Abstract
Iron metabolism might play a crucial role in cytokine release syndrome in COVID-19 patients. Therefore, we assessed iron metabolism markers in COVID-19 patients for their ability to predict disease severity. COVID-19 patients referred to the Heidelberg University Hospital were retrospectively analyzed. Patients were divided into outpatients (cohort A, n = 204), inpatients (cohort B, n = 81), and outpatients later admitted to hospital because of health deterioration (cohort C, n = 23). Iron metabolism parameters were severely altered in patients of cohort B and C compared to cohort A. In multivariate regression analysis including age, gender, CRP and iron-related parameters only serum iron and ferritin were significantly associated with hospitalization. ROC analysis revealed an AUC for serum iron of 0.894 and an iron concentration <6 μmol/l as the best cutoff-point predicting hospitalization with a sensitivity of 94.7% and a specificity of 67.9%. When stratifying inpatients in a low- and high oxygen demand group serum iron levels differed significantly between these two groups and showed a high negative correlation with the inflammatory parameters IL-6, procalcitonin, and CRP. Unexpectedly, serum iron levels poorly correlate with hepcidin. We conclude that measurement of serum iron can help predicting the severity of COVID-19. The differences in serum iron availability observed between the low and high oxygen demand group suggest that disturbed iron metabolism likely plays a causal role in the pathophysiology leading to lung injury.
Introduction
Since iron is a critical cofactor for proteins involved in many fundamental biological processes, including DNA/RNA synthesis and ATP generation, viruses, most likely including Coronaviruses, essentially rely on iron to replicate in host cells.1 Because both, host and pathogen require iron, the host innate immune response carefully orchestrates iron metabolism to limit iron availability during times of infection and especially during infection-related critical illness.2 Previous data suggest that parameters of iron metabolism, particularly transferrin saturation reflecting on serum iron availability, are strong outcome predictors in ICU patients.3 This finding was recently supported by a small, retrospective cohort study of 50 hospitalized Coronavirus disease 2019 (COVID-19) patients in which low serum iron levels are associated with mortality and disease severity.4 Beyond these findings little is known about the interaction of the newly discovered coronavirus SARS-CoV-2 with host iron metabolism during COVID-19.
Patients infected with SARS-CoV-2 may die due to an excessive response of their immune system, hallmarked by an abnormally high release of circulating cytokines, termed cytokine release syndrome (CRS). CRS plays a major role in the deterioration of COVID-19 patients, from pneumonia through acute respiratory distress syndrome (ARDS), cumulating in systemic inflammation and ultimately multi-system organ failure.5 This phenomenon of a plethora of cytokines wreaking havoc throughout the body is vividly referred to as “cytokine storm”. Cytokines involved in the “cytokine storm” in COVID-19 patients, include IL-6, IL-1, IL-2, IL-10, and TNF-α. However, IL-6 seems to play the most prominent role, whereby increased levels in the serum correlate with respiratory failure, ARDS, and adverse clinical outcomes.6 IL-6 is a pleiotropic cytokine involved in eliciting the acute-phase response in the liver, in B-cell proliferation and antibody production, in T-cell differentiation and cytotoxicity, and in hepcidin synthesis in the liver.7 Hepcidin is the master regulator of iron homeostasis. By degrading its target receptor ferroportin, hepcidin controls dietary iron absorption and iron release from iron-recycling macrophages.8 During states of infection or inflammation hepcidin levels increase restricting iron availability in the plasma. The resulting hypoferremia is an integral part of the host defense mechanism. Other than IL-6, several other cytokines such as IL-1,7 IL-22,9 and interferon α,10 contribute to increasing hepcidin expression.
In addition to the regulation of iron metabolism via hepcidin, circulating cytokines such as IL-1 and tumor necrosis factor (TNF) increase synthesis of the iron storage protein ferritin.11,12 Consequently, more iron is retained predominantly in the reticuloendothelial system that handles most of the iron recycled from damaged red blood cells. The resulting hypoferremia disturbs erythropoiesis and iron uptake in most organs.13,14
As the control of iron metabolism is crucial during infections in general and because several cytokines involved in the “cytokine storm” in COVID-19 are strong regulators of iron metabolism, understanding of iron homeostasis in COVID-19 is likely to be a relevant piece in the puzzle of COVID-19 disease. In this study, we noted that severe COVID-19 cases not only show higher levels of inflammatory markers than mild cases, but also that iron metabolism parameters greatly differed depending on disease severity.
Therefore, we focused our attention on iron-related parameters in COVID-19 patients and showed that (1) severely ill COVID-19 patients show marked hypoferremia, (2) the level of hypoferremia predicts disease severity (reflected in hospitalization requirement), (3) hypoferremia only partially correlates with hepcidin and inflammatory markers like C-reactive protein (CRP) and IL-6, and (4) immunomodulatory therapeutic agents (e.g. IL-1R blockade, IL-6R blockade and immunoglobulins)6,15 show a rapid and strong effect on hypoferremia in COVID-19 patients.
Results
Baseline characteristics of patient cohorts
Our outpatient cohort (cohort A) consists of 204 outpatients who remained home throughout the entire course of the disease. 23 patients were initially followed as outpatients but later had to be admitted to the hospital because of clinical worsening. These patients are summarized as cohort C. In addition, we analyzed an inpatient cohort (cohort B) consisting of 81 patients. Table 1 shows the baseline characteristics and treatments of all three cohorts. In cohort B, 48 patients (59.3%) had low oxygen demand (no or low-flow oxygen requirement) while 33 patients (40.7%) showed high oxygen demand (high flow oxygen or invasive ventilation). 14 (17.3%) inpatients of cohort B died, while all patients in cohort A and C survived. Table 2 depicts lab values of patients in all three cohorts. Some of the outpatients of cohort A and C were followed on more than one outpatient visit and data of all time-points are included in laboratory analyses shown in Table 2. This explains the results of 415 visits in 204 patients of cohort A and 48 visits in 23 patients of cohort C. The inpatient cohort B differs significantly from Cohort A in all laboratory parameters analyzed.
