Abstract
The present study investigates if the total replacement of dietary fishmeal (FM) with poultry by-product meal (PBM), supplemented with methionine influences the muscle fatty acids composition, normal gut morphology, histological traits of the liver, muscle, and gill, liver enzymes, immune and antioxidant response, and stress-related gene in juvenile barramundi, Lates calcarifer in relation to growth and feed utilization. Barramundi (3.58±0.01g) were randomly distributed into six 300 L seawater recirculating tanks (25 fish/tank) and fed two formulated isonitrogenous and isolipidic diets for 6 weeks. The control diet had FM as the sole animal protein source, whereas other test diet had only PBM as an animal protein source. Dietary PBM affected the fish performance and feed utilization. Regarding muscle fatty acid profile, total saturated fatty acids and monounsaturated fatty acids elevated while total PUFA particularly n-3 LC-PUFA and EPA decreased in PBM fed fish than control diet fed fish. Liver, muscle, gill, and intestinal histology showed no obvious alteration in control diet fed fish, however, more lipid droplets and hepatic vacuolization in the liver, necrotic myotome in muscle, hyperplasia in secondary lamellae in gill and short and broken folds in the intestine were observed in PBM fed fish. Similar to light microscopy observation of intestinal morphology, the transmission electron microscopy (TEM) analysis revealed shorter and smaller microvilli in fish fed PBM. Histopathological alterations in the liver of PBM fed fish were further associated with the elevated levels of aspartate aminotransferase (AST) and glutamate dehydrogenase (GLDH) and the significant upregulation of stress-related genes, HSP70 and HSP90. Also, a negative influence on lysozyme activity, and antioxidant enzymatic activities were recorded in fish fed PBM. Overall, it can be concluded that a total substitution of FM protein by methionine supplemented PBM negatively influenced the growth performance, liver health, histological traits of different organs, immune and antioxidant response, and expression of stress-related genes in juvenile barramundi.
Introduction
One of the major bottlenecks for carnivorous aquafeed production is the inconsistence supply of global fishmeal (FM) and escalated prices. Therefore, efforts have been exerted over several decades to investigate the feasibility of alternative dietary protein sources replacing FM in carnivore finfish aquaculture [1–4]. Presently, three main categories of FM replacements including terrestrial plant meals, rendered animal by-products, and seafood processing wastes are commercially available and used [5]. However, more than 50% of substitution of FM is now regularly achieved commercially in most carnivorous species [5], including barramundi or Asian sea bass, Lates calcarifer a commercial important carnivorous fish species [6]. Barramundi has good meat quality, ability to tolerate a wide range of salinity, and ability to adapt to the versatile farming environment [7]. It is popularly cultivated both in freshwater and seawater in Malaysia, Thailand, Taiwan, Indonesia, Saudi Arabia, and Australia, contributing USD 320 million globally [8, 9]. In Australia, barramundi farming is heavily dependent on imported FM resulting in incurring around 40% diet related cost which is the main impediment to increase the profitability [10]. Hence, nutritional studies on barramundi have commenced since the 1980s [11] and many of the studies have dedicated to replacing the FM with rendered animal meals [7, 10, 12–18] or plant meals [12, 13, 19–24].
Poultry by-product meal (PBM), an economical and easily available ingredient compared to FM contains a higher level of protein and most of the indispensable amino acids except for lysine and methionine [25–27]. Although, significant research in Australia and New Zealand has been conducted to commercially utilize PBM in various industries, its worldwide utilization is controlled by several regulations, for example the ban in European Union that has been recently lifted to allow the utilization of non-ruminant processed animal protein for aquaculture species [5]. Like other animal-based protein, another major limitation regarding the utilization of PBM is the variable digestibility due to variability in its composition and quality [28]. There have been several studies examining the effect of PBM on barramundi but results are mixed. For instance, Glencross, Blyth [29] reported that the inclusion of poultry meals up to 338g/kg does not influence the growth but beyond this level had a deleterious effect. Besides, a recent study of Simon, Salini [8] found that barramundi growth was impaired despite feeding balanced poultry protein concentrate (5–20%) while PBM along with supplementation of tuna hydrolysate could replace 90% of FM without impairing the growth [16]. In our earlier study, regardless of full-fat black soldier fly larvae supplementation, barramundi fed 90% PBM impacted the growth performance [18]. Similarly, the utilization of PBM above 50% affected the welfare of some marine fish species [25, 26, 30–32]. Imbalanced dietary essential amino acid (EAA) particularly methionine and lysine in PBM based diets are one of the major causes resulting growth depression in many fish [28]. The methionine requirement for barramundi was reported to be 2.2% [11]. In this study, methionine was supplemented to PBM to investigate if supplementation of deficient EAA in PBM could substitute FM totally.
In addition to assessing the growth performance related to non-FM protein ingredients, health aspects including the changes in serum biochemical assays, immune responses, and stress-related oxidative biomarkers are also crucial parameters of interest to aquaculturists. Nutritional factors can influence the production of oxidative enzymes [33]. Oxidative stress is characterized by an increase in malondialdehyde (MDA) and a decrease in glutathione peroxidase (GPx) [34]. The imbalance between antioxidant defences and free radical generation may cause cell damage, which may provoke the leaking of liver enzymes particularly ALT and GLDH in fish [35], associated with liver cell damage. Although published data are available on the effects of plant protein on oxidative biomarkers of barramundi [21, 22, 36, 37], there is less information on the effects of animal protein inclusions. The dietary inclusion of PBM impacted the liver health of barramundi by increasing the levels of ALT and GLDH [18], and animal protein ingredients elevated the levels of AST and ALT in hybrid grouper, Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂ [38]. Moreover, the equilibrium between oxidants and antioxidants is also important for immune cell function since it preserves the integrity and functionality of the cell membrane. Hence, it is crucial to understand the correlation between the antioxidants, liver enzymes, and immune response when entire dietary FM is replaced by any alternate- protein ingredients.
During dietary modification, it is important to consider that replacement of FM with potential ingredients do not exert adverse effects on the welfare of the tested species, as the welfare of fish in captive condition has been a growing concern over the decades [39–41]. The histological approach is one of the important frontline tools applied to assess the health status that can be achieved by evaluating the morphological status of different organs. The liver is the biggest organs involved in nutrient metabolism and producing biochemical compounds required for digestion. Muscle structure is also important as it reflects the nutritional condition and agility of the fish. The intestine is a primary immune organ in fish participated in digestion and absorption of nutrients as well as defence mechanism against microbes [42]. The evaluation of histopathological changes in these organs is important to assess the non-FM diet. Therefore, the present study aimed to investigate whether methionine supplemented PBM based diet has an ability to substitute FM completely without compromising growth performance, fatty acids composition, histological traits of different organs, serum biochemical response, stress-related genes expression, and antioxidant activities in barramundi.
Materials and methods
Animal ethical statement
The experiment was conducted at Curtin Aquatic Research Laboratory (CARL) in Curtin University, Australia in compliance with relevant guidelines and regulations set by the Australian Code of Practice for the care and use of animals for scientific purposes. All methods involving fish were reviewed and approved by the Curtin University Animal Ethics Committee (ARE2018-37). Prior to handling fish, AQUI-S® was used as anaesthesia and an overdose of AQUI-S was used as euthanasia to minimise stress, pain, and discomfort to the fish following the protocol of the Curtin Research Laboratories standard operating procedure (SOP) of anaesthetizing and euthanizing of fish.
Experimental diets
Except PBM, all the ingredients required for formulating test diets were purchased from the Special Feeds, 3150 Great Eastern Hwy, Glen Forrest, WA. Two isonitrogenous and isolipidic containing approximately 48% crude protein and 13% crude lipid were prepared to meet the nutritional requirement of barramundi [43]. FM and PBM were used as the main protein source and canola oil and cod liver oil were used as lipid sources. A control diet was prepared based on FM and another diet was formulated by replacing 100% of FM with PBM supplemented with 0.40% methionine (Table 1) to meet the established methionine requirement for normal growth of barramundi [11, 44]. The diets were formulated in compliance with the standard protocol of CARL. Briefly, all the dry ingredients were mixed homogeneously using a food mixture (Hobart Food equipment, Australia) before blending with fish oil and distilled warm water to make a stiff dough. The dough was passed through a mincer to make 3 mm pellets, then spread out and dried in an oven at 60°C for 36 hours. After drying, pellets were sealed in plastic bags before refrigerating at 4°C until used in the feeding trial. The fatty acid and amino acid profile of test diets and PBM is shown in Table 2 and Table 3, respectively.
Table 1. Formulation and proximate composition of test diets for barramundi.
| Ingredientsa (g/100g DM) | Control | 100PBM |
|---|---|---|
| †FM | 72.00 | 0.00 |
| ‡PBMb | 0.00 | 69.50 |
| Canola oil | 1.00 | 3.00 |
| Cod liver oil | 0.50 | 6.00 |
| Corn/wheat starch | 7.00 | 7.00 |
| wheat (10 CP) | 16.90 | 11.50 |
| Lecithin—Soy (70%) | 1.00 | 1.00 |
| Vitamin C | 0.05 | 0.05 |
| Dicalcium Phosphate | 0.05 | 0.05 |
| Methionine | 0.00 | 0.40 |
| Vitamin and mineral premix | 0.50 | 0.50 |
| Salt (NaCl) | 1.00 | 1.00 |
| Proximate composition (% dry weight)c | ||
| Moisture | 14.96 | 13.98 |
| Crude Protein | 47.88 | 47.86 |
| Crude Lipid | 12.59 | 12.71 |
| Ash | 9.67 | 10.24 |
| Gross energy (MJ/kg) | 20.23 | 19.95 |
a Specialty Feeds, Glen Forrest Stockfeeders, 3150 Great Eastern Highway, Glen Forrest, Western Australia 6071.
bKindly provided by Derby Industries Pty Ltd T/A, Talloman Lot Lakes Rd, Hazelmere WA 6055.
cAnalysed according to Association of Official Analytical Chemists (AOAC) [45].
