Abstract
Objectives:
The conditioned-medium derived from corneal mesenchymal stromal cells (cMSCs) has been shown to have wound healing and immunomodulatory effects in corneal injury models. Here, the therapeutic effects of lyophilized cMSC conditioned-medium were compared with fresh conditioned-medium.
Methods:
The epithelial wound healing effects of fresh and lyophilized cMSC conditioned-medium were compared with conditioned-medium from non-MSC cells (corneal epithelial cells) using scratch assay. To evaluate the anti-inflammatory effects of fresh and lyophilized cMSC conditioned-media, macrophages were stimulated by a Toll-Like Receptor (TLR) ligand followed by treatment with the conditioned-media and measuring the expression of inflammatory genes. In vivo wound healing effects of fresh and lyophilized cMSC conditioned-media were assessed in a murine model of cornea epithelial injury.
Results:
Both fresh and lyophilized cMSCs-derived conditioned-medium induced significantly faster closure of in vitro epithelial wounds compared to conditioned-medium from non-MSC cells (P<0.0001). Treating stimulated macrophages with fresh or lyophilized cMSCs-derived conditioned-media significantly decreased the expression of inflammatory genes compared to control (P<0.0001). Murine corneal epithelial wounds were healed by 87.6±2.7% and 86.2±4.6% following treatment with fresh and lyophilized cMSC conditioned-media, respectively, while the control was healed by 64.7±16.8% (P<0.05).
Conclusion:
Lyophilized cMSC-derived conditioned-medium is as effective as fresh conditioned-medium in promoting wound healing and modulating inflammation. The results of this study support the application of lyophilized cMSCs-derived conditioned-medium, which allows for more extended storage, as a promising non-invasive option in the treatment of corneal wounds.
Keywords: Human Cornea, Mesenchymal Stromal Cell, Wound Healing, Conditioned Media, conditioned-medium, Lyophilization
Introduction:
The past two decades has seen increased interest in regenerative therapies using mesenchymal stromal cells (MSCs)1 based on their anti-inflammatory and trophic properties2. Earlier studies in rabbit3 and rat4–6 models of alkali corneal injury have demonstrated that topical application4, intravenous administration3, surgical grafting5, and subconjunctival injection6 of MSCs substantially promoted corneal wound healing, while inhibiting neovascularization and inflammation3,5,6. These wound healing and anti-inflammatory properties are shared by MSCs derived from different anatomical origins (e.g. adipose, bone marrow) 4,7, however, MSCs also have distinct qualities befitting their tissue of origin. Whereas MSCs derived from bone marrow and adipose tissue secrete more proangiogenic factors (e.g. upregulation of VEGF, MCP-1) 3,8–14, cornea-derived mesenchymal stromal cells (cMSCs) upregulate antiangiogenic factors specific to their native role in maintaining corneal avascularity15.
Although the therapeutic effects of MSCs were previously attributed to engrafting and differentiating to directly replace the damaged tissue, recent studies have found that their beneficial effects are largely derived from their secreted bioactive factors 7. In particular, MSC-derived conditioned media (CM) was found to be comparable to transplanted MSCs in effectiveness8. Studies in rat models have shown enhanced wound healing after topical application of MSC- conditioned-medium in the form of CM from human uterine cervical stem cells16 and adipose-derived mesenchymal stem cells17. These findings have profound implications, as cell-free therapies such as MSC- conditioned-medium circumvent major challenges of stem-cell based therapies, including immunogenic and tumorigenic risks, difficult storage and maintenance, and high costs8,16–18. In particular, lyophilized (i.e. freeze-dried) MSC- conditioned-medium can be stored for extended periods, transported conveniently, distributed in consistent concentrations, and readily adjusted for potency19.
In this study, we investigated the effect of lyophilized cMSC-derived conditioned-medium using in vitro and in vivo models of corneal epithelial wound healing. In particular, we aim to determine whether cMSC-derived conditioned-medium retains its therapeutic properties after lyophilization. Our results may support the feasibility and clinical applicability of this treatment options for corneal epithelial wound healing disorders.
Material and Methods:
MSC isolation and culture
cMSCs were obtained from healthy human cadaver corneas, which were generously provided by the Eversight eye bank (Chicago, IL) and stored in Optisol corneal storage medium (Chiron Ophthalmics, Irvine, California). According to the guidelines of the Office of Protection of Research Subjects (OPRS) at the University of Illinois at Chicago, IRB approval was waived because the corneas were obtained from human cadaver donors and used without any identifiers. All experiments were performed in biosafety cabinets under sterile conditions. After removal from storage media, the human cadaver corneas were washed three times with sterile phosphate-buffered saline (PBS) containing 1% Penicillin/Streptomycin and 1% gentamicin. The central corneas were removed using an 8-mm trephine. The remaining corneoscleral rim was cut into 4 pieces and each piece was cultured with epithelial surface up in one well of a 6-well tissue culture plate containing MEM-α media supplemented with 10% fetal bovine serum, 1% Penicillin-Streptomycin, L-Glutamine, and NEAA (all from Corning, Manassas, VA) incubated at 37 °C in a humid atmosphere with 5% CO2 as described before 20. Two hundred μL of media was put on top of each explant for the first few days to decrease the likelihood of explants detaching from the bottom of the dish. After the explants attached, the culture media were gently replaced every other day. After outgrowing the cells (mixed MSCs and epithelial cells) for 7 days, the explants were gently removed using fine-tip iris forceps, and the cells (Passage 0) were detached using 0.05% TrypLE (Thermo Fisher Scientific). The isolated cells which were the mixture of mesenchymal and epithelial cells, were expanded in cell culture media, which was changed every 2 to 3 days. After two passage in cultured media, only MSCs grew as previously defined 20. Passage 4 cMSCs were used to harvest conditioned-medium.
