Abstract
Objectives
The aim of the current study is to determine if baseline serum adiponectin levels predict the development of rheumatoid arthritis (RA).
Methods
The current report includes 3693 individuals from the Swedish Obese Subjects (SOS). The original SOS study is a longitudinal non-randomized controlled study aiming to assess the effect of bariatric surgery on obesity-related mortality and morbidity. Participants included in the present report had adiponectin measurement available at baseline and no prevalent RA. The diagnosis of RA was retrieved through the Swedish National Patient Register.
Results
During a follow-up for up to 29 years, 82 study participants developed RA. Elevated baseline adiponectin levels were associated with a higher risk of developing RA independently of other factors, including C-reactive protein (CRP) and smoking (hazard ratio, HR 1.70, 95% confidence interval, CI 1.12–2.60 for an increase in adiponectin of 10 mg/L, P=0.01). After stratifying the population according to adiponectin and CRP median at baseline, study participants with both adiponectin and CRP above the median had a higher risk of developing RA compared to subjects with adiponectin and CRP below the median (HR 2.80, 95% CI 1.25–6.31, P=0.01).
Conclusions
In this cohort of subjects with obesity followed up for up to 29 years, high serum adiponectin levels at baseline were associated with an increased risk for RA. Moreover, subjects with both high adiponectin and CRP levels at baseline were at particular risk of developing RA.
ClinicalTrials.gov Identifier: NCT01479452.
Adiponectin is a relatively abundant adipokine in plasma, with concentrations ranging from 2 to 30 mg/L and accounting for up to 0.05% of total plasma proteins (1, 2). Although mostly produced by the adipose tissue, adiponectin levels are inversely correlated with fat mass and are low in subjects with obesity (3, 4). Low serum adiponectin associates with an unhealthy metabolic profile, including a higher risk for type 2 diabetes and insulin resistance (5–7). It has been hypothesized that the low adiponectin levels observed in obesity and its comorbidities are a consequence of the chronic low-grade inflammation that affects the adipose tissue under those circumstances (8, 9). On the other hand, recent studies show that adiponectin levels are elevated in inflammatory and autoimmune diseases (10–12). Indeed, in patients with established rheumatoid arthritis (RA), adiponectin is increased in both serum and synovia, and also subjects with early treatment-naïve RA have higher circulating adiponectin levels than controls. Moreover, plasma adiponectin associates with pro-inflammatory cytokines in a population without RA but at high risk to develop the disease (13), suggesting that adiponectin may play a role in the early phases of disease development (14–17). This hypothesis finds support in earlier studies showing that adiponectin stimulates the production of pro-inflammatory factors by different cell-lines, including fibroblast-like synoviocytes and macrophages, (18–20) although so far adiponectin has been mostly known to induce anti-inflammatory responses (21–23).
The Swedish Obese Subjects (SOS) study is a longitudinal non-randomized controlled study designed to examine the effect of bariatric surgery on obesity-associated morbidity and overall mortality (24–26). In this cohort of subjects with obesity, we have recently analysed the effect of bariatric surgery on the future development of RA, since obesity is thought to be a risk factor for the development of the disease, in particular if combined with other factors (27, 28). However, we were not able to detect any association between bariatric surgery and the incidence of RA (29). Serum adiponectin levels have been measured in SOS study participants at baseline and at 2-and 10-year follow-up, and this has allowed us to study the association between circulating adiponectin and the development of RA in subjects with obesity. Thus, the aim of the current report was to determine if elevated serum adiponectin levels predict the development of RA in those subjects.
Method
Design of the current report
To study the association between circulating adiponectin levels and the future risk of developing RA we selected a subgroup of participants from the SOS study, a clinical trial on bariatric surgery enrolling 4047 subjects with obesity from Sweden. Eleven subjects had prevalent RA at baseline and were therefore excluded. The subjects included in the current report are 3693 SOS participants who did not have a diagnosis of RA and that had available adiponectin measurement at baseline.
SOS study design
The SOS is a longitudinal, non-randomized, controlled study that enrolled 4047 subjects with obesity at 25 surgical departments and 480 primary health care centres in Sweden between 1987 and 2001 (24, 25). Inclusion criteria were age 37–60 years and body-mass index (BMI) ≥34 for men and ≥38 for women. Exclusion criteria were previous surgery for gastric or duodenal ulcer, earlier bariatric surgery, gastric ulcer during the previous 6 months, ongoing or active malignancy during the past 5 years, myocardial infarction during the past 6 months, bulimic eating pattern, drug or alcohol abuse, psychiatric or cooperative problems contraindicating bariatric surgery or other uncommon conditions (25).
