Abstract
Background: Evidence-based guidelines are needed for effective delivery of home oxygen therapy to appropriate patients with chronic obstructive pulmonary disease (COPD) and interstitial lung disease (ILD).
Methods: The multidisciplinary panel created six research questions using a modified Delphi approach. A systematic review of the literature was completed, and the Grading of Recommendations Assessment, Development and Evaluation approach was used to formulate clinical recommendations.
Recommendations: The panel found varying quality and availability of evidence and made the following judgments: 1) strong recommendations for long-term oxygen use in patients with COPD (moderate-quality evidence) or ILD (low-quality evidence) with severe chronic resting hypoxemia, 2) a conditional recommendation against long-term oxygen use in patients with COPD with moderate chronic resting hypoxemia, 3) conditional recommendations for ambulatory oxygen use in patients with COPD (moderate-quality evidence) or ILD (low-quality evidence) with severe exertional hypoxemia, 4) a conditional recommendation for ambulatory liquid-oxygen use in patients who are mobile outside the home and require >3 L/min of continuous-flow oxygen during exertion (very-low-quality evidence), and 5) a recommendation that patients and their caregivers receive education on oxygen equipment and safety (best-practice statement).
Conclusions: These guidelines provide the basis for evidence-based use of home oxygen therapy in adults with COPD or ILD but also highlight the need for additional research to guide clinical practice.
Keywords: mobility, hypoxemia, quality of life, chronic obstructive pulmonary disease, interstitial lung disease
Contents
-
Summary of Recommendations
Chronic Obstructive Pulmonary Disease
Interstitial Lung Disease
Liquid Oxygen
Education and Safety
Introduction
Methods
-
Results
Question 1: Should long-term oxygen be prescribed for adults with COPD who have severe chronic resting room air hypoxemia?
Question 2: Should long-term oxygen be prescribed for adults with COPD who have moderate chronic resting room air hypoxemia?
Question 3: Should ambulatory oxygen be prescribed for adults with COPD who have severe exertional room air hypoxemia?
Question 4: Should long-term oxygen be prescribed for adults with ILD who have severe chronic resting room air hypoxemia?
Question 5: Should ambulatory oxygen be prescribed to adults with ILD who have severe exertional room air hypoxemia?
Question 6: Should portable LOX be provided for adults with chronic lung disease who are prescribed continuous oxygen flow rates of >3 L/min during exertion?
Education and Safety Considerations
Panel Discussion
ATS Recommendation
Conclusions
Summary of Recommendations
Chronic Obstructive Pulmonary Disease
-
•
In adults with chronic obstructive pulmonary disease (COPD) who have severe chronic resting room air hypoxemia,* we recommend prescribing long-term oxygen therapy (LTOT) for at least 15 h/d (strong recommendation, moderate-quality evidence).
*Severe hypoxemia is defined as meeting either of the following criteria: 1) PaO2 ≤ 55 mm Hg (7.3 kPa) or oxygen saturation as measured by pulse oximetry (SpO2) ≤ 88%; 2) PaO2 = 56–59 mm Hg (7.5–7.9 kPa) or SpO2 = 89% plus one of the following: edema, hematocrit ≥ 55%, or P pulmonale on an ECG.
-
•
In adults with COPD who have moderate chronic resting room air hypoxemia,* we suggest not prescribing LTOT (conditional recommendation, low-quality evidence).
*Moderate hypoxemia is defined as an SpO2 of 89–93%.
-
•
In adults with COPD who have severe exertional room air hypoxemia, we suggest prescribing ambulatory oxygen (conditional recommendation, moderate-quality evidence).
Interstitial Lung Disease
-
•
For adults with interstitial lung disease (ILD) who have severe chronic resting room air hypoxemia, we recommend prescribing LTOT for at least 15 h/d (strong recommendation, very-low-quality evidence).
-
•
For adults with ILD who have severe exertional room air hypoxemia, we suggest prescribing ambulatory oxygen (conditional recommendation, low-quality evidence).
Liquid Oxygen
-
•
In patients with chronic lung disease who are mobile outside of the home and require continuous oxygen flow rates of >3 L/min during exertion, we suggest prescribing portable liquid oxygen (LOX) (conditional recommendation, very-low-quality evidence).
Education and Safety
-
•
For patients prescribed home oxygen therapy, we recommend that the patient and their caregivers receive instruction and training on the use and maintenance of all oxygen equipment and education on oxygen safety, including smoking cessation, fire prevention, and tripping hazards (best-practice statement).
Introduction
Five million adults live with chronic lung disease in the United States, with more than one million prescribed LTOT (1, 2), defined as oxygen prescribed for at least 15 h/d. The rationale for the provision of LTOT in adults is based on the survival benefit reported by two randomized clinical trials (RCTs) published over three decades ago in patients with COPD and severe, chronic hypoxemia (3, 4). Since then, an additional clinical trial has examined the role of home oxygen therapy in patients with COPD and moderate resting hypoxemia or exertion-only hypoxemia (LOTT [Long-Term Oxygen Therapy Trial]) (5).
Although several professional societies and groups have published clinical practice guidelines for home oxygen therapy (6–12), most have not incorporated the recent LOTT results (5). Recent data highlight significant differences in home oxygen needs and experiences across patients with different lung diseases, lifestyles, and oxygen supply requirements (13–16). For example, the physiologic mechanisms of hypoxemia differ between obstructive and restrictive lung diseases. The rapid and steep rate of exertional desaturation for patients with ILD differs from that of those with COPD (17, 18). These considerations highlight the need for guidelines specific to individuals with COPD and ILD, the two major diagnosis entities for which oxygen therapy is prescribed (19).
The 2017 American Thoracic Society (ATS) workshop on optimizing home oxygen therapy identified the lack of evidence-based clinical practice guidelines for appropriate use of home oxygen as a critical gap in the care of patients (20). Workshop proceedings suggested a need for additional research on portable oxygen technology, advocacy for improved financing of oxygen therapy, and updated guidelines to guide policy, advocacy, and practice, as none currently exist in the United States.
Our aim was to conduct a rigorous and systematic review and develop clinical guidelines targeting healthcare providers who care for adults living with chronic lung disease who need oxygen in the community outside of inpatient and emergency settings. In applying these guidelines, clinicians should use an interactive, shared decision-making approach to ensure oxygen prescriptions meet the needs of individual patients by considering physiology, lifestyle, and treatment preferences. The systematic review underpinning this guideline did not specifically address supplemental oxygen use for patients with acute hypoxemia or for patients with signs of cor pulmonale, pulmonary hypertension (PH), or polycythemia. However, the panel agreed that clinical guidance related to hypoxemia and PH should be included when pertinent to each question.
Methods
This clinical guideline was developed in accordance with policies and procedures of the ATS. The guideline panel included 4 co-chairs and 18 voting members: 11 pulmonary and/or critical care physicians, 4 nurses, 1 registered respiratory therapist, and 1 physiotherapist. To capture the critical input of an oxygen user, the panel included a patient representative (Box 1). We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to appraise the quality of evidence and to formulate and grade recommendations (Tables 1 and 2) (21). We adopted a published terminology for home oxygen therapy (Table 3) (22). For the systematic review to June 2019, we defined severe hypoxemia as having an SpO2 ≤ 88% as assessed by pulse oximetry or having an PaO2 ≤ 55 mm Hg (7.3 kPa) as assessed by blood-gas sampling, and we defined moderate hypoxemia as having an SpO2 88–93% or PaO2 56–60 mm Hg (7.5–7.8 kPa). We defined severe exertional hypoxemia as having SpO2 ≤ 88% on exertion. However, we found substantial variability in definitions for severe hypoxemia across studies, and the data were not reported in a way that would allow reanalysis of outcomes at different thresholds. Thus, we also considered studies using different thresholds and reported the definitions of severe and moderate hypoxemia used by study authors. We have provided suggested thresholds for hypoxemia in the implementation-consideration sections. Potential conflicts of interest were disclosed and managed in accordance with the policies and procedures of the ATS (see Table E1 in the online supplement). The online supplement provides a detailed description of the methods.
Table 1.
Certainty of Evidence
| Evidence Quality | Definition |
|---|---|
| High | High confidence that the estimated effect is close to the true effect. |
| Moderate | Moderate confidence that the estimated effect is close to the true effect, but with a chance that the true effect is considerably different. |
| Low | Low confidence in the estimated effect. Higher likelihood that the true effect is considerably different from the estimated effect. |
| Very low | Very low confidence in the estimated effect. High likelihood that the true effect is considerably different from the estimated effect. |
Table 2.
Implications of Clinical Guideline Recommendations by Stakeholder
| Stakeholder | Strong Recommendation | Conditional Recommendation |
|---|---|---|
| Patient | The majority of patients would want the recommended course of action in this situation, and only a small number would not. | Many patients in this situation would prefer the recommendation, but a substantial number may not. This is an opportunity for shared decision-making between the clinician and patient. |
| Clinician | Most individuals should receive the course of action that is recommended. There is a low chance that additional formal decision aids are needed to help individuals make decisions consistent with their values and preferences, and adherence to this recommendation could be used as a performance indicator or quality criterion. | Different choices will be applicable to different patients, and additional factors will need to be considered in addition to the recommendation in order for a patient to make a decision according to their values and preferences. Decision aids may be needed to assist individuals in making their best choice. This is an opportunity for shared decision-making between the clinician and patient. |
| Policy-maker | The recommendation can be widely adapted as policy and can be used for performance indicators. | Policy-making will require substantial additional debate and involvement of many and/or additional stakeholders. The likelihood of regional variance is also higher, and performance indicators would need to take into consideration any additional deliberation that has occurred. |
Table 3.
Terminology for Home Oxygen Therapy
| Term | Definition |
|---|---|
| Ambulatory oxygen | Oxygen delivered during exercise or activities of daily living. |
| Continuous-flow oxygen | Oxygen delivered at a constant flow rate, regardless of the respiratory rate, in contrast to pulse-dose oxygen (see below). |
| Continuous oxygen | Oxygen prescribed 24 h/d. |
| Home oxygen | Oxygen delivered in a home, also known as domiciliary oxygen. It includes not only long-term oxygen but also short-term, nocturnal, palliative, ambulatory, and short-burst oxygen. It excludes oxygen use in healthcare and emergency settings. |
| Long-term oxygen | Oxygen that is delivered to patients with chronic hypoxemia, in most cases for the remainder of the patient’s life. Long-term oxygen therapy is prescribed for at least 15 h/d. |
| Nocturnal oxygen | Oxygen delivered during sleep time only. |
| Palliative oxygen | Oxygen to relieve dyspnea. Palliative oxygen may be provided continuously, nocturnally, or during ambulation. Short-burst oxygen therapy falls into this category. |
| Portable oxygen | Oxygen delivered through systems that are sufficiently lightweight so that they can be carried or pulled by patients and allow them to leave their home (e.g., oxygen cylinders or canisters carried or pulled in trolleys or portable oxygen concentrators). |
| Pulse-dose oxygen | Oxygen delivered during inspiration only in such a way that the quantity of oxygen administered is influenced by the respiratory rate. The delivery system is at rest while the patient is exhaling. |
| Short-burst oxygen | Brief and intermittent oxygen administration before and/or after exercise, generally used as needed, in the absence of known hypoxemia. |
| Short-term oxygen therapy | Oxygen provided temporarily, during a period of severe hypoxemia (e.g., during the course of and shortly after an exacerbation of COPD). |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
There are several types of home oxygen therapy. This table is provided to assist in standardizing the terminology and is adapted by permission from Reference 22.
Box 1
“The ability to get out of the house and continue my activities is top of the chart in importance! There is no way I want to become a couch potato . . . All the oxygen equipment was ‘dumped’ on me. I knew nothing and was in a daze. I am sure that the delivery guy gave me some instructions when it was delivered but I retained nothing. . . . My first concern was to find a better solution than the shoulder carry bag that the oxygen company provided. I needed to be hands free to play tennis. . . . I spent a couple of years perfecting my system of how to carry enough tanks to a tennis match (requires 6–8 tanks). I did a lot of Internet research to find carts or carrying cases for tanks. I have settled on a rolling cart that was designed to carry wine bottles to tasting parties. Perfect size for 6 tanks . . . It is a pain to have to plan out a day of activities with oxygen. What is the elevation, how far will I have to walk, how many tanks do I need, where can I recharge my POC [portable oxygen concentrator]? There may come a day when you can't do these things so enjoy every minute you have. When I don’t get enough tanks it makes me mad as hell . . . I still do not let down my guard down around the supplier. I never know when their business decisions will again affect my life.”
—Supplemental home oxygen user
Results
After a systematic literature review, the guideline panel created final recommendations on the basis of the available evidence. Table 4 summarizes these findings for questions related to patients with COPD and ILD as well as for questions related to the use of LOX. A best-practice statement was included to address education and safety needs for all home oxygen users.
Table 4.
Summary of ATS Recommendations
| Question | ATS Recommendation | Strength of Recommendation and Level of Evidence |
|---|---|---|
| COPD | ||
| Question 1: Should long-term oxygen be prescribed for adults with COPD who have severe* chronic resting room air hypoxemia? | In adults with COPD who have severe chronic resting room air hypoxemia, we recommend prescribing LTOT for at least 15 h/d. | Strong recommendation, moderate-quality evidence |
| Question 2: Should long-term oxygen be prescribed for adults with COPD who have moderate† chronic resting room air hypoxemia? | In adults with COPD who have moderate chronic resting room air hypoxemia, we suggest not prescribing LTOT. | Conditional recommendation, low-quality evidence |
| Question 3: Should ambulatory oxygen be prescribed for adults with COPD who have severe exertional room air hypoxemia? | In adults with COPD who have severe exertional room air hypoxemia, we suggest prescribing ambulatory oxygen. | Conditional recommendation, moderate-quality evidence |
| ILD | ||
| Question 4: Should long-term oxygen be prescribed for adults with ILD who have severe chronic resting room air hypoxemia? | For adults with ILD who have severe chronic resting room air hypoxemia, we recommend prescribing LTOT for at least 15 h/d. | Strong recommendation, very-low-quality evidence |
| Question 5: Should ambulatory oxygen be prescribed for adults with ILD who have severe exertional room air hypoxemia? | For adults with ILD who have severe exertional room air hypoxemia, we suggest prescribing ambulatory oxygen. | Conditional recommendation, low-quality evidence |
| Liquid oxygen | ||
| Question 6: Should portable liquid oxygen be provided for adults with chronic lung disease who are prescribed continuous oxygen flow rates of >3 L/min during exertion? | In patients with chronic lung disease who are mobile outside of the home and require continuous oxygen flow rates of >3 L/min during exertion, we suggest prescribing portable liquid oxygen. | Conditional recommendation, very-low-quality evidence |
| Education | ||
| Education and safety for patients and caregivers | For all patients prescribed home oxygen therapy, we recommend that the patient and their caregivers receive instruction and training on the use and maintenance of all oxygen equipment and education on oxygen safety, including smoking cessation, fire prevention, and tripping hazards. | Best-practice statement |
Definition of abbreviations: ATS = American Thoracic Society; COPD = chronic obstructive pulmonary disease; ILD = interstitial lung disease; LTOT = long-term oxygen therapy.
