Abstract
The purpose of our cohort study was to quantify olfactory deficits in Coronavirus disease 2019 (COVID-19) patients using Sniffin’ Sticks and a pre-post design to evaluate olfactory recovery. Thirty adult patients with laboratory-confirmed mild to moderate forms of COVID-19 underwent a quantitative olfactory test performed with the Sniffin’ Sticks test (SST; Burghardt, Wedel, Germany), considering olfactory threshold (T), odor discrimination (D), and odor identification (I). Results were presented as a composite TDI score (range 1–48) that used to define functional anosmia (TDI ≤ 16.5), hyposmia (16.5 < TDI < 30.5), or functionally normal ability to smell (TDI ≥ 30.5). Patients also self-evaluated their olfactory function by rating their ability to smell on a visual analogue scale (Visual Analog Scale rating) and answering a validated Italian questionnaire (Hyposmia Rating Scale). Patients were tested during hospitalization and about 2 months after symptoms onset. During the hospitalization, the overall TDI score indicated that our cohort had impairments in their olfactory ability (10% was diagnosed with anosmia and more than 50% were hyposmic). Almost all patients showed a significant improvement at around 1 month following the first test and for all the parts of the SST except for odor identification. None of the subjects at 1 month was still diagnosed with anosmia. We also quantified the improvement in the TDI score based on initial diagnosis. Anosmic subjects showed a greater improvement than hyposmic and normosmic subjects. In conclusion, within a month time window and 2 months after symptoms’ onset, in our cohort of patients we observed a substantial improvement in the olfactory abilities.
Keywords: COVID-19, olfactory deficits, olfactory test, Sniffin’ Sticks test—SST
Introduction
The novel Coronavirus disease 2019 (COVID-19) has grown to be a global public-health emergency since patients were first detected in Wuhan, China, in December 2019. The disease burden of COVID-19 caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) has been increasing continuously with more than 2 676 300 confirmed cases as of 6 September 2020 (World Health Organization n.d.). SARS-CoV-2 typically causes common cold symptoms with a wide range of clinical manifestations, but can also cause severe pneumonia, respiratory failure, and death (Gandhi et al. 2020). Presently, many reports have summarized the clinical features of patients infected with SARS-CoV-2, revealing that most patients developed smell and taste alterations, namely ageusia and anosmia (Hopkins et al. 2020; Paderno et al. 2020). Total or partial loss of olfactory perception has been proposed as an early marker of SARS-CoV-2 infection (Gerkin et al. 2020; Moein, Hashemian, Mansourafshar, et al. 2020).
Interestingly, chemosensory dysfunction is not associated with nasal symptoms such as rhinorrhea and nasal obstruction (Mercante et al. 2020; Parma et al. 2020) and may be caused by different and yet unidentified factors. Mechanisms behind olfactory loss could include the “cytokine storm” or the direct damage of the olfactory epithelium expressing host receptors required for efficient SARS-CoV-2 infection in humans (Butowt and Bilinska 2020; Cooper et al. 2020).
Recent investigations about smell and taste dysfunction in COVID-19 patients, even if conducted on large cohorts of subjects, are mostly based on interviews and surveys (Giacomelli et al. 2020; Mercante et al. 2020; Menni et al. 2020; Parma et al. 2020). Using objective evaluation methods, fewer studies reported a recovery of olfactory abilities after recovery from SARS-CoV-2 infection. Some authors reported, using surveys, an early smell recovery. For example, Lechien, Chiesa-Estomba, et al. (2020), in a study on 417 COVID-19 patients, 25% reported smell recovery 2 weeks after disease resolution, Lee et al. (2020) described complete recovery within 3 weeks with a median time of improvement of 7 days.
The purpose of our study was to quantify olfactory deficits in COVID-19 patients using Sniffin’ Sticks and to study the recovery of olfactory impairments.
Subjects and Methods
Participants
The study included 34 adult patients admitted to the COVID-Hospital of the Policlinico Hospital of Bari, Italy, between 22 April and 6 May 2020. Four patients dropped-out after the first step of examination: 3 of them did not shown up to the second control and 1 needed intensive care. All patients were positive to nasopharyngeal swabs for SARS-CoV-2 molecular tests and suffered of mild to moderate symptoms (Tables 1 and 2) (Gandhi et al. 2020; Lechien, Chiesa-Estomba, et al. 2020). Patients were enrolled on a voluntary basis and signed the informed consent form. All tests were performed with the highest regard for examiner safety with appropriate personal protective equipment. Exclusion criteria included patients with olfactory dysfunctions before the epidemic, patients affected by rhino-sinusal pathologies, previous rhino-sinusal surgery, current or previous use of recreational drugs by inhalation, recent use of nasal topical vasoconstrictors, head injuries, previous head–neck radiotherapy, neurodegenerative pathology, and psychiatric pathology. In addition, noncompliant patients unable to fully understand the aims of the study as well patients needing invasive or noninvasive ventilation were excluded.
Table 1.
