Abstract
Objectives
Managing participants and their data are fundamental for the success of a clinical trial. Our review identifies and describes processes that deal with management of trial participants and highlights information technology (IT) assistance for clinical research in the context of participant management.
Methods
A scoping literature review design, based on the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement, was used to identify literature on trial participant-related proceedings, work procedures, or workflows, and assisting electronic systems.
Results
The literature search identified 1329 articles of which 111 were included for analysis. Participant-related procedures were categorized into 4 major trial processes: recruitment, obtaining informed consent, managing identities, and managing administrative data. Our results demonstrated that management of trial participants is considered in nearly every step of clinical trials, and that IT was successfully introduced to all participant-related areas of a clinical trial to facilitate processes.
Discussion
There is no precise definition of participant management, so a broad search strategy was necessary, resulting in a high number of articles that had to be excluded. Nevertheless, this review provides a comprehensive overview of participant management-related components, which was lacking so far. The review contributes to a better understanding of how computer-assisted management of participants in clinical trials is possible.
Keywords: clinical trials as topic, information systems, data management, research subjects, medical informatics
LAY SUMMARY
Clinical trials involving participants play a critical role in the development of new treatments. Management of participants and their data is fundamental for the success of a clinical trial. Information technology (IT) offers opportunities to approach clinical trial methodology in new ways, but so far there have been no comprehensive reviews of how computer-supported management of participants’ data is disseminated in trial management processes. We identified 4 main process categories of participant-related management and evaluated their supporting IT systems: recruitment, administration of informed consent, management of participants’ identities, and management of administrative participant data. Our results demonstrated that management of trial participants is considered in every step of clinical trials. Furthermore, we found that IT was successfully introduced to all participant-related areas of a clinical trial to facilitate processes. Our review contributes to a better understanding of how computer-assisted management of participants is possible. We anticipate our review to be a starting point for further research, providing a comprehensive overview of participant management-related components by categorizing existing literature in the field, and thereby giving an assessment of potential size and scope of available research literature.
INTRODUCTION
Clinical research is a key component of medical progress. Clinical trials are used to test efficacy and safety of drugs, new forms of treatment, medical interventions, or medical devices. After the trial planning and design phase, it is necessary to recruit volunteers as participants and inform them about the planned research with its aims, potential risks, and advantages. Tasks of the staff at a study site include the administration of contact data and obtaining informed consent of participants. Furthermore, scheduling and organization of examinations in the study context have to be managed. At many stages of the starting clinical trial, collaboration of the participants is necessary. The management of participants is, therefore, a core component of every successful person-related research project.
The organization and execution of modern clinical trials is not possible without the use of IT-assisted procedures.1–3 Clinical Data Management Systems have become an accepted part of clinical trial data management and are considered efficient in terms of data management requirements.4–8 For other trial management processes, such as management of participants, it is likely that new technologies to facilitate and accelerate workflows are used as well. However, to date, there have been no comprehensive reviews of how computer-supported management of participants is disseminated in trial management processes, and it is currently unknown what evidence exists to substantiate the presumed benefits of a computer-supported participant management.
This scoping review aimed to (1) identify and describe processes that deal with management of trial participants and (2) highlight IT assistance for clinical research in the context of participant management along the process chain. It provides a broad overview of published research regarding the management of participants for clinical trials, providing a basis for an in-depth analysis.
METHODS
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines9 for searching and selecting relevant publications. In 4 phases, articles were first identified by a literature database search, then pre-selected based on title and abstract, checked for suitability by means of the entire text, and finally the validated publications were included in the literature analysis.
Search strategy
A systematic search of literature regarding participant management in clinical research is challenging, due to a lack of consistently used standard terms. Medical Subject Headings, which is the controlled vocabulary thesaurus of the United States National Library of Medicine (NLM), defines “Persons who are enrolled in research studies or who are otherwise the subjects of research” as human subjects or as its preferred term research subjects.10 On the other hand, the website ClinicalTrials.gov, a database for clinical studies conducted around the world, and also provided by NLM, defines in their protocol registration data element definitions, that the term participant is used to refer to human subjects.11 When we started with a broad internet search to identify further keywords and indexing terms, we have seen the use of the terms subject, study-, trial-, research-participant or -patient, and -volunteer to describe someone who enrolls in a clinical research study. Which term is most appropriate will not be discussed here, but it shows that many keywords have to be included in the search strategy.
From the keywords we identified, we developed a database search strategy (Table 1). Databases were searched using a combination of (1) keywords for process flows in the management of participants, (2) participant and similar search terms, and lastly (3) clinical trial and synonyms. Additionally, we included keywords for electronic workflows that we already assigned to the topic of participant management, like electronic informed consent, recruitment system, or pseudonymization services. The challenge was to include all relevant keywords but avoid generic search terms such as informatics or data management, which yielded a too massive result as also off-topic literature was included. We searched a medical and a multidisciplinary science database to find relevant articles for this study: PubMed and Web of Science. The search strategy was mapped to the respective search query syntax and rules of the databases (Supplementary Appendix SA). As search categories, [Title/Abstract] in PubMed and [TS=Topic] in Web of Science were chosen to keep the search broad and include all relevant literature. The search was conducted at the end of July 2019.
Table 1.
Database search strategy
| 1 | (“participant management” OR “trial patient management” OR “study patient management” OR “volunteer management” OR “subject management” OR “trial management” OR “research management” OR “study management” OR “workflow management” OR “task management” OR “project management” OR “identity management” OR “pseudonymization service*” OR “pseudonymisation service*” OR “trusted third party” OR “consent management” OR “electronic consent” OR “electronic informed consent” OR “digital consent” OR “digital informed consent” OR “econsent” OR “e-consent” OR “participant management system*” OR “patient management system*” OR “recruitment system*” OR “enrollment system*” OR “eClinical”) |
| AND ( | |
| 2 | (participant* AND (“clinical study” OR “clinical studies” OR “clinical trial*” OR “clinical research”) OR patient* AND (study OR studies OR trial* OR research)) OR volunteer* AND (“clinical study” OR “clinical studies” OR “clinical trial*” OR “clinical research”) OR “human subject*” OR “research subject*”) |
| 3 | OR (“clinical trial*” OR “clinical research” OR “clinical study” OR “clinical studies”) |
| ) |
In addition, we looked for practice guidelines and available software products, and searched sources of gray literature. The gray literature search was based on the methodology outlined by Godin et al12 for which the same keywords were used as for the systematic literature search, but the search was customized to the syntax of the Google search engine.