On the basis of two clinical trials (3, 4), severe hypoxemia is defined as meeting either of the following criteria: 1) PaO2 ≤ 55 mm Hg (7.3 kPa) or oxygen saturation as measured by pulse oximetry (SpO2) ≤ 88% or 2) PaO2 = 56–59 mm Hg (7.5–7.9 kPa) or SpO2 = 89% plus one of the following: edema, hematocrit ≥ 55%, or P pulmonale on an ECG.
On the basis of a single clinical trial (5), moderate hypoxemia is defined as an SpO2 of 89–93%. The corresponding PaO2 was not reported in that study.
Question 1: Should long-term oxygen be prescribed for adults with COPD who have severe chronic resting room air hypoxemia?
Background
Hypoxemia is common in people with COPD, particularly those with more advanced disease, because of worsening mismatch and decreased diffusion capacity. In some patients, hypoxemia can be sufficiently severe to occur at rest and is associated with dyspnea, worsening neurocognitive function, PH, and mortality (22, 23).
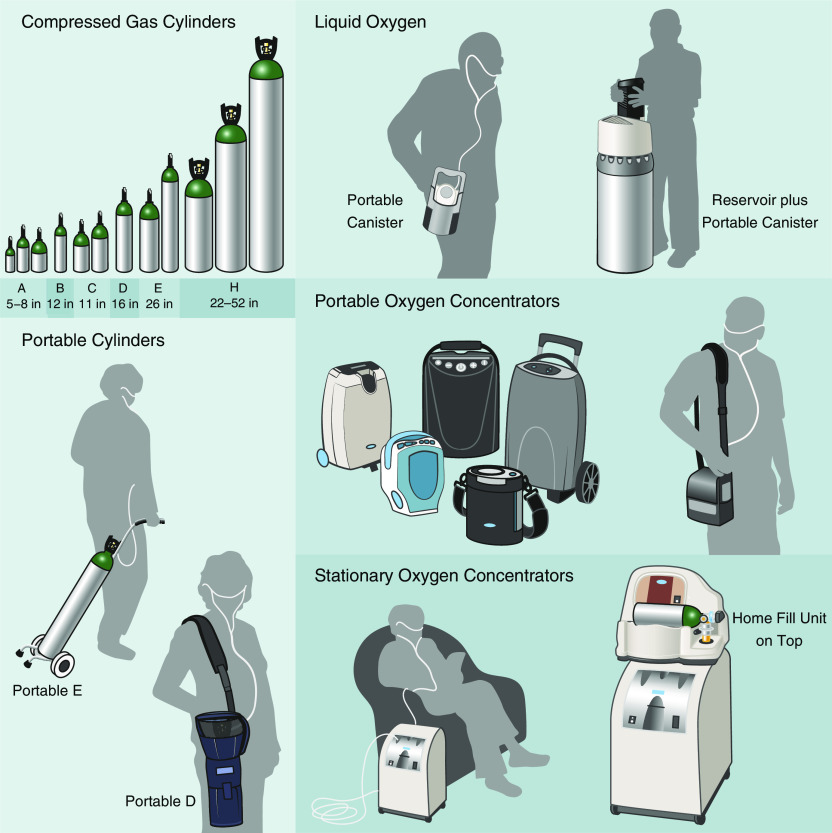
In 1917, Haldane described the therapeutic potential of providing supplemental oxygen (24). Since then, several studies have examined the role of oxygen therapy in patients with COPD. “LTOT” refers to supplemental oxygen for a period of years, and in most cases for the remainder of the patient’s life (Table 3). LTOT can be delivered through a combination of stationary equipment (e.g., stationary oxygen concentrator and liquid reservoirs) and ambulatory oxygen equipment (e.g., compressed-oxygen cylinders, portable oxygen concentrators [POCs], and LOX canisters) (Figure 1). In this section, we discuss the role of LTOT for adults with COPD and severe resting hypoxemia. The critical outcome for this question was mortality, and important outcomes included dyspnea, healthcare resource use, exercise capacity, fatigue, health-related quality of life (HRQL), physical activity, and safety.
Figure 1.
Examples of stationary and portable oxygen devices in the United States. Illustration by Patricia Ferrer Beals.
Description of the evidence and its quality
We included five studies (two RCTs [3, 4]), one pre- versus postintervention study (25), and two observational studies (26, 27) (see the Summary of Studies and Table E2 in the online supplement). Severe resting hypoxemia was defined as a PaO2 ≤ 55 mm Hg (7.3 kPa) or a PaO2 ≤ 59 mm Hg (7.9 kPa) plus one of the following: edema, hematocrit ≥ 55%, or P pulmonale on ECG in the NOTT (Nocturnal Oxygen Therapy Trial) (3); or was defined as a PaO2 of 40–60 mm Hg (5.3–8.0 kPa) in patients with at least one prior episode of ankle edema in the MRC (Medical Research Council) study (4). In the pre- versus postintervention study, eligibility criteria were not reported; participants (n = 6) had a PaO2 of 41.5–46.5 mm Hg (5.5–6.2 kPa) (25). The observational studies enrolled individuals with a PaO2 ≤ 55 mm Hg (7.3 kPa) (26, 27).
There was moderate-quality evidence on the effects of LTOT on mortality in adults with COPD who have severe, chronic resting room air hypoxemia. The RCTs did not employ masking (blinding), but the guideline panel did not judge this to be a serious risk of bias because the critical outcome (mortality) was objective. However, there was substantial imprecision in estimating the treatment effects (Table E2). The NOTT (3) study of 203 participants indicates a 2-year mortality-risk reduction of 55% in those prescribed LTOT (24 h/d) compared with control subjects prescribed only nocturnal oxygen (relative risk [RR], 0.45; 95% confidence interval [CI], 0.25–0.81). Similarly, the MRC study (4) in 87 participants indicates a 5-year mortality-risk reduction of 59% in those with LTOT versus no oxygen (RR, 0.41; 95% CI, 0.17–0.98). Data from the NOTT and MRC studies were not pooled, as they employed different thresholds to define severe hypoxemia, examined different durations of home oxygen therapy (prescribed 24 h/d vs. at least 15 h/d), employed different comparators (nocturnal oxygen in NOTT; no oxygen in MRC), and reported mortality at different time points (1- and 2-yr risk [3] and 5-yr risk [4], respectively).
In the NOTT study (3), subgroup analysis suggested that LTOT improved survival compared with nocturnal oxygen in patients with a higher PaCO2 (PaCO2 ≥ 43 mm Hg [5.7 kPa]: 21.7% vs. 41.5%; P = 0.002), lower arterial pH (pH < 7.40: 16.0% vs. 42.2%; P = 0.004), lower FVC (FVC < 1.89 L: 20.8% vs. 43.5%; P = 0.01), more severe nocturnal hypoxemia (mean room air SaO2 < 85%: 24.4% vs. 50.0%; P = 0.02), lower hematocrit (hematocrit < 47.4%: 21.7% vs. 41.5%; P = 0.03), lower mean pulmonary arterial pressure (PAP; mean PAP < 27 mm Hg: 17.5% vs. 37.0%, P = 0.03), and lower pulmonary vascular resistance (PVR; PVR < 279 dyn ⋅ s/cm5: 12.8% vs. 33.3%; P = 0.03). The NOTT authors were surprised to find smaller differences in mortality, only bordering on statistical significance, between continuous versus nocturnal oxygen in those with higher baseline hematocrit (hematocrit ≥ 47.4%: 24.5% vs. 38.8%; P = 0.20), higher PAP (mean PAP ≥ 27 mm Hg: 24.0% vs. 39.6%; P = 0.14), and higher PVR (PVR ≥ 279 dyn ⋅ s/cm5: 38.6% vs. 45.2%; P = 0.11). Of note however, the direction in the trend toward improved mortality in these individuals was similar to the trend in those with less impaired hemodynamics, and the mean PAP threshold used to separate subgroups (overall group median, 27 mm Hg) was higher than the one used in the currently accepted definition of PH. In the NOTT study, continuous oxygen therapy was associated with a reduction in PVR levels compared with nocturnal oxygen, but a relationship between greater PVR decreases and reduced mortality was not seen, while subgroup numbers were small. The MRC study did not report mortality benefits according to baseline pulmonary hemodynamic characteristics (4) and was presumably enriched for patients with PH (at least one previous episode of ankle edema in the inclusion criteria), but a higher PaCO2 and red-cell mass were associated with greater mortality. The MRC report (4) also incorrectly stated (p. 685) that participants in the NOTT study with higher baseline hematocrit or PAP derived the most benefit from LTOT versus nocturnal oxygen. In light of the caveats listed above, it is not possible to draw firm conclusions on the differential effects of LTOT in patients with concomitant PH. A particular unanswered question is whether a lower threshold for starting LTOT could be of benefit for patients with early pulmonary hemodynamic impairment, an area in need of further research.
There was very-low-quality evidence on the effects of LTOT on healthcare use. One retrospective study (26) found fewer hospitalizations over 3 years for participants using LTOT compared with conventional therapy (mean difference [MD], −1.17; 95% CI, −1.73 to −0.59). An observational study (27) did not find a reduction in admission risk once patients began receiving LTOT (RR, 0.70; 95% CI, 0.15 to 3.30). There was a 35% reduction in hospital-bed days per patient year of follow-up in patients receiving LTOT, but this was not statistically significant (RR, 0.65; 95% CI, 0.40 to 1.05).
For the outcome of safety, the systematic review identified cases of fires, burns from smoking around oxygen equipment, nosebleeds, and tripping over the equipment (28). For all COPD Medicare beneficiaries who used home oxygen (LTOT, exertion only or sleep only), those who had an emergency room visit for a burn injury were twice (odds ratio [OR], 2.43; 95% CI, 1.57–3.78) as likely to be prescribed oxygen in the preceding 90 days compared with those without burn injury (29). The LOTT trial found that for every 100 person-years the rate of fires was 0.08, the rate of burns from smoking around oxygen equipment was 0.12, the rate of burns from oxygen around an open flame was 0.04, the rate of burns from LOX frost was 0.16, the rate of nosebleeds was 0.35, and the rate of tripping or falling over oxygen equipment was 0.90 (5). These safety data from LOTT were for participants with moderate hypoxemia who were prescribed continuous oxygen or oxygen during both exertion and sleep.
Panel judgments
Desirable consequences and their magnitudes (benefits)
The panel concluded that the size of the desirable anticipated effects on mortality is large. LTOT was associated with decreased 2-year and 5-year mortality (critical outcome). There was insufficient evidence to evaluate the effects of LTOT on healthcare use or other important outcomes.
Undesirable consequences and their magnitudes (harms)
The panel concluded that there is a moderate level of undesirable anticipated effects of LTOT. Patients report a physical and mental burden of using oxygen equipment with reduced ability to travel outside of the home, difficulty obtaining information about appropriate access to oxygen equipment during travel, and equipment noise from the use of stationary oxygen equipment, affecting sleep (10, 30).
Rationale for the recommendation
The panel concluded that the balance of desirable and undesirable effects supported the use of LTOT in patients with COPD associated with severe resting hypoxemia. The NOTT trial (3) reported that patients with severe hypoxemia associated with ventilatory compromise (on the basis of PaCO2, arterial pH, and FVC) and milder disturbances in pulmonary hemodynamics (on the basis of PVR and PAP) may be more likely to benefit from LTOT. However, these were subgroup analyses, and similar analyses were not performed in the MRC study (4), so the panel concluded that there is insufficient evidence to recommend preferentially prescribing LTOT to specific subgroups of patients with COPD and severe hypoxemia.
Given the variability in reimbursement rates for medical expenses in the United States, it is difficult to project the true cost per person for LTOT. In the United States, Medicare typically covers 80% of the Medicare-approved amount (31). However, costs may vary depending on the payor. The incremental cost-effectiveness ratio for LTOT was $16,124 per quality-adjusted life year in the United States, which is within the bounds considered to be cost-effective (32). Cost variables were based on the Medicare reimbursement rate for the 2009 published study and on appropriate sources (32). On the basis of these considerations, the panel concluded that cost-effectiveness favors the use of LTOT. Because COPD disproportionately affects minority and low-income populations, a standardized approach to prescribing LTOT will probably increase health equity.
LTOT is probably acceptable to most patients with COPD and severe chronic hypoxemia. LTOT is a widely recognized and recommended therapy for patients with COPD and severe resting hypoxemia (7, 11, 12). Although oxygen is generally available, the main barrier in the United States is cost. This varies internationally, with some countries reporting higher direct costs of oxygen for COPD care than others (33). In addition, reimbursement can vary by region, particularly because of the requirements that must be met for funding (34, 35).
The NOTT and MRC studies tested two different durations of LTOT and two different comparators for LTOT (NOTT: LTOT prescribed for a duration of 24 h/d vs. LTOT prescribed for a duration 12 h/d; MRC: LTOT prescribed for a duration of at least 15 h/d vs. no oxygen prescribed) in patients with COPD and severe resting hypoxemia. From these two studies, we know that prescribing LTOT for at least 15 h/d is superior to prescribing no oxygen and that prescribing LTOT for 24 h/d is likely superior to prescribing the therapy for 12 h/d, as assessed by mortality. We do not know if prescribing LTOT for at least 15 h/d is superior to prescribing it for 12 h/d (no studies compared these two interventions directly) on the basis of mortality. On the basis of these considerations and the potential for a longer duration of LTOT use per day to be a patient burden, the panel believes that prescribing LTOT for at least 15 h/d is justified in patients with COPD and severe resting hypoxemia.
ATS recommendation
In adults with COPD who have severe chronic resting room air hypoxemia, we recommend prescribing LTOT for at least 15 h/d (strong recommendation, moderate-quality evidence).
What others are saying
A 2015 British Thoracic Society (BTS) guideline recommends that patients with stable COPD and severe resting hypoxemia (PaO2 ≤ 55 mm Hg [7.3 kPa]), or with a resting PaO2 ≤ 60 mm Hg (8.0 kPa) with evidence of peripheral edema, hematocrit ≥ 55, or PH, should be considered for LTOT because of its survival benefits and potential to improve pulmonary hemodynamics (36). They recommend LTOT be used for 15–24 h/d (36). The Thoracic Society of Australia and New Zealand (TSANZ) recommends titrating oxygen to maintain a PaO2 > 60 mm Hg (8.0 kPa) or an SpO2 > 90% during waking at rest (9, 37). The Global Initiative for Chronic Lung Disease (GOLD) report recommends LTOT if SpO2 is ≤88% and recommends oxygen titration to maintain a saturation of ≥90% in patients with COPD (12).