Demographic and anamnestic characteristics of patients
| Number of patients (%) | |
|---|---|
| Sample size | 30 |
| Age (mean ± SD) | 47.47 ± 13 |
| Gender | 16 F (53.3) |
| 14 M (46.7) | |
| Current smoker | 2 (6.7) |
| Ex-smoker | 7 (23.3) |
| Never a smoker | 21 (70) |
| Hypertension | 7 (23.3) |
| Thyroid related | 3 (10) |
| Diabetes | 2 (6.7) |
| Neoplastic diseases | 3 (10) |
| Previous pulmonary embolism | 1 (3.3) |
| Fibromyalgia | 1 (3.3) |
| Polycystic ovary | 1 (3.3) |
| Allergy | 9 (30) |
Table 2.
Otolaryngology, flu-like, and neurological symptoms
| Number of patients (%) | |
|---|---|
| Flu-like symptoms | 30 (100) |
| Nasal obstruction | 7 (23.3) |
| Epistaxis | 4 (13.3) |
| Nasal discharge | 5 (16.7) |
| Neurological symptoms | 9 (30) |
| Headache | 8 (26.7)a |
| Nausea | 2 (6.7)a |
| Dizziness | 1 (3.3)a |
aSymptoms are not mutually exclusive.
During hospitalization, an average of 25 days after COVID-19 diagnosis, we collected an accurate medical history and performed quantitative olfactory testing using the Sniffin’ Sticks test (SST) (Burghardt, Wedel, Germany) (Hummel et al. 2007; Oleszkiewicz et al. 2019).
Patient follow-ups (after COVID-19), using the same test, were carried out a month after the first evaluation, about 2 months after symptom onset. The study was approved by the Ethics Committee of “Azienda Ospedaliero Universitaria Policlinico of Bari,” Italy (n.623/2020).
Psychophysical olfactory function
We assessed olfactory function using the SST including olfactory threshold (T), odor discrimination (D), and odor identification (I). The maximum score for each of the 3 subsections of the SST is 16. Results from the 3 tests were presented as a composite TDI score (range 1–48) (Rumeau et al. 2016) and then used to define functional anosmia (TDI ≤ 16.5), functional hyposmia (16.5 < TDI < 30.5), or functionally normal ability to smell (TDI ≥ 30.5) (Hummel et al. 2007; Oleszkiewicz et al. 2019). These values were in the 10th percentile for the reference group (Hummel et al. 2007 updated in Oleszkiewicz et al. 2019).
SSTs were administered first during hospitalization, when patients had been tested positive for SARS-CoV 2 (during COVID-19), and after all but one tested negative using viral swab (after COVID-19).
Subjective evaluation of smell
Rating olfactory abilities on a Visual Analogue Scale
Participants were asked to quantify their olfactory abilities by rating on a Visual Analogue Scale. The examiner asked patients to quantify their olfactory function by putting a mark on a millimetric horizontal line ranging from 0, indicating the perception of a totally impaired olfactory function, to 100, indicative excellent sense of smell. This evaluation was referred to the time of tests (during and after COVID-19) and retrospectively to the onset of symptoms (onset). Results were then calculated by measuring the distance from 0 in millimeter.
Questionnaire
The Hyposmia Rating Scale (HRS) was developed for assessing olfactory dysfunction in patients with Parkinson’s disease. It is a questionnaire based on 6 questions related to the ability to smell different odors (flowers, gas, garbage, perfume, sweat [body odor], cooked food) (Millar Vernetti et al. 2012). Each item is measured by a Likert scale from 1 to 5, where the value 1 corresponds to “no difficulty or absence of disturbance” and the value 5 corresponds to “maximum difficulty or maximum intensity disturbance” (HRS range 6–30). The questionnaire was administered as described above.
Statistical analysis
Data analysis was performed with R via Rstudio (R Core Team 2018). Jamovi (The Jamovi Project 2020) was used for repeated-measures ANOVA. Post hoc comparisons were performed as stated in the figure legends. In our analysis, we do not report the effect of age on our results as by correcting for the effect of age we obtained the same significance level as reported in Results. In our sample, we did not consider sex as between-subject factor because of our relatively small number of participants (see Table 1).
Results
Sniffin’ Sticks
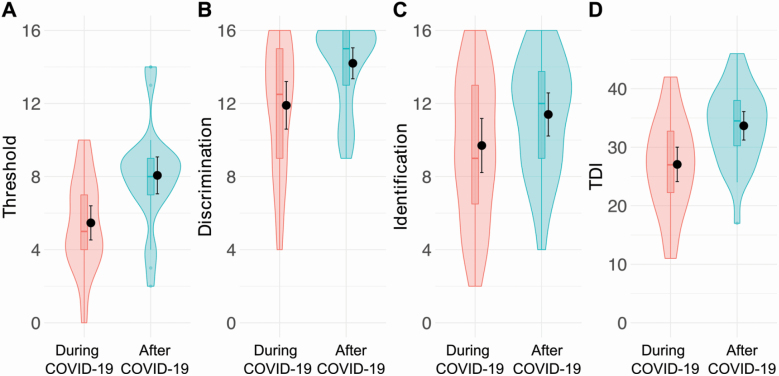
We were able to directly asses the olfactory abilities of patients, by performing SST, at 2 time points of the disease, in particular during hospitalization, when patients had tested positive for SARS-CoV-2 (during COVID-19), and after they had tested negative for the viral swab (after COVID-19). Figure 1 shows several parameters that we evaluated and they revealed significant differences between the score during and after COVID-19. First, during the hospitalization, the overall TDI score (mean = 27.07, SD = 7.88 and 95% confidence interval [CI] [24.8, 30.6]) indicated that our cohort had impairments in their olfactory ability (Table 3 and Figure 1, during COVID-19). Ten percentage of our patients was diagnosed with anosmia based on their TDI score being lower than 16 (score set by Hummel et al. 2007) and updated in 2019 in their normative data (Oleszkiewicz et al. 2019) and more than 50% were hyposmic (Figure 2B). In particular, the olfactory threshold (mean = 5.47, SD = 2.50, and 95% CI [4.5, 6.4]) and Identification (mean = 9.7, SD = 3.97, and 95% CI [8.2, 11.2]) were indicative of this group of patients presenting severe hyposmia.
