Abstract
Females of many species mate with multiple males, thereby inciting competition among ejaculates from rival males for fertilization. In response to increasing sperm competition, males are predicted to enhance their investment in sperm production. This prediction is so widespread that testes size (correcting for body size) is commonly used as a proxy of sperm competition, even in the absence of any other information about a species' reproductive behaviour. By contrast, a debate about whether sperm competition selects for smaller or larger sperm has persisted for nearly three decades, with empirical studies demonstrating every possible response. Here, we synthesize nearly 40 years of sperm competition research in a meta-analytical framework to determine how the evolution of sperm number (i.e. testes size) and sperm size (i.e. sperm head, midpiece, flagellum and total length) is influenced by varying levels of sperm competition across species. Our findings support the long-held assumption that higher levels of sperm competition are associated with relatively larger testes. We also find clear evidence that sperm competition is associated with increases in all components of sperm length. We discuss these results in the context of different theoretical predictions and general patterns in the breeding biology and selective environment of sperm.
This article is part of the theme issue ‘Fifty years of sperm competition’.
Keywords: promiscuity, sperm design, multiple paternity, extrapair paternity, comparative study
1. Introduction
Parker's [1] recognition that female multiple mating leads to competition among sperm from rival males for fertilization greatly expanded Darwin's [2] interpretation of sexual selection as the competition among members of the mate-limited sex (usually males) for mating opportunities. In the 50 years since Parker's [1] insights, research on sexual selection has revealed how widespread female multiple mating is throughout the animal kingdom [3,4], making it clear that sexual selection is better understood as the competition over limited gametes, a definition that encompasses both mating and fertilization success [5]. By broadening this perspective, Parker [1] spurred a wealth of studies examining the contribution of both pre- and postmating sexual selection to individual and population fitness, and to the evolution of reproductive traits [3,4,6,7]. In this article, we will discuss primarily postmating sexual selection that has been the focus of Parker's numerous influential contributions.
One of the cornerstones of sperm competition theory is the prediction that males transferring more sperm should gain a numerical competitive advantage, particularly when fertilization approximates a random process [8–11]. If so, males are predicted to enhance their investment in ejaculate production as the level of sperm competition increases (reviewed in [12]). Generally, there are two avenues for increasing sperm numbers: (i) growing larger and more productive testes [13] and (ii) producing smaller sperm [8]. Both of these evolutionary responses are now fundamental components of most sperm competition models [12].
The first prediction—that elevated sperm competition levels should result in relatively larger testes—has gained broad empirical support in comparative studies across species (reviewed in [14,15]) or among intraspecific populations [16–18], in experimental evolution studies in which mating systems are manipulated [19–21] and in species with alternative reproductive tactics [22,23]. This evidence, combined with the observation that relative sperm numbers can predict a male's paternity share [24–26], has led to the almost universal use of relative testes size (body size-corrected testes size; hereafter RTS) as an index of the level of sperm competition, typically without empirical verification. Yet, without a quantitative evaluation of this key assumption in the literature, it remains unclear if the use of RTS as a proxy for sperm competition is justified. Indeed, several experimental evolution studies of Drosophila [27–29] and comparative studies in a diversity of taxa (e.g. [30–33]) have found no increase in RTS at higher sperm competition levels, raising the question of why deviations from the widely accepted pattern occur. Fortunately, there is now a sufficient abundance of studies of the relationship between RTS and sperm competition to allow a detailed quantitative meta-analysis to identify potential causes of variation in effect sizes between taxa.
The second prediction, that sperm competition should favour smaller sperm, has also found empirical support [24–26] and is particularly intriguing as it helps explain the evolution and maintenance of anisogamy [8,34] and, by extension, the evolution of mating systems [35–37]. Yet, not all males transfer vast numbers of tiny sperm, and postcopulatory sexual selection can favour larger and more complex sperm. Such positive selection on sperm size could occur through intersexual selection equivalent to ‘peacocks' tails on the cellular level' [38–40] or through co-diversification with important dimensions of the female reproductive tract (e.g. [41–45]). A more widespread explanation, mostly derived from studies of vertebrate sperm, is that sperm length and the (relative) dimensions of functional sperm components, like sperm midpiece and flagellum, reflect responses to selection for faster sperm swimming [46–49]. Although such functional links are more often assumed than directly tested, numerous comparative studies framed in this context have explored potential responses of sperm length to varying levels of sperm competition. Interestingly, these studies have reported both a positive, negative or no significant trend, depending on the taxon (reviewed in [14,50,51]). The contrasting, albeit non-mutually exclusive [52], predictions and reported patterns about the response of sperm size to sperm competition call for a meta-analytic approach to determine whether general patterns arise from previous studies.
Given the rapidly accumulating literature on the evolution of both testes size and sperm morphology since Parker's [1] formative insights into the evolutionary importance of sperm competition, we used meta-analyses to explore what general trends have emerged across these studies. We restricted our analyses to interspecific studies for the sake of comparability and data accessibility for all traits of interest. We then addressed two distinct meta-analytic hypotheses. First, we asked if male investment in RTS varies in response to behavioural and genetic proxy measures of sperm competition levels among species (henceforth called the testes meta-analysis). Second, we determined whether interspecific variation in sperm total length, as well as its constituent components (i.e. sperm head, midpiece and flagellum), are influenced by alternative proxy measures of sperm competition, including behavioural, genetic and RTS metrics (henceforth called the sperm meta-analysis).
2. Methods
(a). Literature search
We searched the published literature for comparative studies (i.e. two or more species examined) that addressed the two distinct meta-analytic hypotheses about the response of RTS and sperm length, respectively, to the strength of sperm competition. We performed a systematic literature search following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA [53]) guidelines (electronic supplementary material §1). This search initially identified 1258 unique papers for the testes meta-analysis and 1420 for the sperm meta-analysis, of which 2237 were unique across both analyses and were screened for inclusion in the meta-analyses. After reviewing titles and abstracts, we removed 1896 papers from further consideration and screened the full text of the remaining 341 papers based on our inclusion criteria (electronic supplementary material, §1).
(b). Effect size dataset
For each included paper, we extracted test statistics and sample sizes and used these to calculate Fisher's Zr values (electronic supplementary material, §2). Each entry was double-checked by a second author. Available data were confined to studies from two phyla, Arthropoda and Chordata, with 69% of effect size estimates originating from studies of birds (class Aves) and mammals (class Mammalia) in both the testes and sperm meta-analyses (table 1).
Table 1.