Selection of articles and synthesis of results
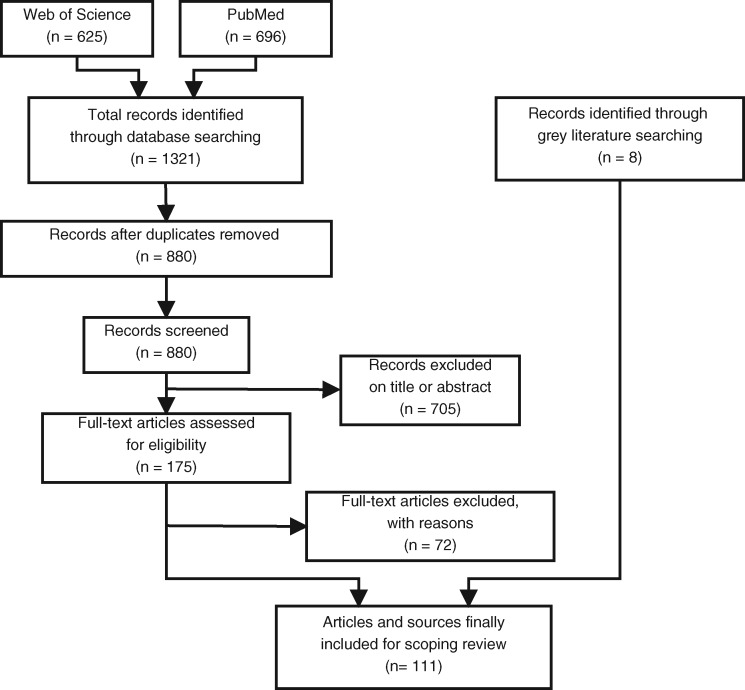
The literature selection process according to PRISMA is shown in Figure 1. Web of Science and PubMed searches identified the potentially relevant articles. A reference management software was used to manage the results of the literature search and the subsequent selection process of relevant articles. After removing duplicates from the results, the articles were screened by titles and abstracts. Articles were kept for further analysis, if they met one or more of the following 3 inclusion criteria:
Figure 1.
Flow diagram of the literature selection process.
Processes: We included articles that described processes, work procedures, or workflows from clinical trials that are trial participant-related. We excluded studies that only mentioned the use of participant management but did not describe how it was conducted. Processes in clinical trials vary depending on the design and aim of the study. Therefore, only procedures common across trials are taken into account.
IT assistance: We were interested in articles that described or evaluated electronic systems used in an administrative context with trial participants. This included systems that were either standalone systems or software as part of a bundled system.
Publication type: We were looking for systematic reviews and original articles in English.
Articles that did not meet the inclusion criteria were excluded. Articles meeting the inclusion criteria and, thereby, considered relevant for this review, were read and tagged with key terms of content to classify articles regarding the clinical trial processes that were covered. This classification was used to identify the main trial processes. In addition, gray literature allowed us to identify topics that are not covered by scientific publications.
RESULTS
The searches from Web of Science and PubMed yielded 1321 potentially relevant articles. Of those, 175 were selected for full-text screening, resulting in a total of 103 articles to be finally included in the review. Most of the excluded articles were not within our scope, did not describe the process of a clinical study but routine care, or described managing of patients or participants in an unrelated context for this review. In addition, 8 results of the gray literature search were included in the review,13–20 which provided information that were not accessible via scientific publications.
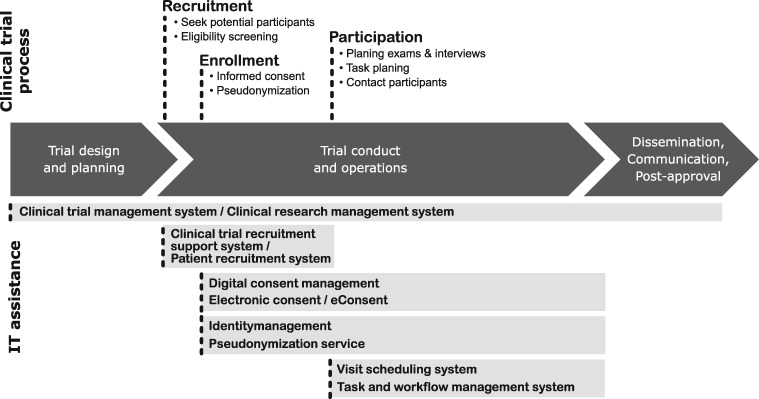
The screened articles were grouped into categories that represent the processes related to management of trial participants throughout a clinical trial execution, from concept development to trial result reporting. In Figure 2, we summarized these processes and highlight possible IT assistance for clinical research in the context of participant management along the process chain. Management of trial participants needs to be considered in nearly every step of the clinical trial—from recruitment of eligible participants and obtaining informed consent, to planning their participation by scheduling tasks and exams, to managing their contact data to inform them about study results and possible follow-up periods.
Figure 2.
Clinical trial management process from start to finish, highlighting processes with trial participant involvement over time. IT systems provide support for the corresponding processes.
The characteristics of the reviewed articles are presented in Table 2. Out of the 103 selected articles, 36 concerned recruitment workflows, 36 dealt with informed consent processes during enrollment, 12 described pseudonymization workflows during enrollment, and 19 articles dealt with processes during trial participation. As shown in Table 2, articles considered different types of IT assistance: for example, recruitment support system, digital consent management, and identity management.
Table 2.
Characteristics of reviewed articles
| Article | Trial process | IT assistance |
|---|---|---|
| Afrin et al,21 Ahmad et al,22 Breitfeld et al,23 Butte et al,24 Cuggia et al,25 Dowling et al,26 Dugas et al,27,28 Ferranti et al,29 Fink et al,30 Grundmeier et al,31 Harris et al,32 Heinemann et al,33 Khosropour et al,34 Kost et al,35 Kotoulas et al,36 Köpcke et al,37 Lagor et al,38 McDonald et al,39,40 Mattingly et al,41 Nielsen et al,42 Rollman et al,43 Schmickl et al,44 Schreiweis et al,45–47 Straube et al,48 Sully et al,49 Thadani et al,50 Thompson et al,51 Trinczek et al,52,53 Treweek et al,54 Walters et al,55 Zimmerman et al56 | Recruitment | Recruitment support system |
| Bergmann et al,57 Bethune et al,58 Bialke et al,59 Boutin et al,60 Buckley et al,61 Chen et al,62 Chhin et al,63 Doerr et al,64 Fink et al,65 Grady et al,66 Greenhalgh et al,67 Harle et al,68,69 Haussen et al,70 Iafrate et al,71 Kim et al,72 Kondylakis et al,73 Nijhawan et al,74 Phillippi et al,75 Ramos,76 Rothwell et al,77 Schreiweis et al,78 Shelton,79 Simon et al,80–82 Sommer et al,83 Soni et al,84 St John et al,85 Stevens et al,86 Suarez et al,87 Vanaken,88 Vanaken and Masand,89 Warriner et al,90 Welch et al,91 Wilbanks92 | Enrollment | Electronic informed consent, digital consent management |
| Aamot et al,93 Bialke et al,94 Bruland et al,95 Chevrier et al,96 Deserno et al,97 Ebner et al,98 Jonas et al,99 Lablans et al,100 Lautenschläger et al,101 Nurmi et al,102 Sahi et al,103 Schwaneberg et al104 | Enrollment | Identity management, pseudonymization service |
| Abshire et al,105 Almeida et al,106 Berard et al,107 Bose and Das,108 Campion et al,109 Cramon et al,110 Durkalski et al,111 Geyer et al,112 Gupta et al,113 Leroux et al,114 Müller et al,115 Nadkarni et al,116 Park et al,117 Raptis et al,118 Schobel et al,119 Schwanke et al,120 Solomon et al,121 Weng et al,122 Zhao and Pauls123 | Participation—managing administrative data | Task- and workflow management system, visit scheduling system, clinical trial management system, clinical research management system |
The selected literature addresses the various aspects of participant involvement in clinical trial processes. In the following chapters, we will reflect each process within the clinical trial workflow taken from the grouped articles with background information on the execution and how IT assistance can contribute.
