Abstract
CD44 is a transmembrane glycoprotein involved in numerous cellular functions, including cell adhesion and extracellular matrix interactions. It is known to be functionally diverse, with alternative splice variants increasingly implicated as a marker for tumor-initiating stem cells associated with poor prognosis. Here, we evaluate CD44 as a potential marker of long-term breast cancer outcomes. Tissue specimens from patients treated on the National Cancer Institute 79-C-0111 randomized trial of breast conservation versus mastectomy between 1979 and 1987 were collected, and immunohistochemistry was performed using the standard isoform of CD44. Specimens were correlated with patient characteristics and outcomes. Survival analysis was performed using the log rank test. Fifty-one patients had evaluable tumor sections and available long-term clinical follow up data at a median follow up of 25.7 years. Significant predictors of OS were tumor size (median OFS 25.4 years for ≤2 cm vs. 7.5 years for >2 cm, p = 0.001), nodal status (median OS 17.2 years for node-negative patients vs. 6.7 years for node positive patients, p = 0.017), and CD44 expression (median OS 18.9 years for CD44 positive patients vs. 8.6 years for CD44 negative patients, p = 0.049). There was a trend toward increased PFS for patients with CD44 positive tumors (median PFS 17.9 vs. 4.3 years, p = 0.17), but this did not reach statistical significance. These findings illustrate the potential utility of CD44 as a prognostic marker for early stage breast cancer. Subgroup analysis in patients with lymph node involvement revealed CD44 positivity to be most strongly associated with increased survival, suggesting a potential role of CD44 in decision making for axillary management. As there is increasing interest in CD44 as a therapeutic target in ongoing clinical trials, the results of this study suggest additional investigation regarding the role CD44 in breast cancer is warranted.
Keywords: Breast cancer, Breast conservation, CD44, Biomarker, Randomized trial
Introduction
Despite advances in screening and staging studies for treatment stratification, early stage breast cancer patients continue to have varying outcomes and responses to standard cancer treatments. Due to the heterogeneity of outcomes in these patients, increasing efforts have been made to personalize cancer treatment based on grade and molecular subtyping, with the recent development of commercially available multigene assays for clinical use. Two such assays, Oncotype Dx and Mammaprint, have been prospectively evaluated and are routinely used to aid decision making regarding the use of adjuvant chemotherapy [1, 2]. Although these assays provide valuable information and can even be cost saving or cost-effective through reduced utilization of chemotherapy [3], widespread use has been limited due to high cost, lack of insurance coverage, and delays in the initiation of adjuvant treatment [4]. In addition, due to the relatively recent availability of these technologies, there is lack of data regarding their usefulness to predict for long-term outcomes and late recurrences.
In contrast to these multigene panels, simpler protein-based expression assays utilizing immunohistochemistry have long been used to aid treatment decisions for patients with breast cancer [5]. Due to their accessibility and simplicity, they are widely implemented in clinical laboratories for a number of disease sites. Immunohistochemical detection of ER, PR, and HER-2 receptor status is currently part of the standard of care and help guide adjuvant treatment decisions [6]. Recent data may even suggest molecular subtyping by immunohistochemistry may outperform gene expression analysis in the neoadjuvant setting [7]. Other molecules, such as Ki-67, are more controversial, but may have a role in providing valuable prognostic information [8]. Given the heterogeneity of breast cancer, further investigation into novel protein biomarkers predictive of long-term clinical outcomes in selected patient subgroups is needed.
CD44 is an intriguing biomarker that has been increasingly studied in invasive breast cancer. It is a transmembrane glycoprotein of the hyaluronate receptor family that is primarily involved in cell adhesion and extracellular matrix interactions [9]. It acts as a ligand receptor and coreceptor, capable of modulating the function of other membrane tyrosine receptors. Recently, it has also been implicated as a surrogate marker for tumor stem cells [10]. Early preclinical studies have suggested CD44 positive cells exhibit the ability to initiate tumors, serially propagate, and demonstrate the ability for self-renewal [11–13]. However, when translated clinically, a substantial body of clinical data has shown conflicting outcomes in regards to CD44 expression, with a number of studies showing both favorable and unfavorable clinical outcomes associated with increased CD44 expression [14–20].
As patients with early stage breast cancer generally have an excellent prognosis, we sought to utilize a protein-based expression assay to determine the long-term prognostic value of CD44 using tissue samples from patients treated on a large, randomized trial from the National Cancer Institute comparing breast-conserving therapy versus modified radical mastectomy.
Materials and methods
Information regarding the study design, preplanned hypotheses, patient and specimen characteristics, assay methods, and statistical analysis is reported as per REMARK criteria [21]. In an IRB approved protocol, tissue specimens from patients enrolled in the National Cancer Institute trial 79-C-0111 were collected and analyzed for immunohistochemical detection of the standard variant of the membrane marker CD44. Detailed descriptions of the study design, eligibility requirements, radiotherapeutic and surgical techniques, and comparison of the characteristics of the patients in each treatment group have been described previously [22, 23]. Briefly, 237 patients at the NCI between 1979 and 1987 with biopsy proven clinical Stage I or Stage II primary breast cancer were randomized to receive either modified radical mastectomy or lumpectomy and adjuvant radiation to the entire breast followed by a boost to the tumor bed.
