Abstract
Background/Objectives:
Gastrointestinal phenotypes have previously been associated with obesity, however it is unknown if these phenotypes are a cause or a consequence of obesity and weight gain. Our aim was to assess whether these gastrointestinal phenotypes are associated with future weight gain in younger adults.
Subjects/Methods:
At baseline, 126 adult participants under the age of 35 were weighed and underwent measurement of gastrointestinal phenotypes including gastric emptying (GE), gastric volume, satiation, satiety and gastrointestinal hormones. Patients were re-appraised after median 4.4 years unless, during the period of follow up, they participated in a formal weight loss program, received obesity-weight loss interventions, or developed a health condition likely to affect weight. Participants were dichotomized into two groups for each phenotype at the median of each phenotype.
Results:
In total, 60 participants met criteria for inclusion and were evaluated after a median of 4.4 years [IQR: 3.5 to 5],36 participants were excluded due to conditions that would abnormally affect weight during study period including pregnancy and weight loss treatment, and 30 participants were lost to prospective follow-up. Faster GE was significantly associated with weight gain. Those with faster GE at baseline (n=30) gained a median of 9.6 kg [3.1 to 14.9] compared to those with slower GE at baseline (n=30) who gained a median of 2.8 kg [−4.6 to 9.2] (p=0.03), over the follow-up period. There was no association between the other phenotypes and weight gain.
Conclusions:
In adults ≤35 years old, faster gastric emptying is associated with significantly increased weight gain over the medium term. This provides supportive evidence for the role of gastric emptying in weight gain and development of obesity.
INTRODUCTION
Despite an expanding range of therapeutic options for obesity (1), the prevalence of obesity continues to increase (2). Through better understanding of the pathophysiology, and identification of sub-phenotypes of obesity, it has been proposed that obesity could be treated, or possibly prevented, based on approaches targeting specific obesity phenotypes (3–5), and those hypotheses are the objectives of current prospective studies (6).
There is growing evidence of a relationship between phenotypes associated with energy intake and obesity; the phenotypes are different quantitative measurements of the brain gut axis that have the potential to regulate food intake. Obesity has previously been associated with the following energy intake phenotypes: Accelerated gastric emptying, increased fasting gastric volume, impaired satiety, and impaired satiation (5) About one third of patients with obesity have accelerated gastric emptying (5) and patients with accelerated gastric emptying have significantly better weight loss response to treatments that retard gastric emptying, including glucagon-like peptide-1 (GLP-1) agonists or analogs (7, 8) and intragastric balloons (9). To date, there are no prospective studies assessing whether these phenotypes predict future weight gain. Therefore, it is currently unknown if these phenotypes cause weight gain and obesity or if these physiologic changes develop only as a consequence of weight gain and obesity.
During the transition from adolescence into young adulthood (e.g. between 18 and 35 years of age), there is often rapid weight gain. Data from NHANES shows that, of any 10 year period, the incidence of major weight gain is highest between ages 25–34 (10). Furthermore, weight gain during this age period is associated with the highest lifetime risk of developing diabetes and cardiovascular disease (11, 12). Therefore, weight control during this age period is deemed to be critical (13). The need to prevent rapid weight gain in young adults is emphasized by the consortium of Early Adult Reduction of weight through LifestYle intervention (EARLY) trials (14, 15). Therefore, further understanding of the pathophysiology or risk factors of weight gain in this age group is of particular interest and a focus of this study.
Our aim was primarily to assess whether energy intake phenotypes measured at the start of this cohort study are predictive of future weight gain in younger adults, and to assess the influence of gender and baseline BMI on the association of any significant phenotype and weight gain.
METHODS
Participant Cohort and Baseline Measurements
The study protocol was approved by Mayo Clinic’s Institutional Review Board and informed consent to access medical records for research was obtained from all participants.
Between 2 011 and 2 013, a cohort of 126 overweight or obese but otherwise generally healthy younger adults without adverse gastrointestinal symptoms were recruited from the local community and underwent standardized measurements of energy intake phenotypes in our laboratory as described in greater detail in prior studies (5). Briefly, gastric emptying was measured by scintigraphy after eating a radiolabeled egg meal (296kcal, 30% fat): the primary endpoint was time for half of the standardized meal to empty from the stomach (GE T1/2). Gastric volume during fasting and after ingestion of a 300kcal liquid nutrient meal (Ensure®, 1kcal/mL) was measured by previously validated single photon emission tomography (SPECT) method (16). Satiation, measured as the volume required to reach fullness (VTF) and the maximum tolerated volume (MTV), was assessed by our previously validated standardized nutrient drink test (17). Satiety, interpreted as the persistence of fullness after a fully satiating meal, was assessed (as in prior studies) by using visual analog scale for appetite after a caloric intake in an ad libitum buffet meal ingested 4 hours after the standard 300 mL of Ensure® ingested during the gastric volume measurement (18). Plasma gastrointestinal hormones GLP-1, PYY, CCK, and ghrelin were measured by RIA/ELISA (Millipore, Sigma) at fasting, 15, 45, and 90 minutes postprandially after standard breakfast for gastric emptying test. Peak hormonal level being the primary endpoint.
