Highlights
-
•
NK cell infiltration to solid tumors independently predicts improved OS.
-
•
We reviewed 53 studies and meta-analyzed hazard ratios from 30.
-
•
Meta-analysis revealed that NK cell infiltration predicts decreased risk of death.
-
•
NK prognostic value is related to identifying marker and subtumor location.
-
•
NK cell infiltration may be associated with tumor stage and grade.
Keywords: Natural killer cells, Solid tumor, Tumor infiltration, Tumor microenvironment, Immuno-oncology
Abstract
The immune landscape of a tumor is highly connected to patient prognosis and response to treatment, but little is known about how natural killer (NK) cells predict overall survival (OS) among patients with solid tumors. We present the first meta-analysis on NK cell infiltration into solid tumors as a prognostic indicator for OS, considering cancer types independently, and together. Samples were collected from 1973 to 2016 with results published between 1989 and 2020. From 53 studies, we found that NK cell infiltration corresponds with decreased risk of death (HR=0.34, 95% CI: 0.26–0.46; p<0.0001). Among studies that investigated the prognostic potential of NK cells in specific regions of the tumor, intraepithelial infiltration was better predictive of OS than NK infiltration in the tumor-adjacent stroma. Generally, NK cell infiltration is lower in advanced-stage and lower-grade tumors; nevertheless, it remains prognostically beneficial. This meta-analysis highlights an important prognostic role of NK cells in solid tumors, but exposes that few studies have considered the contributions of NK cells. Toward NK cell-based immunotherapies, it will be important to understand the conditions under which NK cells can be effective agents of tumor control.
Introduction
Infiltration of solid tumors by immune cells with anti-tumor activity is both a strong prognostic factor and a therapeutic goal1. Specific immune populations can have anti- or pro-tumor roles; the balance of their activity conditions the tumor “microenvironment” (TME) and can predict responses to treatment and overall survival (OS)2. Characteristics of the tumor itself, including underlying mutations, progression (stage and grade), vascularization, metabolism and the soluble factors it produces also contribute to the TME with impacts on immune cell infiltration and activation3,4. Interactions in the TME are complex, and identifying key features for prognostic and therapeutic targeting is key to developing effective immunotherapies for solid tumors. Here, we present a meta-analysis that demonstrates NK cell infiltration is correlated with decreased risk of death across solid tumor origins, grades and stages.
Tumors can be considered as two structural compartments: the epithelial region, which encompasses the malignant cells within an epithelial lining, and the stromal region, which represents its supportive tissue5. The TME and immune reactivity varies within these compartments, which can result in different functional associations for the same leukocyte population. For example, a study conducted in early-stage tongue cancer found lymphocytes infiltrating the intraepithelial compartment frequently expressed the immune checkpoint receptors, PD-1 and NKG2A, but their counterparts in the stroma did not6. This underscores the importance of quantifying immune cells within the context of tumor compartments, which requires in situ analysis of intact tissue.
Select immune cell populations, mainly T cells, have been the focus in immuno-oncology7. A number of systematic reviews and meta-analyses have been conducted to explore the prognostic value of T cell infiltration in a variety of solid tumors[8], [9], [10]. T cell infiltration generally predicts better survival, and further phenotyping of T cell subsets can reveal more informative associations11. For example, infiltration of regulatory T cells (FOXP3+) can be associated with both improved and poorer survival, while the infiltration of cytotoxic T cells (CD8+) is strongly positively correlated with improved OS[8], [9], [10], [11]. Other assessments have revealed prognostic benefits of B cells and M1 macrophages but relatively few studies, in specific cancer subtypes, have analyzed the impact of other lymphocyte populations, including NK cells[11], [12], [13], [14], [15]. As innate controllers of cancer and emergent targets for immunotherapy, understanding the prognostic value of NK cells in solid tumors is overdue.
NK cells are innate lymphocytes that originate in the bone marrow from the common lymphoid progenitor and comprise 5–15% of the total peripheral lymphocyte population16. They can adapt in response to challenge, but do not require sensitization or specific antigens to mount an effective immune response. NK cells recognize danger signals, or stress induced ligands, upregulated in response to DNA damage17, trauma or proinflammatory cytokines18, which may underlie tumor development. Human NK cells are most often defined and experimentally marked as CD56+CD3− cells16, and broadly characterized based on CD56 expression as either circulating, cytokine-producing NK cells (CD57lowCD56bright/CD16−), or tissue-infiltrating, cytotoxic NK cells (CD57brightCD56dim/CD16+)[19], [20], [21]. In reality, NK cells are a diverse collection of functionally dynamic lymphocytes, with up to 30,000 unique NK cell phenotypes comprising the repertoire of each individual22.
NK cells express and co-express an array of germline-encoded receptors to engage with putative target cells18,23. The outcomes of these interactions can be activating, regulatory or inhibitory; “self” human leukocyte antigen (HLA) is a major signal for inhibition19,20. Since HLA negatively regulates NK cell activation, loss of “self” HLA lowers the threshold for NK cell activation. This function is important in recognition of tumors. Additional and important anti-tumor roles played by NK cells involve conditioning of the TME for inflammation, and antibody-dependent cell cytotoxicity (ADCC) to facilitate tumor control by monoclonal antibody therapies such as Trastuzumab24, Dinutuximab[25], [26], [27], or Cetuximab28.
The ability of NK cells to control cancer is underscored by their importance in hematopoietic cell transplantation, where NK immunogenetic configurations predict leukemia control or relapse29,30. These observations prompted clinical trials based on adoptive transfer of unmodified or ex vivo-expanded NK cells31, including NK cells genetically modified to express a chimeric antigen receptor (CAR)32. Current clinical trials investigating adoptive NK cell transfer to treat solid tumors include melanoma (NCT00328861, NCT03470922), kidney cancer (NCT00328861), head and neck cancers (NCT02643550), glioblastoma (NCT02658981), gynecologic malignancies (NCT02459301), and other metastatic (NCT03415100) and non-metastatic solid tumors (NCT01875601, NCT01212341, NCT03940820, NCT02671435, NCT01968109). Compared with hematologic malignancies, solid tumors may present an additional challenge, where infiltration will be key to tumor control33.
We retrieved, compiled and meta-analyzed studies that associated NK cell infiltration with survival outcomes. We found that increased NK cell infiltration is associated with a decreased risk of dying in patients with solid tumors. Our findings highlight associations between OS and NK cell sub-tumor localization, grade and stage, endorse assessments of NK cells for prognostication of solid tumors and inform more precise NK-based immunotherapy.
Methodology
Data sources and search strategy
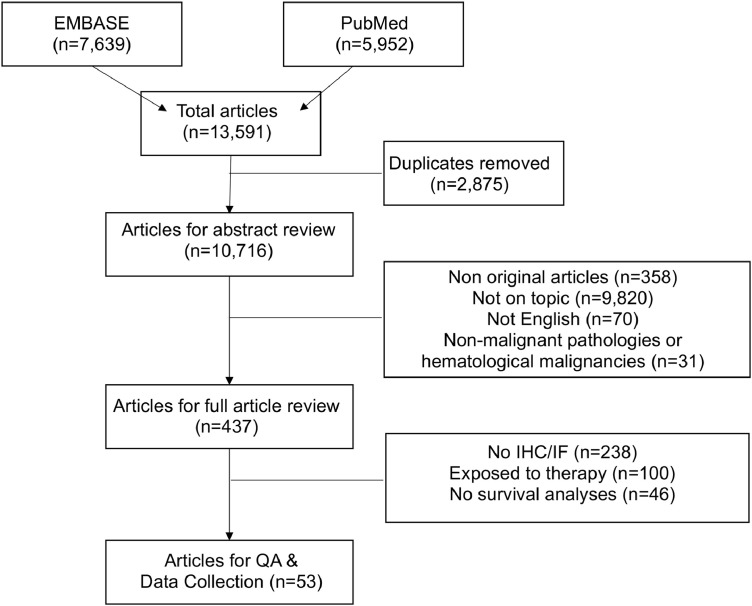
We devised a comprehensive search strategy based on the following three key terms (1) prognostic value, (2) natural killer cell and (3) tumor. Terms used to represent prognostic value included mortality, survival or outcome. Natural killer cells were searched using the terms natural killer cells, NK cells or innate lymphoid cells. To limit our search to those applicable to cancer we searched tumor (tumor), tumor (tumor) infiltration or neoplasm (for full search terms, see Supplemental Data 1). We applied these search terms to EMBASE and PubMed on February 11th, 2020. Through our search we captured a total of 13,591 peer-reviewed studies; 7639 from EMBASE and 5952 from PubMed (Fig. 1). The title, author and study details, from each database were exported and pooled in Microsoft Excel 365.
