Abstract
Coral reefs are degrading globally due to increased environmental stressors including warming and elevated levels of pollutants. These stressors affect not only habitat-forming organisms, such as corals, but they may also directly affect the organisms that inhabit these ecosystems. Here, we explore how the dual threat of habitat degradation and microplastic exposure may affect the behaviour and survival of coral reef fish in the field. Fish were caught prior to settlement and pulse-fed polystyrene microplastics six times over 4 days, then placed in the field on live or dead-degraded coral patches. Exposure to microplastics or dead coral led fish to be bolder, more active and stray further from shelter compared to control fish. Effect sizes indicated that plastic exposure had a greater effect on behaviour than degraded habitat, and we found no evidence of synergistic effects. This pattern was also displayed in their survival in the field. Our results highlight that attaining low concentrations of microplastic in the environment will be a useful management strategy, since minimizing microplastic intake by fishes may work concurrently with reef restoration strategies to enhance the resilience of coral reef populations.
Keywords: habitat degradation, microplastics, coral reef fish, predator–prey, pollution, climate change
1. Introduction
Organisms all over the world are subject to increasing levels of stress as a consequence of exponentially increasing human populations and the push for economic growth [1,2]. For aquatic species, some stressors, such as elevated temperature, lowered pH and increased levels of pollutants, have direct impacts on the physiology of organisms and the viability of their progeny [3]. Other stressors have indirect consequences for individuals, species and communities, including habitat modifications or changes to the quality of available resources [4]. Historically, when researchers have explored the impact of stressors on the biology and ecology of organisms, they have looked at stressors in isolation [5]. However, stressors are most likely to occur concurrently and this co-occurrence can lead to additive, antagonistic or synergistic effects [6–11]. Accordingly, combined stressor effects have become an increasing focus in recent years [12,13]. Given the complexity of natural systems, manipulative experiments have proven key for characterizing multi-stressor interactions, and these findings inform us of the potential trajectory of future communities.
Species inhabiting tropical latitudes are particularly sensitive to new and increasing stressors because they have evolved in relatively stable environmental conditions [14]. For example, tropical hard corals, which often exist at the edge of their thermal maxima, are particularly sensitive to increasing water temperatures. Recent warming events (2016, 2017 and 2019) have led to large-scale coral bleaching and mortality [15–17] with subsequent losses in coral fecundity, which can undermine the recovery of reefs through time [18]. Because corals are ecosystem engineers, this has led to a degradation of the habitat, with flow-on effects for all organisms that live within this ecosystem [19–21]. Recent studies have found that many fishes that live on coral reefs are adversely affected by the changes in chemistry that accompany shifts from coral to algal-dominated seascapes. Research shows that some species can no longer use critically important alarm cue mediated mechanisms of gauging threats and learning new dangers or updating information on risk, leading to increased mortality rates on degraded coral habitats [22–24].
In addition to the threats of warming waters and habitat degradation, tropical ecosystems are under increasing pressure from rapid population and economic growth and with that come the escalating problem of waste disposal [25]. Plastic waste has been generated at an exponential rate over the past decades with 60% of the world's mismanaged plastics waste originating from tropical East Asia-Pacific countries (data 2010, [26–28]). Once in the marine environment, larger plastic fragments break up into smaller more numerous pieces via mechanical processes, photo-oxidation and biodegradation [29]. In addition, small plastic particles, used to increase abrasion in beauty and cleaning products (i.e. microbeads), also end up in aquatic environments in large quantities via wastewater systems [30]. Smaller particles have a larger relative surface area [31], resulting in the increased potential to leach chemicals that are either contained within them or adsorbed to their surface from the surrounding water [32,33]. Two decades of marine research demonstrates that these small particles or microplastics (less than 5 mm in size, Kroon et al. [34]) are common at all trophic levels, and both oceanic surveys and experiments clearly show microplastic transfer between trophic levels [28,35,36]. Ingestion of large plastic items may lead to occlusion or abrasion of the alimentary tract [35] and even small plastic particles that are able to pass through the gut are capable of producing adverse physiological and behavioural effects that may lead to greater mortality in the wild [33,37–39].
The goal of the present study was to explore, for the first time, how two key stressors facing tropical ecosystems—habitat degradation and microplastic pollution—may interact to affect the behaviour of coral reef fish and their survival in the field. Pre-settlement coral reef fish were pulse-fed polystyrene microplastics over a 4-day period and then placed in the field on live or dead-degraded coral patches where their behaviour and survival was monitored over 3 days. The experimental scenario used in the present study will likely be analogous to the microplastic levels found near inshore fringing reefs and inshore reefs near urban areas within the more populated parts of the tropical Indo-Pacific. Our predictions from previous research were that (i) dead coral would negatively impact risk assessment and survival, (ii) microplastics consumption may have a similar effect due to toxins or a starvation effect and (iii) together, the stressors may interact to further reduce the fitness of the affected individuals.
2. Material and methods
(a). Study system and species
Our current study was conducted at Lizard Island (14°40′ S, 145°28′ E), on the northern Great Barrier Reef, Australia. Fish were collected at the end of their larval phase using light traps at night, returned to the laboratory in covered 60 l tanks at dawn, sorted by species and transferred to 35 l tanks of aerated flow-through seawater. Light traps were moored at least 30 m from the nearest reef edge and the fish caught had not yet experienced the specific predators that awaited them upon settlement [40]. The study species, the Ambon damselfish Pomacentrus amboinensis, is a common damselfish within coral reef fish communities of the Indo-Pacific. It is planktivorous when it first settles to the reef and rapidly becomes omnivorous feeding on a mixture of planktonic and benthic food items [41,42]. Pomacentrus amboinensis are 10.3–15.1 mm long (standard length, SL) and 15–23 days old at settlement [43]. They are commonly preyed upon by a variety of predatory fishes, including the sand lizardfish (Synodus dermatogenys), dusky dottyback (Pseudochromis fuscus) and the moonwrasse (Thalassoma lunare) [44–47].
One day after capture in light traps, fish were batch tagged with a fluorescent elastomer (following Hoey and McCormick [48]) so that they could be identified from natural recruits that may have settled on to the same patch reef. Individually tagged recruit-sized damselfishes exhibit minimal movement between patches, and so any loss from the patch reefs can be attributed to predation [49–51]. All research was conducted in accordance with the James Cook University Animal Ethics guidelines with approval from the JCU Animal Ethics Committee (approval A2408). Ethical details concerning transport and tagging of fish can be found in the electronic supplementary material file (Part 1).
