Abstract
Abstract
Individuals born extremely preterm are at significant risk for impaired neurodevelopment. After discharge from the neonatal intensive care, associations between the child’s well-being and factors in the home and social environment become increasingly apparent. Mothers’ prenatal health and socioeconomic status are associated with neurodevelopmental outcomes, and emotional and behavioral problems. Research on early life risk factors and on mechanisms underlying inter-individual differences in neurodevelopment later in life can inform the design of personalized approaches to prevention. Here, we review early life predictors of inter-individual differences in later life neurodevelopment among those born extremely preterm. Among biological mechanisms that mediate relationships between early life predictors and later neurodevelopmental outcomes, we highlight evidence for disrupted placental processes and regulated at least in part via epigenetic mechanisms, as well as perinatal inflammation. In relation to these mechanisms, we focus on four prenatal antecedents of impaired neurodevelopment, namely, (1) fetal growth restriction, (2) maternal obesity, (3) placental microorganisms, and (4) socioeconomic adversity. In the future, this knowledge may inform efforts to detect and prevent adverse outcomes in infants born extremely preterm.
Impact
This review highlights early life risk factors and mechanisms underlying inter-individual differences in neurodevelopment later in life.
The review emphasizes research on early life risk factors (fetal growth restriction, maternal obesity, placental microorganisms, and socioeconomic adversity) and on mechanisms (disrupted placental processes and perinatal inflammation) underlying inter-individual differences in neurodevelopment later in life.
The findings highlighted here may inform efforts to detect and prevent adverse outcomes in infants born extremely preterm.
Background
Extremely preterm birth (birth before 28 weeks of gestation) accounts for <1% of US births; however, due to their greatly increased risk of chronic health and developmental disorders, individuals born extremely preterm contribute a disproportionate fraction of children with cerebral palsy, cognitive impairment, epilepsy, and autism spectrum disorder. Early life predictors of chronic health and developmental disorders among survivors of extreme prematurity is the focus of this review of findings from the Extremely Low Gestational Age Newborn (ELGAN) Study and similar cohorts. The ELGAN cohort was recruited in the years 2002–2004, at 14 hospitals in five states in the United States, and has been evaluated through 15 years of age, although this review will be restricted to findings through 10 years of follow-up. The premises of this review are that: (1) more effective promotion of positive health outcomes among individuals born preterm depends on greater understanding of risk factors for chronic disorders and the mechanisms that link these risk factors to adverse outcomes and (2) interventions that target early life risk factors hold greater potential benefit than those which target factors later in life. For this reason, we focus on prenatal risk factors and highlight biological mechanisms including placenta reprogramming and perinatal systemic inflammation that may be targeted to interrupt the relationships between exposures during fetal life to childhood health outcomes.
Over the past half century in which advances in obstetrical and neonatal care resulted in dramatic improvements in the survival of babies born extremely preterm, the major focus of epidemiological studies (both observational and interventional) has been neonatal morbidities attributable to immaturity of multiple organs, including lungs, brain, eyes, kidneys, and gastrointestinal tract.1 Related to this immaturity are high risks of acute disorders, such as respiratory distress, necrotizing enterocolitis (NEC), sepsis, and more chronic conditions such as bronchopulmonary dysplasia (BPD), perinatal brain injury, and severe retinopathy of prematurity (ROP).2,3 Each of these neonatal morbidities is predictive of adverse neurodevelopmental impairment.3–11 To the extent that neonatal morbidities lie on a causal pathway connecting extremely preterm birth to adverse child health and neurodevelopmental outcomes, interventions that target antecedents of BPD, NEC, sepsis, severe brain injury, and ROP have the potential for increasing the likelihood that an extremely preterm birth will remain free from chronic health or developmental problems.
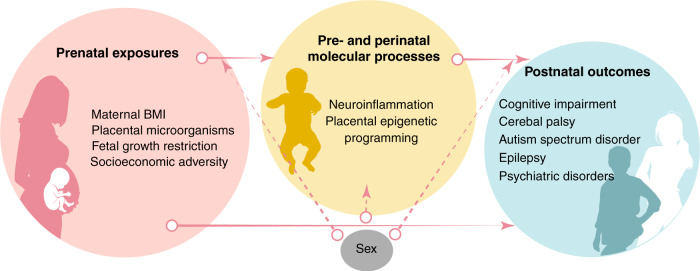
In comparison to the neonatal morbidities, prenatal antecedents of adverse health and developmental outcomes among extremely preterm neonates have been relatively understudied. Prenatal risk factors for adverse outcomes potentially could be linked to both neonatal morbidity, as a mediating factor, and also could have direct links to adverse outcomes, without involving neonatal morbidity as mediator. As examples of prenatal antecedents of neonatal morbidities and/or chronic disorders of health or development, we will review four that have been investigated within the ELGAN cohort: (1) fetal growth restriction (FGR), (2) maternal obesity, (3) placental microorganisms, and (4) socioeconomic adversity (Fig. 1). By considering ELGAN Study findings about these four antecedents, we also illustrate that two mechanisms, namely, placental reprogramming and perinatal inflammation, may underlie the developmental origins of health and disease (DOHaD). By modifying the prevalence of these antecedents and/or targeting mechanistic links between early life predictors and later life outcomes, health and development can be optimized for individuals born extremely preterm.
Fig. 1. Early life risk factors and mechanisms underlying inter-individual differences in neurodevelopment later in life.
Arrows represent associations between pre- or perinatal risk factors, early in life health outcomes (birth–4 months), and later in life health outcomes (middle childhood–early childhood) as observed in the literature. Solid arrows represent associations between risk factors and outcomes that are topics of discussion in this review. Dashed arrows represent links that are not addressed in the current review.
A detailed discussion of the methods used to identify neurodevelopment impairments is beyond the scope of this review, but can be found elsewhere.12,13 Nonetheless, it is important to emphasize that in many studies of neurodevelopmental impairment among extremely preterm infants, the follow-up period extended only through late infancy, when the predictive accuracy of assessments is at best modest.14,15 Thus, an important research priority are studies of adults born extremely preterm.16–18
Mechanism 1: perinatal inflammation and neurodevelopment
The central hypothesis of the ELGAN Study that perinatal inflammation contributes to the neurodevelopmental impairments, which disproportionately affect children born extremely preterm, had its origins >45 years ago when Floyd Gilles and Alan Leviton observed a 34-fold increase in the odds of autopsy-confirmed perinatal white matter damage among infants with postmortem bacteremia, despite the finding of no bacteria in their brains.19 In preclinical models, animals treated with lipopolysaccharide (LPS) or viral mimetics develop systemic inflammation that can then lead to microglial activation and increased local transcription of chemokines in the brain.20–22 Chemokine induction leads to a transient recruitment of neutrophils and monocytes to the brain, and accumulation of macrophages and other immune cells that can extravasate from blood vessels and/or cerebrospinal fluid to infiltrate the brain parenchyma.23 Experimental (e.g., LPS-induced) neuroinflammation leads to neonatal cerebral white matter damage in kittens, rodents, rabbits, dogs, pigs, and non-human primate.21,22,24–26 The propensity to develop neuroinflammation in association with peripheral immune activation is influenced by genetic background, sex, and postnatal age.27
In humans, antecedents of perinatal brain injury and resultant neurodevelopmental impairments that might be mediated by systemic inflammation include maternal factors, such as socioeconomic status (SES) indicators,28 pre-pregnancy obesity,29,30 and FGR;31 perinatal infections, such as sepsis;32 tissue damage, as can occur with NEC33 and ventilator-induced lung injury;34 and pre- and postnatal exposure to environmental chemicals, as well as treatments given to neonates as a component of neonatal intensive care.28,34,35 The heightened inflammatory response to LPS in male neonates, as compared to females, might contribute to males’ higher risk of neurodevelopmental impairments.36
As has been found in preclinical models, human neonates with sustained or multiple intermittent episodes of inflammation are at increased risk for perinatal brain injury as compared to those with a single episode of inflammation.25 Consistent with this possibility is the finding that in the ELGAN Study, infants with both placenta inflammation and neonatal systemic inflammation were more likely to develop brain ultrasound indicators of cerebral white matter damage and developmental impairments at 24 months of age than were infants who had only placenta inflammation or only neonatal systemic inflammation.37 Similarly, among infants born before 33 weeks of gestation, three or more infections was associated with a higher likelihood of magnetic resonance imaging-detectable white matter abnormalities lower scores on developmental assessments.38 A molecular mechanism that might underlie the apparent “sensitization” of the brain by an initial exposure to inflammation is the increased expression of Toll-like receptors that follows LPS treatment,39 and, as a consequence, increased sensitivity to inflammation in life.40
Additional, albeit indirect, evidence that early life inflammation contributes to the risk of neurodevelopmental impairment comes from studies of genetic polymorphisms in inflammatory genes. Single-nucleotide polymorphisms (SNPs) in the genes for interleukin-8 (IL-8),41 tumor necrosis factor-α (TNF-α), and IL-1β42 have been associated with an increased risk of cerebral palsy among very preterm infants, and a SNP in the mannose-binding lectin gene has been associated with worse neurodevelopmental outcome.43 Consistent with inflammation having a mediating role between early life antecedents and neurodevelopment outcome, the recovery of Lactobacillus from placenta was associated with variation in placental DNA CpG methylation of inflammation-related genes,44 decreased neonatal systemic inflammation,45 and decreased risk of cognitive impairment among children born extremely preterm.46
