Abstract
The coordination between mitochondrial and nuclear genes is crucial to eukaryotic organisms. Predicting the nature of these epistatic interactions can be difficult because of the transmission asymmetry of the genes involved. While autosomes and X-linked genes are transmitted through both sexes, genes on the Y chromosome and in the mitochondrial genome are uniparentally transmitted through males and females, respectively. Here, we generate 36 otherwise isogenic Drosophila melanogaster strains differing only in the geographical origin of their mitochondrial genome and Y chromosome, to experimentally examine the effects of the uniparentally inherited parts of the genome, as well as their interaction, in males. We assay longevity and gene expression through RNA-sequencing. We detect an important role for both mitochondrial and Y-linked genes, as well as extensive mitochondrial-Y chromosome epistasis. In particular, genes involved in male reproduction appear to be especially sensitive to such interactions, and variation on the Y chromosome is associated with differences in longevity. Despite these interactions, we find no evidence that the mitochondrial genome and Y chromosome are co-adapted within a geographical region. Overall, our study demonstrates a key role for the uniparentally inherited parts of the genome for male biology, but also that mito-nuclear interactions are complex and not easily predicted from simple transmission asymmetries.
Keywords: uniparental inheritance, sexual antagonism, longevity, gene expression
1. Introduction
The coevolution between mitochondrial and nuclear genes is one of the oldest and best-studied examples of symbiosis [1–3]. Orchestrated interaction between the two genomes is essential for a number of eukaryotic traits, especially metabolism and energy production [4–7], and this intimate coordination has been taken as evidence for positive selection for cooperative mito-nuclear combinations [8,9]. Moreover, there has also been a well-documented transfer of genes from the mitochondrial to the nuclear genome [10–12] and bilaterian animal mitochondrial genomes, with a few exceptions, contain only 37 genes. Finally, the case for the importance of adaptive mito-nuclear epistasis is further strengthened by the observation that placing mitochondrial genomes on novel nuclear backgrounds is often, though not always, associated with adverse fitness effect (see Reinhardt et al. [13] and Eyre-Walker [14] for alternative perspectives).
A key factor governing mito-nuclear coevolution is their difference in transmission. Because the mitochondrial genome is almost exclusively maternally inherited [15], mutations that are deleterious in males can spread in a population, given that they are beneficial or neutral in females [16–21]. The occurrence of male deleterious mitochondrial mutations is particularly well studied in plants [22–25], where such mutations usually prevent pollen production in hermaphroditic plants, essentially rendering individuals female, guaranteeing the mutation's transmission through ovules. This phenomenon is therefore called cytoplasmic male sterility. In the zoological literature, following Gemmell et al. [16], the presence of mitochondrial mutations with deleterious effects in males is known as the Mother's Curse.
Other than the mitochondrial genome, other uniparentally inherited genes are those located on the sex-determining chromosome: on the Y in an XY system, where males are the heterogametic sex, and on the W in a ZW female heterogametic system. The strict paternal inheritance makes the Y an especially interesting candidate for mito-nuclear epistasis. In particular, it has been suggested to be an ideal location for modifiers that counteract the male deleterious effects of mitochondrial mutations [26,27], a scenario formally modelled by Ågren et al. [28]. Despite its heterochromatic structure and paucity of protein-coding genes, the Y chromosome is now recognized as being able to affect a wide variety of traits [29–31]. For example, in Drosophila it underlies variation in traits ranging from susceptibility to bacterial infection [32], male reproductive success [33], to sex-specific ageing [34].
The extent of mitochondrial-Y (mito-Y) interactions and their importance to male fitness remain poorly understood. Some suggestive evidence comes from empirical work by Innocenti et al. [35], who found that loci in the mitochondrial genome can affect the expression of a large number of autosomal loci in male, but not in female Drosophila melanogaster. Such sexual dimorphism in expression is consistent with a sex-specific selective sieve being central to mitochondrial genome evolution. Furthermore, several of the loci identified by Innocenti et al. [35] to be sensitive to mitochondrial variation overlap with loci shown by Lemos et al. [30] to be sensitive to a variation on the Y chromosome [36] . The extent to which male autosomal gene expression is subject to mito-Y interactions, however, is unclear.
Recent attempts to empirically address these questions in the fruit fly D. melanogaster have revealed some suggestive patterns. Dean et al. [27] found that both mitochondrial and Y-linked genes independently affected male locomotor activity, but only under certain diets and social environments. Similarly, Yee et al. [26] used combinations from three populations (a total of nine mito-Y combinations) to provide the proof-of-concept evidence of how mito-Y combinations may affect aspects of male fitness. The latter was also interested in testing for evidence of coevolution between mitochondrial and Y-linked genes. If coevolution is important, the prediction is that males who carry a Y-chromosome and a mitochondrial genome that have co-evolved in the same sympatric population may differ from males where the mitochondrial genome and Y chromosome are from diverged populations and therefore represent a novel mito-Y combination. However, in this relatively low-powered study, Yee et al. [26] did not find evidence that males with sympatric mito-Y combinations had higher fitness than males with novel combinations.
We extend these studies in three ways. First, we increase the sample size considerably, by including 36 mito-Y combinations of D. melanogaster males with mitochondrial and Y chromosomes sampled from five worldwide locations (table 1). Second, we assay longevity, another major fitness trait previously shown to be sensitive to mito-nuclear epistasis [37–40]. Finally, we performed RNA-sequencing on all lines to identify the importance of mitochondrial and Y chromosome variation, as well as mito-Y epistasis, for differential gene expression. In line with Dean et al. [27] and Yee et al. [26], we found an important role for both mitochondrial and Y-linked genes and evidence of mito-Y chromosome epistasis.
Table 1.
Geographical origin of strains used to generate mito-Y combinations. (Sympatric combinations are shown in italics and novel in bold.)
| Y chromosome genotype |
|||||||
|---|---|---|---|---|---|---|---|
| mitochondrial genotype | B04 Beijing | B11 Beijing | N03 Netherlands | N07 Netherlands | ZWH23 Zimbabwe | ZW139 Zimbabwe | |
| B38 Beijing | Beijing × Beijing | Beijing × Beijing | Beijing × Netherlands | Beijing × Netherlands | Beijing × Zimbabwe | Beijing × Zimbabwe | |
| B39 Beijing | Beijing × Beijing | Beijing × Beijing | Beijing × Netherlands | Beijing × Netherlands | Beijing × Zimbabwe | Beijing × Zimbabwe | |
| I02 Ithaca | Ithaca × Beijing | Ithaca × Beijing | Ithaca × Netherlands | Ithaca × Netherlands | Ithaca × Zimbabwe | Ithaca × Zimbabwe | |
| N01 Netherlands | Netherlands × Beijing | Netherlands × Beijing | Netherlands × Netherlands | Netherlands × Netherlands | Netherlands × Zimbabwe | Netherlands × Zimbabwe | |
| N02 Netherlands | Netherlands × Beijing | Netherlands × Beijing | Netherlands × Netherlands | Netherlands × Netherlands | Netherlands × Zimbabwe | Netherlands × Zimbabwe | |
| N23 Netherlands | Netherlands × Beijing | Netherlands × Beijing | Netherlands × Netherlands | Netherlands × Netherlands | Netherlands × Zimbabwe | Netherlands × Zimbabwe | |
2. Methods
(a). Drosophila melanogaster strains
Both the longevity and the gene expression assays were performed on isogenic D. melanogaster males differing only in the geographical origin of their mitochondrial genome and their Y chromosome (referred to as mito-Y combinations throughout). These lines were constructed by crosses to an isogenic strain of Bloomington stock 4361, which has recessive markers on all chromosomes (y1; bw1; e4; ci1 eyR; see the electronic supplementary material for details). Each Y chromosome was crossed into the isogenic 4361 genetic background in a two-generation cross, avoiding recombination between the original Global Diversity Lines (GDL) strain and the 4361 strain by only having them in a heterozygous state in male parents. These lines were Illumina-sequenced to 1x depth, and across all the lines we only detected residual heterozygosity at 196 sites. The confidence in the background replacement of these lines is therefore high (details in Kelsey & Clark [41]). The mitochondrial replacement lines we constructed by recurrent backcrossing to the 4361 stock for 10 generations. In this case, there could be recombination between the GDL and 4361 backgrounds, but the expected removal of the GDL background by dilution exceeded one-half each generation because of the selectable markers. We applied simple computer simulation to determine that more than 99% of the 1000 independent trials had no residual heterozygosity, and in the cases where the replacement was not complete, the expected portion of the genome with residual heterozygosity was 0.0088 (see the electronic supplementary material for details).