Figure 1.
Plots representing ratings for threshold (A), discrimination (B), identification (C), and combined TDI score (D) during (pink) and after (blue) COVID-19. Within each subplot (from left to right), boxplots show the first to third quartiles, the horizontal line denotes the median, and whiskers denote 1.5 times the interquartile range. The density distribution of the data shows the proportions of given ratings. Black dots and lines in each subplot represent the mean and the 95% confidence interval.
Table 3.
Sniffin’ Sticks score means, standard deviation (SD), standard error (SE), and 95% confidence interval (CI)
| Threshold | Discrimination | Identification | TDI | |||||
|---|---|---|---|---|---|---|---|---|
| During COVID-19 | After COVID-19 | During COVID-19 | After COVID-19 | During COVID-19 | After COVID-19 | During COVID-19 | After COVID-19 | |
| N | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Mean | 5.47 | 8.07 | 11.90 | 14.20 | 9.70 | 11.40 | 27.07 | 33.67 |
| SD | 2.50 | 2.70 | 3.49 | 2.27 | 3.97 | 3.16 | 7.88 | 6.52 |
| SE | 0.46 | 0.49 | 0.64 | 0.41 | 0.72 | 0.58 | 1.44 | 1.19 |
| CI | [4.5, 6.4] | [7.1, 9.1] | [10.6, 13.2] | [13.8, 15.0] | [8.2, 11.2] | [10.2, 12.6] | [24.8, 30.6] | [31.2, 36.1] |
| P | 0.003 | 0.015 | 0.252 | 0.001 | ||||
P values are from post hoc Bonferroni comparisons after repeated-measures ANOVA F (7,203) = 291.5, P < 0.05.
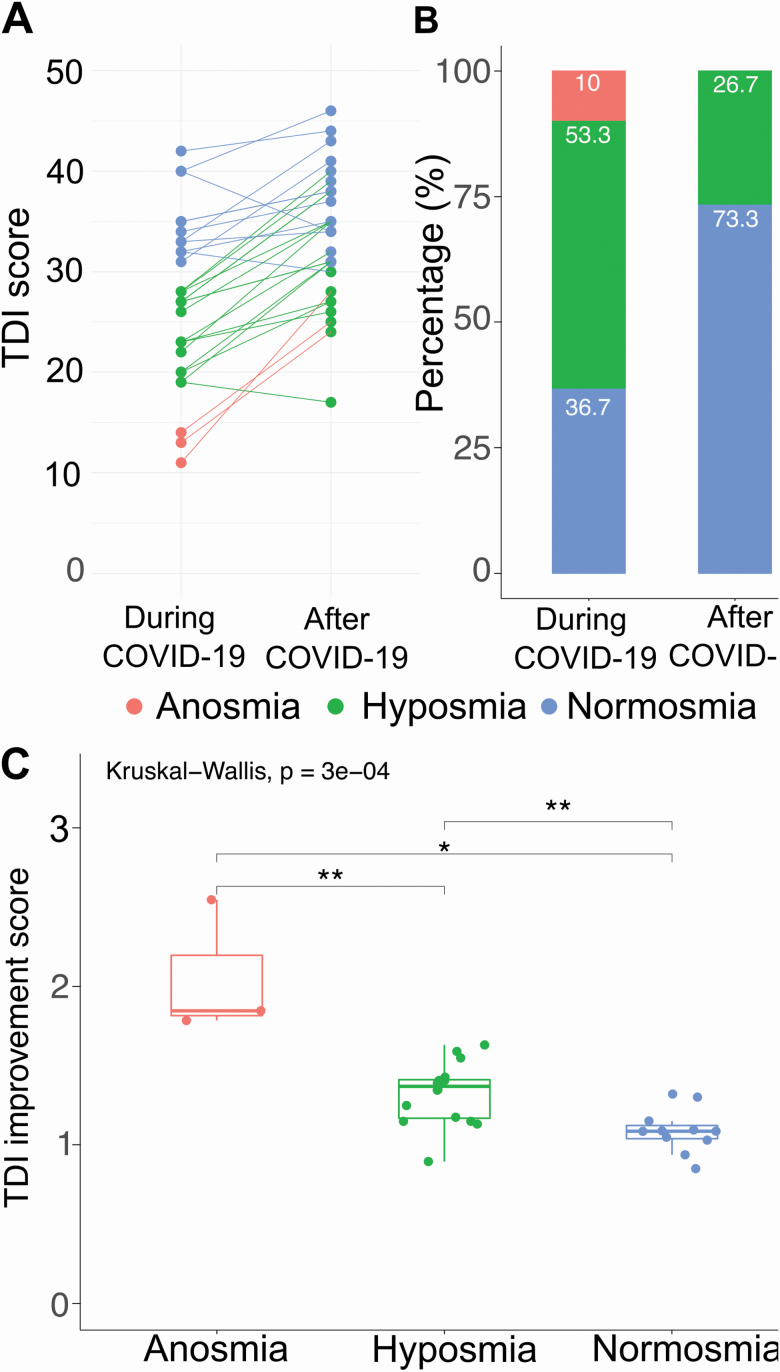
Figure 2.