Producing Fresh and Lyophilized Conditioned-Medium
Human corneal-limbal epithelial (HCLE) cell line (kindly provided by Ilene Gipson, PhD) were used as a non-MSC source for producing a control conditioned-medium. HCLE cells were grown in keratinocyte serum-free medium (KSFM, Invitrogen, Grand Island, NY, USA). When the cells (cMSCs or HCLE cells) reached 80–90% confluency, they were ready for obtaining conditioned-medium. The cells were washed three times with pre-warmed PBS after which the media was changed to phenol red-free MEM-α without serum (containing L-Glutamine, NEAA and 1% Penicillin-Streptomycin, 20 mL in a T-175 flask) and incubated for 48 hours. The supernatant was then collected and centrifuged at 500 ×g for 15 minutes at 4 °C to eliminate any cell contamination. The resultant supernatant after centrifugation, fresh conditioned media (CM) containing cell conditioned-medium, was stored in a 50-mL sterile tubes at 4 °C and used within 2–3 days.
To obtain freeze-dried conditioned-media, fresh cMSC-derived conditioned-medium was snap-frozen in liquid nitrogen and transferred to a lyophilizer (a.k.a. freeze-dryer, LABCONCO, USA) and lyophilized at −55°C and <100 mBar for 48 hours. Each 1 mL of fresh conditioned media yielded 10 mg of lyophilized conditioned-medium in powder form. In order to yield a direct comparison, the same donor cells were used to generate the lyophilized and fresh conditioned-media used in our study. The freeze-dried conditioned-medium in powder form was stored at −80°C, up to 3 months. At the time of experiments, the lyophilized powder was dissolved in deionized water and then filtered through a 0.2 μm filter to produce a sterile final product and used right away.
Scratch Assay
The therapeutic effects of the conditioned-media derived from lyophilized and fresh cMSCs and fresh HCLE were compared using an in vitro wound healing (scratch) assay 21. In brief, HCLEs were cultured in 6-well plates using KSFM media. After 100% confluency, a plus-shaped wound (two perpendicular scratch lines) was gently made by hand using a sterile 20–200 μL pipette tip at a 90-degree angle, thereby forming precise edges (SJ). The loosened epithelial cells were removed by washing each well three times with pre-warmed PBS. Then the cells were treated is the following combination of media and conditioned-media (N=3 wells per each combination): (1) 1 mL of KSFM plus 2 mL of freshly derived conditioned-medium from HCLE cells, (2) 1 mL of KSFM plus 2 mL of MEM-α as control (containing L-Glutamine, NEAA and 1% Penicillin-Streptomycin), (3) 1 ml KSFM plus 2 ml of dissolved lyophilized cMSCs-derived conditioned-medium in pre-warmed deionized water (10 mg/ml and 0.2 μm-filter sterilized), or (4) 1 mL of KSFM plus 2 mL of freshly derived conditioned-medium from cMSCs. This scratch assay was replicated ten times. The migration of HCLEs and closure of the created wounds was evaluated every hour for 24 hours using an inverted microscope with digital camera (Carl Zeiss, Germany). The captured photos of the two 6-well plates were analyzed using ImageJ software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997–2018.).