Table 1.
Baseline Characteristics of Study Population. Results are Presented as Numbers (With %) or Median (With Interquartile Range).
| Cohort A (n = 204) | Cohort B (n = 81) | p value∗ | Cohort C (n = 23) | p value† | |
|---|---|---|---|---|---|
| Age (years) | 51 (36–62) | 64 (54–75) | < 0.001 | 58 (52–68) | 0.029 |
| Gender (male) | 67 (32.8%) | 53 (65.4%) | < 0.001 | 15 (65.2%) | 0.008 |
| Event of death | 0 | 14 (17.3%) | 0 | ||
| No oxygen | 204 (100%) | 8 (19.5%) | 23 (100%) | ||
| Lowflow therapy | 40 (49.4%) | ||||
| Highflow therapy | 12 (14.8%) | 0 | |||
| Invasive ventilation | 21 (25.9%) | ||||
| ARDS | |||||
| mild | 0 | 5 (6.2%) | 0 | ||
| moderate | 0 | 15 (18.5%) | 0 | ||
| severe | 0 | 13 (16.0%) | 0 | ||
| Treatments | |||||
| no treatment | 204 (100%) | 0 | 23 (100%) | ||
| antibiotic/antifungal treatment | 81 (100%) | ||||
| maraviroc | 48 (59.3%) | ||||
| hydroxychloroquine | 52 (65%) | ||||
| anakinra | 6 (7.4%) | ||||
| tocilizumab | 4 (4.9%) | ||||
| immunoglobulines (IVIG) | 6 (7.4%) | ||||
| plasmapheresis | 3 (3.7%) | ||||
| cytosorb® | 2 (2.5%) | ||||
| convalescent serum | 2 (2.5%) | ||||
Comparison of p values between cohort A and B.
Comparison of p values between cohort A and C.
Table 2.
Laboratory Findings of the Study Cohorts. Results are Presented as Median (With Interquartile Range).
| Normal range | Cohort A (n = 204) | Cohort B (n = 81) | p value∗ | Cohort C (n = 23) | p value† | |
|---|---|---|---|---|---|---|
| Hemoglobin, g/dL | 13–17 | 14 (13.2–14.9) | 13.3 (11.9–14.7) | 0.003 | 14.3 (13.3–15.4) | n.s. |
| White blood cell count, ×109 per L | 4–10 | 5.8 (4.6–7.3) | 6.4 (4.6–9.0) | 0.033 | 5.3 (3.4–7.3) | n.s. |
| Lymphocyte count, ×109 per L | 1.0–4.8 | 1.5 (1.1–1.9) | 0.8 (0.6–1.1) | <0.001 | 1.0 (0.7–1.4) | <0.001 |
| CRP, mg/L | <5 | 13.2 (2.0–40.8) | 89.3 (37.6–148.1) | <0.001 | 46.4 (21.8–106.8) | <0.001 |
| Pprocalcitonin, ng/mL | < 0.05 | 0.05 (0.05–0.06) | 0.10 (0.06–0.30) | <0.001 | 0.06 (0.05–0.11) | <0.001 |
| Interleukin-6, pg/mL | <15 | n.a | 43.1 (15.8–83.8) | n.a. | ||
| Ferritin, μg/L | 30–300 | 227 (83–569) | 777 (341–1339) | <0.001 | 741 (404–935) | <0.001 |
| Iron, μmol/L | 14–32 | 8.6 (5.0–14.9) | 2.6 (1.8–3.9) | <0.001 | 3.2 (2.4–4.6) | <0.001 |
| Transferrin, g/L | 2.0–3.6 | 1.9 (1.6–2.3) | 1.5 (1.2–1.7) | <0.001 | 1.7 (1.6–1.9) | 0.012 |
| TfSat, % | 16–45 | 19 (12–28) | 7 (5–11) | <0.001 | 8 (6–10) | <0.001 |
| Hepcidin, ng/mL | 91.4 (59.6–133.7) | |||||
| LDH, U/L | <317 | 286 (232–360) | 384 (308–508) | <0.001 | 348 (272–374) | 0.005 |
| AST, U/L | <37 | n.a. | 42 (32–77) | n.a. | ||
| ALT, U/L | <35 | n.a. | 36 (27–57) | n.a. | ||
| Creatinine, mg/dL | 0.6–1.2 | 0.74 (0.65–0.88) | 0.88 (0.69–1.04) | <0.001 | 0.92 (0.79 – 1.11) | <0.001 |
| BUN, mg/dL | <45 | 25 (20–31) | 28 (21–44) | 0.001 | 29 (24 – 34) | 0.005 |
| NT-pro-BNP, ng/L | <125 | 99 (54–185) | 247 (109–849) | <0.001 | 85 (46–154) | n.s. |
| Troponin-T, pg/mL | <14 | na | 11.5 (6.0–22.3) | n.a. |
Laboratory values in cohort B were obtained at day of admission. Laboratory values in cohort A and C were obtained during outpatient visits. Cohort A and C: All available values were included in analysis. Cohort B: only values of the day of admission were included in the analysis.
