Abstract
HIV infection affects an estimated 38 million people. Approximately 50% of HIV patients exhibit neurocognitive dysfunction termed HIV-Associated Neurocognitive Disorder (HAND). HAND is a consequence of chronic low-level neuroinflammation due to HIV entry into the brain. Initially, monocytes become activated in circulation and traffic to the brain. Monocytes, when activated, become susceptible to infection by HIV and can then carry the virus across the blood brain barrier. Once in the brain, activated monocytes secrete chemokines, which recruit virus-specific CD8+ T cells into the brain to further promote neuroinflammation. HAND is closely linked to systemic inflammation driven, in part, by HIV but is also due to persistent translocation of microorganisms across the GI tract. Persistent anti-viral responses in the GI tract compromise microbial barrier integrity. Indeed, HIV patients can exhibit remarkably high levels of activated (CD16+) monocytes in circulation. Recent studies, including our own, show that HIV patients using medical marijuana exhibit lower levels of circulating CD16+ monocytes than non-cannabis using HIV patients. Cannabis is a known immune modulator, including anti-inflammatory properties, mediated, in part, by Δ9-tetrahydrocannabinol (THC), as well as less characterized minor cannabinoids, such as cannabidiol (CBD), terpenes and presumably other cannabis constituents. The immune modulating activity of THC is largely mediated through cannabinoid receptors (CB) 1 and 2, with CB1 also responsible for the psychotropic properties of cannabis. Here we discuss the anti-inflammatory properties of cannabinoids in the context of HIV and propose CB2 as a putative therapeutic target for the treatment of neuroinflammation.
Graphical abstract description
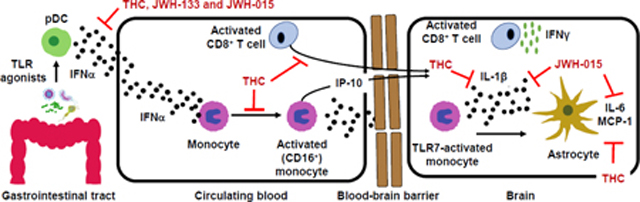
HIV-associated neurocognitive disorder is a systemic inflammatory disease leading to activation of plasmacytoid dendritic cells, monocytes and T cells. Monocyte and CD8 T cell migration across the BBB and interaction with astrocytes promotes neurotoxic inflammatory mediators release. CB2 ligands are proposed as therapeutics capable of suppressing systemic and localized inflammation.
Graphical Abstract
Introduction
HIV infection affects an estimated 38 million people worldwide according to the World Health Organization. With antiretroviral therapy (ART), a major research priority is to understand the health complications that arise in patients on ART. ART is successful in suppressing viral replication and restoring CD4+ T cell levels, turning infection by HIV into a manageable condition and increasing life expectancy. Consequently, serious health complications remain in ART-treated patients, which include HIV-associated neurocognitive disorders (HAND) and cardiovascular diseases (Cardoso et al., 2013).
Approximately 50% of HIV patients exhibit neurocognitive dysfunction, from mild to severe forms (Heaton et al., 2011; Rumbaugh and Tyor, 2015; Saylor et al., 2016). HAND is due to HIV entry into the central nervous system (CNS) and the chronic low-level neuroinflammation that ensues (Kaul et al., 2001; Valcour et al., 2012; Hong and Banks, 2015). A major contributing mechanism to HAND is chronic, systemic peripheral inflammation in which leukocytes are activated and infected by HIV in the periphery prior to migrate across the blood-brain barrier (BBB) (Clay et al., 2007; Ellery et al., 2007; Campbell et al., 2014b). Peripheral chronic immune activation during HIV infection occurs through several mechanisms including gut microbial translocation and residual HIV (Anzinger et al., 2014). Importantly, pathogen associated products (e.g., LPS and HIV associated proteins) released into circulation lead to activation of circulating leukocytes via interaction with pattern-recognition receptors (e.g. TLRs) (Kawai and Akira, 2006; Jiang et al., 2009; Vassallo et al., 2012; Anzinger et al., 2014; Cha et al., 2014; Donninelli et al., 2016). HIV entry into the CNS raises serious problems for ART-treated patients as many antiretroviral drugs have poor penetrance through the BBB, creating a privileged site for HIV replication and survival (Letendre et al., 2008).
There is a growing body of evidence that monocytes and CD8+ T cells are key contributors to neuroinflammation due to their transport of HIV into the CNS and secretion of inflammatory factors (Clay et al., 2007; Anzinger et al., 2014; Campbell et al., 2014a; Campbell et al., 2014b; Williams et al., 2014a). Consequently, the leukocyte-derived inflammatory factors can directly promote neuronal injury (e.g., IFNγ-inducible protein 10 (IP-10)) and indirectly through causing astrocyte dysfunction (e.g., IL-1β), including impaired glutamate uptake and dysregulated secretion of inflammatory proteins (Andjelkovic et al., 2000; Kaul et al., 2001; Gonzalez-Scarano and Martin-Garcia, 2005; Brabers and Nottet, 2006; Sui et al., 2006; Rizzo et al., 2019).
The anti-viral cytokine, interferon-α (IFNα), is chronically produced in response to microbial translocation and residual HIV, and has been identified to have an important role in chronic immune activation (Cha et al., 2014). Specifically, chronic IFNα production in HIV patients has been linked to neurocognitive impairment and disease progression (Rho, 1995; Sas et al., 2009; Anderson et al., 2017). Recent studies have demonstrated a type I IFN gene signature in leukocytes (e.g., monocytes) of HIV patients suggesting exposure to IFNα in vivo (Rempel et al., 2010; Pulliam et al., 2011; Cha et al., 2014). Recently, we have shown that IFNα directly induces monocyte expression of the proinflammatory/activation markers, CD16 and CD163, from HIV- and HIV+ individuals (Rizzo et al., 2018), which is important as the expression of these markers are elevated on monocytes in post-mortem brain tissue of HIV patients with HAND (Fischer-Smith et al., 2001; Fischer-Smith et al., 2008b; Tavazzi et al., 2014). Furthermore, IFNα is a potent signal for promoting IFNγ secretion by CD8+ T cells, a process implicated in HAND (Schrier et al., 2015).
Cannabis use is common amongst HIV-infected individuals in the United States, with an estimated prevalence of 23–56% (Ware et al., 2003; Okafor et al., 2017; Pacek et al., 2018). Constituents of cannabis (e.g., Δ9-tetrahydrocannabinol (THC)) have been shown to exhibit immune suppressive and anti-inflammatory activity (Croxford and Yamamura, 2005; Turcotte et al., 2015). Recent evidence has emerged that HIV+ marijuana-users (MJ+) have fewer circulating CD16+ monocytes and activated CD8+ T cells compared to HIV+MJ- (Manuzak et al., 2018; Rizzo et al., 2018). As cannabinoid receptor (CB1/CB2) activation has been identified to promote anti-inflammatory effects, there has been increased interest in CBRs as therapeutic targets for treatment of inflammation (Cabral and Griffin-Thomas, 2009; Dhopeshwarkar and Mackie, 2014). Specifically, CB2 has garnered attention over CB1, as it does not induce psychotropic effects (Dhopeshwarkar and Mackie, 2014). The canonical ligand for cannabinoid receptors (CB), THC, does not discriminate between the psychotropic-mediating CB1 and non-psychotropic, CB2 (Pertwee, 2005; McPartland et al., 2007), which has limited the utility of THC as an anti-inflammatory. Therefore, CB2 selective agonists present a novel strategy for the treatment of HIV-associated neuroinflammation. The objective of this article is to propose cannabinoid receptor 2 as a therapeutic target for attenuating detrimental leukocyte inflammatory functions that contribute to HIV-associated neuroinflammation.
General Background:
HIV-associated neurocognitive disorder (HAND)
Since HIV patients are living longer, how patients progress from mild to more severe forms of HAND as they age has become an important question. A 5-year follow-up study demonstrated that 63% of patients had persistent neurocognitive impairment (Tozzi et al., 2007) and another revealed that patients already diagnosed with a minor form of HAND had a 2-fold to 6-fold higher risk of developing a more severe form compared to patients with normal cognition (Grant et al., 2014). Older HIV-infected patients have a higher risk of memory deficits compared to younger individuals (Tan et al., 2013). These studies suggest that patients with milder forms of HAND are increasingly likely to experience worsening impairment as they age, which may be governed by the severity of central and peripheral inflammation.
The role of peripheral leukocyte-mediated neuroinflammation in HAND
HAND is due to accumulating damage to neurons; however, HIV does not infect neurons (Wiley et al., 1986). Strong evidence supports low-level chronic neuroinflammation is a key event in HAND development. HIV-induced neuroinflammation is characterized by BBB dysregulation, persistent HIV entry, leukocyte infiltration into the CNS, and activation/HIV-infection of CNS-resident astrocytes, microglia and macrophages (Gonzalez-Scarano and Martin-Garcia, 2005; Strazza et al., 2011; Hong and Banks, 2015).
