Abstract
Background
Olfactory dysfunction is common in aging and associated with dementia and mortality. However, longitudinal studies tracking change in olfactory ability are scarce. We sought to identify predictors of interindividual differences in rate of olfactory identification change in aging.
Method
Participants were 1780 individuals, without dementia at baseline and with at least 2 olfactory assessments over 12 years of follow-up (mean age = 70.5 years; 61.9% female), from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K). Odor identification was assessed with the Sniffin’ Sticks. We estimated the impact of demographic, health, and genetic factors on rate of olfactory change with linear mixed effect models.
Results
Advancing age, manufacturing profession, history of cerebrovascular disease, higher cardiovascular disease burden, diabetes, slower walking speed, higher number of medications, and the APOE ε4 allele were associated with accelerated odor identification decline (ps < .014). Multi-adjusted analyses showed unique associations of age, diabetes, and ε4 to olfactory decline (ps < .017). In 1531 participants who remained free of dementia (DSM IV criteria) during follow-up, age, cardiovascular disease burden, and diabetes were associated with accelerated decline (ps < .011). Of these, age and diabetes remained statistically significant in the multi-adjusted model (ps < .001).
Conclusion
Demographic, vascular, and genetic factors are linked to rate of decline in odor identification in aging. Although some olfactory loss may be an inevitable part of aging, our results highlight the importance of vascular factors for the integrity of the olfactory system, even in the absence of dementia.
Keywords: Cognitive aging, Epidemiology, Olfactory, Olfactory impairment
In recent years, olfactory dysfunction (OD) has received increasing attention as a geriatric research topic. Reasons for this increase in interest are at least threefold. First, cross-sectional studies suggest that OD is common in the general older population and associated with adverse outcomes such as a diminished quality of life (1,2). Second, key brain regions of the olfactory system are among the first to be affected by Alzheimer’s disease (AD) and, indeed, OD is associated with an increased risk of dementia in older adults who are cognitively healthy at baseline (3–5). Third, individuals with OD are more likely to suffer from comorbidities and to die within the next few years compared to their normosmic peers (6–8).
Although olfactory processing encompasses several peripheral as well as higher cognitive aspects, research on OD in relation to aging and dementia has so far mainly focused the ability to identify odors. Previous cross-sectional studies have identified correlates to deficits in olfactory identification in a wide array of domains. For example, OD has been associated with demographic (age, male sex, and education), health (history of cancer, nasal dysfunctions, cardiovascular and cerebrovascular disease), and behavioral (smoking or having worked in a manufacturing occupation) factors (1,9). However, relatively few longitudinal studies have examined olfactory change following the same individuals over an extended time period. With follow-up times ranging between 3 and 10 years, these studies have consistently found olfactory function to decline with increasing age (10,11). Apart from age, identified risk factors of olfactory decline include male sex (11) and increased carotid artery intima media thickness, which is a biomarker of generalized atherosclerosis (12). Furthermore, carrying the ε4 allele of the apolipoprotein E gene (APOE), the most prominent genetic risk factor for the development of dementia, may accelerate olfactory loss (13,14), whereas the met allele of the brain-derived neurotrophic factor gene (BDNF) may attenuate olfactory decline (15).
Variations in olfactory ability are large, even between adults within the same age cohorts (16). This suggests that OD is not an inevitable part of aging and that there are factors that could slow down or accelerate its development. Given that longitudinal studies are sparse, the aim of this study was to investigate predictors that may affect rate of change in olfactory identification ability in aging. For this purpose, we followed 1780 cognitively healthy participants, derived from the general population, over a time span of up to 12 years. Sensitivity analyses investigated whether the observed associations remained in (a) participants that had not received a dementia diagnosis during follow-up, (b) participants without complete loss of smell (anosmia) at baseline, and (c) age-stratified samples of younger (≤72 years) and older (≤78 years) participants.
Method
Participants
Participants were from the Swedish National Study on Aging and Care in Kungsholmen (SNAC-K), a population-based study on aging and health that started in 2001. The original study population consisted of 4590 persons who were randomly drawn from the population registry of Kungsholmen, a central area of Stockholm, Sweden. Of these, 3363 took part in the baseline examination. Participants belonged to 11 prespecified age cohorts: 60, 66, 72, 78, 81, 84, 87, 90, 93, 96, and 99 years and older. To better capture the rapid changes in cognitive and health status of the oldest groups, participants ≥78 years are invited back every 3 years, whereas younger participants (60–72 years) are re-examined every 6 years. At each test wave, participants underwent comprehensive clinical, functional, and cognitive assessments (protocol available from http://www.snac-k-se). This examination involved a social interview and assessment of physical functioning; a clinical assessment of geriatric, neurological, and psychiatric information, and neuropsychological testing. In total, 2848 participants completed the neuropsychological test battery, which also included olfactory assessment (17). As cognitive impairment may impact performance in odor identification, we excluded participants with a baseline diagnosis of dementia, Parkinson’s disease, schizophrenia, or developmental disorder (see Supplementary Figure S1 for an overview of the study inclusion procedure). We further excluded participants with a baseline Mini-Mental State Examination (MMSE) score below 24, indicative of cognitive impairment. Out of the remaining 2669 participants, 2475 (92.7%) completed the olfactory assessment at baseline. Given the longitudinal focus of our study, we further excluded 695 persons (age = 75.1 [10.6]; years of education = 11.2 [4.1]; 59.1 % female; baseline odor identification score = 10.6 [3.6]) who did not participate in at least one additional olfactory assessment during the 12-year follow-up. The final sample consisted of 1780 participants, of whom 524 (29.4%) had participated in 1, 900 (50.6%) in 2, 269 (15.1%) in 3, and 87 (4,9%) in 4 follow-up assessments in addition to the baseline examination. Mean number of follow-up assessments was 1.95 (SD = 0.8). Comparisons between the final sample and participants who were excluded due to lack of follow-up showed that the latter group was significantly older (t = −9.96, p < .001, Hedges’s g = −0.47), had fewer years of education (t = 5.4, p < .001, Hedges’s g = 0.25), and lower olfactory scores (t = 9.8, p < .001, Hedges’s g = 0.49). There was no difference between these groups regarding sex distribution (χ 2 = 1.65, p = .199).
