Abstract
The secretome is defined as the set of molecules and biological factors that are secreted by cells into the extracellular space. In the past decade, secretome-based therapies have emerged as a promising approach to overcome the limitations associated with cell-based therapies for tissue and organ regeneration. Considering the growing number of recent publications related to secretome-based therapies, this review takes a step-by-step engineering approach to evaluate the role of the stem cell secretome in regenerative engineering. We discuss the functional benefits of the secretome, the techniques used to engineer the secretome and tailor its therapeutic effects, and the delivery systems and strategies that have been developed to use the secretome for tissue regeneration.
The Stem Cell Secretome: a New Paradigm for Cell-Free Regenerative Engineering
As the population continues to age, there is a critical clinical need to develop therapeutic strategies to repair and regenerate damaged organs and tissues and to restore their normal functions [1]. Regenerative engineering is a transdisciplinary approach that is defined as the convergence of advanced materials sciences, stem cell sciences, physics, developmental biology, and clinical translation to regenerate complex tissues and organs [2–6]. The capacity to converge the latest advancements made in each of these respective fields can lead to highly translational technologies and solutions that overcome the need for organ transplantation. Thus far, regenerative engineering strategies have primarily focused on using materials science and engineering tools to develop biomaterials that can modulate cellular functions and harness the body’s innate regenerative potential [7–12]. Within the regenerative engineering paradigm, however, the role of progenitor/stem cells and bioactive factors is becoming increasingly evident and of central importance [13].
Cell therapy relies on the delivery of cells, either autologous or allogeneic, to promote tissue repair and regeneration. Cell therapy using differentiated cells is constricted by the limited availability and low proliferative capacity of the cells [14]. Stem cells, however, are superior in that they can be isolated from adult tissues and expanded in large quantities for use. They are integral in cell-based therapies for a variety of tissues secondary to their multipotent differentiation capacity, immunomodulatory properties, and anti-inflammatory effects [15–17].
The therapeutic effects of stem cells are regulated by three main mechanisms: homing, that is, migration to the site of injury; differentiation into various cell types that can engraft to the damaged tissue for repair; and secretion of bioactive factors [18]. While promising, however, the use of stem cells in tissue repair faces several challenges, including immune compatibility, tumorigenicity, and transmission of infections [19,20]. The large quantity of cells that is required for cell therapy mandates continuous growth and passaging of the cells outside the body in in vitro culture conditions, which may result in spontaneous alterations in the behavior and properties of the stem cells [21]. What is more, diverging from earlier studies attesting that the therapeutic benefits of stem cells are due to their engraftment and differentiation at damaged tissue sites, recent studies now suggest that it is the paracrine factors secreted from these cells that are mainly responsible for their therapeutic effects [22–25]. These paracrine factors are collectively termed the secretome and can be individually isolated for therapeutic purposes.
The secretome is defined as the repertoire of molecules and biological factors that are secreted from cells into the extracellular space. These secretory factors play important roles in many biological functions, including homeostasis (see Glossary), development, signaling, immunomodulation, inflammation, angiogenesis, apoptosis, proteolysis, adhesion, and extracellular matrix (ECM) organization [26,27]. The secretome comprises various serum proteins, growth factors, angiogenic factors, hormones, cytokines, ECM proteins and proteases, and even in low abundance, lipid mediators, and genetic material. It is broadly categorized into soluble factors (growth factors, cytokines, chemokines, and enzymes) and extracellular vesicles that transport lipids, proteins, and RNA and DNA subtypes (Figure 1) [28–31]. The composition of the secretome is dynamic, depending on the cell type and microenvironmental stimuli. However, generally, the stem cell secretome is known to have therapeutic benefits for tissue repair including proangiogenic, antiapoptotic, antifibrotic, anti-inflammatory, and immunomodulatory effects [32–34]. In addition to the biological benefits and obviating many of the safety concerns surrounding the direct use of cells, cell-free secretome-based therapies have several logistical advantages for clinical use, including scalability, availability, and longer shelf-lives [18,35].
Figure 1. Schematic Representation of the Secretome and Its Therapeutic Effects.
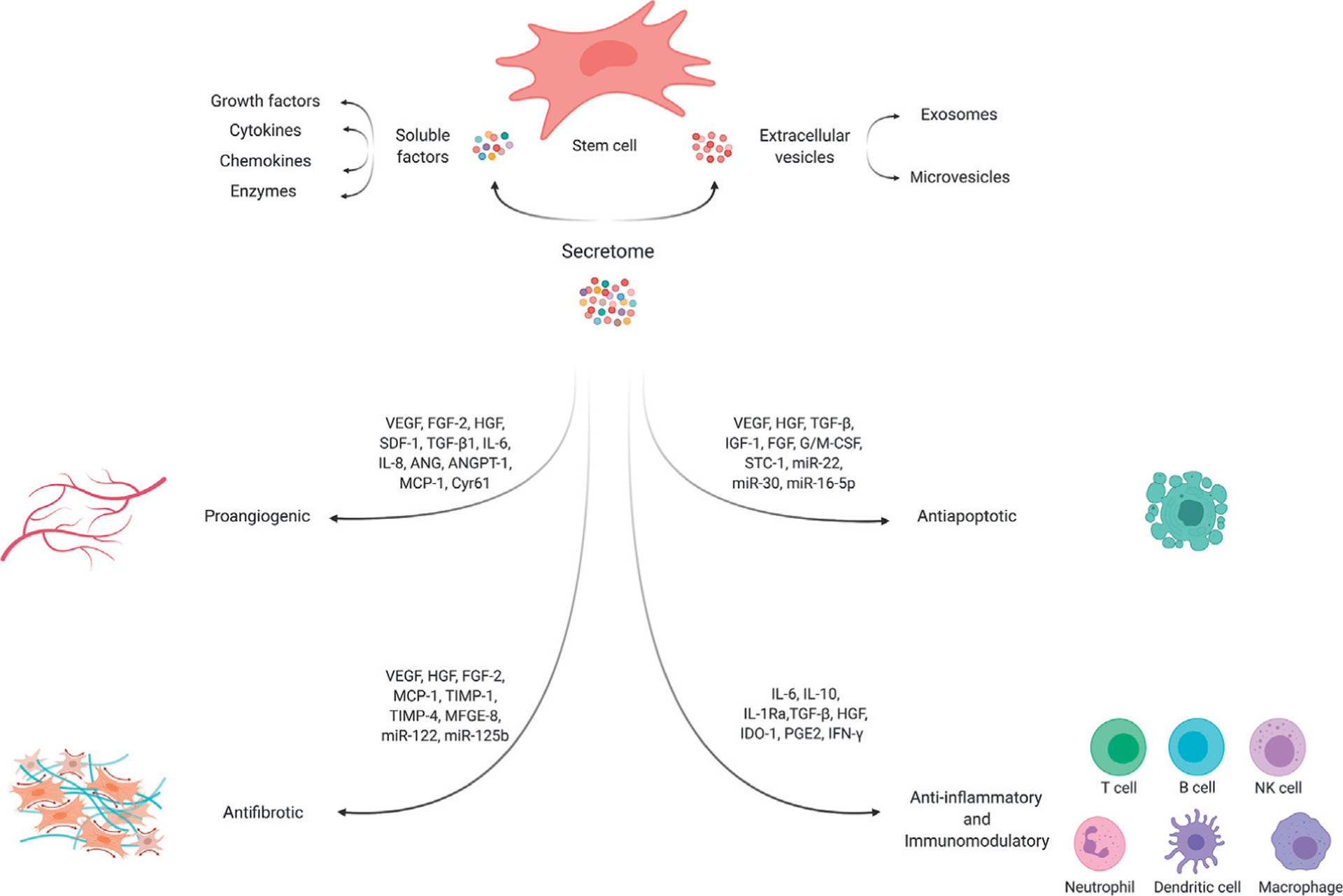
Stem cells secrete various soluble factors and extracellular vehicles (including exosomes and microvesicles) that are collectively termed the secretome. These biologically active factors exert therapeutic effects through their proangiogenic, antifibrotic, antiapoptotic, anti-inflammatory, and immunomodulatory properties. Abbreviations: ANG, angiogenin; ANGPT-1, angiopoietin-1; Cyr61, cysteine-rich protein 61; FGF-2, fibroblast growth factor 2; G/M-CSF, granulocyte/macrophage colony stimulating factor; HGF, hepatocyte growth factor; IDO-1, indolamine 2,3-dioxygenase-1; IFN-γ, interferon-γ; IGF-1, insulin-like growth factor-1; IL-1Ra, IL-1 receptor antagonist; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; MFGE-8, milk fat globule-EGF factor 8; miR-, miRNAs found within the extracellular vesicles; PGE2, prostaglandin E2; SDF-1, stromal-derived factor-1; STC-1, stanniocalcin-1; TGF-β1, transforming growth factor-β1; TIMP-1, tissue inhibitors of metalloproteinase-1; VEGF, vascular endothelial growth factor.