AST = alanine aminotransferase, AST = aspartate aminotransferase, BUN = blood urea nitrogen, CRP = C-reactive protein, LDH = lactate dehydrogenase, n.a. = not analyzed, PCT = procalcitonin, TfSat = transferrin saturation.
Comparison of p values between cohort A and B.
Comparison of p values between cohort A and C.
Serum iron levels predict hospitalization in COVID-19 patients
COVID-19 patients show strongly elevated inflammatory markers, such as CRP and IL-6, confirming results of previous studies.16 In addition, the iron-related parameters analyzed (ferritin, serum iron, TfSat, transferrin, and hepcidin) severely deviated from normal in all cohorts. When comparing our cohorts, outpatients of cohort A showed less severe alterations compared to those outpatients that had to be admitted to the hospital because of clinical worsening (cohort C) (Table 2). Similarly, iron markers were altered more severely in the inpatient cohort B compared to outpatients in cohort A (Table 2). The differences in iron-related parameters in part were gender-specific, with females of cohort A deviating less from the normal range for serum iron, transferrin and ferritin compared to males, while in cohort B and C only ferritin was significantly higher in males (Supplements Fig. 1).
Utilizing this unique cohort set up of in- and outpatients, we performed univariate logistic regression analysis for age, gender, CRP, and iron markers with respect to hospitalization requirement of COVID-19 patients reflecting upon disease severity (Table 3). Interestingly, in univariate analysis, all iron metabolism parameters were significantly associated with admission status. In multivariate regression analysis including age, gender, CRP and iron markers only serum iron and ferritin were significantly associated with hospitalization, whereby doubling of serum iron was associated with a 6.7-fold lower odd of hospitalization (adjusted OR per log2 increase in serum iron: 0.15, 95% CI 0.09–0.26, p < 0.001) (Table 3).
Table 3.
Univariate and Multivariable Logistic Regression Analysis for Admission Status of COVID-19 Patients (cohort A + B).
| COVID-19 outpatient (n = 204) vs inpatient (n = 81) | ||
|---|---|---|
| Covariate, effect | Univariate analysis, OR (95% CI), p | Multivariate analysis, aOR (95% CI), p |
| Age, ≥ 60 vs <60 years | 2.26 (1.39–3.70), 0.001 | 0.60 (0.28–1.31), n.s. |
| gender, m vs f | 3.38 (2.05–5.57), <0.001 | 1.37 (0.65–2.89), n.s. |
| CRP, per log2 increase∗ | 2.14 (1.78–2.59), <0.001 | 1.20 (0.87–1.66), n.s. |
| Serum ferritin, per log2 increase∗ | 1.64 (0.14–1.93), <0.001 | 1.57 (1.14–2.14), 0.005 |
| Serum iron, per log2 increase∗ | 0.15 (0.10–0.23), <0.001 | 0.15 (0.09–0.26), <0.001 |
| transferrin | 0.15 (0.08–0.28), <0.001 | 0.90 (0.26–0.32), n.s. |
| Transferrin saturation, per log2 increase∗ | 0.20 (0.14–0.29), <0.001 | |
| Goodness-of- fit test† | Χ2 = 12.97 (8 df), p = 0.11 | |
aOR = adjusted odds ratio; CI = confidence interval; COVID-19 = Coronavirus-disease 2019; df = degrees of freedom; OR = odds ratio.
Each one unit increase in log2 corresponds to a doubling in the corresponding parameter.
Hosmer-Lemeshow test.
To determine the best discriminating cut-off predicting hospitalization we performed ROC-analysis (Fig. 1A and B). An iron concentration < 6 μmol/l was identified as the best cutoff-point predicting hospitalization. The sensitivity of iron levels at this value was 94.7% with a specificity of 67.9%. The predictive power of iron, expressed as area under the ROC curve (AUC), was 0.894, with a 95% confidence interval of 0.858–0.931. The ROC curve AUCs for TfSat, transferrin, ferritin, and CRP were lower compared to iron (0.863 (CI 0.824–0.903), 0.735 (CI 0.676–0.795), 0.725 (CI 0.667–0.784), and 0.838 (CI 0.790–0.886), respectively).
Figure 1.
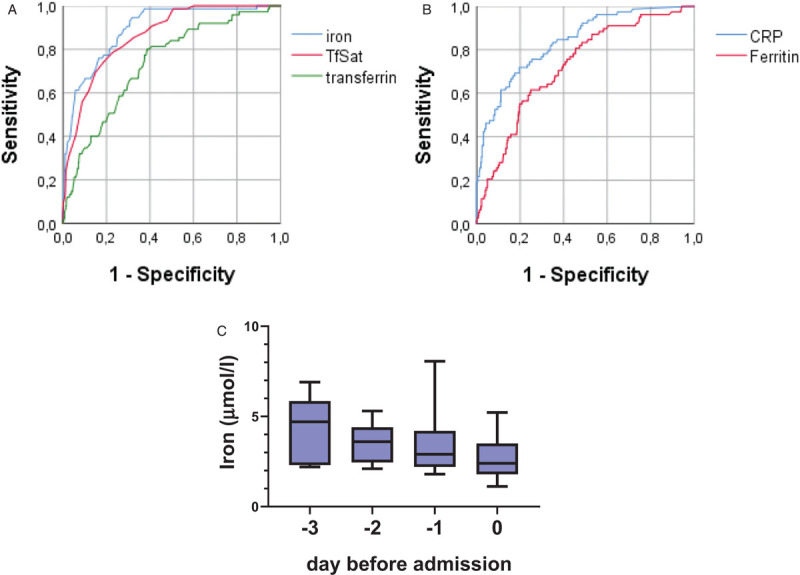
A: ROC curve for cohort A and B for predicting hospitalization for iron, transferrin and TfSat with AUCs of 0.894, 0.735, and 0.863, respectively (and confidence intervals of 0.858–0.931, 0.676–0.795, and 0.824–0.903, respectively). B: ROC curve for cohort A and B for predicting hospitalization for ferritin and CRP with AUCs of 0.725 (CI 0.667–0.784), and 0.838 (CI 0.790–0.886), respectively. C: Course of iron pre-hospitalization for cohort C. Data are presented as boxplot (median ± interquartile range).