Study participants were recruited through a campaign and 6905 subjects who showed interest in the study completed a matching examination. Among these, 2010 eligible individuals who electively chose to undergo bariatric surgery constituted the surgery group. A matched control group of 2037 subjects was created based on 18 different variables from the matching examination (sex, age, weight, height, hip circumference, waist circumference, systolic blood pressure, triglycerides, total cholesterol, postmenopausal status, daily smoking, diabetes, four psychosocial variables associated with death risk and two personality traits related to treatment preferences). To perform the matching, the method of sequential assignment treatment was used, as previously described (30). The surgery patient and the matched conventionally treated patient started the study on the same day, i.e. the day of the surgery. As previously reported, the matching process unexpectedly created a surgery group having a higher mean body weight, a younger age and more severe risk factors than the control group (24).
Subjects from the bariatric surgery group underwent vertical banded gastroplasty (68%), gastric banding (19%), or gastric bypass (13%). The control group received conventional non-surgical treatment for obesity at their centres of registration, which ranged from intensive lifestyle modifications to no treatment, with no attempt to standardize the non-surgical treatment (31).
The study protocol was approved by seven Swedish local ethics review boards and all the participants gave their written or oral informed consent to join the study. The study protocol was approved by seven Swedish local ethics review boards and all the participants gave their written or oral informed consent to join the study. The SOS trial is registered at ClinicalTrials.gov Identifier: NCT01479452.
Clinical and biochemical assessments
Centralized laboratory measurements were performed at matching, at baseline, and at 2-, 10-, 15-and 20-year follow-up at the Central Laboratory of Sahlgrenska University Hospital. Erythrocyte sedimentation rate (ESR) was measured at the participants’ health care centres at the time of health examination visits. Measurements of serum concentrations of adiponectin at baseline and at the 2-and 10-year follow-up were consecutively performed at the German Diabetes Center, Duesseldorf, Germany from November 2010 to April 2011 (6). Total adiponectin was measured using the Quantikine enzyme-linked immunosorbent assay (ELISA) kit from Bio-Techne, Minneapolis, MN, USA (previously R&D Systems, Wiesbaden, Germany). Mean intra-assay and inter-assay coefficients of variation were 3 and 13%, respectively. All samples gave values above the limit of detection (3.9 ng/mL). C-reactive protein (CRP) levels at baseline, and at the 2-and 10-years follow-up were measured with an ultrasensitive immunoturbidimetric method (Sentinel, Milan, Italy) using the Architect c8200 analyzer (Abbott Laboratories, Abbott Park, IL) in Helsinki, Finland, between October 2010 and April 2011. Sufficient amount of serum samples for the measurement of adiponectin and CRP were available for 3693 participants at baseline, 3242 at the 2-year follow-up, and 2605 at the 10-year follow-up, when excluding subjects with prevalent RA at baseline.
BMI was calculated as weight in kilograms divided by the square of the height in meters. Type 2 diabetes was defined as fasting blood glucose ≥110 mg/dl and/or self-reported therapy with glucose-lowering medications.
Diagnoses of RA
The incidence of RA was not among the predefined endpoints of the SOS study (24). RA diagnoses were retrieved through the Swedish National Patient Register by searching for the following International Classification of Diseases (ICD) codes: 712.38 and 712.39 (ICD-8), 714.0–2 (ICD-9) and M05, M06.0, M06.8 and M06.9 (ICD-10) (29). Medical records from inpatient and outpatient visits in Sweden are recorded in the National Patient Register. Recording of inpatient visits started in 1964 but did not reach national coverage until 1987, whereas the non-primary outpatient register, including visits to hospital-based medical specialists, became nationwide in 2001. Participants were followed up from inclusion until diagnosis of RA, death, migration or end of follow-up (31st December 2016), whichever came first. Information on death or migration was obtained from the Cause of Death Register and the Register of the Total Population (32). The median follow-up time for the current report was 21 years, ranging from 0 to 29 years (75000 person-years of follow-up). Median follow-up time to the diagnosis of RA was 14 years, ranging from 1 to 27 years.
Statistical analysis
Baseline characteristics of the study population are shown as mean ± standard deviation. Continuous variables have been compared by linear regression model test. Categorical variables were compared by χ-square tests. Time to incident RA after inclusion was assessed by Kaplan-Meier estimates of cumulative incidence rates. Comparisons between groups were performed by log-rank test. Hazard ratios (HR) and corresponding 95% CIs for the risk of RA were calculated with Cox proportional hazard models after adjustment for preselected risk factors. Proportional hazard assumptions were satisfied for all the variables in the model. Spearman test was used to assess the correlation between serum adiponectin and CRP. Changes in adiponectin and CRP at the 2-and the 10-year follow-up were calculated as [(value at follow-up – baseline value)/baseline value] x100. Changes are shown as mean and 95% confidence intervals (CI).
All P values are two-sided and P-values <0.05 were considered significant. Statistical analyses were performed with the Statistical Package for Social Science (version 24.0; SPSS, Chicago, IL).
