Abstract
Background
MDR bacteria represent an urgent threat to human health globally. Polymyxins are a last-line therapy against life-threatening Gram-negative ‘superbugs’, including Acinetobacter baumannii. Polymyxins exert antimicrobial activity primarily via permeabilizing the bacterial outer membrane (OM); however, the mechanism of interaction between polymyxins and the OM remains unclear at the atomic level.
Methods
We constructed a lipid A-based OM model of A. baumannii using quantitative membrane lipidomics data and employed all-atom molecular dynamics simulations with umbrella sampling techniques to elucidate the structure–interaction relationship and thermodynamics governing the penetration of polymyxins [B1 and E1 (i.e. colistin A) representing the two clinically used polymyxins] into the OM.
Results
Polymyxin B1 and colistin A bound to the A. baumannii OM by the initial electrostatic interactions between the Dab residues of polymyxins and the phosphates of lipid A, competitively displacing the cations from the headgroup region of the OM. Both polymyxin B1 and colistin A formed a unique folded conformation upon approaching the hydrophobic centre of the OM, consistent with previous experimental observations. Polymyxin penetration induced reorientation of the headgroups of the OM lipids near the penetration site and caused local membrane disorganization, thereby significantly increasing membrane permeability and promoting the subsequent penetration of polymyxin molecules into the OM and periplasmic space.
Conclusions
The thermodynamics governing the penetration of polymyxins through the outer leaflet of the A. baumannii OM were examined and novel structure–interaction relationship information was obtained at the atomic and membrane level. Our findings will facilitate the discovery of novel polymyxins against MDR Gram-negative pathogens.
Introduction
The world is facing a serious health challenge due to antimicrobial resistance. The WHO and government sectors have developed a global action plan to mitigate antimicrobial resistance.1 MDR Acinetobacter baumannii is a particularly problematic pathogen due to its resistance to almost all available antibiotics and sits atop the WHO priority pathogen list for research and development of new antibiotics.2 Due to the lack of new antibiotics under development, polymyxins (i.e. polymyxin B and colistin; Figure 1a) are increasingly used as a last-line option against life-threatening Gram-negative pathogens, including A. baumannii.3 Although the interaction of polymyxins with bacterial membranes has been investigated over the last few decades,4–7 the exact mechanism by which polymyxins penetrate into the bacterial outer membrane (OM) remains unclear at the atomic scale.
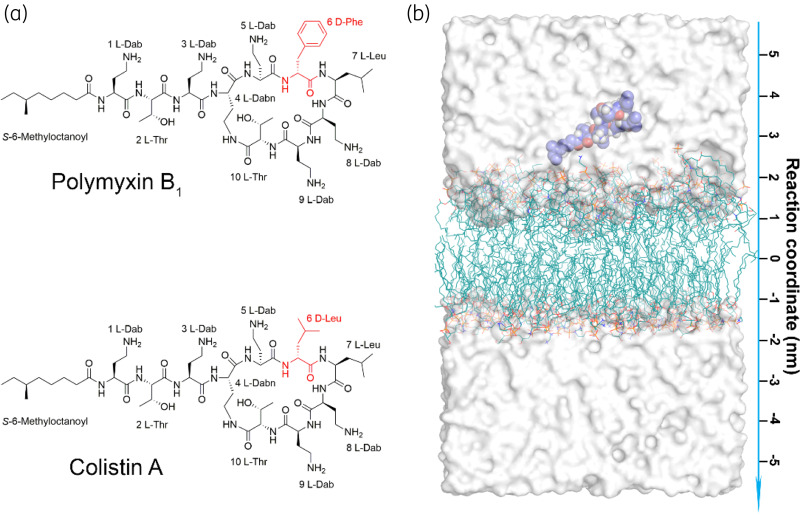
Figure 1.
MD simulations system. (a) Chemical structures of polymyxin B1 and colistin A. Polymyxins consist of an N-terminal fatty acyl tail, a linear tripeptide and a cyclic heptapeptide. The different residues at position 6 are depicted in red. (b) The simulation system includes a lipid bilayer, a single polymyxin molecule, water molecules and ions. The reaction coordinate is the distance between the centre of mass of the polymyxin molecule and the OM. The polymyxin molecule and OM lipids (including lipid A and phospholipids) are shown as blue spheres and teal lines, respectively. Water molecules are depicted by the white surface and ions are not shown for clarity. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
It is proposed that polymyxins cross the bacterial OM via ‘self-promoted uptake’, during which the displacement of divalent cations and membrane permeabilization play important roles.8–10 There are several models for the interaction of antimicrobial cationic peptides with the bacterial cytoplasmic membrane, including the barrel-stave, toroidal pore, carpet and aggregate models;11,12 however, none explains how the polymyxins penetrate the bacterial OM. Lipid A-based structure–activity relationship models were developed for polymyxins based on NMR data.13,14 However, a major limitation of these models is that each polymyxin molecule only interacts with a single lipid A molecule, whereas biochemical assays indicate that the interaction is substantially more complicated.15 This prevents a comprehensive illustration of the mode of action of polymyxins at the OM level.14,16 Although the interactions of polymyxins with bacterial membranes have been investigated by various approaches, including fluorescent titration, neutron reflectometry and X-ray scattering,17–19 they have failed to offer a dynamic view of the permeabilization of the bacterial OM by polymyxins. Molecular dynamics (MD) simulations, coupled with umbrella sampling techniques, provide a powerful approach to examine the exact mode of action of antimicrobial peptides with superior temporal and spatial resolution.12,20,21 Limited by the conformational sampling ability of conventional MD simulations, previous simulation studies on polymyxins mainly characterized their interactions with the headgroups of membrane lipids without tracking their penetration into the hydrophobic region of the membrane.5,6 Importantly, these previous studies often simplified the lipid composition of the membrane and neglected the importance of membrane heterogeneity for the interaction with polymyxins. Here, we developed an A. baumannii OM model using quantitative membrane lipidomics data22 and employed all-atom MD simulations and umbrella sampling to elucidate how polymyxins interact with the OM of A. baumannii and the thermodynamics governing the penetration into the OM.
