Abstract
Objectives
Clinical studies of chloroquine (CQ) and hydroxychloroquine (HCQ) in COVID-19 disease reported conflicting results. We sought to systematically evaluate the effect of CQ and HCQ with or without azithromycin on outcomes of COVID-19 patients.
Methods
We searched multiple databases, preprints and grey literature up to 17 July 2020. We pooled only adjusted-effect estimates of mortality using a random-effect model. We summarized the effect of CQ or HCQ on viral clearance, ICU admission/mechanical ventilation and hospitalization.
Results
Seven randomized clinical trials (RCTs) and 14 cohort studies were included (20 979 patients). Thirteen studies (1 RCT and 12 cohort studies) with 15 938 hospitalized patients examined the effect of HCQ on short-term mortality. The pooled adjusted OR was 1.05 (95% CI 0.96–1.15, I2 = 0%). Six cohort studies examined the effect of the HCQ+azithromycin combination with a pooled adjusted OR of 1.32 (95% CI 1.00–1.75, I2 = 68.1%). Two cohort studies and four RCTs found no effect of HCQ on viral clearance. One small RCT demonstrated improved viral clearance with CQ and HCQ. Three cohort studies found that HCQ had no significant effect on mechanical ventilation/ICU admission. Two RCTs found no effect for HCQ on hospitalization risk in outpatients with COVID-19.
Conclusions
Moderate certainty evidence suggests that HCQ, with or without azithromycin, lacks efficacy in reducing short-term mortality in patients hospitalized with COVID-19 or risk of hospitalization in outpatients with COVID-19.
Introduction
The COVID-19 pandemic has claimed hundreds of thousands of human lives and caused enormous economic damage. While the race to develop an effective vaccine continues, repurposing of approved drugs remains the most logical treatment approach for SARS-CoV-2 infection and its complications.
Since the discovery of the antiviral effects of chloroquine (CQ) and hydroxychloroquine (HCQ) more than 50 years ago, interest in exploring their therapeutic potential against various viral infections has continued relentlessly.1 CQ/HCQ have been tested against numerous viruses, such as HIV-1, SARS, MERS-CoV, influenza, dengue, Ebola, Zika, Chikungunya and other viruses.2–10
Several mechanisms have been proposed for the anti-SARS-CoV-2 effects of CQ/HCQ. All of which are secondary to their ability to raise intracellular pH, which particularly affects endosome function.11,12 CQ/HCQ can interfere with all stages of the viral life cycle.11 They have the potential to hinder SARS-CoV-2 binding to its cell membrane receptor, ACE2, through their interference with the glycosylation process of the ACE2 protein that results in reducing its binding affinity to SARS-CoV-2 virus. CQ/HCQ could also prevent fusion of the viral particles to the host cell membrane and prevent their cell entry. Furthermore, CQ/HCQ can also inhibit viral replication, assembly and release of viral particles from the host cells.11
CQ/HCQ also alter endosomal antigen processing and modulate both the innate and adaptive immune responses.11,12 This leads to decreased production of pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6. Additionally, CQ/HCQ improve endothelial function and reduce the prothrombotic state.13 These properties could have favourable effects in patients with severe COVID-19 disease.
With the emergence of SARS-CoV-2 virus and its rapid spread across the globe, it was natural to test the antiviral effects of CQ/HCQ against this new threatening infection. The enthusiasm for their widespread clinical use in the treatment of COVID-19 disease escalated with the early studies reporting their effective in vitro antiviral effects against SARS-CoV-2 virus.14–16
An early interim analysis of 100 COVID-19 patients was reported by a group from China where they found that CQ therapy was associated with less severe pneumonia, shorter disease course and faster viral clearance.17 Another small non-randomized study of 20 patients from France revealed reduced nasopharyngeal viral carrier state at 6 days after the initiation of treatment with HCQ and azithromycin.18 These limited data along with the political support for CQ/HCQ use led clinicians worldwide to use them indiscriminately and to include them in their institutional protocols and guidelines for the treatment of COVID-19 disease as a monotherapy or in combination with azithromycin. This rapid adoption of CQ/HCQ was associated with an astronomical increase in CQ/HCQ prescription of approximately 2000%.19
While numerous large randomized clinical trials (RCTs) were started in different countries worldwide, several observational studies addressing the efficacy and safety of CQ/HCQ in the treatment of COVID-19 disease got published along with preliminary results from some RCTs. These studies have different methodologies and sample sizes, and produced mixed results, ranging from reduced mortality and improved other clinical outcomes to increased mortality among COVID-19 patients.
The absence of robust clinical evidence for their efficacy, as well as the potential serious drug-induced adverse events associated with CQ/HCQ use, call for rigorously conducted systematic reviews/meta-analyses of the available clinical data to present a clearer picture about their efficacy and provide a data-informed view regarding their utility in the treatment of COVID-19. In this study, we set out to perform a systematic review and meta-analysis of the literature regarding the efficacy of CQ or HCQ in patients with COVID-19.
Methods
Inclusion and exclusion criteria
We followed PRISMA (‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’)20 guidelines for reporting a systematic review and meta-analysis of observational studies. We included (i) RCTs or (ii) cohort or case–control studies reporting on adjusted-effect estimates of the association between HCQ or CQ with or without azithromycin and the following endpoints: (i) short-term mortality, (ii) mechanical ventilation/ICU admission and (iii) viral clearance among hospitalized patients with COVID-19 and (iv) risk for hospitalization among outpatients with COVID-19.
Literature search
The literature was searched by a medical librarian for the concepts of CQ or HCQ combined with COVID-19. The search strategies were created using a combination of keywords and standardized index terms. Searches were run up to 17 July 2020 in Ovid EBM Reviews, Ovid Embase (1974+), Ovid Medline (1946+ including epub ahead of print, in-process and other non-indexed citations), Scopus (1970+) and Web of Science (1975+). Search strategies are provided in Tables S1 to S4 (available as Supplementary data at JAC Online). We also searched for unpublished manuscripts using the medRxiv services operated by Cold Spring Harbor Laboratory and Research Square preprints. In addition, we searched Google Scholar and the references of eligible studies and review articles.
Two reviewers independently identified eligible studies (Z.K. and O.A.) and four reviewers (Z.K., M.A.G., O.A. and H.T.) extracted the data into a pre-specified data collection form. A senior reviewer verified all data included in the analyses (I.M.T.).
