Abstract
The DNA-barcoding and chromosomal study of the eastern water bat, Myotis petax Hollister, 1912, from the earlier unexplored localities in the Russian Far East are carried out. The COI barcoding obtained for 18 from a total of 19 individuals captured in five localities in the Russian Far East showed the low nucleotide variability with the prevalence of the central, the most abundant haplotype. The chromosomal characteristics of eight M. petax specimens (2n = 44, NFa = 52) in the Russian Far East are clarified. The number and localization of NOR in karyotype of M. petax is described at the first time and differ from distributional patterns of NOR in the sibling species M. daubentonii Kuhl, 1819 that can be used as diagnostic feature. The considerable intraspecific variability in the distribution of heterochromatin material revealed is not typical of the genus Myotis, but it has been found in other species of the family Vespertilionidae.
Keywords: bats, chromosome, COI, heterochromatin, Myotis , NOR
Introduction
The eastern water bat, Myotis petax Hollister, 1912, is a common Eastern Palaearctic bat species. The range of M. petax includes the near-water habitats throughout forest, forest-steppe and steppe zones from Western Siberia to the Russian Far East (including Sakhalin and the Kuril Islands) and, outside of Russia – in northern Mongolia, NE China, Korea and Japan (Kruskop 2012). It was first described as a distinct species from the village Kosh-Agach in the Altai Mountains (Hollister 1912). However, starting from Ognev (1928) and until the early 2000s, M. petax had been considered as part of the widespread polytypic species Myotis daubentonii Kuhl, 1819 which had included about 3 to 6 subspecies according to various estimates (Kuzaykin 1950; Gromov 1963; Tiunov 1984, 1997; Yoshiyuki 1989; Bogdanowicz 1994; Koopman 1994).
The morphological heterogeneity and the presence of two major groups of forms in M. daubentonii complex: the “western” and the “eastern” (including the Altai form M. d. petax) has been shown by Kruskop (2004). The species rank of M. petax was finally confirmed by the genetic and morphological data with the using SINEs as genetic markers. A total of 6 specimens of M. daubentonii and 7 specimens of M. petax (including only one bat from the Far East) were examined by molecular method (Matveev et al. 2005). It was shown by the molecular studies based on cyt b and ND1 sequences that M. petax is closer related to M. macrodactylus (Temminck, 1840), M. pilosus Peters, 1869 and M. fimbriatus Peters, 1871 than M. daubentonii. The closest related species for M. daubentonii are M. bechsteinii Kuhl, 1817, M. longicaudatus Ognev, 1927 and M. frater G. Allen, 1823 (Kawai et al. 2002, 2003; Ruedi et al. 2013; Ruedi et al. 2015).
The DNA-barcoding based on 657 bp length sequences of cytochrome c oxidase I (COI) gene has been studied for the 23 M. petax individuals including 6 specimens from the Far East, i.e. 5 bats from Sakhalin and 1 animal from the Primorsky Krai. It was revealed that the intraspecific distances for M. petax are amounted to 0.28% to 1.16% while interspecific distance between M. petax and M. daubentonii is 12% (Kruskop et al. 2012). The differences between cyt b sequences of M. petax from the Far East (n = 1) and China (n = 17) were amounted to 0.2% (Wang et al. 2010). In addition, the partial sequence of control region for one specimen from China had been studied (Zhang et al. 2009) and the full mitochondrial genomes of 4 individuals from South Korea had been obtained (Hwang et al. 2016). Otherwise, the genetics of Myotis petax in Far Eastern populations still remains poorly studied.
Karyotype features are essential diagnostic characteristics of many mammalian species (Vorontsov 1958; Matthey 1973; Orlov and Bulatova 1983; Mazzoleni et al. 2018). The chromosomal data are successfully applied to clarify species affinity and interspecific relationships between species of the order Chiroptera (Volleth 1987; Volleth and Heller 1994, 2012; Volleth et al. 2001; Kearney et al. 2002; Volleth et al. 2006). It was shown by our review that the karyology of Far Eastern bats is studied insufficiently (Gorobeyko and Kartavtseva 2019).
For the genus Myotis Kaup, 1829 the position and number of the nucleolus organizer regions (NORs) and the amount and location of heterochromatic material on chromosomes are species-specific characteristics (Harada and Yosida 1978; Ando et al. 1980; Volleth 1987; Ando et al. 1987; Ono and Obara 1994; Volleth and Heller 2012). The NOR distribution has been studied for 4 out of 6 Far Eastern Myotis species and varied from 5 to 13 centromeric NORs on the acrocentric chromosomes (Ono and Obara 1994). Only 3 NORs were found in karyotype of M. daubentonii on acrocentric pairs Nos. 8 to 10 (Volleth 1987; Volleth and Heller 2012). It is likely that the number and location of NOR on the M. petax and M. daubentonii chromosomes should be different. A small intercalary heterochromatic band in the proximal part of the long arm of X chromosome and largely heterochromatic submetacentric Y chromosome was detected in Myotis daubentonii karyotype (Volleth and Heller 2012). The pattern of heterochromatic material on M. petax chromosomes is still unknown.
Only conventional staining karyotypes of M. petax have been studied from the Primorsky Kray, Russian Far East (Korablev et al. 1989), and from South Korea (Yoo and Yoon 1992). The diploid number of M. petax did not differ from other Myotis species (2n = 44), but the number of autosomal arms (NFa) was different in two works and amounted to 50 or 52, respectively. The feature of genus Myotis the fundamental number of autosomal arms is 52 due to the short euchromatic arms on the autosomal pair No. 7 (Volleth and Heller 1994, 2012)
Thus, the aim of present paper is to study DNA barcodes and chromosomes of Myotis petax from the localities of the Russian Far East that are not covered by previous studies, and to compare obtained results with these data for the species. It is important to investigate the position and number of the NORs and the amount and location of heterochromatic material on chromosomes to clarify chromosomal characteristics of M. petax and to find the differences with the karyotypes of other Myotis species.
Materials and methods
There are 19 specimens of M. petax caught in the Primorsky Krai (n = 7), Khabarovsky Krai (n = 4), Amur Oblast (n = 8) studied in this paper. Bats were caught using mist nets (6–7 m × 2.5 m, Ecotone, Poland) in swarming site and near summer roosts, handling in hibernation sites. The geographical origin of the examined animals and coordinates listed in Table 1. The other collecting data see Suppl. file 1. The samples used in the present study are deposited in the Genetic Mammalian Tissue Collection of the Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far East Branch, Russian Academy of Sciences (Vladivostok, Russia). All applicable international, national and institutional ethics statements when using animals in research have been followed.