Tumor specimens obtained from initial surgery were stored as formalin-fixed, paraffin-embedded tissue blocks, however, since excisional biopsies were standard for initial diagnosis at the time and may have been performed at outside hospitals, these patients were excluded. Specimens without evaluable tumor on reaccessioning were excluded. Immunohistochemical staining was performed using a monoclonal antibody to the standard variant isoform (CD44s) of CD44 (clone DF1485, Dako Corp. Carpinteria, CA 1:50 dilution). Following heat-induced antigen retrieval in modified citrate buffer, pH 6.1, detection was performed utilizing the Dako EnVision visualization system (EnVision™+, Dual Link) with diaminobenzidine (DAB) as the chromogen. The presence of CD44 in areas of tumor was scored utilizing a binary scoring system with negative scoring for the absence of CD44 and positive scoring for having any amount of CD44 present. Scoring was read by two independent pathologists blinded to any patient outcomes data.
Clinical endpoints included progression-free survival (PFS) and overall survival (OS). PFS was defined as the length of time from entry onto the study defined by the NCI diagnosis, until recurrence of the original breast cancer or death. Development of a contralateral breast cancer or a nonbreast cancer was considered a competing risk for disease progression (i.e., led to censorship from PFS calculation). OS was measured from the date of NCI breast cancer diagnosis.
Fisher’s exact test was used to compare patient characteristics after grouping by CD44 expression. Bivariate tests for predictors of PFS and OS were performed using log rank testing. CD44 expression was also tested as a predictor of PFS and OS in patient subsets after grouping by known prognostic factors (tumor size and nodal status). Statistical analyses were performed in SPSS version 18 (SPSS; Chicago, IL).
Results
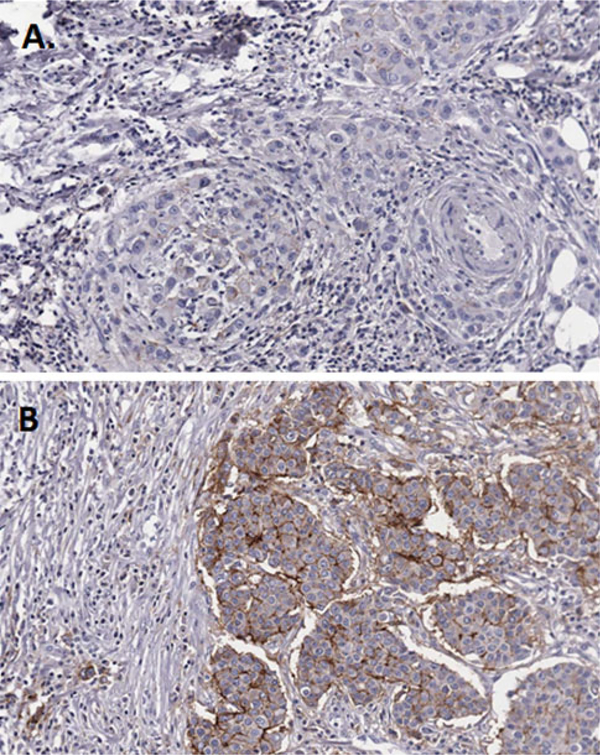
Evaluation of tumor specimens was performed for all 238 patients initially enrolled on the randomized trial; however, adequate tumor sections were obtained from 51 randomized patients. Median follow up for living patients was 27.3 years. Patient characteristics are summarized in Table 1. Mean age at diagnosis was 53 years. 41 % of patients was premenopausal, 53 % of patients had high grade disease, 57 % had lymph node involvement, and 51 % received chemotherapy. Twenty-two patients (43 %) were found to be CD44 positive. Figure 1 displays immunohistochemical examples of CD44 negative and CD44 positive tissue samples. CD44 positivity was not significantly correlated with any clinical characteristics using Fisher’s exact test.
Table 1.
Patient characteristics
| Characteristic | CD44 negative | CD44 positive | p |
|---|---|---|---|
| Age | 0.410 | ||
| ≤50 | 11 | 11 | |
| >50 | 18 | 11 | |
| Menopausal status | 1.000 | ||
| Pre | 12 | 9 | |
| Post | 17 | 13 | |
| Tumor size | 0.242 | ||
| ≤2 cm | 8 | 10 | |
| >2 cm | 21 | 12 | |
| Tumor grade | 0.368 | ||
| 1 | 3 | 2 | |
| 2 | 8 | 10 | |
| 3 | 18 | 9 | |
| Nodal status | 0.410 | ||
| Negative | 11 | 11 | |
| Positive | 18 | 11 | |
| ER status | 0.787 | ||
| Negative | 8 | 6 | |
| Positive | 16 | 14 | |
| Unknown | 5 | 2 | |
| Treatment arm | 0.782 | ||
| MRM | 13 | 11 | |
| BCT | 16 | 11 | |
| Chemotherapy use | 0.577 | ||
| No | 13 | 12 | |
| Yes | 16 | 10 |
Fig. 1.