Implementation considerations
Severe hypoxemia was defined using different thresholds in the two RCTs that reported our critical outcome of survival (PaO2 ≤ 55 mm Hg [7.3 kPa] or PaO2 ≤ 59 mm Hg [7.9 kPa] plus one of the following: edema, hematocrit ≥ 55%, or P pulmonale on ECG [3]; vs. PaO2 40–60 mm Hg [5.3–8.0 kPa] [4]). Because a mortality benefit was demonstrated in both studies, the panel concluded that either definition of severe hypoxemia is clinically justified.
Neither clinical trial reported SpO2-based thresholds for severe hypoxemia. We recognize that the relationship between SpO2 and PaO2 can vary because of an individual’s pH, 2,3-diphosphoglycerate concentration, PaCO2, and temperature. However, the guideline panel concluded that providing approximate thresholds for SpO2 that correspond to the PaO2 thresholds used in the NOTT and MRC studies would improve the usability of the guideline report in circumstances in which arterial blood gas measurements were not available. The panel suggests titrating the level of LTOT to achieve a target saturation of 90%, as opposed to 88% in some guidelines, to avoid prolonged episodes of desaturation with minimal activity.
In addition, the NOTT and MRC trials used slightly different definitions of “chronic.” In the NOTT study (3), chronic was defined as meeting the definition of severe hypoxemia on “at least two occasions more than 1 week apart over a 3-week observation period” while the patient was free of exacerbations. In the MRC study (4), chronic was defined as meeting the PaO2-based criteria for hypoxemia on “two repeated measurements at least 3 weeks apart.”
For implementation purposes, we define chronic resting hypoxemia as resting hypoxemia in the absence of a reversible cause. Although the NOTT and MRC trials used definitions that required repeated measures 3 weeks apart, this may not be possible or necessary in clinical practice (for instance, for a patient with idiopathic pulmonary fibrosis [IPF] who has resting hypoxemia as a result of progressive lung disease, without reversible cause). This differs from the patient who is discharged from the hospital after a COPD exacerbation, in which case resolution of resting hypoxemia may occur over time and reassessment of oxygen needs is important to avoid unnecessary treatment with oxygen.
Reassessment of patients’ oxygen needs after an acute exacerbation is critical. Respiratory exacerbations (in some cases accompanied by pneumonia, pulmonary embolism, or heart failure) usually increase oxygen requirements (e.g., require higher flows to maintain an SpO2 of at least 90%). In addition, the pace at which patients recover partially or fully from their respiratory exacerbations varies and can range from days to months, and their oxygen requirements may therefore vary over time. Moreover, a substantial proportion (perhaps as many as 50%) of patients who are initiated on home oxygen at the time of a respiratory exacerbation may recover sufficiently to no longer have a clinical indication for home oxygen (38, 39). Patient education (e.g., how to use new equipment, how to titrate oxygen flow to evolving requirements), oxygen prescriptions, and communication with other providers (e.g., primary care and home health agencies) would need to match the requirements for home oxygen after a respiratory exacerbation.
Proper reassessment of home oxygen needs is so important that it has been identified as one of the top five areas for further improvement in adult respiratory medicine by the Choosing Wisely Campaign (40). According to the TSANZ guideline, patients who commence LTOT after a COPD exacerbation should be reassessed 4–8 weeks after hospital discharge to ensure continued eligibility (9). In patients prescribed home oxygen for severe chronic resting room air hypoxemia, the 2020 GOLD initiative recommends reassessing the need for oxygen after 60–90 days; when home oxygen is started to treat severe hypoxemia after a COPD exacerbation, the GOLD initiative recommends reassessing the home oxygen requirement at 1–4 weeks and again at 12–16 weeks to update the oxygen prescription as clinically indicated (12). At the time of reassessments, SpO2 (or arterial blood gases) at rest and with exertion should be considered. Ideally, reevaluation should occur at home to capture context and barriers to use. Expert opinion suggests that patients should be monitored, at minimum, every 6 months to confirm continued oxygen use, a current oxygen prescription, and adequacy of the equipment used.
Values and preferences
This recommendation places a high value on reducing mortality and a lower value on cost and resource use.
Research needs
The practice of initiating short-term oxygen therapy on hospital discharge in patients with severe hypoxemia is based on indirect evidence from the NOTT and MRC clinical trial populations with chronic hypoxemia. The harms and benefits of prescribing short-term oxygen therapy on hospital discharge deserves further study. The panel recommends studies to develop and test the acceptability and effectiveness of easy-to-use ambulatory oxygen equipment that can facilitate LTOT use by patients, to identify strategies that improve adherence to LTOT, and to develop and test strategies to discontinue home oxygen in patients who recover sufficiently after an exacerbation or no longer have a clinical indication for its use. Studies to examine whether some subgroups of patients with COPD and severe hypoxemia (e.g., higher hematocrit, higher mean PAP, early hemodynamic impairment) are more or less likely to benefit from LTOT are also needed.
Question 2: Should long-term oxygen be prescribed for adults with COPD who have moderate chronic resting room air hypoxemia?
Background
Some patients with COPD develop moderate resting room air hypoxemia that may be asymptomatic or associated with dyspnea. Moderate hypoxemia is rarely mentioned in clinical guidelines. In this section, we discuss the role of LTOT for adults with COPD associated with moderate resting hypoxemia. For this question, mortality was the critical outcome, and important outcomes were dyspnea, COPD exacerbation, HRQL, fatigue, physical activity, healthcare resource use, and safety.
Description of the evidence and its quality
There was only one study, and the quality of evidence was considered low because of imprecision in the estimate of treatment effects for the critical and important outcomes (see the Summary of Studies and Table E3 in the online supplement). The LOTT study included participants who had moderate hypoxemia at rest (defined as room air SpO2 of 89–93%, no PaO2 threshold specified) as well as those with no hypoxemia at rest but desaturation only on exertion (defined as an SpO2 ≥ 80% for ≥5 min and <90% for ≥10 s during a 6-min-walk test [6MWT]). No information was provided on the presence of PH. Study participants with moderate hypoxemia at rest were randomly allocated to continuous LTOT or no oxygen. Those with isolated exertional hypoxemia were randomly allocated to LTOT during both exertion and sleep or to no oxygen. Unlike the NOTT and MRC trials, chronicity (hypoxemia sustained over 3 wk in stable condition) was not assessed. As with the NOTT and MRC trials, the intervention was not masked (blinded).
At the request of the guideline panel, the LOTT group conducted additional analyses comparing the risk of death with and without LTOT in the subgroup of participants who had moderate resting hypoxemia (n = 419, 57% of LOTT participants) (Table E3). The results indicated no difference between groups in time to death (hazard ratio [HR], 0.95; 95% CI, 0.59–1.50). A previous smaller RCT of 135 patients with COPD by Górecka and colleagues (41) also reported no effect on mortality with LTOT versus no oxygen in patients with PaO2 56–65 mm Hg (7.5–8.7 kPa); the relative hazard of survival was 0.92 (95% CI, 0.57–1.47). This smaller study defined moderate hypoxemia on the basis of PaO2 of 56–65 mm Hg (no SpO2 thresholds specified) and study participants received monthly home visits by a respiratory nurse. Because moderate hypoxemia was defined differently in the LOTT and Górecka and colleagues studies, and the study by Górecka and colleagues included intensive home-based follow-up, inclusion in the evidence tables and a meta-analysis that included both studies was not considered appropriate.
The panel concluded the quality of evidence for effects on HRQL was moderate. The St. George’s Respiratory Questionnaire (SGRQ) favored the use of LTOT at 4-month follow-up in those with both moderate resting and exertional desaturation (MD, −3.30; 95% CI, −6.50 to −0.10). However, no significant differences were found at 12-month follow-up or in those with only resting desaturation. There were no differences between groups in the Quality of Well-Being Scale (5).
In the LOTT study, the composite endpoint of time to death or first hospitalization was not significantly different between those in the LTOT group compared with those in the no-LTOT group in those with moderate hypoxemia at rest only (HR, 0.96; 95% CI, 0.79–1.12) or in those with moderate hypoxemia at rest and desaturation with ambulation (HR, 0.95; 95% CI, 0.72–1.27). No study that met our inclusion criteria directly reported on the effects of LTOT on other outcomes considered “important” by the guideline panel (dyspnea, physical activity, fatigue, or healthcare resource use) in participants with COPD and moderate hypoxemia at rest.
The LOTT trial reported fires, burns, nosebleeds, and tripping or falling over oxygen equipment in the study participants prescribed LTOT or oxygen during exertion and sleep (5); see description of results in question 1.
Panel judgments
Desirable consequences and their magnitudes (benefits)
The panel concluded that the size of the desirable anticipated effects on mortality (critical outcome) was not clinically meaningful. Likewise, the effect on HRQL at 12 months was not clinically meaningful. Data were insufficient to evaluate other important outcomes (5).
Undesirable consequences and their magnitudes (harms)
In a judgment similar to that developed for question 1, the panel concluded that there is a moderate level of undesirable anticipated effects of LTOT.
Rationale for the recommendation
The panel concluded that the certainty of evidence was moderate and was based on a single clinical trial that used SpO2 of 89–93% to define moderate hypoxemia. Similar results were noted in a trial by Górecka and colleagues (41), which compared LTOT plus monthly home visits by a nurse versus monthly home visits by a nurse alone in patients with moderate resting hypoxemia as defined by a PaO2 of 56–65 mm Hg. There was probably no important uncertainty or variability about how much people value mortality as the critical outcome. Some patients may not experience any value added on additional life years if they are very ill. However, this is less likely in patients with moderate hypoxemia, as they tend to have less severe COPD than those with severe resting hypoxemia. The balance between desirable and undesirable effects does not favor LTOT in those with moderate hypoxemia.
ATS recommendation
In adults with COPD who have moderate chronic resting room air hypoxemia, we suggest not prescribing LTOT (conditional recommendation, low-quality evidence).
What others are saying
Only one guideline was found that makes recommendations regarding LTOT in patients with moderate resting hypoxemia. The 2020 GOLD document states, “In patients with stable COPD and resting or exercise-induced moderate desaturation, long-term oxygen treatment should not be prescribed routinely. However, individual patient factors must be considered when evaluating the patient’s need for supplemental oxygen” (12).
Implementation considerations
On the basis of the LOTT study, we defined moderate resting hypoxemia as an SpO2 of 89–93%. The corresponding PaO2 was not reported (5). The costs and burden of the treatment outweigh the minimal benefit of LTOT in adults with COPD who have moderate resting room air hypoxemia. Patients likely would not choose LTOT on the basis of the lack of benefit; they may decide to defer using oxygen at rest until their resting hypoxemia worsens. The LOTT study did not report any data on pulmonary hemodynamics, and it is therefore not possible to conclude whether effects of LTOT in this group of patients differs according to the presence of PH. When patients have moderate resting hypoxemia, it is an opportunity for shared decision-making between the clinician and patient (see Table 2, which discusses the situations that provide an opportunity for shared decision-making).
Values and preferences
This recommendation against LTOT in this setting places a high value on the absence of a mortality reduction and a lower value on short-term improvement in HRQL (which was observed at 4 mo but not at 12 mo).
Research needs
The panel recommends studies on the use of shared decision-making to personalize the use of home oxygen in participants with moderate resting hypoxemia, as well as studies evaluating the discontinuation of supplemental oxygen in patients who previously had severe resting room air hypoxemia but now have moderate resting room air hypoxemia. Confirmatory evidence is needed to determine the effect of LTOT on dyspnea and other outcomes for patients with moderate resting hypoxemia.
Question 3: Should ambulatory oxygen be prescribed for adults with COPD who have severe exertional room air hypoxemia?
Background
Exertional hypoxemia occurs in up to 40% of people with moderate to severe COPD who have normoxemia at rest (42). It is seen most frequently in those with low lung function (FEV1 < 45%, DlCO < 50%), in those with low resting saturation (<95%), and in women (42). The imbalance between oxygen delivery and demand is a major contributor (43). Exertional hypoxemia is linked to more rapid decline in lung function, worse HRQL (44), and increased mortality. In 576 people with severe COPD followed for at least 3 years, mortality in those with isolated exertional hypoxemia on a 6MWT was 2.63-fold higher than in those without exertional hypoxemia (95% CI, 1.53–4.51) (45).
“Ambulatory oxygen” is defined as oxygen delivered during exercise or activities of daily living when the individual is walking freely (Table 3) (22). It is prescribed for people with COPD to improve oxygen delivery during exertion, reduce symptoms, and enhance physical capacity. It may be prescribed for individuals using LTOT who require a portable oxygen supply when leaving the house or for those with isolated exertional hypoxemia. Although the beneficial effects of supplemental oxygen during laboratory-based exercise tests have been reported in COPD (46–49), clinical trials of ambulatory oxygen used during daily life have had less consistent results (50–52). There is variation in prescribing practices and access to ambulatory oxygen across jurisdictions (10, 51, 52).
The prespecified critical outcome for this question was HRQL; important outcomes included dyspnea, fatigue, exercise capacity, physical activity, mortality, healthcare resource use, and safety.
Description of the evidence and its quality
Several studies assessed patients with COPD who had severe exertional desaturation (46, 47, 49–58), but cohorts varied from those who were on or eligible for LTOT (46, 49, 53–55) to those who had isolated exertional desaturation (47, 50–52, 56–58) (Table E4). The quality of evidence was low. Most studies reported the acute effects of oxygen during exercise testing, with only four studies evaluating use of oxygen during daily life (50–52, 55), of which two were crossover trials (50, 52). There were only two parallel-group RCTs of ambulatory oxygen (51, 55), of which only one included blinding to the intervention (51). Three additional RCTS (48, 59, 60) reported on the effects of oxygen supplementation during exercise training, so these were not directly relevant to our question; however, two of them (48, 59) reported baseline data for the acute effects of oxygen during an exercise test that we were able to include in our analysis. The LOTT study was not included in this review, as the patients with exertion-only desaturation were required to wear oxygen during sleep as well as during exercise, thus not meeting our definition of ambulatory oxygen (5). One additional study was identified in which the degree of exertional desaturation was not specified, but the participants were users of LTOT (61). As it is likely that these participants would have met our inclusion criteria, this study was included in the narrative review as indirect evidence.
Evidence for patients with isolated exertional desaturation
Several different tools were used to measure HRQL. Meta-analysis of three studies (50–52) found a small but significant improvement in the dyspnea-related quality-of-life domain of the Chronic Respiratory Disease Questionnaire (CRQ) (standardized mean difference, 0.42; 95% CI, 0.04–0.79; I2 = 12%; n = 211) in favor of ambulatory oxygen (62). However, the effect size was small, and the mean number of changes for the CRQ dyspnea domain was generally less than the minimal clinically important difference (MCID) (63) and was of uncertain clinical significance. The number of participants in whom changes exceeded the MCID was not reported in any study. Improvements with oxygen in individual participants could not be predicted by participant characteristics (degree of exertional desaturation, severity of airflow obstruction or dyspnea, volume or exercise response to hyperoxia, and sex) (51). The emotion, fatigue, and mastery domains were not different between the groups. A sensitivity analysis stratified by study design (crossover trial vs. parallel groups) found that the results remained significant for the dyspnea domain (Table E4 and Figures E1 and E2).