Table 1.
Sampling localities and GenBank sequencing data of Myotis petax. specimen – the number of animal in Genetic Mammalian Tissue Collection of the FSCEATB FEB RAS or in Collection of Zoological Museum of Moscow University. 2n/ NFa – the diploid number of chromosome and the fundamental number of autosomal arms, X and Y – morphology of sex chromosomes: M – metacentric, SM – submetacentric, M-SM – biarmed chromosome, A – acrocentric, conv – conventional staining.
| Code | Locality | Coordinates | GenBank | Specimen | Sex | 2n/ NFa | X | Y | Chromosomal stainings |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Primorsky Krai, Primorsky Velican Cave | 43°17.133'N, 133°36.8'E | MT383996 | 3240 | m | - | |||
| MT383997 | 3400 | f | 44/52 | M-SM | - | conv, GTG, AgNOR | |||
| MT383998 | 3865 | f | 44/52 | M-SM | - | GTG, CBG | |||
| – | 3867 | m | 44/52 | M-SM | A | GTG, CBG | |||
| MT383999 | 3869 | f | - | ||||||
| 2 | Primorsky Krai, Spasskaya Cave | 44°34.883'N, 132°46.083'E | MT384000 | 3258 | m | 44/52 | M-SM | A | conv, AgNOR |
| MT384001 | 3259 | m | 44/52 | M-SM | A | conv, GTG, CBG, AgNOR | |||
| 3 | Khabarovsky Krai, Komsomolsk Nature Reserve | 50°50.1'N, 137°28.7'E | MT384002 | UG16-18 | m | - | |||
| MT384004 | UG21-18 | m | - | ||||||
| 4 | Khabarovsky Krai, Komsomolsk-on-Amur City | 50°42.114'N, 137°12.291'E | MT384003 | UG28-18 | m | - | |||
| MT384005 | UG36-18 | f | - | ||||||
| 5 | Amur Oblast, Zeya City | 53°41.767'N, 127°4.317'E | MT384006 | 3332 | m | - | |||
| MT384007 | 3333 | m | 44/52 | M-SM | A | conv | |||
| MT384008 | 3334 | f | - | ||||||
| MT384009 | 3335 | f | - | ||||||
| MT384010 | 3336 | f | 44/52 | M-SM | - | conv, CBG, AgNOR | |||
| MT384011 | 3337 | m | - | ||||||
| MT384012 | 3338 | f | 44/52 | M-SM | - | conv, GTG, CBG, AgNOR | |||
| MT384013 | 3339 | f | - | ||||||
| GenBank sequencing data of Myotis petax | |||||||||
| Code | Locality | Coordinates | GenBank | Specimen | Sex | Reference | |||
| 6 | Primorsky Krai, Priiskovaya Cave | 44°22.767'N, 133°12.283'E | JF443025 | S173255 | m | Kruskop et al. 2012 | |||
| 7 | Sakhalin Oblast | 46°22.3'N, 141°52.217'E | JF443019, JF443032–JF443035 | S175221-25 | - | Kruskop et al. 2012 | |||
| 8 | Transbaikal Krai | 53°22.5'N, 121°10.38'E | JF443026 | S182081 | m | Kruskop et al. 2012 | |||
| 9 | Transbaikal Krai | 53°25.2'N, 120°19.8'E | JF443028 | S175362 | m | Kruskop et al. 2012 | |||
| 10 | Mongolia | 47°5.783'N, 102°46.38'E | JX008075–JX008077 | S187466-68 | - | Kruskop et al. 2012 | |||
| 11 | Tuva Republic | 50°02'N, 95°04'E | JF443020, JF443029–JF443031, JF443036– JF443038 | S167627, S167738, S168602-03, S168637, S168648-49 | - | Kruskop et al. 2012 | |||
| 12 | Altai Republic | 51°22.2'N, 84°43.8'E | JF443024 | S171621 | m | Kruskop et al. 2012 | |||
| 13 | Altai | 51°21.9'N, 84°42.9'E | JF443021 | S171624 | f | Kruskop et al. 2012 | |||
| 14 | Altai | 51°17.22'N, 84°43.92'E | JF443039, JF443040 | S184155-56 | 2f | Kruskop et al. 2012 | |||
| 15 | South Korea | 36°31'N, 127°48'E | KT199099–KT199102 | KW001-004 | - | Hwang et al. 2016 | |||
In addition, the 26 COI sequences of Myotis petax (Table 1) and 19 COI sequences of five Far Eastern Myotis species (M. macrodactylus, M. longicaudatus, M. bombinus Thomas, 1906, M. ikonnikovi Ognev, 1912, M. sibirica Kastschenko, 1905) and M. daubentonii from GenBank were analyzed. The COI sequence of Murina hilgendorfi Peters, 1880 was used as outgroup in phylogenetic analysis.
M. macrodactylus: HQ580337, HQ580338 (International Barcode of Life, 2010), KT862813, KT862814 (GenBank), M. longicaudatus: JF442982, JF442983, JF442989 (Kruskop et al. 2012), M. bombinus: HQ580336 (International Barcode of Life, 2010), JF442874, JF442876 (Kruskop et al. 2012), M. ikonnikovi: HQ974651, HQ974652 (International Barcode of Life, 2010), JF442993 (Kruskop et al. 2012), M. sibirica: JF442902, JF442905, JF442926 (Kruskop et al. 2012), M. daubentonii: JF442939, JF442942, JF442943 (Kruskop et al. 2012), and Murina hilgendorfi: JF442830 (Kruskop et al. 2012).
DNA extraction, amplification and sequencing
Total DNA was isolated from ethanol-fixed tissues by the method of saline extraction (Aljanabi and Martinez 1997). For the DNA-barcoding we used the part of COI from 49 to 705 nucleotides, 657 bp length. The COI gene was amplified and sequenced by polymerase chain reaction and sequenced using the forward MPCO+ (5’-ATTTGCAATTCAATGTGTATT-3’) and reverse MPCO- (3’-ATAGCTCATACCATTCCTAT-5’). The both primers were designed in this study. Amplification was carried out in a 25 μL reaction mixture, which included 3–4 μg of total DNA, 2.5 μL 10× buffer, 2.5 μL of 20 mM dNTP mixture, 2 μL of each primer, 0.5 units Taq-polymerase (Sibenzim, Russia), and deionized water. The COI gene was amplified under the following conditions: 5 min DNA denaturation at 95 °C, 35 cycles of amplification (95 °C for 10 s, 47.5 °C for 60 s and 72 °C for 60 s) and 7 min chain completion at 72 °C. PCR products were purified and sequenced with the forward and reverse primers using the Big Dye Terminator series 3.1 kit (Applied Biosystems, United States). The nucleotide sequences were analyzed with the ABI Prizm 3130 sequencer (Applied Biosystems, United States) in the Federal Scientific Center of the East Asia Terrestrial Biodiversity, Far East Branch, Russian Academy of Sciences (Vladivostok, Russia).