Entry of activated immune cells into the CNS is suggested to be a major contributor to HIV-associated neuroinflammation (Williams et al., 2014a; Hong and Banks, 2015). Once in the brain, activated immune cells release a host of inflammatory factors including HIV viral proteins, reactive species and cytokines (TNFα, IL-1β and IL-6), which activate brain-resident cells including microglia and astrocytes (Gonzalez-Scarano and Martin-Garcia, 2005; Ton and Xiong, 2013; Campbell et al., 2014a; Williams et al., 2014a; Hong and Banks, 2015; Scutari et al., 2017). The milieu of host and viral factors can directly promote neuronal injury or indirectly through the dysregulation of adjacent cells including astrocytes (Kaul et al., 2001; Gonzalez-Scarano and Martin-Garcia, 2005; Ton and Xiong, 2013; Rizzo et al., 2019). HIV viral proteins, gp120 and transactivator of transcription (Tat), have been shown to promote cell death of primary human neurons in vitro (Jana and Pahan, 2004). Furthermore, elevated IP-10 levels, in vitro, directly promotes neuronal apoptosis (Sui et al., 2006). By contrast, TNFα and IL-1β suppress glutamate uptake by astrocytes, which leads to increased extracellular glutamate and neuronal excitotoxicity (Brabers and Nottet, 2006). IL-1β and TNFα also promote astrocyte secretion of a battery of cytokines and chemokines including IL-6, MCP-1 and IP-10 (Aloisi et al., 1992; Choi et al., 2014). The chronic release of these factors promotes a positive feedback of inflammation via the recruitment of additional activated/infected immune cells from circulation (Ramesh et al., 2013). Overall, prolonged production of neuroinflammatory factors, leukocyte recruitment and impaired glutamate control are major mechanisms involved in driving neuronal dysfunction and HAND (Kaul et al., 2001; Gonzalez-Scarano and Martin-Garcia, 2005; Ton and Xiong, 2013; Hong and Banks, 2015).
The role of monocytes in HIV-associated neuroinflammation
Monocytes, especially those expressing CD16, are thought to contribute to HIV-associated neuroinflammation (Campbell et al., 2014a; Williams et al., 2014a; Hong and Banks, 2015). Specifically, increased levels of activated CD16+ monocytes in circulation have been observed in patients with chronic HIV infection and HIV-associated dementia (Thieblemont et al., 1995; Pulliam et al., 1997; Han et al., 2009). Circulating CD16+ monocytes are highly susceptible to HIV infection when compared to CD16− monocytes, which seems in part, due to elevated expression of the HIV co-receptor, CCR5 (Ellery et al., 2007; Hijdra et al., 2013). Furthermore, the CD16+ subset has been shown to be the primary monocyte population to harbor HIV in vivo despite patients receiving ART (Ellery et al., 2007). Studies involving animal models and post-mortem HAND patients have identified an increased level of CD16+ monocytes in the CNS, which may be due to surface expression of CCR2 (Fischer-Smith et al., 2001; Neuenburg et al., 2005; Clay et al., 2007; Fischer-Smith et al., 2008b; Williams et al., 2013; Tavazzi et al., 2014; Williams et al., 2014b). Specifically, the CCR2 expression level on CD16+ monocytes has been shown to correlate with HAND (Williams et al., 2014b) and be an important contributor to HIV-infected CD16+ monocytes crossing an in vitro BBB (Williams et al., 2013). Moreover, CCL2, the ligand for CCR2, is elevated in the plasma and CSF of HIV patients and correlates with neuronal injury and cognitive impairment (Eugenin et al., 2006; Kamat et al., 2012a; Williams et al., 2013; Anderson et al., 2015; Yuan et al., 2015).
CD16+ monocytes are thought to be a major transport mechanism for HIV into the brain (Gras and Kaul, 2010; Williams et al., 2014a) as these cells have been fluorescently tracked from the blood to the brain in a simian immunodeficiency virus (SIV) model, which was paralleled with HIV entry into the brain (Clay et al., 2007). In addition, CD16+ monocytes detected in post-mortem human brain stain positive for HIV viral proteins and were associated with higher CSF viral loads (Fischer-Smith et al., 2001; Neuenburg et al., 2005). Furthermore, blocking leukocyte trafficking into tissues with an anti-α4 antibody in a SIV model resulted in a decline in virally infected monocytes in the brain and was paralleled with less neuronal injury compared to untreated control animals (Campbell et al., 2014b).
Once in the CNS, monocytes differentiate into long-lived macrophages and serve as a viral reservoir (Fischer-Smith et al., 2001; Williams et al., 2001; Ancuta et al., 2006; Campbell et al., 2014a). Monocyte-derived macrophages release inflammatory factors leading to activation and HIV-infection of microglia, astrocytes and macrophages (Gonzalez-Scarano and Martin-Garcia, 2005; Williams et al., 2014a; Scutari et al., 2017). As noted in the previous section, the inflammatory factors can directly promote neuronal injury. In addition, monocyte-derived factors (e.g. IL-1β) in the brain may also have indirect effects on neuronal functions through promoting astrocyte activation and dysfunction (Gonzalez-Scarano and Martin-Garcia, 2005; Williams et al., 2014a; Scutari et al., 2017).
In addition to CD16, another surface protein expressed by monocytes is CD163 and may be an important marker on CNS-bound CD16+ monocytes during HIV infection (Kristiansen et al., 2001; Kim et al., 2006). CD163 is a scavenging receptor for hemoglobin-haptoglobin complexes that is almost exclusively expressed on monocyte/macrophages (Pulford et al., 1992; Kristiansen et al., 2001). In addition, CD163 has been shown to have an important role for monocyte adherence to endothelial cells while also serving as an immune receptor to detect bacteria (Wenzel et al., 1996; Fabriek et al., 2009). Co-expression of CD16 and CD163 on monocytes has been observed in post-mortem brain tissue of HIV+ individuals with cognitive impairment (Fischer-Smith et al., 2008b; Tavazzi et al., 2014). CD16+CD163+ monocytes are also elevated in circulation of HIV+ individuals with detectable viral loads (Fischer-Smith et al., 2008a), suggesting that CD163 is expressed on CD16+ monocytes before entry into the brain. Other surface markers that may be indicative of monocyte trafficking into the CNS and/or neurocognitive impairment include CCR2 and the cell adhesion molecules, junctional adhesion molecule-A (JAM-A) and activated leukocyte cell adhesion molecule (ALCAM) (Williams et al., 2013; Williams et al., 2014b).
Although outside the scope of this review, it is important to emphasize that microglia also play a critical role in promoting neuroinflammation. Indeed, there are many similarities in the repertoire of pro-inflammatory mediators between monocyte/macrophage and microglia, such as IL-1β and IL-6 release (Reviewed in (Saijo and Glass, 2011)). These cells can also be identified by the expression of CD16 and CD163 (David and Kroner, 2011). These similarities are owed to the shared common myeloid lineage between microglia and monocytes/macrophages. Further, these cells can initiate as well as exacerbate neuroinflammation in response to various insults such as pathogen associated molecular patterns (PAMPs) and neuronal injury (Saijo and Glass, 2011). Similar to activated monocytes that migrate into the brain, microglia also have the potential to traffic to various regions of the brain in response to inflammation and injury (Faden et al., 2016). However, unlike activated monocytes in the brain, microglia have the potential to exert both inflammatory as well as anti-inflammatory/regenerative properties depending on their environment (Bessis et al., 2007; David and Kroner, 2011). In summary, it is well established that cells of the myeloid lineage play a critical role in regulating neuroinflammation and represent a potential therapeutic target.
The role of CD8+ T cells in HIV-induced neuroinflammation and HAND development
CD8+ T cells account for a large portion of the adaptive immune response to HIV infection (Benito et al., 2004; Walker and McMichael, 2012), can cross the BBB (Young et al., 2011; Smolders et al., 2013) and are detectable in the CSF and CNS of HIV patients (Miller et al., 2004; Vendrely et al., 2005). Following activation, HIV-specific CD8+ T cells traffic to sites of viral replication along increasing gradients of viral antigen (von Geldern et al., 2007) and chemokines (Shacklett et al., 2004). Upon antigen reencounter, virus-specific CD8+ T cells exert multiple effector functions to limit viral spread by killing infected cells (Doherty et al., 1997; Topham et al., 1997). Effector functions are classified as cytotoxic or cytokine-mediated (IL-2, IFNγ, and TNFα) (Doherty et al., 1997; Topham et al., 1997). CD8+ T cell cytotoxic effector function is primarily mediated by directed release of cytolytic granules onto target cells (Doherty et al., 1997; Topham et al., 1997). These granules contain membrane-permeating perforin and proteolytic granzymes facilitating the death of virally infected cells (Doherty et al., 1997; Topham et al., 1997). Cytolytic function by CD8+ T cells is considered protective, as the control of viral spread in ART individuals reduces systemic inflammation (Giorgi et al., 1993; Giorgi et al., 1999). In contrast to CD8+ T cell cytolytic function, the production of effector cytokines has been associated with inflammatory diseases of the CNS, including multiple sclerosis (Giunti et al., 2003). Of the cytokines produced by CD8+ effectors, IFNγ is critically involved in inflammatory disease progression (Giunti et al., 2003; Mehla et al., 2012). CD8+ T cell effector function is determined by the immune environment. Considering the three signal hypothesis for T cell activation (Curtsinger and Mescher, 2010), IFNα is a potent signal three cytokine (Curtsinger and Mescher, 2010; Hervas-Stubbs et al., 2010; Manion et al., 2012; Tough, 2012). IFNα promotes cellular proliferation (Manion et al., 2012) and enhances development of polyfunctional CD8+ T cell, through the acquisition of effector cytokine secretion (Curtsinger and Mescher, 2010; Hervas-Stubbs et al., 2010; Tough, 2012).