We used a 6 × 6 crossing design (table 1), crossing females from six mitochondrial replacement lines with males from six Y chromosome replacement lines, resulting in male offspring with 36 mito-Y combinations. Mitochondrial genomes came from Beijing, China (two lines; B38 and B39), Ithaca, NY, USA (one line; I02) and the Netherlands (three lines; N01, N02 and N23); the Y chromosomes from Beijing, China (two lines; B04 and B11), the Netherlands (two lines; N03 and N07) and Zimbabwe (two lines; ZWH123 and ZW139). These populations represent deeply divergent mitochondrial and Y chromosome clades chosen from the GDL [42]. The central aim of this study is to assess the extent of mito-Y epistasis. Following Yee et al. [26], a secondary aim is to investigate if there is any evidence of mito-Y co-adaptation. If the mitochondrial DNA (mtDNA) and Y chromosome were from the same geographical region, they are therefore labelled ‘sympatric’ and otherwise they are labelled ‘novel’.
(b). Longevity assay
(i). Scoring survival
Individual lifespan was quantified for all 36 mito-Y combinations. For each cross, four males and four females were put into a single vial. Flies were collected upon eclosion, sexed and placed into vials. Because collections took place over several days, the starting number of individuals per vial varied and so did the number of vials per cross. Most vials had around 12 individuals (electronic supplementary material, figure S9) and most crosses had seven replicate vials (electronic supplementary material, table S1). The total number of flies per cross ranged from 74 to 138, with an average of 107. Seven crosses had less than 90 individuals. Every other day, flies were transferred without using CO2 to vials with fresh sucrose-yeast medium, and individual deaths were recorded. Investigators scoring deaths were blind to which mito-Y combination a given vial contained. Vials were kept in climate-controlled growth chambers at 25°C, and at a 12 : 12 h light : dark cycle.
In total, approximately 4500 individuals were scored. Because we were interested in documenting differences in intrinsic mortality, we excluded individuals that died within the first 11 days of the experiment (electronic supplementary material, table S1and S15). In total, 671 individuals lived less than 11 days, which represents just under 15% of all individuals. There appears to be little correlation between the starting number of individuals in a vial and the number of individuals in a vial that died before 11 days (Kendall rank correlation = 0.056). In addition, with the exception of the few vials with only a couple of individuals, there seems to be no correlation between the starting number in a vial and average lifespan of individuals in a given vial (Kendall rank correlation coefficient = 0.11; electronic supplementary material, figure S10).
(c). Differential gene expression
(i). RNA extraction and sequencing
Three- to 5-day-old males from each mito-Y combination were maintained in vials on standard sucrose-yeast medium in climate-controlled growth chambers with a 12 : 12 h light : dark cycle at 25°C. For each mito-Y combination, 10 males were flash frozen. We used two biological replicates for each combination, with individuals for each replicate being collected from separate crosses between females from the mitochondrial replacement and males from the Y-replacement parental lines performed on the same day.
RNA was extracted from the 10 pooled whole-fly males and RNA-seq libraries were prepared using the Lexogen QuantSeq 3′ mRNA-Seq Library Prep Kit FWD. Samples were sequenced in a single-end 75 bp run on a NextSeq500 at the Genomics Facility in the Cornell Biotechnology Resource Center.
(ii). Read processing and alignment
The quality of the RNA sequences was assessed using FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Trimmomatic was then used to clip adapters, the leading 10 bp, and reads once the average quality of a sliding window of 4 bp dropped below 20 [43]. Reads shorter than 20 bp were dropped from subsequent analysis. Reads were aligned to the D. melanogaster reference genome (Release 6; [44]) using STAR [45] and HTseq-count was used to determine the raw number of read counts per gene [46].
(iii). Quantifying gene expression
Differential transcript abundance was analysed using DESeq2 [47]. Read counts were normalized using DESeq2's internal normalization function estimateSizeFactors, which corrects for both library size and RNA composition bias. Lowly expressed genes were removed from subsequent analysis, such that a given gene was only kept in the dataset if it had at least 20 normalized counts in at least half of the samples. After filtering, 9533 out of 17 324 (approx. 55%) of the originally identified genes were kept for subsequent analysis.
To identify nuclear genes sensitive to variation in the mitochondrial genome, the Y chromosome, and mito-Y combination, we conducted several differential expression analyses using linear models. We characterized a gene as being differentially expressed if they maintain significance after performing independent filtering for multiple test corrections using the Benjamini–Hochberg method set with a false discovery rate of 0.05 (see the electronic supplementary material, tables S2–S4). To deal with a potential batch effect, suggested by principal component analyses (electronic supplementary material, figure S2), we used a surrogate variable approach ([48]; see the electronic supplementary material).
We performed a gene enrichment analysis to determine whether certain gene families were differentially expressed across Y haplotypes, mitochondrial haplogroups and mito-Y interactions. We used the R Bioconductor package ‘goseq’ [49] to perform gene ontology (GO) analyses. Taking length bias into account, we identified GO categories as either significantly over- or under-represented using a 0.05 false discovery rate cutoff. REVIGO [50] was used to semantically cluster the lists of enriched GO terms to find a representative subset that could be easily analysed and visualized (see the electronic supplementary material, tables S5–S7).
For each set of differentially expressed genes, we identified enriched transcription factor-binding motifs using the R Bioconductor package ‘RcisTarget’ [51]. We used the motif ranking file of Drosophila species ‘dm6–5kb-upstream-full-tx-11species.mc8nr.feather’ (accessed on 20 April 2020) with a search space of 5kb upstream of transcription start site and transcript introns. We searched each gene set independently with parameters aucMacRank = 5% and nesThreshold = 3. We consider high confidence transcription factor annotations, those directly inferred or inferred by orthology, to determine which transcription factors bind to enriched binding motifs. The full list of enriched transcription factor-binding motifs and a list of the annotated transcription factors for these motifs can be found in the electronic supplementary material, tables S8–S13.