(b). Laboratory conditioning
In this study, we decided to pulse expose fish to microplastic concentrations that may be similar to an area of reef offshore of an urban centre (see electronic supplementary material, Part 2 for logic). Still, the employed concentrations are low compared to the majority of past microplastic exposure studies (e.g. [52]).
Fish were held in 35 l tanks for 1 day prior to being randomly allocated to groups of 10 individuals within 1.2 l glass tanks of aerated seawater that had been filtered through a 1 µm bag-filter. Each day, over a 4-day period, four tanks were set up; two tanks had approximately 1000 newly hatched Artemia spp. nauplii and 200 microplastics (200–300 µm transparent polystyrene spherical beads, the density of 1.04 g/ml; Polysciences Inc. Lot 577635; catalogue number 19825) added to them at each feeding time (i.e. a microplastic density of 167 particles per litre), while the remaining two tanks only had 1000 Artemia spp. added to them. Microplastic beads were used to reduce the number of variables being manipulated and thereby enhance interpretation. The microbeads were used as received. Chemical analyses support the manufacture's claim that these beads are composed of polystyrene. Leaching of organic contaminants was not observed over the time frame of the experiment (electronic supplementary material, Part 3). Feeding occurred each day at approximately 08.30 and 16.30 h. After 15 min, fish were carefully transferred to separate tanks while the holding tank was rinsed and refilled with filtered seawater. The remaining food and beads in the tanks were not quantified, but we did notice on several occasions that there was food remaining in the jar. This seemed to occur more frequently on overcast days. Fish were fed using this protocol for six consecutive feedings with the final feeding occurring in the morning of the fourth day.
Gut analysis of fish that had been exposed to microplastic spheres for 1 h (at densities of 50 particles per litre with no additional Artemia, electronic supplementary material, file, Part 4) in a pilot study found that 85% of the fish consumed plastic spheres (51 out of 60 fish), with ingestions rates ranging from 1 to 33 particles (out of a possible 40 particles accessible to four fish in 0.8 l of water; electronic supplementary material, figure S4) and a mean of 4.5 spheres per fish (median = 1.0). The distribution was markedly right skewed with 60% of fish eating three or fewer spheres (electronic supplementary material, figure S4). Serial sampling of the same life stage of a congeneric species (Pomacentrus chrysurus) that had been exposed to the spheres for 1 h and then placed in clean seawater (electronic supplementary material, Part 5), showed that fish can pass the particles through their alimentary tracts, taking up to 14 h to achieve egestion of all particles (electronic supplementary material, figure S5).
(c). Field behavioural assessment
One hour after the final feeding event, fish were placed into individually numbered 1 l plastic clip-seal bags containing aerated water and photographed against a 1 cm grid to obtain a size estimate. Fish in bags were then placed in a 60 l container of seawater, covered and taken by boat to the edge of a shallow fringing reef. Individual fish were placed by divers (MIM, MB) on one of approximately 50 small numbered patch reefs (approx. 25 × 15 × 20 cm; one per patch) made of either live-healthy or dead-degraded Pocillopora damicornis, a bushy hard coral commonly used as a settlement habitat by P. amboinensis [42,53]. The live coral and degraded corals used had similar structural complexity. For our purpose, we define a degraded habitat as Poc. damicornis that had been dead for approximately 3 months to 1 year and had a similar structural complexity to live coral, but was covered with a variety of sessile invertebrates and algae (e.g. see Fig. 1 in [54]). Substrata were sourced from the base of the main reef edge and were vacant of all fishes and mobile invertebrates. A small cage (11 mm mesh size; 30 × 30 × 30 cm) was placed over the patches to allow acclimation to the reef without the threat of predation for the duration of 40 to 60 min. Treatments were systematically placed down the reef to avoid any possible confound with reef position. Patch reefs were approximately 4 m apart and 4 m from the continuous reef edge with 32 to 42 replicate fish per treatment combination. Due to time limitations, behavioural observations were not undertaken on all fish (n = 28–32 per treatment).
After the cage was carefully removed, activity and space use was assessed using a well-established protocol [51] by a single observer (MIM) who was blind to the microplastic feeding treatment. Briefly, fish behaviour was assessed in situ over a 3 min period by the observer on SCUBA that was approximately 1.5 m away from the patch reef with the aid of a magnifying glass. Four aspects of activity and space use were assessed: (i) bite rate; (ii) total distance moved (estimated from knowing the length of each reef); (iii) maximum distance ventured (max DV) from the habitat patch and (iv) boldness. Boldness was quantified using a continuous scale between 0 and 3 from the 3 min observation period, after which the focal fish was approached by a pencil tip and its response observed (electronic supplementary material, Part 6 for details).
(d). Survival monitoring
Tagged fish on live or dead-degraded hard coral patch reefs were monitored three times per day for 72 h (approx. 07.00, 12.00, 17.00 h). If fish were missing from a reef, then a search of the neighbouring reef edge was conducted, but none were found. Previous studies have found that movement is rare, even when the density on patch reefs is high [49]. Observations during behavioural observations and censuses highlighted that when fish strayed too far from shelter they were quickly subjected to predation by predators such as lizardfish or wrasses. On the rare occasion that other fishes naturally settled onto the experimental patch reefs, they were removed with a small dip net at the time of census, ensuring there was minimal disturbance to the focal fish.