Number of effect sizes, studies and the mean (range) sample size of species analysed in the (a) testes and (b) sperm meta-analyses. The total dataset is summarized separately for each meta-analysis followed by a summary that illustrates the distribution of data based on phylum (Arthropoda versus Chordata) and class/subclass of animals included in the analyses.
| meta-analysis | effect sizes | studies | mean n (range) | |
|---|---|---|---|---|
| (a) testes meta-analysis | 99 | 58a | 35.3 (2–776) | |
| phylum | class/subclass | |||
| Arthropoda | Arachnida | 1 | 1 | 8 |
| Insecta | 7 | 5 | 14.6 (2–28) | |
| Chordata | Actinopterygii | 15 | 7 | 27.5 (2–111) |
| Amphibia | 6 | 5 | 40.8 (24–65) | |
| Aves | 24 | 17 | 61.2 (2–776) | |
| Chondrichthyes | 2 | 1 | 12 | |
| Mammalia | 44 | 23 | 28 (2–141) | |
| (b) sperm meta-analysis | 186 | 74 | 42.3 (2–323) | |
| phylum | class/subclass | |||
| Arthropoda | Arachnida | 2 | 1 | 8 |
| Insecta | 23 | 19 | 21.6 (2–127) | |
| Chordata | Actinopterygii | 9 | 5 | 19.3 (7–46) |
| Amphibia | 16 | 4 | 56.2 (22–130) | |
| Aves | 54 | 18 | 48.2 (3–232) | |
| Chondrichthyes | 4 | 1 | 16.5 (16–18) | |
| Mammalia | 74 | 29 | 47.5 (2–226) | |
| Reptilia | 4 | 1 | 25 | |
aNote that the total number of studies is one less than the sum of the number of studies when divided by phylum and class/subclass as one study presented data from two animal classes.
For the testes meta-analysis, we extracted 99 effect sizes from 58 studies spanning 38 years of research (table 1). Most studies assessed sperm competition risk (i.e. the probability that sperm will compete for fertilization) using behavioural proxy measures (64 effect sizes from 43 studies) including (i) species-specific mating system classifications, i.e. species with monogamous/polygynous (low risk) versus polyandrous/promiscuous (high risk) mating systems; (ii) pair (low risk) versus group (high risk) living/reproducing species; (iii) the number of reproductively active adults in a breeding group; (iv) parasitic spawning behaviours; or (v) metrics that ranked species from low to high sperm competition risk (note that some metrics included behavioural and genetic data, but because genetic data were not available for every species, we treat these studies as behavioural proxy measures of sperm competition risk). Only one study used behavioural data to estimate sperm competition intensity (i.e. the number of mated males; 2 effect sizes from 1 study). We also considered genetic measures of sperm competition risk (21 effect sizes from 13 studies), including studies that quantified multiple paternity (the percentage of broods/clutches/nests/litters where offspring are sired by two or more males) or extrapair paternity (the frequency that broods contain individuals sired by males other than the social mate). Genetic measures that quantified the number of males competing to fertilize eggs were used as indices of sperm competition intensity (e.g. number of sires per brood, number of males that a female mates with, 12 effect sizes from 2 studies).
For the sperm meta-analysis, we extracted 186 effect sizes from 74 studies spanning 30 years of research (table 1). Sperm total length was the most commonly examined sperm morphological trait, constituting roughly half the available effect sizes (94 effect sizes from 64 studies, compared to 92 effect sizes for all other sperm morphology components). The numbers of studies assessing sperm head, midpiece and flagellum length were roughly equivalent (sperm head: 30 effect sizes from 23 studies; sperm midpiece: 28 effect sizes from 21 studies; sperm flagellum: 34 effect sizes from 26 studies). Most effect sizes (78%) estimated the effect of sperm competition level using body size-corrected testes size (145 effect sizes from 60 studies), a proxy measure that we validated in the testes meta-analysis. Behavioural measures of sperm competition (33 effect sizes from 17 studies) were based on (i) mating system classifications, (ii) pair versus group mating, (iii) female remating rate and (iv) metrics that ranked species from low to high sperm competition risk (as defined above). Genetic measures of sperm competition risk (i.e. multiple paternity and extrapair paternity rates) were comparatively less common (8 effect sizes from 6 studies). Sperm competition intensity was only considered in two studies examining sperm morphology (sperm total length), representing three effect sizes.
(c). Statistical analyses
We fit meta-analytic and multi-level meta-regression models using the rma.mv function in the R package metafor [54]. To account for non-independence of the data when deriving multiple values from a single paper, all models included a unique observation-level identifier for each effect size and the paper identity as random effects. We used meta-analytic models to determine if grand effect sizes differed significantly from zero. Then, to test the effect of alternative proxy measures of sperm competition level on male investment in testicular tissue and sperm morphology, we constructed meta-regression models with the proxy measure of sperm competition (i.e. behavioural and genetic estimates, additionally including RTS in the sperm meta-analysis) as a moderator. When modelling how alternative proxy measures of sperm competition influence sperm morphology, we focused on total sperm length, as this metric made up roughly 50% of the sperm morphology data (see above). Finally, we constructed a meta-regression model to compare effect sizes among sperm morphology components (sperm head, midpiece, flagellum and total length). We estimated model heterogeneity using I2 and used the omnibus test of parameters, Qm, as test statistic for models that included moderator variables. We examined whether the results were sensitive to a range of biological and analytical factors, including fertilization mode (external versus internal), phylum (Arthropoda versus Chordata), whether the studies were phylogenetically controlled or not, the number of covariates in the models, the taxonomic level of analysis (genus versus species) and possible bias introduced by having the same species present in different studies. We assessed publication bias through visual inspection of funnel plots of meta-analytic residuals, applying Egger's test on the meta-analytic residuals [55], using trim-and-fill methods to identify potentially missing data [56] and by assessing time-lag bias [57].
3. Results
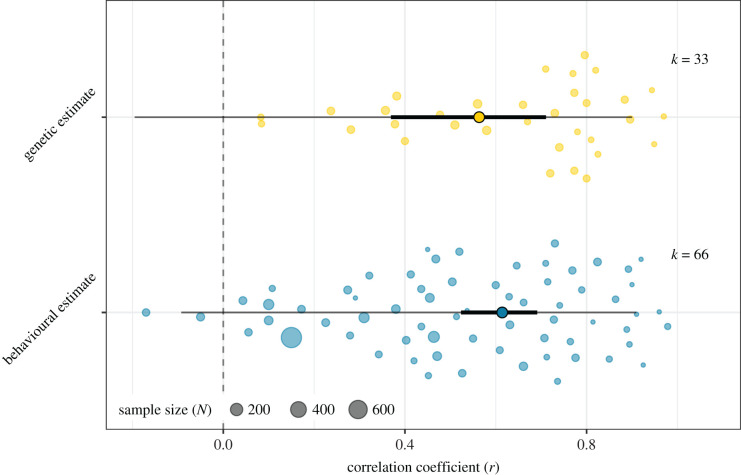
The relationship between RTS and sperm competition levels was positive across all sperm competition metrics (Fisher's Zr [95%CI] = 0.70 [0.58; 0.82], k = 99, Z = 11.6, p < 0.001, electronic supplementary material, §3.1). This translates into a grand mean correlation between RTS and sperm competition of r = 0.60, indicating broad support for the prediction that sperm competition selects for increased investment in sperm-producing tissue. This positive relationship did not differ between behavioural or genetic estimates of sperm competition (Qm,d.f.=1 = 0.29, k = 99, p = 0.59, electronic supplementary material, §3.2.1), which were both significantly positive (figure 1). Effect size estimates of the relationship between RTS and sperm competition level were significantly greater in internal (r = 0.76) compared to external fertilizers (r = 0.45; Qm,df=1 = 4.09, k = 90, p = 0.04, electronic supplementary material, §3.2.2), and significantly weaker in studies that corrected for phylogenetic effects (r = 0.57) compared to those that did not (r = 0.81; Qm,df=1 = 5.8, k = 99, p = 0.02, electronic supplementary material, §3.2.3). The relationship between RTS and sperm competition levels did not differ between Arthropoda and Chordata (Qm,df=1 = 2.97, k = 99, p = 0.09, electronic supplementary material, §3.2.4)
Figure 1.
