Abstract
Background
Myotonic dystrophy type 1 involves cardiac conduction disorders. Cardiac conduction disease can cause fatal arrhythmias or sudden death in patients with myotonic dystrophy type 1.
Methods and Results
This study enrolled 506 patients with myotonic dystrophy type 1 (aged ≥15 years; >50 cytosine‐thymine‐guanine repeats) and was treated in 9 Japanese hospitals for neuromuscular diseases from January 2006 to August 2016. We investigated genetic and clinical backgrounds including health care, activities of daily living, dietary intake, cardiac involvement, and respiratory involvement during follow‐up. The cause of death or the occurrence of composite cardiac events (ie, ventricular arrhythmias, advanced atrioventricular blocks, and device implantations) were evaluated as significant outcomes. During a median follow‐up period of 87 months (Q1–Q3, 37–138 months), 71 patients expired. In the univariate analysis, pacemaker implantations (hazard ratio [HR], 4.35; 95% CI, 1.22–15.50) were associated with sudden death. In contrast, PQ interval ≥240 ms, QRS duration ≥120 ms, nutrition, or respiratory failure were not associated with the incidence of sudden death. The multivariable analysis revealed that a PQ interval ≥240 ms (HR, 2.79; 95% CI, 1.9–7.19, P<0.05) or QRS duration ≥120 ms (HR, 9.41; 95% CI, 2.62–33.77, P < 0.01) were independent factors associated with a higher occurrence of cardiac events than those observed with a PQ interval <240 ms or QRS duration <120 ms; these cardiac conduction parameters were not related to sudden death.
Conclusions
Cardiac conduction disorders are independent markers associated with cardiac events. Further investigation on the prediction of occurrence of sudden death is warranted.
Keywords: conduction disturbance, myotonic dystrophy, sudden death, ventricular tachycardia
Subject Categories: Sudden Cardiac Death, Arrhythmias, Ventricular Fibrillation
Nonstandard Abbreviations and Acronyms
- ADL
activities of daily living
- DM1
myotonic dystrophy type 1
- HR
hazard ratio
Clinical Perspective
What Is New?
This retrospective study, which enrolled 506 patients with myotonic dystrophy type 1 in Japan, showed that cardiac conduction disorders are independent markers associated with cardiac events but not sudden death.
The incidence of sudden death was lower in Japan despite the lower frequency of pacemaker implantations versus France or the United States.
The clinical factors associated with the occurrence of sudden death in patients with myotonic dystrophy type 1 remain unclear.
What Are the Clinical Implications?
Treatment for conduction disorders alone fails to prevent sudden death attributable to myotonic dystrophy type 1.
Intervention against respiratory disease may be the most crucial target for the prevention of sudden death in patients with myotonic dystrophy.
Myotonic dystrophy is a neuromuscular disease of autosomal dominant inheritance characterized by multi‐organ involvements. Cardiac conduction diseases are considered major involvements in myotonic dystrophy type 1 (DM1). This event has been associated with sudden death, which occurs commonly in patients with DM1. 1 , 2 Recent research showed that the optimization of medical and device therapies recovered the cardiac function and reduced the number of outpatient clinic visits or cardiac‐related hospitalizations in patients with DM1. 2 Notably, implantation of a pacemaker device following an evaluation through electrophysiological study has been reported to prevent sudden death in patients with DM1. 3 On the other hand, it remains unclear whether only cardiac conduction diseases increase the risk of sudden death in DM1. 4 , 5 , 6 The objective of this multicenter study conducted in Japan was to determine whether cardiac conduction disorders are associated with cardiac events and sudden death in patients with DM1.
Methods
The institutional review board of each participating center approved this retrospective study. This study was publicly declared and written informed consent was waived because of its retrospective design. None of the patients refused to participate in the study. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Subjects
The present study enrolled patients who had undergone genetic testing, with multivariable data including cardiac and respiratory involvement, activities of daily living (ADL), dietary intake, and basic clinical information. A total of 528 patients with DM1 with a CTG length >50 repeats treated in 9 Japanese centers specializing in neuromuscular diseases from January 1, 2006 to August 31, 2016 were recruited. Twenty‐two patients were excluded from this study: 20 patients with DM1 were aged <15 years, 1 patient was also diagnosed with Charcot–Marie–Tooth disease, and 1 patient was registered twice. Finally, 506 patients with DM1 (249 men and 257 women) with a median age of 40 years (Q1–Q3, 32–49 years) at the initiation of the follow‐up were studied (Table 1). These patients with DM1 were retrospectively analyzed during a median follow‐up period of 87 months (Q1–Q3, 37–138 months).
