Abstract
Purpose
To optimize the direct production of 68Ga on a cyclotron, via the 68Zn(p,n)68Ga reaction using a liquid cyclotron target. We Investigated the yield of cyclotron-produced 68Ga, extraction of [68Ga]GaCl3 and subsequent [68Ga]Ga-PSMA-11 labeling using an automated synthesis module.
Methods
Irradiations of a 1.0 M solution of [68Zn]Zn(NO3)2 in dilute (0.2–0.3 M) HNO3 were conducted using GE PETtrace cyclotrons and GE 68Ga liquid targets. The proton beam energy was degraded to a nominal 14.3 MeV to minimize the co-production of 67Ga through the 68Zn(p,2n)67Ga reaction without unduly compromising 68Ga yields. We also evaluated the effects of varying beam times (50–75 min) and beam currents (27–40 μA). Crude 68Ga production was measured. The extraction of [68Ga]GaCl3 was performed using a 2 column solid phase method on the GE FASTlab Developer platform. Extracted [68Ga]GaCl3 was used to label [68Ga]Ga-PSMA-11 that was intended for clinical use.
Results
The decay corrected yield of 68Ga at EOB was typically > 3.7 GBq (100 mCi) for a 60 min beam, with irradiations of [68Zn]Zn(NO3)2 at 0.3 M HNO3. Target/chemistry performance was more consistent when compared with 0.2 M HNO3. Radionuclidic purity of 68Ga was typically > 99.8% at EOB and met the requirements specified in the European Pharmacopoeia (< 2% combined 66/67Ga) for a practical clinical product shelf-life. The activity yield of [68Ga]GaCl3 was typically > 50% (~ 1.85 GBq, 50 mCi); yields improved as processes were optimized. Labeling yields for [68Ga]Ga-PSMA-11 were near quantitative (~ 1.67 GBq, 45 mCi) at EOS. Cyclotron produced [68Ga]Ga-PSMA-11 underwent full quality control, stability and sterility testing, and was implemented for human use at the University of Michigan as an Investigational New Drug through the US FDA and also at the Royal Prince Alfred Hospital (RPA).
Conclusion
Direct cyclotron irradiation of a liquid target provides clinically relevant quantities of [68Ga]Ga-PSMA-11 and is a viable alternative to traditional 68Ge/68Ga generators.
Keywords: Gallium-68, Cyclotron targetry, Positron emission tomography, PSMA
Introduction
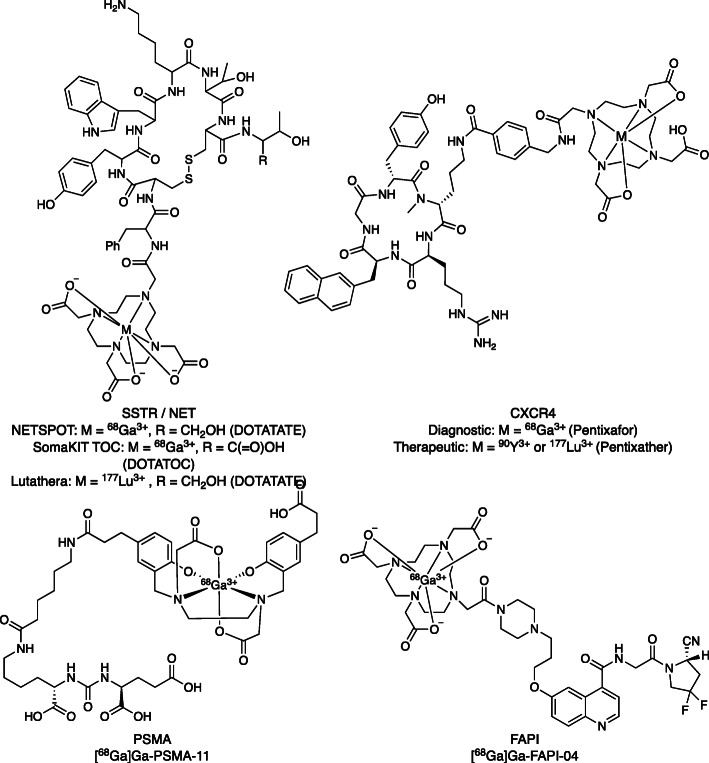
The medicinal use of 68Ga was first described over 4 decades ago albeit with a very small clinical footprint for much of that time (Eder et al. 2014; Graham et al. 2017; Lenzo et al. 2018; Velikyan 2018). Over the past 15 years, there has been a surge in 68Ga radiopharmaceutical development, exceeding that of other radiotracers, with a 100-fold increase in the number of 68Ga publications. Over the last decade, there has also been a marked increase in the clinical use of 68Ga radiotracers that has been attributed to the ease of acquiring 68Ga from 68Ge/68Ga generators and the development and approval of new theranostic tracers (see Fig. 1 for some recent examples) (Baum and Kulkarni 2012). The diagnostic applications of 68Ga vary across jurisdictions/countries, but initial development was driven by development of theranostic agents targeting somatostatin receptors (SSTRs) for the PET imaging (68Ga, 64Cu) and either alpha (213Bi, 225Ac) or beta (90Y, 177Lu) therapy of neuroendocrine tumors (NETs) (Graham et al. 2017; Jackson et al. 2017). In the United States, DOTA-TATE labeled with 68Ga (NETSPOT) and 177Lu (Lutathera) are approved by the U.S. Food and Drug Administration for NET diagnosis and therapy, while the European Union approved [68Ga]Ga-DOTA-TOC (SomaKIT TOC) and Lutathera. Subsequent development of theranostic agents for infection/inflammation (Velikyan 2018), prostate cancer (Eder et al. 2014; Lenzo et al. 2018; Ruangma et al. 2018), C-X-C chemokine receptor type 4 (CXCR4) (Gourni et al. 2011; Herrmann et al. 2016) and, most recently, fibroblast activation protein inhibitors (FAPI) (Kratochwil et al. 2019) is further driving demand and highlights the need for access to a reliable (and economical) supply of 68Ga8 that is the focus of this paper. Analogous development of a reliable pipeline of therapeutic radionuclides is also an urgent need for the nuclear medicine community (Herrmann et al. 2020), but beyond the scope of this article.
Fig. 1.
Theranostic Radiopharmaceuticals
Gallium-68 is usually eluted from a 68Ge/68Ga generator, and thus can be readily implemented in PET facilities that do not own a cyclotron. There are also many additional attributes of 68Ga that make it a desirable PET radionuclide and the first widely available PET radioactive metal ion (radiometal) for routine use globally. 68Ga is a positron emitting (89% β+) radionuclide with a 68 min half-life that, although relatively short, is compatible with distribution. The 68Ga3+ cation is small with an ionic radius of 0.62 Å, which behaves as a relatively hard Lewis acid with an affinity for binding ligands containing oxygen and nitrogen donors, and is suitable for conjugation to various biomolecular vectors using bifunctional chelators and various macromolecules including small molecules with rapid pharmacokinetic profiles, such as peptides and peptidomimetics (Blower et al. 2019; Martiniova et al. 2016; Smith et al. 2013). This synthetic diversity provides the ability for 68Ga kit development.
The most recent main contributor to the expansion of 68Ga-based PET has been imaging of the prostate specific membrane antigen (PSMA) with a host of new radiopharmaceuticals (Ruangma et al. 2018). Of these new agents, [68Ga]Ga-PSMA-11 has been most widely used to date (Hope et al. 2017). Prostate cancer is the second most common cancer found in men in the United States and the second most prevalent cause of cancer death in men (Prostate Cancer: Statistics 2020). Survival rates depend on the type of prostate cancer and the stage at diagnosis. Men with localized disease have a 5-year survival rate of nearly 100%. However, 20–40% of these patients develop biochemical recurrence (BCR) and the recurrent disease can be loco-regional or more widespread. Patients with metastatic disease have a markedly decreased 5-year survival rate of 30% (Prostate Cancer: Statistics 2020). The early and accurate identification of tumor recurrence and metastatic disease is essential for optimal patient management, but this remains a major challenge for traditional imaging methods with anatomical imaging and bone scintigraphy.