Outpatients that were monitored in the days before being admitted to hospital because of increasing disease severity showed a decline of serum iron in the days before admission (Fig. 1C). However, cohort C (n = 23) is small and patients were not monitored at each individual time point; thus, this observation did not reach statistical significance.
Of note, compared to the widely used CRP value, serum iron was predictive in multivariate regression analysis while CRP was not and in ROC analysis the AUC was better for iron than CRP in discriminating in- and outpatients. In conclusion, iron levels are highly predictive for disease progression and hospitalization of COVID19 patients.
Course of iron-related biomarkers and markers of inflammation during hospitalization
The dynamics of blood parameter alterations may provide interesting insights into COVID-19 disease pathology. Therefore, we focused our analyses on the inpatient cohort and analyzed blood parameters on a daily basis starting from the time of admission (d0) until day 6 (d6). This inpatient cohort was further stratified in a low- (n = 48) and a high oxygen demand group (n = 33). We investigated whether iron and inflammatory markers differ between patients with low vs high oxygen demand (Fig. 2). At the day of admission serum iron levels were low in both groups, but increased over the course of the disease in the low-oxygen demand group. By contrast, serum iron levels remained low in the high oxygen demand group. As a result, iron levels differed significantly depending on the oxygen requirement of patients from day three after admission TfSat, an additional indicator of systemic iron availability follows a similar pattern even though the difference does not reach statistical significance at any time point. Severe iron deficiency frequently contributes to the generation of anemia. Consistently, hemoglobin levels are decreased in high oxygen demand patients. Additionally, transferrin levels are significantly decreased at the time of hospitalization, which persists over time.
Figure 2.
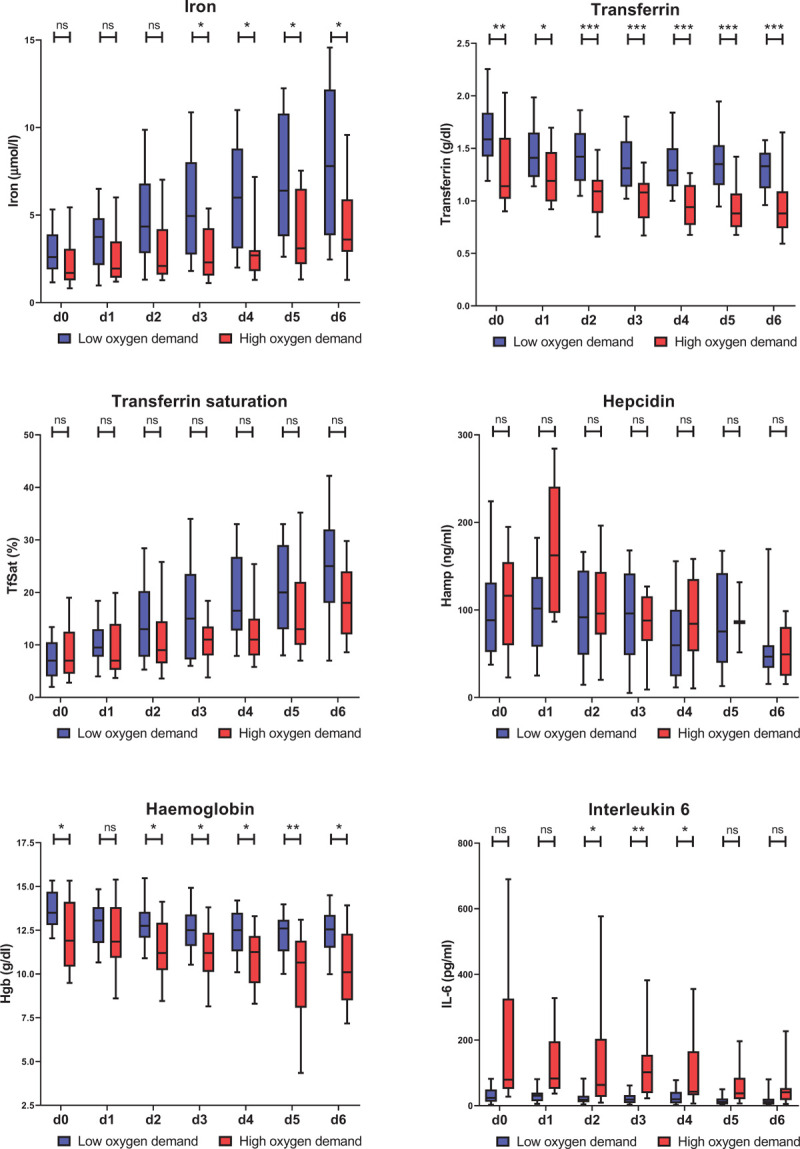
Course of iron and inflammatory parameters in inpatients during the disease course. Serum iron, transferrin, transferrin saturation, hepcidin, hemoglobin (Hgb), and IL-6 have been measured at the time of admission (d0) and for 6 following days (d1-d6). Box and whiskers plot represent median with 25th and 75th percentiles (boxes) together with 10th and 90th percentiles (whiskers). Statistical analysis was performed with the Mann-Whitney U test and the p value has been corrected for multiple comparisons with the Holm method. ns = not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
The cytokine IL-6 activates the expression of the peptide hormone hepcidin that causes hypoferremia by blocking iron export from macrophages. Consistently, COVID-19 patients show significantly increased IL-6 and hepcidin levels and a significant correlation of these two parameters. Unexpectedly, hepcidin levels only poorly inversely correlated with serum iron levels. In addition, hepcidin was unable to discriminate between the low and high oxygen demand group, despite the fact that the pro-inflammatory cytokine IL6 and the inflammatory marker CRP are elevated to a higher degree in patients with high oxygen demand compared to those with low oxygen demand. Ferritin levels are highly variable and therefore do not significantly differentiate patients with high and low oxygen demand (Supplements Fig. 2). Overall, serum iron levels show a high negative correlation with the inflammatory parameters IL-6 and PCT from d0 to d6, while correlation with CRP increases over the time course (Fig. 3). As expected, iron positively correlates with transferrin saturation and hemoglobin.