Table 1.
Baseline Characteristics of Participants
| Patients, n | 506 |
| No. of CTG repeats | 830 (499–1331) |
| Age, y | 40 (32–49) |
|
Female sex, % Male sex, % |
50.8 49.2 |
| Bed rest, % | 2.4 |
| Nutrition support, % | 3.8 |
| Oxygen administration, % | 3.6 |
| Tracheotomy, % | 3.4 |
| Arrhythmia, % | |
| AF | 2.3 |
| VT | 0.2 |
| Pacemaker Implantation, % | 0.6 |
| ECG | |
| Heart rate, bpm | 70.1 (60.3–79.5) |
| Prolonged PQ interval, % | 11.8 |
| Wide QRS duration, % | 20.3 |
| Prolonged QTc interval, % | 33.0 |
Values are expressed as the median (Q1–Q3) for continuous variables and % for categorical variables. AF indicates atrial fibrillation; and VT, ventricular fibrillation.
Clinical Characteristics at Baseline
This study was approved and performed under the guidelines of the institutional ethics committee at each participating center. We collected genetic and clinical information (Table 2), and a total of 4979 ECGs were analyzed. We measured the following ECG parameters: RR interval, PQ interval, QRS duration, QT interval, and JT interval with a speed of 25 mm/s and per each setting in the leads II and III, aVF, or V5 (if lead II was unavailable). ECGs with pacing rhythm were excluded from the analysis. Ventricular tachycardia was defined as collapsed circulation with documented ECG >30 s. Cardiac events were defined as documented ventricular tachycardia, conduction disorders including advanced atrioventricular blocks, sick sinus syndrome, or device implantations.
Table 2.
Clinical Characteristics of Recruited Participants
| No. of CTG repeats |
| Health care |
| Ambulatory |
| Residential care |
| Long‐term hospitalization |
| ADL |
| Walking |
| Manual wheelchair |
| Electric wheelchair |
| Bedridden |
| Dietary intake |
| Ingestion |
| Enteral nutrition |
| Gastrostomy |
| Cardiac involvement |
| ECG |
| 24‐holter ECG |
| Echocardiography |
| BNP value |
| Respiratory involvement |
| Oxygen |
| Tracheotomy |
| Respiratory support (NPPV or ventilator) |
| Medical treatment |
ADL indicates activities of daily living; BNP, B‐type natriuretic peptide; and NPPV, non‐invasive positive pressure ventilation.
Sudden death was defined as death occurring suddenly and unexpectedly in patients who were stable before the event. 1 Witnessed deaths were classified as sudden only if death occurred within 1 hour after the onset of new symptoms. Unwitnessed deaths were considered sudden if the patient was alive and stable within 24 hours before the event. In patients in whom the temporal sequence was consistent with sudden death, but a specific cause of death other than arrhythmia was confirmed, the death was classified as non‐sudden.
Statistical Analysis
We used demographics, clinical characteristics (ADL categories [being bedridden or not], nutrition categories [receiving nutrition support or not], oxygen therapy [yes or no], tracheotomy [yes or no]), history of diabetes mellitus or arrhythmia (atrial fibrillation and ventricular tachycardia), pacemaker implantation, and ECG parameters (heart rate, prolonged PQ interval [defined as PQ interval ≥240 ms], wide QRS duration [defined as QRS duration QRS ≥120 ms], and prolonged QTc interval [defined as ≥440 ms for men and ≥460 ms for women]). The relative risk of death from any causes and respiratory failure, sudden death, and cardiac events, including ventricular tachyarrhythmia, conduction disease, and device implantation, were estimated for individual covariates using the Cox proportional‐hazards model. Cardiac events were analyzed in the subset of patients without a history of sustained ventricular tachycardia and pacemaker implantation. Given the low number of deaths and events, the multivariable models were limited to variables emerging with a P<0.20 or P<0.10 from the univariate Cox potential‐hazards model. 7 The proportionality of hazard assumption for all outcomes was statistically assessed on the basis of the Schoenfeld residuals; there was found no violation of the hazard assumption’s proportionality. The follow‐up time was the interval from the baseline to the most recent assessment, death, or diagnosis of the initial cardiac events. Patients for whom data on outcomes were not available were censored at the most recent evaluation. Changes in characteristics during the follow‐up were incorporated as time‐dependent covariates. The analyses were performed using the STATA 14.0 software (StataCorp LP, College Station, TX, USA). Two‐tailed P<0.05 denoted statistical significance.