Expression of Pro-Inflammatory Mediators
The anti-inflammatory effects of MSC conditioned-medium was evaluated using an assay involving Toll-like receptor (TLR) 3 -induced inflammatory gene expression in macrophages 22. Macrophage cells (J774.2 Cell Line, mouse BALB/C monocyte macrophage, kindly provided by Richard Novak, MD) were grown in 6-well plates in RPMI media (Cellgro) containing 1% Penicillin-Streptomycin, 1% L-Glutamine, 1% HEPES and 10% FBS. After reaching 80% confluency, the cells were starved in serum-free MEM-α media overnight. The cells were then stimulated with the TLR 3 ligand Polyinosinic:polycytidylic acid (PIC, Sigma) 100 μg/mL dissolved in MEM-α media containing 5% FBS for 4 hours at 37 °C. After that, the cells were washed with pre-warmed PBS and each well was treated with (1) 1 ml of MEM-α media plus 2 mL of fresh cMSC conditioned-medium, (2) 1 ml of MEM-α media plus 2 ml of dissolved lyophilized cMSC conditioned-medium (10 mg/mL dissolved in deionized water and filtered through a 0.2 μm filter), or (3) 3 ml of MEM-α media as control. The treated cells were incubated at 37 °C in a humid atmosphere with 5% CO2 for 4 hours. After 4 hours of treatment, the cells were removed by scraping from each well, and the supernatant from each well was collected and centrifuged. The pellets were then lysed with RNA lysis buffer for RNA extraction. The RNA was extracted from cells according to the protocol described previously23 per manufacturer’s instructions (RNeasy Protect Mini Kit, Qiagen). Reverse-transcriptase (RT) reaction was performed using extracted mRNA and a cDNA synthesis kit (Super Script First-Strand Synthesis System, Invitrogen, Carlsbad, CA). Relative quantitative polymerase chain reaction (qPCR) was performed using intron spanning primers for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ICAM1, TLR3, IL-6, IL-8 and TNF-alpha according to the manufacturer’s protocol (Fast SYBER Green Master Mix, Applied Biosystems) (Table 1). Production of these mediators of inflammation and angiogenesis have been inhibited by MSCs in previous experiments on corneal wound healing7,8,17,22. All samples were run in triplicates, and each experiment was repeated three times. Negative controls using samples without reverse transcriptase were included in the qPCR step to confirm that the results were not affected by DNA contaminants. Quantified results of each reaction were analyzed. The relative ΔΔCT mRNA expression was measured by normalization to GAPDH.
Table 1:
The sequence of primers used in qRT-PCR
| Gene | Primer -forward | Primer-reverse |
|---|---|---|
| ICAM-1 | 5’ TGT TTC CTG CCT CTG AAG C 3’ | 5’ CTT CGT TTG TGA TCC TCC G 3’ |
| IL 6 | 5’ CCG GAG AGG AGA CTT CAC AG 3’ | 5’ GGA AAT TGG GGT AGG AAG GA 3’ |
| IL 8 | 5’ CAC CTC AAG AAC ATC CAG AGC T 3’ | 5’ CAA GCA GAA CTG AAC TAC CAT CG 3’ |
| TNF-alpha | 5’ TAC TGA ACT TCG GGG TGA TTG GTC C 3’ | 5’ CAG CCT TGT CCC TTG AAG AGA ACC 3’ |
| GAPDH | 5’ ACC ACA GTC CAT GCC ATC AC 3’ | 5’ CAC CAC CCT GTT GCT GTA GCC 3’ |
in vivo Model of Corneal Epithelial Wound Healing
All animal experiments in this study were conducted in compliance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The animal protocols were approved by the Animal Care Committee (ACC) at the University of Illinois at Chicago (Chicago, IL).
Thirty male C57BL/6J mice (5–6 months-old) were anesthetized with intraperitoneal injection of ketamine (100 mg/kg) and xylazine (5 mg/kg). Anesthetized mice were positioned under a surgical microscope, one drop of 0.5% proparacaine was applied to the eye, and a 2.5 mm diameter area was demarcated using a 2.5 mm trephine. The corneal epithelial layer in the demarcated area was removed gently using an AlgerBrush II (The Alger Companies, Lago Vista, TX). A baseline photograph of the fluorescein-stained eye using a Nikon FS-2 slit lamp biomicroscope was taken. Pilot studies determined the optimal concentration of the lyophilized CM for in vivo assays to be 15mg/ml (10, 15 and 20mg/ml were compared). Fresh conditioned-media (N=10, one eye per mouse) or 15 mg/mL lyophilized conditioned-media (N=10 per each concentration, one eye per mouse) were topically applied as an eye drop. MEM-α was applied with the same pattern in the control group (N=10, one eye per mouse). The treated eyes were evaluated after 22 hours and photographed. The wounded area in captured photos was measured using ImageJ software and the percentage of wound closure in comparison to baseline was calculated.
Statistical Analysis
The results of this study are presented as mean ± standard deviation (SD). GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, California USA, www.graphpad.com) was used for statistical analyses. One-way ANOVA test with Tukey’s post-hoc performed for analyses of mean difference in continuous data (scratch assay results and in vivo 2.5-mm corneal epithelial wound model results). A p-value of <0.05 was considered statistically significant.