To determine whether any differentially expressed genes showed testis- or accessory gland-biased expression, we downloaded data from FlyAtlas, which measures the expression levels of a gene in each adult male [52]. The bias metric used is simply the expression of the gene in the tissue of interest divided by the sum of expression, in males, over all other tissues. We considered a gene biased for expression in a tissue of interest if the bias metric for that tissue is greater than 0.5, as that indicates that more than half the reads collected for that gene come from that tissue. Electronic supplementary material, figures S6–S8 highlight all genes found to show biased expression in male reproductive tissue in both the Y and mitochondrial differential expression analysis.
All scripts used for the RNA-sequencing analysis are on GitHub (https://github.com/mam737/mitoY_RNASEQ).
3. Results and discussion
(a). Extensive variation in lifespan across mitochondrial-Y combinations
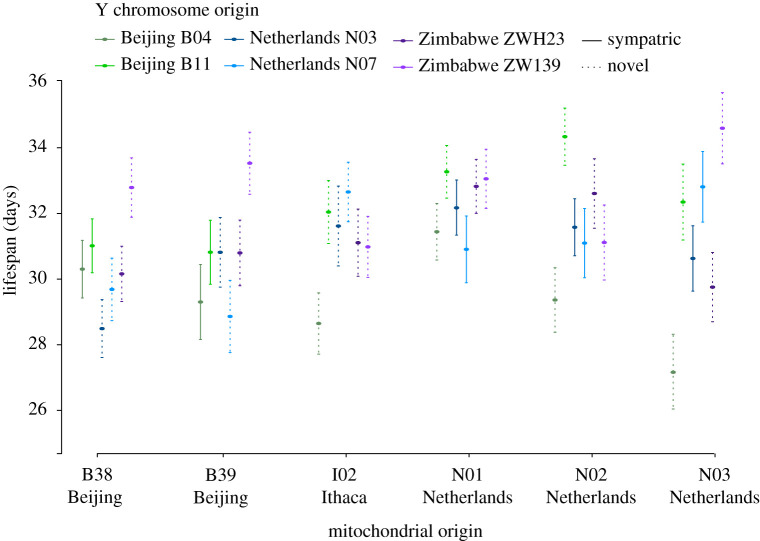
We detected extensive variation in average lifespan across the 36 mito-Y combinations (figure 1; electronic supplementary material, table S1). To assess the contribution of mitochondrial type and Y chromosome and their interaction, we performed several types of analysis. First, we performed a two-way ANOVA based on vial means (table 2). We performed the analysis with and without individuals that lived less than 11 days, and with and without vials with fewer than six individuals. The results were qualitatively the same and reveal a role for a variation on the Y chromosome in governing longevity (F = 3.5, p = 0.00836; individuals with lifespan less than 11 days and vials with less than six individuals not included). These results also hold if we include the starting number of individuals per vial as a covariate in the linear model. In these models, the only significant effect remains the Y chromosome. Second, we also performed a mixed-model ANOVA including vial as a random factor nested within the mtDNA × Y interaction. Again, we performed the analysis with and without individuals with lifespan less than 11 days. These results were qualitatively the same, and found a significant effect of the Y chromosome (F = 3.7, p = 0.0034; individuals with lifespan less than 11 days not included). Finally, we pooled all individuals into two categories, sympatric (n = 1235) and novel (n = 2618), and found no evidence that individuals with a sympatric mito-Y combination live longer than individuals with a novel combination (Wilcoxon rank sum test, W = 1536800, p = 0.5114).
Figure 1.
Mean lifespan (days) ± standard error across 36 mito-Y combinations. Geographical origin of the mitochondrial genome is stated at the bottom and colour coded for the Y chromosome. Solid and dashed lines represent sympatric and novel mito-Y combinations, respectively. (Online version in colour.)
Table 2.
Two-way ANOVA for mito-Y interactions in vial mean longevity.a
| source | degrees of freedom | F | p |
|---|---|---|---|
| mitochondrial genome | 5 | 1.1 | 0.3473 |
| Y chromosome | 5 | 3.5 | 0.0044 |
| mito-Y interaction | 25 | 0.7 | 0.8415 |
| residuals | 206 |
aIndividuals with lifespan less than 11 days not included.
To assess how mortality changed over time, we fitted a number of survival functions based on different assumptions (Gompertz, Gompertz-Makeham, Logistic and Logistic-Makeham models; see the electronic supplementary material for details). This comparison revealed significant variation across lines, but no difference between novel and sympatric mito-Y combinations (electronic supplementary material, figure S1).
As in most species, male Drosophila live shorter lives than females [53,54], and recent studies have demonstrated a central role for both the mitochondria [55] and the Y chromosome [34] in explaining this difference. We find that the Y chromosome contributes to variation in longevity. Consistent with previous work on mito-Y interactions [26,27], our results also reveal no evidence that males with sympatric mito-Y combinations differed in these traits compared to males with their mitochondrial genome and Y chromosome sampled from different populations.
(b). Expression of many nuclear genes is sensitive to a variation on the Y chromosome, in the mitochondrial genome or both
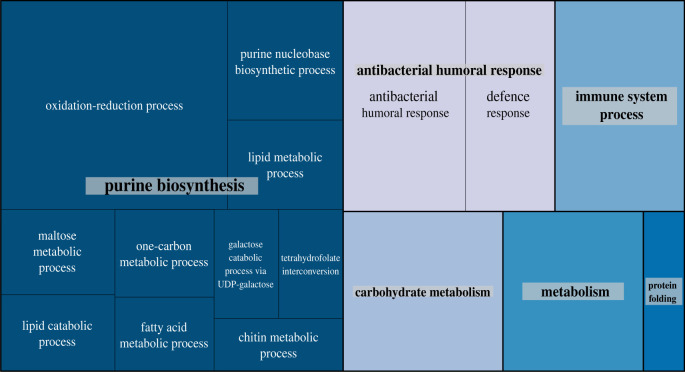
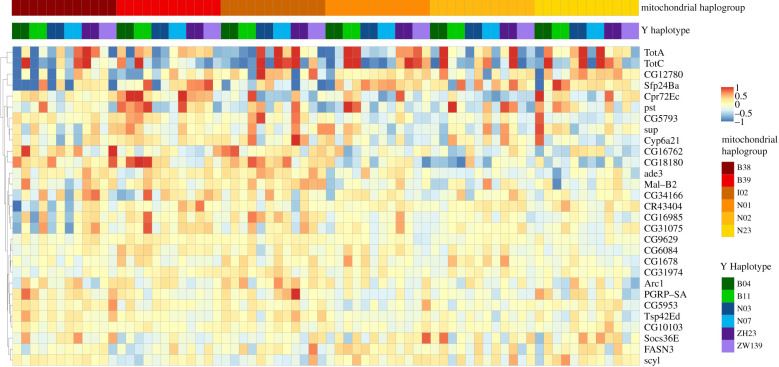
We detected 71, 760 and 29 genes whose expression was sensitive to a variation on the Y chromosome, in the mitochondrial genome, and mito-Y epistasis, respectively. To investigate the biological function of these genes, we searched for GO terms [56,57] that were either over- or under-represented among our significant hits. We found that genes associated with visual perception (GO:0007601; Padj = 0.006), response to stimulus (GO:0050896; Padj = 0.0144), and rhabdomere, a central compartment in compound eyes (GO:0016028; Padj = 0.0435) were over-represented among genes sensitive to the Y haplotype. For those sensitive to mitochondrial haplotype, we find 44 over- and 3 under-represented GO categories with enrichment of terms belonging to categories such as purine biosynthesis, metabolism, and immune responses (figure 2). Whereas genes sensitive to mito-Y interactions show substantial variation in expression across samples (figure 4), we detect no enrichment or depletion of GO categories among the genes sensitive to mito-Y epistasis.
