Abstract
Background
Previous studies have indicated coronavirus disease 2019 (COVID-19) patients with cancer have a high fatality rate.
Methods
We conducted a systematic review of studies that reported fatalities in COVID-19 patients with cancer. A comprehensive meta-analysis that assessed the overall case fatality rate and associated risk factors was performed. Using individual patient data, univariate and multivariable logistic regression analyses were used to estimate odds ratios (OR) for each variable with outcomes.
Results
We included 15 studies with 3019 patients, of which 1628 were men; 41.0% were from the United Kingdom and Europe, followed by the United States and Canada (35.7%), and Asia (China, 23.3%). The overall case fatality rate of COVID-19 patients with cancer measured 22.4% (95% confidence interval [CI] = 17.3% to 28.0%). Univariate analysis revealed age (OR = 3.57, 95% CI = 1.80 to 7.06), male sex (OR = 2.10, 95% CI = 1.07 to 4.13), and comorbidity (OR = 2.00, 95% CI = 1.04 to 3.85) were associated with increased risk of severe events (defined as the individuals being admitted to the intensive care unit, or requiring invasive ventilation, or death). In multivariable analysis, only age greater than 65 years (OR = 3.16, 95% CI = 1.45 to 6.88) and being male (OR = 2.29, 95% CI = 1.07 to 4.87) were associated with increased risk of severe events.
Conclusions
Our analysis demonstrated that COVID-19 patients with cancer have a higher fatality rate compared with that of COVID-19 patients without cancer. Age and sex appear to be risk factors associated with a poorer prognosis.
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread rapidly around the world since it emerged in late 2019 in China (1-3). In early March 2020, the World Health Organization declared the COVID-19 outbreak a global pandemic and as of July 15, this virus has infected nearly 14 million individuals with 580 000 deaths (4). As the number of new cases continues to rise, challenges to the global healthcare system remain.
Cancer is one of the leading causes of death, with an estimated 9.6 million deaths worldwide in 2018 (5). Compared with the general population, cancer patients are more vulnerable to infection (6,7). In addition to being in immunosuppressive states directly caused by cancer itself as well as cytotoxic treatments, this population presents an overall poorer health status, tends to be older, and has coexisting medical conditions. These are all risk factors that could contribute to severe COVID-19 infection (3,8-10) and lead to a potentially poorer prognosis and an increased risk of death.
The challenges facing the healthcare and research community have been unprecedented in response to the COVID-19 pandemic, prompting several national and international collective efforts dedicated to understanding the impact of COVID-19 on cancer patients (11-13). For instance, the COVID-19 and Cancer Consortium (CCC19) is a multicenter registry that includes more than 90 institutions from the United States, Canada, and Spain (12). It aims to collect and analyze observational data from cancer patients with COVD-19 to inform clinical practice in real time. In addition, clinicians and scientists from over 28 countries initiated another global consortium, The Thoracic Cancers International COVID-19 Collaboration (TERAVOLT) registry, dedicated to studying the effects of COVID-19 on patients with thoracic malignancies (11).
The effects of the COVID-19 pandemic on patients with cancer have been detrimental and profound (12). Current evidence based on individual reports from China suggests that cancer patients infected with COVID-19 may be at an increased risk of severe events, including hospitalization, admission to the intensive care unit, requiring invasive ventilation, or death (6,14,15). Preliminary analysis of data from the CCC19 and TERAVOLT cohorts also suggest a higher risk of fatality in cancer patients (10,16).
Although these individual reports provide valuable data on the clinical outcome of cancer patients with COVID-19, the majority of the studies analyzed have a small sample size or are limited by geographical regions (eg, either China, Europe, or the United States) or 1 cancer type (thoracic malignancies in the TERAVOLT). It is crucial to assess the data collected from these individual studies and evaluate them in a systematic manner.
Using data from 15 cohort studies involving 3019 patients, this meta-analysis aims to comprehensively characterize the clinical features, outcome of cancer patients with COVID-19, and potential risk factors contributing to higher fatality. Findings from this analysis will improve our understanding of the impact of the COVID-19 pandemic on cancer patients and highlight the urgent need to provide optimal clinical management to this vulnerable patient population.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (17).
Types of Studies
We included all studies that reported case fatality or severe events in cancer patients with COVID-19. Clinical practice guidelines and recommendations, editorials, commentaries, and review articles were excluded. Publications that were not subject to peer review were also excluded.
Search Strategy and Review Method
Databases including PubMed, Embase, and conference proceedings were searched on June 9 2020 using the following terms: “(coronavirus OR COVID-19 OR SARS-CoV-2 or COVID-2019) AND (cancer OR carcinoma OR neoplasm OR malignancy).” No limitation was placed regarding publication language, but publication date was restricted to 2019 and 2020 considering the timeline of the COVID-19 outbreak. Duplicate records were excluded. References from review articles, commentaries, editorials, included studies, and conference publications of relevant medical societies were reviewed and cross-referenced to ensure completeness. Full-text articles considered eligible in the initial screening were reevaluated. H.Z. and H.H. performed study selection independently and extracted all the data. T.H. conducted independent verification.
Study characteristics, including first author, patient number, age, sex, geographical region of residence, comorbidity (hypertension, cardiovascular disease, and diabetes), common COVID-19 symptoms (fever, dyspnea and cough), cancer type (lung, other solid tumor [breast, prostate, gastrointestinal, gynecological, renal cell, endocrine, melanoma, head and neck, sarcoma, nervous system] and hematological malignancies [lymphoid neoplasms, multiple myeloma, non-Hodgkin lymphoma, myeloid neoplasms, acute myeloid leukemia, acute lymphoblastic leukemia]), cancer treatment type (surgery, radiotherapy, chemotherapy, immunotherapy, and targeted therapy), and clinical outcomes (survivor or nonsurvivor or severe events) were extracted.