Results:
In vitro Effects of cMSCs Conditioned-Medium on Epithelial Wound Healing
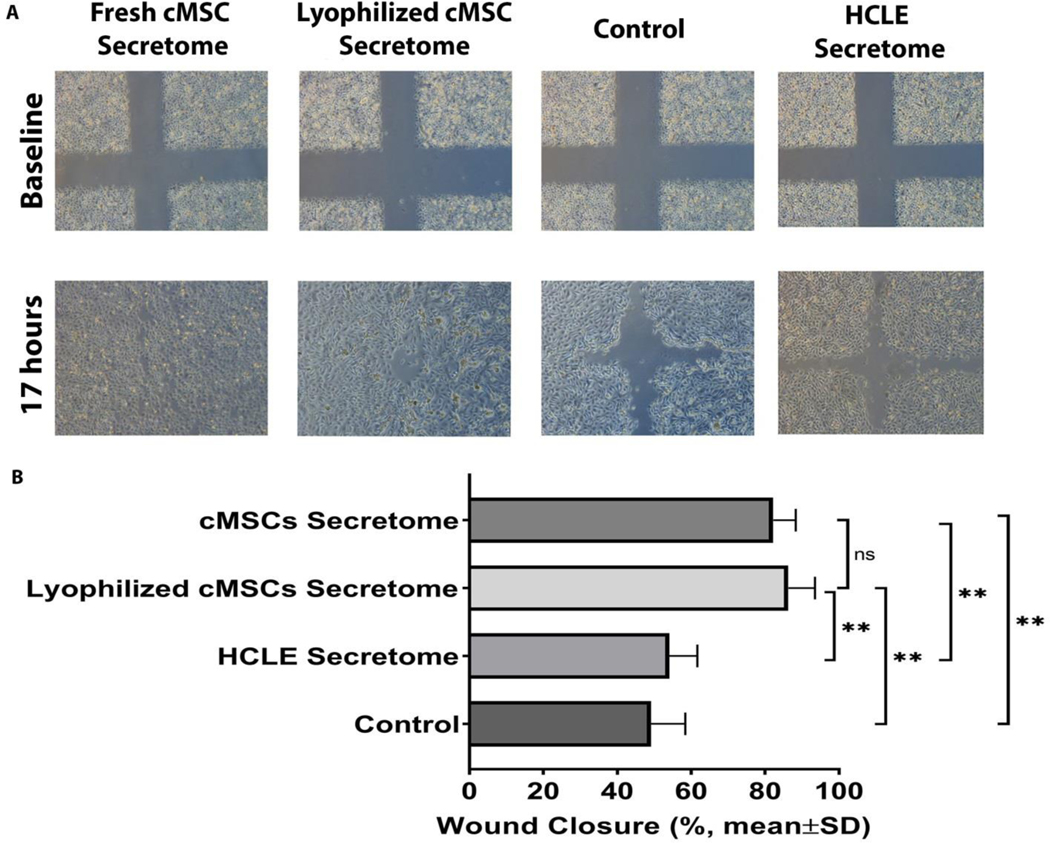
Fresh and lyophilized conditioned-media from cMSC and fresh conditioned-medium from HCLE cells were tested in a scratch assay to evaluate their in vitro wound healing effects and to determine if the therapeutic effect of cMSCs-derived conditioned-medium is seen in conditioned-media derived from other cell types as well. After 17 hours, the wounds treated by HCLE cells-derived conditioned-medium and unconditioned media (control) were closed by 54.1±7.6, and 49.1 ± 9.3%, compared to 82.2 ± 6.1% and 86.3 ± 7.2% in wounds treated with fresh and lyophilized cMSCs conditioned-media, respectively (P<0.0001, N=3 wells per group, Figure 1).
Figure 1:
The healing effects of lyophilized and fresh cMSCs-derived conditioned-medium compared with HCLE-derived conditioned-medium and unconditioned media (control) on in vitro scratch (wound healing) assay of HCLE cells. The representative images (A) show faster wound closure in wounds treated with lyophilized and fresh cMSC-derived conditioned-medium compared with HCLE-derived conditioned-medium and control. Quantitative comparison (B) showed that after 17 hours, wounds treated with fresh and lyophilized cMSCs’ conditioned-medium were closed by 82.2 ± 6.1% and 86.3 ± 7.2%, respectively, while HCLE-derived conditioned-medium and control wounds were closed by 54.1±7.6% and 49.1 ± 9.3%, respectively. There was no significant difference between fresh and lyophilized cMSCs’ conditioned-medium (****, P<0.0001, ns, Not Significant, N=3 wells per each group).
Effect of Fresh/Lyophilized cMSC Conditioned-Medium on the Expression of Pro-Inflammatory Genes
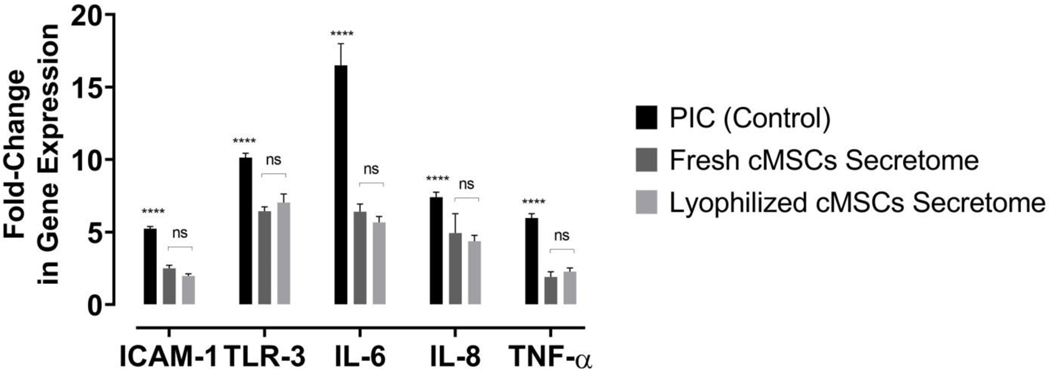
The TLR assay was performed to evaluate the anti-inflammatory effects of freshly derived cMSCs conditioned-medium and compare the results with the anti-inflammatory effects of lyophilized cMSCs-derived conditioned-medium. Therefore, the mRNA expression of the inflammatory genes including ICAM 1, TLR3, IL-6, IL-8, and TNF alpha was measured in lymphocytes stimulated with PIC (Toll-like receptor ligand) and following treatment with fresh and lyophilized cMSC conditioned-media. As shown in Figure 2, the mRNA expressions of all evaluated inflammatory genes were significantly lower in PIC-stimulated macrophage cells treated with fresh or lyophilized cMSC conditioned-medium compared with control (P<0.0001, Figure 2). Moreover, there was no significant difference between the anti-inflammatory effects of fresh and lyophilized cMSCs conditioned-medium (Figure 2).