Methods
System preparation
The heterogeneity of biological membranes plays an essential role in their interactions with antimicrobial peptides.20,23 To build a realistic bacterial OM, quantitative membrane lipidomics data of A. baumannii were employed to set the lipid composition and chemotypes of its OM.22 The methods of lipidomics analysis are available as Supplementary data at JAC Online. The molar ratio of various lipids in the outer leaflet of the OM contained 75% hepta-acyl lipid A (12:0, 12:0, 12:0, 14:0/12:0, 12:0, 14:0), 18% phosphatidylethanolamine (PE; 16:0/18:1), 4% phosphatidylglycerol (PG; 16:0/18:1) and 3% cardiolipin (CL; 16:0, 18:1/16:0, 18:1); the inner leaflet was composed of 72% PE, 16% PG and 12% CL. This lipidomics-informed membrane composition mimicked the OM of A. baumannii. Lipid A represents the conserved component of LPS and the major interaction target of polymyxins.13 As our study aimed to examine the mechanism of interaction between polymyxins and the OM of susceptible isolates at the atomic level, WT lipid A was employed in our MD simulations. The topology parameters of these lipids are from CHARMM-GUI based on the all-atom CHARMM36 force field and the asymmetric membrane model was constructed using CHARMM-GUI Membrane Builder in a tetragonal box.24 TIP3P water molecules and counter-ions were added to hydrate and neutralize the simulation system, respectively.
The structure of polymyxins was built using Chem3D and energy minimization was performed to relieve potential intramolecular steric clashes. We simulated polymyxin B1 molecules in solution and found that their conformations were consistent with the reported conformational ensembles (Figure S1, available as Supplementary data at JAC Online).6 The topology parameters of polymyxins were generated using the SwissParam server.25 The polymyxin molecule was placed above the bilayer using the internal Gromacs tool gmx insert-molecules by replacing water molecules.26 The dimensions of the simulation box were approximately 7.6 × 7.6 × 12.7 nm. The details of the MD simulation systems are listed in Table S1.
Steered MD simulations
Steered MD simulations were employed in the present study to accelerate the penetration of polymyxins into the bacterial OM. This method has been widely used in examining antimicrobial peptide–membrane interactions.12,20,27,28 In our simulations, a harmonic potential with a force constant of 500 kJ/mol/nm2 was applied between the centre of mass of the polymyxin molecule and the membrane along the Z-axis,29 which allowed the polymyxin molecule to penetrate the bacterial OM smoothly and achieve the unique conformational transition consistent with previous experimental observations.14,16 For each OM–polymyxin interaction system, three independent simulation replicates were performed to examine the reproducibility of the simulation results. Figure 1(b) shows the initial configuration of the steered MD simulation system.
Umbrella sampling
Umbrella sampling simulations were conducted to calculate the free energy profile during penetration into the bacterial OM.30,31 Snapshots from the steered MD simulations were generated and employed to initiate a series of simulation windows in which the centre of mass of the polymyxin molecule was harmonically restrained at a series of fixed Z values. The configuration used for each window was selected every 0.2 nm in the range Z = 3.21/2.85 nm (above the outer leaflet) to Z = 0.01/−0.15 nm (the hydrophobic centre), resulting in 17 and 16 simulation windows in the polymyxin B1 and colistin A systems, respectively. Table S2 shows the details of simulation windows and the free energy profiles were calculated according to the WHAM integration algorithm.32
Simulation parameters
GROMACS 5.1.2 was used to perform all MD simulations with the CHARMM36 all-atom force field with the default deprotonated lipid A in CHARMM-GUI.26,33 A recent study revealed that the protonation state of lipid A was a key factor in regulating the OM properties;34 our preliminary simulation results showed that the protonation state of lipid A did not significantly affect the free energy profiles of the penetration of colistin A through the outer leaflet of lipid A-based OM (Figure S2). Energy minimization was performed using the steepest descent algorithm. A six-step equilibration process was carried out by gradually turning off the position restraints on lipid molecules. Periodic boundary conditions were considered and all simulations were conducted at constant temperature (313 K) and pressure (1 bar) using the Nosé–Hoover and semi-isotropic pressure coupling approach with Parrinello–Rhaman barostat.35–37 The time constant of coupling for temperature and pressure was 1 and 5 ps, respectively. The Particle Mesh Ewald (‘PME’) method was employed to treat long-range electrostatic interactions with a short-range cut-off of 1.2 nm,38 while the shifted Lennard–Jones potential algorithm was used to calculate the Van der Waals interactions with a general cut-off of 1.2 nm and a shifting cut-off of 1.0 nm. The trajectory in production simulations was recorded every 10 ps.