Fig. 1.
Flow-diagram outlining the process of study selection.
Eligibility criteria
Once a final list of articles was obtained and duplicates removed, 10,716 studies remained. These studies then underwent abstract review to assess article suitability on the following exclusion criteria: not written in English, not full primary research articles or not within the scope of this review (not on topic) were removed (i.e. studies in animals, on nucleic acids exclusively or that otherwise did not meet our requirement for NK cell evaluation in solid human tumors). Following this, the remaining articles were assessed to ensure they met all of the inclusion criteria: 1) employed immunohistochemistry (IHC) for NK cells using an appropriate marker/markers on intact solid tumor tissue; 2) studied treatment-naïve adult tumors; and 3) reported an endpoint survival analyses with correlated NK cell infiltration data.
Data extraction and quality assessment
From each of the final articles (n = 53), we collected the following information: name of first author, DOI, year of publication, number of patients, female:male ratio (biologic sex), tumor stage, tumor grade, NK cell marker used for IHC, other markers analyzed, method of NK cell quantification and stratification (definition of “high” versus “low” NK infiltration), mean number of NK cells, hazard ratio (HR) for death (95% CI and p-value) and survival-based outcome (OS), disease-free survival (DFS), progression-free survival (PFS) and associated p-values (Table 1). The overall quality of the article was evaluated but due to the low number of studies, we did not exclude studies based on quality assessment (Table 1 and Supplementary Table 1). The studies were given a quality score of 12 based on the inclusion of: subtype identification, sex ratios, age (average or range), stage identification, period of cohort collection, ethics reported, >1 IHC antibody, quantification strategy, stratification strategy, pathologist validation, HR for NK cell infiltration, and p-value for NK cell infiltration (Supplemental Table 1).
Table 1.
Study characteristics. (Studies conducted on multiple cancer tissue sites were separated into respective cancer categories).
| Study | Year | Period of Sample Collection | Cancer Type | Cancer Subtype | n | Sex Ratio (F:M) | Age (Mean (Range)) | NK cell Marker (clone) | Stratification | NK Cell Number | HR of Death (95% CI, p-value), multivariate analysis | Impact on survival High NK cell population |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cho et al.34 | 2003 | 1989 – 1999 | Head & Neck Cancer | Esophageal squamous cell carcinoma | 122 | 17:105 | 62.3 (NR) | CD57 (Leu 7) | Quantified in the stroma into 4 groups: • most abundant • abundant • moderate • scanty |
Median = 0.9NK cells/ HPF | NR | No change, OS (p = 0.47) |
| Fang et al.35 | 2017 | 2007 – 2009 | Head & Neck Cancer | Oral squamous cell carcinoma | 78 | 21:57 | 60 (24–82) | CD57 (ab82749) | Mean NK cell number: • 〈 15.75 NK cells • 〉 15.75 NK cells |
Median = 15.75 cells/ HPF | NR | Improved, OS (p < 0.001) |
| Hsia et al.36 | 2005 | 1994 – 1996 | Head & Neck Cancer | Esophageal squamous cell carcinoma | 38 | 0:38 | NR | CD57 (NR) | Median NK cell number: • 〈 25 NK cells/ 25 fields • 〉 25 NK cells/ 25 fields |
Median = 25 cells/ 25 fields | 0.61 (0.21 – 1.82, p = 0.378) | Improved, OS (p = 0.007) |
| Lazaris et al.37 | 2007 | NR | Head & Neck Cancer | Laryngeal carcinoma | 31 | 1:30 | 61.3 (42–75) | CD56 (T119), CD16 (VIFcRII) | Quantified in the parenchyma: • low, <5% of lymphocytes • intermediate, 5–20% • high, >20% |
NR | NR | No change, DFS (p = 0.66) |
| Lu et al.38 | 2017 | 2002 – 2003 | Head & Neck Cancer | Nasopharyngeal carcinoma | 197 | 51:146 | 45.22 (NR) | CD56 (NR) | Median NK cell number | NR | 0.46 (0.27 – 0.77, p = 0.004) | Improved, OS (p – 0.001) |
| Lv et al.39 | 2011 | 2002 – 2003 | Head & Neck Cancer | Esophageal squamous cell carcinoma | 181 | 40:141 | 56 (33–79) | CD57 (NR) | Median NK cell number | NR | NR | Improved, OS (p = 0.002) |
| Schoenfeld et al.40 | 2017 | 2004 – 2013 | Head & Neck Cancer | Oropharyngeal squamous cell carcinoma | 81 | 17:64 | 64 (49–87) | CD56 (NR) | NK cell presence; • present • absent |
NR | NR | No change, OS (NR) |
| Svensson et al.41 | 2017 | 2006 – 2010 | Head & Neck Cancer | Esophageal squamous cell carcinoma | 97 | NR | NR | NKp46 (NR) | Median NK cell number | Median (based on age) = 1.89 (<avg age) or 1.93 (> avg age) NK cells/ field Median (based on gender) = 1.99 (female) or 1.89 (male) cells/ field |
0.49 (0.28 – 0.86, p = 0.012) | Improved, OS (p = 0.008) |
| Taghavi et al.42 | 2016 | NR | Head & Neck Cancer | Oral squamous cell carcinoma | 57 | 30:27 | 62.89 (34–91) | CD57 (2H7), CD16 (NK-1) | Median NK cell number: • low <25 cells/ 25 fields • high >25 cells/ 25 fields |
Median = 25 cells/ 25 fields | 0.058 (0.013 – 0.26, p <0.001) | Improved, OS (p = 0.001) |
| Tsuchikawa et al.43 | 2011 | 1989 – 1999 | Head & Neck Cancer | Esophageal squamous cell carcinoma | 98 | 14:84 | 62.9 (53.9–71.9) | CD57 (Leu 7) | Quantified in the stroma: • abundant • scanty |
Median = 0.9 NK cells/ 200x field | NR | No change, OS (p = 0.31) |
| Wagner et al.44 | 2016 | 2000 – 2009 | Head & Neck Cancer | Oropharyngeal squamous cell carcinoma | 140 | 34:105 | 59 (38–84) | CD56 (1B6) | Quantified as: • CD56+ tumor & stroma • CD56+ stroma • CD56+ tumor • CD56+ absent |
NR | 0.32 (0.10 – 0.96, p = 0.042) | Improved, OS (p = 0.038) |
| Xu et al.45 | 2016 | 2006 – 2011 | Head & Neck Cancer | Esophageal squamous cell carcinoma | 138 | 36:102 | NR | CD57 (NR) | Quantified in the stroma: • Gr 3 (massive infiltration) • Gr 2 (abundant infiltration) • Gr 1 (moderate infiltration) • Gr 0 (scanty) |
NR | 0.60 (0.39 – 0.91, p = 0.016) | Improved, OS (p = 0.019) |