Engineering the Secretome
The composition of the stem cell secretome can vary based on numerous factors including the species, tissue source, and isolation procedure, and the microenvironment and chemical and physical stimuli to which the cells are exposed to. Although this dynamic nature may seem challenging for progression toward large-scale clinical applications, a thorough systematic and comparative analysis of the influence of the aforementioned factors can pave the way to select the secretome most appropriate for the intended use. More notably, by amplifying or suppressing certain biomolecules, a wide range of possibilities is unraveled to engineer the secretome and customize its therapeutic effects (Box 1).
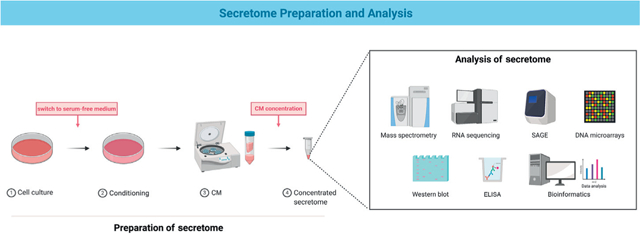
Box 1. Secretome Preparation and Analysis.
The secretome is isolated through a series of steps. (1) The stem cells are cultured in serum-containing culture medium, usually until 70–80% confluency. (2) To remove any remnants of serum proteins that may contaminate and interfere with the detection of minuscule amounts of cell-produced proteins, the cells are washed extensively and cultured in serum-free medium, usually for 12–48 h. This step may also include conditioning the cells using chemical or physical stimuli to obtain specific attributes from the secretome. (3) The conditioned medium (CM) consisting of the soluble components of the secretome and extracellular vesicles is collected by centrifugation to remove any dead cells or cell debris. (4) To increase the analytic resolution, the CM proteins are concentrated using centrifugation or lyophilization, and/or precipitated by ultrafiltration or trichloroacetic acid [33,105–107].
The secreted proteins of the secretome can be characterized using a targeted proteomic approach or a shotgun-based proteomic approach. The targeted approach involves protein microarrays such as antibody-based arrays (ELISA and western blotting) or bead-based arrays. The shotgun approach includes the following: gel-based (e.g., 2D gel electrophoresis) or gel-free (liquid chromatography) methods to separate the proteins in the secretome followed by mass spectroscopy to identify the proteins based on database knowledge; serial analysis of gene expression (SAGE), a short sequence-based method that quantitatively measures global gene expression patterns of tagged sites; RNA sequencing, a high throughput method that analyzes the transcriptome and can provide larger sequence information than SAGE; DNA microarray, a high-throughput method that measures differential gene expression based on known cDNA or oligonucleotide sequences; and secretion traps, a functional method that provides the sequence information of proteins. Bioinformatics tools (software and databases) can be used in combination with these approaches to manage and analyze the secretome/proteome results [33,105,108,109].
Figure I. Preparation and Analysis of the Secretome.
First, mesenchymal stem cells (MSCs) isolated from different tissue origins present variations in their secretory profile that can be used to the advantage. For instance, the secretome of MSCs from adipose tissue (adipose-derived stem cells; ADSCs) consists of a broader range of angiogenic factors and thus may be preferred over the secretome of bone marrow MSCs (BMSCs) for angiogenesis-mediated tissue regeneration [36]. The ADSC-secretome may also be preferred for neuroregenerative applications due to its stronger capabilities in promoting neuronal axonal growth [37,38]. The secretome of MSCs from the Wharton’s jelly is similarly better suited for neurogenesis and angiogenesis compared to that of BMSCs [37,39]. The secretome of MSCs isolated from the placenta and bone marrow have different effects on the functional properties of endothelial progenitor cells (EPCs); one of the important cell populations in neovascularization. While the secretome of placental MSCs enhanced the migration of EPCs, the BMSC-secretome has more of an effect on the invasion and vessel-forming capacity of the cells [40]. These differences highlight the important considerations that are required when selecting a cell source for secretome isolation.
The stem cell secretome can be modulated by genetically modifying the stem cells. Through various techniques, the stem cells can be modified to overexpress or underexpress certain factors leading to a secretome that is tailored in its therapeutic potential. Both BMSCs and ADSCs have been genetically modified to produce greater amounts of angiogenic, antiapoptotic and cardioprotective factors such as vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-1, hepatocyte growth factor (HGF), and basic fibroblast growth factor (b-FGF) that have functional relevance for angiogenesis, cell survival, wound healing, and for preventing tissue fibrosis and inflammation [41–43]. Additionally, genetic modification can be avoided through engineering microparticles that target a central regulatory cellular pathway. This approach was particularly found effective in inflamed MSCs and through targeting the nuclear factor (NF)-κB central regulatory pathway, which promotes the secretion of proinflammatory factors. Intracellular delivery of an NF-κB inhibitor loaded into microparticles of 1–2 μm, sustainably inhibited NF-κB activation and attenuated secretion of proinflammatory factors under inflammatory conditions [44].