Recruitment
Patient recruitment describes the process of finding suitable participants for clinical trials. Potential trial participants are usually identified by the treating physician, the study nurse, or the principal investigator. Conventional clinical trials often occur within the confines of health care settings, through the identification of participants during their clinical encounters, or prior to visits via electronic and paper healthcare records. Other channels to recruit participants are used as well such as advertisements, phone calls, use of third-party data, processing information from clinical trial registries or patient registries. Prior to formal enrollment in clinical trials, patients, interested in participating, will go through a screening process. The eligibility criteria, defined in the study protocol, describe characteristics that must be met by all participants.
A common problem with clinical trials is the achievement of the recruitment target. Trials often fail to achieve the required recruitment numbers or to recruit the necessary number of patients within the planned recruitment time.39 Between 50% and 60% of randomized clinical trials do not meet their original recruitment targets or face significant delays.49,55 Poor recruitment can lead to the prolongation of trials, increasing the costs, and can lead to an underpowered study from which wrong conclusions may be drawn. Several potential limiting factors have been identified in the literature, including organizational, financial, or trial management-related difficulties.40,48 Barriers to patients’ involvement include lack of knowledge, general lack of trust in trials, and disagreement with the assignment to a certain treatment group.40 There are several proposals how to increase recruitment rates in trials. A recent systematic review of methods to improve recruitment numbers in randomized controlled trials compared 68 recruitment strategies.54
IT assistance offers the potential to improve the recruiting processes and meet the recruitment target. Clinical Trial Recruitment Support Systems (CTRSS), or sometimes called Patient Recruitment Systems, have been designed to support the patient recruitment process by suggesting potentially suitable study participants. These CTRSS use electronic patient data, typically from electronic health records, to assess patient eligibility for a clinical trial. The system alerts of a patients’ study eligibility or provides a list of potential trial participants to a study investigator. Our search provided a total of 30 relevant articles on CTRSS,21–38,41–47,50–53,56 which indicates that CTRSS are potentially beneficial in managing trial participants.
A comprehensive review of employing IT for the recruitment for clinical trials was done by Köpcke and Prokosch.37 Compared to a previous review paper by Cuggia et al,25 which analyzed 28 CTRSS from articles published before October 2009, Köpcke and Prokosch reviewed 101 papers on 79 different systems and created an overview of all CTRSS reported until the end of 2013. They distinguished between 3 types of CTRSS: (1) systems for the retrospective identification of trial participants based on existing clinical data, (2) systems that monitored the appearance of a key event of an existing health IT component in which the occurrence of the event triggered an eligibility test of a patient, and (3) independent systems that required a user to enter patient data into an interface to trigger an eligibility assessment.
While in scientific publications the non-commercial part of clinical research is mainly considered, there is also an industrial view of patient recruitment. For profit-oriented companies—such as pharmaceutical and medical device enterprises—a short time to market is crucial. Accelerating patient recruitment for clinical trials with CTRSSs can be one effective component to reduce time until revenues are generated. The patient recruitment industry is valued at a total of $19 billion per year.15 Some companies outsource their patient recruitment-related needs to specialty service providers.14 IT has impacted this branch as well. Several companies have built proprietary software for a range of activities that rely on patient recruitment. The website capterra.com, an online peer-review site that aims to help businesses find software solutions, lists 36 products for Recruiting Management in the category Clinical Trial Software.13
Enrollment—informed consent
Before a participant can enroll in a clinical trial, they must be recruited, screened, and must give their informed consent.16 Informed consent is one of the founding principles of modern research ethics. It is a process by which “a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate,” as stated in the International Council for Harmonisation’s (ICH) Good Clinical Practice guidelines (GCP).17 Informed consent is not only required for clinical trials, but is an essential prerequisite before enrolling a participant in any type of research involving human subjects.74 The process of obtaining informed consent is tightly regulated. Requirements are defined in federal laws and regulations as well as in a worldwide harmonization approach, the ICH-GCP guidelines, and the guidelines for Data Protection of the Organisation for Economic Co-operation and Development.17,18 Informed consent is documented by means of a written, signed, and dated informed consent form (ICF). In accordance with GCP, the signed ICF has to be stored in an Investigator Site File.17
In recent years, there has been a greater use of electronic methods to gain informed consent (called eIC or e-consent) in research studies.57–65,67–92 The New England Journal of Medicine published in “The Changing Face of Informed Consent” innovative approaches to improve and expand the electronic informed consent process for researchers and participants.66 e-Consents can include multimedia information (graphics or videos) and interactive components to increase the understanding of the study purpose and is less focused on signing legal documents. e-Consents can be divided into 3 levels of consent management maturity; from plain paper-based consent to combining paper and electronic means to solely relying on e-consent (Table 3).20 e-Consent management solutions enable to manage a large number and variety of research projects in a hospital due to process automation.59
Table 3.
e-Consent management divided into 3 levels, describing the options of collecting and using structured data in e-consent
| Level | Type | Structure | Explanation |
|---|---|---|---|
| Level I | Paper consent form | No structured data | Consent on a paper form, which is scanned and kept electronically |
| Level II | Paper and e-consent form | Some structured data | Consent on a paper form, which is transferred into an electronic format (manually or by automatic parsing of paper-based consent scans) |
| Level III | e-consent form | Structured data | Consent in an electronic form. IT systems can interpret and process participants consent decisions from structured data |
Note: In accordance with ref.20
Positive results using e-consent are increasingly reported by clinical studies, in which electronic management is perceived by, both, participants and staff members as straightforward, efficient, and simplifying workflows.77,88 As a reaction to the increasing use, the Food and Drug Administration and the Office of Human Research Protections published a joint guidance for the use of e-consent for institutional review boards, investigators, and sponsors in December 2016.19 e-Consent has been accepted by central institutional review boards in the United States and some other countries. However, launching of e-consent is challenging in international trials as each country has different legal requirements for the use of e-consent.89
Enrollment—identity management and pseudonymization service
Preserving participants’ privacy is crucial in clinical research.102 De-identification and anonymization are the 2 most common terms used to refer to the technical approaches that protect privacy.96 However, anonymous data collection are only possible in very few clinical trials. Many clinical study designs require to capture and integrate data from heterogeneous data sources or from different locations, which requires the identification of participants. Here, one of the most important requirements is keeping acquired clinical data and personal information of participants apart to ensure confidentiality and data protection.98,100,101 Therefore, an identification code (pseudonym) is assigned to each participant upon enrollment. The identification code is saved in a confidential list [subject identification code list (ICL)], also including the name of the participant, the date informed consent was obtained, and all additional information needed to relate a participant to their identification code (eg, gender, date of birth). Clinical data, documented in case report forms, are encoded with the identification code and cannot be directly related to the participant’s identity. Only by means of the ICL can the acquired clinical data be connected to identifying information. Linking data and identity, however, is a necessity, as stated in the GCP guidelines,17 to allow contacting participants in case medical irregularities during a trial emerge.
