Abstract
Background & Aims:
Clinical remission, defined by a composite of patient reported outcomes and Mayo endoscopy subscore (MES) 0 or 1 is a recommended treatment target in patients with ulcerative colitis (UC). We estimated whether incorporating more rigorous remission definitions, of endoscopic remission (MES 0) and histologic remission, affects risk of relapse.
Methods:
Through a systematic review, we identified cohort studies in adults with UC in clinical remission that reported a minimum 12-month risk of clinical relapse, based on MES (0 vs 1) and/or histologic disease activity, in patients with endoscopic remission. Using random effects meta-analysis, we calculated relative and absolute risk of clinical relapse in patients with UC achieving different treatment targets.
Results:
In a meta-analysis of 17 studies that included 2608 patients with UC in clinical remission, compared to patients achieving MES 1, patients achieving MES 0 had a 52% lower risk of clinical relapse (relative risk, 0.48; 95% CI, 0.37–0.62). The median 12-month risk of clinical relapse in patients with MES 1 was 28.7%; the estimated annual risk of clinical relapse in patients with MES 0 was 13.7% (95% CI, 10.6–17.9). In a meta-analysis of 10 studies in patients in endoscopic remission (MES 0), patients who achieved histologic remission had a 63% lower risk of clinical relapse vs patients with persistent histologic activity (relative risk, 0.37; 95% CI, 0.24–0.56). Estimated annual risk of clinical relapse in who achieved achieving histologic remission was 5.0% (95% CI, 3.3–7.7).
Conclusions:
In a systematic review and meta-analysis of patients with UC in clinical remission, we observed that patients achieving more rigorous treatment endpoints (endoscopic and histologic remission) have a substantially lower risk of clinical relapse compared with patients achieving clinical remission.
Keywords: inflammatory bowel disease, treat-to-target, biopsy, IBD
Clinical remission, a composite outcome defined by the patient-reported outcomes (PRO) of resolution of rectal bleeding and near normalisation of stool frequency and endoscopic healing based on Mayo endoscopy subscore (MES) 0 or 1, is a consensus treatment target in patients with ulcerative colitis (UC) based upon recent American and European guidelines.1, 2 Moreover, this definition also serves as the primary endpoint for regulatory approval of new drugs in controlled trials.
In recent years, multiple observational studies have suggested that patients with UC who achieve endoscopic remission (MES 0) and/or histologic remission may have a lower risk of clinical relapse and disease-related complications than those who achieve conventionally defined remission. In addition, the notion of mucosal healing has been evolved from an endoscopic-based definition to a composite of endoscopy and histopathology. It is relevant that the most recently approved biological agent for the treatment of UC (ustekinumab) has evaluated and achieved a label for both endoscopic and histologic remission as trial endpoints based upon a definition of “histo-endoscopic mucosal healing” defined as both histologic improvement (defined as neutrophil infiltration in <5% of crypts, no crypt destruction, and no erosions, ulcerations, or granulation tissue) and endoscopic improvement.3 Notwithstanding such evolutionary changes in the clinical trial landscape, it is important to recognize that the potential benefits of achieving more rigorous endoscopic and histologic remission definitions have not been fully evaluated in either the clinical trial or practice environments. Specifically, the magnitude of the potential benefit of treating to a more rigorous target definition has not been accurately estimated. Previous studies and meta-analyses have focused on comparing relapse rates in patients in histologic remission to those with persistently active histologic disease, regardless of clinical and/or endoscopic status.4 Therefore, the relative and absolute magnitude of the benefit associated with achievement of the more rigorous composite targets that incorporate endoscopic remission (MES 0) and/or histologic remission is unknown.
Hence, we performed a systematic review with meta-analysis to estimate the relative and absolute risk of clinical relapse in patients with UC in conventionally-defined clinical remission relative to those who achieve endoscopic remission (MES 0), and histologic remission.
METHODS
This systematic review followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) standards, and was conducted according to a predefined protocol.5
Selection Criteria
We included cohort studies that evaluated adult patients with UC in clinical remission, (based on PROs and endoscopic healing, defined as MES 0 or 1), with a minimum one year follow-up, that compared the risk of clinical relapse in patients achieving (1) endoscopic remission (MES 0) vs. mildly active disease (MES 1), and/or (2) histologic remission vs. persistent histologic activity.
We excluded 1) induction studies performed in patients with active UC, 2) those with a mean follow-up of < 12 months, 3) cross-sectional or case-control studies, and 4) studies where the available data were considered inadequate to allow comparisons of interest.
Search Strategy
First, we conducted a comprehensive search of multiple electronic databases from inception to September 4th, 2019. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Ovid EMBASE, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian (LJP) with input from the principal investigator, using controlled vocabulary supplemented with keywords, without language restrictions. The detailed strategy listing is reported in the Supplementary Appendix. Two study investigators (HY and SS) independently reviewed the title and abstract of studies identified in the search to exclude studies that did not address the research question of interest based on pre-specified inclusion and exclusion criteria. The full text of the remaining articles was examined to determine whether it contained relevant information. Conflicts in study selection at this stage were resolved by consensus, referring back to the original article, in consultation with a third investigator (WJS). Second, we conducted a recursive search of the bibliographies of these selected articles and systematic reviews to identify any additional studies. Third, we conducted a manual search of abstracts from major gastroenterology conferences (Digestive Disease Week, American College of Gastroenterology annual meeting, European Crohn’s and Colitis Organization annual meeting) from 2015 to 2019 to identify additional abstracts on the topic.
Data Abstraction and Exposure Definitions
Data on study-, participant-, disease-, and treatment-related characteristics were abstracted onto a standardized form, by two authors (HY and SS) independently and discrepancies were resolved by consensus, referring to the original article, in consultation with a third reviewer (WJS). Specifically, we abstracted data on indices and definitions of clinical remission and relapse, endoscopic healing and remission, and histological remission.
Clinical remission was defined based upon study-specific criteria that included a composite of PRO criteria and an endoscopic definition. Endoscopic healing was defined as MES 0 or MES 1. In comparison of histologic remission and activity, for studies that used indices other than MES, we used study-defined criteria for endoscopic healing if they were concordant and translatable to MES categories. Although histologic remission was variably defined based on several indices for data synthesis, we standardized the remission definition as absence of neutrophils in the epithelium, corresponding to Geboes’ score < 3.1. This convention is most commonly accepted definition in the literature 6,7
Risk of Bias Assessment
Risk of bias was assessed using the Quality In Prognosis Studies tool.8 It is comprised of 6 domains: participation, attrition, prognostic factor measurement, confounding measurement and account, outcome measurement, and analysis and reporting. The specific criteria used to rate risk of bias across each domain is reported in the Supplementary Appendix. Specifically, risk of bias in outcome measurement was rated as low if study clearly and appropriately defined outcomes as clinical relapse (based on validated disease activity index) and relapse determination was made without knowledge of histological status (without knowledge of endoscopic status for studies regarding MES 0 vs. 1); moderate risk of bias if study used subjective or pragmatic definition of clinical relapse (worsening of symptoms, modification or escalation of medication, hospitalization or surgery) or unblinded determination of relapse, and high-risk of bias if both of the mentioned criteria were not fulfilled.
Outcomes and Subgroup Analysis
The primary outcome of interest was clinical relapse, based upon study-specific criteria, that consisted of a composite of PRO- and endoscopic defined definitions and the need of treatment intensification for active UC. When outcomes were reported at multiple time points, we preferentially extracted 12-month results for analysis.