Measurements at the end of follow-up
At the conclusion of the cohort study in 2017, participants were invited to return to the laboratory for repeat measurement of energy intake for the purpose of analyzing how these traits may have changed in association with their weight change over the study period.
Weight Documentation and Inclusion Criteria
Most recent weights and identification of any exclusion criteria were determined from review of updated clinical records and/or a follow-up questionnaire sent to participants in 2017.
Each participant’s electronic medical record was accessed and reviewed for most recent weight, as documented during clinic visits, and meeting of any exclusion criteria. A comprehensive weight management questionnaire asking for current weight, experience with various weight control measures, other medical conditions, physical activity, and eating patterns was mailed to all participants. Upon receipt of a completed questionnaire, the participant’s most recent weight and meeting of any exclusion criteria were merged with data obtained from the review of the participant’s electronic medical record.
Inclusion/Exclusion Criteria
The study was approved by Mayo Clinic Institutional Review Board (protocol number 10–007083). Participants had consented to the use of their medical records for research. To be included, participants were required to either 1) have sufficient data available in their electronic medical record (as evidenced by both a weight measured a minimum of 2 years following baseline measurements and clinic visit notes in this interval time period documenting ongoing medical care) or 2) have completed and returned the questionnaire. Participants who did not meet either of these two criteria were considered lost to follow-up and not included in the final study cohort. The majority of participants still resided within 90 miles of Mayo Clinic Rochester and received ongoing routine primary and specialty care documented in their electronic medical record. Participation in any experimental trials through our institution is documented in the medical record.
The exclusion criteria included the following developments during the period of follow-up: participation in a formal weight loss program, experimental treatment trial, or specific obesity treatment such as medication or surgery; medical conditions considered likely to abnormally affect a participant’s weight during the follow-up period such as pregnancy, malignancy, poorly controlled depression or anxiety, polycystic ovarian syndrome, ongoing illicit drug use, and treatment with glucocorticoids; and loss to follow-up. In the event that the initiation of an exclusion criterion occurred greater than 2 years following baseline measurement (e.g. initiation of a weight loss medication, participation in a weight loss trial, or becoming pregnant), the participant was included in the final cohort using their weight immediately prior to the disqualifying factor for calculating weight gain, and the date of the eligible weight recording was used for calculating length of follow-up.
Weight Gain and Weight Gain per Year
Weight gain was calculated by most recent weight minus baseline weight (at the time of measurement of gastric emptying). Length of follow-up was calculated by the number of years between date of most recent weight and date of measurement of baseline weight. Weight gain per year was calculated by weight gain divided by length of follow-up.
Categories of Energy Intake Phenotypes
Participants were divided into two groups for each phenotypes based on their baseline measurement of that trait. This includes: faster gastric emptying and slower gastric emptying based on the cohort’s median value for gender-specific GE T1/2 for solids: 103.8 min for females and 97.5 min for males, greater and lesser gastric volume/accommodation by the cohort’s median gastric accommodation ratio of 2.8, greater and lesser satiation based on median VTF and MTV of 630 mL and 1228 mL, respectively, and greater and lesser satiety by the median buffet meal intake of 920 kcal. The gastric emptying groups were divided by the gender specific value because men have faster gastric emptying than women (19). In a post-hoc analysis, weight gain was also assessed based on quartiles of the GE T1/2 for solids, gender specific GE groups, and by baseline BMI.
Statistical Analysis
We analyzed the association of weight gain and participants’ demographics and energy intake phenotypes using Mann-Whitney Rank Sum test. The analysis of the weight change for the four GE quartiles was done using analysis of variance (ANOVA) for the overall association and Dunn’s method for pairwise comparisons. Correlations were done using spearman analysis. Data for all analyses are plotted as median (IQR). Analyses were performed with JMP and SigmaPlot.