Quality Assessment of Study
The quality of included cohort studies was assessed by 2 researchers independently using the Newcastle-Ottawa Scale (NOS) (18). Specifically, the NOS scale evaluates the following criteria: Selection: representativeness of the exposed cohort, selection of the nonexposed cohort, ascertainment of exposure, and demonstration that outcome of interest was not present at start of study; Comparability: comparability of cohorts on the basis of the design or analysis; and Outcome: assessment of outcome, whether the follow-up was long enough for outcomes to occur, and adequacy of follow up of cohorts. The NOS assessment was performed per each cohort study, which was assigned scores based on these 3 criteria (selection, comparability, outcomes). The maximum score was 9 and the minimum score was 0. A score of 7 or more was reflective of high methodological quality, a score of 5 or 6 indicated moderate quality, and a score of 4 or less indicated low quality.
Statistical Analysis
Meta-analysis was performed using R statistical software (version 3.6.1). Random-effects analysis was used for all meta-analyses due to the clinical heterogeneity inherent in the data and the different effect sizes of included studies. We used the DerSimonian and Laird (19) method for the estimator of between-study variance τ2. In the random effects model, weights are equal to the inverse of the sum of within-study variance plus the between-study variance; weights are similar among studies, and therefore large studies lose influence and small studies gain influence for the overall effect (20). The Cochran’s Q statistic is a type of χ2 test that identifies the presence of heterogeneity at P less than .05. The statistic I2 describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) and was calculated to quantify the heterogeneity across studies (21). A value of 0.0% indicates no observed heterogeneity, and larger values show increasing heterogeneity (21); I2 greater than 75.0% is considered high heterogeneity. I2 is equal to 100.0% × (Q − df)/Q, where Q is Cochran's Q statistic and df the degrees of freedom. We report Cochran’s Q statistic, the P value, and I2 per meta-analysis.
Case fatality rate (calculated as the ratio of the number of deaths to the number of cases) or severe event rate (calculated as the ratio of the number of patients with severe events to the number of cases) and its 95% confidence interval (CI) for the overall population and subgroup analyses in an individual study were calculated. A composite “severe event” endpoint was defined here based on the individual being admitted to the intensive care unit, or requiring invasive ventilation, or death. Patients with missing values were excluded from analysis.
Publication bias was assessed by the funnel plots and Egger’s test (22) (P < .05 was considered to be suggestive of a statistically significant publication bias). Based on the quality assessment of study, sensitivity analysis was performed excluding low-quality studies (case series studies) or the cohort studies with moderate quality. In addition, a separate sensitivity analysis using “leaving-one-out” per time approach was performed.
Individual patient data on demographic or clinical characteristics including age, sex, comorbidities (hypertension, diabetes, or cardiovascular disease), cancer type (hematological malignancy, lung, or other solid cancer) and treatment (chemotherapy, immunotherapy, targeted therapy, radiotherapy, or surgery), and associated outcomes (survivor or nonsurvivor, or severe events) were extracted from 3 available studies (6,14,15). In the individual patient data analyses, univariate and multivariable logistic regression analysis were used to estimate odds ratio (OR) and 95% CIs of the association of each variable with the outcomes of cancer patients with COVID-19. The variables (demographic and clinical characteristics as listed above) were prespecified by investigators. Variables with P less than .05 from univariate analysis are considered as candidates for the multivariable logistic model, which assessed their association with outcome. All reported P values are 2-sided, and P less than .05 was used to indicate statistical significance.
Results
Literature Search Results
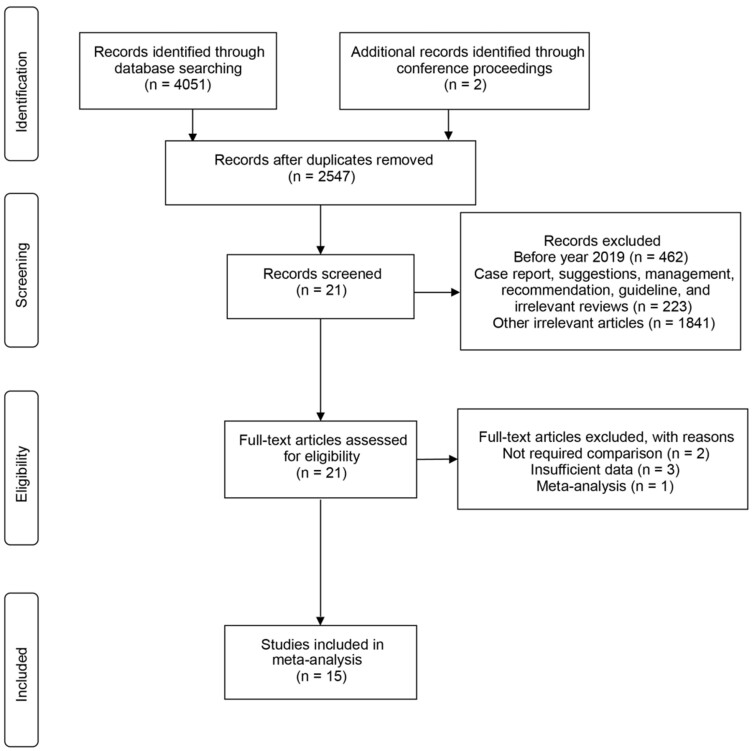
The literature search identified 4051 references through database mining and 2 from conference proceedings. After a full-text review of 21 studies, we identified 15 relevant studies, including 2 from the 2020 American Association for Cancer Research (AACR) Annual Meeting proceedings for the meta-analysis (Figure 1). These 15 studies, comprising of 5 case series (7,15,23-25) and 10 cohort studies (6,10,14,16,26-31), included patient data from institutions located in Europe, the United States, and Asia. Patients included in these 15 studies were with active or previous malignancy, including solid tumors or hematological malignancies, which have either a laboratory-confirmed SARS-CoV-2 infection or a presumptive diagnosis of COVID-19.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram. Flow chart of screen and study selection.