The imaging of PSMA expression with [68Ga]Ga-PSMA-11 and PET/CT has proven to be a highly effective and sensitive tool for patient management (Blower et al. 2019). While the primary use of [68Ga]Ga-PSMA-11 has been for detecting recurrent disease, it has also been successful at staging primary prostate cancer, and useful for guiding biopsies to improve sample accuracy, guiding surgery, and monitoring treatment response (Lenzo et al. 2018). There has been a positive clinical impact of [68Ga]Ga-PSMA-11 at the University of Michigan with a change in patient care management in 72% of the scanned patient population. A similar high impact has been reported in a large Australian study that cited a 51% change in care management (62% for BCR patients and 21% for primary staging) (Roach et al. 2018). In 2016 a study from Belgium reported that in patients who underwent a [68Ga]Ga-PSMA-11 scan there was a 76% impact in patient care management (Albisinni et al. 2017). A 2017 study from the University of California San Francisco reported a 53% change in patient management (Hope et al. 2017). Additionally, [68Ga]Ga-PSMA-11 has been used theranostically in conjunction with complementary 177Lu (i.e. β-) or 225Ac (i.e. α) therapeutic PSMA targeting agents. Such PSMA targeted therapies are currently undergoing evaluation in clinical trials in patients with castrate-resistant metastatic prostate cancer (Fendler et al. 2017; Kratochwil et al. 2018).
Since [68Ga]Ga-PSMA-11 has higher accuracy and sensitivity in detecting metastatic disease than [18F]fluorocholine, [11C]choline, and CT (Afshar-Oromieh et al. 2014; Blower et al. 2019; McCormick et al. 2019; Schwenck et al. 2017), a superior detection rate to [18F]fluciclovine (Calais et al. 2018), and overall superior clinical performance (Lenzo et al. 2018; McCormick et al. 2019; Schwenck et al. 2017), it is rapidly becoming the most commonly used radiotracer for prostate cancer management. This presents challenges in meeting the expected demand for the agent once regulatory approval is gained. To put this into context, [11C]choline has been one of the most widely used prostate cancer radiotracers in the US since its FDA approval in 2012 (Evans et al. 2018), and the busiest cancer centers reportedly perform 10–15 [11C]choline scans daily (Lowe and Kwon 2015). It has been possible to service this volume of patients given high yielding [11C]choline syntheses (> 7.4 GBq (200 mCi) /batch) (Shao et al. 2011; Shao et al. 2010), coupled with the ability to run production multiple times per day (limited only by cyclotron and synthesis module availability). Contrastingly, transferring such a patient population to exclusively [68Ga]Ga-PSMA-11 PET is not feasible in a workflow relying entirely on 68Ge/68Ga generators. While 68Ge/68Ga generators offer workflow simplicity for tracer production there are a number of limitations: a) current GMP generators have a maximum activity of 1.85 GBq (50 mCi) and are restricted to elutions every 3–4-h increments, which in practice typically means 2 production runs per day with 2–4 doses per day; b) two or more generators increase the number of patients doses to 6 or more, but still less than the requirements of busy cancer centers; c) commercial supply has not kept pace with the clinical demand and lead times for generator delivery can be up to 18 months in some markets (Cutler and Minoshima 2018); d) the eluted activity constantly declines over time and so to ensure a regular clinical supply of [68Ga]Ga-PSMA-11, multiple sequential and overlapping generators must be purchased throughout the year and; e) there is the potential for long lived parent 68Ge contamination and/or breakthrough. To this end, an additional source of 68Ga needs to be explored and implemented into the clinical setting to meet the current and future patient demand (Cutler and Minoshima 2018).
An attractive alternative to diversifying the supply of 68Ga is the direct production of 68Ga on a cyclotron, via the 68Zn(p,n)68Ga reaction. This alternative approach has garnered significant interest by the community, including publication of a European Pharmacopeia monograph for the direct accelerator-based production of [68Ga]GaCl3 which was published in draft form in 2018 and finalized in 2020 (Gallium (68Ga) chloride (accelerator-produced) solution for radiolabeling 2020) and a technical document published by the IAEA in support of direct production of 68Ga via liquid and solid targets (International Atomic Energy Agency 2019). There are two strategies for producing 68Ga via the 68Zn(p,n)68Ga reaction on a cyclotron - namely, liquid (Alves et al. 2017; do Carmo et al. 2020; International Atomic Energy Agency 2019; Jensen and Clark 2011; Nair et al. 2017; Oehlke et al. 2015; Pandey et al. 2019; Pandey et al. 2014; Pandey and DeGrado 2019) and solid targets (Boschi et al. 2019; Engle et al. 2012; Lin et al. 2018; Sadeghi et al. 2009; Schweinsberg et al. 2019; Tolmachev and Lundqvist 1996; Zeisler et al. 2019). Liquid targets offer implementation simplicity for sites familiar with [18F]FDG production as they present a similar workflow to production of [18F]fluoride and are compatible with laboratory set-ups in existing PET radiopharmaceutical production centers. Solid targets, however, typically impose increased requirements on infrastructure and/or local site expertise but offer more than an order of magnitude higher 68Ga yields (e.g. several GBq/Ci) (Lin et al. 2018; Schweinsberg et al. 2019). Regardless of opting for liquid or solid targets an efficient means for purifying the 68Ga from the irradiated 68Zn is required. The limitations of cyclotron produced 68Ga are obviously: a) a cyclotron with suitable targets, b) the co-production of 67Ga and 66Ga and, c) the potential for residual levels of 68Zn and other metal impurities affecting labeling efficiencies. These factors place stringent demands on the proton energy, the target material and reagent quality, and finally 68Zn/68Ga separation methods.
We present results of the liquid target-based production of 68Ga on GE PETtrace cyclotrons, with focus on yield of 68Ga and extraction of [68Ga]GaCl3 using the GE FASTlab Developer platform. Furthermore, to demonstrate the clinical relevance of this direct production method, a single FASTlab cassette was used to perform the 68Zn/68Ga purification and subsequent labeling of [68Ga]Ga-PSMA-11. The cyclotron produced [68Ga]Ga-PSMA-11 underwent full quality control, stability and sterility testing, and has been used in humans at the University of Michigan (UM, Ann Arbor Mi, USA) under the FDA’s Investigational New Drug (IND) program, and at Royal Prince Alfred Hospital (RPA, Sydney, Australia) under exemption of the Therapeutic Goods Act (TGA) in a TGA GMP-licensed facility. The results from UM, GEMS (GE Healthcare Uppsala, Sweden) and RPA are presented.
Materials and methods
Liquid target irradiations
The GE 68Ga PETtrace Liquid Target (Fig. 2) is a water-cooled, gridded target without requiring He cooling of foils, designed specifically for 68Ga production. The target comprises a 200 μm thick aluminum energy degrader, a 25 μm Havar foil for support, and a 25 μm niobium foil for chemical inertness with the target media, thus rendering a nominal 14.3 MeV incident proton energy on the target media. Including the target lines/dead volume, the total target fill volume is approximately 2.2 mL.
Fig. 2.
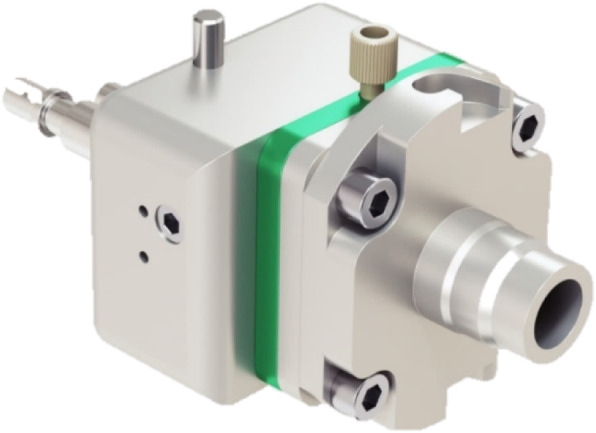
GE Gallium-68 Liquid Target
The target media was prepared from isotopically enriched [68Zn]ZnO (Isoflex, USA) with addition of water (Ultrapur or 18 MΩ-cm) and 70% nitric acid (> 99.999% trace metal basis) to yield a 1.0 M solution of [68Zn]Zn(NO3)2 with an excess 0.2 M or 0.3 M HNO3 (both concentrations tested). All irradiations at UM and GEMS and the majority of irradiations at RPA employed the same lot of enriched 68Zn – namely: 64Zn (0.03%), 66Zn (0.16%), 67Zn (0.62%), 68Zn (99.16%), and 70Zn (0.03%), and from a chemical perspective, comprised 1 ppm iron. Recent irradiations at RPA used a different lot of enriched 68Zn – namely: 64Zn (0.1%), 66Zn (0.18%), 67Zn (0.96%), 68Zn (98.20%), and 70Zn (0.56%), and 3.1 ppm iron.