RESULTS
Study Participants
Sixty of 126 participants (95% white) met criteria for inclusion; Figure 1 shows participant disposition in the follow-up cohort. Five participants were excluded because they did not complete baseline measurement of all studied gastrointestinal phenotypes. Eight participants were included who had their weight immediately prior to a disqualifying factor used for calculation of weight gain. Table 1 shows demographics and baseline characteristics. Participants were followed for a median of 4.4 years [IQR: 3.5 to 5]; median weight gain for the entire cohort was 4.8 kg [0.1 to 12.7] or 1.2 kg/year [0 to 2.9].
Figure 1:
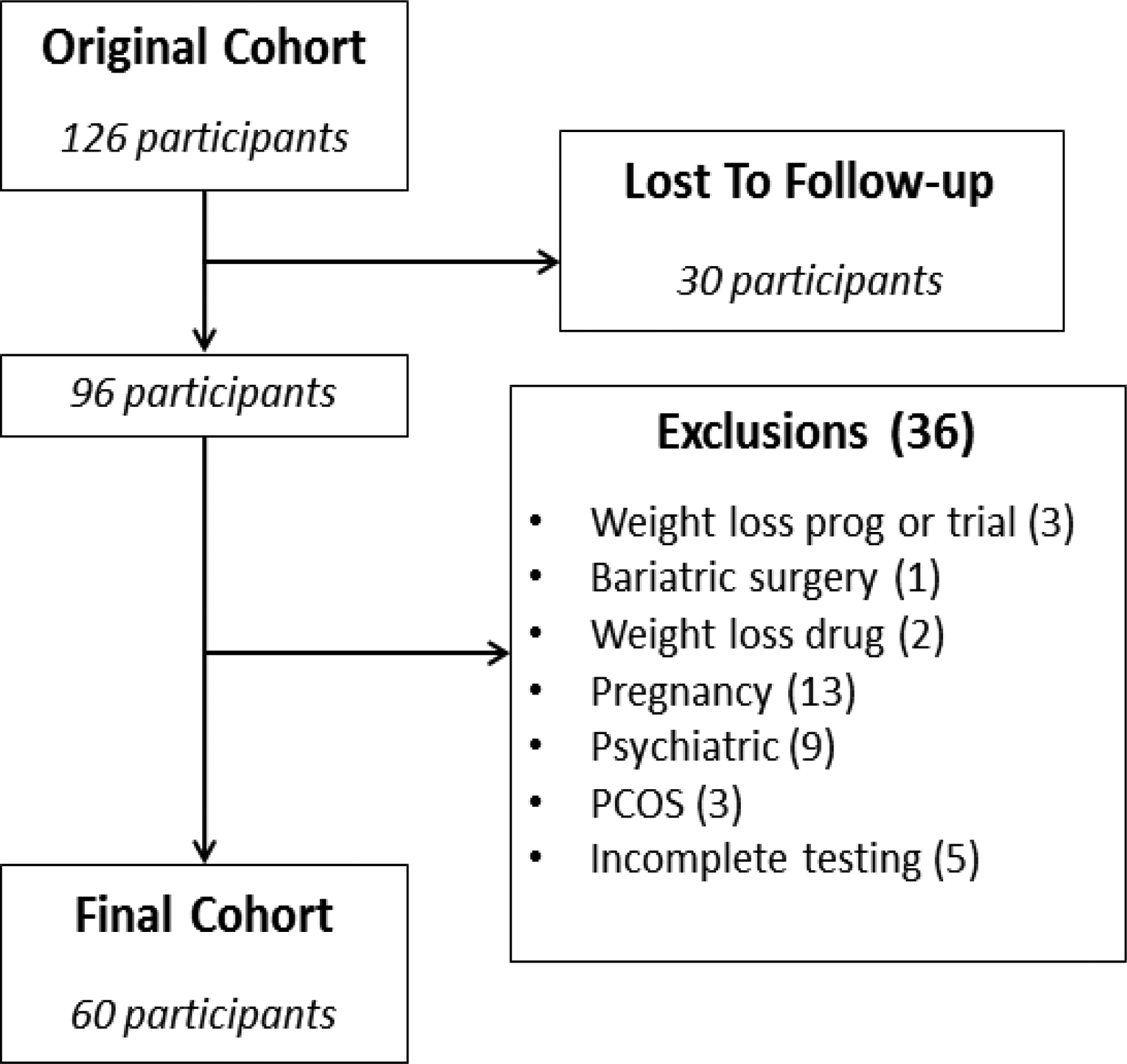
Study cohort and exclusions.