† FM (Fishmeal): 64.0% crude protein, 10.76% crude lipid and 19.12% ash.
‡ PBM (Poultry by-product meal): 67.13% crude protein, 13.52% crude lipid and 13.34% ash.
Table 2. Fatty acids (mg/100g of dry sample) composition of control and test diet replacing FM totally with PBM in barramundi.
| Experimental diets | |||
|---|---|---|---|
| Fatty acids | Control | 100PBM | PBM |
| C12:0 | 2.73 | 7.11 | 9.39 |
| C14:0 | 131.63 | 342.45 | 73.69 |
| C16:0 | 1161.21 | 2090.88 | 2336.27 |
| ∑SFA1 | 1981.40 | 3216.29 | 3344.73 |
| C14:1n5 | 1.52 | 11.32 | 16.26 |
| C16:1n7 | 165.22 | 435.58 | 540.27 |
| C18:1cis+trans | 1158.94 | 3800.14 | 4410.64 |
| C20:1 | 79.86 | 483.71 | 60.84 |
| ∑MUFA2 | 1482.21 | 4873.58 | 5057.95 |
| C18:3n3 | 120.20 | 285.67 | 260.39 |
| C20:5n3 (EPA) | 178.50 | 278.99 | 16.79 |
| C22:5n3# | 63.30 | 64.60 | 36.67 |
| C22:6n3 (DHA) | 908.53 | 455.23 | 27.47 |
| ∑n-3 PUFA3 | 1309.12 | 1240.95 | 358.96 |
| C18:3n6 | 8.84 | 10.58 | 23.07 |
| C20:3n6 | 15.50 | 18.76 | 56.76 |
| C20:4n6 | 112.83 | 43.18 | 180.13 |
| C22:4n6# | 91.14 | 16.39 | 4.78 |
| ∑n-6 PUFA | 228.31 | 88.91 | 264.74 |
| ∑n-3/n-6 | 5.73 | 13.96 | 1.36 |
| ∑PUFA4 | 2184.01 | 2437.48 | 2386.97 |
| ∑n-3 LC-PUFA | 1158.61 | 806.57 | 85.36 |
1Contains 10:0, 13:0, 15:0, 17:0, 18:0, 20:0, 21:0, 22:0 and 23:0.
2Contains C15:1, C17:1, C22:1n9 and C24:1.
3Contains C18:4n3, C20:3n3.
4Contains C18:2 trans, C18:2 cis, C20:2, C22:2.
Poultry by-product meal, PBM; saturated fatty acids, SFA; monounsaturated fatty acids, MUFA; polyunsaturated fatty acids, PUFA.
Eicosapentaenoic acid, EPA; DHA, docosahexaenoic acid, sum of saturated fatty acids, ∑SFA; sum of monounsaturated fatty acids, ∑MUFA; sum of polyunsaturated fatty acids, ∑PUFA; sum of omega-3 polyunsaturated fatty acids, ∑n-3 PUFA; sum of omega-6 polyunsaturated fatty acids, ∑n-6 PUFA and LC-PUFA, long-chain polyunsaturated fatty acids (sum of 20:3n-3, 20:5n-3, 22:5n-3 and 22:6n-3).
Table 3. Amino acids (g/100g on dry matter basis) composition of test diets and PBM.
| Experimental diets | |||
|---|---|---|---|
| Amino acidsa | Control | 100PBM | PBM |
| Hydroxyproline | 1.7 | 3.2 | 3.2 |
| Histidine | 2.4 | 1.8 | 1.8 |
| Taurine | 0.5 | 0.5 | 0.4 |
| Serine | 5.3 | 5.0 | 5.0 |
| Arginine | 4.5 | 4.8 | 5.1 |
| Glycine | 13.2 | 16.4 | 16.5 |
| Aspartic acid | 8.8 | 7.8 | 7.8 |
| Glutamic acid | 11.7 | 12.1 | 11.5 |
| Threonine | 4.9 | 4.2 | 4.3 |
| Alanine | 9.4 | 9.1 | 9.1 |
| Proline | 6.1 | 7.3 | 7.0 |
| Lysine | 6.2 | 5.3 | 5.5 |
| Tyrosine | 2.0 | 1.8 | 2.0 |
| Methionine | 2.4 | 2.2 | 1.8 |
| Valine | 5.6 | 5.2 | 5.1 |
| Isoleucine | 4.3 | 3.8 | 3.8 |
| Leucine | 7.6 | 6.4 | 6.9 |
| Phenylalanine | 3.3 | 3.0 | 3.0 |
aDetermined including hydroxyproline and taurine analysis following our earlier study [46].
Fish husbandry and management
Three hundred and fifty barramundi were obtained from the Australian Centre for Applied Aquaculture Research (ACAAR), Fremantle, Australia in oxygenated plastic bags. Prior to commencing the trial, all fish were stocked into two fiberglass tanks (300 L) filled with ocean water and fed a commercial diet (470 g protein kg−1 diet and 20.0 MJ kg−1dietary gross energy) twice daily for two weeks to acclimate them to CARL experimental facilities and conditions. Following acclimation, 150 normal and visually healthy fish averaging (3.58±0.01g) were randomly distributed into six 300-L tanks, containing 250 L water in each tank. Therefore, stocking number of barramundi in each tank was 25. Each tank was equipped with an aerator, electric heater, and external bio-filter (Astro® 2212, China) to maintain DO, temperature, and other water quality parameters at an optimal level. Hence, the temperature was maintained at 27.90–29.20°C, dissolved oxygen (DO) at 5.92–7.42 mgL−1, salinity at 32–36 ppt, and photoperiod as 14:10 h LD. Commercial test kits were used to test ammonia nitrogen (<0.50 mgL−1) and nitrite (<0.50 mgL−1) level regularly. Each test diet had three replicates and fed by hand twice daily at 8.00 am and 6.00 pm to visual satiety levels for 42 days. Uneaten feed, if any, was collected by siphoning to calculate feed intake, and the number of dead fish were monitored daily to assess the fish survival rate. After 42 days, all fish were starved for 24 h prior to weighing total biomass to analyse the growth performance.
Fatty acids profile
Fish muscles in the form of three samples per dietary treatment were used for fatty acids analysis. Four fish muscle was filleted, wrapped with aluminium foil, freeze-dried, and pooled together. The fatty acids profile of experimental diets and fish flesh was carried out following the protocol of O'Fallon, Busboom [47], and Siddik, Chungu [7]. Approximately 0.5g of sample was hydrolysed at 55°C for 1.5 hrs with 0.1ml of internal standard (1.2g nonadecanoic acid in 100ml chloroform), 0.7ml of 10N KOH and 5.3ml of methanol. The sample was then methylated at 55°C for 1.5hrs with 0.6mL of 24N of sulphuric acid. The FAMES was extracted into 1ml of hexane and then quantified gas chromatography with flame ionization detection. The column used was a capillary column HP INNOWax GC column (60m x 0.25mm ID film 0.50 micron) with hydrogen as the carrier gas. Each sample were run in triplicate and results are expressed as an average.
Histological and Transmission Electron Micrograph (TEM) analysis
After 42 days of feeding, one fish from each tank was randomly euthanized with AQUI-S at 175 mg/L to excise liver, muscle, gill, and intestine for histological and TEM evaluation in response to test diets. For histological analysis, samples of all organs were fixed immediately in 10% buffered formalin, subsequently dehydrated with series of ethanol, infiltrated in xylene, and embedded in paraffin wax, as per standard histological protocols. Section of approximately 5 μm thickness was stained with Periodic Acid-Schiff (PAS) and digitally photographed under a light microscope (BX40F4, Olympus, Tokyo, Japan).
For TEM analysis, freshly collected intestinal samples washed in 2.5% glutaraldehyde buffered in 1x PBS at pH 7.4 before performing secondary fixation in 1% OsO4 (80 W 2 min on, 2 min off, 2 min on), dehydrating in ethanol (50, 70, 95 and 100% at 250 W, 40 seach) and infiltrating finally with epoxy resin in acetone (Procure 812, Proscitech) (1:3, 1:1, 3:1ratios at 250 W, 3 min each). Samples were processed as described in the earlier study in our lab [16] and screened a LaB6 TEM (JEOL2100, Japan) at 120 kV. The electron micrographs obtained from TEM analysis at 30,000 magnification were analysed using ImageJ (National Institute of Health, USA) to determine microvilli length and diameter.
Antioxidant status assessment
The enzyme activities of serum malondialdehyde (MDA) was determined using commercial assay kits following the manufacturer's instructions (Bockit, BIOQUOCHEM SL, 33428 Llanera-Asturias, Spain) and glutathione peroxidase (GPx) was measured with the Randox Laboratories test combination (Ransel, Antrim, United Kingdom) following the protocol of earlier study in our laboratory [22].
Serum biochemistry and immunity
Fish were captured gently at 42 days post-feeding, immediately dipped in a bucket containing 8 mg l−1 of AQUI-S®, and blood was taken by puncturing caudal vessels using 1 mL non-heparinized syringes (22G). Blood was allowed to clot for 24 h at 4°C, centrifuged for 15 min at 3000 rpm and 4°C, the serum collected and stored immediately at—80°C for the analysis of serum biochemical parameters, oxidative biomarkers, and immune parameters. Serum clinical chemistry and immune-related parameters were analysed according to the protocol of our earlier study [18, 46].