For these assessments, the comparisons of interest in patients in conventional clinical remission were (1) endoscopic remission (MES 0 vs. mild endoscopic activity (MES 1), and (2) histologic remission vs. persistent histologic activity in endoscopic healing (MES 0/1 or equivalent). In addition, the incremental benefit of achieving histologic remission among patients achieving endoscopic remission, was evaluated by comparing the annual risk of relapse in patients with histologic remission to those with persistent histological activity in a subset of patients with UC in endoscopic remission (MES 0).
To evaluate stability of association and identify potential sources of heterogeneity, we performed subgroup analyses based on duration of follow-up (12 months vs. > 12 months), study design (prospective vs. retrospective), publication type (full-text vs. abstracts), geographic location (Western vs. Asian), medications used to induce and maintain clinical remission (5-aminosalicylates [5-ASA] only vs. 5-ASA and/or immunosuppressive therapies) and histological indices used (standardized histologic index vs. non-standard index). In addition, post-hoc subgroup analyses was also performed based on bias in endoscopic reading (local vs. central reading; endoscopic reading was classified as local reading if single endoscopist assessed endoscopy result, and as central reading if endoscopy images or video was re-reviewed by >1 endoscopist) and risk of bias in outcome measurement (low vs. moderate-high risk of bias, as defined above). Post-hoc sensitivity analyses based on studies in which histologic remission was defined based on Geboes’ score <3.1 was also performed. Finally, meta-regression was performed to evaluate the effects of study-level prevalence of endoscopic remission and histologic remission.
Statistical Analysis
Dichotomous outcome data (relapse vs. no relapse) based on “exposure” vs. control (MES 0 vs. MES 1 and histologic remission vs. histologic activity) were extracted from each study. When studies reported both unadjusted rates of relapse, as well as risk adjusted for confounding variables, we selectively used the adjusted risks. The estimated relative risk (RR) and 95% confidence intervals (CI) of relapse were calculated using the DerSimonian and Laird random-effects model.9 Statistical heterogeneity was assessed using the I2 statistic with a I2 ≥ 50% considered substantial heterogeneity.10 To evaluate stability of association and identify sources of heterogeneity, between-study heterogeneity was investigated using subgroup analyses by stratifying original estimates according to study characteristics as described above. On mixed-effects model, a p-value for differences between subgroups on of <0.10 was considered statistically significant. Small study effects (publication bias) was assessed visually using funnel plots, and statistically using Egger’s regression test.11 Additionally, when publication bias was assessed, Duval and Tweedie’s trim and fill test was performed to estimate true effect estimates.12 All statistical analyses were performed using the Comprehensive Meta-Analysis software package, Version 3.0 (Biostat, Englewood, NY).
Absolute Magnitude of Effect
For studies that reported the 12-month risk of relapse, we calculated the median risk of clinical relapse in patients with mild endoscopic activity (MES 1). We estimated absolute risk of clinical relapse in patients with MES 0, by multiplying 12-month risk of clinical relapse in the patients with MES 1 with relative risk reduction in the patients with MES 0 vs. MES 1. Subsequently, we used this calculated value of annual risk of relapse in patients with UC in endoscopic remission; we multiplied this with relative risk reduction in the patients with histologic remission vs. persistent histologic activity in a subset of patients in endoscopic remission. This value represents the estimated absolute risk of relapse in patients with UC in both endoscopic and histologic remission.
RESULTS
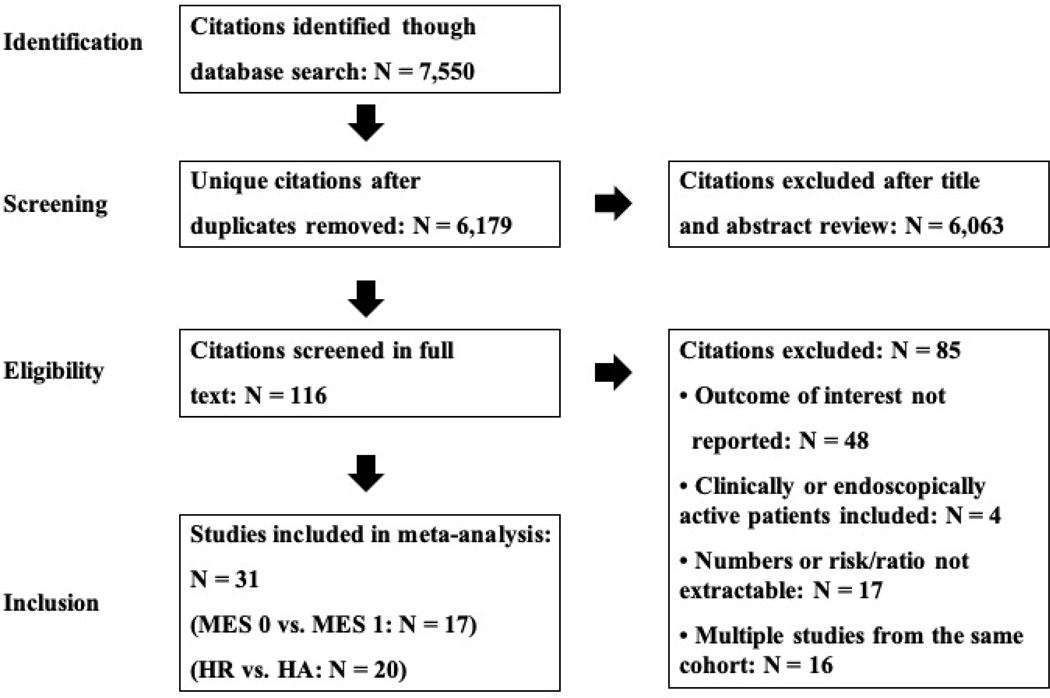
A total of 6,179 unique articles were identified through systematic literature review. After reviewing title and abstracts, 116 articles were selected for full text review, and 31 unique studies were included in the final quantitative synthesis, including one unpublished study from our group (Jangi et al).13–42 Study selection flowsheet is summarized in Figure 1.
Figure 1.
Flow diagram of systematic review and meta-analysis process
Study characteristics and quality assessment
Table 1 and Supplementary Table 1 show the characteristics of included studies. Overall, 17 studies reporting on 2,608 patients with UC in clinical remission, were included in the comparison of risk of relapse in patients with MES 0 vs MES 1.18, 23–26, 28–31, 33, 35–38, 40, 41 (personal communication with Jangi et al) Amongst these studies, a median of 57% (interquartile range [IQR], 51–66%) of patients in clinical remission were in endoscopic remission (MES 0). Twenty studies, that evaluated 2,265 patients with UC in clinical remission and endoscopic healing (MES 0/1 or equivalent), were included in the comparison of risk of relapse in patients with histologic remission vs persistent histologic activity.13–17, 19–22, 27, 29, 31, 32, 34–36, 39, 41, 42 (personal communication with Jangi et al) Amongst these studies, a median of 73% (IQR, 56–76%) of patients were in histologic remission. Of these, 8 studies exclusively evaluated patients in endoscopic remission (MES 0). Separate data for patients with MES 0 was extractable in two additional studies.
Table 1.