| Zancope et al.46 | 2010 | NR | Head & Neck Cancer | Oral and lip squamous carcinoma, Oral: 40 Lip: 30 |
70 |
64:36 | NR | CD57 (NK1) | Median NK cell number | Oral Epi: 14 cells/mm2 Oral Str: 145 cells/mm2 Lip Epi: 32 cells/mm2 |
NR | No change, OS Intra-epithelial (p = 0.70) Stromal (p = 0.69) |
| Honkanen et al.47 | 2017 | 2009 – 2014 | Breast Cancer | HER2+ | 48 | 48:0 | NR | CD56 (MRQ-42) | Quantified as: • low <17 cells/mm2 • high >17 cells/mm2 |
NR | NR | No change, OS (NR) |
| Muntasell et al.48 | 2018 | 2008 – 2016 | Breast Cancer | HER2+ | 113 DC: 42 VC: 71 |
113:0 | 57 (36–88) | CD56 (123C3) |
Quantified as: • low <1 cell • high >1 cell |
DC: 1.5 cells/ 50x VC: 2 cells/ 50x |
DC: 0.07 (0.01 – 0.60, p = 0.01) VC: 0.30 (0.08 – 1.30, p = 0.10) |
Improved, DFS (DC: p = 0.01, VC: p = 0.10) |
| Park et al.49 | 2012 | 1997 – 2002 | Breast Cancer | Invasive ductal carcinoma | 204 | 204:0 |
46= 113 patients >46= 85 patients |
CD57 (TB01) | Quantified as: • low (absent) • High (otherwise) |
Str: 1.10 cells/ | NR | No change, OS (p = 0.167) DFS (p = 0.358) |
| Rathore et al.50 | 2014 | NR | Breast Cancer | Invasive ductal carcinoma | 175 | 175:0 | 49.13 (25–86) | CD56 (NR) | Quantified in Str and Epi each as: • low <25 cells/ 25 fields • high >25 cells/ 25 fields |
NR | 1.92 (1.08 – 3.57, p = 0.05) | Poorer, OS (p = 0.05) |
| Tian et al.51 | 2016 | 2006 – 2008 | Breast Cancer | Invasive ductal carcinoma | 278 | 278:0 | Mean NR (28–75) | NKp46 (ab199128) | Quantified in Str based on density: • 0 (absent) • 3 (dense) |
NR | 0.54 (0.39 – 0.74, p = 0.001) | Improved, OS (p = 0.018) Improved DFS, (p < 0.001) |
| Triki et al.52 | 2019 | NR | Breast Cancer | NR | 158 | 158:0 | 118 patients>40 40 patients <40 |
CD56 (1BC) | Quantified as: • low (negative or weak infiltration) • high (moderate or strong infiltration) |
NR | 0.17 (0.039 – 0.73, p = 0.017) | Improved, OS ER+: p = 0.007 PR+: p = 0.018 HER+ p = 0.287 |
| Vgenopoulou et al.53 | 2003 | NR | Breast Cancer | Invasive ductal carcinoma | 64 | 64:0 | NR | CD57 (TB01) | Quantified based on staining intensity as: • Weak-moderate • Strong |
NR | NR | No change, DFS (NR) |
| Wang et al.54 | 2014 | 2006 – 2007 | Breast Cancer | ALDH1 high | 212 high, 379 low | 591:0 | 49 (23–87) | CD56 (NR) | Quantified as: • CD56 low <5 CD56+ cells • CD56 high >5 CD56+ cells |
NR | 1.10 (0.50 – 2.44, p = 0.81) | No change, OS (NR) |
| ALDH1 low | 0.11 (0.014 – 0.81, p = 0.031) | |||||||||||
| Alderdice et al.55 | 2017 | DC: 2004 – 2013 VC: 2001 - 2005 |
Colorectal Cancer | Locally advanced rectal cancer | 150 | NR | NR | CD56 (NCL-l-CD56–1B6) | Quantified as: • 〈 4 CD56+ cells • 〉 4 CD56+ cells |
NR | 0.28 (0.11 – 0.73, p = 0.005) | Improved, OS (NR) |
| Coca et al.56 | 1997 | 1977–1990 | Colorectal Cancer | 157 | 76:110 | 63.4 (29–84) | CD57 (IOT-10, Immunotech, SA) | NK cell infiltration classified as little (<50 NK cells), moderate (50–150 NK cells), and extensive (>150 NK cells) | NR | NR | Improved, OS and DFS (p < 0.01) | |
| Lim et al.57 | 2014 | 1998 – 2007 | Colorectal Cancer | Locally advanced rectal carcinoma | 52 | 18:34 | 63 (NR) | CD56 (NR) CD57 (NR) |
Median NK cell number | Median = 12 NK cells/1200x | NR | No change, OS (NR) |
| Liska et al.58 | 2012 | 2004 – 2007 | Colorectal Cancer | 150 | 53:97 | 66.33 (NR) | CD57 (NK1) | Quantified as: • 〈 4 NK cells • 〉 4 NK cells |
NR | 0.4 | Improved, OS (p = 0.035) |
|
| Menon et al.59 | 2004 | NR | Colorectal Cancer | 93 | 37:56 | 69 (26 – 85) | CD56 (123C3) CD57 (HNK1) |
Quantified based on staining intensity as: • None-poor • Moderate-marked |
CD56+ Epi: 5 cells/mm2 CD57+ Epi: 2 cells/mm2 |
0.43 (0.17 – 1.01, p = 0.03) | Improved, DFS (p = 0.05) |
|
| Sconocchia et al.60 | 2011 | NR | Colorectal Cancer | Mucinous (1301) Non-mucinous (174) |
1420 | 741:673 (3 NR) | 71 (30 – 96) | CD16 (NR) CD56 (NR) CD57 (NR) |
Quantified as: • <4 cells • >4 cells |
Mean = 0.14 ± 0.07 cells/HPF | 0.43 (0.3 – 0.7, p = 0.002) | No change, OS (NR) |
| Sconocchia et al.61 | 2014 | NR | Colorectal Cancer | 1410 | NR | NR | CD56 (NR) CD57 (NR) |
Quantified as: • 〈 4 CD56+ cells • 〉 4 CD56+ cells |
NR | NR | Improved, OS (p = 0.039) |
|
| Amoueian et al.62 | 2011 | 2004 – 2008 | Gastric Cancer | 50 | 12:38 | 68 (NR) | CD56 (123C3) | Quantified at both low (100x) and high (400x) power as: • low • high |
Mean = 8 cells/ 400x | NR | Improved, OS (NS) |
|
| Ishigami et al.63 | 2000 | 1988 – 1996 | Gastric Cancer | 169 | 48:121 | 63.8 (30 – 87) | CD57 (NR) | Quantified as: • low <25 cells/ 25 fields • high >25 cells/ 25 fields |
Mean = 0.9 cells/ 400x | NR | Improved, OS (p < 0.05) |
|
| Pernot et al.64 | 2020 | NR | Gastric Cancer | 40 | NR | 64 (34 – 83) | CD57 (NK1) | Quantified as: • low < 17% • high >17% |
Mean = 2.8 cells/mm2 | 0.40 (0.15 – 1.06, p = 0.04) | Improved, OS (p = 0.02) |
|
| Rusakiewicz et al.65 | 2013 | NR | Gastric Cancer | Gastrointestinal stromal tumors | 91 | 39:52 | 57 (NR) | NKp46 (195,314) | Median NK cell number | Epi: 3.7 cells/ 200x Str: 12.3 cells/ 200x |
0.2 (NR) | Improved, OS (p = 0.0001) |
| Svensson et al.41 | 2017 | 2006 – 2010 | Gastric Cancer | Gastric adenocarcinoma | 75 | NR | NR | NKp46 (NR) | Median NK cell number | Median (based on age) = 1.89 (<avg age) or 1.93 (> avg age) NK cells/ field Median (based on gender) = 1.99 (female) or 1.89 (male) cells/ field |
0.84 (0.41 – 1.70, p = 0.619) | No change, OS (p = 0.38) |
| Platonova et al.66 | 2011 | NR | Lung Cancer | Non-small cell lung carcinoma, squamous cell | 86 | 35:51 | 63.5 (39 –76) | NK46 (NR) | Quantified as: • low <9 cell • high >10 cell |
Mean Intratumoral NK cell = 15 cell/mm2 Mean stromal NK cells = 21 cells/mm2 |
NR | No change, OS (NR) |
| Takanami et al.67 | 2001 | 1989 – 1994 | Lung Cancer | Pulmonary adenocarcinoma | 150 | 67:83 | 61 (30 – 81) | CD57 (IOT-10) | Mean NK cell number | Mean = 32 cells/ field | 0.41 (p = 0.12) | Improved, OS (p = 0.0002) |