Figure 3.
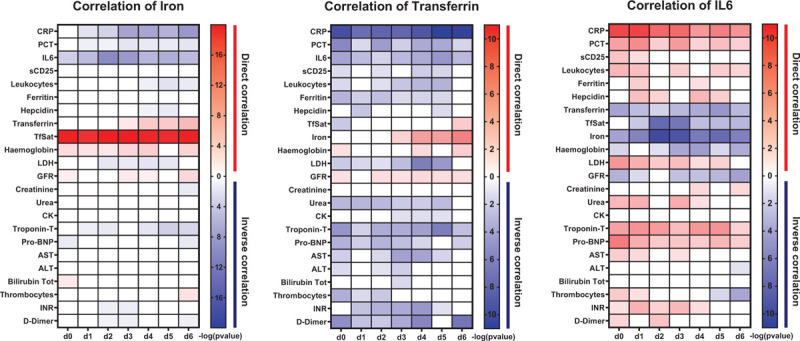
Heatmap of the Spearman's r correlation analysis of iron, transferrin and IL-6 with the parameters indicated. White boxes indicate a lack of correlation (p > 0.05) while in red and blue are reported statistically significant direct and indirect correlations, respectively. The intensity of the color indicates the –log10 (p value).
We next asked whether hypoferremia correlates with markers for organ damage. Interestingly, low serum iron levels inversely correlate with the cardiac injury marker Troponin-T, while correlations for kidney, liver damage or coagulation were not observed. In addition, transferrin shows a significant inverse correlation with CRP, IL-6, procalcitonin, ferritin, and D-dimers as well as with heart damage parameters Troponin-T and NT-pro-BNP. IL-6 has a similar correlation pattern like transferrin but in addition also negatively correlates with the iron metabolism parameters analyzed.
Immunomodulatory therapies increase iron availability
Current management of COVID-19 is mainly supportive and approved treatments based on scientific evidence are not available. Main causes of death include ARDS and cytokine storm syndrome therefore therapy with intravenous immunoglobulins (IVIG) or with agents blocking cytokines, like IL-6 receptor antagonist tocilizumab and the IL-1 receptor antagonist anakinra may be efficient.15,17
All three treatments have a high anti-inflammatory potential, which allowed us to study the influence of these therapies on iron biomarkers.
In Figure 4 the course of iron metabolism parameters before immunomodulatory treatment (defined as day 0) and in the 6 days after treatment is shown. We observed strong effects on serum iron levels and transferrin saturation following therapy with anakinra, tocilizumab and immunoglobulins. When comparing the mean of serum iron levels and transferrin saturation of all patients per treatment group at d1 and d0 these increased 1.8- and 2.1-fold, respectively, after anakinra treatment, 2.0- and 2.2-fold, respectively for tocilizumab treatment, and 2.3- and 2.5-fold, respectively, after immunoglobulin treatment.
Figure 4.
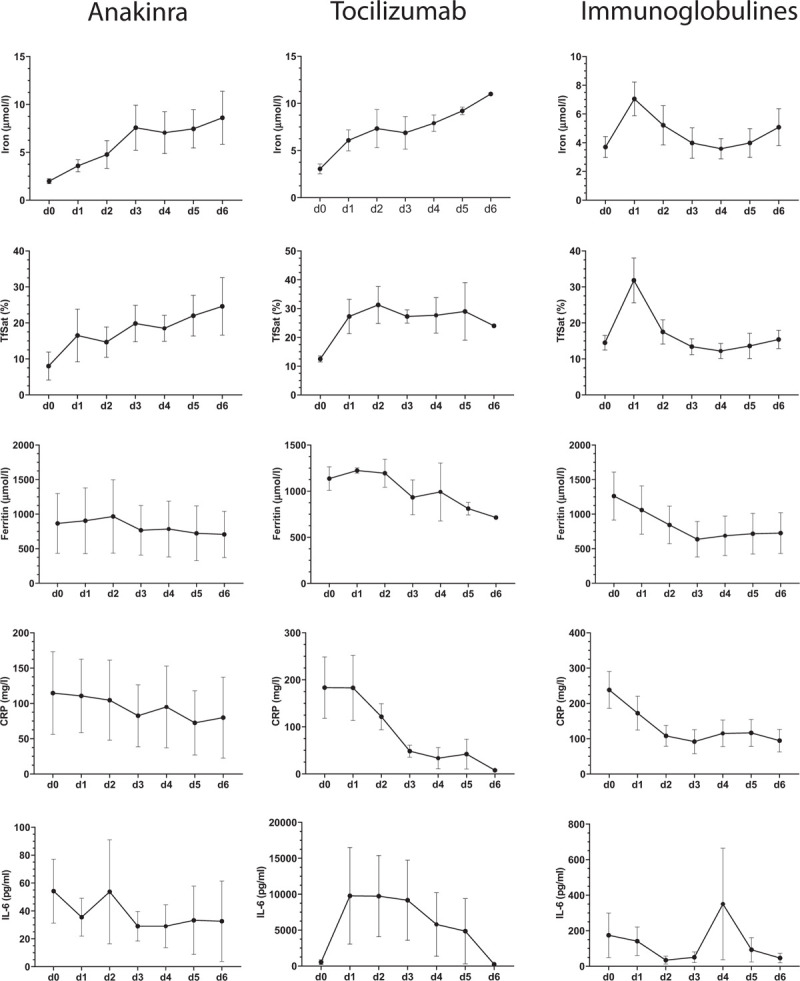
Iron metabolism parameters and CRP before (day 0) and in the days after administration of immunomodulatory medication. Displayed are the courses of serum iron, TfSat, ferritin, CRP, and IL-6 for each individual patient under the three different therapies: Anakinra (n = 6 patients); Tocilizumab (n = 4 patients); intravenous immunoglobulins (IVIG) (n = 6 patients). Data are represented as mean ± s.e.m.