RNA extraction and qRT-PCR analysis
Liver from control and PBM fed fish were aseptically collected after euthanizing (AQUI-S, 175 mg l−1) the fish and preserved in RNA Later (Sigma-Aldrich, Germany) at—80°C until RNA extraction. Five milligrams of liver tissue stored in RNA Later was used for RNA extraction using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to manufacturer protocol. The quality of RNA was checked by gel electrophoresis and, the purity and quantity were determined gel electrophoresis before synthesizing complementary DNA (cDNA) from 1 μg of total RNA using Omnicript RT kit (Qiagen, Hilden, Germany) following the instruction of manufacturer’s company. qRT-PCR on stress-related genes were performed by PowerUpTM Cyber Green Master Mix (Thermo Scientific, USA) with 7500 Real-Time PCR System (Applied Biosystems, USA) and data were normalised against housekeeping genes, 18S rRNA and Ef1-a, (Table 4) and analysed using REST© software [48].
Table 4. Primers of qPCR used in the experiment.
| Genes | Sequences (5ʹ - 3ʹ) | Product size | Tm (°C) | |
|---|---|---|---|---|
| Heat shock protein kDa70, HSP70 | F: AAGGCAGAGGATGATGTC | 186 | 59 | Mohd-Shaharuddin, Mohd-Adnan [48] |
| R: TGCAGTCTGGTTCTTGTC | ||||
| Heat shock protein kDa90, HSP90 | F: ACCTCCCTCACAGAATACC | 197 | 59 | Mohd-Shaharuddin, Mohd-Adnan [48] |
| R: CTCTTGCCATCAAACTCC | ||||
| 18S rRNA, 18S | F: TGGTTAATTCCGATAACGAACGA | 94 | 59/60 | Mohd-Shaharuddin, Mohd-Adnan [48] |
| R: CGCCACTTGTCCCTCTAAGAA | ||||
| Elongation factor-1α, ef1α | F: AAATTGGCGGTATTGGAAC | 83 | 59/60 | Mohd-Shaharuddin, Mohd-Adnan [48] |
| R: GGGAGCAAAGGTGACGAC |
Calculation and statistics
Specific growth rate (SGR), feed conversion ratio (FCR) and total feed intake (TFI) were calculated using the following equations-
All data were represented as mean±SE. The differences between control and PBM fed fish in all data were determined by unpaired student t-test at the significance level of 0.05 < P < 0.001. Percent survival at the termination of the feeding trial was plotted using the Kaplan-Meier survival method with the Log-rank (Mantel-Cox) test.
Results
Growth performance, feed utilization and survival
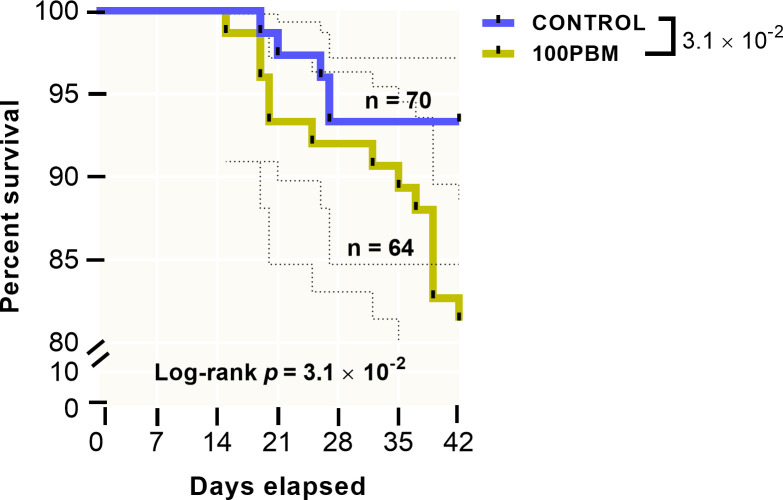
Fish growth, feed intake, and survival rate in response to 42 days feeding trial are presented in Table 5 and Fig 1. The mean final body weight (FBW) and specific growth rate (SGR) of fish fed PBM were significantly lower than the FBG and SGR of fish fed the control diet. FCR in PBM fed fish increased with lower feed intake in PBM fed fish. Survival rate (Fig 1), as drawn by Kaplan-Meier survival analysis with 95% confidence at the end of the 42 days trial decreased significantly in PBM fed fish (81.33%) than the control (93.33%) (χ2100PBM = 4.514, df = 1, P = 0.034).
Table 5. Fish performance including Final Body Weight (FBW), Weight Gain (WG), Specific Growth Rate (SGR), Feed Intake (FI), and Feed Conversion Ratio (FCR) of barramundi when fed control and PBM based diet over a period of 42 days.
| Growth performance | Experimental diets | Unpaired t-test | ||
|---|---|---|---|---|
| Control | 100PBM | t-value | P-value | |
| IW (g) | 3.52±0.02 | 3.49±0.06 | 0.82 | 0.46 |
| FBW (g) | 54.91±0.55a | 32.67±0.23b | 37.38 | 0.00 |
| WG (g) | 51.39±0.53a | 29.18±0.23b | 38.93 | 0.00 |
| SGR (%/d) | 6.54±0.01a | 5.33±0.02b | 55.36 | 0.00 |
| FI (g/fish d-1) | 1.21±0.12a | 0.86±0.01b | 3.051 | 0.04 |
| FCR (FCR) | 0.99±0.15a | 1.24±0.01b | -2.97 | 0.04 |
Results are expressed as mean ± SE (standard error) (n = 3).
Fig 1. Survival rate based on Kaplan-Meier survival analysis with Log-rank (Mantel-Cox) test of barramundi after 42 days feeding with either basal diet or PBM based diet.
Dotted line in survival plot indicates 95% confidence interval and P value indicate significant at 0.05.
Muscle fatty acids composition
The FAs profile of barramundi muscle at the termination of 42 days trial was influenced by the PBM based diet (Table 6). The dietary inclusion of PBM significantly augmented total SFA. All SFA including capric acid (C10:0), lauric acid (C12:0), myristic acid (C14:0), palmitic acid (C16:0), margaric acid (C17:0), stearic acid (C18:0), arachidic acid (C20:0), heneicosylic acid (C21:0), behenic acid (C22:0) and tricosylic acid (C23:0) except for tridecylic acid (C13:0), and pentadecylic acid (C15:0) were significantly higher in the muscles of fish fed PBM. Similarly, total MUFA concentration increased in the muscle of PBM fed fish. PUFA differed significantly between the test diets, with lower concentration of n-3 LC-PUFA and C22:4n6 in PBM fed fish than the fish fed the control diet. Similar result was recorded in ∑n-3/∑n-6 ratio.
Table 6. Fatty acids (mg/100g on dry matter basis) of barramundi muscle when fed control and PBM based diet over a period of 42 days.
| Fatty acid | Experimental diets | Unpaired t-test | ||
|---|---|---|---|---|
| Control | 100PBM | t-value | P-value | |
| C12:0 | 1.02±0.09a | 291.96±1.27b | -228.29 | 0.00 |
| C14:0 | 67.46±0.77a | 213.36±0.48b | -158.64 | 0.00 |
| C16:0 | 713.65±10.19a | 1253.24±68.93b | -7.75 | 0.00 |
| ∑SFA1 | 1153.45±15.15 | 2320.11±65.41 | -17.38 | 0.00 |
| C16:1n7 | 130.78±1.90a | 286.88±2.21b | -53.82 | 0.00 |
| C20:1 | 36.62±0.38a | 111.68±2.97b | -25.07 | 0.00 |
| C14:1n5 | 0.97±0.03a | 6.30±0.06b | -84.46 | 0.00 |
| C18:1cis+trans | 859.76±5.73a | 2961.30±69.99b | -29.93 | 0.00 |
| ∑MUFA2 | 1066.66±8.25a | 3412.55±74.01b | -31.50 | 0.00 |
| C18:3n3 | 55.12±0.38a | 213.57±3.10b | -50.94 | 0.00 |
| C20:5n3 (EPA) | 109.31±1.78 | 113.69±1.31 | -1.97 | 0.12 |
| C22:5n3 | 71.50±0.95a | 78.82±0.44b | -7.14 | 0.00 |
| C22:6n3 (DHA) | 683.13±12.43a | 370.84±1.99b | 24.82 | 0.00 |
| ∑n-3 PUFA3 | 940.67±15.93a | 821.03±11.05b | 5.24 | 0.01 |
| C20:3n6 | 25.68±0.57a | 49.16±2.84b | -8.07 | 0.00 |
| C20:4n6 | 92.29±1.90a | 114.06±3.54b | -5.43 | 0.01 |
| C18:3n6 | 17.51±1.42a | 51.44±6.52b | -5.08 | 0.01 |
| C22:4n6 | 62.67±1.07a | 23.06±0.26b | 36.20 | 0.00 |
| ∑n-6 PUFA | 198.15±3.86a | 237.72±12.61b | -9.15 | 0.00 |
| ∑n-3/∑n-6 | 4.75±0.04a | 3.47±0.18b | 6.78 | 0.00 |
| ∑PUFA4 | 1485.98±24.56 | 1311.18±9.36 | 2.86 | 0.05 |
| ∑n-3 LC-PUFA3 | 868.55±15.25 | 569.45±2.78 | 19.29 | 0.00 |
Results are expressed as mean ± SE (standard error) (n = 3).
1Contains 10:0, 13:0, 15:0, 17:0, 18:0, 20:0, 21:0, 22:0 and 23:0.
2Contains C15:1, C17:1, C22:1n9 and C24:1.
3Contains C18:4n3, C20:3n3.
4Contains C18:2 trans, C18:2 cis, C20:2, C22:2.
Poultry by-product meal, PBM; saturated fatty acids, SFA; monounsaturated fatty acids, MUFA; polyunsaturated fatty acids, PUFA and LC-PUFA, long-chain polyunsaturated fatty acids (sum of 20:3n-3, 20:5n-3, 22:5n-3 and 22:6n-3).
Different superscripts letter indicate significant difference at P < 0.05, 0.01 and 0.001, followed by an unpaired student t-test.
