 11 years.
Table 3.
The estimated direct effect and indirect effect mediated by maternal thyroid dysfunctiona during pregnancy on the association between prenatal PFAS exposures and behavioral difficulties in a subset children.
| SDQ by parents at 7 years (N=846) |
SDQ by parents at 11 years (N=754) |
SDQ by children at 11 years (N=758) |
||||
|---|---|---|---|---|---|---|
| PFAS | Controlled direct effectb (OR (95% CI))* | Natural indirect effectc (OR (95% CI))* | Controlled direct effectb (OR (95% CI))* | Natural indirect effectc (OR (95% CI))* | Controlled direct effectb (OR (95% CI))* | Natural indirect effectc (OR (95% CI))* |
| Total difficulties | ||||||
| PFOS | 1.32 (0.98, 1.79) | 1.03 (1.01, 1.06) | 1.34 (0.97, 1.86) | 1.00 (0.98, 1.03) | 1.28 (0.93, 1.75) | 0.98 (0.95, 1.02) |
| PFOA | 1.30 (0.94, 1.81) | 1.03 (1.00, 1.05) | 0.86 (0.61, 1.21) | 1.00 (0.98, 1.03) | 1.17 (0.84, 1.64) | 0.99 (0.96, 1.02) |
| PFDA | 0.93 (0.71, 1.23) | 1.03 (0.99, 1.05) | 1.20 (0.88, 1.63) | 1.00 (0.99, 1.01) | 1.06 (0.80, 1.42) | 0.99 (0.98, 1.01) |
| PFNA | 1.08 (0.86, 1.35) | 1.02 (1.00, 1.03) | 1.06 (0.83, 1.35) | 1.00 (0.99, 1.01) | 1.18 (0.92, 1.51) | 1.00 (0.98, 1.01) |
| PFHxS | 1.15 (0.87, 1.51) | 1.01 (0.99, 1.03) | 0.84 (0.61, 1.15) | 1.00 (0.99, 1.01) | 1.00 (0.74, 1.36) | 1.00 (0.98, 1.01) |
| PFHpS | 1.38 (1.00, 1.92) | 1.03 (1.00, 1.06) | 1.30 (0.92, 1.83) | 1.00 (0.98, 1.03) | 1.18 (0.86, 1.64) | 0.99 (0.96, 1.02) |
| Internalizing difficulties | ||||||
| PFOS | 1.20 (0.87, 1.64) | 1.02 (0.99, 1.05) | 1.05 (0.78, 1.40) | 1.01 (0.99, 1.04) | 1.30 (0.95, 1.76) | 1.02 (0.99, 1.04) |
| PFOA | 1.12 (0.79, 1.57) | 1.02 (0.99, 1.04) | 0.89 (0.66, 1.21) | 1.01 (0.99, 1.03) | 1.15 (0.83, 1.60) | 1.01 (0.99, 1.03) |
| PFDA | 0.80 (0.60, 1.05) | 1.01 (0.99, 1.04) | 1.11 (0.85, 1.46) | 1.01 (0.99, 1.02) | 1.22 (0.91, 1.64) | 1.01 (0.99, 1.02) |
| PFNA | 0.82 (0.66, 1.02) | 1.01 (0.99, 1.03) | 1.06 (0.85, 1.32) | 1.00 (0.99, 1.01) | 1.11 (0.87, 1.42) | 1.00 (0.99, 1.02) |
| PFHxS | 1.18 (0.88, 1.57) | 1.00 (0.99, 1.02) | 0.71 (0.54, 0.94) | 1.00 (0.99, 1.01) | 0.84 (0.63, 1.13) | 1.00 (0.99, 1.02) |
| PFHpS | 1.25 (0.89, 1.76) | 1.02 (0.99, 1.04) | 1.03 (0.76, 1.40) | 1.01 (0.99, 1.03) | 1.24 (0.90, 1.70) | 1.01 (0.99, 1.04) |
| Externalizing difficulties | ||||||
| PFOS | 1.34 (0.99, 1.82) | 1.02 (1.00, 1.05) | 1.04 (0.80, 1.36) | 1.02 (0.99, 1.04) | 1.14 (0.85, 1.54) | 0.99 (0.96, 1.02) |
| PFOA | 1.18 (0.85, 1.63) | 1.02 (1.00, 1.05) | 0.92 (0.68, 1.25) | 1.02 (0.99, 1.04) | 1.16 (0.85, 1.60) | 0.99 (0.97, 1.02) |
| PFDA | 1.05 (0.79, 1.40) | 1.02 (1.00, 1.04) | 1.04 (0.80, 1.36) | 1.01 (0.99, 1.02) | 1.05 (0.80, 1.38) | 1.00 (0.98, 1.01) |
| PFNA | 1.34 (1.06, 1.71) | 1.01 (1.00, 1.03) | 1.17 (0.88, 1.56) | 1.01 (0.99, 1.02) | 1.22 (0.96, 1.55) | 1.00 (0.99, 1.01) |
| PFHxS | 1.05 (0.80, 1.39) | 1.01 (0.99, 1.02) | 0.93 (0.71, 1.22) | 1.00 (0.99, 1.02) | 1.16 (0.87, 1.54) | 1.00 (0.99, 1.01) |
| PFHpS | 1.32 (0.95, 1.84) | 1.02 (1.00, 1.05) | 1.06 (0.79, 1.43) | 1.02 (0.99, 1.04) | 1.07 (0.79, 1.46) | 0.99 (0.97, 1.02) |
Maternal thyroid dysfunction is defined as either TSH or free T4 levels were outside the gestational week specific reference range.