IFNγ promotes neuroinflammation and cell damage in the CNS
IFNγ is critical for the control of intracellular pathogens and is primarily produced by T cells (Doherty et al., 1997; Topham et al., 1997). However, there is evidence that IFNγ is not correlative with HIV infection control (Zajac et al., 1998; Boasso et al., 2009; Hersperger et al., 2010; Makedonas and Betts, 2011). Despite this, IFNγ is implicated in CNS inflammation and pathogenesis (Giunti et al., 2003). IFNγ is detectable in the CSF and CNS of HAND patients (Giunti et al., 2003; Mehla et al., 2012). The role of IFNγ has been relatively understudied in the context of HAND due to the fact that astrocytes and other immune cells in the brain do not produce IFNγ except under certain ischemic conditions (Lau and Yu, 2001). However, a strong correlation was found between the number of IFNγ+ CD8+ T cells in the CSF and cognitive decline in HIV patients (Schrier et al., 2015). IFNγ potentiates the neurotoxic effects of HIV and inflammation through direct and indirect mechanisms. For example, mixed brain cell cultures infected with HIV showed minimal cytopathic effects (Mehla et al., 2012) yet cell death increased with exogenous IFNγ (Mehla et al., 2012). The explanation for decreased cell viability is the ability of IFNγ to drive IP-10 production by astrocytes, which can trigger neuronal death (Mehla et al., 2012). Enhanced IP-10 production by astrocytes enhances CNS infiltration by monocytes and CD8+ T cells to enhance inflammation (Shacklett et al., 2004; Mehla et al., 2012). In addition, astrocytes are refractory to HIV infection and viral replication (Carroll-Anzinger and Al-Harthi, 2006); however, IFNγ inhibits the beta catenin pathway causing astrocyte susceptibility to HIV infection and release of neurotoxic HIV proteins (Carroll-Anzinger and Al-Harthi, 2006; Li et al., 2011).
The role of interferon alpha on monocyte activation in circulation and the brain
IFNα is a potent cytokine produced during viral infection and when secreted, protects the host by inducing anti-viral defense mechanisms in neighboring cells (Ivashkiv and Donlin, 2014). IFNα secretion by pDC can be promoted by HIV itself and through microbial products derived from the compromised intestinal barrier (Cha et al., 2014). IFNα is a central component of the acute immune response against HIV infection, but sustained levels during chronic stages of infection contributes to immune activation and dysfunction (Cha et al., 2014). Specifically, interferon-stimulated genes (ISGs) have been shown to be upregulated in several different immune populations during chronic HIV infection including T cells, dendritic cells and monocytes (Cha et al., 2014). Furthermore, IFNα can be elevated in the cerebrospinal fluid (CSF) of HIV patients and is thought to be an important contributor to HAND as it correlates with neuronal damage and neurocognitive impairment (Rho, 1995; Perrella et al., 2001; Fritz-French and Tyor, 2012; Anderson et al., 2017). Studies using mouse models of HIV encephalitis treated with IFNα neutralizing antibodies have found a significant role of IFNα on neuronal damage and cognitive decline (Sas et al., 2009; Kessing and Tyor, 2015; Koneru et al., 2018).
CD16− monocytes transition into the CD16+ phenotype in circulation and this process is of interest due to the pathogenic nature of the CD16+ monocyte subset during HIV infection (Ziegler-Heitbrock, 2007; Williams et al., 2014a). However, the specific mechanism(s) of enhanced CD16− monocyte transition to CD16+ during HIV infection remains unclear. Previous reports have identified a type I interferon gene signature in monocytes isolated from HIV-infected individuals, suggesting exposure to IFNα in vivo (Rempel et al., 2010; Pulliam et al., 2011). Additionally, the use of IFNα as a vaccine adjuvant in humans increased the percentage of CD16+ monocytes (Arico et al., 2011). Collectively, the body of literature suggests a key role for IFNα on monocyte activation in the periphery, including increasing the frequency of CD16+ monocytes, and brain during HIV infection.
The role of plasmacytoid dendritic cells in chronic immune activation and HAND
As previously discussed, HIV-infected individuals display elevated levels of circulating IFNα, which is linked to immune cell activation. One of the major sources of IFNα are plasmacytoid dendritic cells (pDC), which produce 1000 times more IFNα than any other immune cell type. pDC represent between 0.2–0.5% of the circulating peripheral blood mononuclear cells yet play a central role in initiating host immune responses against a wide variety of pathogens. In the context of chronic immune activation and HAND, pDC through expression of a range of TLRs, recognize HIV directly as well as commensally-derived microbial products due to a comprised intestinal barrier (i.e., leaky gut). Upon TLR activation, pDC produce inflammatory cytokines that activate other immune cells to initiate specific immune responses. During chronic HIV infection, prolonged secretion of IFNα by pDC has been implicated as a key event driving chronic inflammation, which contributes to etiology of HAND. Specifically, pDC have been shown to localize to the gut during SIV infection where they are believed to produce large amounts of IFNα as well as other cytokines (e.g., TNFα) in response to HIV and microbiota-derived products. In addition, pDC may play a role in facilitating HIV infection of gut resident T cells and likely play a key role in the activation of monocytes and T cells. In fact, an augmented IFNα response by pDC has been correlated with the development of HIV-associated neurocognitive decline.
Chronic monocyte and CD8+ T cell activation in HIV patients
The immune environment present during chronic HIV infection is characterized by increased systemic inflammation and immune cell activation (Jiang et al., 2009; Younas et al., 2016). Specifically, monocyte activation in circulation is readily identifiable in HIV patients and correlates with disease progression and neurocognitive impairment (Fischer-Smith et al., 2008a; Pulliam et al., 2011; Sandler et al., 2011; Kamat et al., 2012b; Burdo et al., 2013; Williams et al., 2014b). Chronic monocyte activation during HIV infection is multi-faceted including; (a) gut microbial translocation (leaky gut syndrome); (b) residual HIV viremia; and (c) co-infection with opportunistic pathogens (Anzinger et al., 2014), all of which can signal through the family of toll-like receptors (TLRs). IFNα is a major contributor to monocyte activation during HIV infection (Pulliam et al., 2011; Cha et al., 2014). For example, chronic IFNα production in HIV patients has been linked to neurocognitive impairment and disease progression (Rho, 1995; Sas et al., 2009). Further, elevated IFNα in the CSF correlated with neuronal injury and cognitive decline in HIV patients (Anderson et al., 2017). Moreover, an enrichment of activated (CD16+) monocytes was observed in the CSF of ART-treated patients (Neuenburg et al., 2005). Recently, we have shown that IFNα treatment of human primary monocytes induces expression of the proinflammatory marker, CD16 (Rizzo et al., 2018), an observation consistent with the IFNα gene signature in monocytes from HIV patients (Rempel et al., 2010; Pulliam et al., 2011; Swiecki and Colonna, 2015; Rizzo et al., 2018). In parallel, IFNα also activates CD8+ T cells, which are recruited from systemic circulation to cross the BBB (Young et al., 2011; Smolders et al., 2013). Furthermore, chronic IFNα production in HIV patients has been linked to neurocognitive impairment (Rho, 1995; Sas et al., 2009). Once in the perivascular space, activated monocytes and CD8+ T cells interact with astrocytes to drive a chronic neuroinflammatory response leading to destruction of neurons and declining cognitive function.
Likewise, CD8+ T cells from HIV patients have a highly activated phenotype (i.e., CD38 expression) (Giorgi et al., 1993; Shacklett et al., 2004). Enhanced CD38 expression was observed on CD8+ but not CD4+ T cells in PBMC isolated from HIV patients treated with exogenous IFNα (Manion et al., 2012). Further, it has been well documented that CD8+ T cells in the brain gradually lose cytolytic effector function over time (Aubert et al., 2011; Shan et al., 2012) and this is likely due to the lack of CD4+ T cells in the CNS given their inability to cross the BBB (Barcia et al., 2006). However, HIV-specific CD8+ T cells maintain their ability to secrete effector cytokines including IFNγ (Schrier et al., 2015). Presently, it is unknown how T cells maintain their ability to secrete IFNγ despite persistent antigen and lack of CD4+ T cells.
The major constituents of cannabis
Cannabis, also known as Cannabis Sativa, is made up over 500 chemicals with 104 of them being defined as phytocannabinoids (ElSohly and Gul, 2014). In addition to cannabinoids, there are other compounds present in cannabis including terpenes and flavonoids (National Academies of Sciences and Medicine, 2017). Two cannabinoids receiving significant attention for their putative medicinal properties are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). THC concentrations can vary widely between cannabis strains, typically 18–33%. By contrast, CBD in most strains is a minor cannabinoid accounting approximately 0.2% of the plant material (ElSohly et al., 2016). However, due to increasing interest in CBD, cannabis strains possessing higher CBD content are increasingly abundant. THC but not CBD, is the main psychotropic component of cannabis, as it impairs aspects of cognition including short-term memory (Atakan, 2012). However, THC and CBD do display similar effects elsewhere in the body, with both shown to have immune modulating/anti-inflammatory, antiemetic and antiepileptic activity (Atakan, 2012). Moreover, it is noteworthy that a number of minor cannabinoids including CBD and cannabinol are known to possess immune modulatory and anti-inflammatory activity; however, because they possess low binding affinity for CB1 or CB2, and therefore do not mediate their activity through CB1 or CB2, they will not be discussed but have been recently reviewed (Pertwee, 1999; Bergamaschi et al., 2011; Iffland and Grotenhermen, 2017; Huestis et al., 2019).