Immunohistochemical staining for CD44. Examples of negative (a) and positive (b) staining are shown
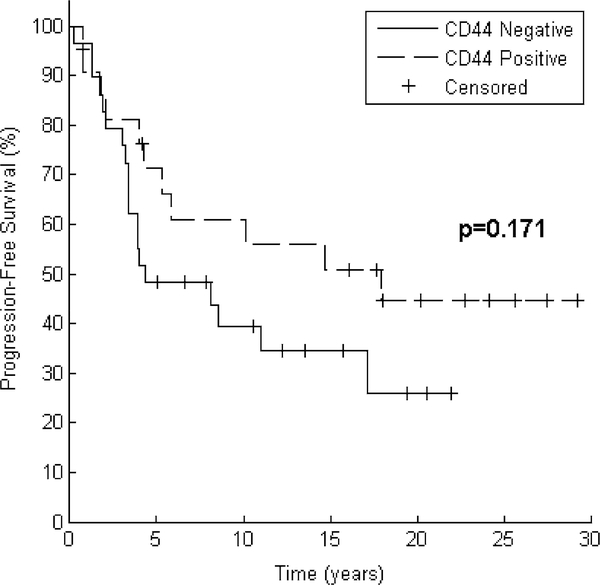
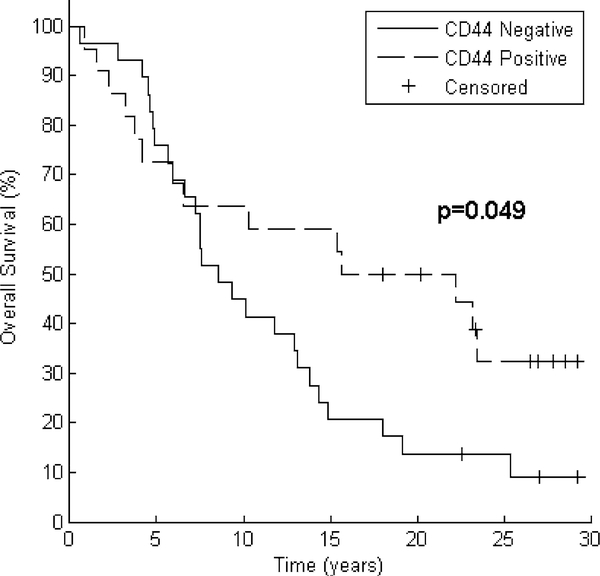
Bivariate analyses for predictors of PFS and OS are noted in Table 2. Significant (p < 0.05 using Log Rank test) predictors of PFS were tumor size (median PFS not reached for ≤2 cm vs. 4.2 years for >2 cm, p = 0.003) and nodal status (median PFS 17.9 years for node-negative patients vs. 4.0 years for node positive patients, p = 0.036). There was a trend toward increased PFS for patients with CD44 positive tumors (median PFS 17.9 vs. 4.3 years, p = 0.171, Fig. 2). Significant predictors of OS were tumor size (median OS 25.4 years for ≤2 cm vs. 7.5 years for >2 cm, p = 0.001), nodal status (median OS 17.2 years for node-negative patients vs. 6.7 years for node positive patients, p = 0.017), and CD44 expression (median OS 18.9 years for CD44 positive patients vs. 8.6 years for CD44 negative patients, p = 0.049, Fig. 3).
Table 2.
Bivariate analyses for predictors of PFS and OS
| Characteristic | Median PFS (years) | p | Median OS (years) | p |
|---|---|---|---|---|
| Age | 0.957 | 0.344 | ||
| ≤50 (n = 22) | 10.2 | 11.5 | ||
| >50 (n = 29) | 8.5 | 10.3 | ||
| Menopausal status | 0.943 | 0.499 | ||
| Pre (n = 21) | 8.5 | 10.2 | ||
| Post (n = 30) | 10.2 | 11.8 | ||
| Tumor size | 0.003 | 0.001 | ||
| ≤2 cm (n = 18) | Not reached | 25.4 | ||
| >2 cm (n = 33) | 4.2 | 7.5 | ||
| Nodal status | 0.036 | 0.017 | ||
| Negative (n = 22) | 17.9 | 17.2 | ||
| Positive (n = 29) | 4.0 | 6.7 | ||
| ER status | 0.912 | 0.374 | ||
| Negative (n = 14) | 8.1 | 9.8 | ||
| Positive (n = 30) | 10.2 | 12.9 | ||
| Unknown (n = 7) | 8.5 | 7.6 | ||
| Treatment arm | 0.064 | 0.103 | ||
| MRM (n = 24) | 17.9 | 14.9 | ||
| BCT (n = 27) | 4.2 | 7.6 | ||
| Chemotherapy use | 0.015 | 0.074 | ||
| No (n = 25) | 17.9 | 14.4 | ||
| Yes (n = 26) | 4.0 | 7.1 | ||
| CD44 expression | 0.171 | 0.049 | ||
| Negative (n = 29) | 4.3 | 8.6 | ||
| Positive (n = 22) | 17.9 | 18.9 |
Median PFS and OS values are Kaplan–Meier estimates. p values obtained using Log Rank test
Bold values indicate a statiscally significant result (i.e., p< 0.05)
Fig. 2.
Progression-free survival trend correlates with CD44 positivity. After grouping by CD44 expression, Kaplan–Meier curves for 51 patients demonstrate a trend toward improved progression-free survival. Log Rank test reveals p = 0.171
Fig. 3.