One study assessed HRQL using the SGRQ (50). No differences for the administration of supplemental oxygen versus compressed room air were observed. However, using the 36-Item Short-Form Health Survey, Eaton and colleagues (52) observed a significant difference in favor of ambulatory oxygen in the domains of physical role (MD, 16.8; 95% CI, 6.02–27.58), general health (MD, 6.1; 95% CI, 0.42–11.78), social functioning (MD, 10.5; 95% CI, 0.31–20.69), and emotional role (MD, 18.3; 95% CI, 3.21–33.39), which exceed the reported MCID for all domains except social functioning (64).
The acute effects of oxygen on functional exercise capacity were assessed using multiple tests. We meta-analyzed the results from two studies (52, 56) using the 6MWT, one of which used cylinder room air and the other of which used room air as the comparator. An MD of 28.9 m (95% CI, 16.1–41.9 m; I2 = 0%) was found in favor of the oxygen group. Oxygen acutely increased exercise endurance time on a cycle ergometer by 5.8 minutes (95% CI, 2.23–9.37 min) compared with room air (58). Peak workload on a stationary bike was acutely improved with ambulatory oxygen (MD, 17.9 W; 95% CI, 8.10–27.70 W) (47). Ambulatory oxygen increased the number of steps walked in 5 minutes compared with cylinder room air (MD, 14.90; 95% CI, 0.85–28.94) (50). Meta-analysis of three studies reporting on the Borg dyspnea score at the end of exercise (52, 56, 58) showed a reduction of 1.11 U (95% CI, 0.53–1.69 U; I2 = 39%) in favor of ambulatory oxygen. This remained significant when we did a sensitivity analysis by study design (crossover trial vs. parallel groups; Table E4). No studies reported the long-term effects of ambulatory oxygen on exercise capacity beyond acute laboratory or field tests, and no studies reported effects on physical activity in daily life.
Evidence for patients with resting hypoxemia and severe exertional desaturation
For the subgroup of studies in which patients had both resting and exertional hypoxemia, six RCTs (46, 48, 49, 53–55) met our inclusion criteria; however, none included results for HRQL, our critical outcome. In one randomized crossover study of LTOT users (n = 24) in which the degree of exertional desaturation was not specified, ambulatory oxygen had no effect on the CRQ dyspnea domain (mean change over the 3-month treatment period, 0.0 U; 95% CI, −0.3 to 0.2 U) (61). A single-blind RCT found that ambulatory oxygen at 2 and 4 L/min acutely increased the distance patients walked in 12 minutes compared with room air if they were not using a walker or shopping trolley, as carrying those devices eliminated any gains in exercise tolerance (49). Baseline data from an RCT of pulmonary rehabilitation found that ambulatory oxygen acutely improved the distance walked compared with room air, measured with the incremental shuttle walk test (MD, 27.3 m; 95% CI, 14.7 to 39.8 m) (48). In a crossover study, ambulatory oxygen acutely improved endurance time by 4.70 minutes compared with room air (95% CI, 3.76 to 5.64 min) (46). We meta-analyzed the results from three studies reporting on the Borg dyspnea score (46, 48, 54) and found a reduction of 0.59 U (95% CI, 0.18 to 0.99; I2 = 25%) in favor of ambulatory oxygen; the MCID is reported to be 1.0 U (65).
No studies in our review examined safety in patients with COPD using only ambulatory portable oxygen systems.
Panel judgments
Desirable consequences and their magnitudes (benefits)
For people with COPD and severe exertional hypoxemia, we did not find consistent evidence that ambulatory oxygen delivered clinically significant improvements in the critical outcome of HRQL, whereas effects generally favored ambulatory oxygen (moderate GRADE evidence). Acute improvements in exercise capacity were seen both in those with isolated exertional hypoxemia and in those eligible for LTOT. The effects of ambulatory oxygen on dyspnea during exercise testing were inconsistent. Mortality risks were not reported. Fatigue and physical activity in daily life were not reported.
Undesirable consequences and their magnitudes (harms)
There is a substantial body of evidence regarding the patient and caregiver burden associated with the use of ambulatory oxygen, including managing the weight and bulk of equipment, embarrassment and perceived stigma, fear of cylinders running out, reduced ability to travel outside the home, equipment noise that may affect social activities, difficulty obtaining POCs, and poor access to information about effective use of oxygen equipment (14, 66, 67).
Rationale for the recommendation
There is some evidence supporting the use of ambulatory oxygen in people with COPD, which is complicated by the potential burden of this therapy. Effects on the critical outcome of HRQL, which may or may not be clinically significant, tended to favor ambulatory oxygen. Ambulatory oxygen acutely improves exercise capacity and may reduce breathlessness during exercise testing. There is little evidence regarding the effects of ambulatory oxygen when used in daily life. Given this uncertainty, together with the known burden of ambulatory oxygen, it is likely that some patients will choose not to use ambulatory oxygen.
ATS recommendation
In adults with COPD who have severe exertional room air hypoxemia, we suggest prescribing ambulatory oxygen (conditional recommendation, low-quality evidence).
What others are saying
A 2015 guideline by the BTS states that ambulatory oxygen should not be routinely offered to patients with isolated exertional hypoxemia and should only be offered to those eligible for LTOT if they are mobile outdoors (7). The 2020 GOLD strategy makes no recommendations regarding ambulatory oxygen (12).
Implementation considerations
We defined exertional hypoxemia as an SpO2 ≤ 88%. Ambulatory oxygen seems to have similar effects in patients who are eligible for LTOT and in those who have isolated exertional hypoxemia. In patients who are eligible for LTOT, prescription of ambulatory oxygen may be important to increase the daily hours of oxygen usage (68). Individuals who experience a reduction in dyspnea or increased activity levels with ambulatory oxygen may be more likely to benefit from, and adhere to, this therapy. Standardization of the level of exertion is critical when assessing the effects of oxygen on dyspnea (69, 70). Ambulatory oxygen devices vary in terms of portability, volume, and flow; shared decision-making between the patient and provider is necessary to ensure that the device prescription meets the patient’s needs and goals.
Values and preferences
This recommendation places a high value on increasing HRQL and the potential for facilitating physical activity outside the home and places a lower value on cost, inconvenience, and resource use.
Research needs
Given the well-documented burden of ambulatory oxygen, there is an urgent need for new ambulatory oxygen devices that increase portability (improved battery life, weight, flow rates, wireless connections, etc.). Although we found several studies evaluating the effects of oxygen during laboratory testing, few studies evaluated ambulatory oxygen during daily life activities, the context for which it is prescribed. Future studies of ambulatory oxygen should address patient-centered outcomes such as HRQL and physical activity in daily life, together with outcomes pertinent to cost-effectiveness, such as productivity, days missed from work, and hospital readmissions. Future studies should also capture costs of care for other health conditions that may occur because of physical inactivity, which could be reduced with ambulatory oxygen.
Question 4: Should long-term oxygen be prescribed for adults with ILD who have severe chronic resting room air hypoxemia?
Background
Severe resting hypoxemia is highly prevalent in adults living with ILD. Those with fibrotic forms of ILD, such as IPF, often experience a progressive course characterized by breathlessness, cough, hypoxemia, episodes of acute respiratory worsening, and early death. When present, severe resting hypoxemia often contributes to disabling and distressing breathlessness, which is common in ILD. With the exception of lung transplantation, supplemental oxygen is the only treatment that improves hypoxemia that persists despite optimal medical management of the underlying disease.
The critical outcome for this question was mortality; important outcomes were dyspnea, fatigue, HRQL, physical activity in daily life, healthcare resource use, exercise capacity, and safety.
Description of the evidence and its quality
No studies were found that met our inclusion criteria. A 2001 Cochrane systematic review (71) reported the results from one unpublished RCT in which severe resting room air hypoxemia was defined as a PaO2 of 45–60 mm Hg (6.0–8.0 kPa), slightly above our prespecified cutoff of PaO2 ≤ 55 mm Hg (7.3 kPa) (Table E5). Because no other study was found reporting on our outcomes of interest, we elected to report these results. The study included 62 participants, 49 of whom had IPF. No significant difference in mortality between the LTOT and room air groups was observed after 1 year (OR, 0.50; 95% CI, 0.15–1.61), 2 years (OR, 1.76; 95% CI, 0.64–4.86), or 3 years (OR, 0.99; 95% CI, 0.16–6.26). As this is an unpublished RCT, there is a high risk of bias and a very low GRADE quality of evidence for this outcome (Table E5).
No studies reported on dyspnea, fatigue, HRQL, physical activity, or healthcare resource use.
Because of the paucity of any direct evidence regarding patients with ILD, we chose to consider indirect evidence from our first population, intervention, comparison, and outcome question that considered patients with COPD and severe resting room air hypoxemia (6, 72–74).
Safety data specific to LTOT in patients with ILD were scarce. A qualitative study reported tripping as a hardship after being on oxygen for 9–12 months (75). The panel agreed that the safety data for COPD related to risks of tripping, burns, fires, nosebleeds, and transporting oxygen cylinders would also be potential safety concerns for patients with ILD on LTOT.
Panel judgments
Desirable consequences and their magnitudes (benefits)
Despite the absence of any published randomized trial data, the panel judged the perceived benefits of LTOT to treat severe resting hypoxemia to be substantial for most adults with ILD. As noted above, LTOT for severe resting hypoxemia may confer a mortality benefit in COPD. LTOT may also prevent organ dysfunction due to severe sustained hypoxemia, including prevention of PH. Other benefits may include relief of breathlessness (76) as well as improvements in disability and HRQL. These desirable consequences were considered to be substantial.
Undesirable consequences and their magnitudes (harms)
The primary undesirable consequences are listed in question 1, including patient burden and cost. Overall, the panel deemed the substantial desirable consequences of LTOT to outweigh the undesirable consequences of untreated severe resting hypoxemia. Notably, despite the very-low-quality evidence available to the panel, ethical concerns about withholding LTOT were strong factors in our decision-making.
Rationale for the recommendation
The absence of published studies examining effects of LTOT in ILD led the panel to incorporate evidence from COPD trials together with their clinical experience in the development of the recommendation. Despite the burden of therapy, the panel concluded that LTOT is likely to confer desirable benefits for many patients with severe resting hypoxemia.
ATS recommendation
For adults with ILD who have severe chronic resting room air hypoxemia, we recommend prescribing LTOT for at least 15 h/d (strong recommendation, very-low-quality evidence).
What others are saying
Because of the lack of direct evidence, the majority of guidelines made their recommendations on the basis of expert opinion or extrapolated from COPD literature. A 2011 multisociety guideline on IPF recommends the use of LTOT in patients with severe resting room hypoxemia (77). The panel made a strong recommendation in favor of LTOT, as they placed a high value on evidence from other chronic lung diseases and low value on cost and inconvenience to patients. A 2015 BTS guideline on home oxygen use suggests a higher threshold of 60 mm Hg (8.0 kPa) for those who have concomitant PH. They also recommend LTOT in patients with ILD with a PaO2 ≤ 55 mm Hg (7.3 kPa) but noted that the evidence base is extrapolated from evidence in COPD (7).
Implementation considerations
For patients with ILD, we have applied the same definition of severe resting hypoxemia as in those with COPD (question 1), as our recommendation was based on indirect evidence from COPD trials. The assessment of PH in patients with ILD should be considered, as it predicts worsening lung function and functional status, increased oxygen needs, and risk of acute exacerbation in ILD and is a predictor of increased mortality in IPF (6, 72–74). Some patients may not experience dyspnea, despite severe hypoxemia at rest, or may have strong personal preferences against the use of LTOT. The panel encourages healthcare providers to educate patients about the harms of severe chronic hypoxemia and to partner with patients and their caregivers to select the oxygen delivery system that best meets their medical and lifestyle needs.
Values and preferences
This recommendation places a high value on indirect evidence of decreased mortality, the relief and prevention of distressing symptoms, and a perceived likelihood of overall improved health status by prevention of organ dysfunction.
Research needs
The panel acknowledges that RCTs of LTOT to treat severe hypoxemia in ILD may face challenges, including a lack of perceived equipoise. We encourage the research community to consider innovative approaches to studying the benefits and harms of LTOT in ILD, including clinical trials and quasiexperimental trial designs. Such trials may minimize harms and be more ethically acceptable to patients, caregivers, healthcare providers, and researchers. Trials of short duration may need to be performed before longer-term trials.
Question 5: Should ambulatory oxygen be prescribed to adults with ILD who have severe exertional room air hypoxemia?
Background
Exertional hypoxemia is a hallmark of ILD, occurring in more than half of patients evaluated at a tertiary ILD service and in over 80% of patients with an FVC < 50% of that predicted (78). The magnitude of exertional hypoxemia is generally greater in people with ILD than in people with COPD (18). People with ILD who desaturate to an SpO2 ≤ 88% on a 6MWT have a fourfold greater risk of death than those who do not, after adjusting for age, sex, smoking, respiratory function, and resting saturation (79). Exertional desaturation is an independent predictor of PH (80), which is itself a strong predictor of mortality (81). Greater exertional desaturation is strongly associated with reduced physical activity (82). These data provide a strong rationale for treatment of exertional hypoxemia in ILD to improve daily functioning and long-term outcomes.
The prespecified critical outcome for this key question was HRQL; important outcomes were dyspnea, fatigue, exercise capacity, physical activity, mortality, healthcare resource use, and safety.
Description of the evidence and its quality for participants with exertional desaturation
The AmbOx (Ambulatory Oxygen in Fibrotic Lung Disease) trial (83) examined the effects of ambulatory oxygen used during daily activities on HRQL in a randomized 2-week crossover trial conducted in 84 patients with fibrotic ILD and isolated exertional hypoxemia (defined as an SpO2 ≤ 88% during a 6MWT). The control group did not use a sham device, and there was no blinding of assessment. For the King’s Brief ILD questionnaire, there was a significant improvement in favor of ambulatory oxygen for the total score (MD, 3.7; 95% CI, 1.8 to 5.6), breathlessness and activities score (MD, 8.6; 95% CI, 4.7 to 12.5), and the chest symptoms score (MD, 7.6; 95% CI, 1.9 to 13.2), whereas no significant difference was observed for the psychological symptoms score. The MCID for the total King’s Brief ILD score has been recently estimated as 3.9 (84, 85). The SGRQ demonstrated a significant improvement in the total score (MD, −3.6; 95% CI, −6.7 to −0.6) and activity score (MD, −7.5; 95% CI, −12.4 to −2.5), with no significant difference in the impact and symptoms scores. The MCID for the SGRQ is 4 U (86). Dyspnea associated with activities of daily living was assessed using the University of California, San Diego, Shortness of Breath Questionnaire, showing an improvement of 8 U (95% CI, 3.6 to 12.4). The MCID is 5 U (87). Despite the clear challenges posed by ambulatory oxygen, highlighted in the qualitative component of the trial, two-thirds of patients decided to continue with ambulatory oxygen at the end of the study. Improvements with oxygen in individual participants could not be predicted by participant characteristics, but preference to continue on oxygen after the trial was influenced by the patient perception of benefit for breathlessness or walking ability (83).