Cannabinoid receptors, CB1 and CB2, in immune modulation
Two cannabinoid receptors (CB) have been cloned, CB1 and CB2. CB1 is expressed primarily in the CNS but is found in most peripheral tissues (Matsuda et al., 1990). CB2 is found primarily in the periphery, with the exception of microglial cells (Carlisle et al., 2002), and is expressed most notably in immune cells (Munro et al., 1993). Measurements of CB1 and CB2 mRNA expression in lymphoid tissues and leukocyte preparations suggest that CB2 is the predominant cannabinoid receptor expressed within the immune system (Bouaboula et al., 1993; Munro et al., 1993; Schatz et al., 1997). Despite the known presence of cannabinoid receptors in immune cells, the role of these receptors in immune modulation is elusive. Since the first identification of cannabinoid receptor expression within the immune system by my laboratory (Kaminski et al., 1992), our major goals have been to define the role of CB1 and CB2 in immune modulation and to elucidate the signal transduction pathways and target genes affected by cannabinoids. Based upon 30+ years of research, virtually every immune cell-type has been found to respond to cannabinoids. In addition, biological activity can occur through CB1 and/or CB2-dependent or –independent mechanisms. Other putative cannabinoid receptors have been postulated but the evidence for their role remains equivocal and will not be discussed here.
Cannabinoid modulation of plasmacytoid dendritic cell secretion of IFNα and the involvement of CB2
As pDC-derived IFNα has been implicated in chronic inflammation and HAND, we have recently explored the ability of cannabinoids, both plant-derived and synthetic, to modulate the production of this cytokine. Toward this end, we reported that THC suppressed TLR9-mediated induction of IFNα by pDC isolated from HIV-negative and HIV+ individuals. Furthermore, pDC from HIV+ individuals exhibited greater sensitivity to THC-mediated suppression than pDC from HIV-negative individuals. One explanation for the observed difference in cannabinoid-sensitivity may be differential expression of cannabinoid receptors between HIV-negative and HIV+ individuals. Indeed, PBMC from HIV+ patients showed augmented expression of both CB1 and CB2. This is concordant with prior literature demonstrating that inflammatory cytokine stimulation induces expression of cannabinoid receptors. As CB2 is the primary cannabinoid receptor expressed by pDC, CB2 is an appealing target for limiting the release of pDC-derived inflammatory mediators. Toward this end, we have demonstrated that the CB2-selective agonists, JWH-015 and JWH-133, impaired TLR9-induced secretion of IFNα by pDC from HIV-negative individuals exhibiting a similar profile to THC. Collectively, these findings suggest that by impairing pDC-derived IFNα, CB2-selective agonists may attenuate critical events contributing to the initiation and progression of HAND, specifically, the acquisition of inflammatory effector functions of immune cells (e.g., CD16+ monocytes and IFNγ+ CD8+ T cells).
Cannabinoid modulation of human monocyte function and the putative involvement of CB2
Purified human monocytes exhibit higher CB2 mRNA expression in comparison to CB1 (Roth et al., 2015). Likewise, microglia express higher CB2 mRNA levels compared to CB1 (Ashton and Glass, 2007; Stella, 2009). Several reports have shown human primary monocytes to be sensitive to THC treatment, with the evidence suggesting a CB2-mediated mode of action. Specifically, in vitro THC treatment was able to inhibit monocyte differentiation into mature dendritic cells, ultimately decreasing monocyte-mediated activation of T cells (Roth et al., 2015). Using specific CB2 agonists and antagonists, CB2 was suggested to be the predominant cannabinoid receptor responsible for THC modulation of monocyte function (Roth et al., 2015). In addition, in vitro treatment with JWH-015 or JWH-133, both CB2 selective agonists, impaired human monocyte chemotaxis in response to CCL2 and CCL3, with the suspected mechanism being a CB2-mediated reduction in the chemokine receptors, CCR2 and CCR1, and the cell adhesion molecule, intercellular adhesion molecule 1 (ICAM-1) (Montecucco et al., 2008).
In the context of chronic immune activation and neuroinflammation during HIV infection, several reports have suggested anti-inflammatory activity by cannabinoids on specific monocyte processes implicated in HAND. Specifically, we and others, have demonstrated that HIV-infected individuals utilizing cannabis display a lower level and/or frequency of CD16+ monocytes compared to HIV+ non-cannabis users (Manuzak et al., 2018; Rizzo et al., 2018; Castro et al., 2019). Albeit not statistically significant, we also observed a lower level of CD16+CD163+ monocytes in HIV-infected cannabis users compared to non-users (Rizzo et al., 2018). In addition, we have in vitro evidence demonstrating that THC suppresses monocyte transition from CD16− to CD16+ when stimulated with IFNα as well as the frequency of CD16+CD163+ monocytes, which was observed in monocytes isolated from HIV-negative and HIV-infected individuals (Rizzo et al., 2018). However, CBD, which displays minimal binding affinity to CB1 and CB2, had no effect on the IFNα-mediated increase in CD16+ monocytes, suggesting cannabinoid receptor involvement (Rizzo et al., 2018) most likely involving a CB2-mediated mechanism. Future studies will be needed to test this hypothesis. As CD16+ monocytes are more permissive to HIV infection compared to classical (CD16−) monocytes and are implicated in neuroinflammation, the decrease in CD16+ monocytes observed with cannabis use would be presumed to be beneficial. In fact, Williams and colleagues have shown that in vitro THC decreased monocyte susceptibility to HIV infection during differentiation into macrophages suggesting CB2 involvement (Williams et al., 2014c). The proposed mechanism of THC was suggested to be through a reduction in expression of HIV co-receptors, CD4, CCR5 and CXCR4. Furthermore, THC, albeit at a high concentration (30μM), reduced monocyte expression of CD16 and CD163 during differentiation (Williams et al., 2014c).
In addition to the observation with cannabis use on CD16+ monocytes, we also found decreased plasma IP-10 in cannabis using HIV+ individuals compared to HIV+ non-users (Rizzo et al., 2018). This decrease in plasma IP-10 with cannabis use may also be beneficial as IP-10 is suggested to be an important contributor to disease progression during HIV infection (Lei et al., 2019). For instance, IP-10 has been shown to stimulate HIV replication in monocyte-derived macrophages and blood lymphocytes (Lane et al., 2003). In addition, IP-10 has been identified to be elevated in the blood and CSF of patients with HAND, and to be inversely correlated with N-acetylaspartate, a marker of neuronal injury (Pulliam et al., 2011; Yuan et al., 2013). In vitro experiments have also revealed direct neurotoxic effects of IP-10 as evidenced by apoptosis of neurons (Sui et al., 2006). In peripheral blood mononuclear cells, monocytes have been identified as the major producers of IP-10 when stimulated with HIV or IFNα (Simmons et al., 2013; Rizzo et al., 2018). Accompanying the observation of decreased plasma IP-10 in HIV+ cannabis users, we found that THC treatment, at relevant concentrations observed with cannabis use (Huestis, 2007), suppressed IFNα-mediated secretion of IP-10 by monocytes that were isolated from HIV+ donors (Rizzo et al., 2018). Future studies will be required to examine whether CB2 is responsible for the THC-mediated suppression of monocyte-derived IP-10.
A consequence of HIV-associated neuroinflammation is astrocyte dysfunction, which can be caused by release of immune-cell derived inflammatory cytokines, reactive species and HIV virions/viral proteins (Genis et al., 1992; Andjelkovic et al., 2000; Gonzalez-Scarano and Martin-Garcia, 2005; Sofroniew and Vinters, 2010; Ton and Xiong, 2013; Rizzo et al., 2019). The resulting effects on astrocytes include secretion of inflammatory cytokines and chemokines, and impaired glutamate uptake (Ton and Xiong, 2013). Cytokine/chemokine secretion by astrocytes further promotes recruitment of blood-derived monocytes and CD8+ T cells while promoting the production of ROS and NO by microglia and macrophages (Kaul et al., 2001; Hong and Banks, 2015; Scutari et al., 2017).
Recently, we developed a human primary co-culture system to address the role of activated monocytes on astrocyte inflammatory responses and subsequent effects of cannabinoids (Rizzo et al., 2019). Specifically, we investigated the effect of TLR7-activated monocytes on astrocyte production of MCP-1, IL-6 and IP-10, as these factors have been shown to be increased in the plasma and/or cerebrospinal fluid (CSF) during HIV infection and associated with neuronal injury and/or cognitive impairment (Pulliam et al., 2011; Kamat et al., 2012a; Yuan et al., 2013; Ramirez et al., 2014; Anderson et al., 2015; Lake et al., 2015; Yuan et al., 2015; Rizzo et al., 2018). When chronically elevated, these factors contribute to ongoing leukocyte infiltration, cytokine secretion and direct neuronal injury (Kaul et al., 2001; Gonzalez-Scarano and Martin-Garcia, 2005; Sui et al., 2006; Sofroniew, 2015; Scutari et al., 2017). We found that TLR7 activation of monocytes (HIV-negative) induced astrocyte production of MCP-1 and IL-6, and when in combination with IFNα, induced IP-10 secretion as well (Rizzo et al., 2019). In addition, monocyte-derived IL-1β was identified to be the critical factor governing the astrocyte response (Rizzo et al., 2019), highlighting a potential mechanism of astrocyte dysfunction during HAND.