Plots representing TDI score ratings (A) for each subject during (left) and after (right) COVID-19. (B) Bar plots represent the percentage of individuals classified as anosmic, hyposmic, and normosmic during (left) and after (right) COVID-19. (C) Boxplots show the first to third quartiles, the horizontal line denotes the median, and whiskers denote 1.5 times the interquartile range. Dots represent single subjects in each group. For the differences between the groups: Krustal–Wallis test followed by Wilcox–Mann U test with Bonferroni correction. P values for the pairwise comparisons are P = 0.0085 for anosmia versus hyposmia, P = 0.0126 and P = 0.0015 for hyposmia versus normosmia.
Interestingly, olfactory threshold, odor discrimination, and total score (TDI) were significantly different between during and after COVID-19, whereas odor identification was not (repeated-measures ANOVA F (7,203) = 291.5, P < 0.05 followed by post hoc Bonferroni). Overall, the TDI score After COVID-19 (mean = 33.67, SD = 6.52, and 95% CI [31.2, 36.1]) indicated a significant improvement of the olfactory abilities (Figure 1D). The olfactory threshold after COVID-19 (mean = 8.07, SD = 2.6, and 95% CI [7.1, 9.1]) improved almost 2-fold while less of an improvement was observed in odor discrimination (around 1.2-fold, now mean = 14.20, SD = 2.27, and 95% CI [13.8, 15.0]) and odor identification (after COVID-19 mean = 11.4, SD = 3.6, and 95% CI [10.2, 12.6]). Among our patients all but three had an improvement in their TDI score, and interestingly, one of these subjects reporting the lowest score after 1 month, was still positive for the virus. None of the subjects at 1 month was still diagnosed with anosmia. Also, we observed a decrease in patients considered to be hyposmic (Figure 2B, from 53.3% to 26.7% of the total number of patients) and an increased in normosmic patients (Figure 2B, from 36.7% to 73.3%). We also quantified the improvement in the TDI score based on the initial diagnosis. Anosmic subjects showed a greater improvement than hyposmic and normosmic subjects (Figure 2C). Also, the improvement in the group initially considered hyposmic was significantly larger than that of the normosmic (which was not significantly different from 1, P = 0.05).
Visual Analog Scale and HRS rating
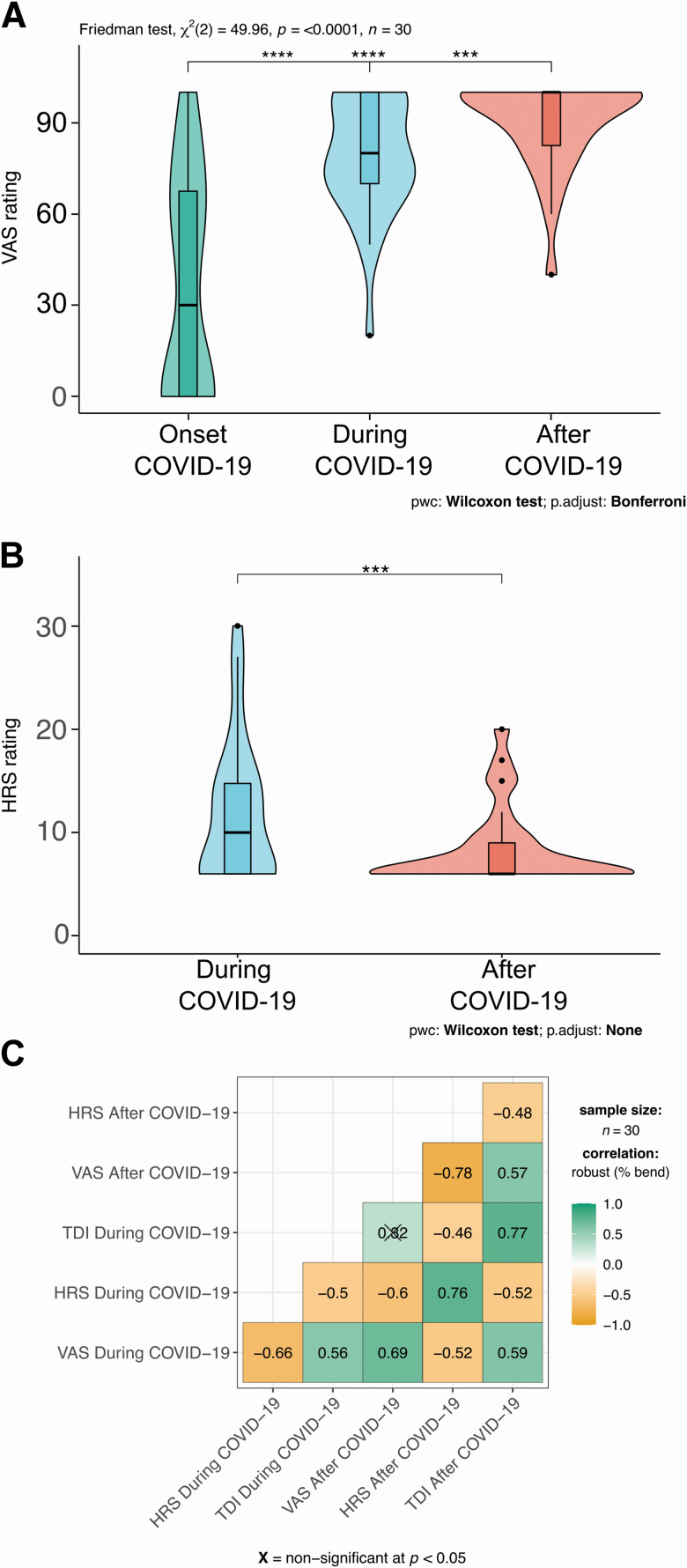
Although we could directly test our participants by using the SSTs, we also asked them to self-rate their olfactory abilities using a Visual Analog Scale (VAS rating) and to self-report the abilities to smell common odors as described by the HRS questionnaire (see Subjects and Methods). By using a VAS rating, we could also obtain information about how that subjects self-reported their olfactory abilities at the onset of the disease. Therefore, we had 3 time points at which to evaluate self-reported rating: onset, during, and after. We could observe a progressive increase in ratings (Figure 3A), being significantly lower at the onset (median = 30 with interquartile rage [iqr] = 67.5; respectively, P = 0.00002 and P = 0.00001 after Bonferroni correction) compared with the other 2 time points. Also, a complete subjective recovery was reported at 1 month (after COVID-19: median = 100, iqr = 17.5) and being significantly improved compared with the rating during COVID-19 (median = 80, iqr = 30, and P = 0.0004 after Bonferroni correction). Also, HRS ratings (during: median = 10, iqr = 8.75 and after: median = 6, iqr = 3 and P = 0.0009) showed a significant decrease in the score indicative of an improvement of the self-rated olfactory abilities (Figure 3B, during: median = 10, iqr = 8.75 and after: median = 6, iqr = 3).
