Abstract
Introduction:
Gene delivery technologies are being developed for an increasing number of biomedical applications, with delivery vehicles including viruses and non-viral materials. Among biomaterials used for non-viral gene delivery, poly(beta-amino ester)s (PBAEs), a class of synthetic, biodegradable polymers, have risen as a leading gene delivery vehicle that has been used for multiple applications in vitro and in vivo.
Areas covered:
This review summarizes the key properties of PBAEs and their development, including a discussion of the advantages and disadvantages of PBAEs for gene delivery applications. The use of PBAEs to improve the properties of other drug delivery vehicles is also summarized.
Expert opinion:
PBAEs are designed to have multiple characteristics that are ideal for gene delivery, including their reversible positive charge, which promotes binding to nucleic acids as well as imparting high buffering capacity, and their rapid degradability under mild conditions. Simultaneously, some of their properties also lead to nanoparticle instability and low transfection efficiency in physiological environments. The ease with which PBAEs can be chemically modified as well as non-covalently blended with other materials, however, allows them to be customized specifically to overcome delivery barriers for varied applications.
Keywords: Biomaterials, gene delivery, nanoparticles, nucleic acids
1. Introduction
Gene therapy has the potential to treat a variety of diseases, including inherited genetic disorders, cancer, and infectious diseases, and ongoing research seeks to develop better viral and non-viral vehicles to safely and efficiently deliver genetic material into cells. Viral vectors are the most widely explored gene delivery vehicles because of their natural ability to efficiently deliver genetic material intracellularly [1]. Viral gene therapies have been used for decades and are beginning to enter the marketplace, including for ex vivo engineering of patient cells for chimeric antigen receptor (CAR) T-cell therapy [2] and for in vivo programming, a direct subretinal in vivo injection of adeno-associated vectors (AAVs) to treat an inherited retinal mutation [3]. However, several key limitations still hamper the translation of other viral therapies to the clinic, including safety concerns, such as immunogenicity, potential side effects, and insertional mutagenesis, as well as other hurdles, such as complex manufacturing processes, limited cargo capacity, and difficulty reaching certain cells and tissues [4].
Non-viral delivery systems are typically less efficient than viral vectors but generally are safer, more cost-effective, less immunogenic, and can carry larger genetic cargo [5]. A broad range of biomaterials have been developed for gene delivery, including lipid-based systems [6], cyclodextrins [7], dendrimers [8], peptides [9–11], and a variety of polymers [12]. Cationic polymers are particularly suitable as gene delivery vehicles due to their ability to electrostatically interact with negatively charged nucleic acids, forming nanoparticles that can be efficiently internalized by cells. Early studies on polymeric gene delivery used materials such as poly(l-lysine) (PLL), a polypeptide with high positive charge, and polyethylenimine (PEI). Both of these on their own are hampered by the inability to overcome certain delivery barriers or by toxicity [13,14], challenges that will be discussed further below, although chemically modified versions of these materials, including catechol-modified PLL, have been successful in gene delivery [15]. Synthetic poly(amido amine)s (PAMAMs), frequently used as dendrimers, have been developed to improve transfection efficacy via their branching chemical structure and high charge density, though they must also be chemically modified to address delivery issues, including toxicity [16,17]. Other polymers used for gene delivery include those based on polysaccharides, such as cationic chitosan [18], a natural material that is generally low in toxicity, though it is limited by certain chemical properties, such as low solubility in water.
This review focuses on poly(beta-amino ester)s (PBAEs), a class of cationic, biodegradable polymers that have been engineered for their ability to overcome gene delivery hurdles (Figure 1). As will be described in more detail in the sections below, PBAEs can compact nucleic acids into nanoscale particles that can be internalized into cells due to the positive charge of the polymer; they facilitate escape from the endolysosomal compartment once inside cells; and they can release their nucleic acid cargo into the appropriate cellular compartment for gene delivery via a variety of targeted degradation mechanisms [19,20]. An important feature of PBAEs is their vast potential for structural diversity, which was explored in early work through the synthesis of a combinatorial library of PBAEs in order to evaluate their properties for gene delivery [21]. Chemical properties like the molecular weight, hydrophobicity, degradability, and the linear or branched structure of PBAEs can be exploited to address the unique challenges of delivering different nucleic acids, including plasmid DNA, mRNA, siRNA, immunostimulatory RNA (isRNA), and cyclic dinucleotides (CDN) [22]. In this review we discuss the benefits and limitations of PBAE nanoparticles for gene delivery and how these challenges can be addressed to develop more efficient delivery vehicles. In addition, although non-viral gene delivery has been slow to enter clinical use, we will also briefly discuss currently approved non-viral gene delivery technologies and the steps that must be undertaken in order for PBAEs to be translated to the clinic.
Figure 1.
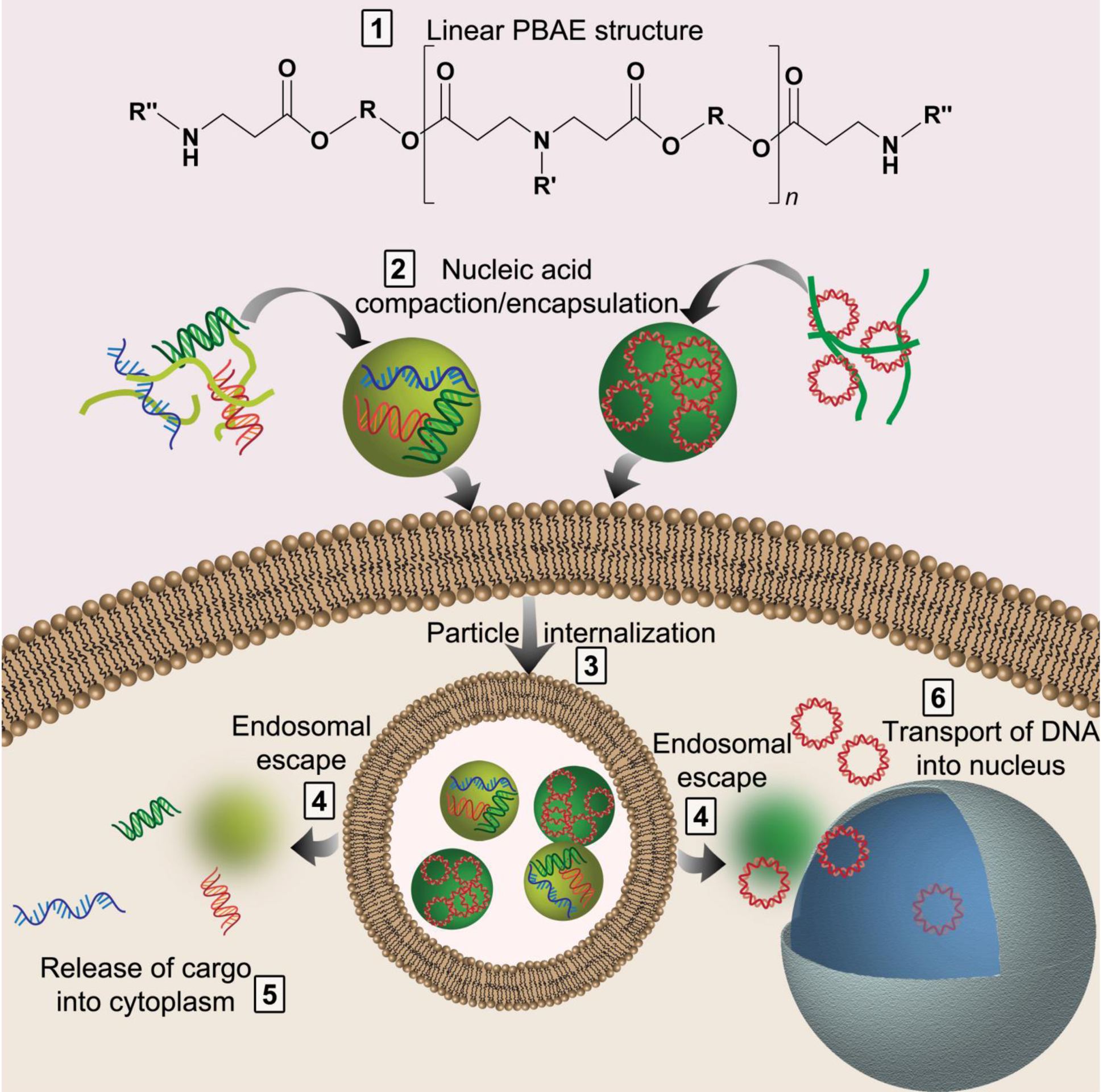
Nucleic acids must overcome numerous hurdles for successful transfection by polymers like PBAEs (1). The cargo must be encapsulated and compacted into nanoparticles (2); the nanoparticles are internalized into the cells (3); the nanoparticles must escape the endosome (4); and the nucleic acid must be released and trafficked to the correct cellular compartment (5–6).
2. Benefits and limitations of PBAE nanocarriers for nucleic acid delivery
2.1. Properties of PBAEs and PBAE-based nanocarriers
2.1.1. PBAE synthesis
PBAEs are generally prepared via either step-growth polymerization, e.g., Michael addition (MA) reaction, or ring opening polymerization (ROP) (Figure 2A–B). Both allow step-economical synthesis of either one or two steps, with stoichiometry used to control polymer size. The MA reaction is most commonly used, being relative simple and versatile, in which linear PBAEs are synthesized by conjugation of primary amines or secondary diamines of the side chain monomer(s) to the bis(acrylate ester) backbone monomer to form a base polymer with acrylate termini. In subsequent synthesis step, an excess of end-capping monomer is added for which amines are conjugated to the acrylate groups of the base polymer. Through this synthesis route, libraries of monomers can be combined to form PBAEs with desired chemical properties. High-throughput testing of combinatorial libraries can then be used to identify polymers that deliver nucleic acids with high efficiency and low toxicity (Figure 2C) [21,23,24]. Kowalski et al. used tertiary amino-alcohols to initiate ROP of lactones to obtain PBAEs with diverse chemical properties to explore tissue- and cell-specific delivery of mRNA as payload [25]. Similarly, Eltoukhy et al. also used high-throughput testing of PBAEs to demonstrate that the molecular weight strongly influenced DNA transfection in HeLa cells, in which an intermediate polymer length mediated the greatest transfection [26].
Figure 2.