When examining the impact of THC on the TLR7-stimulated co-culture response, THC suppressed astrocyte secretion of both MCP-1 and IL-6 (Rizzo et al., 2019), suggesting beneficial anti-inflammatory effects of cannabinoid receptor activation in the context of HAND. Furthermore, we identified that THC targeted both cell types, suppressing TLR7-mediated monocyte secretion of IL-1β and IL-1β+TLR7-mediated astrocyte production of MCP-1 and IL-6 (Rizzo et al., 2019). Furthermore, the CB2 selective agonist, JWH-015, suppressed monocyte secretion of IL-1β in a similar manner as THC, suggesting CB2 receptor-dependence (Rizzo et al., 2019). In addition, these findings provide support for CB2 as a therapeutic target (Cabral and Griffin-Thomas, 2009; Dhopeshwarkar and Mackie, 2014).
Indeed there are significant parallels in response of microglia and monocytes to cannabinoids. This is evidenced by marked reductions in pro-inflammatory cytokine secretion (i.e. IL-1β) by cannabinoid treatment (Lu et al., 2015; Malek et al., 2015; Wen et al., 2015). In both cases, cannabinoid receptors couple primarily to Gαi/o to inhibit adenylyl cyclase and cyclic AMP signaling cascade (Lutz, 2002). Unlike monocytes however, cannabinoid treatment of microglia also appears to skew these cells toward an anti-inflammatory/regenerative phenotype as evidenced by their production of IL-10 and brain-derived neurotrophic factor (BDNF) (Tolon et al., 2009; Correa et al., 2010; Mecha et al., 2016). Despite the breadth of work on microglia and their modulation by cannabinoid receptors, given the practical limitations in acquiring primary human microglia, a large portion of investigations have been conducted in animal or cell systems. It is noteworthy that there are fundamental differences between species in the C terminus of the CB2 receptor amino acid sequence. For example, murine CB2 is 13 amino acids shorter, while the rat is 50 amino acids longer compared to human CB2 (Atwood and Mackie, 2010). As such, care should be taken in the interpretation of results involving CB2 across species.
The conclusions drawn based on the current body of evidence presented here is that THC, potentially through CB2, suppresses specific human monocyte inflammatory processes implicated in HAND; including peripheral IFNα-mediated monocyte activation and TLR-activated monocyte-mediated astrocyte production of cytokines and chemokines.
Modulation of T Cells by cannabinoids
As mentioned, published studies indicate that IFNγ+ HIV-specific cytotoxic lymphocytes (CTL) in the CSF of HIV patients have a strong correlation with HAND (Schrier et al., 2015). Naïve CD8+ T cells residing in the secondary lymphoid organs become activated following intercalation and recognition of antigen presenting cells (APC), driving them to become HIV-specific CTL (Doherty et al., 1997). During this time, the immune environment present during T cell activation greatly influences the magnitude and type of effector function virus-specific CTL adopt. As mentioned previously, secreted IFNα enhances proliferation of virus-specific CTL in murine models (Jennings et al., 2014) and in human studies of CTL isolated from HIV-infected individuals treated with IFNα (Manion et al., 2012). Further, exposure to IFNα during activation increases effector function of virus-specific CTL (Curtsinger and Mescher, 2010; Jennings et al., 2014). As with monocytes, it has been widely established that cannabinoids also regulate CD8+ T cell activation and effector function, and that this regulation is mediated, in part, through the CB2 receptor (Robinson et al., 2015).
One of the most potent effects of cannabinoids on CD8+ T cells is the suppression of T cell proliferation (Klein et al., 1985). This has been demonstrated in both murine and human models using exogenous mitogens as well as antigen-specific proliferation (Yuan et al., 2002; Borner et al., 2009). Given the link between CD8+ T cell proliferation and acquisition of effector function, it is not surprising that cannabinoids potently inhibit CD8+ T cell cytokine secretion, IFNγ specifically (Newton et al., 1994; Klein et al., 2000). This is likely due to the hierarchal nature of the acquisition of effector function by CD8+ T cells (Rothenberg et al., 1991; Kaech et al., 2002). As CD8+ T cells proliferate, promoter regions of effector cytokines become more accessible for transcription factor binding, such as AP-1 (Chen et al., 2018). For example, naïve CD8+ T cells readily secrete IL-2 upon activation but minimal IFNγ (Tham et al., 2002). Following prolonged proliferation, CD8+ T cells begin to secrete IFNγ and eventually other inflammatory cytokines such as TNFα (Pennock et al., 2013). The effects of cannabinoids on CD8+ T cell cytolytic granule release have been less studied. However, evidence exists in rodent models that cannabinoids, THC in particular, may also suppress cytolysis, but has yet to be explored in human models (Klein et al., 1991).
While it has been widely established that cannabinoids suppress CD8+ T cell activation, proliferation, and IFNγ secretion, unfortunately few studies have been conducted on the specific effects of cannabinoids in the context of HAND (Schrier et al., 2015). It has been recently reported that IFNα treatment of CD8+ T cells from HIV+ individuals resulted in enhanced IL-7R signaling and STAT5 phosphorylation (Henriquez et al., 2018). Further, IFNα treatment directly led to increased proliferation of CD8+ T cells, and this enhancement was suppressed by THC treatment, even in CD8+ T cells from HIV+ individuals (Henriquez et al., 2018). While not yet tested, it is tempting to speculate that cytokine secretion by CD8+ T cells may likely be impacted due, in part, to reduced T cell proliferation. Given the established role of HIV-specific CD8+ T cells and IFNγ in exacerbating and sustaining HIV-induced neuroinflammation, there is need for further studies investigating the potential utility of CB2 specific agonists in ameliorating some of these effects.
CB2 as a target in the treatment of HIV-associated neuroinflammation
As discussed here, it is well established that systemic peripheral immune activation is both a major consequence of HIV infection and also a mechanism contributing to the promotion of HIV-associated neuroinflammation and cognitive impairment (Anzinger et al., 2014; Hong and Banks, 2015; Lewis and Couturier, 2019). Indeed, chronic systemic inflammation, including monocyte/microglial activation, is readily identifiable in HIV patients, even in the ART era, and correlates with disease progression and neurocognitive impairment (Fischer-Smith et al., 2008a; Pulliam et al., 2011; Sandler et al., 2011; Kamat et al., 2012b; Burdo et al., 2013; Williams et al., 2014b). Likewise, it is widely established that cannabinoid receptor agonists possess immune modulating activity, and most relevant to this discussion, anti-inflammatory properties. Through the examination of HIV+ cannabis using and non-using individuals several laboratories, including our own, have observed evidence of marked reductions in activated circulating leukocytes, in particular CD16+ monocytes, which have been implicated in promoting neuroinflammation. Unfortunately, due to its psychotropic properties, cannabis remains a highly controversial therapeutic modality. By contrast, CB2 selective agonists such as JWH-015 or JWH-133 have been demonstrated to possess anti-inflammatory properties and do not possess psychotropic activity. Indeed, targeting CB2 to reduce both systemic and CNS localized inflammation could prove to be effective in the management of HIV-associated chronic inflammation and, in particular, in slowing the progression of HAND. Moreover, it is important to emphasize that because neuroinflammation plays a critical role in the etiology and progression of a number of neurocognitive disorders including, Alzheimer’s Disease, Parkinson’s Disease, and Multiple Sclerosis, targeting CB2 may have broader therapeutic applications which go beyond treatment of HAND. Despite the exquisite anti-inflammatory properties of CB2 agonists in in vitro and animal models, these findings have yet to be confirmed in a clinical setting, especially in the context of the neurocognitive disorders discussed in this review.
Acknowledgments
Funding: The National Institutes of Drug Abuse Grant R01 DA047180 and the National Institutes of Environmental Health Sciences Training Grant T32 ES07255 supported this work.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: None of the authors present on this paper report any conflict of interest.