Two reviewers (Z.K. and O.A.) independently assessed risk of bias for each study using RoB 2 of the Cochrane risk-of-bias tool for randomized trials21 and the Newcastle–Ottawa scale for cohort studies and case–control studies.22 Reviewers judged each criterion for risk of bias and resolved any disagreements by discussion with a third senior reviewer. We assessed the certainty of evidence for each of our outcomes using the GRADE (‘Grading of Recommendations Assessment, Development and Evaluations’) approach.23,24 This method evaluates the certainty of evidence by assessing the following domains: limitations, indirectness, inconsistency, imprecision and publication bias.
Statistical analysis
We pooled studies using the DerSimonian–Laird random-effects model (and constructed corresponding forest plots). Pooled adjusted-effect estimates (ORs and HRs) were obtained by combining the estimates of log adjusted-effect estimate from each study. Endpoints that we considered a priori for the meta-analysis were: (i) short-term mortality, (ii) mechanical ventilation/ICU admission and (iii) viral clearance among hospitalized patients with COVID-19 and (iv) risk for hospitalization among outpatients with COVID-19. We evaluated heterogeneity using the I2 statistic, which estimates the variability percentage in effect estimates that is due to heterogeneity rather than to chance—the larger the I2, the greater the heterogeneity. We conducted sensitivity analyses to assess the impact of (i) risk of bias in included studies and (ii) the selection of study population (general populations versus specific populations) on the overall estimate of effect. We constructed funnel plots and performed an Egger precision-weighted linear regression test as a statistical test of funnel plot asymmetry and publication bias. All analyses were conducted using Stata version 16 statistical software (StataCorp, College Station, TX, USA).
Results
Included studies
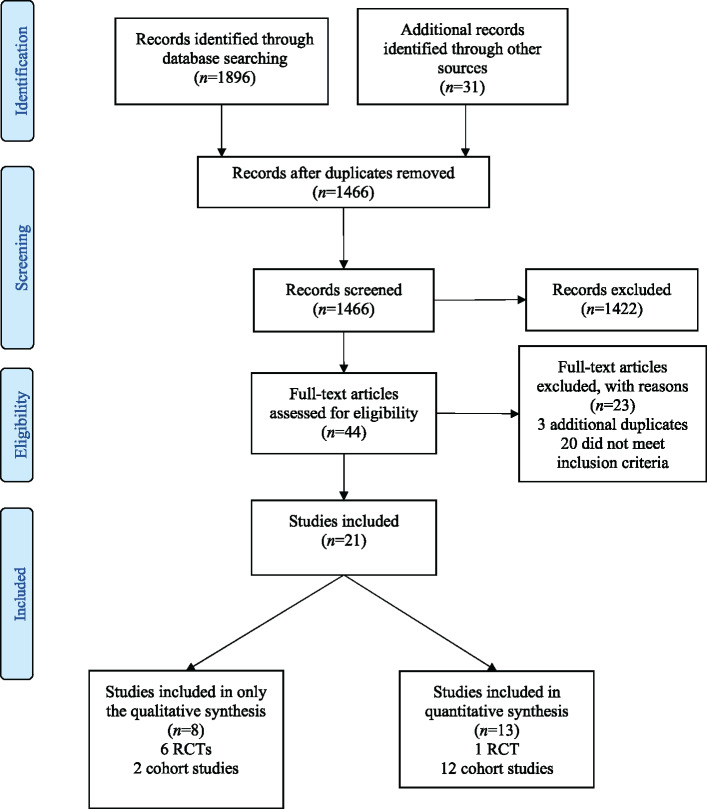
Out of 1896 papers screened for eligibility, 7 RCTs25–31 and 14 cohort studies32–45 were included (Figure 1) with a total of 20 979 patients. Characteristics of included studies are described in Tables 1 and 2. Characteristics of the patients in each study can be found in Table S5. Nineteen studies (5 RCTs and 14 cohort studies)25–29,33–45 with 20 263 patients reported on cases hospitalized with COVID-19. Two RCTs30,31 studied 358 participants in the outpatient setting and one cohort study32 included both inpatients and outpatients (57 versus 37, respectively). Most of the studies included patients presenting with varying levels of disease severity. Yu et al.43 only studied patients with critical disease, whereas Geleris et al.34 and Mahévas et al.41 excluded them.
Figure 1.
PRISMA flow diagram of eligible studies. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Table 1.
Characteristics of RCTs
| Study/year | Country/setting | Number of patients | Case selection | Control selection | Intervention/exposure | Comparator | Outcome |
|---|---|---|---|---|---|---|---|
| Chen J et al./2020 | China/inpatient | 30 | confirmed COVID-19 cases | NA | HCQ | conventional therapy | primary: viral clearance by day 7 or death |
| Chen L et al./2020 | China/inpatient | 48 | PCR-confirmed infection or lung changes characteristic of COVID-19 | patients were assigned to one of three groups using a computer-generated randomization number | HCQ or CQ | SOC | primary: time to clinical recovery; secondary: time to SARS-CoV-2 RNA negativity |
| Horby et al./2020 | UK/inpatient | 4674 | suspected or confirmed COVID-19 cases | random assignment | HCQ 800 mg every 6 h for 2 doses followed by 400 mg after 6 h; then 400 mg twice daily for 9 days | SOC | primary: 28 day mortality |
| Huang et al./2020 | China/inpatient | 22 | PCR-confirmed SARS-CoV-2 | patients randomized to receive lopinavir/ritonavir | CQ 500 mg orally twice daily for 10 days | lopinavir/ ritonavir | primary: time to viral clearance and rate of viral clearance at days 10 and 14; secondary: rate of hospital discharge at day 14, clinical recovery at day 10 and radiological recovery at days 10 and 14 |
| Tang et al./2020 | China/inpatient | 150 | PCR-confirmed SARS-CoV-2 | random assignment with stratification | HCQ loading dose of 1200 mg followed by 200 mg 3 times a day for up to 3 weeks | SOC | primary: negative conversion of SARS-CoV-2 by 28 days and clinical improvement within 28 days in patients with severe infection |
| Mitja et al./2020 | Spain/outpatient | 293 | PCR-confirmed SARS- CoV-2 patients with mild symptoms | randomized to either HCQ or SOC | HCQ 800 mg followed by 400 mg daily for 6 days | SOC | primary: viral RNA load at 3 and 7 days; secondary: hospitalization |
| Skipper et al./2020 | USA and Canada/ outpatient | 491 | PCR-confirmed SARS-COVID-19 infection or probable infection with high-risk exposure within 4 days | patients were randomized | HCQ 800 mg followed by 600 mg in 6–8 h then daily for 4 more days | placebo | primary: change in symptom severity score over 14 days; secondary: hospitalization |
NA, not applicable; SOC, standard of care.