A 2016 Cochrane systematic review (88) included three crossover RCTs evaluating the acute effects of oxygen during exercise testing in patients with ILD. No change was found in 6-minute-walk distance with ambulatory oxygen compared with cylinder room air (89) or in the endurance shuttle walk test distance with oxygen compared with room air (90). There was no change in dyspnea, measured with the Borg dyspnea score (89). One study reported an acute improvement in endurance time by 118.7 seconds (95% CI, 23.9–213.5 s) (91) with ambulatory oxygen compared with room air. Two other systematic reviews assessing the ambulatory oxygen in ILD were subsequently published (92, 93) but did not meet our inclusion criteria.
We identified additional studies that met our inclusion criteria; all used 6MWTs. Meta-analysis of three studies (83, 89, 94) showed that oxygen acutely improved 6-minute-walk distance by 18.57 m (95% CI, 11.14 to 25.99 m; I2 = 0%) compared with room or cylinder air (Table E6 and Figure E5). We pooled the results of three studies (83, 89, 94) that reported the Borg dyspnea score at the end of a 6MWT, one of which was not included in the Cochrane systematic review (83); no significant difference was found (MD, −0.72; 95% CI, −1.70 to 0.27; I2 = 73.28%). Meta-analysis of three studies (83, 89, 94) found a significant reduction in the Borg perceived-exertion score at the end of a 6MWT in favor of ambulatory oxygen (0.37 U; 95% CI, 0.19 to 0.54 U; I2 = 0%). We meta-analyzed the results from two studies (90, 95) and found that exercise duration on cardiopulmonary exercise testing improved by 57.67 seconds using oxygen compared with room air (95% CI, 0.22 to 115.12; I2 = 0%). No significant improvement was observed in the maximal work rate (MD, 10.34 W; 95% CI, −3.59 to 24.26 W; I2 = 0%).
No studies reported the effects of ambulatory oxygen on fatigue, exercise capacity in the long term, physical activity in daily life, or mortality. The quality of evidence was low, with no parallel-group RCTs and only one study evaluating use of ambulatory oxygen in daily life (83): a crossover trial that did not include blinding of participants or researchers. See Table E6 for an evidence profile.
Safety data specific to ambulatory oxygen in ILD were rarely reported. A Cochrane review of the effects of ambulatory or short-burst oxygen in ILD did not report any serious adverse events or side effects (88). However, the panel agreed that risks of transporting cylinders, burns, fires, and tripping would be potential safety concerns for patients with ILD using ambulatory oxygen.
Panel judgments
Desirable consequences and their magnitudes (benefits)
For people with ILD and exertional hypoxemia, ambulatory oxygen resulted in improvements in the critical outcome of HRQL that may be clinically important. However, there was only one crossover trial with a 2-week treatment period, so the long-term impact on HRQL is unknown. In laboratory studies, the improvements in exercise capacity tended to favor ambulatory oxygen but were generally small. Physical activity in daily life and mortality were not reported.
Undesirable consequences and their magnitudes (harms)
Qualitative studies in patients with ILD report negative physical and psychosocial impacts of ambulatory oxygen therapy, which persist despite acceptance that this treatment may be inevitable as disease progresses (96). Patients and caregivers report that the equipment is challenging to use, that there may be unmet expectations for symptom relief (particularly dyspnea), and that challenges related to use of cumbersome or complicated equipment, embarrassment when using ambulatory oxygen in public, reduced independence for patients, and increased caregiver burden mark an important trade-off between benefits and inconvenience (75, 96, 97).
Rationale for the recommendation
Weak evidence supports the use of ambulatory oxygen in people with ILD, suggesting benefits in HRQL; certainty is low, as the medium- to long-term effects are unknown. Ambulatory oxygen may improve exercise capacity, but effects on physical activity in daily life have not been examined. There is uncertainty regarding symptom benefits. Given this uncertainty, together with the known burden of ambulatory oxygen, it is likely that some patients will choose not to use ambulatory oxygen.
ATS recommendation
For adults with ILD who have severe exertional room air hypoxemia, we suggest prescribing ambulatory oxygen (conditional recommendation, low-quality evidence).
What others are saying
A “good practice point” in the 2015 BTS guideline acknowledges that ambulatory oxygen therapy may be useful in patients with ILD and disabling breathlessness, and it could be prescribed if there is evidence of benefit and ongoing adherence (7). Guidelines from TSANZ state that in the absence of trial-based evidence, the benefit of ambulatory oxygen in individual patients should be established by comparing exercise endurance, oxygen saturation, and dyspnea during a blinded exercise test on oxygen versus air (9). Current ATS/European Respiratory Society/Japanese Respiratory Society/Asociación Latinoamericana de Tórax guidelines on management of IPF do not mention ambulatory oxygen therapy (6).
Implementation considerations
We defined exertional hypoxemia as an SpO2 ≤ 88%. In ILD, exertional hypoxemia frequently worsens as the disease progresses and may be profound in those with severe disease; ambulatory oxygen is likely to be particularly important for these patients. It may also be important for those with functional limitation and those being considered for lung transplantation. The panel noted that the need for high-flow ambulatory devices is greater in people with ILD than in those with COPD because of the magnitude of exertional hypoxemia. It is prudent to ensure that ambulatory oxygen devices with appropriate flow capacity are prescribed to patients on the basis of their needs. The panel noted that the majority of evidence is related to laboratory tests that may not be indicative of activities of daily living. Standardization of the level of exertion is necessary to assess the effects of oxygen on dyspnea (69, 70), which may be particularly important when determining the eligibility for and likely benefits of ambulatory oxygen.
Values and preferences
This recommendation places a high value on increasing HRQL and facilitating physical activity outside of the home and places a lower value on cost and resource use.
Research needs
Randomized parallel-group trials that evaluate the impact of ambulatory oxygen on patient-centered outcomes, daily life activities, disease progression, and mortality are required. These trials should address outcomes pertinent to cost-effectiveness, such as productivity, days missed from work, and hospital readmissions. Although we found several studies evaluating the effects of oxygen during laboratory testing, there was only one study that evaluated ambulatory oxygen during daily life activities, the context for which it is prescribed. There is an urgent need for ambulatory oxygen devices that can better meet the high flow requirements of people with ILD during exercise. Novel devices should also increase portability through improvements in weight, maneuverability, and battery life.
Question 6: Should portable LOX be provided for adults with chronic lung disease who are prescribed continuous oxygen flow rates of >3 L/min during exertion?
Background
Three modes of portable oxygen delivery are available for patients’ use outside of the home: metal cylinders of compressed gaseous oxygen, POCs, and LOX canisters. Each varies in size, weight, levels of pulse- or continuous-flow settings, oxygen delivery capacity, and duration of supply (Figure 1 and Tables 5 and E11). In the past, LOX has been used for those with higher flow requirements to facilitate mobility and increase time spent outside of the home, but relative efficacy is unclear.
Table 5.
Characteristics of Portable Oxygen Devices
| Metal Oxygen Cylinders | POCs | LOX | |
|---|---|---|---|
| Size and weight | Available in multiple sizes from 2.5 to 9 kg (E cylinder in United States, which requires a trolley)* | Vary in weight (1.5–10 kg), noise, battery life, oxygen purity (87–95%), maximum breath rates, and settings (pulse flow, continuous flow, or both)†‡ | Medium to large canister ranges between 2.5 and 4 kg |
| Filling | Some stationary concentrators allow patients to fill smaller oxygen cylinders in their home, (home-fill units), but these last <1 h on continuous-flow rates >3 L/min and therefore are inadequate for high-flow patients | No filling; POCs “concentrate” oxygen by extracting nitrogen from ambient air. They run off of a battery and can be recharged | Patients refill portable canisters from a larger home reservoir of LOX |
| One liter of LOX expands to 860 L of gaseous oxygen | |||
| Pulse setting or continuous-flow capacity§ | Oxygen-conserving devices using pulse-flow technology can be attached to metal cylinders to prolong the duration of supply by releasing oxygen only during inspiration | At a given pulse-flow setting, POCs differ as to the volume of oxygen (ml) per pulse, inspiratory time, and triggering sensitivity and may not consistently sense patients’ inspiratory efforts to trigger the device* | Portable LOX technology allows delivery of continuous-flow oxygen up to 15 L/min via a lighter and longer-duration device |
| Because of differences in an individual patient’s ability to trigger a pulse dose of oxygen, and the volume delivered with each pulse at different respiratory rates, they may be insufficient for patients who require continuous oxygen with exertion at >3 L/min, such as those with interstitial lung disease, lung transplantation candidates, and others with severe hypoxemia | Pulse settings are based on an oxygen volume unique to each device, not a standardized L/min methodology | ||
| Duration of supply | A single E tank with a stroller will last approximately 1.9 h on 6 L/min. Multiple cylinders are needed for high-flow (>3 L/min) patients to be out of the home >2–4 h‖ | All POCs depend on a battery supply that depletes more rapidly with higher settings, higher respiratory rates, and the use of continuous-flow settings | A medium LOX canister will last 3 h at 6 L/min of continuous flow |
| Cost | Metal oxygen cylinders range from US$50 to US$100; additional costs for a regulator or oxygen-conserving device. Commonly supplied by U.S. DME companies | In the United States, many DME companies offer POCs as a portable option together with a stationary concentrator; individuals can also purchase them for US$2,000–4,000 | Cost estimates are approximately four times higher per patient compared with POCs or metal-cylinder options because of the requirements for DME companies to access and store LOX, use specially outfitted delivery trucks, and provide weekly refill servicing¶ |
| Travel | Metal cylinders not allowed for air travel | POCs are the only carry-on portable oxygen device allowed by the Federal Aviation Administration for air travel; some airlines may provide oxygen cylinders for emergency in-flight use only** | Liquid oxygen not allowed for air travel |
Definition of abbreviations: DME = durable medical equipment; LOX = liquid oxygen; POCs = portable oxygen concentrators.
The availability of different oxygen devices varies by geographic region, and some jurisdictions do not have smaller metal oxygen cylinders.
The few POCs that currently provide a maximum of 3 L/min on a continuous-flow setting weigh over 9 kg and require a trolley.
For all devices, if an oxygen-conserving device is used, the patient should be tested using that device during exertion, similar to what they would do in daily life, to ensure adequate oxygenation. A continuous-flow setting of 5 L/min and a pulse-flow setting of “5” may not deliver equivalent volumes of oxygen, despite direct marketing claims.
Patients depend on their DME company to deliver an adequate number of cylinders per week or month.
LOX costs are higher than costs for POCs or metal cylinders (103).
The Federal Aviation Administration stipulates which POCs are allowed for use during air travel (119).
Since the implementation of the Medicare National Competitive Bidding Program by the Centers for Medicare and Medicaid Services (CMS) in the United States in 2011, Medicare beneficiary claims for portable LOX declined from 966,846 in 2004 to 97,690 in 2016 (98). This decrease reflects lower CMS reimbursement to durable medical equipment (DME) companies with subsequent elimination of LOX, and other cost-saving changes including transitioning patients to “nondelivery” home-fill oxygen systems (99–101) and heavier E cylinders.
The impact of this decline in the availability and adequacy of portable oxygen devices in the United States has been profound. Supplemental oxygen users reported numerous problems, with the overarching theme being restricted mobility and isolation due to inadequate portable options (13, 14, 16, 20, 102). The panel agreed that portable LOX for individuals requiring >3 L/min of continuous-flow oxygen was an important problem because limited mobility affects our critical outcome of HRQL. The symptoms associated with severe exertional hypoxemia in patients with high-flow oxygen needs may be substantial, but these have not been assessed in clinical trials.
The prespecified critical outcome for this key question was HRQL; important outcomes were oxygen saturation during exertion, dyspnea, exercise capacity, physical activity, adherence, and safety.
Description of evidence and its quality
The literature search did not yield any studies that met our inclusion criteria, which specified that patients be prescribed continuous oxygen flow rates of >3 L/min during exertion. Because of the absence of other forms of evidence, we considered indirect evidence and synthesized the literature for six studies in patients with COPD (54, 55, 103–106) with lower or unreported flow rates. Five of those studies tested subjects on continuous-flow LOX (54, 55, 103–105), whereas one study (106) used pulse-flow LOX (Table E7).
A multicenter RCT (103) compared the use of stationary oxygen concentrators plus small oxygen cylinders or LOX (stationary and portable) in patients with COPD (n = 51). They reported HRQL measured with the Sickness Impact Profile (SIP) and the EuroQol instruments. There was no difference between groups in EuroQol scores, but there were significant differences in favor of LOX for the SIP domains of mobility (MD, −4.57; P = 0.043), body care (MD, −5.83; P = 0.011), ambulation (MD, −8.46; P = 0.017), social interaction (MD, −5.27; P = 0.023), and total SIP score (MD, −3.38; P = 0.018) (103). The MCID for SIP is a change of 5 U (107).
No difference in oxygen saturation was observed during the 6MWT (54, 106) and 2-minute-walk test (105) between LOX and concentrators. The Borg dyspnea score did not differ after the 6MWT (MD, −0.10; 95% CI, −1.23 to 1.03) (54) or 2-minute-walk test (MD, −0.40; 95% CI, −1.36 to 0.56) (105).
A retrospective study (n = 146) compared adherence in users of stationary and portable LOX to a group that used a stationary oxygen concentrator plus small portable metal cylinders (105). The LOX group used stationary and portable oxygen for 6.50 h/d (95% CI, 4.43–8.57 h/d) longer; 92% of the concentrator group did not use their provided portable cylinders. However, the majority of patients in the concentrator group were sicker (presence of tracheostomy or use of home ventilation) compared with the LOX group and were therefore less likely to use portable oxygen outside of the home. An RCT (55) (n = 159) found that portable oxygen increased patients’ daily duration of oxygen therapy compared with those solely using stationary devices (MD, 3.00 h; 95% CI, 1.97–4.03 h); 24% more patients who used oxygen for greater than 18 h/d were LOX users than cylinder users. In a crossover RCT in which all participants were LTOT users (n = 15), participants who were allocated metal cylinders spent more time each week using their home stationary oxygen concentrator than those using LOX spent (13.1 h longer; 95% CI, 1.57–27.92 h longer) (104). LOX users spent more time outside the home than metal-cylinder users (MD, 4.0 h; 95% CI, 0.9–7.1 h) (104, 105) and were more likely to leave the home (105).
Our systematic review did not identify safety data specific to LOX, with the exception of guidance provided by the manufacturer (108).
Panel judgments
Desirable consequences and their magnitudes (benefits)
Despite the absence of studies examining continuous-flow LOX at >3 L/min, the panel judged that the desirable consequences and benefits outweighed the undesirable consequences and harms of portable LOX therapy. Indirect evidence demonstrated improvements in some domains of HRQL, improved adherence, and increased time spent outside the home. LOX provides opportunity to leave the home for patients with severe hypoxemia who would otherwise require multiple, heavy, metal oxygen cylinders to access the community. Patients who are caregivers for others, have paid employment or classroom education needs outside the home, or are attending pulmonary rehabilitation sessions to prepare for lung transplantation would have a means to engage in such activities by having a longer duration of oxygen supply.