The controlled direct effect estimates the effect of prenatal PFAS exposures when maternal thyroid function status is fixed at the reference group (defined as both TSH and free T4 levels were within the gestational week specific reference range), while prenatal PFAS exposure changes from a reference level (25th) to an index level (75th) .
The natural indirect effect measures how much the outcome would change on average when the PFAS exposure were set at an index level (75th), while the thyroid dysfunction were changed from the level it would take at the reference exposure level (25th) to the level it would take at the index exposure level (75th).
Models were adjusted for sex, birth year, maternal age, parity, maternal social-occupational status, maternal pre-pregnancy BMI, gestational week of blood draw, smoking during the 1st trimester, alcohol intake during the 1st trimester, and parent’s behavioral scores.
Discussion
In this study, we found that increasing prenatal PFNA exposure was associated with externalizing difficulties at 7 and 11 years reported by parents and children. PFNA and other PFAS, including PFDA and PFHpS, were suggestively associated with internalizing difficulties reported by children at 11 years. We found no evidence of sex differences in the PFAS exposure and behavioral outcomes associations evaluated. A weak mediating effect of abnormal maternal thyroid function for multiple PFAS was observed for behavioral difficulties at 7 years warrant further investigation.
Earlier epidemiological studies have often focused on PFOS and PFOA only, the two most commonly detected PFAS, while other types of PFAS compounds might also require further scrutiny for their neurotoxicity potentials. In the present study, prenatal PFNA was most consistently associated with child’s externalizing behaviors. This corroborates the finding from another study that analyzed three European cohorts, reporting prenatal PFNA and PFDA to be associated with parent-reported SDQ externalizing difficulties between ages 5 and 9 (Hoyer et al. 2018), while findings for PFOS and PFOA were less conclusive. Similar to epidemiological studies, experimental studies were also mostly focused on PFOS and PFOA and evidence for other types of PFAS compounds were sparse. Only a few zebrafish studies have indicated that PFNA can induce thyroid-disrupting effects (Liu et al. 2011), alter gene expression, and increase aggressive behaviors (Jantzen et al. 2016). Prenatal PFNA and PFDA levels were also associated with internalizing behaviors at 11 years reported by children in our study. Emotional and internalizing behaviors in young adolescence could be less observable than externalizing behaviors, thus might be less accurately characterized in parental reports (De Los Reyes and Kazdin 2005; van der Meer et al. 2008). It is unclear why these behavioral outcomes in childhood were specifically associated with prenatal PFNA and PFDA but not with other PFAS. Mechanistic studies are needed to explore the neurotoxicity difference across various PFAS compounds (Slotkin et al. 2008). Exposure levels to PFNA and also PFDA in the U.S and in Scandinavia were increasing from 2000 to 2013 (Bjerregaard-Olesen et al. 2016a; Kato et al. 2011; Nost et al. 2014) and continuing biomonitoring efforts of their exposure trends are needed.