Discussion
SARS-CoV-2 infection leads to a broad spectrum of clinical outcomes, spanning from asymptomatic to lethal.18 Most of the patients show only moderate symptoms such as weakness, sore throat and fever but in some, the disease progresses to ARDS which in extreme cases can be fatal. Therefore a clinical marker predicting outcome for timely intervention is in high demand. Most studies so far focused on immunologic characteristics as potential markers for monitoring disease severity in COVID-19 patients.19
Although iron metabolism plays a central role in the outcome of infections, knowledge about the dynamics of players controlling iron homeostasis in COVID-19 patients is scarce. Our study reveals the predictive value of iron-related markers in patients with confirmed COVID-19 disease. We focus on these parameters as early indicators for disease progression and guidance for therapy decisions in severely affected patients.
Recently, a small, retrospective cohort study in 50 hospitalized COVID-19 patients has shown an association between low serum iron levels with mortality and disease severity.4 We significantly extend these findings by additionally analyzing less severely ill outpatients that enable much improved risk prediction, by analyzing a larger cohort size and by providing information on a large panel of iron-related markers, including hepcidin analysis. The analyses of diagnostic data from our unique patient cohort that is separated into (i) patients with less severe disease taken care of by clinical staff in an outpatient setting (cohort A), (ii) patients initially taken care of in the outpatient setting, but that later deteriorated and had to be admitted to the hospital (cohort C) and (iii) severely ill inpatients (Cohort B). This unique cohort allowed us to determine biomarkers that predict for disease severity reflected by the need for hospitalization and oxygen requirement.
Iron metabolism parameters are disturbed in outpatients and inpatients. Compared to mildly affected patients that remained at an outpatient level, the inpatient cohort shows a significantly more pronounced derangement of iron metabolism parameters. Of note, we observed a gender difference for ferritin in all three cohorts and for serum iron as well as transferrin levels in cohort A, with males being more affected than females. As for COVID-19 a gender difference in disease severity is well recognized, we speculate that lower serum iron levels as well as higher ferritin levels in male patients may be linked with more severe COVID-19 disease in male patients.
In multivariate analysis only serum iron levels and ferritin are significantly predicting disease severity, while CRP, age, gender, as well as transferrin and its saturation are not. Based on a multiple regression analysis model, the odds ratio for hospital admission was 6.7-fold lower per two-fold increase in serum iron.
Additionally, ROC analysis shows that serum iron levels discriminate outpatients from inpatients, accurately predicting their need for hospitalization. For predicting disease severity reflected in admission status ROC analysis reveals a higher AUC for serum iron (AUC 0.894 with a 95% confidence interval of 0.858–0.931) compared to CRP and ferritin levels, that are previously reported COVID-19 severity markers.20 An iron concentration <6 μmol/l was identified as the best cutoff-point predicting hospitalization. The sensitivity of iron levels at this value is 94.7% with an acceptable specificity for a screening marker of 67.9%. In summary, we suggest to assess serum iron parameters in outpatients regularly in order to detect deterioration at an early time point.
The analysis of plasma parameters in 81 patients with COVID-19 hospitalized in the University Hospital Heidelberg provides interesting insights into COVID-19 disease pathology. In this “in-patient” cohort, we analyzed blood parameters on a daily basis starting from the time of admission (d0) until day 6 (d6). COVID-19 patients with high oxygen demand show consistently low serum iron levels as well as strongly decreases and over the course further decreasing transferrin levels. While literature links inflammation with decreased serum transferrin,21,22 the molecular mechanism and biological meaning of this phenomenon are not clear.
By analyzing ferritin and hepcidin levels this study provides insight into mechanisms how hypoferremia may be caused in COVID-19 patients. It is well established that hypoferremia in response to inflammation is predominantly triggered by iron retention in reticuloendothelial macrophages that recycle large amounts of iron from red blood cells. Iron retention in macrophages can be due to (1) increased levels of the iron storage protein ferritin, as demonstrated in the COVID-19 patients analyzed here (2) inhibition of iron export via decreased transcription of the iron exporter ferroportin or (3) by ferroportin protein degradation mediated by the iron-regulated hormone hepcidin. In response to inflammation hepcidin expression is activated in the liver by the cytokine IL-6 via the JAK/STAT signaling pathway. Consistently, during the 6-day time course analyzed in cohort B hepcidin and IL-6 significantly correlate. Unexpectedly, Spearman's correlation failed to reveal a correlation between serum iron and hepcidin levels. This finding suggests that alternative mechanisms as outlined above may contribute to the generation of hypoferremia in COVID-19 patients. An alternative explanation may be that the hypoxic state in COVID-19 patients triggers erythropoietin-dependent expression of the blood hormone erythroferrone in erythroid precursor cells, which in turn represses hepcidin in hepatocytes explaining the poor correlation between hepcidin levels and the degree of hypoferremia in these patients.