Table 2.
Characteristics of cohort studies
| Study/year | Country/setting | Number of patients | Case selection | Control selection | Intervention/exposure | Comparator | Outcome |
|---|---|---|---|---|---|---|---|
| Alberici et al. 2020 | Italy/inpatient+ outpatient | 94 | PCR-confirmed SARS-CoV-2 | NR | HCQ | lopinavir/ritonavir and darunavir/ritonavir | primary: clinical deterioration |
| An et al. 2020 | China/inpatient | 40 | PCR-confirmed SARS-CoV-2 | NR | HCQ | SOC | primary: time to viral clearance |
| Geleris et al. 2020 | USA/inpatient | 1376 | PCR-confirmed SARS-CoV-2 | NR | HCQ | no HCQ | primary: intubation or death |
| Ip et al. 2020 | USA/inpatient | 2512 | PCR-confirmed SARS-CoV-2 | selected from convenience sample |
1-HCQ 800 mg po od on day one and 400 mg on days 2–5 2-HCQ+AZM 3-AZM |
SOC | primary: mortality |
| Magagnoli et al. 2020 | USA/inpatient | 368 | PCR-confirmed SARS-CoV-2 | NR | HCQ or HCQ+AZM | standard supportive care | primary: death and the need for mechanical ventilation |
| Mahevas et al. 2020 | France/inpatient | 173 | PCR-confirmed SARS-CoV-2 and requiring oxygen | patients with no HCQ | HCQ (600 mg/day) within 48 h of admission | SOC/no HCQ | primary: survival without transfer to the ICU at day 21; secondary: overall survival, survival without acute respiratory distress syndrome and discharge from hospital by day 21 |
| Mallat et al. 2020 | UAE/inpatient | 34 | PCR-confirmed SARS-CoV-2 | NR | HCQ 400 mg twice daily for 1 day, followed by 400 mg daily for 10 days | no HCQ | primary: time to negative nasopharyngeal swab |
| Paccoud et al. 2020 | France/inpatient | 84 | patients hospitalized with PCR-confirmed COVID-19 infection | patients hospitalized before decision to treat all patients with HCQ was made | HCQ 200 mg 3 times daily for 10 days | SOC | primary: mortality, ICU admission; secondary: time to death or discharge, symptoms after 5 days |
| Rosenberg et al. 2020 | USA/inpatient | 1438 | PCR-confirmed SARS-CoV-2 | random sampling |
1-HCQ 400 mg po bd then 200 mg po bd+AZM 2-HCQ alone 3-AZM alone |
SOC | primary: in-hospital mortality; secondary: cardiac arrest and abnormal ECG findings (arrhythmia or QT prolongation) |
| Sánchez- Álvarez et al. 2020 | Spain/dialysis patients | 868 | documented SARS-CoV-2 coronavirus infection | NR | HCQ (85%) and the combination of lopinavir/ritonavir (40%); a third of the patients received the three drugs together; steroids, interferon and tocilizumab were used less frequently | NA | primary: mortality |
| Sbidian et al. 2020 | France | 4642 | patients hospitalized with PCR-confirmed COVID-19 infection | NR | HCQ 600 mg on day 1, followed by 400 mg daily for 9 additional days; AZM 500 mg on day 1 followed by 250 mg for 4 more days | SOC | primary: all-cause 28 day mortality |
| Singh et al. 2020 | USA/inpatient | 3372 | diagnosed with COVID-19 | NR | HCQ+AZM | no HCQ | primary: mortality, need for mechanical ventilation |
| Yu et al. 2020 | China/ICU+inpatient | 568 | all patients with lab-confirmed SARS-CoV-2 infection and a medical history and imaging characteristic of COVID-19 | NR | HCQ (200 mg twice per day) for 7–10 days | no HCQ | primary: in-hospital death and hospital stay time (days) |
AZM, azithromycin; bd, twice daily; NA, not applicable; NR, not reported; od, once daily; po, orally; SOC, standard of care.
The quality of the observational studies was assessed using the Newcastle–Ottawa scale (Table S11). With regards to patient selection, all but four studies had adequate representation of a general population with COVID-19 infection. Sánchez-Álvarez et al.33 and Alberici et al.32 studied haemodialysis patients, Yu et al.43 studied patients with severe COVID-19 infection and Kuderer et al.36 studied patients with cancer. With regards to comparability, three studies32,33,44 did not adequately adjust for confounders in their analyses. Given the relatively short course of disease, all studies were considered to have had a satisfactory follow-up duration. Three studies32,33,43 that assessed mortality were considered at high risk of bias and a sensitivity analysis was performed by excluding these studies to assess heterogeneity. The results of the Cochrane RoB 2 assessment for RCTs can be found in Table S12. Of the five included RCTs, three were considered high risk,27–29 one was low risk25 and one had no available manuscript for quality assessment.26
Some studies were initially included in our review as eligible studies, but were later excluded for various reasons. Chen et al.46 only reported time to clinical recovery and changes in radiological parameters and Feng et al.47 reported disease progression in patients receiving CQ. Four studies were excluded over serious methodology concerns48–51 and one study was retracted due to concerns over the validity of the patient information.52 GRADE was used to assess the certainty of evidence for each outcome. The findings were then summarized, along with the overall certainty of evidence (Table 3).
Table 3.