Irradiations were performed on GE PETtrace cyclotrons using the 68Ga Liquid Target and were typically 50–70 min in duration with beam currents of ~ 30–40 μA. Whenever possible, within the routine daily production schedule, a “cleaning” irradiation at 30–35 μA of typically 10–60 min was performed with dilute nitric acid (0.6 M) after irradiation of the [68Zn]Zn(NO3)2 solution.
Chemical isolation on the FASTlab
Delivery to the FASTlab
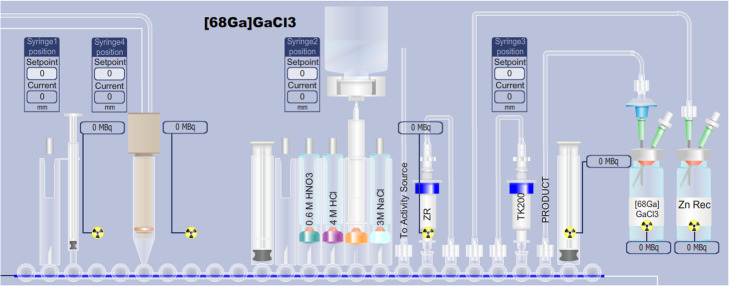
To facilitate use of the same FASTlab for both 68Ga and 18F processing and the dilution of the delivered 68Ga target solution, the irradiated target media was delivered from the cyclotron into an external 10 mL V-vial with connections to the FASTlab (Fig. 3). Thus, delivery of 68Ga target material completely bypasses the incoming activity plunger of the FASTlab module avoiding potential cross contamination between 18F and 68Ga target deliveries when the module is used for both types of targets. In this activity receiving vial, the 68Ga target solution was automatically diluted with water from the synthesis unit to achieve a nitric acid concentration of < 0.1 M required for subsequent processing. The diluted target solution is automatically loaded onto the cassette by nitrogen overpressure.
Fig. 3.
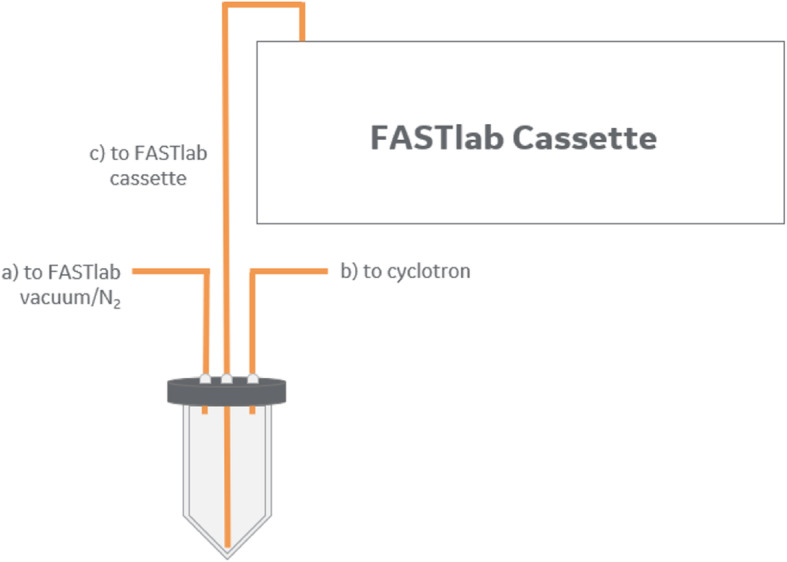
External vial for collection of the irradiated 68Zn solution
Chemical isolation of [68Ga]GaCl3
A primary goal of this effort was to develop a FASTlab cassette which allowed for [68Ga]GaCl3 extraction in a formulation comparable with existing generators. Additionally, on-line column conditioning, the use of minimum quantities of acid, and the exclusion of organic solvents or base-mediated pH adjustments were desired. Chemical isolation of [68Ga]GaCl3 was implemented on the GE FASTlab Developer platform. The process is based on the 2-column approach we have previously reported for liquid targets (Nair et al. 2017) and recently repeated by Riga and colleagues (Riga et al. 2018). The process is shown in Fig. 4. Initial separation of 68Ga from 68Zn is performed by trapping the 68Ga on a hydroxamate-based resin (ZR resin, Triskem) cartridge. Further purification, concentration and acid reduction is realized by using a TOPO-based resin (TK200 resin, Triskem) cartridge.
Fig. 4.
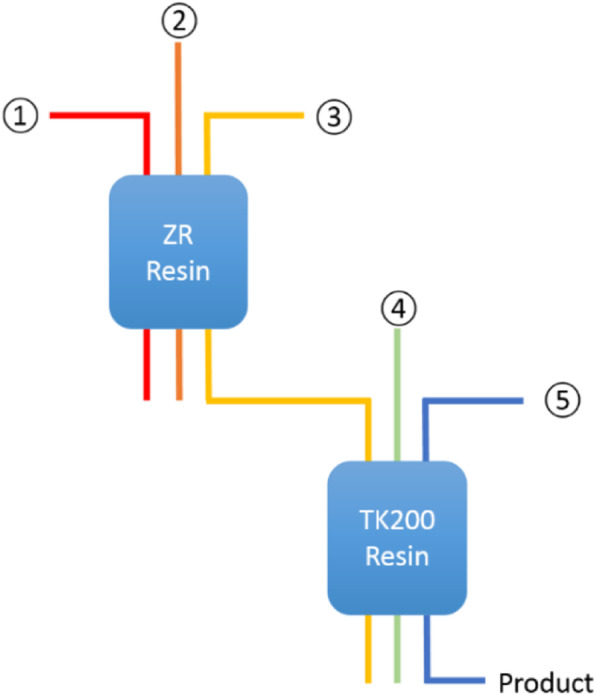
Two-column approach for 68Ga chemical separation
In our initial efforts, elution of the TK200 resin with water (Scheme A in Table 1) resulted in a [68Ga]GaCl3 solution containing approximately 0.6 M HCl due to residual HCl content in the cartridge. Implementation of a NaCl/HCl rinse (Mueller et al. 2012) for reduction of residual acid achieved a final [68Ga]GaCl3 formulation of 0.1 M HCl in 5 mL (Scheme B in Table 1). This formulation is directly comparable to commercially available 68Ge/68Ga generators and is compatible with formulations required for pharmaceutical cold kit labeling.
Table 1.
High level schemes of [68Ga]GaCl3 purifications
| Scheme A* | Scheme B | |
|---|---|---|
 ZR Load ZR Load |
< 0.1 M HNO3 | |
 ZR Wash ZR Wash |
15 mL 0.1 M HNO3 | |
 ZR Elution / Trapping on TK200 ZR Elution / Trapping on TK200 |
5–6 mL ~ 1.75 M HCl | |
 TK Wash TK Wash |
– | 3.5 mL 2.0 M NaCl in 0.13 M HCl |
 TK Elution TK Elution |
H2O | 1–2 mL H2O followed by dilute HCl to formulate |
*Process as reported previously (Nair et al. 2017)
Process steps:
-
0.
Condition ZR cartridge with 0.1 M HNO3 (7 mL) and TK200 resin with sterile water for injection (7 mL) followed by 1.75 M HCl (4 mL) prior to use.
-
1.
Trapping of 68Ga on a hydroxamate-based resin (2 mL [~ 700 mg] ZR resin, Triskem)
-
2.
Rinsing of the resin to remove residual zinc
-
3.
Elution onto a TOPO-based resin (2 mL [~ 700 mg] TK200 resin, Triskem)
-
4.
Wash to decrease residual acid content, and
-
5.