RESULTS
Baseline characteristics
Baseline characteristics of the SOS study cohort have been described elsewhere (24, 26). This report included 3693 participants from the SOS study with available serum adiponectin measurement at baseline, all of them without prevalent RA. Mean serum adiponectin in the cohort was 7.9±4.6 mg/L (Table 1). As expected, serum adiponectin levels were higher in women, smokers, and study participants who were older, had lower BMI and no type 2 diabetes. The control group had higher serum adiponectin, which reflects the fact that participants from the control group had a more beneficial metabolic profile at baseline including lower BMI compared to the surgery group, as previously shown (24). Study participants with lower baseline CRP and higher ESR had higher levels of adiponectin. Subjects who developed RA during follow-up had higher baseline serum adiponectin levels (Table 1). In Supplementary Table S1, we show the baseline characteristics of the study population stratified by incident RA during follow-up; both baseline adiponectin and CRP levels were higher in subjects that would develop RA.
Table 1.
Serum adiponectin levels stratified by baseline characteristics.
| Adiponectin, mg/L | P value† | |
|---|---|---|
| SOS cohort (no.=3693) | 7.9±4.6 | - |
| Men (no.=1103) | 6.0±3.3 | <0.001 |
| Women (no.=2590) | 8.6±4.8 | |
| Age ≤ median (no.=1844) | 7.0±3.8 | <0.001 |
| Age > median (no.=1849) | 8.7±5.0 | |
| BMI ≤ median (no.=1849) | 7.9±4.7 | 0.01 |
| BMI > median (no.=1844) | 7.8±4.4 | |
| Surgery (no.=1852) | 7.5±4.2 | 0.001 |
| Control (no.=1841) | 8.2±4.8 | |
| Diabetes*, yes (no.=564) | 6.2±3.9 | <0.001 |
| Diabetes, no (no.=3119) | 8.2±4.6 | |
| Incident RA (no.=82) | 9.0±4.8 | 0.03 |
| No incident RA (no.=3611) | 7.8±4.5 | |
| CRP ≤ median (no.=1852) | 8.2±4.8 | <0.001 |
| CRP > median (no.=1841) | 7.5±4.2 | |
| ESR** ≤ median (no.=1820) | 7.7±4.5 | 0.01 |
| ESR > median (no.=1796) | 8.0±4.6 | |
| Current or former smoking, yes (no.=2369) | 7.5±4.1 | <0.001 |
| Current or former smoking, no (no.=1324) | 8.6±5.1 |
Median age was 48 years, median BMI was 41, median CRP was 5 mg/L, and median ESR was 12 mm/h.
among SOS study participants included in the present report, 10 were missing information about type 2 diabetes at baseline and
77 subjects did not have ESR measured ad baseline.
P values are adjusted for age, sex and BMI.
BMI, body-mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate.
Potential predictors of RA - multivariable analysis
Out of 3693 subjects included in the current report, 82 developed RA during a follow-up for up to 29 years. In a multivariable analysis including risk factors for RA, baseline adiponectin levels were associated with a higher risk of developing RA in the SOS cohort independently of other factors (HR 1.70, 95% CI 1.12–2.60, P=0.01, Table 2). As recently shown (29), bariatric surgery was not associated with the incidence of the RA in the SOS study, whereas serum CRP levels and current or former smoking were associated with an increased risk for the disease (Table 2). Neither sex, ESR, nor BMI were risk factors for RA in this cohort of subjects with obesity. Unadjusted HR for the incidence of RA are shown in Supplementary table S2.
Table 2.
Potential predictors of RA - multivariable analysis.
| HR (95% CI) | P Value | |
|---|---|---|
| Adiponectin, per 10 mg/L | 1.70 (1.12–2.60) | 0.01 |
| Surgery vs control group | 0.95 (0.60–1.50) | 0.82 |
| Men vs women | 0.75 (0.42–1.34) | 0.33 |
| Age, per 10 years | 0.97 (0.67–1.41) | 0.87 |
| BMI, per 10 kg/m2 | 1.09 (0.68–1.77) | 0.72 |
| ESR, per 10 mm/h | 1.04 (0.85–1.27) | 0.71 |
| CRP, per 10 mg/L | 1.30 (1.09–1.56) | 0.005 |
| Current or former smoking, yes vs no | 1.71 (1.04–2.82) | 0.04 |
The adjusted hazard ratios (HR) were calculated using a Cox proportional hazards models based on baseline data.
BMI, body-mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; CI, confidence interval.