Table 1:
Demographics and baseline characteristics [median (IQR)]
| n | 60 |
| Age | 29 (25 – 32.7) |
| Gender, M:F | 15 (25%); 45 (75%) |
| Ethnicity | n (%) |
| Weight (kg) | 93.9 (82.4 – 103.4) |
| BMI kg/m2 | 32.3 (29.3 – 35.6) |
| Solid Gastric Emptying T1/2 (min) | 100.7 (88.1 – 118) |
| Gastric Accommodation Ratio | 2.8 (2.5 – 3.3) |
| VTF (mL) | 630 (510 – 900) |
| MTV (mL) | 1228.5 (948 – 1424.3) |
| Buffet Meal Intake (kCal) | 920.3 (774.5 – 1113.3) |
| Ghrelin fasting, pg/mL | 60 (43 – 89.5) |
| CCK peak, pmol/L | 6.1 (3.8 – 9.8) |
| GLP-1 peak, pM | 14 (9.1 – 20) |
| PYY peak, pg/mL | 147 (106 – 193) |
| Follow-up (y) | 4.4 (3.5 – 5) |
Overall Assessment of Weight Gain and Associated Factors
There was significant association between weight gain and gastric emptying (r=0.37, p<0.01). There was no significant association between weight and gastric volume, satiety (VAS), satiation (VTF, MTV), or GI hormones, either by linear association or by group comparisons (Table 2).
Table 2:
Weight gain according to Energy Intake Phenotypes [median (IQR)] and correlations examined between weight gain and measured variables
| Measurement | Variable dichotomized | n | Total weight gain (kg) | p | correlation | |
|---|---|---|---|---|---|---|
| R | P | |||||
| Gastric Emptying T1/2 | Faster | 30 | 9.6 (3.1 – 14.9) | 0.03 | 0.37 | 0.003 |
| Slower | 30 | 2.8 (−4.6 – 9.2) | ||||
| Volume to Usual Fullness (VTF) | Less | 30 | 3.9 (−2.9 – 12.4) | 0.28 | 0.09 | 0.47 |
| More | 30 | 8.1 (2.4 – 16.1) | ||||
| Maximal Tolerated Volume (MTV) | Less | 30 | 4 (−0.8 – 11.2) | 0.49 | 0.08 | 0.53 |
| More | 30 | 7.2 (0.2 – 15.8) | ||||
| VAS Fullness | Less | 30 | 4.7 (−3.4 – 12.5) | 0.78 | 0.1 | 0.37 |
| More | 30 | 5 (1.8 – 14.6) | ||||
| Gastric Accommodation, Ratio | Less | 30 | 4.8 (0.8 – 10.5) | 0.24 | 0.03 | 0.84 |
| More | 30 | 5.4 (−0.7 – 18.1) | ||||
| Ad Libitum Buffet Meal | Less | 30 | 4.3 (−0.4 – 12.6) | 0.70 | 0.08 | 0.56 |
| More | 30 | 6.4 (−0.5 – 12.9) | ||||
| Ghrelin fasting, pg/mL | Less | 30 | 5.2 (0.6 – 15) | 0.78 | 0.03 | 0.76 |
| More | 30 | 4.6 (−1.4 – 12.5) | ||||
| CCK peak, pmol/L | Less | 30 | 4.8 (−2.1 – 13.2) | 0.91 | 0.04 | 0.71 |
| More | 30 | 4.3 (0.6 −13.5) | ||||
| GLP-1 peak, pM | Less | 30 | 7.9 (−2.4 – 12) | 0.67 | 0.1 | 0.40 |
| More | 30 | 4.2 (0.6 – 15.8) | ||||
| PYY peak, pg/mL | Less | 30 | 8.4 (0.5 – 13) | 0.60 | 0.01 | 0.97 |
| More | 30 | 3.8 (−2.5 – 14.8) | ||||
Weight Gain Based on Gastric Emptying
Participants with faster gastric emptying at baseline (n=30) gained a median of 9.6 kg [3.1 to 14.9] or an average of 2.2 kg/year [0.7 to 3.1], compared to those with slower GE at baseline (n=30) who gained a median of 2.8 kg [−4.6 to 9.2] (p=0.03) or an average of 0.7 kg/year [−1.6 to 2] (p=0.03) [Figure 2-A].