It is well established by previous research that inflammatory hypoferremia causes iron accumulation in macrophages.23 Future studies will have to investigate whether macrophage iron deposition contributes to enhanced inflammation and tissue damage in COVID-19 patients as has been suggested previously.
Another important finding of this study is that iron levels negatively correlate with the myocardial damage marker Troponin-T, suggesting that reduced iron availability could be a co-factor for cardiac stress. The heart is particularly rich in mitochondria that rely on a large amount of iron to produce energy via oxidative phosphorylation. Consistently, iron deficiency has been widely reported as a possible comorbidity for cardiac injury.24,25
Similar to hypoferremia, transferrin and IL6 display a strong correlation with inflammatory markers (CRP, PCR and Leukocyte number), markers of tissue-damage (LDH) and cardiac injury (Troponin-T and NT-pro-BNP). However, in addition, these 2 markers correlate with the glomerular filtration rate and serum urea levels, even though these parameters do not reach pathological level (Table 3).
Under low oxygen conditions, erythropoietin stimulates erythropoiesis, a process that requires high amounts of iron.8 We next questioned whether the immense reduction in systemic iron availability in COVID-19 patients may be involved in the worsening of ARDS. Using a retrospective approach, we divided cohort B into 2 subgroups, those with high and those with low oxygen demand. We observed that serum iron levels are lower in patients with high oxygen demand already at the time of admission to the hospital, a difference that increases during the time course and becomes significant three days later. A similar tendency is observed for transferrin saturation at most time points analyzed. Taken together, our data suggest that reduced iron availability is associated with and might even contribute to the progression of ARDS in COVID-19 patients. Additional studies are required to address how iron availability contributes to ARDS.
As hypoferremia is correlated with CRP- and IL-6 -levels we wanted to understand the influence of immunomodulatory therapies on iron levels. Of interest, all 3 analyzed anti-inflammatory treatments showed a rapid and profound influence on serum iron levels already one day after therapy. The fact that serum iron levels increased after all three anti-inflammatory treatments is in line with our hypothesis that inflammation is the major driver of hypoferremia. Interestingly, the influence of anakinra and tocilizumab on ferritin levels was only mild. This may be explained by immune dysregulation in severe COVID-19 patients that has previously been shown to be only partially normalized by tocilizumab.26 The fact that in our studies hypoferremia reacts differently than CRP- and IL-6 levels shows that immune dysregulation in COVID-19 is complex and has to be further analyzed in future studies.
One limitation of our study is the retrospective approach. Future studies in a prospective setting and with higher patient numbers are needed to validate our findings.
In summary, we show that measurement of serum iron levels can help in predicting the severity of COVID-19 disease. We propose that both, hypoferremia as well as the expected iron accumulation in macrophages may play a causal role in the pathophysiology of this disease leading to lung injury.
Patients and methods
Patient selection
All patients with laboratory-confirmed SARS-CoV-2 infection with an age of 18 years or older that were followed as an out- or inpatient at the University hospital of Heidelberg between March 18th and April 23rd, 2020 were considered for inclusion. Data analysis was approved (number S-148/2020) by the Ethics Committee of the Medical Faculty Heidelberg and was conducted in accordance with the Declaration of Helsinki.
Diagnosis of SARS-CoV-2 infection was based on a positive reverse-transcriptase quantitative polymerase chain reaction (RT-qPCR) to detect the viral genome from individual throat swabs or airway surface liquid.
The outpatient cohort consisted of patients who were visited at regular intervals by medically trained staff at their homes, because they reported relevant symptoms like dyspnea or continuously high fever. The outpatients could either stay at home for the entire course of the disease (cohort A) or were admitted to our hospital because of relevant deterioration (cohort C).
All patients that were admitted to the Internal Medicine department of the University hospital Heidelberg due to SARS-CoV-2 infection were considered for inclusion into the in-patient cohort (cohort B). Additional inclusion criteria for the inpatient cohort were severe symptoms (like severe dyspnea or neurological symptoms) and/or a severe or critical course of COVID-19 at the admission time point. Severe COVID-19 was defined as patients showing one or more of the following characteristics at admission to hospital: respiratory rate ≥30/min, blood oxygen saturation ≤93%, ratio of partial pressure of oxygen in arterial blood over the fraction of inspired oxygen <300 mmHg. Patients that had been primarily treated in other hospitals and were secondarily transferred after an external treatment period of >48 hours were not included in the study cohort. In addition, patients that were only hospitalized for quarantine or psychological reasons were also excluded from the analysis. Based on these criteria 81 inpatients were included for further analysis and 12 patients were excluded.
For all patients’ baseline characteristics, treatments, oxygen requirement, laboratory values, and outcome measures were obtained. Inpatients were stratified in a low and high oxygen demand group. All patients requiring High-Flow-Nasal-Oxygen (HFNO) or invasive ventilation in order to reach a blood oxygen saturation >93% were stratified in the high-oxygen demand group, and all remaining patient in the low-oxygen demand group. Furthermore, hepcidin levels were measured using a commercially available ELISA assay according to the manufacturer's instructions (DRG Diagnostics).
