Abstract
Severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) is the virus that causes coronavirus disease 2019 (COVID-19); a worldwide pandemic as declared by the World Health Organization (WHO). SARS-CoV-2 appears to infect cells by first binding and priming its viral-spike proteins with membrane-associated angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2). Through the coordinated actions of ACE2 and TMPRSS2, SARS-CoV-2 spike proteins fuse with plasma membranes and ultimately the virus enters cells. ACE2 is integral to the renin-angiotensin-aldosterone system (RAAS), and SARS-CoV-2 down-regulates protein expression levels of ACE2. Once infected, patients typically develop acute respiratory distress syndrome (ARDS) and a number of other severe complications that result in a high rate of fatality, especially in older (>60 years) adults and in people with pre-existing medical conditions. Data now indicate clearly that among people of all age groups, COVID-19 fatalities are higher in men than women. Here, attention is focused on these sex differences and posit a role of estrogen in these differences as well as possible therapeutic and protective actions of 17β-estradiol against COVID-19.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel virus in the coronaviridae family that causes the disease now termed coronavirus 2019 (COVID-19) [1,2]. SARS-CoV-2 is a large single-stranded RNA enveloped virus [3] that reportedly originated in China [3] and that has now spread quickly worldwide leaving behind severe medical complications, high levels of fatality, and serious disruptions in normal daily living and global economies [4]. Accordingly, the WHO declared COVID-19 to be a pandemic.
SARS-CoV-2 infects cells by binding viral-spike proteins with membrane-associated angiotensin-converting enzyme 2 (ACE2) and priming of spike proteins by transmembrane protease serine 2 (TMPRSS2). Together ACE2 and TMPRSS2 facilitate the entry of SARS-CoV2 into cells by promoting the fusion of viral proteins with host plasma membranes [5,6]. The ACE2 receptor is expressed on different types of cells and tissues including human airway epithelia, lung parenchyma, vascular endothelia, kidney cells, central nervous system (CNS) cells, and small intestinal cells [7,8]. However, SARS-CoV-2 primarily infects airway epithelial cells and induces severe symptoms in infected people including acute respiratory distress syndrome (ARDS) [9,10].
ACE2, the presumed receptor for SARS-CoV-2, is involved in the regulation of the renin-angiotensin-aldosterone system (RAAS) that, among other things, catalyzes the conversion of angiotensin II to Ang 1–7. Ang-(1–7) binds to its mas receptor (MasR) that plays an important role in controlling the homeostatically regulated ACE/Ang-II/AT1 receptor axis [11–14]. ACE2 is protective against various severe pathological complications including pulmonary disease, acute respiratory distress syndrome [15–17], asthma [18,19], chronic obstructive pulmonary disease [20,21], vasoconstriction [22,23], oxidative stress [24,25], diabetes [26,27], and inflammation [28,29]. A different coronavirus, SARS-CoV, has been shown to hijack the same ACE2 receptor for viral entry into cells and SARS-CoV infection can result in acute lung injury by damaging the RAAS system [30]. Similarly, SARS-CoV-2 can cause downregulation of ACE2, damage to the RAAS system, and clinical development of ARDS [31]. ARDS is a consequence of an inflammatory storm, a key mechanism underlying the high fatality rate associated with COVID-19 [32].
Worldwide, in all age groups, more men are dying from COVID-19 than are women [33–37]. Data from New York City demonstrated that 61% of COVID-19 related deaths were men [38]. In China and Italy, COVID-19 related death rates of men were about double those of women [39]. In Australia, similar results were observed, but in their case, the cohort was restricted to people 70–85 years of age [40]. For other coronaviruses too including SARS-CoV and Middle East respiratory syndrome-coronavirus (MERS-CoV), fatalities are higher in men compared to women [41,42]. This might be related specifically to coronaviruses and not all viruses because, for example, no clear sex-based differences were observed with HIV-1 [43].
Men might be more susceptible to coronavirus-induced illnesses because of less robust immune responses [44–46]. On the other hand, women might be less susceptible because of strong innate and adaptive immune responses [47–50]. One explanation for these differences includes the presence of female hormones, which have been found to protect against infection by multiple viruses including influenza [51–53], MERS-CoV, and SARS-CoV [54]. Therefore, it is important to know the extent to which hormones and other factors such as the immune system, behavior, and genes account for the higher susceptibility rates of men versus women for COVID-19 related deaths. Accordingly, we review the literature about the possible protective roles of 17β-estradiol against COVID-19.
Protective Actions of 17β-estradiol
17β-estradiol is a female sex hormone that plays essential roles in the development and maintenance of the female reproductive system and women’s secondary sex characteristics [55]. The physiological effects of 17β-estradiol are mediated by a family of receptors including estrogen-receptor-α (ER-α), estrogen receptor-β (ER-β), and GPR30/GPER-1 (membrane-bound G protein-coupled estrogen receptor) [56]. ER-α and ER-β are typically considered to be nuclear steroid receptors, but are in fact associated with plasma membranes, cytoplasm as well as the nucleus. Both ER-α and ER-β are involved in both cellular signaling and the regulation of gene expression through induction of ligand-activated transcription factors and direct binding to promoter-associated estrogen response elements (ERE) of target genes [57–59]. 17β-estradiol protects against multiple pathological complications including ARDS [60–64], hypertension [65,66], atherosclerosis [67], vasoconstriction [68,69], fibrosis [70,71], inflammation [72–74], autoimmune diseases [75–77], viral infections [53,78–80], and neurological disorders [81–83]. Because of such wide-ranging effects, it is important to consider the extent to which 17β-estradiol might control SARS-CoV-2 and the expression of this virus’s associated disease COVID-19 [84–88] through its ability to affect the RAAS system, anti-inflammatory and anti-viral responses, and upregulation of endolysosomal degradation pathways.
Sex and RAAS
RAAS is a hormone/enzyme system that regulates functions of multiple organs including lungs, heart, brain, vasculature, kidneys, liver, and pancreas [89]. RAAS is composed of the classical ACE/Ang-II/AT1 axis and the non-classical ACE2/ang-(1–7)/Mas axis [90]. The renin-catalyzed conversion of angiotensin to angiotensin-I is followed by the ACE-catalyzed conversion of Ang I to Ang II; Ang II activates the AT1 (Angiotensin II type 1) receptor. The non-classical pathway consists of ACE2, Ang 1–7 (Angiotensin I-7)-Ang II receptor AT2, and the Angiotensin II receptor type 2-Mas axis. The ACE2/ang-(1–7)/Mas axis is a master regulator of the RAAS system; it controls the ACE/Ang-II/AT1R axis [90,91]. However, when dysfunctional the ACE2/ang-(1–7)/Mas axis can lead to ARDS [16,17,92], hypertension [11,91,93], and inflammation [28,94].
The RAAS system is differentially regulated in a sex-dependent manner [95–97]. Men have higher expression levels of the ACE/Ang-II/AT1R axis, whereas the ACE2/ang-(1–7)/Mas is more active in women [97,98]. Indeed, in women, treatment with 17β-estradiol enhanced the ACE2/ang-(1–7)/Mas receptor axis [95,99–102]. In contrast, testosterone was less effective than was 17β-estradiol even though testosterone has been shown to downregulate angiotensin II type 2 receptor by androgen-receptor-mediated signaling pathways [95,99,103–105]. Levels of 17β-estradiol decline with age in post-menopausal women [106] and so do activity levels of the 17β-estradiol-controlled ACE2/ang-(1–7)/Mas axis. The consequence of these age-related changes is greater susceptibility to RAAS-related pathologies [105,107–109] including ARDS and other acute lung diseases [110,111], hypertension [112], cardiovascular [113], inflammation [109,114], and fibrosis [115].
