Abstract
Background
No data are available on sex disparities in prevalence and survival for primary malignant cardiac tumors (PMCT). This study aimed to compare male and female PMCT prevalence and long‐term survival rates.
Methods and Results
We utilized the Surveillance, Epidemiology, and End Results (SEER) 18 database from the National Cancer Institute for all PMCTs diagnosed between 1973 and 2015. From a total of 7 384 580 cases of cancer registered in SEER, we identified 327 men and 367 women with PMCTs. The majority (78%) of patients were white. Sarcoma was the most common type of PMCT in both men and women (≈60%). Individuals diagnosed with lymphoma exhibited better survival than those with other types of PMCTs. Men were diagnosed at a younger age than women; however, there was no significant difference in overall survival between the sexes. Men diagnosed with PMCT between the ages of 51 and 65 years demonstrated prolonged survival compared with those diagnosed at younger or older ages. There was no difference in survival rates among women based on age at diagnosis.
Conclusions
PMCTs are rare in both men and women. Tumors tend to be diagnosed at an earlier age in men compared with women, but there is no sex disparity in survival rate. Sarcoma is the most common type of PMCT, and lymphoma is associated with the highest survival rate among both sexes.
Keywords: cardiac tumors; malignancy; sex; Surveillance, Epidemiology, and End Results; survival rate
Subject Categories: Smooth Muscle Proliferation and Differentiation, Epidemiology, Mortality/Survival, Women
Clinical Perspective
What Is New?
Primary malignant cardiac tumors present at a younger age in men compared with women; however, no other sex disparities are associated with these neoplasms.
Sex has no effect on primary malignant cardiac tumor survival.
Among the subtypes of primary malignant cardiac tumors, lymphoma is associated with the highest survival rate in both men and women.
What Are the Clinical Implications?
Primary malignant cardiac tumors are rare; however, these tumors are associated with poor outcomes in both sexes.
More than a quarter of men with primary malignant cardiac tumors are diagnosed before age 50 years; therefore, a prompt and thorough evaluation should be performed in young men presenting with suspicious symptoms.
Resection of primary malignant cardiac tumors may be curative, and improved outcomes can be achieved through early detection and surgical intervention.
Introduction
Primary malignant cardiac tumors (PMCTs) are rare, representing ≈10% to 25% of all primary cardiac neoplasms. Individuals diagnosed with PMCTs typically face a very poor prognosis.1, 2 More than 50% of PMCTs are sarcomas; the other 2 types of PMCT, lymphoma and mesothelioma, are far less common. The prevalence of PMCTs has increased over the past 5 decades. Interestingly, the overall survival rate has also improved during this period. Nevertheless, compared with extracardiac cancers, patients with PMTCs have worse outcomes.1 The 1‐year survival rate is ≈10% without surgical resection.3
Sex disparities have been reported previously in the setting of other cardiovascular diseases, such as valvular heart disease, peripheral arterial disease, coronary artery disease, and hypertrophic cardiomyopathy.1, 2, 4, 5, 6 However, the relationship between sex and PMCT outcomes remains to be definitively established. Although PMCT prevalence, demographics, surgical outcomes, and survival have been studied, survival differences between men and women remain to be investigated.1, 7, 8, 9 We used the Surveillance, Epidemiology, and End Results (SEER) 18 database—the largest cancer database in the United States—to evaluate sex disparities in PMCT prevalence and survival among individuals diagnosed between 1973 and 2015.
Methods
The SEER database is publicly available. Additional information on the database, the method used in the analysis, and the materials will be made available on request for purposes of reproducing the results or replicating the procedure.
