Supplemental Digital Content is available in the text
Keywords: angiotensin-converting enzyme inhibitors, COVID-19, mortality, systematic review
Abstract
Background:
Interest exists concerning the use of angiotensin-converting enzyme inhibitors (ACEis) in patients with COVID-19 disease.
Objectives:
The aim of the study was to perform a systematic review on mortality associated to the use of ACEi in patients with COVID-19 disease.
Methods:
Search in Medline (PubMed), in ISI Web of Knowledge and in medRxiv database; use of other sources.
Results:
A total of 33 articles were evaluated. Concerning the papers used to produce the meta-analyses, 7 studies were selected, 5 of which were used. These 5 studies involved a total number of 944 patients treated with ACEi and 5173 not treated with ACEi. Increased mortality was seen in association to the use of ACEi in the context of COVID-19 disease (ACEi users vs nonusers; odds ratio, 1.48; 95% confidence interval, 1.02–2.15; P = .04). When compared to mortality in patients treated with angiotensin receptor blockers, mortality of patients treated with ACEi was not significantly different (odds ratio, 0.96; 95% confidence interval, 0.76–1.21; P = .74). Concerning the remaining reports, different types of data adjustments were used by several authors, after which increased mortality was not seen in association to the use of ACEi in this context.
Conclusions:
ACEi use could act as a marker of increased mortality risk in some but not all COVID-19 disease settings. The data now presented do not prove a causal relation but argue in favor of carrying out clinical trials studying ACEi in COVID-19 patients, to establish the safety of ACEi use in this context.
Introduction
An epidemic of viral disease caused by a new Coronavirus, Sars-Cov-2, is currently underway in most regions of the world. There is interest concerning angiotensin-converting enzyme inhibitors (ACEis) use in this context, since the virus appears to interact with the angiotensin-converting enzyme type 2 (ACE2).1
Although ACEi and angiotensin receptor blockers (ARBs) are sometimes evaluated together, they do not have a common mechanism of action, and therefore a separate evaluation of ACEi use in this context may be of interest. In the present report, a systematic review was carried out, looking at published reports studying the association between ACEi use and mortality in patients with COVID-19, the disease caused by the new Coronavirus. The aim of the study was to use currently available data to tentatively evaluate if a relation exists between ACEi use and patient mortality in this context.
Methods
Search strategy
The study started with a search on Medline (PubMed), in ISI Web of Knowledge and in medRxiv databases, using the query “Covid-19” AND “ACE inhibitor” AND “mortality” (first query) and “Covid-19” AND “angiotensin-converting enzyme inhibitor” AND “mortality” (second query). The search took place on June 16 to 21, 2020, and no articles were excluded based on publication date. The queries resulted in different sets of articles being found, as presented in Figure 1, prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Further additional studies were identified in other relevant sources, including the sites of major medical journals (Fig. 1).
Figure 1.

Flow diagram of studies selection.
Inclusion criteria
Only human studies with original data were included.
Exclusion criteria
Excluded were mechanistic studies; animal studies; case reports; editorials; review papers; study protocols; duplicate studies, if found; systematic reviews and/or meta-analyses; guidelines; and genetic or pathological studies.
Statistical analysis
Meta-analysis was carried out by using the Comprehensive Meta-analysis Software, V.2.0 (Biostat, New Jersey). Fixed effects or random effects analyses were carried out, depending on the degree of heterogeneity of the data (fixed effects for I squared values <50; random effects otherwise). Mortality was the only parameter under study, and the odds ratio was calculated. A level of significance of 0.05 was used.
Quality assessment of studies and data extraction
Global article quality assessment was carried out according to the method used by Haffar et al,2 concerning the articles used for the meta-analyses.
Results
A total of 33 articles were identified and selected for further study (listed in Supplementary Table 1;).
Reports under meta-analyses
A number of articles failed to include the data of interest for the present purpose, and this was the major reason to exclude reports from entering meta-analyses. The precise number of deaths or specific information on ACEi users were among the data not presented in some reports.
One article, initially selected, was retracted by the authors on June 4, 2020, and was therefore excluded, leaving a total of 7 selected studies. Two of the selected reports (presented in Table 1) had no fatalities in one of the groups, making these data unsuitable to be used by the meta-analysis software.3,4 Five studies were used to produce the meta-analyses5,6,7,8,9 (Figs. 2 and 3). These 5 studies involved a total number of 944 patients treated with ACEi and 5173 not treated with ACEi. The main results concerning ACEi use and mortality are presented in Table 1. Some reports presented data concerning only hypertensive patients, whereas others did not (Table 1).