Improved OS correlates with CD44 positivity. After grouping by CD44 expression, Kaplan–Meier curves for 51 patients demonstrate improved overall survival. Log Rank test reveals p = 0.049
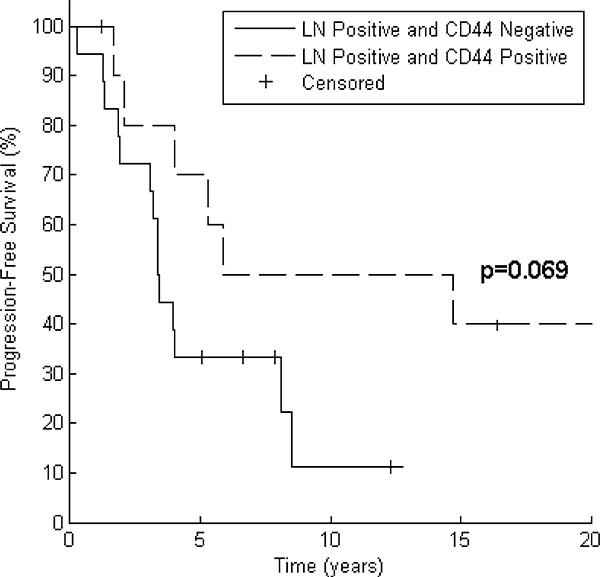
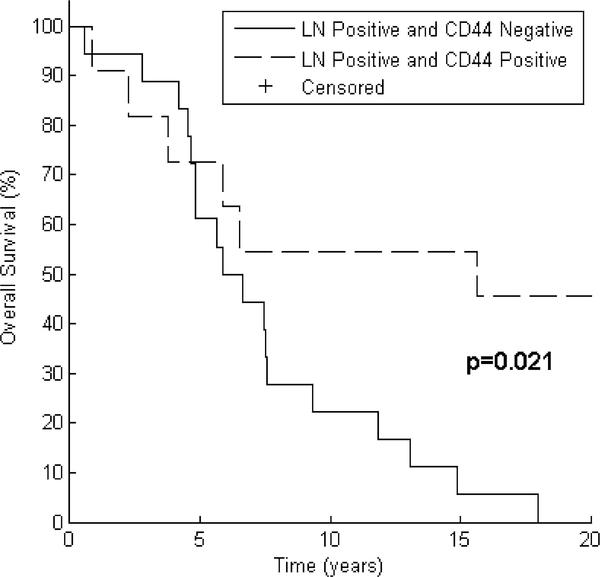
Table 3 depicts the results of testing CD44 expression as a predictor of PFS and OS after grouping patients by tumor size and nodal status. In all patient subsets, median PFS and OS were numerically greater for CD44 positive patients than for CD44 negative patients. This relationship reached statistical significance in the PFS analysis for the subset of patients with tumor size ≤2 cm (median PFS not reached years for CD44 positive vs. 11.0 years for CD44 negative, p = 0.019). CD44 expression was also associated with a significant increase in OS in the subset of patients with lymph node involvement (median OS 15.6 years for CD44 positive vs. 6.3 years for CD44 negative, p = 0.021). Node positive patients who were CD44 positive also experienced prolonged progression-free survival (median PFS 10.3 years for CD44 positive vs. 3.4 years for CD44 negative), but this did not reach statistical significance (p = 0.069). These results are displayed graphically in Figs. 4 and 5.
Table 3.
Tests of CD44 expression as a predictor of PFS and OS after grouping patients by tumor size and nodal status
| Characteristic | Median PFS (years) | p | Median OS (years) | p |
|---|---|---|---|---|
| Tumor size ≤2 cm | 0.019 | 0.133 | ||
| CD44 negative (n = 8) | 11.0 | 9.4 | ||
| CD44 positive (n = 10) | Not reached | Not reached | ||
| Tumor size >2 cm | 0.800 | 0.346 | ||
| CD44 negative (n = 21) | 3.9 | 7.5 | ||
| CD44 positive (n = 12) | 5.3 | 8.4 | ||
| Node negative | 0.997 | 0.776 | ||
| CD44 negative (n = 11) | 17.1 | 14.4 | ||
| CD44 positive (n = 11) | 17.9 | 22.2 | ||
| Node positive | 0.069 | 0.021 | ||
| CD44 negative (n = 18) | 3.4 | 6.3 | ||
| CD44 positive (n = 11) | 10.3 | 15.6 |
Median PFS and OS values are Kaplan–Meier estimates. p values obtained using Log Rank test
Bold values indicate a statiscally significant result (i.e., p< 0.05)
Fig. 4.
Lymph node positive patients with CD44 positivity have a trend toward improved progression-free survival. After grouping by CD44 expression, Kaplan–Meier curves for 29 patients demonstrate a trend toward improved progression-free survival in patients with nodal involvement. Log Rank test reveals p = 0.069
Fig. 5.
Lymph node positive patients with CD44 positivity have improved overall survival. After grouping by CD44 expression, Kaplan–Meier curves for 29 patients demonstrate improved OS in patients with nodal involvement. Log Rank test reveals p = 0.021
Discussion
Breast cancer is one of the few disease entities in which personalized treatment based on molecular subtypes has led to significant improvements in OS [24]. Despite having excellent prognosis overall, patients with early stage breast cancer have tremendous variation in clinical outcomes. A simple, protein-based assay would be useful in identifying high risk patients, particularly those at risk for late events. By identifying patients better suited for adjuvant treatment, opportunities for treatment de-escalation and toxicity sparing are also made available for investigation.