Odor Identification Assessment
We assessed odor identification with the Sniffin’ Sticks battery, a well-established and norm-referenced olfactory test kit with high test-retest reliability (18). Participants were presented with 16 household odors and instructed to freely identify the odor by providing a verbal descriptor. If they failed to give a correct label, they were presented with 4 written response alternatives (1 target and 3 foils) from which they were instructed to choose the label that best matched the odor. In a few cases, individual items were skipped, due to, for example mistakes during test administration, participant refusal, and allergy to a specific compound. In these cases, participants received a score of 0.25, representing chance-level performance. If participants were unable to perceive the presented odor, they received a score of 0 for that item (19). We calculated the odor identification score as the number of correctly identified odors with either free or cued identification (range 0–16).
To minimize test-order effects, the cognitive test battery was administered in 2 different orders across participants. In one, the odor test was administered at the end of the session. In the other, the odor test was administered in the middle of the session. We did not find test-order differences for odor identification performance at baseline (p = .42). To minimize possible effects of presentation order on odor identification performance, participants were randomly assigned to one of 3 test versions, with different presentation orders of the odors. At the next follow-up, the participant would receive a different version in order to minimize retest effects at follow-up occasions (18). Comparisons between test versions at baseline showed that average performance was better for version 1 compared to the other 2 versions for odor identification (p < .01). As test version was randomized across participants, these differences should not have impacted the results. However, we included test version as a covariate in the analyses to certify that the results were not affected by differences on this variable.
Demographic Factors
Data on age, sex, and years of education were collected following standard protocols. Age was measured as years since birth and education as years of formal schooling. We centered these variables on their respective means. Participants’ longest held professional occupation was obtained through self-reports and dichotomized into manufacturing (“blue-collar”) or non-manufacturing (“white-collar”) work.
Vocabulary
Given that the odor identification test involves matching olfactory input to word labels, performance in this test may be affected by word knowledge. We therefore included a Swedish vocabulary test, SRB1 (20). Participants are here presented with 30 words together with 5 additional words and the task is to choose the correct synonym in forced-choice format, similar to the odor identification test.
Health
Chronic medical conditions were diagnosed by physicians and based on the clinical assessment, self-report, medications lists, laboratory data, and information from the computerized Stockholm inpatient register. If not otherwise specified, diagnoses were coded according to the International Classification of Diseases, 10th revision (ICD-10) and dichotomized into present (1) or absent (0).
Vascular Health
Vascular diagnoses included cerebrovascular disease (ICD-10 I60-I69) and cardiovascular disease burden (total number of cardiovascular diseases, including atrial fibrillation [ICD-10 I48], heart failure [ICD-10 I50], and ischaemic heart disease [ICD-10 I20–I25]; range 0–3). Diagnosis of diabetes type 1 or 2 was obtained from medical history, use of diabetes drugs (ATC code A10), diagnosis in the Stockholm inpatient register (ICD-10 code E11), or HbA1c ≥ 6.5% (48 mmol/mol) (21). Only 5 participants had a diagnosis of diabetes type I, which rendered separate statistical analysis according to diabetes type unfeasible (Table 1). We therefore combined type I and II diabetes into one diabetes variable. Hypertension stage 2 (≥ 160/100 mm Hg or current use of antihypertensive medication), smoking (derived from self-reports and categorized into former smokers, current smokers, and never smoked), and obesity (≥30 kg/m2, based on body mass index obtained through weight and height measurements) were also assessed.
Table 1.
Sample Characteristics Derived From Baseline, If Not Stated Otherwise
| Total Sample, N = 1780 | Age ≤ 72, n = 1185 | Age ≥ 78, n = 595 | |
|---|---|---|---|
| Number of follow-up assessments | 3.0 (0.8) | 2.9 (0.6) | 3.2 (1.1) |
| Odor identification score, mean (SD) | 12.1 (2.8) | 12.8 (2.3) | 10.6 (3.1) |
| Age, mean (SD) | 71.0 (9.4) | 65.3 (4.8) | 82.5 (4.6) |
| Female % | 61.9 | 58.6 | 68.4 |
| Years of education, mean (SD) | 12.6 (4.2) | 13.5 (4.2) | 10.8 (3.8) |
| Manufacturing profession, % | 17.9 | 13.8 | 27.7 |
| Vocabulary (SRB1), mean (SD) | 23.5 (4.6) | 24.3 (4.1) | 21.8 (5.0) |
| History of cerebrovascular disease, % | 7 | 3.9 | 12.9 |
| Cardiovascular disease burden, mean (SD) | 0.3 (0.7) | 0.2 (0.5) | 0.6 (0.9) |
| Diabetes, % | 7.6 | 6.9 | 8.9 |
| Hypertension stage 2, % | 48.3 | 40.2 | 64.4 |
| Smoking, %: currently/ever/never | 13/41/46 | 15/44/41 | 9/35/56 |
| Obesity, % | 13.4 | 14.4 | 12.6 |
| Depression, % | 3 | 2 | 5.0 |
| History of head trauma, % | 13.3 | 14.9 | 9.9 |
| Number of medications, mean (SD) | 3.4 (3.1) | 2.8 (2.8) | 4.7 (3.3) |
| Gait speed, mean (SD) | 1.15 (0.4) | 1.3 (0.3) | 0.9 (0.4) |
| APOE ε4 carrier, % | 28.5 | 30.2 | 25.2 |
| BDNF met carrier, % | 33.5 | 33.6 | 33.3 |
| Development of dementia at follow-up, % | 14.0 | 6.0 | 29.9 |
Note: N = sample size. Percentage missing values: 0.1% for manufacturing profession, 0.5% for vocabulary, 0.1% for hypertension, 0.6% for smoking, 1.3% for obesity, 1.4% for gait speed, 1.4% for APOE, and 4.0% for BDNF.