A few epidemiological studies have evaluated the associations between prenatal PFAS exposure and child’s behaviors assessed by SDQ, but most of the studies have limited sample size (Fei and Olsen 2011; Hoyer et al. 2018; Oulhote et al. 2016). These studies included a previous report from the DNBC, which assessed parent-reported SDQ at 7 years and only evaluated prenatal PFOS and PFOA in 787 participants (Fei and Olsen 2011). A more recent investigation for ADHD risk in childhood that combined nine European population-based studies including some samples from the DNBC were also reported (Forns et al. 2020), but the study only investigated two PFAS compounds. The statistical power and precision of estimates were enhanced by using pooled samples in the DNBC, and we further evaluated additional 4 types of PFAS and repeated SDQ measures at 7 and 11 years of follow-up. For ubiquitous environmental contaminants where there is no real “unexposed” comparison, a larger sample size might be needed to detect even a moderate association between the exposure and the outcome.
Numerous statistical methods have been proposed to analyze complex environmental mixtures in health studies, but methods of choice should be carefully guided based on research questions of interests (Braun et al. 2016; Gibson et al. 2019). In our study, we conducted multiple PFAS adjustment to disentangle the possible effect of each of the PFAS compound, and we used the WQS regression to estimate a joint exposure effect of the PFAS mixture from all six compounds (Carrico et al. 2015; Cluett et al. 2019). Our overall findings are consistent in the single and multiple pollutants and mixture analyses, suggesting PFNA to be most consistently associated with externalizing difficulties reported by parents and children, and a few other types of PFAS possibly associated with internalizing difficulties reported by children.
Behavioral measures in childhood might be dependent on age of assessment (Tottenham et al. 2011), gender of the child (Madsen et al. 2018), and the informants (van der Meer et al. 2008). To-date, only one other study has evaluated repeated SDQ measures at 5 and 7 years based on parental reports in relation to PFAS exposure (Oulhote et al. 2016). Our study extended the literature and simultaneously compared parent-rated SDQ at 7 and 11 years, and parent- and child-rated SDQ at 11 years. Consistent with previous studies (De Los Reyes and Kazdin 2005), the parent-child agreement in our sample was also higher for externalizing than internalizing behavioral difficulties, possibly because externalizing problems are more observable by the parents than internalizing problems (De Los Reyes and Kazdin 2005). Despite a moderate to high parent-child agreement for SDQ measures, there are considerable non-shared variances between the two informants, suggesting that parents and children each might provide unique information about the child’s behavioral and emotional problems.
Our study has also evaluated the potential mediating role of maternal thyroid function anomalies for prenatal PFAS exposures and behavioral difficulties in childhood. In this cohort, maternal abnormal thyroid function classified using gestational week-specific cut-off has been associated with adverse cognitive, motor and behavioral function of the offspring assessed at 5 years of age (Andersen et al. 2018). We observed weak mediation effects of maternal thyroid dysfunction, mainly in the total and externalizing SDQ scores at 7 years only. The estimated proportions mediated by maternal thyroid dysfunction ranges from 9% to 20% for several types of PFAS and externalizing difficulties at 7 years. However, the mediation effects were not consistent across the three SDQ measures and thus was inconclusive overall. Our mediation analyses were based on a smaller subset and only one-time measure of thyroid hormones in early gestations. Moreover, thyroid peroxidase antibody status was not measured in the DNBC and we were not able to evaluate possible thyroid-altering effects of PFAS through autoimmune damage that has been suggested recently in other cohorts (Preston et al. 2018; Webster et al. 2014). Few women were classified as overt or subclinical thyroid dysfunction in our sample (Andersen et al. 2018) and our study did not have sufficient sample size to investigate the mediation role of specific thyroid disorders.