Results
Penetration of polymyxins into the OM of A. baumannii
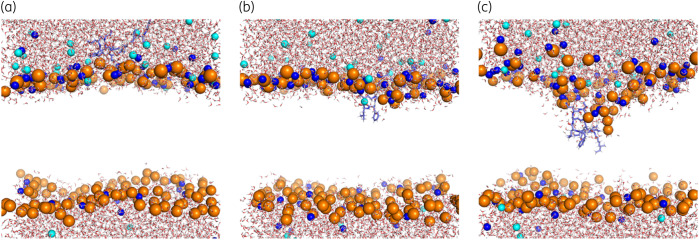
Through a steered MD simulation technique, the penetration trajectories of polymyxin B1 (Figure 2a) and colistin A (Figure 2b) molecules into the bacterial OM were tracked. Following the initial placement of polymyxin molecules above the OM surface, they subsequently attached to the headgroup region of the OM and orientated their hydrophobic segments (i.e. fatty acyl tail, d-Phe6/Leu6 and l-Leu7) to face toward the OM centre. Successively, polymyxin B1 and colistin A translocated to the hydrophobic centre of the OM and formed a folded conformation. During this process, the hydrophobic segments of each polymyxin molecule adopted an inward orientation and were surrounded by the hydrocarbon tails of the lipid A and phospholipids; meanwhile, the polar residues of polymyxins retained the interactions with the headgroups of lipid A and phospholipids in the outer leaflet of the OM. Notably, this folded conformation of polymyxins was also found in previous NMR studies of polymyxin–LPS interaction, although the observed conformation of the polymyxin molecule in the presence of only lipid A was slightly more compacted than observed in membranes with simulations.14,16
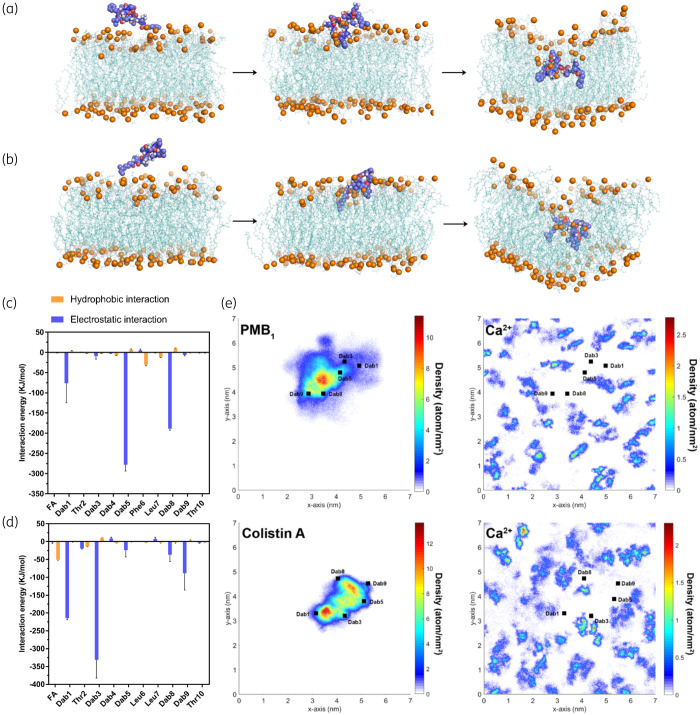
Figure 2.
Polymyxin penetration into the bacterial OM. Representative snapshots showing the penetration of (a) polymyxin B1 and (b) colistin A into the Gram-negative bacterial OM. Polymyxin molecules are shown as blue spheres with red oxygen atoms and white hydrogen atoms. The OM is shown as teal lines. The phosphate atoms are shown as orange spheres to indicate the position of the polymyxin molecule relative to the OM surface. Interaction energy between each residue of (c) polymyxin B1 and (d) colistin A and the OM. (e) Density map of polymyxin molecules and Ca2+ on the x–y plane of the OM. FA, fatty acyl. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The interaction energy results clearly show that the electrostatic interaction between the positively charged Dab residues of the polymyxin molecule and the negatively charged phosphate groups on the OM was the key player for their initial interaction. Although the relative energy contribution of each residue varied slightly in different simulation replicates, l-Dab1, l-Dab5 and l-Dab8 were the major contributors to the interactions observed with polymyxin B1 (Figure 2c and Figure S3) and l-Dab1, l-Dab3 and l-Dab9 were the major contributors to the interactions observed with colistin A (Figure 2d and Figure S3). Due to the repulsive effect between the water and hydrophobic residues, when polymyxins attached to the OM surface, the d-Phe6 in polymyxin B1 was closer to the hydrophobic region of the lipid A molecules compared with d-Leu6 in colistin A. This slight difference in orientation may increase the possibility of l-Dab5 (sits adjacent to d-Phe6) in polymyxin B1 interacting with the headgroups of lipid A molecules in the OM. As a result, l-Dab5 formed the strongest interaction with the OM among the polymyxin B1 residues. Interestingly, the Ca2+ density was lowered in the binding area of polymyxin molecules on the x–y plane of the OM, indicating that both polymyxin B1 and colistin A competitively displaced the Ca2+ from the OM when their Dab side chains bound to the OM headgroup region (Figure 2e). As divalent cations generally stabilize the bacterial OM as a major cross-linking agent between the neighbouring LPS molecules,39,40 the displacement of Ca2+ by polymyxin B1 and colistin A rendered the OM less stable and possibly enhanced the OM permeabilization.