Figure 2.
Semantic clustering of over-represented biological process GO terms found among genes sensitive to mitochondrial haplotype. Each rectangle represents a single cluster, which are joined into larger ‘superclusters’ as visualized by colour. Size of the rectangles indicates p-value significance (absolute value of the log10 transformed p-value). (Online version in colour.)
Figure 4.
Expression profile of genes sensitive to mitochondrial-Y interactions. Samples (36 mito-Y combinations with two replicates, labelled A and B, for each) and genes of interest are listed along the x- and y-axis respectively. Colour indicates either overexpression (red) or underexpression (blue) compared to the mean expression level of that gene across all samples. (Online version in colour.)
We also identified any substantial enrichment of transcription factor-binding motifs among differentially expressed genes. We found 156, 207 and 379 motifs over-represented among genes sensitive to either Y haplotype, mitochondrial haplogroup or mito-Y interactions, respectively. Several of these binding motifs contained high confidence transcription factor annotations (electronic supplementary material, tables S9, S11 and S13). Notably, all gene sets showed enrichment of motifs binding all five GATA transcription factors–GATAd, GATAe, grn, pnr and srp [58], which play a well-documented role in cell differentiation and proliferation [59]. GATA transcription factors have also been demonstrated to influence the effects of dietary amino acids on lifespan, and it is suspected that they operate in a sex-specific manner [60]. Additionally, transcription factors annotated for motifs enriched among Y sensitive genes include several related to photoreception and neuronal processes such as oc, lola [61,62] and X. Below, we discuss certain biological trends that emerge among our most significant hits.
(i). Mitochondrial haplotype influences the expression of metabolic genes
Many metabolic reactions involve mitochondria, so while the emergence of genes involved in several metabolic processes showing differential expression may not be surprising, the sheer number of nuclear genes whose expression is affected by mitochondrial variation is (figure 2). Some of these genes exhibit consistent differences between the haplogroups. Several maltases involved in maltose/carbohydrate metabolism show reduced expression among the Netherlands haplogroups compared to the others, whereas three of the four enzyme-encoding genes involved in the reduction of NADP to NADPH (Men, Idh and Zw) show reduced expression in the Beijing B38 haplogroup (electronic supplementary material, figure S3).
Transcription factors with motifs enriched among mitochondrial haplogroup sensitive genes also show a demonstrated role in regulating metabolism. Hnf4 regulates both lipid mobilization and fatty acid catabolism [63,64], and it is thought to be required for the transcription of nuclear and mitochondrial genes involved in oxidative phosphorylation [65]. SREBP [66,67] is involved in lipid metabolism and regulates the transcription of lipogenesis when lipid or cholesterol levels drop.
(ii). Genes related to male fertility show sensitivity to both Y and mitochondrial haplotype
Both the Y chromosome and the mitochondrial genome have previously been demonstrated to affect male fertility in D. melanogaster [26,33,68–73]. Whereas there is no statistical enrichment of differentially expressed genes belonging to male fertility processes, there are several notable hits that not only show sensitivity across both Y and mitochondrial haplotypes, but also show elevated expression in both the testis and the accessory glands (electronic supplementary material, figures S5–S8). Among our Y sensitive hits, genes such as Testis EndoG-Like 1 (Tengl1), Adenosine deaminase-related growth factor A2 (Adgf-A2), Male-specific RNA 98CA (Mst98Ca), gonadal (gdl) and Ductus ejaculatorius peptide 99B (Dup99B) show markedly higher, if not exclusive, expression in either the testis or the accessory glands. Furthermore, Adgf-A2, Mst98Ca, and gdl are all thought to be involved with spermiogenesis, spermatogenesis, or sperm function, while Dup99B is a sex peptide that influences the female post-mating response [74–78].
Several genes sensitive to mitochondrial haplogroup also show higher expression in the testis and accessory glands. Whereas many of these genes are not functionally characterized, there are a few worth highlighting. Protamine B (ProtB) packages the paternal genome in sperm during spermiogenesis [79,80], Otefin (Ote) encodes a nuclear membrane-associated protein involved in transcriptional silencing of bag-of-marbles (bam), which is a key protein involved in gametogenesis. Tim17a1, a subunit of the TIM23 complex, is involved in transporting proteins across the inner mitochondrial membrane and shows markedly higher expression in the testis compared to all other tissues [81]. Seminase (Sems), which is expressed predominantly in the accessory glands, is not only transferred during mating but is also thought to be involved in sperm release from storage in females [82]. Finally, we also found several accessory gland proteins, lectin-46Ca, Acp53C14a, Acp33A, CG14034 and CG9029. lectin-46-Ca, Acp53C14a and CG13309 (which is significant, but not enriched in the accessory gland) all show reduced expression among the Netherlands mitochondrial haplogroups compared to the others.
These results add to the growing evidence that male-biased nuclear genes sensitive to mtDNA variation tend to be disproportionately expressed in male reproductive tissue in D. melanogaster. In particular, they are in line with the findings of Innoncenti et al. [35], who found that a significant portion of the genes that they identified with differential expression across mitochondrial haplogroups were exclusively or mostly male-limited transcripts expressed in either the male testes, accessory gland or ejaculatory duct.
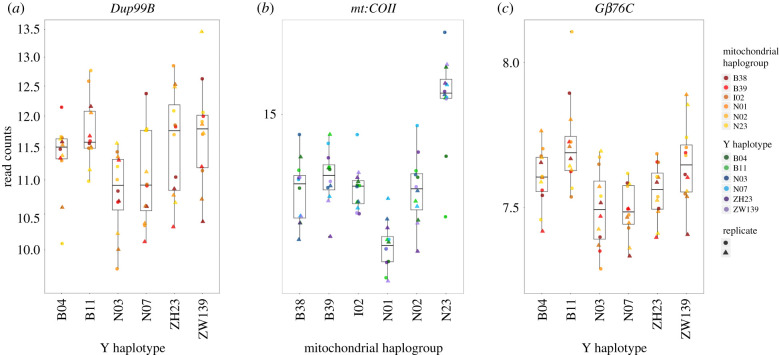
In addition to genes that are enriched for expression in either the testis or the accessory gland, we find a handful of other genes related to male fertility. Particular mutations in mt:COII and mt:Cyt-B have not only been demonstrated to influence male fertility, but also show no effect on females, leading authors to cite them as examples of Mother's Curse variants in Drosophila [73,83–85]. For mt:COII, we see variable expression across mitochondrial haplogroups with the Netherlands N23 haplogroup showing the highest levels of expression (figure 3b). Another top mitochondrial hit, the heat shock protein Hsp83, has been demonstrated to affect spermatid development and differentiation in D. melanogaster [86,87].
Figure 3.