Multiple respiratory viruses including HCoV-NL-63 [116], H5N1 [117], H7N9 [118] and SARS-CoV [119] all cause acute lung injury, decrease protein expression levels of ACE2, and disturb RAAS. 17β-Estradiol increases protein expression levels of ACE2 [91,120] and suppresses lung injuries in influenza [51] and SARS-CoV [54]. Implicated in these effects are decreased inflammation and infiltration of immune cells in lungs, protection of atrial myocardia by modifying RAAS [54,102], and protection against pulmonary arterial hypertension by enhancing ACE2 [91,120]. The notion that 17β-estradiol might be protective is further supported by findings that higher expression of ACE2 protects against various factors including lipopolysaccharides, aging, and comorbid conditions like diabetes [11,121–123]; all linked to RAAS. Hence, 17β-estradiol administration could rescue the SARS-CoV-2 infection caused low levels of ACE2 remains to be further investigated.
Sex-biased Immune Responses
Innate and adaptive immune responses control host-pathogen interactions [124,125], and women appear to have more robust immune responses than do men [44,126]; for viral infections this may be due to more efficient clearance of viruses [51,52]. However, robust immune responses in women may also lead to detrimental outcomes [126–128]. Sex-biased responses to viral infection are dependent on many factors including the presence of disease susceptible genes, different copy numbers of X-linked genes, and sex-dependent steroid hormones [47,126,128–130]. X-linked genes (eg. TLR7) and sex-specific hormones (eg. estrogen and testosterone) can affect adaptive and innate immune responses to pathogens [48–50,73,126,129]. TLR and NLR (NOD-like receptor) are known as pathogen-recognizing receptors (PRRs), which recognize diverse pathogen-associated molecules patterns (PAMPs) [131]. PRRs are expressed in most cells including T lymphocytes, B-cells, dendritic cells, macrophages, and epithelial cells [131,132]. Cellular signaling pathways and transcription factors that regulate inflammation and immune responses are activated by PAMPs and PRRs [131,132]. HIV-1 encoded TLR7 ligands enhance the production of the antiviral factor interferon-α (IFN-α) more robustly in females compared to males [133]. Also, 17β-estradiol enhances the production of TLR-7/9-mediated interferon-α responses in post-menopause women [48,49,134]. However, 17β-estradiol can suppress PAMPs’ responses and restrict inflammation [135–138]. Additionally, 17β-estradiol can abrogate NLRP3-mediated airway inflammation in asthma [139].
Levels of 17β-estradiol’s are higher in women during reproduction and pre-menopause age [140], and this correlates with robust innate immune responses and enhanced ability to clear viruses [52,141]. Lower levels of 17β-estradiol are observed during menopause and this corresponds to decreased immune responses and increased levels of the pro-inflammatory cytokines IL-6, IL-1β, and TNF-α [73,140]. The involvement of 17β-estradiol in these responses is supported by findings that 17β-estradiol supplementation suppresses the production of pro-inflammatory cytokines and boosts immune responses [47,73,142–144]. In contrast, high testosterone levels can reduce immune responses in response of the influenza vaccine [46,128]. Women as well as men with lower levels of testosterone both exhibit higher immune responses and more protection against infections [46]. Although different mechanisms are involved, 17β-estradiol suppresses infection of multiple viruses including influenza [51], HCV [80], Rubella virus [79], HIV-1 [78,133], HSV-1 [141], and SARS-CoV [54]. Experimentally in mice, greater levels of SARS-CoV infection were found in male mice [54] by a mechanism involving estrogen receptor-associated signaling [54], higher accumulation of inflammatory cells, and increased levels of some specific cytokines [IL-6,TNF-α,IL-1β], and chemokines (CXCL-1, CCL2). SARS-CoV2 induces an inflammatory storm in patients with severe symptoms [145], and multiple anti-inflammatory strategies are being tested for their ability to suppress virus-induced severe acute respiratory distress syndromes, including tocilizumab and anti-TNF-α therapy.
IL-6 and TNF-α both play important roles in inflammatory storms and ARDS development [146,147]. Tocilizumab, a humanized anti-human IL-6 receptor monoclonal antibody, has been used against COVID-19 and results show improvement in clinical symptoms and suppression of IL-6-mediated inflammation [148,149]. Similarly, anti-TNF therapies are being tested clinically to protect people at high risk for COVID-19 [149,150]. The anti-inflammatory effects of 17β-estradiol [73,74,151–153] include its ability to decrease ARDS by reducing inflammation and infiltration of immune cells in lungs [154], suppress LPS and burn trauma-induced acute lung injury [61], and attenuate NF-kB-mediated inflammation [63,155]. These might help explain results that women have fewer fatalities in COVID-19 and similarly with SARS-CoV and MERS-CoV.
Upregulation of Endolysosomal Degradation Pathway by 17β-estradiol
Endolysosomes are acidic organelles that participate in the degradation of intracellular and extracellular macromolecules, components of plasma membranes, and cellular fragments [156–159]. In addition to their role in regulating autophagy, endolysosomes help regulate various cellular processes including membrane resealing, cell death, antigen presentation, cellular trafficking, and cell division [160–164]. Functional and structural changes to endolysosomes have been implicated in multiple diseases including cancer, neurodegenerative diseases, and infections [165–168].
Testosterone and 17β-estradiol both affect endolysosomes as well as the process of autophagy [95,169–173]. Testosterone upregulates expression levels of androgen-binding protein (ABP) [171] and androgens inhibit autophagy [172,174]. Moreover, testosterone enhances muscle mass by suppressing autophagy via AMPK inactivation [173]. In contrast, 17β-estradiol upregulates autophagy by diverse mechanisms [169]; it enhances lysosomal catabolic activity [170], promotes phagocytosis [175], and regulates lysosomal activity and autophagy by activating AMPK [169,176]. AMPK regulates RAAS; it enhances the phosphorylation and stability of ACE2 and controls endothelial homeostasis and pulmonary hypertension [177,178]. Additionally, the AMPK-p-ACE2 axis is impaired in human lungs with idiopathic pulmonary arterial hypertension (IPAH) [178]. Therefore, in COVID-19 patients, 17β-estradiol through increasing AMPK activity may up-regulate ACE2 and thereby suppress the development of severe symptoms [176,179–182].
Viral infection typically requires the involvement of endolysosomes [183,184] and SARS-CoV-2 is endocytosed following fusion with cell membranes in a pH-dependent manner [5,185]. Unclear, however, are mechanisms by which endocytosed virus is released from endolysosomes. Regardless, 17β-estradiol may stimulate endolysosomes to promote the degradation of cellular or extracellular materials [186]. Recently, it has been proved that SARS-CoV-2 blocks the autophagy pathway; however, spermidine reduce SARS-COV-2 infection by alleviating the lysosomal degradation pathway [187]. Hence, it needs to validate further that 17β-estradiol may restrict the SARS-CoV-2 infection by promoting the endolysosomal degradation pathway?