(e). Statistical analysis
The four behavioural variables (bite rate, distance travelled, maximum distance ventured, boldness index) were computed into a single synthetic variable using a correlation-based principal component analysis (PCA). Variables were transformed to improve normality (the first three square-root transformed, the last squared) as PCA performs better when data are normally distributed. General linear mixed models were then performed on the first principle component, which accounted for 75% of the variance in fish behaviour, with correlation coefficients for individual variables being high and negative (bite rate −0.73; distance moved −0.88; max distance ventured −0.88; boldness −0.95). Because of the strong negative relationships of the original variables with PC1, the opposite (sign) of the mean scores were represented in the interaction figure to aid interpretation of the difference in behaviour among treatment combinations. The effect of coral environment (fixed: live versus dead) and plastic (fixed: presence versus absence) on fish behaviour was tested with a three-factor nested generalized linear model (GLM), using tank identity as a nested (random) factor in the analysis (type I sums of squares). This random factor took into account the interdependence of the fish maintained and fed in the same tank during the plastic exposure, effectively making tank, not fish, the level of replication for the plastic treatment. Tukey's HSD means comparisons for unequal sample sizes were used to explore the nature of the significant effects found. In the current study, there was an ordinal interaction [55], so the significant main effects were also interpreted. Effect sizes are given as partial-eta-squared (), which represents the proportion of the total variance in a dependent variable that is associated with the membership of different groups [56].
Survival of the P. amboinensis in relation to the four treatments was compared using a Cox proportional hazard analysis. To determine the nature of the significant treatment effect found, data for the ‘live coral–no microplastics' treatment was subsequently dropped and the remaining three treatments were retested for equality. To explore the link between survival (h) and behaviour (as represented by PC1), Spearman's rank order correlation was undertaken because of the bimodal nature of survival. Statistics were undertaken using Statistica (v. 13.0).
3. Results
The behaviour of the fish in the field was affected by a significant interaction between the exposure to plastic and the coral environment in which they were released (F1,16 = 13.42, p = 0.002, figure 1). This interaction stemmed from an inconsistency in the effect of plastic exposure on the behaviour of fish between habitat types (figure 1, Tukey's test). In both coral types, fish pre-exposed to plastic showed elevated behavioural activity compared to those in the no-plastic treatments, as shown by a significant plastic main effect (F1,17 = 27.11, p < 0.0001, electronic supplementary material, table S1). Similarly, there was a clear effect of habitat on the behaviour of fish (F1,16 = 11.17, p < 0.004, electronic supplementary material, table S1), with fish placed on dead coral showing greater behavioural activity compared to those on live coral when fish had not been exposed to microplastics (figure 1, Tukey's tests). Effect sizes indicate that exposure to plastic was the dominant effect, accounting for 30% more of the total variance in behaviour than habitat type, and 25% more variability than the interaction. Trends in PC1 are supported by trends in the original variables (electronic supplementary material, figure S6). There was a significant difference among tanks in the behaviour of fish within the plastic treatments (F19,14 = 2.78, p = 0.03) likely due to the social hierarchies established within the confinement period, and this term also had a large effect size . However, these differences were consistent among habitats (i.e. interaction: p = 0.67).
Figure 1.
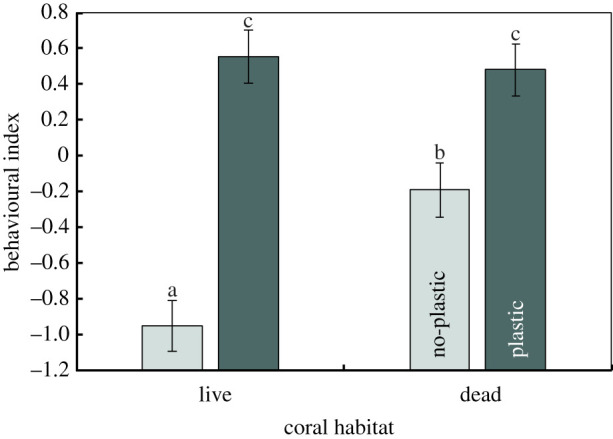
Comparison of the effects of pre-exposure to polystyrene microplastic spheres and the field habitat on which they were subsequently placed (live or dead coral patch) on the behaviour of juvenile Pomacentrus amboinensis. The behavioural index is a composite variable (mean ± s.e.), represented as PC1 of a principal component analysis on bite rate, total distance moved in 3 min, maximum distance ventured from shelter and a boldness index (see text). As represented, the behavioural index is positively related to all variables used in the analysis. All variables were strongly correlated with PC1 (see text). Letters represent Tukey's HSD means comparisons groupings. N (left to right) = 28, 32, 32, 31. (Online version in colour.)
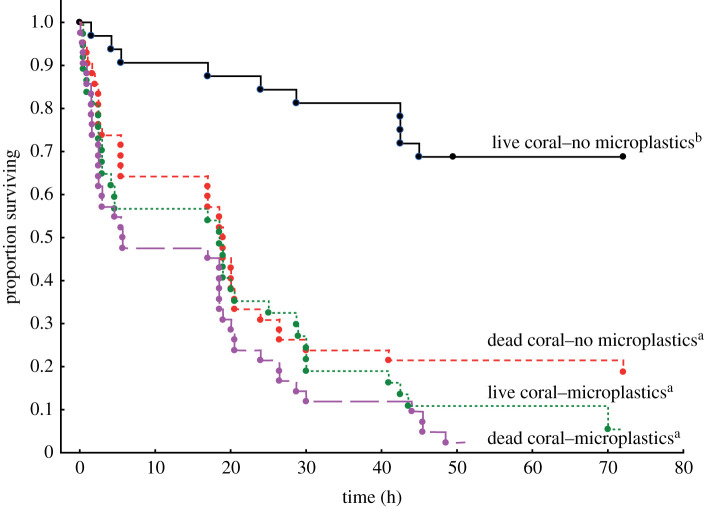
There was a significant difference between the survival trajectories of the four treatments ( p < 0.0001; figure 2). Reanalysis after dropping the live coral–no microplastics treatment from the analysis found no difference between the three remaining treatments ( p = 0.18; figure 2). A Spearman's rank order correlation found a moderately strong positive relationship between the behavioural index and hours surviving (ρ = 0.33, p = 0.001, n = 135). This suggests that survival increases as fish become more conservative in their behaviour (note that there are strong negative relationships between PC1 and behavioural measures, see Statistical analyses above).
Figure 2.
Survival on habitat patches of juvenile Pomacentrus amboinensis that had either been pre-exposed to microplastics or not in the laboratory for 4 days and then placed individually on live or dead-degraded coral patches. Superscript letters represent the grouping of trajectories based on proportional hazard tests (n = 32–42 fish per treatment). (Online version in colour.)