In this study, CD44 expression was found to be a long-term predictor of OS in patients with early stage breast cancer enrolled on a large, randomized, prospective trial. Interestingly, patient subgroup analysis revealed that CD44 positivity was most strongly associated with increased survival in the patients with lymph node involvement, suggesting a possible protective effect of CD44 expression in patients with advanced disease. This has been previously reported in other studies [25, 26]. In addition, this difference in survival was noted only with long-term follow up, with a significant amount of recurrences occurring after 5 years. A survival benefit correlating with CD44 overexpression was validated using gene expression data and survival information of an independent cohort of 1,809 patients. A statistically significant increase in survival in lymph node positive patients in this independent cohort was observed by use of a publicly available integrative data analysis tool [27]. Currently, management of the axilla in early stage disease remains a controversial topic, with studies supporting de-escalation of nodal therapy [28], and others supporting more comprehensive treatment [29]. Biomarkers used in node positive setting would be particularly helpful to stratify patients most likely to benefit from more aggressive treatment.
Although, CD44 has been studied with respect to its association with tumor initiating stem cells in breast cancer [10], it has also been found to be intimately involved in several processes of the cell cytoskeleton and surrounding extracellular matrix. CD44 is a known cell surface receptor for hyaluronic acid and may have complex interactions between tumor suppression and metastasis promotion involving ligand binding, coreceptor signaling, and maintenance of the actin cytoskeleton [30]. Recently, it has been found that CD44-mediated binding of high molecular weight hyaluronic acid may be responsible for early contact inhibition and unusual cancer resistance in the genus Heterocephalus [31].
There has been considerable variation in the literature regarding the prognostic value of CD44. Early studies by Al-Hajj and colleagues identified CD44 as a marker for stem cell-like progenitor breast cancer cells [10], and this has been recapitulated in several in vitro studies [13, 32–34]. However, clinico-pathological correlation in human studies has been more equivocal. While some studies have demonstrated negative prognosis associated with CD44 [16, 18, 35, 36], others have demonstrated CD44 to be independent predictor of both prolonged breast cancer specific survival and metastasis free survival [14]. This positive correlation in survival has also been demonstrated in the node-negative setting as well [17]. In many of these studies, short-term follow up from heterogeneous patient populations was investigated, potentially obscuring results. The current study is unique in that the patient population was derived from a prospective trial with over 25 years of follow up, representing a uniform population of patients with early stage disease meeting specific entry criteria.
Alternative splicing of CD44 into its variant forms may be responsible for the conflicting results in the literature. CD44 is encoded by a 20-exon gene, and can be expressed in standard form (CD44s) or undergo alternative splicing resulting in the production of at least 12 variant isoforms (CD44v1–12) [37]. Several of the variant isoforms have been linked to poor prognosis in animal models [12, 34, 38] and in clinical series [39, 40]. Expression of the CD44v6 isoform has received the most attention to date, with evidence suggesting inferior outcomes in breast cancer [41–43]. However, other studies have shown conflicting data in this arena as well [44, 45]. All variants of CD44 are noted to share a 90 AA amino terminal domain [46], with the stem portion composing the variant isoforms. The standard variant of CD44 is noted to be smallest isoform and contains only this common amino terminal region in its extracellular domain. Interactions through this domain are thought to principally mediate its interactions with hyaluronan [9]. In the current study, a monoclonal antibody to the standard (CD44s) isoform of CD44 was used. In a murine model of knockout CD44s and its variants, implantation of an orthotopic breast tumor resulted in significantly increased pulmonary metastases [47]. Deficiencies in CD44 expression are therefore thought to increase the risk of disease metastasis by disrupting the interactions between CD44 and hyaluronan [48].
Currently, several clinical trials are investigating the role anti-CD44 therapies in the context of cancer treatment [49–53]. Based on our findings and conflicting data in the literature, it should be cautioned that therapeutics targeting variant isoforms of CD44 or stem cell precursors expressing CD44 may exhibit cross reactivity between the constitutively expressed interactive hyaluronan domain and its common epitopes. In multiple murine models of cancer, silencing of CD44 was shown to promote metastasis [47, 54, 55]. Due to the variable data in the literature regarding the role of CD44, careful consideration should be made regarding the inhibition of CD44 and its downstream pathways. Given the common pathways involved in CD44-mediated pathways, future research is needed to investigate the role of each variant isoform and potential downstream effector molecules from these pathways that can be more specifically targeted.
Some limitations of the current study include its retrospective design with a small sample size due to the extended period of follow up. In addition, due to the archival nature of the specimens, it was not technically feasible to perform double staining in order to identify the CD44+/CD24− phenotype commonly associated with breast cancer stem cells [10, 35].
In summary, the current study has linked expression of the standard isoform of CD44 in early stage breast cancer patients with improved OS in the setting of extended follow up. CD44 expression appears to be a significant prognostic marker in early stage patients and may be useful in determine long-term outcomes, warranting future investigation of this molecule. Given the conflicting literature, however, caution should be exercised in the usage of future therapeutics targeting the CD44 receptor due to the extensive overlap with other tumor regulating pathways.
Supplementary Material
Acknowledgments
Research support was in part through the Intramural Research Program of the NIH and also in part by the Kimmel Cancer Center’s NCI Cancer Center Support Grant P30 CA56036.
Footnotes
Conflict of interest The authors declare that they have no conflicts of interest.
Ethical standards IRB approval from the National Cancer Institute was obtained for this study.
Electronic supplementary material The online version of this article (doi:10.1007/s10549-013-2758-9) contains supplementary material, which is available to authorized users.