Figure 2:
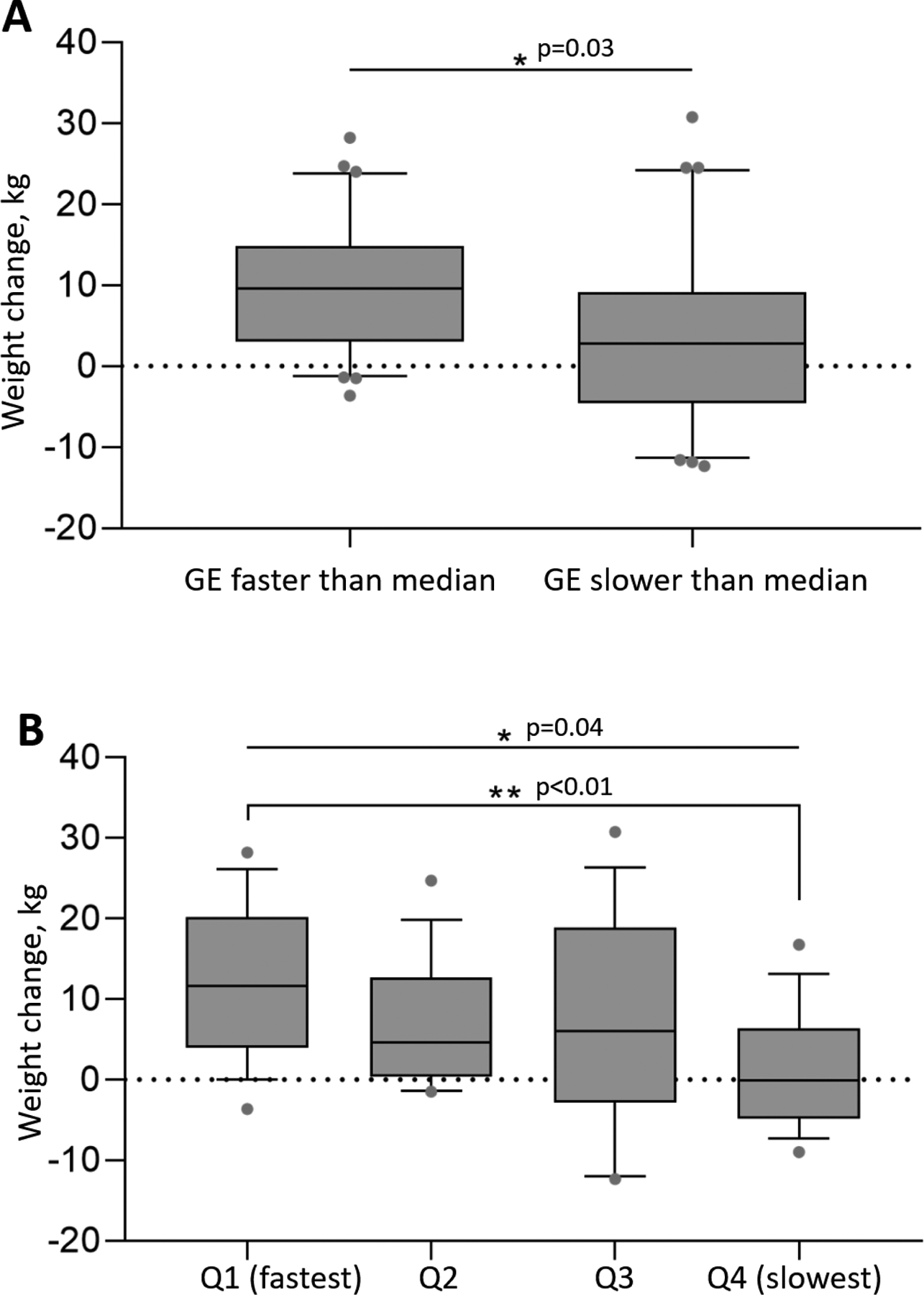
Median weight gain (IQR) based on GE faster or slower than the gender specific median (A) and in subgroups based on solid GE T1/2 quartiles (B).
When considering weight gained based on GE quartiles, the overall differences in weight gain were statistically significant by ANOVA (p=0.04); in a two group comparison, the fastest quartile gained a median of 11.6 kg [4 to 20.2] and the slowest gastric emptying quartile gained a median of −0.1 kg [−4.8 to 6.4] (p<0.01) [Figure 2-B], a difference of 11.7 kg.
Factors Potentially Impacting Weight Gain According to Baseline Gastric Emptying
We assessed whether gender and baseline BMI were associated with the influence of baseline gastric emptying on weight gain at follow-up.