Particularly compelling evidence of a relationship between perinatal inflammation and disrupted brain development comes from studies of the relationship of biomarkers of inflammation, such as TNF-α and IL-1, -6, -8, and -9. In one of the earliest biomarkers studies, neonates born at term with elevated levels of inflammatory biomarkers in the first several postnatal days were more likely to subsequently develop cerebral palsy, with the strongest association being with spastic diplegia.47 In a meta-analysis of 37 studies, the protein biomarkers most consistently predictive of neurodevelopmental impairments were IL-6, IL-8, and, to a lesser extent, TNF-α and IL-1β.48 In the ELGAN Study, elevated levels in neonatal blood of multiple inflammation biomarkers, most prominently IL-8, were associated with neonatal brain ultrasound indicators of white matter damage,49 cognitive impairment,50 cerebral palsy,51 autism spectrum disorder,52 and attention deficit hyperactivity disorder symptoms,53 as well as decreased cortical and deep gray matter, cerebellar, and brainstem volumes as measured with magnetic resonance at 10 years of age.54
A detailed description of the cellular and molecular events that could explain a causal relationship between perinatal inflammation and neurodevelopmental impairments is beyond the scope of this review, and the interested reader is referred to several excellent reviews of this topic.55–57 Briefly, systemic inflammation disrupts the blood–brain barrier and allows movement of molecular inflammation mediators into the brain or by stimulating secretion from endothelial cells of inflammatory mediators into the brain parenchyma. Microglial activation to an immune-responsive state not only increases microglial production of molecules that are toxic to neighboring neurons but also detracts from developmental microglial functions that support axonal connectivity and synaptic formation.55,58 The neuroimmune system is central not only to neuronal injury but also to brain plasticity and manifests as both reparative and pathological activity.56 One implication of these dual roles of neuroinflammation is that great caution is needed when designing therapeutic interventions targeting neuroinflammation.
Mechanism 2: disrupted placental programming and neurodevelopment
Evidence is growing that the mechanisms by which maternal exposure to stressors is associated with later life neurobehavioral dysfunction likely initiate within the placenta. As such, placental development and function are important features of the DOHaD framework. As the conduit between the mother and fetus, the placenta serves as a transient organ, yet the master regulator of fetal growth and development through numerous functions, such as metabolism, neuroendocrine signaling, and immunologic control.59–61
In humans, the decidua, composed of maternal uterine and immune cells, controls the immunological tolerance of the embryo.62 Trophoblast cells of fetal origin predominate in the basal plate where they serve as the source for the synthesis and secretion of endocrine factors into both maternal and fetal circulations. In contrast, chorionic villous trophoblasts located between the maternal and fetal vasculature are critical for the exchange of oxygen, nutrients, and waste enabled through diffusion and macro- and micronutrient transporters.63 Disruption of these critical functions has adverse effects on fetal development, including the brain and primordial germ cells.
Sex-specific reprogramming occurs in response to maternal stress and manifests as sex differences in placental size, gene expression, and CpG methylation. Sex-specific placental abnormalities predict offspring outcome in pregnancies complicated by maternal asthma and preeclampsia.64–68
Evidence of a role for the placenta in relation to child health outcomes in ELGANs are several studies that have integrated placental programming, including gene expression and mechanisms that control gene expression, such as altered DNA (i.e., CpG) methylation signatures with later life health. Specifically, ELGAN researchers have provided evidence of the relationship between CpG methylation and gene expression of genes in critical biological pathways, including genes involved in the hypothalamic-pituitary-adrenal (HPA) axis and health outcomes in children born preterm.69,70 Specifically, placental CpG methylation levels of the glucocorticoid receptor gene, nuclear receptor subfamily group 3C member 1 (NR3C1) and brain-derived neurotropic factor (BDNF) were significantly associated with increased odds in developing moderate/severe adverse cognitive impairment at age 10 years.69 In terms of the mechanistic basis for this relationship, related to placental function, NR3C1 is highly expressed and plays a role in regulating fetal exposure to cortisol. BDNF has been shown to promote trophoblast growth, and cell survival during placental development. Low expression levels of BDNF in the placenta have been associated with pregnancy complications, such as preeclampsia and preterm birth. In support of these data, differences in the methylation and subsequent altered expression of NR3C1 and FKBP5 in the placenta have been associated with adverse neurobehavioral outcomes. CpG methylation in humans occurs at the fifth position of the pyrimidine ring of the cytosine residues within CpG sites to form 5-methylcytosines. The presence of multiple methylated CpG sites in CpG islands of promoters often causes stable silencing of genes,71 although this gene silencing can also be initiated by other mechanisms.71
The placental also serves as a sensor and transducer of environmental signals, such as prenatal exposure to tobacco,72–75 air pollution,76–90 and environmental pollutants,91–94 which have been tied to both neonatal morbidities and perinatal inflammation. Important questions remain in relation to the role of specific biological pathways in the placenta that when perturbed can lead to child health or disease. Through interrogation of molecular signatures in the placenta including the placental epigenomic and transcriptome, we anticipate that novel links between protective prenatal factors, placental molecular functions, and health outcomes in children will be identified.
Below, we discuss four antecedents of neurodevelopmental impairment for which there is at least preliminary evidence of associations with inflammation, placenta epigenetic variation, and neurodevelopmental impairment.
Antecedent 1: FGR
FGR and neurodevelopment
FGR, as reflected in unusually low birth weight for the infant’s gestational age, can arise from pathological conditions within the mother, fetus, or placenta.95 Among neonates born extremely preterm, FGR has been associated with an increased risk of BPD,96 NEC,97 and cerebral white matter injury (identified with ultrasound).98 The association between FGR and BPD is particularly strong among infants with relatively normal pulmonary function in the first 2 weeks of life, among whom the odds ratio (OR) for the association between FGR and BPD was 26 (95% confidence interval (CI) 7–95).96 Interventions that have the potential for mitigating risks associated with FGR include aspirin for women at high risk of developing preeclampsia and calcium supplementation among women with low calcium intake (<800 mg per day).99
In the ELGAN cohort, the disorder that was most strongly associated with FGR was autism spectrum disorder without intellectual deficit.100 FGR also was associated with severe early cognitive impairment (a Bayley Scale Mental Development Index <55) at 2 years of age,101 although the association between FGR and a low intelligence quotient at 10 years of age was not statistically significant when adjusted for confounders.102 Among girls, FGR was associated with delayed motor development at 2 years of age,103 but was not associated with an increased risk of cerebral palsy.98 Sex differences in outcome of fetuses with FGR might result from sex-specific changes in the structure and function of the placenta in response to processes that underlie FGR.104
Fetal growth restriction and inflammation-related proteins
Potential molecular mechanisms that might underlie associations between fetal growth restriction and BPD, NEC, and cerebral white matter include insufficiency of growth factors, such as insulin-like growth factor-1, and neurotrophic factors, such as neurotrophin-4 and brain-derived neurotrophic factor,105,106 as well as increased expression, during the first postnatal month, of inflammatory proteins in neonatal blood, including cytokines (IL-1β, IL-6, TNF-α, and IL-8), chemokines (monocyte chemoattractant protein-4), adhesion molecules (E-selectin and intracellular adhesion molecule-1 and -3), and matrix metalloproteinase-9.31 Although ELGAN Study infants with FGR, as well as those born to mothers with preeclampsia, were less likely than infants without FGR to have systemic inflammation on the first postnatal day, they were more likely to have systemic inflammation in the second week of life.31 In addition, FGR could sensitize the fetal brain so that the adverse effect of postnatal inflammation is accentuated. Consistent with a “two-hit” model of pathogenesis,107,108 among ELGAN cohort infants with FGR, those with elevated blood concentrations of IL-1β, TNF-α, or IL-8 during the first 2 postnatal weeks were at higher risk of severe early cognitive impairment as compared to FGR infants without systemic inflammation and non-FGR infants with systemic inflammation.109
Fetal growth restriction and placental programming
Among pregnancies delivered at term, differences in DNA CpG methylation have been found when comparing placenta from neonates with and without fetal growth restriction.110 However, in human studies it is not possible to definitively determine if altered profiles of DNA methylation are a response of the placenta to the intrauterine environment and/or growth restriction, or whether these methylation differences precede, and contribute to, growth restriction. In the ELGAN cohort, the most common pregnancy complication associated with FGR was preeclampsia.103 Preeclampsia is associated with differential methylation of genes in the transforming growth factor-β signaling pathway, a regulator of placental trophoblast invasion and migration.111 Another epigenetic mediator is microRNA, and in the ELGAN cohort, 268 miRNAs were identified as associated with birth weight,112 some of which regulate important biological pathways, including glycoprotein VI (the major receptor for collagen), human growth, and hepatocyte growth factor signaling. Environmental chemicals such as inorganic arsenic113 and cadmium114 are associated with epigenetic modifications that might mediate associations between these chemical exposures and fetal growth restriction.