| Villegas et al.68 | 2002 | 1986 – 1997 | Lung Cancer | Squamous cell | 50 | 1:49 | 67.2 (50 – 81) | CD57 (NR) | Quantified as: • low <5 cells • high >5 cells |
Epi: 6.74 cells/ field | 0.43 (0.20 – 0.95, p = 0.036) | No change, OS (NR) |
| Yamada et al.69 | 2010 | 2007 – 2008 | Lung Cancer | Malignant Pleural Mesothelioma: Epithelioid: 26 Biphasic: 14 Sarcomatoid: 4 |
44 | 4:40 | 59 (35 – 85) | CD56 (1B6) | Median NK cell number | Median = 1.8 cells/ 400x Mean = 5.4 cells/ 400x |
0.66 (0.25 – 1.78, p = 0.41) | Improved, OS (p = 0.032) |
| Chew et al.70 | 2012 | 1991 – 2009 | Liver Cancer | Hepatocellular Carcinoma | 40 | NR | 59 (20 – 84) | CD56 (NR) | Median NK cell number | Median = 13 cells/ field | 0.12 (0.043 – 0.31, p < 0.001) | Improved, OS (p < 0.001) |
| Wu et al.71 | 2013 | 2000 – 2004 | Liver Cancer | Hepatocellular Carcinoma | 256 | NR | NR | CD57 (NK1) | Median intra-epithelial NK cell number | Median = 7 | 0.63 (0.40 – 0.99, p = 0.046) | Improved, OS and DFS (NR) |
| Zhao et al.72 | 2014 | 2003 – 2004 | Liver Cancer | Hepatocellular Carcinoma | 163 | 32:131 | NR | CD57 (NR) | Median number of CD57+ NK cells | NR | NR | Improved, OS (p = 0.002) |
| Zhu et al.73 | 2009 | 2002 – 2004 2006 – 2007 | Liver Cancer | Hepatocellular Carcinoma | 81 | 6:13, 9:53 | 55 (34 – 75) | CD56 (NR) | Quantified as: • low <1 cell • high >1 cell |
Epi (high) = 11.8 cells/ field Str (high) = 18 cells/ field Epi (low) = 2.3 cells/ field Str (low) = 8.5 cells/ field |
0.38 (0.17 – 0.85, p = 0.019) | Improved, OS (p = 0.005) |
| Henriksen et al.74 | 2019 | 2005 | Ovarian Cancer | High grade serous carcinoma | 283 | 283:0 | 63 (NR) | CD57 (NK1) | Quantified by ROC curve • low <9 cell • high >9 cell |
Median = 5 cells/mm2 | 0.67 (0.46 – 0.98, p = 0.041) | Improved, OS (p = 0.031) |
| Li et al.75 | 2009 | 1993 – 2003 | Ovarian Cancer | Serous, Clear, Transitional, Endometrioid | 82 | 82:0 | 55.3 (26 – 80) | CD57 (NR) | Quantified as: • CD56+ tumor & stroma • CD56+ stroma • CD56+ tumor • CD56+ absent |
Epi: > 1 cell/ 200x field in 61% of samples Str: > 2 cells/ 200x field in 40% of samples |
Epi 0.55 (0.188 – 1.607 p = 0.27) Str: 2.62 (1.007 – 6.818, p = 0.048) |
Epi: Improved, OS (p < 0.05) |
| Ino et al.76 | 2008 | 1992 – 2001 | Endometrial Cancer | 65 | 65:0 | 57.7 (NR) | CD57 (NR) | Quantified as: • low <5 cells • high >5 cells |
Median = 2 cells/ 200x | NR (p = 0.23) | No change, OS (p = 0.17) |
|
| Versluis et al.77 | 2017 | 1984 – 2004 | Endometrial Cancer | 355 | 355:0 | NR | NKp46 (195,314) | NK cell presence; • present • absent |
NR | HLA-E up-regulation 0.074 (0.0094 – 0.58, p = 0.014) HLA-E normal 0.64 (0.37 – 1.11, p = 0.115) |
Improved only if with HLA-3 upregulation (NR) | |
| Zinovkin et al.78 | 2016 | 2008 – 2009 | Endometrial Cancer | 82 | 82:0 | NR (45 – 80) | CD57 (NR) | Patients divided into; • Unfavourable outcome (recurrence or death within 5 years of diagnosis) • Favourable outcome |
Median CD57 in: Unfavourable group = 24.3% Favourable group = 45.6% |
NR | Improved, OS (p = 0.001) | |
| Sznurkowski et al.79 | 2014 | NR | Vulvar Cancer | Squamous cell carcinoma | 76 | 76:0 | 69.5 (36 – 85) | CD56 (123C3) | Median NK cell number | Median = 2 cells/ field | NR | Improved, OS (p = 0.0004) |
| Jasinski-Bergner et al.80 | 2015 | 1998 – 2011 | Kidney Cancer | Clear cell: 345 Papillary: 49 Chromophobe: 29 Other: 17 |
445 | 166:279 | 63.6 (23 – 92) | CD56 (MRQ-42) | Median NK cell number | Mean = 0.55 cells/ 400x field | NR | No change, OS (p = 0.91) |
| Jensen et al.81 | 2009 | 1992 – 2001 | Kidney Cancer | 121 | 61: 74 | 61 (19 – 82) | CD57 (NK1) | Quantified as: • low <28 cells/mm2 • high >28 cells/mm2 |
Median = 28 cells/mm2 | NR | No change, OS (p = 0.22) | |
| Sorbye et al.82 | 2012 | 1973 – 2006 1996 – 2006 |
Sarcoma | Soft tissue | 249 | 139:110 | NR (20 – 60) | CD57 (NR) | Quantified AS: • Gr 3 (20+ cells) • Gr 2 (6 – 19 cells) • Gr 1 (1 – 5 cells) • Gr 0 (no cells) |
NR | NR | No change, OS (p = 0.62) |
| Erdag et al.83 | 2012 | 1982 – 2007 | Melanoma | 147 | 63:84 | 58 (19 – 89) | CD56 | Median NK cell number | Epi = 5.1 cells/ mm2 Str = 2.5 cells/ mm2 |
NR | No change, OS (p = 0.43) | |
| Lundgren et al.84 | 2016 | 2001 – 2013 | Periampullary adenocarcinoma | Intestinal: 65 Pancreatobiliary: 110 |
175 | 82:90 | 67 (38 – 84) | CD56 (MRQ-42) | Quantified as: • low <2.75 cells/core • high >2.75 cells/core |
NR | Intestinal: 0.23 (0.07 – 0.78, p < 0.05) Pancreatobiliary: 0.59 (0.34 – 1.02, NR) |
Improved, OS (p = 0.002) |
| Nakakubo et al.85 | 2003 | 1989 – 1999 | Gallbladder cancer | Primary gallbladder adenocarcinoma | 45 | 28:17 | 66.7 (NR) | CD57 (Leu-7) | Quantified as: • 〈 10 NK cells/ HPF • 〉 10 NK cells/ HPF |
Mean = 1.2 cells/ 200x | 0.56 (0.20 – 1.58, p = 0.27) | No change, OS (p = 0.27) |
| Vaquero et al.86 | 1989 | NR | Glioblastoma | 25 | NR | 55.6 (19 – 69) | CD57 (IOT-10) | Divided into NK cell present or absent groups | Mean = 6 cells/ core | NR | No change, OS (NR) |
Analysis
Studies that reported HR values representing the risk of death in patients with NK cell infiltration were compiled for meta-analysis. We conducted specific sub-studies to evaluate differences that may be attributable to differences in markers or methods used. Meta-analysis was conducted in R Studio (version 3.6.3; for code see Supplemental Data 2) using the R package ‘meta’ and “metaphor” following the random-effects model, specifically the Hartung-Knapp-Sidik-Jonkman method. This method was chosen to account for the maximum study heterogeneity. Interstudy heterogeneity was quantified using the I2 statistic, with an I2 value>50% as our a priori threshold for substantial heterogeneity. All graphical representation of statistical results including Forrest plots and p-value visualization were created using GraphPad Prism 8.