Results
DM1 With Sudden Death
The causes of 69 deaths – excluding those of unknown cause (n=2) – were classified as sudden (26%, n=18) or non‐sudden (74%, n=51). Non‐sudden deaths were subclassified into those related to progressive neuromuscular respiratory failure (38%, n=26) and other deaths (36%, n=25). Progressive neuromuscular respiratory failure was defined as death resulting from respiratory dysfunction related to myotonic dystrophy. Among the 18 sudden deaths, 5 and 13 were witnessed and unwitnessed during the follow‐up period, respectively. The median age at the time of sudden death was 58 years (Q1–Q3, 51–63 years), and 10 of those patients were women. Based on the ECG finding or other clinical information, sudden death was unexpected in 14 cases. The remaining 4 cases had sudden death associated with cardiogenic shock (n=1), device replacement (n=1), and without information at the time of sudden death (n=2).
Figure 1 shows the univariate analysis between the characteristics of the patients and death from any causes, respiratory failure, or sudden death. The age of patients was associated with any causes of death, and the hazard ratio (HR) of sudden death was 1.08 (95% CI, 1.03–1.13) with age. Pacemaker implantation was significantly related to the occurrence of sudden death with an HR of 4.35 (95% CI, 1.22–15.50). In contrast, ECG markers including the prolongation of PQ interval or QRS duration were not associated with the occurrence of sudden death. The conditions of patients, including ADL, nutrition, and respiratory support were also not related to sudden death but death of any causes with HR of 1.96 (95% CI, 1.53–2.51), 2.92 (95% CI, 1.78–4.77) and 3.65 (95% CI, 2.26–5.91) or respiratory failure with HR of 2.09 (95% CI, 1.39–3.12), 3.94 (95% CI, 1.76–8.85) and 7.49 (95% CI, 3.22–17.41), respectively.
Figure 1. Univariate analysis between the characteristics of the patients and death from any causes, respiratory failure, and sudden death.
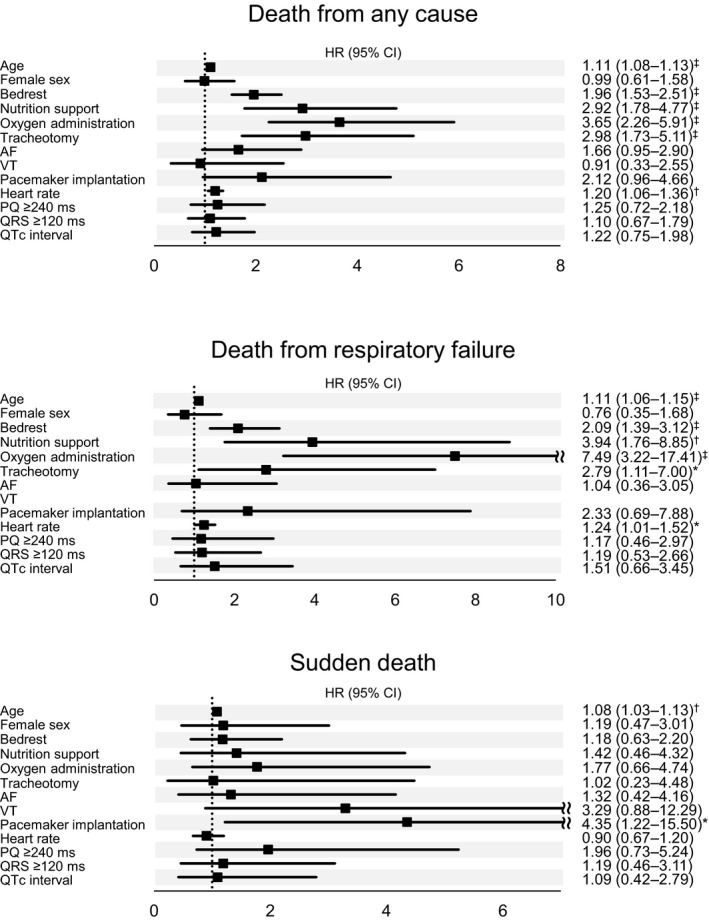
Univariate relative risks and P values were calculated using the Cox proportional‐hazards analysis for time to death from any cause, death from progressive neuromuscular respiratory failure, and sudden death. All covariates, except for age and sex, were time‐dependent. Hazard ratio per 1‐log unit higher in number of CTG repeats. Hazard ratio per 10 bpm higher in heart rate. AF indicates atrial fibrillation; HR indicates hazard ratio; NA, not available; and VT, ventricular fibrillation. P values: *<0.05; †<0.01; ‡<0.001.