One of the more common approaches in secretome engineering has been to expose the cells to biochemical stimuli during the preconditioning stage. Much of the work involving biochemical stimulation of MSCs has been through exposing the cells to various inflammatory mediators [e.g., tumor necrosis factor (TNF)-α), interferon (IFN)-γ, transforming growth factor (TGF)-β, interleukin (IL)-1, and lipopolysaccharides (LPS)] that elicit the cells to produce respondent biomolecules that are useful. These include various cytokines and chemokines (IL-1β, IL-6, and IL-8), proteases (matrix metalloproteases), protease inhibitors, and proangiogenic and prosurvival factors (VEGF, FGF-2, HGF, IGF-1, angiopoietin, and monocyte chemoattractant protein (MCP)-1] that play roles in immunomodulation, angiogenesis, and regeneration, and have anti-inflammatory, antifibrotic, and neuroprotective effects [45–52]. Modulating the secretion of cytokines, chemokines, and exosomes that are involved in immunomodulation, immunosuppression, and allergic responses could have significant implications for immune-associated disorders [53,54].
Physical stimuli can also be applied during the preconditioning stage to modulate the composition of the secretome. Hypoxic culture has been one of the most widely used methods to engineer the secretory profile of MSCs and enhance its therapeutic potential for tissue and organ regeneration. Hypoxic culture promotes the secretion of numerous prosurvival and proangiogenic factors, such as VEGF, angiogenin, b-FGF, FGF-2, HGF, IL-6, MCP-1, MCP-3, IGF-1, TGF-β, platelet-derived growth factor (PDGF)-BB, and epidermal growth factor (EGF), that have shown beneficial effects in promoting angiogenesis and survival in ischemic conditions, and in promoting neurorestoration, cardioprotection, chemotaxis, wound healing, skin regeneration, and hair regrowth [51,55–63]. Of note, the conditions of hypoxic exposure during preconditioning should be chosen with care. Different oxygen levels - typically from 0.1% to 5% O2 - have different effects on the properties of the secretome and can influence the secretome’s functional potential [64–66]. In addition, the duration of exposure is important. For instance, prolonged hypoxic exposure can act in the negative and lead to a secretome that is detrimental for tissue repair [66,67].
Spheroid culture of stem cells provides a more physiologically relevant environment that allows the cells to exhibit improved biological properties. In secretome engineering, spheroid culture can elevate the secretion of proangiogenic factors (VEGF, bFGF, HGF, angiogenin, IL-11), anti-inflammatory markers (IL-1ra, granulocyte-colony stimulating factor, prostaglandin E2 (PGE2)], and antifibrotic molecules that have mostly shown therapeutic implications for angiogenesis and cardiac tissue repair [68–71]. In addition to standard spheroid culture methods, spheroid culture in 3D spinner flasks is particularly shown to produce a secretome enriched in angiogenic and antiapoptotic factors that is clinically relevant and efficacious for improving angiogenesis, blood perfusion, and limb salvage in ischemic limbs [72].
Cells in the body are constantly experiencing mechanical forces. These forces are generally converted into biochemical signals through mechanotransduction that can stimulate different signaling pathways and influence cell behavior [73]. While the majority of studies using mechanical forces have been to regulate stem cell differentiation, it is evident that mechanical stimulation can also be used as a means to modulate the secretome. For bone applications and in bone cells, mechanical loading increases the secretion of various paracrine factors, including nitric oxide and PGE2, two important signaling molecules that mediate the response of bone cells to mechanical forces [74]. However, the secretome produced from the various bone cells or MSCs is distinct, and has different effects on the proliferation, migration, and differentiation of other MSCs or bone cells [75–77]. Of note, mechanical loading can be applied to MSCs undergoing differentiation to produce a secretome that is customized in its regenerative and therapeutic potential. For instance, mechanical stimulation of MSCs during chondrogenic induction amplified the secretion of various proteins and cytokines involved in cartilage development, regeneration, and disease, such as type II collagen, aggrecan, keratan sulfate, TGF-β1, matrix metalloproteinase-13, PDGF-AA, VEGF, and angiogenin [78,79].
Secretome Applications and Delivery Strategies
Numerous delivery systems and strategies have been developed to harness the therapeutic potential of the stem cell secretome for organ/tissue repair and regeneration. Depending on the target site, the secretome or conditioned medium (CM) can be delivered either through direct injections, systemic injections, or through using delivery vehicles such as scaffolds, hydrogels, and microparticles (Table 1).
Table 1.
Strategies to Deliver the Secretome for Regenerative Engineering Applications.
| Delivery systema | Animal model | In vivo effect | Refs |
|---|---|---|---|
| IV injection of human deciduous dental pulp stem cell-CM | Mouse rheumatoid arthritis model |
|
[80] |
| IV injection of human cardiac progenitor cell-exosomes | Rat model of drug-induced cardiotoxicity |
|
[81] |
| IV injection of MSC-EVs | Mouse model of traumatic brain injury |
|
[82] |
| Direct injection of human UC-MSCs into the soleus muscle | Rat model of muscle atrophy |
|
[119] |
| Weekly intra-articular injections of exosomes derived from human embryonic stem cell-derived MSCs | Rat critical-sized osteochondral defect |
|
[85] |
| Weekly subcutaneous secretome injections of human fetal skin-derived stem cells and UC-MSCs | Rat radiation-induced skin injury |
|
[88] |
| Topical administration of human ADSC-CM and HUVEC-CM | Diabetic swine full-thickness wound healing model |
|
[90] |
| Atelocollagen-based sponge soaked with hMSC-CM | Rat calvarial bone defect |
|
[93–95] |
| β-TCP scaffold soaked with hMSC-CM | Rabbit maxillary sinus floor elevation model |
|
[96] |
| β-TCP scaffold soaked with hMSC-CM | Human maxillary sinus floor elevation model |
|
[97] |
| NaOH-treated PLGA membrane soaked with rat BMSC-CM | Rat calvarial bone defect |
|
[99] |
| 3D-printed PLA scaffold loaded with PEI-engineered EVs | Rat calvarial bone defect |
|
[100] |
| Silk fibroin-based hydrogel loaded with human UC-MSC-CM | Rat model of age-related osteoporosis |
|
[102] |
| Gelatin and Laponite-based hydrogel loaded with hADSC-CM from spheroid culture | Rat myocardial infarction |
|
[69] |
| Hydroxyethyl cellulose hydrogel loaded with hADSC-exosomes | Rat excisional wound-splinting model |
|
[120] |
| PLGA microparticles coated with hMSC membrane fragments and loaded with hMSC-secretome | Mouse myocardial infarction |
|
[103] |
| PLGA microparticles coated with hCSC membrane fragments and loaded with hCSC-secretome | Mouse myocardial infarction |
|
[104] |
ADSC, adipose-derived stem cell; β-TCP, β-tricalcium phosphate; CM, conditioned media; CSC, cardiac stem cell; ECM, extracellular matrix; EVs, extracellular vesicles; IL-1β, interleukin-1β; HUVEC, human umbilical vein endothelial cell; IGF-1, insulin-like growth factor; iNOS, inducible NO synthase; MSC, mesenchymal stem cell; PEI, polyethyleneimine; PI3K, phosphatidylinositol 3-kinase; PLA, polylactic acid; PLGA, poly(lactic-co-glycolic acid); s-GAG, s-glycosaminoglycan; TGF-β1, transforming growth factor-β1; TNF-α, tumor necrosis factor-α; UC-MSC, umbilical cord mesenchymal stem cell; VEGF, vascular endothelial growth factor.