Orchard plot [58] showing a positive correlation of testes size with genetic (r = 0.56, Z = 4.99, p < 0.001) and behavioural (r = 0.61, Z = 10.42, p < 0.001) estimates of sperm competition. The coloured dots present individual effect sizes, scaled by their precision, and model estimates are indicated by solid points outlined in black. Effect sizes and model estimates are back-transformed from Zr to r for easier interpretation. The 95% confidence intervals (solid black lines) do not overlap with zero (dotted grey line), and the 95% prediction intervals (solid grey lines) show that the ranges of values that can be expected from future studies are largely positive. (Online version in colour.)
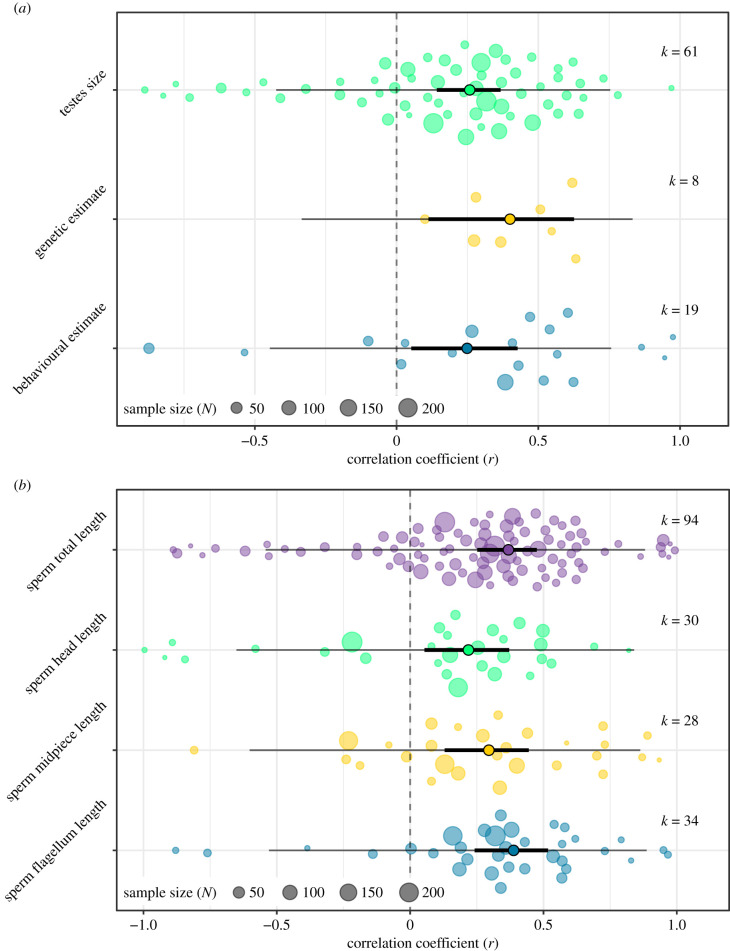
The overall relationship between sperm length (including sperm head, midpiece, flagellum and total length) and sperm competition levels was positive across all sperm competition metrics (Fisher's Zr [95%CI] = 0.36 [0.24; 0.49], k = 186, Z = 5.86, p < 0.001, electronic supplementary material, §4.1). When assessing sperm total length, which constituted approximately half of the available data on sperm morphology, effect size estimates did not differ between RTS, behavioural or genetic estimates of sperm competition (Qm,df=2 = 0.95, k = 88, p = 0.62, electronic supplementary material, §4.2.1), which were all positive with confidence intervals that excluded zero (figure 2a). Therefore, we combined all proxy measures into a single global metric of sperm competition levels for analyses examining responses in each sperm component. There was weak evidence that effect sizes for sperm head, midpiece, flagellum and total length differed from one another (Qm,df=3 = 8.04, k = 186, p = 0.05, electronic supplementary material, §4.2.2), with those for head length being lowest (r = 0.22), followed by midpiece (r = 0.30), flagellum (r = 0.37) and sperm total length (r = 0.39), although all sperm components showed an overall positive relationship with sperm competition. Indeed, all sperm components were significantly positively related with sperm competition levels (figure 2b). Effect size estimates between sperm total length and sperm competition level did not differ based on fertilization mode (Qm,df=1 = 0.33, k = 92, p = 0.57, electronic supplementary material, §4.2.3). Studies that corrected for phylogenetic effects had significantly weaker effect size estimates of the relationship between sperm total length and sperm competition level compared to studies that did not account for phylogenetic effects (with phylogenetic correction: r = 0.26; without phylogenetic correction: r = 0.66; Qm,df=1 = 9.33, k = 94, p = 0.002, electronic supplementary material, §4.2.4). Effect size estimates from Arthropoda were significantly greater than estimates from Chordata (Qm,df=1 = 8.69, k = 94, p = 0.003, electronic supplementary material, §4.2.5).
Figure 2.
(a) Sperm length shows a positive correlation with testes size (r = 0.28, Z = 4.68, p < 0.001), and with genetic (r = 0.40, Z = 2.75, p = 0.006) and behavioural (r = 0.26, Z = 2.63, p = 0.009) estimates of sperm competition. (b) Sperm head length (r = 0.28, Z = 3.07, p = 0.002), midpiece length (r = 0.35, Z = 3.75, p < 0.001), flagellum length (r = 0.41, Z = 4.70, p < 0.001) and total length (r = 0.40, Z = 5.46, p < 0.001) increase with increasing sperm competition. The coloured dots present individual effect sizes, scaled by their precision, and model estimates are indicated by solid points outlined in black. Effect sizes and model estimates are back-transformed from Zr to r for easier interpretation. The 95% confidence intervals (solid black lines) do not overlap with zero (dotted grey line), and the 95% prediction intervals (solid grey lines) show that the ranges of values that can be expected from future studies are mostly positive despite considerable heterogeneity. (Online version in colour.)