Antecedent 2: maternal obesity
Maternal obesity and neurodevelopment
Over a third of all women of childbearing age in the United States are obese (BMI ≥ 30 kg/m2).115 Confirming findings from other cohorts,116–119 among ELGAN Study participants, newborns of obese mothers, as compared to those born to mothers with normal BMIs, were more likely to have Bayley Scales of Mental and Motor scale scores >3 standard deviations below the reference mean (mental: OR = 2.1; 95% CI 1.3, 3.5) (motor: OR = 1.7; 95% CI 1.1, 2.7) and these associations were more prominent in children who did not have intermittent or sustained systemic inflammation (mental: OR = 4.6; 95% CI 1.6, 14) (motor: OR = 3.7; 95% CI 1.5, 8.9).120 Similarly, based on evaluations at ten years of age, individuals in the ELGAN Study whose mothers were obese prior to pregnancy were more likely to have low scores on intelligence tests, measures of processing speed and visual fine motor control, and spelling achievement tests.121 However, ELGAN Study children who were born to mothers with pre-pregnancy obesity were not more likely to develop cerebral palsy.122
In studying associations between maternal obesity and offspring outcomes, a number of potential confounding factors should be considered. Obese women are more likely to experience adversities and exposures arising from low SES, and more often experience micronutrient deficiencies, emotional distress, and mental health dysfunctions.30 Studies of molecular mechanisms, such as inflammation and placenta programming, could increase understanding of the putative link between maternal obesity and offspring outcome.
Maternal obesity and inflammation
Systemic inflammation is one molecular mechanism that might contribute to the observed association between maternal obesity to less favorable neurodevelopmental outcomes. In the ELGAN cohort, among the pregnancies delivered as a result of maternal or fetal indications, such as preeclampsia or severe fetal growth restriction, infants born to mothers who were overweight (BMI >25 but <30) or obese (BMI ≥ 30) were more likely to have elevated levels of protein biomarkers of inflammation, such as C-reactive protein, E-selectin, intracellular adhesion molecule-3, and receptors for TNF and vascular endothelial growth factor.29 In addition, when assessed at 10 years of age, ELGAN individuals who were exposed to pre-pregnancy maternal overweight or obesity were more likely to be overweight or obese, outcomes that would be expected to result in a chronic proinflammatory state123 and could have deleterious effects on brain structure and function across the life span.30,124–129
Maternal obesity and placental programming
Disrupted placenta signaling is a second molecular mechanism that likely plays a role in early life programming of fetuses exposed to maternal obesity. Maternal obesity preceding or during pregnancy is associated with variations in DNA CpG methylation in umbilical cord blood.130,131 A study of siblings born either before or after their mothers underwent bariatric surgery for weight identified 5698 differentially methylated genes, with a disproportionate representation of glucoregulatory, inflammatory, and vascular disease genes.131 One of the largest studies (n = 9340) of the relationship of maternal obesity and offspring epigenetic provides robust evidence of associations between maternal adiposity and variations in newborn blood DNA methylation.132 In most of the CpG sites that were differentially methylated in cord blood, the association with maternal BMI was also found in blood collected during adolescence, suggesting persistence of epigenetic “marks”. About 90% of the associations between maternal BMI and offspring CpG methylation were most likely explained by shared mother–offspring genetic and postnatal environmental factors, but ~10% were most likely attributable to a causal intrauterine mechanism. Other molecular mechanisms that might mediate links between maternal obesity and offspring neurodevelopment include an increase in oxidative stress and altered maternal microbiome, both of which could alter placental immune and metabolic functions.133
Antecedent 3: placental microorganisms
Placenta microorganisms and neurodevelopment
Previously we have described the relationship between placental microorganisms and neurodevelopmental outcomes.134 Microorganisms in the placenta are associated with intrauterine infection and preterm labor.135–138 Pathogenic bacteria can colonize the placenta by hematogenous spread or invasion from the vagina.135 Although somewhat controversial, some researchers posit that even among uncomplicated pregnancies a placental microbiome exists, comprising non-pathogenic commensal microorganisms.139–141 Studies that utilized culture techniques optimized for detection of pathogenic organisms142 might fail to detect commensal organisms, while newer culture-independent techniques, such as 16S ribosomal RNA (rRNA) gene sequencing, can detect a more diverse set of organisms and less abundant organisms,143 but do not differentiate between living and dead bacteria and are susceptible to contamination from dust or commercial reagents.144,145 Notwithstanding this methodological concern, studies using 16S rRNA sequencing have detected microorganisms in the placenta that also are found in the vagina and oral cavity.139,146 In studies of extremely preterm births, conventional culture techniques detected non-pathogenic bacteria in placenta, but these results might not apply to normal pregnancies.147,148
In the ELGAN cohort, the presence of Ureaplasma urealyticum was associated with increased risk of brain ultrasound indicators of intraventricular hemorrhage and cerebral white matter injury.149 The presence of any aerobe in the placenta was associated with a 4-fold increase in the odds of diparesis, and the presence of two or more species of bacteria was associated with a 5.2-fold increase in odds. The recovery of any anaerobe or of two or more species of bacteria were associated with an approximate doubling of the odds of quadriparesis.150 Placental microorganisms were not associated with an increased risk of low scores on the Bayley Scales Mental Development Index, assessed at 2 years of age.151 However, assessments of the ELGAN cohort at 10 years of age indicated that recovery of U. urealyticum, Corynebacterium sp., Escherichia coli, or alpha-Streptococcus from placenta was associated with low scores on mathematics achievement tests, and recovery of U. urealyticum or Staphylococcus was associated with low scores on oral and written language tests.46 In contrast, recovery of Lactobacillus from placenta was associated with a lower risk of cognitive impairment and higher scores on language assessments.46
Placenta microorganisms and inflammation
In the ELGAN cohort, biopsies of the subamniotic placenta parenchyma were taken around the time of delivery and were cultured and evaluated for specific histologic patterns of inflammation in a blinded fashion. Excluding cases with prolonged membrane rupture, microorganisms were recovered from 41% of placentas. High-grade chorionic plate inflammation and fetal vasculitis were found more frequently in placentas from which the following organisms were recovered: Actinomyces, Prevotella bivia, Corynebacterium sp., E. coli, Peptostreptococcus magnus, multiple species of Streptococci, and Mycoplasma sp., including U. urealyticum.152
Consistent with the premise that perinatal inflammation is a mediator of associations between the presence in placenta of specific microorganisms and altered risks of neurodevelopmental impairments, in the ELGAN cohort, the presence in placenta of either U. urealyticum or alpha-Streptococcus was associated with an increased likelihood of elevated levels of IL-8 in neonatal blood on day 1, whereas the presence in placenta of Lactobacillus was associated with a lower likelihood of elevated IL-8 levels.45 Among the 28 inflammation-related proteins measured in neonatal blood, IL-8 was the most strongly associated with neurodevelopmental impairment.
Placenta microorganisms and placental programming
As discussed above, placental microorganisms are associated with both inflammation within the placenta and in the extremely preterm neonate’s blood. Within the placenta, inflammation might alter epigenetic processes, as suggested by the finding that acute chorioamnionitis is associated with altered DNA methylation in placentas from preterm deliveries, with DNA methylation profiles consistent with activation of the innate immune response.153 In the ELGAN cohort, placental microorganisms were associated with differential methylation within genes coding for growth and transcription factors, the immune response, and the inflammatory response, specifically the nuclear factor-κB pathway.44 These observations support the concept that microorganisms in the placenta could influence health and development in the offspring fetal development via altered placenta epigenetic programming. Further support for this concept is being sought in ongoing studies of the relationship of microorganisms to the placental transcriptome within the ELGAN cohort.