The described workflow is often conducted paper-based, especially in local (ie, single site) and small trials. However, the procedure quickly reaches its limits when dealing with larger projects or greater technical requirements. Technical requirements increase, for example, for collaborative acquisition of data (eg, in multi-site studies), which has become a core element of biomedical research over the last years. To advance data management and improve data quality in clinical trials, electronic data capture (EDC) systems are increasingly used.4–8 Integrating pseudonymization services into EDC systems is, therefore, a chance to improve and facilitate the described procedure by making use of IT infrastructures.95,97,104 In this context, digital solutions that are compliant with data protection legislation are needed to ensure (1) that participants who appear in multiple institutions obtain the same pseudonym across sites and are not handled as 2 different individuals (identity management) and that (2) unambiguous pseudonyms are assigned for each participant and each separate system (pseudonymization service). A comprehensive overview of current methods and applications for pseudonymization services can be found in the literature.93,103 For identity management and pseudonymization services, a trusted third party (TTP) is often assigned to handle the ICLs.94 Participants can then only be identified by the person in charge of the identifying data. A recent study has investigated a novel approach to pseudonymization without the necessary use of a TTP, but requires a higher accountability of the participant.99
Managing administrative participant data
Commitment of study participants is essential to ensure the power and internal validity of longitudinal research. One of the most commonly used strategies to avoid losing participants are reminder, contact, and regular scheduling methods.105 The participant’s contact information is securely stored at each clinical site for internal use during the study. The information is collected on a so-called locator form—a working document of all contact information that can possibly help the researcher team find the participant when needed.121 At the end of the study, all records will be kept in a secure location for as long as dictated by local regulations. The clinical research staff is in charge of scheduling appointments for trial visits and may send reminders to participants. Depending on the trial protocol, the trial visits could include lab tests, X-rays, computed tomography scans, physical exams, etc.
Using a task and workflow management system for clinical studies, all the processes associated with a health research study can be simplified.106 These systems support the setup of clinical trials, the management of participants, as well as the overall governance process. Electronic clinical research visit scheduling systems provide the potential of coordinating trial visits with patient care visits. Thereby, the efficiency at clinical sites as well as the likelihood of participants keeping their trial appointments can be increased.122 Unnecessary or redundant visits or tests for patients, and a considerable administrative burden for involved institutions can, thus, be avoided. The ability to manage participant schedules displayed in calendar form can be a feature of a Clinical Trial Management System (CTMS).117 Managing administrative participant data in software systems is often not a standalone application. CTMS107,108,110–112,114,117–119,123 or Clinical Research Management Systems (CRMS)109,115,116 support the whole clinical trial process. Although the functional scope of a CTMS or a CRMS is larger and varies from product to product, these systems also manage trial participants and support tasks in the administration of study participants. Systems that were intended for a different purpose, however, can be used for the management of study participants as well, such as custom-build case management systems for participant data113 or customer relationship management systems for the management of study participants.120
DISCUSSION
Trial participant management in its generic concept stands for a number of processes that handle participants and their data in clinical research. However, there is no precise definition of participant management as a distinct subset of processes in clinical research. For the purposes of this review, we selected articles that either describe processes that deal with management of trial participants, or in which IT assistance for clinical research in the context of participants is used. This made a broad search strategy necessary, including many keywords (Table 1). In our opinion, using the determined terms was sufficient to provide a broad range of participant-related processes and workflows. Disadvantage of the broad search was the high number of articles (n = 656) that had to be excluded. Many of the excluded articles described clinical trials on specific diseases and focused on their results, but were only casually mentioning the participant managing processes. Based on the included articles, we identified the key components of participant management in clinical research, divided into 4 processes: recruitment, obtaining informed consent, managing identities, and managing administrative data. We found that IT was successfully introduced to all participant-related areas of clinical trials. However, our review is limited insofar that the processes identified are based on the literature and information publically accessible. In our experience, clinical trials use far more IT assistance than is mentioned in the available literature on clinical trials. Most medical publications, however, describe little about methods or systems that have supported the data collection in an operational context. Additionally, traditional methodologies that rely on paper-based processes are also rarely published. We intended to emphasize the benefits of using IT-based solutions and technologies, but similar to missing process descriptive literature, studies comparing paper-based and IT-assisted management are also lacking.
A good overview of research activities was found for the recruitment process of clinical trials. It shows the importance of the topic for medical research—patient recruitment is vital to the success of any trial. The use of CTRSSs dates back to the early 1990s. Since then, CTRSSs have become even more popular and numerous publications have appeared. Although CTRSSs have been established, some of the problems in recruitment still persist, which require further research.
The combination of organizational, legal, ethical, and technical approaches is necessary to protect health data. The first and most important basis is established by gaining informed consent. Working with pseudonymous data is another important element to protect privacy and confidentiality. Nevertheless, a central question that researchers have to explore is how to protect privacy and confidentiality in constantly changing clinical research practice, technical possibilities, and legislation. For instance, getting ethical approval for studies varies enormously across European countries.124
After conducting the literature review, we noted that few publications deal with the managing of administrative participant data in a clinical trial. This is surprising as these processes must be conducted in every clinical trial. It can be assumed that administrative patient management processes are often managed with the common office IT tools.
IT can assist clinical research in the context of participant management. However, the degree of digitalization depends on the respective clinical trial. While the biopharmaceutical industry often uses more complex software solutions to manage the whole process of a clinical trial, the non-commercial clinical study research is generally less well equipped.8,125–127 This is also reflected in the fact that commercial software products were not mentioned at all in scientific publications, although there is a strong market for these products.
Attention has been paid to each participant-related topic individually in the literature, but a synthesis addressing all aspects of participant management in clinical research was lacking so far. This review provides a comprehensive overview of participant management-related components.
CONCLUSION
Conducting a clinical trial typically implies management processes handling participants and their data. Participant-related processes include recruitment, administration of informed consent, management participants’ identities with pseudonyms, and the management of administrative participant data. Effective use of available technologies is a crucial advantage. This article categorizes existing literature in the field, and thereby gives an assessment of potential size and scope of available research literature. It contributes to a better understanding of how computer-supported management of participants is disseminated in trial management processes. Further research should focus on availability, interconnectivity, and costs of IT assistance for participant management presented here. Additionally, the comparison of various participant management methods and their impact on the trial success as well as the comparison of management in different trial types should find more attention in future studies.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. This publication was supported by the Open Access Publication Funds of the Göttingen University.