Characteristics of Identified Studies
Tables 1-4 list the main characteristics and clinical outcomes (survivor or nonsurvivor) of the 15 studies. In total, 3019 patients were included, of which 1628 (54.0%) were men and 1388 (46.0%) were women; more than one-half were over 65 years of age (Table 1). All cohorts enrolled patients within the past 6 months since the outbreak of COVID-19, and all studies were published in the past 4 months. The most common geographical region of residence was the United Kingdom and Europe (41.0%), followed by the United States and Canada (35.7%), and Asia (China, 23.3%) (Table 1). Among the patients with reported comorbidity data, the 3 most common were hypertension (37.7%), diabetes (18.1%), and cardiovascular disease (13.1%) (Table 1). The most common presenting symptoms at time of hospital admission were cough and fever (Table 2). Two of the 15 studies (24,28) included patients with hematological malignancy only, whereas 3 of them (7,10,15) were comprised of patients with solid tumor only (Table 3). The rest were mixed with both cancer types (Table 4). All 15 studies reported at least 1 clinical outcome of cancer patients with COVID-19 as either survivor or nonsurvivor (14 studies) or severe events (1 study) (Table 4). Of note, 3 of 15 studies reported both clinical outcomes (survivor or nonsurvivor and severe events) (Table 4). Severe events were defined by investigators as a composite endpoint defined based on the individual being admitted to the intensive care unit, requiring invasive ventilation, or death (6,14-16).
Table 1.
Demographic characteristics of the 15 studies included in the meta-analysis
| Study | Patients, No. | Age, median (range or IQR), y | Female/male, No. | Region of residency |
|---|---|---|---|---|
| Yu et al., 2020 (7) | 12 | 66 (48-78) | 2/10 | Asia |
| Barlesi et al., 2020 (23) | 137 | 61 (21-90) | 79/58 | Europe |
| Garassino et al., 2020 (10) | 200 | 68 (62-75)a | 59/141 | Europe, USA, Asia |
| Dai et al., 2020 (14) | 105 | 64 (55-69)a | 48/57 | Asia |
| Martin-Moro et al., 2020 (24) | 34 | 73 (59-83)a | 15/19 | Europe |
| Liang et al., 2020 (6) | 18 | 62 (56-68)a | 6/12 | Asia |
| Mehta et al., 2020 (26) | 218 | 69 (10-92) | 91/127 | USA |
| Yang et al., 2020 (25) | 52 | 63 (34-98) | 24/28 | Asia |
| Ma et al., 2020 (27) | 37 | 62 (59-70)a | 17/20 | Asia |
| Zhang et al., 2020 (15) | 28 | 65 (56-70)a | 11/17 | Asia |
| He et al., 2020 (28) | 13 | 35 (23-53)a | 6/7 | Asia |
| Tian et al., 2020 (29) | 232 | 64 (58-69)a | 113/119 | Asia |
| Yang et al., 2020 (30) | 205 | 63 (56-70)a | 109/96 | Asia |
| Kuderer et al., 2020 (16) | 928 | 66 (57-76)a | 412/516 | USA, Canada, Europe |
| Lee et al., 2020 (31) | 800 | 69 (59-76)a | 349/449 | UK |
Denotes IQR used to determine median age, otherwise shown as range. IQR = interquartile range.
Table 2.
Reported comorbidities and COVID-19 symptoms of individuals included in the meta-analysisa
| Study | Comorbidity, No. (%) |
Symptoms, No. (%) |
||||
|---|---|---|---|---|---|---|
| Hypertension | Cardiovascular disease | Diabetes | Fever | Dyspnea | Cough | |
| Yu et al., 2020 (7) | NR | NR | NR | 12 (100.0) | 3 (25.0) | 3 (25.0) |
| Barlesi et al., 2020 (23) | 47 (34.3) | 19 (13.9) | 27 (19.7) | 65 (47.4) | 45 (32.8) | 63 (46.0) |
| Garassino et al., 2020 (10) | 93 (46.5) | 30 (15.0) | 29 (14.5) | 127 (63.5) | 106 (53) | 103 (51.5) |
| Dai et al., 2020 (14) | 30 (28.6) | 12 (11.4) | 7 (6.7) | 68 (64.8) | NR | 57 (54.3) |
| Martin-Moro et al., 2020 (24) | NR | NR | NR | 31 (91.2) | 19 (55.9) | 20 (58.8) |
| Liang et al., 2020 (6) | 2 (11.1) | 0 (0.0) | 2 (11.1) | NR | 8 (44.4) | NR |
| Mehta et al., 2020 (26) | 147 (67.4) | 43 (19.7) | 80 (36.7) | NR | NR | NR |
| Yang et al., 2020 (25) | 17 (32.7) | 5 (9.6) | 7 (13.5) | 13 (25) | 3 (5.8) | 9 (17.3) |
| Ma et al., 2020 (27) | NR | NR | NR | 28 (75.7) | 12 (32.4) | 21 (56.8) |
| Zhang et al., 2020 (15) | 4 (14.3) | 4 (14.3) | 23 (82.1) | 14 (50.0) | 22 (78.6) | |
| He et al., 2020 (28) | 0 (0.0) | 3 (23.1) | 0 (0.0) | 12 (92.3) | 10 (76.9) | 12 (92.3) |
| Tian et al., 2020 (29) | 96 (41.4) | 22 (9.5) | 55 (23.7) | 150 (64.7) | 63 (27.2) | 119 (51.3) |
| Yang et al., 2020 (30) | 67 (32.7) | 16 (7.8) | 22 (10.7) | 159 (77.6) | 39 (19.0) | 151 (73.7) |
| Kuderer et al., 2020 (16) | NR | NR | NR | NR | NR | NR |
| Lee et al., 2020 (31) | 247 (30.9) | 109 (13.6) | 131 (16.4) | NR | NR | NR |
NR = not reported.