We used SEER*STAT v8.2.1 (Division of Cancer Control and Population Sciences, National Cancer Institute [Calverton, MD] https://seer.cancer.gov) to extract data on PMCTs diagnosed between 1973 and 2015 from the National Cancer Institute's SEER 18 database. SEER 18 obtains data from 18 cancer registries in the United States (San Francisco, Connecticut, Detroit, California, Kentucky, Louisiana, New Jersey, Greater Georgia, Hawaii, Iowa, New Mexico, Seattle, Utah, Alaska, San Jose–Monterey, Los Angeles, Rural Georgia, and Metropolitan Atlanta) who had a histologic and/or radiographically confirmed diagnosis of PMCT. The SEER site code C38.0 (heart) was used to identify cases with pathology limited to the heart. The International Classification of Diseases for Oncology, 3rd Edition (ICD‐O‐3; World Health Organization 2008) was used as the primary diagnostic criterion. ICD‐O‐3 is used in tumor registries for coding the site (topography) and the histology of neoplasms. An index registry was used to classify patients into various geographic regions: Midwestern (Detroit and Iowa), Western (California, Los Angeles, San Francisco, Hawaii, New Mexico, Seattle, Utah, Alaska, and San Jose‐Monterey), Southern (Rural Georgia, Kentucky, Louisiana, Metropolitan Atlanta, and Greater Georgia), and North Eastern (New Jersey and Connecticut). The SEER registries continuously code and submit American Joint Committee on Cancer (AJCC) 6th and 7th edition stages for all cancers diagnosed in 2010 and beyond; patients diagnosed before 2010 are staged using the AJCC 6th edition only. The AJCC 6th edition was used in this study to increase the sample size by including all patients diagnosed before 2010. Exclusion criteria included (1) age <18 years, (2) stage 0 or in situ tumor, (3) unknown site of primary tumor, (4) patient deceased and cause of death unknown, and (5) history of previous cancer. Figure 1 shows a flowchart for patient selection. Institutional review board approval was not required because SEER 18 data are deidentified and publicly available.
Figure 1.
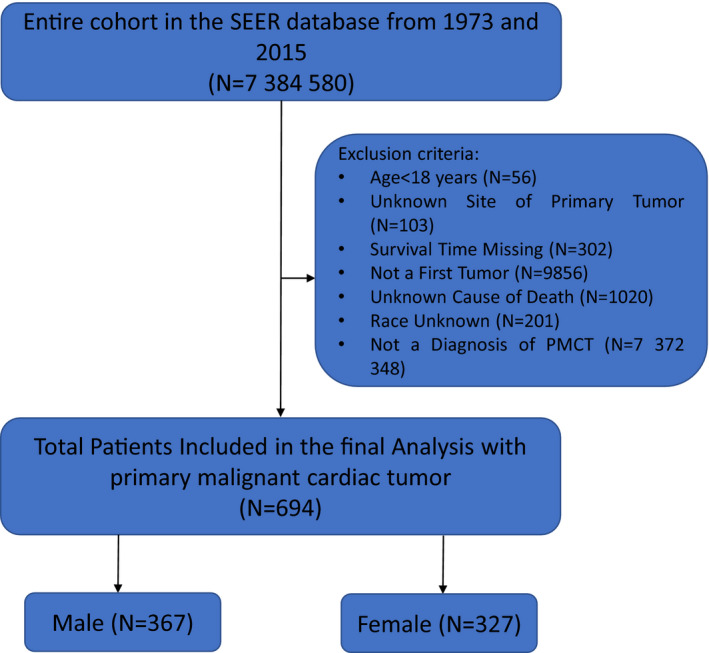
Flow chart for the patient selection with exclusion criteria SEER: Surveillance, Epidemiology and End Results.
Statistical Analysis
We performed a sex‐specific analysis of the effect of age group and tumor type (PMCT). The baseline characteristics and group differences were compared using the Pearson χ2 test for proportions.10, 11 The Kaplan–Meier method was used for survival analysis and the log‐rank test for equality of survival functions, including assessing survival differences.12 Continuous data were analyzed using a t test.11, 13 P<0.05 was deemed statistically significant in this study. All statistical analyses were performed using Stata v14.2 (StataCorp).
Results
The SEER 18 database included a total of 7 384 580 cases of cancer diagnosed between 1973 and 2015. We identified 327 men and 367 women with PMCTs (Figure 1). Patient characteristics, tumor characteristics, and a comparison of male and female survival are outlined in Table. There was a statistically significant difference in the age at diagnosis; specifically, the average age of diagnosis was younger in men than women (Figure 2). The incidence of PMCT decreased after age 50 years. Approximately 78% of all patients diagnosed with PMCT were white; black patients represented the second most frequently affected ethnic group. The overall incidence of PMCTs was higher in men, regardless of race (Figure S1).