Free energy profiles during the OM penetration of polymyxins
The overall trends of the free energy profiles were similar between polymyxin B1 and colistin A (Figure 3a). During the initial movement of the polymyxin molecule from the water environment into the headgroup region of the OM, there was a slight decrease in free energy (ΔGPMB = −4.08 kcal/mol; ΔGcolistin = −3.01 kcal/mol), reflective of the favourable electrostatic interactions between the cationic Dab residues on the polymyxin molecule and the negatively charged phosphate groups on lipid A in the OM. Subsequently, the free energy gradually increased as the polymyxin molecule passed through the headgroup region of the OM outer leaflet. After this, the free energy substantially increased as the polymyxin molecule traversed the hydrophobic layer of the OM. Notably, there was a major difference in free energy profiles between polymyxin B1 and colistin A. In the range from 0.91 to 1.67 nm, equivalent to the headgroup region of the OM outer leaflet, the free energy was approximately 55% higher with polymyxin B1 than colistin A (e.g. 23.2 versus 15.0 kcal/mol at Z = 1 nm), indicating that colistin A traversed the polar headgroup region more readily than polymyxin B1. This difference is very likely due to the different hydrophobicity and steric hindrance at position 6 (L-Phe6 in polymyxin B1 versus l-Leu6 in colistin A; Figure 1a), which is the only structural difference between the two polymyxins.
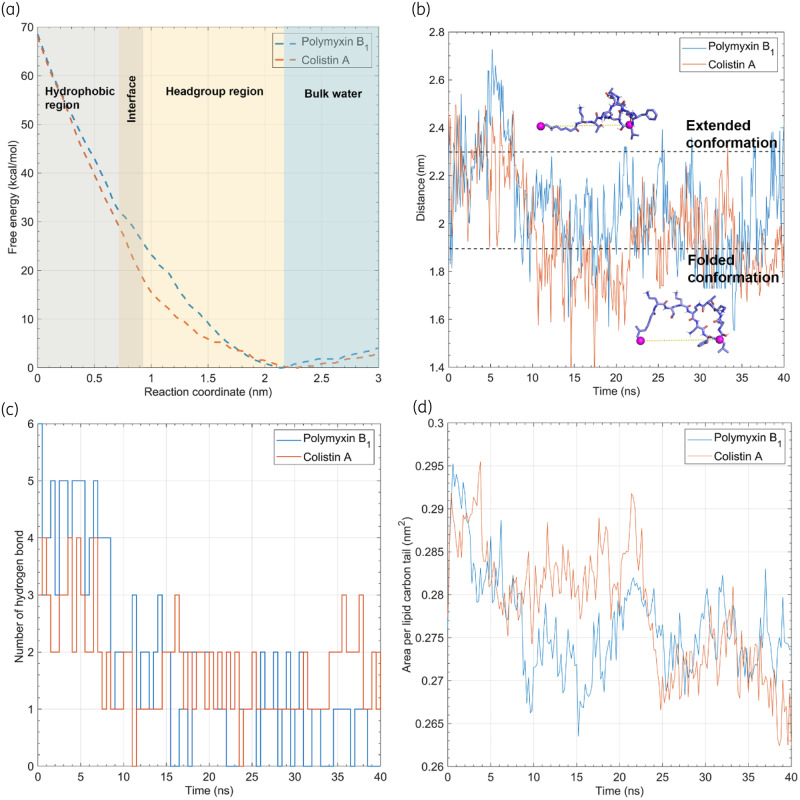
Figure 3.
Thermodynamics governing the penetration of polymyxins. (a) Free energy profiles of polymyxin penetration into the OM. The free energy minimum is shifted to zero in the profiles. (b) Geometric distance between the atom pairs of polymyxins. The atoms used for the distance calculation are shown as purple spheres in the polymyxin B1 structure. (c) Number of hydrogen bonds between the lipids that interact with the polymyxins and the rest of the OM. (d) Area per lipid carbon tail is calculated to indicate the packing density in the hydrophobic region of the OM. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
As mentioned above, both polymyxin B1 and colistin A adopted a folded conformation inside the OM. To characterize their conformational dynamics, the distance between the last carbon atom of the fatty acyl tail and the Cα of l-Leu7 of the polymyxin molecule was calculated (Figure 3b). When polymyxins entered the hydrophobic region of the OM, they experienced a significant conformational transition from an extended to a folded conformation with the latter being maintained during the subsequent penetration process, most likely due to the highly hydrophobic environment of the OM centre. This folded conformation was reproducible in different simulation replicates for polymyxin B1 and colistin A (Figure S4) and allowed the amphipathic polymyxin molecule to specifically interact with the negatively charged headgroups of lipid A and hydrophobic core of the OM.41
We further examined the number of intermolecular hydrogen bonds between the lipids interacting with the polymyxin molecule and the other lipids in the OM. Not surprisingly, the number of hydrogen bonds decreased from 3–5 to 0–2 upon the penetration of polymyxin B1 and from 2–4 to 1–2 upon OM penetration of colistin A (Figure 3c), indicating a reduction in the intermolecular interactions between the lipid headgroups near the polymyxin penetration site on the OM. Meanwhile, the area per lipid carbon tail also decreased as the polymyxins penetrated into the OM (Figure 3d), reflective of the conformational constraint of the lipid A and phospholipid tails. These results indicate that the penetration of polymyxins primarily disorganized the headgroup region of the OM, while they increased the packing of the OM hydrophobic region, which is consistent with an earlier report.42
Atomic structure–interaction relationship of polymyxins with the OM of A. baumannii
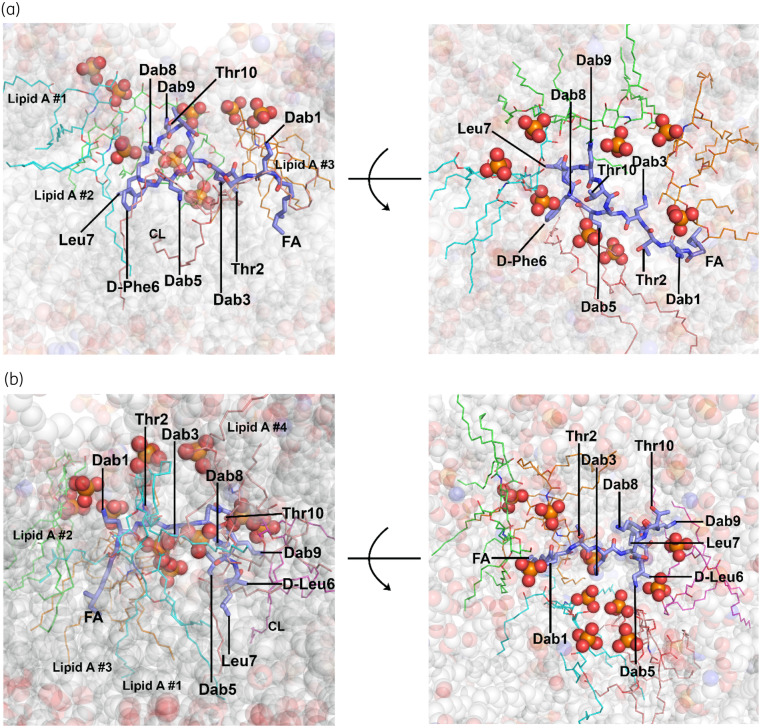