Several limitations of the study should be noted. First, the study sample 1 only had information about PFOS and PFOA. The lack of information about other types of PFAS reduce the statistical power in analysis. Second, we also cannot rule out residual confounding from other unmeasured or unknown confounders, such as hemodynamic factors including albumin and glomerular filtration rate (GFR) that could have affected the PFAS measure during pregnancy (Verner et al. 2015), maternal dietary habits, lifestyles and household factors (Halldorsson et al. 2008). However, multiple types of PFAS were studied while only a few were found to be associated with the repeated outcome measures. Thus, if these associations were because of uncontrolled confounding, the confounding structure has also to be specific to these associations. Third, we do not have measures of other persistent organic pollutants (POPs). Studies have reported that PFAS are only weakly correlated with other classes of POPs (Fisher et al. 2016), thus potential confounding from these other persistent chemicals are expected to be minimal. However, if these other POPs also affect similar biological pathways and fetal neurodevelopment, the joint exposure effects could be larger than assessing each of the exposure separately (Braun et al. 2016). Despite our efforts in controlling difference in samplings using IPSW technique, possible batch effects of PFAS measures from combining these study samples could still occur and might contribute random errors to the effect estimates. Finally, child blood samples are not available in the DNBC thus we were not able to study postnatal PFAS exposure effects in this cohort.
CONCLUSION
In summary, we found that prenatal PFNA exposure was consistently associated with externalizing behavioral difficulties in childhood using repeated parental-rated SDQ measures at 7 and 11 years and child self-rated measure at 11 years. Possible associations were also found for PFNA, PFDA and PFHpS and internalizing difficulties reported by children at 11 years. Only a weak mediation effect of maternal thyroid dysfunction was found for externalizing behaviors at 7 years but not for other endpoints. Our findings call for further research of possible developmental neurotoxicity of exposure to other types of PFAS in addition to PFOS and PFOA.
Supplementary Material
Highlights.
We evaluated prenatal exposure to six PFAS and child neurobehavioral development.
Child behavrioal difficulties were assessed repeatedly at age 7 and 11 years.
Prenatal perfluorononanoic acid (PFNA) was associated with externalizing behaviors in childhood.
Possible mediating effect of PFAS via maternal thyroid dysfunction warrant further research.
Acknowledgement:
The Danish National Birth Cohort was established with a significant grant from the Danish National Research Foundation. Additional support was obtained from the Danish Regional Committees, the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Health Foundation and other minor grants. The DNBC Biobank has been supported by the Novo Nordisk Foundation and the Lundbeck Foundation.
Funding: This work is supported by the NIH/NIEHS Pathway to Independence Award (R00ES026729) and the Danish Council for Strategic Research (10-092818, http://www.fetotox.au.dk).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosures: The authors declare no actual or potential competing financial interests.
REFERENCE
- Andersen SL, Olsen J. 2017. Early pregnancy thyroid function test abnormalities in biobank sera from women clinically diagnosed with thyroid dysfunction before or after pregnancy. Thyroid 27:451–459. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Andersen S, Liew Z, Vestergaard P, Olsen J. 2018. Maternal thyroid function in early pregnancy and neuropsychological performance of the child at 5 years of age. J Clin Endocrinol Metab 103:660–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros V, Costa O, Iniguez C, Fletcher T, Ballester F, Lopez-Espinosa MJ. 2017. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environ Int 99:15–28. [DOI] [PubMed] [Google Scholar]
- Bjerregaard-Olesen C, Bach CC, Long M, Ghisari M, Bossi R, Bech BH, et al. 2016a. Time trends of perfluorinated alkyl acids in serum from danish pregnant women 2008–2013. Environ Int 91:14–21. [DOI] [PubMed] [Google Scholar]
- Bjerregaard-Olesen C, Ghisari M, Bonefeld-Jorgensen EC. 2016b. Activation of the estrogen receptor by human serum extracts containing mixtures of perfluorinated alkyl acids from pregnant women. Environ Res 151:71–79. [DOI] [PubMed] [Google Scholar]