Systemic/Local Direct Administration
Systemic administration is a convenient approach that relies on rapidly increasing the concentration of the agent in the systemic circulation for distribution to sites of action. In a mouse model of rheumatoid arthritis, intravenous injections of CM from human deciduous dental pulp stem cells markedly improved arthritis symptoms and joint destruction [80]. Intravenous (IV) injections of exosomes derived from the CM of cardiac progenitor cells exerted cardioprotective effects in a rat model of drug-induced cardiotoxicity [81]. In a mouse model of traumatic brain injury, IV injecions of the exosomes collected from MSCs suppressed neuroinflammation and rescued the cognitive impairments [82]. A recent study evaluated the efficacy of IL-1α-primed MSC-derived CM on brain injury and recovery after cerebral ischemia in mice. The composition of the secretome was engineered by priming the MSCs using IL-1α toward a more anti-inflammatory and proreparative phenotype. Subcutaneous injections of the CM were performed to produce systemic effects against stroke, which included significant neuroprotective effects and improved functional recovery [83]. While there have not been many studies comparing local versus systemic injections for secretome delivery, the protective effects of the MSC secretome in reducing fatty degeneration and atrophy of the rotator cuff muscles was shown through both delivery routes in a rat massive rotator cuff tear model [84].
The more common approach taken for delivering the secretome to a target organ has been through direct injections at the site of injury. For example, the efficacy of direct injections of MSC-derived exosomes in promoting cartilage repair has been shown in a few studies. In an immunocompetent rat model of osteochondral defects, weekly intra-articular injections of exosomes from human embryonic stem cell-derived MSCs induced the regeneration of the cartilage and subchondral bone tissue in as early as 2 weeks and led to orderly regeneration of both tissues by 12 weeks [85]. The exosomes from human embryonic stem cell-derived MSCs can also impede cartilage destruction in osteoarthritic mice through balancing the synthesis and degradation of the ECM [86]. Interestingly, exosomes from induced pluripotent stem cell-derived MSCs were shown to have greater therapeutic efficiency against osteoarthritis than those from synovial membrane-derived MSCs, possibly due to their greater stimulatory effects on chondrocyte migration and proliferation [87].
The benefits of local secretome administration have also been investigated for wound healing applications. Weekly injections of the secretome from both human fetal skin-derived stem cells and human umbilical cord MSCs effectively enhanced wound healing and angiogenesis in radiation-induced skin injuries in rats [88]. In diabetic rats, BMSC-CM effectively improved wound closure rates and the quality of healed skin in chronic diabetic wounds, through modulating the behavior of fibroblasts, angiogenesis, and the inflammatory and immune responses [89]. In a larger diabetic swine model, topical application of ADSC-CM or human umbilical vein endothelial cell-CM accelerated wound closure, possibly through the secretome’s angiogenic and immunomodulatory effects [90]. The paracrine effects of human adipose tissue for wound healing applications was evaluated by preparing a cell-free liquid extract from the tissue and directly injecting it into the wound bed of mice. The extract, which was collected from the liquid portion of lipoaspirates after centrifugation, contained as many as 1975 human proteins (including bFGF, EGF, TGF-β1, VEGF, HGF, and PDGF) that were mainly from the cytoplasm, followed by the nucleus, extracellular space, and plasma membrane. Direct injections of this minimally processed tissue extract increased wound healing rates, angiogenesis and adipogenesis in full-thickness wounds [91].
Delivery Vehicles
Direct injections often result in a rapid clearance rate from the delivery site; therefore, it is important to develop carriers that prolong secretome retention for more efficacious therapeutic effects [92]. The use of the stem cell secretome in combination with biomaterials for bone regeneration has been extensively investigated by Katagiri and colleagues. The addition of MSC-CM to atelocollagen (Terudermis) scaffolds implanted into the calvarial defect of rats increased new bone regeneration, which was shown to be due to the secretome’s proangiogenic properties and ability to induce early migration of endogenous stem cells to the defect site [93,94]. A following study showed that a cytokine cocktail of only IGF-1, VEGF-A, and TGF-β1 at concentrations similar to that of the MSC-CM was sufficient to induce bone regeneration at comparable levels to the MSC-CM [95]. β-Tricalcium phosphate (β-TCP) scaffolds soaked in MSC-CM for 5 min effectively delivered the secretome to promote early bone regeneration in a rabbit maxillary sinus floor elevation model [96]. Katagiri and colleagues have further evaluated the safety and regenerative capacity of the MSC-CM in two small human studies. Patients requiring maxillary sinus floor elevation and bone grafts received β-TCP implants that were either soaked with MSC-CM (four patients) or as is (control, two patients). There was greater new bone formation in patients receiving the MSC-CM, particularly in the center of the augmented area [97]. In patients needing bone augmentation prior to dental implants also β-TCP- or atelocollagen-based scaffolds soaked with MSC-CM demonstrated great osteogenic potential and were found safe, causing no systemic or local complications [98].
Synthetic polymer-based scaffolds have also been used for secretome delivery. To increase hydrophilicity and improve CM immobilization, poly(lactic-co-glycolic acid) membranes were treated with NaOH before CM loading. NaOH treatment increased the immobilization of CM on the membranes which in turn led to greater BMSC proliferation and alkaline phosphatase activity on the membranes in vitro, and more new bone formation in rat calvarial defects [99]. Another study used polyethyleneimine to incorporate the extracellular vesicles of human gingival MSCs onto 3D-printed polylactic acid (PLA) scaffolds. Implantation of the PLA+polyethyleneimine extracellular vesicles scaffolds into rat calvarial defects led to complete bridging and repair of the defects, and greater new bone formation and vascularization (Box 2) [100].