Figure 2:
The anti-inflammatory effects of fresh and lyophilized cMSCs-derived conditioned-media on the mRNA expression of pro-inflammatory cytokines in stimulated macrophages with Toll-Like Receptor-3 ligand (PIC) (****, P<0.0001 compared to the expression of same gene, ns, Not Significant, N=3).
In vivo Wound Healing effects of Lyophilized cMSCs Conditioned-Medium
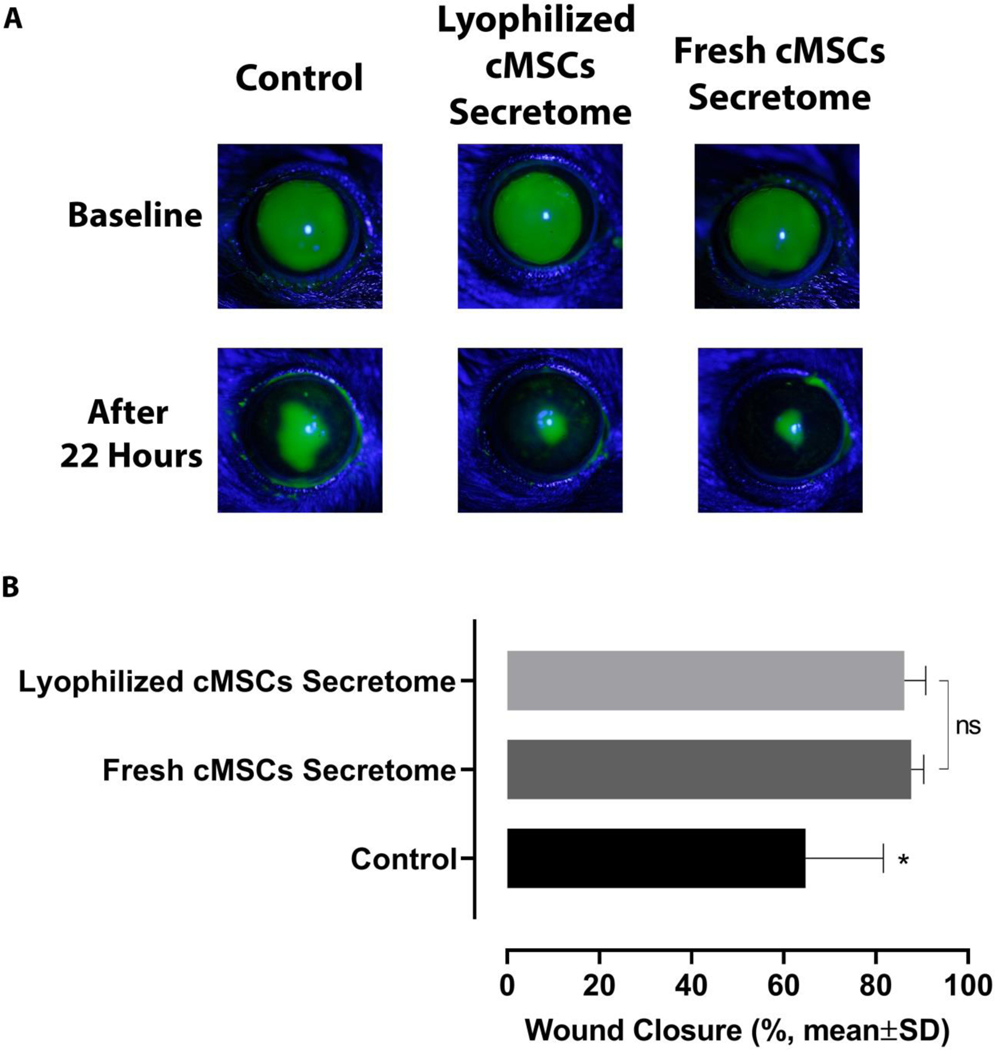
To evaluate the in vivo corneal epithelial wound healing effects of both fresh and lyophilized cMSCs conditioned-medium, the samples were applied (as eye-drop-12ul) on the ocular surface after 2.5 mm corneal epithelial debridement wounds. The closure of the corneal wounds was followed-up by fluorescein staining 22 hours later. The results of in vivo corneal epithelial wound healing following treatment with lyophilized (15 mg/ml) or fresh cMSC conditioned-medium showed that the average healed (re-epithelialized) area in mice treated with fresh conditioned-medium or lyophilized conditioned-media were significantly higher compared with controls after 22 hours (87.6±2.7%, 86.1±4.6% and 64.7±16.8%, respectively, P<0.05, N=10 eyes per group, Figure 3), while there was no significant difference between the in vivo wound healing effect of fresh and lyophilized cMSCs conditioned-medium (Figure 3).