Phylogenetic analysis
All sequences were aligned using the software program BioEdit, version 7.0.9.0 and deposited in the GenBank database. The accession numbers of our and sequences obtained from GenBank are reported in the Table 1.
The interspecific nucleotide diversity (π) and haplotype diversity (P) were calculated using DnaSP6 (Hall 1999). A search for the best model of nucleotide evolution was performed using Modeltest: Hasegava-Kishino-Yano including invariant sites (HKY+I) (Nei and Kumar 2000, Kumar et al. 2018). The phylogenetic analysis was based on Maximum Likelihood (ML) method and run in MEGA-X 10.1.7 with 1000 bootstrap replicates (Kumar et al. 2018). To calculate pairwise genetic p-distances the MEGA-X 10.1.7 software used. To construct the haplotype network by the “median joining” method the Network 10 software used (https//www.fluxus-engineering.com).
Chromosomal analysis
Chromosome preparations were obtained from in vivo bone marrow method (Ford and Hamerton 1956), as well as from short-term cell cultures established from spleen and bone marrow (Graphodatsky and Rajabli 1988). GTG-banding procedure was carried out according to Seabright (1971). Chromosomes were numbered using Bickham’s scheme, in which ordinal numbers have been given to all of the autosomal arms based on GTG-banding patterns (Bickham 1979). The locations of nucleolus organizer regions (NORs) were detected by sequential using of silver staining method (Bloom and Goodpasture 1976) and GTG-banding of chromosomes. Heterochromatic material was detected using C-banding (Sumner 1972). To determine the locations of heterochromatic bands on chromosomes, we used sequential GTG-staining and C-staining. The mean value of active NORs per chromosomal pair and cell was calculated according to Volleth (1987), where each distinct NOR was counted as 1.0 and indistinct one as 0.5. The greatest possible value of NORs per chromosomal pair and cell was 2.0 (Volleth 1987).
The results of differential staining were analyzed with an AXIOSKOP 2 Plus microscope (Zeiss). The microimage registration and adjustment was performed with a CCD camera with software (META Systems GmbH, Germany) of the Joint-Use Center «Biotechnology & Genetic Engineering» in the Federal Scientific Center of the East Asia Terrestrial Biodiversity Far East Branch Russian Academy of Sciences (Vladivostok, Russia).
Results and discussion
DNA-barcoding and phylogenetic analysis
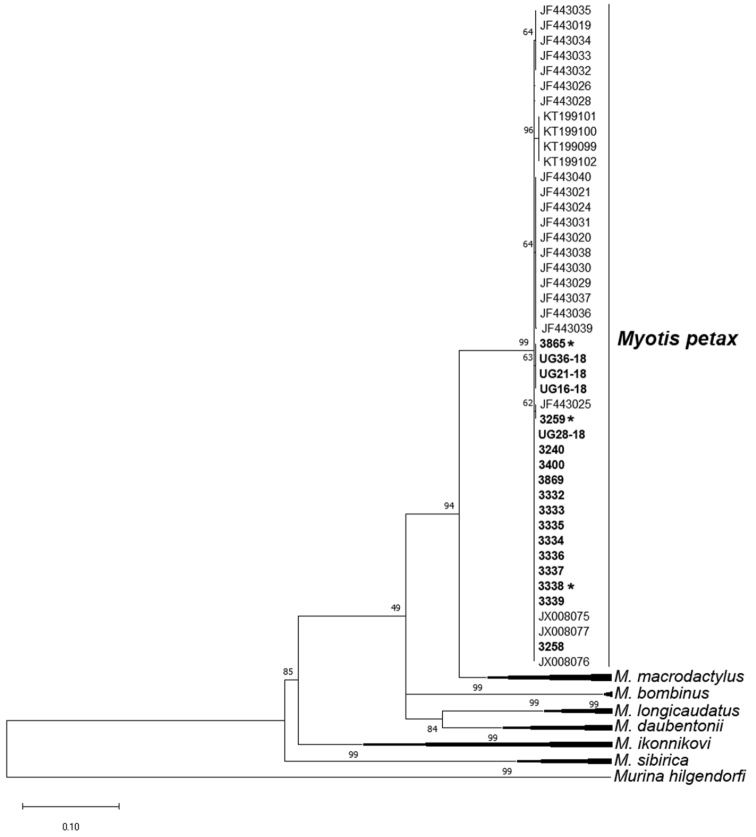
The DNA barcodes are obtained for 18 from a total of 19 M. petax individuals captured in five localities in the Russian Far East. To identify the species the sequences have compared with the 45 DNA barcodes of 7 Myotis species (M. petax, M. macrodactylus, M. longicaudatus, M. bombinus, M. ikonnikovi, M. sibirica, M. daubentonii) from GenBank. All of the obtained sequences have highest similarity with sequences of M. petax from GenBank (Fig. 1).
Figure 1.
Maximal Likelihood tree of the cytochrome oxidase I gene. ML tree based on 64 COI sequences of Myotis species and outgroup. The bold numbers marked our data. Asterisks marked individuals for which the CBG-banding karyotype is studied.
A pairwise genetic distances between the specimens of M. petax studied vary from 0 to 0.8%. The obtained values are within the range of interspecific distances (0.28–1.16%) previously described for M. petax (Kruskop et al. 2012). A mean genetic p-distances between the individuals from the Primorsky Krai and South Korea is 0.54% (less than 1000 km), while a mean genetic p-distances between the Altai Mountains and the Primorsky Krai specimens is only 0.26% (approximately 3000–3500 km). This means that a geographically closer South Korean population is genetically more distant from the population of M. petax in the Primorsky Krai.