The overall positive relationships between RTS/sperm morphology and sperm competition levels remained qualitatively similar under a broad range of analytical conditions. In both meta-analyses, estimates were not influenced by the number of covariates included in the models (electronic supplementary material, §3.2.5 and 4.2.6) and whether analyses were performed at the genus or species level (electronic supplementary material, §3.2.6 and 4.2.7). Responses in testes size and sperm morphology were consistent when sperm competition was assessed using either sperm competition risk (the proportion of matings involving two or more males) or sperm competition intensity (the number of males involved in a mating) [12,13] metrics (electronic supplementary material, §§3.2.7 and 4.2.8) and when using different measures of testes size (i.e. testes mass, length and volume; electronic supplementary material, §§3.2.8 and 4.2.9). Within each sperm component, effect size estimates did not differ when different measures were used (i.e. length, area and volume; electronic supplementary material, §4.2.10).
There was significant publication bias, but no year effect, among studies in the testes meta-analysis, with an excess of studies with small sample sizes showing a positive effect between RTS and sperm competition (electronic supplementary material, §5.1). However, when accounting for this bias analytically, our overall findings remained qualitatively unchanged (electronic supplementary material, §5.1.2). There was no evidence for publication bias in the sperm meta-analysis (electronic supplementary material, §5.2). Within each meta-analysis, however, different studies often shared one or more species, which violates the assumption of independence among studies. To account for this issue, we performed a series of sensitivity analyses using subsets of our full dataset that included only those studies with the largest sample size on an animal class, or on an order (mammals) or family (passerine birds), from which most taxon-specific effect sizes were derived (electronic supplementary material, §6). These sensitivity analyses, which ensured that no species contributed to more than one effect size, produced results that are broadly consistent with those presented in the main text (electronic supplementary material, §6).
4. Discussion
Consistent with Parker's series of sperm competition models (reviewed in [12]), our testis meta-analysis confirmed that males of species with higher levels of sperm competition invest relatively more in their gonads, with little difference between behavioural and genetic estimates of sperm competition. Further, we found an overall trend for longer rather than shorter sperm in response to elevated sperm competition, with a similar pattern for each of the three main sperm components. Interestingly, the overall effect of sperm competition estimates was considerably higher on testes size compared to sperm length (back-transformed r = 0.60 versus r = 0.39), partly because the latter also had many more negative effect sizes (testes: 2 out of 99; sperm length: 20 out of 94). This difference is likely to result from sperm competition selecting on both sperm size and number. By encapsulating both ejaculate traits, testes can respond positively to the selection regardless of which trait is under stronger selection [59]. By contrast, if the selection is stronger on sperm number than on sperm size, as directly shown in multiple taxa [59–61], the effect of sperm competition on sperm length would necessarily weaken or even become negative as observed. Such taxon-specific differences in the reproductive biology, in selection pressures or in trait responses (discussed in more detail in the following sections) might also be responsible for the relatively high heterogeneity in both meta-analyses (I2 = 84.0 and 90.5 for testes and sperm meta-analyses, respectively; electronic supplementary material, §7).
Another general pattern across both our meta-analyses, and possibly another contributor to heterogeneity in the main analyses, was that effect sizes were lower for studies that accounted for phylogenetic non-independence. This difference can be attributed in part to traditional analyses overestimating relationships between traits by not reducing the weight of data points that derive from species with shared ancestry [62]. Further, the use of phylogenetic independent contrasts [63] in older studies sometimes considerably reduced the number of independent data points owing to poorly resolved phylogenies (i.e. many polytomies), thereby weakening effects compared to equal studies without phylogenetic control. Finally, at least in the testes meta-analysis, the non-phylogenetically controlled set of studies was dominated by small studies, where effect sizes can often be high even if the relationships themselves are not statistically significant.
In the following, we discuss our findings of the two meta-analyses in turn.
(a). Evolution of testes size
That males should enhance their investment in ejaculate production as the level of sperm competition increases is arguably one of the most fundamental and intuitive predictions of sperm competition theory. Here, we have provided a robust meta-analytical test of this central prediction. By examining only interspecific comparative studies, we were able to extract 99 effect sizes, of which only two were negative and few were weak, while all others were moderately to strongly positive. Although this strong overall effect was expected, it is critical to quantitatively evaluate the validity of RTS as a proxy of sperm competition, particularly given how frequently its use is justified by citing examples from the literature, even where these come from unrelated taxa. Our analysis did identify a significant publication bias favouring positive effects among small comparative studies, which potentially inflates the ubiquity of the assumed pattern. Nevertheless, the positive effect remained even after accounting for the observed publication bias. The empirical evidence is therefore that the response of testes size to sperm competition holds for most taxa at the interspecific level. However, our results do not necessarily translate to single-species studies, and intraspecific patterns should separately undergo a systematic meta-analytic evaluation.
Even with this strong support of Parker's predictions, not all taxon-specific effects were statistically significant. Broadly, the results with genetic estimates of sperm competition appeared more consistent than the behavioural estimates. Apart from often small sample sizes, some of this difference could also be owing to coarse classifications of sperm competition levels among the behavioural estimates (e.g. monogamy versus polygamy), which ignore potentially considerable variation in the degree of sperm competition within each category. Overall, genetic estimates of the magnitude of sperm competition are preferable as they quantify the extent of sperm competition and skew among males more directly and precisely, although it is also important to note that genetic estimates can only provide a minimum estimate for the level of multiple mating (i.e. postcopulatory sexual selection may favour one or only a very small subset of mated males [64]). However, such data can often be more challenging and time-consuming to obtain with adequate taxonomic coverage.
Regardless of the sperm competition estimate, it is important to note that sources of variation in RTS other than sperm competition could also contribute to the wide distribution of effect sizes among studies and taxa. For example, in addition to sperm competition, selection for relatively larger testes can also result from a higher male mating rate [15,27,65]. Further, our analysis revealed stronger relationships of RTS with sperm competition in internal compared to external fertilizers. This difference could result from a higher risk of sperm limitation in external fertilizers, particularly broadcast spawners [66], compared to internal fertilizers with more targeted sperm release [7,67]. If so, even if total selection on testes size is stronger in external than internal fertilizers, sperm competition would explain a relatively smaller portion of RTS variation, thereby weakening the relationship between the total variation in RTS and measures of sperm competition. However, a more refined analysis might be needed to fully understand the contribution of sperm limitation to the response in the evolution of testes size. There is considerable variation in the risk of sperm dilution between broadcast-spawning fish and foam-nesting frogs [7,60,67], as well as between different internal fertilizers owing to their enormous variation in the volume of female reproductive tracts [61,67].
It is also important to recognize that testes size does not always reflect sperm production alone, which could further influence relationships with indices of sperm competition. For example, in vertebrates in particular, testes can have varying portions of their volume dedicated to the production of androgens and other non-spermatogenic functions [68–71], reducing the sperm-producing tissue to less than a third of the entire testes in some species [68,72]. Within the spermatogenic tissue itself, the efficiency of sperm production can also vary considerably among species in response to sperm demand [73–75]. This raises the intriguing question of whether such variation in the proportion of seminiferous tissue among closely related (and so jointly studied) species might also contribute to the weakly lower overall effect of sperm competition on RTS in vertebrate taxa compared to the arthropods, in addition to other factors such as sperm limitation (see above). Thus, starting to understand how different testicular components and their function respond to sexual selection would be an important avenue for future research.