Contributor Information
T. Dan, Department of Radiation Oncology, Bodine Center for Cancer Treatment, Kimmel Cancer Center, Jefferson Medical College of Thomas Jefferson University, 111 S. 11th Street G-301G, Philadelphia, PA 19107, USA
S. M. Hewitt, Pathology Branch, National Cancer Institute, Bethesda, MD, USA
N. Ohri, Department of Radiation Oncology, Bodine Center for Cancer Treatment, Kimmel Cancer Center, Jefferson Medical College of Thomas Jefferson University, 111 S. 11th Street G-301G, Philadelphia, PA 19107, USA
D. Ly, Radiation Oncology Branch, National Cancer Institute, Bethesda, MD, USA
B. P. Soule, Radiation Biology Branch, National Cancer Institute, Bethesda, MD, USA
S. L. Smith, Radiation Oncology Branch, National Cancer Institute, Bethesda, MD, USA
K. Matsuda, Pathology Branch, National Cancer Institute, Bethesda, MD, USA
C. Council, Radiation Oncology Branch, National Cancer Institute, Bethesda, MD, USA
U. Shankavaram, Radiation Oncology Branch, National Cancer Institute, Bethesda, MD, USA
M. E. Lippman, University of Miami Miller School of Medicine, Miami, FL, USA
J. B. Mitchell, Radiation Biology Branch, National Cancer Institute, Bethesda, MD, USA
K. Camphausen, Radiation Oncology Branch, National Cancer Institute, Bethesda, MD, USA
N. L. Simone, Department of Radiation Oncology, Bodine Center for Cancer Treatment, Kimmel Cancer Center, Jefferson Medical College of Thomas Jefferson University, 111 S. 11th Street G-301G, Philadelphia, PA 19107, USA Radiation Oncology Branch, National Cancer Institute, Bethesda, MD, USA.
References
- 1.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL,Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826. doi: 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 2.Drukker CA, Bueno-de-Mesquita JM, Retèl VP, van Harten WH, van Tinteren H, Wesseling J, Roumen RMH, Knauer M, van ‘t Veer LJ, Sonke GS, Rutgers EJT, van de Vijver MJ, Linn SC (2013) A prospective evaluation of a breast cancer prognosis signature in the observational RASTER study. Int J Cancer 133(4):929–936. doi: 10.1002/ijc.28082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouzier R, Pronzato P, Chéreau E, Carlson J, Hunt B, Valentine WJ (2013) Multigene assays and molecular markers in breast cancer: systematic review of health economic analyses. Breast Cancer Res Treat 139(3):621–637. doi: 10.1007/s10549-013-2559-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weldon CB, Trosman JR, Gradishar WJ, Benson AB 3rd, Schink JC (2012) Barriers to the use of personalized medicine in breast cancer. J Oncol Pract 8(4):e24–e31. doi: 10.1200/JOP.2011.000448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology (1996). J Clin Oncol 14 (10):2843–2877 [DOI] [PubMed] [Google Scholar]
- 6.Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364 [DOI] [PubMed] [Google Scholar]
- 7.Lips EH, Mulder L, Ronde JJ, Mandjes IAM, Koolen BB, Wessels LFA, Rodenhuis S, Wesseling J (2013) Breast cancer subtyping by immunohistochemistry and histological grade outperforms breast cancer intrinsic subtypes in predicting neoadjuvant chemotherapy response. Breast Cancer Res Treat 140(1):63–71. doi: 10.1007/s10549-013-2620-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inwald EC, Klinkhammer-Schalke M, Hofstadter F, Zeman F,Koller M, Gerstenhauer M, Ortmann O (2013) Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat 139(2):539–552. doi: 10.1007/s10549-013-2560-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherman L, Sleeman J, Herrlich P, Ponta H (1994) Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol 6(5):726–733 [DOI] [PubMed] [Google Scholar]
- 10.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7):3983–3988. doi: 10.1073/pnas.0530291100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG (2005) Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 65(13):5506–5511. doi: 10.1158/0008-5472.CAN-05-0626 [DOI] [PubMed] [Google Scholar]
- 12.Afify A, Purnell P, Nguyen L (2009) Role of CD44s and CD44v6 on human breast cancer cell adhesion, migration, and invasion. Exp Mol Pathol 86(2):95–100. doi: 10.1016/j.yexmp.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 13.Hiraga T, Ito S, Nakamura H (2013) Cancer stem-like cell marker CD44 promotes bone metastases by enhancing tumorigenicity, cell motility, and hyaluronan production. Cancer Res 73(13):4112–4122. doi: 10.1158/0008-5472.can-12-3801 [DOI] [PubMed] [Google Scholar]
- 14.Ahmed MAH, Aleskandarany MA, Rakha EA, Moustafa RZA, Benhasouna A, Nolan C, Green AR, Ilyas M, Ellis IO (2011) A CD44−/CD24+ phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res Treat 133(3):979–995. doi: 10.1007/s10549-011-1865-8 [DOI] [PubMed] [Google Scholar]
- 15.Friedrichs K, Franke F, Lisboa BW, Kugler G, Gille I, Terpe HJ,Holzel F, Maass H, Gunthert U (1995) CD44 isoforms correlate with cellular differentiation but not with prognosis in human breast cancer. Cancer Res 55(22):5424–5433 [PubMed] [Google Scholar]
