Abstract
Existing drug delivery methods have not led to a significant increase in survival for patients with malignant primary brain tumors. While the combination of conventional therapies consisting of surgery, radiotherapy, and chemotherapy has improved survival for some types of brain tumors (e.g., WNT medulloblastoma), other types of brain tumors (e.g., glioblastoma and diffuse midline glioma) still have a poor prognosis. The reason for the differences in response can be largely attributed to the blood–brain barrier (BBB), a specialized structure at the microvasculature level that regulates the transport of molecules across the blood vessels into the brain parenchyma. This structure hampers the delivery of most chemotherapeutic agents for the treatment of primary brain tumors. Several drug delivery methods such as nanoparticles, convection enhanced delivery, focused ultrasound, intranasal delivery, and intra-arterial delivery have been developed to overcome the BBB in primary brain tumors. However, prognosis of most primary brain tumors still remains poor. The heterogeneity of the BBB in primary brain tumors and the distinct vasculature of tumors make it difficult to design a drug delivery method that targets the entire tumor. Drug delivery methods that combine strategies such as focused ultrasound and nanoparticles might be a more successful approach. However, more research is needed to optimize and develop new drug delivery techniques to improve survival of patients with primary brain tumors.
Key Points
| Drug delivery methods such as nanoparticles, convection enhanced delivery, focused ultrasound, intranasal delivery, and intra-arterial delivery have not yet led to a significant increase in survival for most patients with a malignant primary brain tumor. |
| Blood–brain barrier disruption is heterogeneous within and between primary brain tumors in both adult glioblastoma and pediatric brain tumors. |
| A multimodal drug delivery approach might be more effective than a single drug delivery method. |
Introduction
Most primary brain tumors, such as high-grade glioma, have an exceedingly poor prognosis due to their tumor location and fast development in both adult and pediatric patients [1–4]. The presence of the blood–brain barrier (BBB) is an important obstacle for drug delivery in most brain cancers [2, 5–8]. The BBB is a complex interplay between endothelial cells, astrocytes, pericytes, basal lamina, and extracellular matrix (ECM). These components, together with smooth muscle cells and neurons, form the neurovascular unit (NVU), which in turn regulates cerebral blood flow and BBB function [9–11]. The consequence of this tightly regulated barrier is that toxins and drugs, including chemotherapy, do not readily cross the BBB, posing a problem for drug delivery into the brain.
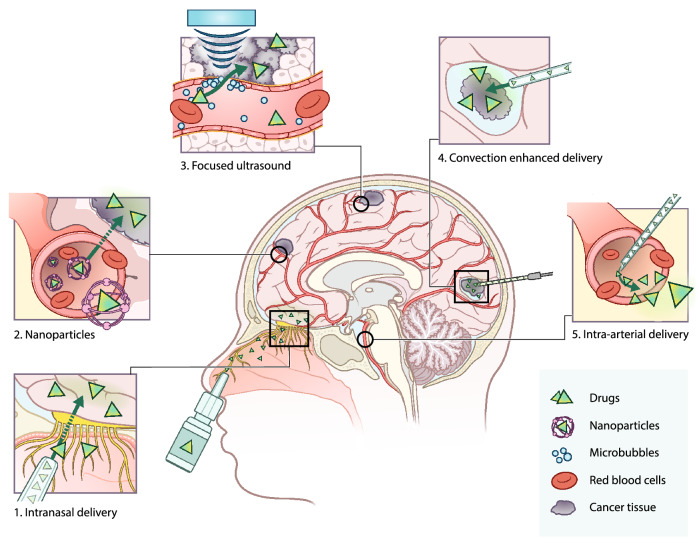
In order for a therapeutic intervention to be effective, chemotherapy must be capable of traversing the BBB and penetrating the brain parenchyma [5, 6, 12]. Systemic delivery of drugs (via the blood stream) is possible for molecules with a molecular weight of < 500 Dalton (Da) and a high lipophilicity [13, 14]. However, as only 5% of drugs meet these requirements, adequate drug delivery methods are needed to efficiently deliver the remaining 95% of drugs into the brain [12, 15, 16]. Current research on central nervous system (CNS) drug development predominantly focuses on either optimizing systemic drug delivery to the brain, or on circumventing the BBB (Fig. 1) [5, 6, 17–19]. Systemic delivery can be achieved with nanomedicine [20, 21]; drugs that are not likely to cross the BBB can be adapted or packed into liposomes to make them more lipophilic [22, 23]. Nanomedicine can also be used for targeted therapy where nanoparticles can be equipped with specific proteins to target the tumor [23]. In contrast, the BBB can also be disrupted or circumvented by microbubble-mediated focused ultrasound (FUS), convection enhanced delivery (CED), intranasal delivery, and intra-arterial delivery [24–27]. Microbubble-mediated FUS uses microbubbles to locally and temporarily open the BBB, enabling drugs to accumulate in the brain parenchyma, while CED is a more invasive method for bypassing the BBB using surgically implanted catheters to administer drugs locally into the tumor [24, 25]. Intranasal delivery uses the direct anatomical relationship of the olfactory neuro-epithelium to the brain to circumvent the BBB, whereas intra-arterial delivery locally administers the drug in the artery [26, 27].
Fig. 1.
Overview of current drug delivery methods for the treatment of primary brain tumors. Panel 1 intranasal drug delivery: drug is formulated in spray particles that enter the brain through the nasal cavity via the neuroepithelium. Here, the drug can enter without interference of the blood–brain barrier (BBB). Panel 2 nanoparticles: nanoparticles encapsulate drugs to increase plasma half-life and allow entry to the brain parenchyma by the enhanced permeability and retention (EPR) effect, endocytosis, and receptor-mediated transcytosis. Panel 3 microbubble-mediated focused ultrasound: microbubbles are intravenously administered and upon the application of focused ultrasound, microbubbles start to oscillate. The oscillation disrupts the BBB, temporarily opening it to allow drugs to enter the brain parenchyma. Panel 4 convection enhanced delivery (CED): surgical placement of catheters in the brain to administer the drug directly in the tumor site. Panel 5 intra-arterial drug delivery: catherization of the blood vessel and injection of drugs directly in the vicinity of the tumor, sometimes in combination with hyperosmolar drugs that open the BBB
As promising as all these methods are, they have not led to a significant improvement of drug delivery for most malignant primary brain tumors. The phenotypic heterogeneity of the BBB across primary brain tumors makes it difficult to determine the best drug delivery method. Therefore, knowledge about BBB pathology is essential to determine the optimal drug delivery method. In the following paragraphs we will elaborate more on BBB pathology and the different methods for drug delivery in primary brain tumors.