Characteristics of included studies
| First author | Publication year | Country | Format | Study design | No. of patients | Medications | Outcome† | Follow-up (months) ‡ |
|---|---|---|---|---|---|---|---|---|
| Riley13 | 1991 | U.K. | full article | prospective | 82 | 5-ASA | B | 12 |
| Bitton14 | 2001 | U.S., Canada | full article | prospective | 74 | 5-ASA, IMM, none | B | 12 |
| Nishio15 | 2006 | Japan | full article | prospective | 113 | 5-ASA, CS, none | B, C | 12 |
| Azad16 | 2011 | India | full article | prospective | 26 | N/A | B | 12 |
| Bessissow17 | 2012 | Belgium | full article | retrospective | 75 | 5-ASA, IMM, anti-TNF, VDZ | B, C | 12 |
| Inoue18 | 2013 | Japan | abstract | retrospective | 254 | N/A | A | 39 (24–49) |
| Wolff19 | 2013 | Europe* | full article | retrospective | 630 | 5-ASA | B | 12 |
| Jauregui- Amezaga20 |
2014 | Spain | full article | prospective | 64 | 5-ASA, IMM, anti-TNF | B, C | 12 |
| Li21 | 2014 | China | full article | prospective | 43 | 5-ASA | B | 12 |
| Nishiyama22 | 2015 | Japan | full article | prospective | 24 | 5-ASA, IMM, anti-TNF, CS, none | B, C | 15 ± 3 |
| Barreiro-de Acosta23 | 2016 | Spain | full article | prospective | 187 | 5-ASA, IMM, anti-TNF, none | A | 12 |
| Boal Carvalho24 | 2016 | Portugal | full article | retrospective | 138 | 5-ASA, IMM, anti-TNF | A | 12 |
| Kim25 | 2016 | Korea | full article | retrospective | 200 | 5-ASA, IMM, anti-TNF | A | 80 (12–118) |
| Nakarai26 | 2016 | Japan | full article | retrospective | 151 | 5-ASA, IMM, biologics, CS | A | 90 |
| Theede27 | 2016 | Denmark | full article | prospective | 70 | 5-ASA, IMM, anti-TNF | B, C | 12 |
| Yoshino28 | 2016 | Japan | article | retrospective | 88 | 5-ASA, IMM, anti-TNF, GMAA, none | A | 16 (1–90) |
| Calafat29 | 2017 | Spain | full article | retrospective | 113 | 5-ASA, IMM, anti-TNF | A, B | 12 |
| Christensen30 | 2017 | U.S. | full article | retrospective | 230 | 5-ASA, IMM, anti-TNF, CS | A | 22 (14–34) |
| Frieri31 | 2017 | Italy | full article | retrospective | 52 | 5-ASA | A, B, C | 36 |
| Gubatan32 | 2017 | U.S. | full article | prospective | 61 | 5-ASA, IMM, anti-TNF | B | 12 |
| Lopez-Diaz33 | 2017 | Spain | abstract | prospective | 57 | 5-ASA | A | 12 |
| Takeuchi34 | 2017 | Japan | abstract | retrospective | 103 | 5-ASA, IMM, biologics | B, C | 27 (7–211) |
| Lobaton35 | 2018 | Belgium/Spain | full article | prospective | 96 | 5-ASA, IMM, anti-TNF | A, B, C | 12 |
| Narang36 | 2018 | India | full article | prospective | 46 | 5-ASA, IMM | A, B | 12 |
| Ozaki37 | 2018 | Japan | full article | retrospective | 194 | 5-ASA, IMM, anti-TNF, CS | A | 20.3 |
| Yamamoto38 | 2018 | Japan | full article | prospective | 164 | 5-ASA | A | 12 |
| Cushing39 | 2019 | U.S. | full article | prospective | 83 | IMM, anti-TNF, VDZ, CS | B, C | 24 |
| Hosomi40 | 2019 | Japan | abstract | retrospective | 202 | 5-ASA, IMM | A | 60 |
| Kanazawa41 | 2019 | Japan | full article | retrospective | 166 | 5-ASA | A, B | 100 |
| Wang42 | 2019 | Australia | abstract | retrospective | 74 | N/A | B | 42 (26–63) |
| Jangi | 2020 | U.S. | abstract | retrospective | 270 | 5-ASA, IMM, anti-TNF, VDZ, tofacitinib | A, B, C | 12 |
Croatia, Czech, Estonia, Hungary, Germany, Israel, Latvia, Lithuania, Poland, Russia, Slovak Republic, Slovenia, Ukraine
A: MES 0 vs. MES 1; B: histologic remission vs. histologic activity in endoscopic remission; C: histologic remission vs. histologic activity in MES 0
Presented with mean ± SD or median (range or IQR)
5-ASA: 5-aminosalicylic acid; CS: corticosteroid; GMAA: granulocyte monocyte adsorption apheresis; IMM: immunomodulators (azathioprine, 6-mercaptopurine, methotrexate, tacrolimus); N/A: non-available; U.K.: United Kingdom; U.S.: United States.; VDZ: vedolizumab
Table 2 shows the definition of remission and relapse used in the studies. The most commonly used clinical disease activity index was the partial Mayo clinic score (13 studies); 10 studies met the STRIDE-defined target of PRO remission (resolution of rectal bleeding and near-normalization of bowel frequency). Definitions for clinical relapse were slightly variable amongst the studies. In most studies, endoscopy was read locally; central reading was performed in only 5 studies.20, 25, 26, 30, 41 For studies comparing risk of clinical relapse in patients with histologic remission to those with persistent histologic activity, the majority used the MES for assessing endoscopic healing/remission (14/20 studies), and 15/20 used standardized indices for assessing histologic activity (Geboes’ score, 7 studies;16, 17, 21, 32, 35, 36, 39 Matts classification, 3 studies;22, 34, 41 Harpaz index, 2 studies;27, 31 Riley score, 2 studies;13, 20 Nancy index, 1 study).42
Table 2.
Definition of remission and relapse in included studies
| First author | Clinical remission | Endoscopic healing/remission* | Histologic remission | Clinical relapse |
|---|---|---|---|---|
| Riley | Normal SF, no RB for 4 weeks | Baron ≤ 1 | No neutrophils in lamina propria |
Symptomatic deterioration with hemorrhagic in endoscopy |
| Bitton | Baseline bowel function, no RB |
Endoscopic Riley ≤ 1 | No neutrophils in lamina propria |
Worsening of bowel function plus RB with Endoscopic Riley > 1 |