In males (n=15), those with faster gastric emptying at baseline (n=8) gained a median of 13 kg [5.5 to 23.4] compared to those with slower gastric emptying at baseline (n=7) who gained a median of −4.2 kg [−11.6 to 7.9] (p<0.01). In females (n=45), those with faster gastric emptying at baseline (n=22) gained a median of 6.8 kg [0.6 to 13.2] compared to those with slower gastric emptying at baseline (n=23) who gained a median of 2.9 kg [−3.3 to 10.7] (p=0.44).
There was no significant difference in the amount of weight gained based on baseline BMI, which was <30 kg/m2 in 20 participants, 30–35 kg/m2 in 23 participants, and >35 kg/m2 in 17 participants.
DISCUSSION
In this cohort of overweight or obese younger adults with a follow-up period of median 4.4 years, faster baseline gastric emptying is associated with greater weight gain. In contrast to the association with gastric emptying, there is no observed association between weight gain and other phenotypes associated with obesity such as gastric volume, satiation, satiety, or gastrointestinal hormones. These findings further support the role of gastric emptying in obesity and weight gain.
Previously we reported an association between faster gastric emptying and obesity compared to normal weight controls (5). In the current study, we found that the physiologic parameter of faster gastric emptying at baseline predicts weight gain in adults younger than 35 years old. This observation, in a complex, multifactorial, and highly heterogeneous disease such as obesity appears to be clinically significant, as thus far, the proposed predictors of weight gain have been either equivocal or difficult to quantify, as covered in detail by Loos and Janssens, who showed that models based on established genetic variants have poorer predictive ability than traditional predictors, such as family history of obesity and childhood obesity (20). The magnitude of difference in weight gain of 6.8 kg (median) over approximately 4 years between faster gastric emptying and slower gastric emptying groups is also notable, suggesting that in comparison to several other previously studied factors contributing to weight gain in younger adults, baseline gastric emptying can be a stronger predictor. The literature has documented association of additional weight gain with fast food or sweetened beverage consumption, and demonstrated lower median weight increases over a similar follow-up period. Thus, in a prospective study of 3394 participants aged 18–30 years old, increased fast food consumption was associated with an additional weight gain of 1.4 kg over 3 years compared to those without increased fast food consumption (21). Similarly, in a prospective analysis of a combined cohort of Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study participants, increased intake of sugar sweetened beverages resulted in an adjusted additional weight gain of 0.6 kg over a 4 year period, and there was no significant difference in weight gain based on differences in absolute physical activity level (22).
If our findings are reproduced, these observations would support the measurement of gastric emptying in high-risk younger adults and potentially administering the FDA-approved GLP-1 agonists or analogs to significantly delay gastric emptying (7) and induce satiety (23), or other medications that slows gastric emptying such as amylin analogs(24). Given that prior studies have found patients with accelerated gastric emptying have significantly better weight loss response to GLP-1 agonists or analogs (7, 8), identification and intervention in these high-risk younger adults with accelerated gastric emptying could potentially be effective in preventing long-term obesity-related comorbidities that impose a substantial burden on personal health (25) and have significant economic implications to individuals and to society as a whole (20).
It is unknown why obesity is associated with accelerated gastric emptying and why accelerated gastric emptying predicts weight gain. One possible explanation is the fact that faster gastric emptying will result in a more rapid return to an “empty” stomach. This is associated with an increase in ghrelin secretion and consequently increased hunger, which could lead to an increase in overall daily caloric intake (3, 26). Other possible explanations for increase in meal caloric intake are a larger fasting or postprandial increase in volume to fullness (VTF), lower satiety, lower satiation, or lower levels of the hormones associated with decreased appetite such as GLP-1 and PYY (27); in the current study, these factors were not significantly associated with weight gain. However, larger studies are required to exclude a type II error in appraising the totality of the phenotypes studied here. It is also worth noting that slower gastric emptying was associated with a decrease in caloric intake (27) and that pharmacological retardation of gastric emptying is associated with decreased food intake (7, 23).
While there is little evidence to support a genetic susceptibility to induce faster gastric emptying in obesity, it is commonly reported that young adults with early onset and/or extreme cases of obesity might be enriched for common genetic variants that contribute to their weight gain (20, 28, 29). Thus, this high risk population, defined by faster gastric emptying, may be a population of interest for a subsequent genetic analysis study. There are preliminary observations in support of such a research approach. For example, the rs17782313 SNP in melanocortin 4 receptor gene was associated with increased scores of nausea and satiation symptoms after a fully satiating meal and slower gastric emptying (30). Similarly, variation in rs7903146 in the transcription factor 7-like 2 (TCF7L2) gene, a regulator of proglucagon processing, was associated with accelerated gastric emptying of liquids but not solids, and had no association with satiation or postprandial symptoms (31).