Undesirable consequences and their magnitudes (harms)
The panel agreed that some of the undesirable consequences of LOX were similar to those previously described, including equipment management, unmet expectations for symptom relief, embarrassment, reduced independence, and increased caregiver burden. However, LOX may have less impact on mobility because of smaller, longer-lasting, and more lightweight canisters and the ability to provide continuous high-flow oxygen. Use of LOX requires manual ability to fill portable canisters from a large reservoir, which is not required with other portable oxygen systems. There is also a risk of skin burns from frost leaks when filling portable canisters that is unique to LOX.
Rationale for the recommendation
The panel made a conditional recommendation because although LOX is relevant to a subgroup of patients, there are limited data to guide patient selection. However, the panel considered that, on the basis of the limited capacity of POCs and portable metal cylinders, the prescription of LOX is critically important for patients who require high flow rates and who need to spend extended periods of time outside the home. Patients who require continuous-flow oxygen at >3 L/min cannot spend >2 hours outside the home with a single E tank. LOX at 3–4 L/min may allow patients to spend up to 5 hours away from home, which is reduced to 4 hours if using LOX at 5 L/min (Table E11).
ATS recommendation
In patients with chronic lung disease who are mobile outside of the home and require continuous flow rates of >3 L/min during exertion, we suggest prescribing portable LOX (conditional recommendation, very-low-quality evidence).
What others are saying
The National Institute for Clinical Excellence guidelines for COPD management (109) state that “small lightweight cylinders, oxygen-conserving devices and portable liquid oxygen systems should be available for the treatment of patients with COPD. A choice about the nature of equipment prescribed should take into account the hours of ambulatory oxygen use required by the patient and the oxygen flow rate required.” They suggest that compressed gas cylinders be used for no less than 90 minutes and that LOX be used for >4 hours or >30 minutes, for flow rates higher than 2 L/min (109).
Guidelines by the U.S. Department of Veterans Affairs state that LOX should be “prescribed for ambulatory patients who use an extensive amount of oxygen from portable sources” (110). They further state that “The most efficient and medically appropriate system for providing oxygen will be determined by the prescribing physician based upon the flow rate (per minute) desired, the daily period of usage, the patient’s physical condition and daily activities” (110).
Although individual coverage varies across insurance companies, one national insurer’s policy for portable oxygen, including ambulatory LOX, says it is “Considered medically necessary for members who occasionally go beyond the limits of a stationary oxygen delivery system with 50-ft. tubing for >2 hours per day for most days of the week (minimum 2 hours/week)” (111).
Finally, a health technology assessment in Quebec, Canada, assessing the evidence behind portable LOX concluded that “advantages are likely to be most beneficial to a select group of patients who are relatively active and compliant with therapy, while there are no agreed social or clinical indicators that would be reliable predictors of use or benefit” (112).
Implementation considerations
Costs associated with LOX are higher than for other delivery systems. DME suppliers in the United States are reluctant to provide LOX under current CMS funding, as it does not fully reimburse costs associated with a special delivery truck, frequent deliveries, and related equipment. In other countries, provision of LOX varies in its costs, availability, and patient eligibility requirements. The feasibility of implementing LOX will vary across geographic areas and reimbursement policies.
Values and preferences
This recommendation places a high value on HRQL related to mobility outside of the home and places a lower value on costs and resource use.
Research needs
The lack of any studies meeting our evidence criteria underscores the need for future research on the benefits of LOX in those with ILD and in other patients who require high-flow oxygen and are mobile outside the home. Outcomes such as change in mobility, depressive symptoms, use of healthcare resources, and survival require further investigation. Technologic and device-development research is needed to develop lighter-weight and longer-lasting portable oxygen devices.
Education and Safety Considerations
Panel Discussion
The panel agreed that for all patients receiving home oxygen therapy, there was no acceptable alternative to providing patients and their caregivers appropriate education related to adherence to their oxygen prescription, proper use and troubleshooting of oxygen equipment, oxygen safety education, and education on self-management. These were recurring topics of discussion for all population, intervention, comparison, and outcome questions. The panel agreed that these recommendations applied to patients using LTOT as well as to ambulatory oxygen users. A best-practice statement was included to address these recommendations.
Safety education should be provided to patients and caregivers to avoid tripping and falls and to decrease fire risk by not smoking or allowing smoking inside the home, to avoid the use of inline devices, to avoid activities around an open flame or spark, and to avoid the use of nonpetroleum nasal products (5, 28). For LOX users, patients should be provided product information that includes instructions on avoiding skin burns from contact with any of the frosted parts on LOX-device connectors (108). The panel agreed that patients also need guidance on transporting and traveling safely with oxygen (14).
Access to oxygen for patients who continue to smoke varies globally. In some regions, smoking is an absolute contraindication to home oxygen therapy (9); the BTS recommends advising the patient that oxygen provides limited clinical benefit for those who continue to smoke (36). Smoking safety is now inclusive of e-cigarettes, or vaping, on the basis of reported burn accidents in e-cigarette smokers receiving home oxygen therapy (113). Included in safety concerns is establishment of back-up devices for emergencies or loss of power. Current smokers or caregiver smokers should receive education and support for treatment of tobacco dependence (referral to appropriate resources).
Educational support is necessary for patients and caregivers and should be tailored to patients’ health literacy and cultural contexts. Current practice does not consistently include effective evaluation and support of the oxygen-using patient by healthcare providers or oxygen delivery personnel to ensure adequate education and return demonstration of their ability to use their prescribed devices (teaching back) both in the home and ambulatory settings (13). Other considerations include access to appropriate equipment based on patients’ physical, physiologic, and lifestyle and/or mobility needs. Clinical support for monitoring at home by nurses and respiratory therapists is rare in the United States but common in other regions (19). The panel agreed that patients should be advised to bring their portable device to healthcare visits to assess its effectiveness and to reinforce self-management.
All panelists agreed on the need for ongoing reassessment of patients’ increased or decreased oxygen needs and acknowledged that the frequency would vary by disease characteristics, rate of progression, or posthospitalization status. The high priority for posthospitalization reassessment of the ongoing need for oxygen is in agreement with the ATS/American College of Chest Physicians policy statement (40) and others findings (39).
ATS Recommendation
For patients prescribed home oxygen therapy, we recommend that the patient and their caregivers receive instruction and training on adherence to their oxygen prescription; the use, maintenance and troubleshooting of all oxygen equipment; and education on oxygen safety, including smoking cessation, fire prevention, and tripping hazards (best-practice statement).
Conclusions
Our systematic review reveals that the quality and quantity of clinical trial evidence is low, thereby leaving significant gaps in available data regarding prescription of supplemental oxygen. The need for guidance is high; the prescription of supplemental oxygen is common. The recommendations in this document reflect an integration of current evidence and clinical experience by a multidisciplinary expert panel.
For patients with severe resting hypoxemia, the prescription of LTOT to improve survival is supported by historical trials in patients with COPD. The panel also strongly recommends prescribing oxygen for patients with ILD with severe resting hypoxemia. Existing evidence and panel consensus suggest not prescribing LTOT for patients with COPD with moderate resting hypoxemia. The practice of initiating short-term oxygen therapy on hospital discharge in patients with severe hypoxemia is based on indirect evidence from the NOTT and MRC clinical trial populations with chronic hypoxemia. The harms and benefits of prescribing short-term oxygen therapy on hospital discharge deserves further study. Further research is needed on the appropriate use of shared decision-making between patients and their clinicians for decisions regarding home oxygen therapy and on approaches to discontinue home oxygen in patients who no longer have severe resting hypoxemia.
This review confirmed scarce and inconclusive data to support the prescription of oxygen in patients who have normoxemia at rest but desaturate (sometimes markedly) with exertion. Emerging evidence suggests that ambulatory oxygen may improve HRQL in patients with ILD in the short term, but longer-term data are needed. This was identified as a critical research need. The urgency is underscored by the treatment’s cumbersome nature, associated risks, and complex effects on the patients, families, and caregivers.
No studies met the panel’s criteria for the evaluation of LOX in patients who use >3 L/min of continuous-flow oxygen and spend regular and frequent time outside the home. The panel concluded that although an E tank, or other large metal cylinder, can adequately provide oxygen at up to 5 or 6 L/min, the patient would be restricted by the need to carry multiple E tanks to leave home for anything more than a very short time period. Thus, this population is unintentionally denied the necessary mobility to travel, work, socialize, or attend pulmonary rehabilitation—critical contributors to HRQL. The panel unanimously agreed that LOX should be offered to active patients on high-flow oxygen and that policies to accommodate this subgroup should be moved forward.
Finally, the minimal standard of care for all patients receiving home oxygen therapy must include education and training related to their oxygen equipment, oxygen safety, and self-management.
We urge the research community and funding agencies to work together to develop a stronger evidence base that will guide clinical practice for oxygen prescription. Of critical importance is the involvement of engineers and those in related fields who can combine creativity with applied science to develop methods of raising arterial blood oxygen content to normal levels, even during intense exercise, without the burdens associated with current oxygen delivery systems.
Supplementary Material
Acknowledgments
This official clinical practice guideline was prepared by an ad hoc subcommittee of the ATS Assembly on Nursing.
Members of the subcommittee are as follows:
Susan S. Jacobs, R.N., M.S.1 (Co-Chair)
Anne E. Holland, P.T., Ph.D.2 (Co-Chair)
Jerry A. Krishnan, M.D., Ph.D.3 (Co-Chair)
David J. Lederer, M.D.4,5 (Co-Chair)
Brian Carlin, M.D.6
M. Bradley Drummond, M.D., M.H.S.7
Magnus Ekström, M.D., Ph.D.8
Chris Garvey, F.N.P., M.S.N., M.P.A.9
Marya Ghazipura, Ph.D., M.S.10*
Bridget A. Graney, M.D.11
Tanzib Hossain, M.D., M.S.10‡
Beverly Jackson, M.S.12§
Thomas Kallstrom, M.B.A., R.R.T.13
Shandra L. Knight, M.S.14‖
Kathleen Lindell, Ph.D., R.N.15
Valentin Prieto-Centurion, M.D.3
Elisabetta A. Renzoni, M.D., Ph.D.16
Christopher J. Ryerson, M.D., M.A.S.17
Ann Schneidman, M.S., C.N.S., C.H.P.N.18
Jeffrey Swigris, D.O., M.S.14
Ai-Yui M. Tan, M.D.3‡
Dona Upson, M.D., M.A.19
*Lead methodologist.
‡Methodologist.
§Patient representative.
‖Medical librarian.
1Stanford University, Stanford, California; 2Alfred Health, Monash University, Melbourne, Victoria, Australia; 3University of Illinois at Chicago, Chicago, Illinois; 4Regeneron Pharmaceuticals, Inc., Tarrytown, New York; 5Columbia University, New York, New York; 6Sleep Medicine and Lung Health Consultants, Sewickley, Pennsylvania; 7University of North Carolina at Chapel Hill, Chapel Hill, North Carolina; 8Lund University, Lund, Sweden; 9University of California, San Francisco, San Francisco, California; 10New York University Langone Health, New York University, New York, New York; 11Anschutz Medical Campus, University of Colorado, Aurora, Colorado; 12LAM Foundation, Cincinnati, Ohio; 13American Association for Respiratory Care, Irving, Texas; 14National Jewish Health, Denver, Colorado; 15University of Pittsburgh, Pittsburgh, Pennsylvania; 16Royal Brompton Hospital, London, United Kingdom; 17University of British Columbia, Vancouver, British Columbia, Canada; 18Hospice of the Valley, Phoenix, Arizona; and 19New Mexico Veterans Affairs Health Care System, Albuquerque, New Mexico
Author Disclosures: S.S.J. served on an advisory committee for the Pulmonary Fibrosis Foundation. A.E.H. served as a speaker for AstraZeneca and received research support from Air Liquide and Linde Healthcare. J.A.K. served on a data safety and monitoring board for Sanofi and received research support from Inogen, Patient-Centered Outcomes Research Institute, and ResMed. D.J.L. served on an advisory committee for Boehringer Ingelheim, Fibrogen, Galapagos, Genentech/Roche, and Veracyte; served as a consultant for Fibrogen, Galapagos, Galecto, Genentech/Roche, Patara, the Pulmonary Fibrosis Foundation, Sanofi, and Veracyte; received research support from Boehringer Ingelheim, Fibrogen, and Global Blood Therapeutics; and is an employee of and owns stock and stock options with Regeneron. B.C. served on an advisory committee for AstraZeneca, GlaxoSmithKline, Monaghan, and Sunovion; served as a consultant for GlaxoSmithKline and Monaghan; and served as a speaker for GlaxoSmithKline, Monaghan, and Sunovion. M.B.D. served on an advisory committee for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Mylan; served as a consultant for AstraZeneca, Enterprise Therapeutics, GlaxoSmithKline, NovaVax, Parion, Philips Respironics, and Theravance; received research support from Boehringer Ingelheim; and received author royalties from Karger Publishing. C.G. served on an advisory committee, as a consultant, and as a speaker for Boehringer Ingelheim. V.P.-C. received research support from ResMed and is an employee of Vertex. E.A.R. served on an advisory committee for Roche; served as a speaker for Boehringer Ingelheim, Mundipharma, and Roche; and received travel support from Boehringer Ingelheim. C.J.R. served on an advisory committee for Boehringer Ingelheim and received research support from and served as a speaker for Boehringer Ingelheim and Roche. J.S. served as a consultant for Boehringer Ingelheim; served on an advisory committee, served as a speaker for, and received research support from Boehringer Ingelheim and Genentech; and served on the board of directors and has an intellectual property/patent unsold for Live Fully, Inc. M.E., M.G., B.A.G., T.H., B.J., T.K., S.L.K., K.L., A.S., A.-Y.M.T., and D.U. reported no relationships with relevant commercial interests.
Acknowledgment
The guideline panel thanks the ATS staff for their organization and support, which was critical to this project’s completion and success. The authors also thank panel member Ms. Beverly Jackson for her insightful contributions as an oxygen user and as an advocate for those using home oxygen. The panel thanks the LOTT authors’ sharing of their important data on patients with exertion-only hypoxemia and thanks the authors of the Moore and colleagues 2011 study (51) for providing additional study information.
Footnotes
This official clinical practice guideline of the American Thoracic Society was approved September 2020
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1164/rccm.202009-3608ST.