Table 3.
Cancer types and cancer treatment of individuals included in the meta-analysis
| Study | Cancer type, No. (%) |
Cancer treatment, No. (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Lung cancer | Other solid cancer | Hematological cancer | Surgery | Radiotherapy | Chemotherapy | Targeted therapy | Immunotherapy | |
| Yu et al., 2020 (7) | 7 (58.3) | 5 (41.7) | 0 (0.0) | 0 (0.0) | 3 (25.0) | 3 (25.0) | 1 (8.3) | 2 (16.7) |
| Barlesi et al., 2020 (23) | 12 (8.8) | 107 (78.1) | 24 (17.5) | 0 (0.0) | 0 (0.0) | 48 (35.0) | 18 (13.1) | 12 (8.8) |
| Garassino et al., 2020 (10)a | 200 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 68 (34.0) | 28 (14.0) | 54 (27.0) |
| Dai et al., 2020 (14) | 22 (21.0) | 74 (70.5) | 9 (8.6) | 8 (7.6) | 13 (12.4) | 17 (16.2) | 4 (3.8) | 6 (5.7) |
| Martin-Moro et al., 2020 (24) | 0 (0.0) | 0 (0.0) | 34 (100) | NR | NR | NR | NR | NR |
| Liang et al., 2020 (6) | 5 (27.8) | 12 (66.7) | 1 (5.6) | 1 (5.6) | 0 (0.0) | 2 (11.1) | 2 (11.1) | 1 (5.6) |
| Mehta et al., 2020 (26) | 11 (5.0) | 153 (70.2) | 54 (24.8) | 0 (0.0) | 49 (22.5) | 42 (19.3) | 0 (0.0) | 5 (2.3) |
| Yang et al., 2020 (25) | 10 (19.2) | 42 (80.8) | 0 (0.0) | 2 (3.8) | 0 (0.0) | 6 (11.5) | 0 (0.0) | 0 (0.0) |
| Ma et al., 2020 (27) | 8 (21.6) | 29 (78.4) | 0 (0.0) | NR | NR | NR | NR | NR |
| Zhang et al., 2020 (15) | 7 (25.0) | 21 (75.0) | 0 (0.0) | 5 (17.9) | 4 (14.3) | 10 (35.7) | 3 (10.7) | 1 (3.6) |
| He et al., 2020 (28) | 0 (0.0) | 0 (0.0) | 13 (100.0) | 0 (0.0) | 0 (0.0) | 6 (46.2) | 1 (7.7) | 0 (0.0) |
| Tian et al., 2020 (29) | 23 (9.9) | 197 (84.9) | 12 (5.2) | 197 (84.9) | 214 (92.2) | 32 (13.8) | ||
| Yang et al., 2020 (30) | 24 (11.7) | 159 (77.6) | 22 (10.7) | 4 (2.0) | 9 (4.4) | 31 (15.1) | 12 (5.9) | 4 (2.0) |
| Kuderer et al., 2020 (16) | 654 (70.5) | 167 (18.0) | 32 (3.4) | 12 (1.3) | 160 (17.2) | 75 (8.1) | 38 (4.1) | |
| Lee et al., 2020 (31) | 584 (73.0) | 169 (21.1) | 29 (3.6) | 76 (9.5) | 281 (35.1) | 72 (9.0) | 44 (5.5) | |
This study included other types of thoracic cancer such as thymic carcinoma (n = 8), mesothelioma (n = 8), and carcinoid (n = 4). NR = not reported.
Table 4.
Cancer status and outcomes of individuals included in the meta-analysis
| Study | Cancer status | Severe event, No. (%) |
Fatality, No. (%) |
||
|---|---|---|---|---|---|
| Yes | No | Survivor | Nonsurvivor | ||
| Yu et al., 2020 (7) | Active | NR | NR | 9 (75) | 3 (25) |
| Barlesi et al., 2020 (23) | Mixeda | NR | NR | 117 (85.4) | 20 (14.6) |
| Garassino et al., 2020 (10) | Active | NR | NR | 125 (62.5) | 66 (33.0) |
| Dai et al., 2020 (14) | Mixed | 40 (38.1) | 65 (61.9) | 93 (88.6) | 12 (11.4) |
| Martin-Moro et al., 2020 (24) | Active | NR | NR | 23 (67.6) | 11 (32.4) |
| Liang et al., 2020 (6) | Mixed | 9 (50.0) | 9 (50.0) | NR | NR |
| Mehta et al., 2020 (26) | Mixed | NR | NR | 157 (72.0) | 61 (28.0) |
| Yang et al., 2020 (25) | Active | NR | NR | 41 (78.8) | 11 (21.2) |
| Ma et al., 2020 (27) | Active | NR | NR | 32 (86.5) | 5 (13.5) |
| Zhang et al., 2020 (15) | Mixed | 15 (53.6) | 13 (46.4) | 20 (71.4) | 8 (28.6) |
| He et al., 2020 (28) | Active | NR | NR | 5 (38.5) | 8 (61.5) |
| Tian et al., 2020 (29) | Active | NR | NR | 186 (80.2) | 46 (19.8) |
| Yang et al., 2020 (30) | Mixed | NR | NR | 165 (80.5) | 40 (19.5) |
| Kuderer et al., 2020 (16) | Mixed | 611 (65.8) | 317 (34.2) | 807 (87.0) | 121 (13.0) |
| Lee et al., 2020 (31) | Active | NR | NR | 574 (71.8) | 226 (28.2) |
Mixed status included patients with active and previous malignancies. NR = not reported.