References
- Aloisi F, Care A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C (1992) Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol 149:2358–2366. [PubMed] [Google Scholar]
- Ancuta P, Kunstman KJ, Autissier P, Zaman T, Stone D, Wolinsky SM, Gabuzda D (2006) CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology 344:267–276. [DOI] [PubMed] [Google Scholar]
- Anderson AM, Lennox JL, Mulligan MM, Loring DW, Zetterberg H, Blennow K, Kessing C, Koneru R, Easley K, Tyor WR (2017) Cerebrospinal fluid interferon alpha levels correlate with neurocognitive impairment in ambulatory HIV-Infected individuals. J Neurovirol 23:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AM, Harezlak J, Bharti A, Mi D, Taylor MJ, Daar ES, Schifitto G, Zhong J, Alger JR, Brown MS, Singer EJ, Campbell TB, McMahon DD, Buchthal S, Cohen R, Yiannoutsos C, Letendre SL, Navia BA, Consortium HIVN (2015) Plasma and Cerebrospinal Fluid Biomarkers Predict Cerebral Injury in HIV-Infected Individuals on Stable Combination Antiretroviral Therapy. J Acquir Immune Defic Syndr 69:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic AV, Kerkovich D, Pachter JS (2000) Monocyte:astrocyte interactions regulate MCP-1 expression in both cell types. J Leukoc Biol 68:545–552. [PubMed] [Google Scholar]
- Anzinger JJ, Butterfield TR, Angelovich TA, Crowe SM, Palmer CS (2014) Monocytes as regulators of inflammation and HIV-related comorbidities during cART. J Immunol Res 2014:569819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arico E, Castiello L, Urbani F, Rizza P, Panelli MC, Wang E, Marincola FM, Belardelli F (2011) Concomitant detection of IFNalpha signature and activated monocyte/dendritic cell precursors in the peripheral blood of IFNalpha-treated subjects at early times after repeated local cytokine treatments. J Transl Med 9:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Glass M (2007) The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr Neuropharmacol 5:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atakan Z (2012) Cannabis, a complex plant: different compounds and different effects on individuals. Ther Adv Psychopharmacol 2:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Mackie K (2010) CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha SJ, Barber DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R (2011) Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A 108:21182–21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, Thomas CE, Curtin JF, King GD, Wawrowsky K, Candolfi M, Xiong WD, Liu C, Kroeger K, Boyer O, Kupiec-Weglinski J, Klatzmann D, Castro MG, Lowenstein PR (2006) In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J Exp Med 203:2095–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito JM, Lopez M, Soriano V (2004) The role of CD8+ T-cell response in HIV infection. AIDS Rev 6:79–88. [PubMed] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Zuardi AW, Crippa JA (2011) Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf 6:237–249. [DOI] [PubMed] [Google Scholar]
- Bessis A, Bechade C, Bernard D, Roumier A (2007) Microglial control of neuronal death and synaptic properties. Glia 55:233–238. [DOI] [PubMed] [Google Scholar]
- Boasso A, Shearer GM, Chougnet C (2009) Immune dysregulation in human immunodeficiency virus infection: know it, fix it, prevent it? J Intern Med 265:78–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner C, Smida M, Hollt V, Schraven B, Kraus J (2009) Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J Biol Chem 284:35450–35460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, Le Fur G, Casellas P (1993) Cannabinoid-receptor expression in human leukocytes. Eur J Biochem 214:173–180. [DOI] [PubMed] [Google Scholar]
- Brabers NA, Nottet HS (2006) Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest 36:447–458. [DOI] [PubMed] [Google Scholar]
- Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC (2013) Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 27:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, Griffin-Thomas L (2009) Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med 11:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JH, Hearps AC, Martin GE, Williams KC, Crowe SM (2014a) The importance of monocytes and macrophages in HIV pathogenesis, treatment, and cure. AIDS 28:2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JH, Ratai EM, Autissier P, Nolan DJ, Tse S, Miller AD, Gonzalez RG, Salemi M, Burdo TH, Williams KC (2014b) Anti-alpha4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog 10:e1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso SW, Torres TS, Santini-Oliveira M, Marins LM, Veloso VG, Grinsztejn B (2013) Aging with HIV: a practical review. Braz J Infect Dis 17:464–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, Cabral GA (2002) Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol 2:69–82. [DOI] [PubMed] [Google Scholar]
- Carroll-Anzinger D, Al-Harthi L (2006) Gamma interferon primes productive human immunodeficiency virus infection in astrocytes. J Virol 80:541–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro FOF, Silva JM, Dorneles GP, Barros JBS, Ribeiro CB, Noronha I, Barbosa GR, Souza LCS, Guilarde A, Pereira A, Guimaraes RF, Oliveira TF, Oliveira SEF, Peres A, Romao PRT, Pfrimer IAH, Fonseca SGD (2019) Distinct inflammatory profiles in HIV-infected individuals under ART using cannabis, cocaine or cannabis plus cocaine. AIDS. [DOI] [PubMed] [Google Scholar]
- Cha L, Berry CM, Nolan D, Castley A, Fernandez S, French MA (2014) Interferon-alpha, immune activation and immune dysfunction in treated HIV infection. Clin Transl Immunology 3:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zander R, Khatun A, Schauder DM, Cui W (2018) Transcriptional and Epigenetic Regulation of Effector and Memory CD8 T Cell Differentiation. Front Immunol 9:2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SS, Lee HJ, Lim I, Satoh J, Kim SU (2014) Human astrocytes: secretome profiles of cytokines and chemokines. PLoS One 9:e92325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay CC, Rodrigues DS, Ho YS, Fallert BA, Janatpour K, Reinhart TA, Esser U (2007) Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J Virol 81:12040–12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa F, Hernangomez M, Mestre L, Loria F, Spagnolo A, Docagne F, Di Marzo V, Guaza C (2010) Anandamide enhances IL-10 production in activated microglia by targeting CB(2) receptors: roles of ERK1/2, JNK, and NF-kappaB. Glia 58:135–147. [DOI] [PubMed] [Google Scholar]
- Croxford JL, Yamamura T (2005) Cannabinoids and the immune system: potential for the treatment of inflammatory diseases? J Neuroimmunol 166:3–18. [DOI] [PubMed] [Google Scholar]
- Curtsinger JM, Mescher MF (2010) Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol 22:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388–399. [DOI] [PubMed] [Google Scholar]
- Dhopeshwarkar A, Mackie K (2014) CB2 Cannabinoid Receptors as a Therapeutic Target—What Does the Future Hold? Molecular pharmacology 86:430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG (1997) Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev 159:105–117. [DOI] [PubMed] [Google Scholar]
- Donninelli G, Gessani S, Del Corno M (2016) Interplay between HIV-1 and Toll-like receptors in human myeloid cells: friend or foe in HIV-1 pathogenesis? J Leukoc Biol 99:97–105. [DOI] [PubMed] [Google Scholar]
- Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM (2007) The CD16+ Monocyte Subset Is More Permissive to Infection and Preferentially Harbors HIV-1 In Vivo. The Journal of Immunology 178:6581–6589. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Gul W (2014) Constituents of Cannabis Sativa In: Handbook of Cannabis. Oxford: Oxford University Press. [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC (2016) Changes in Cannabis Potency Over the Last 2 Decades (1995–2014): Analysis of Current Data in the United States. Biol Psychiatry 79:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW (2006) CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci 26:1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K, Vloet RP, Dijkstra CD, van den Berg TK (2009) The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 113:887–892. [DOI] [PubMed] [Google Scholar]
- Faden AI, Wu J, Stoica BA, Loane DJ (2016) Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol 173:681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Tedaldi EM, Rappaport J (2008a) CD163/CD16 coexpression by circulating monocytes/macrophages in HIV: potential biomarkers for HIV infection and AIDS progression. AIDS Res Hum Retroviruses 24:417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Bell C, Croul S, Lewis M, Rappaport J (2008b) Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol 14:318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J (2001) CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol 7:528–541. [DOI] [PubMed] [Google Scholar]
- Fritz-French C, Tyor W (2012) Interferon-alpha (IFNalpha) neurotoxicity. Cytokine Growth Factor Rev 23:7–14. [DOI] [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ, et al. (1992) Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med 176:1703–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R (1993) Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 6:904–912. [PubMed] [Google Scholar]
- Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM, Detels R (1999) Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis 179:859–870. [DOI] [PubMed] [Google Scholar]
- Giunti D, Borsellino G, Benelli R, Marchese M, Capello E, Valle MT, Pedemonte E, Noonan D, Albini A, Bernardi G, Mancardi GL, Battistini L, Uccelli A (2003) Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J Leukoc Biol 73:584–590. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J (2005) The neuropathogenesis of AIDS. Nat Rev Immunol 5:69–81. [DOI] [PubMed] [Google Scholar]
- Grant I et al. (2014) Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology 82:2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras G, Kaul M (2010) Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Wang B, Han N, Zhao Y, Song C, Feng X, Mao Y, Zhang F, Zhao H, Zeng H (2009) CD14(high)CD16(+) rather than CD14(low)CD16(+) monocytes correlate with disease progression in chronic HIV-infected patients. J Acquir Immune Defic Syndr 52:553–559. [DOI] [PubMed] [Google Scholar]
- Heaton RK et al. (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez JE, Rizzo MD, Crawford RB, Gulick P, Kaminski NE (2018) Interferon-alpha-Mediated Activation of T Cells from Healthy and HIV-Infected Individuals Is Suppressed by Delta(9)-Tetrahydrocannabinol. J Pharmacol Exp Ther 367:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, Kovacs CM, Rodriguez B, Sieg SF, Teixeira-Johnson L, Gudonis D, Goepfert PA, Lederman MM, Frank I, Makedonas G, Kaul R, Walker BD, Betts MR (2010) Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog 6:e1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas-Stubbs S, Riezu-Boj JI, Gonzalez I, Mancheno U, Dubrot J, Azpilicueta A, Gabari I, Palazon A, Aranguren A, Ruiz J, Prieto J, Larrea E, Melero I (2010) Effects of IFN-alpha as a signal-3 cytokine on human naive and antigen-experienced CD8(+) T cells. Eur J Immunol 40:3389–3402. [DOI] [PubMed] [Google Scholar]