Final elution with water and dilute hydrochloric acid, volumes of which can be varied, to yield [68Ga]GaCl3 in the desired formulation (e.g. 5 mL of 0.1 M HCl)
The process was optimized over time (with regards to flow rates, volumes, cassette rinsing, etc), thus not all runs were identical with regards to time lists on the FASTlab. Nevertheless, the chemical process can be categorized into two primary schemes (as noted in Table 1). Building on Scheme A, Scheme B includes a wash step of the TK200 resin in order to reduce the residual acid content in the [68Ga]GaCl3 eluate. The purification time is approximately 30 min.
In comparison to recent literature, this method requires less acid and does not involve organic solvents or base-mediated pH adjustments, which is highlighted in Table 2.
Table 2.
Comparison of FASTlab [68Ga]GaCl3 purification vs. recent literature
| Reference | HNO3a [mmol] | HCl [mmol] | Organic solvents | Base-mediated pH adjustment? |
|---|---|---|---|---|
| This work | 2.4 | 16 | No | No |
| (Oehlke et al. 2015) | – | 886 | Yes (Methanol) | No |
| (Alves et al. 2018) | – | 265 | Yes (HBr/acetone) | No |
| (Pandey and DeGrado 2019) | 0.25 | 38 | Yes (Acetonitrile) | Yes |
aDoes not account for HNO3 in the liquid target
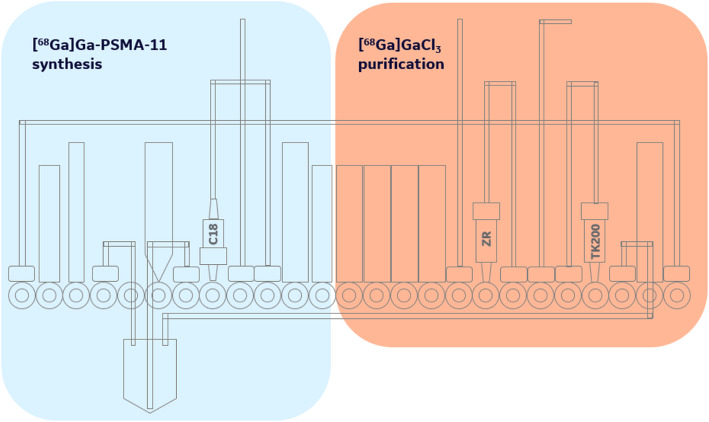
The cassette layout for the automated [68Ga]GaCl3 separation on the FASTlab is given in Fig. 5, noting that the [68Ga]GaCl3 chemistry is reserved to the right-hand side of the cassette. The left-hand side was kept vacant to enable subsequent on-cassette labeling (e.g. PSMA, NET tracers, etc), including C18 cartridge purification (Fig. 6). The line labeled “to activity source” is connected to the activity receiving vial (Fig. 3). Where applicable during the process, the vials of 0.6 M HNO3, 4 M HCl, and 3 M NaCl were automatically diluted and/or mixed to the desired concentrations by the FASTlab.
Fig. 5.
FASTlab cassette layout – applicable to Schemes “A&B” of Table 1
Fig. 6.
Partitioning of FASTlab cassette: Right-hand side is reserved for [68Ga]GaCl3 purification, and the left-hand side accommodates the [68Ga]Ga-PSMA-11 synthesis and C18 cartridge based purification
In advance of receipt of the activity the columns were automatically conditioned on the FASTlab, the ZR resin was conditioned with 0.1 M HNO3 (7 mL) and the TK200 was conditioned with both water (7 mL) followed by 1.75 M HCl (4 mL). Recycling of 68Zn is not presently being performed given the current availability and cost of 68Zn (approximately US$100 per target fill), however, the 68Zn solution is collected separately to facilitate future recycling.
Synthesis of [68Ga]Ga-PSMA-11
The direct cyclotron-based production of [68Ga]Ga-PSMA-11 was executed at the UM, GEMS and RPA using a single FASTlab cassette in a continuous process to perform both the [68Ga]GaCl3 isolation chemistry and subsequent PSMA-11 labeling, including C18 purification. Cassettes were prepared at each institution based on the FASTlab developer cassettes and accessories.
Initial labeling tests (UM) employed [68Ga]GaCl3 separation scheme “A”, with 10 μg PSMA-11 precursor in 1.5 M Hepes (1 mL) and 3 M NaOAc (1.3 mL) buffer. Scheme “B” developed and implemented at GEMS and RPA used 10 μg PSMA-11 precursor in 1.5 mL/1.0 M NaOAc (GEMS) or 1.3 mL/1.5 M NaOAc buffer (RPA) adjusted to pH 4.5–4.8. Approximately 3–4 mg L-ascorbic acid was also added (GEMS/RPA) to the precursor vial to minimize radiolysis during synthesis. An additional 20–21 mg of L-ascorbic acid (0.44 mL; 0.25 M) is also added directly into the product line at RPA as stabilizer of the final product. Labeling occurred for 5 min at 50 °C. At UM and RPA, the final product was reformulated into Phosphate Buffered Saline (PBS) using a C18 cartridge (preconditioned with 1.2 mL EtOH followed by 4.5 mL sterile water for injection). Briefly, at UM the crude reaction mixture was passed through the C18 cartridge to trap [68Ga]Ga-PSMA-11. The reaction vessel was rinsed with water (2.4 mL) and the rinse passed over the C18 cartridge. [68Ga]Ga-PSMA-11 was eluted from the C18 cartridge with EtOH/H2O (1:1 v:v, 2 mL), sterile filtered (Millipore Cathivex-GV, 0.22 μm) and diluted with PBS solution (14.5 mL) to give the final formulated product that was submitted for quality control testing.
Quality control of [68Ga]Ga-PSMA-11
Quality control testing of [68Ga]Ga-PSMA-11 doses was conducted according to the guidelines outlined in the U.S. and European Pharmacopeias using standard methods (visual inspection, pH, radionuclidic identity, sterile filter integrity, bacterial endotoxin analysis, and sterility testing) previously described (Mossine et al. 2020), as well as procedures specific for [68Ga]Ga-PSMA-11 and described below.
Representative TLC analysis of [68Ga]Ga-PSMA-11
At UM, radiochemical purity was determined by thin layer chromatography (TLC) using silica-backed 7.5 cm glass or paper chromatography plates and an Eckert and Ziegler AR-2000 TLC scanner. The plate was spotted with a sample of [68Ga]Ga-PSMA-11 product. The developing solution (mobile phase), 77 g/L ammonium acetate in a 50/50 water/methanol. The plate was developed in this solvent system, dried using a warm laboratory hotplate, and placed on the TLC scanner to determine radiochemical purity, which must be ≥90%.
Representative radio-HPLC analysis of [68Ga]Ga-PSMA-11
At UM, chemical and radiochemical purities/identities were analyzed using a Shimadzu LC2010 HPLC equipped with a radioactivity detector and an ultraviolet (UV) detector (column: Phenomenex C18(2) 250 × 4.6 mm column; mobile phase A: 0.1% trifluoroacetic acid in MeCN; mobile phase B: 0.1% trifluoroacetic acid in milli-Q water; Gradient: 0 min A:B = 5:95–10 min A:B = 30:70–11 min A:B = 30:70–11.1 min A:B = 5:95–15 min A:B = 5:95; flow rate: 1.0 mL/min; UV: 205 nm). On this QC HPLC system, [68Ga]Ga-PSMA-11 has a retention time of ~ 11 min. Radiochemical purity for doses was confirmed to be > 90%, and identity was confirmed by comparing the retention time of the radiolabelled product with that of the corresponding unlabelled reference standard (40 μg/mL).
Representative Gallium-66 and Gallium-67 concentration (Radionuclidic purity)
At UM, cyclotron-produced [68Ga]Ga-PSMA-11 was tested for 66Ga and 67Ga weekly to determine the fitness of the target produced 68Ga for use in production of clinical doses of [68Ga]Ga-PSMA-11 for the following week. A small sample of [68Ga]Ga-PSMA-11 was assayed via a dose calibrator to determine an initial radioactivity profile. The sample was then stored in a shielded environment for 24–72 h to allow 68Ga to decay to near-zero, and the sample analyzed by gamma spectroscopy. Decay-corrected values for the amount of 66Ga and 67Ga were determined and used in conjunction with the initial radioactivity profile value to determine the relative amount of both radionuclides in the final product. The recent EU Monograph on cyclotron-produced requires ≤2% combined 66Ga and 67Ga in doses until the end of the shelf-life.