Summary
SARS-CoV-2 infects men and women at the same rate, but men have a higher risk of developing severe complications and death. SARS-CoV-2 infects men and women at the same rate, but men have a higher risk of developing severe complications and death. Immune-compromised patients with hypertension, diabetes, cancer, and HIV-1 are at higher risk of being infected with the virus and developing severe symptoms including ARDS and death. 17β-estradiol might decrease SARS-CoV-2 infection by controlling RAAS, suppressing inflammatory storms, inducing anti-viral immune responses, and enhancing the virus’ degradation in endolysosomes by promoting the fusion of endosomes and lysosomes. High-risk patients may benefit from strategies designed to increase levels of 17β-estradiol by consuming estrogen pills and 17β-estradiol-enriched herbs [188,189].
Finally, it is important to address albeit briefly why postmenopausal women who have low levels of estradiol are still exhibiting lower death rates than are men from COVID-19. One reason might be the presence of catalytically-active mature natural killer (NK) cells (CD56dim); these cells are more plentiful in women than in men at ages greater than age 70 [37,190] and these cells may participate in suppression of SARS-CoV-2 infection. Other possible mechanisms may too be involved in protection of women from COVID-19.
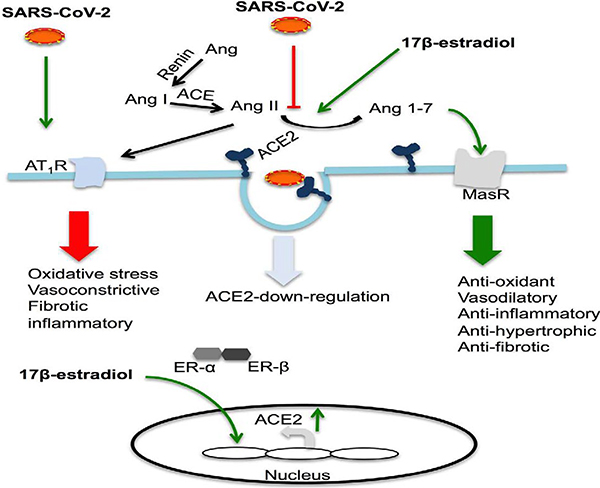
Figure 1: 17β-estradiol regulates the RAAS system by enhancing ACE2 expression.
SARS-CoV-2 may activate the ACE/AT1 axis by down-regulating ACE2 and thereby promoting the development of ARDS by inducing an inflammatory storm and increasing oxidative stress. 17β-estradiol may enhance expression levels of ACE2 (ACE2/Mas axis) and reduce ARDS. Activation of estrogen receptors regulates 17β-estradiol-mediated cellular signaling and gene expression. ACE catalyzes the conversion of Ang I to Ang II, which activates AT1 receptors. ACE2 catalyzes the conversion of Ang II to Ang I-7, which activates the Mas receptor. (SARS-CoV-2, severe acute respiratory syndrome-coronavirus 2; ER-α, estrogen receptor-α; ER-β, estrogen receptor-β; AT1R, angiotensin II type 1 (AT1) receptor; ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; MasR, Mas receptor; Ang II, angiotensin II; Ang 1–7, angiotensin 1–7).
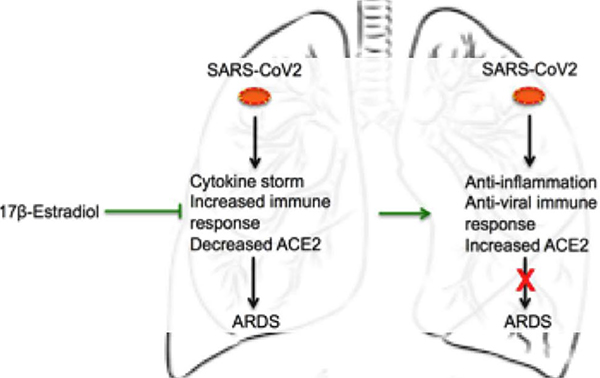
Figure 2: 17β-estradiol might suppress ARDS by enhancing anti-viral and anti-inflammatory immune responses.
SARS-CoV-2 develops severe complications in infected people including ARDS, by the down-regulating ACE2 expression and inducing a massive inflammatory storm. However, 17β-estradiol might suppress ARDS by, for example, controlling the RAAS system, and enhancing anti-inflammatory and anti-viral immune responses. (SARS-CoV-2: Severe Acute Respiratory Syndrome-Coronavirus-2; ACE2: Angiotensin-Converting Enzyme; ARDS: Acute Respiratory Distress Syndrome).
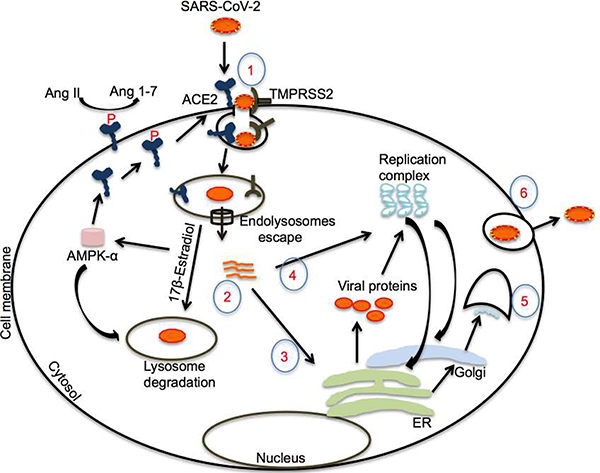
Figure 3: Upregulation of endolysosomal degradation pathway by 17β-estradiol.
The virus infects the cell by binding spike proteins with ACE2 on cell membranes following priming by TMPRSS2. Following endolysosome escape, RNA can accumulate in the cytosol, where it participates in protein translation. Translated proteins produce a replication complex to make viral RNA. 17β-estradiol may enhance the endolysosome’s degradation of the virus by enhancing the fusion of endosomes and lysosomes, probably by increasing AMPK activity and other possible mechanisms. Moreover, 17β-estradiol-mediated AMPK activation may enhance ACE2 stability by inducing phosphorylation and reduce ARDS and pulmonary hypertension. (SARS-CoV2: Severe Acute Respiratory Syndrome-Coronavirus 2; ACE2: Angiotensin-Converting Enzyme 2; p-ACE2: Phosphor-Angiotensin-Converting Enzyme 2; TMPRSS2: Transmembrane Protease, Serine 2; Ang II: Angiotensin II; Ang 1–7: Angiotensin 1–7; AMPKα: Adenosine Monophosphate Kinase-α; ER: Endoplasmic Reticulum).
Acknowledgment
I am thankful to Prof. Jonathan D. Geiger (University of North Dakota) for his on-going efforts on my behalf including intellectual support and the editing of this manuscript.
Funding
This work was partly supported by NIH grant RO1 (MH119000).
Footnotes
Conflict of Interest
The author declares no conflict of interest.