Table 1.
Major data from the selected papers
| Authors | Population | ACEi users | Non-ACEi users | ARB users |
|---|---|---|---|---|
| Li et al | Observational single-center case series of the 1178 hospitalized patients with COVID-19 infections at the Central Hospital of Wuhan, China, from January 15 to March 15, 2020. Data reported for 362 patients with arterial hypertension. Median age 55.5 years (interquartile range, 38–67 years). Overall mortality: 11.03% (21.27% for patients with arterial hypertension). Criterion: use of drugs at the time of admission that continued through hospitalization | 35 ACEi users 28 Survivors 7 Nonsurvivors | 327 Non-ACEi users 257 Survivors 70 Nonsurvivors | 83 ARB users. 68 Survivors 15 Nonsurvivors |
| Richardson et al | Observational case series of 5700 patients with COVID-19 admitted to 12 hospitals in New York. Median age 63 years, (interquartile range 52–75 years). Data reported for patients with arterial hypertension. Overall mortality: 20.99% (28.11% for patients with arterial hypertension). Criterion: home medication at admission. | 168 ACEi users 113 Patients discharged 55 Nonsurvivors | 1198 Non-ACEi users 869 Patients discharged; 329 nonsurvivors | 245 ARB users 170 Patients discharged 75 Nonsurvivors |
| Giorgi Rossi et al | Observational study of all 2653 symptomatic patients who tested positive for SARS-CoV-2 from February 27 to April 2, 2020 in the province of Reggio Emilia. Mean age 63.2 years. Overall mortality: 8.18%. Criterion: use of drugs in previous year. | 450 ACEi users 56 Nonsurvivors | 2203 Non-ACEi users 161 Nonsurvivors | 368 ARB users 52 Nonsurvivors |
| Jung et al | Nationwide cohort study using the Korean Health Insurance Review and Assessment database: 5179 patients with COVID-19; 1954 patients hospitalized. Mean age: 44.6 years overall cohort, 62.5 years patients using either ARB or ACEi. Overall in-hospital mortality: 4.3%. Criterion: drug use at 1–30 days before the index date. | 45 ACEi users 0 Nonsurvivors | 5134 Non-ACEi users 84 Nonsurvivors | 732 ARB users 33 Nonsurvivors |
| Felice et al | Single center study of 133 hypertensive subjects with COVID-19 disease in March 2020. Mean age 73.1 years for ACEi users, 69.0 years for ARB users. Overall mortality: 24.8%. Criterion: chronic use of drugs. | 40 ACEi users 8 Nonsurvivors | 93 Non-ACEi users 25 Nonsurvivors | 42 ARB users 7 Nonsurvivors |
| Meng et al | Observational study of 476 patients recruited from January 1 to February 15, 2020 at 3 hospitals in Wuhan, Shanghai and Anhui. Median age of analyzed subjects 64.5 years (interquartile range, 55.8–69.0 years). Data from 42 patients receiving antihypertensive therapy. Overall mortality: 2.38%. | 2 ACEi users 0 Nonsurvivors | 40 Non-ACEi users 1 Nonsurvivor | 14 ARB users 0 Nonsurvivors |
| Bravi et al | Observational study of 1603 adults with SARS-CoV-2 infection from 2 Italian provinces. Mean age of 58.0 years. Overall mortality: 9.6%. Criterion: background pharmacological treatment up to the previous 2 years (from prescription database), integrated with Clinical chart information for hospitalized subjects. | 251 ACEi users 45 Nonsurvivors | 1352 Non-ACEi users 109 Nonsurvivors | 228 ARB users 46 Nonsurvivors |
ACEi = angiotensin-converting enzyme inhibitors, ARB = angiotensin receptor blockers. For references see text.
Figure 2.

Meta-analysis comparing mortality in patients with COVID-19 disease treated or not treated with angiotensin-converting inhibitors (ACEi). For references see text. CI = confidence interval.
Figure 3.
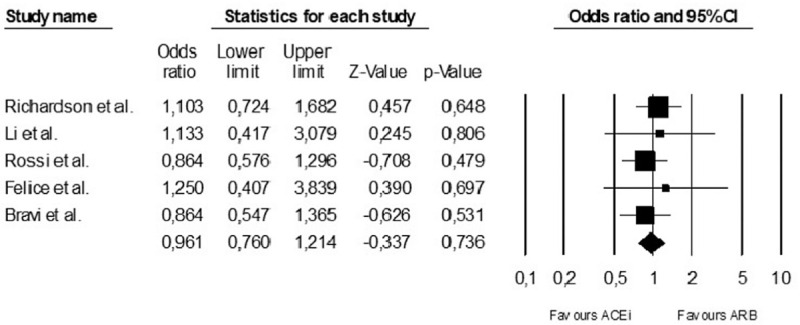
Meta-analysis comparing mortality in patients under angiotensin-converting inhibitors (ACEi) or angiotensin receptor blockers (ARB) in patients with COVID-19 disease. For references see text. CI = confidence interval.