Antecedent 4: socioeconomic adversity
Socioeconomic adversity and neurodevelopment
In analyzing a child’s health, the concept of socioeconomic adversity illustrates aspects of the network of disadvantages leading to poor health outcomes. In the ELGAN cohort, factors indicative of socioeconomic disadvantage are associated with short and long health outcomes.28,154–164 Indicators of mother’s low SES that are associated with an increased risk of executive dysfunctions include young age at the time of the delivery, not married, low level of educational achievement, eligibility for government-provided medical-care insurance, and smoking cigarettes during pregnancy.165 Low education status at time of birth is also associated with substantial neurodevelopmental impairment with scores ≥2 standard deviations below normative expectation.155 Conversely, maternal educational advancement in child’s first 10 years of life is associated with modestly improved neurocognitive outcomes, even when adjusting for confounders, including gestational age, fetal growth restriction, maternal IQ, and minority ethnic/racial status. In the ELGAN Study maternal educational status serves as a proxy measure of SES and is strongly associated with household income, as reflected by eligibility for public (government-provided) health insurance (i.e., Medicaid).155 Follow-up studies of adults born prematurely indicate that maternal socioeconomic hardship at birth are associated with worse cognitive outcomes.166–168
Socioeconomic adversity and inflammation
Recent research has focused on the potential biological mechanisms linking socioeconomic adversity and poor outcomes; much of this work has pointed to systemic inflammation.169 In the ELGAN Study, indicators of socioeconomic disadvantage (education) are associated with modestly increased risk of systemic inflammation in postnatal blood during the first postnatal month and with a slightly reduced risk of a neurotrophic signal, but do not confound relationships between inflammatory proteins and outcomes.170–174
Socioeconomic adversity and placental programming
Advances in the developmental origins of chronic illness suggest that multiple environmental stressors are linked to variations in fetal–placental development,175 which involve DNA that can alter gene expression and set pathways linked to later life illness.66,176–180 In utero exposure to environmental stressors (i.e., socioeconomic adversity) can alter the expression of HPA axis-associated genes.181,182 Furthermore, adversity in fetal life can shape the maturation of stress-regulating pathways, leading to altered stress responsivity during adulthood.183–187 Although associations between epigenetic changes and health outcomes have been established, the extent to which maternal socioeconomic adversity affects CpG methylation in extremely preterm children is rarely demonstrated. In an epigenome-wide DNA methylation in 426 placentas from ELGAN cohort, we found that DNA methylation in 33 CpG sites (representing 21 genes) associated with either a summative socioeconomic adversity cumulative score or individual component exposures, including marital status, maternal education, and food security.188 Placentas from female pregnancies showed more robust differential CpG methylation than placentas from male pregnancies. Maternal socioeconomic adversity was associated with differential methylation of genes involved in gene transcription and placental function potentially altering immunity and stress response. These findings suggest that socioeconomic adversity is associated with imprints on the epigenome and may be linked to biological embedding of socioeconomic adversity, which could affect long-term child outcomes.188
Summary
Early life factors that influence health and development of individuals born extremely preterm include prenatal factors such as maternal BMI, placental microorganisms, fetal growth restrictions, and lower maternal socioeconomic status.101,120,121,189–192 The body of evidence reviewed here suggests at least two broad causal pathways between early life predictors and childhood outcomes. These factors, which are not mutually exclusive, include: (1) increased neonatal systemic inflammation, which consistently has been associated with later life impairments; and (2) disrupted placental programming that may be controlled, at least in part, through epigenetic mechanisms.
Novel findings from the ELGAN cohort that support the hypothesis that placental CpG methylation is an intermediate linking prenatal exposures to later life neurodevelopmental outcomes include: (1) prenatal factors, such as maternal health and socioeconomic adversity, are predictive of placental CpG methylation,193 and (2) placental CpG methylation is predictive of neurodevelopmental outcomes.69,70 With regard to sex differences in outcome among individuals born extremely preterm, an important research question is whether increased susceptibility of males is mediated by sexual dimorphism in the placenta68 and/or greater susceptibility to neonatal morbidities and the systemic inflammation that accompanies these morbidities. Increased understanding of pathways and mechanisms associating early life factors to childhood outcomes could inform the design of interventions to improve childhood health and development for individuals born extremely preterm.
Acknowledgements
This review was supported by grants from the National Institutes of Health (NIH), the Office of the NIH Director (5UH3OD023348-05), the National Institute of Environmental Health Sciences (T32-ES007018), National Institute of Nursing Research (K23NR017898), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD092374).
Author contributions
J.T.B., H.H., H.P.S., T.M.O., and R.C.F. contributed to the content design of the review and provided critical edits to the manuscript.
Competing interests
The authors declare no competing interests.
Patient consent
Patient consent was required for participation in the Extremely Low Gestational Age Newborn Study (ELGAN) highlighted in this review.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Swamy GK, Østbye T, Skjærven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. JAMA. 2008;299:1429–1436. doi: 10.1001/jama.299.12.1429. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt B, et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 monthsresults from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289:1124–1129. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- 3.Bassler D, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics. 2009;123:313–318. doi: 10.1542/peds.2008-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hintz SR, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 5.Laughon M, et al. Chronic lung disease and the risk of developmental delay at two years of age in children born before 28 weeks postmenstrual age. Pediatrics. 2009;124:637–648. doi: 10.1542/peds.2008-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt B, et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months. JAMA. 2003;289:1124–1129. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- 7.Hintz, S. R. et al. Preterm neuroimaging and school-age cognitive outcomes. Pediatrics142, 10.1542/peds.2017-4058 (2018). [DOI] [PMC free article] [PubMed]
- 8.Van Marter LJ, et al. Does bronchopulmonary dysplasia contribute to the occurrence of cerebral palsy among infants born before 28 weeks of gestation? Arch. Dis. Child Fetal Neonatal Ed. 2011;96:F20–F29. doi: 10.1136/adc.2010.183012. [DOI] [PubMed] [Google Scholar]
- 9.Kuban KC, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J. Child Neurol. 2009;24:63–72. doi: 10.1177/0883073808321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Shea TM, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122:E662–E669. doi: 10.1542/peds.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hintz SR, et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135:e32–e42. doi: 10.1542/peds.2014-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Shea TM, Goldstein DJ. Follow-up data—their use in evidence-based decision-making. Clin. Perinatol. 2003;30:217–250. doi: 10.1016/s0095-5108(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 13.Marlow N. Measuring neurodevelopmental outcome in neonatal trials: a continuing and increasing challenge. Arch. Dis. Child Fetal Neonatal Ed. 2013;98:F554–F558. doi: 10.1136/archdischild-2012-302970. [DOI] [PubMed] [Google Scholar]
- 14.O’Shea TJ, et al. ELGAN Study Investigators. Accuracy of the Bayley-II Mental Development Index at 2 years as a predictor of cognitive impairment at school age among children born extremely preterm. J. Perinatol. 2018;38:908–916. doi: 10.1038/s41372-017-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hack M, et al. Improved cognitive function of extreme-low-birth-weight (ELBW, < 1kg) children at age 8 years: poor predictive validity of the Bayley II Mental Developmental Index (MDI) Pediatr. Res. 2004;55:504A–504A. [Google Scholar]
- 16.O’Reilly, H., Johnson, S., Ni, Y., Wolke, D. & Marlow, N. Neuropsychological outcomes at 19 years of age following extremely preterm birth. Pediatrics145, 10.1542/peds.2019-2087 (2020). [DOI] [PubMed]