4. Discussion
This is the first study to examine the outcome of a scenario that may be increasingly common in the future—one of living in a degraded marine environment in the presence of plastic pollution. Our study shows that negative effects can arise when fish are exposed to both stressors alone or concurrently. When fish were living in a habitat of healthy live coral they stayed closer to shelter and took less risk compared to their dead-degraded coral counterparts, leading to higher survival from predation. However, when fish had a history of potentially feeding on microplastic particles for a small portion of their early life, their behaviour became even more risk-prone and they strayed even further from shelter than fish on dead-degraded coral without plastic exposure. A positive relationship between mortality and travelling further from shelter emphasizes that risky behaviour leads to higher mortality. Exposure to microplastics accounted for more variability in behaviour than living in degraded habitats. Moreover, fish that had a history of exposure to microplastics exhibited six times lower survival than those that had not been exposed to microplastics when they were living in a live coral-dominated habitat. The results of both the behavioural and survival analyses revealed a similar antagonistic interaction between coral habitat and plastic treatments, which likely stem from a ceiling truncation. Indeed, fish fed plastic exhibited an increase in risk-taking behaviours, which was more extreme than those observed in fish placed in dead coral alone. We also failed to see a habitat difference between the two groups of plastic-fed fish, which indicates that these fish displayed maximal risk-prone behaviours. Similarly, fish fed plastic and fish placed in dead coral suffered approximately 90% mortality, a number that is mathematically capped at 100%. It is also interesting to note that the plastic–dead coral fish were the only ones to reach the 100% mortality, and that they did so well before the end of the monitoring period, supporting our ceiling truncation hypothesis. What these results imply, however, is that plastic consumption has a behavioural (and potentially a survival) impact that is at least as, if not more, severe than coral degradation. Our results suggest that a reduction of microplastics in the environment may be a useful management strategy, since minimizing microplastic intake by fishes will work in concert with reef restoration strategies to enhance the resilience of coral reef populations.
A number of studies have examined the interactive effects of microplastic consumption with other stressors, such as herbicides and antibiotics [57]. However, few studies to date have explored the interactive effect of microplastics and other environmental stressors on fish behaviour [57,58] and those that have, typically used plastic concentrations that are intended to cause gross physiological effects, rather than represent ecologically relevant concentrations (e.g. Ferreira et al. [59]). Compared to most previous research, our study used relatively conservative concentrations of microplastics [52] in an attempt to tease out interactive effects of microplastic pollution and habitat degradation. The present study found what appears to be an antagonistic effect between consuming microplastics and habitat quality in that the effect of habitat degradation and microplastic exposure together was actually less than the sum of the independent effects. However, as mentioned our study likely underestimates the combined effect of the two stressors because of the constrained nature of the variables measured (e.g. mortality cannot be higher than 100%). However, there being a large influence of habitat degradation on behaviour and survival as predicted [22,23,60], when fish had consumed microplastics, they behaved in a similar way regardless of habitat and displayed similarly low survival. Fish exposed to microplastics moved further from shelter and took more risks, exposing themselves to the predators that have high feeding rates and are highly selective for juvenile fish that stray from shelter [44,46]. This high risk behaviour dramatically reduced survival compared to fish not exposed to plastic and living on live coral. Interestingly, studies of interactive effects of stressors on fish life history and growth have generally found synergistic effects, rather than antagonistic or additive [61–63]; however, studies measuring growth rates, in juveniles especially, may not suffer from ceiling truncation issues.
Only one other study has fed microplastics to juvenile fishes and examined survival in realistic predator–prey scenario. Jacob et al. [64] fed small (90 µm) microplastics at high concentrations to settlement stage manini surgeonfish (Acanthurus triostegus) for 8 days and then exposed them to four lionfish (Pterois radiata) to find that there was no effect of microplastic consumption on survival. This result may have been due to lionfish being largely invisible to their prey [65,66], having efficient mechanisms of prey capture [67,68] and being crepuscular feeders [69], all of which would promote non-selective feeding among a group of similar sized prey. Clearly more studies are required that put microplastic-treated organisms into realistic scenarios to determine how consumption may impact biological interactions and the transfer of energy and toxins through the community.
The current study gave fish the opportunity to ingest microplastics in 15 min pulses twice a day for a total of six feeding episodes. The exact levels of microplastic capture for each individual were unknown due to the rapid passage of plastics through their guts. However, gut samples from our supplementary studies of microplastic-exposed fish suggest that the levels of ingestion were highly variable at the individual level. There is a paucity of information currently on the levels of microplastics within tropical environments (electronic supplementary material, Part 1) and their availability to marine organisms of different life stages [70]. Concentrations of plastic waste are likely to be greater in the shallow productive coastal areas that border urban areas (e.g. [71]) due to terrestrial sources and coastal current regimes. They are also likely to be greater during periods of heavy rain and wind in tropical areas [72], causing the inputs of new microplastics into offshore waters and resuspending existing loads from the sediment [73]. At an individual level, we have shown that fish ingest microplastics when available, but it is likely that ingestion within a population will be right skewed with only some individuals within the population focusing on the consumption of microplastics, possibly as a result of a search image making foraging for like-items more efficient [74], or due to individuality of food preferences [75].
Despite high variation in the likely number of microplastic particles consumed among individuals, we found there were marked differences in behaviour and mortality at the treatment (i.e. sub-population) level. This suggests that exposure to microplastics for a relatively short duration is enough to alter their behaviour and survival. Since we know that these small fish are able to evacuate ingested microplastic particles (electronic supplementary material, figure S5), it is possible that all fish within the treatment may have at some stage ingested particles that rapidly passed though the gut. Alternatively, the water that has been in contact with microplastics may itself have become toxic and lead to the behavioural changes; a possibility demonstrated for the intertidal gastropod Littorina littorea [38], though this study used degraded beach plastic that may have absorbed additional contaminates. Other studies have found toxic effects of free monomer styrene on a number of invertebrates and fish (e.g. [76]). Chemical analyses of the leachates from the beads used in the current study found that they were inert, suggesting that toxic effects are unlikely in the present study. Evidence suggests that our results are more likely due to nutritional stress promoting risk-taking over predator vigilance [77] (possibly due to the extremely high metabolic rates of these juvenile fish [78]), rather than a toxicity-induced behavioural syndrome [79]. If weathered particles had been used in the study we may have found additional influences of products released from the fragmented particles [80], which may have changed the likelihood that they would be ingested by fishes [80]. Clearly, we are only just beginning to understand the impacts that the ingestion of microplastics may have on animals, and the role that microplastic weathering plays is a key component that warrants further study.