| Nishio | MCS ≤ 3 | Inactive mucosal appearance, obscure vascular patterns, granularity of the mucosa or erythema | No or mild chronic inflammatory cell infiltrate without tissue destruction | MCS increased by ≥ 3 points |
| Azad | Baseline bowel function, no RB, no CS | Endoscopic Riley ≤ 1 | Geboes’ score < 3.1 | Worsening of bowel function associated with RB |
| Bessissow | PMS ≤ 2 | MES = 0 | Geboes’ score < 3.1 | PMS ≥ 3 |
| Inoue | N/A | Increase in BM plus reappearance of RB at 2 consecutive visits | ||
| Wolff | RCAI ≤ 4 | Rachmilewitz Endoscopic Index ≤ 3 | No histologic activity | CAI > 4 and an increase of 3 points from baseline |
| Jauregui- Amezaga |
PMS ≤1: normal SF, no RB for 3 months | MES = 0 | No neutrophilic infiltrate | Presence of RB confirmed by a MES ≥ 1 and histologic corroboration |
| Li | SCCAI < 5 | No active inflammation (erosion, ulcer, or spontaneous bleeding) | Geboes’ score < 3.1 | SSCAI ≥ 5 |
| Nishiyama | N/A | MES = 0 | Matts classification ≤ 2 | CAI > 4 (bloody diarrhea for 1 week or more, abdominal pain) |
| Barreiro-de Acosta | PMS ≤ 2 | RB with remission induction treatment, treatment escalation, hospitalization or colectomy | ||
| Boal Carvalho |
SF ≤ 1 and RB 0 | Need for intensification or modification of medication, admission or surgery | ||
| Kim | PMS ≤ 1, allowing only SF ≤ 1 | PMS ≥ 3 | ||
| Nakarai | PMS ≤ 2, no individual subscore > 1 | Increase or modification of concomitant medications due to a worsening of symptoms | ||
| Theede | MCS ≤ 1 for 3 months | MES = 0 | Harpaz index = 0 | symptom demanding adjustment of actual or initiation of new treatment |
| Yoshino | Lichtiger index < 4 under CS and GMAA- free conditions |
Any recurrence of symptoms that required additional treatment | ||
| Calafat | PMS ≤ 1, no RB | MES ≤ 1 | No acute histologic activity | Symptom with the need for treatment optimization |
| Christensen | SCCAI ≤ 2 and subscore of ≤ 1 for SF or RB | SCCAI > 2, subscore >1 for SF or RB, or medication escalation for symptom, hospitalization, or colectomy |
||
| Frieri | PMS ≤ 1 | MES = 0 | Harpaz index = 0 | Relapses needing CS, immunomodulators and/or biological drugs |
| Gubatan | SCCAI ≤ 2 | MES ≤ 1 | Geboes’ score < 3.1 | SCCAI > 2, medication intensification, hospitalization |
| Lopez-Diaz | N/A | Need for remission induction treatment, any treatment escalation, hospitalization or colectomy | ||
| Takeuchi | MCS ≤ 1, no RB | MES = 0 | Matts classification ≤ 2 | Symptom needing medical intervention |
| Lobaton | MCS ≤ 2 | MES ≤ 1 | Geboes’ score < 3.1 | PMS ≥ 3, introducing CS or any other treatment escalation. |
| Narang | SCCAI ≤ 2 | MES ≤ 1 | Geboes’ score < 3.1 | N/A |
| Ozaki | PMS ≤ 2 | PMS ≥ 3, or modification of treatment | ||
| Yamamoto | normal SF, no RB | Worsening of SF and/or RB with the MES > 1 | ||
| Cushing | N/A | Baron = 0 | Geboes’ score < 3.1 | Treatment change (dose escalation, class change, need for systemic CS), hospitalization or surgery |
| Hosomi | SF ≤ and RB 0 | Exacerbation of symptom requiring treatment intensification, including adjustment of 5-ASA and/or IMM | ||
| Kanazawa | RCAI ≤ 4 | MES ≤ 1 | Matts classification ≤ 2 | RCAI ≥ 5 |
| Wang | PMS ≤ 1 | MES ≤ 1 | Nancy index ≤ 1 | PMS ≥ 3, initiation of CS, hospitalization, and escalation or alteration of therapy |
| Jangi | PRO2 ≤ 1 | MES ≤ 1 | Normal mucosa or architectural change without | New symptoms of diarrhea and/or RB |
This is presented only in the studies that compared the risk of relapse in patients with histologic remission vs. persistent histologic activity; the definition of endoscopic healing and endoscopic remission in the studies that compared the risk of relapse in patients with MES 0 vs. MES 1 is MES ≤ 1 and MES = 0 per se, respectively.
CS: corticosteroid; GMAA: granulocyte monocyte adsorption apheresis; MCS: Mayo clinic score; MES: Mayo endoscopic score; N/A: non-available; PMS: partial Mayo clinic score; RB: rectal bleeding; RCAI: Rachmilewitz clinical activity index; SCCAI; simple clinical colitis activity index; SF: stool frequency
Study-level risk of bias assessment is summarized in Supplementary Table 2. Overall, 45–55% studies were at high risk of bias for domains of outcome measurement (unblinded assessment of relapse and use of non-validated disease activity index for defining relapse), study confounding (failure to adjust for key clinical characteristics and medication use), and statistical analysis and reporting (reporting unadjusted rates of relapse).
Clinical Relapse in Patients in Clinical Remission in Endoscopic Remission vs. Mild Endoscopic Activity
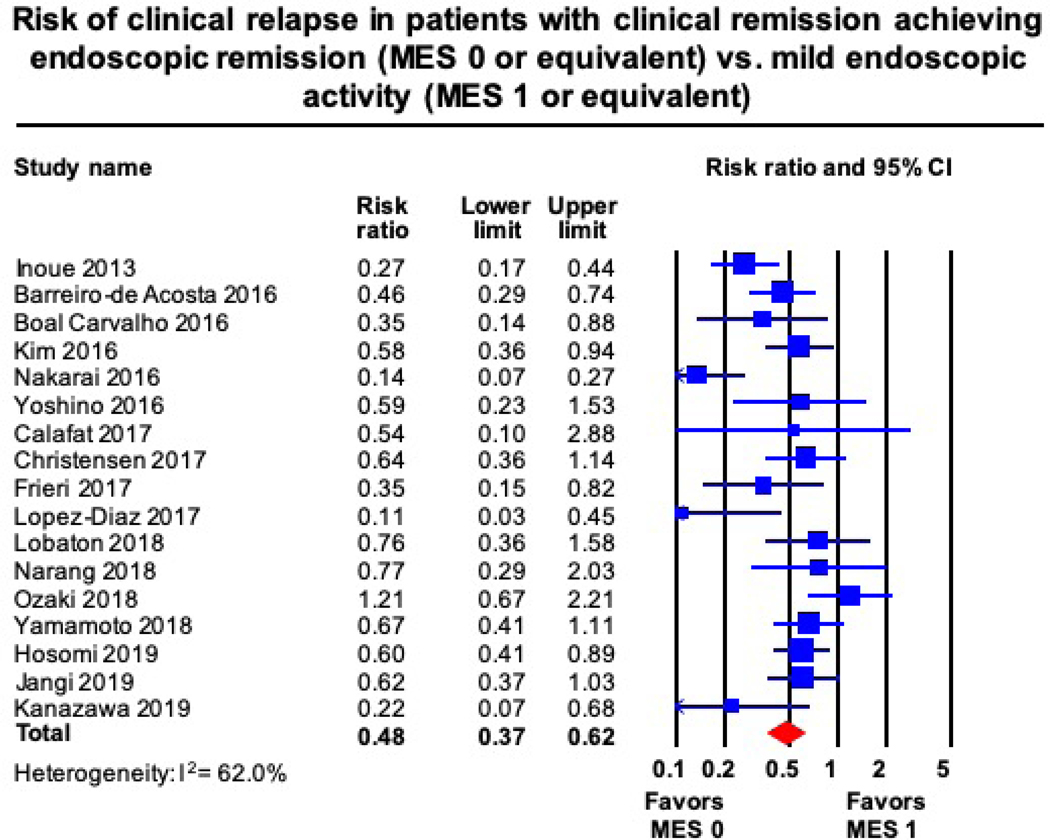
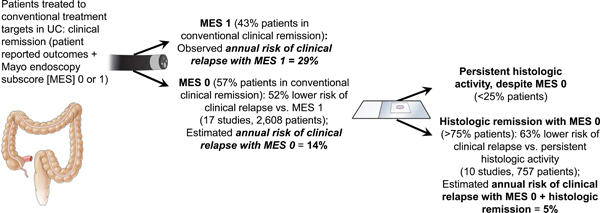
Based upon meta-analysis of 17 studies including 2,608 patients in clinical remission, achieving endoscopic remission (MES 0) had a 52% lower risk of clinical relapse (RR, 0.48 [95% CI, 0.37–0.62]) than those with mild endoscopic activity (MES 1). Substantial heterogeneity was identified for this estimate (I2 = 62%) (Figure 2). In 8 studies, median 12-month risk of clinical relapse in patients with UC with mild endoscopic activity was 28.7%. Based on this, the estimated annual risk of clinical relapse in patients achieving endoscopic remission would be 13.7% (95% CI, 10.6–17.9).
Figure 2.