- 17.Linsell L, et al. Trajectories of behavior, attention, social and emotional problems from childhood to early adulthood following extremely preterm birth: a prospective cohort study. Eur. Child Adolesc. Psychiatry. 2019;28:531–542. doi: 10.1007/s00787-018-1219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson S, O’Reilly H, Ni Y, Wolke D, Marlow N. Psychiatric symptoms and disorders in extremely preterm young adults at 19 years of age and longitudinal findings from middle childhood. J. Am. Acad. Child Adolesc. Psychiatry. 2019;58:820–826. e826. doi: 10.1016/j.jaac.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Leviton A, Gilles F, Neff R, Yaney P. Multivariate analysis of risk of perinatal telencephalic leucoencephalopathy. Am. J. Epidemiol. 1976;104:621–626. doi: 10.1093/oxfordjournals.aje.a112340. [DOI] [PubMed] [Google Scholar]
- 20.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann. Neurol. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 21.Hagberg H, et al. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 2015;11:192–208. doi: 10.1038/nrneurol.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Favrais G, et al. Systemic inflammation disrupts the developmental program of white matter. Ann. Neurol. 2011;70:550–565. doi: 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- 23.Thomson CA, McColl A, Graham GJ, Cavanagh J. Sustained exposure to systemic endotoxin triggers chemokine induction in the brain followed by a rapid influx of leukocytes. J. Neuroinflamm. 2020;17:94. doi: 10.1186/s12974-020-01759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilles F, Leviton A, Kerr CS. Endotoxin leucoencephalopathy in the telencephalon of the newborn kitten. J. Neurol. Sci. 1976;27:183–191. doi: 10.1016/0022-510x(76)90060-5. [DOI] [PubMed] [Google Scholar]
- 25.Fleiss B, et al. Inflammation-induced sensitization of the brain in term infants. Dev. Med. Child Neurol. 2015;57:17–28. doi: 10.1111/dmcn.12723. [DOI] [PubMed] [Google Scholar]
- 26.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal infection regulates BDNF and NGF expression in fetal and neonatal brain and maternal-fetal unit of the rat. J. Neuroimmunol. 2003;138:49–55. doi: 10.1016/s0165-5728(03)00095-x. [DOI] [PubMed] [Google Scholar]
- 27.Bruce M, et al. Acute peripheral immune activation alters cytokine expression and glial activation in the early postnatal rat brain. J. Neuroinflamm. 2019;16:200. doi: 10.1186/s12974-019-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leviton A, et al. Socioeconomic status and early blood concentrations of inflammation-related and neurotrophic proteins among extremely preterm newborns. PLoS ONE. 2019;14:e0214154. doi: 10.1371/journal.pone.0214154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Burg JW, et al. Is maternal obesity associated with sustained inflammation in extremely low gestational age newborns? Early Hum. Dev. 2013;89:949–955. doi: 10.1016/j.earlhumdev.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 30.van der Burg JW, et al. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr. Res. 2016;79:3–12. doi: 10.1038/pr.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McElrath TF, Allred EN, Van ML, Fichorova RN, Leviton A. Perinatal systemic inflammatory responses of growth-restricted preterm newborns. Acta Paediatr. 2013;102:e439–e442. doi: 10.1111/apa.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leviton A, et al. Systemic responses of preterm newborns with presumed or documented bacteraemia. Acta Paediatr. 2012;101:355–359. doi: 10.1111/j.1651-2227.2011.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin CR, Bellomy M, Allred EN, Fichorova RN, Leviton A. Systemic inflammation associated with severe intestinal injury in extremely low gestational age newborns. Fetal Pediatr. Pathol. 2013;32:222–234. doi: 10.3109/15513815.2012.721477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bose C, et al. Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine. 2013;61:315–322. doi: 10.1016/j.cyto.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bilbo SD, Block CL, Bolton JL, Hanamsagar R, Tran PK. Beyond infection—maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 2018;299:241–251. doi: 10.1016/j.expneurol.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim-Fine S, et al. Male gender promotes an increased inflammatory response to lipopolysaccharide in umbilical vein blood. J. Matern. Fetal Neonatal Med. 2012;25:2470–2474. doi: 10.3109/14767058.2012.684165. [DOI] [PubMed] [Google Scholar]
- 37.Yanni D, et al. Both antenatal and postnatal inflammation contribute information about the risk of brain damage in extremely preterm newborns. Pediatr. Res. 2017;82:691–696. doi: 10.1038/pr.2017.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glass TJA, et al. Multiple postnatal infections in newborns born preterm predict delayed maturation of motor pathways at term-equivalent age with poorer motor outcomes at 3 years. J. Pediatr. 2018;196:91–97. e91. doi: 10.1016/j.jpeds.2017.12.041. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, et al. Lipopolysaccharide sensitizes neonatal hypoxic-ischemic brain injury in a MyD88-dependent manner. J. Immunol. 2009;183:7471–7477. doi: 10.4049/jimmunol.0900762. [DOI] [PubMed] [Google Scholar]
- 40.Bilbo SD, et al. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav. Neurosci. 2005;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- 41.Kallankari H, et al. Cerebral palsy and polymorphism of the chemokine CCL18 in very preterm children. Neonatology. 2015;108:124–129. doi: 10.1159/000430765. [DOI] [PubMed] [Google Scholar]
- 42.Kapitanovic Vidak H, Catela Ivkovic T, Jokic M, Spaventi R, Kapitanovic S. The association between proinflammatory cytokine polymorphisms and cerebral palsy in very preterm infants. Cytokine. 2012;58:57–64. doi: 10.1016/j.cyto.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Auriti C, et al. MBL2 gene polymorphisms increase the risk of adverse neurological outcome in preterm infants: a preliminary prospective study. Pediatr. Res. 2014;76:464–469. doi: 10.1038/pr.2014.118. [DOI] [PubMed] [Google Scholar]
- 44.Tomlinson MS, et al. Microorganisms in the human placenta are associated with altered CpG methylation of immune and inflammation-related genes. PLoS ONE. 2017;12:e0188664. doi: 10.1371/journal.pone.0188664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fichorova RN, et al. Maternal microbe-specific modulation of inflammatory response in extremely low-gestational-age newborns. Mbio. 2011;2:e00280–00210. doi: 10.1128/mBio.00280-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomlinson MS, et al. Neurocognitive and social-communicative function of children born very preterm at 10 years of age: associations with microorganisms recovered from the placenta parenchyma. J. Perinatol. 2020;40:306–315. doi: 10.1038/s41372-019-0505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann. Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 48.Nist MD. & Pickler, R. H. An integrative review of cytokine/chemokine predictors of neurodevelopment in preterm infants. Biol. Res. Nurs. 2019;21:366–376. doi: 10.1177/1099800419852766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leviton A, et al. Circulating biomarkers in extremely preterm infants associated with ultrasound indicators of brain damage. Eur. J. Paediatr. Neurol. 2018;22:440–450. doi: 10.1016/j.ejpn.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuban KJ. Extremely Low Gestational Age Newborn (ELGAN) Study Investigators. Circulating inflammatory-associated proteins in the first month of life and cognitive impairment at age 10 years in children born extremely preterm. J. Pediatr. 2017;180:116–123. doi: 10.1016/j.jpeds.2016.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuban KC, et al. Systemic inflammation and cerebral palsy risk in extremely preterm infants. J. Child Neurol. 2014;29:1692–1698. doi: 10.1177/0883073813513335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korzeniewski SJ, Allred EN, O’Shea TM, Leviton A, Kuban KCK. Elevated protein concentrations in newborn blood and the risks of autism spectrum disorder, and of social impairment, at age 10 years among infants born before the 28th week of gestation. Transl. Psychiatry. 2018;8:1–10. doi: 10.1038/s41398-018-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allred EN, et al. Systemic inflammation during the first postnatal month and the risk of attention deficit hyperactivity disorder characteristics among 10 year-old children born extremely preterm. J. Neuroimmune Pharmacol. 2017;12:531–543. doi: 10.1007/s11481-017-9742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuban KCK, et al. Association of circulating proinflammatory and anti-inflammatory protein biomarkers in extremely preterm born children with subsequent brain magnetic resonance imaging volumes and cognitive function at age 10 years. J. Pediatr. 2019;210:81–90. e83. doi: 10.1016/j.jpeds.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fleiss B, Gressens P, Stolp HB. Cortical gray matter injury in encephalopathy of prematurity: link to neurodevelopmental disorders. Front. Neurol. 2020;11:575. doi: 10.3389/fneur.2020.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Reilly ML, Tom VJ. Neuroimmune system as a driving force for plasticity following CNS injury. Front. Cell Neurosci. 2020;14:187. doi: 10.3389/fncel.2020.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bokobza C, et al. Neuroinflammation in preterm babies and autism spectrum disorders. Pediatr. Res. 2019;85:155–165. doi: 10.1038/s41390-018-0208-4. [DOI] [PubMed] [Google Scholar]
- 58.Michels M, et al. The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav. Immun. 2015;43:54–59. doi: 10.1016/j.bbi.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Kay, H. H., Nelson, D. M. & Wang, Y. M. D. The Placenta: From Development To Disease. (Wiley-Blackwell, 2011).
