Abstract
Polychlorinated biphenyls (PCBs) are one of the original twelve classes of toxic chemicals covered by the Stockholm Convention on Persistent Organic Pollutants (POP), an international environmental treaty signed in 2001. PCBs are present in the environment as mixtures of multiple isomers at different degree of chlorination. These compounds are manmade and possess useful industrial properties including extreme longevity under harsh conditions, heat absorbance, and the ability to form an oily liquid at room temperature that is useful for electrical utilities and in other industrial applications. They have been widely used for a wide range of industrial purposes over the decades. Despite a ban in production in 1979 in the US and many other countries, they remain persistent and ubiquitous in environment as contaminants due to their improper disposal. Humans, independent of where they live, are therefore exposed to PCBs, which are routinely found in random surveys of human and animal tissues. The prolonged exposures to PCBs have been associated with the development of different diseases and disorders, and they are classified as endocrine disruptors. Due to its ability to interact with thyroid hormone, metabolism and function, they are thought to be implicated in the global rise of obesity diabetes, and their potential toxicity for neurodevelopment and disorders, an example of gene by environmental interaction (GxE). The current review is primarily intended to summarize the evidence for the association of PCB exposures with increased risks for metabolic dysfunctions and neurobehavioral disorders. In particular, we present evidence of gene expression alterations in PCB-exposed populations to construct the underlying pathways that may lead to those diseases and disorders in course of life. We conclude the review with future perspectives on biomarker-based research to identify susceptible individuals and populations.
Keywords: Polychlorinated Biphenyls (PCBs), Exposure, Gene expression, Biomarkers, Gene Validation
1. Introduction: Polychlorinated Biphenyls in Humans
Polychlorinated biphenyls (PCBs) are a class of chlorinated organic chemicals that are resistant to environmental/metabolic degradation and therefore considered as persistent organic pollutants (POPs). PCBs were widely used from the 1930’s through the 1980’s and later in some countries, with an estimated cumulative production of about 1.3 million metric tons worldwide (Breivik et al., 2002). PCB production was banned by the United States Congress in 1979 and by the Stockholm Convention on Persistent Organic Pollutants in 2001 (http://www.pops.int/documents/convtext/convtext_en.pdf). Even though the production was banned, exposure continued from the leakage of transformers and capacitors, volatilization of improperly disposed PCBs, and combustion of materials containing PCBs (Dyke et al., 2003). Due to their persistence for long periods in the environment and their highly lipophilic nature, PCBs remained detectable at significant levels in humans even after the bans, as they tend to bioaccumulate in the adipose tissue of animals and humans through contaminated food, water, and air (Giera et al., 2011; Schecter et al., 2010).
Evaluation of PCB levels in sera from adults from a major manufacturing site in Anniston, Alabama, collected from 1996 to 1999, indicated that this was one of the most highly exposed communities in the world ((ATSDR), 2000; Silverstone et al., 2012). In another highly contaminated area General Electric (GE) discharged PCBs into the Upper Hudson River for over 30 years, ending in 1977. As a result, a 200-mile stretch of the Hudson River and the NY/NJ Harbor was one of the most serious PCB contaminated area in the world (Sandy et al., 2012). Many countries worldwide reported the presence of PCBs in human milk and are well documented in the neonatal period of the children and the concentration PCBs varies with the duration of breast feeding (Čechová et al., 2017; Chovancová et al., 2011; Lancz et al., 2015; Yu et al., 2007). Also, PCBs contaminated foods are considered as pathways of exposure of these developmental neurotoxins, Interestingly, significant level of PCBs (during 2004-2005) were reported in the breast milk of mothers residing around an open dumping site in Kolkata, India where fishes were the potential dietary source of exposure (Someya et al., 2010). The biomonitoring data from the National Health and Nutritional Examination Survey (NHANES) to characterize both individual and multiple chemical exposures in the U.S. detected PCBs and related compounds (e.g. PCBs, 4–8 ng/g lipid; PBDEs, 5–23 ng/g lipid) in 99-100% of pregnant women (Woodruff et al., 2011).
Several unfortunate occurrences of acute PCB poisoning have been reported. Notably, in 1968 Yusho in western Japan, accidental human exposure to rice oil "Kanemi rice oil contaminated with PCBs caused food poisoning in many people and led to the development of Yusho oil disease (Fukushi et al., 2016; Kamio et al., 2020; Miyawaki et al., 2015). Even after half a century passed since the Yusho incident, and although inflammatory disorders such as suppuration have been observed in Yusho patients, the etiology of this inflammation susceptibility remains murky. Recent study on the mechanisms of susceptibility to inflammation in Yusho patients divulge that the comparative proportion of natural killer cells was higher in Yusho patients than in healthy subjects, while the amount of regulatory T cells did not differ among groups suggests that the innate immune response has been triggered in Yusho patients. Increased numbers of NK cells in Yusho patients indicates that the innate immune response has been activated in Yusho patients. The seemingly paradoxical results for CTLA-4 and IFN-γ may reflect counterbalancing mechanisms preventing excessive NK cell activation. This dysregulation of innate immunity may perhaps add to the inflammation observed in Yusho patients (Kamio et al., 2020). In 1979, about 2000 people in central Taiwan were intoxicated via rice oil consumption that was contaminated with PCBs. This "Yu-cheng" incident was one of the two known major human PCB intoxication episodes (Lung SC, 2005). These patterns reflecting distinctive exposure scenarios are valuable for the future epidemiologic studies when linking exposure with specific health effect (Hsu et al., 2005). The clinical manifestations associated with the people who fell ill after eating rice-bran oil contaminated with PCBs fluids (Onozuka et al., 2011) included low birth weights (Tsukimori et al., 2012), chloracne, and hyperpigmentation (Hashiguchi et al., 2011), especially in newborns (Hsu et al., 1995; Yoshimura, 2003).
Effects of PCBs on chronic diseases have also been widely reported. Although diabetes has not usually been considered to be an environmentally induced disease, there is a growing body of evidence that environmental exposure to POPs is associated with an increased prevalence of this disease (Langer et al., 2014). Elevated prevalence of diabetes has been demonstrated following exposure to dioxin, a related compound, in Seveso, Italy (Bertazzi et al., 1998; Pesatori et al., 1998). Cramer et al. (Cranmer et al., 2000) found that plasma insulin concentrations were elevated in individuals who had elevated levels of dioxin, and they concluded that dioxin exposure leads to insulin resistance. Additional studies (Longnecker and Daniels, 2001b; Longnecker et al., 2001a; Radikova et al., 2004) showed the dose-dependent relationships between diabetes or fasting-glucose levels and PCBs. Perhaps most compelling are the reports (Everett et al., 2007; Lee et al., 2006; Lee et al., 2007), which demonstrated dose-response relationships between serum concentrations of different organochlorine compounds and the increased prevalence of diabetes (Eden et al., 2016; John C et al., 2018; Son et al., 2010; Wolf et al., 2019).
Despite the previous bans on production in all Stockholm Convention signatory countries, PCBs still occupy an important position as human toxicants as they ranked 5th out of more than 275 chemicals and chemical classes, given their frequency, toxicity, and potential for human exposure ((ATSDR), 2018). In the remainder of this review, we focus on the biomedical consequences of the worldwide production of PCBs and their long-lasting legacy in the environment.
2. PCBs Exposure in Relation to Metabolic Disorders and Neurobehavioral Disease Development
2.1. Obesity and Diabetes
Over the last several decades, the prevalence of obesity had raised sharply in most countries worldwide (Saklayen, 2018; Yach et al., 2006). In 2016, the World Health Organization estimated that more than 650 million adults were obese, a prevalence rate of 13% of the world's population that has nearly tripled since 1975 (WHO 2020). Among the many factors for the cause of obesity, POPs such as organochlorine (OC) pesticides and PCBs might be particularly interesting because several epidemiological and experimental studies have shown that the low dose OC pesticides or PCBs were strongly linked to type 2 diabetes, insulin resistance, and metabolic syndrome (Aminov and Carpenter, 2020; Lee et al., 2017; Suarez-Lopez et al., 2015; Wolf et al., 2019; Zani et al., 2019), in all of which obesity was believed to play a critical role (Lee et al., 2006).
2.1.1. Endocrine Disruption
Endocrine disruptors are exogenous compounds with the potential to disturb hormonal regulation and the normal endocrine system, consequently affecting health and reproduction in animals and humans. Endocrine disruptors can interfere with the production, release, metabolism, and elimination of or can mimic the occurrence of natural hormones (Tabb and Blumberg, 2006). This concern is further amplified by two factors, the expansion in chemical production with a global sale estimated at US dollars 3.47 trillion in 2017 (ECIC, 2018), and the increased pollution from chemicals (Casals–Casas and Desvergne, 2011).
There is growing evidence that PCB-related perturbations of endocrine regulatory systems in early gestation may contribute to the development of obesity in later life (Philibert et al., 2009; Turyk et al., 2009a; Turyk et al., 2009b; Vasiliu et al., 2006; Wang et al., 2008). Conversely, PCBs with more chlorine atoms tended to show inverse associations with obesity (Arrebola JP et al., 2010; Dirinck et al., 2011), and some effects are more pronounced in men than in women, or vice versa (Elobeid et al., 2010). The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study found that environmental exposure to some POPs substantially increased risk of future type 2 diabetes in an elderly population (Lee et al., 2011). Investigations on the same PIVUS study, using both a cross-sectional and a prospective approach, demonstrated that the less chlorinated PCBs, some OC pesticides including p,p′-DDE, and dioxin had all showed an increased risk of abdominal obesity (Lee et al., 2012).