Our results are in keeping with other studies that have found higher mortality in dead-degraded coral compared to live coral-dominated habitats for fish that often associate with live coral [51,81,82]. Results from monitoring studies show major shifts in community composition upon the loss of live coral [19,21,83]. Experimental studies have shown that fish on dead-degraded coral tend to stay further away from the coral habitat [51,81,84], thereby exposing themselves to a greater threat of predation. The reason underlying this movement away from a shelter is debatable but may include: being repelled by the smell of coral necrosis [51], an attempt to enhance crypsis due to the upwelling yellow light from the surrounding sand [51,85], or a willingness to take more risk due to a loss of the chemical associative learning mechanisms of learning and updating threat information [22,23,54].
The marine environment is a system under increasing stress from plastic pollution, but also from the global impact of ocean warming which has caused the widespread degradation of one of the Earth's most biologically diverse ecosystems, coral reefs. The predictions of increasing warming, storm frequency and severity [86] have led to a prognosis of a general decline in the quality of coral reefs globally [87]. Evidence from our study suggests that the consumption of microplastics may have a detrimental effect on juvenile fish that is of similar magnitude, at least over small spatial and temporal scales, to living in association with a degraded habitat. Our study also suggests that microplastic consumption may exacerbate the detrimental effects of coral reef degradation to impact the survival of newly recruiting fishes. Understanding how organisms respond to the co-occurrence of local stressors, such as pollutants like microplastics, with stressors such as global warming that are regulated by factors outside geographic boundaries, is central to determining useful management strategies that will promote the resilience of community members that resist change.
Supplementary Material
Acknowledgements
We are grateful to all the staff at the Lizard Island Research Station, and all the students and volunteers that helped with the light traps and sorting of fishes.
Data accessibility
Data available from: https://doi.org/10.6084/m9.figshare.12072021 or Tropical Data Hub doi:10.25903/5cff2e614a5d5.
Authors' contributions
M.I.M., B.J.M.A., D.P.C. and M.C.O.F. conceived and designed the study. D.P.C. undertook the laboratory conditioning of fish. G.B.N. and C.R. conducted ingestion and egestion trials. C.R., G.B.N., M.C.O.F. and B.J.M.A. dissected fish and counted particles. M.I.M., M.I.B. and E.P.F. undertook the field study. M.I.M. and M.C.O.F. analysed the data. G.V. and A.M.G. undertook chemical assays of the microplastics. M.I.M. wrote the first draft. All authors contributed substantially to the writing of the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by the Australian Research Council Centre of Excellence for Coral Reef Studies (EI140100117; M.I.M.) and an ARC Discovery grant no. (DP170103372; M.I.M., M.C.O.F., D.P.C.). B.J.M.A.'s research was supported by a Rossi Foundation plastic pollution grant and a grant from the Lizard Island Research Station. G.B.N. was supported by the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 700838.
References
- 1.Steffen W, Crutzen PJ, McNeill JR. 2007. The Anthropocene: are humans now overwhelming the great forces of nature? BioOne 36, 614–621. ( 10.1579/0044-7447(2007)36[614:TAAHNO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 2.McNeill JR, Engelke P. 2016. The great acceleration: an environmental history of the anthropocene since 1945. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Bonga SE.W. 1997. The stress response in fish. Physiol. Rev. 77, 591–625. ( 10.1152/physrev.1997.77.3.591) [DOI] [PubMed] [Google Scholar]
- 4.Wilson SK, Fisher R, Pratchett MS, Graham NAJ, Dulvy NK, Turner RA, Cakacaka A, Polunin NVC, Rushton SP. 2008. Exploitation and habitat degradation as agents of change within coral reef fish communities. Global Change Biol. 14, 2796–2809. ( 10.1111/j.1365-2486.2008.01696.x) [DOI] [Google Scholar]
- 5.O'Brien AL, Dafforn KA, Chariton AA, Johnston EL, Mayer-Pinto M. 2019. After decades of stressor research in urban estuarine ecosystems the focus is still on single stressors: a systematic literature review and meta-analysis. Sci. Total Environ. 684, 753–764. ( 10.1016/j.scitotenv.2019.02.131) [DOI] [PubMed] [Google Scholar]
- 6.Montoya JM, Raffaelli D. 2010. Climate change, biotic interactions and ecosystem services. Phil. Trans. R. Soc. B 365, 2013–2018. ( 10.1098/rstb.2010.0114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ormerod SJ, Dobson M, Hildrew AG, Townsend CR. 2010. Multiple stressors in freshwater ecosystems. Freshwat. Biol. 55, 1–4. ( 10.1111/j.1365-2427.2009.02395.x) [DOI] [Google Scholar]
- 8.Ferrari MCO, et al. 2015. Interactive effects of ocean acidification and rising sea temperatures alter predation rate and predator selectivity in reef fish communities. Global Change Biol. 21, 1848–1855. ( 10.1111/gcb.12818) [DOI] [PubMed] [Google Scholar]
- 9.Crain CM, Kroeker K, Halpern BS. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecol. Lett. 11, 1304–1315. ( 10.1111/j.1461-0248.2008.01253.x) [DOI] [PubMed] [Google Scholar]
- 10.Darling ES, Cote IM. 2008. Quantifying the evidence for ecological synergies. Ecol. Lett. 11, 1278–1286. ( 10.1111/j.1461-0248.2008.01243.x) [DOI] [PubMed] [Google Scholar]