The funnel plot (Supplementary Figure 1, available online) and Egger’s test (P = .17) showed no publication bias in the overall population. The quality of the cohort studies was assessed according to the NOS tool, which showed that 8 studies had high quality (score ranging between 7 and 9) (Supplementary Table 1, available online), whereas 2 studies were of moderate quality (score ranging between 5 and 6). The 5 case series studies were considered low quality.
Primary Analysis
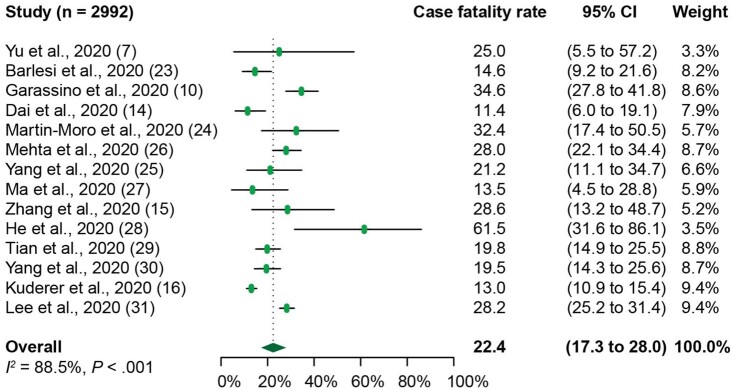
The meta-analysis of 14 of the 15 studies with reported death as outcome demonstrated the overall case fatality rate among COVID-19 patients with cancer was 22.4% (95% CI = 17.3% to 28.0%) (Figure 2). We next sought to compare the case fatality rate of COVID-19 patients with cancer with that of COVID-19 patients without cancer by using 3 studies where death was recorded in both of these populations (14,28,29). Our analysis revealed that the case fatality rate of COVID-19 patients with cancer was 23.4% (95% CI = 9.7% to 40.5%) (Supplementary Figure 2, A, available online), whereas that of COVID-19 patients without cancer was 5.9% (95% CI = 1.9% to 11.7%) (Supplementary Figure 2, B, available online). COVID-19 patients with cancer have a higher case fatality rate than COVID-19 patients without cancer (Supplementary Figure 2, A and B, available online) as well as higher than that of the general patient population with COVID-19 from recent published work (1,32,33).
Figure 2.
Forest plot of overall case fatality rate of patients with cancer and Coronavirus disease 2019 (COVID-19) from 14 individual studies analyzed. The Cochran’s Q test was used to identify heterogeneity and to calculate the P values. Heterogeneity was further quantified using I2 as described in the Methods. CI = confidence interval.
Of note, the case fatality rate in COVID-19 patients with cancer in Asia (China) is 20.0% (95% CI = 14.5% to 26.2%) (Supplementary Figure 2, C, available online), which is similar to that of the rest of the world (Europe, the United Kingdom, and the United States) at 24.1% (95% CI = 16.1% to 33.2%) (Supplementary Figure 2, D, available online). There was no difference observed in case fatality rate between Asia and the rest of the world. Statistically significant heterogeneity was demonstrated among the case fatality rates (Q = 113.5, P <.001, I2 = 88.5%). The case fatality rate of 61.5% (95% CI = 31.6% to 86.1%) in He et al. (28) was the highest among all the individual studies. Potential contributing factors identified through this cohort include hematological malignancy, severe COVID-19 disease classification, and coinfections.
The sensitivity analysis conducted by excluding 5 case series studies showed that the case fatality rate of COVID-19 patients with cancer was 21.6% (95% CI = 15.7% to 28.2%). Because 2 of the cohort studies had moderate quality based on the NOS scores (scoring ranging 5 to 6) (Supplementary Table 1, available online), we performed a further sensitivity analysis excluding these 2 studies. This showed that the case fatality rate of COVID-19 patients with cancer was 22.5% (95% CI = 16.2% to 29.4%). Neither of these sensitivity analyses demonstrated an obvious change in case fatality rate of COVID-19 patients with cancer. In addition, the sensitivity analysis performed by using the “leave-one-out” approach did not change our result (Supplementary Figure 3, available online).