Figure 3.
Plots representing ratings for VAS (A) at the onset of (left), during (middle), and after (right) COVID-19. HRS (B) rating during (left) and after (right) COVID-19. Within each subplot, boxplots show the first to third quartiles, the horizontal line denotes the median, and whiskers denote 1.5 times the interquartile range. The density distribution of the data shows the proportions of given ratings. The pairwise comparisons test used are annotated under the graphs in (A) and (B). (C) Correlation matrices for the different methods we used to quantify olfactory abilities of the patients before and after COVID-19. Crosses indicate nonsignificant correlations.
Are these methods reporting a real improvement? We correlated the VAS and HRS questionnaires with the TDI score at different time points (Figure 3C). We found a significant anticorrelation between VAS and HRS ratings during COVID-19 (−0.66, P < 0.05) and between VAS and HRS after COVID-19 (−0.78, P < 0.05). Also, a lower but significant correlation between TDI score and VAS during COVID-19 (0.56, P < 0.05) emerged. TDI score and VAS rating after COVID-19 were correlated (0.59, P < 0.05).
There was a significant anticorrelation between TDI score and HRS during COVID-19 (−0.50, P < 0.05) and between TDI score and HRS after COVID-19 (−0.48, P < 0.05).
Discussion
During the developing COVID-19 pandemic, it quickly emerged from early anecdotal reports to large-scale studies that the sense of smell is severely impaired in affected subjects. Several reports addressed the degree of the impairments by using self-reported surveys that may be unable to precisely characterize the degree of loss in the absence of objective olfactory testing (Welge-Lüssen 2005; Hoffman et al. 2016; Lötsch and Hummel 2019). It is worth noticing, though, that due to complete lockdown procedures and isolation of patients these could be the only methods to address and quantify the degree of the olfactory loss during that time.
We were able to overcome the problem of the isolation and used a more objective testing method: the SST. In our clinic, we had initially 34 mild to moderate hospitalized patients; among which, 30 were tested around 20 days after hospitalization and with positive SARS-CoV-2 viral swabs.
We administered the complete battery of tests and found that 10% of our subjects could be classified as anosmic based on their TDI score and more than half of our participants were hyposmic during COVID-19. During the COVID-19 pandemic, it has been reported that the SST is more sensitive in detecting anosmia and hyposmia in comparison to self-reporting or taking a medical history (Hornuss et al. 2020), making the SST an appropriate test to use. In our case, we could identify 63% of participants with reduced olfactory ability.
Lechien, Cabaraux, et al. (2020), also using the SST on 86 patients with COVID-19, found a very similar percentage of participants with olfactory deficits (48% anosmic, 14% hyposmic, 62% total) compared with our study. Other studies used different olfactory tests and found different percentages, in particular highly variable were the proportion of anosmic and hyposmic participants (Bocksberger et al. 2020; Tsivgoulis et al. 2020; Vaira, Hopkins, et al. 2020).