- 11.Tekin E, Diamant ES, Cruz-Loya M, Enriquez V, Singh N, Savage VM, Yeh PJ. 2020. Using a newly introduced framework to measure ecological stressor interactions. Ecol. Lett. 23, 1391–1403. ( 10.1111/ele.13533) [DOI] [PubMed] [Google Scholar]
- 12.Côté I.M., Darling ES, Brown CJ. 2016. Interactions among ecosystem stressors and their importance in conservation. Proc. R. Soc. B 283, 20152592 ( 10.1098/rspb.2015.2592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson MC, Loewen CJ, Vinebrooke RD, Chimimba CT. 2016. Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Global Change Biol. 22, 180–189. ( 10.1111/gcb.13028) [DOI] [PubMed] [Google Scholar]
- 14.Stevens GC. 1989. The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat. 133, 240–256. ( 10.1086/284913) [DOI] [Google Scholar]
- 15.Hughes TP, et al. 2017. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. ( 10.1038/nature21707) [DOI] [PubMed] [Google Scholar]
- 16.Hughes TP, et al. 2018. Global warming transforms coral reef assemblages. Nature 556, 492–497. ( 10.1038/s41586-018-0041-2) [DOI] [PubMed] [Google Scholar]
- 17.Harrison HB, Álvarez-Noriega M, Baird AH, Heron SF, MacDonald C, Hughes TP. 2019. Back-to-back coral bleaching events on isolated atolls in the Coral Sea. Coral Reefs 38, 713–719. [Google Scholar]
- 18.Hughes TP, et al. 2019. Global warming impairs stock–recruitment dynamics of corals. Nature 568, 387–390. ( 10.1038/s41586-019-1081-y) [DOI] [PubMed] [Google Scholar]
- 19.Stuart-Smith RD, Brown CJ, Ceccarelli DM, Edgar GJ. 2018. Ecosystem restructuring along the Great Barrier Reef following mass coral bleaching. Nature 560, 92 ( 10.1038/s41586-018-0359-9). [DOI] [PubMed] [Google Scholar]
- 20.Pratchett MS, Munday PL, Wilson SK, Graham NA.J., Cinner JE, Bellwood DR, Jones GP, Polunin NVC, McClanahan TR. 2008. Effects of climate-induced coral bleaching on coral-reef fishes — ecological and economic consequences. Oceanogr. Mar. Biol. 46, 251–296. ( 10.1201/9781420065756.ch6) [DOI] [Google Scholar]
- 21.Jones GP, McCormick MI, Srinivasan M, Eagle JV. 2004. Coral decline threatens fish biodiversity in marine reserves. PNAS 101, 8251–8253. ( 10.1073/pnas.0401277101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrari MC.O., McCormick MI, Allan BJ.M, Chivers DP. 2017. Not equal in the face of habitat change: closely related fishes differ in their ability to use predation-related information in degraded coral. Proc. R. Soc. B 284, 20162758 ( 10.1098/rspb.2016.2758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick MI, Barry R, Allan BJ.M. 2017. Algae associated with coral degradation affects risk assessment in coral reef fishes. Sci. Rep. 7, 16937 ( 10.1038/s41598-017-17197-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chivers DP, McCormick MI, Fakan EP, Barry RP, Edmiston J, Ferrari MC.O. 2019. Coral degradation alters predator odour signatures and influences prey learning and survival. Proc. R. Soc. B 286, 20190562 ( 10.1098/rspb.2019.0562) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebreton L, Andrady A. 2019. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 5, 6 ( 10.1057/s41599-018-0212-7) [DOI] [Google Scholar]
- 26.Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. 2015. Plastic waste inputs from land into the ocean. Science 347, 768–771. ( 10.1126/science.1260352) [DOI] [PubMed] [Google Scholar]
- 27.Purba NP, Handyman DIW, Pribadi TD, Syakti AD, Pranowo WS, Harvey A, Ihsan YN. 2019. Marine debris in Indonesia: a review of research and status. Mar. Pollut. Bull. 146, 134–144. ( 10.1016/j.marpolbul.2019.05.057) [DOI] [PubMed] [Google Scholar]
- 28.Rhodes CJ. 2018. Plastic pollution and potential solutions. Sci. Prog. 101, 207–260. ( 10.3184/003685018X15294876706211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ter Halle A, Ladirat L, Gendre X, Goudouneche D, Pusineri C, Routaboul C, Tenailleau C, Duployer B, Perez E. 2016. Understanding the fragmentation pattern of marine plastic debris. Environ. Sci. Technol. 50, 5668–5675. ( 10.1021/acs.est.6b00594) [DOI] [PubMed] [Google Scholar]
- 30.Eriksen M, Lebreton LCM, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J. 2014. Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 9, e111913 ( 10.1371/journal.pone.0111913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberdörster G, Oberdörster E, Oberdörster J. 2005. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 113, 823–839. ( 10.1289/ehp.7339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T. 2001. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 35, 318–324. ( 10.1021/es0010498) [DOI] [PubMed] [Google Scholar]
- 33.Galloway TS, Cole M, Lewis C. 2017. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 1, 0116 ( 10.1038/s41559-017-0116) [DOI] [PubMed] [Google Scholar]
- 34.Kroon FJ, Motti CE, Jensen LH, Berry KLE. 2018. Classification of marine microdebris: a review and case study on fish from the Great Barrier Reef, Australia. Sci. Rep. 8, 15 ( 10.1038/s41598-018-34590-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Derraik JGB. 2002. The pollution of the marine environment by plastic debris: a review. Mar. Pollut. Bull. 44, 842–852. ( 10.1016/S0025-326X(02)00220-5) [DOI] [PubMed] [Google Scholar]
- 36.Bucci K, Tulio M, Rochman CM. 2020. What is known and unknown about the effects of plastic pollution: a meta-analysis and systematic review. Ecol. Appl. 30, 2044 ( 10.1002/eap.2044) [DOI] [PubMed] [Google Scholar]