(b). Evolution of sperm morphology
Parker's original models [8] included the prediction that selection through sperm competition should favour vast numbers of tiny sperm. Subsequent models [9], however, have suggested that sperm size itself could also increase under certain circumstances, such as (i) in the presence of constraints on sperm number, or if sperm size confers (ii) greater competitive benefits at higher sperm density, (iii) sperm survival benefits during prolonged sperm storage or (iv) competitive advantages at the cost of sperm survival when fertilization occurs relatively shortly after sperm release. As revealed in our meta-analysis, many comparative studies have indeed indicated selection for longer sperm, resulting in a strongly positive overall trend. Hence, in addition to maximizing sperm numbers, it appears that producing longer sperm may often add further fitness benefits, although the processes of selection for longer sperm are likely to differ between taxa. For example, the density-related advantage of longer sperm (Parker's [9] second prediction, modelled more specifically in [52]) is most likely to manifest itself in small organisms such as many insects, in which sperm competition occurs in the form of displacing rival sperm from densely packed sperm-storage organs [76,77]. In fact, this mode of sperm competition can even exert stronger selection on sperm length than on sperm number [52,59], often associated with co-diversification between sperm size and the female reproductive tract [41,43–45,78,79]. These factors might also explain why, on average, arthropods showed tighter relationships between sperm length and measures of sperm competition (electronic supplementary material, §4.2.11) and generally exhibit much greater intra-taxonomic variation in sperm size than chordates [80].
By contrast, in many vertebrate taxa, a competitive advantage of longer sperm has been attributed to swimming faster to ova or female sperm-storage structures [46–49]. Following Parker's [9] fourth prediction above, this advantage of faster sperm swimming could outweigh any benefits of prolonged sperm survival in most fish and many mammalian taxa that lack prolonged sperm storage [81]. Yet, even in birds, the evolution of longer sperm under sperm competition is more likely to be driven by selection for faster sperm swimming to the specialized female storage structures than prolonged sperm survival within them, given that extended sperm storage is associated with shorter or slower-swimming sperm [33,82,83]. In addition, it has been suggested that sperm survival within the female storage structures might be aided by female secretions [84] (but see [85]). We are not aware of comparative studies supporting Parker's [9] first and third predictions, but taxa with extended sperm storage, such as reptiles [81], are still very poorly studied.
In addition to sperm total length, our meta-analysis revealed that, overall, selection under sperm competition also appears to favour longer sperm heads, midpieces and flagella. That all sperm components showed a similar pattern could result from underlying genetic covariation, concerted evolution under simultaneous selection on these different traits, and/or extrinsic functional or intrinsic mechanistic constraints [86]. The increase in both midpiece and flagellum length is consistent with the predicted selection for faster sperm, in that the flagellum is the component propelling the sperm forward, and the midpiece generates the necessary energy (reviewed in [50,87]). Even though selection should, in principle, favour a relatively small head relative to flagellum length [87], the positive effect of sperm head length is not in conflict with this prediction as our meta-analysis informs solely on the strength of the relationships between sperm dimensions and indices of sperm competition, and not on the allometries between sperm components. Thus, sperm flagellum length could still increase at a faster rate than sperm head length, consistent with the generally much greater variation in sperm flagellum length than sperm head length [51].
(c). The next 50 years of sperm competition research
Our meta-analyses have revealed overarching patterns across a wealth of studies generated in the 50 years since Parker's [1] influential paper. However, they also highlight the strong taxonomic bias in the study of testes and sperm evolution (table 1, electronic supplementary material, §§3.2.4 and 4.2.5). The vast majority of effect sizes were derived from birds (and here strongly biased toward passerines) and mammals (primarily primates and rodents), with the more speciose vertebrate taxa (bony fishes and reptiles) being represented by a mere handful of studies or absent entirely (e.g. reptiles in testes meta-analysis). Even less information is available on the invertebrates, although arthropods alone dwarf the speciosity of all other animal phyla combined and exhibit both astounding diversity in sperm morphology [51,80] and rampant sperm competition [3]. Consequently, many novel patterns of reproductive evolution are likely to be discovered in future endeavours.
In addition to broadening the taxonomic breadth in studies of sperm competition, it is important to recognize that reproductive traits rarely evolve independently of one another, of other sexual and non-sexual traits, or the environment in which sperm competition occurs. Since sperm morphology is often an indirect target of selection via sperm performance (e.g. [47]), knowledge of how variation in sperm morphology translates into fitness within the context of other ejaculate traits seems critical for understanding sperm evolution itself (reviewed in [14,50,80]). Progress in this direction has been made by studying the joint evolution of sperm size and number, which has revealed how the selective environment modifies the relative strength and direction of natural and sexual selection on either trait [32,61,62,73]. However, other sperm and seminal fluid parameters are yet to be integrated into such studies. Similarly, a better understanding is needed of the operation of sperm within their selective environment, including the forms of selection or contributions of sperm–female interactions as potential drivers of sperm diversification [80]. This is particularly true for internal fertilizers, where sperm size and shape are likely to co-diversify with the female reproductive tract morphology and sperm-use patterns. Since these female traits themselves are extremely diverse, however, it might be difficult to compare female contributions to sperm evolution in a systematic manner among species.
Overall, the past 50 years have exposed critical patterns of male reproductive trait evolution that we summarized here. Yet, we are still a long way from understanding the full complexity of selective processes on these traits. Nevertheless, methodological and analytical advances continue to deepen our knowledge of sperm competition and its contributions to reproductive diversity, and we look forward to the next 50 years for yet further clarity in our understanding of the evolutionary significance of sperm competition.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Nina Wedell and Leigh Simmons for inviting us to contribute to this special issue, the reviewers for their constructive comments, and Geoff Parker for his enormous contributions to our field that have motivated much of our academic careers.
Data accessibility
All data are provided in the electronic supplementary material.
Authors' contributions
All authors contributed to the conception of the study and data collection. R.A.d.B. analysed the data and prepared the figures and supplementary material; S.L. and J.L.F. drafted the article; all authors edited and approved its final version.
Competing interests
We declare we have no competing interests.
Funding
S.L. was supported by the Swiss National Science Foundation (PP00P3_170669), R.A.d.B. by a Carl Tryggers Postdoctoral Fellowship, and J.L.F by a Knut and Alice Wallenberg Academy Fellowship.