This document has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Nursing
References
- 1.Doherty DE, Petty TL, Bailey W, Carlin B, Cassaburi R, Christopher K, et al. Recommendations of the 6th long-term oxygen therapy consensus conference. Respir Care. 2006;51:519–525. [PubMed] [Google Scholar]
- 2.Croxton TL, Bailey WC. Long-term oxygen treatment in chronic obstructive pulmonary disease: recommendations for future research: an NHLBI workshop report. Am J Respir Crit Care Med. 2006;174:373–378. doi: 10.1164/rccm.200507-1161WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 4.Stuart-Harris C. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: report of the Medical Research Council Working Party. Lancet. 1981;1:681–686. [PubMed] [Google Scholar]
- 5.Albert RK, Au DH, Blackford AL, Casaburi R, Cooper JA, Jr, Criner GJ, et al. Long-Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617–1627. doi: 10.1056/NEJMoa1604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardinge M, Suntharalingam J, Wilkinson T British Thoracic Society. Guideline update: the British Thoracic Society Guidelines on home oxygen use in adults. Thorax. 2015;70:589–591. doi: 10.1136/thoraxjnl-2015-206918. [DOI] [PubMed] [Google Scholar]
- 8.Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. British Thoracic Society Interstitial Lung Disease Guideline Group, British Thoracic Society Standards of Care Committee; Thoracic Society of Australia; New Zealand Thoracic Society; Irish Thoracic Society. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63:v1–v58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 9.McDonald CF, Whyte K, Jenkins S, Serginson J, Frith P. Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology. 2016;21:76–78. doi: 10.1111/resp.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacasse Y, Bernard S, Maltais F. Eligibility for home oxygen programs and funding across Canada. Can Respir J. 2015;22:324–330. doi: 10.1155/2015/280604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 12.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Fontana, WI: Global Initiative for Chronic Obstructive Lung Disease; 2020. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report) [accessed 2020 Jul 16]. Available from: https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf. [Google Scholar]
- 13.Jacobs SS, Lindell KO, Collins EG, Garvey CM, Hernandez C, McLaughlin S, et al. Patient perceptions of the adequacy of supplemental oxygen therapy: results of the American Thoracic Society Nursing Assembly Oxygen Working Group Survey. Ann Am Thorac Soc. 2018;15:24–32. doi: 10.1513/AnnalsATS.201703-209OC. [DOI] [PubMed] [Google Scholar]
- 14.AlMutairi HJ, Mussa CC, Lambert CT, Vines DL, Strickland SL. Perspectives from COPD subjects on portable long-term oxygen therapy devices. Respir Care. 2018;63:1321–1330. doi: 10.4187/respcare.05916. [DOI] [PubMed] [Google Scholar]
- 15.Dobson A, Heath S, Kilby D, Hu J, DaVanzo JE. Vienna, VA: Dobson DaVanzo & Associates; 2017. Access to home medical equipment: survey of beneficiary, case manager and supplier experiences—understanding the impact of competitive bidding. [accessed 2017 Oct 10]. Available from: https://s3.amazonaws.com/aafh/downloads/1287/Dobson_DaVanzo_Patient_Access_Survey_Final_Report_10.11.17_FINAL.pdf?1507750324. [Google Scholar]
- 16.Lindell KO, Collins EG, Catanzarite L, Garvey CM, Hernandez C, Mclaughlin S, et al. Equipment, access and worry about running short of oxygen: key concerns in the ATS patient supplemental oxygen survey. Heart Lung. 2019;48:245–249. doi: 10.1016/j.hrtlng.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Swigris J. Caution against extrapolating results from the trial of long-term oxygen for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14:296. doi: 10.1513/AnnalsATS.201611-933LE. [DOI] [PubMed] [Google Scholar]
- 18.Du Plessis JP, Fernandes S, Jamal R, Camp P, Johannson K, Schaeffer M, et al. Exertional hypoxemia is more severe in fibrotic interstitial lung disease than in COPD. Respirology. 2018;23:392–398. doi: 10.1111/resp.13226. [DOI] [PubMed] [Google Scholar]
- 19.Ekström M, Ahmadi Z, Larsson H, Nilsson T, Wahlberg J, Ström KE, et al. A nationwide structure for valid long-term oxygen therapy: 29-year prospective data in Sweden. Int J Chron Obstruct Pulmon Dis. 2017;12:3159–3169. doi: 10.2147/COPD.S140264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs SS, Lederer DJ, Garvey CM, Hernandez C, Lindell KO, McLaughlin S, et al. Optimizing home oxygen therapy: an official American Thoracic Society workshop report. Ann Am Thorac Soc. 2018;15:1369–1381. doi: 10.1513/AnnalsATS.201809-627WS. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacasse Y, Tan AM, Maltais F, Krishnan JA. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:1254–1264. doi: 10.1164/rccm.201802-0382CI. [DOI] [PubMed] [Google Scholar]
- 23.Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208. doi: 10.2147/COPD.S10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haldane JS. The therapeutic administration of oxygen. BMJ. 1917;1:181–183. doi: 10.1136/bmj.1.2928.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine BE, Bigelow DB, Hamstra RD, Beckwitt HJ, Mitchell RS, Nett LM, et al. The role of long-term continuous oxygen administration in patients with chronic airway obstruction with hypoxemia. Ann Intern Med. 1967;66:639–650. doi: 10.7326/0003-4819-66-4-639. [DOI] [PubMed] [Google Scholar]
- 26.Bao H, Wang J, Zhou D, Han Z, Zhang Y, Su L, et al. Community physician-guided long-term domiciliary oxygen therapy combined with conventional therapy in stage IV COPD patients. Rehabil Nurs. 2017;42:268–273. doi: 10.1002/rnj.233. [DOI] [PubMed] [Google Scholar]
- 27.Crockett AJ, Moss JR, Cranston JM, Alpers JH. The effects of home oxygen therapy on hospital admission rates in chronic obstructive airways disease. Monaldi Arch Chest Dis. 1993;48:445–446. [PubMed] [Google Scholar]
- 28.Tanash HA, Huss F, Ekström M. The risk of burn injury during long-term oxygen therapy: a 17-year longitudinal national study in Sweden. Int J Chron Obstruct Pulmon Dis. 2015;10:2479–2484. doi: 10.2147/COPD.S91508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma G, Meena R, Goodwin JS, Zhang W, Kuo YF, Duarte AG. Burn injury associated with home oxygen use in patients with chronic obstructive pulmonary disease. Mayo Clin Proc. 2015;90:492–499. doi: 10.1016/j.mayocp.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacasse Y, LaForge J, Maltais F. Got a match? Home oxygen therapy in current smokers. Thorax. 2006;61:374–375. doi: 10.1136/thx.2006.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medicare.gov Your Medicare coverage: oxygen equipment and accessories Lawrence, KS: Medicare.gov; 2020[accessed 2020 Jul 16]. Available from: https://www.medicare.gov/coverage/oxygen-equipment-accessories [Google Scholar]
- 32.Oba Y. Cost-effectiveness of long-term oxygen therapy for chronic obstructive disease. Am J Manag Care. 2009;15:97–104. [PubMed] [Google Scholar]
- 33.Foo J, Landis SH, Maskell J, Oh YM, van der Molen T, Han MK, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11:e0152618. doi: 10.1371/journal.pone.0152618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Government of Alberta Alberta health: Alberta aids to daily living—general policy and procedures manual[accessed 2017 Apr]. Edmonton, AB, Canada: Government of Alberta; 2017. Available from: https://open.alberta.ca/dataset/8476d5ff-7280-495f-a858-f14286566406/resource/0b2d2918-5660-4af5-b5ed-1b844bc5fb22/download/aadl-policy-procedures.pdf [Google Scholar]
- 35.Saskatchewan Aids to Independent Living (SAIL) Universal Benefits Program. Government of Saskatchewan; 2019[accessed 2020 Oct 14]. Available from: https://www.saskatchewan.ca/residents/health/accessing-health-care-services/health-services-for-people-with-disabilities/sail#universal-benefits-programs
- 36.Hardinge M, Annandale J, Bourne S, Cooper B, Evans A, Freeman D, et al. British Thoracic Society Home Oxygen Guideline Development Group; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for home oxygen use in adults. Thorax. 2015;70:i1–i43. doi: 10.1136/thoraxjnl-2015-206865. [DOI] [PubMed] [Google Scholar]
- 37.Beasley R, Chien J, Douglas J, Eastlake L, Farah C, King G, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘swimming between the flags’. Respirology. 2015;20:1182–1191. doi: 10.1111/resp.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levi-Valensi P, Weitzenblum E, Pedinielli JL, Racineux JL, Duwoos H. Three-month follow-up of arterial blood gas determinations in candidates for long-term oxygen therapy: a multicentric study. Am Rev Respir Dis. 1986;133:547–551. doi: 10.1164/arrd.1986.133.4.547. [DOI] [PubMed] [Google Scholar]
- 39.Eaton TE, Grey C, Garrett JE. An evaluation of short-term oxygen therapy: the prescription of oxygen to patients with chronic lung disease hypoxic at discharge from hospital. Respir Med. 2001;95:582–587. doi: 10.1053/rmed.2001.1106. [DOI] [PubMed] [Google Scholar]
- 40.Wiener RS, Ouellette DR, Diamond E, Fan VS, Maurer JR, Mularski RA, et al. An official American Thoracic Society/American College of Chest Physicians policy statement: the Choosing Wisely top five list in adult pulmonary medicine. Chest. 2014;145:1383–1391. doi: 10.1378/chest.14-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Górecka D, Gorzelak K, Sliwiński P, Tobiasz M, Zieliński J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax. 1997;52:674–679. doi: 10.1136/thx.52.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrianopoulos V, Franssen FM, Peeters JP, Ubachs TJ, Bukari H, Groenen M, et al. Exercise-induced oxygen desaturation in COPD patients without resting hypoxemia. Respir Physiol Neurobiol. 2014;190:40–46. doi: 10.1016/j.resp.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Panos RJ, Eschenbacher W. Exertional desaturation in patients with chronic obstructive pulmonary disease. COPD. 2009;6:478–487. doi: 10.3109/15412550903341497. [DOI] [PubMed] [Google Scholar]
- 44.Kim C, Seo JB, Lee SM, Lee JS, Huh JW, Lee JH, et al. Exertional desaturation as a predictor of rapid lung function decline in COPD. Respiration. 2013;86:109–116. doi: 10.1159/000342891. [DOI] [PubMed] [Google Scholar]
- 45.Casanova C, Cote C, Marin JM, Pinto-Plata V, de Torres JP, Aguirre-Jaíme A, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest. 2008;134:746–752. doi: 10.1378/chest.08-0520. [DOI] [PubMed] [Google Scholar]
- 46.O’Donnell DE, D’Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:892–898. doi: 10.1164/ajrccm.163.4.2007026. [DOI] [PubMed] [Google Scholar]
- 47.Mitlehner W, Kerb W. Exercise hypoxemia and the effects of increased inspiratory oxygen concentration in severe chronic obstructive pulmonary disease. Respiration. 1994;61:255–262. doi: 10.1159/000196348. [DOI] [PubMed] [Google Scholar]
- 48.Garrod R, Paul EA, Wedzicha JA. Supplemental oxygen during pulmonary rehabilitation in patients with COPD with exercise hypoxaemia. Thorax. 2000;55:539–543. doi: 10.1136/thorax.55.7.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leggett RJ, Flenley DC. Portable oxygen and exercise tolerance in patients with chronic hypoxic cor pulmonale. BMJ. 1977;2:84–86. doi: 10.1136/bmj.2.6079.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nonoyama ML, Brooks D, Guyatt GH, Goldstein RS. Effect of oxygen on health quality of life in patients with chronic obstructive pulmonary disease with transient exertional hypoxemia. Am J Respir Crit Care Med. 2007;176:343–349. doi: 10.1164/rccm.200702-308OC. [DOI] [PubMed] [Google Scholar]
- 51.Moore RP, Berlowitz DJ, Denehy L, Pretto JJ, Brazzale DJ, Sharpe K, et al. A randomised trial of domiciliary, ambulatory oxygen in patients with COPD and dyspnoea but without resting hypoxaemia. Thorax. 2011;66:32–37. doi: 10.1136/thx.2009.132522. [DOI] [PubMed] [Google Scholar]
- 52.Eaton T, Garrett JE, Young P, Fergusson W, Kolbe J, Rudkin S, et al. Ambulatory oxygen improves quality of life of COPD patients: a randomised controlled study. Eur Respir J. 2002;20:306–312. doi: 10.1183/09031936.02.00301002. [DOI] [PubMed] [Google Scholar]
- 53.McKeon JL, Tomlinson JC, Tarrant EP, Mitchell CA. Portable oxygen in patients with severe chronic obstructive pulmonary disease. Aust N Z J Med. 1988;18:125–129. [Google Scholar]
- 54.Nasilowski J, Przybylowski T, Zielinski J, Chazan R. Comparing supplementary oxygen benefits from a portable oxygen concentrator and a liquid oxygen portable device during a walk test in COPD patients on long-term oxygen therapy. Respir Med. 2008;102:1021–1025. doi: 10.1016/j.rmed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Vergeret J, Brambilla C, Mounier L. Portable oxygen therapy: use and benefit in hypoxaemic COPD patients on long-term oxygen therapy. Eur Respir J. 1989;2:20–25. [PubMed] [Google Scholar]
- 56.Jarosch I, Gloeckl R, Damm E, Schwedhelm AL, Buhrow D, Jerrentrup A, et al. Short-term effects of supplemental oxygen on 6-min walk test outcomes in patients with COPD: a randomized, placebo-controlled, single-blind, crossover trial. Chest. 2017;151:795–803. doi: 10.1016/j.chest.2016.11.044. [DOI] [PubMed] [Google Scholar]
- 57.Light RW, Mahutte CK, Stansbury DW, Fischer CE, Brown SE. Relationship between improvement in exercise performance with supplemental oxygen and hypoxic ventilatory drive in patients with chronic airflow obstruction. Chest. 1989;95:751–756. doi: 10.1378/chest.95.4.751. [DOI] [PubMed] [Google Scholar]
- 58.Porszasz J, Cao R, Morishige R, van Eykern LA, Stenzler A, Casaburi R. Physiologic effects of an ambulatory ventilation system in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:334–342. doi: 10.1164/rccm.201210-1773OC. [DOI] [PubMed] [Google Scholar]
- 59.Dyer F, Callaghan J, Cheema K, Bott J. Ambulatory oxygen improves the effectiveness of pulmonary rehabilitation in selected patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2012;9:83–91. doi: 10.1177/1479972312438702. [DOI] [PubMed] [Google Scholar]
- 60.Ringbaek T, Martinez G, Lange P. The long-term effect of ambulatory oxygen in normoxaemic COPD patients: a randomised study. Chron Respir Dis. 2013;10:77–84. doi: 10.1177/1479972312473135. [DOI] [PubMed] [Google Scholar]