- Hijdra D, Vorselaars AD, Grutters JC, Claessen AM, Rijkers GT (2013) Phenotypic characterization of human intermediate monocytes. Front Immunol 4:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Banks WA (2015) Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun 45:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA (2007) Human cannabinoid pharmacokinetics. Chem Biodivers 4:1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardo FP (2019) Cannabidiol Adverse Effects and Toxicity. Curr Neuropharmacol 17:974–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iffland K, Grotenhermen F (2017) An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res 2:139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv LB, Donlin LT (2014) Regulation of type I interferon responses. Nat Rev Immunol 14:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana A, Pahan K (2004) Human immunodeficiency virus type 1 gp120 induces apoptosis in human primary neurons through redox-regulated activation of neutral sphingomyelinase. J Neurosci 24:9531–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings RN, Grayson JM, Barton ES (2014) Type I interferon signaling enhances CD8+ T cell effector function and differentiation during murine gammaherpesvirus 68 infection. J Virol 88:14040–14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI, Deeks SG, Douek DC, Brenchley JM (2009) Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 199:1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R (2002) Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol 2:251–262. [DOI] [PubMed] [Google Scholar]
- Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D (2012a) Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr 60:234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat A, Misra V, Cassol E, Ancuta P, Yan Z, Li C, Morgello S, Gabuzda D (2012b) A plasma biomarker signature of immune activation in HIV patients on antiretroviral therapy. PLoS One 7:e30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski NE, Abood ME, Kessler FK, Martin BR, Schatz AR (1992) Identification of a functionally relevant cannabinoid receptor on mouse spleen cells involved in cannabinoid-mediated immune modulation. Mol Pharmacol 42:736–742. [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988–994. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S (2006) TLR signaling. Cell Death Differ 13:816–825. [DOI] [PubMed] [Google Scholar]
- Kessing CF, Tyor WR (2015) Interferon-alpha induces neurotoxicity through activation of the type I receptor and the GluN2A subunit of the NMDA receptor. J Interferon Cytokine Res 35:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WK, Alvarez X, Fisher J, Bronfin B, Westmoreland S, McLaurin J, Williams K (2006) CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am J Pathol 168:822–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TW, Newton CA, Widen R, Friedman H (1985) The effect of delta-9-tetrahydrocannabinol and 11-hydroxy-delta-9-tetrahydrocannabinol on T-lymphocyte and B-lymphocyte mitogen responses. J Immunopharmacol 7:451–466. [DOI] [PubMed] [Google Scholar]
- Klein TW, Kawakami Y, Newton C, Friedman H (1991) Marijuana components suppress induction and cytolytic function of murine cytotoxic T cells in vitro and in vivo. J Toxicol Environ Health 32:465–477. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton CA, Nakachi N, Friedman H (2000) Delta 9-tetrahydrocannabinol treatment suppresses immunity and early IFN-gamma, IL-12, and IL-12 receptor beta 2 responses to Legionella pneumophila infection. J Immunol 164:6461–6466. [DOI] [PubMed] [Google Scholar]
- Koneru R, Bimonte-Nelson H, Ciavatta V, Haile W, Elmore K, Ward J, Maroun L, Tyor WR (2018) Reversing interferon-alpha neurotoxicity in a HIV-associated neurocognitive disorder mouse model. AIDS 32:1403–1411. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK (2001) Identification of the haemoglobin scavenger receptor. Nature 409:198–201. [DOI] [PubMed] [Google Scholar]
- Lake JE, Vo QT, Jacobson LP, Sacktor N, Miller EN, Post WS, Becker JT, Palella FJ Jr., Ragin A, Martin E, Munro CA, Brown TT (2015) Adiponectin and interleukin-6, but not adipose tissue, are associated with worse neurocognitive function in HIV-infected men. Antivir Ther 20:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane BR, King SR, Bock PJ, Strieter RM, Coffey MJ, Markovitz DM (2003) The C-X-C chemokine IP-10 stimulates HIV-1 replication. Virology 307:122–134. [DOI] [PubMed] [Google Scholar]
- Lau LT, Yu AC (2001) Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma 18:351–359. [DOI] [PubMed] [Google Scholar]
- Lei J, Yin X, Shang H, Jiang Y (2019) IP-10 is highly involved in HIV infection. Cytokine 115:97–103. [DOI] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ, Group C (2008) Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DE, Couturier JP (2019) Chapter 6 - Chronic Inflammation in HIV Pathogenesis: Effects On Immune Cells, Organ Systems, and Systemic Consequences In: Translational Inflammation (Actor JK, Smith KC, eds), pp 111–131: Academic Press. [Google Scholar]
- Li W, Henderson LJ, Major EO, Al-Harthi L (2011) IFN-gamma mediates enhancement of HIV replication in astrocytes by inducing an antagonist of the beta-catenin pathway (DKK1) in a STAT 3-dependent manner. J Immunol 186:6771–6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Liu Y, Sun B, Sun Y, Hou B, Zhang Y, Ma Z, Gu X (2015) Intrathecal Injection of JWH-015 Attenuates Bone Cancer Pain Via Time-Dependent Modification of Pro-inflammatory Cytokines Expression and Astrocytes Activity in Spinal Cord. Inflammation 38:1880–1890. [DOI] [PubMed] [Google Scholar]
- Lutz B (2002) Molecular biology of cannabinoid receptors. Prostaglandins Leukot Essent Fatty Acids 66:123–142. [DOI] [PubMed] [Google Scholar]
- Makedonas G, Betts MR (2011) Living in a house of cards: re-evaluating CD8+ T-cell immune correlates against HIV. Immunol Rev 239:109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek N, Popiolek-Barczyk K, Mika J, Przewlocka B, Starowicz K (2015) Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures. Neural Plast 2015:130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manion M, Rodriguez B, Medvik K, Hardy G, Harding CV, Schooley RT, Pollard R, Asmuth D, Murphy R, Barker E, Brady KE, Landay A, Funderburg N, Sieg SF, Lederman MM (2012) Interferon-alpha administration enhances CD8+ T cell activation in HIV infection. PLoS One 7:e30306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuzak JA, Gott TM, Kirkwood JS, Coronado E, Hensley-McBain T, Miller C, Cheu RK, Collier AC, Funderburg NT, Martin JN, Wu MC, Isoherranen N, Hunt PW, Klatt NR (2018) Heavy Cannabis Use Associated With Reduction in Activated and Inflammatory Immune Cell Frequencies in Antiretroviral Therapy-Treated Human Immunodeficiency Virus-Infected Individuals. Clin Infect Dis 66:1872–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature (London) 346:561–564. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Glass M, Pertwee RG (2007) Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol 152:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha M, Carrillo-Salinas FJ, Feliu A, Mestre L, Guaza C (2016) Microglia activation states and cannabinoid system: Therapeutic implications. Pharmacol Ther 166:40–55. [DOI] [PubMed] [Google Scholar]
- Mehla R, Bivalkar-Mehla S, Nagarkatti M, Chauhan A (2012) Programming of neurotoxic cofactor CXCL-10 in HIV-1-associated dementia: abrogation of CXCL-10-induced neuro-glial toxicity in vitro by PKC activator. J Neuroinflammation 9:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RF, Isaacson PG, Hall-Craggs M, Lucas S, Gray F, Scaravilli F, An SF (2004) Cerebral CD8+ lymphocytosis in HIV-1 infected patients with immune restoration induced by HAART. Acta Neuropathol 108:17–23. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Burger F, Mach F, Steffens S (2008) CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Heart Circ Physiol 294:H1145–1155. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of peripheral receptor for cannabinoids. Nature 365:61–65. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences E, Medicine (2017) The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Neuenburg JK, Furlan S, Bacchetti P, Price RW, Grant RM (2005) Enrichment of activated monocytes in cerebrospinal fluid during antiretroviral therapy. AIDS 19:1351–1359. [DOI] [PubMed] [Google Scholar]
- Newton CA, Klein TW, Friedman H (1994) Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infect Immun 62:4015–4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor CN, Zhou Z, Burrell LE 2nd, Kelso NE, Whitehead NE, Harman JS, Cook CL, Cook RL (2017) Marijuana use and viral suppression in persons receiving medical care for HIV-infection. Am J Drug Alcohol Abuse 43:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Towe SL, Hobkirk AL, Nash D, Goodwin RD (2018) Frequency of Cannabis Use and Medical Cannabis Use Among Persons Living With HIV in the United States: Findings From a Nationally Representative Sample. AIDS Educ Prev 30:169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock ND, White JT, Cross EW, Cheney EE, Tamburini BA, Kedl RM (2013) T cell responses: naive to memory and everything in between. Adv Physiol Educ 37:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrella O, Carreiri PB, Perrella A, Sbreglia C, Gorga F, Guarnaccia D, Tarantino G (2001) Transforming growth factor beta-1 and interferon-alpha in the AIDS dementia complex (ADC): possible relationship with cerebral viral load? Eur Cytokine Netw 12:51–55. [PubMed] [Google Scholar]
- Pertwee RG (1999) Pharmacology of cannabinoid receptor ligands. Curr Med Chem 6:635–664. [PubMed] [Google Scholar]
- Pertwee RG (2005) Pharmacological actions of cannabinoids. Handb Exp Pharmacol:1–51. [DOI] [PubMed] [Google Scholar]
- Pulford K, Micklem K, McCarthy S, Cordell J, Jones M, Mason DY (1992) A monocyte/macrophage antigen recognized by the four antibodies GHI/61, Ber-MAC3, Ki-M8 and SM4. Immunology 75:588–595. [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS (1997) Unique monocyte subset in patients with AIDS dementia. The Lancet 349:692–695. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Rempel H, Sun B, Abadjian L, Calosing C, Meyerhoff DJ (2011) A peripheral monocyte interferon phenotype in HIV infection correlates with a decrease in magnetic resonance spectroscopy metabolite concentrations. AIDS 25:1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, MacLean AG, Philipp MT (2013) Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm 2013:480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez LA, Arango TA, Thompson E, Naji M, Tebas P, Boyer JD (2014) High IP-10 levels decrease T cell function in HIV-1-infected individuals on ART. J Leukoc Biol 96:1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel H, Sun B, Calosing C, Pillai SK, Pulliam L (2010) Interferon-alpha drives monocyte gene expression in chronic unsuppressed HIV-1 infection. AIDS 24:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho MBW S; Glass JD; McArthur JC; Choi S; Griffin J; Tyor WR (1995) A Potential Role for Interferon-α in the Pathogenesis of HIV-Associated Dementia. Brain Behav Immun 9:366–377. [DOI] [PubMed] [Google Scholar]