Box and scatter plots visualizing differential expression of genes sensitive to (a) Y haplotype, (b) mitochondrial haplogroup and (c) mitochondrial-Y interactions. Normalized read counts for (a) Dup99B, a male sex peptide, which shows a reduced expression in the Netherlands haplotypes, (b) mt:COII, a mitochondrial subunit of cytochrome oxidase II, which shows variable expression across all six mitochondrial haplotypes, (c) Gβ76C, a Y-sensitive hit, linked to visual perception that shows significant differences in expression across all six Y haplotypes. (Online version in colour.)
We also find transcription factors involved in male reproduction with binding motifs enriched among genes differentially expressed across both Y haplotype and mitochondrial haplogroup. Enriched among Y sensitive genes are motifs for SOX100B, which is required for testis differentiation [88], and FoxP, which influences levels of courtship behaviour in males [89]. For mitochondrial sensitive genes, motifs for ERR [90,91], a transcription factor directly involved in testicular development and spermatogenesis in Drosophila males, are over-represented.
Despite several genes related to male fertility showing sensitivity to both Y and mitochondrial haplotype, we see little evidence for epistatic interactions between them. Simply looking at the overlap of genes that show sensitivity to Y or mitochondrial haplotype yields only one gene: mino. Furthermore, when specifically testing for mito-Y epistasis, the only gene related to male fertility with significant differential expression is the seminal fluid protein Sfp24Ba.
Overall, our analysis identifies several differentially expressed genes that present a strong potential to have downstream consequences for male fertility. The pattern of expression levels seen in figure 4 suggests no simple additive role of mitochondrial and Y variants, but instead points to specific genotypic combinations having the most aberrant expression.
(iii). Visual perception and nervous system processes show Y haplotype sensitivity
Genes belonging to both visual perception (GO:0007601) and rhabdomere (GO:0016028), a key compartment in compound eyes, are not only enriched among our Y sensitive hits but they are also some of our most significant hits. We see less expression of these genes among the Netherlands haplotypes, yet markedly higher expression of these genes for the Beijing B11 haplotype (electronic supplementary material, figure S4). Previous work has shown that variable expression in Arr1, Arr2, Rh4, Gβ76C, trpl and Gγ30A, all of which show differential expression, may lead to phenotypic differences in rhodopsin inactivation, phototransduction and the photoresponse [92–99]. Further, we find several binding motifs for transcription factors regulating vision including oc, lola and Optix. Oc is required for photoreceptor development and rhabdomere morphogenesis [100–102], and Optix is involved in eye formation and development of the optic lobe [103,104] . Lola is not only involved in axon growth but also acts during photoreceptor neuron differentiation [105,106]. By contrast, we are not aware of any previous work linking variation on the Y chromosome to variation in visual perception in Drosophila or any other system.
4. Conclusion
In this study, we used 36 mito-Y combinations to experimentally examine the effects of the uniparentally inherited parts of the genome on male D. melanogaster. We detect an important role for Y-linked genes but little sign that individuals with their Y chromosome and mitochondrial genome sampled from the same population being any different than individuals where the two are from geographically isolated populations. We also detect many genes that are sensitive to a variation on the Y chromosome, in the mitochondrial genome or both. The biological function of these genes ranges from metabolic to visual and neuronal phenotypes, with the strongest effect being for genes involved in male reproduction. These results highlight the opportunity for these uniparentally inherited segments of the genome to influence male biology.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Amanda Manfredo for help with laboratory work and Yasir Ahmed-Braimah for guidance on the RNA-sequencing analysis. We thank Angela Early for construction of the mtDNA replacement lines and Keegan Kelsey for construction of the Y replacement lines. We thank the editor and three reviewers for comments that improved the manuscript, especially for the suggestion analysis of transcription factor-binding motifs.
Data accessibility
The RNA-seq data reported here are posted on the GEO resource with reference number GSE155395. Fly lines are available on request to A.G.C. All scripts used for the RNA-sequencing analysis are on GitHub (https://github.com/mam737/mitoY_RNASEQ).
Authors' contributions
J.A.Å., M.M. and A.G.C. conceived the study. J.A.Å. performed the fly work and analysed the longevity data. M.M. analysed the expression data. J.A.Å. wrote the first draft and all authors contributed to the writing of the manuscript. A.G.C. supervised the project.
Competing interests
We declare we have no competing interests.
Funding
J.A.Å. was funded by fellowships from the Sweden-America Foundation and the Wenner-Gren Foundations. This work was also supported by grant R01 GM119125 to Dan Barbash and A.G.C.
References
- 1.Gillham NW. 1994. Organelle genes and genomes. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Rand DM, Haney RA, Fry AJ. 2004. Cytonuclear coevolution: the genomics of cooperation. Trends Ecol. Evol. 19, 645–653. ( 10.1016/j.tree.2004.10.003) [DOI] [PubMed] [Google Scholar]
- 3.Lane N. 2017. Origin of the eukaryotic cell. Mol. Front. J. 01, 108–120. ( 10.1142/S2529732517400120) [DOI] [Google Scholar]
- 4.Blier PU, Dufresne F, Burton RS. 2001. Natural selection and the evolution of mtDNA-encoded peptides: evidence for intergenomic co-adaptation. Trends Genet. 17, 400–406. ( 10.1016/S0168-9525(01)02338-1) [DOI] [PubMed] [Google Scholar]
- 5.Hill GE. 2015. Mitonuclear ecology. Mol. Biol. Evol. 32, 1917–1927. ( 10.1093/molbev/msv104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton RS, Pereira RJ, Barreto FS. 2013. Cytonuclear genomic interactions and hybrid breakdown. Annu. Rev. Ecol. Evol. Syst. 44, 281–302. ( 10.1146/annurev-ecolsys-110512-135758) [DOI] [Google Scholar]
- 7.Ghiselli F, Milani L. 2020. Linking the mitochondrial genotype to phenotype: a complex endeavour. Phil. Trans. R. Soc. B 375, 20190169 ( 10.1098/rstb.2019.0169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wade MJ, Goodnight CJ. 2006. Cyto-nuclear epistasis: two-locus random genetic drift in hermaphroditic and dioecious species. Evolution 60, 643–659. ( 10.1111/j.0014-3820.2006.tb01146.x) [DOI] [PubMed] [Google Scholar]