Table 1.
Demographics and Baseline Characteristics in Patients With PMCTs
| Variable | Female | Male | P Value |
|---|---|---|---|
| Age, y, mean±SD | 57.5±19.1 | 52.6±18.5 | 0.001 |
| Age group | |||
| 18–50 | 115 (35.2) | 183 (49.9) | 0.001 |
| 51–65 | 87 (26.6) | 84 (22.9) | |
| 66–80 | 84 (25.7) | 64 (17.4) | |
| >80 | 41 (12.5) | 36 (9.8) | |
| Race | |||
| White | 254 (77.7) | 291 (79.3) | 0.583 |
| Black | 34 (10.4) | 41 (11.2) | |
| Others | 39 (11.9) | 35 (9.5) | |
| Region | |||
| Northeastern | 51 (15.6) | 42 (11.4) | 0.233 |
| Midwestern | 46 (14.1) | 42 (11.4) | |
| Western | 178 (54.4) | 221 (60.2) | |
| Southern | 52 (15.9) | 62 (68.9) | |
| SEER historic stages | |||
| Localized | 70 (21.4) | 68 (18.5) | 0.738 |
| Regional | 65 (19.9) | 71 (19.4) | |
| Distant | 84 (25.7) | 95 (25.9) | |
| Unstaged | 108 (33.0) | 133 (36.2) | |
| Staging | |||
| I | 31 (9.5) | 38 (10.4) | 0.432 |
| II | 29 (8.9) | 23 (6.3) | |
| III | 19 (5.8) | 25 (6.8) | |
| IV | 72 (22.0) | 67 (18.3) | |
| Unknown | 176 (53.8) | 214 (58.3) | |
| Type of tumor | |||
| Lymphoma | 78 (23.9) | 100 (27.3) | 0.748 |
| Sarcoma | 202 (61.8) | 213 (58.0) | |
| Mesothelioma | 19 (5.8) | 21 (5.7) | |
| Other | 28 (8.6) | 33 (9.0) | |
| Radiation | |||
| Yes | 59 (18.0) | 66 (18.0) | 1.00 |
| No | 268 (82.0) | 301 (82.0) | |
| Surgery | |||
| Yes | 165 (50.5) | 158 (43.1) | 0.051 |
| No | 162 (49.5) | 209 (57.0) | |
| Radiation and surgery | 36 (11.0) | 39 (10.6) | 0.871 |
| Diagnostic confirmation | |||
| Histology | 287 (87.8) | 319 (86.9) | 0.814 |
| Cytology | 25 (7.7) | 27 (7.4) | |
| Clinical | 4 (1.2) | 3 (0.8) | |
| Direct visualization | 0 (0.0) | 1 (0.3) | |
| Radiography only | 8 (2.5) | 14 (3.8) | |
| Unknown | 3 (0.9) | 3 (0.8) | |
| Survival | |||
| Alive | 49 (15.0) | 65 (17.7) | 0.333 |
| Dead | 278 (85.0) | 302 (82.3) | |
| Histopathology | |||
| Lymphoma | 78 (43.8) | 100 (56.2) | 0.102 |
| Sarcoma | 202 (48.7) | 213 (51.3) | 0.588 |
| Mesothelioma | 19 (47.5) | 21 (52.5) | 0.752 |
| Other | 28 (45.9) | 33 (54.1) | 0.523 |
Data are shown as n (%) except as noted. PMCT indicates primary malignant cardiac tumor; SEER, Surveillance, Epidemiology, and End Results.
Figure 2.