- 37.Critchell K, Hoogenboom MO. 2018. Effects of microplastic exposure on the body condition and behaviour of planktivorous reef fish (Acanthochromis polyacanthus). PLoS ONE 13, 19 ( 10.1371/journal.pone.0193308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seuront L. 2018. Microplastic leachates impair behavioural vigilance and predator avoidance in a temperate intertidal gastropod. Biol. Lett. 14, 20180453 ( 10.1098/rsbl.2018.0453) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barboza LGA, et al. 2020. Microplastics in wild fish from North East Atlantic Ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Sci. Total Environ. 717, 14 ( 10.1016/j.scitotenv.2019.134625) [DOI] [PubMed] [Google Scholar]
- 40.Lönnstedt OM, McCormick MI, Meekan MG, Ferrari MC.O, Chivers DP. 2012. Learn and live: the role of predator experience in influencing prey behaviour and survival. Proc R. Soc. B 279, 2091–2098. ( 10.1098/rspb.2011.2516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCormick MI. 2003. Consumption of coral propagules after mass spawning enhances larval quality of a damselfish through maternal effects. Oecologia 136, 37–45. ( 10.1007/s00442-003-1247-y) [DOI] [PubMed] [Google Scholar]
- 42.McCormick MI, Weaver CJ. 2012. It pays to be pushy: intracohort interference competition between two reef fishes. PLoS ONE 7, e42590 ( 10.1371/journal.pone.0042590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerrigan BA. 1996. Temporal patterns in the size and condition of settlement in two tropical reef fishes (Pomacentridae: Pomacentrus amboinensis and P. nagasakiensis). Mar. Ecol. Prog. Ser. 135, 27–41. ( 10.3354/meps135027) [DOI] [Google Scholar]
- 44.Sweatman HPA. 1984. A field study of the predatory behaviour and feeding rate of a piscivorous coral reef fish, the lizardfish Synodus englemani. Copeia 1984, 187–194. ( 10.2307/1445051) [DOI] [Google Scholar]
- 45.Holmes T, McCormick MI. 2010. Size-selectivity of predatory reef fish on juvenile prey. Mar. Ecol. Prog. Ser. 399, 273–283. ( 10.3354/meps08337) [DOI] [Google Scholar]
- 46.Feeney WE, Lönnstedt OM, Bosiger YJ, Martin J, Jones GP, Rowe RJ, McCormick MI. 2012. High rate of prey consumption in a small predatory fish on coral reefs. Coral Reefs 31, 909–918. ( 10.1007/s00338-012-0894-z) [DOI] [Google Scholar]
- 47.Holmes TH, Wilson SK, Vanderklift M, Babcock R, Fraser M. 2012. The role of Thalassoma lunare as a predator of juvenile fish on a sub-tropical coral reef. Coral Reefs 31, 1113–1123. ( 10.1007/s00338-012-0934-8) [DOI] [Google Scholar]
- 48.Hoey AS, McCormick MI. 2006. Effects of subcutaneous fluorescent tags on the growth and survival of a newly settled coral reef fish, Pomacentrus amboinensis (Pomacentridae). In Proceedings of the 10th International Coral Reefs Symposium, pp. 420–425. [Google Scholar]
- 49.Hoey AS, McCormick MI. 2004. Selective predation for low body condition at the larval–juvenile transition of a coral reef fish. Oecologia 139, 23–29. ( 10.1007/s00442-004-1489-3) [DOI] [PubMed] [Google Scholar]
- 50.McCormick MI, Meekan MG. 2007. Social facilitation of selective mortality. Ecology 88, 1562–1570. ( 10.1890/06-0830) [DOI] [PubMed] [Google Scholar]
- 51.McCormick MI. 2009. Behaviourally mediated phenotypic selection in a disturbed coral reef environment. PLoS ONE 4, e7096 ( 10.1371/journal.pone.0007096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phuong NN, Zalouk-Vergnoux A, Poirier L, Kamari A, Châtel A, Mouneyrac C, Lagarde F. 2016. Is there any consistency between the microplastics found in the field and those used in laboratory experiments? Environ. Pollut. 211, 111–123. ( 10.1016/j.envpol.2015.12.035) [DOI] [PubMed] [Google Scholar]
- 53.McCormick MI, Moore JAY, Munday PL. 2010. Influence of habitat degradation on fish replenishment. Coral Reefs 29, 537–546. ( 10.1007/s00338-010-0620-7) [DOI] [Google Scholar]
- 54.McCormick MI, Lönnstedt OM. 2016. Disrupted learning: habitat degradation impairs crucial antipredator responses in naive prey. Proc. R. Soc. B 283, 20160441 ( 10.1098/rspb.2016.0441) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Widaman KF, Helm JL, Castro-Schilo L, Pluess M, Stallings MC, Belsky J. 2012. Distinguishing ordinal and disordinal interactions. Psychol. Methods 17, 615–622. ( 10.1037/a0030003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richardson JT. 2011. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 6, 135–147. ( 10.1016/j.edurev.2010.12.001) [DOI] [Google Scholar]
- 57.de Sá LC, Oliveira M, Ribeiro F, Rocha TL, Futter MN. 2018. Studies of the effects of microplastics on aquatic organisms: what do we know and where should we focus our efforts in the future? Sci. Total Environ. 645, 1029–1039. ( 10.1016/j.scitotenv.2018.07.207) [DOI] [PubMed] [Google Scholar]
- 58.Fonte E, Ferreira P, Guilhermino L. 2016. Temperature rise and microplastics interact with the toxicity of the antibiotic cefalexin to juveniles of the common goby (Pomatoschistus microps): post-exposure predatory behaviour, acetylcholinesterase activity and lipid peroxidation. Aquat. Toxicol. 180, 173–185. ( 10.1016/j.aquatox.2016.09.015) [DOI] [PubMed] [Google Scholar]
- 59.Ferreira P, Fonte E, Soares ME, Carvalho F, Guilhermino L. 2016. Effects of multi-stressors on juveniles of the marine fish Pomatoschistus microps: gold nanoparticles, microplastics and temperature. Aquat. Toxicol. 170, 89–103. ( 10.1016/j.aquatox.2015.11.011) [DOI] [PubMed] [Google Scholar]
- 60.Lönnstedt OM, McCormick MI, Chivers DP, Ferrari MCO. 2014. Habitat degradation is threatening reef replenishment by making fish fearless. J. Anim. Ecol. 83, 1178–1185. ( 10.1111/1365-2656.12209) [DOI] [PubMed] [Google Scholar]
- 61.Harvey BP, Gwynn-Jones D, Moore PJ. 2013. Meta-analysis reveals complex marine biological responses to the interactive effects of ocean acidification and warming. Ecol. Evol. 3, 1016–1030. ( 10.1002/ece3.516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Przeslawski R, Byrne M, Mellin C. 2015. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Global Change Biol. 21, 2122–2140. ( 10.1111/gcb.12833) [DOI] [PubMed] [Google Scholar]