RESULTS
NK cell infiltration predicts improved OS in patients with solid tumors
We identified 53 studies representing a total of 9624 patients and encompassing a variety of tissue origins and cancer subtypes. The details of these studies including cancer subtype, sample size, IHC marker, infiltrating NK cell stratification, HR values and impact on survival are summarized in Table 1.
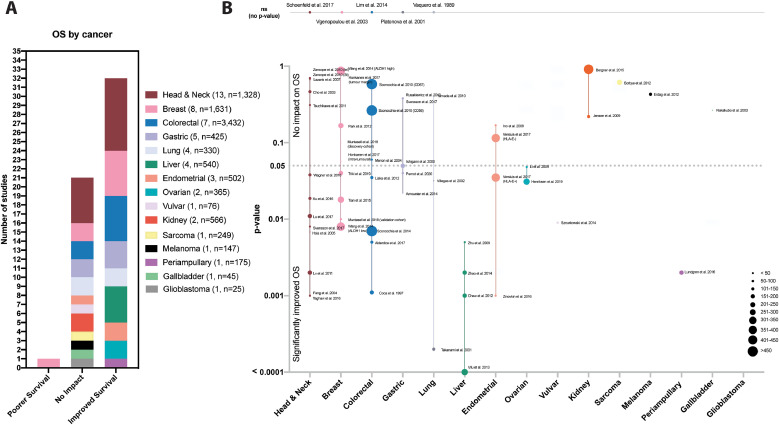
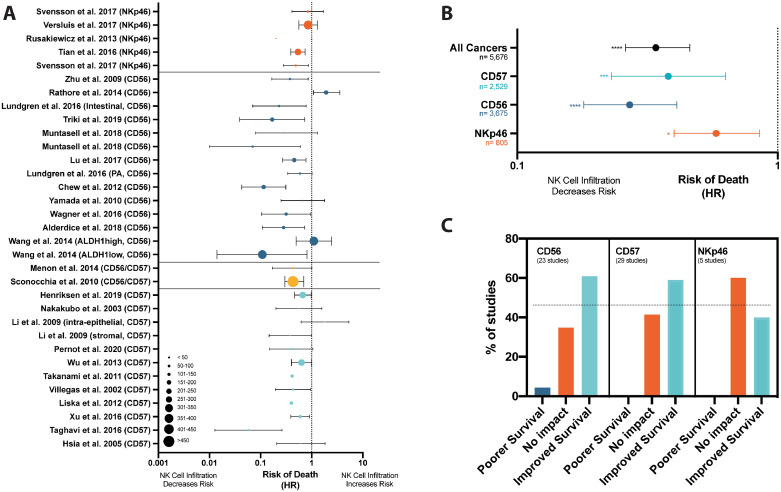
Cancer types explored in the studies included head and neck[34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], breast[47], [48], [49], [50], [51], [52], [53], [54], colorectal[55], [56], [57], [58], [59], [60], [61], gastric41,[62], [63], [64], [65], lung[66], [67], [68], [69], liver[70], [71], [72], [73], ovarian74,75, endometrial[76], [77], [78], vulvar79, kidney80,81, sarcoma82, melanoma83, periampullary adenocarcinoma84, gallbladder85and glioblastoma86 . Most studies identified NK cells using antibodies against CD57 (56.6%) followed by CD56 (41.5%) and NKp46 (9.4%); four of the 53 studies used both CD57 and CD56. All 53 studies (54 studies separating the esophageal and gastric patients in Svensson et al.41) reported the impact of NK cell infiltration on survival measures (Fig. 2A). The majority of studies (n = 32, 59.3%) reported significantly improved OS, while 21 (38.9%) reported no significant impact on survival. Of the studies that did not achieve statistical significance by dichotomizing between high and low NK cell infiltration, four reported p values <0.10, demonstrating trends towards improved OS in the NK cell high groups (Fig. 2B). Notably, only one study (1.9%) reported significantly poorer OS with higher NK cell infiltration50.
Fig. 2.
Studies evaluating the associations between NK cell infiltration and overall survival. (A) Bar graph representing the distribution of conclusions from studies assessed in this review. From the 53 studies reviewed, the majority of studies (32 (59.3%)) reported significantly improved OS, 21 (38.9%) reported no significant impact on survival and one (1.9%) reported significantly poorer OS. (B) When p-values were provided (y-axis, line at 0.05) we noted them on this visualization scatter graph organized by tumor type (x-axis). Those that did not provide p-value are included above the graph. Dot size indicates the number of patients in each study (or arm of study when applicable).
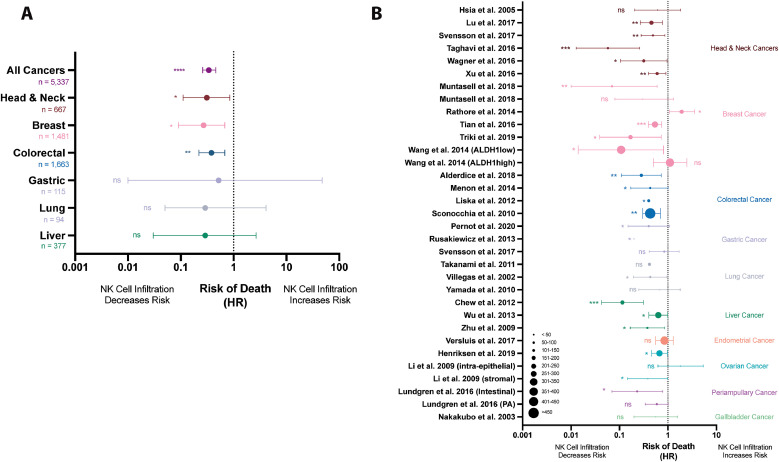
Thirty studies reported HR values, 95% confidence intervals and p-values and therefore could be included in our meta-analysis. Importantly, these studies employed similar, but not identical, methods of NK cell quantification and stratification (dichotomizing or grouping patient populations based on NK cell infiltration). Meta-analyzed, these studies demonstrated a lower risk of dying in populations with increased NK cell infiltrations (HR=0.34, 95% CI: 0.26–0.46, p<0.0001) (Table 2, Fig. 3A). Notably, this specific meta-analysis yielded a high level of heterogeneity (I2 = 86.9%, Table 2, Fig. 3B). This statistic indicates that while the majority of studies, across tumor types, found NK cells to be associated with improved OS, additional sub-meta-analyses is warranted by tissue compartment, marker, or within cancer types to strengthen and validate conclusions.
Table 2.