Risk of clinical relapse in patients with clinical remission, in endoscopic remission (MES 0) vs. with mild endoscopic activity (MES 1)
On subgroup analyses, heterogeneity could be partly explained based on duration of follow-up and risk of bias in outcomes measurement (Table 3). Overall, the relative risk of relapse in patients with MES 0 vs. MES 1 was significantly lower in studies in which mean or median follow-up period was >12 months vs. 12 months (RR, 0.39 vs. RR, 0.60; P=0.097), and in studies at high risk of bias in outcome measurement (RR, 0.31 vs. RR, 0.62, P<0.01). No significant difference in magnitude of risk of relapse was observed in studies in which endoscopy was locally vs. centrally read. The results of the meta-regression indicated that prevalence of endoscopic remission in each study did not significantly influence the relative risk of relapse. No publication bias was observed (Egger’s test P = 0.29) (Supplementary Figure 1)
Table 3.
Sub-group analyses: Degree of endoscopic healing (MES 0 vs. MES 1) and risk of clinical relapse in patients with ulcerative colitis in clinical remission
| Groups | Categories | No. of studies | Relative risk of relapse (95% CI) | Heterogeneity within groups (I2) | Pinteraction |
|---|---|---|---|---|---|
| Publication type | Full text | 13 | 0.51 (0.37–0.70) |
59 | 0.45 |
| Abstract | 4 | 0.40 (0.22–0.70) |
75 | ||
| Study design | Prospective | 5 | 0.55 (0.35–0.84) |
45 | 0.53 |
| Retrospective | 12 | 0.46 (0.33–0.64) |
68 | ||
| Study location | Western | 8 | 0.50 (0.38–0.67) |
17 | 0.85 |
| Asia | 9 | 0.48 (0.31–0.73) |
76 | ||
| Medication* | 5-ASA only | 4 | 0.33 (0.16–0.69) |
63 | 0.20 |
| Others | 12 | 0.55 (0.42–0.74) |
57 | ||
| Endoscopic reading | Central | 4 | 0.34 (0.16–0.73) |
80 | 0.29 |
| Local | 13 | 0.53 (0.40–0.69) |
51 | ||
| Follow-up period | 12 months | 9 | 0.60 (0.44–0.83) |
44 | 0.10 |
| > 12 months | 8 | 0.39 (0.26–0.58) |
69 | ||
| Risk of bias in outcome measurement |
Moderate | 9 | 0.62 (0.51–0.76) |
20 | 0.005 |
| High | 8 | 0.31 (0.20–0.48) |
48 |
Type of medication used to induce and maintain initial clinical remission; data extraction was not available in one study.CI: Confidence intervals
Clinical Relapse in Patients in Clinical Remission in Histologic Remission vs. Persistent Histological Activity
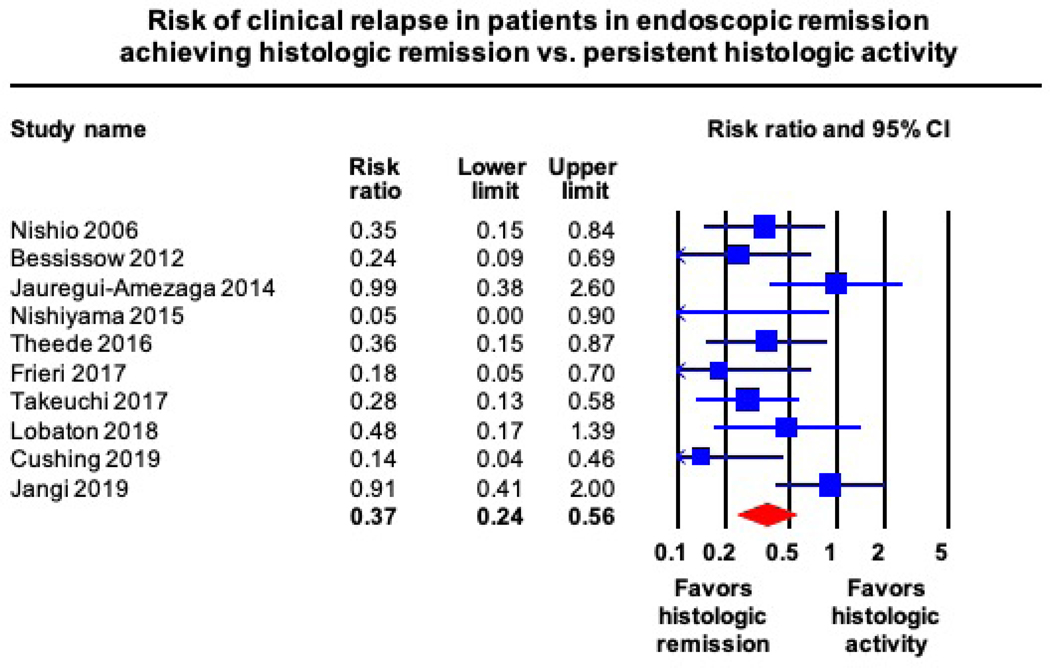
Based upon a meta-analysis of 20 studies including 2,265 patients with UC in clinical remission and endoscopic healing/remission, patients with histologic remission had a 61% lower risk of clinical relapse (RR, 0.39 [95% CI, 0.31–0.51]), than those with persistent histologic activity. Substantial heterogeneity was identified for these analyses (I2 = 62%) (Supplementary Figure 2). A sub-analysis of 10 studies that included 757 patients with UC in endoscopic remission (MES 0 or equivalent), patients with histologic remission had a 63% lower risk of clinical relapse (RR, 0.37 [95% CI, 0.24–0.56]), than patients with persistent histologic activity. Moderate statistical heterogeneity (I2 = 43%) was identified in this analysis (Figure 3). With calculated 13.7% annual risk of clinical relapse in patients with UC in endoscopic remission (MES 0), the estimated annual risk of clinical relapse in patients achieving endoscopic and histologic remission would be 5.0% (95% CI, 3.3–7.7).
Figure 3.
Risk of clinical relapse in patients with clinical remission with endoscopic remission (MES 0) in histologic remission vs. persistent histologic activity
On subgroup analyses, relative risk of relapse in patients with histologic remission relative to those with persistent histologic activity was significantly lower in studies in which the mean or median duration of follow-up was > 12 months (RR, 0.22 vs. RR, 0.48; P<0.01), and in which standardized histologic disease activity indices were used (RR, 0.33 vs. RR, 0.62, P<0.01) (Table 4). No significant difference in magnitude of risk of relapse was observed in studies at moderate vs. high risk of bias in outcome measurement. In 7 studies that used Geboes’ score for assessing histologic activity, patients with Geboes’ score<3.1 had a 70% lower risk of clinical relapse (RR, 0.30 [95% CI, 0.17–0.53]), than those with Geboes’ score≥3.1 (I2 = 76%). Based upon meta-regression, the prevalence of histologic remission in each study did not significantly influence the relative risk estimate. Visual assessment of the funnel plot, and Egger’s test (P < 0.01) suggested publication bias (Supplementary Figure 3). The conclusion of the trim-and-fill-test suggested 8 potential unpublished studies, with an adjusted RR for clinical relapse of 0.51 (95% CI, 0.40–0.65).
Table 4.