Incidence of RA stratified by adiponectin and CRP levels at baseline
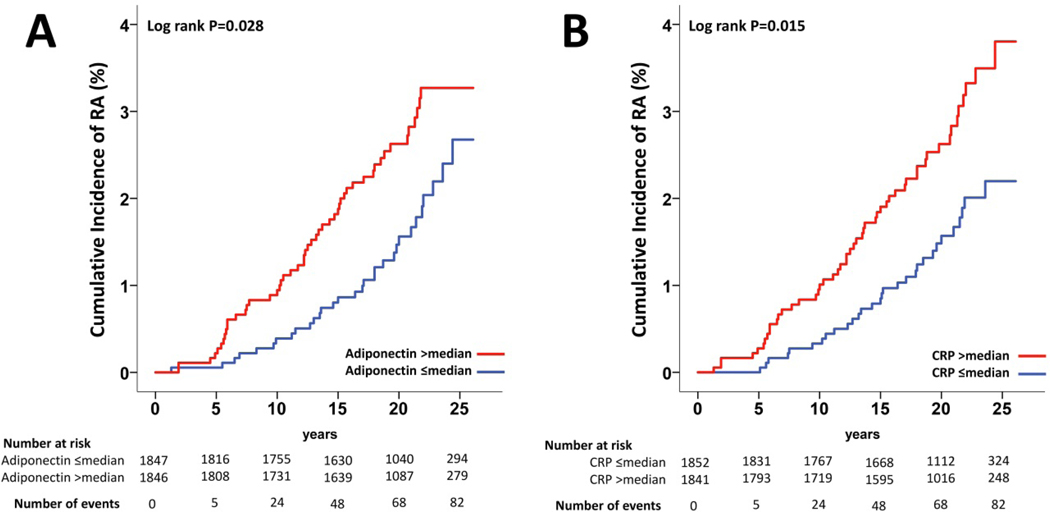
To confirm the association between adiponectin and CRP and the incidence of RA we stratified the population according to the median of adiponectin and CRP serum levels at baseline (6.8 mg/L and 5.1 mg/L, respectively). In the subgroup with adiponectin below or equal to the median (no.=1847), 31 subjects (1.7%) developed RA whereas in the subgroup with adiponectin above the median (no.=1846), 51 subjects (2.8%) developed RA during follow-up. Baseline adiponectin levels were associated with the incidence of RA during follow-up (log-rank P=0.028, Figure 1A). In the subgroup with CRP below or equal to the median (no.=1852), 31 subjects (1.7%) developed RA whereas in the subgroup with CRP above the median (no.=1841), 51 subjects (2.8%) developed RA during follow-up. Baseline CRP levels were also associated with the incidence of RA during follow-up (log-rank P=0.015, Figure 1B).
Figure 1.
(A) Cumulative incidence of RA in the SOS study stratified by median of baseline serum adiponectin and (B) C-reactive protein CR.
Median serum adiponectin and CRP at baseline were 6.8 mg/L and 5.1 mg/L respectively.
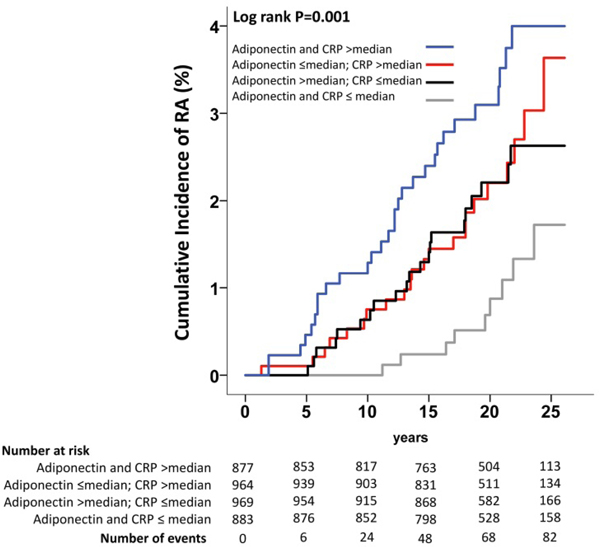
The interaction between baseline levels of adiponectin and CRP in modulating the incidence of RA was not significant (P=0.34). The correlation between serum adiponectin and CRP was weakly negative, although significant (r=−0.08; P<0.001). This means that the subgroup having baseline adiponectin levels above the median only partially overlapped with the subgroup having CRP below the median (Supplementary table S3). We therefore stratified the SOS cohort in four groups, depending on baseline adiponectin and CRP serum levels (i.e. both adiponectin and CRP above the median; adiponectin below the median and CRP above the median; adiponectin above the median and CRP below the median; both adiponectin and CRP below the median). Higher baseline levels of adiponectin and CRP were positively associated with the incidence of RA during follow-up (log-rank P=0.001, Figure 2). Subjects with both baseline adiponectin and CRP above the median had a greater risk of developing RA compared to subjects with both adiponectin and CRP below or equal to the median (adjusted HR 2.80, 95% CI 1.25–6.31, P=0.01, Table 3, Model 2).
Figure 2.
Cumulative incidence of RA in the SOS study stratified by baseline serum adiponectin and CRP levels.
Median serum adiponectin and C-reactive protein (CRP) at baseline were 6.8 mg/L and 5.1 mg/L respectively.
Table 3.