Figure 3:
The effects of lyophilized or fresh cMSCs-derived conditioned-medium on corneal epithelial wound healing following corneal epithelial debridement (2.5 mm) in mouse model compared with control. Representative images (A) show greater closure of wound in eyes treated with fresh or freeze-dried cMSCs-derived conditioned-media compared with control after 22 hours. Quantitative measurement of healed epithelial wounds (B) shows that topically applied fresh or lyophilized cMSCs conditioned-medium resulted in 87.6±2.7% and 86.1±4.6% closure, respectively; while, the mean wound closure percentage was 64.7±16.8% in control eyes. There was no significant difference between fresh and lyophilized cMSCs’ conditioned-medium (*, P<0.05, ns, Not Significant, N=10 eyes per group).
Discussion:
In this study, we aimed to evaluate the therapeutic potential of lyophilized cMSC conditioned-medium in the treatment of corneal epithelial wound healing. We demonstrated that lyophilized cMSC conditioned-medium has preserved wound healing efficacy in both in vitro and in vivo epithelial wound healing models compared to fresh formulations. We also demonstrated that the conditioned-medium produced by cMSCs has higher in vitro efficacy than that secreted by HCLE cells. Ultimately, both lyophilized and fresh formulations of conditioned-medium were found to have similar efficacy in reducing inflammatory gene expression as well as in vitro wound healing by the scratch assay. Finally, application of lyophilized and fresh conditioned-medium to in vivo wounds in our murine model demonstrated similar efficacy and had promising effects when compared to untreated controls.
The use of cMSCs in the treatment of corneal epithelial wounds has been demonstrated in prior studies16,21. Further, the conditioned-medium produced by cMSCs is thought to confer a significant portion of cMSC therapeutic effect24, 25, and the use of the conditioned-medium rather than the cMSCs themselves may allow for advantages over cell-based therapies with regards to shelf life, handling conditions, and immunologic rejection considerations. The lyophilization of the conditioned-medium would confer even further improvements in product stability, as lyophilized bioactive products can have a shelf life on the order of years26. Lyophilized products can be easily stored and the concentration can easily be controlled upon - important considerations when transitioning to clinical use. Moreover, the use of lyophilized conditioned-medium allows for the ability to use the same batch of product for future testing. Lyophilized conditioned media from various MSC sources have already demonstrated efficacy in various applications, including regeneration of bone27, lung28, skin29, and cornea19. Our study suggests that the lyophilization process does not appear to affect the biochemical activity of the conditioned-medium in the case of cMSCs. The extended shelf life of lyophilized conditioned-medium can be confirmed by determining the stability and efficacy of the product at various storage times, which may be a subject of further study. Additionally, the optimal concentration of lyophilized conditioned-medium may be evaluated in future studies, along with different delivery vehicles. Of note, we found 1x concentration (10mg/ml) to be effective in in vitro experiments while in vivo 1.5x (15mg/ml) was more effective than the 1x concentration (data not shown), highlighting the advantage of lyophilized conditioned-medium in adjusting the final concentration.
Local tissue-derived MSCs, such as cMSCs, may have specific beneficial effects on local processes when compared to MSCs derived from other tissue. Specifically, cMSC conditioned-medium has been shown to have direct antiangiogenic properties16 in addition to modulation of macrophages to anti-inflammatory phenotype 21,30. These properties are thought to be conferred by the activity of such cytokines as pigment epithelium-derived factor (PEDF) and soluble fms-like tyrosine kinase-1 (sFLT-1). The increased epithelial cell proliferation, migration and wound healing functions followed by treating with stem cell-derived secretome has been reported in previous studies 31–34. The accelerated proliferation has been reported due to immunomodulatory effects of conditioned media and reducing inflammatory profile 35, as well as activation of protein kinase B (Akt) pathway 36, as one of the major pathways in regulating the epithelial cell migration 37. In addition, higher concentrations of fibroblast growth factor (FGF) which is implicated in wound healing process and regeneration, has been found in stem cell-derived conditioned media 34.
Moreover, perhaps one reason that epithelial conditioned media did not have any effect is that the epithelial cells (in vitro scratch assay) or the injured epithelium (in vivo experiment), may already be exposed to their own secreted factors so adding additional epithelial derived secretome may not activate any new pathways. The importance of mesenchymal signals in promoting epithelial healing has been demonstrated in many studies in the past (30–36).