- Braun JM, Gennings C, Hauser R, Webster TF. 2016. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Persp 124:A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM. 2017. Early-life exposure to edcs: Role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr Environ Assess Manag 7:513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. 2015. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat 20:100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluett R, Seshasayee SM, Rokoff LB, Rifas-Shiman SL, Ye X, Calafat AM, et al. 2019. Per- and polyfluoroalkyl substance plasma concentrations and bone mineral density in midchildhood: A cross-sectional study (project viva, united states). Environ Health Perspect 127:87006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Eon JC, Mabury SA. 2011. Is indirect exposure a significant contributor to the burden of perfluorinated acids observed in humans? Environ Sci Technol 45:7974–7984. [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Kazdin AE. 2005. Informant discrepancies in the assessment of childhood psychopathology: A critical review, theoretical framework, and recommendations for further study. Psychol Bull 131:483–509. [DOI] [PubMed] [Google Scholar]
- Ehresman DJ, Froehlich JW, Olsen GW, Chang SC, Butenhoff JL. 2007. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (pfos), perfluorooctanoate (pfoa), and other fluorochemicals. Environ Res 103:176–184. [DOI] [PubMed] [Google Scholar]
- Ernst A, Brix N, Lauridsen LLB, Olsen J, Parner ET, Liew Z, et al. 2019. Exposure to perfluoroalkyl substances during fetal life and pubertal development in boys and girls from the danish national birth cohort. Environ Health Perspect 127:17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. 2007. Perfluorinated chemicals and fetal growth: A study within the danish national birth cohort. Environ Health Perspect 115:1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, Olsen J. 2011. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ Health Perspect 119:573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Liang CL, LeBlanc A, Gaudreau E, Foster WG, et al. 2016. Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (mirec) cohort study. Environ Health 15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Verner M-A, Iszatt N, Nowack N, Bach CC, Vrijheid M, et al. 2020. Early life exposure to perfluoroalkyl substances (pfas) and adhd: A meta-analysis of nine european population-based studies. Environmental Health Perspectives 128:057002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EA, Nunez Y, Abuawad A, Zota AR, Renzetti S, Devick KL, et al. 2019. An overview of methods to address distinct research questions on environmental mixtures: An application to persistent organic pollutants and leukocyte telomere length. Environ Health 18:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman A, Goodman R. 2009. Strengths and difficulties questionnaire as a dimensional measure of child mental health. J Am Acad Child Adolesc Psychiatry 48:400–403. [DOI] [PubMed] [Google Scholar]
- Goodman A, Lamping DL, Ploubidis GB. 2010. When to use broader internalising and externalising subscales instead of the hypothesised five subscales on the strengths and difficulties questionnaire (sdq): Data from british parents, teachers and children. J Abnorm Child Psychol 38:1179–1191. [DOI] [PubMed] [Google Scholar]
- Goodman R 1997. The strengths and difficulties questionnaire: A research note. J Child Psychol Psychiatry 38:581–586. [DOI] [PubMed] [Google Scholar]
- Gump BB, Wu Q, Dumas AK, Kannan K. 2011. Perfluorochemical (pfc) exposure in children: Associations with impaired response inhibition. Environ Sci Technol 45:8151–8159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK, Olsen SF. 2008. Dietary predictors of perfluorinated chemicals: A study from the danish national birth cohort. Environ Sci Technol 42:8971–8977. [DOI] [PubMed] [Google Scholar]
- Henrichs J, Bongers-Schokking JJ, Schenk JJ, Ghassabian A, Schmidt HG, Visser TJ, et al. 2010. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: The generation r study. J Clin Endocrinol Metab 95:4227–4234. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. 2010. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in u.S. Children 12–15 years of age. Environ Health Perspect 118:1762–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. 2006. Biological monitoring of polyfluoroalkyl substances: A review. Environ Sci Technol 40:3463–3473. [DOI] [PubMed] [Google Scholar]