Hazard ratios for RA according to adiponectin and CRP levels
| Unadjusted HR (95% CI) | Model 1* HR (95% CI) | Model 2** HR (95% CI) | |
|---|---|---|---|
| Adiponectin and CRP ≤median | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Adiponectin >median-CRP ≤median | 2.26 (1.04–4.91) | 2.19 (0.99–4.88) | 2.03 (0.91–4.55) |
| Adiponectin ≤median-CRP >median | 2.38 (1.10–5.17) | 2.25 (1.01–5.02) | 2.15 (0.95–4.87) |
| Adiponectin and CRP >median | 3.39 (1.60–7.16) | 3.20 (1.44–7.08) | 2.80 (1.25–6.31) |
In Model 1, HR are adjusted for age, sex, and BMI
In Model 2, HR are adjusted for age, sex, BMI, treatment (surgery yes/no), current or former smoking, ESR, diabetes and year of inclusion in the study.
CRP, C-reactive protein; HR, hazard ratio; CI, confidence interval; BMI, body-mass index.
Changes in adiponectin and CRP levels during follow-up and the incidence of RA
As previously reported (6), adiponectin levels increased after bariatric surgery, whereas they remained stable in the control group, both at the 2-(increase 73%, 95% CI 69 to 77 vs. increase 1%, 95% −1 to 3 respectively, P<0.001) and the 10-year follow-up (63%, 95% CI 59 to 68 vs. 12%, 95% 8 to 15 respectively, P<0.001; Supplementary figure S1A). During follow-up, CRP levels decreased in the surgery group by −41% (95% CI −49 to −33) at the 2-year follow-up, whereas they increased in the control group by 34% (95% CI 27 to 42, P<0.001; Supplementary figure S1B). A similar trend was observed at the 10-year follow-up, with the surgery group having a decrease in CRP levels (−19%, 95% CI −27 to −12) and the control group having an increase in CRP (56%, 95% CI 42 to 71, P<0.001. Supplementary figure S1B) compared with baseline.
Since adiponectin and CRP levels at the 2-year follow-up are highly affected by bariatric surgery, we tested if the 2-year changes in adiponectin and CRP associated with the incidence of RA. As shown in Supplementary Table S4, changes in adiponectin after baseline were not associated with the incidence of RA in neither the control nor the surgery group. In the surgery group, 2-year changes in CRP was associated with a marginally higher risk for RA (HR for 10% increase in CRP 1.01, 95% CI 1.00–1.02, P=0.04).
Discussion
This exploratory study shows that high serum adiponectin levels at baseline were associated with an increased risk for RA in a cohort of subjects with obesity followed up for up to 29 years. This association was independent of CRP, whose levels were also associated with the incidence of RA as previously shown (33–35). Subjects having both baseline adiponectin and CRP levels above the median had an almost 3-times increased risk of developing RA during follow-up.
In subjects with preclinical RA, auto-antibodies, chemokines, and cytokines are elevated in blood up to several years before the onset of the disease suggesting an early activation of the immune system (33, 36). CRP levels are also increased years before the diagnosis of RA (29, 33–35). CRP and adiponectin levels showed poor correlation, suggesting different mechanisms behind the regulation of those proteins (37). SOS study participants with both serum adiponectin and CRP above the median had an almost 3-times increased risk of developing RA compared to subjects with low adiponectin and CRP and this association was independent of known risk factors for RA, including smoking.
When first discovered in the 1990’s, adiponectin raised interest because its circulating levels are low in subjects with obesity, although this adipokine is almost only produced by the adipose tissue. Moreover, serum adiponectin is also low in subjects with metabolic syndrome and type 2 diabetes whereas high circulating adiponectin levels associate with a favourable metabolic and cardiovascular profile (3–5, 7). In vitro and in vivo studies have also shown that adiponectin has anti-inflammatory properties by inhibiting the production of pro-inflammatory cytokines and chemokines, as well as anti-atherogenic effects (38–40). However, most Mendelian randomization studies have failed to detect a causal connection between adiponectin and metabolic traits or cardiovascular disease (41–43). Recent studies have shown that circulating adiponectin is elevated in subjects with autoimmune and inflammatory diseases, including RA (10–12, 14, 15, 17, 18). A possible explanation of this paradox is that adiponectin is downregulated in the case of low-grade inflammation -as for metabolic syndrome-through an adipocyte-mediated suppressive mechanism, and upregulated in conditions of overt systemic inflammation, as for RA (12). The mechanisms of this upregulation are still unknown; possibly adiponectin levels are increased in systemic inflammatory conditions as an ineffective attempt to counter-regulate the ongoing inflammation (44).