In summary, we have demonstrated the efficacy of lyophilized cMSC conditioned-medium for the treatment of corneal epithelial wounds. Lyophilization of the conditioned-medium extends many of the conditioned-medium’s existing advantages over cell-based therapies, and improves stability and therefore clinical applicability.
Acknowledgments
This work was supported by R01 EY024349 (ARD) and Core Grant for Vision Research EY01792 (MIR) from NEI/NIH; unrestricted grant to the department from Research to Prevent Blindness, and Eversight (providing both seed funding and human corneal research tissue). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References:
- 1.Savvatis K, van Linthout S, Miteva K, Pappritz K, Westermann D, Schefold JC, Fusch G, Weithäuser A, Rauch U, Becher PM, et al. Mesenchymal stromal cells but not cardiac fibroblasts exert beneficial systemic immunomodulatory effects in experimental myocarditis. PLoS One. 2012;7(7):e41047. doi: 10.1371/journal.pone.0041047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray IR, Péault B. Q&A: Mesenchymal stem cells - where do they come from and is it important? BMC Biol. BMC Biol. 2015; 23;13:99. doi: 10.1186/s12915-015-0212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 4.Jia Z, Jiao C, Zhao S, Li X, Ren X, Zhang L, Han ZC, Zhang X. Immunomodulatory effects of mesenchymal stem cells in a rat corneal allograft rejection model. Exp Eye Res. 2012;102:44–49. doi: 10.1016/j.exer.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Ye J, Yao K, Kim JC. Mesenchymal stem cell transplantation in a rabbit corneal alkali burn model: engraftment and involvement in wound healing. Eye (Lond). 2006;20:482–490. doi: 10.1038/sj.eye.6701913. [DOI] [PubMed] [Google Scholar]
- 6.Jiang T-S, Cai L, Ji W-Y, Hui YN, Wang YS, Hu D, Zhu J. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis. 2010;14; 16:1304–1316. [PMC free article] [PubMed] [Google Scholar]
- 7.Yao L, Li Z, Su W, Li YP, Lin ML, Zhang WX, Liu Y, Wan Q, Liang D. Role of mesenchymal stem cells on cornea wound healing induced by acute alkali burn. PLoS One. 2012;7(2):e30842. doi: 10.1371/journal.pone.0030842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, Rosa RH Jr, Prockop DJ. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-α stimulated gene/protein 6. Stem Cells. 2011;29 (10):1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 9.Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017; 25;18(9). pii: E1852. doi: 10.3390/ijms18091852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira JR, Teixeira GQ, Santos SG, Barbosa MA, Almeida-Porada G, Gonçalves RM. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front Immunol. 2018; 9:2837. doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bortolotti F, Ukovich L, Razban V, Martinelli V, Ruozi G, Pelos B, Dore F, Giacca M, Zacchigna S. In vivo therapeutic potential of mesenchymal stromal cells depends on the source and the isolation procedure. Stem Cell Reports. 2015; 4:332–339. doi: 10.1016/j.stemcr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pires AO, Mendes-Pinheiro B, Teixeira FG, Anjo SI, Ribeiro-Samy S, Gomes ED, Serra SC, Silva NA, Manadas B, Sousa N, et al. Unveiling the Differences of Secretome of Human Bone Marrow Mesenchymal Stem Cells, Adipose Tissue-Derived Stem Cells, and Human Umbilical Cord Perivascular Cells: A Proteomic Analysis. Stem Cells Dev. 2016;25:1073–1083. doi: 10.1089/scd.2016.0048. [DOI] [PubMed] [Google Scholar]
- 13.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 14.Estrada R, Li N, Sarojini H, An J, Lee MJ, Wang E. Secretome from mesenchymal stem cells induces angiogenesis via Cyr61. J Cell Physiol. 2009;219:563–571. doi: 10.1002/jcp.21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS ONE. 2012;7:e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eslani M, Putra I, Shen X, Hamouie J, Afsharkhamseh N, Besharat S, Rosenblatt MI, Dana R, Hematti P, Djalilian AR. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Invest Ophthalmol Vis Sci. 2017;58:5507–5517. doi: 10.1167/iovs.17-22680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermudez MA, Sendon-Lago J, Eiro N, Treviño M, Gonzalez F, Yebra-Pimentel E, Giraldez MJ, Macia M, Lamelas ML, Saa J, et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Invest Ophthalmol Vis Sci. 2015;56:983–992. doi: 10.1167/iovs.14-15859. [DOI] [PubMed] [Google Scholar]