2.1.2. PCB Exposures and Diabetes
Type 2 Diabetes Mellitus (T2DM), the predominant form of diabetes, is a chronic, multifactorial, metabolic disorder characterized by hyperglycemia and has become a public health challenge worldwide (Bar-Tana, 2020; Lee et al., 2020). Beta cell dysfunction, impaired insulin secretion, and increased insulin resistance cause the chronic dysregulation of the hyperglycemia state and are critical in pathophysiological determinants of the disease’s progression and pathogenesis (Cohrs et al., 2020; Min-Kyung et al., 2020). T2DM can lead to life-threatening complications, viz., neuropathy, cerebrovascular disease, nephropathy, peripheral vascular disease, coronary artery disease, and retinopathy (Einarson et al., 2018; Muñoz-Torres et al., 2020). Rapid Industrialization and urbanization, high caloric diet, decreased physical activities, and genetic predispositions, alone or aggregated cannot fully suffice the sharp rise of T2DM globally. Environmental factors are believed to account for a substantial burden of T2DM.
Recent evidence supports the hypothesis that exposure to some commonly encountered environmental contaminants might also contribute to Type II diabetes, particularly organochlorine (OC) exposure (Carpenter, 2008; Eden et al., 2016; Lee et al., 2012; Lee et al., 2011; Silverstone et al., 2012; Son et al., 2010; Ukropec et al., 2010; Wolf et al., 2019). Several PCB congeners and chlorinated pesticides have also been associated with increased diabetes risk (Alonso-Magdalena P et al., 2011; Dirinck et al., 2011; Ghosh et al., 2014; Ghosh et al., 2011a; Ghosh et al., 2015; Ghosh et al., 2013; Ghosh et al., 2011b; Rignell-Hydbom et al., 2010; Turyk et al., 2009a; Turyk et al., 2009b; Wang et al., 2008). Until recently diabetes has not been considered to be an environmentally induced disease, but associations between serum PCB and pesticide levels and Type II diabetes have been observed in the past decade (Carpenter, 2011; Codru et al., 2007; Eden et al., 2016; Everett et al., 2011; Lee et al., 2011; Patel et al., 2010; Persky et al., 2011; Silverstone et al., 2012; Son et al., 2010; Wolf et al., 2019). Additionally, Bisphenol-A (BPA) is one of the most prevalent endocrine disrupting chemicals (EDCs). It is applied as the base compound in the manufacture of polycarbonate and other plastics present in many consumer products. Humans are consistently exposed to BPA and, in consequence, this compound has been detected in the majority of individuals examined (Lind and Lind, 2018). While endocrine disrupting chemicals (EDCs) like bisphenol-A (BPA) have received special attention for their mechanistic role in metabolic disruption, clinical diabetes setting to demonstrate an association of increased BPA levels with cellular senescence, proinflammation, poor glycemic control, insulin resistance, and shortened telomeres in patients with T2DM (Soundararajan et al., 2019), and has given a solid support for the role of BPA in the etiology of diabetes and other metabolic disorders (Tudurí et al., 2018). These results do not establish cause and effect, but there is a growing body of evidence that environmental exposure to persistent organochlorine compounds is associated with an elevated incidence of Type II diabetes (Baillie-Hamilton, 2002; Crinnion, 2011).
Studies supporting the associations of metabolic diseases to PCB exposures are growing and substantial. Of the components of metabolic syndrome, high blood pressure, elevated triglycerides, and glucose intolerance were most closely associated with these pollutants (Uemura et al., 2009). A study of selected POPs in the NHANES 1999–2002 data set has reported an association between waist circumferences and BMI in subjects with detectable levels of POPs, perhaps suggesting that these chemicals are plausible contributors to the obesity epidemic (Elobeid et al., 2010). Supporting that hypothesis, a prospective analysis in the Nurses’ Health Study based on two prospective studies and Meta-analysis indicate that higher plasma HCB and total PCB concentrations at baseline were significantly associated with increased incidence of diabetes development (Wu et al., 2012). In the Nurses’ Health Study II, a nested case-controlled study involving middle-aged US women conducted on plasma-POPs (including 3 organochlorine pesticides [OCPs] and 20 polychlorinated biphenyls [PCBs]) indicated a higher risk of Type 2 diabetes during more than 11 years of follow up. Age, breastfeeding history, previous weight change, and concurrent body weight were among the primary determinants of circulating POP concentrations (Zong et al., 2018). A Belgian study observed a lag in breast development with higher current serum concentrations of dioxin-like compounds. A relation concerning lower insulin secretion in puberty related to higher prenatal dioxin prenatal and lactational PCDD/F exposure and later initiation of breast development was seen. A delay in initiation of breast development was found in girls with higher prenatal and PCDD/F exposure (Leijs et al., 2008). Studies also demonstrated that timing of pubertal onset can be altered by prenatal exposure to dioxins or PCBs (Humblet et al., 2011).
2.1.3. Potential Mechanisms by which PCBs Might Induce Diabetes
Several studies have shed light on possible biological mechanisms underlying the epidemiological evidence described above. For example, it has been proposed that associations between PCB exposure and diabetes in post-menopausal women might reflect pancreatic β-cell function rather than insulin resistance (Persky et al., 2011). Lee et al. (Lee et al., 2017) addressed this mechanism in an experimental study that evaluated the exposure of low dose POPs on the insulin secretary function of the β-cells in human and in vitro cells: they found that serum concentrations of POPs may increase the risk of T2DM by primarily affecting pancreatic β-cell function rather than insulin resistance. La Merill et al. (La Merrill et al., 2019) studied the influence of prior exposure to POP’s on the diabetes risk of Asian Indians’ even after they have immigrated to the US. Their results showed increased insulin resistance in people with higher levels of DDT in their blood and suggested explanation is that it occurs through excess hepatic fat levels independent of obesity leading to an increased risk of diabetes.
2.2. Neurobehavioral and Cognitive Effects
The incidence of neurodevelopmental and neurodegenerative diseases globally has significantly increased over the last decades (Cannon and Greenamyre, 2011; Feigin and Vos, 2019). However, the etiology remains unclear. Evidence is growing that exposure to persistent organic pollutants during complex neurodevelopmental periods such as early life may be a potent risk factor, predisposing the individual to disease development in life in the future. Epidemiological studies have associated environmentally persistent organic pollutant exposure to brain disorders viz., neuropathies, cognitive, motor, and sensory impairments; neurodevelopmental disorders such as autism spectrum disorder (ASD) (Sealey et al., 2016), attention-deficit hyperactivity disorder (ADHD) (Rossignol et al., 2014); and neurodegenerative diseases including Alzheimer’s disease (Singh et al., 2012), Parkinson’s disease (Singh et al., 2014), and amyotrophic lateral sclerosis (ALS) (Tran and Miyake, 2017). In many ways, this expands the conventional “Developmental Origins of Health and Disease” paradigm to include exposure to pollutants (Nathalie et al., 2019). Epidemiological studies on PCB exposure have also identified associations on possible long-term effects on diseases such as Alzheimer’s, attention-deficit hyperactivity disorder (ADHD) and autism spectrum disorder (Nathalie et al., 2019).
2.2.1. Low Dose Prenatal Exposures & Neurodevelopment
It is estimated that nearly 12 million U.S. children (17%) under age 18 suffer from one or more learning, developmental or behavioral disabilities, according to the U.S. Centers for Disease Control and Prevention (Bloom et al., 2011). These disabilities are clearly the result of complex interactions among genetic, environmental and social factors that impact children during vulnerable periods of development (Schettler et al., 2000). There is evidence, summarized here, implicating PCB exposures in these important health issues.
In one study, children with higher prenatal mono-ortho-substituted PCB exposures performed more poorly on the Bayley Scales, suggesting impaired brain development in utero (Park et al., 2010). Furthermore, studies on neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally divulged an association between organochlorines (mainly PCBs) and neuropsychological measures of attention among boys but not girls (Sagiv et al., 2012). The negative effects of exposure to PCBs on early psychomotor development suggest that the potential neurotoxic effects of these compounds may be evident even at low doses (Forns et al., 2012a; Forns et al., 2012b). Cohorts with lower exposure levels have demonstrated mixed results (Forns et al., 2012a; Forns et al., 2012b; Stewart et al., 2012). On the other hand, prenatal exposure to low-level concentrations of PCBs, particularly PCB153, was associated with an overall deleterious effect on neuropsychological development at 4 years of age, including negative effects on executive function, verbal functions and visuospatial abilities (Forns et al., 2012a; Forns et al., 2012b). A recent mother child cohort study reported between prenatal BPA exposure and differences in children's brain microstructure, which appeared to mediate the association between this exposure and children's behavioral symptoms (Mustieles and Fernández, 2020). Postnatal BPA is also linked with increasing executive function difficulties in preschool children (England-Mason et al., 2020).
Such PCB-induced effects might be of even greater significance for children genetically predisposed to behavioral problems such as attention-deficit hyperactivity disorder (Verner et al., 2010). Studies with Mohawk Indian adolescents, living in a PCB-contaminated area of New York State, suggested that they had experienced continuing or recent environmental exposure to PCBs that was sufficient to result in detectable cognitive decrements (Fitzgerald et al., 2008; Fitzgerald et al., 2012; Newman et al., 2006; Newman et al., 2009). Several other investigations concluded that exposure to PCBs may be associated with some measures of memory and learning and depression among adults of age 55-74 years in upper Hudson river communities whose current body burdens are similar to those of the general population (Fitzgerald et al., 2008; Fitzgerald et al., 2012).
2.2.2. Associations of PCBs with Risks of Parkinson’s and Alzheimer’s Diseases
The long-term effects on PCBs exposure have also been linked to risk of adult-onset neurodegenerative diseases such as Parkinson’s and Alzheimer’s, and to broader cognitive declines in older persons. In terms of Parkinson’s disease, some studies have suggested greater susceptibility of females to the effects of PCB congeners 153 and 180, and they were significantly elevated in the brains of Parkinson’s disease patients (Hatcher-Martin et al., 2012). A retrospective mortality study of PCB-exposed workers at electrical capacitor plants showed that the rate of Parkinson’s disease-related mortality is twice that of controls (US population), and sex specific analysis revealed that the women are at a 3-fold higher risk (Steenland et al., 2006).