On the other hand, our results showing that serum adiponectin levels associate with the future risk of developing RA may suggest that adiponectin could be actively implicated in the pro-inflammatory processes leading to RA. This hypothesis, which cannot be confirmed or rejected due to the limitations of the current study, is in line with previous reports showing that adiponectin is also able to stimulate pro-inflammatory response in different cell lines involved in RA pathogenesis such as lymphocytes, osteoblasts, chondrocytes and fibroblasts-like synoviocytes (20, 45–49). Moreover, circulating adiponectin levels associate with pro-inflammatory cytokines in a population at high risk of developing RA (13). A possible explanation for the pro-and anti-inflammatory effects of adiponectin may be that different forms of adiponectin play different roles in modulating the inflammatory response. In fact, adiponectin has been shown to circulate in blood in three major complexes, i.e. as a trimer (low-molecular weight), a hexamer (medium-molecular weight), or a high-molecular weight multimer (50). Adiponectin forms may exert different actions in different tissues. It has been hypothesized that high-molecular weight adiponectin is the most relevant biomarker of metabolism whereas low-molecular weight adiponectin plays a role in inflammation (51). This hypothesis cannot be tested in the current study since only total adiponectin measurements are available in the SOS trial.
Smoking is a well-known risk factor for the development of RA (28). In our cohort of subjects with obesity, smoking was associated with the risk of developing RA together with adiponectin and CRP levels. Interestingly, as also previously shown (52), smokers had lower baseline levels of adiponectin compared to non-smokers, and, yet, both smokers and subjects with high adiponectin levels had an increased risk for RA. This might suggest that the increase in adiponectin levels preceding the development of RA is independent of the pro-inflammatory mechanisms associated with smoking habit.
As previously shown, bariatric surgery was associated with a significant increase in adiponectin levels in our cohort of subjects with obesity (6). Moreover, CRP levels were reduced in subjects who underwent bariatric surgery. Our current results show that changes in adiponectin and CRP levels did not significantly affect the incidence of RA in our cohort in neither the surgery nor the control group. However, it is important to point out that the regulation of the immune system in inflammatory diseases is highly complex, and this cannot be reflected by the assessment of a few serum parameters, that may not necessarily have a causal effect on the pathogenesis of the disease.
As stated before (29), one of the main limitations of this report is that RA was not among the predefined outcomes of the SOS study and that the SOS study was not designed to investigate the association between adiponectin and RA. Hence it is not surprising that the number of subjects who developed RA is low. Moreover, our study is performed in a cohort of subjects with obesity, thus making it difficult to generalize our findings to the general population. Other studies are warranted to confirm the association between serum adiponectin and future development of RA at a general population level. Another limitation of the study, as previously acknowledged (29), is that the diagnosis of RA was retrieved only by screening of the ICD codes in the Swedish National Patient Register and we may have therefore missed some RA diagnoses.
This exploratory study suggests that high serum adiponectin predicts the development of RA independently of other risk factors in a cohort of subjects with obesity followed up for up to 29 years. Further studies are necessary to clarify the relevance of this adipokine in the early development of RA.
Supplementary Material
Supplementary table S1. Baseline characteristics of study participants included in the present report stratified by RA diagnosis during follow-up.
Supplementary table S2. Unadjusted HR for the incidence of RA.
Supplementary table S3. Stratification of the study population according to adiponectin and CRP levels.
Supplementary table S4. Association between 2-year changes in adiponectin or CRP and the incidence of RA
Supplementary Figure S1. Changes in adiponectin and CRP in the control and surgery group during follow-up.
Acknowledgments
We thank the staff members at 480 primary health care centres and 25 surgical departments in Sweden who participated in the study. Christina Torefalk is acknowledged for valuable administrative support.
This work was supported by the Knut and Alice Wallenberg Foundation and the Wallenberg Centre for Molecular and Translational Medicine at the University of Gothenburg, Sweden. It has also been supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (grant number R01DK105948), the Swedish Research Council (grant number 2017–01707), the Sahlgrenska University Hospital Regional Agreement on Medical Education and Research research grants, and the Ministry of Culture and Science of the State of North Rhine-Westphalia and the German Federal Ministry of Health. This study was also partially supported by a grant from the German Federal Ministry of Education and Research to the German Center for Diabetes Research (DZD).
Disclosure statement
YZ, MP, JAA, PAS, CH, AR and CM declare that they do not have any competing financial interests that may be relevant to the submitted work. LC reports receiving lecture fees from AstraZeneca and Johnson & Johnson. This study was presented at the 2018 American College of Rheumatology (ACR) Annual Meeting (abstract number 1907) and at the 2019 European League Against Rheumatism (EULAR) congress (abstract number THU0088).