- 18.Shibata S, Hayashi R, Okubo T, Kudo Y, Baba K, Honma Y, Nishida K. The secretome of adipose-derived mesenchymal stem cells attenuates epithelial-mesenchymal transition in human corneal epithelium. Regen Ther. 2019;11:114–122. doi: 10.1016/j.reth.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes-Cunha GM, Na K-S, Putra I, Lee HJ, Hull S, Cheng YC, Blanco IJ, Eslani M, Djalilian AR, Myung D. Corneal Wound Healing Effects of Mesenchymal Stem Cell Secretome Delivered Within a Viscoelastic Gel Carrier. Stem Cells Transl Med. 2019;8:478–489. doi: 10.1002/sctm.18-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabbehdari S, Yazdanpanah G, Kanu LN, Anwar KN, Shen X, Rabiee B, Putra I, Eslani M, Rosenblatt MI, Hematti P, et al. Reproducible Derivation and Expansion of Corneal Mesenchymal Stromal Cells for Therapeutic Applications. Transl Vis Sci Technol. 2020;9(3):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eslani M, Putra I, Shen X, Hamouie J, Tadepalli A, Anwar KN, Kink JA, Ghassemi S, Agnihotri G, Reshetylo S, et al. Cornea-Derived Mesenchymal Stromal Cells Therapeutically Modulate Macrophage Immunophenotype and Angiogenic Function. Stem Cells. 2018;36:775–784. doi: 10.1002/stem.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53(3):248–56. [DOI] [PubMed] [Google Scholar]
- 23.Djalilian AR, Namavari A, Ito A, Balali S, Afshar A, Lavker RM, Yue BY. Down-regulation of Notch signaling during corneal epithelial proliferation. Mol Vis. 2008;14:1041–1049. [PMC free article] [PubMed] [Google Scholar]
- 24.Konala VBR, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy. 2016;18:13–24. doi: 10.1016/j.jcyt.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P, Eslani M, Djalilian AR. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Invest Ophthalmol Vis Sci. 2018;59:5194–5200. doi: 10.1167/iovs.18-24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuijen B, Bouma M, Henrar REC, Floriano P, Jimeno JM, Talsma H, Kettenes-van den Bosch JJ, Heck AJ, Bult A, Beijnen JH. Pharmaceutical Development of a Parenteral Lyophilized Formulation of the Novel Antitumor Agent Aplidine. PDA J Pharm Sci Technol. 2000; 54:193–208. [PubMed] [Google Scholar]
- 27.Katagiri W, Osugi M, Kawai T, Hibi H. First-in-human study and clinical case reports of the alveolar bone regeneration with the secretome from human mesenchymal stem cells. Head Face Med. 2016;12:5. doi: 10.1186/s13005-016-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cargnoni A, Ressel L, Rossi D, Poli A, Arienti D, Lombardi G, Parolini O. Conditioned medium from amniotic mesenchymal tissue cells reduces progression of bleomycin-induced lung fibrosis. Cytotherapy. 2012;14:153–161. doi: 10.3109/14653249.2011.613930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Y, Xuan M, Zou J, Liu H, Zhuo Z, Wan Y, Cheng B. Freeze-Dried Rat Bone Marrow Mesenchymal Stem Cell Paracrine Factors: A Simplified Novel Material for Skin Wound Therapy. Tissue Eng Part A. 2015;21:1036–1046. doi: 10.1089/ten.tea.2014.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stojanovic S, Najman S. The effect of conditioned media of stem cells derived from lipoma and adipose tissue on macrophages’ response and wound healing in indirect co-culture system in vitro. Int J Mol Sci. 2019;20:1671. doi: 10.3390/ijms20071671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SR, Kim JW, Jun HS, Roh JY, Lee HY, Hong IS. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol Ther. 2018; 26:606–617. doi: 10.1016/j.ymthe.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Zhao J, Hu L, Liu J, Gong N, Chen L. The effects of cytokines in adipose stem cell-conditioned medium on the migration and proliferation of skin fibroblasts in vitro. Biomed Res Int. 2013;2013:578479. doi: 10.1155/2013/578479. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Noverina R, Widowati W, Ayuningtyas W, Kurniawan D, Afifah E, Laksmitawati DR, Rinendyaputri R, Rilianawati R, Faried A, Bachtiar I, et al. Growth factors profile in conditioned medium human adipose tissue-derived mesenchymal stem cells (cm-hatmscs) Clin Nutr Exp. 2019;24:34–44. doi: 10.1016/j.yclnex.2019.01.002. [DOI] [Google Scholar]
- 35.Irons RF, Cahill KW, Rattigan DA, Marcotte JH, Fromer MW, Chang S, Zhang P, Behling EM, Behling KC, Caputo FJ. Acceleration of diabetic wound healing with adipose-derived stem cells, endothelial-differentiated stem cells, and topical conditioned medium therapy in a swine model. J Vasc Surg. 2018;68:115S–125S. doi: 10.1016/j.jvs.2018.01.065. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira ADF, Cunha PDS, Carregal VM, da Silva PC, de Miranda MC, Kunrath-Lima M, de Melo MIA, Faraco CCF, Barbosa JL, Frezard F, et al. Extracellular vesicles from adipose-derived mesenchymal stem/stromal cells accelerate migration and activate akt pathway in human keratinocytes and fibroblasts independently of mir-205 activity. Stem Cells Int. 2017;2017:9841035. doi: 10.1155/2017/9841035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue G, Hemmings BA. PKB/Akt-dependent regulation of cell motility. J Natl Cancer Inst. 2013;105:393–404. doi: 10.1093/jnci/djs648. [DOI] [PubMed] [Google Scholar]