By using University of Pennsylvania Identification Tests (UPSIT), Moein, Hashemian, Tabarsi, et al. (2020) reported some degree of smell loss in 96% of their tested COVID-19 participants. The differences between studies using objective methods could stem from the relative smaller sample size (as in our case) and the different timing of testing during the disease progression. Indeed, in our work, we tested at around 20 days since disease onset compared with shorter onset in other studies (Bocksberger et al. 2020; Moein, Hashemian, Tabarsi, et al. 2020; Tsivgoulis et al. 2020; Vaira, Hopkins, et al. 2020). Despite the delayed timing of our tests, our anosmic participants were around 10%, similar to the 8% reported by Le Bon et al. (2020) who tested their participants 5 weeks after the onset of olfactory loss and more or less 15 days after their confirmed COVID-19 diagnosis obtained either by RT-PCR on nasopharyngeal swab or by serology testing.
Finally, a recent meta-analysis deposited as pre-print reported that studies that used objective methods (i.e., Sniffing Sticks) (Vaira, Deiana, et al. 2020; Vaira, Hopkins, et al. 2020; Vaira, Salzano, et al. 2020; Iravani et al. 2020; Moein, Hashemian, Tabarsi, et al. 2020) to asses olfactory deficits were, in general, more sensitive than those that used subjective methods (i.e., questionnaires) and on average 77% of COVID-19 patients had been found with olfactory deficits (with a 95% CI of 61.4–89.2) (Hannum et al. 2020). Again, our data are in line with these results.
Olfactory thresholds during COVID-19 had, as observed also for TDI, a score lower than the cutoff value for hyposmia (Oleszkiewicz et al. 2019). Interestingly, olfactory threshold score was also found to affect the lower overall TDI of a cohort of 72 patients that tested positive (either via viral swab or via serological tests) to COVID-19 (Le Bon et al. 2020).
The olfactory threshold part of the test is mainly dependent on the peripheral olfactory system (i.e., olfactory epithelium in the nasal cavity, which is most easily reached by the virus), and, in our case, it diminished to a larger degree than odor identification and discrimination which are more strongly related to higher cognitive processes (Hummel et al. 2016; Oleszkiewicz et al. 2019). This might point to a preferential peripheral damage to olfactory perception. This would be consistent with the findings on animal models showing that SARS-CoV-2 through its Spike glycoprotein can bind ACE2 receptors abundantly expressed in the sustentacular cells of the olfactory epithelium and most likely start inflammatory processes, a so-called “cytokines storm” that could exacerbate the immune response. In the olfactory epithelium, a variety of cytokines are secreted by infiltrating leukocytes and those can affect olfactory receptor neurons and the stem cell niche, this impairing both the odorant responses and the regeneration capability (Sultan et al. 2011; Chen et al. 2019; Cooper et al. 2020).
Although sustentacular cells are not directly involved in the conversion of a chemical odor signal into an electrical nerve signal by olfactory receptor neurons that is then sent to the brain, they regulate several aspects of the tissue homeostasis, which in turn assures a normal function of olfactory receptor neurons (Dibattista et al. 2020). All these events could drive a partial or complete loss of smell. It has been reported recently that a French woman affected by COVID-19, who was tested for olfactory sensitivity and found to be anosmic, presented obstructive bilateral inflammation in the olfactory cleft. Although local tissue inflammation was present, it is not clear whether it affected the integrity of the olfactory epithelium (Eliezer et al. 2020).
It has been suggested that the combined assessment of odor detection threshold and odor identification would be the most appropriate test to detect olfactory impairments in COVID-19 patients (Le Bon et al. 2020). Although in our participants odor discrimination is higher than 10th percentile (cutoff value for hyposmia), identification is the other score worth mentioning because of its lower values during COVID-19. It is dependent on semantic memory and involves more difficult cognitive tasks than olfactory threshold and therefore requires intact cognitive skills. So, does this mean that also cognitive skills are impaired in COVID-19 patients? Not necessarily so, as we could consider that these patients, affected by a peripheral olfactory loss, require active relearning of odor identification, which is potentially a more complex and therefore slower process than odor discrimination (Hummel et al. 2016).
Another hypothesis could be that the deficit in odor identification and in particular the lack of a significant improvement could be indicative of more extensive nonfunctional pathways due to the potential neuroinvasiveness of the SARS-CoV-2. At our understanding, although neurological symptoms have been reported, this area is subject to an extensive debate (Mao et al. 2020).
We also used a self-reported questionnaire to gather more information about how participants self-evaluated their sense of smell and also to obtain retrospective data (i.e., VAS at the onset).
Interestingly, we found correlations for VAS ratings at different time points and between VAS and HRS (anticorrelation since the HRS scoring system is reversed, i.e., lower score better olfaction). Still significant, although milder, was the correlations between the TDI scores and the questionnaire ratings, indicating that VAS and HRS with their scoring system based on self-evaluation could be a good proxy to test olfactory abilities.