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem 1996;120:803–12. [DOI] [PubMed] [Google Scholar]
- 2.Yanai H, Yoshida H. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. Int J Mol Sci 2019;20. pii: E1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 1996;271:10697–703. [DOI] [PubMed] [Google Scholar]
- 4.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 1999;257:79–83. [DOI] [PubMed] [Google Scholar]
- 5.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 2000;20:1595–9. [DOI] [PubMed] [Google Scholar]
- 6.Herder C, Peltonen M, Svensson PA, Carstensen M, Jacobson P, Roden M, et al. Adiponectin and bariatric surgery: associations with diabetes and cardiovascular disease in the Swedish Obese Subjects Study. Diabetes Care 2014;37:1401–9. [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 2001;86:1930–5. [DOI] [PubMed] [Google Scholar]
- 8.Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, et al. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun 2003;301:1045–50. [DOI] [PubMed] [Google Scholar]
- 9.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 2010;314:1–16. [DOI] [PubMed] [Google Scholar]
- 10.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis 2006;12:100–5. [DOI] [PubMed] [Google Scholar]
- 11.Cantarini L, Brucato A, Simonini G, Imazio M, Cumetti D, Cimaz R, et al. Leptin, adiponectin, resistin, visfatin serum levels and idiopathic recurrent pericarditis: biomarkers of disease activity? A preliminary report. Clin Exp Rheumatol 2013;31:207–12. [PubMed] [Google Scholar]
- 12.Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine 2013;64:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes-Austin JM, Deane KD, Giles JT, Derber LA, Zerbe GO, Dabelea DM, et al. Plasma adiponectin levels are associated with circulating inflammatory cytokines in autoantibody positive first-degree relatives of rheumatoid arthritis patients. PLoS One 2018;13:e0199578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurberg TB, Frystyk J, Ellingsen T, Hansen IT, Jorgensen A, Tarp U, et al. Plasma adiponectin in patients with active, early, and chronic rheumatoid arthritis who are steroid-and disease-modifying antirheumatic drug-naive compared with patients with osteoarthritis and controls. J Rheumatol 2009;36:1885–91. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Gay MA, Llorca J, Garcia-Unzueta MT, Gonzalez-Juanatey C, De Matias JM, Martin J, et al. High-grade inflammation, circulating adiponectin concentrations and cardiovascular risk factors in severe rheumatoid arthritis. Clin Exp Rheumatol 2008;26:596–603. [PubMed] [Google Scholar]
- 16.Del Prete A, Salvi V, Sozzani S. Adipokines as potential biomarkers in rheumatoid arthritis. Mediators Inflamm 2014;2014:425068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YH, Bae SC. Circulating adiponectin and visfatin levels in rheumatoid arthritis and their correlation with disease activity: A meta-analysis. Int J Rheum Dis 2018;21:664–72. [DOI] [PubMed] [Google Scholar]
- 18.Choi HM, Lee YA, Lee SH, Hong SJ, Hahm DH, Choi SY, et al. Adiponectin may contribute to synovitis and joint destruction in rheumatoid arthritis by stimulating vascular endothelial growth factor, matrix metalloproteinase-1, and matrix metalloproteinase-13 expression in fibroblast-like synoviocytes more than proinflammatory mediators. Arthritis Res Ther 2009;11:R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu R, Zhao P, Zhang Q, Che N, Xu L, Qian J, et al. Adiponectin promotes fibroblast-like synoviocytes producing IL-6 to enhance T follicular helper cells response in rheumatoid arthritis. Clin Exp Rheumatol 2020;38:11–18. [PubMed] [Google Scholar]
- 20.Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, et al. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun 2005;335:1254–63. [DOI] [PubMed] [Google Scholar]
- 21.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 1999;100:2473–6. [DOI] [PubMed] [Google Scholar]
- 22.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 2000;102:1296–301. [DOI] [PubMed] [Google Scholar]
- 23.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun 2004;323:630–5. [DOI] [PubMed] [Google Scholar]
- 24.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52. [DOI] [PubMed] [Google Scholar]
- 25.Sjöström L, Larsson B, Backman L, Bengtsson C, Bouchard C, Dahlgren S, et al. Swedish obese subjects (SOS). Recruitment for an intervention study and a selected description of the obese state. Int J Obes Relat Metab Disord 1992;16:465–79. [PubMed] [Google Scholar]
- 26.Carlsson LM, Peltonen M, Ahlin S, Anveden Å, Bouchard C, Carlsson B, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 2012;367:695–704. [DOI] [PubMed] [Google Scholar]
- 27.Tedeschi SK, Cui J, Arkema EV, Robinson WH, Sokolove J, Lingampalli N, et al. Elevated BMI and antibodies to citrullinated proteins interact to increase rheumatoid arthritis risk and shorten time to diagnosis: A nested case-control study of women in the Nurses’ Health Studies. Semin Arthritis Rheum 2017;46:692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Hair MJ, Landewe RB, van de Sande MG, van Schaardenburg D, van Baarsen LG, Gerlag DM, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis 2013;72:1654–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maglio C, Zhang Y, Peltonen M, Andersson-Assarsson J, Svensson PA, Herder C, et al. Bariatric surgery and the incidence of rheumatoid arthritis - a Swedish Obese Subjects study. Rheumatology (Oxford) 2019;59:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–15. [PubMed] [Google Scholar]