Blood–Brain Barrier (BBB) Physiology
The tightly regulated BBB is impermeable for most conventional chemotherapeutics [28]. Transport across the BBB is restricted by specialized endothelial cells [29, 30], which have specific characteristic properties that create an impermeable barrier. The first is the presence of tight junctions, which prevent paracellular passage of molecules. The main tight junction proteins are occludins, tricellulins, claudins, and junctional adhesion molecules [29]. Second, CNS endothelial cells express efflux transporters that regulate the movement of substrates across the BBB [31]. Various drugs are substrates for efflux transporters, thus hampering drug accumulation in the brain parenchyma. These efflux transporters belong to the class of ATP-binding cassette (ABC) transporters, with the most important transporters being multidrug resistance receptors (MDRs, ABCB), multidrug resistance proteins (MRPs, ABCC), and the breast cancer resistance protein (BCRP/ABCG2) [32]. In the brain, MDR1 (ABCB1, P-glycoprotein [P-gp])—the most extensively researched ABC transporter—plays a role in the efflux of numerous drugs [33]. Third, transcytosis by pinocytic and endocytotic vesicles is limited due to the low density of these vesicles in CNS endothelial cells [34]. Finally, CNS endothelial cells are able to limit the entry of immune cells into the brain due to their low expression of leukocyte adhesion molecules, consequently hampering immunotherapy [29].
Proper function of the BBB also requires other cells such as astrocytes, pericytes, basal lamina, neurons, and the ECM. Astrocytes are vital for BBB formation and maintenance [29], being closely linked to the endothelial cells by astrocytic endfeet, which cover more than 99% of the capillaries [35]. The endfeet produce a variety of proteins that regulate the composition of the ECM, immune cell infiltration, BBB permeability, and BBB integrity [29, 33, 36]. Astrocytic endfeet are important for maintaining junctional complexes regulating BBB permeability [36]. Pericytes are multi-functional cells that are key regulators of BBB permeability and vascular function, regulating vessel formation and vessel maturation [29, 30, 36]. The basal lamina, formed by endothelial cells, astrocytes, and pericytes, consists of an ECM that is both responsible and influential to proper BBB function [36]. Microglia are resident immune cells that act as a first line of defense in the CNS by screening the brain parenchyma for blood–borne substances and potential inflammatory stimuli [37]. Due to their low turnover rate, these cells exist as permanent populations within the brain [38]. Microglia, in combination with macrophages, also play a role in the regulation of vascular growth by secreting various signals [39]. Together these structures create an impermeable barrier.
BBB/Blood–Tumor Barrier (BTB) Pathology
The presence of a brain tumor disrupts the regulation of the BBB, resulting in an altered BBB phenotype that is referred to as the blood–tumor barrier (BTB) [36, 40]. Characterization of the BBB/BTB phenotypes of different tumors is important to understand the extent of effectiveness of drug delivery for the treatment of primary brain tumors. In the following paragraphs, the BBB/BTB of several primary brain tumors is discussed.
Adult Glioblastoma
The most common malignant primary brain tumor in adults is glioblastoma (GBM), with patients having a median survival of one year. It is believed that the presence of the BBB/BTB is a major influence on the effectiveness of drug delivery [41, 42]. GBM is a highly heterogeneous malignancy characterized by aggressive and invasive growth, and is one of the most hypoxic and angiogenic brain tumors [43, 44]. The microenvironment of GBM consists of specialized niches, each of which display different BBB properties [37, 43]. In the core of the tumor, higher oxygen demands lead to severe hypoxia and necrosis, and subsequent BBB/BTB defects, especially in late-stage disease. Glioma cells that have migrated further into the brain parenchyma reside behind an intact BBB, demonstrating the heterogeneity of GBM [37, 44]. This heterogeneity can be visualized by magnetic resonance imaging (MRI), where the core of the tumor is enhanced by contrast agent on MRI, indicating a disrupted BBB/BTB, while (often large) areas are not enhanced on MRI, showing a mostly intact BBB of the diffusely growing tumor [44].
GBM vessel areas that are contrast-enhanced on MRI are characterized by aberrant and disorganized angiogenesis, resulting in permeable vessels with defective pericyte coverage and an abnormal basement membrane—all suggestive of BBB breakdown [43, 45, 46]. The disrupted BBB is typified by a disturbed organization of permeable endothelial cell junctions due to the downregulation of claudin-5, claudin-3, and occludin [37, 47, 48]. In vitro, it was shown that GBM cells disrupt the BBB by secreting soluble factors that break down tight junctions [49]. The BBB is then further degraded via displacement of non-neoplastic astrocytes by tumor cells, that in turn allow tumor-derived chemokines and cytokines to cross the BBB [50]. Furthermore, the loss of aquaporin 4 (AQP4) results in the polarization of astrocytic endfeet, reducing the astrocytic endfeet coverage of the endothelial cells, resulting in BBB disruption [51, 52]. A study showed that relocation of AQP4 in GBM coincides with a redistributed or diminished expression of argin, which is associated with the loss of several tight junction proteins [53, 54].
The presence of ABC transporters and organic anion transporting polypeptides (OATP) transporters influences the resistance of tumor cells to chemotherapeutics [55]. These transporters have been identified in both GBM tumor vasculature and tumor cells [55, 56]. Remarkably, MDR1, MRP4, and MRP5 are expressed in glioma cells and astrocytes, while these receptors are usually not expressed by glial cells and astrocytes [55, 56]. In addition, BCRP expression is also increased in GBM cells [56, 57]. OATPA2 and OATP2B1 have been detected on the luminal membrane of endothelial cells in both the BBB and BTB, but not in glioma cells themselves [55].
Pediatric Brain Tumors
In children, primary brain tumors are the leading cause of cancer-related morbidity and mortality [3, 4]. The most common malignant pediatric primary brain tumors are medulloblastoma, ependymoma, diffuse intrinsic pontine glioma (DIPG), and atypical teratoid/rhabdoid tumor (AT/RT) [3, 4, 58]. Even though advances in surgery, adjuvant therapy, and research have resulted in the increase of survival rates of some of these brain tumor types, such as medulloblastoma, other tumor types still have a dismal prognosis [4, 58, 59]. Like for adult brain tumors, the poor prognosis can in part be attributed to the BBB phenotype of malignant pediatric brain cancers.
Medulloblastoma
Medulloblastoma is the most common malignant embryonal brain tumor [3, 58, 60]. These tumors can be classified based on histology (distinguishing classic, desmoplastic, and large-cell medulloblastoma), or molecular signature (WNT, Sonic hedgehog [SHH], Group 3 and Group 4) [60, 61]. Despite the general survival of medulloblastoma patients having increased significantly in the past decades, a subset of patients still have poor outcomes, partly due to compromised vasculature and the presence of an intact BBB [3, 7, 58]. Medulloblastomas have a low capillary permeability and blood flow compared with normal cerebellum, although the capillary density is heterogeneous throughout the tumor [62, 63]. Some types of medulloblastoma show an absence of astrocytes while other types show disruption of astrocytes from endothelial cells in the tumor parenchyma [63]. In addition, over 40% of medulloblastomas demonstrate the expression of MDR1 efflux transporters in the tumor, indicating an effective BTB [64]. Group 3 medulloblastomas specifically overexpress BCRP, MRP7 (ABCC10), MRP5 (ABCC5), and MRP1 [65]. As an exception, WNT medulloblastomas, which have the best prognosis of the different subgroups, lack a functional BBB [3, 58, 60]. This BBB dysfunction is likely due to aberrant, antagonistic medulloblastoma-endothelial cell WNT signaling, which renders the non-CNS vasculature porous, resulting in a hemorrhagic vasculature, aberrant fenestration, and higher vascular density compared with the other subtypes [7]. Overall, the BBB/BTB and the vasculature are affected in medulloblastoma, influencing the outcome of therapy.