- Hoyer BB, Bonde JP, Tottenborg SS, Ramlau-Hansen CH, Lindh C, Pedersen HS, et al. 2018. Exposure to perfluoroalkyl substances during pregnancy and child behaviour at 5 to 9years of age. Horm Behav 101:105–112. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ritz B, Andersen SL, Ramlau-Hansen CH, Hoyer BB, Bech BH, et al. 2019. Perfluoroalkyl substances and maternal thyroid hormones in early pregnancy; findings in the danish national birth cohort. Environ Health Perspect 127:117002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen CE, Annunziato KM, Cooper KR. 2016. Behavioral, morphometric, and gene expression effects in adult zebrafish (danio rerio) embryonically exposed to pfoa, pfos, and pfna. Aquat Toxicol 180:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the u.S. Population: 1999–2008. Environ Sci Technol 45:8037–8045. [DOI] [PubMed] [Google Scholar]
- Kesmodel US, Underbjerg M, Kilburn TR, Bakketeig L, Mortensen EL, Landro NI, et al. 2010. Lifestyle during pregnancy: Neurodevelopmental effects at 5 years of age. The design and implementation of a prospective follow-up study. Scand J Public Health 38:208–219. [DOI] [PubMed] [Google Scholar]
- Korevaar TI, Muetzel R, Medici M, Chaker L, Jaddoe VW, de Rijke YB, et al. 2016. Association of maternal thyroid function during early pregnancy with offspring iq and brain morphology in childhood: A population-based prospective cohort study. Lancet Diabetes Endocrinol 4:35–43. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol Sci 99:366–394. [DOI] [PubMed] [Google Scholar]
- Laurberg P, Andersen SL, Hindersson P, Nohr EA, Olsen J. 2016. Dynamics and predictors of serum tsh and ft4 reference limits in early pregnancy: A study within the danish national birth cohort. J Clin Endocrinol Metab 101:2484–2492. [DOI] [PubMed] [Google Scholar]
- Lien GW, Huang CC, Shiu JS, Chen MH, Hsieh WS, Guo YL, et al. 2016. Perfluoroalkyl substances in cord blood and attention deficit/hyperactivity disorder symptoms in seven-year-old children. Chemosphere 156:118–127. [DOI] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Bonefeld-Jorgensen EC, Henriksen TB, Nohr EA, Bech BH, et al. 2014. Prenatal exposure to perfluoroalkyl substances and the risk of congenital cerebral palsy in children. Am J Epidemiol 180:574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, et al. 2015. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: A nested case-control study in the danish national birth cohort. Environ Health Perspect 123:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Goudarzi H, Oulhote Y. 2018a. Developmental exposures to perfluoroalkyl substances (pfass): An update of associated health outcomes. Curr Environ Health Rep 5:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Bach CC, Asarnow RF, Bech BH, Nohr EA, et al. 2018b. Prenatal exposure to perfluoroalkyl substances and iq scores at age 5; a study in the danish national birth cohort. Environ Health Perspect 126:067004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang J, Fang X, Zhang H, Dai J. 2011. The thyroid-disrupting effects of long-term perfluorononanoate exposure on zebrafish (danio rerio). Ecotoxicology 20:47–55. [DOI] [PubMed] [Google Scholar]
- Long M, Ghisari M, Kjeldsen L, Wielsoe M, Norgaard-Pedersen B, Mortensen EL, et al. 2019. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: A case-control study. Mol Autism 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Yau VM, Hansen R, Kharrazi M, Yoshida CK, Calafat AM, et al. 2018. Prenatal maternal serum concentrations of per- and polyfluoroalkyl substances in association with autism spectrum disorder and intellectual disability. Environ Health Perspect 126:017001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KB, Ravn MH, Arnfred J, Olsen J, Rask CU, Obel C. 2018. Characteristics of undiagnosed children with parent-reported adhd behaviour. Eur Child Adolesc Psychiatry 27:149–158. [DOI] [PubMed] [Google Scholar]
- Meng Q, Inoue K, Ritz B, Olsen J, Liew Z. 2018. Prenatal exposure to perfluoroalkyl substances and birth outcomes; an updated analysis from the danish national birth cohort. Int J Environ Res Public Health 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niclasen J, Teasdale TW, Andersen A-MN, Skovgaard AM, Elberling H, Obel C. 2012. Psychometric properties of the danish strength and difficulties questionnaire: The sdq assessed for more than 70,000 raters in four different cohorts. PloS one 7:e32025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nost TH, Vestergren R, Berg V, Nieboer E, Odland JO, Sandanger TM. 2014. Repeated measurements of per- and polyfluoroalkyl substances (pfass) from 1979 to 2007 in males from northern norway: Assessing time trends, compound correlations and relations to age/birth cohort. Environ Int 67:43–53. [DOI] [PubMed] [Google Scholar]