AUTHOR CONTRIBUTIONS
JP and OR developed the study design. JP was responsible for data acquisition, analysis, and interpretation, and wrote the first draft of the manuscript. OR participated in the revision and approved the final version of the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Krishnankutty B, Bellary S, Kumar NBR, et al. Data management in clinical research: an overview. Indian J Pharmacol 2012; 44 (2): 168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhatti P, Schemitsch EH, Bhandari M. Managing data in surgical trials: a guide to modern-day data management systems. J Bone Joint Surg Am 2012; 94 (Suppl 1): 45–8. [DOI] [PubMed] [Google Scholar]
- 3. Payne PRO. The clinical research environment In: Richesson RL, Andrews JE, eds. Clinical Research Informatics. Cham: Springer International Publishing; 2019: 27–47. [Google Scholar]
- 4. Sahoo U, Bhatt A. Electronic data capture (EDC)—a new mantra for clinical trials. Quality Assurance 2004; 10 (3–4): 117–21. [DOI] [PubMed] [Google Scholar]
- 5. El Emam K, Jonker E, Sampson M, et al. The use of electronic data capture tools in clinical trials: web-survey of 259 Canadian trials. J Med Internet Res 2009; 11 (1): e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schrimpf D, Haag M, Pilz LR. Possible combinations of electronic data capture and randomization systems. Methods Inf Med 2014; 53 (3): 202–7. [DOI] [PubMed] [Google Scholar]
- 7. Ohmann C, Kuchinke W, Canham S, et al. ; the ECRIN Working Group on Data Centres. Standard requirements for GCP-compliant data management in multinational clinical trials. Trials 2011; 12 (1): 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohmann C, Speer R. Klinische Studien. In: Drepper J, Semler SC, eds. IT-Infrastrukturen in Der Patientenorientierten Forschung Aktueller Stand Und Handlungsbedarf - 2016. Berlin: Akademische Verlagsgesellschaft AKA GmbH; 2016: 25–47. [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, et al. ; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6 (7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medical Subject Headings (MeSH): Heading: Research Subjects, Unique ID: D035842. https://meshb.nlm.nih.gov/record/ui?ui=D035842 Accessed July 26, 2019.
- 11.ClinicalTrials.gov: Protocol Registration Data Element Definitions for Interventional and Observational Studies. https://prsinfo.clinicaltrials.gov/definitions.html Accessed July 26, 2019.
- 12. Godin K, Stapleton J, Kirkpatrick SI, et al. Applying systematic review search methods to the grey literature: a case study examining guidelines for school-based breakfast programs in Canada. Syst Rev 2015; 4 (1): 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.capterra.com. https://www.capterra.com/clinical-trial-management-software/?utf8=%E2%9C%93&feature%5B7%5D=30992&commit=Apply+Filters Accessed September 05, 2019.
- 14.PR News Wire. Patient Recruitment and Retention Services Market, 2019-2030 2019. https://www.prnewswire.com/news-releases/patient-recruitment-and-retention-services-market-2019-2030-300834895.html Accessed September 05, 2019.
- 15.PR News Wire. Outcome Health Introduces Clinical Trial Solution to Boost Patient Recruitment 2017. https://www.prnewswire.com/news-releases/outcome-health-introduces-clinical-trial-solution-to-boost-patient-recruitment-300395346.html Accessed September 05, 2019.
- 16.EUPATI - European Patients’ Academy on Therapeutic Innovation. Enrolling in clinical trials 2015. https://www.eupati.eu/clinical-development-and-trials/enrolling-clinical-trials/ Accessed September 05, 2019.
- 17.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Guideline for Good Clinical Practice ICH E6 (R2), 2nd ed. 2016. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf Accessed January 25, 2019.
- 18.The Organisation for Economic Co-operation and Development (OECD). Guidelines on the Protection of Privacy and Transborder Flows of Personal Data: The OECD Privacy Framework 2013. http://www.oecd.org/sti/ieconomy/oecd_privacy_framework.pdf Accessed January 29, 2019.
- 19.U.S. Department of Health and Human Services Office for Human Research Protections (OHRP) Food and Drug Administration (FDA). Use of Electronic Informed Consent. Questions and Answers: Guidance for Institutional Review Boards, Investigators, and Sponsors 2016. https://www.fda.gov/downloads/drugs/guidances/ucm436811.pdf Accessed January 29, 2019.
- 20.MITRE Corporation. Electronic Consent Management: Landscape Assessment, Challenges, and Technology, 1st ed. 2014. https://www.healthit.gov/sites/default/files/privacy-security/ecm_finalreport_forrelease62415.pdf Accessed February 16, 2019.
- 21. Afrin LB, Oates JC, Boyd CK, et al. Leveraging of open EMR architecture for clinical trial accrual. AMIA Annu Symp Proc 2003; 2003: 16–20. [PMC free article] [PubMed] [Google Scholar]
- 22. Ahmad F, Gupta R, Kurz M. Real time electronic patient study enrollment system in emergency room. AMIA Annu Symp Proc 2005; 2005: 881. [PMC free article] [PubMed] [Google Scholar]
- 23. Breitfeld PP, Ullrich F, Anderson J, et al. Web-based decision support for clinical trial eligibility determination in an international clinical trials network. Control Clin Trials 2003; 24 (6): 702–10. [DOI] [PubMed] [Google Scholar]
- 24. Butte AJ, Weinstein DA, Kohane IS. Enrolling patients into clinical trials faster using RealTime Recuiting. Proc AMIA Symp 2000; 111–5. [PMC free article] [PubMed] [Google Scholar]
- 25. Cuggia M, Besana P, Glasspool D. Comparing semi-automatic systems for recruitment of patients to clinical trials. Int J Med Inform 2011; 80 (6): 371–88. [DOI] [PubMed] [Google Scholar]
- 26. Dowling NM, Olson N, Mish T, et al. A model for the design and implementation of a participant recruitment registry for clinical studies of older adults. Clinical Trials 2012; 9 (2): 204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dugas M, Amler S, Lange M, et al. Estimation of patient accrual rates in clinical trials based on routine data from hospital information systems. Methods Inf Med 2009; 48 (03): 263–6. [DOI] [PubMed] [Google Scholar]
- 28. Dugas M, Lange M, Berdel WE, et al. Workflow to improve patient recruitment for clinical trials within hospital information systems—a case-study. Trials 2008; 9 (1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferranti JM, Gilbert W, McCall J, et al. The design and implementation of an open-source, data-driven cohort recruitment system: the Duke Integrated Subject Cohort and Enrollment Research Network (DISCERN). J Am Med Inform Assoc 2012; 19 (e1): e68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fink E, Hall LO, Goldgof DB, eds., et al. Experiments on the automated selection of patients for clinical trials. In: SMC’03 Conference Proceedings. 2003 IEEE International Conference on Systems, Man and Cybernetics. Conference Theme—System Security and Assurance (Cat. No.03CH37483) 2003; Washington, DC.