Ependymoma
Ependymomas are slow-growing tumors that are treated with surgery and local fractionated radiotherapy, while the potential benefit of chemotherapy is still under debate [3, 4, 58]. Ependymomas overexpress vascular endothelial growth factor (VEGF), a main marker of angiogenesis [68]. Ependymomas exert an aberrant vasculature, the extent of which is dependent on tumor grade [66, 67]. The mean vessel area is larger for myxopapillary ependymoma grade I, low-grade ependymoma grade II, and anaplastic ependymoma grade III compared with normal cerebral and cerebellar tissue [66]. In addition, grade III has a higher blood vessel density compared with grade II, but the diameter of blood vessels for grade II was found to be larger than that for grade III [66, 67]. Subependymoma grade I has fewer vessels than normal cerebral tissue [66]. In contrast, Wagemakers et al. found that microvessel density in neovasculature does not differ by age, gender, tumor location, or tumor grade. The microvessel density of ependymoma was increased and comparable to GBM [68]. Little is known about the expression of BBB proteins and transporters in ependymomas. MDR1, BCRP, and ABCB1 were not significantly altered in grade II and III ependymomas [69, 70]. Hence, the different ependymoma grades have characteristic vasculature profiles, however little is known about the BBB.
Diffuse Intrinsic Pontine Glioma
DIPG is a high-grade glioma of the brainstem with a median survival of 11 months [59]. The poor prognosis is mainly due to the tumor location making complete resection of the tumor impossible [71]. At diagnosis, many patients have an absence of contrast enhancement on MRI [72]. Little is known about the BBB/BTB pathology in DIPG. One study indicated highly active SHH signaling which decreased BBB permeability in DIPG [73, 74]. Furthermore, efflux transporters MDR1, BCRP, and MRP1 are present in endothelial cells, and MRP1 is expressed in tumor cells [75]. Not only the tumor, but also its location can affect the BBB phenotype. In healthy non-human primates, BBB heterogeneity was observed with differential penetration of temozolomide between the pontine region, the cortex, and the CSF [72, 76]. Since the brain region already has an influence on the BBB permeability, a tumor in the pontine region might have a different effect compared with other supratentorial tumors. However, there is no direct evidence that the BBB is intact in DIPG.
Atypical Teratoid/Rhabdoid Tumor
AT/RT tumors are highly aggressive embryonal tumors most commonly found in infants and young children [3, 77]. The overall median survival is 17 months [78]. Improvement of treatment protocols has resulted in only a small increase of survival, as only a subset of patients respond to treatment [77, 79, 80]. MRI images of AT/RT patients show contrast enhancement, indicating BBB disruption [79, 81]. However, little is known about the BBB/BTB and the vasculature in AT/RT. Only one paper has been published on the vasculature and BBB alterations in AT/RT, showing a significant decrease in vessel density and an increase in vessel diameter of the tumor vasculature [79]. Endothelial cells in existing blood vessels maintained expression of claudin-5 but showed displacement of claudin-5 localization compared with healthy tissue [79]. In neovasculature, expression of claudin-5 was lost. In contrast, glucose transporter 1 (GLUT1) was lost in both existing endothelial cells as well as in neovasculature [79]. In order to improve median survival, more research is needed to determine to what extent the BBB/BTB is compromised, and how this can be used for efficient drug delivery.
Drug Delivery Methods to Overcome the BBB
Several drug delivery methods have been used to overcome the BBB. Nanoparticles can be used to modify the drug permeability of an existing drug. Other methods such as FUS, CED, intranasal and intra-arterial delivery can be used to temporarily disrupt or bypass the BBB to deliver drugs into the brain parenchyma.
Nanoparticles
Nanoparticles are particles created from different packaging materials such as lipids, polymers, and metals that can be utilized as a proxy to efficiently deliver drugs. These particles can be designed in various compositions that, for example, increase their half-life or ability to target a specific receptor [23, 36]. Nanoparticles have been successfully used in the treatment of several types of cancer [21].
Nanoparticles cross the BBB in a variety of ways, including endocytosis, receptor-mediated transcytosis, or the enhanced permeability and retention (EPR) effect [82–84]. The EPR effect exploits the leaky vasculature of solid tumors where the nanoparticle can extravasate locally into the tumor [83]. After the nanoparticle is extravasated, the encapsulated drugs are slowly released into the tissue. Since nanoparticles are not able to cross normal vasculature in most organs, this reduces both peripheral and systemic toxicities [6, 21, 84].
Nanoparticles are able to cross the leaky BBB, which could be a potential method for drug delivery for brain tumors. However, in clinical trials nanoparticles have not been able to reach therapeutic concentrations in the tumor [20, 36]. For example, GBM is characterized by both intact and disrupted BBB niches. Due to the heterogenous BBB/BTB in GBM, drugs are not homogenously distributed in the tumor leaving parts of the tumor untreated. In addition, GBM is characterized by high interstitial pressure and hypoxia which negatively influences the passage of nanoparticles in these areas [84]. Therefore, the use of nanoparticles as a delivery method for the treatment of brain cancer has not yet been successful [36]. However, nanoparticles exhibiting beneficial properties, such as sustained release of a drug over a prolonged period of time, could potentially be useful in combination with other drug delivery methods for treatment of brain tumors [85, 86].
Focused Ultrasound
Microbubble-mediated focused ultrasound (FUS), or sonoporation, is a minimally/non-invasive method for targeted drug delivery into the brain tumor [25, 87, 88]. Upon acoustic pressure from a transducer, microbubbles are pressed against the endothelial cell wall and start to vibrate. The vibration induces stress on the endothelial cell wall resulting in the temporary and local disruption of the BBB [89]. The combination of ultrasound with microbubbles is considered to be safe, since no neuronal damage, apoptosis, ischemia, or long-term vascular damage has been observed upon treatment [87]. The combination of FUS with conventional chemotherapeutics, antibodies, nanoparticles, and gene-based therapies allows for a range of possibilities [36, 85, 90].
The therapeutic window of microbubble-mediated FUS is dependent on the closure dynamics of the BBB after disruption. The BBB slowly closes within several hours, whereas larger molecules such as nanoparticles have a shorter therapeutic window compared with smaller molecules [91]. In addition, drug half-life and penetration depth after sonoporation is also drug dependent. Therefore, the pharmacokinetic profile of the drug is an important parameter for treatment success [92]. The heterogeneous nature of the BBB phenotype poses less of a problem for FUS since the focused ultrasound can be applied over the entire tumor area. Furthermore, the vasculature is important for the delivery of microbubbles and drugs since the blood vessels are key to deliver microbubbles and drugs to the tumor. Brain tumors with a low vessel density might be less suited for focused ultrasound. Moreover, efflux transporters hamper drug accumulation in the brain. However, it was recently discovered that FUS suppresses MDR1, which could aid in the increase of drug accumulation in the tumor tissue [93]. Many brain tumors such as GBM, medulloblastoma, and DIPG express ABC transporters. The use of FUS could potentially increase the accumulation of drugs in these tumors.