Analysis of a population-based study on aged 65+ years which included 669 clinically assessed subjects followed up for 10 years in the Canadian Study of Health and Aging (CSHA) - a national cohort study of dementia – revealed that the exposure to PCB congeners 118, 153, 156 and 163, and two OC pesticides, p,p′-DDT and p,p′-DDE, reduced the mean cognitive performances of adults in the cohort (Medehouenou et al., 2019). Path analysis used on the data from the U.S. National Health and Nutrition and Examination Survey (1999–2002) revealed association between PCB exposure, specifically PCB 146, and lower cognitive functioning especially in older adults (Przybyla et al., 2017). An evaluation of the neuropsychological status and low-level PCB exposure among older adults aged 55-74 years revealed memory and learning deficits in persons with higher serum PCB concentrations (Fitzgerald et al., 2008).
Little awareness has been paid to neurotoxicants in relation to the risk of dementia. Exposure to known neurotoxicants such as PCBs and organochlorine pesticides is suspected to have adverse cognitive effects (Medehouenou et al., 2019). Early studies have reported negative effects associated with prenatal or perinatal exposure to PCBs on aspects of cognitive functioning in children (Klocke et al., 2020; Zhang et al., 2017), Alzheimer's disease and cognitive decline in an older population (Medehouenou et al., 2019) with considerable variation in findings of different studies which could be due to the particular congeners comprising the different exposures in studies of PCBs and cognition. The elimination of organochlorines and other persistent chemical agents from the body is hard. Due to their long retention time (even for life) these chemicals have an adverse neurodevelopmental impact as a result of various pathophysiologic mechanisms including neuronal mitochondrial toxicity, disruption of neurotransmitter regulation and potentially widespread cause of declining brain function and dementia (Genuis and Kelln, 2015).
Biomarker Discovery and Validation Work
3.1. Gene-Expression Based Biomarkers Identify Disease Susceptibility
Gene–environment interactions (GxE) play an important role in determining the relationship between exposure to environmental chemicals and risk to human health. A growing body of research is beginning to suggest that many chronic adult diseases and disorders, including asthma, diabetes and obesity, may be traced back to exposures that occur during development which enable flexibility include epigenetic processes, affecting the expression of genes associated with regulatory pathways (Gluckman et al., 2010). Currently, there are still many roadblocks to evaluating such disease risks associated with this large group of 209 PCB congeners all of which have different physiochemical properties, variable fate and transport mechanisms in the environment, and a different range of ability for persistence, bioaccumulation, and biological activity (Birnbaum and Staskal-Wikoff, 2010). Nevertheless, there are recent developments in research methods and study designs that may offer a way forward towards solving these conundrums.
During the last two decades, there has been increasing interest in the use of biomarkers in epidemiological research to enhance exposure assessment (Vineis et al., 2013), to gain insight into a disease mechanism, and to understand acquired or inherited susceptibility (Zetzsche et al., 2010). The validation of biomarkers as early predictors of clinical disease can enhance health risk assessment and contribute to effective new disease prevention policies in environmental and occupational settings (Bonassi et al., 2001). Given that gene-environment interaction underlines almost all human disease, the significance of genomic research on the public health is huge. The research on non-communicable chronic diseases with modifiable environmental risk are based not necessarily on finding the genetic “causes” but on improving existing approaches to identify environmental risk factors to better prevent and treat the disease. Such applied genomic research for environmentally influenced disease is important because; 1) it could help in stratifying disease risk (especially early diagnosis) and differentiating interventions with the greatest potential for achieving population health benefits; 2) it could help to identify new environmental risk factors for diseases or help to confirm suspected environmental risk factors; and 3) it could aid our understanding of disease occurrence in terms of transmission (mother to infant in longitudinal studies), and severity (highly-exposed population groups compared to others).
A collaborative study was executed in the authors’ laboratories to develop signature disease biomarkers (genes in pathways) with a focus on metabolic and neurological dysfunction/disease of a PCB-exposed human population. We prioritized the identification of biological response (exposure/disease biomarkers), indicators of environmental stress to develop early disease biomarkers. Our research used biospecimens (venous blood with minimal risks to the research participants) along with gene expression tools to study the early pathogenesis of the disease with an eye towards early interventions for multiple chronic diseases. The population selected for the study was based on PCB-Cohort (within a larger cohort design) from two districts of Eastern Slovakia: (a) Michalovce (high exposure), and (b) Svidnik District (lower exposure), where PCB 153 and PCB 138 were the most abundant PCBs in the blood samples of persons living in this environmentally contaminated region (Hertz-Picciotto et al., 2003; Wimmerová et al., 2015). In our gene expression studies, this was the key aspect of the study design, i.e. to select the comparative exposure groups based on the total PCB concentration (ng/g total serum lipid).
Earlier studies on PCB-Cohort (Hertz-Picciotto et al., 2003) established that even though the newborn child and mother had somehow similar concentrations of PCBs in their blood samples, the PCB load of the children increased after birth as they acquired more PCBs through breast feeding (Lancz et al., 2015), and then after weaning consumed fat from locally produced food of animal origin (Sonneborn et al., 2008), suggesting that additional pathways of environmental exposure were important determinants of PCB body burden.
As PCB cohort of children continued to be followed and monitored medically, we analyzed blood samples collected at 45 months of age. Microarrays (on Affymetrix platform) were used for measurement of global gene expression, pathway analysis was performed with Ingenuity Pathway Analysis (IPA). Briefly, data sets containing gene identifiers and corresponding expression values (fold change) from the microarray experiment were uploaded into Ingenuity Pathway Analysis software (Ingenuity® Systems, www.ingenuity.com). Each gene identifier was plotted to its similar gene entity in the Ingenuity Pathways Knowledge Base Genes differentially expressed with p<0.05 were overlaid onto global molecular networks developed from information contained in the IPA knowledge base. Networks were then algorithmically generated based on their connectivity. Networks were “named” on the most prevalent functional group(s) present. Canonical Pathway (CP) analysis identified function specific genes significantly present within the networks. To advance our pathway analysis, we used the data sources from Ingenuity expert findings and used the “Core Analysis” function to interpret the data in the perspective of biological processes, pathways, and networks to capture an overall view. Differentially expressed gene identifiers were defined as value parameters for analysis and identified the relationship between gene expression alterations and related changes in biofunctions under the subcategories of Molecular and Cellular Functions, Physiological System Development and Function, and Disease and Disorders (only shown here, Fig. 1.).
Fig. 1.
Comparison of top biofunctions in disease and disorder development with the in vitro studies (PCBs mixed, PCB-153 and PCB-138 alone), and from the 45-month-old Slovak children’s cohort, as generated through IPA analysis. The gene sets from each analysis were processed with same filter settings and were uploaded and run through the IPA Comparative Analysis module. The important disease and disorders that are represented here were at or above the threshold value (corresponding to a p value of 0.05, comparing high- and low-exposure groups). Fischer’s exact test was used to calculate this p-value determining the probability that each biological function and/or disease assigned to that dataset is due to chance alone.
The analysis of the gene expression levels with IPA yielded the top disease and disease disorders among which the Endocrine System Disorder (Warembourg et al., 2016), Neurological Disease (Colter et al., 2018), Cardiovascular Disease (Pavuk et al., 2019; Raffetti et al., 2020), which is well established are represented that also corroborated well with other studies discussed earlier. Besides them, at the interest of the researchers, we are reporting here that the analysis also yielded important other future disease risks viz., Cancer (Ghosh et al., 2018; Mikhaylov et al., 2020), Reproductive System Disease (Neblett et al., 2017), Developmental Disorder (Polańska et al., 2013), Skeletal and Muscular Disorder (Mitra et al., 2012; Sisto et al., 2015; Williams et al., 2020), Infection Mechanism (Waugh et al., 2018), Renal & Urological Disease (Grice et al., 2017), Dermatological Disease (Leijs et al., 2018), Gastrointestinal Disease (Cheng et al., 2018), Inflammatory Disease and Inflammatory Response (Wang et al., 2019), Connective Tissue Disorder (Abella V et al., 2016; Lee et al., 2007) etc., in their respective canonical pathways which is very much consistent and corroborated with the other studies (shown in parenthesis) on the future risk of disease and disorder development of these exposed children in Slovakia (Fig. 1.)
Those experiments helped us to identify some potential signature biomarkers of PCB exposure effects, namely RRAD, MYC, CD3, CYP1A2, PON1, CYP2D6, ARNT, BCL2, LEPR, LPR12, ENTPD3, ITGB1, NPPB, and TRAP1. In turn, these genes (p <0.0.5, with Fold Change +/−1.5) were mapped to their respective pathways, i.e. Cardiovascular, Obesity, Diabetes, Cancers, and Neurobehavioral Deficits (Ghosh et al., 2018; Ghosh et al., 2014; Ghosh et al., 2011a; Ghosh et al., 2015; Ghosh et al., 2013; Ghosh et al., 2011b; Mitra et al., 2012). The studies cited above showed that there are significant similarities in vitro and population studies (Slovak exposed population) in functionality, pathway, and disease & disorder development across this cohort (Ghosh et al., 2011a; Ghosh et al., 2011b).
Looking more closely at these genes and their pathways reveals interesting molecular pathways associated with increasing risks of metabolic dysfunctions, neurobehavioral, and other disease risks in course of life. PCBs exposures-For example, the genes RRAD (Ras-related associated with Diabetes), PON1 (Human paraoxonase-1,a ), and LEPR (Leptin Receptor) have been identified as three putative biomarkers of PCB exposures that map to the canonical, biofunctional pathways of Type II Diabetes Mellitus Signaling, Insulin Receptor Signaling, and Type I Diabetes Mellitus Signaling, respectively (Ghosh et al., 2014; Ghosh et al., 2013). Diving deeper into one of the potential mechanisms represented in those results, the adipokine molecule leptin has received significant interest as a potential programming factor; alterations in the profile of leptin (LEPR mostly down-regulated in our population based study) in early life are associated with altered susceptibility to obesity and metabolic disorders in adulthood (Ghosh et al., 2014). Maintenance of a critical leptin level during early development facilitates the normal maturation of tissues and signaling pathways involved in metabolic homeostasis (Vickers and Sloboda, 2012).