References
- 1.Li Q An outbreak of NCIP (2019-nCoV) infection in China—wuhan, Hubei province, 2019− 2020. China CDC Weekly. 2020;2(5):79–80. [PMC free article] [PubMed] [Google Scholar]
- 2.Tang JW, Tambyah PA, Hui DS. Emergence of a novel coronavirus causing respiratory illness from Wuhan, China. Journal of Infection. 2020;80(3):350–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan JF-W, Kok K-H, Zhu Z, Chu H, To KK-W, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes & Infections. 2020;9(1):221–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al. Spread of SARS-CoV-2 in the Icelandic Population. New England Journal of Medicine. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertension Research. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. Journal of Pathology. 2004;203(2):631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gembardt F, Sterner-Kock A, Imboden H, Spalteholz M, Reibitz F, Schultheiss H-P, et al. Organ-specific distribution of ACE2 mRNA and correlating peptidase activity in rodents. Peptides. 2005;26(7):1270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. The EMBO Journal.n/a(n/a):e105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tikellis C, Thomas MC. Angiotensin-Converting Enzyme 2 (ACE2) Is a Key Modulator of the Renin Angiotensin System in Health and Disease. International Journal of Peptides. 2012;2012:256294–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel VB, Zhong J-C, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1–7 Axis of the Renin–Angiotensin System in Heart Failure. Circulation Research. 2016;118(8):1313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Current Hypertension Reports. 2010;12(3):170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke NE, Turner AJ. Angiotensin-converting enzyme 2: the first decade. International Journal of Hypertension. 2012;2012:307315–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Critical Care. 2017;21(1):305–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai Y, Kuba K, Penninger JM. The renin-angiotensin system in acute respiratory distress syndrome. Drug Discovery Today: Disease Mechanisms. 2006;3(2):225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wösten-van Asperen RM, Lutter R, Specht PA, Moll GN, van Woensel JB, van der Loos CM, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1–7) or an angiotensin II receptor antagonist. Journal of Pathology. 2011;225(4):618–27. [DOI] [PubMed] [Google Scholar]
- 18.Dhawale VS, Amara VR, Karpe PA, Malek V, Patel D, Tikoo K. Activation of angiotensin-converting enzyme 2 (ACE2) attenuates allergic airway inflammation in rat asthma model. Toxicology and Applied Pharmacology. 2016;306:17–26. [DOI] [PubMed] [Google Scholar]
- 19.Leung JM, Yang CX, Tam A, Shaipanich T, Hackett T-L, Singhera GK, et al. ACE-2 Expression in the Small Airway Epithelia of Smokers and COPD Patients: Implications for COVID-19. European Respiratory Journal. 2020:2000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue T, Wei N, Xin Z, Qingyu X. Angiotensin-converting enzyme-2 overexpression attenuates inflammation in rat model of chronic obstructive pulmonary disease. Inhalation Toxicology. 2014;26(1):14–22. [DOI] [PubMed] [Google Scholar]
- 21.Kaparianos A, Argyropoulou E. Local Renin-Angiotensin II Systems, Angiotensin-Converting Enzyme and its Homologue ACE2: Their Potential Role in the Pathogenesis of Chronic Obstructive Pulmonary Diseases, Pulmonary Hypertension and Acute Respiratory Distress Syndrome. Current Medicinal Chemistry. 2011;18(23):3506–15. [DOI] [PubMed] [Google Scholar]
- 22.Chamsi-Pasha MAR, Shao Z, Tang WHW . Angiotensin-converting enzyme 2 as a therapeutic target for heart failure. Current Heart Failure Reports. 2014;11(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo J, Huang Z, Lin L, Lv J. Coronavirus Disease 2019 (COVID‐19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin‐Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Journal of the American Heart Association. 2020;9(7):e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang G, Chu P-L, Rump LC, Le TH, Stegbauer J. ACE2 and the Homolog Collectrin in the Modulation of Nitric Oxide and Oxidative Stress in Blood Pressure Homeostasis and Vascular Injury. Antioxidants & Redox Signaling. 2016;26(12):645–59. [DOI] [PubMed] [Google Scholar]
- 25.Fang Y, Gao F, Liu Z. Angiotensin-converting enzyme 2 attenuates inflammatory response and oxidative stress in hyperoxic lung injury by regulating NF-κB and Nrf2 pathways. QJM: An International Journal of Medicine. 2019;112(12):914–24. [DOI] [PubMed] [Google Scholar]
- 26.Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin I–Converting Enzyme Type 2 (ACE2) Gene Therapy Improves Glycemic Control in Diabetic Mice. Diabetes. 2010;59(10):2540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal R, Bhansali A. COVID-19, diabetes mellitus and ACE2: The conundrum. Diabetes Research and Clinical Practice. 2020;162:108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravinder Reddy G, Stephen C, Madhav B. ACE and ACE2 in Inflammation: A Tale of Two Enzymes. Inflammation & Allergy - Drug Targets (Discontinued). 2014;13(4):224–34. [DOI] [PubMed] [Google Scholar]
- 29.Thiago Ruiz Rodrigues P, Natalia Pessoa R, Aline Silva M, Antônio Lúcio T, Ana Cristina S-e-S. The Anti-Inflammatory Potential of ACE2/Angiotensin-(1–7)/Mas Receptor Axis: Evidence from Basic and Clinical Research. Current Drug Targets. 2017;18(11):1301–13. [DOI] [PubMed] [Google Scholar]
- 30.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nature Medicine. 2005;11(8):875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS-CoV-2 receptor. Pharmacological Research. 2020;157:104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chau VQ, Oliveros E, Mahmood K, Singhvi A, Lala A, Moss N, et al. The Imperfect Cytokine Storm: Severe COVID-19 with ARDS in Patient on Durable LVAD Support. JACC Case Rep. 2020: 10.1016/j.jaccas.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin J-M, Bai P, He W, Wu F, Liu X-F, Han D-M, et al. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Frontiers in Public Health. 2020;8(152). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng Y, Wu P, Lu W, Liu K, Ma K, Huang L, et al. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: A retrospective study of 168 severe patients. PLOS Pathogens. 2020;16(4):e1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iyer SP, Ensor J, Anand K, Hwu P, Subbiah V, Flowers C, et al. Higher mortality in men from COVID19 infection-understanding the factors that drive the differences between the biological sexes. medRxiv. 2020:2020.04.19.20062174. [Google Scholar]
- 36.La Vignera S, Cannarella R, Condorelli RA, Torre F, Aversa A, Calogero AE. Sex-Specific SARS-CoV-2 Mortality: Among Hormone-Modulated ACE2 Expression, Risk of Venous Thromboembolism and Hypovitaminosis D. International Journal of Molecular Sciences. 2020;21(8):2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chakravarty D, Nair SS, Hammouda N, Ratnani P, Gharib Y, Wagaskar V, et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Communications Biology. 2020;3(1):374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infectious Diseases. 2020:S1473–3099(20)30243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nasiri MJ, Haddadi S, Tahvildari A, Farsi Y, Arbabi M, Hasanzadeh S, et al. COVID-19 clinical characteristics, and sex-specific risk of mortality: Systematic Review and Meta-analysis. medRxiv. 2020:2020.03.24.20042903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karlberg J, Chong DSY, Lai WYY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? American Journal of Epidemiology. 2004;159(3):229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mobaraki K, Ahmadzadeh J. Current epidemiological status of Middle East respiratory syndrome coronavirus in the world from 1.1.2017 to 17.1.2018: a cross-sectional study. BMC Infectious Diseases. 2019;19(1):351–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castilho JL, Melekhin VV, Sterling TR. Sex differences in HIV outcomes in the highly active antiretroviral therapy era: a systematic review. AIDS research and human retroviruses. 2014;30(5):446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biology of Reproduction. 2008;78(3):432–7. [DOI] [PubMed] [Google Scholar]