References
- 1.Parker GA. 1970. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 526–567. ( 10.1111/j.1469-185X.1970.tb01176.x) [DOI] [Google Scholar]
- 2.Darwin C. 1871. The descent of Man and selection in relation to Sex. London, UK: John Murray. [Google Scholar]
- 3.Birkhead TR, Møller AP. 1998. Sperm competition and sexual selection. San Diego, CA: Academic Press. [Google Scholar]
- 4.Birkhead TR, Hosken DJ, Pitnick S. 2009. Sperm biology: an evolutionary perspective. San Diego, CA: Academic Press. [Google Scholar]
- 5.Jennions MD, Kokko H. 2010. Sexual selection. In Evolutionary behavioral ecology (eds Westneat DF, Fox CW), pp. 343–364. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Evans JP, Garcia-Gonzalez F. 2016. The total opportunity for sexual selection and the integration of pre- and post-mating episodes of sexual selection in a complex world. J. Evol. Biol. 29, 2338–2361. ( 10.1111/jeb.12960) [DOI] [PubMed] [Google Scholar]
- 7.Parker GA. 2014. The sexual cascade and the rise of pre-ejaculatory (Darwinian) sexual selection, sex roles, and sexual conflict. Cold Spring Harb. Perspect. Biol. 6, a017509 ( 10.1101/cshperspect.a017509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker GA. 1982. Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J. Theor. Biol. 96, 281–294. ( 10.1016/0022-5193(82)90225-9) [DOI] [PubMed] [Google Scholar]
- 9.Parker GA. 1993. Sperm competition games: sperm size and sperm number under adult control. Proc. R. Soc. B 253, 245–254. ( 10.1098/rspb.1993.0110) [DOI] [PubMed] [Google Scholar]
- 10.Parker GA. 1998. Sperm competition and the evolution of ejaculates: towards a theory base. In Sperm competition and sexual selection (eds Birkhead TR, Møller AP), pp. 3–54. London, UK: Academic Press. [Google Scholar]
- 11.Parker GA, Begon ME. 1993. Sperm competition games: sperm size and sperm number under gametic control. Proc. R. Soc. B 253, 255–262. ( 10.1098/rspb.1993.0111) [DOI] [PubMed] [Google Scholar]
- 12.Parker GA, Pizzari T. 2010. Sperm competition and ejaculate economics. Biol. Rev. 85, 897–934. ( 10.1111/j.1469-185X.2010.00140.x) [DOI] [PubMed] [Google Scholar]
- 13.Parker GA, Ball GF. 2005. Sperm competition, mating rate and the evolution of testis and ejaculate sizes: a population model. Biol. Lett. 1, 235–238. ( 10.1098/rsbl.2004.0273) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmons LW, Fitzpatrick JL. 2012. Sperm wars and the evolution of male fertility. Reproduction 144, 519–534. ( 10.1530/REP-12-0285) [DOI] [PubMed] [Google Scholar]
- 15.Vahed K, Parker DJ. 2012. The evolution of large testes: sperm competition or male mating rate? Ethology 118, 107–117. ( 10.1111/j.1439-0310.2011.01991.x) [DOI] [Google Scholar]
- 16.Gage MJG. 1995. Continuous variation in reproductive strategy as an adaptive response to population density in the moth Plodia interpunctella. Proc. R. Soc. B 261, 25–30. ( 10.1098/rspb.1995.0112) [DOI] [Google Scholar]
- 17.Dziminski MA, Roberts JD, Beveridge M, Simmons LW. 2010. Among-population covariation between sperm competition and ejaculate expenditure in frogs. Behav. Ecol. 21, 322–328. ( 10.1093/beheco/arp191) [DOI] [Google Scholar]
- 18.Firman RC, Simmons LW. 2008. The frequency of multiple paternity predicts variation in testes size among island populations of house mice. J. Evol. Biol. 21, 1524–1533. ( 10.1111/j.1420-9101.2008.01612.x) [DOI] [PubMed] [Google Scholar]
- 19.Hosken DJ, Ward PI. 2001. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 4, 10–13. ( 10.1046/j.1461-0248.2001.00198.x) [DOI] [Google Scholar]
- 20.Simmons LW, García-González F. 2008. Evolutionary reduction in testes size and competitive fertilization success in response to the experimental removal of sexual selection in dung beetles. Evolution 62, 2580–2591. ( 10.1111/j.1558-5646.2008.00479.x) [DOI] [PubMed] [Google Scholar]
- 21.Pitnick S, Miller GT, Reagan J, Holland B. 2001. Males' evolutionary responses to experimental removal of sexual selection. Proc. R. Soc. B 268, 1071–1080. ( 10.1098/rspb.2001.1621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simmons LW, Emlen DJ, Tomkins JL. 2007. Sperm competition games between sneaks and guards: a comparative analysis using dimorphic male beetles. Evolution 61, 2684–2692. ( 10.1111/j.1558-5646.2007.00243.x) [DOI] [PubMed] [Google Scholar]
- 23.Montgomerie R, Fitzpatrick JL. 2009. Testis size, sperm size and sperm competition. In Reproductive biology and phylogeny of fishes (ed. Jamieson BGM.), pp. 1–53. Enfield, NH: Science Publishers. [Google Scholar]
- 24.Martin PA, Reimers TJ, Lodge JR, Dziuk PJ. 1974. Effect of ratios and numbers of spermatozoa mixed from two males on proportions of offspring. J. Reprod. Fertil. 39, 251–258. ( 10.1530/jrf.0.0390251) [DOI] [PubMed] [Google Scholar]
- 25.Gage MJG, Morrow EH. 2003. Experimental evidence for the evolution of numerous, tiny sperm via sperm competition. Curr. Biol. 13, 754–757. ( 10.1016/S0960-9822(03)00282-3) [DOI] [PubMed] [Google Scholar]
- 26.García-González F, Simmons LW. 2007. Shorter sperm confer higher competitive fertilization success. Evolution 61, 816–824. ( 10.1111/j.1558-5646.2007.00084.x) [DOI] [PubMed] [Google Scholar]
- 27.Crudgington HS, Fellows S, Badcock NS, Snook RR. 2009. Experimental manipulation of sexual selection promotes greater male mating capacity but does not alter sperm investment. Evolution 63, 926–938. ( 10.1111/j.1558-5646.2008.00601.x) [DOI] [PubMed] [Google Scholar]
- 28.Wigby S, Chapman T. 2004. Sperm competition. Curr. Biol. 14, 100–103. ( 10.1016/j.cub.2004.01.013) [DOI] [PubMed] [Google Scholar]
- 29.Chechi TS, Ali Syed Z, Prasad NG. 2017. Virility does not imply immensity: testis size, accessory gland size and ejaculate depletion pattern do not evolve in response to experimental manipulation of sex ratio in Drosophila melanogaster. J. Insect Physiol. 98, 67–73. ( 10.1016/j.jinsphys.2016.11.012) [DOI] [PubMed] [Google Scholar]
- 30.Pyron M. 2000. Testes mass and reproductive mode of minnows. Behav. Ecol. Sociobiol. 48, 132–136. ( 10.1007/s002650000191) [DOI] [Google Scholar]