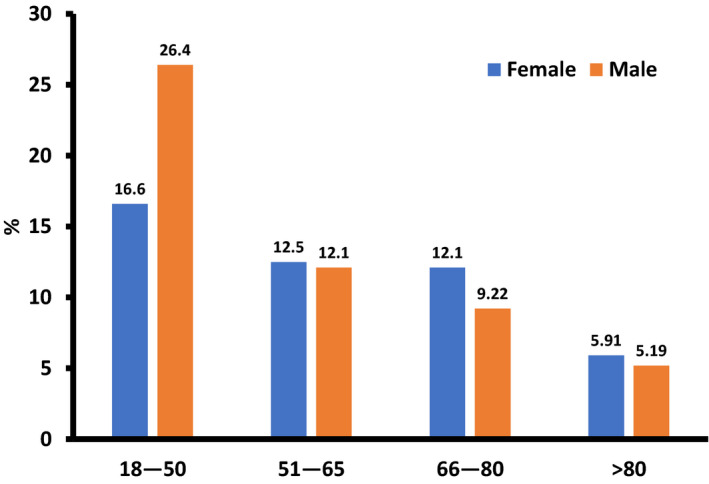
Age at diagnosis for primary malignant cardiac tumors, stratified by sex. There was statistically significant difference in the incidence of PMCTs between the ages of 18 and 50. After 50 years, males had lower incidence compared with females which was not statistically significant. SEER indicates The Surveillance, Epidemiology, and End Results; PMCT, primary malignant cardiac tumors.
More than half (60.2% of men and 54.4% of women) of the patients with PMCT were diagnosed in the western part of the United States. The histologic stage was not known in >30% of the cases, and the clinical stage was unknown in approximately half of the cases. The most common type of tumor was sarcoma (61.8% of women and 58% of men). The second and third most common types of PMCT were lymphoma and mesothelioma, respectively. Only about 18% of patients underwent radiation therapy. Surgical resection was performed in 50.5% of women and 43% of men with PMCT, and 11% of all patients with PMCT underwent combination surgery and radiotherapy. The overall prevalence of PMCTs was 0.005% for men and 0.004% for women. Diagnostic confirmation of PMCT was established via histology in ≈87% of patients. PMCT was diagnosed with cytology, radiography, clinical evaluation, and direct visualization in the remaining cases.
The overall survival rate was 17.7% for men and 15% for women. Median overall survival by sex was 16 and 14 months for men and women, respectively. However, the difference in the survival rate was not deemed statistically significant, as shown in Figure 3. The overall survival rate was highest among individuals diagnosed between the ages of 18 and 50 years, regardless of sex (Figures S2 and S3). Sarcoma was the most common type of PMCT (≈60%), followed by lymphoma, mesothelioma, and others. Lymphoma was associated with the highest survival rate; the lowest survival rate was observed among individuals diagnosed with mesothelioma (Figure S4). Lymphoma was associated with prolonged survival among both sexes (Figure 4A and 4B). There were no statistically significant sex‐specific differences in the histopathology of the tumors (Table).
Figure 3.
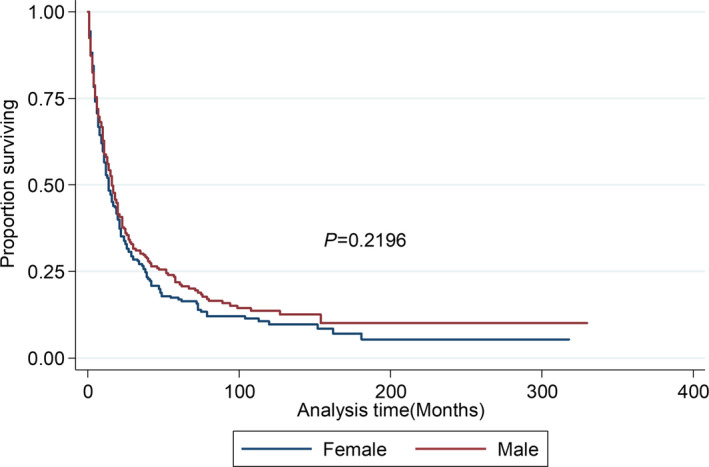
Kaplan‐‐Meier survival estimates for overall survival: stratified by sex. There is no difference in overall survival between both sexes.
Figure 4.