Other Health Factors
Neurological/psychiatric predictors encompassed current diagnosis of depression (ICD-10 F32) and history of head trauma (ICD-10 S06). Gait speed (m/s) was included as a measure of mobility and general health status (22) and assessed by asking participants to walk 6 m at their usual speed. Participants who reported difficulties walking, or for whom the assessment had to be carried out in a restricted space, were asked to walk 2.4 m. We further considered polypharmacy, measured as total number of medications (23).
Genotyping
Genotyping of APOE (rs429358) and BDNF (rs6265) was obtained using MALDI-TOF analysis on the Sequenom MassARRAY platform at the Mutation Analysis Facility, Karolinska Institutet. Participants were grouped as either carriers or noncarriers of the APOE ε4 allele throughout all main analyses, whereas follow-up analyses also considered the effects of carrying 2 ε4 alleles. For BDNF, participants were grouped into homozygotic val carriers versus carriers of any met allele.
Dementia Diagnosis
Sensitivity analyses involved conversion to dementia during the study period. At each assessment, all-cause dementia was diagnosed using DSM-IV criteria. The examining physician and a second physician made an independent preliminary diagnoses of dementia based on the clinical examination. A third senior neurologist was consulted to reach consensus in cases of disagreement. Among participants who died during the follow-up period, dementia diagnoses were made by consulting death certificates and medical records.
Statistical Analysis
We assessed change in olfactory function over the follow-up period using linear mixed-effect models. Time in study, measured in years from baseline, was used as the time scale. We fitted separate models for each predictor variable, assessing its effect on the intercept and rate of change on the odor identification task. The fixed effects included factorial indicator variables for the categorical predictors or continuous variables for the continuous predictors, linear annual follow-up time, and a term for the interaction between the predictor and time. The random effects included random intercept and slope, allowing for individual differences at baseline and across time. We used unstructured variance–covariance matrices for all models. After having fitted separate models for every predictor variable, we included all predictors that were found to be significantly associated with olfactory change into one competing model, together with their interaction terms with time. This allowed us to analyze which variables were associated with olfactory change independent of the other predictors.
To determine whether an observed association of the predictor variable with olfactory change may have been driven by preclinical dementia pathology, sensitivity analyses included participants who remained free of dementia during follow-up (n = 1531). Furthermore, we considered the possibility that our results may be confounded by participants that already exhibited severe OD at baseline. We therefore repeated our analyses after having excluded participants with a baseline olfactory score of <7 (n = 1698), indicative of anosmia (9). Third, different mechanisms may underlie olfactory change at different ages. We therefore stratified our sample according to age at baseline to investigate predictors of olfactory change in subsamples of younger and older participants. As participants in SNAC-K belong to prespecified age cohorts, we stratified our sample into the following groups of younger (≤72 years, n = 1185) and older (≥78 years, n = 595) participants.
Results
Table 1 provides information on baseline characteristics of our study population. Of the 1780 participants, 558 were reassessed at wave 2, 1532 at wave 3, 447 at wave 4, and 932 at wave 5. Coefficients of intercorrelations between the odor identification assessments at each testing phase were statistically significant and generally high (Table 2).
Table 2.
Correlations Between Odor Identification Scores at Each Assessment Wave
| Odor Identification Score | ||||
|---|---|---|---|---|
| Odor Identification Score | Baseline | Wave 2 | Wave 3 | Wave 4 |
| Baseline | – | |||
| Wave 2 | 0.64** | – | ||
| Wave 3 | 0.63** | 0.64** | – | |
| Wave 4 | 0.60** | 0.68** | 0.70** | – |
| Wave 5 | 0.49** | 0.46** | 0.58** | 0.64** |
Note: **Significance < .001.
Change in Olfactory Identification
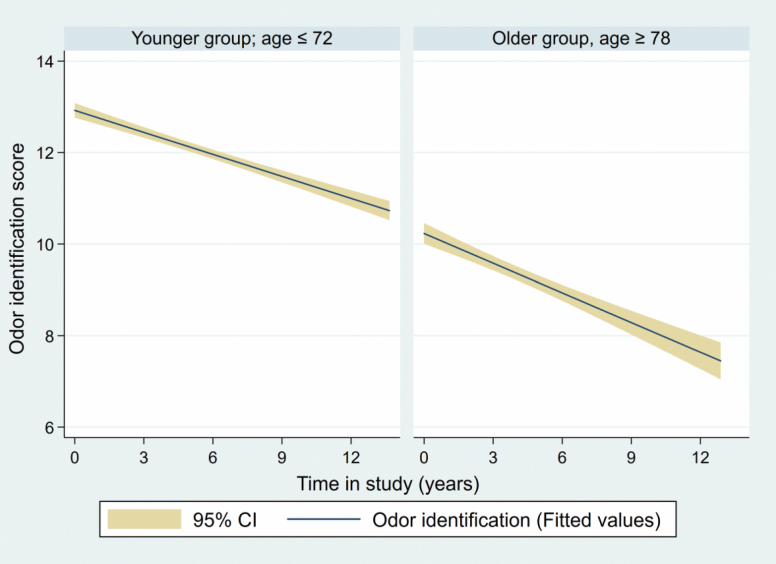
Without taking any other variables into account except time, participants showed significant decline in olfactory identification ability for each year in the study (β = −0.213 [95% CI −0.23 to −0.196], p < .001; Cohen’s ƒ 2 = 0.40). Decline trajectories were attenuated by excluding participants who developed dementia during follow-up but remained statistically significant (β = −0.194 [95% CI −0.209 to −0.179], p < .001; Cohen’s ƒ 2 = 0.21). Results of age-stratified analyses (Figure 1) showed statistically significant decline trajectories for both older (≥78 years; β = −0.348 [95% CI −0.386 to −0.31], p < .001; Cohen’s ƒ 2 = 0.38) and younger (≤72 years; β = −0.168 [95% CI −0.186 to −0.149], p < .001; Cohen’s ƒ 2 = 0.04) participants.
Figure 1.
Estimated decline trajectories in odor identification ability (dark line), along with 95% CIs (shadowed area), during the studied period. Stratified by age of the participants at baseline.