For diffuse infiltrating tumors such as GBM and DIPG, FUS is a promising technique since it is a non-invasive drug delivery method that can target the tumor. Recently, the first clinical MRI-guided FUS study was concluded for GBM patients [94]. A 1.5- to sevenfold increased concentration of temozolomide in sonicated versus unsonicated tumor tissue was observed in two patients whose tissue could be analyzed. The treatment was well tolerated in all patients [94]. Implanted ultrasound devices have been studied in several clinical studies: CarThera (SonoCloud) is an implantable ultrasound device that has been used in phase I clinical studies in combination with systemic administration of carboplatin [95, 96]. Treatment via FUS prolonged progression-free survival in 11 GBM patients presenting BBB disruption compared with 8 patients with intact BBB [95, 96]. The downside of implanted ultrasound devices is the requirement of invasive surgery, they do not specifically target the tumor site, and are mostly suitable for superficial brain tumors. As such, these devices will be limited for treating DIPG as they do not reach the pontine region. Furthermore, combining FUS with immunotherapy might be a powerful combination for the treatment of brain cancer. Immune cells are not able to cross the BBB since CNS endothelial cells have a low expression of leukocyte adhesion molecules [29]. Immune cells can extravasate after the BBB is disrupted with FUS. In vivo studies are now investigating the possibilities of immunotherapy in combination with FUS [36]. The non-invasive nature of FUS in combination with numerous drugs makes FUS a versatile and promising technique for drug and/or immune therapy delivery for various brain tumors.
Convection Enhanced Delivery
CED has been proposed as a promising strategy for drug delivery to the CNS. This method involves placing one or more intracranial catheters connected to an external infusion pump, which allows for the direct delivery of therapeutic agents into target tissues via an established pressure gradient [24, 97, 98]. The local infusion ensures a higher therapeutic concentration in the brain parenchyma with less systemic toxicity [24, 97]. The pressure-driven bulk flow of the desired drug solution allows for more uniform infusion over larger volumes [98–101]. The infusion volume is not dependent on molecule size and weight [98, 101]. The drug can travel for a few centimeters, increasing the volume treated, and makes it suitable for tumors with a low vascular density [101]. However, this technique also has some caveats. Highly vascular tumors are potentially less suited since the infused drugs can be excreted/absorbed into the vasculature [102]. The catheters should not be placed inside or around necrotic tissues since the drug can pool together in this necrotic area, thereby not exposing the entire tumor to the drug [101].
CED is in clinical trials for multiple brain cancers, with most trials focusing on GBM and DIPG [100, 103, 104]. However, clinical trials have had major setbacks and so far only one phase III trial has been completed [101, 104, 105]. In this multicenter phase III study, which included 276 patients with recurrent GBM, no difference in median survival was observed between treatment with CED using cintredekin besudotox (IL13-PE38QQR) and GLIADEL wafers, a carmustine implant [105]. For DIPG, only phase I studies have been conducted but without significant improvement in survival. While CED in combination with IL13-PE38QQR reported that tumor coverage was not optimal, 124I-8H9 administered with CED did show good tumor coverage with a single dose [103, 106]. The study was not powered for survival and a follow-up phase II study will soon be initiated [103]. Besides the fact that the optimal CED drug with the highest therapeutic index has yet to be found, the limited success of CED can be attributed to a number of factors. First, CED causes a heterogeneous pressure gradient in the tumor, resulting in a non-uniform drug distribution and inhomogeneous drug concentrations in the treated area [99]. Second, catheter-induced tissue damage and reflux can be seen contiguous to the catheter, with ‘intrinsic’ backflow of solute and air bubbles [99]. Third, the mixed-tissue environment can cause rapid efflux of many drugs, lowering the concentration of drugs in the brain [107]. Additionally, other factors may affect efficient delivery, such as high and varying tumor interstitial fluid pressure, which are reviewed elsewhere [99, 100]. Provided that these problems can be resolved, CED represents a suitable technique to overcome the BBB.
Intranasal Delivery
Intranasal delivery is an alternative method to overcome the BBB. The nasal cavity provides access to the brain parenchyma without interference of the BBB. The drugs are delivered to the brain via paracellular, transcellular, and neuronal transport from the neuroepithelium of the nasal cavity to the CNS. However, not all drugs are suitable for intranasal drug delivery, since specific physicochemical properties and formulations determine the bioavailability of the drug in the brain. Generally, lipophilic drugs with low molecular weight show a more ready bioavailability after nasal administration than charged hydrophilic drugs. Drug formulations can be modified to increase drug bioavailability with, for example, liposomes, cyclodextrans, and nanoparticles. In addition, the advantage of drug delivery through the nose is that the drugs are not metabolized by first-pass metabolism. However, a disadvantage is the small volume that can be administered via intranasal delivery [26, 108, 109].
Only limited results have been published on clinical trials using intranasal drug delivery. Perillyl alcohol has been used as an intranasal drug for the treatment of malignant glioma [110, 111]. The four-times daily administration of perillyl alcohol resulted in a 6-month progression-free survival in ~ 45% of a limited number of cases [110, 111]. Potential problems with intranasal drug delivery are the non-specificity of drugs, which can result in toxicity. Toxicity can be minimized by targeting tumor cells. For example, GRN163 has been investigated in vivo, which specifically targets telomerase. The treatment resulted in specific targeting of the tumor and minimal toxicity [112]. Other ways to decrease toxicity to surrounding brain tissue is the combination of intranasal drug delivery with microbubble-mediated FUS. The combination of these methods has shown an increased and specific drug uptake in the targeted tumor region [113, 114]. Only a few studies have investigated the use of intranasal drug delivery for the treatment of primary brain tumors; therefore, it is difficult to conclude if intranasal drug delivery is a suitable technique to overcome the BBB.
Intra-Arterial Drug Delivery
Intra-arterial drug delivery administers drugs directly into an artery in the proximity of the tumor [27, 115]. After the targeted area is cannulated, the drug is released into the blood vessel. In addition to the drug, a hyperosmolar drug such as mannitol can be administered to open the BBB locally [115, 116]. This technique has been successful in the treatment of retinoblastoma and liver cancer [27]. However, several clinical trials and cases did not report significant improvement in survival. Intra-arterial drug delivery in a small set of medulloblastoma patients treated with celyvir (autologous mesenchymal stem cells infected with adenovirus ICOVIR5), a 7-day treatment course of oral procarbazine, intravenous vincristine in combination with four cycles of intra-arterial carmustine, or conventional chemotherapeutics in combination with mannitol, did not lead to remission of the disease in most patients [117–119]. In ependymoma, a small cohort of patients were treated with intra-arterial drug delivery with carmustine, bevacizumab, and cetuximab, and responded to the treatment [120, 121]. However, toxicity concerns arose for the treatment with carmustine, epipodophyllotoxin, and cisplatin [120]. Several clinical studies investigated the use of intra-arterial drug delivery for GBM patients. The reported survival ranged from 20 weeks to 10 months following treatment with nimustine, bevacizumab, or carboplatin in combination with other conventional chemotherapy [122–125]. Toxicity and low drug efficacy seem to hamper the use of intra-arterial drug delivery in primary brain tumors [27, 115, 120, 126].