The nucleotide diversity for the whole species is amounted to 0.00227±0.00032 with the haplotype diversity P = 0.801 ± 0.040. The nucleotide diversity and haplotypic diversity for specimens from the mainland part of the Russian Far East are amounted to P = 0.503 ± 0.113, π = 0.00084 ± 0.00022. These values are close to the values of haplotype diversity for the COI gene described for M. ikonnikovi from South Korea (P = 0.5–0.8667) which are characterized by high genetic diversity of mitochondrial genes compared to other Myotis species (Park et al. 2019). The similar values of haplotype diversity have found for control region of two Northern American bat species M. lucifugus (P = 0.812–0.845) and M. septentrionalis (P = 0.827–0.910) (Johnson et al. 2015). At the same time the haplotype diversity of cyt b gene described for European M. myotis was amounted to P = 0.491 (Ruedi and Castella 2003), and for M. dasycneme was P = 0.335–0.868 (Andersen et al. 2018). On the other hand, the nucleotide diversity of M. petax is lower than that of M. ikonnikovi (π between 0.00163 to 0.00878) and is comparable with the nucleotide diversity of cyt b of M. myotis (π between 0.0003 to 0.0028) and M. dasycneme (π between 0.0004 to 0.0029) (Ruedi and Castella 2003, Andersen et al. 2018, Park et al. 2019).
A total of 9 COI haplotypes found in all specimens of M. petax studied including GenBank data (G1–9) but only 3 COI haplotypes detected in 18 M. petax individuals from the Russian Far East (G1–3). The G2 haplotype revealed at the first time.
The relationship among a total of 9 haplotypes reflected in the median‐joining network (Fig. 2) revealed a close relationship between the all M. petax studied, expect the Korean bats which are more distantly related to other populations. The most common haplotype, G1, is observed in the waist territory from Baikal Lake to Pacific Ocean coast. It is found in 16 of the 44 specimens studied. Khabarovsky Krai and Primorsky Krai shared haplotype G2 which is found in the 4 individuals. The third haplotype observed in the Primorsky Krai is a G3 haplotype found in 2 specimens. Two individuals from Transbaikal Krai have two different haplotype G5 and G6, and 5 bats from the Sakhalin Island have G4 haplotype.
Figure 2.
Distributional range and COI haplotypes of Myotis petaxA map showing approximate range and capture sites of M. petax (for this paper and previous studies) B median-joining network of COI haplotypes are colour-coded based on capture sites, circle size corresponds to number of samples CM. petax (Russia, Buryatia Republic, 2014), photo by Denis V. Kazakov.
Haplotypes G7 and G8 form a separate branch on the network and are found only in 8 specimens from Tuva and the Altai. The G8 haplotype revealed in the one specimen from the Altai differed from G7 on one nucleotide substitution and from G1 on two nucleotide substitution. The spread of G7 and G8 is apparently coincides with the distribution of nominative subspecies.
The other differential branch on the network is a G9 haplotype differed from G1 on three nucleotide substitution. It is found only in 4 individuals from South Korea. The distinct subspecies for M. petax from Korea has not been described previously.
Most of the haplotypes represented in the samples are separated by G1 just one mutation creating a starlike network characteristic for expanding populations that have been through a bottleneck or been founded recently. However, COI gene is conservative and is not suitable for studying population events.
Karyotype, differential staining and chromosomal polymorphism of M. petax from the Far East
The conventional staining karyotypes of eight M. petax specimens from Primorsky Krai and Amur Oblast have no differences and shows 2n = 44 with the NFa = 52 (Table 1). There are composed of three large (1/2, 3/4, 5/6) and one small (16/17) metacentric pair, 17 acrocentric-subtelocentric autosomal pairs and one pair of sex chromosomes (X, Y). The X is a medium-sized biarmed chromosome. The small-sized Y chromosome is acrocentric and largely heterochromatic.
It was previously reported for the specimens from the Primorsky Krai the fundamental number of autosomal arms was 50 (Korablev et al. 1989). We already noted that variations of fundamental number in the different studies can be explained by different approaches to the taking into account short euchromatic arms on the seventh autosomal pair or the including the additional heterochromatic short arms on 24 or 25 pairs of acrocentrics in NFa (Kartavtseva et al. 2014, Gorobeyko and Kartavtseva 2019). While the karyotype of M. petax from South Korea (NFa = 52) showed short arms on 24 or 25 pair of acrocentric (Yoo and Yoon 1992), the all Far Eastern specimens studied have no short arms on these autosomal pairs. The image of M. petax chromosomes from the Primorsky Krai is not given and there is no mention of the presence or absence short arms on any autosomal pairs in the paper (Korablev et al. 1989).
The X chromosome is biarmed and it was not possible to determine whether this is a submetacentric or metacentric. At the same time the previously examined individuals from the Primorsky Krai have shown clearly a metacentric X chromosome. It is possible that these karyotypic differences are due to the methodological difficulties, such as the various spiralization of metacentric chromosomes or the lack of metaphase plates on the preparation often occurred in the analysis of chromosome suspensions obtained in vivo.
The patterns of NOR and the heterochromatic segments in karyotype of M. petax are described at the first time. Figure 3 demonstrated the sequential GTG- and AgNOR-banding of Myotis petax chromosomes. The distribution of active NORs in the four M. petax specimens is shown in Table 2. All four specimens showed active NORs in the minute short arms of chromosomes Nos. 7, 9, 10, 12, 13, 15, 18, 20, 21, 23-25.
Figure 3.
The sequential GTG- and AgNOR-banding of Myotis petax chromosomes A the AgNOR-banded karyotype of male 3259. Arrows indicate the NOR-bearing xcrocentric chromosome. Ordinal numbers indicate autosomal arm numbers revealed by GTG-banding B the GTG-banded karyotype of male 3259.
Table 2.
Distribution of nucleolus organizer regions: mean value of active NORs per chromosomal arm and cell. ID – identification number of specimen. No cells – number of cells analyzed. The numbers before ID (1, 2, 5) indicate sampling localities, the abbreviations see in Table 1.
| ID | No cells | chromosomal arm no. | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | ||
| 1-3400 | 11 | 0,41 | 0,5 | 0,27 | 0,14 | 0,09 | 0,27 | 0,86 | 0,55 | 0,27 | 0,27 | 0,27 | 0,09 | |||||
| 2-3259 | 20 | 0,78 | 0,93 | 0,48 | 0,2 | 0,33 | 0,45 | 1 | 0,8 | 0,8 | 0,13 | 0,2 | 0,2 | |||||
| 5-3336 | 22 | 0,16 | 0,38 | 0,13 | 0,19 | 0,17 | 0,27 | 0,36 | 0,33 | 0,38 | 0,38 | 0,14 | 0,2 | |||||
| 5-3338 | 63 | 0,34 | 0,63 | 0,41 | 0,45 | 0,32 | 0,68 | 0,9 | 0,88 | 0,56 | 0,18 | 0,1 | 0,06 | |||||
On average only 4.7 NOR per cell from 24 potential sites is detected that illustrated the low NOR activity of all the specimens studied. In many cells only one homologue of a chromosomal pair is shown to bear an active NOR. A similar low NOR-activity was shown for M. myotis, M. capaccinii, M. bechsteinii (Volleth 1987) and for M. bombinus, M. longicaudatus, M. macrodactylus (Ono and Obara 1994). All these species including M. petax have small multiple centromeric NORs on chromosomes.