Individual Predictors of Olfactory Change
Results from univariate mixed models are summarized in Table 3. Older age at baseline was associated with lower olfactory ability (p < .001) and a steeper rate of olfactory decline (p < .001). Years of education were positively associated with olfactory performance at baseline (p < .008), but not with olfactory change (p = .212). Similarly, women outperformed men at baseline (p < .001), but there was no significant Sex × Time interaction (p = .553), indicating parallel olfactory-decline trajectories for women and men, despite differences in starting points. Compared to non-manufacturing work, manufacturing profession was significantly associated with a steeper olfactory decline (p = .014), despite similar baseline performance (p = .302). Better performance on the vocabulary test (SRB1) was related to higher olfactory baseline performance (p < .001) and slower rate of olfactory change (p = .026).
Table 3.
Results of Univariate Mixed Models for Each of the Predictor Variables in the Total Sample, Adjusted for Baseline Age, Sex, Education, and Test Version
| Cross-sectional | Predictor × Time | |
|---|---|---|
| β (95% CI); p-Value | β (95% CI); p-Value | |
| Age | −0.109 (−0.122 to −0.096); <.001 | −0.012 (−0.014 to −0.01); <.001 |
| Female | 0.801 (0.554 to 1.048); <.001 | 0.011 (−0.025 to 0.046); .553 |
| Years of education | 0.04 (0.011 to 0.07); .008 | 0.003 (−0.001 to 0.007); .212 |
| Manufacturing profession | −0.174 (−0.503 to 0.156); .302 | −0.058 (−0.104 to −0.012); .014 |
| Vocabulary (SRB1) | 0.069 (0.04 to 0.097); <.001 | 0.004 (0.001 to 0.008); .026 |
| History of cerebrovascular disease | −0.056 (−0.532 to 0.419); .816 | −0.127 (−0.206 to −0.047); .002 |
| Cardiovascular disease burden | −0.103 (−0.294 to 0.089); .294 | −0.057 (−0.087 to −0.026); <.001 |
| Diabetes | −0.173 (−0.625 to 0.279); .454 | −0.114 (−0.182 to −0.047); .001 |
| Hypertension stage 2 | 0.185 (−0.619 to 0.433); .142 | −0.027 (−0.061 to 0.008); .129 |
| Obesity | −0.133 (−0.486 to 0.220); .460 | 0.03 (−0.02 to 0.081); .234 |
| Smoking (previously) | −0.165 (−0.425 to 0.096); .215 | 0.012 (−0.025 to 0.049); .517 |
| Smoking (currently) | −0.344 (−0.723 to 0.035); .076 | −0.011 (−0.066 to 0.043); .679 |
| History of head trauma | −0.305 (−0.659 to 0.049); .092 | −0.011 (−0.061 to 0.039); .647 |
| Depression | −0.821 (−1.513 to −0.13); .02 | −0.031 (−0.14 to 0.078); .577 |
| Number of medications | −0.004 (−0.044 to 0.037); .863 | −0.009 (−0.015 to −0.003); .002 |
| Walking speed | 0.589 (0.188 to 0.99); .004 | 0.127 (0.075 to 0.179); <.001 |
| APOE ε4 carrier | −0.31 (−0.577 to −0.044); .023 | −0.057 (−0.095 to −0.019); .003 |
| BDNF met carrier | 0.256 (0.001 to 0.511); .049 | −0.028 (−0.065 to 0.01); .146 |
History of cerebrovascular disease was associated with a steeper rate of olfactory decline (p = .002), as were cardiovascular disease burden (p < .001) and diabetes (p = .001). These conditions were not significantly associated with olfactory ability cross-sectionally. Apart from diabetes, we did not find statistically significant associations between cardiovascular risk factors (ie, hypertension, obesity, and smoking) and baseline performance or rate of olfactory change. History of head trauma did not affect olfactory function in our sample, neither at baseline nor across time. Depression was associated with a lower olfactory score at baseline (p = .02), but not with differences in olfactory-change trajectories. Faster walking speed was related to both better olfactory performance at baseline (p = .004) and to a less steep olfactory-decline trajectory (p < .001). Total number of medications was not linked to differences in olfactory baseline scores, but was predictive of accelerated olfactory decline (p = .002).
Carriers of the APOE ε4 allele had significantly lower odor identification scores at baseline (p = .023) and exhibited a steeper rate of olfactory decline (p = .003). Follow-up analyses considering number of risk alleles showed that both carriers of 1 (p = .010) and 2 (p = .040) ε4-alleles showed a steeper rate of olfactory decline. However, olfactory-decline trajectories were not significantly steeper for carriers of 2 as compared to one ε4 allele (p = .247). We did not find significant differences between carriers and non-carriers of the BDNF met allele in olfactory ability change. However, BDNF met carriers had significantly higher baseline odor identification scores (p = .049).
Multi-adjusted Predictors of Olfactory Change
To test which predictors were uniquely associated with olfactory change, we ran a multi-adjusted model in which we entered all variables that were significant predictors of olfactory change in the univariate models, together with their interaction terms with time. Thus, this model included manufacturing profession, vocabulary, cerebrovascular disease, cardiovascular disease burden, diabetes, walking speed, number of medications, and APOE ε4. These analyses controlled for sex, education, and test version at baseline. Results from the multi-adjusted models are summarized in Table 4. We found that older age (p < .001), APOE ε4 (p = .001), and diabetes (p = .007) were associated with accelerated olfactory decline, independent of the other predictors.
Table 4.