- 31. Grundmeier RW, Swietlik M, Bell LM. Research subject enrollment by primary care pediatricians using an electronic health record. AMIA Annu Symp Proc 2007; 2007: 289–93. [PMC free article] [PubMed] [Google Scholar]
- 32. Harris PA, Scott KW, Lebo L, et al. ResearchMatch: a national registry to recruit volunteers for clinical research. Acad Med 2012; 87 (1): 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heinemann S, Thuring S, Wedeken S, et al. A clinical trial alert tool to recruit large patient samples and assess selection bias in general practice research. BMC Med Res Methodol 2011; 11 (1): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khosropour CM, Dombrowksi JC, Hughes JP, et al. Evaluation of a computer-based recruitment system for enrolling men who have sex with men into an observational HIV behavioral risk study. Am J Epidemiol 2016; 184 (6): 477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kost RG, Corregano LM, Rainer T-L, et al. A data-rich recruitment core to support translational clinical research. Clin Transl Sci 2015; 8 (2): 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kotoulas A, Lambrou G, Koutsouris D-D. Design and virtual implementation of a biomedical registry framework for the enhancement of clinical trials: colorectal cancer example. BMJ Health Care Inform 2019; 26 (1): e100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Köpcke F, Prokosch H-U. Employing computers for the recruitment into clinical trials: a comprehensive systematic review. J Med Internet Res 2014; 16 (7): e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lagor C, Aronsky D, Fiszman M, et al. Automatic identification of patients eligible for a pneumonia guideline: comparing the diagnostic accuracy of two decision support models. Stud Health Technol Inform 2001; 84 (Pt 1): 493–7. [PubMed] [Google Scholar]
- 39. McDonald AM, Knight RC, Campbell MK, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 2006; 7 (1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McDonald AM, Treweek S, Shakur H, et al. Using a business model approach and marketing techniques for recruitment to clinical trials. Trials 2011; 12 (1): 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mattingly WA, Kelley RR, Wiemken TL, et al. Real-time enrollment dashboard for multisite clinical trials. Contemp Clin Trials Commun 2015; 1: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nielsen JS, Thomsen RW, Steffensen C, et al. The Danish Centre for Strategic Research in Type 2 Diabetes (DD2) study: implementation of a nationwide patient enrollment system. Clin Epidemiol 2012; 4 (Suppl 1): 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rollman BL, Fischer GS, Zhu F, et al. Comparison of electronic physician prompts versus waitroom case-finding on clinical trial enrollment. J Gen Intern Med 2008; 23 (4): 447–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmickl CN, Li M, Li G, et al. The accuracy and efficiency of electronic screening for recruitment into a clinical trial on COPD. Respir Med 2011; 105 (10): 1501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schreiweis B, Bergh B. Requirements for a patient recruitment system. Stud Health Technol Inform 2015; 210: 521–5. [PubMed] [Google Scholar]
- 46. Schreiweis B, Gruber G, Bergh B. First experiences in implementing a software-based support for patient recruitment at Heidelberg university hospital. Stud Health Technol Inform 2012; 180: 1147–9. [PubMed] [Google Scholar]
- 47. Schreiweis B, Trinczek B, Kopcke F, et al. Comparison of electronic health record system functionalities to support the patient recruitment process in clinical trials. Int J Med Inform 2014; 83 (11): 860–8. [DOI] [PubMed] [Google Scholar]
- 48. Straube C, Herschbach P, Combs SE. Which obstacles prevent us from recruiting into clinical trials: a survey about the environment for clinical studies at a German University Hospital in a Comprehensive Cancer Center. Front Oncol 2017; 7: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sully BGO, Julious SA, Nicholl J. A reinvestigation of recruitment to randomised, controlled, multicenter trials: a review of trials funded by two UK funding agencies. Trials 2013; 14 (1): 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thadani SR, Weng C, Bigger JT, et al. Electronic screening improves efficiency in clinical trial recruitment. J Am Med Inform Assoc 2009; 16 (6): 869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thompson DS, Oberteuffer R, Dorman T. Sepsis alert and diagnostic system: integrating clinical systems to enhance study coordinator efficiency. Comput Inform Nurs 2003; 21 (1): 22–6. [DOI] [PubMed] [Google Scholar]
- 52. Trinczek B, Köpcke F, Leusch T, et al. Design and multicentric implementation of a generic software architecture for patient recruitment systems re-using existing HIS tools and routine patient data. Appl Clin Inform 2014; 5 (1): 264–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trinczek B, Schulte B, Breil B, et al. Patient recruitment workflow with and without a patient recruitment system. Stud Health Technol Inform 2013; 192: 1124. [PubMed] [Google Scholar]
- 54. Treweek S, Pitkethly M, Cook J, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev 2018; (2): 1465–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Walters SJ, Bonacho dos Anjos Henriques-Cadby I, Bortolami O, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 2017; 7 (3): e015276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zimmerman LP, Goel S, Sathar S, et al. A novel patient recruitment strategy: patient selection directly from the community through linkage to clinical data. Appl Clin Inform 2018; 09 (1): 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bergmann J, Bott OJ, Hoffmann I, et al. An eConsent-based system architecture supporting cooperation in integrated healthcare networks. Stud Health Technol Inform 2005; 116: 961–6. [PubMed] [Google Scholar]
- 58. Bethune A, Davila-Foyo M, Valli M, et al. e-Consent: approaching surgical consent with mobile technology. Can J Surg 2018; 61 (5): 339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bialke M, Bahls T, Geidel L, et al. MAGIC: once upon a time in consent management—a FHIR tale. J Transl Med 2018; 16 (1): 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boutin TN, Mathieu K, Hoffnagle GA, et al. Implementation of electronic consent at a biobank: an opportunity for precision medicine research. J Pers Med 2016; 6 (2): 2075–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Buckley MT, Lengfellner JM, Koch MJ, et al. The Memorial Sloan Kettering (MSK) electronic informed consent (eIC) platform for clinical trials: an operational model and suite of tools for obtaining informed consent, and managing consent documents. J Clin Oncol 2018; 36 (15_suppl): e18577. [Google Scholar]
- 62. Chen C, Turner SP, Sholle ET, et al. Evaluation of a REDCap-based workflow for supporting federal guidance for electronic informed consent. AMIA Jt Summits Transl Sci Proc 2019; 2019: 163–72. [PMC free article] [PubMed] [Google Scholar]
- 63. Chhin V, Roussos J, Michaelson T, et al. Leveraging mobile technology to improve efficiency of the consent-to-treatment process. JCO Clin Cancer Inform 2017; 1 (1): 1–8. [DOI] [PubMed] [Google Scholar]