Unlike previous NMR studies on LPS–polymyxin interaction,14,16 in our MD systems the polymyxin molecule interacted with several different lipid A and phospholipid molecules in the OM (Figure 4). For polymyxin B1, the five cationic Dab side chains formed polar contacts with the phosphate groups of three lipid A molecules and one cardiolipin molecule, whereas the hydrophobic triad (i.e. fatty acyl tail, d-Phe6 and l-Leu7) formed hydrophobic contacts with the hydrocarbon tails of the surrounding lipid A and phospholipid molecules (Figure 4a). Similarly, the five cationic Dab side chains of colistin A were close to the phosphate groups of four lipid A molecules and one cardiolipin molecule, with the hydrophobic triad embedded in the fatty acyl tails of lipid A and phospholipid molecules. The structure–interaction models generated from independent simulation replicates revealed that one polymyxin molecule primarily interacted with 3–4 lipid A molecules in the OM (Figure S4). Notably, in our OM-based interaction model the side chains of l-Dab3 and l-Dab5 of polymyxin B1 and colistin A orientated towards the middle of the polymyxin molecule and were partly shielded by the hydrophobic triad, while l-Dab1, l-Dab8 and l-Dab9 were located at the edge of the polymyxin molecule and interacted with the phosphate groups of adjacent lipid A molecules, inducing reorientation of the headgroups of these lipid A molecules. Thus, the folded conformation of polymyxins allows effective interactions with both polar headgroups and hydrophobic carbon tails of multiple lipid A molecules and is thus a major intermediate step in the disorganization of the bacterial OM.
Figure 4.
Interactions of (a) polymyxin B1 and (b) colistin A with the OM. Polymyxin molecules are shown as blue stick models. The lipid A and phospholipid molecules interacting with polymyxins are shown as coloured line models, while their phosphate groups are presented as sphere models. The OM is shown as transparent sphere models. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Polymyxins enhance the permeability of the OM of A. baumannii
The integral OM structure constituted a permeability barrier, preventing water molecules from entering into its hydrophobic region (Figure 5a). Along with the penetration of the polymyxin molecule, a local membrane deformation gradually formed, due to the strong electrostatic interactions between the polymyxin Dab side chains and the lipid A phosphate groups (Figure 5b and c and Figure S5). Consequently, water molecules and calcium ions entered the hydrophobic region of the OM readily via the formed pore, significantly increasing the membrane permeability.
Figure 5.
Pore formation in the OM during the penetration of polymyxin B1. Water molecules are shown as sticks with red oxygen and white hydrogen atoms, polymyxin B1 is shown as blue sticks and Ca2+ and Cl− are represented by blue and cyan spheres, respectively. The hydrocarbon tails of the membrane lipids are not shown for clarity. Phosphate atoms are shown as orange spheres to represent the positions of the lipid headgroups. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
To test whether induced membrane pores promote the penetration of antimicrobial peptides,43 we employed the polymyxin-treated OM (with a pore) and performed unconstrained MD simulations in which four additional colistin A molecules were added above the OM surface. As expected, the pore on the OM was persistent over the entire 100 ns simulation period. Importantly, we found that a newly added colistin A molecule entered the pore in the OM spontaneously (Figure S6a). By contrast, in the same timescale with the untreated bacterial OM, the colistin A molecules only bound to the OM surface and none of the colistin A molecules inserted into the OM (Figure S6b). These results revealed that polymyxin-induced pores in the OM not only increased the permeability of water and ions, but also promoted the penetration of other polymyxin molecules, supporting the ‘self-promoted uptake’ theory of polymyxin activity proposed in the literature.44–46
Discussion
To the best of our knowledge, the present study is the first to employ steered MD simulations and umbrella sampling to reveal, at the all-atomic scale, the unique thermodynamics governing the penetration of polymyxins into the OM of Gram-negative A. baumannii. Due to the limited ability of conformational sampling, classical MD simulations cannot track the penetration process of polymyxins into the bacterial OM.5,6 Enhanced sampling techniques, including umbrella sampling MD simulations and coarse-grained MD simulations, are essential to achieve the penetration of polymyxins within the timescale of simulations.42 Notably, it is not feasible to investigate the atomic interaction of polymyxins with bacterial membranes using the coarse-grained method, because multiple atoms are considered as one bead in coarse-grained MD.42 By contrast, all-atom MD simulations21,23,30 combined with umbrella sampling techniques allow characterization of the interaction of polymyxins with the membrane with an atomic resolution.