- 9.Wade MJ, Drown DM. 2016. Nuclear-mitochondrial epistasis: a gene's eye view of genomic conflict. Ecol. Evol. 6, 6460–6472. ( 10.1002/ece3.2345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams KL, Palmer JD. 2003. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol. Phylogenet. Evol. 29, 380–395. ( 10.1016/S1055-7903(03)00194-5) [DOI] [PubMed] [Google Scholar]
- 11.Berg OG, Kurland CG. 2000. Why mitochondrial genes are most often found in nuclei. Mol. Biol. Evol. 17, 951–961. ( 10.1093/oxfordjournals.molbev.a026376) [DOI] [PubMed] [Google Scholar]
- 12.Lotz C, et al. 2014. Characterization, design, and function of the mitochondrial proteome: from organs to organisms. J. Proteome Res. 13, 433–446. ( 10.1021/pr400539j) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinhardt K, Dowling DK, Morrow EH. 2013. Medicine. Mitochondrial replacement, evolution, and the clinic. Science 341, 1345–1346. ( 10.1126/science.1237146) [DOI] [PubMed] [Google Scholar]
- 14.Eyre-Walker A. 2017. Mitochondrial replacement therapy: are mito-nuclear interactions likely to be a problem? Genetics 205, 1365–1372. ( 10.1534/genetics.116.196436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birky CW., Jr 1995. Uniparental inheritance of mitochondrial and chloroplast genes: mechanisms and evolution. Proc. Natl Acad. Sci. USA 92, 11 331–11 338. ( 10.1073/pnas.92.25.11331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gemmell NJ, Metcalf VJ, Allendorf FW. 2004. Mother's Curse: the effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 19, 238–244. ( 10.1016/j.tree.2004.02.002) [DOI] [PubMed] [Google Scholar]
- 17.Frank SA, Hurst LD. 1996. Mitochondria and male disease. Nature 383, 224 ( 10.1038/383224a0) [DOI] [PubMed] [Google Scholar]
- 18.Charlesworth B, Charlesworth D. 1978. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997. ( 10.1086/283342) [DOI] [Google Scholar]
- 19.Frank SA. 1989. The evolutionary dynamics of cytoplasmic male sterility. Am. Nat. 133, 345–376. ( 10.1086/284923) [DOI] [Google Scholar]
- 20.Lewis D. 1941. Male sterility in natural populations of hermaphrodite plants. The equilibrium between females and hermaphrodites to be expected with different types of inheritance. New Phytol. 46, 56–63. ( 10.1111/j.1469-8137.1941.tb07028.x) [DOI] [Google Scholar]
- 21.Connallon T, Camus MF, Morrow EH, Dowling DK. 2018. Coadaptation of mitochondrial and nuclear genes, and the cost of mother's curse. R. Soc. Proc. B 285, 20172257 ( 10.1098/rspb.2017.2257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budar F, Touzet P, De Paepe R.. 2003. The nucleo-mitochondrial conflict in cytoplasmic male sterilities revisited. Genetica 117, 3–16. ( 10.1023/A:1022381016145) [DOI] [PubMed] [Google Scholar]
- 23.Budar F, Pelletier G. 2001. Male sterility in plants: occurrence, determinism, significance and use. C. R. Acad. Sci. III 324, 543–550. ( 10.1016/S0764-4469(01)01324-5) [DOI] [PubMed] [Google Scholar]
- 24.Kaul MLH. 1988. Male sterility in higher plants. Berlin Heidelberg; Germany: Springer-Verlag. [Google Scholar]
- 25.Havird JC, Forsythe ES, Williams AM, Werren JH, Dowling DK, Sloan DB. 2019. Selfish mitonuclear conflict. Curr. Biol. 29, R496–R511. ( 10.1016/j.cub.2019.03.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yee WKW, Rogell B, Lemos B, Dowling DK. 2015. Intergenomic interactions between mitochondrial and Y-linked genes shape male mating patterns and fertility in Drosophila melanogaster. Evolution 69, 2876–2890. ( 10.1111/evo.12788) [DOI] [PubMed] [Google Scholar]
- 27.Dean R, Lemos B, Dowling DK. 2015. Context-dependent effects of Y chromosome and mitochondrial haplotype on male locomotive activity in Drosophila melanogaster. J. Evol. Biol. 28, 1861–1871. ( 10.1111/jeb.12702) [DOI] [PubMed] [Google Scholar]
- 28.Ågren JA, Munasinghe M, Clark AG. 2019. Sexual conflict through Mother's Curse and Father's Curse. Theor. Popul. Biol. 129, 9–17. ( 10.1016/j.tpb.2018.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemogs B, Branco AT, Hartl DL. 2010. Epigenetic effects of polymorphic Y chromosomes modulate chromatin components, immune response, and sexual conflict. Proc. Natl Acad. Sci. USA 107, 15 826–15 831. ( 10.1073/pnas.1010383107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemos B, Araripe LO, Hartl DL. 2008. Polymorphic Y chromosomes harbor cryptic variation with manifold functional consequences. Science 319, 91–93. ( 10.1126/science.1148861) [DOI] [PubMed] [Google Scholar]
- 31.Jiang P-P, Hartl DL, Lemos B. 2010. Y not a dead end: epistatic interactions between Y-linked regulatory polymorphisms and genetic background affect global gene expression in Drosophila melanogaster. Genetics 186, 109–118. ( 10.1534/genetics.110.118109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kutch IC, Fedorka KM. 2017. A test for Y-linked additive and epistatic effects on surviving bacterial infections in Drosophila melanogaster. J. Evol. Biol. 30, 1400–1408. ( 10.1111/jeb.13118) [DOI] [PubMed] [Google Scholar]
- 33.Chippindale AK, Rice WR. 2001. Y chromosome polymorphism is a strong determinant of male fitness in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 98, 5677–5682. ( 10.1073/pnas.101456898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown EJ, Nguyen AH, Bachtrog D. 2020. The Y chromosome may contribute to sex-specific ageing in Drosophila. Nat. Ecol. Evol. 4, 853–862. ( 10.1038/s41559-020-1179-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Innocenti P, Morrow EH, Dowling DK. 2011. Experimental evidence supports a sex-specific selective sieve in mitochondrial genome evolution. Science 332, 845–848. ( 10.1126/science.1201157) [DOI] [PubMed] [Google Scholar]
- 36.Rogell B, Dean R, Lemos B, Dowling DK. 2014. Mito-nuclear interactions as drivers of gene movement on and off the X-chromosome. BMC Genomics 15, 330 ( 10.1186/1471-2164-15-330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rand DM, Fry A, Sheldahl L. 2006. Nuclear-mitochondrial epistasis and Drosophila aging: introgression of Drosophila simulans mtDNA modifies longevity in D. melanogaster nuclear backgrounds. Genetics 172, 329–341. ( 10.1534/genetics.105.046698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu C-T, Ingelmo P, Rand DM. 2014. G×G×E for lifespan in Drosophila: mitochondrial, nuclear, and dietary interactions that modify longevity. PLoS Genet. 10, e1004354 ( 10.1371/journal.pgen.1004354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clancy DJ. 2008. Variation in mitochondrial genotype has substantial lifespan effects which may be modulated by nuclear background. Aging Cell 7, 795–804. ( 10.1111/j.1474-9726.2008.00428.x) [DOI] [PubMed] [Google Scholar]
- 40.Tower J. 2017. Sex-specific gene expression and life span regulation. Trends Endocrinol. Metab. 28, 735–747. ( 10.1016/j.tem.2017.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelsey KJP, Clark AG. In preparation Functional consequences of variation in Y chromosome heterochromatin in Drosophila.