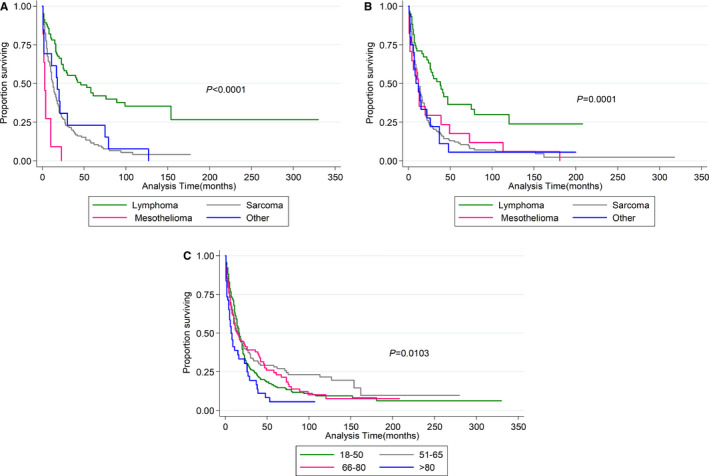
Kaplan–Meier survival estimates by type of tumor. For males (A) and females (B), lymphoma has highest survival rate among all the primary malignant cardiac tumors. C, overall survival for entire cohort by age group; (C) demonstrates that patients between 51‐65 years have the highest survival rate compared with other age groups.
Statistically significant sex disparities in survival were not observed. The sex difference in survival was also tested by sex and age as an interaction term, with no difference in survival (P=0.392). This was again tested by introducing an interaction term between sex and tumor type, and similar results were observed (P=0.850). Men diagnosed with PMCT between the ages of 51 and 65 years demonstrated prolonged survival compared with younger and older men (P=0.0103; Figure 4C). There was no difference in survival among women based on age at diagnosis.
Discussion
Our study describes the influence of sex on the baseline characteristics, histopathology, staging, diagnosis, treatment, and survival rate among patients diagnosed with PMCT. PMCTs are exceedingly rare, accounting for only 0.008% of all malignancies reported in the SEER database between 1973 and 2011.1 We evaluated new data added to the database between 2011 and 2015 and found a modest increase in the prevalence of PMCTs; these uncommon tumors now represent 0.009% of all cancers included in the SEER database. PMCTs represent ≈9.4% of all primary cardiac tumors. Prevalence peaks in the fifth decade of life.
We did not observe any significant sex disparity in long‐term survival; however, there was a slight female predilection in PMCT prevalence (52.9%). It has been hypothesized that the increased prevalence of PMCTs among women is due to exposure to chest radiation for breast cancer and the established tumorigenic effect of estrogen.14, 15 The mean age of diagnosis was slightly younger among men than women. White patients were disproportionately affected compared with members of other ethnic groups. These findings are consistent with previous investigations and may be related to underreporting due to racial inequities in healthcare access.16 It is unknown if there are genetic or environmental predispositions to explain this observation. No difference was noted in the distribution of tumor type between the sexes; the ratio of sarcomas, lymphomas, and mesotheliomas was approximately equivalent. There was also no significant sex difference in the type of treatment provided.