Increased mortality was seen in association to the use of ACEi in the context of COVID-19 disease [ACEi users vs nonusers; random effects; odds ratio, 1.48; 95% confidence interval (CI), 1.02–2.15; P = .04; Fig. 2].
When compared to mortality in patients treated with ARBs, mortality of patients treated with ACEi was not significantly different (fixed effects, odds ratio, 0.96; 95% CI, 0.76–1.21; P = .74; Fig. 3).
The population studied in the selected reports had different mean or median patient ages (Table 1). Overall mortality also differed when the selected reports were compared, with mortality rates ranging from <10% to >20% (Table 1).
The 5 reports used for the meta-analyses were evaluated for global quality, and the results are presented in the Supplementary Table 2;.2
Giorgi Rossi et al6 indicate a numerical increase in mortality with previous ACEi use, although the authors carried out a data adjustment for age, sex, and Charlson Comorbidity Index, and state that “previous use of ACE inhibitors has no effect on risk of death (hazard ratio 0.97, 95% CI 0.69–1.34)”. Richardson et al5 also show a numerical increase in mortality with ACEi use. Bravi et al9 stated that “In multivariable analyses restricted to hypertensive subjects…, the treatment with ARBs and/or ACE inhibitors never increased the likelihood of severe or very severe/lethal disease”.
Reports not used for the meta-analyses
A number of reports presented data on ACEi and/or ARB use in COVID-19 patients but did not contain data that could be used for the meta-analyses (listed in Supplementary Table 1; some of which presented below).
Feng et al10 reported on 476 patients from China, and stated that “more patients were taking ACEis/angiotensin II receptor blockers in the moderate group than in the severe and critical groups.” Huang et al11 reported on 50 hospitalized hypertension patients, and stated that there was no significant difference in clinical severity, clinical course, and in-hospital mortality between patients either taking or not taking renin-angiotensin system blocking drugs. Zhang et al12 reported on 1128 adult patients with hypertension, and stated that “inpatient use of ACEI/ARB was associated with lower risk of all-cause mortality compared with ACEI/ARB nonusers.” These authors used adjusted data, ARB users were in greater number (157 patients) than ACEi users (31 patients) and 34% of patients with hypertension did not receive antihypertensive drugs during hospitalization.12 Zhou et al13 reported on a lower death rate in association to in-hospital use of ACEi or ARB therapy in COVID-19 patients with hypertension, coronary artery disease, or both.
Bean et al14 reported on 1200 patients from the United Kingdom, and 399 COVID-19 patients were taking ACEi or ARB. The primary endpoint of death or transfer to a critical care unit was reached less often in this latter group (adjusted data).
Mancia et al15 reported on 6272 COVID-19 patients from Italy, as well as on a control population. The authors showed that both ARB and ACEi were more frequently prescribed in case patients than in controls. After adjustment, ARB and ACEi had no significant association with the risk of COVID-19 disease.
The report by Ip et al16 described favorable results for ACEi in the context of COVID-19, and the authors stated that mortality rates were lower for hypertensive patients prescribed ACEi. Argenziano et al17 reported on 1000 American COVID-19 patients. Hypertension was seen in 60.1% of patients, and 28.4% of patients were taking either ACEi or ARB. Reynolds et al18 reported on 5894 COVID-19 patients, including 2573 hypertensive patients. The authors found no association between medication class and either an increased likelihood of a positive test or of severe illness.
Khera et al19 studied both an outpatient and an inpatient cohort of hypertensive patients with COVID-19 disease, based on administrative data. The use of ACEi was not associated to an increased mortality risk.19
Discussion
In the present report, a systematic review was carried out, concerning the use of ACEi and a possible association to a change in mortality in COVID-19 disease. Only observational reports were found, with no clinical trial data.
In the meta-analysis, ACEi use was associated to increased mortality in the setting of COVID-19 disease. ACEi use could act as a marker of increased mortality risk in patients with COVID-19 disease—even if not causally related. ACEi use could act as a proxy for the presence of arterial hypertension, and perhaps also for the presence, in patients with arterial hypertension, of further medical conditions with an increased mortality risk – such as heart failure, chronic kidney disease, coronary artery disease, or atrial fibrillation.20 ACEi are also used in patients with these conditions in the absence of hypertension.