Conclusions
Is there a way to overcome the BBB to give modern therapy a chance? So far, we have discussed several drug delivery techniques that have been developed to overcome the BBB. However, most techniques have not led to a significant increase in survival in patients with primary brain tumors. One of the main reasons that drug delivery techniques have not been successful is the limited knowledge of the BBB/BTB and vasculature in both adult and pediatric brain tumors. We have reviewed several BBB pathologies, of which almost all have incomplete information regarding the BBB pathology of specific tumors. Pediatric medulloblastoma has illustrated the importance of knowledge about the characterization of the BBB to improve survival. Medulloblastoma WNT subtype has a dysfunctional and high vascular density compared with the other subtypes, making this tumor subtype treatable with conventional therapies. Understanding BBB/BTB properties and challenges can provide more insight into the optimization of drug delivery techniques. For example, highly vascularized tumors might benefit more from FUS, as this technique requires the systemic administration of microbubbles and drugs. Furthermore, FUS can suppress efflux transporters, which could potentially benefit the accumulation and retainment of drugs in the brain parenchyma. In contrast, CED is especially suited for tumors that have a low vascular density and an intact BBB to prevent ‘leakage’ of infused drugs from the tumor site. A multimodal approach might even be necessary to treat brain tumors by combining strategies such as FUS with nanoparticles or immunotherapy. We therefore urge the collaboration of physicians, researchers, and biotechnical companies to characterize BBB/BTB from patient samples to help personalize the chemotherapy delivery method.
Acknowledgements
The authors would like to thank Dr John Bianco for critically reviewing the manuscript and Amanda Gautier and Madeleine G. van Mackelenbergh for graphic support.
Declarations
Funding
Funding for this work was received by KWF-STW (Project number: 15184) and KiKa (Children Cancer Free Foundation). The open access fee was paid by Amsterdam UMC (Vrije Universiteit Amsterdam).
Conflicts of interest
Rianne Haumann, Jessica Carvalho Videira, Gertjan J.L. Kaspers, Dannis G. van Vuurden, and Esther Hulleman declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Author contributions
RH: concept and writing, JCV: drafted the initial version of the manuscript, GJLK: editing manuscript, DGvV: editing manuscript, EH: concept and editing manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
- 1.Howlader NNA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, et al. SEER cancer statistics review 1975–2016. National Cancer Institute.
- 2.Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells EM, Packer RJ. Pediatric brain tumors. Continuum. 2015;21(2):373–396. doi: 10.1212/01.CON.0000464176.96311.d1. [DOI] [PubMed] [Google Scholar]
- 4.Karajannis M, Allen JC, Newcomb EW. Treatment of pediatric brain tumors. J Cell Physiol. 2008;217(3):584–589. doi: 10.1002/jcp.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel MM, Patel BM. Crossing the blood–brain barrier: recent advances in drug delivery to the brain. CNS Drugs. 2017;31(2):109–133. doi: 10.1007/s40263-016-0405-9. [DOI] [PubMed] [Google Scholar]
- 6.Dong X. Current strategies for brain drug delivery. Theranostics. 2018;8(6):1481–1493. doi: 10.7150/thno.21254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phoenix TN, Patmore DM, Boop S, Boulos N, Jacus MO, Patel YT, et al. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell. 2016;29(4):508–522. doi: 10.1016/j.ccell.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci. 1998;95(8):4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57(2):173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 10.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 11.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat Rev Neurosci. 2006;7(1):41. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 12.Partridge W. The blood–brain barrier: bottleneck in brain development. J Am Soc Exp Neuro Ther. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Tu M, Kelly RS, Chen C, Smith BJ. Development of a computational approach to predict blood–brain barrier permeability. Drug Metab Dispos. 2004;32(1):132–139. doi: 10.1124/dmd.32.1.132. [DOI] [PubMed] [Google Scholar]
- 15.Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J Combin Chem. 1999;1(1):55–68. doi: 10.1021/cc9800071. [DOI] [PubMed] [Google Scholar]
- 17.Azad TD, Pan J, Connolly ID, Remington A, Wilson CM, Grant GA. Therapeutic strategies to improve drug delivery across the blood–brain barrier. Neurosurg Focus. 2015;38(3):E9. doi: 10.3171/2014.12.FOCUS14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhan C, Lu W. The blood–brain/tumor barriers: challenges and chances for malignant gliomas targeted drug delivery. Curr Pharm Biotechnol. 2012;13(12):2380–2387. doi: 10.2174/138920112803341798. [DOI] [PubMed] [Google Scholar]
- 19.Pardridge WM. blood–brain barrier delivery. Drug Discov Today. 2007;12(1–2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 21.Lockman PR, Mumper RJ, Khan MA, Allen DD. Nanoparticle technology for drug delivery across the blood–brain barrier. Drug Dev Ind Pharm. 2002;28(1):1–13. doi: 10.1081/ddc-120001481. [DOI] [PubMed] [Google Scholar]
- 22.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12):6387–6392. [PubMed] [Google Scholar]
- 23.Gaillard PJ, Appeldoorn CC, Dorland R, van Kregten J, Manca F, Vugts DJ, et al. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101) PLoS ONE. 2014;9(1):e82331. doi: 10.1371/journal.pone.0082331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging–guided focal opening of the blood–brain barrier in rabbits. Radiology. 2001;220(3):640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 26.Pires A, Fortuna A, Alves G, Falcão A. Intranasal drug delivery: how, why and what for? J Pharm Pharmac Sci. 2009;12(3):288–311. doi: 10.18433/j3nc79. [DOI] [PubMed] [Google Scholar]
- 27.Joshi S, Ellis JA, Ornstein E, Bruce JN. Intraarterial drug delivery for glioblastoma mutiforme. J Neurooncol. 2015;124(3):333–343. doi: 10.1007/s11060-015-1846-6. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee S, Bhat MA. Neuron-glial interactions in blood–brain barrier formation. Annu Rev Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langen UH, Ayloo S, Gu C. Development and cell biology of the blood–brain barrier. Annu Rev Cell Dev Biol. 2019;35:591–613. doi: 10.1146/annurev-cellbio-100617-062608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daneman R, Engelhardt B. Brain barriers in health and disease. Neurobiol Dis. 2017;107:1–3. doi: 10.1016/j.nbd.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Löscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6(8):591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 32.Löscher W, Potschka H. Blood–brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2(1):86–98. doi: 10.1602/neurorx.2.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bart J, Groen H, Hendrikse N, Van der Graaf W, Vaalburg W, De Vries E. The blood–brain barrier and oncology: new insights into function and modulation. Cancer Treat Rev. 2000;26(6):449–462. doi: 10.1053/ctrv.2000.0194. [DOI] [PubMed] [Google Scholar]