Histopathology of liver, muscle, gill and intestine
Total replacement of FM with PBM dysregulated the histological structure of liver, muscle, gills, and intestine (Fig 2A–2H). The liver of control (Fig 2A) fed fish showed higher pigmentation of hepatocyte cytoplasm, indicating a higher amount of glycogen, while the liver of PBM fed fish (Fig 2B) showed less hepatocyte cytoplasm pigmentation, indicating less amount of glycogen with more lipid vacuolization. Healthy and normal myotome were observed in the muscle of the fish fed the control diet (Fig 2C) but necrotic myotome was found in the fish fed PBM diet (Fig 2D). Control fed fish showed normal gill structure (Fig 2E) but hyperplasia in secondary lamellae was recorded in PBM fed fish (Fig 2F). All the examined fish fed control (Fig 2G) presented normal intestinal structure whilst broken and short fold were found in fish fed PBM (Fig 2H).
Fig 2.
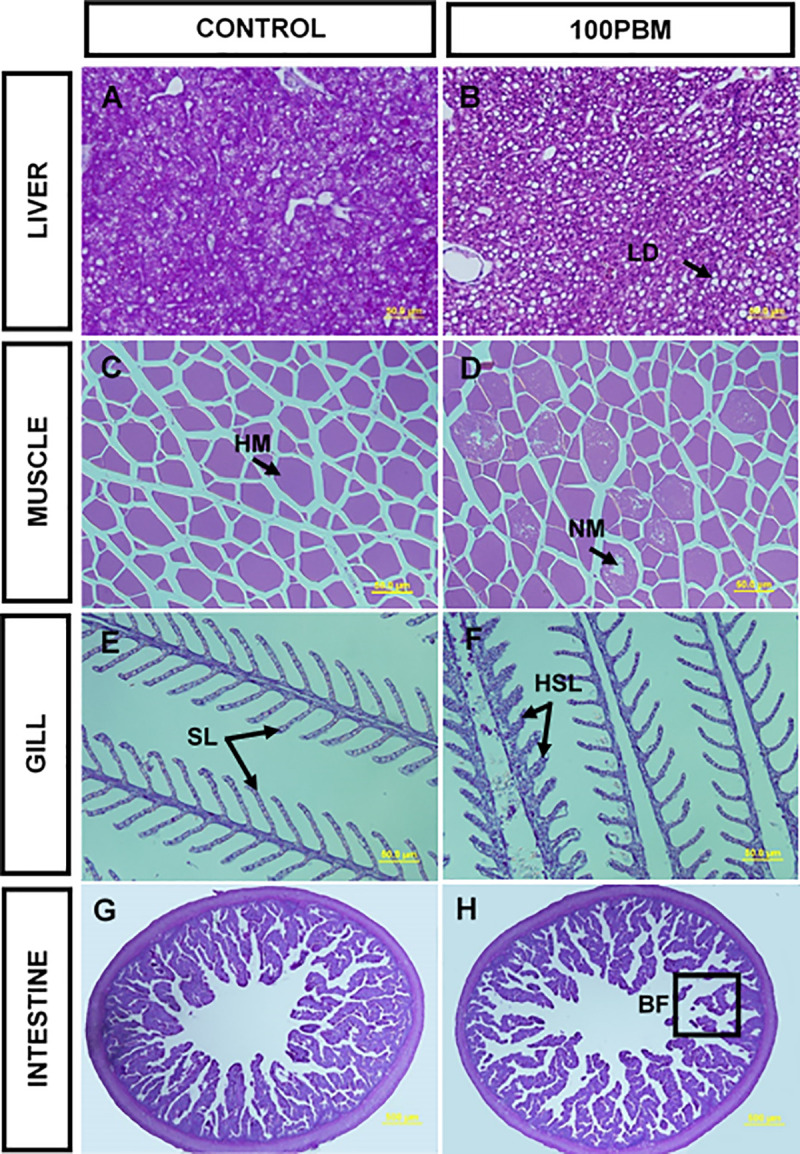
Liver (A-B), muscle (C-D), gill (E-F) (PAS stain; 40 × magnification; scale bar = 50 μm) and distal intestine (G-H) (PAS stain; 4 × magnification; scale bar = 500 μm) sections of barramundi fed control and PBM based diet at the end of 42 days of feeding trial. Lipid droplet, LD; healthy myotome, HM; necrotic myotome, NM; secondary lamellae, SL; hyperplasia in secondary gill lamellae, HSL and broken fold, BF.
Intestinal morphology
The distal intestine of barramundi fed control (Fig 3A) and PBM (Fig 3B) were examined by transmission electron microscope. Microvilli height (Fig 3D) (t = 6.727, df = 28, P < 0.0001) and diameter (Fig 3E) (t = 3.494, df = 28, P = 0.0016) of barramundi fed PBM diet was significantly lower than barramundi fed control diet.
Fig 3.
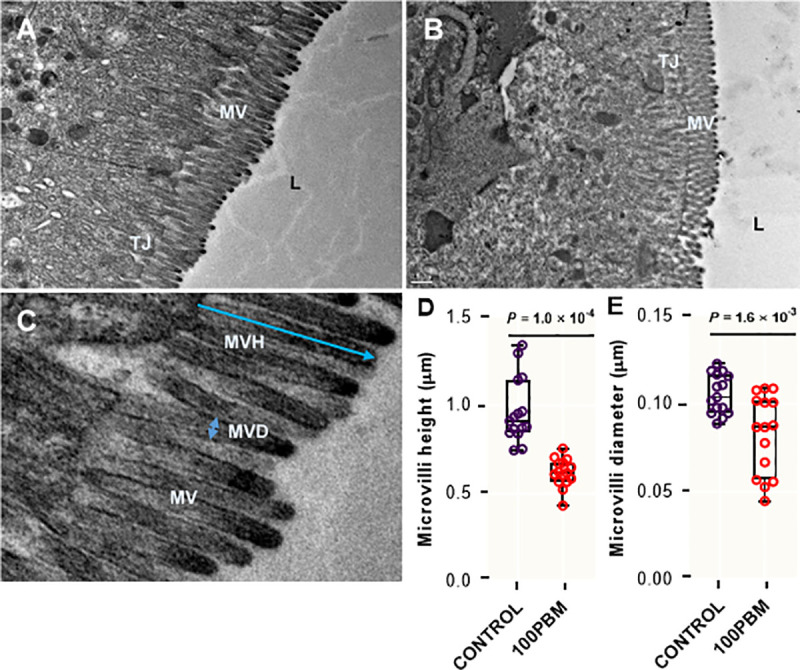
Observation of TEM in the intestine of barramundi fed Control (A) and PBM (B) at the end of 42 days of feeding trial. (C) Microvilli height and diameter measurement and comparison of microvilli height and diameter (panel D & E), performed by an unpaired student t-test at P <0.05 and 0.01. Microvilli, MV; Microvilli height, MVH; tight junction, TJ; microvilli diameter, MVD.
Liver enzymes, immunity and stress related genes
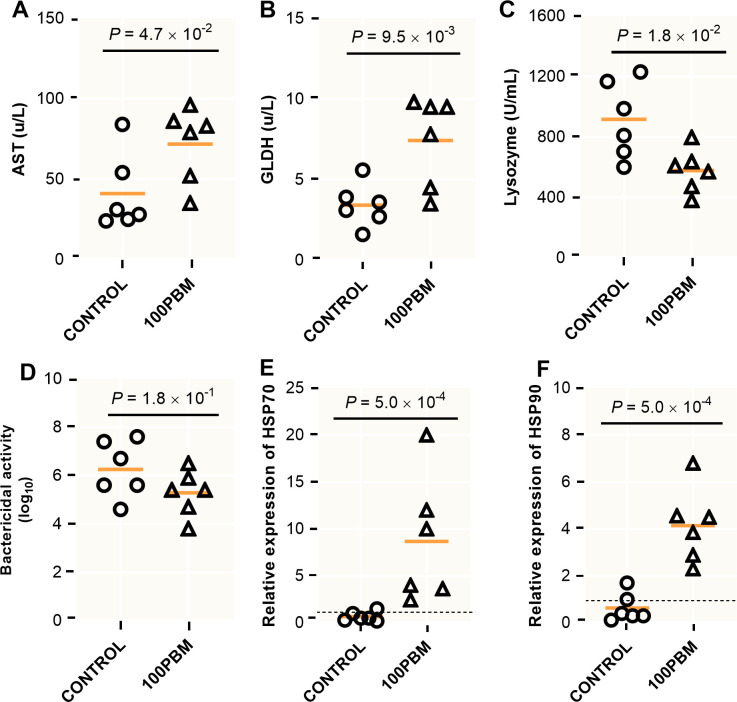
Liver enzymes (AST and GLDH), immune response including serum lysozyme and bactericidal activity and stress related genes (HSP70 and HSP90) were significantly induced by the experimental diets (Fig 4). AST and GLDH in PBM fed fish was significantly higher than the control (t = 2.268, df = 10, P = 0.047 and t = 3.199, df = 10, P = 0.010) (Fig 4A and 4B), while serum lysozyme decreased significantly in PBM fed fish compared to control (t = 2.842, df = 10, P = 0.018) (Fig 4C). Meanwhile, none of the diets had significant effects on bactericidal activity (t = 1.572, df = 10, P = 0.147) (Fig 4D). In line with liver enzymes, similar results were observed in HSP70 and HSP90 when compared with control (t = 2.905, df = 10, P = 0.016 and t = 5.102, df = 10, P = 0.001) (Fig 4E and 4F).
Fig 4.
AST, aspartate aminotransferase (A) and GLDH, glutamate dehydrogenase (B), lysozyme (C), bactericidal activity (D) and heat shock related gene including HSP70 (E) and HSP90 (F) in barramundi after 42 days feeding with either control diet or PBM based diet. P values indicate significant at P < 0.05, 0.01 and 0.001, followed by an unpaired student t-test.