Results of Multi-adjusted Mixed Models With Subsample-Specific Significant Predictors as Competing Covariates, in Interaction With Time
| Total Sample (N = 1780) |
No Dementia During Follow-up (n = 1531) |
Age ≤ 72 (n = 1185) |
Age ≥ 78 (n = 595) |
|
|---|---|---|---|---|
| Predictor × Time | Predictor × Time | Predictor × Time | Predictor × Time | |
| β (95% CI) | β (95% CI) | β (95% CI) | β (95% CI) | |
| Age | −0.012 (−0.014 to −0.01); <.001 | −0.01 (−0.012 to −0.007); <.001 | −0.013 (−0.017 to −0.01); <.001 | N.a. |
| Manufacturing profession | −0.007 (−0.054 to 0.04); .766 | N.a. | N.a. | N.a. |
| Vocabulary | −0.001 (−0.005 to 0.003); .695 | N.a. | N.a. | N.a. |
| Cerebrovascular disease | −0.077 (−0.155 to 0.002); .055 | N.a. | −0.072 (−0.017 to 0.026); .150 | N.a. |
| Cardiovascular disease burden | −0.009 (−0.041 to 0.023); .574 | −0.022 (−0.056 to 0.012); .203 | N.a. | N.a. |
| Diabetes | −0.093 (−0.161 to −0.026); .007 | −0.128 (−0.20 to −0.06); <.001 | −0.106 (−0.179 to −0.033); .004 | N.a. |
| Hypertension | N.a. | N.a. | N.a. | 0.084 (0.005–0.162); .037 |
| Number of medications | 0.004 (−0.003 to 0.01); .249 | 0.003 (−0.003 to 0.01); .307 | N.a. | N.a. |
| Gait speed | −0.005(−0.064 to 0.054); .873 | −0.024 (−0.084 to 0.036); .436 | N.a. | N.a. |
| APOE ε4 | −0.064 (−0.101 to −0.028); .001 | N.a. | −0.073 (−0.112 to −0.034); <.001 | N.a. |
| BDNF met | N.a. | N.a. | N.a. | −0.102 (−0.182 to −0.021); .014 |
Note: N.a. = not applicable (ie, predictor was not statistically significant in the univariate mixed models within these samples and therefore not included in the multi-adjusted model). Adjusted for age, sex, education, and test version.
Sensitivity Analyses
Results of univariate sensitivity analyses with mixed models in subsamples are summarized in Supplementary Table S1. During the 12-year follow-up, 14% (n = 249) of participants developed dementia. Sensitivity analyses in a subsample without prodromal dementia (n = 1531) yielded a different pattern of results as compared to the total sample. Manufacturing profession, vocabulary, cerebrovascular disease, and the ε4 allele were no longer significant predictors of olfactory decline. By contrast, age, cardiovascular disease burden, diabetes, number of medications, and walking speed remained significantly associated with olfactory change (ps < .011). Again, we were interested in which variables remained as unique predictors of olfactory decline. Results from the multi-adjusted model with age, cardiovascular disease burden, diabetes, number of medications, and walking speed as competing variables showed that age and diabetes remained uniquely associated with olfactory decline in the dementia-free subsample (ps < .001; Table 4). At baseline, 4.6 % (n = 82) of participants had an odor identification score below 7 and were classified as anosmic (9). In a subsample free from anosmia (n = 1698), we found the same predictors to be associated with olfactory decline as in the initial sample, both in univariate and multi-adjusted analyses.
Finally, results of univariate models in subsamples with younger (≤72; n = 1185) and older (≥78; n = 595) participants yielded different patterns for predictors of olfactory change. In the younger sample, rate of olfactory decline was significantly associated with advancing age, cerebrovascular disease, cardiovascular disease burden, diabetes, and APOE ε4 (ps < .025). Age, diabetes, and ε4 persisted as predictors in the multi-adjusted model (ps < .004; Table 4). In the older group, participants with hypertension and BDNF val homozygotes (ps < .038) exhibited less accelerated olfactory decline. These predictors remained in the multi-adjusted model (ps < .037; Table 4).
Discussion
We studied intraindividual change in odor identification in older adults over a follow-up period of 12 years. Participants in our study declined significantly in olfactory function over the study period, which is in line with the few studies that have previously examined olfactory abilities in aging longitudinally. The average decline was approximately 0.21 items per year in the odor identification test, and the effect size for time was quite large. Age-stratified comparisons of effect sizes indicated a markedly steeper effect of time on olfactory function in the older as compared to the younger cohorts. Albeit somewhat higher, the annual rates of change seen for the younger and older age groups in our sample were comparable to rates obtained in middle-aged and older adults in a previous study based on a similar olfactory test and follow-up time span (14).
Advancing age was a significant predictor of accelerated olfactory identification decline. Notably, we found that age remained statistically significant as a predictor in the multi-adjusted model, as well as in sensitivity analyses of participants that remained free from dementia. Given the large differences in olfactory performance across age cohorts, aging likely constitutes one of the most important determinants of olfactory loss (24). Intact olfaction relies heavily on neuronal turnover (25), a feature that renders it sensitive to age-related decreases in neural plasticity (26). These deficits may affect both peripheral sensory and higher olfactory functions. In addition, cumulative effects of sinonasal diseases or upper respiratory infections in the olfactory sense are directly linked to advancing age (24). Furthermore, risk factors of OD likely accumulate in older age where they may exacerbate olfactory decline related to normal aging. Interestingly, our age-stratified analyses showed that age was no longer predictive of olfactory change in adults aged 78+, despite steeper decline trajectories in this sample as compared to the younger participants. These findings suggest that significant damage to the olfactory system, catalyzing olfactory loss, may already have taken place in older age. Importantly, none of the variables that emerged as risk factors for olfactory decline in the total sample were associated with decline in the older sample, although they were predictive of olfactory change among the younger participants. These findings may partly have resulted from diminished power in the older group due to smaller sample size, but also reflect selective survival. On the other hand, the fact that modifiable variables were not associated with olfactory decline in the oldest cohorts may also suggest that olfactory function is more easily influenced at younger ages. From this perspective, attempts to ameliorate olfactory decline through the management of risk factors should be implemented as early as possible.
Women in our sample outperformed men in baseline odor identification, which is in line with results from previous cross-sectional studies (27,28). Contrary to the results of one longitudinal study (11) but in line with another (13), being female was not associated with an attenuated rate of decline over time. Whereas female sex may be linked to better olfactory performance throughout the lifespan, our results indicate that it does not protect from olfactory decline. We found a similar pattern for years of education, where higher educational level was linked to better baseline performance, but not to rate of olfactory change.