- 34.Reese T, Karnovsky MJ. Fine structural localization of a blood–brain barrier to exogenous peroxidase. The Journal of cell biology. 1967;34(1):207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 36.Arvanitis CD, Ferraro GB, Jain RK. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2019;2:1–16. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood–brain barrier in health and disease. Acta Neuropathol. 2018;2:1–26. doi: 10.1007/s00401-018-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59(8):1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- 39.Engelhardt B, Liebner S. Novel insights into the development and maintenance of the blood–brain barrier. Cell Tissue Res. 2014;355(3):687–699. doi: 10.1007/s00441-014-1811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei X, Chen X, Ying M, Lu W. Brain tumor-targeted drug delivery strategies. Acta Pharmac Sin B. 2014;4(3):193–201. doi: 10.1016/j.apsb.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncol. 2017;19(5):1–88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu XM, Zhang QP, Mu YG, Zhang XH, Sai K, Pang JCS, et al. Clinical significance of vasculogenic mimicry in human gliomas. J Neuro-Oncol. 2011;105(2):173–179. doi: 10.1007/s11060-011-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hambardzumyan D, Bergers G. Glioblastoma: defining tumor niches. Trends Cancer. 2015;1(4):252–265. doi: 10.1016/j.trecan.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Tellingen O, Yetkin-Arik B, De Gooijer M, Wesseling P, Wurdinger T, De Vries H. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist Updates. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nat Rev Drug Discov. 2016;15(6):385. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 46.Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell. 2017;31(3):326–341. doi: 10.1016/j.ccell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liebner S, Fischmann A, Rascher G, Duffner F, Grote E-H, Kalbacher H, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100(3):323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 48.Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, et al. Localization of claudin-3 in tight junctions of the blood–brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105(6):586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- 49.Schneider SW, Ludwig T, Tatenhorst L, Braune S, Oberleithner H, Senner V, et al. Glioblastoma cells release factors that disrupt blood–brain barrier features. Acta Neuropathol. 2004;107(3):272–276. doi: 10.1007/s00401-003-0810-2. [DOI] [PubMed] [Google Scholar]
- 50.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro-Oncol. 2005;7(4):452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicchia G, Nico B, Camassa L, Mola M, Loh N, Dermietzel R, et al. The role of aquaporin-4 in the blood–brain barrier development and integrity: studies in animal and cell culture models. Neuroscience. 2004;129(4):935–944. doi: 10.1016/j.neuroscience.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 52.Mader S, Brimberg L. Aquaporin-4 water channel in the brain and its implication for health and disease. Cells. 2019;8(2):90. doi: 10.3390/cells8020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warth A, Kröger S, Wolburg H. Redistribution of aquaporin-4 in human glioblastoma correlates with loss of agrin immunoreactivity from brain capillary basal laminae. Acta Neuropathol. 2004;107(4):311–318. doi: 10.1007/s00401-003-0812-0. [DOI] [PubMed] [Google Scholar]
- 54.Rascher G, Fischmann A, Kröger S, Duffner F, Grote E-H, Wolburg H. Extracellular matrix and the blood–brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Acta Neuropathol. 2002;104(1):85–91. doi: 10.1007/s00401-002-0524-x. [DOI] [PubMed] [Google Scholar]
- 55.Bronger H, König J, Kopplow K, Steiner H-H, Ahmadi R, Herold-Mende C, et al. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Can Res. 2005;65(24):11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- 56.Wijaya J, Fukuda Y, Schuetz JD. Obstacles to brain tumor therapy: key ABC transporters. Int J Mol Sci. 2017;18(12):2544. doi: 10.3390/ijms18122544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhatia P, Bernier M, Sanghvi M, Moaddel R, Schwarting R, Ramamoorthy A, et al. Breast cancer resistance protein (BCRP/ABCG2) localises to the nucleus in glioblastoma multiforme cells. Xenobiotica. 2012;42(8):748–755. doi: 10.3109/00498254.2012.662726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pollack IF, Agnihotri S, Broniscer A. Childhood brain tumors: current management, biological insights, and future directions: JNSPG 75th anniversary invited review article. J Neurosurg Pediatr. 2019;23(3):261–273. doi: 10.3171/2018.10.PEDS18377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffman LM, Van Zanten SEV, Colditz N, Baugh J, Chaney B, Hoffmann M, et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic Pontine Glioma (DIPG): a collaborative report from the international and European Society for Pediatric Oncology DIPG registries. J Clin Oncol. 2018;36(19):1963. doi: 10.1200/JCO.2017.75.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408. doi: 10.1200/JCO.2009.27.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho Y-J, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warnke PC, Kopitzki K, Timmer J, Ostertag CB. Capillary physiology of human medulloblastoma: impact on chemotherapy. Cancer. 2006;107(9):2223–2227. doi: 10.1002/cncr.22212. [DOI] [PubMed] [Google Scholar]
- 63.Hong CS, Ho W, Piazza MG, Ray-Chaudhury A, Zhuang Z, Heiss JD. Characterization of the blood brain barrier in pediatric central nervous system neoplasms. J Interdiscip Histopathol. 2016;4(2):29. doi: 10.5455/jihp.20160623053540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Othman RT, Kimishi I, Bradshaw TD, Storer LC, Korshunov A, Pfister SM, et al. Overcoming multiple drug resistance mechanisms in medulloblastoma. Acta neuropathologica communications. 2014;2(1):57. doi: 10.1186/2051-5960-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morfouace M, Cheepala S, Jackson S, Fukuda Y, Patel YT, Fatima S, et al. ABCG2 transporter expression impacts group 3 medulloblastoma response to chemotherapy. Can Res. 2015;75(18):3879–3889. doi: 10.1158/0008-5472.CAN-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gilhuis HJ, van der Laak JA, Pomp J, Kappelle AC, Gijtenbeek JM, Wesseling P. Three-dimensional (3D) reconstruction and quantitative analysis of the microvasculature in medulloblastoma and ependymoma subtypes. Angiogenesis. 2006;9(4):201–208. doi: 10.1007/s10456-006-9054-9. [DOI] [PubMed] [Google Scholar]
- 67.Duda-Szymanska J, Papierz W. Morphological analysis of vascular density in ependymomas. Folia Neuropathol. 2007;45(3):115. [PubMed] [Google Scholar]
- 68.Wagemakers M, Sie M, Hoving EW, Molema G, de Bont ES, den Dunnen WF. Tumor vessel biology in pediatric intracranial ependymoma. J Neurosurg Pediatr. 2010;5(4):335–341. doi: 10.3171/2009.11.PEDS09260. [DOI] [PubMed] [Google Scholar]
- 69.Chou PM, Barquin N, Gonzalez-Crussi F, Sanz CR, Tomita T, Reyes-Mugica M. Ependymomas in children express the multidrug resistance gene: immunohistochemical and molecular biologic study. Pediatr Pathol Lab Med. 1996;16(4):551–561. [PubMed] [Google Scholar]
- 70.Ginguené C, Champier J, Maallem S, Strazielle N, Jouvet A, Fèvre-Montange M, et al. P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) localize in the microvessels forming the blood-tumor barrier in ependymomas. Brain Pathol. 2010;20(5):926–935. doi: 10.1111/j.1750-3639.2010.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jansen MH, Lagerweij T, Sewing ACP, Vugts DJ, Van Vuurden DG, Molthoff CF, et al. Bevacizumab targeting diffuse intrinsic pontine glioma: results of 89Zr-bevacizumab PET imaging in brain tumor models. Mol Cancer Ther. 2016;15(9):2166–2174. doi: 10.1158/1535-7163.MCT-15-0558. [DOI] [PubMed] [Google Scholar]
- 72.Warren KE. Beyond the blood: brain barrier: the importance of central nervous system (CNS) pharmacokinetics for the treatment of CNS tumors, including diffuse intrinsic pontine glioma. Front Oncol. 2018;8:239. doi: 10.3389/fonc.2018.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sajesh B, On N, Donald M, Kazina C, Serletis D, Del-Bigio M et al. DIPG-18. Sonic hedgehog (SHH) signalling promotes blood brain barrier (BBB) integrity in diffuse intrinsic pontine glioma (DIPG). Neuro-Oncology. 2019;21(Suppl 2):ii72.