Box 2. Extracellular Vesicle Based-Therapeutics.
Extracellular vesicles (EVs) are nanosized membrane-enclosed vesicles that are released by the cells into the extracellular space. Such vesicles include exosomes (40–150 nm), formed by the fusion of intracellular multivesicular bodies with the plasma membrane, and microvesicles (50–1000 nm), which are shed directly from the plasma membrane. EVs contain various bioactive components such as nucleic acids (RNA, mRNA, miRNA, and DNA), proteins, cytokines, and lipids and are a significant means of communication between not only adjacent cells but also distant cells, to which they travel to through blood and bodily fluids. EVs have been shown to play important roles in regulating physiological and pathological processes including homeostasis, immunomodulation, regenerative processes, and tumorigenesis [110–113].
For many years considered as inert cellular debris, EVs are now receiving increasing attention as therapeutic delivery systems due to their natural ability to robustly transport biologically active components and genetic material to target cells, near or far. The EV delivery systems are used through loading the cargo either endogenously, that is, engineering the secretome for desired biomolecules and subsequently isolating and purifying the produced EVs, or exogenously, that is, encapsulating other drugs into already isolated EVs [114–116]. As a rapidly evolving and expanding field, EV-based therapies have so far been used toward the preclinical treatment or regeneration of various diseases and conditions including those of the respiratory, renal, hepatic, neurological, cardiovascular, and musculoskeletal systems. There are also clinical trials and applications involving the use of EVs particularly for cancer therapy and immunotherapy [117,118].
Hydrogels are another class of biomaterials that can be used to retain the secretome for a controlled release profile. Injectable hydrogels, specifically, provide a minimally invasive means for localized delivery to target organs. One of the earliest works on secretome delivery was based on developing an injectable peptide-based hydrogel sponge to soak up the secretome in situ, and subsequently release it in the therapeutic environment [101]. Silk fibroin-based injectable hydrogels were used to deliver the secretome of human umbilical cord MSCs into the bone marrow of osteoporotic rats. These hydrogels were shown to provide a slow and sustained release for a period of 30 days in vitro. Intratibial injections localized the secretome at sites of osteoporosis within the bone, enabling the secretome to exert its antiaging effects and attenuate the drastic loss of bone [102]. To treat myocardial infarction (MI) in rats, an injectable hydrogel composed of gelatin and Laponite was developed. Laponite was chosen due to its high protein adsorption capacity, and contribution to the shear-thinning behavior of the hydrogels. The secretome from spheroid culture of human ADSCs was loaded and delivered to the peri-infarct regions of the heart through intramyocardial injections. Secretome delivery improved overall cardiac function and vascularization, and reduced fibrosis and scar tissue formation [69].
One of the more interesting approaches for secretome delivery has been to fabricate secretome-loaded microparticles the size of stem cells and to coat them with fragments of the stem cell membrane to create ‘synthetic MSCs’ or ‘cell-mimicking particles’. These microparticles provide a controlled and sustained release for up to 7 days in vitro. In a mouse model of acute MI, intramyocardial injection of the microparticles that were loaded with the secretome of BMSCs significantly reduced the infarct area and promoted endogenous heart repair [103]. The injection of similar microparticles loaded with the secretome of cardiac stem cells (CSCs), rather than BMSCs, in a similar model of acute MI also improved cardiac function. It is important to note that the cardioprotective effects of the secretome-loaded particles were similar to that of CSC injections, however, CSC injections caused severe immune rejection and substantial T cell infiltration, which were found to be negligible in the secretome group [104].
Concluding Remarks and Future Prospects
Secretome-based therapies have emerged as a promising alternative to cell-based therapies. The ability to be manufactured, stored, and used as off-the-shelf ready-to-go products while maintaining the therapeutic benefits of stem cells but with fewer safety concerns have situated the secretome at the forefront of next-generation tissue and organ-regenerative engineering applications.
There are many challenges, however, that need to be overcome to translate this promise to the clinic (see Outstanding Questions). There is a need to develop guidelines and standardize the methods and procedures used in cell isolation and culture, and the techniques used to extract and purify the secretome. As discussed, the secretome is dynamic and responsive to microenvironmental changes and its composition can vary depending on its source of origin and culture conditions. Thus, it is imperative to consider the implications of these factors as variations in each may influence outcomes and obscure findings. This threat can, however, be used to advantage by modulating the composition of the secretome and tailoring its therapeutic effects for target applications. The methods described in secretome engineering can be used in combination to synergistically produce a secretome more fitting and efficient in its therapeutic effects. With this, it is important to note that the dynamic concentrations and combinations of proteins in the secretome respond differently to microenvironmental cues and that the effects of biochemical and physical stimuli should be considered on the entirety of the secretome, and not just a few biomolecules. As an example, there are components in the secretome such as some inflammatory mediators that are not necessarily beneficial for tissue regeneration and that through secretome engineering may become to adversely impact the secretome’s therapeutic potential, and thus must be considered in secretome manipulation. The technological advancements and improvements in the methodologies and tools that are used in proteomics and secretomics to decipher the secretome and have a global understanding of the secreted factors will be integral for further progress and to further define the safety and efficacy of secretome-based therapeutics.
Outstanding Questions.
How can we develop guidelines and standardize the procedures for secretome production?
How can we develop high-throughput systems for the large-scale production of the secretome?
How can we best engineer and customize the secretome for targeted therapeutic benefits?
What are the technical advances necessary to propel this technology to its next stage and progress into clinical practice?
What are the safety and regulatory concerns that need to be addressed?
Will secretome-based therapies provide long-term function and safety in patients?
Highlights.
The stem cell secretome has emerged as a promising cell-free alternative to cell-based therapies.
Secretome-based therapies have many of the therapeutic benefits of cell-based therapies, while obviating many of the safety and logistical concerns associated with directly using stem cells.
The secretome is highly dynamic and its therapeutic effects can be engineered and customized according to its intended application.
Delivery systems and strategies have been developed to harness the therapeutic benefits of the secretome for the repair and regeneration of various tissues and organs.
Acknowledgments
We would like to acknowledge funds from NIH DP1 AR068147, NIH R01 AR063698, and NSF/EFRI #1332329. Dr. Laurencin is a recipient of the National Medal of Technology and Innovation. All figures were created with BioRender.com.
Glossary
- Apoptosis
a regulated and programmed process of cell death and self destruction.
- Chemotaxis
directed movement and migration of cells in response to a chemical signal.
- Chemokines (chemoattractant cytokines)
a subgroup of cytokines that induce chemotaxis in other cells.
- Clearance
drug elimination from an organ.
- Cytokines
a large family of small signaling protein molecules that are secreted by cells for cell signaling and cell-cell communication.
- Homeostasis
the ability of the body or a cell to regulate and maintain a steady and stable physiological condition.