- Rizzo MD, Crawford RB, Bach A, Sermet S, Amalfitano A, Kaminski NE (2019) Imiquimod and interferon-alpha augment monocyte-mediated astrocyte secretion of MCP-1, IL-6 and IP-10 in a human co-culture system. Journal of Neuroimmunology:576969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MD, Crawford RB, Henriquez JE, Aldhamen YA, Gulick P, Amalfitano A, Kaminski NE (2018) HIV-infected cannabis users have lower circulating CD16+ monocytes and IFN-gamma-inducible protein 10 levels compared with nonusing HIV patients. AIDS 32:419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RH, Meissler JJ, Fan X, Yu D, Adler MW, Eisenstein TK (2015) A CB2-Selective Cannabinoid Suppresses T-Cell Activities and Increases Tregs and IL-10. J Neuroimmune Pharmacol 10:318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MD, Castaneda JT, Kiertscher SM (2015) Exposure to Delta9-Tetrahydrocannabinol Impairs the Differentiation of Human Monocyte-derived Dendritic Cells and their Capacity for T cell Activation. J Neuroimmune Pharmacol 10:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg EV, Chen D, Diamond RA, Dohadwala M, Novak TJ, White PM, Yang-Snyder JA (1991) Acquisition of mature functional responsiveness in T cells: programming for function via signaling. Adv Exp Med Biol 292:71–83. [DOI] [PubMed] [Google Scholar]
- Rumbaugh JA, Tyor W (2015) HIV-associated neurocognitive disorders: Five new things. Neurol Clin Pract 5:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Glass CK (2011) Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol 11:775–787. [DOI] [PubMed] [Google Scholar]
- Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC, Group ISS (2011) Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 203:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas AR, Bimonte-Nelson H, Smothers CT, Woodward J, Tyor WR (2009) Interferon-alpha causes neuronal dysfunction in encephalitis. J Neurosci 29:3948–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC (2016) HIV-associated neurocognitive disorder--pathogenesis and prospects for treatment. Nat Rev Neurol 12:234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz AR, Lee M, Condie RB, Pulaski JT, Kaminski NE (1997) Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol Appl Pharmacol 142:278–287. [DOI] [PubMed] [Google Scholar]
- Schrier RD, Hong S, Crescini M, Ellis R, Perez-Santiago J, Spina C, Letendre S, Group H (2015) Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS One 10:e0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scutari R, Alteri C, Perno CF, Svicher V, Aquaro S (2017) The Role of HIV Infection in Neurologic Injury. Brain Sci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacklett BL, Cox CA, Wilkens DT, Karl Karlsson R, Nilsson A, Nixon DF, Price RW (2004) Increased adhesion molecule and chemokine receptor expression on CD8+ T cells trafficking to cerebrospinal fluid in HIV-1 infection. J Infect Dis 189:2202–2212. [DOI] [PubMed] [Google Scholar]
- Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF (2012) Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RP, Scully EP, Groden EE, Arnold KB, Chang JJ, Lane K, Lifson J, Rosenberg E, Lauffenburger DA, Altfeld M (2013) HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. AIDS 27:2505–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders J, Remmerswaal EB, Schuurman KG, Melief J, van Eden CG, van Lier RA, Huitinga I, Hamann J (2013) Characteristics of differentiated CD8(+) and CD4 (+) T cells present in the human brain. Acta Neuropathol 126:525–535. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV (2015) Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16:249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N (2009) Endocannabinoid signaling in microglial cells. Neuropharmacology 56 Suppl 1:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazza M, Pirrone V, Wigdahl B, Nonnemacher MR (2011) Breaking down the barrier: the effects of HIV-1 on the blood-brain barrier. Brain Res 1399:96–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Y, Stehno-Bittel L, Li S, Loganathan R, Dhillon NK, Pinson D, Nath A, Kolson D, Narayan O, Buch S (2006) CXCL10-induced cell death in neurons: role of calcium dysregulation. Eur J Neurosci 23:957–964. [DOI] [PubMed] [Google Scholar]
- Swiecki M, Colonna M (2015) The multifaceted biology of plasmacytoid dendritic cells. Nature Reviews Immunology 15:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan IL, Smith BR, Hammond E, Vornbrock-Roosa H, Creighton J, Selnes O, McArthur JC, Sacktor N (2013) Older individuals with HIV infection have greater memory deficits than younger individuals. J Neurovirol 19:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazzi E, Morrison D, Sullivan P, Morgello S, Fischer T (2014) Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Curr HIV Res 12:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham EL, Shrikant P, Mescher MF (2002) Activation-induced nonresponsiveness: a Th-dependent regulatory checkpoint in the CTL response. J Immunol 168:1190–1197. [DOI] [PubMed] [Google Scholar]
- Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N (1995) CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol 25:3418–3424. [DOI] [PubMed] [Google Scholar]
- Tolon RM, Nunez E, Pazos MR, Benito C, Castillo AI, Martinez-Orgado JA, Romero J (2009) The activation of cannabinoid CB2 receptors stimulates in situ and in vitro beta-amyloid removal by human macrophages. Brain Res 1283:148–154. [DOI] [PubMed] [Google Scholar]
- Ton H, Xiong H (2013) Astrocyte Dysfunctions and HIV-1 Neurotoxicity. J AIDS Clin Res 4:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham DJ, Tripp RA, Doherty PC (1997) CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol 159:5197–5200. [PubMed] [Google Scholar]
- Tough DF (2012) Modulation of T-cell function by type I interferon. Immunol Cell Biol 90:492–497. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P (2007) Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 45:174–182. [DOI] [PubMed] [Google Scholar]
- Turcotte C, Chouinard F, Lefebvre JS, Flamand N (2015) Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J Leukoc Biol 97:1049–1070. [DOI] [PubMed] [Google Scholar]
- Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J, Group RSS (2012) Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 206:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassallo M, Mercie P, Cottalorda J, Ticchioni M, Dellamonica P (2012) The role of lipopolysaccharide as a marker of immune activation in HIV-1 infected patients: a systematic literature review. Virol J 9:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrely A, Bienvenu B, Gasnault J, Thiebault JB, Salmon D, Gray F (2005) Fulminant inflammatory leukoencephalopathy associated with HAART-induced immune restoration in AIDS-related progressive multifocal leukoencephalopathy. Acta Neuropathol 109:449–455. [DOI] [PubMed] [Google Scholar]
- von Geldern G, Cepok S, Nolting T, Du Y, Grummel V, Adams O, Hartung HP, Arendt G, Hemmer B (2007) CD8 T-cell subsets and viral load in the cerebrospinal fluid of therapy-naive HIV-infected individuals. AIDS 21:250–253. [DOI] [PubMed] [Google Scholar]
- Walker B, McMichael A (2012) The T-cell response to HIV. Cold Spring Harb Perspect Med 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware MA, Rueda S, Singer J, Kilby D (2003) Cannabis Use by Persons Living with HIV/AIDS: Patterns and Prevalence of Use. Journal of Cannabis Therapeutics 3:3–15. [Google Scholar]
- Wen J, Ribeiro R, Tanaka M, Zhang Y (2015) Activation of CB2 receptor is required for the therapeutic effect of ABHD6 inhibition in experimental autoimmune encephalomyelitis. Neuropharmacology 99:196–209. [DOI] [PubMed] [Google Scholar]
- Wenzel I, Roth J, Sorg C (1996) Identification of a novel surface molecule, RM3/1, that contributes to the adhesion of glucocorticoid-induced human monocytes to endothelial cells. Eur J Immunol 26:2758–2763. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB (1986) Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A 83:7089–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW (2014a) Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res 12:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW (2014b) CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol Neuroimmunol Neuroinflamm 1:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, Morgello S, Berman JW (2013) Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One 8:e69270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JC, Appelberg S, Goldberger BA, Klein TW, Sleasman JW, Goodenow MM (2014c) Delta(9)-Tetrahydrocannabinol treatment during human monocyte differentiation reduces macrophage susceptibility to HIV-1 infection. J Neuroimmune Pharmacol 9:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA (2001) Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med 193:905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younas M, Psomas C, Reynes J, Corbeau P (2016) Immune activation in the course of HIV-1 infection: Causes, phenotypes and persistence under therapy. HIV Med 17:89–105. [DOI] [PubMed] [Google Scholar]
- Young KG, Maclean S, Dudani R, Krishnan L, Sad S (2011) CD8+ T cells primed in the periphery provide time-bound immune-surveillance to the central nervous system. J Immunol 187:1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Liu A, Qiao L, Sheng B, Xu M, Li W, Chen D (2015) The Relationship of CSF and Plasma Cytokine Levels in HIV Infected Patients with Neurocognitive Impairment. BioMed Research International 2015:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Qiao L, Wei F, Yin J, Liu L, Ji Y, Smith D, Li N, Chen D (2013) Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J Neurovirol 19:144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Kiertscher SM, Cheng Q, Zoumalan R, Tashkin DP, Roth MD (2002) Delta 9-Tetrahydrocannabinol regulates Th1/Th2 cytokine balance in activated human T cells. J Neuroimmunol 133:124–131. [DOI] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R (1998) Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 188:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L (2007) The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 81:584–592. [DOI] [PubMed] [Google Scholar]