- 45.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Human Reproduction Update. 2005;11(4):411–23. [DOI] [PubMed] [Google Scholar]
- 46.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(2):869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taneja V Sex Hormones Determine Immune Response. Frontiers in Immunology. 2018;9(1931). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 Ligands Induce Higher IFN-α Production in Females. The Journal of Immunology. 2006;177(4):2088–96. [DOI] [PubMed] [Google Scholar]
- 49.Laffont S, Rouquié N, Azar P, Seillet C, Plumas J, Aspord C, et al. X-Chromosome Complement and Estrogen Receptor Signaling Independently Contribute to the Enhanced TLR7-Mediated IFN-α Production of Plasmacytoid Dendritic Cells from Women. The Journal of Immunology. 2014;193(11):5444–52. [DOI] [PubMed] [Google Scholar]
- 50.Seillet C, Laffont S, Trémollières F, Rouquié N, Ribot C, Arnal J-F, et al. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor α signaling. Blood. 2012;119(2):454–64. [DOI] [PubMed] [Google Scholar]
- 51.Robinson DP, Lorenzo ME, Jian W, Klein SL. Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses. PLOS Pathogens. 2011;7(7):e1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17β-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. Journal of virology. 2014;88(9):4711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peretz J, Pekosz A, Lane AP, Klein SL. Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2016;310(5):L415–L25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. Journal of Immunology. 2017;198(10):4046–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrison RF, Bonnar J. Clinical uses of estrogens. Pharmacology & Therapeutics. 1980;11(2):451–67. [DOI] [PubMed] [Google Scholar]
- 56.Yaşar P, Ayaz G, User SD, Güpür G, Muyan M. Molecular mechanism of estrogen-estrogen receptor signaling. Reproductive Medicine and Biology. 2016;16(1):4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deroo BJ, Korach KS. Estrogen receptors and human disease. Journal of Clinical Investigation. 2006;116(3):561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahmoodzadeh S, Dworatzek E. The Role of 17β-Estradiol and Estrogen Receptors in Regulation of Ca2+ Channels and Mitochondrial Function in Cardiomyocytes. Frontiers in Endocrinology. 2019;10(310). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Björnström L, Sjöberg M. Mechanisms of Estrogen Receptor Signaling: Convergence of Genomic and Nongenomic Actions on Target Genes. Molecular Endocrinology. 2005;19(4):833–42. [DOI] [PubMed] [Google Scholar]
- 60.Hamidi S, Dickman K, Berisha H, Said S. 17 beta-Estradiol Protects the Lung against Acute Injury: Possible Mediation by Vasoactive Intestinal Polypeptide. Endocrinology. 2011;152:4729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qi D, He J, Wang D, Deng W, Zhao Y, Ye Y, et al. 17β-estradiol suppresses lipopolysaccharide-induced acute lung injury through PI3K/Akt/SGK1 mediated up-regulation of epithelial sodium channel (ENaC) in vivo and in vitro. Respiratory Research. 2014;15(1):159–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Speyer CL, Rancilio NJ, McClintock SD, Crawford JD, Gao H, Sarma JV, et al. Regulatory effects of estrogen on acute lung inflammation in mice. American Journal of Physiology-Cell Physiology. 2005;288(4):C881–C90. [DOI] [PubMed] [Google Scholar]
- 63.Yao X, Wigginton JG, Maass DL, Ma L, Carlson D, Wolf SE, et al. Estrogen-provided cardiac protection following burn trauma is mediated through a reduction in mitochondria-derived DAMPs. American Journal of Physiology-Heart and Circulatory Physiology. 2014;306(6):H882–H94. [DOI] [PubMed] [Google Scholar]
- 64.Assaggaf H, Felty Q. Gender, Estrogen, and Obliterative Lesions in the Lung. International Journal of Endocrinology. 2017;2017:8475701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ashraf MS, Vongpatanasin W. Estrogen and hypertension. Current Hypertension Reports. 2006;8(5):368–76. [DOI] [PubMed] [Google Scholar]
- 66.Gilbert EL, Mathis KW, Ryan MJ. 17β-Estradiol protects against the progression of hypertension during adulthood in a mouse model of systemic lupus erythematosus. Hypertension. 2014;63(3):616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nathan and L, Chaudhuri G. ESTROGENS AND ATHEROSCLEROSIS. Annual Review of Pharmacology and Toxicology. 1997;37(1):477–515. [DOI] [PubMed] [Google Scholar]
- 68.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacology Review. 2008;60(2):210–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tostes RC, Nigro D, Fortes ZB, Carvalho MHC. Effects of estrogen on the vascular system. Brazilian Journal of Medical and Biological Research. 2003;36:1143–58. [DOI] [PubMed] [Google Scholar]
- 70.Tzouvelekis A, Bouros D. Estrogen Signaling and MicroRNAs in Lung Fibrosis. Sex, Hormones, and Rock Scars. American Journal of Respiratory and Critical Care Medicine. 2019;200(10):1199–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59(4):1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monteiro R, Teixeira D, Calhau C. Estrogen Signaling in Metabolic Inflammation. Mediators of Inflammation. 2014;2014:615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Straub RH. The Complex Role of Estrogens in Inflammation. Endocrine Reviews. 2007;28(5):521–74. [DOI] [PubMed] [Google Scholar]
- 74.Draijer C, Hylkema MN, Boorsma CE, Klok PA, Robbe P, Timens W, et al. Sexual maturation protects against development of lung inflammation through estrogen. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2015;310(2):L166–L74. [DOI] [PubMed] [Google Scholar]
- 75.Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, et al. Estrogens and Autoimmune Diseases. Annals of the New York Academy of Sciences. 2006;1089(1):538–47. [DOI] [PubMed] [Google Scholar]
- 76.Moulton VR. Sex Hormones in Acquired Immunity and Autoimmune Disease. Frontiers in Immunology. 2018;9:2279–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Merrheim J, Villegas J, Van Wassenhove J, Khansa R, Berrih-Aknin S, le Panse R, et al. Estrogen, estrogen-like molecules and autoimmune diseases. Autoimmunity Reviews. 2020;19(3):102468. [DOI] [PubMed] [Google Scholar]
- 78.Szotek EL, Narasipura SD, Al-Harthi L. 17β-Estradiol inhibits HIV-1 by inducing a complex formation between β-catenin and estrogen receptor α on the HIV promoter to suppress HIV transcription. Virology. 2013;443(2):375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roehrig JT, Brawner TA, Riggs HG Jr. Effects of 17beta-estradiol on the replication of rubella virus in an estrogen-responsive, continuous cell line. Journal of Virology. 1979;29(1):417–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Magri A, Barbaglia MN, Foglia CZ, Boccato E, Burlone ME, Cole S, et al. 17,β-estradiol inhibits hepatitis C virus mainly by interference with the release phase of its life cycle. Liver International. 2017;37(5):669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raghava N, Das BC, Ray SK. Neuroprotective effects of estrogen in CNS injuries: insights from animal models. Neuroscience and Neuroeconomics. 2017;6:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heron P, Turchan-Cholewo J, Bruce-Keller A, Wilson M. Estrogen Receptor Alpha Inhibits the Estrogen-Mediated Suppression of HIV Transcription in Astrocytes: Implications for Estrogen Neuroprotection in HIV Dementia. AIDS Research and Human Retroviruses. 2009;25:1071–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wise PM, Dubal DB, Wilson ME, Rau SW, Böttner M. Minireview: Neuroprotective Effects of Estrogen—New Insights into Mechanisms of Action. Endocrinology. 2001;142(3):969–73. [DOI] [PubMed] [Google Scholar]