- 60.Gude NM, Roberts CT, Kalionis B, King RG. Growth and function of the normal human placenta. Thrombosis Res. 2004;114:397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 61.Sahay, A., Sundrani, D. & Joshi, S. In Vitamins and Hormones Vol. 104, 243–261 (Elsevier, 2017). [DOI] [PubMed]
- 62.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat. Med. 2013;19:548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- 63.Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- 64.Buckberry S, Bianco-Miotto T, Bent SJ, Dekker GA, Roberts CT. Integrative transcriptome meta-analysis reveals widespread sex-biased gene expression at the human fetal-maternal interface. Mol. Hum. Reprod. 2014;20:810–819. doi: 10.1093/molehr/gau035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl.):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 66.Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol. Sex. Differences. 2013;4:5. doi: 10.1186/2042-6410-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc. Natl Acad. Sci. USA. 2006;103:5478–5483. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin, E. et al. Sexual epigenetic dimorphism in the human placenta: implications for susceptibility during the prenatal period. Epigenomics9, 267–278 (2017). [DOI] [PMC free article] [PubMed]
- 69.Meakin, C. J. et al. Placental CpG methylation of HPA-axis genes is associated with cognitive impairment at age 10 among children born extremely preterm. Horm. Behav.10.1016/j.yhbeh.2018.02.007 (2018). [DOI] [PMC free article] [PubMed]
- 70.Tilley SK, et al. Placental CpG methylation of infants born extremely preterm predicts cognitive impairment later in life. PLoS ONE. 2018;13:e0193271. doi: 10.1371/journal.pone.0193271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 72.Indredavik MS, Brubakk AM, Romundstad P, Vik T. Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatr. 2007;96:377–382. doi: 10.1111/j.1651-2227.2006.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim KM, et al. Associations between urinary cotinine and symptoms of attention deficit/hyperactivity disorder and autism spectrum disorder. Environ. Res. 2018;166:481–486. doi: 10.1016/j.envres.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 74.Caramaschi D, et al. Maternal smoking during pregnancy and autism: using causal inference methods in a birth cohort study. Transl. Psychiatry. 2018;8:262. doi: 10.1038/s41398-018-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohamed NN, Loy SL, Lim PY, Al Mamun A, Jan Mohamed HJ. Early life secondhand smoke exposure assessed by hair nicotine biomarker may reduce children’s neurodevelopment at 2years of age. Sci. Total Environ. 2018;610-611:147–153. doi: 10.1016/j.scitotenv.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 76.Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles county, California. Environ. Health Perspect. 2013;121:380–386. doi: 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Transl. Neurosci. 2016;7:24–30. doi: 10.1515/tnsci-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costa, L. G., Cole, T. B., Dao, K., Chang, Y. C. & Garrick, J. M. Developmental impact of air pollution on brain function. Neurochem. Int.10.1016/j.neuint.2019.104580 (2019). [DOI] [PMC free article] [PubMed]
- 79.Dix-Cooper L, Eskenazi B, Romero C, Balmes J, Smith KR. Neurodevelopmental performance among school age children in rural Guatemala is associated with prenatal and postnatal exposure to carbon monoxide, a marker for exposure to woodsmoke. Neurotoxicology. 2012;33:246–254. doi: 10.1016/j.neuro.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Ehsanifar M, et al. Prenatal exposure to diesel exhaust particles causes anxiety, spatial memory disorders with alters expression of hippocampal pro-inflammatory cytokines and NMDA receptor subunits in adult male mice offspring. Ecotoxicol. Environ. Saf. 2019;176:34–41. doi: 10.1016/j.ecoenv.2019.03.090. [DOI] [PubMed] [Google Scholar]
- 81.Ehsanifar M, et al. Exposure to nanoscale diesel exhaust particles: oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicol. Environ. Saf. 2019;168:338–347. doi: 10.1016/j.ecoenv.2018.10.090. [DOI] [PubMed] [Google Scholar]
- 82.Forns J, et al. Air pollution exposure during pregnancy and symptoms of attention deficit and hyperactivity disorder in children in Europe. Epidemiology. 2018;29:618–626. doi: 10.1097/EDE.0000000000000874. [DOI] [PubMed] [Google Scholar]
- 83.Guxens M, et al. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol. Psychiatry. 2018;84:295–303. doi: 10.1016/j.biopsych.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 84.Jo H, et al. Sex-specific associations of autism spectrum disorder with residential air pollution exposure in a large Southern California pregnancy cohort. Environ. Pollut. 2019;254:113010. doi: 10.1016/j.envpol.2019.113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lam J, et al. A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PLoS ONE. 2016;11:e0161851. doi: 10.1371/journal.pone.0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lertxundi A, et al. Prenatal exposure to PM2.5 and NO2 and sex-dependent infant cognitive and motor development. Environ. Res. 2019;174:114–121. doi: 10.1016/j.envres.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 87.Lett, L. A., Stingone, J. A. & Claudio, L. The combined influence of air pollution and home learning environment on early cognitive skills in children. Int. J. Environ. Res. Public Health14, 10.3390/ijerph14111295 (2017). [DOI] [PMC free article] [PubMed]
- 88.Oudin A, et al. Prenatal exposure to air pollution as a potential risk factor for autism and ADHD. Environ. Int. 2019;133:105149. doi: 10.1016/j.envint.2019.105149. [DOI] [PubMed] [Google Scholar]
- 89.Perera F, Ashrafi A, Kinney P, Mills D. Towards a fuller assessment of benefits to children’s health of reducing air pollution and mitigating climate change due to fossil fuel combustion. Environ. Res. 2019;172:55–72. doi: 10.1016/j.envres.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 90.Ritz, B. et al. Air pollution and autism in Denmark. Environ. Epidemiol. 2, 10.1097/EE9.0000000000000028 (2018). [DOI] [PMC free article] [PubMed]
- 91.Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 92.Bellinger DC. Prenatal exposures to environmental chemicals and children’s neurodevelopment: an update. Saf. Health Work. 2013;4:1–11. doi: 10.5491/SHAW.2013.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fruh V, et al. Prenatal lead exposure and childhood executive function and behavioral difficulties in project viva. Neurotoxicology. 2019;75:105–115. doi: 10.1016/j.neuro.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weiss B, Bellinger DC. Social ecology of children’s vulnerability to environmental pollutants. Environ. Health Perspect. 2006;114:1479–1485. doi: 10.1289/ehp.9101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meler E, Sisterna S, Borrell A. Genetic syndromes associated with isolated fetal growth restriction. Prenat. Diagn. 2020;40:432–446. doi: 10.1002/pd.5635. [DOI] [PubMed] [Google Scholar]
- 96.Laughon M, et al. Antecedents of chronic lung disease following three patterns of early respiratory disease in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 2011;96:F114–F120. doi: 10.1136/adc.2010.182865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rose AT, Patel RM. A critical analysis of risk factors for necrotizing enterocolitis. Semin. Fetal Neonatal Med. 2018;23:374–379. doi: 10.1016/j.siny.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McElrath TF, et al. Maternal antenatal complications and the risk of neonatal cerebral white matter damage and later cerebral palsy in children born at an extremely low gestational age. Am. J. Epidemiol. 2009;170:819–828. doi: 10.1093/aje/kwp206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Poon LC, et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019;145:1–33. doi: 10.1002/ijgo.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Joseph RM, et al. Extremely low gestational age and very low birthweight for gestational age are risk factors for autism spectrum disorder in a large cohort study of 10-year-old children born at 23–27 weeks’ gestation. Am. J. Obstet. Gynecol. 2017;216:304 e301–304.e316. doi: 10.1016/j.ajog.2016.11.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Helderman JB, et al. Antenatal antecedents of cognitive impairment at 24 months in extremely low gestational age newborns. Pediatrics. 2012;129:494–502. doi: 10.1542/peds.2011-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Korzeniewski SJ, et al. Neurodevelopment at age 10 years of children born< 28 weeks with fetal growth restriction. Pediatrics. 2017;140:e20170697. doi: 10.1542/peds.2017-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Streimish IG, et al. Birth weight- and fetal weight-growth restriction: Impact on neurodevelopment. Early Hum. Dev. 2012;88:765–771. doi: 10.1016/j.earlhumdev.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sundrani DP, Roy SS, Jadhav AT, Joshi SR. Sex-specific differences and developmental programming for diseases in later life. Reprod. Fertil. Dev. 2017;29:2085–2099. doi: 10.1071/RD16265. [DOI] [PubMed] [Google Scholar]