Subgroup Analysis
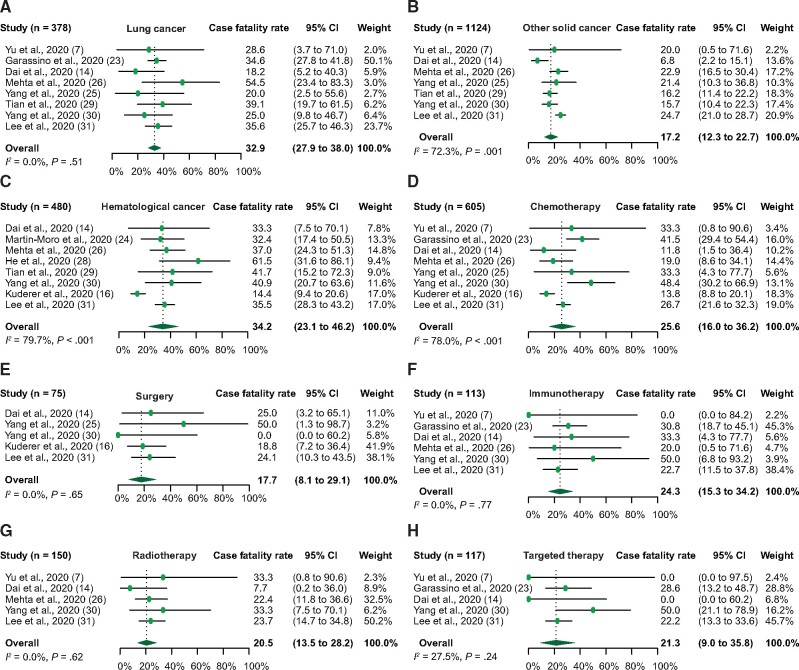
We performed a number of subgroup analyses on case fatality or severe event rate according to cancer type (lung, other solid, or hematological cancer) and treatment type (chemotherapy, immunotherapy, and radiotherapy). The overall case fatality rate in the lung cancer patients with COVID-19 was 32.9% (95% CI = 27.9% to 38.0%) (Figure 3, A), and the severe event rate was 57.2% (95% CI = 31.4% to 81.3%) (Supplementary Figure 2, E, available online). In other types of solid cancer excluding lung, the overall case fatality and severe event rates were 17.2% (95% CI = 12.3% to 22.7%) (Figure 3, B) and 59.7% (95% CI = 53.5% to 65.9%) (Supplementary Figure 2 , F, available online), respectively. In addition, the overall case fatality rate in hematological cancer patients was 34.2% (95% CI = 23.1% to 46.2%) (Figure 3, C). No statistically significant heterogeneity was demonstrated among the case fatality rates in lung cancer patients with COVID-19 (Q = 6.3, P = .51, I2 = 0.0%), whereas heterogeneity was observed in other types of solid cancer (Q = 21.6, P = .001, I2 = 72.3%) or hematological cancer patients (Q = 34.6, P < .001, I2 = 79.7%). It is worth noting that the case fatality rate in lung cancer is comparable with that of hematological cancer patients with COVID-19, whereas it is lower in other types of solid cancer. Furthermore, we examined the case fatality rate in the patients from individual studies, who underwent chemotherapy, surgery, immunotherapy, radiotherapy, and targeted therapy actively or within the past 3 months when diagnosed with COVID-19. In patients treated with chemotherapy, surgery, or immunotherapy, the case fatality rate was 25.6% (95% CI = 16.0% to 36.2%) (Figure 3, D), 17.7% (95% CI = 8.1% to 29.1%) (Figure 3, E), and 24.3% (95% CI = 15.3% to 34.2%) (Figure 3, F). The case fatality rate for patients under treatment of radiotherapy or targeted therapy was 20.5% (95% CI = 13.5% to 28.2%) (Figure 3, G) and 21.3% (95% CI = 9.0% to 35.8%) (Figure 3, H), respectively. In comparison, the case fatality rate was similar among patients who were given different types of treatment. Statistically significant heterogeneity was observed among the case fatality rates in patients treated with chemotherapy (Q=31.8, P < .001, I2 = 78.0%) whereas no substantial heterogeneity was demonstrated in patients receiving treatment with surgery (Q = 2.46, P = .65, I2 = 0.0%), immunotherapy (Q=2.52, P = .77, I2 = 0.0%), radiotherapy (Q=2.62, P = .62, I2 = 0.0%), or targeted therapy (Q=2.52, P = .24, I2 = 27.5%).
Figure 3.
Forest plot of overall case fatality rate in the subgroup analysis. Overall case fatality rate in different cancer types: (A) lung cancer, (B) other solid cancer, and (C) hematological cancer; and in different cancer treatment: (D) chemotherapy, (E) surgery, (F) immunotherapy, (G) radiotherapy, and (H) targeted therapy. The Cochran’s Q test was used to identify heterogeneity and to calculate the P values. Heterogeneity was further quantified using I2 as described in the Methods. CI = confidence interval.
Individual Patient Data Analysis
Next, we extracted the individual patient data of 150 patients from available studies (6,14,15) and analyzed the associations between demographic or clinical characteristics and outcomes (survivor or nonsurvivor, or severe events). Univariate logistic regression was used to estimate the odds ratios of each variable, including age, sex, comorbidities (hypertension, diabetes, or cardiovascular disease), or cancer type (hematological malignancy, lung, or other solid cancer), or treatment (chemotherapy, immunotherapy, targeted therapy, radiotherapy or surgery) with outcomes (Table 5). Factors, including age, sex, hypertension, and diabetes, that showed a statistically significant association with outcomes from univariate analysis were included in the multivariable analysis (Table 5).
Table 5.