Our in vitro model system (in HepG2) in deciphering the pathways related to mode of action to the PCBs exposure is an important advance in the sense that it accommodates the mixtures of chemicals and group of gene(s) in their respective pathways (pathway specific) (Ghosh et al., 2018), in contrast to most of the studies on gene-environment interaction that typically have focused on a single exposure or candidate gene (Bookman et al., 2011). Our study paved the way towards developing a genomic classifier for early disease risk as a consequence of earlier life exposures. What is needed next in this progression of knowledge is validation of the findings, which we address in the next section of this review.
3.2. Validation of Signature Biomarkers by High-Throughput Taqman® Low Density Array (TLDA) application: The Next Step Forward
A biomarker must be validated before it can have a meaningful impact on health risk assessment. An impressive amount of literature has been published on the concept of biomarker validity and the various aspects of the validation process, which outlines the main steps of the validation process of biomarkers of risk (Bonassi et al., 2001; Tahara et al., 2009). The first step in such a process is biomarker development, which we discussed in the section above. The second step considered possible perturbation in the association between putative biomarker of risk and outcome. The third step aimed at assessing the presence of a causal relationship between a biomarker and its associated disease using epidemiologic studies. A general recommendation is that the validation effort concentrate on those biomarkers that are directly involved in the causal pathway of disease, since closer is the biomarkers to the causal pathway, the more precisely it will predict disease. The overall process of biomarker and discovery validation, in the context of our cohort of pre-natally exposed children is shown in Fig. 2.
Fig. 2.
Authors’ perspective on a proposed model of how PCB exposures pre- and post-natal can lead to effects on gene expression alterations in early childhood. In this proposed model, neurobehavioral deficits (mental development index in the figure) in the children are considered as exposure outcomes.
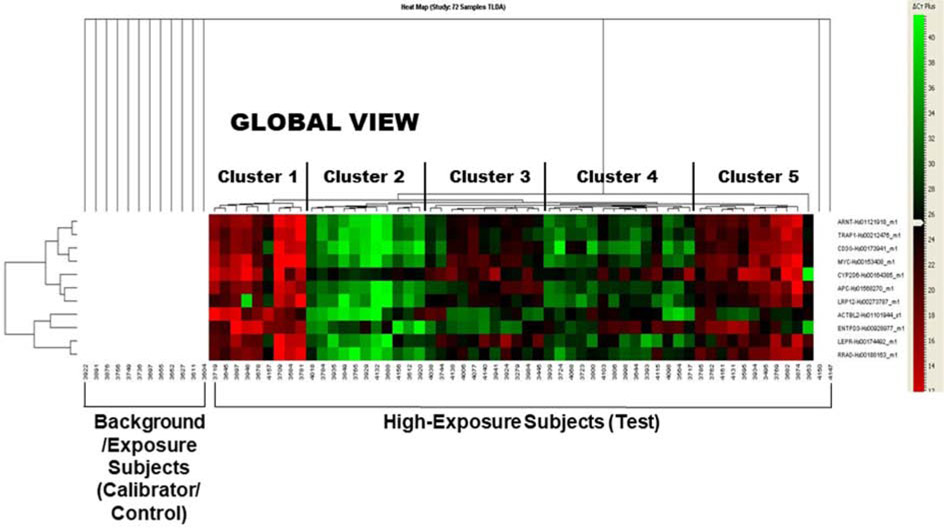
With epidemiological data in hand and the identification of putative biomarkers related to disease and disorder development, to further refine and validate the signatures of PCB exposure, we randomly choose 72 subjects from the high exposure (subgroup from the Slovak PCB cohort, based on median child serum concentrations of PCB-153 >121 ng/g lipid). We designed a 16-format TLDA microfluidic card with our signature gene panel (described in section 3.1, above): the cards were configured in a 384-well format and then subjected to high-throughput qRT-PCR. This was essentially a reverse transcriptase polymerase chain reaction process using the ABI Prism 7900 HT Sequence Detection System (Applied Biosystems) that allowed us to amplify each gene in the panel and compared the intensity (fold-difference) of the signals from the high exposure group with the control (calibrator) subjects (subjects with low or no detectable PCB exposures in the cohort) (Total n=72). The preliminary results were quite encouraging. The unsupervised clustering of these gene expression levels indicated that the subjects could be reasonably grouped into five clusters depending on the expression of the candidate genes (Fig. 3.), segregating the high exposure group from the control groups. Examination of the heat map suggested a clear separation of the clusters, providing a clue that this “gene fingerprinting” approach could indeed lead us to identify altered disease risk genes related to the PCB load and suggestive of future health risks. Further mapping the gene(s) from to their respective pathophysiogical pathways (e.g. diabetes, neurobehavioral) allowed us to collapse a large number of potential single genes to a relatively small and consolidated group of disease pathways. It is reassuring that our gene expression results from the blood samples also correlated with those from the cultured PBMCs (Ghosh et al., 2018; Ghosh et al., 2014; Ghosh et al., 2015; Ghosh et al., 2013; Mitra et al., 2012). Larger population validation studies, our approach will have the ability to investigate disease association of a single gene over the whole number of subjects.
Fig. 3.
This heat map shows the Gene Expression and Hierarchical Clustering in the study population, as revealed by analysis of the Taqman Low Density Array® (TLDA). The results were obtained from building the hierarchical clustering from qRT-PCR data upon validation of the selected signature genes from 72 subjects from Slovakia with varied (high vs. low) PCBs exposures. We have used the expression results from the ABI platform using SDS RQ Manager (v.1.2.1.) and DataAssist™ v2.0. The analyses of global expression of the validating genes have indicated that the subjects are typically grouped into five (5) clusters depending on the expression of the candidate genes and segregating the high and low exposure groups.
In the context of an environmentally exposed PCB cohort who are under continuing medical surveillance, our ability to flag certain pathways of concern provides a way to stratify them into subgroups most in need to intensive monitoring for adverse health conditions, well before clinical symptoms arise. This cutting-edge approach is also scalable, using a high-throughput method over a single platform in thousands of subjects at a time. The results we stated above do show promise that large scale evaluation of changes in gene expression using microarrays, combined with a primary validation through high-throughput TLDA, may become a useful tool for toxicity evaluation, biomarker validation, and will empower us to study the process of development of diseases and aid in our understanding the potential health risk of PCBs
4. Conclusions and Implications for Future Studies
Over the past 20 years, knowledge of the genome and its functions have increased dramatically, but disease risk assessment methodologies using such knowledge have not advanced as quickly. In the field of environmental toxicity assessment, the need for in-time risk management decisions requires setting up a battery of standardized and relatively easy to perform tests, allowing quick answers to pressing regulatory questions, but these approaches typically address acute health endpoints. Several new health research approaches are developed that use new technologies to modernize toxicity testing towards the prediction, and ultimately the prevention, of future, chronic disease processes including risks of cancer, metabolic syndrome, and neurodegenerative diseases.
In the context of widespread environmental contaminants such as PCBs, to which entire populations may be exposed, it would be frustrating to follow persons for 60 years or more to see if they develop diseases based on what they were exposed to before birth, as opposed to acting on knowledge to prevent future disease. But it is increasingly recognized that even seemingly low-level exposures during early development can lead to functional deficits and increased disease risks later in life. The developmental period is also the most sensitive time for the development of the epigenetic system. If the “developmental basis of disease” hypothesis proves true, then there is an even greater need to change our focus from treating diseases after they are detected to prevention of future disease.
Fortunately, we are now in the era of “Omics” technologies (i.e. Toxicogenomics, Proteomics, Transcriptomics, Metabolomics, and others), which can yield personalized measures of exposure and disease risk. The genomic approach we highlighted above from our own studies of PCBs illustrate the development and validation of robust biomarkers that can provide a way to stratify highly vulnerable groups for more intensive surveillance, including early diagnosis and disease detection. Prediction of long-term effect of chemical exposures using genomic classifiers will facilitate assignment of treatment and development of more effective therapy, as illustrated in Fig. 4. which displays our own approach and a generalizable model appropriate to other exposure scenarios. Whichever approach is undertaken, the downstream goals should include ways to help inform policy decisions, address health disparities, and improve public health.
Fig. 4.
By using our overall approach that deploys genomic classifiers in relation to PCB exposures in our Slovak population, we were able to develop a method for discovering and validating biomarkers to predict future disease risks (as shown on the left). On the right, we generalize the approach for use in other types of populations and exposure scenarios.
The future holds for population studies of PCBs and other toxicants of concern. Targeted, hypothesis-driven studies that address the life span will be increasingly important (Fig. 5.). The integration of environmental sciences, genetics, epigenetics, and multiple omics technologies in the same study is highly likely to be much more informative than any single approach by itself, as there is mounting proof, for example, of epigenetic changes due to environmental exposures. Thus, epigenetics can act as a mediator of environmental exposure through genetic regulation, which is not included in this review, but readers should consider this fact while investigating the gene-environment interactions under any particular exposure. Ultimately, such a scientific model, together with systems biology and expertise in bioinformatics and biostatistics, holds great promise for identifying and ameliorating the harmful effects of environmental toxicants.
Fig. 5.
PCBs exposure pathways from the factory to the environment, and subsequently to the human fetus and finally to disease & disorder development in children and in adults. Such an exposure scenario across the lifespan has important implications for the development of biomarkers to monitor for disordered metabolic and other pathophysiological pathways that can signal future disease risks.
Acknowledgements
Authors are indebted to Dr. Vijay Chandra, Primus Hospital, New Delhi, India for his careful review of this manuscript. The contents of this report are solely the responsibility of the authors.