The penetration process of polymyxins into the bacterial OM can be divided into three major stages according to the free energy profiles (Figure 3a). First, polymyxin molecules bind to the headgroups of the OM lipids via electrostatic interaction between the cationic Dab residues of polymyxins and the phosphate groups of lipid A (Figure 2c and d). Therefore, it is a common strategy for Gram-negative bacteria to develop polymyxin resistance via modifying the lipid A phosphate groups with moieties (e.g. 4-amino-4-deoxy-l-arabinose and phosphoethanolamine), diminishing the initial interactions with polymyxins.47,48 Secondly, polymyxin molecules pass across the headgroup region of the OM, during which the free energy increases slowly to approximately 18 kcal/mol for colistin A and approximately 30 kcal/mol for polymyxin B1. Thirdly, polymyxin molecules traverse the hydrocarbon region of the outer leaflet of the OM, during which the free energy sharply increases to approximately 68 kcal/mol for both polymyxin B1 and colistin A. During penetration, the polymyxin molecules adopted a folded conformation (Figure 3b), disrupted the cross-linking interactions between adjacent lipid A molecules (Figure 3c) and increased the order of the hydrophobic region of the OM (Figure 3d). These thermodynamic changes represent the entropic penalties of both the polymyxin molecules and the fatty acyl tails of lipid A and OM phospholipids and the enthalpic penalty of the headgroups of lipid A and OM phospholipids, which together constitute the free energy barrier of polymyxin penetration into the bacterial OM.20,42
Jefferies et al.42 reported that the free energy for polymyxin penetration into the OM centre was only approximately 10 kcal/mol, lower than the free energy barrier of approximately 68 kcal/mol calculated in the present study. The discrepancy in the free energy barrier likely results from at least two major factors. First, in the previous simulation the polymyxin B1 molecule did not completely penetrate into the hydrophobic centre of the OM, but primarily stayed in the headgroup region of the OM.42 However, our results revealed that the free energy barrier for traversing the polar headgroup region is less than a half of the total free energy barrier (Figure 3a). Secondly, the hydrocarbon tails of lipid A were simplified as two or three beads in the coarse-grained models employed in the previous study,42 which substantially decreased the thickness and complexity of the OM hydrophobic layer, thus reducing the free energy barrier of polymyxin penetration significantly. In reality, a large number of polymyxin molecules can simultaneously bind to the bacterial OM and disorganize the OM structure cooperatively,13 which promotes the penetration of polymyxins into the bacterial OM.
With this unique folded conformation (Figure 4), the amphipathic polymyxin molecule specifically interacts with the negatively charged headgroups of lipid A and non-specifically interacts with the hydrophobic core of lipid A and the OM phospholipids.41 Previous NMR studies also discovered this folded conformation of polymyxins in the presence of a single lipid A molecule,14,16 indicating that the folded conformation of polymyxins is a key intermediate state in the penetration into the bacterial OM. However, we should note that the measured distance between the fatty acyl group and Leu7 in the folded conformation of polymyxin B1 was approximately 1.9 nm in our MD simulations (Figure 3b) and approximately 1.3 nm in a previous NMR study.49 In the NMR experiments only one lipid A molecule was employed to characterize the interaction mode with polymyxins; therefore, all polar and hydrophobic segments of polymyxin were restrained in the space of a single lipid A molecule. In contrast, in our OM-based all-atom simulations the Dab residues and hydrophobic segments of the polymyxin molecule were able to interact with 3–4 lipid A and phospholipid molecules (Figure 4 and Figure S4). These findings highlight the importance of using lipidomics-informed OM systems in examining the interaction with polymyxins to develop a novel membrane-based structure–activity relationship model of polymyxins.
A recent simulation study examined the permeabilizing effect of polymyxin B;50 unfortunately, a coarse-grained, symmetric Re-LPS [KDO2-lipid A, consisting of lipid A and 2–3 molecules of 3-deoxy-d-manno-2-octulosonic acid (KDO)] bilayer was used to mimic the bacterial OM,50 which ignores the heterogeneity of the asymmetric bacterial OM. In contrast, we constructed the A. baumannii OM model based on the quantitative lipidomics data.22 Furthermore, the complete penetration of the polymyxin molecule into the hydrophobic centre of the OM was not examined in previous simulation studies;5,6,50 therefore, the folded conformation of polymyxins was not observed in those studies, which significantly limits the understanding of the atomic interaction of polymyxins with the bacterial OM. Interestingly, our results revealed that formed pores in the OM promoted the penetration of other polymyxin molecules (Figure S6), supporting a recent experimental study that showed membrane deformation caused by a polymyxin molecule cooperatively enhances the subsequent interactions of other molecules with the bacterial OM.51 Collectively, our results provide strong support for the ‘self-promoted uptake’ mechanism of polymyxins in Gram-negative bacteria.4,8
To the best of our knowledge, this is the first all-atom study to uncover the thermodynamics of the penetration of the polymyxin molecule into the Gram-negative bacterial OM. Unlike previous studies based on model membranes, the present study employed quantitative membrane lipidomics results to develop an A. baumannii OM model for MD simulations. Excitingly, our all-atom simulation results have been utilized to develop a novel membrane-based structure–activity relationship model for polymyxins, which may facilitate the discovery of new-generation polymyxins to combat life-threatening Gram-negative ‘superbugs’.