- 31.Byrne PG, Roberts JD, Simmons LW. 2002. Sperm competition selects for increased testes mass in Australian frogs. J. Evol. Biol. 15, 347–355. ( 10.1046/j.1420-9101.2002.00409.x) [DOI] [Google Scholar]
- 32.Fitzpatrick JL, Almbro M, Gonzalez-Voyer A, Kolm N, Simmons LW. 2012. Male contest competition and the coevolution of weaponry and testes in pinnipeds. Evolution 66, 3595–3604. ( 10.1111/j.1558-5646.2012.01713.x) [DOI] [PubMed] [Google Scholar]
- 33.Liao WB, Zhong MJ, Lüpold S. 2019. Sperm quality and quantity evolve through different selective processes in the Phasianidae. Sci. Rep. 9, 19278 ( 10.1038/s41598-019-55822-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker GA. 1978. Selection on non-random fusion of gametes during the evolution of anisogamy. J. Theor. Biol. 73, 1–28. ( 10.1016/0022-5193(78)90177-7) [DOI] [PubMed] [Google Scholar]
- 35.Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity 2, 349–368. ( 10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- 36.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of Man 1871–1971 (ed. Campbell B.), pp. 136–179. Chicago, IL: Aldine-Atherton. [Google Scholar]
- 37.Arnold SJ. 1994. Bateman's principles and the measurement of sexual selection in plants and animals. Am. Nat. 144, S126–S149. ( 10.1086/285656) [DOI] [Google Scholar]
- 38.Sivinski J. 1984. Sperm in competition. In Sperm competition and the evolution of animal mating systems (ed. Smith RL.), pp. 85–115. New York, NY: Academic Press. [Google Scholar]
- 39.Bjork A, Pitnick S. 2006. Intensity of sexual selection along the anisogamy–isogamy continuum. Nature 441, 742–745. ( 10.1038/nature04683) [DOI] [PubMed] [Google Scholar]
- 40.Lüpold S, Manier MK, Puniamoorthy N, Schoff C, Starmer WT, Luepold SHB, Belote JM, Pitnick S. 2016. How sexual selection can drive the evolution of costly sperm ornamentation. Nature 533, 535–538. ( 10.1038/nature18005) [DOI] [PubMed] [Google Scholar]
- 41.Dybas LK, Dybas HS. 1981. Coadaptation and taxonomic differentiation of sperm and spermathecae in featherwing beetles. Evolution 35, 168–174. ( 10.1111/j.1558-5646.1981.tb04869.x) [DOI] [PubMed] [Google Scholar]
- 42.Briskie JV, Montgomerie R. 1992. Sperm size and sperm competition in birds. Proc. R. Soc. B 247, 89–95. ( 10.1098/rspb.1992.0013) [DOI] [PubMed] [Google Scholar]
- 43.Morrow EH, Gage MJG. 2000. The evolution of sperm length in moths. Proc. R. Soc. B 267, 307–313. ( 10.1098/rspb.2000.1001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minder AM, Hosken DJ, Ward PI. 2005. Co-evolution of male and female reproductive characters across the Scathophagidae (Diptera). J. Evol. Biol. 18, 60–69. ( 10.1111/j.1420-9101.2004.00799.x) [DOI] [PubMed] [Google Scholar]
- 45.Higginson DM, Miller KB, Segraves KA, Pitnick S. 2012. Female reproductive tract form drives the evolution of complex sperm morphology. Proc. Natl Acad. Sci. USA 109, 4538–4543. ( 10.1073/pnas.1111474109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gomendio M, Roldan ERS. 1991. Sperm competition influences sperm size in mammals. Proc. R. Soc. B 243, 181–185. ( 10.1098/rspb.1991.0029) [DOI] [PubMed] [Google Scholar]
- 47.Fitzpatrick JL, Montgomerie R, Desjardins JK, Stiver KA, Kolm N, Balshine S. 2009. Female promiscuity promotes the evolution of faster sperm in cichlid fishes. Proc. Natl Acad. Sci. USA 106, 1128–1132. ( 10.1073/pnas.0809990106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lüpold S, Calhim S, Immler S, Birkhead TR. 2009. Sperm morphology and sperm velocity in passerine birds. Proc. R. Soc. B 276, 1175–1181. ( 10.1098/rspb.2008.1645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gómez Montoto L, Sánchez MV, Tourmente M, Martín-Coello J, Luque-Larena JJ, Gomendio M, Roldan ERS. 2011. Sperm competition differentially affects swimming velocity and size of spermatozoa from closely related muroid rodents: head first. Reproduction 142, 819–830. ( 10.1530/REP-11-0232) [DOI] [PubMed] [Google Scholar]
- 50.Fitzpatrick JL, Lüpold S. 2014. Sexual selection and the evolution of sperm quality. Mol. Hum. Reprod. 20, 1180–1189. ( 10.1093/molehr/gau067) [DOI] [PubMed] [Google Scholar]
- 51.Pitnick S, Hosken DJ, Birkhead TR. 2009. Sperm morphological diversity. In Sperm biology: an evolutionary perspective (eds Birkhead TR, Hosken DJ, Pitnick S), pp. 69–149. San Diego, CA: Academic Press. [Google Scholar]
- 52.Parker GA, Immler S, Pitnick S, Birkhead TR. 2010. Sperm competition games: Sperm size (mass) and number under raffle and displacement, and the evolution of P2. J. Theor. Biol. 264, 1003–1023. ( 10.1016/j.jtbi.2010.03.003) [DOI] [PubMed] [Google Scholar]
- 53.Moher D. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264 ( 10.7326/0003-4819-151-4-200908180-00135) [DOI] [PubMed] [Google Scholar]
- 54.Viechtbauer W. 2010. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48. ( 10.18637/jss.v036.i03) [DOI] [Google Scholar]
- 55.Nakagawa S, Santos ESA. 2012. Methodological issues and advances in biological meta-analysis. Evol. Ecol. 26, 1253–1274. ( 10.1007/s10682-012-9555-5) [DOI] [Google Scholar]
- 56.Duval S, Tweedie R. 2000. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463. ( 10.1111/j.0006-341X.2000.00455.x) [DOI] [PubMed] [Google Scholar]
- 57.Jennions MD, Møller AP. 2002. Relationships fade with time: a meta-analysis of temporal trends in publication in ecology and evolution. Proc. R. Soc. B 269, 43–48. ( 10.1098/rspb.2001.1832) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakagawa S, Lagisz M, O'Dea RE, Rutkowska J, Yang Y, Noble DWA, Senior AM. 2020. The orchard plot: cultivating a forest plot for use in ecology, evolution, and beyond. Res. Synth. Methods. ( 10.1002/jrsm.1424) [DOI] [PubMed] [Google Scholar]
- 59.Immler S, Pitnick S, Parker GA, Durrant KL, Lüpold S, Calhim S, Birkhead TR. 2011. Resolving variation in the reproductive tradeoff between sperm size and number. Proc. Natl Acad. Sci. USA 108, 5325–5330. ( 10.1073/pnas.1009059108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liao WB, Huang Y, Zeng Y, Zhong MJ, Luo Y, Lüpold S. 2018. Ejaculate evolution in external fertilizers: influenced by sperm competition or sperm limitation? Evolution 72, 4–17. ( 10.1111/evo.13372) [DOI] [PubMed] [Google Scholar]