Funding Source
The authors are indebted for generous support by the 2-U54 MD00759731 grant (PI: Southerland from the National Institute of Minority Health and Disparity (NIMHD/NIH)), 1-P20 CA262617-01 (PI: Ghosh), U.S. NIH Grant R03-TW007152, The European Commission through the 7FP project OBELIX (No. 227391), Ministry of Health, Slovak Republic through projects 2007/07-SZU-03, 2012/41-SZU-05 and 2012/47-SZU-11, Slovak Research and Development Agency through projects APVV-0571-12 & APVV-0444-11, the project “Center of Excellence of Environmental Health”, based on the supporting Operational Research and Development Program financed from the European Regional Development Fund (ITMS No. 26240120033).
Footnotes
Conflict of Interest
There is no conflict of interest among the authors.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Statement on Human Participants Research
Some of the studies that include human research participants, mentioned herein, were undertaken with the prior approval by the Howard University Institutional Review Board (IRB-07-GSAS-30), and the informed consent were obtained from volunteers as per the approval of the Institutional Review Board (IRB).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- (ATSDR), A. for T.S. and D.R., 2000. Toxicological profile for Polychlorinated Biphenyls (PCBs). [PubMed] [Google Scholar]
- (ATSDR), A. for T.S. and D.R., 2018. Substance Priority List. [Google Scholar]
- Abella V, Pérez T, Scotece M, Conde J, Pirozzi C, Pino J, Lago F, González-Gay MÁ, Mera A, Gómez R, Gualillo O, 2016. Pollutants make rheumatic diseases worse: Facts on polychlorinated biphenyls (PCBs) exposure and rheumatic diseases. Life Sci. 157. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Quesada I, Nadal A, 2011. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol 7, 346–353. [DOI] [PubMed] [Google Scholar]
- Aminov Z, Carpenter DO, 2020. Serum concentrations of persistent organic pollutants and the metabolic syndrome in Akwesasne Mohawks, a Native American community. Env. Pollut 260, 114004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrebola JP, Fernandez MF, Porta M, Rosell J, de la Ossa RM, Olea N, Martin-Olmedo P, 2010. Multivariate models to predict human adipose tissue PCB concentrations in Southern Spain. Env. Int 36, 705–713. [DOI] [PubMed] [Google Scholar]
- Baillie-Hamilton PF, 2002. Chemical Toxins: A Hypothesis to Explain the Global Obesity. Epidemic J Altern Complement Med. 8, 185–192. [DOI] [PubMed] [Google Scholar]
- Bar-Tana J, 2020. Type 2 diabetes–unmet need, unresolved pathogenesis, mTORC1-centric paradigm. Rev Endocr Metab Dis. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertazzi PA, Bernucci I, Brambilla G, Consonni D, Pesatori AC, 1998. The Seveso studies on early and long-term effects of dioxin exposure: a review. Env. Heal. Perspect 106, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal-Wikoff DS, 2010. 5th International PCB Workshop--summary and implications. Env. Int 36, 814–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B, Cohen RA, Freeman G, 2011. Summary health statistics for U.S. children: National Health Interview Survey, 2010. National Center for Health Statistics. . Vital Heal. Stat 10 [PubMed] [Google Scholar]
- Bonassi S, Neri M, Puntoni R, 2001. Validation of biomarkers as early predictors of disease. Mutat Res 480–481, 349–358. [DOI] [PubMed] [Google Scholar]
- Bookman EB, McAllister K, Gillanders E, Wanke K, Balshaw D, Rutter J, Reedy J, Shaughnessy D, Agurs-Collins T, Paltoo D, Atienza A, Bierut L, Kraft P, Fallin MD, Perera F, Turkheimer E, Boardman J, Marazita ML, Rappaport SM, Boerwinkle E, Suomi SJ, Caporaso NE, Hertz-Picciotto I, Jacobson KC, Lowe WL, Goldman LR, Duggal P, Gunnar MR, Manolio TA, Green ED, Olster DH, Birnbaum LS, 2011. Gene-environment interplay in common complex diseases: forging an integrative model-recommendations from an NIH workshop. Genet Epidemiol 35, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivik K, Sweetman A, Pacyna JM, Jones KC, 2002. Towards a global historical emission inventory for selected PCB congeners--a mass balance approach. 1. Global production and consumption. Sci Total Env. 290, 181–198. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT, 2011. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicol. Sci 124, 225–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DO, 2008. Environmental contaminants as risk factors for developing diabetes. Environ. Heal 23, 59–74. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, 2011. Health effects of persistent organic pollutants: the challenge for the Pacific Basin and for the world. Rev Env. Heal 26, 61–69. [DOI] [PubMed] [Google Scholar]
- Casals-Casas C, Desvergne B, 2011. Endocrine disruptors: from endocrine to metabolic disruption. Annu Rev Physiol 73, 135–162. [DOI] [PubMed] [Google Scholar]
- Čechová E, Vojta Š, Kukučka P, Kočan A, Trnovec T, Murínová L’P, de Cock M, van de Bor M, Askevold J, Eggesbø M, M. S, 2017. Legacy and alternative halogenated flame retardants in human milk in Europe: Implications for children’s health. Env. Int. 108, 137–145. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Li X, Lehmler HJ, Phillips B, Shen D, Cui JY, 2018. Gut Microbiota Modulates Interactions Between Polychlorinated Biphenyls and Bile Acid Homeostasis. Toxicol Sci. 166, 269–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovancová J, Čonka K, Kočan A, Sejáková ZS, 2011. PCDD, PCDF, PCB and PBDE concentrations in breast milk of mothers residing in selected areas of Slovakia. Chemosphere 83, 1383–1390. [DOI] [PubMed] [Google Scholar]
- Codru N, Schymura MJ, Negoita S, Rej R, Carpenter DO, 2007. Diabetes in relation to serum levels of polychlorinated biphenyls and chlorinated pesticides in adult Native Americans. Env. Heal. Perspect 115, 1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs CM, Panzer JK, Drotar DM, Enos SJ, Kipke N, Chen C, Brennand A, 2020. Dysfunction of persisting β cells is a key feature of early type 2 diabetes pathogenesis. Cell Rep. 31, 107469. [DOI] [PubMed] [Google Scholar]
- Colter BT, Garber HF, Fleming SM, Fowler JP, Harding GD, Hooven MK, Howes AA, Infante SK, Lang AL, MacDougall MC, Stegman M, Taylor KR, Curran CP, 2018. Ahr and Cyp1a2 genotypes both affect susceptibility to motor deficits following gestational and lactational exposure to polychlorinated biphenyls. Neurotoxicology 65, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranmer M, Louie S, Kennedy RH, Kern PA, Fonseca VA, 2000. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is associated with hyperinsulinemia and insulin resistance. Toxicol Sci 56, 431–436. [DOI] [PubMed] [Google Scholar]
- Crinnion WJ, 2011. The role of persistent organic pollutants in the worldwide epidemic of type 2 diabetes mellitus and the possible connection to Farmed Atlantic Salmon (Salmo salar). Altern Med Rev 16, 301–313. [PubMed] [Google Scholar]
- Dirinck E, Jorens PG, Covaci A, Geens T, Roosens L, Neels H, Mertens I, Van Gaal L, 2011. Obesity and persistent organic pollutants: possible obesogenic effect of organochlorine pesticides and polychlorinated biphenyls. Obes. (Silver Spring) 19, 709–714. [DOI] [PubMed] [Google Scholar]
- Dyke PH, Foan C, Fiedler H, 2003. PCB and PAH releases from power stations and waste incineration processes in the UK. Chemosphere 50, 469–480. [DOI] [PubMed] [Google Scholar]
- ECIC, 2018. Facts & Figures of the European Chemical Industry. Eur. Chem. Ind. Counc [Google Scholar]
- Eden PR, Meek EC, Wills RW, Olsen EV, Crow JA, Chambers JE, 2016. Association of type 2 diabetes mellitus with plasma organochlorine compound concentrations. J Expo Sci Env. Epidemiol 26, 207–213. [DOI] [PubMed] [Google Scholar]
- Einarson TR, Acs A, Ludwig C, UH P, 2018. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol 17, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elobeid MA, Padilla MA, Brock DW, Ruden DM, Allison DB, 2010. Endocrine disruptors and obesity: an examination of selected persistent organic pollutants in the NHANES 1999-2002 data Int J Env. Res Public Heal; 7, 2988–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England-Mason G, Liu J, Martin JW, GF G, Letourneau N, Dewey D, Team, Ap.S., 2020. Postnatal BPA is associated with increasing executive function difficulties in preschool children. Pediatr Res. 10.1038/s41390-020-0922-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett CJ, Frithsen IL, Diaz VA, Koopman RJ, Simpson WM, Mainous AG, 2007. Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl and DDT with diabetes in the 1999–2002. Env. Res 103, 413–418. [DOI] [PubMed] [Google Scholar]
- Everett CJ, Frithsen I, Player M, 2011. Relationship of polychlorinated biphenyls with type 2 diabetes and hypertension. J Env. Monit 13, 241–251. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Vos T, 2019. Global Burden of Neurological Disorders: From Global Burden of Disease Estimates to Actions. Neuroepidemiology 52, 1–2. [DOI] [PubMed] [Google Scholar]
- Fitzgerald EF, Belanger EE, Gomez MI, Cayo M, McCaffrey RJ, Seegal RF, Jansing RL, Hwang SA, Hicks HE, 2008. Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Env. Heal. Perspect 116, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Shrestha S, Gomez MI, McCaffrey RJ, Zimmerman EA, Kannan K, Hwang SA, 2012. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and neuropsychological status among older adults in New York. Neurotoxicology 33, 8–15. [DOI] [PubMed] [Google Scholar]