Data were available for 3 distinct histologic subtypes of PMCT: lymphoma, sarcoma, and mesothelioma. Of these, sarcomas were the most common type (59.8%), followed by lymphomas (25.6%) and mesotheliomas (5.8%). These proportions are similar to those reported by Oliveira and colleagues, 1 who observed that 64.8%, 27%, and 8% of PMCTs were sarcomas, lymphomas, and mesotheliomas, respectively. However, other investigators have described a significantly lower prevalence of lymphomas.3, 17, 18 The underlying cause of the increasing prevalence of lymphoma remains to be elucidated, but it has been hypothesized that Epstein–Barr virus–induced lymphoproliferative disorders may develop secondary to AIDS or cardiac transplantation.19
Approximately 50% of patients underwent surgery, which has been established as the most effective therapy for PMCTs.20, 21 Less than 20% of patients underwent radiation therapy; this may be due to the risk of radiation‐induced cardiac toxicity, which typically manifests as pericarditis or cardiomyopathy. Furthermore, sarcomas are not highly radiosensitive, and thus a moderate level of radiotherapy is minimally effective.22
PMCTs are generally associated with poor outcomes, and only half of the patients in our analysis were alive at the end of 25 months. However, survival was improved compared with previous analyses.1 The age at diagnosis influenced the survival of patients with PMCTs; 100‐month survival was the lowest in those aged >80 years. There were no significant survival differences between men and women. Interestingly, men demonstrated a significant difference based on age: men diagnosed between the ages of 51 and 65 years survived longer than their older and younger counterparts. In contrast, female survival was unaffected by age at diagnosis. In a comparison of the 3 main tumor types, there was a significant survival advantage for individuals diagnosed with lymphoma. It has been postulated that the improved outcomes among individuals with lymphoma‐type PMCTs were due to the potential to achieve full recovery with combined surgery and postoperative chemotherapy.23 Mean 5‐year survival was ≈40%, which represents a 1% decrease in survival in the 4 years between 2011 and 2015.1
This study utilized the SEER database, which is considered a highly reliable source of epidemiologic information. Nevertheless, human error in data collection is unavoidable, and we were unable to determine if mortality was specifically related to PMCT or if other contributory factors were present. Furthermore, we were also unable to determine whether PMCTs with distant metastases affected the morality rate or if mortality was caused by cardiovascular compromise (eg, lethal arrhythmias, diminished cardiac index, or increased thromboembolic events in the setting of malignancy). The SEER database also does not report specific chemotherapy regimens; therefore, we were unable to control for the influence of systemic toxicities of various chemotherapy regimens. As data were collected over a longer period, it is conceivable that there were confounders related to classification and/or treatment modalities. Finally, “California data” include California excluding San Francisco, San Jose–Monterey, and Los Angeles.
Conclusions
PMCTs are rare and associated with poor prognosis. There are no sex disparities in the distribution of PMCTs by type or treatment modality. There is also no significant difference in survival between men and women. Knowledge regarding the influence of sex, age, and race on PMCT survival may help facilitate the development of targeted therapies for at‐risk subgroups and help frame advance care planning conversations between patients and providers.
Disclosures
None.
Supporting information
Figure S1. Racial difference in the incidence of primary malignant cardiac tumors, stratified by sex.
Figure S2. Overall survival rate in men with primary malignant cardiac tumors stratified by age.
Figure S3. Overall survival rate in women with primary malignant cardiac tumors, stratified by age.
Figure S4. Overall survival rate by tumor type.
(J Am Heart Assoc. 2020;9:e014846 DOI: 10.1161/JAHA.119.014846.)
This article was handled independently by Carol Ann Remme, MD, PhD as a guest editor. The editors had no role in the evaluation of the article or in the decision about its acceptance.
References
- 1. Oliveira GH, Al‐Kindi SG, Hoimes C, Park SJ. Characteristics and survival of malignant cardiac tumors: a 40‐year analysis of >500 patients. Circulation. 2015;132:2395–2402. [DOI] [PubMed] [Google Scholar]