Summary of outcomes, key findings and certainty of evidence
| Treatment | Outcome | Study design: no. of studies | Findings and magnitude of effect | Strength of evidence |
|---|---|---|---|---|
| HCQ | mortality | RCT: 1; cohort: 12 |
1-studies with moderate and high risk of bias, with consistent but imprecise EEs, found no significant association between HCQ and mortality; EEs ranged from 0.32 (0.16–0.62) to 2.61 (1.10–6.17) 2-pooled adjusted OR from nine cohort studies at moderate risk of bias and one RCT at low risk of bias found no significant association between HCQ and mortality [1.05 (95% CI 0.96–1.15 I2=0%, P=0.647)], with no heterogeneity or evidence of publication bias |
moderate (no effect) |
| viral clearance | RCTs: 3; cohort: 2 | studies with low and high risk of bias and inconsistent and imprecise EEs found no association between HCQ and viral clearance; EEs ranged from 0.46 (95% CI 0.04–5.75) to 5.68 (95% CI 1.05–10.08) | very low | |
| mechanical ventilation/ ICU admission | cohort: 3 | studies with moderate risk of bias and consistent and precise results found no significant association between HCQ and the composite outcome; EEs ranged from 0.81 (95% CI 0.55–1.18) to 1.43 (95% CI 0.53–3.79) | very low | |
| hospitalization | RCTs: 2 | studies with low and moderate risk of bias and inconsistent and imprecise EEs found no significant effect of HCQ on risk of hospitalization in outpatients | low | |
| HCQ+azithromycin | mortality | cohort: 6 |
1-studies with moderate risk of bias showed a trend towards increased mortality; AEEs ranged from 0.98 (0.75-1.28) to 2.93 (1.79-4.79) 2-pooled adjusted OR=1.15 (95% CI 0.99–1.34, I2=0.0%) from five cohort studies at moderate risk of bias |
low (higher mortality) |
| mechanical ventilation/ ICU admission | cohort: 2 | studies with moderate risk of bias and consistent and precise results found no significant association between HCQ+azithromycin and the composite outcome | very low | |
| CQ | viral clearance | RCTs: 2 | one RCT with high risk of bias and inconsistent and imprecise EEs showed no significant effect of CQ on viral clearance [EE 1.07 (0.44–2.56)]; one RCT with high risk of bias demonstrated shorted time to viral clearance in the CQ group (median 2.5 days; IQR 2–3.8) versus the control group (median 7 days; IQR 2–10) | very low |
AEE, adjusted-effect estimate; EE, effect estimate.
Treatment efficacy
Outcome data and analytical methods reported by included studies are summarized in Tables S6 to S10.
Mortality
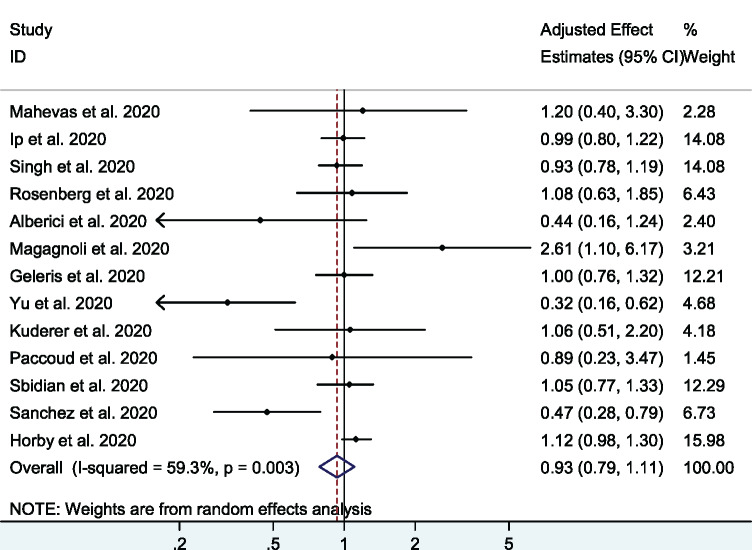
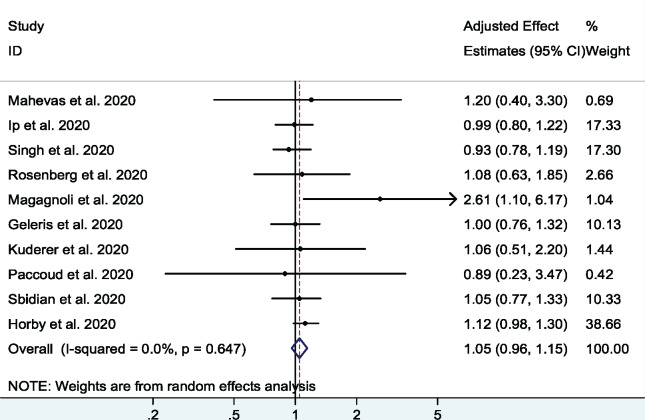
A total of 13 studies26,32–43 (1 RCT and 12 cohort studies) with 19 573 patients examined the effect of HCQ on short-term mortality in hospitalized COVID-19 patients. The pooled adjusted OR was 0.93 (95% CI 0.79–1.11, I2 = 59.3%), indicating no significant association between HCQ and mortality (Figure 2). There was moderate heterogeneity among the included studies. In a sensitivity analysis, after excluding the three studies with high risk of bias32,33,43 the pooled adjusted OR was 1.05 (95% CI 0.96–1.15, I2 = 0%) (Figure 3). In an analysis restricted to cohort studies, the pooled adjusted OR was 0.90 (95% CI 0.73–1.09, I2 = 57%) (Figure S1).
Figure 2.
Association between HCQ and short-term mortality in COVID-19 patients (all cohort studies and one RCT). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 3.
Association between HCQ and short-term mortality in COVID-19 patients (excluding studies at high risk of bias). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
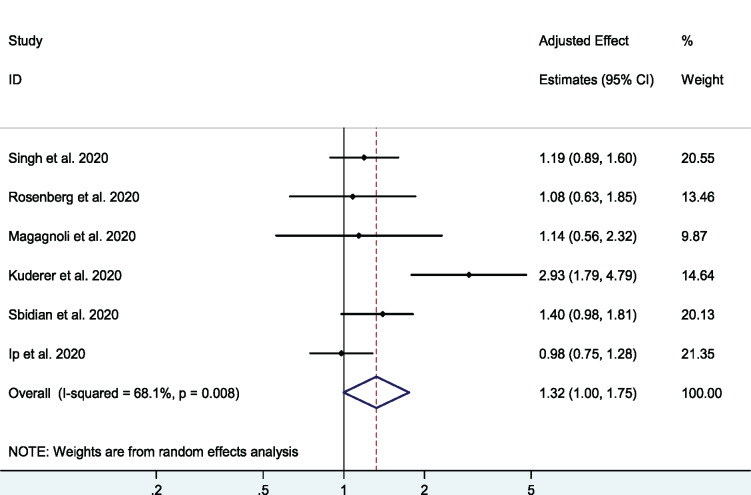
A total of six cohort studies35–40 with 3430 patients examined the effect of the HCQ+azithromycin combination on mortality. The pooled adjusted OR was 1.32 (95% CI 1.00–1.75, I2 = 68.1%), with a higher odds for mortality in the combination therapy group compared with the control group (Figure 4). Excluding the study of Kuderer et al.,36 that included only patients with cancers, eliminated this heterogeneity (adjusted OR = 1.15, 95% CI 0.99–1.34, I2 = 0.0%).