- 61.Lüpold S, Fitzpatrick JL. 2015. Sperm number trumps sperm size in mammalian ejaculate evolution. Proc. R. Soc. B 282, 20152122 ( 10.1098/rspb.2015.2122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garamszegi LZ. 2014. Modern phylogenetic comparative methods and their application in evolutionary biology. Berlin, Germany: Springer. [Google Scholar]
- 63.Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125, 1–15. ( 10.1086/284325) [DOI] [Google Scholar]
- 64.Collet JM, Dean RF, Worley K, Richardson DS, Pizzari T. 2014. The measure and significance of Bateman's principles. Proc. R. Soc. B 281, 20132973 ( 10.1098/rspb.2013.2973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reuter M, Linklater JR, Lehmann L, Fowler K, Chapman T, Hurst GDD. 2008. Adaptation to experimental alterations of the operational sex ratio in populations of Drosophila melanogaster. Evolution 62, 401–412. ( 10.1111/j.1558-5646.2007.00300.x) [DOI] [PubMed] [Google Scholar]
- 66.Evans JP, Lymbery RA. 2020. Sexual selection after gamete release in broadcast spawning invertebrates. Phil. Trans. R. Soc. B 375, 20200069 ( 10.1098/rstb.2020.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parker GA. 2016. The evolution of expenditure on testes. J. Zool. 298, 3–19. ( 10.1111/jzo.12297) [DOI] [Google Scholar]
- 68.Russell LD, Ren HP, Hikim IS, Schulze W, Hikim APS. 1990. A comparative study in twelve mammalian species of volume densities, volumes, and numerical densities of selected testis components, emphasizing those related to the Sertoli cell. Am. J. Anat. 188, 21–30. ( 10.1002/aja.1001880104) [DOI] [PubMed] [Google Scholar]
- 69.Lüpold S, Linz GM, Rivers JW, Westneat DF, Birkhead TR. 2009. Sperm competition selects beyond relative testes size in birds. Evolution 63, 391–402. ( 10.1111/j.1558-5646.2008.00571.x) [DOI] [PubMed] [Google Scholar]
- 70.Rowe M, Pruett-Jones S. 2011. Sperm competition selects for sperm quantity and quality in the Australian Maluridae. PLoS ONE 6, e15720 ( 10.1371/journal.pone.0015720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gómez Montoto L, Arregui L, Sánchez NM, Gomendio M, Roldan ERS. 2012. Postnatal testicular development in mouse species with different levels of sperm competition. Reproduction 143, 333–346. ( 10.1530/REP-11-0245) [DOI] [PubMed] [Google Scholar]
- 72.Moreira JR, Clarke JR, Macdonald DW. 1997. The testis of capybaras (Hydrochoerus hydrochaeris). J. Mammal. 78, 1096–1100. ( 10.2307/1383052) [DOI] [Google Scholar]
- 73.Wistuba J, Schrod A, Greve B, Hodges JK, Aslam H, Weinbauer GF, Luetjens CM. 2003. Organization of seminiferous epithelium in primates: relationship to spermatogenic efficiency, phylogeny, and mating system. Biol. Reprod. 69, 582–591. ( 10.1095/biolreprod.103.015925) [DOI] [PubMed] [Google Scholar]
- 74.Lüpold S, Wistuba J, Damm OS, Rivers JW, Birkhead TR. 2011. Sperm competition leads to functional adaptations in avian testes to maximize sperm quantity and quality. Reproduction 141, 595–605. ( 10.1530/REP-10-0501) [DOI] [PubMed] [Google Scholar]
- 75.Ramm SA, Stockley P. 2010. Sperm competition and sperm length influence the rate of mammalian spermatogenesis. Biol. Lett. 6, 219–221. ( 10.1098/rsbl.2009.0635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lüpold S, Manier MK, Berben KS, Smith KJ, Daley BD, Buckley SH, Belote JM, Pitnick S. 2012. How multivariate ejaculate traits determine competitive fertilization success in Drosophila melanogaster. Curr. Biol. 22, 1667–1672. ( 10.1016/j.cub.2012.06.059) [DOI] [PubMed] [Google Scholar]
- 77.Manier MK, Belote JM, Berben KS, Lüpold S, Ala-Honkola O, Collins WF, Pitnick S. 2013. Rapid diversification of sperm precedence traits and processes among three sibling Drosophila species. Evolution 67, 2348–2362. ( 10.1111/evo.12117) [DOI] [PubMed] [Google Scholar]
- 78.Joly D, Schiffer M. 2010. Coevolution of male and female reproductive structures in Drosophila. Genetica 138, 105–118. ( 10.1007/s10709-009-9392-9) [DOI] [PubMed] [Google Scholar]
- 79.Rugman-Jones PF, Eady PE. 2008. Co-evolution of male and female reproductive traits across the Bruchidae (Coleoptera). Funct. Ecol. 22, 880–886. ( 10.1111/j.1365-2435.2008.01446.x) [DOI] [Google Scholar]
- 80.Lüpold S, Pitnick S. 2018. Sperm form and function: what do we know about the role of sexual selection? Reproduction 155, R229–R243. ( 10.1530/rep-17-0536) [DOI] [PubMed] [Google Scholar]
- 81.Orr TJ, Brennan PLR. 2015. Sperm storage: distinguishing selective processes and evaluating criteria. Trends Ecol. Evol. 30, 261–272. ( 10.1016/j.tree.2015.03.006) [DOI] [PubMed] [Google Scholar]
- 82.Immler S, Saint-Jalme M, Lesobre L, Sorci G, Roman Y, Birkhead TR. 2007. The evolution of sperm morphometry in pheasants. J. Evol. Biol. 20, 1008–1014. ( 10.1111/j.1420-9101.2007.01302.x) [DOI] [PubMed] [Google Scholar]
- 83.Kleven O, Laskemoen T, Fossøy F, Robertson RJ, Lifjeld JT. 2008. Intraspecific variation in sperm length is negatively related to sperm competition in passerine birds. Evolution 62, 494–499. ( 10.1111/j.1558-5646.2007.00287.x) [DOI] [PubMed] [Google Scholar]
- 84.Sasanami T, Matsuzaki M, Mizushima S, Hiyama G. 2013. Sperm storage in the female reproductive tract in birds. J. Reprod. Dev. 59, 334–338. ( 10.1262/jrd.2013-038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Froman D. 2003. Deduction of a model for sperm storage in the oviduct of the domestic fowl (Gallus domesticus). Biol. Reprod. 69, 248–253. ( 10.1095/biolreprod.102.013482) [DOI] [PubMed] [Google Scholar]
- 86.Immler S, Gonzalez-Voyer A, Birkhead TR. 2012. Distinct evolutionary patterns of morphometric sperm traits in passerine birds. Proc. R. Soc. B 279, 4174–4182. ( 10.1098/rspb.2012.1398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Humphries S, Evans JP, Simmons LW. 2008. Sperm competition: linking form to function. BMC Evol. Biol. 8, 319 ( 10.1186/1471-2148-8-319) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are provided in the electronic supplementary material.