Our results suggested that compared to “white collar” workers, individuals with a manufacturing background exhibited accelerated olfactory decline, independently of sex and education. Findings from previous cross-sectional studies indicate that the exposure to certain work-related chemicals may harm the olfactory system (29,30). Note, however, that manufacturing profession did not survive as a predictor in the multi-adjusted model. This may suggest that, apart from the exposure to harmful chemicals, manufacturing profession may be associated with olfactory loss also due to an increased risk of, for example, cardiovascular conditions or other health factors related to socioeconomic status.
Higher cardiovascular disease burden, cerebrovascular disease, and diabetes were all associated with an accelerated decline in odor identification. These findings agree with previous cross-sectional studies that have linked cardiovascular disease to OD (12,31). However, cardiovascular disease burden was not uniquely associated with olfactory decline, indicating significant overlap with comorbidities. Although cerebrovascular disease was uniquely associated with olfactory decline in the multi-adjusted model, the effect disappeared when excluding participants who developed dementia during follow-up. This suggests an overlap with dementia pathology, which could be of vascular or neurodegenerative origin, or both (32).
We found diabetes to accelerate olfactory loss, independent of dementia and other comorbidities and risk factors. Given that only 5 participants were diagnosed with type I diabetes in our main sample, this association was likely driven by the participants with type II diabetes. Cross-sectional studies have linked type II diabetes to OD, though it should be noted that findings have not been consistent (33,34). We suggest that the effects of type II diabetes on the olfactory system may be cumulative and therefore more likely to be observed in longitudinal settings. Type II diabetes may lead to the development of insulin-resistance in the olfactory system, ultimately disrupting the function of the olfactory bulbs (35). Importantly, type II diabetes also increases the risk of developing cardiovascular disease (36), which in turn may exacerbate olfactory loss. It is important to point out that we found that neither cardiovascular disease burden nor diabetes were associated with olfactory performance differences at baseline. If OD is partially caused by these conditions, effects may be negligible at baseline in a sample of relatively high-performing and healthy participants.
Although some previous cross-sectional findings have linked hypertension to OD (37), we found that hypertension was protective of olfactory decline in participants aged 78+. Strong evidence has linked midlife hypertension to harmful effects on cognitive function, whereas late-life findings are mixed, with some studies suggesting negative associations between high blood pressure and cognitive ability and others reporting opposite or u-shaped associations (38). Furthermore, it is possible that antihypertensive drugs, such as angiotensin II receptor blockers and dihydropyridines, may reduce the risk of cognitive impairment. If antihypertensive treatment protects against cognitive decline, favorable effects on olfaction are conceivable, but more research is needed (39).
We found higher walking speed to attenuate decline in odor identification, an effect that persisted when excluding participants who converted to dementia. Gait speed represents an important marker of general physical condition (22). As such, gait speed may reflect overall health in a similar manner as the state of the olfactory system (40). Indeed, some previous findings suggest a significant overlap between frailty, inflammatory markers, and OD (41,42). Note, however, that gait speed was not associated with olfactory change in multi-adjusted models, again indicating significant overlap with comorbidities. Likewise, number of medications remained predictive in the absence of dementia, but were not uniquely associated with steeper olfactory decline. Although many commonly prescribed medications as well as polypharmacy in itself may affect olfactory function (43), dissociating the effects of treatment from ailment is intrinsically difficult. However, as shown by one previous cross-sectional study, plausible conclusions about mechanistic drug effects on olfactory function may be drawn by using a molecular drug-target-based approach (44).
Carrying the ɛ4 allele accelerated olfactory decline, independent of other risk factors. However, our results suggest that APOE may not affect olfactory change in the absence of dementia, contradicting some previous findings (45). As the ɛ4 allele is associated with deficits in promoting neuritic outgrowth in the olfactory epithelium (46), it may increase the risk of impaired cellular regeneration within the olfactory system independent of dementia pathology. However, it is conceivable that the impact of the ɛ4 allele on olfactory integrity may be smaller in magnitude than its impact on dementia pathology. Indeed, we found that the slope of olfactory decline associated with APOE was smaller in the dementia-free as compared to the total sample. More large-scale analyses are needed in order to clarify whether the olfactory system may be affected by APOE also in healthy aging.
Although we did not find an effect of BDNF on olfactory change in the total sample, met carriers showed better baseline olfactory performance as compared to val homozygotes, as we have shown previously (9). Reasons for why the met allele may be beneficial for olfactory function remain unclear, but it has been speculated that it may act favorably on inhibitory microcircuits in the olfactory bulbs (47). Importantly, results from our age-stratified analyses showed steeper olfactory decline trajectories for met carriers in the older sample, contradicting one previous study with a similar design (15). Previous studies have suggested an age-inversed effect of BDNF on cognitive function, although in the opposite direction to our findings (48). Further studies with large sample sizes in different age cohorts are needed to clarify the impact of BDNF on olfactory function.
A strength of this study is the longitudinal design including 12 years of follow-up, allowing for the identification of predictors of intraindividual olfactory decline over an extended time period. Another strength is the comparatively large sample size and the population-based nature of the sample, which increases generalizability. However, attrition is an inevitable concern in longitudinal research, and we found that participants with a comparatively better sense of smell were more likely to remain in the study to undergo subsequent olfactory testing. This selective drop-out may have affected our results such that the actual rate of olfactory decline, as well as its associations with the investigated predictors, may be underestimated. Cross-sectional findings indicate that olfactory function may remain relatively stable until about the age of 65 after which it declines more rapidly (9,49). This suggests that olfactory loss may not follow a linear rate of decline. Future studies, including an even larger number of follow-up occasions allowing for the investigation of quadratic trends, would enhance our understanding of the shape of olfactory change trajectories in aging. Finally, we were unable to consider the effect of respiratory diseases on change in olfactory ability, due to a lack of objective information regarding ear, nose, and throat conditions.
We found olfactory identification to decline significantly during 12 years of follow-up in older adults. Our results highlight the importance of advancing age, vascular health, and genetic variability for the state of the olfactory system in aging.