M. petax is clearly differ on the number and localization of NORs as from the other Far Eastern Myotis species as from the sibling species M. daubentonii. The comparison of the NOR distributions in the karyotypes of the Far Eastern Myotis species is shown in Table 3. The NOR-distribution of the one Far Eastern species M. sibirica (gracilis) is still unknown. The conventional staining karyotype of this species (2n = 44, NFa = 50) was published by Kartavtseva and Dokuchaev (1998).
Table 3.
Distribution of NORs in karyotypes of the Far Eastern Myotis species. * – distribution of NORs of European species, Myotis daubentonii, is shown to comparison with distribution of NORs in karyotype of M. petax. The abbreviations see in Table 1. cmc – centromere-cap NORs, ST – subtelocentric chromosome.
| Species | 2n | NFa | X | Y | Chromosome arm no. | NOR | Source | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | |||||||
| Myotis bombinus | 44 | 52 | M | A | + | + | + | + | + | + | + | + | + | + | + | 11 cmc | Ono and Obara 1994 | ||||||
| M. longicaudatus | 44 | 52 | M | ST | + | + | + | + | + | + | + | + | + | + | + | + | + | 13 cmc | Ono and Obara 1994 | ||||
| M. ikonnikovi | 44 | 52 | M | A | + | + | + | + | + | 5 cmc | Ono and Obara 1994 | ||||||||||||
| M. macrodactylus | 44 | 52 | M | A | + | + | + | + | + | + | 6 cmc | Ono and Obara 1994 | |||||||||||
| M. petax | 44 | 52 | M-SM | A | + | + | + | + | + | + | + | + | + | + | + | + | 12 cmc | this study | |||||
| M. daubentonii* | 44 | 52 | SM | SM | + | + | + | 3 cmc | Volleth 1987, Volleth and Heller 2012 | ||||||||||||||
The amounts and localizations of heterochromatin bands on chromosomes of three M. petax from the Primorsky Krai and Amur region presented in Figure 4A–C and are clearly different.
Figure 4.
Comparison of C-banded karyotypes of far eastern Myotis petaxA CBG-banded karyotype of specimen 3259 (locality 2) B CBG- banded karyotype of 3865 (locality 1) C C-banded karyotype of specimen 3338 (locality 5) D GTG-banded karyotype of 3865 (locality 1). The abbreviations see in Table 1.
1) The male M. petax (3259) from Spasskaya Cave (locality 2) showed centromeric heterochromatic bands on most of chromosomal pairs. The one or two homologues in chromosome pairs Nos. 7–10, 12–14 and 25 bore large centromeric heterochromatic segment. Small but distinct telomeric heterochromatic bands are found on all biarmed chromosomal pairs and seven acrocentric pairs from 7 to 22. Large intercalary heterochromatic segments are located on chromosome 8, 11 and 18. A heteromorphism in localization of heterochromatin blocks found in nine autosomal pairs 8–12, 14, 18, 21, 24.
2) The female M. petax (3865) from the Primorsky Velican Cave (locality 1) showed centromeric heterochromatic bands on most of the acrocentric pairs, on the metacentric pair 16/17 and X chromosome. The large heterochromatic centromeric segments are found in 8 and 9 autosomal pairs. The telomeric heterochromatic segments are presented on all biarmed chromosomal pairs and acrocentric pairs Nos. 11 and 21. A heteromorphism in localization of heterochromatin blocks is found in four autosomal pairs 8, 25 and 16/17. There were no intercalary heterochromatic bands in karyotype of M. petax from the Primorsky Velican Cave. The GTG-banded karyotype of 3865 showed in Figure 4D.
3) In karyotype of the female M. petax (3338) from Zeya (locality 5) small and slightly stained heterochromatic centromeric bands are found on nine acrocentric pairs from 7 to 25, metacentric pair 16/17 and X chromosome. Three autosomal pairs 7, 14 and 22 showed a heteromorphism on amount heterochromatic material. This specimen had no telomeric or intercalary heterochromatic bands.
The distinct telomeric heterochromatic segments found on several chromosomes of both individuals from the Primorsky Krai were previously described only for the Chinese Myotis species such as M. altarium Thomas, 1911 (Li et al. 2007), M. cf. siligorensis (published as “M. dividii), M. cf. daubentonii (Peng et al. 2011), M. fimbriatus (Peters, 1871) (Wang et al. 2009). The intercalary heterochromatic segments were observed in karyotypes of Eurasian Myotis species (Volleth and Heller 2012), but no one have intercalary heterochromatin bands on acrocentric pairs Nos. 8, 11, 18 found in the specimen 3259.
All individuals studied had the heteromorphic chromosome pairs. The similar intraspecific heteromorphism of several heterochromatic segments was previously observed in a few Eurasian Myotis species (Harada and Yosida 1978; Volleth and Heller 2012). Intraspecific polymorphism of the several heterochromatic segments in karyotypes of a few Eurasian Myotis species is illustrated in the Table 4. Nevertheless, a variability of the heterochromatic material as found in karyotype M. petax is not typical for the most of the Eurasian Myotis species. We have already noted the same significant polymorphism in the amount and location of the heterochromatin bands in the karyotype of two Pipistrellus-like species: Pipistrellus abramus (Temminck, 1840) and Vespertilio sinensis Peters, 1880 (Ando et al. 1980; Harada et al. 1987; Ando et al. 1987; Ono and Obara 1994; Ono and Yoshida 1997; Lin et al. 2002; Wu et al. 2009; Gorobeyko and Kartavtseva 2019).
Table 4.