- Hypoxia
the reduction or lack of oxygen in organs, tissues, and cells. Hypoxic culture refers to performing the cell culture at O2 levels lower than ambient air (21% O2) and typically at 0.1–5% O2.
- Mechanotransduction
the process in which cells sense and respond to mechanical stimuli by converting them into biochemical signals.
- Osteochondral
pertaining to bone and cartilage.
- Preconditioning
exposing the cells to certain physical or chemical stimuli to manipulate cell behavior for a desired response.
- Protease
an enzyme that helps break down proteins or peptides
- Proteolysis
the process in which proteins are degraded, partially or completely, by the activity of proteases.
- Proteome
the entire set of proteins expressed by an organism.
- Proteomics
the large-scale study of the proteome to analyze the structure, function, and interactions of all the proteins of a cell for a global and integrated biological view.
- Secretomics
is a subfield of proteomics that involves the global study of the secretome - proteins that are secreted by organisms.
- Spheroid
3D structures of cells grown in aggregates that recreate the natural in vivo environment of the cells more closely than 2D culture conditions.
References
- 1.Miller RR and Roubenoff R (2019) Emerging interventions for elderly patients - the promise of regenerative medicine. Clin. Pharmacol. Ther 105, 53–60 [DOI] [PubMed] [Google Scholar]
- 2.Laurencin CT and Khan Y (2012) Regenerative engineering. Sci. Transl. Med 4, 160ed9 [DOI] [PubMed] [Google Scholar]
- 3.Laurencin CT and Nair LS (2015) Regenerative engineering: approaches to limb regeneration and other grand challenges. Regen. Eng. Transl. Med 1, 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang X et al. (2019) Skeletal muscle regenerative engineering. Regen. Eng. Transl. Med 5, 233–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo KW-H et al. (2014) Small-molecule based musculoskeletal regenerative engineering. Trends Biotechnol. 32, 74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurencin CT and Nair LS (2016) The quest toward limb regeneration: a regenerative engineering approach. Regen. Biomater 3, 123–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold AM et al. (2019) Phosphate graphene as an intrinsically osteoinductive scaffold for stem cell-driven bone regeneration. Proc. Natl. Acad. Sci 116, 4855–4860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daneshmandi L and Laurencin CT (2020) Regenerative engineered vascularized bone mediated by calcium peroxide. J. Biomed. Mater. Res. A 108, 1045–1057 [DOI] [PubMed] [Google Scholar]
- 9.Laurencin CT and Daneshmandi L (2020) Graphene for tissue and organ regenerative engineering. Int. J. Ceram. Sci. Eng (in press) [Google Scholar]
- 10.Laurencin CT and Nair LS (2014) Nanotechnology and Regenerative Engineering: the Scaffold, CRC Press [Google Scholar]
- 11.Jiang T et al. (2014) Micro-and nanofabrication of chitosan structures for regenerative engineering. Acta Biomater. 10, 1632–1645 [DOI] [PubMed] [Google Scholar]
- 12.Amini AR et al. (2012) Optimally porous and biomechanically compatible scaffolds for large-area bone regeneration. Tissue Eng. A 18, 1376–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayanan G et al. (2017) Musculoskeletal tissue regeneration: the role of the stem cells. Regen. Eng. Transl. Med 3, 133–165 [Google Scholar]
- 14.Mao AS and Mooney DJ (2015) Regenerative medicine: current therapies and future directions. Proc. Natl. Acad. Sci 112, 14452–14459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naji A et al. (2019) Biological functions of mesenchymal stem cells and clinical implications. Cell. Mol. Life Sci 76, 3323–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krueger TE et al. (2018) Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl. Med 7, 651–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vernekar VN et al. (2016) Nanotechnology applications in stem cell science for regenerative engineering. J. Nanosci. Nanotechnol 16, 8953–8965 [Google Scholar]
- 18.Vizoso FJ et al. (2017) Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int. J. Mol. Sci 18, 1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurencin CT and McClinton A (2020) Regenerative cell-based therapies: cutting edge, bleeding edge, and off the edge. Regen. Eng. Transl. Med 6, 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukomska B et al. (2019) Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019, 9628536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turinetto V et al. (2016) Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci 17, 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eggenhofer E et al. (2014) The life and fate of mesenchymal stem cells. Front. Immunol 5, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menasché P (2018) Cell therapy trials for heart regeneration-lessons learned and future directions. Nat. Rev. Cardiol 15, 659–671 [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y et al. (2019) The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J. Clin. Med 8, 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maguire G (2013) Stem cell therapy without the cells. Commun. Integr. Biol 6, e26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konala VBR et al. (2016) The current landscape of the mesenchymal stromal cell secretome: a new paradigm for cell-free regeneration. Cytotherapy 18, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caccia D et al. (2013) Bioinformatics tools for secretome analysis. Biochim. Biophys. Acta Proteins Proteomics 1834, 2442–2453 [DOI] [PubMed] [Google Scholar]
- 28.Harrell CR et al. (2019) Molecular mechanisms responsible for therapeutic potential of mesenchymal stem cell-derived secretome. Cells 8, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eleuteri S and Fierabracci A (2019) Insights into the secretome of mesenchymal stem cells and its potential applications. Int. J. Mol. Sci 20, 4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin L and Du L (2018) The role of secreted factors in stem cells-mediated immune regulation. Cell. Immunol 326, 24–32 [DOI] [PubMed] [Google Scholar]
- 31.Hofer HR and Tuan RS (2016) Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther 7, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusuma GD et al. (2017) Effect of the microenvironment on mesenchymal stem cell paracrine signaling: opportunities to engineer the therapeutic effect. Stem Cells Dev. 26, 617–631 [DOI] [PubMed] [Google Scholar]
- 33.Tran C and Damaser MS (2015) Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv. Drug Deliv. Rev 82, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haque N et al. (2018) Secretome: pharmaceuticals for cell-free regenerative therapy In Stem Cell Drugs - a New Generation of Biopharmaceuticals, pp. 17–35, Springer [Google Scholar]
- 35.Van Pham P et al. (2018) Evolution of stem cell products in medicine: future of off-the-shelf products In Stem Cell Drugs - a New Generation of Biopharmaceuticals, pp. 93–118, Springer [Google Scholar]
- 36.Hsiao ST-F et al. (2011) Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 21, 2189–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrenko Y et al. (2020) A comparative analysis of multipotent mesenchymal stromal cells derived from different sources, with a focus on neuroregenerative potential. Sci. Rep 10, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Assunção-Silva RC et al. (2018) Exploiting the impact of the secretome of MSCs isolated from different tissue sources on neuronal differentiation and axonal growth. Biochimie 155, 83–91 [DOI] [PubMed] [Google Scholar]