Supplementary Material
Acknowledgements
The scientific calculations in this study were performed on the HPC Cloud Platform (National Key Research Program, 2016YFB0201702) at Shandong University and the supercomputer at eResearch, Monash University.
Funding
This research was supported by a research grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01 AI132154). X.J. is a recipient of the 2019 Faculty Bridging Fellowship, Biomedicine Discovery Institute, Monash University. N.A.P. is an Australia National Health and Medical Research Council (NHMRC) ECR Research Fellow. R.E.W.H. is funded by the Canadian Institutes for Health Research FDN-154287 and holds a Canada Research Chair in Health and Genomics and a UBC Killam Professorship. J.L. is an Australia NHMRC Principal Research Fellow.
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
References
- 1.WHO. Antimicrobial Resistance Global Report on Surveillance: 2014. Summary. https://www.who.int/drugresistance/documents/surveillancereport/en/.
- 2.WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discover, and Development of New Antibiotics. https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/.
- 3. Nation RL, Li J, Cars O et al. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 2015; 15: 225–34. [DOI] [PubMed] [Google Scholar]
- 4. Zhang L, Dhillon P, Yan H et al. Interactions of bacterial cationic peptide antibiotics with outer and cytoplasmic membranes of Pseudomonas aeruginosa. Antimicrob Agents Chemother 2000; 44: 3317–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berglund NA, Piggot TJ, Jefferies D et al. Interaction of the antimicrobial peptide polymyxin B1 with both membranes of E. coli: a molecular dynamics study. PLoS Comput Biol 2015; 11: e1004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santos DES, Pol-Fachin L, Lins RD et al. Polymyxin binding to the bacterial outer membrane reveals cation displacement and increasing membrane curvature in susceptible but not in resistant lipopolysaccharide chemotypes. J Chem Inf Model 2017; 57: 2181–93. [DOI] [PubMed] [Google Scholar]
- 7. Domingues MM, Inácio RG, Raimundo JM et al. Biophysical characterization of polymyxin b interaction with LPS aggregates and membrane model systems. Biopolymers 2012; 98: 338–44. [DOI] [PubMed] [Google Scholar]
- 8. Hancock RE. Peptide antibiotics. Lancet 1997; 349: 418–22. [DOI] [PubMed] [Google Scholar]
- 9. Piers KL, Brown MH, Hancock RE. Recombinant DNA procedures for producing small antimicrobial cationic peptides in bacteria. Gene 1993; 134: 7–13. [DOI] [PubMed] [Google Scholar]
- 10. Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev 2006; 19: 491–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vineeth Kumar TV, Sanil G. A review of the mechanism of action of amphibian antimicrobial peptides focusing on peptide-membrane interaction and membrane curvature. Curr Protein Pept Sci 2017; 18: 1263–72. [DOI] [PubMed] [Google Scholar]
- 12. Bennett WF, Hong CK, Wang Y et al. Antimicrobial peptide simulations and the influence of force field on the free energy for pore formation in lipid bilayers. J Chem Theory Comput 2016; 12: 4524–33. [DOI] [PubMed] [Google Scholar]
- 13. Velkov T, Thompson PE, Nation RL et al. Structure–activity relationships of polymyxin antibiotics. J Med Chem 2010; 53: 1898–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Velkov T, Roberts KD, Nation RL et al. Teaching ‘old’ polymyxins new tricks: new-generation lipopeptides targeting gram-negative ‘superbugs’. ACS Chem Biol 2014; 9: 1172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moore RA, Bates NC, Hancock R. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob Agents Chemother 1986; 29: 496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mares J, Kumaran S, Gobbo M et al. Interactions of lipopolysaccharide and polymyxin studied by NMR spectroscopy. J Biol Chem 2009; 284: 11498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McInerney MP, Roberts KD, Thompson PE et al. Quantitation of polymyxin-lipopolysaccharide interactions using an image-based fluorescent probe. J Pharm Sci 2016; 105: 1006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han ML, Velkov T, Zhu Y et al. Polymyxin-induced lipid A deacylation in Pseudomonas aeruginosa perturbs polymyxin penetration and confers high-level resistance. ACS Chem Biol 2018; 13: 121–30. [DOI] [PubMed] [Google Scholar]
- 19. Dupuy FG, Pagano I, Andenoro K et al. Selective interaction of colistin with lipid model membranes. Biophys J 2018; 114: 919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin D, Grossfield A. Thermodynamics of antimicrobial lipopeptide binding to membranes: origins of affinity and selectivity. Biophys J 2014; 107: 1862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang K, Garcia AE. Free energy of translocating an arginine-rich cell-penetrating peptide across a lipid bilayer suggests pore formation. Biophys J 2013; 104: 412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu Y, Lu J, Han ML et al. Polymyxins bind to the cell surface of unculturable Acinetobacter baumannii and cause unique dependent resistance. Adv Sci 2020; doi:10.1002/advs.202000704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khondker A, Dhaliwal AK, Saem S et al. Membrane charge and lipid packing determine polymyxin-induced membrane damage. Commun Biol 2019; 2: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jo S, Kim T, Iyer VG et al. CHARMM‐GUI: a web‐based graphical user interface for CHARMM. J Comput Chem 2008; 29: 1859–65. [DOI] [PubMed] [Google Scholar]