- 105.Leviton A, et al. Early postnatal IGF-1 and IGFBP-1 blood levels in extremely preterm infants: relationships with indicators of placental insufficiency and with systemic inflammation. Am. J. Perinatol. 2019;36:1442–1452. doi: 10.1055/s-0038-1677472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leviton A, et al. Antecedents and correlates of blood concentrations of neurotrophic growth factors in very preterm newborns. Cytokine. 2017;94:21–28. doi: 10.1016/j.cyto.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Korzeniewski SJ, et al. A “multi-hit” model of neonatal white matter injury: cumulative contributions of chronic placental inflammation, acute fetal inflammation and postnatal inflammatory events. J. Perinat. Med. 2014;42:731–743. doi: 10.1515/jpm-2014-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr. Res. 2005;58:112–116. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 109.Leviton A, et al. Two-hit model of brain damage in the very preterm newborn: small for gestational age and postnatal systemic inflammation. Pediatr. Res. 2013;73:362–370. doi: 10.1038/pr.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Banister CE, et al. Infant growth restriction is associated with distinct patterns of DNA methylation in human placentas. Epigenetics. 2011;6:920–927. doi: 10.4161/epi.6.7.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martin E, et al. Epigenetics and preeclampsia: defining functional epimutations in the preeclamptic placenta related to the TGF-beta pathway. PLoS ONE. 2015;10:e0141294. doi: 10.1371/journal.pone.0141294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Payton A, et al. Placental genomic and epigenomic signatures associated with infant birth weight highlight mechanisms involved in collagen and growth factor signaling. Reprod. Toxicol. 2020;96:221–230. doi: 10.1016/j.reprotox.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Winterbottom EF, et al. Transcriptome-wide analysis of changes in the fetal placenta associated with prenatal arsenic exposure in the New Hampshire Birth Cohort Study. Environ. Health. 2019;18:100. doi: 10.1186/s12940-019-0535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Everson TM, et al. Placental expression of imprinted genes, overall and in sex-specific patterns, associated with placental cadmium concentrations and birth size. Environ. Health Perspect. 2019;127:57005. doi: 10.1289/EHP4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 116.Craig WY, Palomaki GE, Neveux LM, Haddow JE. Maternal body mass index during pregnancy and offspring neurocognitive development. Obstet. Med. 2013;6:20–25. doi: 10.1177/1753495X12472643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hinkle SN, et al. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int. J. Obes. 2012;36:1312–1319. doi: 10.1038/ijo.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krakowiak P, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121–e1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Lieshout RJ, Schmidt LA, Robinson M, Niccols A, Boyle MH. Maternal pre-pregnancy body mass index and offspring temperament and behavior at 1 and 2 years of age. Child Psychiatry Hum. Dev. 2013;44:382–390. doi: 10.1007/s10578-012-0332-z. [DOI] [PubMed] [Google Scholar]
- 120.van der Burg JW, et al. Maternal obesity and development of the preterm newborn at 2 years. Acta Paediatr. 2015;104:900–903. doi: 10.1111/apa.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jensen, E. T. et al. The relationship of maternal prepregnancy body mass index and pregnancy weight gain to neurocognitive function at age 10 years among children born extremely preterm. J. Pediatr.10.1016/j.jpeds.2017.02.064 (2017). [DOI] [PMC free article] [PubMed]
- 122.van der Burg JW, et al. Are extremely low gestational age newborns born to obese women at increased risk of cerebral palsy at 2 years? J. Child Neurol. 2018;33:216–224. doi: 10.1177/0883073817751303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Singer K, Eng DS, Lumeng CN, Gebremariam A, J ML. The relationship between body fat mass percentiles and inflammation in children. Obesity. 2014;22:1332–1336. doi: 10.1002/oby.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stillman CM, Weinstein AM, Marsland AL, Gianaros PJ, Erickson KI. Body-brain connections: the effects of obesity and behavioral interventions on neurocognitive aging. Front. Aging Neurosci. 2017;9:115. doi: 10.3389/fnagi.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Smith E, Hay P, Campbell L, Trollor JN. A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes. Rev. 2011;12:740–755. doi: 10.1111/j.1467-789X.2011.00920.x. [DOI] [PubMed] [Google Scholar]
- 126.Miller AL, Lee HJ, Lumeng JC. Obesity-associated biomarkers and executive function in children. Pediatr. Res. 2015;77:143–147. doi: 10.1038/pr.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guillemot-Legris O, Muccioli GG. Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci. 2017;40:237–253. doi: 10.1016/j.tins.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 128.Li Y, Dai Q, Jackson JC, Zhang J. Overweight is associated with decreased cognitive functioning among school-age children and adolescents. Obesity. 2008;16:1809–1815. doi: 10.1038/oby.2008.296. [DOI] [PubMed] [Google Scholar]
- 129.Hanc T, et al. Attention-deficit/hyperactivity disorder is related to decreased weight in the preschool period and to increased rate of overweight in school-age boys. J. Child Adolesc. Psychopharmacol. 2015;25:691–700. doi: 10.1089/cap.2014.0157. [DOI] [PubMed] [Google Scholar]
- 130.Liu X, et al. Maternal preconception body mass index and offspring cord blood DNA methylation: exploration of early life origins of disease. Environ. Mol. Mutagen. 2014;55:223–230. doi: 10.1002/em.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guenard F, et al. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc. Natl Acad. Sci. USA. 2013;110:11439–11444. doi: 10.1073/pnas.1216959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sharp GC, et al. Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum. Mol. Genet. 2017;26:4067–4085. doi: 10.1093/hmg/ddx290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cirulli, F., Musillo, C. & Berry, A. Maternal obesity as a risk factor for brain development and mental health in the offspring. Neuroscience. 10.1016/j.neuroscience.2020.01.023 (2020). [DOI] [PubMed]
- 134.Tomlinson, M. S. et al. Microorganisms in the placenta: links to early-life inflammation and neurodevelopment in children. Clin. Microbiol. Rev.32, 10.1128/CMR.00103-18 (2019). [DOI] [PMC free article] [PubMed]
- 135.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 136.Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am. J. Obstet. Gynecol. 2003;189:861–873. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- 137.Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am. J. Obstet. Gynecol. 2015;213:S53–S69. doi: 10.1016/j.ajog.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Emig OR, Napier JV, Brazie JV. Inflammation of the placenta. Correlation with prematurity and perinatal death. Obstet. Gynecol. 1961;17:743–750. [PubMed] [Google Scholar]
- 139.Aagaard K, et al. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014;6:237ra265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stout MJ, et al. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am. J. Obstet. Gynecol. 2013;208:226 e221–226. e227. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fox C, Eichelberger K. Maternal microbiome and pregnancy outcomes. Fertil. Steril. 2015;104:1358–1363. doi: 10.1016/j.fertnstert.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 142.Bursle E, Robson J. Non-culture methods for detecting infection. Aust. Prescr. 2016;39:171–175. doi: 10.18773/austprescr.2016.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang C, et al. Identification of low abundance microbiome in clinical samples using whole genome sequencing. Genome Biol. 2015;16:265. doi: 10.1186/s13059-015-0821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kliman HJ. Comment on “the placenta harbors a unique microbiome”. Sci. Transl. Med. 2014;6:254le254. doi: 10.1126/scitranslmed.3009864. [DOI] [PubMed] [Google Scholar]
- 145.Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome. 2017;5:48. doi: 10.1186/s40168-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fardini Y, Chung P, Dumm R, Joshi N, Han YW. Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infect. Immun. 2010;78:1789–1796. doi: 10.1128/IAI.01395-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Onderdonk AB, et al. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am. J. Obstet. Gynecol. 2008;198:110 e111–110.e117. doi: 10.1016/j.ajog.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 148.Onderdonk AB, et al. Colonization of second-trimester placenta parenchyma. Am. J. Obstet. Gynecol. 2008;199:52 e51–52 e10. doi: 10.1016/j.ajog.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Olomu IN, et al. Perinatal correlates of ureaplasma urealyticum in placenta parenchyma of singleton pregnancies that end before 28 weeks of gestation. Pediatrics. 2009;123:1329–1336. doi: 10.1542/peds.2008-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Leviton A, et al. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. The ELGAN study. Pediatr. Res. 2010;67:95–101. doi: 10.1203/PDR.0b013e3181bf5fab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Helderman JB, et al. Antenatal antecedents of cognitive impairment at 24 months in extremely low gestational age newborns. Pediatrics. 2012;129:494–502. doi: 10.1542/peds.2011-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hecht JL, et al. Characterization of chorioamnionitis in 2nd-trimester C-section placentas and correlation with microorganism recovery from subamniotic tissues. Pediatr. Dev. Pathol. 2008;11:15–22. doi: 10.2350/07-06-0285.1. [DOI] [PubMed] [Google Scholar]