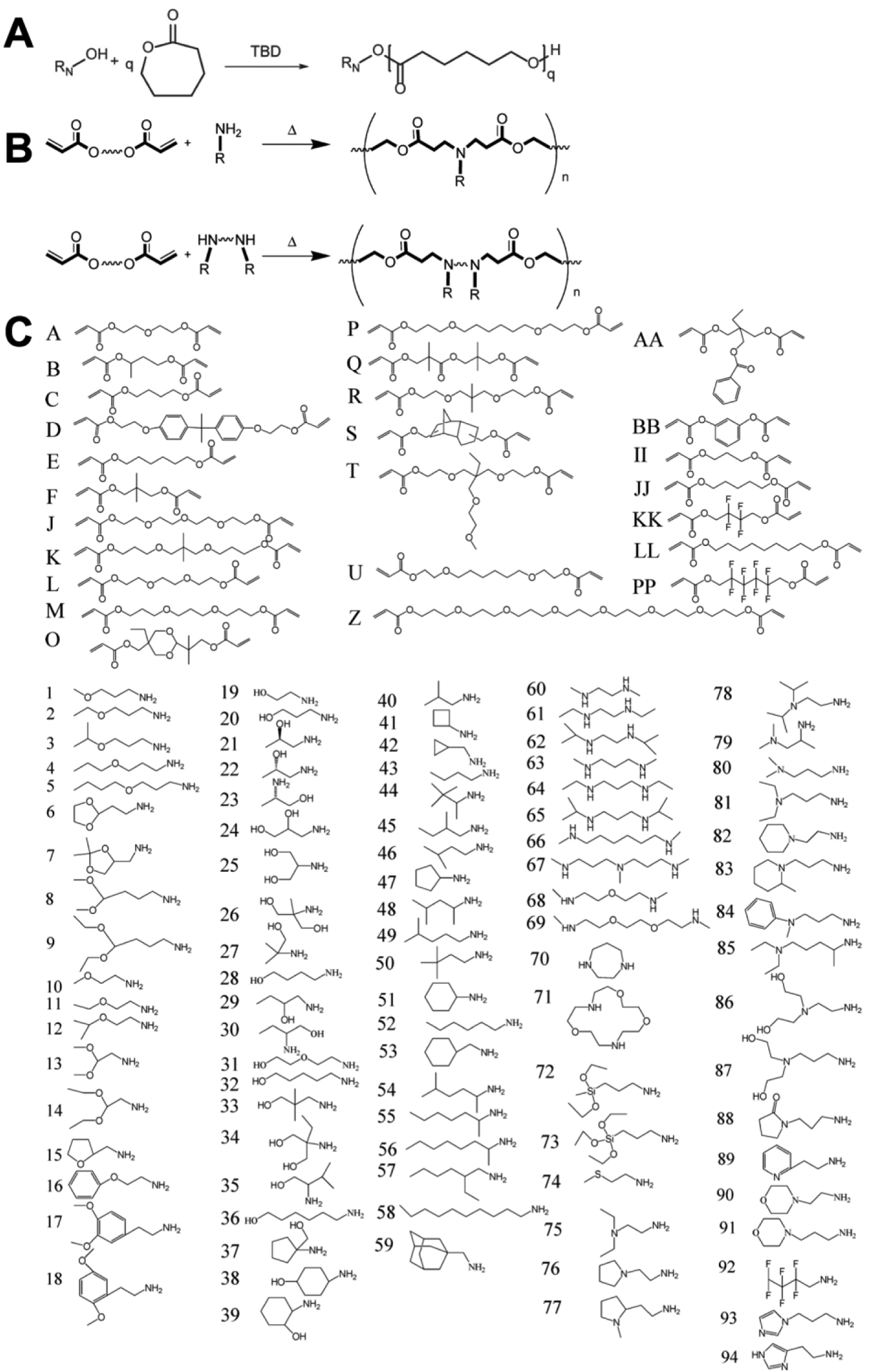
Ring-opening polymerization (ROP) (A) or Michael addition (MA) chemistry can be used to synthesize PBAEs (B). Diacrylate monomers (lettered) and amine-containing monomers (numbered) can be used to generate a vast library of PBAEs with varying chemical structure via combinatorial chemistry using MA (C). (A) was adapted with permission from Kowalski et al. [25] (B)-(C) were adapted with permission from Green et al., “A Combinatorial Polymer Library Approach Yields Insight into Nonviral Gene Delivery,” Accounts of Chemical Research 41(6):749–759. Copyright (2008) American Chemical Society [21].
The ease with which PBAEs can be synthesized with diverse chemical properties allows the preparation of PBAE libraries in a time-efficient manner to explore the chemical properties that are essential for efficient nucleic acid delivery to a target cell type. Polymer end-groups have shown to regulate cell-type specificity of PBAE vehicles for nucleic acid delivery. Sunshine et al. explored DNA delivery using PBAE nanocarriers to a wide range of cells, including cancer cells, immune cells, and human stem cells [27]. Although the mechanism of cell-type specificity was not elucidated in this study, the authors showed that end-group modification using the same base polymer modulated the gene delivery efficiency, with some polymer end-caps highly effective for one cell line while being rather poor at delivery to another.
Studies have also demonstrated that PBAEs can have intrinsic properties that facilitate preferential delivery to cancerous cells over healthy cells. Polymer library screens have been used to identify PBAE structures that preferentially deliver DNA to a range of human and rodent hepatocellular carcinoma (HCC) cells over healthy hepatocytes in vitro [28,29]. Kozielski et al. found PBAE structure that facilitated tumor-specific siRNA delivery [30], with near-complete siRNA-mediated knockdown achieved in patient-derived glioblastoma (GBM) cells and below 25% knockdown in non-cancerous counterparts. Preferential delivery of DNA to brain tumor cells was also demonstrated by Tzeng et al. [31], who showed preferential gene delivery to the GBM cells over non-cancerous brain cells.
2.1.2. Nanoparticle manufacture
In vivo studies have demonstrated the promise of using PBAEs for effective and safe delivery of nucleic acid therapeutics. The polymers can be quickly synthesized with diverse chemical properties to function as nanocarriers for intracellular delivery to different cells. The formation of PBAE/nucleic acid nanoparticles is based on self-assembly between cationic PBAEs and the anionic nucleic acid into nanoscale particles. The ratio between the PBAE and the nucleic acid in nanoparticles, an important parameter that is discussed below, is reported as the mass ratio of polymer to nucleic acid (w/w). Simultaneously, however, PBAEs are also associated with a number of challenges, some of which are tied to their intrinsic beneficial properties. In this section, we discuss beneficial properties that allow PBAEs to be used as vehicles for nucleic acid therapeutics, as well as some of the hurdles facing research on PBAEs and some of the strategies that have been employed to overcome them (summarized in Table 1).
Table 1.
Hurdles faced by PBAEs as gene delivery vehicles.
| Disadvantages | Strategies for Overcoming Challenge |
|---|---|
| Uncontrolled/off-target nucleic acid release | Triggered release by external stimulus [72] |
| Encapsulation of anionic cargo only | Addition of chemical groups to improve hydrogen bonding and hydrophobic interactions with other cargo [54] |
| Toxicity due to excessive positive charge | Blending with anionic or less highly charged polymer [55,113] |
| Poor transport through tissue due to excessive positive charge | Shielding of the surface with ligands, peptides, or PEG [52,57,76,80] |
| Immune response | Selection of applications that require immune activation [60,87] |
| Low nucleic acid binding efficiency | Incorporation of other polymers with high positive charge density [40] |
| Low colloidal stability in physiological fluids | Non-covalent functionalization with PEG [52,80] |
| Poor stability during storage or transport | Lyophilization with lyoprotectants [31,33–38] |
Non-viral gene delivery systems in general, including cationic polymers and lipid-based materials, may face storage, transport, and shelf-stability challenges [32]. For PBAEs, these include aggregation after formation of soft polyplexes and degradation. Pre-clinically, some of these hurdles can be circumvented by simply avoiding long-term storage and instead forming nanoparticles directly before use, but this may be impractical for widespread clinical use. A potential solution to this problem is lyophilization of nanoparticles. This was done for PBAE/DNA nanoparticles using sucrose as a cryo- and lyoprotectant [31] with no loss in transfection efficacy after at least two years of cold storage [33]. This strategy was studied in detail using oligopeptide-modified PBAEs for delivery of plasmid DNA as well as mRNA, with the finding the sucrose as well as the pH buffer HEPES were optimal protectants for lyophilization [34]. This method has since been used for the delivery of DNA for in situ CAR T-cell generation [35], siRNA for direct cancer killing [36], immunostimulatory nucleic acids for tumor immunotherapy [37], and mRNA for protein production in the lung after nanoparticle inhalation [38], raising the potential of this type of technology to be translated to a range of useful clinical products.
2.2. Overcoming intracellular barriers to nucleic acid delivery
2.2.1. Encapsulation and binding efficiency
The binding between the PBAE vehicle and nucleic acid payload is important for the self-assembly into small and stable nanoparticles, which prevents the nucleic acid from being degraded following administration and ensures high cellular uptake efficiency. PBAEs, while cationic, have lower charge density than some other polymers used traditionally for gene delivery, including PEI and PLL, and thus PBAEs must often be used in higher amounts to achieve the same degree of nucleic acid binding as other polymers. This was explored by Sunshine et al. [39], who found that, compared to PEI, a greater mass of PBAE was needed to achieve the same DNA binding and the same buffering capacity; however, due to the lower toxicity of the PBAEs tested, greater transfection efficacy was still achieved by the PBAE groups than by PEI. Other researchers combined a PBAE with PEI and synthesized a hybrid material that would have the advantages of PBAEs as well as improved DNA binding due to the higher charge density from PEI [40]. Though they found this novel material to be more effective at transfecting cells in vitro, it should be noted that, as discussed above, increased charge density may not be advantageous in an in vivo setting.
The relatively low binding strength of PBAEs is more apparent when delivering smaller cargo, such as oligonucleotides, which may suffer from lower binding avidity than larger nucleic acids. Higher PBAE-to-nucleic acid w/w is typically required for oligonucleotides [41], with some studies using 100 w/w or higher PBAE-to-siRNA [30] or -miRNA [42]. As described above, these PBAEs can be engineered to degrade rapidly upon entry into the cytoplasm, reducing concerns about toxicity; however, increasing the polymer-to-nucleic acid mass ratio does reduce the amount of active ingredient that can be injected per mass of polymer. In cases where the injection volume is limited, polymer solubility may limit the dose of nucleic acid that can be administered.
The binding strength between PBAE and nucleic acid can also be adjusted by using different end-caps on the PBAE structure [41,43]. Secondary amines in the end-caps have been demonstrated to improve gene delivery, and a greater number of amine-containing end-caps generally condense the nucleic acid payload more tightly and forms smaller nanoparticles [21], which can be beneficial for cell uptake. Anderson et al. used a library screen to elucidate structure-function relationship of PBAE nanoparticles for DNA delivery to the fibroblast-like cell line COS-7, showing that the greatest transfection was achieved by particles smaller than 150 nm [44]. End-cap modification may also alter both the overall transfection efficacy in vivo as well as the biodistribution [45].
Researchers have overcome the low binding efficiency by engineering nucleic acid dendrimers, with branching siRNA architectures showing higher binding to PBAEs than small linear siRNAs due to an increase in avidity [46]. Alternatively, branched PBAEs have been synthesized to improve nucleic acid binding [47] by using triacrylate in addition to diacrylate monomers to generate polymer backbones (Figure 3A–C). The increase in the number of tertiary amines afforded by branching PBAE structure leads to more protonation in low-pH buffer, resulting in better condensation of DNA, while the greater number of end-groups also leads to more chemical flexibility (Figure 3C) and strong effects on transfection efficacy [48,49] (Figure 3D–E). Because, as discussed above, nucleic acid release is crucial to successful transfection as well as binding, PBAEs have also been further modified to have branching structure that promotes tight initial binding as well as reducible linkages throughout the backbone to allow triggered release of the cargo once inside the cytoplasm of the cell [49], resulting in better siRNA-mediated knockdown. Patel et al. reported a hyperbranched PBAE vehicle for safe and effective mRNA in vivo delivery to lung epithelium following inhalation [38]. In contrast to the linear PBAEs, the hyperbranched PBAE nanoparticles remained stable in size at high mRNA doses, and a lower polymer:RNA ratio was required for encapsulation. Another reason for the improved efficacy of the hyperbranched PBAE was the higher isoelectric point, which ensured greater positive charge at physiological pH than the linear versions.
Figure 3.