- 64. Doerr M, Truong AM, Bot BM, et al. Formative evaluation of participant experience with mobile eConsent in the app-mediated Parkinson mPower study: a mixed methods study. JMIR Mhealth Uhealth 2017; 5 (2): e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fink AS, Prochazka AV, Henderson WG, et al. Enhancement of surgical informed consent by addition of repeat back: a multicenter, randomized controlled clinical trial. Ann Surg 2010; 252 (1): 27–36. [DOI] [PubMed] [Google Scholar]
- 66. Grady C, Cummings SR, Rowbotham MC, et al. Informed consent. N Engl J Med 2017; 376 (9): 856–67. [DOI] [PubMed] [Google Scholar]
- 67. Greenhalgh T, Morris L, Wyatt JC, et al. Introducing a nationally shared electronic patient record: case study comparison of Scotland, England, Wales and Northern Ireland. Int J Med Inform 2013; 82 (5): e125. [DOI] [PubMed] [Google Scholar]
- 68. Harle CA, Golembiewski EH, Rahmanian KP, et al. Patient preferences toward an interactive e-consent application for research using electronic health records. J Am Med Inform Assoc 2018; 25:360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Harle CA, Golembiewski EH, Rahmanian KP, et al. Does an interactive trust-enhanced electronic consent improve patient experiences when asked to share their health records for research? A randomized trial. J Am Med Inform Assoc 2019; 26 (7): 620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haussen DC, Doppelheuer S, Schindler K, et al. Utilization of a smartphone platform for electronic informed consent in acute stroke trials. Stroke 2017; 48 (11): 3156–60. [DOI] [PubMed] [Google Scholar]
- 71. Iafrate RP, Lipori GP, Harle CA, et al. Consent2Share: an integrated broad consenting process for re-contacting potential study subjects. J Clin Transl Res 2017; 2 (4): 113–22. [PMC free article] [PubMed] [Google Scholar]
- 72. Kim H, Bell E, Kim J, et al. iCONCUR: informed consent for clinical data and bio-sample use for research. J Am Med Inform Assoc 2017; 24 (2): 380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kondylakis H, Koumakis L, Hanold S, et al. Donor’s support tool: enabling informed secondary use of patient’s biomaterial and personal data. Int J Med Inform 2017; 97: 282–92. [DOI] [PubMed] [Google Scholar]
- 74. Nijhawan LP, Janodia MD, Muddukrishna BS, et al. Informed consent: Issues and challenges. J Adv Pharm Technol Res 2013; 4 (3): 134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Phillippi JC, Doersam JK, Neal JL, et al. Electronic informed consent to facilitate recruitment of pregnant women into research. J Obstet Gynecol Neonatal Nurs 2018; 47 (4): 529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ramos SR. User-centered design, experience, and usability of an electronic consent user interface to facilitate informed decision-making in an HIV clinic. Comput Inform Nurs 2017; 35 (11): 556–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rothwell E, Wong B, Rose NC, et al. A randomized controlled trial of an electronic informed consent process. J Empir Res Hum Res Ethics 2014; 9 (5): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schreiweis B, Bronsch T, Merzweiler A, et al. Implementing modular research consents using IHE advanced patient privacy consents. Stud Health Technol Inform 2018; 247: 840–4. [PubMed] [Google Scholar]
- 79. Shelton RH. Electronic consent channels: preserving patient privacy without handcuffing researchers. Sci Transl Med 2011; 3 (69): 69cm4. [DOI] [PubMed] [Google Scholar]
- 80. Simon CM, Klein DW, Schartz HA. Traditional and electronic informed consent for biobanking: a survey of U.S. biobanks. Biopreserv Biobank 2014; 12 (6): 423–29. [DOI] [PubMed] [Google Scholar]
- 81. Simon CM, Klein DW, Schartz HA. Interactive multimedia consent for biobanking: a randomized trial. Genet Med 2016; 18 (1): 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Simon CM, Schartz HA, Rosenthal GE, et al. Perspectives on electronic informed consent from patients underrepresented in research in the United States: a focus group study. J Empir Res Hum Res Ethics 2018; 13 (4): 338–48. [DOI] [PubMed] [Google Scholar]
- 83. Sommer C, Zuccolin D, Arnera V, et al. Building clinical trials around patients: evaluation and comparison of decentralized and conventional site models in patients with low back pain. Contemp Clin Trials Commun 2018; 11: 120–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Soni H, Grando A, Murcko A, et al. Current state of electronic consent processes in behavioral health: outcomes from an observational study. AMIA Annu Symp Proc 2017; 2017: 1607–16. [PMC free article] [PubMed] [Google Scholar]
- 85. St John ER, Scott AJ, Irvine TE, et al. Completion of hand-written surgical consent forms is frequently suboptimal and could be improved by using electronically generated, procedure-specific forms. Surgeon 2017; 15 (4): 190–95. [DOI] [PubMed] [Google Scholar]
- 86. Stevens N, Edwards L, Balayah Z, et al. Risk based survey evidence supports electronic informed consent as a recruitment method for UK clinical trials. J Clin Epidemiol 2016; 77: 134–36. [DOI] [PubMed] [Google Scholar]
- 87. Suarez A, Reilly C, Fajgenbaum DC. Quantitative analysis of a rare disease network’s international contact database and E-repository provides insights into biobanking in the electronic consent era. Orphanet J Rare Dis 2019; 14 (1): 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vanaken H. eConsent study provides insights to shape industry adoption. Applied Clinical Trials 2016; 25 (8). http://www.appliedclinicaltrialsonline.com/econsent-study-provides-insights-shape-industry-adoption Accessed February 18, 2019. [Google Scholar]
- 89. Vanaken H, Masand SN. Awareness and collaboration across stakeholder groups important for eConsent achieving value-driven adoption. Ther Innov Regul Sci 2019; 53 (6): 724–735. [DOI] [PubMed] [Google Scholar]
- 90. Warriner AH, Foster PJ, Mudano A, et al. A pragmatic randomized trial comparing tablet computer informed consent to traditional paper-based methods for an osteoporosis study. Contemp Clin Trials Commun 2016; 3: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Welch BM, Marshall E, Qanungo S, et al. Teleconsent: a novel approach to obtain informed consent for research. Contemp Clin Trials Commun 2016; 3: 74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wilbanks J. Design issues in E-consent. J Law Med Ethics 2018; 46 (1): 110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Aamot H, Kohl CD, Richter D, et al. Pseudonymization of patient identifiers for translational research. BMC Med Inform Decis Mak 2013; 13 (1): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bialke M, Penndorf P, Wegner T, et al. A workflow-driven approach to integrate generic software modules in a trusted third party. J Transl Med 2015; 13 (1): 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bruland P, Doods J, Brix T, et al. Connecting healthcare and clinical research: workflow optimizations through seamless integration of EHR, pseudonymization services and EDC systems. Int J Med Inform 2018; 119: 103–08. [DOI] [PubMed] [Google Scholar]