- 42.Grenier JK, Arguello JR, Moreira MC, Gottipati S, Mohammed J, Hackett SR, Boughton R, Greenberg AJ, Clark AG. 2015. Global diversity lines: a five-continent reference panel of sequenced Drosophila melanogaster strains. G3 (Bethesda) 5, 593–603. ( 10.1534/g3.114.015883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. ( 10.1093/bioinformatics/btu170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoskins RA, et al. 2015. The Release 6 reference sequence of the Drosophila melanogaster genome. Genome Res. 25, 445–458. ( 10.1101/gr.185579.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. ( 10.1093/bioinformatics/bts635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anders S, Pyl PT, Huber W. 2015. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169. ( 10.1093/bioinformatics/btu638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 ( 10.1186/s13059-014-0550-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leek JT. 2014. svaseq: removing batch effects and other unwanted noise from sequencing data. Nucleic Acids Res. 42, e161 ( 10.1093/nar/gku864) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young MD, Wakefield MJ, Smyth GK, Oshlack A. 2010. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11, R14 ( 10.1186/gb-2010-11-2-r14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Supek F, Bošnjak M, Škunca N, Šmuc T. 2011. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 6, e21800 ( 10.1371/journal.pone.0021800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aibar S, et al. 2017. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086. ( 10.1038/nmeth.4463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leader DP, Krause SA, Pandit A, Davies SA, Dow JAT. 2018. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Res. 46, D809–D815. ( 10.1093/nar/gkx976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoon JS, Gagen KP, Zhu DL. 1990. Longevity of 68 species of Drosophila. Ohio J. Sci. 90, 16–32. [Google Scholar]
- 54.Tower J, Arbeitman M. 2009. The genetics of gender and life span. J. Biol. 8, 38 ( 10.1186/jbiol141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camus MF, Clancy DJ, Dowling DK. 2012. Mitochondria, maternal inheritance, and male aging. Curr. Biol. 22, 1717–1721. ( 10.1016/j.cub.2012.07.018) [DOI] [PubMed] [Google Scholar]
- 56.Ashburner M, et al. 2000. Gene ontology: tool for the unification of biology. Nat. Genet. 25, 25–29. ( 10.1038/75556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The Gene Ontology Consortium. 2019. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 47, D330–D338. ( 10.1093/nar/gky1055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okumura T, Matsumoto A, Tanimura T, Murakami R. 2005. An endoderm-specific GATA factor gene, dGATAe, is required for the terminal differentiation of the Drosophila endoderm. Dev. Biol. 278, 576–586. ( 10.1016/j.ydbio.2004.11.021) [DOI] [PubMed] [Google Scholar]
- 59.Patient RK, McGhee JD. 2002. The GATA family (vertebrates and invertebrates). Curr. Opin. Genet. Dev. 12, 416–422. ( 10.1016/S0959-437X(02)00319-2) [DOI] [PubMed] [Google Scholar]
- 60.Camus MF, Piper MD, Reuter M. 2019. Sex-specific transcriptomic responses to changes in the nutritional environment. eLife 8, e47262 ( 10.7554/eLife.47262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sato K, Ito H, Yokoyama A, Toba G, Yamamoto D. 2019. Partial proteasomal degradation of Lola triggers the male-to-female switch of a dimorphic courtship circuit. Nat. Commun. 10, 166 ( 10.1038/s41467-018-08146-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spletter ML, Liu J, Liu J, Su H, Giniger E, Komiyama T, Quake S, Luo L. 2007. Lola regulates Drosophila olfactory projection neuron identity and targeting specificity. Neural Dev. 2, 14 ( 10.1186/1749-8104-2-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Storelli G, Nam H-J, Simcox J, Villanueva CJ, Thummel CS. 2019. Drosophila HNF4 directs a switch in lipid metabolism that supports the transition to adulthood. Dev. Cell 48, 200–214.e6. ( 10.1016/j.devcel.2018.11.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palanker L, Tennessen JM, Lam G, Thummel CS. 2009. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 9, 228–239. ( 10.1016/j.cmet.2009.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barry WE, Thummel CS. 2016. The Drosophila HNF4 nuclear receptor promotes glucose-stimulated insulin secretion and mitochondrial function in adults. eLife 5, e11183 ( 10.7554/eLife.11183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim H-Y, Wang W, Wessells RJ, Ocorr K, Bodmer R. 2011. Phospholipid homeostasis regulates lipid metabolism and cardiac function through SREBP signaling in Drosophila. Genes Dev. 25, 189–200. ( 10.1101/gad.1992411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bertolio R, Napoletano F, Mano M, Maurer-Stroh S, Fantuz M, Zannini A, Bicciato S, Sorrentino G, Del Sal G. 2019. Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism. Nat. Commun. 10, 1326 ( 10.1038/s41467-019-09152-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brosseau GE. 1960. Genetic analysis of the male fertility factors on the Y chromosome of Drosophila melanogaster. Genetics 45, 257–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kennison JA. 1981. The genetic and cytological organization of the Y chromosome of Drosophila melanogaster. Genetics 98, 529–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morgan TH. 1910. Sex limited inheritance in Drosophila. Science 32, 120–122. ( 10.1126/science.32.812.120) [DOI] [PubMed] [Google Scholar]
- 71.Carvalho AB, Lazzaro BP, Clark AG. 2000. Y chromosomal fertility factors kl-2 and kl-3 of Drosophila melanogaster encode dynein heavy chain polypeptides. Proc. Natl Acad. Sci. USA 97, 13 239–13 244. ( 10.1073/pnas.230438397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Clark AG. 1990. Two tests of Y chromosomal variation in male fertility of Drosophila melanogaster. Genetics 125, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel MR, et al. 2016. A mitochondrial DNA hypomorph of cytochrome oxidase specifically impairs male fertility in Drosophila melanogaster. eLife 5, e16923 ( 10.7554/eLife.16923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsushita T, Fujii-Taira I, Tanaka Y, Homma KJ, Natori S. 2000. Male-specific IDGF, a novel gene encoding a membrane-bound extracellular signaling molecule expressed exclusively in testis of Drosophila melanogaster. J. Biol. Chem. 275, 36 934–36 941. ( 10.1074/jbc.M003455200) [DOI] [PubMed] [Google Scholar]
- 75.Schäfer M, Börsch D, Hülster A, Schäfer U. 1993. Expression of a gene duplication encoding conserved sperm tail proteins is translationally regulated in Drosophila melanogaster. Mol. Cell. Biol. 13, 1708–1718. ( 10.1128/MCB.13.3.1708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ayyar S, Jiang J, Collu A, White-Cooper H, White RAH. 2003. Drosophila TGIF is essential for developmentally regulated transcription in spermatogenesis. Development 130, 2841–2852. ( 10.1242/dev.00513) [DOI] [PubMed] [Google Scholar]
- 77.Jiang J, White-Cooper H. 2003. Transcriptional activation in Drosophila spermatogenesis involves the mutually dependent function of aly and a novel meiotic arrest gene cookie monster. Development 130, 563–573. ( 10.1242/dev.00246) [DOI] [PubMed] [Google Scholar]