Improvement of the sense of smell
Questions that up to now do not have clear answers are whether anosmic symptom persist and for how long after a person had a negative viral swab. At around 1 month from the first test and more or less 2 months from the onset of COVID-19, we performed a follow-up study with our patients. All but one had been cleared from the viral load and all but three showed an improvement of the TDI score. The higher scores in the follow-up study was overall significantly improved from that compared with during COVID-19 except for odor identification. Therefore, although we could not definitely conclude that after 2 months from the onset of the disease, there is a full recovery of the sense of smell, certainly we could state that in this time window there is a substantial improvement in mild to moderate COVID-19 patients. Our results still showed a portion of participants that remained hyposmic (27%) and similar to the 29% found by Le Bon et al. (2020). A higher percentage of hyposmic was found by Otte et al. (2020) after 7 weeks after COVID-19 onset. Discrepancies could be due to several factors such as different study populations, sample size, duration of initial anosmia (Le Bon et al. 2020), and days since the onset. Indeed, by applying a machine learning algorithm, it has been shown that recovery has the number of days from the onset of the disease as best predictor (Gerkin et al. 2020). We observed a larger improvement in those subjects initially diagnosed as anosmic, which was larger compared with hyposmic and normosmic subjects. Indeed, in the follow-up no one was anosmic and all but 3 (10%) of our patients improved their scores. Interestingly, the only subject still positive for a viral test was the one with the lowest score. The other two that did not improve belonged to the normosmic group that showed less or nonsignificant improvement compared with the anosmic and hyposmic group. The overall improvement was also detected by the VAS and HRS questionnaires. Due the opportunity of asking the patients to rate their sense of smell at the onset, we could observe a progressive increase of the ratings during and after COVID-19. Although participants can both underestimate or overestimate olfactory acuity, the use of VAS to self-rate olfactory abilities during COVID-19 pandemic has been crucial to detect impairments (e.g., Parma et al. 2020; Giacomelli et al. 2020; and summarized by Pellegrino et al. 2020). Also, subjective methods could be used to collect large dataset that are important to create training and testing set of data for machine learning algorithms that could be implemented as diagnostic tools (Gerkin et al. 2020; Menni et al. 2020).
Strengths and limitations
Our work has both strength and limitations. The use of the SST as an objective test to quantify olfactory impairments is a major strength. Few studies have applied objective measurements (to the best of our knowledge 6 vs. 35 and growing studies that used subjective methods). Also, our group was quite homogeneous and balanced for sex (in our case, we could not see an effect of sex in our analysis similar to Le Bon et al. (2020) and we had the opportunity to have a pre-post longitudinal design).
Limitations arise mainly from our sample size, smaller than other studies where objective methods were used (Bocksberger et al. 2020; Iravani et al. 2020; Le Bon et al. 2020; Lechien, Cabaraux, et al. 2020; Moein, Hashemian, Tabarsi, et al. 2020; Tsivgoulis et al. 2020; Vaira, Deiana, et al. 2020; Vaira, Hopkins, et al. 2020; Vaira, Salzano, et al. 2020).
This could limit the generalization of our conclusions and other type of analysis that could reveal more about the relation between COVID-19 symptoms and olfactory deficits. For example, Iravani et al. (2020) found that intensity ratings during COVID-19 is dependent on the number of symptoms (i.e., the more symptoms the less intense was the rating of household odors).
Conclusions
By using a psychophysical test to directly asses the olfactory abilities of the patients, we found a decreased sense of smell of COVID-19-affected patients. The choice of performing the SST allowed us to not rely exclusively on self-reports and to compare our results with normative data sets available for the general population. In addition, by following-up with our subjects we are beginning to answer the question about gaining back the sense of smell. In our cohort, we could show a clear improvement in the olfactory abilities with a negative viral swab within 1 month time window.
References
- World Health Organization n.d. Coronavirus disease 2019 (COVID-19). Situation Report Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200907-weekly-epi-update-4.pdf?sfvrsn=f5f607ee_2.
- Bocksberger S, Wagner W, Hummel T, Guggemos W, Seilmaier M, Hoelscher M, Wendtner CM. 2020. Temporary hyposmia in COVID-19 patients. HNO. 68(6):440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butowt R, Bilinska K. 2020. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci. 11(9):1200–1203. [DOI] [PubMed] [Google Scholar]
- Chen M, Reed RR, Lane AP. 2019. Chronic inflammation directs an olfactory stem cell functional switch from neuroregeneration to immune defense. Cell Stem Cell. 25(4):501–513.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, Weinreb C, Joseph PV, Larson ED, Parma V, et al. 2020. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 107(2):219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibattista M, Pifferi S, Menini A, Reisert J. 2020. Alzheimer’s disease: what can we learn from the peripheral olfactory system? Front Neurosci. 14:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliezer M, Hautefort C, Hamel AL, Verillaud B, Herman P, Houdart E, Eloit C. 2020. Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. 146(7):674–675. doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- Gandhi RT, Lynch JB, Del Rio C. 2020. Mild or moderate Covid-19. N Engl J Med. doi: 10.1056/NEJMcp2009249. Epub ahead of print. PMID: 32329974. [DOI] [PubMed] [Google Scholar]
- Gerkin RC, Ohla K, Veldhuizen MG, Joseph PV, Kelly CE, Bakke AJ, Steele KE, Farruggia MC, Pellegrino R, Pepino MY, et al. 2020. The best COVID-19 predictor is recent smell loss: a cross-sectional study. MedRxiv. doi: 10.1101/2020.07.22.20157263. [DOI] [Google Scholar]
- Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, et al. 2020. Self-reported olfactory and taste disorders in patients with severe acute respiratory Coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 71(15):889–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum ME, Ramirez VA, Lipson SJ, Herriman RD, Toskala AK, Lin C, Joseph PV, Reed DR. 2020. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19 positive patients compared to subjective methods: a systematic review and meta-analysis. medRxiv, 6 July 2020, doi: 10.1101/2020.07.04.20145870, preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HJ, Rawal S, Li CM, Duffy VB. 2016. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord. 17(2):221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C, Surda P, Whitehead E, Kumar BN. 2020. Early recovery following new onset anosmia during the COVID-19 pandemic – an observational cohort study. J Otolaryngol Head Neck Surg. 49(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornuss D, Lange B, Schröter N, Rieg S, Kern WV, Wagner D. 2020. Anosmia in COVID-19 patients. Clin Microbiol Infect. 26:1426–1427. doi: 10.1016/j.cmi.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Kobal G, Gudziol H, Mackay-Sim A. 2007. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 264(3):237–243. [DOI] [PubMed] [Google Scholar]
- Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, Damm M, Frasnelli J, Gudziol H, Gupta N, et al. 2016. Position paper on olfactory dysfunction. Rhinology. 56(1):1–30. [DOI] [PubMed] [Google Scholar]
- Iravani B, Arshamian A, Ravia A, Mishor E, Snitz K, Shushan S, Roth Y, Perl O, Honigstein D, Weissgross R, et al. 2020. Relationship between odor intensity estimates and COVID-19 prevalence prediction in a Swedish population. Chem Senses. 45:449–456. doi: 10.1093/chemse/bjaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bon SD, Pisarski N, Verbeke J, Prunier L, Cavelier G, Thill MP, Rodriguez A, Dequanter D, Lechien JR, Le Bon O, et al. 2020. Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID-19: a prospective cohort study on 72 patients. Eur Arch Otorhinolaryngol. 4:1–8. doi: 10.1007/s00405-020-06267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien JR, Cabaraux P, Chiesa-Estomba CM, Khalife M, Hans S, Calvo-Henriquez C, Martiny D, Journe F, Sowerby L, Saussez S. 2020. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 42(7):1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, et al. 2020. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 277(8):2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Min P, Lee S, Kim SW. 2020. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci. 35(18):e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J, Hummel T. 2019. Machine-learned analysis of side-differences in odor identification performance. Neuroscience. 422:44–53. [DOI] [PubMed] [Google Scholar]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, et al. 2020. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 77(6):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, Ganesh S, Varsavsky T, Cardoso MJ, El-Sayed Moustafa JS, et al. 2020. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 26(7):1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercante G, Ferreli F, De Virgilio A, Gaino F, Di Bari M, Colombo G, Russo E, Costantino A, Pirola F, Cugini G, et al. 2020. Prevalence of taste and smell dysfunction in Coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 146(8):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar Vernetti P, Perez Lloret S, Rossi M, Cerquetti D, Merello M. 2012. Validation of a new scale to assess olfactory dysfunction in patients with Parkinson’s disease. Parkinsonism Relat Disord. 18(4):358–361. [DOI] [PubMed] [Google Scholar]
- Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. 2020. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 10(8):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moein ST, Hashemian SM, Tabarsi P, Doty RL. 2020. Prevalence and reversibility of smell dysfunction measured psychophysically in a cohort of COVID-19 patients. Int Forum Allergy Rhinol. doi: 10.1002/alr.22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. 2019. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 276(3):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte MS, Eckel HNC, Poluschkin L, Klussmann JP, Luers JC. 2020. Olfactory dysfunction in patients after recovering from COVID-19. Acta Otolaryngol. 27:1–4. doi: 10.1080/00016489.2020.1811999. [DOI] [PubMed] [Google Scholar]
- Paderno A, Schreiber A, Grammatica A, Raffetti E, Tomasoni M, Gualtieri T, Taboni S, Zorzi S, Lombardi D, Deganello A, et al. 2020. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol. 10(8):955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, Cooper KW, Bouysset C, Pirastu N, Dibattista M, et al. 2020. More than smell – COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 45:609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino R, Cooper KW, Di Pizio A, Joseph PV, Bhutani S, Parma V. 2020. Corona viruses and the chemical senses: past, present, and future. Chem Senses. 45:415–422. doi: 10.1093/chemse/bjaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2018. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. [Google Scholar]
- The Jamovi Project. 2020. Jamovi (version 1.2) [computer software]. Available from https://www.jamovi.org.
- Rumeau C, Nguyen DT, Jankowski R. 2016. How to assess olfactory performance with the Sniffin’ Sticks test®. Eur Ann Otorhinolaryngol Head Neck Dis. 133(3):203–206. [DOI] [PubMed] [Google Scholar]
- Sultan B, May LA, Lane AP. 2011. The role of TNF-α in inflammatory olfactory loss. Laryngoscope. 121(11):2481–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivgoulis G, Fragkou PC, Delides A, Karofylakis E, Dimopoulou D, Sfikakis PP, Tsiodras S. 2020. Quantitative evaluation of olfactory dysfunction in hospitalized patients with Coronavirus [2] (COVID-19). J Neurol. 267(8):2193–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A, Babudieri S, Petrocelli M, Serra A, Bussu F, et al. 2020. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 42(6):1252–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M, Ferrari M, Gagliardini L, Pipolo C, Deiana G, et al. 2020. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck. 42(7):1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, De Riu G. 2020. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck. 42(7):1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welge-Lüssen A. 2005. Gestörte Riech- und Schmeckfunktion. Therapieoptionen bei Riech- und Schmeckstörungen [Impaired sense of smell and taste. Therapy options in anosmia and dysgeusia]. Laryngorhinootologie. 84(Suppl 1):S92–S100. [DOI] [PubMed] [Google Scholar]