- 2. Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Sex differences in mortality after coronary artery bypass graft surgery. JAMA. 2004;292:40–41; author reply 41 [DOI] [PubMed] [Google Scholar]
- 3. Leja MJ, Shah DJ, Reardon MJ. Primary cardiac tumors. Tex Heart Inst J. 2011;38:261–262. [PMC free article] [PubMed] [Google Scholar]
- 4. Olivotto I, Maron MS, Adabag AS, Casey SA, Vargiu D, Link MS, Udelson JE, Cecchi F, Maron BJ. Gender‐related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:480–487. [DOI] [PubMed] [Google Scholar]
- 5. Meghji Z, Nguyen A, Fatima B, Geske JB, Nishimura RA, Ommen SR, Lahr BD, Dearani JA, Schaff HV. Survival differences in women and men after septal myectomy for obstructive hypertrophic cardiomyopathy. JAMA Cardiol. 2019;4:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doshi R, Shah P, Meraj P. Gender disparities among patients with peripheral arterial disease treated via endovascular approach: a propensity score matched analysis. J Interv Cardiol. 2017;30:604–611. [DOI] [PubMed] [Google Scholar]
- 7. Zhang PJ, Brooks JS, Goldblum JR, Yoder B, Seethala R, Pawel B, Gorman JH, Gorman RC, Huang JH, Acker M, Narula N. Primary cardiac sarcomas: a clinicopathologic analysis of a series with follow‐up information in 17 patients and emphasis on long‐term survival. Hum Pathol. 2008;39:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bear PA, Moodie DS. Malignant primary cardiac tumors. The Cleveland Clinic experience, 1956 to 1986. Chest. 1987;92:860–862. [DOI] [PubMed] [Google Scholar]
- 9. Pacini D, Careddu L, Pantaleo A, Parolari A, Leone O, Daprati A, Gargiulo GD, Di Bartolomeo R. Primary malignant tumors of the heart: outcomes of the surgical treatment. Asian Cardiovasc Thorac Ann. 2015;23:645–651. [DOI] [PubMed] [Google Scholar]
- 10. McHugh ML. The chi‐square test of independence. Biochem Med (Zagreb). 2013;23:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel N, Kalra R, Doshi R, Arora H, Bajaj NS, Arora G, Arora P. Hospitalization rates, prevalence of cardiovascular manifestations, and outcomes associated with sarcoidosis in the United States. J Am Heart Assoc. 2018;7:e007844 DOI: 10.1161/JAHA.117.007844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goel MK, Khanna P, Kishore J. Understanding survival analysis: Kaplan‐Meier estimate. Int J Ayurveda Res. 2010;1:274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nahm FS. Nonparametric statistical tests for the continuous data: the basic concept and the practical use. Korean J Anesthesiol. 2016;69:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramalho J, Nunes S, Marques I, Marques F. Primary cardiac sarcoma after breast cancer. BMJ Case Rep. 2013;2013:bcr2013008947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang J, Shang Y. Estrogen and cancer. Annu Rev Physiol. 2013;75:225–240. [DOI] [PubMed] [Google Scholar]
- 16. Weinick RM, Zuvekas SH, Cohen JW. Racial and ethnic differences in access to and use of health care services, 1977 to 1996. Med Care Res Rev. 2000;57(suppl 1):36–54. [DOI] [PubMed] [Google Scholar]
- 17. Barreiro M, Renilla A, Jimenez JM, Martin M, Al Musa T, Garcia L, Barriales V. Primary cardiac tumors: 32 years of experience from a Spanish tertiary surgical center. Cardiovasc Pathol. 2013;22:424–427. [DOI] [PubMed] [Google Scholar]
- 18. Burazor I, Aviel‐Ronen S, Imazio M, Markel G, Grossman Y, Yosepovich A, Adler Y. Primary malignancies of the heart and pericardium. Clin Cardiol. 2014;37:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6:219–228. [DOI] [PubMed] [Google Scholar]
- 20. Silverman NA. Primary cardiac tumors. Ann Surg. 1980;191:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simpson L, Kumar SK, Okuno SH, Schaff HV, Porrata LF, Buckner JC, Moynihan TJ. Malignant primary cardiac tumors: review of a single institution experience. Cancer. 2008;112:2440–2446. [DOI] [PubMed] [Google Scholar]
- 22. Truong PT, Jones SO, Martens B, Alexander C, Paquette M, Joe H, Hart J, Allan SJ. Treatment and outcomes in adult patients with primary cardiac sarcoma: the British Columbia Cancer Agency experience. Ann Surg Oncol. 2009;16:3358–3365. [DOI] [PubMed] [Google Scholar]
- 23. Jonavicius K, Salcius K, Meskauskas R, Valeviciene N, Tarutis V, Sirvydis V. Primary cardiac lymphoma: two cases and a review of literature. J Cardiothorac Surg. 2015;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Racial difference in the incidence of primary malignant cardiac tumors, stratified by sex.
Figure S2. Overall survival rate in men with primary malignant cardiac tumors stratified by age.
Figure S3. Overall survival rate in women with primary malignant cardiac tumors, stratified by age.
Figure S4. Overall survival rate by tumor type.