- 74.Chapouly C, Guimbal S, Hollier P-L, Renault M-A. Role of hedgehog signaling in vasculature development, differentiation, and maintenance. Int J Mol Sci. 2019;20(12):3076. doi: 10.3390/ijms20123076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veringa SJ, Biesmans D, van Vuurden DG, Jansen MH, Wedekind LE, Horsman I, et al. In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. PLoS ONE. 2013;8:4. doi: 10.1371/journal.pone.0061512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCully CM, Pastakia D, Bacher J, Steffen-Smith EA, Saleem K, Murphy RF, et al. Model for concomitant microdialysis sampling of the pons and cerebral cortex in rhesus macaques (Macaca mulatta) Comp Med. 2013;63(4):355–360. [PMC free article] [PubMed] [Google Scholar]
- 77.Chi SN, Zimmerman MA, Yao X, Cohen KJ, Burger P, Biegel JA, et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009;27(3):385. doi: 10.1200/JCO.2008.18.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ginn KF, Gajjar A. Atypical teratoid rhabdoid tumor: current therapy and future directions. Front Oncol. 2012;2:114. doi: 10.3389/fonc.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meel MH, Guillén Navarro M, de Gooijer MC, Metselaar DS, Waranecki P, Breur M, et al. MEK/MELK inhibition and blood–brain barrier deficiencies in atypical teratoid/rhabdoid tumors. Neuro-oncology. 2020;22(1):58–69. doi: 10.1093/neuonc/noz151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hilden JM, Meerbaum S, Burger P, Finlay J, Janss A, Scheithauer BW, et al. Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol. 2004;22(14):2877–2884. doi: 10.1200/JCO.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 81.Arslanoglu A, Aygun N, Tekhtani D, Aronson L, Cohen K, Burger PC, et al. Imaging findings of CNS atypical teratoid/rhabdoid tumors. Am J Neuroradiol. 2004;25(3):476–480. [PMC free article] [PubMed] [Google Scholar]
- 82.Vieira DB, Gamarra LF. Getting into the brain: liposome-based strategies for effective drug delivery across the blood–brain barrier. Int J Nanomed. 2016;11:5381. doi: 10.2147/IJN.S117210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 84.Zhao M, van Straten D, Broekman ML, Préat V, Schiffelers RM. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics. 2020;10(3):1355. doi: 10.7150/thno.38147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Etame AB, Diaz RJ, Oreilly MA, Smith CA, Mainprize TG, Hynynen K, et al. Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomedicine. 2012;8(7):1133–1142. doi: 10.1016/j.nano.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sawyer AJ, Saucier-Sawyer JK, Booth CJ, Liu J, Patel T, Piepmeier JM, et al. Convection-enhanced delivery of camptothecin-loaded polymer nanoparticles for treatment of intracranial tumors. Drug Deliv Transl Res. 2011;1(1):34–42. doi: 10.1007/s13346-010-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood–brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Can Res. 2012;72(14):3652–3663. doi: 10.1158/0008-5472.CAN-12-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burgess A, Hynynen K. Noninvasive and targeted drug delivery to the brain using focused ultrasound. ACS Chem Neurosci. 2013;4(4):519–526. doi: 10.1021/cn300191b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dasgupta A, Liu M, Ojha T, Storm G, Kiessling F, Lammers T. Ultrasound-mediated drug delivery to the brain: principles, progress and prospects. Drug Discov Today Technol. 2016;20:41–48. doi: 10.1016/j.ddtec.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Etame AB, Diaz RJ, Smith CA, Mainprize TG, Hynynen K, Rutka JT. Focused ultrasound disruption of the blood–brain barrier: a new frontier for therapeutic delivery in molecular neurooncology. Neurosurg Focus. 2012;32(1):E3. doi: 10.3171/2011.10.FOCUS11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O'Reilly MA, Hough O, Hynynen K. Blood–brain barrier closure time after controlled ultrasound-induced opening is independent of opening volume. J Ultrasound Med. 2017;36(3):475–483. doi: 10.7863/ultra.16.02005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arvanitis CD, Askoxylakis V, Guo Y, Datta M, Kloepper J, Ferraro GB, et al. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood–tumor barrier disruption. Proc Natl Acad Sci. 2018;115(37):E8717–E8726. doi: 10.1073/pnas.1807105115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aryal M, Fischer K, Gentile C, Gitto S, Zhang Y-Z, McDannold N. Effects on P-glycoprotein expression after blood–brain barrier disruption using focused ultrasound and microbubbles. PLoS ONE. 2017;12:1. doi: 10.1371/journal.pone.0166061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, et al. Blood–brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep. 2019;9(1):321. doi: 10.1038/s41598-018-36340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, et al. Clinical trial of blood–brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8(343):343. doi: 10.1126/scitranslmed.aaf6086. [DOI] [PubMed] [Google Scholar]
- 96.Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G, et al. Safety and feasibility of repeated and transient blood–brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin Cancer Res. 2019;25(13):3793–3801. doi: 10.1158/1078-0432.CCR-18-3643. [DOI] [PubMed] [Google Scholar]
- 97.De Vries NA, Beijnen JH, Boogerd W, Van Tellingen O. Blood–brain barrier and chemotherapeutic treatment of brain tumors. Expert Rev Neurother. 2006;6(8):1199–1209. doi: 10.1586/14737175.6.8.1199. [DOI] [PubMed] [Google Scholar]
- 98.Lonser RR, Sarntinoranont M, Morrison PF, Oldfield EH. Convection-enhanced delivery to the central nervous system. J Neurosurg. 2015;122(3):697–706. doi: 10.3171/2014.10.JNS14229. [DOI] [PubMed] [Google Scholar]
- 99.Bidros DS, Vogelbaum MA. Novel drug delivery strategies in neuro-oncology. Neurotherapeutics. 2009;6(3):539–546. doi: 10.1016/j.nurt.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jahangiri A, Chin AT, Flanigan PM, Chen R, Bankiewicz K, Aghi MK. Convection-enhanced delivery in glioblastoma: a review of preclinical and clinical studies. J Neurosurg. 2017;126(1):191–200. doi: 10.3171/2016.1.JNS151591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mehta A, Sonabend A, Bruce J. Convection-enhanced delivery. Neurotherapeutics. 2017;14(2):358–371. doi: 10.1007/s13311-017-0520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brady ML, Raghavan R, Alexander A, Kubota K, Sillay K, Emborg ME. Pathways of infusate loss during convection-enhanced delivery into the putamen nucleus. Stereotact Funct Neurosurg. 2013;91(2):69–78. doi: 10.1159/000342492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Souweidane MM, Kramer K, Pandit-Taskar N, Zanzonico P, Zhou Z, Donzelli M et al. A phase I study of convection enhanced delivery (CED) of 124I-8H9 radio-labeled monoclonal antibody in children with diffuse intrinsic pontine glioma (DIPG). American Society of Clinical Oncology; 2017.