Antioxidant activity
Antioxidant activities of blood serum were significantly affected by total inclusion of PBM. Serum GPx activity declined significantly in PBM fed fish (t = 2.833, df = 10, P = 0.017) (Fig 5A), while MDA increased significant in PBM (t = 2.251, df = 10, P = 0.048) (Fig 5B) with respect to control.
Fig 5.
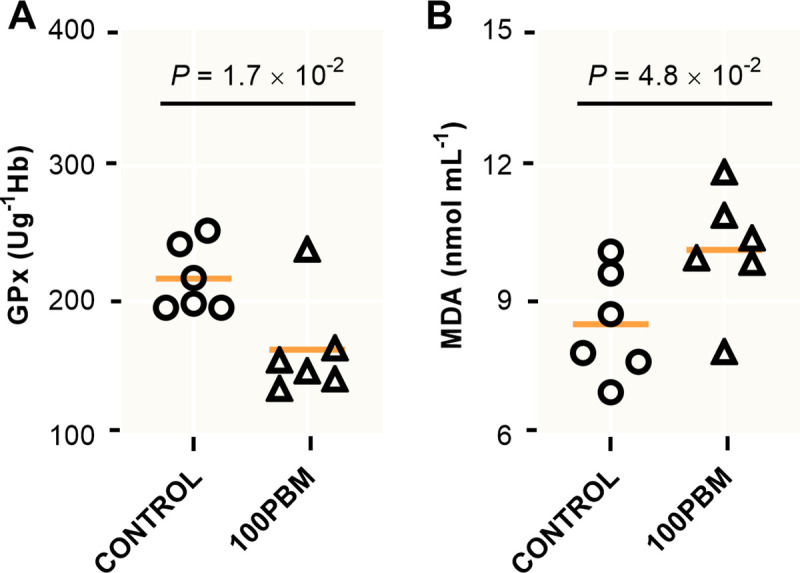
(A) GPx (Ug-1 Hb) and (B) MDA (nmol mL-1) in the serum of barramundi after 42 days feeding with either control diet or PBM based diet. P values indicate significant at P < 0.05, 0.01 and 0.001, followed by an unpaired student t-test.
Discussion
A good number of studies have been devoted over the years to incorporate different levels of PBM, at the expense of FM in the diet of finfish and shellfish aquaculture [28] but most of the studies were performed on the basic nutritional aspects including proximate composition, amino acids, and fatty acid content, and its potential effect on the growth performance of the host fish [49–51]. In depth investigations are still lacking pertaining to the effects of PBM on the integrity of different organs, stress-related genes expression, or antioxidative responses in barramundi.
Establishing protein derived from animal industry as an ideal feed for finfish aquaculture, a series of studies have been conducted in Australia especially on barramundi. For instance, dietary inclusion of high-quality poultry protein concentrate from 5 to 20% demonstrated a reduced growth performance despite providing balanced amino acids in the diet but the reverse trend was observed when supplemented with phosphorous [8]. On the contrary, Siddik, Howieson [16] were able to replace 90% FM with either bioprocessed or unprocessed PBM along with supplementation of fish protein hydrolysate with no apparent effects on the growth performance. In the present study, barramundi fed PBM supplemented with methionine affected the growth, feed utilization, FCR, and survival rate. Similarly, feeding barramundi non-FM based diet containing 450 g kg-1 PBM and 285 g kg-1 soybean meal impacted the growth, feed intake, and FCR despite supplementing taurine and the presumable reasons were palatability [29]. Deterioration in the growth performance of gibel carp, Carassius auratus gibelio was also observed when fed 100% animal protein containing PBM and meat and bone meal despite supplementation with methionine and lysine [52]. The possible reasons were the nutritional superiority or enhanced palatability in FM that could not be met up by PBM and MBM. Methionine, lysine and arginine levels in the 100PBM diet were at optimum level for barramundi growth [11] but histidine, isoleucine, and phenylalanine were lower compare to FM based diet which may suppress the growth performance. Similarly, deficiency of histidine, methionine, isoleucine, lysine, and phenylalanine were identified to reduce the growth performance of spotted rose snapper, Lutjanus guttatus with higher inclusion of PBM [53]. Moreover, the abundance of MUFA and n-6 PUFA coupled with a deficiency of EFA particularly n-3 LC-PUFA, EPA and DHA in PBM were highlighted as one of the reasons for the reduced growth in Totoaba [54], catfish, Ictalurus punctatus [55], black sea turbot, Psetta maeotica [30] and gilthead sea bream, Sparus aurata L [56]. Similarly, higher MUFA content and lower levels of PUFA, in particular, n-3 LC-PUFA and EPA contents were found in 100PBM diets that could be responsible for the negative influence on growth, survival feed utilization and FCR. In addition, n-3 PUFA have been reported as an indispensable FAs for optimum growth and survival of many marine fish species [28, 56]. However, these findings contradict with the results of Panicz, Żochowska‐Kujawska [57], Gunben, Senoo [58] and Shapawi, Ng [32] who reported no adverse effects of 100PBM on the growth and biometry indices of female tenches, Tinca tinca, tiger grouper juveniles, Epinephelus fuscoguttatus and humpback grouper, Cromileptes altivelis. This heterogeneity might be due to use different fish species and culture system or variability in nutritional composition palatability, and digestibility of PBM as it varies from batch to batch or among supplier companies [18, 28].
FAs composition of diet affects the FAs composition of fish muscle or meat which have been reported in many fish species [59–61]. In the present study, FAs of barramundi fillet were affected by the PBM diet. Total muscle SFA concentration was significantly higher in PBM fed fish may be due to the abundance of palmitic acid and myristic acid in fish muscle that are reflected in the PBM diet. A higher concentration of total SFA due to a high abundance of palmitic acid was observed in juvenile black sea bass fed 100PBM [62]. Muscle MUFA content in the present study increased in PBM fed fish which could be due to higher proportion MUFA in the diet. This finding was similar to our earlier study [46]. Lower concentration of n-3 LC-PUFA and adrenic acid in fish muscle resulted in low total PUFA and n-3/n-6 ratio which are similar to the findings in barramundi fed high levels of PBM [7]. Similarly, 100PBM was lacking in essential fatty acids (EFAs) and also worsened the EFAs in the muscle of totoaba juveniles, Totoaba macdonaldi [54]. These results demonstrated that the total substitution of FM with PBM decreased PUFA levels in barramundi, which may consequently affect the nutritional value in terms of fatty acids available for human consumption.
It is well known that AST and GLDH are two important enzymes which primarily exist in liver at lower levels under normal condition but can leak into the blood rapidly when liver cells are damaged due to various stressors [63, 64]. In the present study, the PBM diet significantly increased the levels of AST and GLDH in the serum of barramundi, concomitant with the histopathological damage of liver tissue. Likewise, plasma ALT was negatively impacted by the inclusion of animal protein blend (APB) (20% to 80%) in the diet of hybrid grouper while AST augmented significantly in 80% APB fed fish [38]. However, Panicz, Żochowska‐Kujawska [57] reported no effects on blood biochemical parameters of juvenile tenches, Tinca tinca fed graded levels of PBM (25.7 to 100%).
To further clarify the effects of PBM on the liver function of barramundi, heat shock-related genes including HSP70 and HSP90 were examined. HSP70 and HSP90 are two important stress-related protein and their expression level elevate significantly when fish are exposed to different stressors, including pathogenic infection, crowding, poor water quality, and nutritionally deficient diet [65–67]. In the present study, both HSP70 and HSP90 upregulated significantly in the liver of barramundi that received 100PBM, indicating that 100% inclusion of PBM could act as a stressor.
In fish, immune functions of immune organs are strongly associated with the presence and activity of a unique array of molecules including lysozyme, complement proteins, immunoglobulins [68–70], and bactericidal activity that are influenced by the dietary modifications. Serum lysozyme was negatively triggered by PBM diet that support the findings of Subhadra, Lochmann [71, 72], who reported aggravated levels of complement and lysozyme activity in PBM fed largemouth bass, Micropterus salmoides.
Substitution of 100% FM with PBM resulted in lipidosis with clearly visible inflammation in the liver of juvenile tenches, Tinca tinca [57], supporting our present findings as hepatocyte lipid vacuolization with less amount of glycogen was observed in the liver tissue of barramundi fed PBM. The excessive amount of fat deposition in the liver negatively impacted the growth and immune response of fish [73] that are synchronous with the immunological results in the present study. Similarly, Siddik, Chungu [7] fed juvenile barramundi with different levels of PBM for 42 days and reported irregular liver arrangement with lipid deposition in the 100% PBM and bioprocessed PBM groups. Furthermore, higher administration of animal protein blend affected the morphology of the liver of hybrid grouper, characterized by hepatic vacuoles and a high amounts of lipid droplets which is a sign of hepatic steatosis [38]. The lipid accumulation in the liver may occur when dietary lipid exceeds the capacity of the hepatic cells to oxidize which lead to synthesize and deposit larger amounts of triglyceride in vacuoles [38, 73, 74].
Muscle structure is the determinant of fish growth and can be affected by nutritionally-deprived diet [75]. For example, nutritional deficiency altered the muscle structure of Atlantic salmon, Salmo salar including myodegeneration [76]. Similarly, fish fed PBM diet showed necrosis and fibre degeneration in muscle. Gill is one of the important immune organs in fish and its structure can be affected by stress and diet [77]. In the present study, hyperplasia in secondary gill lamellae was in PBM fed fish but the possible reasons are not well understood, deserving further study.
Evaluating intestinal morphology in response to dietary changes is important to determine the health status and welfare of fish. Intestinal morphology, in particular, villous structure, and microvillus height and diameter is related to absorption and assimilation of nutrient and immunological function [16, 78, 79]. Histological analysis showed that broken and short fold in the present study in PBM fed groups are in line with TEM results, showing significantly smaller with a shorter diameter of microvilli, which are responsible for the lower efficiency of nutrient uptake, thus suppressing the growth and survival. Similar results were reported by Siddik, Howieson [16] who found significantly lower microvilli height in the distal intestine of barramundi after 56 days post-feeding with 10% supplemented 90PBM. Hence total replacement of FM with PBM impacted the welfare of barramundi, as reflected by the histological and TEM analysis.