Figure 4.
Association between HCQ+azithromycin combination and short-term mortality in COVID-19 patients. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
There was no publication bias on visual inspection of funnel plots (Figures S2 to S5). Additionally, Egger’s regression did not detect any significant publication bias (P = 0.276).
Viral clearance
Six studies in our meta-analysis evaluated the effect of therapy on viral clearance, of which five had a high risk of bias and, thus, effect estimates were not pooled together.
Four RCTs (two with a high risk of bias,28,29 one with a moderate risk of bias30 and one with a low risk of bias;25 assessed using Cochrane RoB 2) and two cohort studies44,45 (with a high risk of bias; assessed using the Newcastle–Ottawa scale) assessed the effect of HCQ on viral clearance. One RCT29 demonstrated significant improvement in time to viral clearance (2.0 days; IQR 2.0–3.5), two25,28 didn’t show any significant effect on time to viral clearance [0.46 (95% CI 0.04–5.75) and 0.846 (95% CI 0.58–1.23), respectively] and one study reported no difference in viral clearance at 7 days [−0.7 (95% CI −0.44–0.29)].30 One cohort study demonstrated an association between HCQ and slower viral clearance [adjusted OR 5.68 (95% CI 1.05–10.08)],44 while the other found no significant association [adjusted HR 1.53 (95% CI 0.83–2.94)].45
Two RCTs27,29 with high risk of bias studied the effect of CQ on viral clearance. In one study with 22 patients,27 there was no significant effect on viral clearance [OR 1.07 (95% CI 0.44–2.56)]. Another RCT with 48 patients found a significant difference in time to viral clearance in the CQ group compared with the control group [2.5 days (IQR 2.0–3.8) versus 7.0 days (IQR 3.0–10.0), respectively].29
Mechanical ventilation/ICU admission
Three cohort studies assessed the association between HCQ and the composite outcome of mechanical ventilation or ICU admission.38,40,41 None of the studies found any association between HCQ and the composite outcome [1.1 (95% CI 0.476–2.5), 1.43 (95% CI 0.53–3.79) and 0.81 (95% CI 0.55–1.18), respectively].
Additionally, two cohort studies38,40 failed to demonstrate any significant association between HCQ+azithromycin and the composite outcome of mechanical ventilation or ICU admission [adjusted-effect estimate 0.43 (95% CI 0.16–1.12) and 0.976 (95% CI 0.64–1.49), respectively].
Hospitalization
Two RCTs (one with moderate risk30 and one with low risk31 of bias) reported on the effect of HCQ in outpatients with mild or moderate COVID-19 on risk of hospitalization. Neither study was able to demonstrate a decreased risk of hospitalization. Skipper et al.31 reported 4 hospitalized patients in the treatment group versus 10 in the control group (P = 0.29), whereas Mitjà et al.30 reported a risk ratio of 0.75 (95% CI 0.32–1.77).
Discussion
Main findings
Our systematic review and meta-analysis included 12 cohort studies and 1 RCT, which addressed the association between HCQ therapy and mortality in patients hospitalized with COVID-19 disease. Among a total of 19 573 patients, we found, with a moderate level of certainty, that HCQ monotherapy did not reduce short-term mortality among COVID-19 patients, which remained statistically non-significant even after excluding three studies at high risk of bias. These observations did not change when we included in the model only the cohort studies. Moreover, we found that the use of the combination HCQ+azithromycin was associated with a trend of increased mortality. The study by Kuderer et al.36 analysed cancer patients and demonstrated higher mortality than other studies, creating significant heterogeneity. Excluding this study did not decrease the risk of mortality. Because of the limited number of studies and/or high risk of bias, we could not conduct a meta-analysis of other clinical outcomes, such as viral clearance, risk of ICU admission and need for mechanical ventilation. We also observed that two RCTs found no significant effect for HCQ on hospitalization risk in outpatients with mild or moderate COVID-19.
Our findings of lack of efficacy of HCQ in the inpatient and outpatient clinical setting despite its effective in vitro inhibitory actions against SARS-CoV-2 virus is consistent with previous observations with other viral illnesses. Numerous studies demonstrated significant in vitro inhibitory effects of CQ/HCQ against coronaviruses and non-coronaviruses.11 For example, CQ at EC50 of 8.8 ± 1.2 μM effectively inhibited SARS-CoV replication in Vero E6 cells.3 CQ was also shown to inhibit MERS-CoV and alphacoronavirus HCoV-229E replication in vitro in a dose-dependent manner.4 Likewise, CQ/HCQ exhibited in vitro inhibitory effects against several other viruses, such as HIV-1, influenza, dengue, Ebola, Zika, Chikungunya and other viruses.2,5–10 Although CQ/HCQ have shown consistent broad-spectrum in vitro antiviral effects, their in vivo and clinical antiviral effects were disappointing. For instance, CQ was ineffective in preventing or ameliorating influenza following viral challenge in mouse and ferret models5 and did not prevent influenza infection in a randomized, double-blind placebo-controlled trial in humans.53 CQ also resulted in worse outcomes in a guinea pig model of Ebola infection7 and was shown to enhance Chikungunya viral infections in different animal models, including non-human primates.54 Moreover, CQ was ineffective in improving the course of Chikungunya viral infection in humans.54,55 CQ was also tested in a randomized controlled trial of 307 patients with dengue virus and failed to reduce duration of viraemia or NS1 antigenaemia.56 In the case of HIV-1, the use of CQ/HCQ was inconclusive and hence they were not endorsed for routine use in the treatment of HIV-1 infection.57
Possible reasons for lack of efficacy of HCQ in the treatment of COVID-19 disease
The discrepancy between the observed in vitro anti-SARS-CoV-2 effects of CQ/HCQ and the lack of efficacy in clinical studies, which mirrors previous observations with other viral infections, could be due to three main reasons.