- 84.Di Stadio A, Della Volpe A, Ralli M, Ricci G. Gender differences in COVID-19 infection. The estrogen effect on upper and lower airways. Can it help to figure out a treatment? European Review for Medical and Pharmacological Sciences. 2020;24(10):5195–6. [DOI] [PubMed] [Google Scholar]
- 85.Breithaupt-Faloppa AC, Correia CdJ, Prado CMx, Stilhano RS, Ureshino RP, Moreira LFP. 17≤-Estradiol, a potential ally to alleviate SARS-CoV-2 infection. Clinics. 2020;75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discovery. 2020;6(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shabbir S, Hafeez A, Rafiq MA, Khan MJ. Estrogen shields women from COVID-19 complications by reducing ER stress. Medical Hypotheses. 2020;143:110148–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Costeira R, Lee KA, Murray B, Christiansen C, Castillo-Fernandez J, Ni Lochlainn M, et al. Estrogen and COVID-19 symptoms: associations in women from the COVID Symptom Study. MedRxiv. 2020:2020.07.30.20164921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nehme A, Zouein FA, Zayeri ZD, Zibara K. An Update on the Tissue Renin Angiotensin System and Its Role in Physiology and Pathology. Journal of Cardiovascular Development and Disease. 2019;6(2):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Santos RAS, Oudit GY, Verano-Braga T, Canta G, Steckelings UM, Bader M. The renin-angiotensin system: going beyond the classical paradigms. American Journal of Physiology-Heart and Circulatory Physiology. 2019;316(5):H958–H70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guignabert C, de Man F, Lombès M. ACE2 as therapy for pulmonary arterial hypertension: the good outweighs the bad. European Respiratory Journal. 2018;51(6):1800848. [DOI] [PubMed] [Google Scholar]
- 92.Zambelli V, Grassi A, Bellani G. Role of the Renin-Angiotensin System in ARDS In: Vincent J-L, editor. Annual Update in Intensive Care and Emergency Medicine 2012. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. p. 171–81. [Google Scholar]
- 93.Chappell MC. Does ACE2 contribute to the development of hypertension? Hypertension Research. 2010;33(2):107–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calò LA, Rigato M, Bertoldi G. ACE2/Angiotensin 1–7 protective anti-inflammatory and antioxidant role in hyperoxic lung injury: support from studies in Bartter’s and Gitelman’s syndromes. QJM: An International Journal of Medicine. 2019. [DOI] [PubMed] [Google Scholar]
- 95.Boese AC, Kim SC, Yin K-J, Lee J-P, Hamblin MH. Sex differences in vascular physiology and pathophysiology: estrogen and androgen signaling in health and disease. The American Journal of Physiology: Heart and Circulatory Physiology. 2017;313(3):H524–H45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dalpiaz PLM, Lamas AZ, Caliman IF, Ribeiro RF Jr., Abreu GR, Moyses MR, et al. Sex Hormones Promote Opposite Effects on ACE and ACE2 Activity, Hypertrophy and Cardiac Contractility in Spontaneously Hypertensive Rats. PLoS One. 2015;10(5):e0127515–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hilliard LM, Mirabito KM, Widdop RE, Denton KM. Chapter 17 - Sex Differences in AT2R Expression and Action In: Unger T, Steckelings UM, dos Santos RAS, editors. The Protective Arm of the Renin Angiotensin System (RAS). Boston: Academic Press; 2015. p. 125–30. [Google Scholar]
- 98.White MC, Fleeman R, Arnold AC. Sex differences in the metabolic effects of the renin-angiotensin system. Biology of Sex Differences. 2019;10(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beale Anna L, Meyer P, Marwick Thomas H, Lam Carolyn SP, Kaye David M. Sex Differences in Cardiovascular Pathophysiology. Circulation. 2018;138(2):198–205. [DOI] [PubMed] [Google Scholar]
- 100.Mompeón A, Lázaro-Franco M, Bueno-Betí C, Pérez-Cremades D, Vidal-Gómez X, Monsalve E, et al. Estradiol, acting through ERα, induces endothelial non-classic renin-angiotensin system increasing angiotensin 1–7 production. Molecular and Cellular Endocrinology. 2016;422:1–8. [DOI] [PubMed] [Google Scholar]
- 101.Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen Regulation of Angiotensin-Converting Enzyme mRNA. Hypertension. 1999;33(1):323–8. [DOI] [PubMed] [Google Scholar]
- 102.Bukowska A, Spiller L, Wolke C, Lendeckel U, Weinert S, Hoffmann J, et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Experimental Biology and Medicine (Maywood). 2017;242(14):1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mishra JS, Hankins GD, Kumar S. Testosterone downregulates angiotensin II type-2 receptor via androgen receptor-mediated ERK1/2 MAP kinase pathway in rat aorta. Journal of the Renin-Angiotensin-Aldosterone System. 2016;17(4):1470320316674875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mishra JS, More AS, Gopalakrishnan K, Kumar S. Testosterone plays a permissive role in angiotensin II-induced hypertension and cardiac hypertrophy in male rats. Biology of Reproduction. 2019;100(1):139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramírez-Expósito MJ, Martinez-Martos JM. Hypertension, RAS, and gender: what is the role of aminopeptidases? Heart Failure Reviews. 2008;13(3):355–65. [DOI] [PubMed] [Google Scholar]
- 106.Burger HG. The endocrinology of the menopause. Maturitas. 1996;23(2):129–36. [DOI] [PubMed] [Google Scholar]
- 107.Hilliard LM, Sampson AK, Brown RD, Denton KM. The “his and hers” of the renin-angiotensin system. Current Hypertension Reports. 2013;15(1):71–9. [DOI] [PubMed] [Google Scholar]
- 108.Fernández-Atucha A, Izagirre A, Fraile-Bermúdez AB, Kortajarena M, Larrinaga G, Martinez-Lage P, et al. Sex differences in the aging pattern of renin–angiotensin system serum peptidases. Biology of Sex Differences. 2017;8(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Golomb L, Sagiv A, Pateras IS, Maly A, Krizhanovsky V, Gorgoulis VG, et al. Age-associated inflammation connects RAS-induced senescence to stem cell dysfunction and epidermal malignancy. Cell Death & Differentiation. 2015;22(11):1764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Johnston CJ, Rubenfeld GD, Hudson LD. Effect of Age on the Development of ARDS in Trauma Patients. Chest. 2003;124(2):653–9. [DOI] [PubMed] [Google Scholar]
- 111.Koutsoukou A, Katsiari M, Orfanos S, Rovina N, Dimitrakopoulou C, Kotanidou A. ARDS in Aged Patients: Respiratory System Mechanics and Outcome. Health Science Journal. 2017;11. [Google Scholar]
- 112.Lionakis N, Mendrinos D, Sanidas E, Favatas G, Georgopoulou M. Hypertension in the elderly. World Journal of Cardiology. 2012;4(5):135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yazdanyar A, Newman AB. The burden of cardiovascular disease in the elderly: morbidity, mortality, and costs. Clinics in Geriatric Medicine. 2009;25(4):563–vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chung HY, Kim DH, Lee EK, Chung KW, Chung S, Lee B, et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Diseases. 2019;10(2):367–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miao J, Liu J, Niu J, Zhang Y, Shen W, Luo C, et al. Wnt/β-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell. 2019;18(5):e13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dijkman R, Jebbink M, Deijs M, Milewska A, Pyrc K, Buelow E, et al. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. The Journal of General Virology. 2012;93:1924–9. [DOI] [PubMed] [Google Scholar]