- Forns J, Lertxundi N, Aranbarri A, Murcia M, Gascon M, Martinez D, Grellier J, Lertxundi A, Julvez J, Fano E, Goñi F, Grimalt JO, Ballester F, Sunyer J, Ibarluzea J, 2012a. Prenatal exposure to organochlorine compounds and neuropsychological development up to two years of life. Env. Int 45, 72–77. [DOI] [PubMed] [Google Scholar]
- Forns J, Torrent M, Garcia-Esteban R, Grellier J, Gascon M, Julvez J, Guxens M, Grimalt JO, Sunyer J, 2012b. Prenatal exposure to polychlorinated biphenyls and child neuropsychological development in 4-year-olds: an analysis per congener and specific cognitive domain. Sci Total Env. 432, 338–343. [DOI] [PubMed] [Google Scholar]
- Fukushi J-I, Tokunaga S, Nakashima Y, Motomura G, Mitoma C, Uchi H, Furue M, Iwamoto Y, 2016. Effects of Dioxin-Related Compounds on Bone Mineral Density in Patients Affected by the Yusho Incident. Chemosphere 145, 25–33. [DOI] [PubMed] [Google Scholar]
- Genuis SJ, Kelln KL, 2015. Toxicant exposure and bioaccumulation: a common and potentially reversible cause of cognitive dysfunction and dementia. Behav. Neurol 2015, 620143 10.1155/2015/620143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Loffredo CA, Mitra PS, Trnovec T, Palkovicova Murinova L, Sovcikova E, Hoffman EP, Makambi KH, Dutta SK, 2018. PCB exposure and potential future cancer incidence in Slovak children: an assessment from molecular finger printing by Ingenuity Pathway Analysis (IPA®) derived from experimental and epidemiological investigations. Environ. Sci. Pollut. Res 25, 16493–16507. 10.1007/s11356-017-0149-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Lubica M, Tomas T, Christopher A L, Kareem W, Partha S M, Sisir K D, 2014. Biomarkers linking PCB exposure and obesity. Curr. Pharm. Biotechnol 15, 1058–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Mitra PS, Malve P, Trnovec T, Palkovicova L, Sovcikova E, Hertz-Picciotto I, Sonneborn D, Ghimbovschi S, Hoffman EP, Dutta SK, 2011. Validation of Signature Biomarkers by High-Throughput Taqman® Low Density Array (TLDA) in PCB-exposed Population in ISEE 2011, 13–16 September, Barcelona. Env. Heal. Perspect. [Google Scholar]
- Ghosh S, Mitra PS, Loffredo CA, Trnovec T, Murinova L, Sovcikova E, Ghimbovschi S, Zang S, Hoffman EP, Dutta SK, 2015. Transcriptional profiling and biological pathway analysis of human equivalence PCB exposure in vitro: Indicator of disease and disorder development in humans. Environ. Res 138, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Trnovec T, Palkovicova L, Hoffman EP, Washington K, Dutta SK, 2013. Status of LEPR Gene in PCB-exposed Population: A Quick Look. Int. J. Hum. Genet 13, 27–32. 10.1080/09723757.2013.11886193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Zang S, Mitra PS, Ghimbovschi S, Hoffman EP, Dutta SK, 2011. Global gene expression and Ingenuity biological functions analysis on PCBs 153 and 138 induced human PBMC in vitro reveals differential mode(s) of action in developing toxicities. Env. Intl 37, 838–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT, 2011. Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology 152, 2909–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Buklijas T, 2010. A Conceptual Framework for the Developmental Origins of Health and Disease. J Dev Orig Heal. Dis 1, 6–18. [DOI] [PubMed] [Google Scholar]
- Grice BA, Nelson RG, Williams DE, Knowler WC, Mason C, Hanson RL, Bullard KM, Pavkov ME, 2017. Associations between persistent organic pollutants, type 2 diabetes, diabetic nephropathy and mortality. Occup Env. Med 74, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi I, Yoshimine Y, Maeda H, Gotou Y, Wada N, Fujii S, Tomokiyo A, Saito K, Monnouchi S, Kouno K, Okumura H, Akamine A, 2011. An epidemiologic examination on the prevalence of the periodontal diseases and oral pigmentation in Yusho patients in 2010. Fukuoka Igaku Zasshi 102, 75–80. [PubMed] [Google Scholar]
- Hatcher-Martin JM, Gearing M, Steenland K, Levey AI, Miller GW, Pennell KD, 2012. Association between polychlorinated biphenyls and Parkinson’s disease neuropathology. Neurotoxicology 33, 1298–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Trnovec T, Kocan A, Charles MJ, Ciznar P, Langer P, Šovcikova E, Rebecca J, 2003. PCBs and early childhood development in Slovakia: Study design and background. Environ. Bull 12, 208–214. [Google Scholar]
- Hsu JF, Yue-Liang G, Shu-Yao Y, Pao-Chi L, 2005. Congener profiles of PCBs and PCDD/Fs in Yucheng victims fifteen years after exposure to toxic rice-bran oils and their implications for epidemiologic studies. Chemosphere 61, 1231–1243. [DOI] [PubMed] [Google Scholar]
- Hsu MM, Mak CP, Hsu CC, 1995. Follow-up of skin manifestations in Yu-Cheng children. Br J Dermatol 132, 427–432. [DOI] [PubMed] [Google Scholar]
- Humblet O, Williams PL, Korrick SA, Sergeyev O, Emond C, Birnbaum LS, Burns JS, Altshul L, Patterson DGJ, Turner WE, Lee MM, Revich B, Hauser R, 2011. Dioxin and polychlorinated biphenyl concentrations in mother’s serum and the timing of pubertal onset in sons. Epidemiology 22, 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John C C, Sylvia S S, Michele A La M, Alan E H, Anthony M, Matthew M, Luoping Z, Paul E, Martyn T S, Jaspal K, 2018. Elevated Levels of Organochlorine Pesticides in South Asian Immigrants Are Associated With an Increased Risk of Diabetes. J. Endocr. Soc 2, 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y, Gunge Y, Koike Y, Kuwatsuka Y, Tsuruta K, Yanagihara K, Furue M, Murota H, 2020. Insight into innate immune response in “Yusho”: The impact of natural killer cell and regulatory T cell on inflammatory prone diathesis of Yusho patients. . Env. Res 185, 109415. [DOI] [PubMed] [Google Scholar]
- Klocke C, Sethi S, Lein PJ, 2020. The developmental neurotoxicity of legacy vs. contemporary polychlorinated biphenyls (PCBs): similarities and differences. Env. Sci Pollut Res Int 27, 8885–8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill MA, Johnson CL, Smith MT, Kandula NR, Macherone A, Pennell KD, Kanaya AM, 2019. Exposure to Persistent Organic Pollutants (POPs) and Their Relationship to Hepatic Fat and Insulin Insensitivity among Asian Indian Immigrants in the United States. Environ. Sci. Technol 53, 13906–13918. 10.1021/acs.est.9b03373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancz K, Hertz-Picciotto I, Jusko TA, Murínová L, Wimmerová S, Sovčíková E, Dedík L, Strémy M, Drobná B, Farkašová D, Trnovec T, 2015. Duration of breastfeeding and serum PCB 153 concentrations in children. . Env. Res 136, 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P, Ukropec J, Kocan A, Drobna B, Radikova Z, Huckova M, Imrich R, Gasperikova D, Klimes I, Trnovec T, 2014. Obesogenic and diabetogenic impact of high organochlorine levels (HCB, p, p'-DDE, PCBs) on inhabitants in the highly polluted Eastern Slovakia. Endocr Regul. 48, 17–24. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lee IK, Song K, Steffes M, Toscano W, Baker BA, Jacobs DRJ, 2006. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care 29, 1638–1644. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lind L, Jacobs DRJ, Salihovic S, van Bavel B, Lind PM, 2012. Associations of persistent organic pollutants with abdominal obesity in the elderly: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Env. Int 40, 170–178. [DOI] [PubMed] [Google Scholar]
- Lee DH, Lind PM, Jacobs DRJ, Salihovic S, van Bavel B, Lind L, 2011. Polychlorinated biphenyls and organochlorine pesticides in plasma predict development of type 2 diabetes in the elderly: the prospective investigation of the vasculature in Uppsala Seniors (PIVUS) study. Diabetes Care 34, 1778–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Steffes M, Jacobs DR, 2007. Positive associations of serum concentration of polychlorinated biphenyls or organochlorine pesticides with self-reported arthritis, especially rheumatoid type, in women. Env. Heal. Perspect 115, 883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Han K, Kim MK, Koh ES, Kim ES, Nam GE, Kwon HS, 2020. Changes in metabolic syndrome and its components and the risk of type 2 diabetes: a nationwide cohort study. Sci. Rep 10, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Ha CM, Kim SA, Thoudam T, Yoon YR, Kim DJ, Kim HC, Moon HB, Park S, Lee IK, Lee DH, 2017. Low-Dose Persistent Organic Pollutants Impair Insulin Secretory Function of Pancreatic β-Cells: Human and In Vitro Evidence. Diabetes 66, 2669–2680. [DOI] [PubMed] [Google Scholar]
- Leijs MM, Esser A, Amann PM, Schettgen T, Gube M, Merk HF, Kraus T, Baron JM, 2018. Hyperpigmentation and higher incidence of cutaneous malignancies in moderate-high PCB- and dioxin exposed individuals. Env. Res 164, 221–228. [DOI] [PubMed] [Google Scholar]
- Lind PM, Lind L, 2018. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia 61, 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Daniels JL, 2001b. Environmental contaminants as etiologic factors for diabetes. Environ. Environ. Heal. Perspect 109, 871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Klebanoff MA, Brock JW, Zhou H, 2001a. Collaborative Perinatal Project (CPP) Polychlorinated biphenyl serum levels in pregnant subjects with diabetes. Diabetes Care 24, 1099–1101. [DOI] [PubMed] [Google Scholar]