DM1 With Cardiac Events
Figure 2 shows the univariate analysis between the characteristics of the patients and cardiac events (ie, documented ventricular tachycardia, conduction diseases, or device implantation). Cardiac events occurred frequently in older patients with (HR, 1.06; 95% CI, 1.02–1.10). Worsened ADL was significantly associated with the incidence of cardiac events and patients with DM1 at bed rest had a higher frequency of cardiac events with (HR, 1.79; 95% CI, 1.15–2.79) than those with well‐conditioned ADL. This result was unlike the relationship between sudden death and ADL. Nutrition support was also associated with a significant incidence of cardiac events (HR, 3; 95% CI, 1.29–6.94). Notably, cardiac conduction disorders such as PR prolongation or increased QRS duration were significantly associated with the occurrence of all cardiac events (HR, 4.52; 95% CI, 1.99–10.27 and HR, 9.91; 95% CI, 3.38–29.08, respectively) and each category of composite cardiac events. Higher heart rates were also associated with a lower incidence of cardiac events (HR, 0.58; 95% CI, 0.43–0.78).
Figure 2. Univariate analysis between the characteristics of the patients and cardiac events.
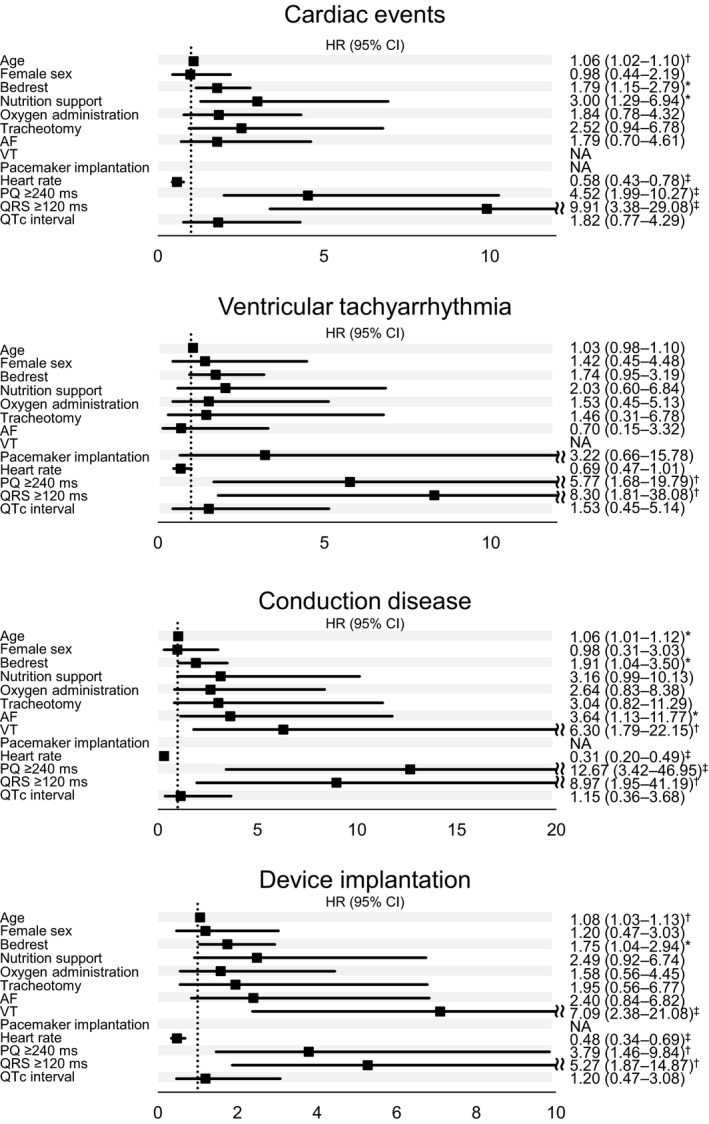
Univariate relative risks and Pvalues were calculated using the Cox proportional‐hazards analysis for time to incidence of ventricular tachyarrhythmia, conduction disease, device implantation, and cardiac events. All covariates, except for age and sex, were time‐dependent. Hazard ratio per 1‐log unit higher in number of CTG repeats. Hazard ratio per 10 bpm increase in heart rate. AF indicates atrial fibrillation; HR, hazard ratio; NA, not available; and VT, ventricular fibrillation. P values: *<0.05; †<0.01; ‡<0.001.