- 117.Zou Z, Yan Y, Shu Y, Gao R, Sun Y, Li X, et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nature Communications. 2014;5:3594–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang P, Gu H, Zhao Z, Wang W, Cao B, Lai C, et al. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Science Reports. 2014;4:7027–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, et al. Differential Downregulation of ACE2 by the Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus NL63. Journal of Virology. 2010;84(2):1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Richards EM, Raizada MK. ACE2 and pACE2: A Pair of Aces for Pulmonary Arterial Hypertension Treatment? American Journal Respiratory Critical Care Medicine. 2018;198(4):422–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Y, Zeng Z, Cao Y, Liu Y, Ping F, Liang M, et al. Angiotensin-converting enzyme 2 prevents lipopolysaccharide-induced rat acute lung injury via suppressing the ERK1/2 and NF-κB signaling pathways. Science Reports. 2016;6:27911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lumbers ER, Pringle KG, Wang Y, Gibson KJ. The renin–angiotensin system from conception to old age: the good, the bad and the ugly. Clinical and Experimental Pharmacology and Physiology. 2013;40(11):743–52. [DOI] [PubMed] [Google Scholar]
- 123.Li Q, Verma A, Han P-Y, Shan Z, Yuan L, Lewin AS, et al. ACE2 Over-Expression Protects Retinal Vascular Dysfunction in an Animal Model of Diabetic Retinopathy. Investigative Ophthalmology & Visual Science. 2009;50(13):4296–. [Google Scholar]
- 124.Chaplin DD. Overview of the immune response. Journal of Allergy and Clinical Immunology. 2010;125(2 Suppl 2):S3–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Braciale TJ, Hahn YS. Immunity to viruses. Immunol Rev. 2013;255(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Klein SL, Flanagan KL. Sex differences in immune responses. Nature Reviews Immunology. 2016;16(10):626. [DOI] [PubMed] [Google Scholar]
- 127.Karnam G, Rygiel TP, Raaben M, Grinwis GCM, Coenjaerts FE, Ressing ME, et al. CD200 receptor controls sex-specific TLR7 responses to viral infection. PLoS pathogens. 2012;8(5):e1002710-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, et al. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biology of Sex Differences. 2011;2:8–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The Effect of Testosterone Replacement on Endogenous Inflammatory Cytokines and Lipid Profiles in Hypogonadal Men. The Journal of Clinical Endocrinology & Metabolism. 2004;89(7):3313–8. [DOI] [PubMed] [Google Scholar]
- 131.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical Microbiology Review. 2009;22(2):240–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leiva-Juárez MM, Kolls JK, Evans SE. Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal Immunology. 2018;11(1):21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nature Medicine. 2009;15(8):955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lélu K, Krust A, et al. 17β-Estradiol Promotes TLR4-Triggered Proinflammatory Mediator Production through Direct Estrogen Receptor α Signaling in Macrophages In Vivo. The Journal of Immunology. 2010;185(2):1169–76. [DOI] [PubMed] [Google Scholar]
- 135.Medina-Estrada I, Alva-Murillo N, López-Meza JE, Ochoa-Zarzosa A. Immunomodulatory Effects of 17β-Estradiol on Epithelial Cells during Bacterial Infections. Journal of Immunology Research. 2018;2018:6098961–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Crane-Godreau MA, Wira CR. Effects of estradiol on lipopolysaccharide and Pam3Cys stimulation of CCL20/macrophage inflammatory protein 3 alpha and tumor necrosis factor alpha production by uterine epithelial cells in culture. Infection and Immunity. 2005;73(7):4231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fahey JV, Wright JA, Shen L, Smith JM, Ghosh M, Rossoll RM, et al. Estradiol selectively regulates innate immune function by polarized human uterine epithelial cells in culture. Mucosal Immunology. 2008;1(4):317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kowsar R, Hambruch N, Liu J, Shimizu T, Pfarrer C, Miyamoto A. Regulation of innate immune function in bovine oviduct epithelial cells in culture: the homeostatic role of epithelial cells in balancing Th1/Th2 response. Journal of Reproduction and Development. 2013;59(5):470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheng C, Wu H, Wang M, Wang L, Zou H, Li S, et al. Estrogen ameliorates allergic airway inflammation by regulating activation of NLRP3 in mice. Bioscience Reports. 2019;39(1):BSR20181117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in Proinflammatory Cytokine Activity after Menopause. Endocrine Reviews. 2002;23(1):90–119. [DOI] [PubMed] [Google Scholar]
- 141.Anipindi VC, Bagri P, Roth K, Dizzell SE, Nguyen PV, Shaler CR, et al. Estradiol Enhances CD4+ T-Cell Anti-Viral Immunity by Priming Vaginal DCs to Induce Th17 Responses via an IL-1-Dependent Pathway. PLOS Pathogens. 2016;12(5):e1005589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Science Reports. 2015;5(1):15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Galien R, Garcia T. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic Acids Research. 1997;25(12):2424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Khan D, Ansar Ahmed S. The Immune System Is a Natural Target for Estrogen Action: Opposing Effects of Estrogen in Two Prototypical Autoimmune Diseases. Frontiers in immunology. 2016;6:635–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virologica Sinica. 2020:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspectives in Biology. 2014;6(10):a016295-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proceedings of the National Academy of Sciences. 2020:202005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao M Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. International Journal of Antimicrobial Agents. 2020:105982–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feldmann M, Maini RN, Woody JN, Holgate ST, Winter G, Rowland M, et al. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395(10234):1407–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Molecular and Cellular Biology. 1995;15(9):4971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.An J, Ribeiro RCJ, Webb P, Gustafsson J-Å, Kushner PJ, Baxter JD, et al. Estradiol repression of tumor necrosis factor-α transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proceedings of the National Academy of Sciences. 1999;96(26):15161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ito A, Bebo BF, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, et al. Estrogen Treatment Down-Regulates TNF-α Production and Reduces the Severity of Experimental Autoimmune Encephalomyelitis in Cytokine Knockout Mice. The Journal of Immunology. 2001;167(1):542–52. [DOI] [PubMed] [Google Scholar]
- 154.Breithaupt-Faloppa AC, Thais Fantozzi E, Romero DC, Rodrigues AdS, de Sousa PTR, Lino Dos Santos Franco A, et al. Acute effects of estradiol on lung inflammation due to intestinal ischemic insult in male rats. Shock. 2014;41(3):208–13. [DOI] [PubMed] [Google Scholar]