Antioxidant status in fish, as determined by several antioxidant enzymes including CAT, SOD, GPx, and MDA have been considered as the first line of defensive biomarkers to protect cells and tissues from oxidative damage, caused by some free radicals such as superoxide anion (O2-), hydrogen peroxide (H2O2) and hydroxyl radical (OH) [80, 81]. Glutathione peroxidase, GPx is an important antioxidant enzyme showing strong radical-scavenging capacity against free radicals and lipid peroxides [82]. The present study detected a significantly lower activity of GPx in the serum of barramundi fed with PBM, which may due to the lower levels of n-3 LC-PUFA and DHA in 100PBM diet. Liu, Mai [83] reported that marine fish are susceptible to oxidative stress due to their high demand for LC-PUFA. However, elevated serum GPx activity in barramundi fed 90% fermented PBM supplemented with tuna hydrolysate [17] might be due to the antioxidant capacity of fish protein hydrolysate [84]. It has been reported that the GPx activity is well correlated with the concentration of MDA [85]. MDA is a natural biomarker and main ending product of lipid peroxidation [86, 87] and its elevation indicates oxidative injury [88] and associates with the pathological state of animals including cell structure damage and function [86, 87]. A lower activity of GPx with the higher level of MDA indicates that PBM based diet may provoke the oxidative damage of barramundi which was further proven by the presence of hepatocyte lipid vacuolization.
In summary, regardless of methionine supplementation, the total replacement of FM with PBM is not nutritionally adequate for barramundi, as indicated by depressed growth performance and immune response. An unfavourable effect of a PBM based diet was observed on antioxidant enzymes. Also, adding PBM induced the lipid droplet in the liver for barramundi via affecting the expression levels of heat shock related genes and liver enzymes. Feeding PBM not only triggered the fiber degeneration and necrosis in muscle and hyperplasia in gills but also induced the intestinal villus morphology by decreasing intestinal microvilli morphology, which may suggest that high levels of PBM could impair the welfare of barramundi. Further studies need to be conducted along with supplementation of other EAA and/or EFA with PBM to investigate the welfare of farmed barramundi.
Acknowledgments
The authors are also thankful to the Australian Centre for Applied Aquaculture Research (ACAAR), Fremantle, Australia for providing fish and Rowan Kleindienst for technical assistance during fish husbandry. We are also sincerely thankful to Dr. Fran Stephens for helping to collect histological samples and prepare histological slides.
Data Availability
All data generated or analyzed during this study have been presented in the forms of figures and tables in the paper.
Funding Statement
Support of the trial was obtained from the Research Training Program (RTP) Stipend Scholarship, funded by Australian Government to Md Reaz Chaklader (No. 19061054-Curtin).
References
- 1.Gatlin DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, et al. Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquaculture Research. 2007;38(6):551–79. 10.1111/j.1365-2109.2007.01704.x [DOI] [Google Scholar]
- 2.Hardy RW. Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquaculture Research. 2010;41(5):770–6. [Google Scholar]
- 3.Tacon AG, Metian M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture. 2008;285(1–4):146–58. [Google Scholar]
- 4.Pham HD, Siddik MA, Fotedar R, Chaklader MR, Foysal MJ, Nguyen CM, et al. Substituting fishmeal with lupin Lupinus angustifolius kernel meal in the diets of cobia Rachycentron canadum: Effects on growth performance, nutrient utilization, haemato-physiological response, and intestinal health. Animal Feed Science and Technology. 2020:114556. [Google Scholar]
- 5.Klinger D, Naylor R. Searching for solutions in aquaculture: charting a sustainable course. Annual Review of Environment and Resources. 2012;37:247–76. [Google Scholar]
- 6.Van Vo B, Siddik MA, Fotedar R, Chaklader MR, Foysal MJ, Pham HD. Digestibility and water quality investigations on the processed peanut (Arachis hypogaea) meal fed barramundi (Lates calcarifer) at various inclusion levels. Aquaculture Reports. 2020;18:100474. [Google Scholar]
- 7.Siddik M, Chungu P, Fotedar R, Howieson J. Bioprocessed poultry by-product meals on growth, gut health and fatty acid synthesis of juvenile barramundi, Lates calcarifer (Bloch). PLoS One. 2019;14(4):e0215025 10.1371/journal.pone.0215025 TN_proquest2208024522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon CJ, Salini MJ, Irvin S, Blyth D, Bourne N, Smullen R. The effect of poultry protein concentrate and phosphorus supplementation on growth, digestibility and nutrient retention efficiency in barramundi Lates calcarifer. Aquaculture. 2019;498:305–14. 10.1016/j.aquaculture.2018.08.069 [DOI] [Google Scholar]
- 9.Van Vo B, Siddik MA, Fotedar R, Chaklader MR, Hanif MA, Foysal MJ, et al. Progressive replacement of fishmeal by raw and enzyme-treated alga, Spirulina platensis influences growth, intestinal micromorphology and stress response in juvenile barramundi, Lates calcarifer. Aquaculture. 2020:735741. [Google Scholar]
- 10.Williams KC, Barlow CG, Rodgers LJ, Ruscoe I. Potential of meat meal to replace fish meal in extruded dry diets for barramundi, Lates calcarifer (Bloch). I. Growth performance. Aquaculture Research. 2003;34(1):23–32. [Google Scholar]
- 11.Glencross B. The nutritional management of barramundi, Lates calcarifer–a review. Aquaculture Nutrition. 2006;12(4):291–309. 10.1111/j.1365-2095.2006.00410.x [DOI] [Google Scholar]
- 12.Glencross B, Rutherford N, Jones B. Evaluating options for fishmeal replacement in diets for juvenile barramundi (Lates calcarifer). Aquaculture Nutrition. 2011;17(3):e722–e32. 10.1111/j.1365-2095.2010.00834.x [DOI] [Google Scholar]
- 13.Glencross B. A comparison of the digestibility of diets and ingredients fed to rainbow trout (Oncorhynchus mykiss) or barramundi (Lates calcarifer)–the potential for inference of digestibility values among species. Aquaculture Nutrition. 2011;17(2):e207–e15. [Google Scholar]
- 14.Williams K, Barlow C, Rodgers L. Efficacy of crystalline and protein‐bound amino acids for amino acid enrichment of diets for barramundi/Asian seabass (Lates calcarifer Bloch). Aquaculture Research. 2001;32:415–29. [Google Scholar]
- 15.Williams KC, Barlow CG, Rodgers L, Hockings I, Agcopra C, Ruscoe I. Asian seabass Lates calcarifer perform well when fed pelleted diets high in protein and lipid. Aquaculture. 2003;225(1–4):191–206. [Google Scholar]
- 16.Siddik MAB, Howieson J, Fotedar R. Beneficial effects of tuna hydrolysate in poultry by-product meal diets on growth, immune response, intestinal health and disease resistance to Vibrio harveyi in juvenile barramundi, Lates calcarifer. Fish and Shellfish Immunology. 2019;89:61–70. 10.1016/j.fsi.2019.03.042 [DOI] [PubMed] [Google Scholar]
- 17.Siddik MAB, Chaklader MR, Foysal MJ, Howieson J, Fotedar R, Gupta SK. Influence of fish protein hydrolysate produced from industrial residues on antioxidant activity, cytokine expression and gut microbial communities in juvenile barramundi Lates calcarifer. Fish & shellfish immunology. 2019;97:465–73. 10.1016/j.fsi.2019.12.057 [DOI] [PubMed] [Google Scholar]
- 18.Chaklader MR, Siddik MAB, Fotedar R, Howieson J. Insect larvae, Hermetia illucens in poultry by-product meal for barramundi, Lates calcarifer modulates histomorphology, immunity and resistance to Vibrio harveyi. Scientific reports. 2019;9:16703 10.1038/s41598-019-53018-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irvin S, Blyth D, Glencross B. Discrete and interactive effects of non‐starch polysaccharides on feed digestibility by barramundi, Lates calcarifer. Aquacult Nutr. 2015;22:1047–54. [Google Scholar]
- 20.Ngo DT, Pirozzi I, Glencross B. Digestibility of canola meals in barramundi (Asian seabass; Lates calcarifer). Aquaculture. 2015;435:442–9. [Google Scholar]
- 21.Ilham, Siddik M, Fotedar R. Effects of Organic Selenium Supplementation on Growth, Accumulation, Haematology and Histopathology of Juvenile Barramundi (Lates calcarifer) Fed High Soybean Meal Diets. 2016. 10.1007/s12011-016-0708-1 [DOI] [PubMed] [Google Scholar]
- 22.Ilham, Fotedar R, Munilkumar S. Effects of organic selenium supplementation on growth, glutathione peroxidase activity and histopathology in juvenile barramundi (Lates calcarifer Bloch 1970) fed high lupin meal-based diets. Aquaculture. 2016;457:15–23. 10.1016/j.aquaculture.2016.02.003 [DOI] [Google Scholar]