Intraspecific variations of heterochromatic material in karyotypes of Myotis species. specimen – identification number (ID) of specimen. The numbers before ID (1, 4, 5) indicate sampling localities, the abbreviations see in Table 1. Symbols: o – totally euchromatic chromosomes, • – totally heterochromatic chromosomes. + – small heterochromatic band in vicinity of the centromere on both homologues in pair, ++ – large heterochromatic band in vicinity of the centromere on both homologues in pair, x/xx – heteromorphic heterochromatic bands in vicinity of the centromere: small and absent / large and small or absent. tel – heterochromatic segment in vicinity of the telomere, int – interstitial heterochromatic band, arm – heterochromatic secondary arm. Bold Italic – heteromorphic chromosomal pair. The abbreviations see in Table 1.
| Specimen/Species | 2n | NFa | Chromosome arm no. | X | Y | Sourse | ||||||||||||||||||||
| 1/2 | 3/4 | 5/6 | 16/17 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | ||||||
| 1-3338 | 44 | 50 | o | o | o | + | x | o | o | + | o | + | o | x | o | + | + | o | o | x | + | ++ | o | + | - | Present study |
| 4-3259 | 44 | 50 | tel | tel | +, tel | +, tel | ++, tel | +, int | xx, tel | xx | +, int | xx, tel | +, tel | xx | +, tel | +, int | +, tel | o | xx | +, tel | + | x | ++ | + | •, A | Present study |
| 5-3865 | 44 | 50 | tel | tel | tel | +, tel | o | xx | ++ | + | +, tel | + | + | o | + | + | +, tel | + | +, tel | + | + | + | x | + | - | Present study |
| M. m. bulgaricus | 44 | 52 | + | + | + | +, int | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | +, arm | + | •, SM | Volleth and Heller 2012 |
| M. daubentonii | 44 | 52 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | int , int | •, SM/ST | Volleth and Heller 2012 |
| M. ikonnikovi | 44 | 52 | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | ++ | ++ | ++ | + | + | + | + | ++ | + | + | x | x | arm, A/M | ++ | - | Harada and Yoshida 1978 |
| M. macrodactylus | 44 | 52 | xx | + | xx | + | + | ++ | ++ | + | ++ | + | + | + | ++ | ++ | + | + | + | ++ | + | + | arm, SM/M | ++ | •, A | Harada and Yoshida 1978 |
The individuals differing in the amounts and localizations of heterochromatin bands on chromosomes are also belonged in different COI haplotypes. The specimen 3331 from Amur Oblast is showed G1 haplotype, while the bats 3259 and 3865 from the Primorsky Krai are belonged to G3 and G2, respectively. Nevertheless, the number of M. petax individuals studied and the differences between the COI haplotypes are insufficient to draw conclusions regarding the relationship between chromosomal and COI variability.
Conclusion
The COI barcoding showed the presence of only 3 COI haplotypes (G1–3) in the Russian Far East from 9 COI haplotypes (G1–9) found in M. petax. The G2 haplotype detected at the first time. This species showed to have the low nucleotide variability with the prevalence of the central, the most abundant haplotype. The distances between individuals do not exceed 0.8%.
The chromosomal characteristics of M. petax from the Russian Far East are clarified. The distributional patterns of NOR and heterochromatic segments on the chromosomes M. petax are described at the first time. The number and localization of NOR in karyotypes of sibling species M. petax and M. daubentonii is different and can be used as diagnostic feature. The significant intraspecific variability in the heterochromatin distribution of revealed in Far Eastern M. petax was not described for the genus Myotis, but it had been found in other vespertilionid species.
Acknowledgements
We would like to thank Elena V. Ignatenko and Sergei Yu. Ignatenko (Zeisky Nature Reserve), Vadim V. Bobrovsky and Polina Van (Komsomolsky Nature Reserve) for their help in mounting the expeditions. This study was partly funded by RFBR according to the research project № 18-34-00285.
Citation
Gorobeyko UV, Kartavtseva IV, Sheremetyeva IN, Kazakov DV, Guskov VYu (2020) DNA-barcoding and a new data about the karyotype of Myotis petax (Chiroptera, Vespertilionidae) in the Russian Far East. CompCytogen 14(4): 483–500. https://doi.org/10.3897/compcytogen.v14.i4.54955
Supplementary materials
The other collecting data for Myotis petax
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Uliana V. Gorobeyko, Irina V. Kartavtseva, Irina N. Sheremetyeva, Denis V. Kazakov, Valentin Yu. Guskov
Data type
species data
References
- Aljanabi S, Martinez I. (1997) Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Research 25: 4692–4693. 10.1093/nar/25.22.4692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen LW, Dirksen R, Nikulina EA, Baagøe HJ, Petersons G, Estók P, Orlov OL, Orlova MV, Gloza‐Rausch F, Göttsche M, Fjederholt ET, Krüger F, Elmeros M. (2018) Conservation genetics of the pond bat (Myotis dasycneme) with special focus on the populations in northwestern Germany and in Jutland, Denmark. Ecology and Evolution 9: 5292–5308. 10.1002/ece3.5119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K, Tagawa T, Uchida TA. (1980) The C-banding pattern of 6 Japanese species of vespertilionine bats (Mammalia: Chiroptera). Experientia 36: 653–653. 10.1007/BF01970118 [DOI] [PubMed] [Google Scholar]
- Ando K, Harada M, Uchida TA. (1987) A karyological study on five Japanese species of Myotis and Pipistrellus, with special attention to composition of their C-band materials. Journal of the Mammalogical Society of Japan 12(1–2): 25–29. [Google Scholar]
- Bickham J. (1979) Chromosomal Variation and Evolutionary Relationships of Vespertilionid Bats. Journal of Mammalogy 60(2): 350–363. 10.2307/1379807 [DOI] [Google Scholar]
- Bloom SE, Goodpasture C. (1976) An improved technique for selective silver staining of nucleolar organizer regions in human chromosomes. Human Genetics 34: 199–206. 10.1007/BF00278889 [DOI] [PubMed] [Google Scholar]