- Lung SC, Guo YL, Chang HY, 2005. Serum concentrations and profiles of polychlorinated biphenyls in Taiwan Yu-cheng victims twenty years after the incident. . Env. Pollut 136, 71–79. [DOI] [PubMed] [Google Scholar]
- Medehouenou TCM, Ayotte P, Carmichael P-H, Kröger E, Verreault R, Lindsay J, Dewailly É, Tyas SL, Bureau A, Laurin D, 2019. Exposure to polychlorinated biphenyls and organochlorine pesticides and risk of dementia, Alzheimer’s disease and cognitive decline in an older population: a prospective analysis from the Canadian Study of Health and Aging. Environ. Health 18, 57 10.1186/s12940-019-0494-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylov R, Wu F, Wang H, Clayton A, Sun C, Xie Z, Liang D, Dong Y, Yuan F, Moschou D, Wu Z, Shen MH, Yang J, Fu Y, Yang Z, Burton C, Errington RJ, Wiltshire M, Yang X, 2020. Development and characterisation of acoustofluidic devices using detachable electrodes made from PCB. Lab Chip. . [DOI] [PubMed] [Google Scholar]
- Min-Kyung L, Kyungdo H, Kim MK, Koh ES, Kim ES, Eun NG, Hyuk-Sang K, 2020. Changes in metabolic syndrome and its components and the risk of type 2 diabetes: a nationwide cohort study. Sci. Rep [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra PS, Ghosh S, Zang S, Sonneborn D, Hertz-Picciotto I, Trnovec T, Palkovicova L, Sovcikova E, Ghimbovschi S, Hoffman EP, Dutta SK, 2012. Analysis of the toxicogenomic effects of exposure to persistent organic pollutants (POPs) in Slovakian girls: Correlations between gene expression and disease risk. Environ. Int 39, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T, Hirakawa S, Todaka T, Hirakawa H, Hori T, Kajiwara J, Hirata T, Uchi H, Furue M, 2015. A Study on Polychlorinated Biphenyls Specifically--Accumulated in Blood of Yusho Patients Collected From Medical Check-Ups in 2012. Fukuoka Igaku Zasshi 106, 160–168. [PubMed] [Google Scholar]
- Muñoz-Torres AV, Medina-Bravo P, Valerio-Pérez BE, Mendoza-Salmeron G, Escobedo-de la Peña J, Velázquez-López L, 2020. Positive health beliefs are associated with improvement of glycated hemoglobin and lipid profiles in Mexican patients with type 2 diabetes mellitus: a cross-sectional study. BMC Public Health 24, 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustieles V, Fernández MF, 2020. Bisphenol A shapes children’s brain and behavior: towards an integrated neurotoxicity assessment including human data. Environ. Health 19, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathalie G, Henri S, Jean-Luc O, Jonathan D T, 2019. Epigenetic and Neurological Impairments Associated with Early Life Exposure to Persistent Organic Pollutants. Int. J. Genomics [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neblett MF 2nd, Curtis SW, Gerkowicz SA, Spencer JB, Terrell ML, Jiang VS, Marder ME, Barr DB, Marcus M, Smith AK, 2017. Examining Reproductive Health Outcomes in Females Exposed to Polychlorinated Biphenyl and Polybrominated Biphenyl. Reprod Toxicol 67, 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J, Aucompaugh AG, Schell LM, Denham M, DeCaprio AP, Gallo MV, Ravenscroft J, Kao CC, Hanover MR, David D, Jacobs AM, Tarbell AM, Worswick P, Environment, A.T.F. on the, 2006. PCBs and cognitive functioning of Mohawk adolescents. Neurotoxicol Teratol 28, 439–445. [DOI] [PubMed] [Google Scholar]
- Newman J, Gallo MV, Schell LM, DeCaprio AP, Denham M, Deane GD, Akwesasne Task Force on Environment, 2009. Analysis of PCB congeners related to cognitive functioning in adolescents. Neurotoxicology 30, 686–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozuka D, Hirata T, Furue M, 2011. Relative survival after exposure to polychlorinated biphenyls and dioxins: a follow-up of Japanese patients affected in the Yusho incident. Sci Total Env. 409, 2361–2365. [DOI] [PubMed] [Google Scholar]
- Park HY, Hertz-Picciotto I, Sovcikova E, Kocan A, Drobna B, Trnovec T, 2010. Neurodevelopmental toxicity of prenatal polychlorinated biphenyls (PCBs) by chemical structure and activity: a birth cohort study. Env. Heal. Perspect 9, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel CJ, Bhattacharya J, Butte AJ, 2010. An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuk M, Serio TC, Cusack C, Cave M, Rosenbaum PF, Birnbaum LS, 2019. Hypertension in Relation to Dioxins and Polychlorinated Biphenyls from the Anniston Community Health Survey Follow-Up. Env. Heal. Perspect 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky V, Piorkowski J, Turyk M, Freels S, Chatterton RJ, Dimos J, Bradlow HL, Chary LK, Burse V, Unterman T, Sepkovic D, McCann K, 2011. Associations of polychlorinated biphenyl exposure and endogenous hormones with diabetes in post-menopausal women previously employed at a capacitor manufacturing plant. Env. Res 111, 817–824. [DOI] [PubMed] [Google Scholar]
- Pesatori AC, Zocchetti C, Guercilena S, Consonni D, Turrini D, Bertazzi PA, 1998. Dioxin exposure and non-malignant health effects: a mortality study. Occup Env. Med 55, 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert A, Schwartz H, Mergler D, 2009. An exploratory study of diabetes in a First Nation community with respect to serum concentrations of p,p′-DDE and PCBs and fish consumption. Int J Env. Res Public Heal 6, 3179–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polańska K, Jurewicz J, Hanke W, 2013. Review of current evidence on the impact of pesticides, polychlorinated biphenyls and selected metals on attention deficit / hyperactivity disorder in children. Int J Occup Med Env. Heal 26, 16–38. [DOI] [PubMed] [Google Scholar]
- Przybyla J, Houseman E A, Smit E, Kile ML, 2017. A path analysis of multiple neurotoxic chemicals and cognitive functioning in older US adults (NHANES 1999-2002). Environ. Health 16, 19 10.1186/s12940-017-0227-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radikova Z, Koska J, Ksinantova L, Imrich R, Kocan A, Petrik J et al. , 2004. Increased frequency of diabetes and other forms of dysglycemia in the population of specific areas of eastern Slovakia chronically exposed to contamination with polychlorinated biphenyls (PCB) Organohalogen Compounds, in: International Symposium on Halogenated Environmental Organic Pollutants and POPs. Berlin, Germany. [Google Scholar]
- Raffetti E, Donato F, De Palma G, Leonardi L, Sileo C, Magoni M, 2020. Polychlorinated biphenyls (PCBs) and risk of hypertension: A population-based cohort study in a North Italian highly polluted area. Sci Total Env. 714. [DOI] [PubMed] [Google Scholar]
- Rignell-Hydbom A, Elfving M, Ivarsson SA, Lindh C, Jönsson BA, Olofsson P, Rylander L, 2010. A nested case-control study of intrauterine exposure to persistent organochlorine pollutants in relation to risk of type 1 diabetes. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Genuis SJ, Frye RE, 2014. Environmental toxicants and autism spectrum disorders: a systematic review. Transl. Psychiatry 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Altshul LM, Korrick SA, 2012. Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Env. Heal. Perspect 120, 904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklayen MG, 2018. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep 20, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy AL, Guo J, Miskewitz RJ, McGillis WR, Rodenburg LA, 2012. Fluxes of polychlorinated biphenyls volatilizing from the Hudson River, New York measured using micrometeorological approaches. Env. Sci Technol 46, 885–891. [DOI] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Haffner D, Patel K, Opel M, Papke O, Birnbaum L, Päpke O, Birnbaum L, 2010. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environ. Heal. Perspect 118, 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettler T, Stein J, Reich F, Valenti M, 2000. In Harm’s Way: Toxic threats to child development. A report by Greater Boston Physicians for Social Responsibility. [Google Scholar]
- Sealey LA, Hughes BW, Sriskanda AN, Guest JR, Gibson AD, Johnson-Williams L, Pace DG, Bagasra O, 2016. Environmental factors in the development of autism spectrum disorder. Env. Int 88, 288–298. [DOI] [PubMed] [Google Scholar]
- Silverstone AE, Rosenbaum PF, Weinstock RS, Batell SM, Foushee HR, Shelton C, Pavuk M, 2012. Polychlorinated Biphenyl (PCB) Exposure and Diabetes: Results from the Anniston Community Health Survey. Env. Heal. Perspect 120, 727–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NK, Banerjee BD, Bala K, Chhillar M, Chhillar N, 2014. Gene-gene and gene-environment interaction on the risk of Parkinson’s disease. Curr Aging Sci 7, 101–109. [DOI] [PubMed] [Google Scholar]
- Singh NK, Chhillar N, Banerjee BD, Bala K, Mukherjee AK, Mustafa MD, Mitrabasu, 2012. Gene-environment interaction in Alzheimer’s disease. Am J Alzheimers Dis Other Demen 27, 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisto R, Moleti A, Palkovičová ML’., Wimmerová S, Lancz K, Tihányi J, Čonka K, Šovčíková E, Hertz-Picciotto I, Jusko TA, Trnovec T, 2015. Environmental exposure to organochlorine pesticides and deficits in cochlear status in children. Env. Sci Pollut Res Int 22, 14570–14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya M, Ohtake M, Kunisue T, Subramanian A, Takahashi S, Chakraborty P, Ramachandran R, Tanabe S, 2010. Persistent organic pollutants in breast milk of mothers residing around an open dumping site in Kolkata, India: specific dioxin-like PCB levels and fish as a potential source. Env. Int 36, 27–35. [DOI] [PubMed] [Google Scholar]