Supplementary Material
Acknowledgments
I.E. and E.J.L. contributed to the conception and design of the study. I.E. conducted the statistical analyses. All the authors contributed to interpretation of the results. I.E. drafted the first version of the manuscript. All the authors critically revised the manuscript for important intellectual content. All the authors made a significant contribution to the research and the development of the manuscript and approved the final version for publication. We thank the participants as well as all staff involved in the data collection and management of the SNAC-K study.
Funding
SNAC-K is financially supported by the Swedish Ministry of Health and Social Affairs, the participating County Councils and Municipalities, and the Swedish Research Council. This work was further funded by a research grant from the Swedish Research Council awarded to E.J.L. (2017-01759), a program grant from the Swedish Foundation for Humanities and Social Sciences awarded to M.L. (M14-0375:1).
Conflict of Interest
None declared.
References
- 1. Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. J Am Med Assoc. 2002;288(18):2307–2312. doi: 10.1001/jama.288.18.2307 [DOI] [PubMed] [Google Scholar]
- 2. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life—an updated review. Chem Senses. 2014;39(3):185–194. doi: 10.1093/chemse/bjt072 [DOI] [PubMed] [Google Scholar]
- 3. Attems J, Lintner F, Jellinger KA. Olfactory involvement in aging and Alzheimer’s disease: an autopsy study. J Alzheimers Dis. 2005;7(2):149–157; discussion 173. doi: 10.3233/jad-2005-7208 [DOI] [PubMed] [Google Scholar]
- 4. Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology. 2015;84(2):182–189. doi: 10.1212/WNL.0000000000001132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stanciu I, Larsson M, Nordin S, Adolfsson R, Nilsson LG, Olofsson JK. Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: a longitudinal, population-based study. J Int Neuropsychol Soc. 2014;20(2):209–217. doi: 10.1017/S1355617713001409 [DOI] [PubMed] [Google Scholar]
- 6. Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114(10):1764–1769. doi: 10.1097/00005537-200410000-00017 [DOI] [PubMed] [Google Scholar]
- 7. Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS ONE. 2014;9(10):e107541. doi: 10.1371/journal.pone.0107541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ekström I, Sjölund S, Nordin S, et al. Smell loss predicts mortality risk regardless of dementia conversion. J Am Geriatr Soc. 2017;65(6):1238–1243. doi: 10.1111/jgs.14770 [DOI] [PubMed] [Google Scholar]
- 9. Seubert J, Laukka EJ, Rizzuto D, et al. Prevalence and correlates of olfactory dysfunction in old age: a population-based study. J Gerontol A Biol Sci Med Sci. 2017;72(8):1072–1079. doi: 10.1093/gerona/glx054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ship JA, Pearson JD, Cruise LJ, Brant LJ, Metter EJ. Longitudinal changes in smell identification. J Gerontol A Biol Sci Med Sci. 1996;51(2):M86–M91. doi: 10.1093/gerona/51a.2.m86 [DOI] [PubMed] [Google Scholar]
- 11. Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK. The rate of age-related olfactory decline among the general population of older U.S. adults. J Gerontol A Biol Sci Med Sci. 2015;70(11):1435–1441. doi: 10.1093/gerona/glv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schubert CR, Cruickshanks KJ, Fischer ME, Klein BE, Klein R, Pinto AA. Inflammatory and vascular markers and olfactory impairment in older adults. Age Ageing. 2015;44(5):878–882. doi: 10.1093/ageing/afv075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ekström I, Josefsson M, Larsson M, Rönnlund M, Nordin S, Olofsson JK. Subjective olfactory loss in older adults concurs with long-term odor identification decline. Chem Senses. 2019;44(2):105–112. doi: 10.1093/chemse/bjy079 [DOI] [PubMed] [Google Scholar]
- 14. Josefsson M, Larsson M, Nordin S, Adolfsson R, Olofsson J. APOE-ɛ4 effects on longitudinal decline in olfactory and non-olfactory cognitive abilities in middle-aged and old adults. Sci Rep. 2017;7(1):1286. doi: 10.1038/s41598-017-01508-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hedner M, Nilsson LG, Olofsson JK, et al. Age-related olfactory decline is associated with the BDNF val66met polymorphism: evidence from a population-based study. Front Aging Neurosci. 2010;2:24. doi: 10.3389/fnagi.2010.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264(3):237–243. doi: 10.1007/s00405-006-0173-0 [DOI] [PubMed] [Google Scholar]
- 17. Larsson M, Hedner M, Papenberg G, Seubert J, Bäckman L, Laukka EJ. Olfactory memory in the old and very old: relations to episodic and semantic memory and APOE genotype. Neurobiol Aging. 2016;38:118–126. doi: 10.1016/j.neurobiolaging.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 18. Croy I, Zehner C, Larsson M, Zucco GM, Hummel T. Test-retest reliability and validity of the Sniffin’ TOM odor memory test. Chem Senses. 2015;40(3):173–179. doi: 10.1093/chemse/bju069 [DOI] [PubMed] [Google Scholar]
- 19. Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39–52. doi: 10.1093/chemse/22.1.39 [DOI] [PubMed] [Google Scholar]
- 20. Dureman I. SRB: 1. Psykologiförlaget; 1960. [Google Scholar]
- 21. Marseglia A, Wang HX, Rizzuto D, Fratiglioni L, Xu W. Participating in mental, social, and physical leisure activities and having a rich social network reduce the incidence of diabetes-related dementia in a cohort of Swedish older adults. Diabetes Care. 2019;42(2):232–239. doi: 10.2337/dc18-1428 [DOI] [PubMed] [Google Scholar]