Sub-group analyses: Histologic remission vs. persistent histologic activity and risk of clinical relapse in patients with ulcerative colitis in clinical remission and endoscopic healing
| Groups | Categories | No. of studies | Relative risk of relapse (95% CI) | Heterogeneity within groups (I2) | Pinteraction |
|---|---|---|---|---|---|
| Publication type | Full text | 17 | 0.40 (0.30–0.53) |
63 | 0.78 |
| Abstract | 3 | 0.36 (0.21–0.64) |
52 | ||
| Study design | Prospective | 12 | 0.40 (0.28–0.57) |
63 | 0.71 |
| Retrospective | 8 | 0.36 (0.23–0.56) |
65 | ||
| Study location | Western | 13 | 0.48 (0.37–0.63) |
57 | 0.02 |
| Asia | 7 | 0.29 (0.21–0.40) |
0 | ||
| Medication* | 5-ASA only | 5 | 0.37 (0.20–0.70) |
71 | 0.88 |
| Others | 13 | 0.40 (0.28–0.55) |
62 | ||
| Endoscopic reading | Central | 2 | 0.51 (0.14–1.90) |
74 | 0.69 |
| Local | 18 | 0.39 (0.30–0.50) |
63 | ||
| Histological disease activity index | Standardized | 15 | 0.33 (0.23–0.46) |
65 | 0.01 |
| Nonstandardized | 5 | 0.60 (0.45–0.82) |
30 | ||
| Follow-up period | 12 months | 14 | 0.48 (0.38–0.62) |
56 | 0.002 |
| > 12 months | 6 | 0.22 (0.15–0.34) |
0 | ||
| Risk of bias in outcome measurement | Low | 1 | 0.14 (0.04–0.55) |
0 | 0.28 |
| Moderate | 9 | 0.44 (0.31–0.61) |
68 | ||
| High | 10 | 0.39 (0.27–0.55) |
38 |
Type of medication used to induce and maintain initial clinical remission; data extraction was not available in two studies.
CI: Confidence intervals
DISCUSSION
Current consensus statements and clinical guidelines recommend clinical remission as a treatment target, defined as a composite of symptomatic remission and endoscopic healing (MES 0 or 1). While this target is attractive because it addresses control of symptoms, which is important from a patient perspective, and specifies objective evaluation of endoscopic disease activity, there is increasing interest in achieving the more rigorous goals of endoscopic remission (MES 0) and histologic remission. However, before these targets can be accepted in either clinical trials or practice, it is necessary to quantify their potential benefits relative to our current standard of clinical remission.
In this systematic review and meta-analysis of 31 cohort studies, we evaluated the relative and absolute magnitude of the incremental benefit associated with achieving more rigorous treatment targets in patients who have achieved the conventionally-defined target of clinical remission. Several conclusions can be drawn from these analyses. First, we observed that patients who achieved endoscopic remission (MES 0) had a 52% lower risk of clinical relapse than those who achieved symptomatic remission with mild endoscopic activity (MES 1). This difference translates into an estimated annual clinical relapse risk of 13.7% in patients with UC achieving symptomatic remission with endoscopic remission, compared with 28.7% in patients with mild endoscopic activity. This absolute difference of 15% is clearly of clinical relevance, and it also has important pharmacoeconomic implications. Second, we observed that among patients with endoscopic remission, those who also achieved histologic remission had a 63% lower risk of clinical relapse, relative to patients with persistent histological activity. An estimated annual clinical relapse risk of only 5% was observed in this patient population compared with 13.7% for those with endoscopic remission alone. Again, the more rigorous remission target was associated with a substantially better prognosis. Collectively, these findings suggest that revising our current treatment targets in UC is a worthy objective. However, controlled trials examining the efficacy of current and future therapies in achieving these stringent end points are warranted to ascertain the population-level feasibility and cost-effectiveness of such strategies. In this regard, the VERDICT (In actiVE ulcerative colitis, a RanDomIzed Controlled Trial for determination of the optimal treatment target) trial has been launched to determine the optimal treatment target for UC (EudraCT Number: 2019–002485-12). In this study, patients with moderate-severely active UC will be randomized to a target of symptomatic remission (rectal bleeding score = 0), symptomatic + endoscopic remission (MES 0/1) or symptomatic + endoscopic + histological remission (Robarts histological index < 3), following a treatment algorithm to guide escalation of therapy to achieve the assigned target.
In the STRIDE consensus statement, while specialists agreed that endoscopic remission might be a preferred treatment target, there was insufficient evidence to recommend it for all patients. This led to the consensus view that “endoscopic healing” was the most appropriate criterion for the endoscopic component of the clinical remission definition. In a recent meta-analysis of randomized placebo controlled trials that evaluated biologics or small-molecules therapy for UC to achieve PRO-defined remission (resolution of rectal bleeding with normalization or near-normalization of stool frequency), the overall prevalence of endoscopic healing (MES 0 or 1) in post-induction and during maintenance therapy was estimated to be 75% and 88%, respectively.43 However, in this study the prevalence of endoscopic remission vs. MES 1 was 25% vs. 52% in post-induction, and 51% vs. 37% during maintenance therapy, respectively. These data underscore that following induction therapy, at a time that corresponds to the STRIDE recommendation for endoscopic re-evaluation initiation, only a minority of patients will achieve endoscopic remission. The reasons for this are likely multi-factorial and include the limited efficacy of current treatments, the absence of a treat to endoscopic remission strategy in the trials, and a relatively short duration of time to achieve the more rigorous endoscopic remission definition.
The findings of our study are consistent with previous observations that histologic remission may be the treatment target that conveys the best long-term prognosis. We observed a significant 61% lower risk of clinical relapse in patients with UC in conventionally-defined clinical remission and who achieved histologic remission, compared with those who had persistent histologic activity. These findings contrast with a prior meta-analysis by Park and colleagues who estimated that the risk of relapse in patients with histologic remission was only 19% lower compared to patients with conventionally-defined clinical remission (symptomatic remission and endoscopic healing). These differences may be due to shorter duration of follow-up in studies included in their synthesis; notably only 20% of the studies included had follow-up > 12 months. In contrast, we limited our synthesis to studies with a minimum follow-up of 12 months. In support of this notion, on sub-group analysis, we observed that studies with longer duration of follow-up were associated with a greater benefit for achieving histologic remission. This observation indicates that prospective treat to target trials should be of sufficient duration to fully evaluate the potential benefits of histologic remission in maintenance therapy.