- 25. Zoete V, Cuendet MA, Grosdidier A et al. SwissParam: a fast force field generation tool for small organic molecules. J Comput Chem 2011; 32: 2359–68. [DOI] [PubMed] [Google Scholar]
- 26. Van Der Spoel D, Lindahl E, Hess B et al. GROMACS: fast, flexible, and free. J Comput Chem 2005; 26: 1701–18. [DOI] [PubMed] [Google Scholar]
- 27. Lin D, Grossfield A. Thermodynamics of micelle formation and membrane fusion modulate antimicrobial lipopeptide activity. Biophys J 2015; 109: 750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deshayes S, Morris M, Divita G et al. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci 2005; 62: 1839–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Babakhani A, Gorfe AA, Kim JE et al. Thermodynamics of peptide insertion and aggregation in a lipid bilayer. J Phys Chem B 2008; 112: 10528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yesylevskyy S, Marrink SJ, Mark AE. Alternative mechanisms for the interaction of the cell-penetrating peptides penetratin and the TAT peptide with lipid bilayers. Biophys J 2009; 97: 40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gu Y, Shrivastava IH, Amara SG et al. Molecular simulations elucidate the substrate translocation pathway in a glutamate transporter. Proc Natl Acad Sci USA 2009; 106: 2589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hub JS, de Groot BL, van der Spoel D. g_wham—a free weighted histogram analysis implementation including robust error and autocorrelation estimates. J Chem Theory Comput 2010; 6: 3713–20. [Google Scholar]
- 33. Klauda JB, Venable RM, Freites JA et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J Phys Chem B 2010; 114: 7830–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rice A, Rooney MT, Greenwood AI et al. Lipopolysaccharide simulations are sensitive to phosphate charge and ion parameterization. J Chem Theory Comput 2020; 16: 1806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parrinello M, Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 1981; 52: 7182–90. [Google Scholar]
- 36. Nosé S. A molecular dynamics method for simulations in the canonical ensemble. Mol Phys 1984; 52: 255–68. [Google Scholar]
- 37. Hoover WG. Canonical dynamics: equilibrium phase-space distributions. Phys Rev A 1985; 31: 1695–7. [DOI] [PubMed] [Google Scholar]
- 38. Darden T, York D, Pedersen L. Particle mesh Ewald: an N⋅log (N) method for Ewald sums in large systems. J Chem Phys 1993; 98: 10089. [Google Scholar]
- 39. Pink D, Truelstrup Hansen L, Gill TA et al. Divalent calcium ions inhibit the penetration of protamine through the polysaccharide brush of the outer membrane of Gram-negative bacteria. Langmuir 2003; 19: 8852–8. [Google Scholar]
- 40. Pontes FJ, Rusu VH, Soares TA et al. The effect of temperature, cations, and number of acyl chains on the lamellar to non-lamellar transition in lipid-A membranes: a microscopic view. J Chem Theory Comput 2012; 8: 3830–8. [DOI] [PubMed] [Google Scholar]
- 41. Hancock REW. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis 2001; 1: 156–64. [DOI] [PubMed] [Google Scholar]
- 42. Jefferies D, Hsu PC, Khalid S. Through the lipopolysaccharide glass: a potent antimicrobial peptide induces phase changes in membranes. Biochemistry 2017; 56: 1672–9. [DOI] [PubMed] [Google Scholar]
- 43. Sun D, Forsman J, Lund M et al. Effect of arginine-rich cell penetrating peptides on membrane pore formation and life-times: a molecular simulation study. Phys Chem Phys 2014; 16: 20785–95. [DOI] [PubMed] [Google Scholar]
- 44. Hancock RE. Alterations in outer membrane permeability. Annu Rev Microbiol 1984; 38: 237–64. [DOI] [PubMed] [Google Scholar]
- 45. Wiese A, Münstermann M, Gutsmann T et al. Molecular mechanisms of polymyxin B-membrane interactions: direct correlation between surface charge density and self-promoted transport. J Membr Biol 1998; 162: 127–38. [DOI] [PubMed] [Google Scholar]
- 46. Clausell A, Garcia-Subirats M, Pujol M et al. Gram-negative outer and inner membrane models: insertion of cyclic cationic lipopeptides. J Phys Chem B 2007; 111: 551–63. [DOI] [PubMed] [Google Scholar]
- 47. Baron S, Hadjadj L, Rolain JM et al. Molecular mechanisms of polymyxin resistance: knowns and unknowns. Int J Antimicrob Agents 2016; 48: 583–91. [DOI] [PubMed] [Google Scholar]
- 48. Jeannot K, Bolard A, Plesiat P. Resistance to polymyxins in Gram-negative organisms. Int J Antimicrob Agents 2017; 49: 526–35. [DOI] [PubMed] [Google Scholar]
- 49. Velkov T, Gallardo-Godoy A, Swarbrick JD et al. Structure, function, and biosynthetic origin of octapeptin antibiotics active against extensively drug-resistant Gram-negative bacteria. Cell Chem Biol 2018; 25: 380–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fu L, Wan M, Zhang S et al. Polymyxin B loosens lipopolysaccharide bilayer but stiffens phospholipid bilayer. Biophys J 2020; 118: 138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Akhoundsadegh N, Belanger CR, Hancock RE. Outer membrane interaction kinetics of new polymyxin B analogs in Gram-negative bacilli. Antimicrob Agents Chemother 2019; 63: e00935–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.