First, most in vitro studies employ pre-treatment protocols, where cells are treated with the drugs before infecting them with the tested virus. In vitro studies that compared pre-treatment and post-infection treatment have shown that CQ/HCQ have less effective antiviral activities if added after infection.4,16,58 For example, Vincent et al.58 showed that CQ at 0.1, 1.0 and 10 μM added 20–24 h before infection with SARS-CoV decreased infectivity by 28%, 53% and 100%. However, if CQ is added 3–5 h after infecting the cells, higher concentrations of CQ of up to 50 μM were needed to decrease infectivity.58 This may raise the possibility that chronic or prophylactic use of CQ/HCQ may reduce the risk of acquiring SARS-CoV-2 infection. However, a recent large population study of 14 250 individuals showed that chronic use of HCQ was not protective against SARS-CoV-2 infection.59
Second, a wide range of EC50 values were reported for CQ and HCQ and, in the case of SARS-CoV-2, the EC50 for CQ ranges between 1.13 and 7.36 μM and between 0.72 and 17.31 μM for HCQ.11 It is worth noting that the lowest EC50 of 0.72 μM for HCQ reported by Yao et al.16 in their post-infection experiments was different from the lowest EC50 of 5.85 μM in their pre-treatment experiments. None of the other investigators reported such a low EC50 for CQ of HCQ with SARS-CoV-2 or any other viruses. Achieving adequate blood and tissue drug concertation is essential for proper antiviral activity. In mice, a high dose of 90 mg/kg twice a day of CQ was necessary to achieve steady-state blood levels of 2.5 μg/mL.8 High doses of CQ/HCQ in COVID-19 patients can be associated with increased adverse events, as shown in a recent RCT, where high-dose CQ was shown to be associated with significant toxicity in COVID-19 patients.60 In addition, optimal CQ or HCQ blood levels for effective antiviral action are unclear, since the suggested levels were based on widely differing in vitro EC50 estimates. In a study of 40 HIV-1 patients treated with HCQ 800 mg/day for 8 weeks, the HCQ blood concentration range was 0.27–1.0 μg/mL.61 Only those HIV-1 patients who achieved the highest HCQ blood concentrations had a favourable response to HCQ.61 There are only two small studies that looked at the pharmacokinetics of HCQ in COVID-19 patients.18,62 In the first study, Gautret et al.18 found that the mean HCQ level in 20 patients treated with HCQ 600 mg/day was 0.46 μg/mL. This blood concentration is lower than the lowest effective in vitro concentration of 0.72 μM. Perinel et al.62 showed that only 61% of 13 patients treated with HCQ 600 mg/day achieved what they considered the minimum therapeutic concentration of 1 μg/mL with a mean time to reach this concentration of 2.7 days. Based on published data and their own, Balevic et al.63 found that the average serum/plasma HCQ concentration was below the lowest antiviral target level for SARS-CoV-2 of 0.48 μg/mL in all studies. These studies indicate that current HCQ dosing is probably suboptimal to achieve adequate blood levels necessary for effective antiviral activity.
Third, antimalarials exhibit anti-inflammatory and immunomodulatory effects, decreasing the production of pro-inflammatory cytokines and improving endothelial function and reducing the prothrombotic state.12,13 These effects would be very beneficial in patients with severe COVID-19 disease; however, HCQ reduces the affinity of toll-like receptor 7 and 9 (TLR7 and TLR9) to viral RNA and also inhibits the cyclic GMP-AMP synthase pathway and hence it inhibits the type I interferon response, which is the first line of defence of the innate immune system against viral infections.12 This effect might counteract the direct antiviral effects of HCQ and reduce its efficacy in treating COVID-19 disease.
Further research is needed to address these important issues to improve the clinical utility of CQ/HCQ for the treatment of COVID-19 disease, which should include exploring alternate administration routes like intranasal application and inhalation therapy.
Safety of CQ/HCQ in the context of COVID-19 disease
Our meta-analysis not only revealed lack of efficacy of HCQ in improving the outcomes of COVID-19 patients, but also suggested possible increased risk of mortality when used in combination with azithromycin. Several studies have shown increased risk of cardiac toxicity among COVID-19 patients treated with CQ/HCQ. Our group have recently conducted a meta-analysis on CQ/HCQ-induced cardiac toxicity in COVID-19 patients,64 which revealed increased risk of QTc prolongation and discontinuation of drug due to QT prolongation. In addition, CQ/HCQ were associated with a clinically significant risk of malignant arrhythmias and cardiac arrest.60
It has also been a common practice to use HCQ in combination with azithromycin for COVID-19 during the current pandemic. Azithromycin has been linked to increased risk of sudden cardiac death.65,66 Hence, the concomitant use of CQ/HCQ and azithromycin or other QT-prolonging agents could potentially increase the risk of serious cardiac arrhythmias and death. Increased risk of 30 day cardiac death, angina and heart failure complications associated with the combination therapy of HCQ+azithromycin has also been reported in a recent preprint of a large population study of 323 122 patients.67 Our findings are consistent with the IDSA recommendations on the use of CQ/HCQ in COVID-19 disease68 and the recent systematic reviews.69–71 However, our study has several advantages over the previous reviews (Table S13). First, it is the only study that included both qualitative and quantitative analyses. Second, it has the largest number of identified studies and therefore patient population because our search is the most up to date. Third, in contrast to all previous studies, we included only studies that reported adjusted-effect estimates and therefore we avoided including studies at high risk of bias due to confounding. Fourth, we provided adequate assessment of the certainty of evidence using the GRADE classification. Finally, we offered a comprehensive discussion regarding the probable mechanisms of lack of efficacy of HCQ in COVID-19, which will inform and stimulate further research in this area.
Strengths and limitations
This meta-analysis has several strengths. Firstly, published and unpublished studies were included, which reduces publication bias. We also employed rigorous methodologies, where we excluded studies that did not report adjusted ORs or HRs and those with poor methodology. We analysed and reported monotherapy and combination therapy separately. We also examined mortality and other clinical outcomes separately and performed sensitivity analyses to eliminate sources of between-study heterogeneity. However, our study has several limitations; all of our included studies except one were observational studies, which are prone to bias; including confounding by allocation, survival bias and residual confounding. Our group and others have shown that survivor bias, which occurs because patients who live longer are more likely to receive treatment than those who die early, could change associations from benefit to harm.72,73 Moreover, as with all observational studies, residual confounding could weaken any observed association74 even with appropriate adjustment or propensity score matching. Nevertheless, the direction of these biases is supposed to be in favour of HCQ efficacy. In addition, our pooled estimates are consistent with the results of the interim report from the RECOVERY trial, which lends support to our findings.