- 16.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H (2005) Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res 11(3):1154–1159 [PubMed] [Google Scholar]
- 17.Diaz LK, Zhou X, Wright ET, Cristofanilli M, Smith T, Yang Y, Sneige N, Sahin A, Gilcrease MZ (2005) CD44 expression is associated with increased survival in node-negative invasive breast carcinoma. Clin Cancer Res 11(9):3309–3314. doi: 10.1158/1078-0432.CCR-04-2184 [DOI] [PubMed] [Google Scholar]
- 18.Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, Nakopoulou L (2008) The clinicopathologic and prognostic significance of CD44+/CD24(−/low) and CD44−/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol 39(7):1096–1102. doi: 10.1016/j.humpath.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Kim MJ, Ahn SH, Son BH, Kim SB, Ahn JH, Noh WC, Gong G (2011) Different prognostic significance of CD24 and CD44 expression in breast cancer according to hormone receptor status. Breast 20(1):78–85. doi: 10.1016/j.breast.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 20.Bane A, Viloria-Petit A, Pinnaduwage D, Mulligan AM, O’Malley FP, Andrulis IL (2013) Clinical–pathologic significance of cancer stem cell marker expression in familial breast cancers. Breast Cancer Res Treat 140(1):195–205. doi: 10.1007/s10549-013-2591-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100(2):229–235. doi: 10.1007/s10549-006-9242-8 [DOI] [PubMed] [Google Scholar]
- 22.Simone NL, Dan T, Shih J, Smith SL, Sciuto L, Lita E, Lippman ME, Glatstein E, Swain SM, Danforth DN, Camphausen K (2011) Twenty-five-year results of the national cancer institute randomized breast conservation trial. Breast Cancer Res Treat. doi: 10.1007/s10549-011-1867-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson JA, Danforth DN, Cowan KH, d’Angelo T, Steinberg SM, Pierce L, Lippman ME, Lichter AS, Glatstein E, Okunieff P (1995) Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med 332(14):907–911. doi: 10.1056/NEJM199504063321402 [DOI] [PubMed] [Google Scholar]
- 24.Senn H-J (2013) St. Gallen Consensus 2013: optimizing and personalizing primary curative therapy of breast cancer worldwide. Breast Care 8(2):101. doi: 10.1159/000351222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adamczyk A, Niemiec JA, Ambicka A, Mucha-Malecka A, Mitus J, Rys J (2013) CD44/CD24 as potential prognostic markers in node-positive invasive ductal breast cancer patients treated with adjuvant chemotherapy. J Mol Histol. doi: 10.1007/s10735-013-9523-6 [DOI] [PubMed] [Google Scholar]
- 26.Horiguchi K, Toi M, Horiguchi S, Sugimoto M, Naito Y, Hayashi Y, Ueno T, Ohno S, Funata N, Kuroi K, Tomita M, Eishi Y (2010) Predictive value of CD24 and CD44 for neoadjuvant chemotherapy response and prognosis in primary breast cancer patients. J Med Dent Sci 57(2):165–175 [PubMed] [Google Scholar]
- 27.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z (2010) An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 123(3):725–731. doi: 10.1007/s10549-009-0674-9 [DOI] [PubMed] [Google Scholar]
- 28.Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305(6):569–575. doi: 10.1001/jama.2011.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whelan NCIC-CTGMA.20: An intergroup trial of regional nodal irradiation in early breast cancer. Journal of Clinical Oncology, 2011 ASCO Annual Meeting Proceedings (Post-Meeting Edition). Vol 29, No 18_suppl (June 20 Supplement), 2011: LBA1003 [Google Scholar]
- 30.Tsukita S, Oishi K, Sato N, Sagara J, Kawai A (1994) ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol 126(2):391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A (2013) High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499(7458):346–349. doi: 10.1038/nature12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsang JYS, Huang Y-H, Luo M-H, Ni Y-B, Chan S-K, Lui PCW, Yu AMC, Tan PH, Tse GM (2012) Cancer stem cell markers are associated with adverse biomarker profiles and molecular subtypes of breast cancer. Breast Cancer Res Treat 136(2):407–417. doi: 10.1007/s10549-012-2271-6 [DOI] [PubMed] [Google Scholar]
- 33.Wright MH, Calcagno A, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L (2008) Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res 10(1):R10. doi: 10.1186/bcr1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagano O, Okazaki S, Saya H (2013) Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene. doi: 10.1038/onc.2012.638 [DOI] [PubMed] [Google Scholar]
- 35.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, Grabau D, Ferno M, Borg A, Hegardt C (2008) The CD44+/CD24− phenotype is enriched in basal-like breast tumors. Breast Cancer Res 10(3):R53. doi: 10.1186/bcr2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer S, Hausen A, Watermann DO, Stamm S, Jäger M, Gitsch G, Stickeler E (2008) Increased soluble CD44 concentrations are associated with larger tumor size and lymph node metastasis in breast cancer patients. J Cancer Res Clin Oncol 134(11):1229–1235. doi: 10.1007/s00432-008-0397-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI (1992) Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci USA 89(24):12160–12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinn HP, Heider KH, Skroch-Angel P, von Minckwitz G, Kaufmann M, Herrlich P, Ponta H (1995) Human mammary carcinomas express homologues of rat metastasis-associated variants of CD44. Breast Cancer Res Treat 36(3):307–313 [DOI] [PubMed] [Google Scholar]