- 104.https://www.clinicaltrials.gov. https://www.clinicaltrials.gov.
- 105.Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro-Oncol. 2010;12(8):871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heiss JD, Jamshidi A, Shah S, Martin S, Wolters PL, Argersinger DP, et al. Phase I trial of convection-enhanced delivery of IL13-Pseudomonas toxin in children with diffuse intrinsic pontine glioma. J Neurosurg Pediatr. 2018;23(3):333–342. doi: 10.3171/2018.9.PEDS17225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muldoon LL, Soussain C, Jahnke K, Johanson C, Siegal T, Smith QR, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25(16):2295–2305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 108.Van Woensel M, Wauthoz N, Rosière R, Amighi K, Mathieu V, Lefranc F, et al. Formulations for intranasal delivery of pharmacological agents to combat brain disease: a new opportunity to tackle GBM? Cancers. 2013;5(3):1020–1048. doi: 10.3390/cancers5031020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.League-Pascual JC, Lester-McCully CM, Shandilya S, Ronner L, Rodgers L, Cruz R, et al. Plasma and cerebrospinal fluid pharmacokinetics of select chemotherapeutic agents following intranasal delivery in a non-human primate model. J Neurooncol. 2017;132(3):401–407. doi: 10.1007/s11060-017-2388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Da Fonseca CO, Linden R, Futuro D, Gattass CR, Quirico-Santos T. Ras pathway activation in gliomas: a strategic target for intranasal administration of perillyl alcohol. Archivum immunologiae et therapiae experimentalis. 2008;56(4):267–276. doi: 10.1007/s00005-008-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Da Fonseca CO, Schwartsmann G, Fischer J, Nagel J, Futuro D, Quirico-Santos T, et al. Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg Neurol. 2008;70(3):259–266. doi: 10.1016/j.surneu.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 112.Hashizume R, Ozawa T, Gryaznov SM, Bollen AW, Lamborn KR, Frey WH, et al. New therapeutic approach for brain tumors: Intranasal delivery of telomerase inhibitor GRN163. Neuro-oncology. 2008;10(2):112–120. doi: 10.1215/15228517-2007-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ye D, Zhang X, Yue Y, Raliya R, Biswas P, Taylor S, et al. Focused ultrasound combined with microbubble-mediated intranasal delivery of gold nanoclusters to the brain. J Control Release. 2018;286:145–153. doi: 10.1016/j.jconrel.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen H, Chen CC, Acosta C, Wu S-Y, Sun T, Konofagou EE. A new brain drug delivery strategy: focused ultrasound-enhanced intranasal drug delivery. PLoS ONE. 2014;9:10. doi: 10.1371/journal.pone.0108880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Warren KE. Novel therapeutic delivery approaches in development for pediatric gliomas. CNS oncology. 2013;2(5):427–435. doi: 10.2217/cns.13.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Basso U, Lonardi S, Brandes AA. Is intra-arterial chemotherapy useful in high-grade gliomas? Expert Rev Anticancer Ther. 2002;2(5):507–519. doi: 10.1586/14737140.2.5.507. [DOI] [PubMed] [Google Scholar]
- 117.Carceller F, Aleu A, Casasco A, Guimaraens L, López-Pino MA, Madero L, et al. Superselective intracerebral catheterization for administration of oncolytic virotherapy in a case of diffuse intrinsic pontine glioma. J Pediatr Hematol Oncol. 2014;36(7):e430–e432. doi: 10.1097/MPH.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 118.Jahnke K, Kraemer DF, Knight KR, Fortin D, Bell S, Doolittle ND, et al. Intraarterial chemotherapy and osmotic blood–brain barrier disruption for patients with embryonal and germ cell tumors of the central nervous system. Cancer. 2008;112(3):581–588. doi: 10.1002/cncr.23221. [DOI] [PubMed] [Google Scholar]
- 119.Watne K, Hager B, Hirschberg H. Intra-arterial BCNU in the treatment of recurrent medulloblastoma. J Neurooncol. 1990;8(2):139–143. doi: 10.1007/BF00177836. [DOI] [PubMed] [Google Scholar]
- 120.David JS, Grahovac Z, Benoit B, Addison D, Richard MT, Dennery J, et al. Intracarotid chemotherapy with a combination of 1, 3-Bis (2-chloroethyl)-1-nitrosourea (BCNU), cis-diaminedichloroplatinum (Cisplatin), and 4'-O-Demethyl-1-O-(4, 6-O-2-thenylidene-β-D-glucopyranosyl) epipodophyllotoxin (VM-26) in the treatment of primary and metastatic brain tumors. Neurosurgery. 1984;15(6):828–833. doi: 10.1227/00006123-198412000-00010. [DOI] [PubMed] [Google Scholar]
- 121.Rajappa P, Krass J, Riina H, Boockvar J, Greenfield JP. Super-selective basilar artery infusion of bevacizumab and cetuximab for multiply recurrent pediatric ependymoma. Interv Neuroradiol. 2011;17(4):459–465. doi: 10.1177/159101991101700410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Happold C, Roth P, Wick W, Steinbach JP, Linnebank M, Weller M, et al. ACNU-based chemotherapy for recurrent glioma in the temozolomide era. J Neurooncol. 2009;92(1):45–48. doi: 10.1007/s11060-008-9728-9. [DOI] [PubMed] [Google Scholar]
- 123.Vega F, Davila L, Chatellier G, Chiras J, Fauchon F, Cornu P, et al. Treatment of malignant gliomas with surgery, intraarterial chemotherapy with ACNU and radiation therapy. J Neurooncol. 1992;13(2):131–135. doi: 10.1007/BF00172762. [DOI] [PubMed] [Google Scholar]
- 124.Newton HB, Slivka MA, Stevens CL, Bourekas EC, Christoforidis GA, Baujan MA, et al. Intra-arterial carboplatin and intravenous etoposide for the treatment of recurrent and progressive non-GBM gliomas. J Neurooncol. 2002;56(1):79–86. doi: 10.1023/a:1014498225405. [DOI] [PubMed] [Google Scholar]
- 125.Burkhardt J-K, Riina H, Shin BJ, Christos P, Kesavabhotla K, Hofstetter CP, et al. Intra-arterial delivery of bevacizumab after blood–brain barrier disruption for the treatment of recurrent glioblastoma: progression-free survival and overall survival. World Neurosurg. 2012;77(1):130–134. doi: 10.1016/j.wneu.2011.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muldoon LL, Pagel MA, Netto JP, Neuwelt EA. Intra-arterial administration improves temozolomide delivery and efficacy in a model of intracerebral metastasis, but has unexpected brain toxicity. J Neurooncol. 2016;126(3):447–454. doi: 10.1007/s11060-015-2000-1. [DOI] [PubMed] [Google Scholar]