Conclusions
This systematic review and meta-analysis indicates, with a moderate level of certainty, that HCQ monotherapy lacks efficacy in reducing short-term mortality in hospitalized patients with COVID-19 or in reducing risk of hospitalization in outpatients with COVID-19. We also found that the use of HCQ in combination with azithromycin is probably associated with increased short-term mortality among hospitalized COVID-19 patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Transparency declarations
None to declare.
Published in the preprint server medRxiv (2020; 2020.07.12.20150110).
Supplementary data
Tables S1 to S13 and Figures S1 to S5 are available as Supplementary data at JAC Online.
Supplementary Material
References
- 1. Inglot AD. Comparison of the antiviral activity in vitro of some non-steroidal anti-inflammatory drugs. J Gen Virol 1969; 4: 203–14. [DOI] [PubMed] [Google Scholar]
- 2. Chiang G, Sassaroli M, Louie M. et al. Inhibition of HIV-1 replication by hydroxychloroquine: mechanism of action and comparison with zidovudine. Clin Ther 1996; 18: 1080–92. [DOI] [PubMed] [Google Scholar]
- 3. Keyaerts E, Vijgen L, Maes P. et al. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun 2004; 323: 264–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Wilde AH, Jochmans D, Posthuma CC. et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother 2014; 58: 4875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vigerust DJ, McCullers JA. Chloroquine is effective against influenza A virus in vitro but not in vivo. Influenza Other Respir Viruses 2007; 1: 189–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farias KJS, Machado PRL, de Almeida Junior RF. et al. Chloroquine interferes with dengue-2 virus replication in U937 cells. Microbiol Immunol 2014; 58: 318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dowall SD, Bosworth A, Watson R. et al. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model. J Gen Virol 2015; 96: 3484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Madrid PB, Chopra S, Manger ID. et al. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One 2013; 8: e60579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delvecchio R, Higa LM, Pezzuto P. et al. Chloroquine, an endocytosis blocking agent, inhibits Zika virus infection in different cell models. Viruses 2016; 8: 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan M, Santhosh SR, Tiwari M. et al. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in Vero cells. J Med Virol 2010; 82: 817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hashem AM, Alghamdi BS, Algaissi AA. et al. Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: a narrative review. Travel Med Infect Dis 2020; 35: 101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meyerowitz EA, Vannier AGL, Friesen MGN. et al. Rethinking the role of hydroxychloroquine in the treatment of COVID-19. FASEB J 2020; 34: 6027–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miranda S, Billoir P, Damian L. et al. Hydroxychloroquine reverses the prothrombotic state in a mouse model of antiphospholipid syndrome: role of reduced inflammation and endothelial dysfunction. PLoS One 2019; 14: e0212614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang M, Cao R, Zhang L. et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020; 30: 269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu J, Cao R, Xu M. et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yao X, Ye F, Zhang M. et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020; 71: 732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020; 14: 72–3. [DOI] [PubMed] [Google Scholar]
- 18. Gautret P, Lagier J, Parola P. et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020; 56: 105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Vaduganathan M, van Meijgaard J, Mehra MR. et al. Prescription fill patterns for commonly used drugs during the COVID-19 pandemic in the United States. JAMA 2020; 323: 2524–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sterne JAC, Savović J, Page MJ. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 22. Wells GA, Shea B, O’Connell D. et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 23. Guyatt GH, Oxman AD, Vist GE. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murad MH. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc 2017; 92: 423–33. [DOI] [PubMed] [Google Scholar]
- 25. Chen J, Liu D, Liu L. et al. [A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19]. Zhejiang Da Xue Bao Yi Xue Ban 2020; 49: 215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horby P, Mafham M, Linsell L. et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv 2020; doi:10.1101/2020.07.15.20151852. [Google Scholar]
- 27. Huang M, Tang T, Pang P. et al. Treating COVID-19 with chloroquine. J Mol Cell Biol 2020; 12: 322–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tang W, Cao Z, Han M. et al. Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomized, controlled trial. medRxiv 2020; doi:10.1101/2020.04.10.20060558. [Google Scholar]
- 29. Chen L, Zhang Z, Fu J. et al. Efficacy and safety of chloroquine or hydroxychloroquine in moderate type of COVID-19: a prospective open-label randomized controlled study. medRxiv 2020; doi:10.1101/2020.06.19.20136093. [Google Scholar]
- 30. Mitjà O, Corbacho-Monné M, Ubals M. et al. Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial. Clin Infect Dis 2020; doi:10.1093/cid/ciaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skipper CP, Pastick KA, Engen NW. et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med 2020; doi:10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alberici F, Delbarba E, Manenti C. et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int 2020; 98: 20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sánchez-Álvarez JE, Pérez Fontán M, Jiménez Martín C. et al. SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 registry of the Spanish Society of Nephrology (SEN). Nefrologia 2020; 40: 272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Geleris J, Sun Y, Platt J. et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 2020; 382: 2411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ip A, Berry DA, Hansen E. et al. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-an observational study. PLoS One 2020; 15: e0237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuderer NM, Choueiri TK, Shah DP. et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020; 395: 1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sbidian E, Josse J, Lemaitre G. et al. Hydroxychloroquine with or without azithromycin and in-hospital mortality or discharge in patients hospitalized for COVID-19 infection: a cohort study of 4,642 in-patients in France. medRxiv 2020; doi:10.1101/2020.06.16.20132597. [Google Scholar]
- 38. Singh S, Khan A, Chowdhry M. et al. Outcomes of hydroxychloroquine treatment among hospitalized COVID-19 patients in the United States- real-world evidence from a federated electronic medical record network. medRxiv 2020; doi:10.1101/2020.05.12.20099028. [Google Scholar]