Results and discussion
68Ga yields
Total 68Ga yields from the target were assessed by: (a) downloading the total irradiated target contents into a vial placed in a dose calibrator without chemical purification and ensuring suitable decay time (90–120 min) or curve fitting to avoid any 13N contribution, or (b) measurement of residual activity of cassette components and product post [68Ga]GaCl3 isolation or post [68Ga]Ga-PSMA-11 labeling chemistry. For the data presented at GEMS, this includes an early series of 9 consecutive 60-min irradiations from 30 to 40 μA (entire target contents), and 20 consecutive irradiations (post-chemistry) following a target rebuild.
Radioactivity yields exceeding 100 mCi (3.7 GBq) at EOB are typical (see Table 3) with irradiation of [68Zn]Zn(NO3)2 at 0.3 M HNO3 yielding more consistent target/chemistry performance. Albeit higher acid concentrations have been reported in the literature (Pandey et al. 2019), we opted to maintain the excess nitric acid as low as possible to minimize corrosive wear on components and facilitate the subsequent chemistry (which requires < 0.1 M HNO3 for ZR resin loading).
Table 3.
Summary of 68Ga productions and total 68Ga radioactivity yield at EOB
| Site | HNO3 [M] |
N | I [uA] |
Beam time [min] |
EOB activity [GBq] |
EOB activity [mCi] | Measurement |
|---|---|---|---|---|---|---|---|
| UM | 0.2 | 13 | 30 | 60 | 4.1 ± 0.6 | 112 ± 16 | Entire target contents |
| 0.2 | 6 | 35 | 60 | 3.9 ± 0.6 | 106 ± 17 | Entire target contents | |
| 0.2 | 6 | 40 | 60 | 3.8 ± 0.4 | 102 ± 11 | Entire target contents | |
| 0.3 | 12 | 34 ± 4 | 60 | 4.6 ± 0.4 | 126 ± 12 | Entire target contents | |
| GEMS | 0.2 | 9 | 36 ± 5 | 60 | 4.5 ± 0.3 | 120 ± 9 | Entire target contents |
| 0.2 | 14 | 30 | 69 ± 7 | 3.5 ± 0.9 | 94 ± 24 | Ʃ of parts post chemistry | |
| 0.3 | 6 | 29 ± 1 | 70 ± 13 | 4.3 ± 0.5 | 115 ± 14 | Ʃ of parts post chemistry | |
| RPA | 0.3 | 25 | 36 ± 2.2 | 60 | 4.0 ± 0.6 | 107 ± 17 | Entire target contents |
| 0.3 | 53 | 35 | 60 | 3.8 ± 0.5 | 104 ± 14 | Ʃ of parts post chemistry |
While it is theoretically possible to increase the target yields by increasing the 68Zn concentration, the 1.0 M solution used here facilitates transfer to the hot cell (i.e. the solution is not too viscous). Should multi-Ci yields of 68Ga be desired, adoption of the proposed method to solid targets as has been reported previously by taking advantage of 68Ga trapping on ZR resin in high HCl concentration loading conditions (Schweinsberg et al. 2019).
[68Ga]GaCl3, [68Ga]Ga-PSMA-11 – yields and quality
Several hundred irradiations and purifications/labelings have been performed throughout the development efforts, however, for sake of brevity, we report herein on several representative subsets of experimental data. These data are summarized in Tables 4, 5 and 6.
Table 4.
High-level summary of 68Ga runs reported herein for UM, GEMS and RPA
| Site | N | comment | |
|---|---|---|---|
| [68Ga]GaCl3 | UM | 27 | 60 min beam current |
| GEMS | 13 | Consecutive productions, 0.2 or 0.3 M HNO3 | |
| RPA | 20 | 60 min 35 μA beam, 0.3 M HNO3 | |
| [68Ga]Ga-PSMA-11 | UM | 3 + 35 | Validation + clinical |
| GEMS | 3 | Consecutive productions | |
| RPA | 8 | Validation + clinical |
Table 5.
Overview of [68Ga]GaCl3 productions (EOS)
| Site | Chemistry Scheme |
HNO3 [mol/L] |
I [μA] |
Beam time [min] |
N | Product activity | |
|---|---|---|---|---|---|---|---|
| [GBq] | [mCi] | ||||||
| UM | A | 0.2 | 30 | 60 | 15 | 2.0 ± 0.3 | 54 ± 8 |
| 35 | 6 | 2.0 ± 0.3 | 55 ± 8 | ||||
| 40 | 6 | 1.9 ± 0.2 | 50 ± 5 | ||||
| GEMS | B | 0.2 | 30 | 64 ± 6 | 10 | 1.7 ± 0.5 | 46 ± 13 |
| 0.3 | 29 ± 1 | 73 ± 6 | 3 | 2.5 ± 0.1 | 67 ± 3 | ||
| RPA | B | 0.3 | 35 | 60 | 20 | 2.0 ± 0.2 | 55 ± 6 |
Table 6.
Overview of [68Ga]Ga-PSMA-11 productions (EOS)
| Site | HNO3 [mol/L] | I [μA] |
Beam time [min] |
N | Product activity | Notes | |
|---|---|---|---|---|---|---|---|
| [GBq] | [mCi] | ||||||
| UM | 0.2 | 30–40 | 60 | 3 | 1.6 ± 0.3 | 43 ± 9 | Validation runs |
| UM | 0.2 | 30–40 | 60 | 35 | 1.7 ± 0.2 | 45 ± 6 | Clinical |
| GEMS | 0.3 | 30 | 64 ± 4 | 3 | 2.1 ± 0.4 | 57 ± 10 | R&D efforts |
| RPA | 0.3 | 35 | 60 | 14 | 1.6 ± 0.1 | 44 ± 3 | Final validation and clinical (5) runs |
Table 5 clearly demonstrates a robust routine production of ~ 1.85 GBq (~ 50 mCi) of [68Ga]GaCl3 via the liquid target cyclotron route. This compares favorably with approximately ~ 1.48 GBq (~ 40 mCi) of [68Ga]GaCl3 from a brand new, highest commercially available activity GMP generator with ~ 1.85 GBq (~ 50 mCi) of 68Ge. Furthermore, the eluted 68Ga activity steadily decreases over time due to the decay of the 68Ge.
[68Ga]Ga-PSMA-11 activity yields at EOS varied slightly across sites (Table 6) which may be at least partly attributed to beam parameters, state of target and slightly different labeling conditions used. At RPA, 3 patients can be readily scanned from a single batch of [68Ga]Ga-PSMA-11 using 2 scanners, which is the same number of patients which can be scanned with [68Ga]Ga-PSMA-11 produced from 2 staggered 68Ge/68Ga generators. As more than 2 productions runs can potentially be performed with the target, the number of patients able to be scanned per day is potentially increased.
Although activity yield is an important parameter to measure process performance, the quality of the cyclotron-produced [68Ga]GaCl3 is of even greater importance as high quality [68Ga]GaCl3 is critical to enable efficient labeling. Therefore, in addition to yield measurement and periodic quality control (QC) assessment, validation studies for [68Ga]GaCl3 and [68Ga]Ga-PSMA-11 were carried out at UM and for [68Ga]Ga-PSMA-11 at RPA. The test methods performed (half-life, radiochemical purity, pH, radionuclidic purity, metal analysis) were in accordance with the Ph. Eur. monograph for [68Ga]GaCl3 (Gallium (68Ga) chloride (accelerator-produced) solution for radiolabeling 2020) and are described in Section 2.2.4, with the exception of testing the Fe and Zn content, for which semi-quantitative colorimetric test strips (e.g. EM-Quant, Merck) and/or ICP-MS were used.
After we were confident with the performance of both the target and synthesis methods, we moved forward and conducted validation runs for both [68Ga]GaCl3 and [68Ga]Ga-PSMA-11. Table 7 reports the QC results for the [68Ga]GaCl3 validation runs carried out at UM, and all of the validation runs met or exceeded the established criteria in the European Pharmacopoeia (Gallium (68Ga) chloride (accelerator-produced) solution for radiolabeling 2020). Subsequently, validation of [68Ga]Ga-PSMA-11 was also undertaken. QC testing results for [68Ga]Ga-PSMA-11 are shown in Table 8 (UM) and Table 9 (RPA), with RCP assessment by radio-TLC and HPLC (see Section 2.2.4). Endotoxin, 4-h stability (data not shown), and sterility testing were also performed for the three validation runs. The 3 validation runs met (or exceeded) all QC criteria at end-of-synthesis and at the 4-h stability time point.