- 22. Grande G, Vetrano DL, Fratiglioni L, et al. Disability trajectories and mortality in older adults with different cognitive and physical profiles. Aging Clin Exp Res. 2020;32(6):1007–1016. doi: 10.1007/s40520-019-01297-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wimmer BC, Bell JS, Fastbom J, Wiese MD, Johnell K. Medication regimen complexity and number of medications as factors associated with unplanned hospitalizations in older people: a population-based cohort study. J Gerontol A Biol Sci Med Sci. 2016;71(6):831–837. doi: 10.1093/gerona/glv219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol. 2014;5:20. doi: 10.3389/fpsyg.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Durante MA, Kurtenbach S, Sargi ZB, et al. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat Neurosci. 2020;23:323–326. doi: 10.1038/s41593-020-0587-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watabe-Rudolph M, Begus-Nahrmann Y, Lechel A, et al. Telomere shortening impairs regeneration of the olfactory epithelium in response to injury but not under homeostatic conditions. PLoS ONE. 2011;6(11):e27801. doi: 10.1371/journal.pone.0027801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larsson M, Finkel D, Pedersen NL. Odor identification: influences of age, gender, cognition, and personality. J Gerontol B Psychol Sci Soc Sci. 2000;55(5):P304–P310. doi: 10.1093/geronb/55.5.p304 [DOI] [PubMed] [Google Scholar]
- 28. Sorokowski P, Karwowski M, Misiak M, et al. Sex differences in human olfaction: a meta-analysis. Front Psychol. 2019;10:242. doi: 10.3389/fpsyg.2019.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park SW, Kang YJ, Eom H, Cho HJ, Ahn J, Lee SG. Work-related olfactory disorder: a case series and review. Ann Occup Environ Med. 2018;30:18. doi: 10.1186/s40557-018-0230-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Werner S, Nies E. Olfactory dysfunction revisited: a reappraisal of work-related olfactory dysfunction caused by chemicals. J Occup Med Toxicol. 2018;13:28. doi: 10.1186/s12995-018-0209-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siegel JK, Wroblewski KE, McClintock MK, Pinto JM. Olfactory dysfunction persists after smoking cessation and signals increased cardiovascular risk. Int Forum Allergy Rhinol. 2019;9(9):977–985. doi: 10.1002/alr.22357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet. 1999;354(9182):919–920. doi: 10.1016/S0140-6736(99)02355-7 [DOI] [PubMed] [Google Scholar]
- 33. Yulug B, Saatci O, Işıklar A, et al. The association between HbA1c levels, olfactory memory and cognition in normal, pre-diabetic and diabetic persons. Endocr Metab Immune Disord Drug Targets. 2020;20(2):198–212. doi: 10.2174/1871530319666190614121738 [DOI] [PubMed] [Google Scholar]
- 34. Zaghloul H, Pallayova M, Al-Nuaimi O, Hovis KR, Taheri S. Association between diabetes mellitus and olfactory dysfunction: current perspectives and future directions. Diabet Med. 2018;35(1):41–52. doi: 10.1111/dme.13542 [DOI] [PubMed] [Google Scholar]
- 35. Lietzau G, Davidsson W, Östenson CG, et al. Type 2 diabetes impairs odour detection, olfactory memory and olfactory neuroplasticity; effects partly reversed by the DPP-4 inhibitor Linagliptin. Acta Neuropathol Commun. 2018;6(1):14. doi: 10.1186/s40478-018-0517-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371(9626):1800–1809. doi: 10.1016/S0140-6736(08)60768-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kunte H, Schmidt F, Kronenberg G, et al. Olfactory dysfunction in patients with idiopathic intracranial hypertension. Neurology. 2013;81(4):379–382. doi: 10.1212/WNL.0b013e31829c5c9d [DOI] [PubMed] [Google Scholar]
- 38. Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016;68(6):e67–e94. doi: 10.1161/HYP.0000000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parsons C, Murad MH, Andersen S, Mookadam F, Labonte H. The effect of antihypertensive treatment on the incidence of stroke and cognitive decline in the elderly: a meta-analysis. Future Cardiol. 2016;12(2):237–248. doi: 10.2217/fca.15.90 [DOI] [PubMed] [Google Scholar]
- 40. Van Regemorter V, Hummel T, Rosenzweig F, Mouraux A, Rombaux P, Huart C. Mechanisms linking olfactory impairment and risk of mortality. Front Neurosci. 2020;14:140. doi: 10.3389/fnins.2020.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Henkin RI, Schmidt L, Velicu I. Interleukin 6 in hyposmia. JAMA Otolaryngol Head Neck Surg. 2013;139(7):728–734. doi: 10.1001/jamaoto.2013.3392 [DOI] [PubMed] [Google Scholar]
- 42. Laudisio A, Navarini L, Margiotta DPE, et al. The association of olfactory dysfunction, frailty, and mortality is mediated by inflammation: results from the InCHIANTI Study. J Immunol Res. 2019;2019:3128231. doi: 10.1155/2019/3128231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ottaviano G, Savietto E, Scarpa B, et al. Influence of number of drugs on olfaction in the elderly. Rhinology. 2018;56(4):351–357. doi: 10.4193/Rhin17.152 [DOI] [PubMed] [Google Scholar]
- 44. Lötsch J, Daiker H, Hähner A, Ultsch A, Hummel T. Drug-target based cross-sectional analysis of olfactory drug effects. Eur J Clin Pharmacol. 2015;71:461–471. doi: 10.1007/s00228-015-1814-2 [DOI] [PubMed] [Google Scholar]
- 45. Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larsson M. Odor identification impairment in carriers of ApoE-varepsilon4 is independent of clinical dementia. Neurobiol Aging. 2010;31(4):567–577. doi: 10.1016/j.neurobiolaging.2008.05.019 [DOI] [PubMed] [Google Scholar]
- 46. Hussain A, Luong M, Pooley A, Nathan BP. Isoform-specific effects of apoE on neurite outgrowth in olfactory epithelium culture. J Biomed Sci. 2013;20(1):49. doi: 10.1186/1423-0127-20-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mandairon N, Peace ST, Boudadi K, et al. Compensatory responses to age-related decline in odor quality acuity: cholinergic neuromodulation and olfactory enrichment. Neurobiol Aging. 2011;32(12):2254–2265. doi: 10.1016/j.neurobiolaging.2009.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miyajima F, Ollier W, Mayes A, et al. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7(4):411–417. doi: 10.1111/j.1601-183X.2007.00363.x [DOI] [PubMed] [Google Scholar]
- 49. Oleszkiewicz A, Schriever VA, Croy I, et al. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 2019;276:719–728. doi: 10.1007/s00405-018-5248-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.