HR values of meta-analysis overall, by cancer site and individual studies.
| Study | n | HR | Lower CI | Upper CI | p-value | I2 Heterogeneity |
|---|---|---|---|---|---|---|
| ALL SOLID TUMORS | 5337 | 0.34 | 0.26 | 0.46 | <0.0001 | 86.9% |
| Head and Neck Cancer | 667 | 0.31 | 0.11 | 0.85 | 0.030 | 94.7% |
| Hsia et al. 2005 | 38 | 0.61 | 0.21 | 1.82 | 0.378 | |
| Lu et al. 2017 | 197 | 0.46 | 0.27 | 0.77 | 0.004 | |
| Svensson et al. 2017 | 97 | 0.49 | 0.28 | 0.86 | 0.012 | |
| Taghavi et al. 2016 | 57 | 0.06 | 0.01 | 0.26 | 0.001 | |
| Wagner et al. 2016 | 140 | 0.32 | 0.10 | 0.96 | 0.042 | |
| Xu et al. 2016 | 138 | 0.60 | 0.39 | 0.91 | 0.016 | |
| Breast Cancer | 1481 | 0.27 | 0.09 | 0.68 | 0.027 | 85% |
| Muntasell et al. 2018 | 71 | 0.07 | 0.01 | 0.60 | 0.01 | |
| Muntasell et al. 2018 | 41 | 0.30 | 0.08 | 1.30 | 0.10 | |
| Rathore et al. 2014 | 175 | 1.92 | 1.08 | 3.57 | 0.05 | |
| Tian et al. 2016 | 278 | 0.54 | 0.39 | 0.74 | 0.001 | |
| Triki et al. 2019 | 158 | 0.17 | 0.04 | 0.73 | 0.017 | |
| Wang et al. 2014 (ALDH1high) | 379 | 1.10 | 0.50 | 2.44 | 0.811 | |
| Wang et al. 2014 (ALDH1low) | 379 | 0.12 | 0.01 | 0.81 | 0.031 | |
| Colorectal Cancer | 1663 | 0.38 | 0.22 | 0.68 | 0.019 | 0% |
| Alderdice et al. 2018 | 150 | 0.28 | 0.11 | 0.73 | 0.005 | |
| Menon et al. 2014 | 93 | 0.43 | 0.17 | 1.01 | 0.030 | |
| Sconocchia et al. 2011 | 1420 | 0.43 | 0.30 | 0.70 | 0.0020 | |
| Gastric Cancer | 115 | 0.52 | 0.01 | 47.35 | 0.32 | 0% |
| Pernot et al. 2020 | 40 | 0.40 | 0.15 | 1.06 | 0.040 | |
| Svensson et al. 2017 | 75 | 0.84 | 0.41 | 1.70 | 0.619 | |
| Lung Cancer | 94 | 0.47 | 0.05 | 4.11 | 0.14 | 0% |
| Villegas et al. 2002 | 50 | 0.43 | 0.20 | 0.95 | 0.036 | |
| Yamada et al. 2010 | 44 | 0.66 | 0.25 | 1.78 | 0.41 | |
| Liver Cancer | 377 | 0.29 | 0.03 | 2.67 | 0.14 | 91.8% |
| Chew et al. 2012 | 40 | 0.12 | 0.04 | 0.31 | 0.0010 | |
| Wu et al. 2013 | 256 | 0.63 | 0.40 | 0.99 | 0.046 | |
| Zhu et al. 2009 | 81 | 0.38 | 0.17 | 0.85 | 0.019 | |
| Ovarian Cancer | 365 | 0.57 | 0.26 | 1.24 | 0.089 | 0% |
| Henriksen et al. 2019 | 283 | 0.67 | 0.46 | 0.98 | 0.031 | |
| Li et al. 2009 (intra-epithelial) | 41 | 0.38 | 0.15 | 0.99 | 0. 048 | |
| Li et al. 2009 (stromal) | 41 | 1.62 | 0.15 | 5.32 | 0.27 | |
| Endometrial Cancer | ||||||
| Versluis et al. 2017 | 355 | 0.85 | 0.56 | 1.29 | 0.445 | |
| Periampullary Cancer | ||||||
| Lundgren et al. 2016 (Intestinal) | 87.5 | 0.23 | 0.07 | 0.78 | 0.05 | |
| Lundgren et al. 2016 (PA) | 87.5 | 0.59 | 0.34 | 1.02 | Not reported | |
| Gallbladder Cancer | ||||||
| Nakakubo et al. 2003 | 45 | 0.56 | 0.20 | 1.58 | 0.2655 | |
Fig. 3.
NK cell infiltration is associated with a decreased risk of dying in patients with solid tumors. (A) A random effects model meta-analysis was conducted on the 30 studies revealing a decreased risk of death in patients with greater NK cell infiltration (HR=0.34, 95% CI: 0.26–0.46; p<0.0001). Forest plots demonstrate pooled meta-analysis results by solid tumor type and pooled meta-analysis results from all solid tumor types. (B) All studies which reported HR values were visualized by Forest plot, grouped by tumor type (dots indicate the size of patient population studied).
NK cell infiltration into intraepithelial compartments is most strongly associated with improved OS
To determine whether the association between NK cells and patient survival extends to different regions within tumors (stromal or epithelial regions), we next examined studies that reported NK cell infiltration at this resolution. Of the 53 studies analyzed in this review, eight (15.1%) explored the relationship of patient prognosis with NK infiltration to specific tumor regions. Locations included intraepithelial (intra-tumoral, cancer cell nest or center of tumor)34,45,47,50,59,75,82,84 (n = 8), peritumoral (at the margin of the tumor)47,59,82 (n = 3) or stromal34,45,50,59,75,84 (n = 6). A general trend was observed that intraepithelial NK cells had a greater impact on survival compared to NK cells in the surrounding regions45,47,50,75,84. For example, patients with advanced stage esophageal cancer had the highest OS concurrent to high NK cell infiltration into the intraepithelial region and low infiltration in the stroma; conversely, the worst OS was found in patients with low NK cell infiltration into the intraepithelial region, and high infiltration in the stroma45. Overall, NK cell infiltration into the cancer nest significantly improved OS (p = 0.019), whereas infiltration into the stroma did not (p = 0.65)45. In another study, patients with ovarian cancer who had only intraepithelial NK cell infiltration had significantly longer OS than those who had stromal infiltration (p<0.05)70; patients with NK cells restricted to the stroma had the lowest OS, even compared with patients who had no NK cell infiltration to their tumors at all70. Noteworthy, only five (6%) of patients were classified in the stroma infiltration only group70. One notable exception was a study in invasive ductal carcinoma where OS was significantly worse when more NK cells infiltrated into the intraepithelial region (p = 0.0029), but no difference was noted when they infiltrated the stromal region (p = 0.21)50. In general, NK cells in closer physical proximity to tumor cells may lead to better survival, though further study across an array of tumor types is required.
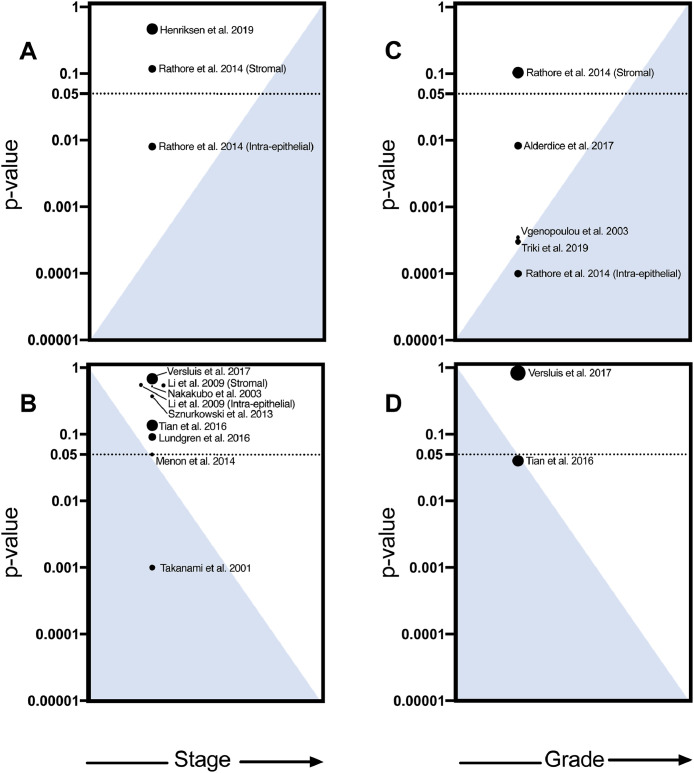
NK cell infiltration is greatest in low stage and high-grade tumors
Tumor staging incorporates tumor size, nodal involvement and metastases to describe tumor progression, inform treatment and predict survival. Later stage tumors are typically larger, metastatic and generally carry poorer prognosis87. Fourteen studies assessed how tumor stage is associated with NK cell infiltration in breast, colorectal, endometrial, gallbladder, head and neck, lung, ovarian, and vulvar cancer. There was a general trend toward decreased NK cell infiltration with increasing tumor stages51,59,67,75,77,84,85,88 (n = 8), of which colorectal (p = 0.00559) and lung cancer (p = 0.00167) reached statistical significance (Fig. 4B). Two studies observed more NK cells in advanced stages of high grade serous ovarian (p = 0.4774) and invasive ductal carcinomas (p = 0.01 (intraepithelial), and p = 0.12 (stromal)50) (Fig. 4A). Comparing within stages, NK cell infiltration was most strongly associated with improved OS among patients with high stage tumors in colorectal cancer (stage I: p = 0.24; stage II: p = 0.11; stage III p = 0.0008)56.