Univariate and multivariable models of factors associated with outcomes
| Variables | Univariate logistic regression analysis |
Multivariable logistic regression analysis |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P a | Odds ratio (95% CI) | P a | |
| Age (>65 y) | 3.57 (1.80 to 7.06) | <.001 | 3.16 (1.45 to 6.88) | .004 |
| Sex (male) | 2.10 (1.07 to 4.13) | .03 | 2.29 (1.07 to 4.87) | .03 |
| Comorbidities (all) | 2.00 (1.04 to 3.85) | .04 | — | — |
| Hypertension vs no | 3.10 (1.38 to 6.99) | .006 | 1.37 (0.51 to 3.71) | .53 |
| Diabetes vs no | 4.16 (1.31 to 13.20) | .02 | 2.73 (0.76 to 9.81) | .13 |
| Cardiovascular disease vs no | 2.13 (0.74 to 6.14) | .16 | — | — |
| Hematological vs lung | 0.68 (0.14 to 3.28) | .63 | — | — |
| Other solid cancer vs lung | 0.80 (0.37 to 1.74) | .58 | — | — |
| Treatment vs no | 0.98 (0.51 to 1.90) | .95 | — | — |
| Chemotherapy vs no | 0.94 (0.40 to 2.20) | .89 | — | — |
| Immunotherapy vs no | 4.00 (0.77 to 20.90) | .10 | — | — |
| Target therapy vs no | 0.38 (0.08 to 1.94) | .25 | — | — |
| Radiotherapy vs no | 0.56 (0.18 to 1.71) | .31 | — | — |
| Surgery vs no | 1.00 (0.32 to 3.12) | 1.00 | — | — |
P values were calculated using the Wald χ22-sided test. CI = confidence interval.
Univariate analysis (Table 5) revealed that being older than 65 years (OR = 3.57, 95% CI = 1.80 to 7.06), being male (OR = 2.10, 95% CI = 1.07 to 4.13), the presence of any comorbidity (OR = 2.00, 95% CI = 1.04 to 3.85), single comorbidity hypertension (OR = 3.10, 95% CI = 1.38 to 6.99), and diabetes (OR = 4.16, 95% CI = 1.31 to 13.20) were associated with increased risk of severe events (a composite endpoint defined based on the individual being admitted to the intensive care unit, or requiring invasive ventilation, or death).
No association was found between cardiovascular disease and increased risk of severe events from COVID-19 compared with those with no comorbidity (Table 5). Similarly, there was no statistically significant difference in the increased risk of severe events comparing patients with lung cancer vs other solid cancer or hematological cancer, respectively (Table 5). In addition, cancer patients on chemotherapy or surgery, or other anticancer treatment, were not associated with increased risk of severe events from COVID-19 compared with those not on active treatment (including patients with active tumor or previous malignancy) (Table 5). Furthermore, in the subgroup of patients with active tumor only, our analysis demonstrated that treatment (including chemotherapy, immunotherapy, targeted therapy, radiotherapy, or surgery) was not associated with a higher risk of a poorer prognosis (Supplementary Table 2, available online). In multivariable analysis, only being older than 65 years (OR = 3.16, 95% CI = 1.45 to 6.88) and being male (OR = 2.29, 95% CI = 1.07 to 4.87) were associated with increased risk of severe events (Table 5).
In summary, the occurrence of severe events, including death, in cancer patients with COVID-19 appears to be primarily accentuated by age, sex, and coexisting comorbidities.
Discussion
The rapid spread of COVID-19 has a substantial impact on cancer patients. Our knowledge of the clinical features and relationship of COVID-19 with cancer and anticancer treatment needs to progress in real time to meet the unprecedented challenges facing the healthcare community and this exceptionally vulnerable population. A systemic review and meta-analysis of individual studies improves our understanding of the effects of COVID-19 on cancer patients and evaluates current evidence to inform clinicians and guide best practice.
To our knowledge, this is the first meta-analysis that systematically examined the largest aggregate of patients with cancer and COVID-19 to date, involving 3019 patients with a broad geographical distribution. These 15 individual cohorts are comprised of a diverse population in terms of age and sex distribution, cancer type, presence of comorbidities, and status of active anticancer treatment. The findings in this study suggest that overall case fatality rate was estimated at 22.4% in the COVID-19 patients with cancer, which is higher than that of the COVID-19 patients without cancer at 5.9% in this study as well as that of general patient population with COVID-19 from recent published work (1,32,33). Age and sex appear to be important risk factors associated with poorer prognosis.
One of the strengths of our meta-analysis is that we performed an extensive systematic review of 15 individual studies covering many geographical regions to reflect the global impact on cancer patients. The patients included in this meta-analysis are well-represented in ethnicity and geographical regions of residence, with nearly one-half from the United Kingdom and Europe (41.0%), followed by the United States and Canada (35.7%) and Asia (China, 23.3%). It is interesting to note that there is no difference in case fatality rate in cancer patients with COVID-19 between China and the rest of the world, highlighting a need for continuous data sharing and international collaboration on a global scale.
While recognizing the daunting fact that cancer patients have a higher death rate, an important question is which cancer types might be most vulnerable among all cases presented in these studies? Subgroup analysis on clinical outcomes in different cancer types indicated that case fatality rate is higher in lung or hematological cancer compared with other solid cancer. Similarly, some studies have also observed individuals with hematological or lung cancer had poorer prognoses than did those with solid tumors (14,30). Another interesting observation is that although the severe events rate is similar in lung cancer patients compared with that of patients with other solid cancer, the case fatality rate of lung cancer is higher than that of other solid cancer. This suggests that lung cancer patients with COVID-19 may experience increased difficulty recovering from this disease once it has progressed to a severe stage.
However, in our subsequent univariate analysis using available individual patient data, no statistically significant associations were found between cancer type and severe events rate, consistent with a recent report (16). Considering the heterogeneity between different tumor subtypes identified as solid tumor in the cohorts analyzed, cautious interpretation of these results is necessary. Future analysis with a larger cohort of individual patient data is worthwhile and might offer new insight in guiding clinical management in high-risk cancer type populations.