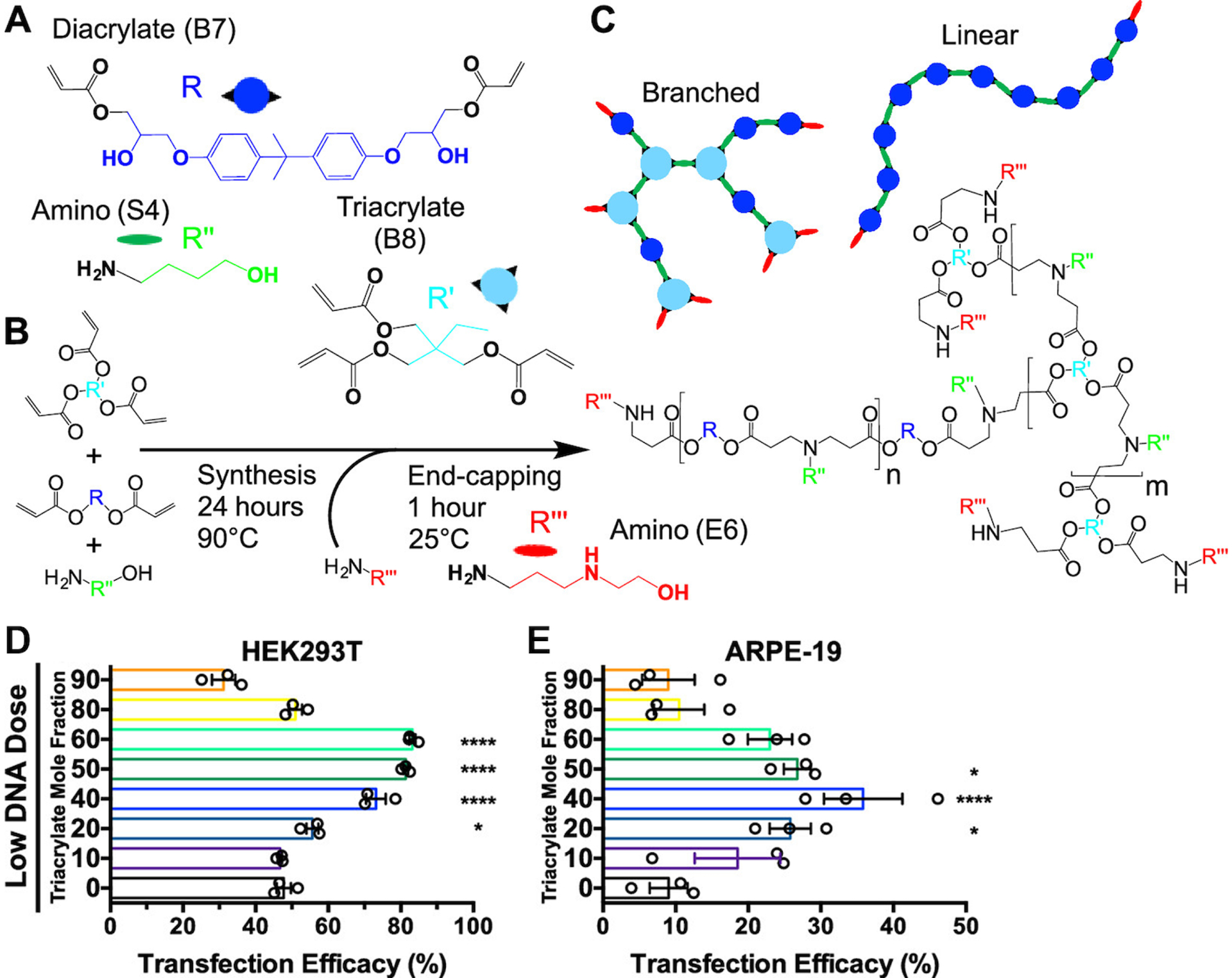
Branched PBAEs can be synthesized using Michael addition of diacrylate and triacrylate monomers with amine-containing monomers (A) to generate a series of acrylate-terminated polymers in a one-pot synthesis (B). The resulting linear or branched polymers can be terminated with two or more end-caps, respectively (C). Under stringent transfection conditions, moderate branching, represented as the triacrylate mole fraction, leads to improved transfection of HEK293T cells (D) and hard-to-transfect ARPE-19 cells (E). Modified with permission from Wilson et al., “Differentially branched ester amine quadpolymers with amphiphilic and pH-sensitive properties for efficient plasmid DNA delivery,” Molecular pharmaceutics 16(2):655–668. Copyright (2019) American Chemical Society [49].
The binding strength can also be controlled by changing the polymer:nucleic acid ratio of the nanoparticle formulation. This was critical for the delivery of the nucleic acid-based adjuvant CpG [50]. CpG needs to be internalized by immune cells and agonize Toll-like receptor 9 (TLR9) to trigger an immune response, thus requiring binding that is tight enough for internalization but weak enough to release CpG subsequently. Wilson et al. used PBAE as nanocarrier for cyclic dinucleotides (CDN) as adjuvants to induce an anti-tumor response [37]. Due to the small size of the CDN payload, a high w/w ratio of PBAE to CDN (500 w/w) was used to form stable nanoparticles. Despite the high polymer content, the formulation was safely tolerated, while nanoparticle-mediated cytosolic delivery of CDN generated the same immunogenicity as 100-fold higher extracellular concentration of CDN without nanocarrier [37]. An assay developed by Bhise et al. using nanoparticle tracking analysis (NTA) can be used to assess the encapsulation efficiency of PBAE nanoparticles carrying DNA [51]. This study demonstrated that both the polymer structure and the polymer:DNA w/w ratio can be modulated to control plasmid loading.
In addition to electrostatic interactions, hydrophobic components in the PBAE structure can be incorporated to improve nanoparticle stability in physiological conditions. Eltoukhy et al. included alkyl side chains in their PBAE design and demonstrated that polyplexes in which greater than 20 mol% alkylamine was incorporated in the PBAE structure maintained their nanoparticle size over 1 h, whereas polyplexes formed by PBAEs lacking alkyl side chains increased in size by about 50% [52].
2.2.2. Cationic surface charge
The cationic nature of PBAE/nucleic acids, while an advantage during nucleic acid encapsulation and cellular uptake, can also lead to additional challenges. First, as the formation of nanoparticles is driven largely by electrostatic interactions, PBAEs are generally limited to the delivery of anionic cargo. While this lends itself well to the delivery of nucleic acids, which are highly negatively charged, it can be limiting for applications that require co-delivery of another type of cargo. For example, CRISPR-Cas9 systems generally require the delivery of, minimally, sequence-specific small guide RNA (sgRNA) and either the Cas9 ribonucleoprotein (RNP) or genes encoding the Cas9 RNP. While PBAEs can be used for gene editing by delivering multiple plasmids [53], to sidestep some of the hurdles involved in delivery of Cas9 RNP as a gene, Rui et al. developed PBAEs end-terminated with carboxylates that were able to co-encapsulate the anionic sgRNA through electrostatic interactions and the Cas9 RNP through hydrogen bonding and hydrophobic effects [54]. As a proof of principle, they showed that this carboxylated PBAE could deliver not only Cas9 but also a variety of other proteins with differing charges, greatly expanding the utility of this type of platform for delivery.
Excessive positive charge can also contribute to cytotoxicity by disrupting cell membranes. Fields et al. addressed this by blending PBAE with a solid polymer, poly(lactic-co-glycolic acid) (PLGA), and coating the surface of the PBAE/PLGA/DNA nanoparticles with a cell-penetrating peptide (CPP) [55]. This caused the overall surface charge to become less positive, improving the safety of this tool, while using the CPP to preserve its ability to enter cells, normally mediated in part by the positive surface charge. Cationic surfaces can also limit the movement of nanoparticles through tissue due to electrostatic interactions with both cells and matrix proteins. For instance, highly cationic polymers like PEI and PLL are immobilized in mucus after delivery to the lung [56], and a study on using the related polymer type PAA to deliver a gene encoding an antigen for a dermal vaccine also found that the high positive charge of the PAA/DNA nanoparticle inhibited antigen expression, which the authors attributed to low mobility of the cationic nanoparticles within the extracellular matrix [57]. In many cases, the authors have modified nanoparticles with polyethylene glycol (PEG) to reduce the surface charge and improve mobility, a strategy that will be discussed further below.
Although synthetic materials are generally thought to be less immunogenic than biological vectors like viruses, it has been found that cationic PBAE nanoparticles, as a biomaterial, may also have immunomodulatory properties. The Jewell group has reported that, while free PBAE in solution is immunologically inert, PBAEs complexed with a polyanion to form nanoparticles can cause activation of antigen-presenting cells (APCs) [58]. Moreover, the degree to which PBAE particles exert immunological activity is dependent on the polymer molecular weight, such that their stimulatory effect decreases as the polymer degrades by hydrolysis, and a macrophage activation pathway independent of Toll-like receptor (TLR) or NF-κB signaling pathways was found [59]. However, for some applications, such as genetic vaccines or gene therapies that require immune activation, these properties of PBAEs can be used as an advantage [60].
2.2.3. Endosomal escape
The limiting factor for many delivery materials is insufficient endosomal escape, leading to degradation of the nucleic acid in the late endosomes/lysosomes or recycling of the cargo out of the cell [61–64]. PBAEs enable endosomal escape by undergoing protonation at the lower pH of the endosomal compartment, leading to osmotic pressure buildup due buffering, which causes endosomal disruption. High-throughput combinatorial library screens of PBAEs for nucleic acid delivery have shown that the presence of tertiary amines improves buffering capacity at low pH and facilitate endosomal escape [21]. A direct comparison between particles using poly(lactide-co-glycolide) (PLGA) and PBAE as nanocarriers for mRNA delivery showed that the PLGA nanoparticles were entrapped in the endosomes/lysosomes [65], whereas fluorescence imaging showed that PBAE nanoparticles had diffused throughout the cell. In a study by Vandenbroucke et al., it was hypothesized that sustained release of siRNA from PBAE polyplexes within endosomes due to this osmotic effect led to prolonged gene knockdown in human liver cells after in vitro transfection [66]. Kilchrist et al. recently developed an assay to study endosomal escape [67] in order to explore how the hydrophobicity of PBAE end-caps can be modulated for nanoparticle-mediated endosomal disruption [54] (Figure 4A). Wilson et al. used a high-throughput imaging assay (Figure 4B) to demonstrate that the degree of branching for PBAE polymers also influences endosomal escape [49] (Figure 4C), as the increased number of secondary- and tertiary amines in the PBAE structure increased the buffering capacity of the PBAE, and the efficiency with which PBAEs overcome intracellular delivery hurdles is highly sensitive to the effective pKa of polymers [68].
Figure 4.