Table 7.
Quality Control Data for three [68Ga]GaCl3 validation runs (UM)a
| TEST | 1 | 2 | 3 | Avg & SD | Release Criteria (Ph. Eur.) |
|---|---|---|---|---|---|
| Radiochemical Purity [68Ga]GaCl3 (iTLC-SG) | 99 | 98 | 98 | 98.3 ± 0.3 | ≥ 95 |
| Rf [68Ga]GaCl3 (TLC) | < 0.2 | < 0.2 | < 0.2 | < 0.2 | ≤ 0.2 |
| Rf Ref Bb (TLC) | > 0.7 | > 0.7 | > 0.7 | > 0.7 | ≥ 0.7 |
| pH | < 2 | < 2 | < 2 | < 2 | < 2 |
| Visual Inspection | Passed | Passed | Passed | N/A | Clear, colorless, no visible particulate |
| Radionuclidic Identity (t½) | 67.2 | 68.8 | 69.1 | 68.4 ± 0.8 | 64.6–71.4 min |
| Endotoxin Analysis | < 2 | < 2 | < 2 | < 2 | ≤ 58.3 EU/mL |
| Fe μg/GBq | < 5 | < 5 | < 5 | < 5 | ≤ 10 μg/GBq |
| Zn μg/GBq | < 1.25 | < 1.25 | < 1.25 | < 1.25 | ≤ 10 μg/GBq |
| RNP at EOB (MCA) | 99.8 | 99.8 | 99.8 | 99.8 | ≥ 98% (at shelf-life) |
aAfter FASTLab isolation; b Reference solution B (Pentetic acid solution) from the European Pharmacopoeia (Gallium (68Ga) chloride (accelerator-produced) solution for radiolabeling 2020)
Table 8.
Quality Control Data for three [68Ga]Ga-PSMA-11 validation runs (UM)
| Tests | 1 | 2 | 3 | Avg & SD | Release Criteria (UM) |
|---|---|---|---|---|---|
| Radiochemical Purity (via TLC) | 99.5 | 99.4 | 99.3 | 99.4 ± 0.1 | ≥ 90% |
| Relative Retention time (via HPLC) | 1.004 | 1.005 | 1.005 | 1.0046 ± 0.0003 | RRT: 0.9–1.1 |
| pH | 7.0 | 7.0 | 7.0 | 7.0 | 4.0–8.0 |
| Visual Inspection | Passed | Passed | Passed | N/A | Clear, colorless, no visible particulate |
| Radionuclidic Identity (t½) | 67.61 | 68.45 | 67.20 | 67.75 ± 0.53 | 64.6–71.4 min |
| Endotoxin Analysis | < 2 | < 2 | < 2 | < 2 | ≤ 10.9 EU/mL |
| Bubble Point (PSI) | 51 | 52 | 53 | 52 ± 1 | ≥ 50 PSI |
| Sterility | Passed | Passed | Passed | Passed | Complies with USP< 71>a |
| RNP at EOB (MCA) | 99.8 | 99.8 | 99.8 | 99.8 | ≥ 98% (at time of use) |
aSee: USP 71 Microbiological Tests/Sterility Tests 2012
Table 9.
Quality Control Data for three [68Ga]Ga-PSMA-11 validation runs (RPA)
| Tests | 1 | 2 | 3 | Avg & SD | Release Criteria (RPA) |
|---|---|---|---|---|---|
| Radiochemical Purity (via TLC) | 99.94 | 99.99 | 99.94 | 99.96 ± 0.03 | ≥ 95% |
| Radiochemical Purity (via HPLC) | 99.94 | 99.97 | 100.0 | 99.97 ± 0.03 | ≥ 95% |
| pH | 5.0 | 5.5 | 5.5 | 5.3 ± 0.3 | 4.0–8.0 |
| Visual Inspection | Passed | Passed | Passed | N/A | Clear, colorless, no visible particulate |
| Radionuclidic Identity (t½) | 67.9 | 68.1 | 67.7 | 67.9 ± 0.20 | 62–74 min |
| Endotoxin Analysis | < 1 | < 1 | < 1 | < 1 | ≤ 17.5 EU/mL |
| Bubble Point (bar) | 4.1 | 4.2 | 4.1 | 4.1 ± 0.06 | ≥ 3.5 bar |
| Sterility | Passed | Passed | Passed | Passed | Sterile – no growth |
| RNP at EOS (Well Counter) | 99.7 | 99.8 | 99.8 | 99.8 ± 0.06 | ≥ 98% (at time of use) |
The validation data shown here demonstrates the high quality of cyclotron-produced [68Ga]GaCl3 and [68Ga]Ga-PSMA-11, and highlights the reliability and reproducibility of both processes. Notably, the reported RNP satisfied the proposed EU Pharmacopoeia limits (≤2% of combined 66Ga + 67Ga for the shelf life of the product) (Gallium (68Ga) chloride (accelerator-produced) solution for radiolabeling 2020). Setting this criteria for duration of shelf life and the varying amounts of 66Ga + 67Ga in each batch mean that the shelf-life will vary batch to batch. In this work the RNP was typically 0.2% at EOS. This allows determination of the shelf life to be approximately 3.5 h, after which time the amount of 66Ga + 67Ga will exceed 2% due to radioactive decay of shorter-lived 68Ga. The dosimetry of the 2% 66Ga + 67Ga limit has been previously reported using worst-case assumptions, such as no biological clearance and rapid organ uptake (Graves et al. 2018). For this scenario, a relative dose increase up to 20% is reported but is typically less than 10% when compared to “pure” 68Ga (i.e. not comparing with generator 68Ga which may contain 68Ge). Overall, the obtained results provided a solid basis for the clinical evaluation of cyclotron-produced [68Ga]Ga-PSMA-11.
Clinical production and use of [68Ga]Ga-PSMA-11
The necessary FDA approval was obtained to translate cyclotron-based [68Ga]Ga-PSMA-11 into clinical use at UM and evaluation began in 2019. At RPA, cyclotron-based 68Ga used for clinical [68Ga]Ga-PSMA-11 production began in 2020. The final manufacturing process takes ~ 2 h from start of irradiation to release of the dose to the clinic. For example, the process at UM proceeds as follows:
Cyclotron irradiation (60 min)
[68Ga]Ga-PSMA-11 synthesis (35 min)
Quality control testing (25 min)
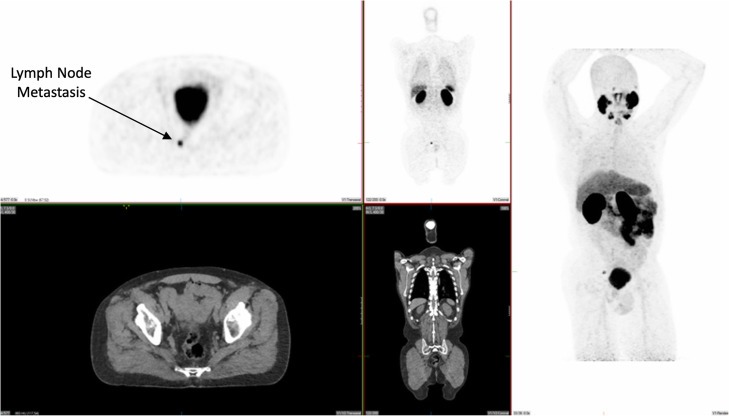
To date, over 700 patients have been scanned with [68Ga]Ga-PSMA-11 at UM under our IND approval. Initially, this was with generator-based [68Ga]Ga-PSMA-11. We amended the IND to include cyclotron-based [68Ga]Ga-PSMA-11, and the first clinical production of cyclotron-based [68Ga]Ga-PSMA-11 from a single FASTlab cassette occurred in February 2019. As of March 2020, 50 clinical batches of cyclotron-produced [68Ga]Ga-PSMA-11 have been manufactured and used to scan more than 90 patients (see Table 6). A representative image from the first patient scanned with cyclotron-based [68Ga]Ga-PSMA-11 is shown in Fig. 7. We have noted no differences in the quality of studies where 68Ga was produced from a cyclotron when compared to studies using 68Ga obtained from a generator, and no pharmacological or physiological changes have been observed after intravenous administration of either generator-based or cyclotron-based [68Ga]Ga-PSMA-11.