Multivariable Association Between the Characteristics of Patients With DM1 and Outcomes
Figure 3 shows the multivariable analysis between the characteristics of patients with DM1 and outcomes including death from any causes, death from respiratory failure, sudden death, or cardiac events. Age was a significant factor associated with the occurrence of sudden death (HR, 1.09; 95% CI, 1.03–1.14) and any causes of death (HR, 1.09; 95% CI, 1.06–1.12). In contrast, bed rest, nutrition support, or oxygen administration corresponding to ADL indicators, nutrition, or respiratory failure were not associated with the incidence of sudden death or cardiac events. Cardiac involvements including ventricular tachycardia, pacemaker implantation, or increased QRS duration did not exhibit a significant association with the occurrence of sudden death; patients with DM1 with a PQ interval ≥240 ms or QRS duration ≥120 ms were linked to a higher incidence of cardiac events than those with a PQ interval <240 ms or QRS duration <120 ms (HRs of 2.79 [95% CI, 1.09–7.19] and 9.41 [95% CI, 2.62–33.77], respectively). As shown in Figure S1, these findings were also observed when the multivariable analysis was limited to variables emerging with a P<0.10.
Figure 3. Multivariable analysis between the characteristics of patients and outcomes.
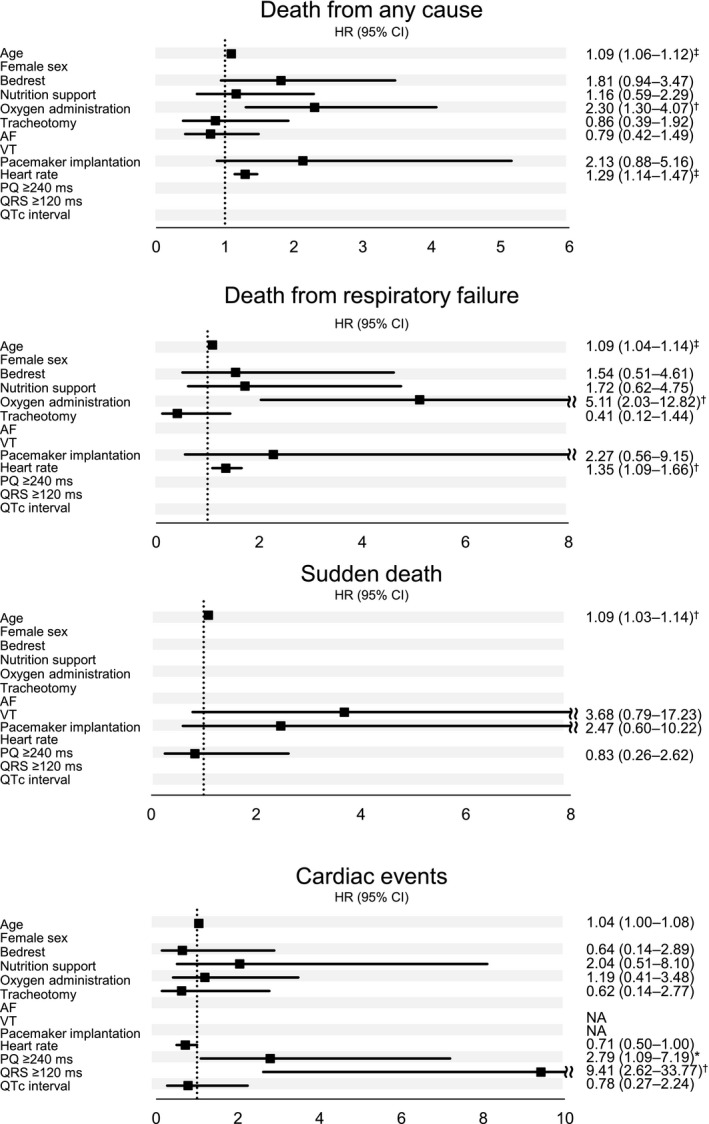
Multivariable relative risks and P values were calculated using the Cox proportional‐hazards model for time to death from any cause, death from progressive neuromuscular respiratory failure, sudden death, and incidence of cardiac events. The multivariable models were limited to variables emerging with a P<0.2 from the univariate analysis. All covariates, except for age, were time‐dependent. Hazard ratio per 10 bpm increase in heart rate. Hazard ratio per year of age. AF indicates atrial fibrillation; HR, hazard ratio; NA, not available; and VT, ventricular fibrillation. P values: *<0.05; †<0.01; ‡<0.001.