- Son HK, Kim SA, Kang JH, Chang YS, Park SK, Lee SK, Jacobs DRJ, Lee DH, 2010. Strong associations between low-dose organochlorine pesticides and type 2 diabetes in Korea. Env. Int 36, 410–414. [DOI] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Babinska K, Palkovicova L, Trnovec T, Kocan A, Nguyen DV, Hertz-Picciotto I, 2008. Serum PCB concentrations in relation to locally produced food items in eastern Slovakia. J Expo Sci Env. Epidemiol 18, 581–587. [DOI] [PubMed] [Google Scholar]
- Soundararajan A, Prabu P, Mohan V, Gibert Y, Balasubramanyam M, 2019. Novel insights of elevated systemic levels of bisphenol-A (BPA) linked to poor glycemic control, accelerated cellular senescence and insulin resistance in patients with type 2 diabetes. Mol Cell Biochem 458, 171–183. [DOI] [PubMed] [Google Scholar]
- Steenland K, Hein MJ, Cassinelli RTII, Prince MM, Nilsen NB, Whelan EA, Waters MA, Ruder AM, Schnorr TM, 2006. Polychlorinated Biphenyls and Neurodegenerative Disease Mortality in an Occupational Cohort. Epidemiology 17, 8–13. 10.1097/01.ede.0000190707.51536.2b [DOI] [PubMed] [Google Scholar]
- Stewart PW, Reihman J, Lonky E, Pagano J, 2012. Issues in the interpretation of associations of PCBs and IQ. Neurotoxicol Teratol 34, 96–107. [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Lee DH, Porta M, Steffes MW, Jacobs DRJ, 2015. Persistent organic pollutants in young adults and changes in glucose related metabolism over a 23-year follow-up. Env. Res 137, 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabb M, Blumberg B, 2006. New modes of action for endocrine-disrupting chemicals. Mol. Endocrinol 20, 475–82. [DOI] [PubMed] [Google Scholar]
- Tahara H, Sato M, Thurin M Butterfield LH, Disis ML, Fox BA, Lee PP, Khleif SN, Wigginton JM, Ambs S, Akutsu Y, Chaussabel D, Doki Y, Eremin O, Fridman WH, Hirohashi Y, Imai K, Jacobson J, Jinushi M, Kanamoto A, Kashani-Sabet M, Kato K, Kawakami Y, Kirkwood JM, Kleen TO, Lehmann PV, Liotta L, Lotze MT, Maio M, Malyguine A, Masucci G, Matsubara H, Mayrand-Chung S, Nakamura K, Nishikawa H, Palucka AK, Petricoin EF, Pos Z, Ribas A, Rivoltini L, Sato N, Shiku H, Slingluff CL, Streicher H, Stroncek DF, Takeuchi H, Toyota M, Wada H, Wu X, Wulfkuhle J, Yaguchi T, Zeskind B, Zhao Y, Zocca MB, Marincola FMJ, W.E., 2009. Emerging concepts in biomarker discovery; the US-Japan Workshop on Immunological Molecular Markers in Oncology. Transl Med 7, 7–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran NQV, Miyake K, 2017. Neurodevelopmental disorders and environmental toxicants: epigenetics as an underlying mechanism. Int. J. Genomics 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukimori K, Uchi H, Mitoma C, Yasukawa F, Chiba T, Todaka T, Kajiwara J, Yoshimura T, Hirata T, Fukushima K, Wake N, Furue M, 2012. Maternal exposure to high levels of dioxins in relation to birth weight in women affected by Yusho disease. Env. Int 38, 79–86. [DOI] [PubMed] [Google Scholar]
- Tudurí E, Marroqui L, Dos Santos RS, Quesada I, Fuentes E, Alonso-Magdalena P, 2018. Timing of Exposure and Bisphenol-A: Implications for Diabetes Development. Front Endocrinol 31, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk M, Anderson H, Knobeloch L, Imm P, Persky V, 2009a. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p0-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere 75, 379–674. [DOI] [PubMed] [Google Scholar]
- Turyk M, Anderson H, Knobeloch L, Imm P, Persky V, 2009b. Organochlorine exposure and incidence of diabetes in a cohort of Great Lakes sport fish consumers. Environ. Heal. Perspect 117, 1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura H, Arisawa K, Hiyoshi M, Kitayama A, Takami H, Sawachika F, Dakeshita S, Nii K, Satoh H, Sumiyoshi Y, Morinaga K, Kodama K, Suzuki T, Nagai M, Suzuki T, 2009. Prevalence of metabolic syndrome associated with body burden levels of dioxin and related compounds among Japan’s general population. Env. Heal. Perspect 117, 568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukropec J, Radikova Z, Huckova M, Koska J, Kocan A, Sebokova E, Drobna B, Trnovec T, Susienkova K, Labudova V, Gasperikova D, Langer P, Klimes I, 2010. High prevalence of prediabetes and diabetes in a population exposed to high levels of an organochlorine cocktail. Diabetologia 53, 899–906. [DOI] [PubMed] [Google Scholar]
- Vasiliu O, Cameron L, Gardiner J, Deguire P, Karmaus W, 2006. Polybrominated biphenyls, polychlorinated biphenyls, body weight, and incidence of adult-onset diabetes mellitus. Epidemiology 17, 352–359. [DOI] [PubMed] [Google Scholar]
- Verner MA, Plusquellec P, Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL, Charbonneau M, Haddad S, 2010. Alteration of infant attention and activity by polychlorinated biphenyls: unravelling critical windows of susceptibility using physiologically based pharmacokinetic modeling. Neurotoxicology 31, 424–431. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Sloboda DM, 2012. Leptin as mediator of the effects of developmental programming. Best Pr. Res Clin Endocrinol Metab 26, 677–687. [DOI] [PubMed] [Google Scholar]
- Vineis P, Karin van V, Marc C-H, Toby J A, 2013. Advancing the Application of Omics-Based Biomarkers in Environmental Epidemiology. Env. Mol Mutagen 54, 461–467. [DOI] [PubMed] [Google Scholar]
- Wang C, Petriello MC, Zhu B, Hennig B, 2019. PCB 126 induces monocyte/macrophage polarization and inflammation through AhR and NF-κB pathways. Toxicol Appl Pharmacol. 367, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Tsai PC, Yang CY, Guo YL, 2008. Increased risk of diabetes and polychlorinated biphenyls and dioxins: A 24-year follow-up study of the Yucheng cohort. Diabetes Care 31, 1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warembourg C, Debost-Legrand A, Bonvallot N, Massart C, Garlantézec R, Monfort C, Gaudreau E, Chevrier C, Cordier S, 2016. Exposure of pregnant women to persistent organic pollutants and cord sex hormone levels. Hum Reprod 31, 190–198. [DOI] [PubMed] [Google Scholar]
- Waugh CA, Arukwe A, Jaspers VLB, 2018. Deregulation of microRNA-155 and its transcription factor NF-kB by polychlorinated biphenyls during viral infections. APMIS 126, 234–240. [DOI] [PubMed] [Google Scholar]
- Williams AE, Watt J, Robertson LW, Gadupudi G, Osborn ML, Soares MJ, Iqbal K, Pedersen KB, Shankar K, Littleton S, Maimone C, Eti NA, Suva LJ, Ronis MJJ, 2020. Skeletal Toxicity of Coplanar Polychlorinated Biphenyl Congener 126 in the Rat is Aryl Hydrocarbon Receptor-Dependent. Toxicol Sci. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmerová S, Watson A, Drobná B, Šovčíková E, Weber R, Lancz K, Patayová H, Richterová D, Koštiaková V, Jurečková D, Závacký P, Strémy M, Jusko TA, Palkovičová ML’, Hertz-Picciotto I, Trnovec T, 2015. The spatial distribution of human exposure to PCBs around a former production site in Slovakia. Env. Sci Pollut Res Int 22, 14405–14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Bongaerts BWC, Schneider A, Huth C, Meisinger C, Peters A, Schneider A, Wittsiepe J, Schramm KW, Greiser KH, Hartwig S, Kluttig A, Rathmann W, 2019. Persistent organic pollutants and the incidence of type 2 diabetes in the CARLA and KORA cohort studies. Env. Int 129, 221–228. [DOI] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM, 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Env. Heal. Perspect 119, 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Bertrand KA, Choi AL, Hu FB, Laden F, Grandjean P, Sun Q, 2012. Persistent Organic Pollutants and Type 2 Diabetes: A Prospective Analysis in the Nurses’ Health Study and Meta-analysis. Env. Heal. Perspect 121, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yach D, Stuckler D, Brownell KD, 2006. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med 12, 62–66. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, 2003. Yusho in Japan. Ind Heal. 41, 139–148. [DOI] [PubMed] [Google Scholar]
- Yu Z, Palkovicova L, Drobna B, Petrik J, Kocan A, Trnovec T, Hertz-Picciotto I, 2007. Comparison of organochlorine compound concentrations in colostrum and mature milk. Chemosphere 66, 1012–1018. [DOI] [PubMed] [Google Scholar]
- Zani C, Magoni M, Speziani F, Leonardi L, Orizio G, Scarcella C, Gaia A, Donato F, 2019. Polychlorinated biphenyl serum levels, thyroid hormones and endocrine and metabolic diseases in people living in a highly polluted area in North Italy: A population-based study. Heliyon 5, e01870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetzsche T, Rujescu D, Hardy J, Hampel H, 2010. Advances and perspectives from genetic research: development of biological markers in Alzheimer’s disease. Expert Rev Mol Diagn 10, 667–690. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yolton K, Webster GM, Sjödin A, Calafat AM, Dietrich KN, Xu Y, Xie C, Braun JM, Lanphear BP, Chen A, 2017. Prenatal PBDE and PCB Exposures and Reading, Cognition, and Externalizing Behavior in Children. Env. Heal. Perspect 125, 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong G, Valvi D, Coull B, Göen T, Hu FB, Nielsen F, Grandjean P, Sun Q, 2018. Persistent organic pollutants and risk of type 2 diabetes: A prospective investigation among middle-aged women in Nurses’ Health Study II. Environ. Int 114, 334–342. 10.1016/j.envint.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]