- 63.Manek AK, Ferrari MCO, Niyogi S, Chivers DP. 2014. The interactive effects of multiple stressors on physiological stress responses and club cell investment in fathead minnows. Sci. Total Environ. 476-477, 90–97. ( 10.1016/j.scitotenv.2013.12.042) [DOI] [PubMed] [Google Scholar]
- 64.Jacob H, Gilson A, Lanctôt C, Besson M, Metian M, Lecchini D. 2019. No effect of polystyrene microplastics on foraging activity and survival in a post-larvae coral-reef fish, Acanthurus triostegus. Bull. Environ. Contam. Toxicol. 102, 457–461. [DOI] [PubMed] [Google Scholar]
- 65.Lönnstedt OM, McCormick MI. 2013. Ultimate predators: lionfish have evolved to circumvent prey risk assessment abilities. PLoS ONE 8, e75781 ( 10.1371/journal.pone.0075781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCormick MI, Allan BJM. 2016. Lionfish misidentification circumvents an optimised escape response by prey. Conservation Physiology 4, cow064 ( 10.1093/conphys/cow064) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Albins MA, Hixon MA. 2013. Worst case scenario: potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ. Biol. Fishes 96, 1151–1157. ( 10.1007/s10641-011-9795-1) [DOI] [Google Scholar]
- 68.Albins MA, Lyons PJ. 2012. Invasive red lionfish Pterois volitans blow directed jets of water at prey fish. Mar. Ecol. Prog. Ser. 448, 1–5. ( 10.3354/meps09580) [DOI] [Google Scholar]
- 69.Green SJ, Akins JL, Côté I.M. 2011. Foraging behaviour and prey consumption in the Indo-Pacific lionfish on Bahamian coral reefs. Mar. Ecol. Prog. Ser. 433, 159–167. ( 10.3354/meps09208) [DOI] [Google Scholar]
- 70.Andrady AL. 2011. Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605. ( 10.1016/j.marpolbul.2011.05.030) [DOI] [PubMed] [Google Scholar]
- 71.Dubaish F, Liebezeit G. 2013. Suspended microplastics and black carbon particles in the Jade system, southern North Sea. Water, Air, Soil Pollut. 224, 1352 ( 10.1007/s11270-012-1352-9) [DOI] [Google Scholar]
- 72.Browne NK, Smithers SG, Perry CT. 2013. Spatial and temporal variations in turbidity on two inshore turbid reefs on the Great Barrier Reef, Australia. Coral Reefs 32, 195–210. ( 10.1007/s00338-012-0965-1) [DOI] [Google Scholar]
- 73.Hitchcock JN. 2020. Storm events as key moments of microplastic contamination in aquatic ecosystems. Sci. Total Environ. 734, 139436 ( 10.1016/j.scitotenv.2020.139436) [DOI] [PubMed] [Google Scholar]
- 74.Kamil AC, Dukas R. 2001. Limited attention: the constraint underlying search image. Behav. Ecol. 12, 192–199. ( 10.1093/beheco/12.2.192) [DOI] [Google Scholar]
- 75.Nanninga GB, Scott A, Manica A. 2020. Microplastic ingestion rates are phenotype-dependent in juvenile anemonefish. Environ. Pollut. 259, 113855 ( 10.1016/j.envpol.2019.113855) [DOI] [PubMed] [Google Scholar]
- 76.Mamaca E, Bechmann RK, Torgrimsen S, Aas E, Bjørnstad A, Baussant T, Le Floch S. 2005. The neutral red lysosomal retention assay and comet assay on haemolymph cells from mussels (Mytilus edulis) and fish (Symphodus melops) exposed to styrene. Aquat. Toxicol. 75, 191–201. ( 10.1016/j.aquatox.2005.08.001) [DOI] [PubMed] [Google Scholar]
- 77.Ferrari MCO, Sih A, Chivers DP. 2009. The paradox of risk allocation: a review and prospectus. Anim. Behav. 78, 579–585. ( 10.1016/j.anbehav.2009.05.034) [DOI] [Google Scholar]
- 78.Nilsson GE, Ostlund-Nilsson S, Penfold R, Grutter AS. 2007. From record performance to hypoxia tolerance: respiratory transition in damselfish larvae settling on a coral reef. Proc. R. Soc. B 274, 79–85. ( 10.1098/rspb.2006.3706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barron MG. 2002. Environmental contaminants altering behaviour. In Behavioural ecotoxicology (ed. Dell'Omo G.), pp. 167–184. Chichester, UK: John Wiley and Sons. [Google Scholar]
- 80.Liu P, Zhan X, Wu XW, Li JL, Wang HY, Gao SX. 2020. Effect of weathering on environmental behavior of microplastics: properties, sorption and potential risks. Chemosphere 242, 125193 ( 10.1016/j.chemosphere.2019.125193) [DOI] [PubMed] [Google Scholar]
- 81.Coker DJ, Pratchett MS, Munday PL. 2009. Coral bleaching and habitat degradation increase susceptibility to predation for coral-dwelling fishes. Behav. Ecol. 20, 1204–1210. ( 10.1093/beheco/arp113) [DOI] [Google Scholar]
- 82.McCormick MI. 2012. Lethal effects of habitat degradation on fishes through changing competitive advantage. Proc. R. Soc. B 279, 3899–3904. ( 10.1098/rspb.2012.0854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Graham NA, et al. 2008. Climate warming, marine protected areas and the ocean-scale integrity of coral reef ecosystems. PLoS ONE 3, e3039 ( 10.1371/journal.pone.0003039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bostrom-Einarsson L, Bonin MC, Munday PL, Jones GP. 2018. Loss of live coral compromises predator-avoidance behaviour in coral reef damselfish. Sci. Rep. 8, 7795 ( 10.1038/s41598-018-26090-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lönnstedt OM, McCormick MI, Chivers DP. 2013. Degraded environments alter prey risk assessment. Ecol. Evol. 3, 38–47. ( 10.1002/ece3.388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.IPCC. 2014. Summary for policymakers. In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds CB Field et al.) See http://www.ipcc.ch/site/assets/uploads/2018/02/ar5_wgII_spm_en.pdf. [Google Scholar]
- 87.Hughes TP, et al. 2017. Coral reefs in the Anthropocene. Nature 546, 82–90. ( 10.1038/nature22901) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available from: https://doi.org/10.6084/m9.figshare.12072021 or Tropical Data Hub doi:10.25903/5cff2e614a5d5.