Fig. 7.
Images from the first patient scanned with [68Ga]Ga-PSMA-11 labeled with cyclotron produced 68Ga at the University of Michigan
Conclusions and outlook
A process for isolating high purity [68Ga]GaCl3 from cyclotron-produced 68Ga and subsequent labeling of PSMA-11 on the GE FASTlab synthesizer with both steps being performed on a single cassette has been developed. The cyclotron-based method offers a reliable source of 68Ga and delivers consistently higher yields than currently available commercial 1.85 GBq (50 mCi) 68Ge/68Ga generators. Furthermore, in contrast to generators, for which 68Ga activity falls over time due to 68Ge decay, cyclotron-based 68Ga activity is consistent with time thereby simplifying patient scheduling. The FASTlab-derived [68Ga]GaCl3 solution for radiolabeling met the requirements in the European Pharmacopeia with the purity of reagents and 68Zn enrichment and purity used at these sites, and validation of [68Ga]GaCl3 and [68Ga]Ga-PSMA-11 for clinical application has been demonstrated by the UM and RPA. The total manufacturing time is approximately 2 h and over 90 patients have been scanned using cyclotron-based [68Ga]Ga-PSMA-11 to date. The process is in routine use to meet the growing demands for PSMA-based PET imaging at UM, and the first clinical studies have also been conducted at RPA. Additional studies to broaden the applicability of the [68Ga]GaCl3 process for labeling with other commonly used chelators such as DOTA have been performed successfully at RPA, and clinical use of [68Ga]Ga-DOTA-TATE labeled with cyclotron-produced 68Ga is ongoing. Similar studies are also currently ongoing at other sites.
Acknowledgements
The authors thank Dr. Ali Afshar-Oromieh MD PhD, Prof. Dr. Klaus Kopka PhD, and their colleagues at the University Hospital of Heidelberg and the German Cancer Research Center (DKFZ) Heidelberg for the valuable assistance in qualifying the University of Michigan for clinical production of [68Ga]Ga-PSMA-11. In addition, the authors thank Steffen Happel at Triskem for valuable suggestions, feedback, and initial resin samples.
Authors’ contributions
MER, MC, BGH and BDH conducted experimental work at UM. CS, DCP, JF and KG conducted experimental work at GE. AK, DS, MJF and SE conducted experimental work at RPA. MA-G and MRP oversaw clinical studies at UM. SE, KG and PJHS had supervisory and project management responsibility. The authors read and approved the final manuscript.
Funding
PJHS acknowledges financial support from Michigan Medicine.
Ethics approval
This article does not contain any original studies with human or animal subjects performed by any of the authors.
Competing interests
KG, JF, DCP, and CS are employees of GE Healthcare. SE, AK, DS, MJF, MER, MC, BGH, BDH, MA-G, MRP and PJHS declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Stefan Eberl, Email: stefan.eberl@sydney.edu.au.
Katherine Gagnon, Email: katherine.gagnon@ge.com.
Peter J. H. Scott, Email: pjhscott@umich.edu
References
- Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. doi: 10.1007/s00259-013-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albisinni S, Artigas C, Aoun F, Biaou I, Grosman J, Gil T, et al. Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: preliminary analysis. BJU Int. 2017;120:197–203. doi: 10.1111/bju.13739. [DOI] [PubMed] [Google Scholar]
- Alves F, Alves VHP, Do Carmo SJC, Neves ACB, Silva M, Abrunhosa AJ. Production of copper-64 and gallium-68 with a medical cyclotron using liquid targets. Mod Phys Lett A. 2017;32:1740013. doi: 10.1142/S0217732317400132. [DOI] [Google Scholar]
- Alves V, do Carmo S, Alves F, Abrunhosa A. Automated Purification of Radiometals Produced by Liquid Targets. Instruments. 2018;2:17. doi: 10.3390/instruments2030017. [DOI] [Google Scholar]
- Baum RP, Kulkarni HR. THERANOSTICS: from molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy - the Bad Berka experience. Theranostics. 2012;2:437–447. doi: 10.7150/thno.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blower JE, Cooper MS, Imberti C, Ma MT, Marshall C, Young JD, et al. The Radiopharmaceutical Chemistry of the Radionuclides of Gallium and Indium. In: Radiopharmaceutical Chemistry by Lewis J, Windhorst A, Zeglis B (Eds). Springer; 2019. p. 255–271.
- Boschi A, Martini P, Costa V, Pagnoni A, Uccelli L, Santini C, et al. Interdisciplinary tasks in the cyclotron production of Radiometals for medical applications. The case of 47Sc as example. Molecules. 2019;24:1–14. doi: 10.3390/molecules24030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calais J, Fendler WP, Herrmann K, Eiber M, Ceci F. Comparison of 68Ga-PSMA-11 and 18F-Fluciclovine PET/CT in a case series of 10 patients with prostate cancer recurrence. J Nucl Med. 2018;59:789–794. doi: 10.2967/jnumed.117.203257. [DOI] [PubMed] [Google Scholar]
- Cutler CS, Minoshima S. Shortage of Germanium68/gallium68 generators in the United States. 2018; Available from: https://s3.amazonaws.com/rdcms-snmmi/files/production/public/Ga68%20shortage%20letter.pdf. Accessed 12 Aug 2020.
- do Carmo SJC, Scott PJH, Alves F. Production of radiometals in liquid targets. EJNMMI Radiopharm Chem. 2020;5:2. [DOI] [PMC free article] [PubMed]
- Eder M, Neels O, Müller M, Bauder-Wüst U, Remde Y, Schäfer M, et al. Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate Cancer. Pharmaceuticals. 2014;7:779–796. doi: 10.3390/ph7070779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle JW, Lopez-Rodriguez V, Gaspar-Carcamo RE, Valdovinos HF, Valle-Gonzalez M, Trejo-Ballado F, et al. Very high specific activity 66/68Ga from zinc targets for PET. Appl Radiat Isot. 2012;70:1792–1796. doi: 10.1016/j.apradiso.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Jethwa KR, Ost P, Williams S, Kwon ED, Lowe VJ, et al. Basic original report prostate cancer-specific PET radiotracers: a review on the clinical utility in recurrent disease. Pract Radiat Oncol. 2018;8:28–39. doi: 10.1016/j.prro.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Fendler WP, Rahbar K, Herrmann K, Kratochwil C, Eiber M. 177Lu-PSMA Radioligand therapy for prostate Cancer. J Nucl Med. 2017;58:1196–1200. doi: 10.2967/jnumed.117.191023. [DOI] [PubMed] [Google Scholar]
- Gallium (68Ga) Chloride (accelerator produced) solution for radiolabelling. European Pharmacopoeia. 2020;10(Suppl. 10.3):4864–4865.
- Gourni E, Demmer O, Schottelius M, D’Alessandria C, Schulz S, Dijkgraaf I, et al. PET of CXCR4 expression by a 68Ga-labeled highly specific targeted contrast agent. J Nucl Med. 2011;52:1803–1810. doi: 10.2967/jnumed.111.098798. [DOI] [PubMed] [Google Scholar]
- Graham MM, Gu X, Ginader T, Breheny P, Sunderland JJ. Ga-DOTATOC imaging of neuroendocrine tumors: a systematic review and Metaanalysis. J Nucl Med. 2017;58:1452–1458. doi: 10.2967/jnumed.117.191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves SA, Engle JW, Eriksson TE, Gagnon K. Dosimetry of cyclotron-produced [68Ga]Ga-PSMA-11, [68Ga]Ga-DOTA-TATE, and [68Ga]Ga-DOTA-TOC. J Nucl Med. 2018;59(Suppl. 1):1003. [Google Scholar]
- Herrmann K, Schottelius M, Lapa C, Osl T, Poschenrieder A, Hänscheid H, et al. First-in-human experience of CXCR4-directed endoradiotherapy with 177Lu-and 90Y-labeled pentixather in advanced-stage multiple myeloma with extensive intra-and extramedullary disease. J Nucl Med. 2016;57:248–251. doi: 10.2967/jnumed.115.167361. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Schwaiger M, Lewis JS, Solomon SB, McNeil BJ, Baumann M, et al. Radiotheranostics: a roadmap for future development. Lancet Oncol. 2020;21:e146–e156. doi: 10.1016/S1470-2045(19)30821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope TA, Aggarwal R, Chee B, Tao D, Greene KL, Cooperberg MR, et al. Impact of 68Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med. 2017;58:1956–1961. doi: 10.2967/jnumed.117.192476. [DOI] [PubMed] [Google Scholar]
- International Atomic Energy Agency. Gallium-68 Cyclotron Production. IAEA-TECDOC-1863. IAEA, Vienna; 2019.