Further study is required to better understand the pathophysiology of obesity associated with rapid gastric emptying, what genetic or environmental factors are associated with faster gastric emptying and weight gain, and whether the observations also apply to other racial groups.
Strengths and Limitations
Strengths of our study include the use of gender-based gastric emptying T1/2 for solids to assess the association between baseline gastric emptying and weight gain. This was necessitated by the well-established difference in gastric emptying rate between genders (19). Another strength of this study is the participants being without dyspepsia, a variable that could abnormally affect results. In clinical practice we have observed some patients with either pathologically rapid or delayed gastric emptying experience weight loss as a result of their associated symptoms of nausea and abdominal pain and consequent reduction in food intake.
A limitation of this study is that over half of the participants already had BMI ≥ 30kg/m2 at baseline. Therefore, while providing supporting evidence, these results cannot be interpreted as proof that faster gastric emptying causes obesity. To provide stronger evidence to support gastric emptying as a cause of obesity, further study should be conducted with a larger cohort of participants having baseline BMI < 25 kg/m2. In our subgroup analysis of the association of baseline gastric emptying with weight gain according to baseline BMI, we were limited by the small sample of participants who were not yet obese (n=20).
Another limitation of our study was in assessing weight gain in association with gastrointestinal traits according to gender due to the limited sample size in these subgroups. Post-hoc subgroup analysis here showed increased weight gain in both males and females with faster gastric emptying but this was only statistically significant in males. Due to the limited sample size, associated risk of type II error, and necessary caution in interpreting subgroup analyses, we do not recommend forming any conclusion on gender influence.
CONCLUSION
In conclusion, in younger adults <35 years old, faster gastric emptying predicts future weight gain over a median follow up of 4.4 years. There was no observed association between weight gain and other gastrointestinal traits associated with obesity including gastric volume, satiation, satiety, or gastrointestinal hormones. This provides additional supporting evidence for the role of gastric emptying in both weight gain and development of obesity. Further study is needed to determine if younger patients with faster gastric emptying would benefit from a therapeutic approach aimed at slowing gastric emptying in order to either promote weight loss or prevent further excess weight gain.
Acknowledgements
Financial support: Dr. Camilleri is supported by NIH RO1-DK67071. Dr. Acosta is supported by NIH (C-Sig P30DK84567, K23-DK114460) and ANMS Career Development Award.
Footnotes
Potential competing interests: Dr. Acosta is a stockholder in Gila Therapeutics, Phenomix Sciences and Lipiquester; he serves as a consultant for Rhythm Pharmaceuticals, General Mills, Gila Therapeutics. Dr. Camilleri is a stockholder in Phenomix Sciences and serves as a consultant to Takeda, Allergan, Rhythm, Salix, Arena, Enterin.
REFERENCES
- 1.Acosta A, Streett S, Kroh MD, Cheskin LJ, Saunders KH, Kurian M, et al. White Paper AGA: POWER - Practice Guide on Obesity and Weight Management, Education, and Resources. Clin Gastroenterol Hepatol 2017; 15(5): 631–649 e10. [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2014; 384(9945): 766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acosta A, Abu Dayyeh BK, Port JD, Camilleri M. Recent advances in clinical practice challenges and opportunities in the management of obesity. Gut 2014; 63(4): 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 2017; 376(3): 254–266. [DOI] [PubMed] [Google Scholar]
- 5.Acosta A, Camilleri M, Shin A, Vazquez-Roque MI, Iturrino J, Burton D, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology 2015; 148(3): 537–546 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilot Study of the Effect of Liraglutide on Weight Loss and Gastric Functions in Obesity - Full Text View - ClinicalTrials.gov. 2018 [Available from: https://clinicaltrials.gov/ct2/show/NCT02647944.