- 96. Chevrier R, Foufi V, Gaudet-Blavignac C, et al. Use and understanding of anonymization and de-identification in the biomedical literature: scoping review. J Med Internet Res 2019; 21 (5): e13484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Deserno TM, Haak D, Brandenburg V, et al. Integrated image data and medical record management for rare disease registries. A general framework and its instantiation to the German Calciphylaxis Registry. J Digit Imaging 2014; 27 (6): 702–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ebner H, Hayn D, Falgenhauer M, et al. Piloting the European Unified Patient Identity Management (EUPID) concept to facilitate secondary use of neuroblastoma data from clinical trials and biobanking. Stud Health Technol Inform 2016; 223: 31–38. [PubMed] [Google Scholar]
- 99. Jonas S, Siewert S, Spreckelsen C. Privacy-preserving record grouping and consent management based on a public-private key signature scheme: theoretical analysis and feasibility study. J Med Internet Res 2019; 21 (4): e12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lablans M, Borg A, Uckert F. A RESTful interface to pseudonymization services in modern web applications. BMC Med Inform Decis Mak 2015; 15 (1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lautenschläger R, Kohlmayer F, Prasser F, et al. A generic solution for web-based management of pseudonymized data. BMC Med Inform Decis Mak 2015; 15 (1): 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nurmi S-M, Kangasniemi M, Halkoaho A, et al. Privacy of clinical research subjects: an integrative literature review. J Empir Res Hum Res Ethics 2019; 14 (1): 33–48. [DOI] [PubMed] [Google Scholar]
- 103. Sahi MA, Abbas H, Saleem K, et al. Privacy preservation in e-healthcare environments: state of the art and future directions. IEEE Access 2018; 6: 464–78. [Google Scholar]
- 104. Schwaneberg T, Weitmann K, Dosch A, et al. Data privacy management and data quality monitoring in the German Centre for Cardiovascular Research’s multicentre TranslatiOnal Registry for CardiomyopatHies (DZHK-TORCH). ESC Heart Fail 2017; 4 (4): 440–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Abshire M, Dinglas VD, Cajita MIA, et al. Participant retention practices in longitudinal clinical research studies with high retention rates. BMC Med Res Methodol 2017; 17 (1): 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Almeida JR, Gini R, Roberto G, et al. TASKA: a modular task management system to support health research studies. BMC Med Inform Decis Mak 2019; 19 (1): 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Berard C, Cloutier LM, Cassivi L. Evaluating clinical trial management systems: a simulation approach. Ind Manage Data Syst 2012; 112 (1): 146–64. [Google Scholar]
- 108. Bose A, Das S. Trial analytics—a tool for clinical trial management. Acta Pol Pharm 2012; 69 (3): 523–33. [PubMed] [Google Scholar]
- 109. Campion TR, Blau JR, Brown VL, SW, et al. Implementing a clinical research management system: one institution’s successful approach following previous failures. AMIA Jt Summits Transl Sci Proc 2014; 2014: 12–17. [PMC free article] [PubMed] [Google Scholar]
- 110. Cramon P, Rasmussen AK, Bonnema SJ, et al. Development and implementation of PROgmatic: a clinical trial management system for pragmatic multi-centre trials, optimised for electronic data capture and patient-reported outcomes. Clinical Trials 2014; 11 (3): 344–54. [DOI] [PubMed] [Google Scholar]
- 111. Durkalski V, Wenle Z, Dillon C, et al. A web-based clinical trial management system for a sham-controlled multicenter clinical trial in depression. Clin Trials 2010; 7 (2): 174–82. [DOI] [PubMed] [Google Scholar]
- 112. Geyer J, Myers K, Vander Stoep A, et al. Implementing a low-cost web-based clinical trial management system for community studies: a case study. Clinical Trials 2011; 8 (5): 634–44. [DOI] [PubMed] [Google Scholar]
- 113. Gupta A, Calfas KJ, Marshall SJ, et al. Clinical trial management of participant recruitment, enrollment, engagement, and retention in the SMART study using a Marketing and Information Technology (MARKIT) model. Contemp Clin Trials 2015; 42: 185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Leroux H, McBride S, Gibson S. On selecting a clinical trial management system for large scale, multi-centre, multi-modal clinical research study. Stud Health Technol Inform 2011; 168: 89–95. [PubMed] [Google Scholar]
- 115. Müller J, Heiss KI, Oberhoffer R. Implementation of an open adoption research data management system for clinical studies. BMC Res Notes 2017; 10 (1): 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Nadkarni PM, Kemp R, Parikh CR. Leveraging a clinical research information system to assist biospecimen data and workflow management: a hybrid approach. J Clin Bioinformatics 2011; 1 (1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Park YR, Yoon YJ, Koo H, et al. Utilization of a clinical trial management system for the whole clinical trial process as an integrated database: system development. J Med Internet Res 2018; 20 (4): e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Raptis DA, Mettler T, Fischer MA, et al. Managing multicentre clinical trials with open source. Inform Health Soc Care 2014; 39 (2): 67–80. [DOI] [PubMed] [Google Scholar]
- 119. Schobel M, Staubert S, Lobe M, et al. Requirements on clinical trial management systems for academic site management organizations. Stud Health Technol Inform 2016; 228: 292–96. [PubMed] [Google Scholar]
- 120. Schwanke J, Rienhoff O, Schulze TG, et al. Suitability of customer relationship management systems for the management of study participants in biomedical research. Methods Inf Med 2013; 52 (4): 340–50. [DOI] [PubMed] [Google Scholar]
- 121. Solomon P, Cavanaugh MM, Draine J. Randomized Controlled Trials. New York: Oxford University Press; 2009. [Google Scholar]
- 122. Weng C, Li Y, Berhe S, et al. An Integrated Model for Patient Care and Clinical Trials (IMPACT) to support clinical research visit scheduling workflow for future learning health systems. J Biomed Inform 2013; 46 (4): 642–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhao W, Pauls K. Architecture design of a generic centralized adjudication module integrated in a web-based clinical trial management system. Clin Trials 2016; 13 (2): 223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. de Lange DW, Guidet B, Andersen FH, et al. Huge variation in obtaining ethical permission for a non-interventional observational study in Europe. BMC Med Ethics 2019; 20 (1): 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lamberti MJ, Kush R, Kubick W, et al. An examination of eClinical technology usage and CDISC standards adoption. Ther Innov Regul Sci 2015; 49 (6): 869–76. [DOI] [PubMed] [Google Scholar]
- 126. Skripcak T, Just U, Simon M, et al. Toward distributed conduction of large-scale studies in radiation therapy and oncology: open-source system integration approach. IEEE J Biomed Health Inform 2016; 20 (5): 1397–403. [Google Scholar]
- 127. Wilkinson M, Young R, Harper B, et al. Baseline assessment of the evolving 2017 eClinical landscape. Ther Innov Regul Sci 2019; 53 (1): 71–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.