- 39. Rosenberg ES, Dufort EM, Udo T. et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA 2020; 323: 2493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Magagnoli J, Narendran S, Pereira F. et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. Med (N Y) 2020; doi:10.1016/j.medj.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahévas M, Tran V, Roumier M. et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ 2020; 369: m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paccoud O, Tubach F, Baptiste A. et al. Compassionate use of hydroxychloroquine in clinical practice for patients with mild to severe Covid-19 in a French university hospital. Clin Infect Dis 2020; doi:10.1093/cid/ciaa791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yu B, Wang DW, Li C. Hydroxychloroquine application is associated with a decreased mortality in critically ill patients with COVID-19. medRxiv 2020; doi:10.1101/2020.04.27.20073379. [Google Scholar]
- 44. Mallat J, Hamed F, Balkis M. et al. Hydroxychloroquine is associated with slower viral clearance in clinical COVID-19 patients with mild to moderate disease: a retrospective study. medRxiv 2020; doi:10.1101/2020.04.27.20082180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. An MH, Kim MS, park Y. et al. Treatment response to hydroxychloroquine and antibiotics for mild to moderate COVID-19: a retrospective cohort study from South Korea. medRxiv 2020; doi:10.1101/2020.07.04.20146548. [Google Scholar]
- 46. Chen Z, Hu J, Zhang Z. et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv 2020; doi:10.1101/2020.03.22.20040758. [Google Scholar]
- 47. Feng Z, Li J, Yao S. et al. The use of adjuvant therapy in preventing progression to severe pneumonia in patients with coronavirus disease 2019: a multicenter data analysis. medRxiv 2020; doi:10.1101/2020.04.08.20057539. [Google Scholar]
- 48. Membrillo de Novales FJ, Ramírez-Olivencia G, Estébanez M. et al. Early hydroxychloroquine is associated with an increase of survival in COVID-19 patients: an observational study. Preprints 2020; 2020050057. doi:10.20944/preprints202005.0057.v1. [Google Scholar]
- 49. Barbosa J, Kaitis D, Le K. et al. Clinical Outcomes of Hydroxychloroquine in Hospitalized Patients With COVID-19: A Quasi-Randomized Comparative Study. 2020. https://aslm.org/wp-content/uploads/2020/06/1589740749-NEJM_Clinical-Outcomes-of-Hydroxychlorquine-in-Patients-with-COVID19.pdf.
- 50. Arshad S, Kilgore P, Chaudhry ZS. et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis 2020; 97: 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mikami T, Miyashita H, Yamada T. et al. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med 2020; doi:10.1007/s11606-020-05983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mehra MR, Desai SS, Ruschitzka F. et al. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet 2020; doi:10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53. Paton NI, Lee L, Xu Y. et al. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect Dis 2011; 11: 677–83. [DOI] [PubMed] [Google Scholar]
- 54. Roques P, Thiberville S, Dupuis-Maguiraga L. et al. Paradoxical effect of chloroquine treatment in enhancing Chikungunya virus infection. Viruses 2018; 10: 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Lamballerie X, Boisson V, Reynier J. et al. On Chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis 2008; 8: 837–9. [DOI] [PubMed] [Google Scholar]
- 56. Tricou V, Minh NN, Van TP. et al. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis 2010; 4: e785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chauhan A, Tikoo A. The enigma of the clandestine association between chloroquine and HIV-1 infection. HIV Med 2015; 16: 585–90. [DOI] [PubMed] [Google Scholar]
- 58. Vincent MJ, Bergeron E, Benjannet S. et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005; 2: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gendelman O, Amital H, Bragazzi NL. et al. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: insights from a large healthcare database analysis. Autoimmun Rev 2020; 19: 102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Borba MGS, Val FFA, Sampaio VS. et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 2020; 3: e208857. [DOI] [PubMed] [Google Scholar]
- 61. Sperber K, Louie M, Kraus T. et al. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin Ther 1995; 17: 622–36. [DOI] [PubMed] [Google Scholar]
- 62. Perinel S, Launay M, Botelho-Nevers É. et al. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. Clin Infect Dis 2020; doi:10.1093/cid/ciaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Balevic SJ, Hornik CP, Green TP. et al. Hydroxychloroquine in patients with rheumatic disease complicated by COVID-19: clarifying target exposures and the need for clinical trials. J Rheumatol 2020; doi:10.3899/jrheum.200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tleyjeh I, Kashour Z, AlDosary O. et al. The cardiac toxicity of chloroquine or hydroxychloroquine in COVID-19 patients: a systematic review and meta-regression analysis. medRxiv 2020; doi:10.1101/2020.06.16.20132878. [Google Scholar]
- 65. Ray WA, Murray KT, Hall K. et al. Azithromycin and the risk of cardiovascular death. N Engl J Med 2012; 366: 1881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang Z, Prinsen JK, Bersell KR. et al. Azithromycin causes a novel proarrhythmic syndrome. Circ Arrhythm Electrophysiol 2017; 10: e003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lane JCE, Weaver J, Kostka K. et al. Safety of hydroxychloroquine, alone and in combination with azithromycin, in light of rapid wide-spread use for COVID-19: a multinational, network cohort and self-controlled case series study. medRxiv 2020; doi:10.1101/2020.04.08.20054551. [Google Scholar]
- 68. Bhimraj A, Morgan RL, Shumaker AH. et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis 2020; doi:10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Liu W, Zhou P, Chen K. et al. Efficacy and safety of antiviral treatment for COVID-19 from evidence in studies of SARS-CoV-2 and other acute viral infections: a systematic review and meta-analysis. Can Med Assoc J 2020; 192: E734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hernandez AV, Roman YM, Pasupuleti V. et al. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med 2020; 173: 287–96. [DOI] [PubMed] [Google Scholar]
- 71. Qaseem A, Yost J, Etxeandia-Ikobaltzeta I. et al. Should clinicians use chloroquine or hydroxychloroquine alone or in combination with azithromycin for the prophylaxis or treatment of COVID-19? Living practice points from the American College of Physicians (version 1). Ann Intern Med 2020; 173: 137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wolkewitz M, Schumacher M. Survival biases lead to flawed conclusions in observational treatment studies of influenza patients. J Clin Epidemiol 2017; 84: 121–9. [DOI] [PubMed] [Google Scholar]
- 73. Tleyjeh IM, Ghomrawi HMK, Steckelberg JM. et al. Conclusion about the association between valve surgery and mortality in an infective endocarditis cohort changed after adjusting for survivor bias. J Clin Epidemiol 2010; 63: 130–5. [DOI] [PubMed] [Google Scholar]
- 74. Fewell Z, Davey Smith G, Sterne JAC. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol 2007; 166: 646–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.