The ability of current therapies to achieve more stringent endpoints, however, needs to be better evaluated before incorporating these endpoints in the treat-to-target paradigm. In a systematic review of pharmacological therapies in randomized controlled trials for UC, we observed that oral 5-ASA may achieve endoscopic and histologic remission in 60% patients with mild to moderately active UC in trials of maintenance therapy.44 In contrast, likelihood of achieving histologic remission in moderate-to-severe UC with biologic therapies and novel small molecule inhibitors is 30–40%, albeit with limited data.45 These mean that the actual likelihood of achieving this rigorous target in patients with moderate-severely active UC is still challenging, even using the newest drugs. In addition, while current studies focused on examining outcomes of patients who achieve endoscopic and/or histologic outcomes under routine clinical practice, whether proactively trying to achieve these endpoints with treatment optimization under the treat-to-target paradigm will result in similar benefits is unknown; furthermore there will be interest in any potential safety implications of treatment escalation in order to achieve more stringent targets. In addition, in implementing treat-to-target, there is increasing emphasis on combining PROs and interim biomarkers such as fecal calprotectin to inform treatment optimization decisions. While integrating PROs with biomarkers may accurately predict the presence of endoscopic healing, it’s performance for predicting the presence of endoscopic remission and histologic remission may be suboptimal and merits further evaluation.45 Recent studies have identified a strong correlation between fecal calprotectin and endoscopic and/or histologic remission; however, thresholds for fecal calprotectin for differentiating histologic remission and activity vary widely, ranging from 40.5 to 250μg/g.46,47 Finally, standardization of measuring and reporting these end points in routine clinical practice, particularly histological remission is warranted.48
Our study has several strengths. First, to inform incremental benefit of achieving endoscopic and/or histologic remission, we focused only on studies in patients with UC in conventionally-defined clinical remission, which makes our study directly applicable in clinical practice. Second, besides relative benefit, we also informed the absolute benefit of achieving these rigorous treatments to help contextualize clinical discussions and research questions. However, our study has several limitations that need to be considered. First, there was variability in definition of exposures, especially histologic remission, and outcome measurements, which led to substantial heterogeneity in some estimates. Studies used various validated and non-validated histological disease activity indices, with different cut-offs. We tried to standardize assessment of histological remission across studies specifically focusing on absence of neutrophils in the epithelium. We also performed sub-group analyses which demonstrated that the magnitude of benefit of achieving histologic remission was higher in studies which defined remission based on standardized indices. Similarly, we observed the studies at moderate risk of bias in outcome measurement (vs. high risk of bias in outcome measurement) reported lower magnitude of benefit of achieving MES 0 vs. MES 1. Second, most studies used MES to define endoscopic healing and remission. STRIDE suggests that UC Endoscopic Index of Severity (UCEIS) may be a better validated tool but recognizes that MES is widely used and easily implemented in practice. However, considering similarity between the two indices, we anticipate that the relative and absolute benefit of achieving UCEIS-defined endoscopic remission would be compared to MES-defined endoscopic remission. Third, since most studies combined medications including immunosuppressive therapies together, we are unable to ascertain whether the magnitude of benefit may be different in patients treated with 5-ASA vs. patients treated with immunosuppressives including biologics and targeted small molecule inhibitors. Forth, we were unable to separately ascertain the potential benefit of achieving these rigorous targets on outcomes such as hospitalization, colectomy and colorectal cancer, as well as potential harms in a quest to achieve more rigorous endoscopic and histologic remission definitions.48
In conclusion, patients with UC achieving treatment end points of endoscopic and/or histologic remission have a substantially lower risk of clinical relapse as compared to patients achieving conventionally-defined clinical remission, with the lowest risk of relapse in patients who achieve combined endoscopic and histologic remission. These end points may be considered as preferred treatment targets, but future studies are needed to evaluate the population-level feasibility and cost-effectiveness of treating patients with UC to these end points.
Supplementary Material
WHAT YOU NEED TO KNOW
Background and Context:
It is not clear whether incorporating endoscopic remission (Mayo endoscopic score [MES] 0) and/or histologic remission endpoints affects risk of relapse in patients with ulcerative colitis (UC) in clinical remission.
New Findings:
In a meta-analysis, patients with UC in clinical remission who achieved MES 0 had a 52% lower risk of relapse compared with patients with MES 1. Among patients with MES 0, those who achieved histologic remission had a 63% lower risk of relapse, compared with patients with histologic activity.
Limitations:
There was substantial heterogeneity among estimates from the different studies; this could be partly accounted for by differences in definition of histologic remission, outcome measurement (clinical relapse), and duration of follow up.
Impact:
Patients who achieve more rigorous treatment endpoints (endoscopic and histologic remission) have a lower risk of clinical relapse than patients with only the conventional definition of defined clinical remission.
LAY SUMMARY
Among patients with UC in clinical remission, those who achieve endoscopic and/or histologic markers of remission have a lower risk of clinical relapse than patients who achieved only clinical remission.
Footnotes
Disclosures: Parambir S. Dulai is supported by an American Gastroenterology Association Research Scholar Award. William Sandborn is supported by the NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515). Siddharth Singh is supported by an American College of Gastroenterology Junior Faculty Development Award #144271, Crohn’s and Colitis Foundation Career Development Award #404614, and the National Institute of Diabetes and Digestive and Kidney Diseases K23DK117058. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest:
• Hyuk Yoon: No conflicts of interest
• Sushrut Jangi: No conflicts of interest
• Parambir Dulaihas received research support from Takeda, Pfizer, Abbvie, Janssen, Polymedco, ALPCO, Buhlmann, and consulting fees from Takeda, Pfizer, Abbvie and Janssen.
• Brigid Boland has received research support from Takeda and Janssen, and consulting fees from Abbvie and Prometheus laboratories.
• Larry Prokop: No conflicts of interest
• Vipul Jairath has received has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert, Celltrion; speaker’s fees from Takeda, Janssen, Shire, Ferring, Abbvie, Pfizer.
• Brian Feagan has received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma, AbbVie, Novartis Pharmaceuticals, Centocor, Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix and Wyeth Pharmaceuticals; consulting fees from Millennium Pharmaceuticals, Merck, Centocor, Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, AstraZeneca, Serono, Genentech, Tillotts Pharma, Unity Pharmaceuticals, Albireo Pharma, Given Imaging, Salix Pharmaceuticals, Novonordisk, GSK, ActoGenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, GiCare Pharma and Sigmoid Pharma; and speaker’s bureau fees from UCB, AbbVie and J&J/Janssen.
• William Sandborn has received research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, Abbvie, Janssen, Takeda, Lilly, Celgene/Receptos,Pfizer, Prometheus Laboratories (now Prometheus Biosciences); consulting fees from Abbvie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Opthotech - consultant, stock options; Progenity - consultant, stock; Oppilan Pharma - employee, stock options; Escalier Biosciences - employee, stock options; Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories) - employee, stock options; Ventyx Biosciences – employee, stock options; Vimalan Biosciences – employee, stock options.
• Siddharth Singh has received research grants from AbbVie and Janssen, and personal fees from Takeda and Pfizer.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 2.Harbord M, Eliakim R, Bettenworth D, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis 2017;11:769–784. [DOI] [PubMed] [Google Scholar]
- 3.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med 2019;381:1201–1214. [DOI] [PubMed] [Google Scholar]
- 4.Park S, Abdi T, Gentry M, et al. Histological Disease Activity as a Predictor of Clinical Relapse Among Patients With Ulcerative Colitis: Systematic Review and Meta-Analysis. Am J Gastroenterol 2016;111:1692–1701. [DOI] [PubMed] [Google Scholar]