- 153.Konwar C, et al. DNA methylation profiling of acute chorioamnionitis-associated placentas and fetal membranes: insights into epigenetic variation in spontaneous preterm births. Epigenet. Chromatin. 2018;11:63. doi: 10.1186/s13072-018-0234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jackson WM, et al. Risk factors for chronic lung disease and asthma differ among children born extremely preterm. Pediatr. Pulmonol. 2018;53:1533–1540. doi: 10.1002/ppul.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Joseph RM, O’Shea TM, Allred EN, Heeren T, Kuban KK. Maternal educational status at birth, maternal educational advancement, and neurocognitive outcomes at age 10 years among children born extremely preterm. Pediatr. Res. 2018;83:767–777. doi: 10.1038/pr.2017.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Singh R, et al. Antecedents of epilepsy and seizures among children born at extremely low gestational age. J. Perinatol. 2019;39:774–783. doi: 10.1038/s41372-019-0355-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bradley RH, Casey PH. Family environment and behavioral development of low-birthweight children. Dev. Med. Child Neurol. 1992;34:822–826. doi: 10.1111/j.1469-8749.1992.tb11520.x. [DOI] [PubMed] [Google Scholar]
- 158.Breslau N, et al. Stability and change in children’s intelligence quotient scores: a comparison of two socioeconomically disparate communities. Am. J. Epidemiol. 2001;154:711–717. doi: 10.1093/aje/154.8.711. [DOI] [PubMed] [Google Scholar]
- 159.Chin-Lun Hung G, et al. Socioeconomic disadvantage and neural development from infancy through early childhood. Int. J. Epidemiol. 2015;44:1889–1899. doi: 10.1093/ije/dyv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Loe IM, Lee ES, Luna F, Feldman HM. Behavior problems of 9-16 year old preterm children: biological, sociodemographic, and intellectual contributions. Early Hum. Dev. 2011;87:247–252. doi: 10.1016/j.earlhumdev.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Noble KG, et al. Socioeconomic disparities in neurocognitive development in the first two years of life. Dev. Psychobiol. 2015;57:535–551. doi: 10.1002/dev.21303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reiss F. Socioeconomic inequalities and mental health problems in children and adolescents: a systematic review. Soc. Sci. Med. 2013;90:24–31. doi: 10.1016/j.socscimed.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 163.Zhou SJ, Baghurst P, Gibson RA, Makrides M. Home environment, not duration of breast-feeding, predicts intelligence quotient of children at four years. Nutrition. 2007;23:236–241. doi: 10.1016/j.nut.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 164.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA Pediatr. 2015;169:1162–1172. doi: 10.1001/jamapediatrics.2015.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leviton A, et al. Antenatal and neonatal antecedents of executive dysfunctions in extremely preterm children. J. Child Neurol. 2018;33:198–208. doi: 10.1177/0883073817750499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Eryigit Madzwamuse S, Baumann N, Jaekel J, Bartmann P, Wolke D. Neuro-cognitive performance of very preterm or very low birth weight adults at 26 years. J. Child Psychol. Psychiatry Allied Discip. 2015;56:857–864. doi: 10.1111/jcpp.12358. [DOI] [PubMed] [Google Scholar]
- 167.Ekeus C, Lindstrom K, Lindblad F, Rasmussen F, Hjern A. Preterm birth, social disadvantage, and cognitive competence in Swedish 18- to 19-year-old men. Pediatrics. 2010;125:e67–e73. doi: 10.1542/peds.2008-3329. [DOI] [PubMed] [Google Scholar]
- 168.Manley BJ, et al. Social variables predict gains in cognitive scores across the preschool years in children with birth weights 500 to 1250 grams. J. Pediatrics. 2015;166:870–876.e872. doi: 10.1016/j.jpeds.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 169.Muscatell, K. A., Brosso, S. N. & Humphreys, K. L. Socioeconomic status and inflammation: a meta-analysis. Mol. Psychiatry. 10.1038/s41380-018-0259-2 (2018). [DOI] [PMC free article] [PubMed]
- 170.Leviton A, et al. Socioeconomic status and early blood concentrations of inflammation-related and neurotrophic proteins among extremely preterm newborns. PLoS ONE. 2019;14:e0214154. doi: 10.1371/journal.pone.0214154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lockwood KG, John-Henderson NA, Marsland AL. Early life socioeconomic status associates with interleukin-6 responses to acute laboratory stress in adulthood. Physiol. Behav. 2018;188:212–220. doi: 10.1016/j.physbeh.2018.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.David J, Measelle J, Ostlund B, Ablow J. Association between early life adversity and inflammation during infancy. Dev. Psychobiol. 2017;59:696–702. doi: 10.1002/dev.21538. [DOI] [PubMed] [Google Scholar]
- 173.Miller GE, et al. Maternal socioeconomic disadvantage is associated with transcriptional indications of greater immune activation and slower tissue maturation in placental biopsies and newborn cord blood. Brain Behav. Immun. 2017;64:276–284. doi: 10.1016/j.bbi.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Janusek LW, Tell D, Gaylord-Harden N, Mathews HL. Relationship of childhood adversity and neighborhood violence to a proinflammatory phenotype in emerging adult African American men: an epigenetic link. Brain Behav. Immun. 2017;60:126–135. doi: 10.1016/j.bbi.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 175.Barker DJ, Thornburg KL. The obstetric origins of health for a lifetime. Clin. Obstet. Gynecol. 2013;56:511–519. doi: 10.1097/GRF.0b013e31829cb9ca. [DOI] [PubMed] [Google Scholar]
- 176.Lester BM, Conradt E, Marsit CJ. Are epigenetic changes in the intrauterine environment related to newborn neurobehavior? Epigenomics. 2014;6:175–178. doi: 10.2217/epi.14.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Robinson J, et al. Placental control of fetal growth. Reprod. Fertil. Dev. 1995;7:333–344. doi: 10.1071/rd9950333. [DOI] [PubMed] [Google Scholar]
- 178.Lewis AJ, Galbally M, Gannon T, Symeonides C. Early life programming as a target for prevention of child and adolescent mental disorders. BMC Med. 2014;12:33. doi: 10.1186/1741-7015-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol. Rev. 2016;96:1509–1565. doi: 10.1152/physrev.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Paquette AG, et al. Placental epigenetic patterning of glucocorticoid response genes is associated with infant neurodevelopment. Epigenomics. 2015;7:767–779. doi: 10.2217/epi.15.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kitraki E, Nalvarte I, Alavian-Ghavanini A, Ruegg J. Developmental exposure to bisphenol A alters expression and DNA methylation of Fkbp5, an important regulator of the stress response. Mol. Cell. Endocrinol. 2015;417:191–199. doi: 10.1016/j.mce.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 182.Maniam J, Antoniadis C, Morris MJ, Early-Life Stress HPA. Axis adaptation, and mechanisms contributing to later health outcomes. Front. Endocrinol. 2014;5:73. doi: 10.3389/fendo.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kinlein SA, Wilson CD, Karatsoreos IN. Dysregulated hypothalamic-pituitary-adrenal axis function contributes to altered endocrine and neurobehavioral responses to acute stress. Front. Psychiatry. 2015;6:31. doi: 10.3389/fpsyt.2015.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lewis AJ, Austin E, Knapp R, Vaiano T, Galbally M. Perinatal maternal mental health, fetal programming and child development. Healthcare. 2015;3:1212–1227. doi: 10.3390/healthcare3041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Seckl JR. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 2004;151:U49–U62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- 186.Parets SE, Bedient CE, Menon R, Smith AK. Preterm birth and its long-term effects: methylation to mechanisms. Biology. 2014;3:498–513. doi: 10.3390/biology3030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Murgatroyd C, Spengler D. Epigenetics of early child development. Front. Psychiatry. 2011;2:16. doi: 10.3389/fpsyt.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nelson CA. Hazards to early development: the biological embedding of early life adversity. Neuron. 2017;96:262–266. doi: 10.1016/j.neuron.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 189.Frazier JA, et al. Antecedents of the child behavior checklist-dysregulation profile in children born extremely preterm. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:816–823. doi: 10.1016/j.jaac.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Leviton A, et al. Antecedents of screening positive for attention deficit hyperactivity disorder in ten-year-old children born extremely preterm. Pediatr. Neurol. 2018;81:25–30. doi: 10.1016/j.pediatrneurol.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Leviton A, Joseph RM, Allred EN, O’Shea TM, Kuban KKC. Antenatal and neonatal antecedents of learning limitations in 10-year old children born extremely preterm. Early Hum. Dev. 2018;118:8–14. doi: 10.1016/j.earlhumdev.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bangma JT, et al. Early life antecedents of positive child health among 10-year-old children born extremely preterm. Pediatr. Res. 2019;86:758–765. doi: 10.1038/s41390-019-0404-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Santos HP, Jr, et al. Epigenome-wide DNA methylation in placentas from preterm infants: association with maternal socioeconomic status. Epigenetics. 2019;14:751–765. doi: 10.1080/15592294.2019.1614743. [DOI] [PMC free article] [PubMed] [Google Scholar]