To study the effects of certain highly immunomodulatory therapeutic agents on iron metabolism, we analyzed defined cohorts of patients before and after treatment with anakinra, tocilizumab, and intravenous immunoglobulins (IVIG).
Statistical analysis
Data in tables are presented as median with interquartile range (IQR) and as numbers (with percentages) in case of categorical data. For comparison between variables, the Mann-Whitney U-test or Wilcoxon test, chi-squared or Fisher exact test were used as appropriate. In case of multiple comparisons, the p-value has been corrected with the Holm method. Spearman correlation was used to determine correlation of laboratory values. Since CRP levels and iron parameters showed a left-skewed distribution, data was log2 transformed for further analysis. A univariate logistic regression analysis was chosen as the screening method to assess the relationships between putative predictive parameters and the severity of COVID-19, that was defined either by the status of the patients (in- vs out-patient) and for the inpatients by the oxygen demand (low vs high oxygen demand). The predictor variables identified as significant by the univariate logistic analysis were then entered into a multiple logistic regression model to establish which of them could most accurately predict COVID-19 severity including age, gender, and CRP as confounders. Model calibration was evaluated by using the Hosmer-Lemeshow goodness-of-fit test.27 The odds ratios (ORs) and their 95% confidence intervals (CIs) for each of the variables were then generated to clarify the respective association of each of the risk factors with the COVID-19 severity. In addition, receiver operating characteristic (ROC) curve analysis with calculation of the area under the curve (AUC) was performed.
Data collection and analyses were performed using SPSS 21 (IBM Corp. Armonk, NY, USA) or with GraphPad prism 8 (GraphPad Software, San Diego, CA, USA). For all tests, a p value < 0.05 was considered to be statistically significant.
Disclosures
MUM acknowledges funding from the Deutsche Forschungsgemeinschaft (SFB1036, SFB1118) and from the Federal Ministry of Education and Research (NephrESA project Nr 031L0191C).
Supplementary Material
Supplementary Material
Footnotes
TH and SA contributed equally to this work.
There are no potential conflicts of interest.
Supplemental Digital Content is available for this article.
References
- 1.Drakesmith H, Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6:541–552. [DOI] [PubMed] [Google Scholar]
- 2.Litton E, Lim J. Iron metabolism: an emerging therapeutic target in critical illness. Crit Care. 2019;23:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tacke F, Nuraldeen R, Koch A, et al. Iron parameters determine the prognosis of critically ill patients. Crit Care Med. 2016;44:1049–1058. [DOI] [PubMed] [Google Scholar]
- 4.Zhao K, Huang J, Dai D, et al. Serum iron level as a potential predictor of coronavirus disease 2019 severity and mortality: a retrospective study. Open Forum Infect Dis. 2020;7:ofaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80:607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee P, Peng H, Gelbart T, et al. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A. 2005;102:1906–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muckenthaler MU, Rivella S, Hentze MW, et al. A red carpet for iron metabolism. Cell. 2017;168:344–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armitage AE, Eddowes LA, Gileadi U, et al. Hepcidin regulation by innate immune and infectious stimuli. Blood. 2011;118:4129–4139. [DOI] [PubMed] [Google Scholar]
- 10.Ryan JD, Altamura S, Devitt E, et al. Pegylated interferon-alpha induced hypoferremia is associated with the immediate response to treatment in hepatitis C. Hepatology. 2012;56:492–500. [DOI] [PubMed] [Google Scholar]
- 11.Torti SV, Kwak EL, Miller SC, et al. The molecular cloning and characterization of murine ferritin heavy chain, a tumor necrosis factor-inducible gene. J Biol Chem. 1988;263:12638–12644. [PubMed] [Google Scholar]
- 12.Wei Y, Miller SC, Tsuji Y, et al. Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun. 1990;169:289–296. [DOI] [PubMed] [Google Scholar]
- 13.Smirnov IM, Bailey K, Flowers CH, et al. Effects of TNF-alpha and IL-1beta on iron metabolism by A549 cells and influence on cytotoxicity. Am J Physiol. 1999;277:L257–263. [DOI] [PubMed] [Google Scholar]
- 14.Tsuji Y, Miller LL, Miller SC, et al. Tumor necrosis factor-alpha and interleukin 1-alpha regulate transferrin receptor in human diploid fibroblasts. Relationship to the induction of ferritin heavy chain. J Biol Chem. 1991;266:7257–7261. [PubMed] [Google Scholar]
- 15.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Zheng KI, Liu S, et al. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbiol Antimicrob. 2020;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aouba A, Baldolli A, Geffray L, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79:1381–1382. [DOI] [PubMed] [Google Scholar]
- 18.Velavan TP, Meyer CG. Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis. 2020;95:304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matusiewicz M, Neubauer K, Lewandowska P, et al. Reduced transferrin levels in active inflammatory bowel disease. Biomed Res Int. 2017;2017:9541370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchie RF, Palomaki GE, Neveux LM, et al. Reference distributions for the negative acute-phase serum proteins, albumin, transferrin and transthyretin: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 1999;13:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera S, Nemeth E, Gabayan V, et al. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106:2196–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzsimons S, Doughty RN. Iron deficiency in patients with heart failure. Eur Heart J Cardiovasc Pharmacother. 2015;1:58–64. [DOI] [PubMed] [Google Scholar]
- 25.Huang CH, Chang CC, Kuo CL, et al. Serum iron concentration, but not hemoglobin, correlates with TIMI risk score and 6-month left ventricular performance after primary angioplasty for acute myocardial infarction. PLoS One. 2014;9:e104495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemeshow S, Hosmer DW., Jr A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