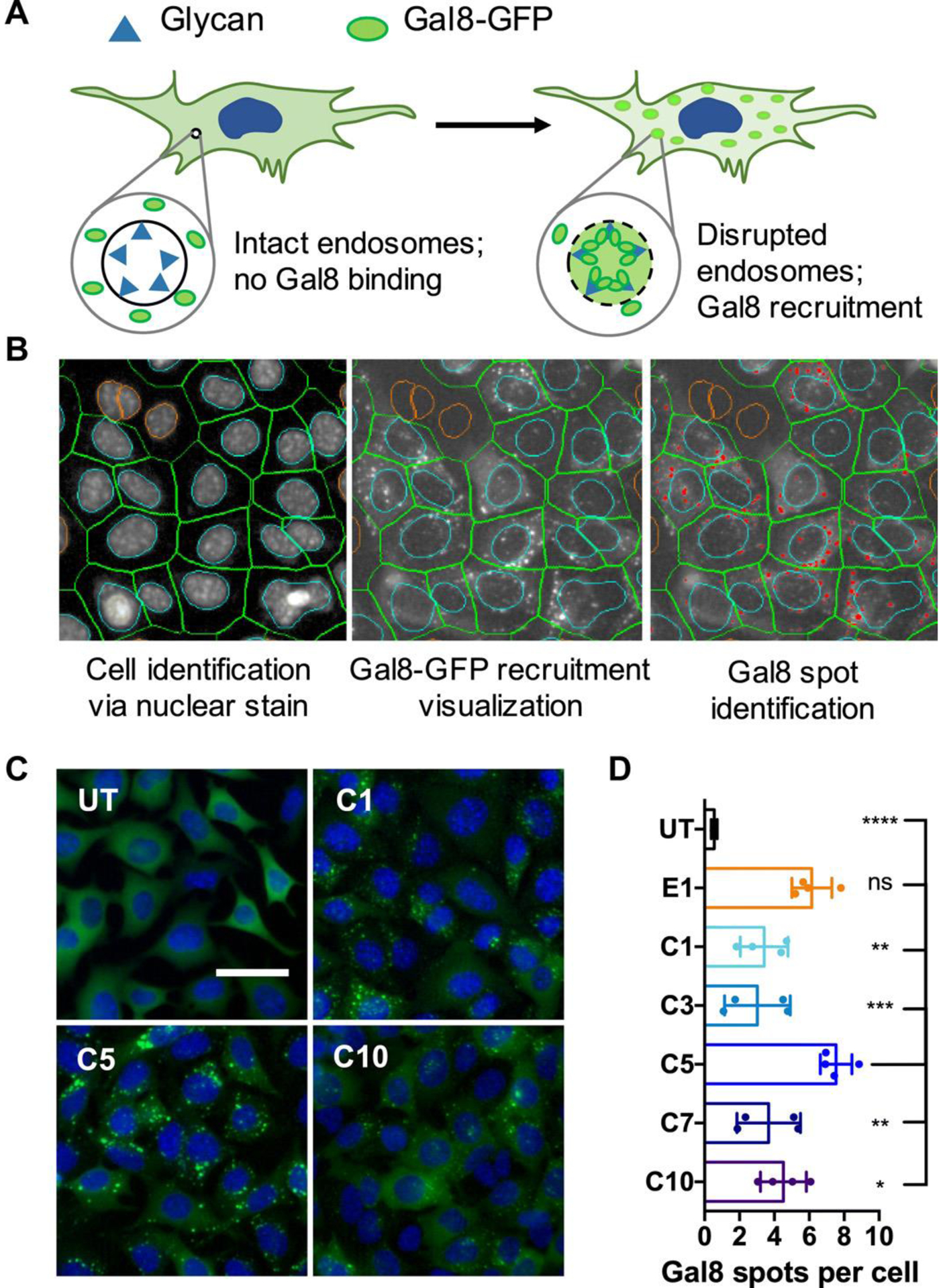
A Gal8 recruitment assay shows PBAE nanoparticle-mediated endosomal disruption. (A) Gal8-GFP is dispersed throughout cells with intact endosomes. In disrupted endosomes, Gal8-GFP binds to endosomal glycans, resulting in punctate fluorescent dots. (B) Image-based analysis of acquired microscope images as shown in (C) can be used to quantify endosomal disruption. Individual cells are identified through nuclear staining (left); Gal8-GFP recruitment is visualized in the green fluorescence channel (middle); and punctate GFP+ spots are identified and counted (red dots). (C) Representative images of Gal8-GFP+ B16 cells left untreated (UT) or treated with carboxylated PBAE/BSA nanoparticles, where C1, C5, and C10 represent particles made using different polymers (scale bar: 50 μm). (D) Endosomal disruption is quantified by the number of Gal8-GFP spots per cell following carboxylated nanoparticle treatment. Particles made from different polymers are listed on the y-axis, where UT is an untreated group. From [54]. Reproduced with permission from American Association for the Advancement of Science (AAAS).
2.2.4. Nucleic acid release
The ester bonds in the backbone structure of PBAEs undergo hydrolysis in aqueous conditions, making PBAEs less toxic than other non-degradable cationic polymers, such as PEI, which has been broadly investigated as a nucleic acid delivery vehicle [19]. The relatively low toxicity of PBAEs allows a higher amount polymer to be used in the nanoparticle formulation compared to non-biodegradable polymers without concerns of toxicity. The hydrolytic degradation of ester bonds also facilitates fast release of the payload. There are different features of the PBAE structure that can be tuned to control the rate of degradation, including polymer molecular weight (Mw) and hydrophobicity [39,69]. PBAEs are also pH sensitive: they undergo protonation of the amine backbone as the pH is lowered, allowing them to bind electrostatically to negatively charged nucleic acids at low pH while remaining closer to neutral at physiological pH. In addition to esters, ketal groups can be incorporated in the PBAE backbone for more pH-dependent hydrolysis, as protonation of amines at pH 5 increases the uptake of water, leading to increased ketal hydrolysis [70]. This strategy results in triggered release at low pH. The reversible protonation of amines at low pH, therefore, can facilitate both initial binding, via charge interactions, and also cargo release, via polymer degradation due to increased hydrophilicity. The incorporation of ketal groups, however, may necessitate an additional hydrophobic spacer to balance hydrophobic and hydrophilic properties to ensure nanoparticle stability at physiological pH [70].
While hydrolysis of PBAEs can cause degradation with a half-life of several hours [39,41], release of nucleic acids due to hydrolysis alone is relatively slow and uncontrolled compared to cargo release by viral gene delivery materials; this is believed to be one reason why non-viral gene delivery materials in general still lag behind viral methods in delivery efficiency, particularly in an in vivo setting [5], To solve this problem, researchers have chemically manipulated PBAEs to promote quick, triggered release of the nucleic acid cargo once inside the cell. These strategies result in rapid degradation of the nanoparticle in response to a stimulus or environmental change, which allows the cargo to be released as well as reducing potential toxicity as the Mw of the polymer decreases, a trend that has been observed for other cationic polymers [71]. Deng et al. introduced a light-sensitive 2-nitrobenzene moiety into the PBAE backbone that could be cleaved by ultraviolet (UV) radiation on a timescale of seconds to minutes [72]. This modified PBAE could then be used to electrostatically complex DNA and then release it quickly upon application of the external stimulus of brief UV exposure.
Another strategy to achieve triggered intracellular release from PBAE nanoparticles is to incorporate disulfide bonds, which can be cleaved and converted to thiol groups within minutes of exposure to the relatively reducing environment of the cytoplasm. Poly(amido amine)s, a class of polymers related to PBAEs but with much slower hydrolytic degradation, can be synthesized with disulfides in the backbone for quick release of DNA in the cytoplasm [73]. Similar strategies have been employed for PBAEs, including the use of disulfide-containing end-groups for siRNA delivery [41,74]. Bioreducible PBAEs with disulfides within the backbone of the polymer have demonstrated efficient intracellular release of miRNA [42] or siRNA. Reducible disulfide-containing PBAEs promoted siRNA release in patient-derived glioblastoma cells with decreased toxicity compared to non-reducible counterparts [30], allowing higher polymer:siRNA w/w ratio to be used to improve transfection while still ensuring safety. A high w/w ratio of bioreducible PBAE:siRNA was reported to promote colloidal nanoparticle stability and to enable a systemic siRNA delivery in an orthotopic brain tumor mouse model without any signs of systemic toxicity [75]. In addition, the increased polymer:siRNA ratio also reduced nanoparticle size to promote transendothelial crossing in a biomimetic blood-brain barrier (BBB) in vitro model. The possibility of increasing polymer concentration is also useful for encapsulating higher doses nucleic acid, and thus combinational cargos targeting different genes can be used [36].
While the use of reducible linkages can be applied to the delivery of many types of nucleic acid, it is particularly well suited for cargoes like siRNA, miRNA, and mRNA, whose site of action is the cytosol. Other nucleic acids, such as plasmid DNA, must first be trafficked into the nucleus, another major barrier to efficient gene delivery that must be overcome for some applications. For instance, Smith et al. found that, although a traditional, non-functionalized PBAE was poorly able to transfect T cells, PBAE/DNA nanoparticles functionalized with a T cell-targeting moiety and a nuclear localization signal (NLS) were able to achieve >80% transfection of T cells, demonstrating the importance of both uptake and nuclear trafficking of the nanoparticle [35].
2.3. Overcoming in vivo delivery barriers
2.3.1. PBAE nanoparticle stability
There are several factors known to influence the colloidal stability of nanoparticles, including surface charge, pH, ionic strength, and the presence of serum proteins. Unmodified PBAE-based nanoparticles tend to have low colloidal stability in physiological fluids. Early work showed that the in vivo transfection efficacy of PBAE/DNA nanoparticles was lower after intravenous (IV) injection than after intraperitoneal (IP) injection, while in vitro transfection was higher in serum-free conditions [45], demonstrating tat the presence of serum can negatively affect the properties of nanoparticles. An established approach to improving colloidal stability is the incorporation of a polymer shield of PEG or polyethylene oxide (PEO) around the particle, known as PEGylation [76], to promote nanoparthicle stability by reducing adsorption of serum proteins. One strategy is to end-cap the PBAE with PEG. Gale et al. showed that their nanoparticle formulation carrying the nucleic acid-based adjuvant polyinosinic-polycytidylic acid [poly(I:C)] had the highest immunogenicity after IP administration when using a 2 kDa PEG-PBAE end capped nanocarrier [77]. The shielding capability of PEGylated PBAEs can also be used to improve transfection in vitro and [78] the in vivo diffusivity of nanoparticles, improving their mobility within tissue. Kim et al. used thiol-PEG to end-cap PBAEs to improve penetration of brain tumors [78]. The engineered PEG-PBAE nanoparticles carried DNA encoding herpes simplex virus-thymidine kinase (HSV-tk) as a suicide gene, and PEG-PBAE nanoparticles penetrated the tumor better than non-PEGylated PBAE nanoparticles. PEGylated PBAE nanoparticles can also help to overcome the mucus barrier, since mucin fibers contain a high density of negatively charged glycans [79]. Mastorakos et al. demonstrated that PEG-end-capped PBAE nanoparticles carrying DNA efficiently penetrated the mucus and resulted in a high level of transfection throughout the mouse lungs [76].
As an alternative approach, PEG can also be incorporated non-covalently by being added after the self-assembly of PBAE and nucleic acid into nanoscale particles. PBAE terpolymers with hydrophobic components have since been developed in order to improve DNA or RNA binding via hydrophobic interactions as well as to non-covalently incorporate PEG shielding to prevent aggregation in serum [52,80]. Successful incorporation of PEG decreases the positive surface charge of PBAE nanoparticle formulations [24]. Kaczmarek et al. reported PEGylated PBAE nanoparticle formulations with high colloidal stability in presence of serum and potent mRNA delivery capacity in vivo [24,81].
Other methods of stabilizing PBAE via blending with other polymers will be discussed below in the section on PBAE-Based Hybrid Materials.
2.3.2. Functionalization for cell targeting
Another strategy for cell targeting is to functionalize PBAE nanoparticles with ligands targeting receptors of cellular membranes for cell type-specific delivery (Table 2). Various design strategies have been used to incorporate ligands into the nanoparticle design. One is covalent attachment of the ligand during polymer synthesis, either as a side-chain moiety or as an end-cap. For instance, PBAEs were synthesized with mannose as end-caps in order to target APCs. Jones et al. demonstrated that their mannosylated PBAEs carrying DNA transfected RAW264.7 better in vitro than non-functionalized PBAEs [82]. Mannosylated PBAEs also elicited a higher antibody titers in vivo than non-functionalized PBAE, and this genetic vaccine also generated higher antibody titers than the standard protein-plus-adjuvant control. Fornaguera et al. reported that oligopeptide end-modified PBAEs enable targeted mRNA transfection APCs, in which the peptide sequence can be modulated to promote interactions with cell membranes and pH buffering capacity [83].