Discussion
This multicenter study examined 506 patients with DM1 in Japan and revealed that cardiac conduction disorders were independent markers associated with the incidence of cardiac events. This is consistent with reports from the United States 1 and France 7 . On the incidence of sudden death in patients with DM1, the present analysis revealed inconsistent results compared with the previous reports. 1 This finding suggests that pacemaker implantation may prevent sudden death in patients with DM1 involved cardiac conduction disorders. At the same time, we need to identify another reason for the sudden death which occurred patients without cardiac involvements including cardiac conduction disorder or pacemaker implantation.
DM1 is a neuromuscular disease with multi‐organ involvement, which is caused by an expansion of CTG repeats in the 3’ untranslated region of the dystrophia myotonica protein kinase gene. The number of CTG repeats has been associated with the age at onset 8 and incidence 9 , 10 of cardiac involvement. Patients with DM1 experience conduction disturbances (ie, atrioventricular block, bundle branch blocks, or sick sinus syndrome) and tachyarrhythmias (ie, atrial fibrillation or ventricular tachycardias) as major cardiac events. 11 , 12 ECG markers such as non‐sinus rhythm, PR interval ≥240 ms, QRS duration ≥120 ms, or second‐ or third‐degree atrioventricular block, should be evaluated daily, as these ECG conditions correspond to cardiac arrhythmias (including fatal ventricular arrhythmias or atrial fibrillation) and sudden death. Moreover, invasive electrophysiological study should be considered for those patients to determine the need for the placement of a pacemaker or implantable cardioverter defibrillator. 13 A prospective electrophysiological study performed by Wahbi et al. 3 reported that an intraventricular conduction time (His‐Ventricle interval) >70 ms should be considered an indication for pacemaker implantation in patients with DM1. Thus, the His‐Ventricle interval is critical for the prognosis, and pacemaker implantation after electrophysiological study may be useful for the primary prevention of sudden death in patients with DM1. Sudden death is commonly observed in such patients, and it was noted that those who need to undergo pacemaker implantations are at a high risk of sudden death. The treatments strategy for cardiac involvements is essential for the prevention of sudden death in patients with DM1.
Cardiac conduction disorders have been associated with sudden death, and cardiac pacemaker therapy may prevent sudden death in patients with DM1. 1 , 3 More recently, a multivariable analysis conducted by Wahbi et al. 3 reported that only left bundle branch block was associated with sudden death. They concluded that a higher ratio of pacemaker implantations compared with that calculated in a previous study (19.7% versus 10%, respectively) may prevent sudden death in patients with DM1. 1 As shown Figure 4, the frequency of pacemaker implantations in this study was only 3.3% (17 of 506 patients). However, the multivariable statistical analysis did not demonstrate a positive correlation between sudden death and cardiac conduction disorders. Furthermore, the majority of the last ECGs before sudden death in each patient with DM1 failed to reveal a conduction problem, with most QRS durations being within normal limits. We noted that the cause of sudden death in patients with DM1 may be attributed to various factors (Figure 5). Especially sudden death in Japanese patients with DM1 had been unexpectedly caused by cardiac conduction disturbance and other involvements. Cardiac conduction disturbance among patients with DM1 who experienced sudden death was observed less commonly in Japan despite the lower frequency of pacemaker implantation compared with that reported in patients from the United States or France. Although we also investigated the underlying mechanism for sudden death through the evaluation of multiple clinical factors except for cardiac involvements, we were unable to reach a convincing conclusion.
Figure 4. Patients’ outcomes in each cohort: the United States, France, and Japan.
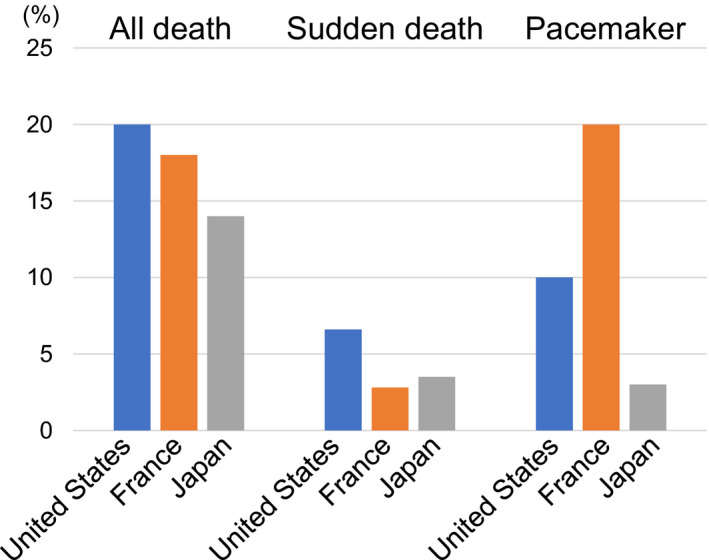
Although the frequency of pacemaker implantations was less common in Japan (3.3%) than the United States (10%) and France (20%), the rate of sudden death was not lower in Japan compared with those observed in the 2 other countries. The data for the United States and France were obtained from the New England Journal of Medicine (2008) 1 and European Heart Journal (2017) 7 .