- 78.Saudan P, et al. 2002. Ductus ejaculatorius peptide 99B (DUP99B), a novel Drosophila melanogaster sex-peptide pheromone. Eur. J. Biochem. 269, 989–997. ( 10.1046/j.0014-2956.2001.02733.x) [DOI] [PubMed] [Google Scholar]
- 79.Rathke C, Barckmann B, Burkhard S, Jayaramaiah-Raja S, Roote J, Renkawitz-Pohl R. 2010. Distinct functions of Mst77F and protamines in nuclear shaping and chromatin condensation during Drosophila spermiogenesis. Eur. J. Cell Biol. 89, 326–338. ( 10.1016/j.ejcb.2009.09.001) [DOI] [PubMed] [Google Scholar]
- 80.Raja SJ, Jayaramaiah Raja S, Renkawitz-Pohl R. 2006. Replacement by Drosophila melanogaster protamines and Mst77F of histones during chromatin condensation in late spermatids and role of sesame in the removal of these proteins from the male pronucleus. Mol. Cell. Biol. 26, 3682 ( 10.1128/MCB.26.9.3682.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thurmond J, et al. 2019. FlyBase 2.0: the next generation. Nucleic Acids Res. 47, D759–D765. ( 10.1093/nar/gky1003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.LaFlamme BA, Ravi Ram K, Wolfner MF. 2012. The Drosophila melanogaster seminal fluid protease ‘Seminase’ regulates proteolytic and post-mating reproductive processes. PLoS Genet. 8, e1002435 ( 10.1371/journal.pgen.1002435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dowling DK, Tompkins DM, Gemmell NJ. 2015. The Trojan female technique for pest control: a candidate mitochondrial mutation confers low male fertility across diverse nuclear backgrounds in Drosophila melanogaster. Evol. Appl. 8, 871–880. ( 10.1111/eva.12297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yee WKW, Sutton KL, Dowling DK. 2013. In vivo male fertility is affected by naturally occurring mitochondrial haplotypes. Curr. Biol. 23, R55–R56. ( 10.1016/j.cub.2012.12.002) [DOI] [PubMed] [Google Scholar]
- 85.Clancy DJ, Hime GR, Shirras AD. 2011. Cytoplasmic male sterility in Drosophila melanogaster associated with a mitochondrial CYTB variant. Heredity 107, 374–376. ( 10.1038/hdy.2011.12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yue L, Karr TL, Nathan DF, Swift H, Srinivasan S, Lindquist S. 1999. Genetic analysis of viable Hsp90 alleles reveals a critical role in Drosophila spermatogenesis. Genetics 151, 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wasbrough ER, Dorus S, Hester S, Howard-Murkin J, Lilley K, Wilkin E, Polpitiya A, Petritis K, Karr TL. 2010. The Drosophila melanogaster sperm proteome-II (DmSP-II). J. Proteomics 73, 2171–2185. ( 10.1016/j.jprot.2010.09.002) [DOI] [PubMed] [Google Scholar]
- 88.Nanda S, DeFalco TJ, Loh SHY, Phochanukul N, Camara N, Van Doren M, Russell S.. 2009. Sox100B, a Drosophila group E Sox-domain gene, is required for somatic testis differentiation. Sex Dev. 3, 26–37. ( 10.1159/000200079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lawton KJ, Wassmer TL, Deitcher DL. 2014. Conserved role of Drosophila melanogaster FoxP in motor coordination and courtship song. Behav. Brain Res. 268, 213–221. ( 10.1016/j.bbr.2014.04.009) [DOI] [PubMed] [Google Scholar]
- 90.Misra S, Pandey AK, Gupta S, Kumar A, Khanna P, Shankar J, Ravi Ram K. 2017. Estrogen related receptor is required for the testicular development and for the normal sperm axoneme/mitochondrial derivatives in Drosophila males. Sci. Rep. 7, 40372 ( 10.1038/srep40372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu J, et al. 2015. Identification of seven genes essential for male fertility through a genome-wide association study of non-obstructive azoospermia and RNA interference-mediated large-scale functional screening in Drosophila. Hum. Mol. Genet. 24, 1493–1503. ( 10.1093/hmg/ddu557) [DOI] [PubMed] [Google Scholar]
- 92.Satoh AK, Ready DF. 2005. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr. Biol. 15, 1722–1733. ( 10.1016/j.cub.2005.08.064) [DOI] [PubMed] [Google Scholar]
- 93.Shieh B-H, Kristaponyte I, Hong Y. 2014. Distinct roles of arrestin 1 protein in photoreceptors during Drosophila development. J. Biol. Chem. 289, 18 526–18 534. ( 10.1074/jbc.M114.571224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katz B, Minke B. 2018. The Drosophila light-activated TRP and TRPL channels: targets of the phosphoinositide signaling cascade. Prog. Retin. Eye Res. 66, 200–219. ( 10.1016/j.preteyeres.2018.05.001) [DOI] [PubMed] [Google Scholar]
- 95.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. 1996. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell 85, 651–659. ( 10.1016/S0092-8674(00)81232-5) [DOI] [PubMed] [Google Scholar]
- 96.Gu Y, Oberwinkler J, Postma M, Hardie RC. 2005. Mechanisms of light adaptation in Drosophila photoreceptors. Curr. Biol. 15, 1228–1234. ( 10.1016/j.cub.2005.05.058) [DOI] [PubMed] [Google Scholar]
- 97.Dolph PJ, Man-Son-Hing H, Yarfitz S, Colley NJ, Deer JR, Spencer M, Hurley JB, Zuker CS. 1994. An eye-specific G beta subunit essential for termination of the phototransduction cascade. Nature 370, 59–61. ( 10.1038/370059a0) [DOI] [PubMed] [Google Scholar]
- 98.Hardie RC, Raghu P. 2001. Visual transduction in Drosophila. Nature 413, 186–193. ( 10.1038/35093002) [DOI] [PubMed] [Google Scholar]
- 99.Hanlon CD, Andrew DJ. 2015. Outside-in signaling: a brief review of GPCR signaling with a focus on the Drosophila GPCR family. J. Cell Sci. 128, 3533–3542. ( 10.1242/jcs.175158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vandendries ER, Johnson D, Reinke R. 1996. orthodenticle is required for photoreceptor cell development in the Drosophila eye. Dev. Biol. 173, 243–255. ( 10.1006/dbio.1996.0020) [DOI] [PubMed] [Google Scholar]
- 101.McDonald EC, Xie B, Workman M, Charlton-Perkins M, Terrell DA, Reischl J, Wimmer EA, Gebelein BA, Cook TA. 2010. Separable transcriptional regulatory domains within Otd control photoreceptor terminal differentiation events. Dev. Biol. 347, 122–132. ( 10.1016/j.ydbio.2010.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mishra M, Oke A, Lebel C, McDonald EC, Plummer Z, Cook TA, Zelhof AC. 2010. Pph13 and orthodenticle define a dual regulatory pathway for photoreceptor cell morphogenesis and function. Development 137, 2895–2904. ( 10.1242/dev.051722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kenyon KL, Yang-Zhou D, Cai CQ, Tran S, Clouser C, Decene G, Ranade S, Pignoni F. 2005. Partner specificity is essential for proper function of the SIX-type homeodomain proteins Sine oculis and Optix during fly eye development. Dev. Biol. 286, 158–168. ( 10.1016/j.ydbio.2005.07.017) [DOI] [PubMed] [Google Scholar]
- 104.Gold KS, Brand AH. 2014. Optix defines a neuroepithelial compartment in the optic lobe of the Drosophila brain. Neural Dev. 9, 18 ( 10.1186/1749-8104-9-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mishra AK, Bargmann BOR, Tsachaki M, Fritsch C, Sprecher SG. 2016. Functional genomics identifies regulators of the phototransduction machinery in the Drosophila larval eye and adult ocelli. Dev. Biol. 410, 164–177. ( 10.1016/j.ydbio.2015.12.026) [DOI] [PubMed] [Google Scholar]
- 106.Giniger E, Tietje K, Jan LY, Jan YN. 1994. lola encodes a putative transcription factor required for axon growth and guidance in Drosophila. Development 120, 1385–1398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data reported here are posted on the GEO resource with reference number GSE155395. Fly lines are available on request to A.G.C. All scripts used for the RNA-sequencing analysis are on GitHub (https://github.com/mam737/mitoY_RNASEQ).