Table 2.
PBAE nanoparticle functionalization strategies
| Design Strategy of Ligand Incorporation | Added Functionality | Ligand |
|---|---|---|
| Polyglutamic (PGA)-coated nanoparticles for electrostatic incorporation | Cell-targeted DNA delivery to primary endothelial cells | RGD peptide [84] |
| T cell-targeted DNA delivery | Anti-CD3e f(ab´)2 [35] | |
| Cell-targeted mRNA delivery to T cells and hematopoietic stem cells (HSCs) | Anti-CD3, anti-CD8, and anti-CD105 [85] | |
| Macrophage-targeted mRNA delivery | Mannose [86] | |
| Covalently incorporated in PBAE structure | Targeted DNA delivery to APCs | Mannose [82,100,103] |
| Targeted mRNA delivery to APCs | Oligopeptide [83] | |
| Improved mucus penetration and DNA transfection in the lungs | PEG (5 kDa) [114] | |
| Improved in vivo transfection of nucleic acid-based adjuvant after IP administration | PEG (2 kDa) [77] | |
| Shielding to enhance tissue diffusivity | PEG [78] | |
| Ligand functionalized lipid incorporated via hydrophobic interactions | Improved cellular internalization and DNA transfection, reduced toxicity, and increased DNA loading | Cell-penetrating peptides (CPPs) mTAT, bPrPp, and MPG [115] |
| Minimized toxicity for safe and effective mRNA transfection of dendritic cells (DCs) | PEGylated lipid [65] | |
| Shielding to improve colloidal stability and prevent aggregation in serum for potent mRNA transfection after systemic administration | PEGylated lipid [25,80,81,97,116] | |
| Steric stabilization | Pluronic F-108 [92] |
Alternatively, the ligand can be added post-nanoparticle formation either via electrostatic- or hydrophobic interactions. Green et al. electrostatically coated the cationic nanoparticle surface with the anionic polyglutamic acid (PGA) conjugated to an RGD peptide for endothelial cell (EC) targeting [84]. The RGD-coated nanoparticles caused receptor-mediated gene delivery to ECs. PGA was also used by Smith et al. to electrostatically attach antibodies against CD3 to PBAE nanoparticles for selective binding of T lymphocytes [35]. The T cell-targeted PBAE nanoparticles carried DNA encoding a leukaemia-targeting CAR that reprogrammed circulating T cells to recognize and combat tumor cells for long-term disease remission. The Stephan lab has also used this nanoparticle design for targeted mRNA delivery to T cells [85] in order to (1) knock out genes in anti-cancer T cells, (2) transfect T cells with mRNA encoding a key transcription factor of memory formation, and (3) reprogram hematopoietic stem cells with improved self-renewal properties. In another study, they electrostatically functionalized PBAEs with mannose for macrophage targeting to reprogram tumor-associated macrophages to a M1 phenotype for an anti-tumor response [86].
Functionalization of PBAEs can also be used to achieve improved accumulation of targeted organ. The incorporation of retinol into particles increases the adsorption of retinol binding protein (RBP) whose receptor is upregulated in the liver. Fornaguera et al. showed that PBAE nanoparticles modified with retinol effected increased mRNA transfection in the liver, and they reported that apolipoproteins in the corona directed the particles to the liver [83].
3. PBAE-Based Hybrid Materials
Hybrid delivery materials can be constructed to overcome challenges associated with PBAE particles including stability and toxicity, as well as provide further enhancements, such as superior transfection efficacy, additional functionalities, and control over gene expression kinetics. Three classes of hybrid gene delivery systems will be discussed: PBAE/polymer blends, lipid-coated PBAE particles, and PBAE coatings.
An emerging class of particles for gene delivery are composed of hybrid biodegradable polymer blends. Polyesters like poly(lactic-co-glycolic acid) (PLGA) are generally recognized as safe (GRAS) and have an extensive history of use as drug carriers. They typically have low transfection efficacy on their own, but their incorporation into PBAE particles increases particle rigidity, improves stability and shelf-life, and introduces a large degree of control over the particle size to exploit passive targeting to different tissues or phagocytic APC populations. Multiple groups have found that particles composed of 75–85% PLGA and 15–25% PBAE are optimal for transfection of APCs in vitro and in vivo, while being relatively nontoxic to cells [55,87,88]. The particles themselves also have an immunostimulatory effect on APCs, making them useful for applications like genetic vaccines [87]. Hybrid particles also support surface functionalization with ligands for active targeting and cell entry. Fields et al. showed nanoparticles formed from a PLGA/PBAE blend could be surface-modified with PEG-lipids and cell-penetrating peptides, leading to more efficient DNA loading, internalization, and transfection efficacy compared to unmodified PLGA/PBAE particles and PLGA particles [55,88]. In addition to increasing the ratio of PBAE to other polymers, varying polymer properties such as lipophilicity has also been shown to enhance transfection [89]. PBAE/polyester particle blends have been used for delivery of other cargo, including small molecule drugs and biologics, exploiting favorable properties of PBAEs like pH-responsive triggered release and rapid degradation [90–93], and PBAE has also been explored as a porogen in PLGA-based scaffolds for the release of multiple agents on different timescales [94–96].
Liposomal transfection reagents are commercially available and have been investigated extensively, but they tend to be unstable and do not support sustained or stimuli-responsive cargo release [6]. When combined with a polymer core, lipid shells can facilitate targeting and binding of particles to cells of interest, which can be particularly advantageous for difficult-to-transfect cells, such as immune cells [65]. Persano et al. demonstrated that PBAE/mRNA polyplexes incased in a lipid bilayer led to 100% transfection in dendritic cell-like DC2.4 cells, while uncoated polyplexes did not detectably transfect DC2.4 cells [97]. Lipid-coated polyplexes were then used to deliver mRNA encoding ovalbumin subcutaneously to immune cells, causing stimulation, measured by IFN-β levels in the serum (Figure 5A) and activation of T cells and cells of the lymph nodes (Figure 5B–C) [97]. This caused antigen-specific killing of B16F10 tumor cells in vitro (Figure 5D), inhibited ovalbumin-expressing B16F10 tumor metastasis to the lungs (Figure 5E), and activated antigen-specific CD8+ T cells in vivo (Figure 5F–G) [97]. Lipid coatings can also shield the cationic polymer core to reduce toxicity due to excessive positive charge, particularly for in vivo applications [81], and liposome-coated PBAE particles have been observed to be minimally toxic in vivo [81,98]. Finally, consideration must be given to the composition of the lipid coating. Lipid and PEG-lipid ratios are key factors influencing transfection efficacy in vivo, and optimization is necessary to maximize transfection efficacy [81].
Figure 5.
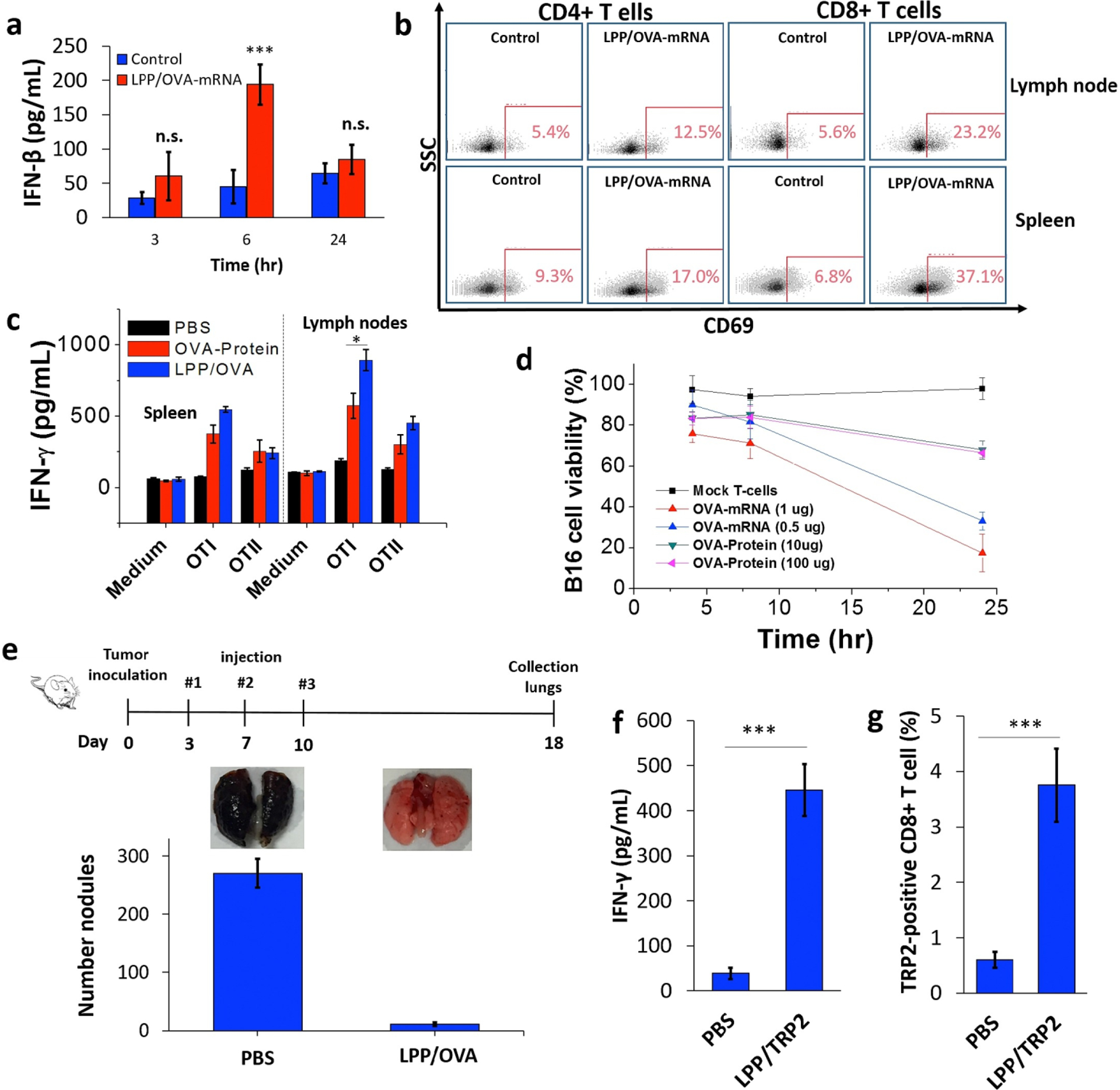
Immunization with lipopolyplexes containing ovalbumin mRNA increased IFN-β levels present in serum six hours after vaccination (A), increased T cell activation, measured as upregulation of CD69 (B), increased IFN-γ secretion by cells from the lymph nodes upon restimulation with OT-I cells (C), and promoted antigen-specific killing of B16-OVA melanoma cells in vitro (D). Vaccination also decreased the number of metastatic tumor nodules in the lungs of mice (E). Vaccination with lipopolyplexes containing mRNA for tumor antigen TRP2 increased IFN-γ production by peripheral blood mononuclear cells (PBMCs), suggesting immune activation (F), and increased the percentage of antigen-specific CD8+ T cells in mice (G). Reprinted from Biomaterials, Vol. 125, Persano et al., “Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination,” pg. 81–89, Copyright Elsevier (2017) [97].