- 7.Halawi H, Khemani D, Eckert D, O’Neill J, Kadouh H, Grothe K, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol 2017; 2(12): 890–899. [DOI] [PubMed] [Google Scholar]
- 8.Acosta A, Camilleri M, Burton D, O’Neill J, Eckert D, Carlson P, et al. Exenatide in obesity with accelerated gastric emptying: a randomized, pharmacodynamics study. Physiol Rep 2015; 3(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez V, Woodman G, Abu Dayyeh BK. Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: Results of a prospective study. Obesity 2016; 24(9): 1849–1853. [DOI] [PubMed] [Google Scholar]
- 10.Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med 1990; 150(3): 665–672. [PubMed] [Google Scholar]
- 11.Lloyd-Jones DM, Dyer AR, Wang R, Daviglus ML, Greenland P. Risk factor burden in middle age and lifetime risks for cardiovascular and non-cardiovascular death (Chicago Heart Association Detection Project in Industry). Am J Cardiol 2007; 99(4): 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd-Jones DM, Liu K, Colangelo LA, Yan LL, Klein L, Loria CM, et al. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: the Coronary Artery Risk Development in Young Adults Study. Circulation 2007; 115(8): 1004–1011. [DOI] [PubMed] [Google Scholar]
- 13.Nelson MC, Story M, Larson NI, Neumark-Sztainer D, Lytle LA. Emerging adulthood and college-aged youth: an overlooked age for weight-related behavior change. Obesity 2008; 16(10): 2205–2211. [DOI] [PubMed] [Google Scholar]
- 14.Lytle LA, Svetkey LP, Patrick K, Belle SH, Fernandez ID, Jakicic JM, et al. The EARLY trials: a consortium of studies targeting weight control in young adults. Transl Behav Med 2014; 4(3): 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lytle LA, Moe SG, Nanney MS, Laska MN, Linde JA. Designing a weight gain prevention trial for young adults: The CHOICES Study. Am J Health Educ 2014; 45(2): 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouras EP, Delgado-Aros S, Camilleri M, Castillo EJ, Burton DD, Thomforde GM, et al. SPECT imaging of the stomach: comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Single photon emission computed tomography. Gut 2002; 51(6): 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chial HJ, Camilleri C, Delgado-Aros S, Burton D, Thomforde G, Ferber I, et al. A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterol Motil 2002; 14(3): 249–253. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez Roque M, Camilleri M, Stephens D, Jensen M, Burton D, Baxter K, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterology 2006; 131(6): 1717–1724. [DOI] [PubMed] [Google Scholar]
- 19.Camilleri M, Iturrino J, Bharucha AE, Burton D, Shin A, Jeong ID, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil 2012; 24(12): 1076–e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loos RJF, Janssens A. Predicting polygenic obesity using genetic information. Cell Metab 2017; 25(3): 535–543. [DOI] [PubMed] [Google Scholar]
- 21.Duffey KJ, Gordon-Larsen P, Jacobs DR Jr., Williams OD, Popkin BM. Differential associations of fast food and restaurant food consumption with 3-y change in body mass index: the Coronary Artery Risk Development in Young Adults Study. Am J Clin Nutr 2007; 85(1): 201–208. [DOI] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med 2011; 364(25): 2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond) 2014; 38(6): 784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vella A, Lee JS, Camilleri M, Szarka LA, Burton DD, Zinsmeister AR, et al. Effects of pramlintide, an amylin analogue, on gastric emptying in type 1 and 2 diabetes mellitus. Neurogastroenterology and motility. 2002;14(2):123–31 [DOI] [PubMed] [Google Scholar]
- 25.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999; 282(16): 1523–1529. [DOI] [PubMed] [Google Scholar]
- 26.Vella A, Camilleri M. The gastrointestinal tract as an integrator of mechanical and hormonal response to nutrient ingestion. Diabetes 2017; 66(11): 2729–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halawi H, Camilleri M, Acosta A, Vazquez-Roque M, Oduyebo I, Burton D, et al. Relationship of gastric emptying or accommodation with satiation, satiety, and postprandial symptoms in health. Am J Physiol Gastrointest Liver Physiol 2017; 313(5): G442–G447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyre D, Delplanque J, Chèvre J-C, Lecoeur C, Lobbens S, Gallina S, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet 2009; 41(2): 157. [DOI] [PubMed] [Google Scholar]
- 29.Ramachandrappa S, Farooqi IS. Genetic approaches to understanding human obesity. J Clin Invest 2011; 121(6): 2080–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acosta A, Camilleri M, Shin A, Carlson P, Burton D, O’Neill J, et al. Association of melanocortin 4 receptor gene variation with satiation and gastric emptying in overweight and obese adults. Genes Nutr 2014; 9(2): 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vazquez-Roque MI, Camilleri M, Vella A, Carlson P, Laugen J, Zinsmeister AR. Association of TCF7L2 allelic variations with gastric function, satiation, and GLP-1 levels. Clin Transl Sci 2011; 4(3): 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]