- 23.Vo BV, Siddik MA, Chaklader MR, Fotedar R, Nahar A, Foysal MJ, et al. Growth and health of juvenile barramundi (Lates calcarifer) challenged with DO hypoxia after feeding various inclusions of germinated, fermented and untreated peanut meals. PLoS One. 2020;15(4):e0232278 10.1371/journal.pone.0232278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Vo B, Bui DP, Nguyen HQ, Fotedar R. Optimized fermented lupin (Lupinus angustifolius) inclusion in juvenile barramundi (Lates calcarifer) diets. Aquaculture. 2015;444:62–9. [Google Scholar]
- 25.González‐Rodríguez Á, Celada JD, Carral JM, Sáez‐Royuela M, García V, Fuertes JB. Evaluation of poultry by‐product meal as partial replacement of fish meal in practical diets for juvenile tench (Tinca tinca L. Aquaculture Research. 2016;47(5):1612–21. 10.1111/are.12622 [DOI] [Google Scholar]
- 26.Zhou Q-C, Zhao J, Li P, Wang H-L, Wang L-G. Evaluation of poultry by-product meal in commercial diets for juvenile cobia (Rachycentron canadum). Aquaculture. 2011;322–323:122–7. 10.1016/j.aquaculture.2011.09.042 [DOI] [Google Scholar]
- 27.Gupta SK, Fotedar R, Foysal MJ, Priyam M, Siddik MA, Chaklader MR, et al. Impact of varied combinatorial mixture of non-fishmeal ingredients on growth, metabolism, immunity and gut microbiota of Lates calcarifer (Bloch, 1790) fry. Scientific Reports. 2020;10(1):1–13. 10.1038/s41598-019-56847-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galkanda‐Arachchige HS, Wilson AE, Davis DA. Success of fishmeal replacement through poultry by‐product meal in aquaculture feed formulations: a meta‐analysis. Reviews in Aquaculture. 2019. 10.1111/raq.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glencross B, Blyth D, Irvin S, Bourne N, Campet M, Boisot P, et al. An evaluation of the complete replacement of both fishmeal and fish oil in diets for juvenile Asian seabass, Lates calcarifer. Aquaculture. 2016;451:298–309. [Google Scholar]
- 30.Yigit M, Erdem M, Koshio S, Ergün S, Türker A, Karaali B. Substituting fish meal with poultry by‐product meal in diets for black Sea turbot Psetta maeotica. Aquaculture Nutrition. 2006;12(5):340–7. 10.1111/j.1365-2095.2006.00409.x [DOI] [Google Scholar]
- 31.Karapanagiotidis IT, Psofakis P, Mente E, Malandrakis E, Golomazou E. Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata). Aquaculture Nutrition. 2019;25(1):3–14. 10.1111/anu.12824 [DOI] [Google Scholar]
- 32.Shapawi R, Ng W-K, Mustafa S. Replacement of fish meal with poultry by-product meal in diets formulated for the humpback grouper, Cromileptes altivelis. Aquaculture. 2007;273(1):118–26. 10.1016/j.aquaculture.2007.09.014 [DOI] [Google Scholar]
- 33.Martínez-Álvarez RM, Morales AE, Sanz A. Antioxidant defenses in fish: biotic and abiotic factors. Reviews in Fish Biology and fisheries. 2005;15(1–2):75–88. [Google Scholar]
- 34.Sinha AK, AbdElgawad H, Giblen T, Zinta G, De Rop M, Asard H, et al. Anti-oxidative defences are modulated differentially in three freshwater teleosts in response to ammonia-induced oxidative stress. PLoS One. 2014;9(4). 10.1371/journal.pone.0095319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoseini SM, Yousefi M, Hoseinifar SH, Van Doan H. Antioxidant, enzymatic and hematological responses of common carp (Cyprinus carpio) fed with myrcene-or menthol-supplemented diets and exposed to ambient ammonia. Aquaculture. 2019;506:246–55. [Google Scholar]
- 36.Ilham I, Fotedar R. Growth, enzymatic glutathione peroxidase activity and biochemical status of juvenile barramundi (Lates calcarifer) fed dietary fermented soybean meal and organic selenium. Fish Physiology and Biochemistry. 2017;43(3):775–90. 10.1007/s10695-016-0331-2 [DOI] [PubMed] [Google Scholar]
- 37.Ilham I, Hapsari F, Fotedar R. Growth, enzymatic glutathione peroxidase activity and biochemical status of juvenile barramundi (Lates calcarifer) fed dietary fermented lupin meal supplemented with organic selenium. Aquaculture Research. 2018;49(1):151–64. 10.1111/are.13444 [DOI] [Google Scholar]
- 38.Ye H, Zhou Y, Su N, Wang A, Tan X, Sun Z, et al. Effects of replacing fish meal with rendered animal protein blend on growth performance, hepatic steatosis and immune status in hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). Aquaculture. 2019;511:734203 10.1016/j.aquaculture.2019.734203 [DOI] [Google Scholar]
- 39.Huntingford FA, Kadri S. Defining, assessing and promoting the welfare of farmed fish. Revue Scientifique et Technique (International Office of Epizootics). 2014;33:pp. 233–44. 10.20506/rst.33.1.2286 [DOI] [PubMed] [Google Scholar]
- 40.Saraiva A, Costa J, Serrão J, Cruz C, Eiras J. A histology-based fish health assessment of farmed seabass (Dicentrarchus labrax L.). Aquaculture. 2015;448:375. [Google Scholar]
- 41.Tort L, Pavlidis MA, Woo NYS. Stress and welfare in sparidfishes. In: Pavlidis M.A., Mylonas C.C. (Eds.), Sparidae Biology and Aquaculture of Gilthead Sea Bream and Other Species. Wiley-Blackwell, Chichester: 2011:pp. 75–94. [Google Scholar]
- 42.Siddik MAB, Howieson J, Partridge GJ, Fotedar R, Gholipourkanani H. Dietary tuna hydrolysate modulates growth performance, immune response, intestinal morphology and resistance to Streptococcus iniae in juvenile barramundi, Lates calcarifer. Sci Rep. 2018;8(1):15942 Epub 2018/10/31. 10.1038/s41598-018-34182-4 PubMed Central PMCID: PMC6206086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.NRC. Nutrient Requirements of Fish and Shrimp: National Academies Press; 2011. [Google Scholar]
- 44.Poppi DA, Glencross BD. Partial utilization efficiencies of protein and methionine by barramundi (Lates calcarifer) in response to dietary methionine source and form. Aquaculture Research. 2018;49(7):2518–26. [Google Scholar]
- 45.AOAC. Official Methods of Analysis, 16th Ed, Association of Official Analytical Chemists, Washington DC, USA. 1995.
- 46.Chaklader MR, Fotedar R, Howieson J, Siddik MA, Foysal J. The ameliorative effects of various fish protein hydrolysates in poultry by-product meal based diets on muscle quality, serum biochemistry and immunity in juvenile barramundi, Lates calcarifer. Fish & Shellfish Immunology. 2020;104:567–78. 10.1016/j.fsi.2020.06.014 [DOI] [PubMed] [Google Scholar]
- 47.O'Fallon JV, Busboom JR, Nelson ML, Gaskins CT. direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. 2007;85(6):1511–21. 10.2527/jas.2006-491 [DOI] [PubMed] [Google Scholar]
- 48.Mohd-Shaharuddin N, Mohd-Adnan A, Kua B-C, Nathan S. Expression profile of immune-related genes in Lates calcarifer infected by Cryptocaryon irritans. Fish & shellfish immunology. 2013;34(3):762–9. 10.1016/j.fsi.2012.11.052 [DOI] [PubMed] [Google Scholar]
- 49.Ayadi FY, Rosentrater KA, Muthukumarappan K. Alternative protein sources for aquaculture feeds. Journal of Aquaculture Feed Science and Nutrition. 2012;4(1):1–26. [Google Scholar]
- 50.Barreto-Curiel F, Parés-Sierra G, Correa-Reyes G, Durazo-Beltrán E, Viana MT. Total and partial fishmeal substitution by poultry by-product meal (petfood grade) and enrichment with acid fish silage in aquafeeds for juveniles of rainbow trout Oncorhynchus mykiss. Latin American Journal of Aquatic Research. 2016;44(2):327–35. [Google Scholar]
- 51.Gümüş E, Aydin B. Effect of poultry by-product meal on growth performance and fatty acid composition of carp (Cyprinus carpio) fry. Turkish Journal of Fisheries and Aquatic Sciences. 2013;13(5):827–34. [Google Scholar]
- 52.Hu M, Wang Y, Wang Q, Zhao M, Xiong B, Qian X, et al. Replacement of fish meal by rendered animal protein ingredients with lysine and methionine supplementation to practical diets for gibel carp, Carassius auratus gibelio. Aquaculture. 2008;275(1–4):260–5. 10.1016/j.aquaculture.2008.01.005 [DOI] [Google Scholar]
- 53.Hernández C, Osuna-Osuna L, Hernandez AB, Sanchez-Gutierrez Y, González-Rodríguez B, Dominguez-Jimenez P. Replacement of fish meal by poultry by-product meal, food grade, in diets for juvenile spotted rose snapper (Lutjanus guttatus). Latin american journal of aquatic research. 2014;42(1):111–20. 10.3856/vol42-issue1-fulltext-8 [DOI] [Google Scholar]
- 54.Zapata DB, Lazo JP, Herzka SZ, Viana MT. The effect of substituting fishmeal with poultry by‐product meal in diets for Totoaba macdonaldi juveniles. Aquaculture Research. 2016;47(6):1778–89. 10.1111/are.12636 [DOI] [Google Scholar]
- 55.García-Pérez OD, Cruz-Valdez JC, Ramírez-Martínez C, Villarreal-Cavazos D, Gamboa-Delgado J. Exploring the contribution of dietary protein from poultry by-product meal and fish meal to the growth of catfish Ictalurus punctatus by means of nitrogen stable isotopes. Latin american journal of aquatic research. 2018;46(1):37–44. [Google Scholar]
- 56.Nengas I, Alexis MN, Davies SJ. High inclusion levels of poultry meals and related byproducts in diets for gilthead seabream Sparus aurata L. Aquaculture. 1999;179(1):13–23. 10.1016/S0044-8486(99)00148-9 [DOI] [Google Scholar]