- 155.Al-Tarrah K, Moiemen N, Lord JM. The influence of sex steroid hormones on the response to trauma and burn injury. Burns Trauma. 2017;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Klumperman J, Raposo G. The complex ultrastructure of the endolysosomal system. Cold Spring Harbor Perspectives in Biology. 2014;6(10):a016857-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hu Y-B, Dammer EB, Ren R-J, Wang G. The endosomal-lysosomal system: from acidification and cargo sorting to neurodegeneration. Translational Neurodegeneration. 2015;4:18–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pryor PR, Luzio JP. Delivery of endocytosed membrane proteins to the lysosome. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2009;1793(4):615–24. [DOI] [PubMed] [Google Scholar]
- 159.Khan N, Lakpa KL, Halcrow PW, Afghah Z, Miller NM, Geiger JD, et al. BK channels regulate extracellular Tat-mediated HIV-1 LTR transactivation. Science Reports. 2019;9(1):12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Afghah Z, Chen X, Geiger JD. Role of endolysosomes and inter-organellar signaling in brain disease. Neurobiology of Disease. 2020;134:104670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Khan N, Haughey NJ, Nath A, Geiger JD. Involvement of organelles and inter-organellar signaling in the pathogenesis of HIV-1 associated neurocognitive disorder and Alzheimer’s disease. Brain Research. 2019;1722:146389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Perera RM, Zoncu R. The Lysosome as a Regulatory Hub. Annual Review of Cell and Developmental Biology. 2016;32(1):223–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Munz C Antigen Processing for MHC Class II Presentation via Autophagy. Frontiers in Immunology. 2012;3(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Khan N, Halcrow PW, Lakpa KL, Afghah Z, Miller NM, Dowdy SF, et al. Two-pore channels regulate Tat endolysosome escape and Tat-mediated HIV-1 LTR transactivation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2020;34(3):4147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Colacurcio DJ, Nixon RA. Disorders of lysosomal acidification—The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Research Reviews. 2016;32:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Colacurcio DJ, Pensalfini A, Jiang Y, Nixon RA. Dysfunction of autophagy and endosomal-lysosomal pathways: Roles in pathogenesis of Down syndrome and Alzheimer’s Disease. Free Radical Biology and Medicine. 2018;114:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Van Acker ZP, Bretou M, Annaert W. Endo-lysosomal dysregulations and late-onset Alzheimer’s disease: impact of genetic risk factors. Molecular Neurodegeneration. 2019;14(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Halcrow P, Khan N, Datta G, Ohm JE, Chen X, Geiger JD. Importance of measuring endolysosome, cytosolic, and extracellular pH in understanding the pathogenesis of and possible treatments for glioblastoma multiforme. Cancer Reports. 2019;2(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xiang J, Liu X, Ren J, Chen K, Wang H-L, Miao Y-Y, et al. How does estrogen work on autophagy? Autophagy. 2019;15(2):197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Burlando B, Marchi B, Panfoli I, Viarengo A. Essential role of Ca2+-dependent phospholipase A2 in estradiol-induced lysosome activation. American Journal of Physiology-Cell Physiology. 2002;283(5):C1461–C8. [DOI] [PubMed] [Google Scholar]
- 171.Ma Y, Yang H-Z, Xu L-M, Huang Y-R, Dai H-L, Kang X-N. Testosterone regulates the autophagic clearance of androgen binding protein in rat Sertoli cells. Sci Reports. 2015;5(1):8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jankauskaitė E, Ambroziak AM, Hajieva P, Ołdak M, Tońska K, Korwin M, et al. Testosterone increases apoptotic cell death and decreases mitophagy in Leber’s hereditary optic neuropathy cells. Journal of Applied Genetics. 2020;61(2):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Serra C, Sandor NL, Jang H, Lee D, Toraldo G, Guarneri T, et al. The effects of testosterone deprivation and supplementation on proteasomal and autophagy activity in the skeletal muscle of the male mouse: differential effects on high-androgen responder and low-androgen responder muscle groups. Endocrinology. 2013;154(12):4594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bennett HL, Fleming JT, O’Prey J, Ryan KM, Leung HY. Androgens modulate autophagy and cell death via regulation of the endoplasmic reticulum chaperone glucose-regulated protein 78/BiP in prostate cancer cells. Cell Death Diseases. 2010;1(9):e72-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lara-Cruz C, Jiménez-Salazar JE, Arteaga M, Arredondo M, Ramón-Gallegos E, Batina N, et al. Gold nanoparticle uptake is enhanced by estradiol in MCF-7 breast cancer cells. International Journal of Nanomedicine. 2019;14:2705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang X, Ge Y, Bukhari A-A-S, Zhu Q, Shen Y, Li M, et al. Estrogen negatively regulates the renal epithelial sodium channel (ENaC) by promoting Derlin-1 expression and AMPK activation. Experimental & Molecular Medicine. 2019;51(5):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu J, Li X, Lu Q, Ren D, Sun X, Rousselle T, et al. AMPK: a balancer of the renin-angiotensin system. Bioscience Reports. 2019;39(9):BSR20181994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang J, Dong J, Martin M, He M, Gongol B, Marin T, et al. AMPK Phosphorylation of ACE2 in Endothelium Mitigates Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine. 2018;198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lipovka Y, Konhilas JP. Estradiol activates AMPK through interaction with extrogen receptor beta (15.4). The FASEB Journal. 2014;28(1_ supplement):15.4. [Google Scholar]
- 180.Lipovka Y, Chen H, Vagner J, Price TJ, Tsao T-S, Konhilas JP. Oestrogen receptors interact with the α-catalytic subunit of AMP-activated protein kinase. Bioscience Reports. 2015;35(5):e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhao X, Zmijewski JW, Lorne E, Liu G, Park Y-J, Tsuruta Y, et al. Activation of AMPK attenuates neutrophil proinflammatory activity and decreases the severity of acute lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2008;295(3):L497–L504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang G, Song Y, Feng W, Liu L, Zhu Y, Xie X, et al. Activation of AMPK attenuates LPS-induced acute lung injury by upregulation of PGC1α and SOD1. Experimental and Therapeutic Medicine. 2016;12(3):1551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Du L, He Y, Zhou Y, Liu S, Zheng B, Jiang S. The spike protein of SARS-CoV - A target for vaccine and therapeutic development. Nature Reviews Microbiology. 2009;7:226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Carter GC, Bernstone L, Baskaran D, James W. HIV-1 infects macrophages by exploiting an endocytic route dependent on dynamin, Rac1 and Pak1. Virology. 2011;409(2):234–50. [DOI] [PubMed] [Google Scholar]
- 185.Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wei Y, Zhou J, Wu J, Huang J. ERβ promotes Aβ degradation via the modulation of autophagy. Cell Death Diseases. 2019;10(8):565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gassen NC, Papies J, Bajaj T, Dethloff F, Emanuel J, Weckmann K, et al. Analysis of SARS-CoV-2-controlled autophagy reveals spermidine, MK-2206, and niclosamide as putative antiviral therapeutics. BioRxiv. 2020:2020.04.15.997254. [Google Scholar]
- 188.Morgan HE, Dillaway D, Edwards TM. Estrogenicity of soybeans (Glycine max) varies by plant organ and developmental stage. Endocrine Disruptors. 2014;2(1):e28490. [Google Scholar]
- 189.Janeczko A, Skoczowski A. Mammalian sex hormones in plants. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society. 2005;43:71–9. [PubMed] [Google Scholar]
- 190.Al-Attar A, Presnell SR, Peterson CA, Thomas DT, Lutz CT. The effect of sex on immune cells in healthy aging: Elderly women have more robust natural killer lymphocytes than do elderly men. Mechanisms of Ageing and Development. 2016;156:25–33. [DOI] [PubMed] [Google Scholar]