- Bogdanowic W. (1994) Myotis daubentonii Mammalian Species 43: 1. 10.2307/3504215 [DOI]
- Ford CE, Hamerton JL. (1956) A colchicine, hypotonic citrate, squash sequence for mammalian chromosomes. Stain Technology 31: 247–251. 10.3109/10520295609113814 [DOI] [PubMed] [Google Scholar]
- Gorobeyko UV, Kartavtseva IV. (2019) Karyology of the Bats from the Russian Far East. In: Larramendy M, Soloneski S (Eds) Cytogenetics – Past, Present and Further Perspectives. IntechOpen, 14 pp 10.5772/intechopen.78767 [DOI] [Google Scholar]
- Graphodatsky AS, Radjabli SI. (1988) Chromosomes of Agricultural and Laboratory Animals: Atlas. Nauka Press, Novosibirsk, 127 pp. [Google Scholar]
- Gromov IM, Gureev AA, Novikov GA, Sokolov AI, Strelkov PP, Chapsky PP. (1963) Mammal Fauna of the USSR, part 1. Keys to the Fauna of Russia, Published by the Zoological Institute of the USSR Academy of Sciences. Moscow, Leningrad, Publishing House of the USSR Academy of Sciences, 640 pp. [Google Scholar]
- Hall TA. (1999) BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Harada M, Yoshida TH. (1978) Karyological study of four Japanese Myotis bats (Chiroptera, Mammalia). Chromosoma (Berlin) 65: 283–291. 10.1007/BF00327623 [DOI] [Google Scholar]
- Harada M, Ando K, Uchida TA, Takada S. (1987) Karyotypic evolution of two Japanese Vespertilio species and its taxonomic implication (Chiroptera: Mammalia). Caryologia 40(3): 175–184. 10.1080/00087114.1987.10797821 [DOI] [Google Scholar]
- Hollister N. (1912) New mammals from the highlands of Siberia. Smithsonian miscellaneous collection 60: 1–6. 10.5962/bhl.part.7637 [DOI] [Google Scholar]
- Hwang JY, Jin GD, Park J, Lee SG, Kim EB. (2016) Complete sequences of eastern water bat, Myotis petax (Chiroptera; Microchiroptera; Vespertilionidae) mitogenome. Mitochondrial DNA Part A DNA Mapping, Sequencing, and Analysis 27(5): 3715–3716. 10.3109/19401736.2015.1079871 [DOI] [PubMed] [Google Scholar]
- Johnson LNL, McLeod BA, Burns LE, Arseneault K, Frasier TR, Broders HG. (2015) Population Genetic Structure Within and among Seasonal Site Types in the Little Brown Bat (Myotis lucifugus) and the Northern Long-Eared Bat (M. septentrionalis). PLoS ONE 10(5): e0126309. 10.1371/journal.pone.0126309 [DOI] [PMC free article] [PubMed]
- Kartavtseva IV, Gorobeiko UV, Tiunov MP. (2014) The current status of chromosomal investigations of bats (Chiroptera) from the Russian Far East. Russian Journal of Zoology. 93(7): 887–900. [Google Scholar]
- Kawai K, Nikaido M, Harada M, Matsumura S, Lin LK, Wu Y, Hasegawa M, Okada N. (2002) Intra- and interfamily relathionships of Vespertilionidae inferred by various molecular markers including SINE insertion data. Journal of Molecular Evolution 5: 284–301. 10.1007/s00239-002-2326-0 [DOI] [PubMed] [Google Scholar]
- Kawai K, Nikaido M, Harada M, Matsumura S, Lin LK, Wu Y, Hasegawa M, Okada N. (2003) The status of the Japanese and East Asian bats of the genus Myotis (Vespertilionidae) based on mitochondrial sequences. Molecular Phylogenetics and Evolution 28(2): 297–307. 10.1016/S1055-7903(03)00121-0 [DOI] [PubMed] [Google Scholar]
- Kearney TC, Volleth M, Contrafatto G, Taylor PG. (2002) Systematic implications of chromosome GTG-band and bacula morphology for Southern African Eptesicus and Pipistrellus and several other species of Vespertilioninae (Chiroptera: Vespertilionidae). Acta Chiropterologica. 4(1): 55–76. 10.3161/001.004.0107 [DOI] [Google Scholar]
- Korablev BP, Yakimenko LV, Tiunov MP. (1989) Karyotypes of bats in the Russian Far East. In: Kryukov AP, Chelomina GN, Pavlenko MV. (Eds) The present-day approached to studies of variability: collection of scientific papers.Vladivostok: The Far Eastern Branch Academy of Sciences of the USSR, 95–98.
- Kruskop SV. (2004) Subspecific structure of Myotis daubentonii (Chiroptera, Vespertilionidae) and composition of the “daubentonii” species group. Mammalia 68(4): 299–306. 10.1515/mamm.2004.029 [DOI] [Google Scholar]
- Kruskop SV. (2004) Order Chiroptera. In: Pavlinov IY, Lissovsky AA. (Eds) The Mammals of Russia: A Taxonomic and Geographic Reference.Moskow: KMK Sci. Press, 73–126.
- Kruskop SV, Borisenko AV, Ivanova NV, Lim BK, Eger JL. (2012) Genetic diversity of northeastern Palaearctic bats as revealed by DNA barcodes. Acta Chiropterologica 14(1): 1–14. 10.3161/150811012X654222 [DOI] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. (2018) MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution 35: 1547–1549. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzyakin AP. (1950) Bats. Moscow: Soviet science, 443 pp.
- Li N, Ao L, He SY, Gu XM. (2007) G-bands and C-bands in 3 species of Vespertilionidae. Chinese Journal of Zoology 42(2): 96–101. [Google Scholar]
- Lin LK, Motokawa M, Harada M. (2002) Karyological study of the house bat Pipistrellus abramus (Mammalia: Chiroptera) from Taiwan with comments on its taxonomic status. The Raffles Bulletin of Zoology 50(2): 507–510. [Google Scholar]
- Matthey R. (1973) The chromosome formulae of eutherian mammals. In: Cytotaxonomy and Vertebrate Evolution. London: Academic Press, 531–616. 10.1515/mamm.1973.37.3.394 [DOI]
- Matveev VA, Kruskop SV, Kramerov DA. (2005) Revalidation of Myotis petax Hollister, 1912 and its new status in connection with M. daubentonii (Kuhl, 1817) (Vespertilionidae, Chiroptera). Acta Chiropterologica 7(1): 23–37. 10.3161/1733-5329(2005)7[23:ROMPHA]2.0.CO;2 [DOI]
- Mazzoleni S, Rovatsos M, Schillaci O, Dumas F. (2018) Evolutionary insight on localization of 18S, 28S rDNA genes on homologous chromosomes in Primates genomes. Comparative Cytogenetics 12(1): 27–40. 10.3897/compcytogen.v12i1.19381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Kumar S. (2000) Molecular Evolution and Phylogenetics. Oxford University Press, New York.