- 57.Panicz R, Żochowska‐Kujawska J, Sadowski J, Sobczak M. Effect of feeding various levels of poultry by‐product meal on the blood parameters, filet composition and structure of female tenches (Tinca tinca. Aquaculture Research. 2017;48(10):5373–84. 10.1111/are.13351 [DOI] [Google Scholar]
- 58.Gunben EM, Senoo S, Yong A, Shapawi R. High potential of poultry by-product meal as a main protein source in the formulated feeds for a commonly cultured grouper in Malaysia (Epinephelus fuscoguttatus). Sains Malaysiana. 2014;43(3):399–405. [Google Scholar]
- 59.Emre Y, Kurtoğlu A, Emre N, Güroy B, Güroy D. Effect of replacing dietary fish oil with soybean oil on growth performance, fatty acid composition and haematological parameters of juvenile meagre, Argyrosomus regius. Aquaculture Research. 2016;47(7):2256–65. 10.1111/are.12677 [DOI] [Google Scholar]
- 60.González-Félix ML, Maldonado-Othón CA, Perez-Velazquez M. Effect of dietary lipid level and replacement of fish oil by soybean oil in compound feeds for the shortfin corvina (Cynoscion parvipinnis). Aquaculture. 2016;454:217–28. 10.1016/j.aquaculture.2015.12.021 [DOI] [Google Scholar]
- 61.Xu H, Zhang Y, Wang J, Zuo R, Mai K, Ai Q. Replacement of Fish Oil with Linseed Oil or Soybean Oil in Feeds for Japanese Seabass, Lateolabrax japonicus: Effects on Growth Performance, Immune Response, and Tissue Fatty Acid Composition. Journal of the World Aquaculture Society. 2015;46(4):349–62. 10.1111/jwas.12205 [DOI] [Google Scholar]
- 62.Dawson MR, Alam MS, Watanabe WO, Carroll PM, Seaton PJ. Evaluation of poultry by‐product meal as an alternative to fish meal in the diet of juvenile Black Sea Bass reared in a recirculating aquaculture system. North American Journal of Aquaculture. 2018;80(1):74–87. [Google Scholar]
- 63.Wells R, McIntyre R, Morgan A, Davie P. Physiological stress responses in big gamefish after capture: observations on plasma chemistry and blood factors. Comparative biochemistry and physiology A, Comparative physiology. 1986;84(3):565–71. 10.1016/0300-9629(86)90366-x [DOI] [PubMed] [Google Scholar]
- 64.Sabbagh M, Schiavone R, Brizzi G, Sicuro B, Zilli L, Vilella S. Poultry by-product meal as an alternative to fish meal in the juvenile gilthead seabream (Sparus aurata) diet. Aquaculture. 2019;511:734220. [Google Scholar]
- 65.Huang L, Ran C, He S, Ren P, Hu J, Zhao X, et al. Effects of dietary Saccharomyces cerevisiae culture or live cells with Bacillus amyloliquefaciens spores on growth performance, gut mucosal morphology, hsp70 gene expression, and disease resistance of juvenile common carp (Cyprinus carpio). Aquaculture. 2015;438:33–8. 10.1016/j.aquaculture.2014.12.029 [DOI] [Google Scholar]
- 66.Rollo A, Sulpizio R, Nardi M, Silvi S, Orpianesi C, Caggiano M, et al. Live microbial feed supplement in aquaculture for improvement of stress tolerance. Fish Physiology and Biochemistry. 2006;32(2):167–77. 10.1007/s10695-006-0009-2 [DOI] [Google Scholar]
- 67.Ming J, Xie J, Xu P, Ge X, Liu W, Ye J. Effects of emodin and vitamin C on growth performance, biochemical parameters and two HSP70s mRNA expression of Wuchang bream (Megalobrama amblycephala Yih) under high temperature stress. Fish and Shellfish Immunology. 2012;32(5):651–61. 10.1016/j.fsi.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 68.Burgos-Aceves MA, Cohen A, Smith Y, Faggio C. Estrogen regulation of gene expression in the teleost fish immune system. Fish and Shellfish Immunology. 2016;58:42–9. 10.1016/j.fsi.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 69.Lauriano ER, Pergolizzi S, Capillo G, Kuciel M, Alesci A, Faggio C. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish and Shellfish Immunology. 2016;59(C):250–5. 10.1016/j.fsi.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 70.Lazado CC, Caipang CMA. Mucosal immunity and probiotics in fish. Fish and Shellfish Immunology. 2014;39(1):78–89. 10.1016/j.fsi.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 71.Subhadra B, Lochmann R, Rawles S, Chen R. Effect of dietary lipid source on the growth, tissue composition and hematological parameters of largemouth bass (Micropterus salmoides). Aquaculture. 2006;255:210–22. [Google Scholar]
- 72.Subhadra B, Lochmann R, Rawles S, Chen R. Effect of fish-meal replacement with poultry by-product meal on the growth, tissue composition and hematological parameters of largemouth bass (Micropterus salmoides) fed diets containing different lipids. Aquaculture. 2006;260(1–4):221–31. 10.1016/j.aquaculture.2006.06.029 [DOI] [Google Scholar]
- 73.Kang-Le L, Wei-Na X, Li-Na W, Ding-Dong Z, Chun-Nuan Z, Wen-Bin L. Hepatic β-oxidation and regulation of carnitine palmitoyltransferase (CPT) I in blunt snout bream Megalobrama amblycephala fed a high fat diet. PLoS ONE. 2014;9(3):e93135 10.1371/journal.pone.0093135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spisni E, Tugnoli M, Ponticelli A, Mordenti T, Tomasi V. Hepatic steatosis in artificially fed marine teleosts. Journal of Fish Diseases. 1998;21(3):177–84. 10.1046/j.1365-2761.1998.00089.x [DOI] [PubMed] [Google Scholar]
- 75.Alami-Durante H, Bazin D, Cluzeaud M, Fontagné-Dicharry S, Kaushik S, Geurden I. Effect of dietary methionine level on muscle growth mechanisms in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture. 2018;483:273–85. 10.1016/j.aquaculture.2017.10.030 [DOI] [Google Scholar]
- 76.Rodger H, Murphy T, Drinan E, Rice D. Acute skeletal myopathy in farmed Atlantic salmon Salmo salar. Diseases of Aquatic Organisms. 1991;12(1):17–23. [Google Scholar]
- 77.Anderson Brunetti R, Débora de Mello Gonçales SA, Jorge Fernandes de A, Luiz Sérgio M, Eduardo José de Almeida A. Alterações do epitélio branquial e das lamelas de tilápias (Oreochromis niloticus) causadas por mudanças do ambiente aquático em tanques de cultivo intensivo The influence of the aquatic environment in tanks sequetially interconnected with PVC pipes on the gill epithelium and lamellas of tilapia (Oreochromis niloticus). Pesquisa Veterinária Brasileira. 2009;29(4):303–11. 10.1590/S0100-736X2009000400005 [DOI] [Google Scholar]
- 78.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, et al. Host-gut microbiota metabolic interactions. Science (New York, NY). 2012;336(6086):1262 10.1126/science.1223813 [DOI] [PubMed] [Google Scholar]
- 79.Urán PA, Schrama JW, Rombout JHWM, Obach A, Jensen L, Koppe W, et al. Soybean meal‐induced enteritis in Atlantic salmon (Salmo salar L.) at different temperatures. Aquaculture Nutrition. 2008;14(4):324–30. 10.1111/j.1365-2095.2007.00534.x [DOI] [Google Scholar]
- 80.Devi G, Harikrishnan R, Paray BA, Al-Sadoon MK, Hoseinifar SH, Balasundaram C. Effect of symbiotic supplemented diet on innate-adaptive immune response, cytokine gene regulation and antioxidant property in Labeo rohita against Aeromonas hydrophila. Fish & shellfish immunology. 2019;89:687–700. 10.1016/j.fsi.2019.04.036 [DOI] [PubMed] [Google Scholar]
- 81.Zheng X, Chi C, Xu C, Liu J, Zhang C, Zhang L, et al. Effects of dietary supplementation with icariin on growth performance, antioxidant capacity and non-specific immunity of Chinese mitten crab (Eriocheir sinensis). Fish & shellfish immunology. 2019;90:264–73. [DOI] [PubMed] [Google Scholar]
- 82.Wang W-N, Zhou J, Wang P, Tian T-T, Zheng Y, Liu Y, et al. Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comparative Biochemistry and Physiology, Part C. 2009;150(4):428–35. 10.1016/j.cbpc.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 83.Liu J, Mai K, Xu W, Zhang Y, Zhou H, Ai Q. Effects of dietary glutamine on survival, growth performance, activities of digestive enzyme, antioxidant status and hypoxia stress resistance of half-smooth tongue sole (Cynoglossus semilaevis Günther) post larvae. Aquaculture. 2015;446:48–56. [Google Scholar]
- 84.Thiansilakul Y, Benjakul S, Shahidi F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi). Food chemistry. 2007;103(4):1385–94. [Google Scholar]
- 85.Sabzi E, Mohammadiazarm H, Salati AP. Effect of dietary l-carnitine and lipid levels on growth performance, blood biochemical parameters and antioxidant status in juvenile common carp (Cyprinus carpio). Aquaculture. 2017;480:89–93. 10.1016/j.aquaculture.2017.08.013 [DOI] [Google Scholar]
- 86.Nugroho R, Fotedar R. Comparing the effects of dietary selenium and mannan oligosaccharide supplementation on the growth, immune function, and antioxidant enzyme activity in the cultured marron Cherax cainii (Austin, 2002). Journal of the European Aquaculture Society. 2014;22(2):585–96. 10.1007/s10499-013-9682-1 [DOI] [Google Scholar]
- 87.Satoshi S, Kiyoji T, Hiroyo K, Fumio N. Exercise-induced lipid peroxidation and leakage of enzymes before and after vitamin E supplementation. International Journal of Biochemistry. 1989;21(8):835–8. 10.1016/0020-711x(89)90280-2 [DOI] [PubMed] [Google Scholar]
- 88.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine, fifth ed. Oxford University Press, Croydon, UK: 2015:pp. 944. [Google Scholar]