- 39.Hsieh J-Y et al. (2013) Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One 8, e72604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamprom W et al. (2016) Effects of mesenchymal stem cell-derived cytokines on the functional properties of endothelial progenitor cells. Eur. J. Cell Biol 95, 153–163 [DOI] [PubMed] [Google Scholar]
- 41.Li H et al. (2010) Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am. J. Phys. Heart Circ. Phys 299, H1772–H1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song S-H et al. (2012) Genetic modification of human adipose-derived stem cells for promoting wound healing. J. Dermatol. Sci 66, 98–107 [DOI] [PubMed] [Google Scholar]
- 43.Gnecchi M et al. (2006) Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 20, 661–669 [DOI] [PubMed] [Google Scholar]
- 44.Ranganath SH et al. (2016) Controlled inhibition of the mesenchymal stromal cell pro-inflammatory secretome via microparticle engineering. Stem Cell Rep. 6, 926–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee MJ et al. (2010) Proteomic analysis of tumor necrosis factor-α-induced secretome of human adipose tissue-derived mesenchymal stem cells. J. Proteome Res 9, 1754–1762 [DOI] [PubMed] [Google Scholar]
- 46.Zubkova ES et al. (2016) Regulation of adipose tissue stem cells angiogenic potential by tumor necrosis factor-alpha. J. Cell. Biochem 117, 180–196 [DOI] [PubMed] [Google Scholar]
- 47.Maffioli E et al. (2017) Proteomic analysis of the secretome of human bone marrow-derived mesenchymal stem cells primed by pro-inflammatory cytokines. J. Proteome 166, 115–126 [DOI] [PubMed] [Google Scholar]
- 48.Redondo-Castro E et al. (2017) Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther 8, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C et al. (2016) Paracrine effect of inflammatory cytokine-activated bone marrow mesenchymal stem cells and its role in osteoblast function. J. Biosci. Bioeng 121, 213–219 [DOI] [PubMed] [Google Scholar]
- 50.Lee SC et al. (2015) Lipopolysaccharide preconditioning of adipose-derived stem cells improves liver-regenerating activity of the secretome. Stem Cell Res Ther 6, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wobma HM et al. (2018) The influence of hypoxia and IFN-γ on the proteome and metabolome of therapeutic mesenchymal stem cells. Biomaterials 167, 226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Z et al. (2018) The paracrine effect of adipose-derived stem cells inhibits IL-1β-induced inflammation in chondrogenic cells through the Wnt/β-catenin signaling pathway. Regen. Eng. Transl. Med 4, 35–41 [Google Scholar]
- 53.Rodríguez TM et al. (2015) Effect of TGF-β1 stimulation on the secretome of human adipose-derived mesenchymal stromal cells. Stem Cells Transl. Med 4, 894–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Q et al. (2018) Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation. J. Cell. Physiol 233, 6832–6840 [DOI] [PubMed] [Google Scholar]
- 55.Rehman J et al. (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109, 1292–1298 [DOI] [PubMed] [Google Scholar]
- 56.Linero I and Chaparro O (2014) Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS One 9, e107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsiao ST et al. (2013) Hypoxic conditioning enhances the angiogenic paracrine activity of human adipose-derived stem cells. Stem Cells Dev. 22, 1614–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moon KM et al. (2012) The effect of secretory factors of adipose-derived stem cells on human keratinocytes. Int. J. Mol. Sci 13, 1239–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee EY et al. (2009) Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 17, 540–547 [DOI] [PubMed] [Google Scholar]
- 60.Park B-S et al. (2010) Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed. Res 31, 27–34 [DOI] [PubMed] [Google Scholar]
- 61.Roth S et al. (2016) Hypoxic-preconditioned bone marrow stem cell medium significantly improves outcome after retinal ischemia in rats. Invest. Ophthalmol. Vis. Sci 57, 3522–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang C-P et al. (2012) Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci 124, 165–176 [DOI] [PubMed] [Google Scholar]
- 63.Song S-W et al. (2016) Proteomic analysis and identification of paracrine factors in mesenchymal stem cell-conditioned media under hypoxia. Cell. Physiol. Biochem 40, 400–410 [DOI] [PubMed] [Google Scholar]
- 64.Frazier TP et al. (2013) Impact of low oxygen on the secretome of human adipose-derived stromal/stem cell primary cultures. Biochimie 95, 2286–2296 [DOI] [PubMed] [Google Scholar]
- 65.Paquet J et al. (2015) Oxygen tension regulates human mesenchymal stem cell paracrine functions. Stem Cells Transl. Med 4, 809–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antebi B et al. (2018) Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res Ther 9, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saraswati S et al. (2015) Prolonged hypoxia induces monocarboxylate transporter-4 expression in mesenchymal stem cells resulting in a secretome that is deleterious to cardiovascular repair. Stem Cells 33, 1333–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potapova IA et al. (2007) Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells 25, 1761–1768 [DOI] [PubMed] [Google Scholar]
- 69.Waters R et al. (2018) Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater. 69, 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Redondo-Castro E et al. (2018) Changes in the secretome of tri-dimensional spheroid-cultured human mesenchymal stem cells in vitro by interleukin-1 priming. Stem Cell Res Ther 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ylöstalo JH et al. (2012) Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 30, 2283–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhang SH et al. (2014) Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol. Ther 22, 862–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolfenson H et al. (2019) Steps in mechanotransduction pathways that control cell morphology. Annu. Rev. Physiol 81, 585–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klein-Nulend J et al. (1997) Pulsating fluid flow stimulates prostaglandin release and inducible prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J. Bone Miner. Res 12, 45–51 [DOI] [PubMed] [Google Scholar]
- 75.Brady RT et al. (2015) Mechanically stimulated bone cells secrete paracrine factors that regulate osteoprogenitor recruitment, proliferation, and differentiation. Biochem. Biophys. Res. Commun 459, 118–123 [DOI] [PubMed] [Google Scholar]
- 76.Tan SD et al. (2007) Osteocytes subjected to fluid flow inhibit osteoclast formation and bone resorption. Bone 41, 745–751 [DOI] [PubMed] [Google Scholar]
- 77.Sanuki R et al. (2010) Compressive force induces osteoclast differentiation via prostaglandin E2 production in MC3T3-E1 cells. Connect. Tissue Res 51, 150–158 [DOI] [PubMed] [Google Scholar]
- 78.Gardner O et al. (2016) Differences in human mesenchymal stem cell secretomes during chondrogenic induction. Eur. Cells Mater 31, 221–235 [DOI] [PubMed] [Google Scholar]
- 79.Ogawa R et al. (2009) The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells. Tissue Eng. A 15, 2937–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishikawa J et al. (2016) Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental rheumatoid arthritis. Bone 83, 210–219 [DOI] [PubMed] [Google Scholar]