PBAEs are useful not only as a nanoparticle core material but also as a coating for other gene delivery vehicles. This strategy leverages the advantages of PBAEs in overcoming gene delivery barriers like cellular internalization and endosomal escape, while an alternative core material can provide stability, theranostic capabilities, or enhanced nucleic acid loading. For example, the rigidity of siRNA compared to plasmid DNA may interfere with its ability to complex with PBAEs and induce gene knockdown, but Lee et al. showed that gold nanoparticles surface-conjugated with siRNA and coated with PBAE induced high levels of knockdown [99]. PBAEs have also been used to coat organic delivery vectors to enhance gene delivery. An interesting example is PBAE coated onto E. coli, with both vectors carrying plasmid DNA encoding ovalbumin [100]. E. coli is an efficient, easily manipulated gene carrier and promotes uptake by APCs via phagocytosis. Immunization with the hybrid vector led to increased anti-ovalbumin antibody titers compared to ovalbumin plasmid DNA or protein administered with an adjuvant. Potential drawbacks of this system include its complexity and potential immunogenicity [101], but it illustrates the potential for synergy between PBAEs and other vectors to deliver genes more effectively than either vector alone [100,102,103].
Cationic polymers like PBAEs can be useful components of layer-by-layer (LbL) coatings, formed through repeated layering of oppositely charged polyelectrolytes onto particles, films, scaffolds, or other structures. LbL coatings support efficient loading and release of one or more nucleic acids and kinetically controlled gene expression. Release of the nucleic acid is dependent on the degradability of polymers within the coating and their electrostatic interactions and can occur over a period of hours or days depending on the composition of the layers [104]. Two nucleic acids can also be loaded and released sequentially from LbL-coated nanoparticles, leading to the expression of two genes on different timescales [105]. LbL techniques utilizing PBAE have also been employed to generate 3D surfaces for gene and protein delivery. Li et al. electrospun fibers from a blend of PBAE and PCL and used an LbL technique to coat the fibrous mat with layers of PBAE and DNA [106]. This created a scaffold that achieved sustained high-dosage release of DNA over a period of 10 hours and improved transfection of glioblastoma cells compared to LbL films based on PBAE alone [106]. PBAE-based multilayer coatings have also been applied to microneedles to deliver genetic vaccines. DeMuth et al. used LbL techniques to coat polymeric microneedles with DNA, isRNA, and PBAE to induce a robust immune response against a model HIV antigen [107]. These same properties also allow other biologics and small molecule drugs to be incorporated into PBAE-based LbL coatings for controlled release [108–110].
Additional materials can enhance the efficacy of PBAE-based vehicles for gene delivery. Incorporation of other polymers substantially alters the rigidity and size of PBAE particles, enhancing their stability and introducing possibilities for surface modification. Lipid bilayers may provide additional means to target particles to cells and help shield the PBAE particle to reduce toxicity. PBAE coatings can impart gene delivery capabilities to a variety of 3D substrates, and PBAE polyplexes can be dispersed throughout hydrogels to generate a 3D gene delivery depot [111]. PBAE-based hybrid materials show significant promise for gene delivery, because one can exercise more freedom in design and exploit the strengths of different materials to achieve the desired therapeutic effect.
4. Conclusion
PBAEs have been designed over the past twenty years to have properties that promote successful delivery of nucleic acids. Their biodegradability improves their safety profile, though it also can limit the ability of PBAEs to sustain delivery over long timespans. Their positive charge allows them to bind well to nucleic acids, and the pH-sensitive amine groups in PBAEs allow them to buffer acidic environments; however, excessive positive charge can also cause toxicity and limit the movement of PBAE nanoparticles throughout tissue. Importantly, PBAEs contain many functional groups that can be used for chemical modifications, and several researchers have taken advantage of this to develop PBAEs that are tailored for delivery to specific cell types or optimized to overcome certain limitations of traditional PBAE structures. This ease of chemical modification allows the design of PBAEs to be continually improved for efficacy while maintaining a favorable safety profile, and the field is continuing to develop novel materials based on PBAEs that are increasingly suitable for gene delivery applications.
5. Expert opinion
Nucleic acid-based therapeutics have tremendous potential to be used for a wide range of unmet therapeutic needs and for personalized medicine. However, carriers are needed to enable nucleic acid delivery across biological barriers into cells, involving encapsulation, colloidal stability, cellular uptake, endosomal escape, intracellular release, and safety. We have in this review highlighted the use of PBAEs to facilitate efficient intracellular delivery, including current understanding of the structure-function relationships that are important for the design of PBAEs. A major advantage over many other delivery materials is that PBAEs are hydrolytically biodegradable in physiological conditions, and they can be synthesized to ensure that degradation byproducts are nontoxic. As described above, preclinical studies have demonstrated that PBAEs can be used for safe delivery.
Another major advantage is the ease of synthesis of PBAEs and that they can be synthesized with diverse chemical properties. This has allowed for high-throughput screens of PBAE nanocarriers for nucleic acid delivery that have identified formulations with effective intracellular delivery to a wide range of cell types while also allowing for cell-specific or environmentally triggered delivery, including cancer-specific delivery. Moreover, the possibility of forming PBAEs with diverse chemical properties has made it possible to form nanoparticle formulations for different types of nucleic acid molecules. The ease of manufacture and potential for long-term storage stability is also beneficial for scaling up their production for future clinical use.
However, there are still challenges that must be overcome in order for PBAEs to enter a clinical setting. Previous studies have shown that a desired therapeutic outcome can be achieved by local administration of PBAE/nucleic acid nanoparticle formulations in a wide range of in vivo models, but local administration is limited to certain clinical settings. In order for PBAE-based nanoparticles to be more broadly applicable, they must often undergo modifications for effective systemic in vivo delivery to overcome hurdles like nanoparticle instability in physiological fluids. There have been important advancements in the development of technologies with which local nanoparticle administration might be combined, such as osmotic pump systems, microneedles, and implants with an inbuilt release, to further advance genetic medicine into clinic; at the same time, however, PBAE nanoparticles are increasingly being engineered for more accessible routes of administration. Accordingly, future studies must establish design criteria for achieving efficient delivery to target sites and cells of interest following intravenous administration, including serum stability, tissue diffusivity, and sufficient circulation time. Other factors that will need to be considered for eventual clinical translation include large-scale manufacture and studies on storage stability. Because PBAEs are hydrolytically degradable and are often designed to break down rapidly in order to facilitate nucleic acid release and biocompatibility, they may require production and storage considerations that would not apply to other nondegradable materials.
An important strategy for improving the translational potential of PBAE nanoparticles is to coat them with ligands for increased functionality. Added functionalities can lead to reduced interactions with serum proteins that might influence the stability of particles, prolonged circulation time, and cell-type targeted delivery. Functionalization with PEG reduces the interaction with serum proteins and blood clearance by the reticuloendothelial system (RES) as well as improving mobility through tissue. Moreover, a variety of materials are being explored in combination with PBAEs to form hybrid nanocarriers for improved delivery efficacy, including inorganic materials, other polymers, surface-active molecules, lipids, and bacteria. These hybrid delivery vectors have shown to add functionalities for improved cell transfection.
The recent approval of Onpattro, a lipid nanoparticle formulation of siRNA to treat liver disease, is a truly exciting breakthrough for the field of non-viral delivery of nucleic acid-based drugs [112]. This opens the door for others in the clinical pipeline and also yields important insights for physicochemical properties of nanoparticles for clinical translation. PBAE nanocarriers have shown promise for realizing the broad potential of nucleic acid therapeutics for a wide range of diseases. Studies up to the present have shown that PBAEs can be synthesized with precise molecular organization and diverse chemical properties; controlled molecular weight; high payload capacity; efficient intracellular delivery and endosomal escape, biodegradability; and low toxicity and immunogenicity. With all this promise, there is great hope that we will see new novel nanoparticle formulations using biodegradable PBAE carriers in a not-too-distant future that will open new avenues of genetic medicine.
Article highlights.
Materials for non-viral gene delivery must overcome many extracellular and intracellular barriers for successful transfection
Poly(beta-amino ester)s (PBAEs) are cationic polymers designed to bind nucleic acids, promote cellular uptake and endosomal escape, and release polymers due to degradation
PBAEs may be hampered by challenges, including their relative instability in physiological fluids and their potential to cause toxicity or immunogenicity due to excessive positive charge
The ease with which PBAEs can be covalently and non-covalently modified allows them to be customized or blended with other materials to overcome delivery challenges and broadens their applicability
Funding
This paper was supported by the National Institutes of Health (P41EB028239, R01CA228133, and R01EY031097), American Autoimmune-Related Diseases Association (grant support), and National Science Foundation (fellowship support).
Footnotes
Declaration of interest
S Y Tzeng is credited with inventorship on related patents received and pending. K R Rhodes is credited with inventorship on related pending patents. J J Green is also a Member of the Board of Directors, a Consultant, Co-Founder and holds Equity in Asclepix Therapeutics; A Managing Member, Co-Founder and holds Equity in Dome Therapeutics: is a Board of Directors Member, Consultant and holds Stock Options in VasoRX: is a Scientific Advisory Board Member and holds Stock Options in Tidal; and is an Investigator for AstraZeneca. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Giacca M, Zacchigna S. Virus-mediated gene delivery for human gene therapy. J Control Release. 2012. July 20;161(2):377–88. [DOI] [PubMed] [Google Scholar]
- 2.Miliotou AN, Papadopoulou LC. CAR T-cell Therapy: A New Era in Cancer Immunotherapy. Curr Pharm Biotechnol. 2018;19(1):5–18. [DOI] [PubMed] [Google Scholar]
- 3.Trapani I, Auricchio A. Seeing the Light after 25 Years of Retinal Gene Therapy. Trends Mol Med. 2018. August;24(8):669–681. [DOI] [PubMed] [Google Scholar]
- 4.Nayerossadat N, Maedeh T, Ali PA. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lostalé-Seijo I, Montenegro J. Synthetic materials at the forefront of gene delivery. Nat Rev Chem. 2018. 2018/10/01;2(10):258–277. [Google Scholar]
- 6.Zylberberg C, Gaskill K, Pasley S, et al. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017. August;24(8):441–452. [DOI] [PubMed] [Google Scholar]
- 7.Lai WF. Cyclodextrins in non-viral gene delivery. Biomaterials. 2014. January;35(1):401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Zhang Q, Chang H, et al. Surface-engineered dendrimers in gene delivery. Chem Rev. 2015. June 10;115(11):5274–300. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed M Peptides, polypeptides and peptide-polymer hybrids as nucleic acid carriers. Biomater Sci. 2017. October 24;5(11):2188–2211. [DOI] [PubMed] [Google Scholar]
- 10.Alipour M, Majidi A, Molaabasi F, et al. In vivo tumor gene delivery using novel peptideticles: pH-responsive and ligand targeted core-shell nanoassembly. Int J Cancer. 2018. October 15;143(8):2017–2028. [DOI] [PubMed] [Google Scholar]
- 11.Sadeghian F, Hosseinkhani S, Alizadeh A, et al. Design, engineering and preparation of a multi-domain fusion vector for gene delivery. Int J Pharm. 2012. May 10;427(2):393–9. [DOI] [PubMed] [Google Scholar]
- 12.Peng L, Wagner E. Polymeric Carriers for Nucleic Acid Delivery: Current Designs and Future Directions. Biomacromolecules. 2019. October 14;20(10):3613–3626. [DOI] [PubMed] [Google Scholar]
- 13.Yue YA, Jin F, Deng R, et al. Revisit complexation between DNA and polyethylenimine - Effect of length of free polycationic chains on gene transfection. J Control Release. 2011. May 30;152(1):143–151. [DOI] [PubMed] [Google Scholar]
- 14.Zhang P, Wagner E. History of Polymeric Gene Delivery Systems. Top Curr Chem (Cham). 2017. April;375(2):26. [DOI] [PubMed] [Google Scholar]
- 15.Shen W, Wang R, Fan Q, et al. Natural Polyphenol Inspired Polycatechols for Efficient siRNA Delivery. CCS Chemistry.2(3):146–157. [Google Scholar]
- 16.Leiro V, Garcia JP, Tomas H, et al. The present and the future of degradable dendrimers and derivatives in theranostics. Bioconjug Chem. 2015. July 15;26(7):1182–97. [DOI] [PubMed] [Google Scholar]
- 17.Huang D, Wu D. Biodegradable dendrimers for drug delivery. Mater Sci Eng C Mater Biol Appl. 2018. September 1;90:713–727. [DOI] [PubMed] [Google Scholar]
- 18.Chuan D, Jin T, Fan R, et al. Chitosan for gene delivery: Methods for improvement and applications. Adv Colloid Interface Sci. 2019. June;268:25–38. [DOI] [PubMed] [Google Scholar]
- 19.Lynn DM, Langer R. Degradable poly (beta-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J Am Chem Soc. 2000;122(44):10761–10768. [Google Scholar]
- 20.Akinc A, Lynn DM, Anderson DG, et al. Parallel synthesis and biophysical characterization of a degradable polymer library for gene delivery. J Am Chem Soc. 2003. May 7;125(18):5316–23. [DOI] [PubMed] [Google Scholar]
- **21.Green JJ, Langer R, Anderson DG. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc Chem Res. 2008. June;41(6):749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the key early descriptions of the potential of PBAEs as gene delivery reagents, this work highlights the ease of chemical manipulation and function of initial generations of PBAEs.