- 5.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 6.Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut 2000;47:404–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchal Bressenot A, Riddell RH, Boulagnon-Rombi C, et al. Review article: the histological assessment of disease activity in ulcerative colitis. Aliment Pharmacol Ther 2015;42:957–67. [DOI] [PubMed] [Google Scholar]
- 8.Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–6. [DOI] [PubMed] [Google Scholar]
- 9.Serghiou S, Goodman SN. Random-Effects Meta-analysis: Summarizing Evidence With Caveats. JAMA 2019;321:301–302. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egger M, Davey Smith G, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 13.Riley SA, Mani V, Goodman MJ, et al. Microscopic activity in ulcerative colitis: what does it mean? Gut 1991;32:174–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bitton A, Peppercorn MA, Antonioli DA, et al. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology 2001;120:13–20. [DOI] [PubMed] [Google Scholar]
- 15.Nishio Y, Ando T, Maeda O, et al. Pit patterns in rectal mucosa assessed by magnifying colonoscope are predictive of relapse in patients with quiescent ulcerative colitis. Gut 2006;55:1768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azad S, Sood N, Sood A. Biological and Histological Parameters as Predictors of Relapse in Ulcerative Colitis: A Prospective Study. Saudi J Gastroenterol 2011;17:194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bessissow T, Lemmens B, Ferrante M, et al. Prognostic Value of Serologic and Histologic Markers on Clinical Relapse in Ulcerative Colitis Patients With Mucosal Healing. Am J Gastroenterol 2012;107:1684–1692. [DOI] [PubMed] [Google Scholar]
- 18.Inoue N, Takabayashi K, Takayama T, et al. Mucosal healing without endoscopic activity predicts long-term clinical remission in patients with ulcerative colitis. Gastroenterology 2013;1):S776–S777. [Google Scholar]
- 19.Wolff S, Terheggen G, Mueller R, et al. Are endoscopic endpoints reliable in therapeutic trials of ulcerative colitis? Inflamm Bowel Dis 2013;19:2611–5. [DOI] [PubMed] [Google Scholar]
- 20.Jauregui-Amezaga A, Lopez-Ceron M, Aceituno M, et al. Accuracy of Advanced Endoscopy and Fecal Calprotectin for Prediction of Relapse in Ulcerative Colitis: A Prospective Study. Inflamm Bowel Dis 2014;20:1187–1193. [DOI] [PubMed] [Google Scholar]
- 21.Li C-Q, Liu J, Ji R, et al. Use of confocal laser endomicroscopy to predict relapse of ulcerative colitis. BMC Gastroenterol 2014;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishiyama S, Oka S, Tanaka S, et al. Clinical usefulness of endocytoscopy in the remission stage of ulcerative colitis: a pilot study. Journal of Gastroenterology 2015;50:1087–1093. [DOI] [PubMed] [Google Scholar]
- 23.Barreiro-de Acosta M, Vallejo N, de la Iglesia D, Uribarri L, et al. Evaluation of the Risk of Relapse in Ulcerative Colitis According to the Degree of Mucosal Healing (Mayo 0 vs 1): A Longitudinal Cohort Study. J Crohns Colitis 2016;10:13–9. [DOI] [PubMed] [Google Scholar]
- 24.Boal Carvalho P, Dias de Castro F, Rosa B, et al. Mucosal Healing in Ulcerative Colitis--When Zero is Better. J Crohns Colitis 2016;10:20–5. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Cheon JH, Park Y, et al. Effect of mucosal healing (Mayo 0) on clinical relapse in patients with ulcerative colitis in clinical remission. Scand J Gastroenterol 2016;51:1069–74. [DOI] [PubMed] [Google Scholar]
- 26.Nakarai A, Kato J, Hiraoka S, et al. Ulcerative colitis patients in clinical remission demonstrate correlations between fecal immunochemical test results, mucosal healing, and risk of relapse. World J Gastroenterol 2016;22:5079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theede K, Holck S, Ibsen P, et al. Fecal Calprotectin Predicts Relapse and Histological Mucosal Healing in Ulcerative Colitis. Inflamm Bowel Dis 2016;22:1042–8. [DOI] [PubMed] [Google Scholar]
- 28.Yoshino T, Yamakawa K, Nishimura S, et al. The predictive variable regarding relapse in patients with ulcerative colitis after achieving endoscopic mucosal healing. Intest Res 2016;14:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calafat M, Lobaton T, Hernandez-Gallego A, et al. Acute histological inflammatory activity is associated with clinical relapse in patients with ulcerative colitis in clinical and endoscopic remission. Dig Liver Dis 2017;49:1327–1331. [DOI] [PubMed] [Google Scholar]
- 30.Christensen B, Hanauer SB, Erlich J, et al. Histologic Normalization Occurs in Ulcerative Colitis and Is Associated With Improved Clinical Outcomes. Clin Gastroenterol Hepatol 2017;15:1557–1564 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frieri G, Galletti B, Di Ruscio M, et al. The prognostic value of histology in ulcerative colitis in clinical remission with mesalazine. Ther Adv Gastroenterol 2017;10:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gubatan J, Mitsuhashi S, Zenlea T, et al. Low Serum Vitamin D During Remission Increases Risk of Clinical Relapse in Patients With Ulcerative Colitis. Clin Gastroenterol Hepatol 2017;15:240–246.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez-Diaz J, Ferreiro R, De La Iglesia D, et al. Efficacy of MMX-mesalamine monotherapy for maintenance of remission in ulcerative colitis patients after mucosal healing. J Crohns Colitis 2017;11 (Supplement 1):S336–S337. [Google Scholar]
- 34.Takeuchi Y, Tashiro T, Arai K, et al. Incidence of clinical flare-up in patients with ulcerative colitis who achieved deep remission and its association factors. Gastroenterology 2017;152 (5 Supplement 1):S360. [Google Scholar]
- 35.Lobaton T, Bessissow T, Ruiz-Cerulla A, et al. Prognostic value of histological activity in patients with ulcerative colitis in deep remission: A prospective multicenter study. United European Gastroenterol J 2018;6:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narang V, Kaur R, Garg B, et al. Association of endoscopic and histological remission with clinical course in patients of ulcerative colitis. Intes Res 2018;16:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozaki R, Kobayashi T, Okabayashi S, et al. Histological Risk Factors to Predict Clinical Relapse in Ulcerative Colitis With Endoscopically Normal Mucosa. J Crohns Colitis 2018;12:1288–1294. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T, Shimoyama T, Umegae S, Matsumoto K. Endoscopic score vs. fecal biomarkers for predicting relapse in patients with ulcerative colitis after clinical remission and mucosal healing. Clin Transl Gastroenterol 2018;9:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cushing KC, Tan W, Alpers DH, et al. Complete histologic normalisation is associated with reduced risk of relapse among patients with ulcerative colitis in complete endoscopic remission. Aliment Pharmacol Ther 2020;51:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hosomi S, Itani S, Nakata R, et al. Does Treatment Intensification for Ulcerative Colitis Patients with Clinical Remission Who Have Mild Endoscopic Activity Improve Long-Term Prognosis? Gastrointest Endosc 2019;89 (6 Supplement):AB70–AB71. [Google Scholar]
- 41.Kanazawa M, Takahashi F, Tominaga K, et al. Relationship between endoscopic mucosal healing and histologic inflammation during remission maintenance phase in ulcerative colitis: a retrospective study. Endoscopy Int Open 2019;7:E568–E575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Fewings I, Bornman L, et al. Histological remission (NANCY index) is superior to endoscopic mucosal healing in predicting relapse-free survival in patients with ulcerative colitis in clinical and endoscopic remission. J Crohns Colitis 2019;13 (Supplement 1):S071. [DOI] [PubMed] [Google Scholar]
- 43.Dulai PS, Singh S, Jairath V, Ma C, et al. Prevalence of endoscopic improvement and remission according to patient-reported outcomes in ulcerative colitis. Aliment Pharmacol Ther 2020;51:435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Battat R, Duijvestein M, Guizzetti L, et al. Histologic Healing Rates of Medical Therapies for Ulcerative Colitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am J Gastroenterol 2019;114:733–745. [DOI] [PubMed] [Google Scholar]
- 45.Dulai PS, Battat R, Barsky M, et al. Incorporating Fecal Calprotectin Into Clinical Practice for Patients With Moderate-to-Severely Active Ulcerative Colitis Treated With Biologics or Small-Molecule Inhibitors. Am J Gastroenterol 2020; doi: 10.14309/ajg.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh A, Kormilitzin A, Hinds C, et al. Defining Faecal Calprotectin Thresholds as a Surrogate for Endoscopic and Histological Disease Activity in Ulcerative Colitis-a Prospective Analysis. J Crohns Colitis 2019;13:424–430 [DOI] [PubMed] [Google Scholar]
- 47.D’Amico F, Bonovas S, Danese S, et al. Review Article: Faecal Calprotectin and Histologic Remission in Ulcerative Colitis. Aliment Pharmacol Ther 2020;51:689–98 [DOI] [PubMed] [Google Scholar]
- 48.Chateau T, Feakins R, Marchal-Bressenot A, et al. Histological Remission in Ulcerative Colitis: Under the Microscope Is the Cure. Am J Gastroenterol 2020;115:179–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.