Figure 5. A schema associated with the etiology of sudden death in DM1.
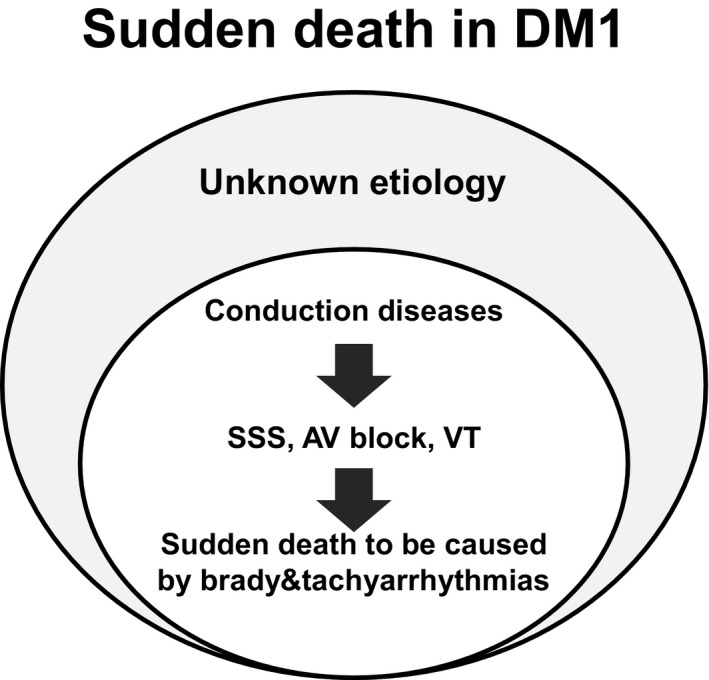
AV, indicates atrioventricular; DM1, myotonic dystrophy type 1; SSS, sick sinus syndrome; and VT, ventricular fibrillation.
The management of cardiac conduction diseases as a major cause of sudden death in DM1 is of crucial importance. Implantable cardioverter defibrillator or cardiac resynchronization therapy for the treatment for bradycardia and ventricular tachyarrhythmias, would benefit the prevention of sudden death in patients with DM1. Cardiac resynchronization therapy has been considered an option in patients with DM with both left ventricular dysfunction and left bundle branch block. 14 On the other hand, it is established that we may be unable to easily predict the risk of sudden death in patients with DM1 because of the complexity of treatment or healthcare resources. Notably, it is insufficient to manage cardiac conduction diseases without addressing the multi‐organ involvement in DM1. Respiratory muscle gradually weakens with the reduction of lung volume in DM1, and some DM1 cases will occasionally require non‐invasive mechanical ventilation for the treatment of respiratory failure. Nocturnal sleep apnea syndrome is frequently observed and can affect the prognosis of DM1. It currently remains unclear whether mechanical support may improve the prognosis of DM1. Nevertheless, respiratory disease increases the complexity related to the cause of sudden death in patients with DM1, and may be the most critical target for the prevention of sudden death in this setting. 4
Conclusions
Sudden death is a critical concern in the management of DM1 and has been associated with cardiac conduction diseases. Appropriate care during hospitalization may assist in resuscitating patients with DM1 from aborted sudden cardiac death because of ventricular tachycardias or critical bradyarrhythmias. In our retrospective analysis, cardiac conduction disorders were associated with documented cardiac events, but not sudden death. This finding emphasizes that physicians should consider the risk of sudden death even in patients with DM1 without conduction disorders.
Sources of Funding
Dr Masanori Takahashi received a research grant from the Japan Agency for Medical Research and Development (JP19ek0109259, JP20ek0109474)
Disclosures
None.
Supporting information
Figure S1
(J Am Heart Assoc. 2020;9:e015709 DOI: 10.1161/JAHA.119.015709.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.015709
For Sources of Funding and Disclosures, see page 9.