- 22.Tzeng SY, Green JJ. Polymeric Nucleic Acid Delivery for Immunoengineering. Curr Opin Biomed Eng. 2018. September;7:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green JJ, Zugates GT, Tedford NC, et al. Combinatorial modification of degradable polymers enables transfection of human cells comparable to adenovirus. Adv Mater. 2007. October 5;19(19):2836–2842. [Google Scholar]
- 24.Anderson DG, Lynn DM, Langer R. Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angew Chem Int Ed. 2003;42(27):3153–3158. [DOI] [PubMed] [Google Scholar]
- 25.Kowalski PS, Palmiero UC, Huang YX, et al. Ionizable Amino-Polyesters Synthesized via Ring Opening Polymerization of Tertiary Amino-Alcohols for Tissue Selective mRNA Delivery. Adv Mater. 2018. August 23;30(34). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *26.Eltoukhy AA, Siegwart DJ, Alabi CA, et al. Effect of molecular weight of amine end-modified poly(beta-amino ester)s on gene delivery efficiency and toxicity. Biomaterials. 2012. May;33(13):3594–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a method for controlling the molecular weight of PBAEs and its influence for efficient DNA delivery.
- 27.Sunshine J, Green JJ, Mahon KP, et al. Small-Molecule End-Groups of Linear Polymer Determine Cell-Type Gene-Delivery Efficacy. Adv Mater. 2009. December 28;21(48):4947–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamboni CG, Kozielski KL, Vaughan HJ, et al. Polymeric nanoparticles as cancer-specific DNA delivery vectors to human hepatocellular carcinoma. Journal of Controlled Release. 2017. October 10;263:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzeng SY, Higgins LJ, Pomper MG, et al. Biomaterial-mediated cancer-specific DNA delivery to liver cell cultures using synthetic poly(beta-amino ester)s. J Biomed Mater Res A. 2013. July;101(7):1837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **30.Kozielski KL, Tzeng SY, De Mendoza BAH, et al. Bioreducible Cationic Polymer-Based Nanoparticles for Efficient and Environmentally Triggered Cytoplasmic siRNA Delivery to Primary Human Brain Cancer Cells. ACS Nano. 2014. April;8(4):3232–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports the synthesis of PBAEs containing disulfide bonds in the backbone stucture for environmentally triggered cytosolic siRNA release.
- 31.Tzeng SY, Guerrero-Cazares H, Martinez EE, et al. Non-viral gene delivery nanoparticles based on poly(beta-amino esters) for treatment of glioblastoma. Biomaterials. 2011. August;32(23):5402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anchordoquy TJ, Koe GS. Physical stability of nonviral plasmid-based therapeutics. J Pharm Sci. 2000. March;89(3):289–96. [DOI] [PubMed] [Google Scholar]
- 33.Guerrero-Cazares H, Tzeng SY, Young NP, et al. Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS Nano. 2014. May 27;8(5):5141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fornaguera C, Castells-Sala C, Lazaro MA, et al. Development of an optimized freeze-drying protocol for OM-PBAE nucleic acid polyplexes. Int J Pharm. 2019. October 5;569:118612. [DOI] [PubMed] [Google Scholar]
- **35.Smith TT, Stephan SB, Moffett HF, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017. August;12(8):813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work demonstrates a simple and easily adaptable method of cellular targeting using PBAEs as well as other modifications that allow in vivo transfection of a cell type that is traditionally considered difficult to transfect.
- 36.Kozielski KL, Ruiz-Valls A, Tzeng SY, et al. Cancer-selective nanoparticles for combinatorial siRNA delivery to primary human GBM in vitro and in vivo. Biomaterials. 2019. July;209:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson DR, Sen R, Sunshine JC, et al. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomedicine. 2018. February;14(2):237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel AK, Kaczmarek JC, Bose S, et al. Inhaled Nanoformulated mRNA Polyplexes for Protein Production in Lung Epithelium. Adv Mater. 2019. February;31(8):e1805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sunshine JC, Peng DY, Green JJ. Uptake and transfection with polymeric nanoparticles are dependent on polymer end-group structure, but largely independent of nanoparticle physical and chemical properties. Mol Pharm. 2012. November 5;9(11):3375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao J, Yang L, Huang P, et al. Synthesis and characterization of low molecular weight polyethyleneimine-terminated Poly(beta-amino ester) for highly efficient gene delivery of minicircle DNA. J Colloid Interface Sci. 2016. February 1;463:93–8. [DOI] [PubMed] [Google Scholar]
- 41.Tzeng SY, Green JJ. Subtle changes to polymer structure and degradation mechanism enable highly effective nanoparticles for siRNA and DNA delivery to human brain cancer. Adv Healthc Mater. 2013. March;2(3):468–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Bertoni H, Kozielski KL, Rui Y, et al. Bioreducible Polymeric Nanoparticles Containing Multiplexed Cancer Stem Cell Regulating miRNAs Inhibit Glioblastoma Growth and Prolong Survival. Nano Lett. 2018. July 11;18(7):4086–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keeney M, Ong SG, Padilla A, et al. Development of poly(beta-amino ester)-based biodegradable nanoparticles for nonviral delivery of minicircle DNA. ACS Nano. 2013. August 27;7(8):7241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson DG, Akinc A, Hossain N, et al. Structure/property studies of polymeric gene delivery using a library of poly(beta-amino esters). Mol Ther. 2005. March;11(3):426–434. [DOI] [PubMed] [Google Scholar]
- 45.Zugates GT, Peng WD, Zumbuehl A, et al. Rapid optimization of gene delivery by parallel end-modification of poly(beta-amino ester)s. Mol Ther. 2007. July;15(7):1306–1312. [DOI] [PubMed] [Google Scholar]
- 46.Hong CA, Eltoukhy AA, Lee H, et al. Dendrimeric siRNA for Efficient Gene Silencing. Angew Chem Int Ed. 2015;54(23):6740–6744. [DOI] [PubMed] [Google Scholar]
- *47.Cutlar L, Zhou D, Gao Y, et al. Highly Branched Poly(β-Amino Esters): Synthesis and Application in Gene Delivery. Biomacromolecules. 2015. 2015/09/14;16(9):2609–2617. [DOI] [PubMed] [Google Scholar]; This paper describes the synthesis of PBAEs with branching architecture and demonstrates certain limitations of linear PBAEs as well as a strategy for overcoming those limitations using synthetic chemistry.
- 48.Zhou D, Cutlar L, Gao Y, et al. The transition from linear to highly branched poly(beta-amino ester)s: Branching matters for gene delivery. Sci Adv. 2016. June;2(6):e1600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson DR, Rui Y, Siddiq K, et al. Differentially Branched Ester Amine Quadpolymers with Amphiphilic and pH-Sensitive Properties for Efficient Plasmid DNA Delivery. Mol Pharm. 2019. February 4;16(2):655–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai SJ, Andorko JI, Zeng XB, et al. Polyplex interaction strength as a driver of potency during cancer immunotherapy. Nano Research. 2018. October;11(10):5642–5656. [Google Scholar]
- 51.Bhise NS, Shmueli RB, Gonzalez J, et al. A Novel Assay for Quantifying the Number of Plasmids Encapsulated by Polymer Nanoparticles. Small. 2012. February 6;8(3):367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eltoukhy AA, Chen DL, Alabi CA, et al. Degradable Terpolymers with Alkyl Side Chains Demonstrate Enhanced Gene Delivery Potency and Nanoparticle Stability. Adv Mater. 2013. March 13;25(10):1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rui Y, Varanasi M, Mendes S, et al. Poly(Beta-Amino Ester) Nanoparticles Enable Nonviral Delivery of CRISPR-Cas9 Plasmids for Gene Knockout and Gene Deletion. Mol Ther Nucleic Acids. 2020. April 21;20:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **54.Rui Y, Wilson DR, Choi J, et al. Carboxylated branched poly(beta-amino ester) nanoparticles enable robust cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci Adv. 2019. December;5(12):eaay3255. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reported the synthesis of carboxylated PBAEs that provided robust CRISPR-Cas9 gene editing in an orthotopic murine glioma model.
- *55.Fields RJ, Cheng CJ, Quijano E, et al. Surface modified poly(beta amino ester)-containing nanoparticles for plasmid DNA delivery. J Control Release. 2012. November 28;164(1):41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report utilizes surface-modified nanoparticles synthesized with varying ratios of PLGA and PBAE to enhance transfection efficacy in cystic fibrosis affected bronchiolar epithelial cells with reduced toxicity.