- 81.Milano G et al. (2020) Intravenous administration of cardiac progenitor cell-derived exosomes protects against doxorubicin/trastuzumab-induced cardiac toxicity. Cardiovasc. Res 116, 383–392 [DOI] [PubMed] [Google Scholar]
- 82.Kim D. k. et al. (2016) Chromatographically isolated CD63+ CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc. Natl. Acad. Sci 113, 170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cunningham CJ et al. (2020) Systemic conditioned medium treatment from interleukin-1 primed mesenchymal stem cells promotes recovery after stroke. Stem Cell Res Ther 11, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sevivas N et al. (2017) Mesenchymal stem cell secretome: a potential tool for the prevention of muscle degenerative changes associated with chronic rotator cuff tears. Am. J. Sports Med 45, 179–188 [DOI] [PubMed] [Google Scholar]
- 85.Zhang S et al. (2018) MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 156, 16–27 [DOI] [PubMed] [Google Scholar]
- 86.Wang Y et al. (2017) Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther 8, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhu Y et al. (2017) Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther 8, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rong X et al. (2019) Human fetal skin-derived stem cell secretome enhances radiation-induced skin injury therapeutic effects by promoting angiogenesis. Stem Cell Res Ther 10, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Saheli M et al. (2019) Human mesenchymal stem cells-conditioned medium improves diabetic wound healing mainly through modulating fibroblast behaviors. Arch. Dermatol. Res Published online November 30, 2019 10.1007/s00403-019-02016-6 [DOI] [PubMed] [Google Scholar]
- 90.Irons RF et al. (2018) Acceleration of diabetic wound healing with adipose-derived stem cells, endothelial-differentiated stem cells, and topical conditioned medium therapy in a swine model. J. Vasc. Surg 68, 115S–125S [DOI] [PubMed] [Google Scholar]
- 91.He Y et al. (2019) Human adipose liquid extract induces angiogenesis and adipogenesis: a novel cell-free therapeutic agent. Stem Cell Res Ther 10, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barajaa MA et al. (2019) Bioinspired scaffold designs for regenerating musculoskeletal tissue interfaces. Regen. Eng. Transl. Med Published online December 17, 2019 10.1007/s40883-019-00132-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katagiri W et al. (2017) Angiogenesis in newly regenerated bone by secretomes of human mesenchymal stem cells. Maxillofac. Plast. Reconstr. Surg 39, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ogata K et al. (2018) Secretomes of mesenchymal stem cells induce early bone regeneration by accelerating migration of stem cells. J. Oral Maxillofac. Surg. Med. Pathol 30, 445–451 [Google Scholar]
- 95.Katagiri W et al. (2017) A defined mix of cytokines mimics conditioned medium from cultures of bone marrow-derived mesenchymal stem cells and elicits bone regeneration. Cell Prolif. 50, e12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katagiri W et al. (2015) Conditioned medium from mesenchymal stem cells enhances early bone regeneration after maxillary sinus floor elevation in rabbits. Implant. Dent 24, 657–663 [DOI] [PubMed] [Google Scholar]
- 97.Katagiri W et al. (2017) Clinical study of bone regeneration by conditioned medium from mesenchymal stem cells after maxillary sinus floor elevation. Implant. Dent 26, 607–612 [DOI] [PubMed] [Google Scholar]
- 98.Katagiri W et al. (2016) First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells. Head Face Med 12, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsuchiya S et al. (2015) An experimental study on guided bone regeneration using a polylactide-co-glycolide membrane-immobilized conditioned medium. Int. J. Oral Maxillofac. Implants 30, 1175–1186 [DOI] [PubMed] [Google Scholar]
- 100.Diomede F et al. (2018) Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res Ther 9, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bakota EL et al. (2011) Injectable multidomain peptide nanofiber hydrogel as a delivery agent for stem cell secretome. Biomacromolecules 12, 1651–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liang M et al. (2019) The therapeutic effect of secretome from human umbilical cord-derived mesenchymal stem cells in age-related osteoporosis. Artif. Cells Nanomed. Biotechnol 47, 1357–1366 [DOI] [PubMed] [Google Scholar]
- 103.Luo L et al. (2017) Fabrication of synthetic mesenchymal stem cells for the treatment of acute myocardial infarction in mice. Circ. Res 120, 1768–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang J et al. (2017) Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat. Commun 8, 13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar P et al. (2019) The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 46, 1–9 [DOI] [PubMed] [Google Scholar]
- 106.Skalnikova HK (2013) Proteomic techniques for characterisation of mesenchymal stem cell secretome. Biochimie 95, 2196–2211 [DOI] [PubMed] [Google Scholar]
- 107.Kapur SK and Katz AJ (2013) Review of the adipose derived stem cell secretome. Biochimie 95, 2222–2228 [DOI] [PubMed] [Google Scholar]
- 108.Mukherjee P and Mani S (2013) Methodologies to decipher the cell secretome. Biochim. Biophys. Acta Proteins Proteomics 1834, 2226–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lavoie JR and Rosu-Myles M (2013) Uncovering the secretes of mesenchymal stem cells. Biochimie 95, 2212–2221 [DOI] [PubMed] [Google Scholar]
- 110.Lener T et al. (2015) Applying extracellular vesicles based therapeutics in clinical trials-an ISEV position paper. J. Extracell. Vesicles 4, 30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Busatto S et al. (2020) The nanostructured secretome. Biomater. Sci 8, 39–63 [DOI] [PubMed] [Google Scholar]
- 112.Dostert G et al. (2017) How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication? Front. Cell Dev. Biol 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Margolis L and Sadovsky Y (2019) The biology of extracellular vesicles: the known unknowns. PLoS Biol. 17, e3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Murphy DE et al. (2019) Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp. Mol. Med 51, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Villa F et al. (2019) Extracellular vesicles as natural, safe and efficient drug delivery systems. Pharmaceutics 11, 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burnouf T et al. (2019) Extracellular vesicles as nanomedicine: hopes and hurdles in clinical translation. Int. J. Nanomedicine 14, 8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wiklander OP et al. (2019) Advances in therapeutic applications of extracellular vesicles. Sci. Transl. Med 11, eaav8521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mentkowski KI et al. (2018) Therapeutic potential of engineered extracellular vesicles. AAPS J. 20, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim MJ et al. (2016) Conditioned medium derived from umbilical cord mesenchymal stem cells regenerates atrophied muscles. Tissue Cell 48, 533–543 [DOI] [PubMed] [Google Scholar]
- 120.Ferreira A.d.F. et al. (2017) Extracellular vesicles from adipose-derived mesenchymal stem/stromal cells accelerate migration and activate AKT pathway in human keratinocytes and fibroblasts independently of miR-205 activity. Stem Cells Int. Published online November 5, 2019 10.1155/2017/9841035 [DOI] [PMC free article] [PubMed] [Google Scholar]