Arterial hypertension has been shown to act as a marker of increased mortality risk in COVID-19 disease.21 The age difference seen in the populations studied by the various authors could modulate the effects of ACEi use in this context. Aging is associated to an increased low-grade chronic inflammatory state,22 decreased muscle mass, increased adiposity, and a state of immune dysregulation. In COVID-19 disease, an increase in inflammatory mediators is seen in patients with more severe disease.23 Increasing age is a known major factor for mortality in COVID-19 disease.23
A number of other reports under analysis failed to show increased mortality associated to ACEi use in the setting of COVID-19 disease, after different types of statistical manipulation of data, and there were even reports presenting favorable results.13,16
Should patients discontinue ACE inhibitor therapy out of a concern that they are at increased risk during the COVID-19 pandemic?24 The evidence currently available does not allow an answer to be given on a firm ground—neither a negative nor a positive one. These drugs may act as markers of increased risk in some but not all COVID-19 disease settings, but that does not mean causality exists.
Limitations
Limitations of the present report are very important. Not only are all the data reviewed of an observational character, but significant differences exist when the different reports are compared, both in reports entering the meta-analyses (as shown in Table 1) and in the remaining reports under evaluation.
Several types of bias could exist in the reports under study. Clear limitations would exist concerning the possible use of the nonadjusted data under review to infer causality. The interpretation of adjusted data, which point in the direction that the use of ACEi was not independently associated with increased mortality, has the limitation that different types of statistical manipulation of data were used by different authors (meaning that other types of data manipulation could lead to different results).
Significant differences exist between mortality rates associated to COVID-19 disease in different countries. The same happens with patterns of antihypertensive drug use.
Conclusions
In conclusion, ACEi use could act as a marker of increased mortality risk in some but not all COVID-19 disease settings. The data now presented do not prove a causal relation but argue in favor of carrying out clinical trials studying ACEi in COVID-19 patients, to establish the safety of ACEi use in this context.
Funding information
No funding received for the preparation of this text.
Conflicts of interest
None to report.
Supplementary Material
References
- [1]. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271.e8–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Haffar S, Shalimar, Kaur RJ, et al. Acute liver failure caused by hepatitis E virus genotype 3 and 4: a systematic review and pooled analysis. Liver Int. 2018;38:1965–1973. [DOI] [PubMed] [Google Scholar]
- [3]. Meng J, Xiao G, Zhang J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9:757–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Jung S-Y, Choi JC, You S-H, Kim W-Y. Association of renin-angiotensin-aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Giorgi Rossi P, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R. The Reggio Emilia COVID-19 Working Group Characteristics and outcomes of a cohort of SARS-CoV-2 patients in the Province of Reggio Emilia, Italy. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;23:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Felice C, Nardin C, Di Tanna GL, et al. Use of RAAS inhibitors and risk of clinical deterioration in COVID-19: results from an Italian cohort of 133 hypertensives. Am J Hypertens. 2020;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Bravi F, Flacco ME, Carradori T, et al. Predictors of severe or lethal COVID-19, including angiotensin converting enzyme inhibitors and Angiotensin II Receptor Blockers, in a sample of infected Italian citizens. PLoS One. 2020;15:e0235248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Huang Z, Cao J, Yao Y, et al. The effect of RAS blockers on the clinical characteristics of COVID-19 patients with hypertension. Ann Transl Med. 2020;8:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Zhou F, Liu Y-M, Xie J, et al. Comparative impacts of angiotensin converting enzyme inhibitors versus angiotensin II receptor blockers on the risk of COVID-19 mortality. Hypertension. 2020;76:e15–e17. [DOI] [PubMed] [Google Scholar]
- [14]. Bean DM, Kraljevic Z, Searle T, et al. ACE-inhibitors and angiotensin-2 receptor blockers are not associated with severe SARS-COVID19 infection in a multi-site UK Acute Hospital Trust. Eur J Heart Fail. 2020;22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Ip A, Parikh K, Parrillo JE, et al. Hypertension and renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. medRxiv. 2020. [Google Scholar]
- [17]. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Khera R, Clark C, Lu Y, et al. Association of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with coronavirus disease-19. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- [21]. Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Chung HY, Kim DH, Lee EK, et al. Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging Dis. 2019;10:367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Jarcho JA, Ingelfinger JR, Hamel MB, D’Agostino RB, Harrington DP. Inhibitors of the renin–angiotensin–aldosterone system and COVID-19. N Engl J Med. 2020;392:2462–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