- 56.Suk JS, Kim AJ, Trehan K, et al. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J Control Release. 2014. March 28;178:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van den Berg JH, Oosterhuis K, Hennink WE, et al. Shielding the cationic charge of nanoparticle-formulated dermal DNA vaccines is essential for antigen expression and immunogenicity. J Control Release. 2010. January 25;141(2):234–40. [DOI] [PubMed] [Google Scholar]
- 58.Andorko JI, Hess KL, Pineault KG, et al. Intrinsic immunogenicity of rapidly-degradable polymers evolves during degradation. Acta Biomater. 2016. March 1;32:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dold NM, Zeng Q, Zeng X, et al. A poly(beta-amino ester) activates macrophages independent of NF-kappaB signaling. Acta Biomater. 2018. March 1;68:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andorko JI, Pineault KG, Jewell CM. Impact of molecular weight on the intrinsic immunogenic activity of poly(beta amino esters). J Biomed Mater Res A. 2017. April;105(4):1219–1229. [DOI] [PubMed] [Google Scholar]
- 61.Martens TF, Remaut K, Demeester J, et al. Intracellular delivery of nanomaterials: How to catch endosomal escape in the act. Nano Today. 2014. 2014/06/01/;9(3):344–364. [Google Scholar]
- 62.Jiang Y, Lu Q, Wang Y, et al. Quantitating Endosomal Escape of a Library of Polymers for mRNA Delivery. Nano Lett. 2020. February 12;20(2):1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sahay G, Querbes W, Alabi C, et al. Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol. 2013. July;31(7):653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilleron J, Querbes W, Zeigerer A, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat Biotechnol. 2013. July;31(7):638–46. [DOI] [PubMed] [Google Scholar]
- 65.Su XF, Fricke J, Kavanagh DG, et al. In Vitro and in Vivo mRNA Delivery Using Lipid-Enveloped pH-Responsive Polymer Nanoparticles. Mol Pharm. 2011. May-Jun;8(3):774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vandenbroucke RE, De Geest BG, Bonne S, et al. Prolonged gene silencing in hepatoma cells and primary hepatocytes after small interfering RNA delivery with biodegradable poly(beta-amino esters). J Gene Med. 2008. July;10(7):783–94. [DOI] [PubMed] [Google Scholar]
- 67.Kilchrist KV, Dimobi SC, Jackson MA, et al. Gal8 Visualization of Endosome Disruption Predicts Carrier-Mediated Biologic Drug Intracellular Bioavailability. ACS Nano. 2019. February;13(2):1136–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Routkevitch D, Sudhakar D, Conge MJ, et al. Efficiency of Cytosolic Delivery with Poly(beta-amino ester) Nanoparticles is Dependent on the Effective pKa of the Polymer. ACS Biomaterials Science & Engineering. 2020. 2020/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karlsson J, Vaughan HJ, Green JJ. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Annu Rev Chem Biomol Eng. 2018. June 7;9:105–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sankaranarayanan J, Mahmoud EA, Kim G, et al. Multiresponse Strategies To Modulate Burst Degradation and Release from Nanoparticles. Acs Nano. 2010. October;4(10):5930–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Monnery BD, Wright M, Cavill R, et al. Cytotoxicity of polycations: Relationship of molecular weight and the hydrolytic theory of the mechanism of toxicity. Int J Pharm. 2017. April 15;521(1–2):249–258. [DOI] [PubMed] [Google Scholar]
- 72.Deng X, Zheng N, Song Z, et al. Trigger-responsive, fast-degradable poly(beta-amino ester)s for enhanced DNA unpackaging and reduced toxicity. Biomaterials. 2014. June;35(18):5006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang B, Ma X, Murdoch W, et al. Bioreducible poly(amido amine)s with different branching degrees as gene delivery vectors. Biotechnol Bioeng. 2013. March;110(3):990–8. [DOI] [PubMed] [Google Scholar]
- 74.Tzeng SY, Hung BP, Grayson WL, et al. Cystamine-terminated poly(beta-amino ester)s for siRNA delivery to human mesenchymal stem cells and enhancement of osteogenic differentiation. Biomaterials. 2012. November;33(32):8142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karlsson J, Rui Y, Kozielski KL, et al. Engineered nanoparticles for systemic siRNA delivery to malignant brain tumours. Nanoscale. 2019. November 14;11(42):20045–20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *76.Mastorakos P, da Silva AL, Chisholm J, et al. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc Natl Acad Sci U S A. 2015. July 14;112(28):8720–5. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper contains a detailed description of the ways in which PEGylation can be crucial for successful in vivo gene delivery, particularly to tissues where nanoparticle movement is normally highly restricted.
- 77.Gale EC, Roth GA, Smith AAA, et al. A Nanoparticle Platform for Improved Potency, Stability, and Adjuvanticity of Poly(I:C). Advanced Therapeutics. 2020. January;3(1). [Google Scholar]
- 78.Kim J, Mondal SK, Tzeng SY, et al. Poly(ethylene glycol)–Poly(beta-amino ester)-Based Nanoparticles for Suicide Gene Therapy Enhance Brain Penetration and Extend Survival in a Preclinical Human Glioblastoma Orthotopic Xenograft Model. ACS Biomaterials Science & Engineering. 2020. 2020/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leal J, Smyth HDC, Ghosh D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int J Pharm. 2017. October 30;532(1):555–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaczmarek JC, Patel AK, Kauffman KJ, et al. Polymer-Lipid Nanoparticles for Systemic Delivery of mRNA to the Lungs. Angew Chem Int Ed Engl. 2016. October 24;55(44):13808–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaczmarek JC, Kauffman KJ, Fenton OS, et al. Optimization of a Degradable Polymer-Lipid Nanoparticle for Potent Systemic Delivery of mRNA to the Lung Endothelium and Immune Cells. Nano Lett. 2018. October;18(10):6449–6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones CH, Chen MF, Ravikrishnan A, et al. Mannosylated poly(beta-amino esters) for targeted antigen presenting cell immune modulation. Biomaterials. 2015. January;37:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fornaguera C, Guerra-Rebollo M, Lazaro MA, et al. In Vivo Retargeting of Poly(beta aminoester) (OM-PBAE) Nanoparticles is Influenced by Protein Corona. Advanced Healthcare Materials. 2019. October;8(19). [DOI] [PubMed] [Google Scholar]
- 84.Green JJ, Chiu E, Leshchiner ES, et al. Electrostatic ligand coatings of nanoparticles enable ligand-specific gene delivery to human primary cells. Nano Lett. 2007. April;7(4):874–879. [DOI] [PubMed] [Google Scholar]
- 85.Moffett HF, Coon ME, Radtke S, et al. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nature Communications. 2017. August 30;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang F, Parayath NN, Ene CI, et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nature Communications. 2019. September 3;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Little SR, Lynn DM, Ge Q, et al. Poly-beta amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc Natl Acad Sci U S A. 2004. June 29;101(26):9534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fields RJ, Quijano E, McNeer NA, et al. Modified poly(lactic-co-glycolic acid) nanoparticles for enhanced cellular uptake and gene editing in the lung. Adv Healthc Mater. 2015. February 18;4(3):361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Capasso Palmiero U, Kaczmarek JC, Fenton OS, et al. Poly(beta-amino ester)-co-poly(caprolactone) Terpolymers as Nonviral Vectors for mRNA Delivery In Vitro and In Vivo. Adv Healthc Mater. 2018. July;7(14):e1800249. [DOI] [PubMed] [Google Scholar]
- 90.van Vlerken LE, Duan Z, Little SR, et al. Biodistribution and pharmacokinetic analysis of Paclitaxel and ceramide administered in multifunctional polymer-blend nanoparticles in drug resistant breast cancer model. Mol Pharm. 2008. Jul-Aug;5(4):516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang J, De G, Yue Q, et al. pH Responsive Polymer Micelles Enhances Inhibitory Efficacy on Metastasis of Murine Breast Cancer Cells. Front Pharmacol. 2018;9:543. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Shenoy D, Little S, Langer R, et al. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. In vitro evaluations. Mol Pharm. 2005. Sep-Oct;2(5):357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tian Z, Xu L, Chen Q, et al. Treatment of Surgical Brain Injury by Immune Tolerance Induced by Peripheral Intravenous Injection of Biotargeting Nanoparticles Loaded With Brain Antigens. Front Immunol. 2019;10:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clark A, Milbrandt TA, Hilt JZ, et al. Mechanical properties and dual drug delivery application of poly(lactic-co-glycolic acid) scaffolds fabricated with a poly(beta-amino ester) porogen. Acta Biomater. 2014. May;10(5):2125–32. [DOI] [PubMed] [Google Scholar]
- 95.Fisher PD, Palomino P, Milbrandt TA, et al. Improved small molecule drug release from in situ forming poly(lactic-co-glycolic acid) scaffolds incorporating poly(beta-amino ester) and hydroxyapatite microparticles. J Biomater Sci Polym Ed. 2014;25(11):1174–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fisher PD, Clemens J, Zach Hilt J, et al. Multifunctional poly(beta-amino ester) hydrogel microparticles in periodontal in situ forming drug delivery systems. Biomed Mater. 2016. March 7;11(2):025002. [DOI] [PubMed] [Google Scholar]
- 97.Persano S, Guevara ML, Li Z, et al. Lipopolyplex potentiates anti-tumor immunity of mRNA-based vaccination. Biomaterials. 2017. May;125:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brito LA, Chandrasekhar S, Little SR, et al. In vitro and in vivo studies of local arterial gene delivery and transfection using lipopolyplexes-embedded stents. J Biomed Mater Res A. 2010. April;93(1):325–36. [DOI] [PubMed] [Google Scholar]
- 99.Lee JS, Green JJ, Love KT, et al. Gold, poly(beta-amino ester) nanoparticles for small interfering RNA delivery. Nano Lett. 2009. June;9(6):2402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jones CH, Ravikrishnan A, Chen M, et al. Hybrid biosynthetic gene therapy vector development and dual engineering capacity. Proc Natl Acad Sci U S A. 2014. August 26;111(34):12360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palffy R, Gardlik R, Hodosy J, et al. Bacteria in gene therapy: bactofection versus alternative gene therapy. Gene Ther. 2006. January;13(2):101–5. [DOI] [PubMed] [Google Scholar]
- 102.Li Y, Beitelshees M, Fang L, et al. In situ pneumococcal vaccine production and delivery through a hybrid biological-biomaterial vector. Sci Adv. 2016. July;2(7):e1600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jones CH, Gollakota A, Chen M, et al. Influence of molecular weight upon mannosylated bio-synthetic hybrids for targeted antigen presenting cell gene delivery. Biomaterials. 2015. July;58:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Flessner RM, Yu Y, Lynn DM. Rapid release of plasmid DNA from polyelectrolyte multilayers: a weak poly(acid) approach. Chem Commun (Camb). 2011. January 7;47(1):550–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bishop CJ, Liu AL, Lee DS, et al. Layer-by-layer inorganic/polymeric nanoparticles for kinetically controlled multigene delivery. J Biomed Mater Res A. 2016. March;104(3):707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li C, Tzeng SY, Tellier LE, et al. (3-aminopropyl)-4-methylpiperazine end-capped poly(1,4-butanediol diacrylate-co-4-amino-1-butanol)-based multilayer films for gene delivery. ACS Appl Mater Interfaces. 2013. July 10;5(13):5947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DeMuth PC, Min Y, Huang B, et al. Polymer multilayer tattooing for enhanced DNA vaccination. Nat Mater. 2013. April;12(4):367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith RC, Riollano M, Leung A, et al. Layer-by-layer platform technology for small-molecule delivery. Angew Chem Int Ed Engl. 2009;48(47):8974–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shah NJ, Macdonald ML, Beben YM, et al. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials. 2011. September;32(26):6183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.DeMuth PC, Moon JJ, Suh H, et al. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano. 2012. September 25;6(9):8041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Keeney M, Onyiah S, Zhang Z, et al. Modulating polymer chemistry to enhance non-viral gene delivery inside hydrogels with tunable matrix stiffness. Biomaterials. 2013. December;34(37):9657–65. [DOI] [PubMed] [Google Scholar]
- 112.Akinc A, Maier MA, Manoharan M, et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nature Nanotechnology. 2019. December;14(12):1084–1087. [DOI] [PubMed] [Google Scholar]
- 113.Zhang C, An T, Wang D, et al. Stepwise pH-responsive nanoparticles containing charge-reversible pullulan-based shells and poly(beta-amino ester)/poly(lactic-co-glycolic acid) cores as carriers of anticancer drugs for combination therapy on hepatocellular carcinoma. J Control Release. 2016. March 28;226:193–204. [DOI] [PubMed] [Google Scholar]
- 114.Mastorakos P, da Silva AL, Chisholm J, et al. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. P Natl Acad Sci USA. 2015. July 14;112(28):8720–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fields RJ, Cheng CJ, Quijano E, et al. Surface modified poly(beta amino ester)-containing nanoparticles for plasmid DNA delivery. J Control Release. 2012. November 28;164(1):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Palmiero UC, Kaczmarek JC, Fenton OS, et al. Poly(beta-amino ester)-co-poly(caprolactone) Terpolymers as Nonviral Vectors for mRNA Delivery In Vitro and In Vivo. Advanced Healthcare Materials. 2018. July 25;7(14). [DOI] [PubMed] [Google Scholar]