One of the most important issues to address is whether patients should be advised to delay any anticancer treatment. It is inconclusive whether patients who received active chemotherapy, targeted therapy, or immunotherapy or underwent surgery (defined as given within 4 weeks of COVID-19 diagnosis in many studies) had a higher risk of death or severe events. Several studies (6,14,30) from China indicated a positive association between treatment and increased deaths or severe events. All these studies are either relatively small cohorts or from a few hospitals restricted to a specific geographical region.
Based on our statistical analysis, we are not able to identify evidence supporting that cancer patients on cytotoxic chemotherapy are at an increased risk of severe events from COVID-19 disease compared with those not on active treatment. Despite the smaller sample size of these patients, similar findings were observed for surgery, immunotherapy, radiotherapy, and targeted therapy. This is consistent with recent reports from several larger national or international cohorts including the CCC19 (16), The TERAVOLT (10), and the UK Coronavirus Cancer Monitoring Project (31). Further evaluation of individual-level data from all 3019 patients in the included 15 cohort studies in this meta-analysis will allow us to draw a clearer conclusion on the relationship between anticancer treatment and its association with cancer and COVID-19. While awaiting further evidence, it might be beneficial to continue curative surgical resections, chemotherapy, and other cancer treatment based on a comprehensive evaluation of individual cases with multidisciplinary team management.
Another key question is what are the potential risk factors associated with COVID-19–related severe events in cancer patients? Of note, recent studies revealed that greater age, being male, or with comorbidities is associated with higher risk of death or severe events in the population of general COVID-19 patients (34-36). In line with the findings from these studies, our univariate analysis revealed being older than 65 years, being male, or the presence of any comorbidity or hypertension or diabetes was associated with an increased risk of severe events. However, in multivariable analysis, only being older than 65 years and being male were at a higher risk of worse outcomes, supporting that age and sex are important predictors associated with poorer prognosis. Based on these findings, we suggest that this high-risk cancer population should be evaluated and treated in a clinical setting equipped with comprehensive medical resources.
There are a number of limitations herein. First, many clinical characteristics were not available (such as tumor staging) from individual studies; thus, we were not able to examine the associations between some crucial factors and risk of death. Additionally, given the lack of information on some key characteristics that might affect the baseline prognosis of the patients, careful interpretation of results is needed. Second, there were heterogeneities in classifying the same variable in included studies, reducing the overall statistical power of the meta-analysis. For instance, 1 study combined chemotherapy and radiotherapy as 1 type of antitumor treatment (29), whereas most listed chemotherapy as a separate type; all studies reported fatality rate, but one described severe event rate (6). Finally, we were not able to access the original data from all of the individuals in the 15 cohorts analyzed. It was therefore not possible to answer some key questions with high confidence, including whether cancer origins or treatment types are important predictors associated with death.
In aggregate, our analysis confirmed that patients with cancer are at increased risk of fatality and severe illness due to COVID-19. The occurrence of severe events and death in cancer patients with COVID-19 appears to be primarily accentuated by age, sex, and coexisting comorbidities. Best practice based on evidence will mitigate the impact of the COVID-19 pandemic on cancer patients and reduce the risk of morbidity or death from COVID-19.
Funding
None.
Notes
Role of the funder: Not applicable.
Disclosures: JS reports his conflicts at https://www.nature.com/onc/editors, none of which are relevant here. No other authors report a conflict of interest.
Author contributions: HZ, HH and TH had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Concept and Design: HZ, HH and KL. Acquisition, Analysis, or Interpretation of Data: all authors. Statistical Analysis: TH, HH and AH. Drafting of the Manuscript: HZ, KL, JS and KW. Final version was edited and approved by all authors.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
Supplementary Material
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239. [DOI] [PubMed] [Google Scholar]
- 4. Dong E, Du H, Gardner L.. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. [DOI] [PubMed] [Google Scholar]
- 6. Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu J, Ouyang W, Chua MLK, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6(7):1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914-922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whisenant JG, Trama A, Torri V, et al. TERAVOLT: thoracic cancers international COVID-19 collaboration. Cancer Cell. 2020;37(6):742-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rubinstein SM, Steinharter JA, Warner J, et al. The COVID-19 and Cancer Consortium: a collaborative effort to understand the effects of COVID-19 on patients with cancer. Cancer Cell. 2020;37(6):738-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singer DS. NCI's work to advance cancer research while responding to the COVID-19 pandemic. Cancer Cell. 2020;37(6):746-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10(6):783-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31(7):894-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907-1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, et al. ; for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells GA, Sb O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. http://www.ohri.ca/Programs/clinical_epidemiology/oxford.asp. Accessed September 27, 2020.
- 19. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 20. Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97-111. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barlesi F, Foulon S, Bayle A, et al. Outcome of cancer patients infected with COVID-19, including toxicity of cancer treatments. Paper presented at: April 28, 2020. Virtual Annual Meeting of the American Association for Cancer Research.
- 24. Martin-Moro F, Marquet J, Piris M, et al. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol. 2020;190(1):e16-e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang F, Shi S, Zhu J, et al. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020;92(10):2067-2073. [DOI] [PubMed] [Google Scholar]
- 26. Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020;10(7):935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma J, Yin J, Qian Y, Wu Y.. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center's retrospective study. J Infect. 2020;81(2):318-356. doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34(6):1637-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):893-903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang K, Sheng Y, Huang C, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21(7):904-913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee LYW, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919-1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Onder G, Rezza G, Brusaferro S.. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775-1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 33. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20(6):669-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;e203596. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grasselli G, Greco M, Zanella A, et al. ; COVID-19 Lombardy ICU Network. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.