Fig. 4.
NK cell infiltration is highest in early stage and high grade tumors. Studies reporting NK cell infiltration at different (A, B) stages and (C, D) grades were included. The blue triangles indicate increasing or decreasing NK cell infiltration as stages and grades advance. Dot size indicates the number of patients in each study. (A) Studies that demonstrate increased NK cell infiltration corresponding with higher stage (n = 3). (B) Studies that demonstrate increased NK cell infiltration corresponding with lower stage (n = 9). (C) Studies that demonstrate increased NK cell infiltration at higher grades (n = 5). (D) Studies that demonstrate increased NK cell infiltration at lower grades (n = 2).
Cancer grade, the microscopic description of the cells and tissues, is also used to classify tumors: the higher the grade, the more abnormal, or undifferentiated, the cells appear87. Five studies found a significant correlation of NK cell infiltration to grading[50], [51], [52], [53],55. Four found that at higher grades, there were more NK cells in advanced rectal cancer (p = 0.0080)55 breast cancer (p = 0.0003052), and invasive ductal carcinoma (p = 0.0003546, 0.0001050,52, (Fig. 4C). Conversely, one study in invasive ductal carcinoma observed a significant association between lower NK cell infiltration and higher grades (p = 0.04051) (Fig. 4D). Although the majority of studies did not report sub-analyses of tumor stage and grade, these pioneering investigations reveal that they should be considered when exploring the prognostic value of immune infiltration.
NK cell identifying marker impacts the interpretation of the importance of NK infiltration
There is no universal marker to define NK cells, and subsets of NK cells express CD56, CD57 and NKp46 differently16. We meta-analyzed HR values from studies based on their primary NK cell marker (Fig. 5A). Studies staining for CD5638,44,48,50,52,54,55,59,60,69,70,73,84 (n = 16, HR=0.27, 95% CI: 0.18–0.41; p = 0.0001) and CD57 36,42,45,58,59,64,67,68,71,74,75,85,89 (n = 12, HR=0.38, 95% CI: 0.23–0.63, p = 0.0014) demonstrated a similarly reduced risk of death with NK cell infiltration. Although studies staining for NKp46 also revealed a significant trend, the prognostic value was weaker41,51,65,77 (n = 4, HR=0.64, 95% CI: 0.41–1.00; p = 0.020) (Fig. 5B). Next, we similarly pooled the studies examining the relationship between NK cell infiltration and survival outcome (improved, no impact or poorer survival). Of the 53 studies, we found CD56 and CD57 yielded consistent conclusions and that the majority of studies (61%) that used these markers indicated improved OS. In contrast, a larger percentage of studies (57%) that evaluated infiltration by NKp46 found no impact on survival (Fig. 5C). Although the importance of this trend remains to be validated and functionally described, the relatively poor predictive capacity of NKp46 could reflect the inclusion of both immunosuppressive ILC subsets and conventional NK cells within the marked populations, the natural variation in expression of NKp46 that occurs between people, or downregulation of NKp46 prompted by the tumor[90], [91], [92], [93], [94].
Fig. 5.
The associations concluded for the impact of NK cell infiltration and may be influenced by IHC marker. (A) Forest plot visualizing the reported HR's of studies organized by marker used; NKp46 (orange), CD56 (dark blue), CD56 & CD57 (yellow) or CD57 (light blue). A random-effects model meta-analyses was conducted on studies using each marker. (B) This Forrest plot demonstrates the difference in the pooled risk of dying was larger in studies staining by CD56 (n = 16, HR:0.27, 0.18–0.41, p = 0.0001) and CD57 (n = 12, HR:0.38, 0.23–0.63, p = 0.0014) than NKp46 (n = 4, HR:0.58, 0.40–0.85, p = 0.020). (C) Bar graph demonstrating difference in proportion of studies finding associations between NK cell infiltration and survival when separated by marker used.
Head & neck cancers (13 studies, n = 1328 patients)
Head and neck cancers represent a heterogenous group of cancers that arise from tissues of the mouth, nose, throat, larynx, sinuses or salivary glands. A benefit of NK cell infiltration in these tumors, including longer DFS, was first reported in 1986 and confirmed in a recent meta-analysis95,96. We identified 13 studies that assessed the value of NK cell infiltration in head and neck cancers including esophageal34,36,39,41,43,45 (n = 6), oropharyngeal40,44 (n = 2), nasopharyngeal38 (n = 1), oral35,42,46 (n = 3), laryngeal37 (n = 1), and lip46 (n = 1) carcinomas (Table 1, Fig. 2B). Of the 13 studies, 8 found that NK cell infiltration was associated with significantly improved OS; the remainder found no significant impact. Six of these studies reported HRs and together represent a total of 667 patients. Meta-analyzed, these studies revealed an overall decreased risk of dying in patients with NK cell infiltration or higher numbers of infiltrating NK cells (HR=0.31; 95% CI: 0.11–0.85, p<0.030, Table 2, Fig. 3A). Based on the extremely high heterogeneity between the studies (I2 = 94.7%), further studies and stratification by specific head and neck cancer subtype are warranted. In our meta-analysis, this could be accomplished for esophageal cancer only.
Esophageal (6 studies, n = 674 patients)
Four studies reported a significant association of NK cell infiltration with improved OS in patients with esophageal cancer (p = 0.00239, 0.00736, 0.00841, 0.01945, Table 1, Fig. 2B). Meta-analysis conducted on the three studies that reported HRs demonstrated decreased risk of dying with high NK cell infiltration (HR=0.55; 95% CI: 0.41–0.74, p = 0.013). These studies demonstrated substantially diminished heterogeneity (I2 = 0%). Hence, NK cells are positive prognostic markers for improved survival in esophageal cancer.
Breast cancer (8 studies, n = 1631 patients)
Breast cancer is highly heterogenous, with targeted treatment informed by molecular subtypes characterized by the expression or constitutive activation of receptors: human epidermal growth factor receptor-2 (HER-2+), estrogen receptor (ER+), progesterone receptor (PR+) or triple negative breast cancer (TNBC), which does not express any of these receptors. The eight breast cancer studies we evaluated included: HER2+52 (n = 211), ER+52 (n = 100), PR+52 (n = 87), and TNBC51 (n = 278). Four studies found that higher NK cell infiltration was associated with significantly improved OS (Table 1, Fig. 2B)47,51,52,54; one reported significantly improved DFS48, and two reported no significant impact on survival49,53. The eighth study did not report patient subtype and was the only one to find NK cells associated with significantly worse survival50. Notably, this study stained for NK cells using a single marker, CD56, with no exclusionary markers and therefore NK cell frequency may be overrepresented50. Meta-analysis of these studies demonstrated a decreased risk of death (HR=0.27, 95% CI: 0.09–0.68, p = 0.027) however with a high level of heterogeneity (I2 = 85%) (Table 2, Fig. 3A). Breast cancer is a multi-faceted disease with significant heterogeneity and as such, it is not surprising that a clear trend was not observed, therefore, further analysis with stratification by subtype may provide a clearer association.