- 61.Lacasse Y, Lecours R, Pelletier C, Bégin R, Maltais F. Randomised trial of ambulatory oxygen in oxygen-dependent COPD. Eur Respir J. 2005;25:1032–1038. doi: 10.1183/09031936.05.00113504. [DOI] [PubMed] [Google Scholar]
- 62.Faraone SV. Interpreting estimates of treatment effects: implications for managed care. P&T. 2008;33:700–711. [PMC free article] [PubMed] [Google Scholar]
- 63.Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189:250–255. doi: 10.1164/rccm.201310-1863PP. [DOI] [PubMed] [Google Scholar]
- 64.Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease. Health Serv Res. 2005;40:577–591. doi: 10.1111/j.1475-6773.2005.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg scale, and visual analog scale. COPD. 2005;2:105–110. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 66.Arnold E, Bruton A, Donovan-Hall M, Fenwick A, Dibb B, Walker E. Ambulatory oxygen: why do COPD patients not use their portable systems as prescribed? A qualitative study. BMC Pulm Med. 2011;11:9. doi: 10.1186/1471-2466-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelly CA, Lynes D, O’Brien MR, Shaw B. A wolf in sheep’s clothing? Patients’ and healthcare professionals’ perceptions of oxygen therapy: an interpretative phenomenological analysis. Clin Respir J. 2018;12:616–632. doi: 10.1111/crj.12571. [DOI] [PubMed] [Google Scholar]
- 68.Petty TL, Bliss PL.Ambulatory oxygen therapy, exercise, and survival with advanced chronic obstructive pulmonary disease (the Nocturnal Oxygen Therapy Trial revisited) Respir Care 200045204–211.[Discussion, pp. 211–213.] [PubMed] [Google Scholar]
- 69.Ekström M. Why treatment efficacy on breathlessness in laboratory but not daily life trials? The importance of standardized exertion. Curr Opin Support Palliat Care. 2019;13:179–183. doi: 10.1097/SPC.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 70.Ekström M, Ringbaek T. Which patients with moderate hypoxemia benefit from long-term oxygen therapy? Ways forward. Int J Chron Obstruct Pulmon Dis. 2018;13:231–235. doi: 10.2147/COPD.S148673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crockett AJ, Cranston JM, Antic N. Domiciliary oxygen for interstitial lung disease. Cochrane Database Syst Rev. 2001;(3):CD002883. doi: 10.1002/14651858.CD002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lv H, Liu J, Pan Q, Cai R, Zhang J. Clinical retrospective analysis of interstitial lung disease patients associated with pulmonary hypertension. Med Sci Monit. 2019;25:7763–7769. doi: 10.12659/MSM.916585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nemoto K, Oh-Ishi S, Akiyama T, Yabuuchi Y, Goto H, Nonaka M, et al. Borderline pulmonary hypertension is associated with exercise intolerance and increased risk for acute exacerbation in patients with interstitial lung disease. BMC Pulm Med. 2019;19:167. doi: 10.1186/s12890-019-0932-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.King CS, Nathan SD. Pulmonary hypertension due to interstitial lung disease. Curr Opin Pulm Med. 2019;25:459–467. doi: 10.1097/MCP.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 75.Graney BA, Wamboldt FS, Baird S, Churney T, Fier K, Korn M, et al. Looking ahead and behind at supplemental oxygen: a qualitative study of patients with pulmonary fibrosis. Heart Lung. 2017;46:387–393. doi: 10.1016/j.hrtlng.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Swinburn CR, Mould H, Stone TN, Corris PA, Gibson GJ. Symptomatic benefit of supplemental oxygen in hypoxemic patients with chronic lung disease. Am Rev Respir Dis. 1991;143:913–915. doi: 10.1164/ajrccm/143.5_Pt_1.913. [DOI] [PubMed] [Google Scholar]
- 77.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis—an update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [Published erratum appears in Am J Respir Crit Care Med 192:644.] [DOI] [PubMed] [Google Scholar]
- 78.Khor YH, Goh NS, Glaspole I, Holland AE, McDonald CF. Exertional desaturation and prescription of ambulatory oxygen therapy in interstitial lung disease. Respir Care. 2019;64:299–306. doi: 10.4187/respcare.06334. [DOI] [PubMed] [Google Scholar]
- 79.Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:1084–1090. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 80.Papakosta D, Pitsiou G, Daniil Z, Dimadi M, Stagaki E, Rapti A, et al. Prevalence of pulmonary hypertension in patients with idiopathic pulmonary fibrosis: correlation with physiological parameters. Lung. 2011;189:391–399. doi: 10.1007/s00408-011-9304-5. [DOI] [PubMed] [Google Scholar]
- 81.Corte TJ, Wort SJ, Wells AU. Pulmonary hypertension in idiopathic pulmonary fibrosis: a review. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26:7–19. [PubMed] [Google Scholar]
- 82.Wallaert B, Monge E, Le Rouzic O, Wémeau-Stervinou L, Salleron J, Grosbois JM. Physical activity in daily life of patients with fibrotic idiopathic interstitial pneumonia. Chest. 2013;144:1652–1658. doi: 10.1378/chest.13-0806. [DOI] [PubMed] [Google Scholar]
- 83.Visca D, Mori L, Tsipouri V, Fleming S, Firouzi A, Bonini M, et al. Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): a prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet Respir Med. 2018;6:759–770. doi: 10.1016/S2213-2600(18)30289-3. [DOI] [PubMed] [Google Scholar]
- 84.Nolan CM, Birring SS, Maddocks M, Maher TM, Patel S, Barker RE, et al. King’s Brief Interstitial Lung Disease Questionnaire: responsiveness and minimum clinically important difference. Eur Respir J. 2019;54:1900281. doi: 10.1183/13993003.00281-2019. [DOI] [PubMed] [Google Scholar]
- 85.Sinha A, Patel AS, Siegert RJ, Bajwah S, Maher TM, Renzoni EA, et al. The King’s Brief Interstitial Lung Disease (KBILD) Questionnaire: an updated minimal clinically important difference. BMJ Open Respir Res. 2019;6:e000363. doi: 10.1136/bmjresp-2018-000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2:75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 87.Kupferberg DH, Kaplan RM, Slymen DJ, Ries AL. Minimal clinically important difference for the UCSD Shortness of Breath Questionnaire. J Cardiopulm Rehabil. 2005;25:370–377. doi: 10.1097/00008483-200511000-00011. [DOI] [PubMed] [Google Scholar]
- 88.Sharp C, Adamali H, Millar AB. Ambulatory and short-burst oxygen for interstitial lung disease. Cochrane Database Syst Rev. 2016;7:CD011716. doi: 10.1002/14651858.CD011716.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nishiyama O, Miyajima H, Fukai Y, Yamazaki R, Satoh R, Yamagata T, et al. Effect of ambulatory oxygen on exertional dyspnea in IPF patients without resting hypoxemia. Respir Med. 2013;107:1241–1246. doi: 10.1016/j.rmed.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 90.Troy L, Young I, Munoz P, Taylor N, Webster S, Lau E, Corte P, et al. Does supplemental oxygen increase exercise endurance in patients with idiopathic pulmonary fibrosis? Respirology. 2014;19:95. [Google Scholar]
- 91.Arizono S, Taniguchi H, Sakamoto K, Kondoh Y, Kimura T, Kataoka K, et al. Benefits of supplemental oxygen on exercise capacity in IPF patients with exercise-induced hypoxemia. Eur Respir J. 2015;46:OA4971. doi: 10.1111/resp.13829. [DOI] [PubMed] [Google Scholar]
- 92.Bell EC, Cox NS, Goh N, Glaspole I, Westall GP, Watson A, et al. Oxygen therapy for interstitial lung disease: a systematic review. Eur Respir Rev. 2017;26:160080. doi: 10.1183/16000617.0080-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bajwah S, Higginson IJ, Ross JR, Wells AU, Birring SS, Riley J, et al. The palliative care needs for fibrotic interstitial lung disease: a qualitative study of patients, informal caregivers and health professionals. Palliat Med. 2013;27:869–876. doi: 10.1177/0269216313497226. [DOI] [PubMed] [Google Scholar]
- 94.Khor YH, McDonald CF, Hazard A, Symons K, Westall G, Glaspole I, et al. Portable oxygen concentrators versus oxygen cylinder during walking in interstitial lung disease: a randomized crossover trial. Respirology. 2017;22:1598–1603. doi: 10.1111/resp.13083. [DOI] [PubMed] [Google Scholar]
- 95.Harris-Eze AO, Sridhar G, Clemens RE, Zintel TA, Gallagher CG, Marciniuk DD. Role of hypoxemia and pulmonary mechanics in exercise limitation in interstitial lung disease. Am J Respir Crit Care Med. 1996;154:994–1001. doi: 10.1164/ajrccm.154.4.8887597. [DOI] [PubMed] [Google Scholar]
- 96.Khor YH, Goh NSL, McDonald CF, Holland AE. Oxygen therapy for interstitial lung disease: a mismatch between patient expectations and experiences. Ann Am Thorac Soc. 2017;14:888–895. doi: 10.1513/AnnalsATS.201611-934OC. [DOI] [PubMed] [Google Scholar]
- 97.Graney BA, Wamboldt FS, Baird S, Churney T, Fier K, Korn M, et al. Informal caregivers experience of supplemental oxygen in pulmonary fibrosis. Health Qual Life Outcomes. 2017;15:133. doi: 10.1186/s12955-017-0710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.U.S. COPD Coalition. Union, ME: U.S. COPD Coalition; 2018. Patient provider comments to CMS re DME oxygen. [updated 2019 Sep 11; accessed 2019 Sep 11]. Available from: https://uscopdcoalition.org/wp-content/uploads/2020/06/Patient-Provider-Comments-to-CMS-re-DME-Oxygen-9-6-18.pdf. [Google Scholar]
- 99.The National Association for Medical Direction of Respiratory Care. Medicare competitive bidding and the supplemental oxygen benefit: how a benefit mired in decades old policy is woven into a new delivery model. Washington Watchline. 2013;23:1–9. [Google Scholar]
- 100.Kacik A Modern Healthcare. Detroit, MI: Crain Communications; 2017. Competitive bidding nets up to $26 billion in savings on medical equipment. [accessed 2020 Jul 16]. Available from: https://www.modernhealthcare.com/article/20170808/NEWS/170809907/competitive-bidding-nets-up-to-26-billion-in-savings-on-medical-equipment. [Google Scholar]
- 101.Centers for Medicare and Medicaid Services. Baltimore, MD: Centers for Medicare and Medicaid Services; 2016. CMS awards contracts for the DMEPOS competitive bidding program round 2 recompete and national mail-order recompete. [accessed 2020 Jul 16]. CMS Fact Sheet, CMS Media Relations. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/DMEPOSCompetitiveBid/Electronic-Mailing-List-Message-Archive-Items/2016-03-15-DMEPOS-Suppliers.html. [Google Scholar]
- 102.Hanlon P. CMS cuts detrimental to home oxygen patients, industry. RT Magazine 2016 July 31 [accessed 2016 Jul]. Available from: http://www.rtmagazine.com/2016/07/cms-cuts-detrimental-home-oxygen-patients-industry/
- 103.Andersson A, Ström K, Brodin H, Alton M, Boman G, Jakobsson P, et al. Domiciliary liquid oxygen versus concentrator treatment in chronic hypoxaemia: a cost-utility analysis. Eur Respir J. 1998;12:1284–1289. doi: 10.1183/09031936.98.12061284. [DOI] [PubMed] [Google Scholar]
- 104.Lock SH, Blower G, Prynne M, Wedzicha JA. Comparison of liquid and gaseous oxygen for domiciliary portable use. Thorax. 1992;47:98–100. doi: 10.1136/thx.47.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su C-L, Lee C-N, Chen H-C, Feng L-P, Lin H-W, Chiang L-L. Comparison of domiciliary oxygen using liquid oxygen and concentrator in northern Taiwan. J Formos Med Assoc. 2014;113:23–32. doi: 10.1016/j.jfma.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 106.Strickland SL, Hogan TM, Hogan RG, Sohal HS, McKenzie WN, Petroski GF. A randomized multi-arm repeated-measures prospective study of several modalities of portable oxygen delivery during assessment of functional exercise capacity. Respir Care. 2009;54:344–349. [PubMed] [Google Scholar]
- 107.MacKenzie CR, Charlson ME, DiGioia D, Kelley K. Can the Sickness Impact Profile measure change? An example of scale assessment. J Chronic Dis. 1986;39:429–438. doi: 10.1016/0021-9681(86)90110-4. [DOI] [PubMed] [Google Scholar]
- 108.Apria Healthcare. Morrisville, NC: Apria Healthcare; 2016. Patient/caregiver instructions: liquid oxygen. [accessed 2019 Oct 29]. Available from: https://www.apria.com/wp-content/uploads/RES-2002_Manual_Liquid-Oxygen_01-16_v21_ONLINE.pdf. [Google Scholar]
- 109.National Clinical Guideline Centre, National Institute for Health and Clinical Excellence. Guidance: chronic obstructive pulmonary disease—management of chronic obstructive pulmonary disease in adults in primary and secondary care. London, UK: Royal College of Physicians; 2010. [PubMed] [Google Scholar]
- 110.U.S. Department of Veterans Affairs. VHA handbook 1173.13: home respiratory care program. Washington, DC: U.S. Department of Veteran Affairs; 2000. [Google Scholar]
- 111.Aetna. Hartford, CT: Aetna; 2004. Oxygen for home use. Clinical Policy Bulletins. [Google Scholar]
- 112.Law S Agence d’Évaluation des Technologies et des Modes d’Intervention en Santé. Montreal, QC, Canada: Agence d’Évaluation des Technologies et des Modes d’Intervention en Santé; 2005. Liquid oxygen therapy at home. [Google Scholar]
- 113.Lacasse Y, Légaré M, Maltais F. E-cigarette use in patients receiving home oxygen therapy. Can Respir J. 2015;22:83–85. doi: 10.1155/2015/215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McCoy RW. Options for home oxygen therapy equipment: storage and metering of oxygen in the home. Respir Care. 2013;58:65–85. doi: 10.4187/respcare.01932. [DOI] [PubMed] [Google Scholar]
- 115.Gloeckl R, Osadnik C, Bies L, Leitl D, Koczulla AR, Kenn K. Comparison of continuous flow versus demand oxygen delivery systems in patients with COPD: a systematic review and meta-analysis. Respirology. 2019;24:329–337. doi: 10.1111/resp.13457. [DOI] [PubMed] [Google Scholar]
- 116.Carlin B, McCoy R, Diesem R. 2 is not 2 is not 2. Respir Ther. 2018;13:29–32. [Google Scholar]
- 117.Branson RD, King A, Giordano SP. Home oxygen therapy devices: providing the prescription. Respir Care. 2019;64:230–232. doi: 10.4187/respcare.06850. [DOI] [PubMed] [Google Scholar]
- 118.Chen JZ, Katz IM, Pichelin M, Zhu K, Caillibotte G, Finlay WH, et al. In vitro-In silico comparison of pulsed oxygen delivery from portable oxygen concentrators versus continuous flow oxygen delivery. Respir Care. 2019;64:117–129. doi: 10.4187/respcare.06359. [DOI] [PubMed] [Google Scholar]
- 119.Federal Aviation Administration. Acceptance criteria for portable oxygen concentrators. Washington, DC: Federal Aviation Administration; 2015. [accessed 2019 Aug 9]. Available from: https://www.faa.gov/about/initiatives/cabin_safety/portable_oxygen/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.