- 39.Kaufmann M, Heider KH, Sinn HP, von Minckwitz G, Ponta H, Herrlich P (1995) CD44 variant exon epitopes in primary breast cancer and length of survival. Lancet 345(8950):615–619. doi:S0140–6736(95)90521–9 [DOI] [PubMed] [Google Scholar]
- 40.Ma W, Deng Y, Zhou L (2005) The prognostic value of adhesion molecule CD44v6 in women with primary breast carcinoma: a clinicopathologic study. Clin Oncol 17(4):258–263. doi: 10.1016/j.clon.2005.02.007 [DOI] [PubMed] [Google Scholar]
- 41.Tempfer C, Losch A, Heinzl H, Hausler G, Hanzal E, Kolbl H, Breitenecker G, Kainz C (1996) Prognostic value of immunohistochemically detected CD44 isoforms CD44v5, CD44v6 and CD44v7–8 in human breast cancer. Eur J Cancer 32A(11):2023–2025 [DOI] [PubMed] [Google Scholar]
- 42.Afify A, McNiel MA, Braggin J, Bailey H, Paulino AF (2008) Expression of CD44s, CD44v6, and hyaluronan across the spectrum of normal-hyperplasia-carcinoma in breast. Appl Immunohistochem Mol Morphol 16(2):121–127. doi: 10.1097/PAI.0b013e318047df6d [DOI] [PubMed] [Google Scholar]
- 43.Yu P, Zhou L, Ke W, Li K (2010) Clinical significance of pAKT and CD44v6 overexpression with breast cancer. J Cancer Res Clin Oncol 136(8):1283–1292. doi: 10.1007/s00432-010-0779-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foekens JA, Dall P, Klijn JG, Skroch-Angel P, Claassen CJ, Look MP, Ponta H, Van Putten WL, Herrlich P, Henzen-Logmans SC (1999) Prognostic value of CD44 variant expression in primary breast cancer. Int J Cancer 84(3):209–215. doi: [DOI] [PubMed] [Google Scholar]
- 45.Jansen RH, Joosten-Achjanie SR, Arends JW, Volovics A, Hupperets PS, Schouten HC, Hillen HF (1998) CD44v6 is not a prognostic factor in primary breast cancer. Ann Oncol 9(1):109–111 [DOI] [PubMed] [Google Scholar]
- 46.Ponta H, Sherman L, Herrlich PA (2003) CD44: From adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4(1):33–45. doi: 10.1038/nrm1004 [DOI] [PubMed] [Google Scholar]
- 47.Lopez JI, Camenisch TD, Stevens MV, Sands BJ, McDonald J, Schroeder JA (2005) CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res 65(15):6755–6763. doi: 10.1158/0008-5472.CAN-05-0863 [DOI] [PubMed] [Google Scholar]
- 48.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, Wakeham A, Shahinian A, Catzavelos C, Rak J, Furlonger C, Zakarian A, Simard JJ, Ohashi PS, Paige CJ, Gutierrez-Ramos JC, Mak TW (1997) CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood 90(6):2217–2233 [PubMed] [Google Scholar]
- 49.McClements L, Yakkundi A, Papaspyropoulos A, Harrison H, Ablett MP, Jithesh PV, McKeen HD, Bennett R, Donley C, Kissenpfennig A, McIntosh S, McCarthy HO, O’Neill E, Clarke RB, Robson T (2013) Targeting treatment-resistant breast cancer stem cells with FKBPL and its peptide derivative, AD-01, via the CD44 pathway. Clin Cancer Res 19(14):3881–3893. doi: 10.1158/1078-0432.ccr-13-0595 [DOI] [PubMed] [Google Scholar]
- 50.Koppe M, Schaijk F, Roos J, Leeuwen P, Heider KH, Kuthan H, Bleichrodt R (2004) Safety, pharmacokinetics, immunogenicity, and biodistribution of (186)Re-labeled humanized monoclonal antibody BIWA 4 (Bivatuzumab) in patients with early-stage breast cancer. Cancer Biother Radiopharm 19(6):720–729. doi: 10.1089/cbr.2004.19.720 [DOI] [PubMed] [Google Scholar]
- 51.Liu S, Wicha MS (2010) Targeting breast cancer stem cells. J Clin Oncol 28(25):4006–4012. doi: 10.1200/JCO.2009.27.5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orian-Rousseau V (2010) CD44, a therapeutic target for metastasising tumours. Eur J Cancer 46(7):1271–1277. doi: 10.1016/j.ejca.2010.02.024 [DOI] [PubMed] [Google Scholar]
- 53.Rupp U, Schoendorf-Holland E, Eichbaum M, Schuetz F, Lauschner I, Schmidt P, Staab A, Hanft G, Huober J, Sinn HP, Sohn C, Schneeweiss A (2007) Safety and pharmacokinetics of bivatuzumab mertansine in patients with CD44v6-positive metastatic breast cancer: final results of a phase I study. Anticancer Drugs 18(4):477–485. doi: 10.1097/CAD.0b013e32801403f4 [DOI] [PubMed] [Google Scholar]
- 54.Gvozdenovic A, Arlt MJ, Campanile C, Brennecke P, Husmann K, Born W, Muff R, Fuchs B (2013) Silencing of CD44 gene expression in human 143-B osteosarcoma cells promotes metastasis of intratibial tumors in SCID mice. PLoS ONE 8(4):e60329. doi: 10.1371/journal.pone.0060329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao AC, Lou W, Dong JT, Isaacs JT (1997) CD44 is a metastasis suppressor gene for prostatic cancer located on human chromosome 11p13. Cancer Res 57(5):846–849 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.