- 31.Zentenius E, Andersson-Assarsson JC, Carlsson LMS, Svensson PA, Larsson I. Self-Reported Weight-Loss Methods and Weight Change: Ten-Year Analysis in the Swedish Obese Subjects Study Control Group. Obesity (Silver Spring) 2018;26:1137–43. [DOI] [PubMed] [Google Scholar]
- 32.Ludvigsson JF, Almqvist C, Bonamy AE, Ljung R, Michaëlsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol 2016;31:125–36. [DOI] [PubMed] [Google Scholar]
- 33.Deane KD, O’Donnell CI, Hueber W, Majka DS, Lazar AA, Derber LA, et al. The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age-dependent manner. Arthritis Rheum 2010;62:3161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masi AT, Rehman AA, Elmore KB, Aldag JC. Serum acute phase protein and inflammatory cytokine network correlations: comparison of a pre-rheumatoid arthritis and non-rheumatoid arthritis community cohort. J Innate Immun 2013;5:100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielen MM, van Schaardenburg D, Reesink HW, Twisk JW, van de Stadt RJ, van der Horst-Bruinsma IE, et al. Increased levels of C-reactive protein in serum from blood donors before the onset of rheumatoid arthritis. Arthritis Rheum 2004;50:2423–7. [DOI] [PubMed] [Google Scholar]
- 36.Johansson L, Pratesi F, Brink M, Arlestig L, D’Amato C, Bartaloni D, et al. Antibodies directed against endogenous and exogenous citrullinated antigens pre-date the onset of rheumatoid arthritis. Arthritis Res Ther 2016;18:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herder C, Hauner H, Haastert B, Rohrig K, Koenig W, Kolb H, et al. Hypoadiponectinemia and proinflammatory state: two sides of the same coin?: results from the Cooperative Health Research in the Region of Augsburg Survey 4 (KORA S4). Diabetes Care 2006;29:1626–31. [DOI] [PubMed] [Google Scholar]
- 38.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol 2005;288:R1220–5. [DOI] [PubMed] [Google Scholar]
- 39.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 2004;316:924–9. [DOI] [PubMed] [Google Scholar]
- 40.Villarreal-Molina MT, Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie 2012;94:2143–9. [DOI] [PubMed] [Google Scholar]
- 41.Yaghootkar H, Lamina C, Scott RA, Dastani Z, Hivert MF, Warren LL, et al. Mendelian randomization studies do not support a causal role for reduced circulating adiponectin levels in insulin resistance and type 2 diabetes. Diabetes 2013;62:3589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Au Yeung SL, Schooling CM. Adiponectin and coronary artery disease risk: A bi-directional Mendelian randomization study. Int J Cardiol 2018;268:222–6. [DOI] [PubMed] [Google Scholar]
- 43.Borges MC, Barros AJD, Ferreira DLS, Casas JP, Horta BL, Kivimaki M, et al. Metabolic Profiling of Adiponectin Levels in Adults: Mendelian Randomization Analysis. Circ Cardiovasc Genet 2017;10:pii e001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rathmann W, Herder C. Adiponectin and cardiovascular mortality: evidence for “reverse epidemiology”. Horm Metab Res 2007;39:1–2. [DOI] [PubMed] [Google Scholar]
- 45.Neumeier M, Weigert J, Schaffler A, Wehrwein G, Muller-Ladner U, Scholmerich J, et al. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol 2006;79:803–8. [DOI] [PubMed] [Google Scholar]
- 46.Lee YA, Ji HI, Lee SH, Hong SJ, Yang HI, Chul Yoo M, et al. The role of adiponectin in the production of IL-6, IL-8, VEGF and MMPs in human endothelial cells and osteoblasts: implications for arthritic joints. Exp Mol Med 2014;46:e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez R, Scotece M, Conde J, Gomez-Reino JJ, Lago F, Gualillo O. Adiponectin and leptin increase IL-8 production in human chondrocytes. Ann Rheum Dis 2011;70:2052–4. [DOI] [PubMed] [Google Scholar]
- 48.Kitahara K, Kusunoki N, Kakiuchi T, Suguro T, Kawai S. Adiponectin stimulates IL-8 production by rheumatoid synovial fibroblasts. Biochem Biophys Res Commun 2009;378:218–23. [DOI] [PubMed] [Google Scholar]
- 49.Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J Immunol 2007;179:5483–92. [DOI] [PubMed] [Google Scholar]
- 50.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 2006;116:1784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto Y, Toyomasu K, Uchimura N, Ishitake T. Low-molecular-weight adiponectin is more closely associated with episodes of asthma than high-molecular-weight adiponectin. Endocr J 2013;60:119–25. [DOI] [PubMed] [Google Scholar]
- 52.Miyazaki T, Shimada K, Mokuno H, Daida H. Adipocyte derived plasma protein, adiponectin, is associated with smoking status in patients with coronary artery disease. Heart 2003;89:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table S1. Baseline characteristics of study participants included in the present report stratified by RA diagnosis during follow-up.
Supplementary table S2. Unadjusted HR for the incidence of RA.
Supplementary table S3. Stratification of the study population according to adiponectin and CRP levels.
Supplementary table S4. Association between 2-year changes in adiponectin or CRP and the incidence of RA
Supplementary Figure S1. Changes in adiponectin and CRP in the control and surgery group during follow-up.