References
- 1. Groh WJ, Groh MR, Saha C, Kincaid JC, Simmons Z, Ciafaloni E, Pourmand R, Otten RF, Bhakta D, Nair GV, et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med. 2008;2688–2697. [DOI] [PubMed] [Google Scholar]
- 2. Nikhanj A, Yogasundaram H, Miskew Nichols B, Richman‐Eisenstat J, Phan C, Bakal JA, Siddiqi ZA, Oudit GY. Cardiac intervention improves heart disease and clinical outcomes in patients with muscular dystrophy in a multidisciplinary care setting. J Am Heart Assoc. 2020;e014004 DOI: 10.1161/JAHA.119.014004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wahbi K, Meune C, Porcher R, Bécane HM, Lazarus A, Laforêt P, Stojkovic T, Béhin A, Radvanyi‐Hoffmann H, Eymard B, et al. Electrophysiological study with prophylactic pacing and survival in adults with myotonic dystrophy and conduction system disease. JAMA. 2012;1292–1301. [DOI] [PubMed] [Google Scholar]
- 4. Dello Russo A, Pace M, Bellocci F. Sudden death in myotonic dystrophy. N Engl J Med. 2008;1626. [DOI] [PubMed] [Google Scholar]
- 5. Petri H, Vissing J, Witting N, Bundgaard H, Køber L. Cardiac manifestations of myotonic dystrophy type 1. Int J Cardiol. 2012;82–88. [DOI] [PubMed] [Google Scholar]
- 6. Merino JL, Carmona JR, Fernández‐Lozano I, Peinado R, Basterra N, Sobrino JA. Mechanisms of sustained ventricular tachycardia in myotonic dystrophy: implications for catheter ablation. Circulation. 1998;541–546. [DOI] [PubMed] [Google Scholar]
- 7. Wahbi K, Babuty D, Probst V, Wissocque L, Labombarda F, Porcher R, Bécane HM, Lazarus A, Béhin A, Laforêt P, et al. Incidence and predictors of sudden death, major conduction defects and sustained ventricular tachyarrhythmias in 1388 patients with myotonic dystrophy type 1. Eur Heart J. 2017;751–758. [DOI] [PubMed] [Google Scholar]
- 8. Merlevede K, Vermander D, Theys P, Legius E, Ector H, Robberecht W. Cardiac involvement and CTG expansion in myotonic dystrophy. J Neurol. 2002;693–698. [DOI] [PubMed] [Google Scholar]
- 9. Melacini P, Villanova C, Menegazzo E, Novelli G, Danieli G, Rizzoli G, Fasoli G, Angelini C, Buja G, Miorelli M, et al. Correlation between cardiac involvement and CTG trinucleotide repeat length in myotonic dystrophy. J Am Coll Cardiol. 1995;239–245. [DOI] [PubMed] [Google Scholar]
- 10. Chong‐Nguyen C, Wahbi K, Algalarrondo V, Bécane HM, Radvanyi‐Hoffman H, Arnaud P, Furling D, Lazarus A, Bassez G, Béhin A, et al. Association between mutation size and cardiac involvement in myotonic dystrophy type 1: an analysis of the DM1‐heart registry. Circ Cardiovasc Genet. 2017;9:e001526 10.1161/CIRCGENETICS.116.001526. [DOI] [PubMed] [Google Scholar]
- 11. Nikhanj A, Sivakumaran S., Nikhanj A, Sivakumaran S, Miskew‐Nichols B, Siddiqi ZA, Oudit GY. Ventricular tachycardia in patients with type 1 myotonic dystrophy: a case series. Eur Heart J Case Rep. 2019;ytz095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. NcNally EM, Mann DL, Pinto Y, Bhakta D, Tomaselli G, Nazarian S, Groh WJ, Tamura T, Duboc D, Itoh H, et al. Clinical care recommendations for cardiologists treating adults with myotonic dystrophy. J Am Heart Assoc. 2020;9:e014006 DOI: 10.1161/JAHA.119.014006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feingold B, Mahle WT, Auerbach S, Clemens P, Domenighetti AA, Jefferies JL, Judge DP, Lal AK, Markham LW, Parks WJ. et al. Management of cardiac involvement associated with neuromuscular diseases: A scientific statement from the american heart association. Circulation. 2017;9:e200–e231 DOI: 10.1161/CIR.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 14. Nikhanj A, Sivakumaran S, Yogasundaram H, Becher H, Kimber S, Siddiqi ZA, Oudit GY. Comparison of usefulness of cardiac resynchronization therapy in patients with type 1 myotonic dystrophy with versus without left bundle branch block. Am J Cardiol. 2019;1770–1774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