- Jackson IM, Scott PJH, Thompson S. Clinical applications of radiolabeled peptides for PET. Semin Nucl Med. 2017;47:493–523. doi: 10.1053/j.semnuclmed.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Jensen M, Clark JC. Direct production of Ga-68 from proton bombardment of concentrated aqueous solutions of [Zn-68] Zinc Chloride. In The 13th International Workshop on Targetry and Target Chemistry (July 26-28, 2010) Proceedings by Horoun S, Givskov and Jensen M (Eds). Risø DTU, National Laboratory for Sustainable Energy, Technical University of Denmark. Roskilde. 2011; 288–292. Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/43/006/43006750.pdf. Accessed 12-Aug-2020.
- Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. Targeted a-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802. doi: 10.2967/jnumed.117.203539. [DOI] [PubMed] [Google Scholar]
- Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of Cancer. J Nucl Med. 2019;60:801–805. doi: 10.2967/jnumed.119.227967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzo N, Meyrick D, Turner J, Lenzo NP, Meyrick D, Turner JH. Review of gallium-68 PSMA PET/CT imaging in the management of prostate cancer. Diagnostics. 2018;8:16. doi: 10.3390/diagnostics8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Waligorski GJ, Lepera CG. Production of curie quantities of 68Ga with a medical cyclotron via the 68Zn(p,n)68Ga reaction. Appl Radiat Isot. 2018;133:1–3. doi: 10.1016/j.apradiso.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Lowe VJ, Kwon ED. PET in Prostate Cancer: A Focus on C-11 Choline. Pathways Clin. Trials Netw. Newsl. 2015;5:1–4. Available from: http://s3.amazonaws.com/rdcms-snmmi/files/production/public/images/Publications/Jan%202015%20Pathways_web.pdf. Accessed 12 Aug 2020.
- Martiniova L, De Palatis L, Etchebehere E, Ravizzini G. Gallium-68 in Medical Imaging. Curr Radiopharm. 2016;9:187–207. doi: 10.2174/1874471009666161028150654. [DOI] [PubMed] [Google Scholar]
- McCormick B, Mahmoud A, Williams S, Davis J. Biochemical recurrence after radical prostatectomy: current status of its use as a treatment endpoint and early management strategies. Indian J Urol. 2019;35:6–17. doi: 10.4103/iju.IJU_355_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossine AV, Tanzey SS, Brooks AF, Makaravage KJ, Ichiishi N, Miller JM, et al. Synthesis of high-molar-activity [18F]6-fluoro-l-DOPA suitable for human use via cu-mediated fluorination of a BPin precursor. Nat Protoc. 2020;15:1742–1759. doi: 10.1038/s41596-020-0305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Klette I, Baum RP, Gottschaldt M, Schultz MK, Breeman WAP. Simplified NaCl based 68Ga concentration and labeling procedure for rapid synthesis of 68Ga radiopharmaceuticals in high radiochemical purity. Bioconjug Chem. 2012;23:1712–1717. doi: 10.1021/bc300103t. [DOI] [PubMed] [Google Scholar]
- Nair M, Happel S, Eriksson T, Pandey M, DeGrado T, Gagnon K. Cyclotron production and automated new 2-column processing of [68Ga]GaCl3. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 2):S119–S956. [Google Scholar]
- Oehlke E, Hoehr C, Hou X, Hanemaayer V, Zeisler S, Adam MJ, et al. Production of Y-86 and other radiometals for research purposes using a solution target system. Nucl Med Biol. 2015;42:842–849. doi: 10.1016/j.nucmedbio.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Pandey MK, Byrne JF, Jiang H, Packard AB, DeGrado TR. Cyclotron production of 68Ga via the 68Zn(p,n)68Ga reaction in aqueous solution. Am J Nucl Med Mol Imaging. 2014;4:303–310. [PMC free article] [PubMed] [Google Scholar]
- Pandey MK, Byrne JF, Schlasner KN, Schmit NR, DeGrado TR. Cyclotron production of 68Ga in a liquid target: Effects of solution composition and irradiation parameters. Nucl. Med. Biol. 2019;74/75:49–55. doi: 10.1016/j.nucmedbio.2019.03.002. [DOI] [PubMed] [Google Scholar]
- Pandey MK, DeGrado TR. Rapid Isolation of Cyclotron-Produced Gallium-68. 2019; WO2018039662.
- Prostate Cancer: Statistics. 2020; Available from: https://www.cancer.net/cancer-types/prostate-cancer/statistics. Accessed 12 Aug 2020.
- Riga S, Cicoria G, Pancaldi D, Zagni F, Vichi S, Dassenno M, et al. Production of Ga-68 with a General Electric PETtrace cyclotron by liquid target. Phys Medica. 2018;55:116–126. doi: 10.1016/j.ejmp.2018.10.018. [DOI] [PubMed] [Google Scholar]
- Roach PJ, Francis R, Emmett L, Hsiao E, Kneebone A, Hruby G, et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: results of an australian prospective multicenter study. J Nucl Med. 2018;59:82–88. doi: 10.2967/jnumed.117.197160. [DOI] [PubMed] [Google Scholar]
- Ruangma A, Kijprayoon S, Ngokpol S. PSMA for PET imaging of prostate cancer. Bangkok Med J. 2018;14:95–100. doi: 10.31524/bkkmedj.2018.09.016. [DOI] [Google Scholar]
- Sadeghi M, Kakavand T, Rajabifar S, Mokhtari L, Rahimi-Nezhad A. Cyclotron production of 68Ga via proton-induced reaction on 68Zn target. Nukleonika. 2009;54:25–28. [Google Scholar]
- Schweinsberg C, Johayem A, Llamazares A, Gagnon K. The first curie-quantity production of [68Ga]Ga-PSMA-HBED-CC. J Label Compd Radiopharm 2019;62(S1):P121.
- Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:92–101. doi: 10.1007/s00259-016-3490-6. [DOI] [PubMed] [Google Scholar]
- Shao X, Hoareau R, Runkle AC, Tluczek LJM, Hockley BG, Henderson BD, et al. Highlighting the versatility of the Tracerlab synthesis modules. Part 2: fully automated production of [11C]-labeled radiopharmaceuticals using a Tracerlab FXC-Pro. J Label Compd Radiopharm. 2011;54:819–838. doi: 10.1002/jlcr.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Hockley BG, El Hoareau R, Schnau PL, Scott PJH. Fully automated preparation of [11C]choline and [18F]fluoromethylcholine using TracerLab synthesis modules and facilitated quality control using analytical HPLC. Appl Radiat Isot. 2010;69:403–9. [DOI] [PubMed]
- Smith DL, Breeman WAP, Sims-Mourtada J. The untapped potential of gallium 68-PET: the next wave of 68Ga-agents. Appl Radiat Isot Pergamon. 2013;76:14–23. doi: 10.1016/j.apradiso.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Tolmachev V, Lundqvist H. Rapid separation of gallium from zinc targets by thermal diffusion. Appl Radiat Isot. 1996;47:297–299. doi: 10.1016/0969-8043(95)00290-1. [DOI] [Google Scholar]
- USP 71 Microbiological Tests/Sterility Tests . United States Pharmacopeial Conv. 2012. [Google Scholar]
- Velikyan I. Prospective of 68Ga radionuclide contribution to the development of imaging agents for infection and inflammation. Contrast Media Mol Imaging Hindawi. 2018;2018:1–24. doi: 10.1155/2018/9713691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisler S, Limoges A, Kumlin J, Siikanen J, Hoehr C. Fused zinc target for the production of gallium radioisotopes. Instruments. 2019;3:10. doi: 10.3390/instruments3010010. [DOI] [Google Scholar]