- Obel C, Heiervang E, Rodriguez A, Heyerdahl S, Smedje H, Sourander A, et al. 2004. The strengths and difficulties questionnaire in the nordic countries. Eur Child Adolesc Psychiatry 13 Suppl 2:II32–39. [DOI] [PubMed] [Google Scholar]
- Ode A, Kallen K, Gustafsson P, Rylander L, Jonsson BA, Olofsson P, et al. 2014. Fetal exposure to perfluorinated compounds and attention deficit hyperactivity disorder in childhood. PLoS One 9:e95891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, et al. 2001. The danish national birth cohort--its background, structure and aim. Scand J Public Health 29:300–307. [DOI] [PubMed] [Google Scholar]
- Oulhote Y, Steuerwald U, Debes F, Weihe P, Grandjean P. 2016. Behavioral difficulties in 7-year old children in relation to developmental exposure to perfluorinated alkyl substances. Environ Int 97:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston EV, Webster TF, Oken E, Claus Henn B, McClean MD, Rifas-Shiman SL, et al. 2018. Maternal plasma per- and polyfluoroalkyl substance concentrations in early pregnancy and maternal and neonatal thyroid function in a prospective birth cohort: Project viva (USA). Environ Health Perspect 126:027013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Melnick RL, Thayer KA, Seidler FJ. 2008. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro. Environ Health Perspect 116:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CR, Savitz DA. 2011. Serum perfluorinated compound concentration and attention deficit/hyperactivity disorder in children 5–18 years of age. Environ Health Perspect 119:1466–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom M, Hansen S, Olsen SF, Haug LS, Rantakokko P, Kiviranta H, et al. 2014. Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes--a prospective study with long-term follow-up. Environ Int 68:41–48. [DOI] [PubMed] [Google Scholar]
- Thapar A, Cooper M, Rutter M. 2017. Neurodevelopmental disorders. Lancet Psychiatry 4:339–346. [DOI] [PubMed] [Google Scholar]
- Toms LM, Thompson J, Rotander A, Hobson P, Calafat AM, Kato K, et al. 2014. Decline in perfluorooctane sulfonate and perfluorooctanoate serum concentrations in an australian population from 2002 to 2011. Environ Int 71:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Casey BJ. 2011. Behavioral assessment of emotion discrimination, emotion regulation, and cognitive control in childhood, adolescence, and adulthood. Front Psychol 2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri L, Vanderweele TJ. 2013. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: Theoretical assumptions and implementation with sas and spss macros. Psychol Methods 18:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer M, Dixon A, Rose D. 2008. Parent and child agreement on reports of problem behaviour obtained from a screening questionnaire, the sdq. Eur Child Adolesc Psychiatry 17:491–497. [DOI] [PubMed] [Google Scholar]
- Verner MA, Loccisano AE, Morken NH, Yoon M, Wu HL, McDougall R, et al. 2015. Associations of perfluoroalkyl substances (pfas) with lower birth weight: An evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (pbpk). Environmental Health Perspectives 123:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Wang Z, Xie C, Webster GM, Ye X, et al. 2018. Childhood perfluoroalkyl substance exposure and executive function in children at 8years. Environ Int 119:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Xie C, Dietrich KN, Braun JM, Webster GM, et al. 2019. Prenatal and childhood exposure to poly- and perfluoroalkyl substances (pfas) and cognitive development in children at age 8 years. Environ Res 172:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen PC, Lien GW, Chen HY, Tseng YC, et al. 2014. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan maternal and infant cohort study. Environ Health Perspect 122:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Venners SA, Mattman A, Martin JW. 2014. Associations between perfluoroalkyl acids (pfass) and maternal thyroid hormones in early pregnancy: A population-based cohort study. Environ Res 133:338–347. [DOI] [PubMed] [Google Scholar]
- Yuan YC. 2010. Multiple imputation for missing data: Concepts and new development (version 9.0). SAS Institute Inc, Rockville, MD: 49:1–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