- Ognev SV. (1928) Animals of Eastern Europe and North Asia. T. 1. Moscow, Leningrad, GIZ, 631 pp. [Google Scholar]
- Ono T, Obara Y. (1994) Karyotypes and Ag-NOR variations in Japanese vespertilionid bats (Mammalia: Chiroptera). Zoological Science 11(3): 473–484. [Google Scholar]
- Ono T, Yoshida MC. (1997) Differences in the chromosomal distribution of telomeric (TTAGGG)n sequences in two species of the vespertilionid bats. Chromosome Research 5: 203–212. 10.1023/A:1018403215999 [DOI] [PubMed] [Google Scholar]
- Orlov VN, Bulatova NSh. (1983) Comparative cytogenetics and karyosystematics of mammals: a training manual. Moscow: Science, 406 pp.
- Park S, Noh P, Choi YS, Joo S, Jeong G, Kim SS. (2019) Population genetic structure based on mitochondrial DNA analysis of Ikonnikov’s whiskered bat (Myotis ikonnikovi, Chiroptera: Vespertilionidae) from Korea. Journal of Ecology and Environment 43: 1–45. 10.1186/s41610-019-0140-5 [DOI] [Google Scholar]
- Peng YQ, Li GH, Gu XM. (2011) Study on the karyotypes, G-bands and C-bands of two Myotis species. Sichuan Journal of Zoology 30: 882–885. [Google Scholar]
- Ruedi M, Castella V. (2003) Genetic consequences of the ice ages on nurseries of the bat Myotis myotis: a mitochondrial and nuclear survey. Molecular Ecology 12(6): 1527–1540. 10.1046/j.1365-294X.2003.01828.x [DOI] [PubMed] [Google Scholar]
- Ruedi M, Stadelmann B, Gager Y, Douzery EJP, Francis CM, Lin LK, Guillén-Servent A, Cibois A. (2013) Molecular phylogenetic reconstructions identify East Asia as the cradle for the evolution of the cosmopolitan genus Myotis (Mammalia, Chiroptera). Molecuar Phylogenetics and Evolution 69: 437–449. 10.1016/j.ympev.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Ruedi M, Csorba G, Lin LK, Chou CH. (2015) Molecular phylogeny and morphological revision of Myotis bats (Chiroptera: Vespertilionidae) from Taiwan and adjacent China. Zootaxa 3920(1): 301–342. 10.11646/zootaxa.3920.2.6 [DOI] [PubMed] [Google Scholar]
- Seabright M. (1971) A rapid banding technique for human chromosomes. Lancet 2: 971–972. 10.1016/S0140-6736(71)90287-X [DOI] [PubMed] [Google Scholar]
- Sumner AT. (1972) A simple technique for demonstraiting centrometric heterochromatin. Experimental Cell Research 83: 438–442. 10.1016/0014-4827(74)90366-8 [DOI] [PubMed] [Google Scholar]
- Tiunov MP. (1997) Bats of the Russian Far East. Vladivostok: Dal’nauka Press, 134 pp. Tiunov MP (1984) Order Chiroptera Blumenbach, 1779 – Bats. In: Krivosheev VG. (Eds) Terrestrial mammals of the Far East.Nauka, Moscow, 73–102.
- Volleth M, Heller KG. (2012) Variations on a theme: Karyotype comparison in Eurasian Myotis species and implications for phylogeny. Vespertilio 16: 329–350. ISSN 1213-6123
- Volleth M, Bronner G, Gopfert MC, Heller KG, von Helversen O, Yong HS. (2001) Karyotype comparison and phylogenetic relationships of Pipistrellus-like bats (Vespertilionidae; Chiroptera; Mammalia). Chromosome Research 9: 25–46. 10.1023/A:1026787515840 [DOI] [PubMed] [Google Scholar]
- Volleth M, Heller KG, Fahr J. (2006) Phylogenetic relationships of three “Nycticeiini” genera (Vespertilionidae, Chiroptera, Mammalia) as revealed by karyological analysis. Mammalian Biology – Zeitschrift für Säugetierkunde 71(1): 1–12. 10.1016/j.mambio.2005.09.001 [DOI] [Google Scholar]
- Volleth M. (1987) Differences in the location of nucleolus organizer regions in European vespertilionid bats. Cytogenetics and Cell Genetics 44: 186–197. 10.1159/000132371 [DOI] [PubMed] [Google Scholar]
- Volleth M, Heller KG. (1994) Phylogenetic relationships of vespertilionid genera (Mammalia: Chiroptera) as revealed by karyological analysis. Zeitschrift für Zoologische Systematik und Evolutionsforschung 32: 11–34. 10.1111/j.1439-0469.1994.tb00467.x [DOI] [Google Scholar]
- Vorontsov NN. (1958) The importance of chromosomal sets for mammalian taxonomy. Bulletin of the Moscow Society of Naturalists 6(2): 5–36. [Google Scholar]
- Wang H, Li N, Ao L, Gu XM. (2009) A study on karyotypes, G-bands and C-bands of Myotis fimbriatus. Journal of Guizhou Normal University (Natural Science) 27(2): 13–14. 10.7150/ijbs.5.659 [In Chinese with English summary] [DOI] [Google Scholar]
- Wang L, Jiang TL, Sun KP, Wang YX, Tiunov MP, Feng J. (2010) Morphological description and taxonomical status of Myotis petax. Acta Zootaxonomica Sinica 35(2): 360–365. [Google Scholar]
- Wu Y, Motokawa M, Li YC, Harada M, Chen Z, Lin LK. (2009) Karyology of eight species of bats (Mammalia: Chiroptera) from Hainan Island, China. International Journal of Biological Sciences 5: 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo DH, Yoon MH. (1992) A karyotypic study on six Korean vespertilionid bats. Korean Journal of Zoology 35(4): 489–496. [Google Scholar]
- Yoshiyuki M. (1989) A Systematic Study of the Japanese Chiroptera. National Science Museum, Tokyo, 242 pp. [Google Scholar]
- Zhang Z, Tan X, Sun K, Liu S, Xu L, Feng J. (2009) Molecular systematics of the Chinese Myotis (Chiroptera, Vespertilionidae) inferred from cytochrome-b sequences. Mammalia 73: 323–330. 10.1515/MAMM.2009.058 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The other collecting data for Myotis petax
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Uliana V. Gorobeyko, Irina V. Kartavtseva, Irina N. Sheremetyeva, Denis V. Kazakov, Valentin Yu. Guskov
Data type
species data