Colorectal cancer (7 studies, n = 3432 patients)
Colorectal cancer is highly heterogenous, with a large number of hereditary predispositions and environmental risk factors that both contribute to a high mutational burden97. We evaluated seven studies that investigated the impact of NK cell infiltration into colorectal tumors on OS. Two studies identified the subtype as either large bowel adenocarcinoma56 (n = 157) or mucinous (n = 119)60 and nonmucinous (n = 1301)60; the other five did not disclose a subtype55,[57], [58], [59],61 (n = 1855). Two of the studies identified patients as having locally advanced rectal cancer (n = 202)55,57. The studies included the NK cell markers CD56 and CD57. Of the seven studies, five observed a significantly improved OS with high NK cell infiltration (Table 1, Fig. 2B)55,56,[58], [59], [60], [61]. Sconnochia et al. (2011)60 observed a trend towards better OS in those with greater NK cell infiltration, and Lim et al. (2014)57 did not identify any associations between OS and NK cell infiltration. Meta-analysis of the three studies that reported HR revealed a significant decrease in risk of death with high NK cell infiltration with little heterogeneity between studies (HR=0.38; 95% CI: 0.22–0.68, p = 0.019, I2 = 0%, Table 2, Fig. 3A)51,55,60. Noteworthy, Menon et al. (2004)59 observed that women and older patients had the highest NK cell infiltration into the stroma. The prognostic implications of this were not explored, though these observations indicate that the role of sex and age should be assessed in the further study of NK cell infiltration into tumor regions. Patient survival with colorectal cancer has strong evidence to be influenced by NK cell infiltration, and therefore further analysis should be completed to understand this relationship.
Gastric cancer (5 studies, n = 425 patients)
Gastric cancer is an aggressive adenocarcinoma largely affecting the lining of the stomach and exemplified by genetic heterogeneity98. Several subtypes were represented across the five studies examined, including intestinal-type gastric adenocarcinoma64 (n = 50), and gastrointestinal stromal65 (n = 91); the remaining samples were of unspecified subtype41,99 (n = 284). Three of the five studies identified NK cells by staining for CD56 or CD57 and found that NK cell infiltration significantly improved OS62,64,99(Table 1, Fig. 2B). Meta-analysis of the gastric cancer studies reporting a HR (n = 2; patients n = 115) did not reach significance for an overall decreased risk of dying when NK cells were present or found at high frequencies within the tumor (HR=0.52, 95% CI:0.01–47.35, p = 0.32, Table 2, Fig. 3A). Two of the five studies included in this meta-analysis used NKp46 as their NK cell-defining marker; with increasing NK infiltration, one demonstrated a trend toward improved OS41, and the other observed significantly improved progression free survival, but not OS64. NK cell infiltration into gastric cancer can act as a positive prognostic marker for OS; these associations may be made clearer with further studies and careful choice of NK marker.
Lung cancer (4 studies, n = 330 patients)
Lung cancer, the leading cause of cancer death worldwide, is divided into two major categories: non-small cell (>80% of cases) and small cell lung carcinomas100,101. We identified four lung cancer studies (n = 330 patients) that evaluated the association between NK cell infiltration and OS. The lung cancer subtypes examined included malignant pleural mesothelioma (n = 44)69, squamous cell carcinoma (n = 67)66,68, and adenocarcinoma (n = 219)66,67. Of the three studies that identified tumor stage, 126 (51.6%) patients were stage 1, 47 (19.3%) were stage 2, 68 (27.9%) were stage 3, and 3 (1.2%) were stage 4. Two of the studies found that high CD57+ NK cell infiltration into the tumor was associated with significantly improved OS67,68. Two studies did not find associations between NK cell infiltration and prognosis: one stained using CD56 and their cohort included more than 50% of patients at advanced stages69; the other used NKp46 to mark NK cells and found them mostly localizing to the invasive margin of the tumor66. Our meta-analysis included two lung cancer studies and found a non-significant trend towards a decreased risk of death with NK cell infiltration (HR=0.47, 95% CI: 0.05–4.11, p = 0.14, Table 2, Fig. 3A)68,69. There may be associations between NK cell infiltration and lung cancer, but additional studies are required to understand how NK cells can be used as a prognostic factor, and whether this differs with subsets of lung cancer.
Liver cancer (4 studies, n = 540 patients)
Liver cancer is most often preceded by cirrhosis and chronic liver disease102. This precancerous chronic disease is associated with decreased numbers of circulating NK cells compared with age- and sex-matched individuals without cancer103. Four studies of five patient cohorts examined the value of infiltrating NK cells in hepatocellular carcinoma (n = 540) using either CD56 or CD57 to identify NK cells[70], [71], [72], [73] (Table 1, Fig. 2B). All four studies found NK cell infiltration was significantly associated with improved OS. Meta-analysis of the liver cancer studies (n = 3; n = 377 patients) revealed a trend towards decreased risk of death when NK cells were present within the tumor (HR=0.29, 95% CI:0.03–2.67, p = 0.14, Table 2, Fig. 3A). While these studies all agree that NK cells are prognostically beneficial in liver cancer, a high level of heterogeneity (I2 = 91.8%) supports further investigation to clarify the variability observed.
Gynecological cancers
All gynecologic cancers similarly involve the female reproductive tract, but they arise from various tissues. The impact of NK cell infiltration on survival has been studied in ovarian74,75, endometrial[76], [77], [78], and vulvar cancer79.
Ovarian cancer (2 studies, n = 365 patients)
The majority of ovarian cancers arise from the epithelial lining of the fallopian tubes104. They are subclassified into serous, endometrioid, mucinous, clear cell, or undifferentiated; however, tumors can also arise from germ and stromal cells105. Two studies explored the impact of NK cell infiltration in ovarian cancer using CD57 as their principal NK cell marker and observed a decreased risk of dying in patients with greater NK cell infiltration74, or more infiltration of NK cells into the tumor epithelium (Table 1, Fig. 2B)75. Meta-analysis of these two studies demonstrate a trend towards a decrease in risk of death for those with higher NK cell infiltration (HR=0.57, 95% CI:0.26–1.24, p = 0.089, Table 2). Taken together, these studies suggest NK cell infiltration, particularly into the intra-epithelial region, appears to be associated with better prognosis in ovarian cancer.
Endometrial cancer (3 studies, n = 502 patients)
Unopposed estrogen stimulation can lead to the rapid proliferation of the endometrial lining, resulting in endometrial cancer106. Three studies evaluated NK cell infiltration in endometrial cancer; PFS trended towards improvement in patients with NK cell counts greater than five but did not reach significance (p = 0.17) (Table 1, Fig. 2B)77. Overall survival was significantly higher in the population with a greater proportional infiltration of CD57+ NK cells (p = 0.001)78. In the third study, DFS was significantly improved with NKp46+ NK cell presence but only in the context of HLA-E overexpression (p = 0.035)77. A HR was only reported in this last study and was not significant (Table 2, Fig. 3B)77. The mixed results between studies highlight that further investigation is warranted to confirm the trend towards improved prognosis with greater NK cell infiltration.
Vulvar cancer (1 study, n = 76 patients)
Unlike many other solid tumors, presence of T cells does not affect the prognosis of vulvar cancer88. Sznurkowski et al. (2014) used CD56 as a NK cell marker along with Granzyme B to mark cytotoxicity (Table 1, Fig. 2B)79. No significant improvement in OS was observed between high and low NK-infiltrated cases, both generally and in metastatic cases79. Although it appears that NK cell infiltration may not be associated with better prognosis in vulvar cancer, further studies will be required to confirm this finding.
Kidney cancer (2 studies, n = 566 patients)
Kidney cancer occurs in the renal tubular epithelium and includes a heterogenous group with various cancer subtypes, including clear cell, papillary, and chromophobe renal cell carcinoma107. The value of infiltrating NK cells in renal cell carcinoma (including subtypes) was examined in two studies (n = 566) with staining for CD5680 or CD5772 (Table 1, Fig. 2B). In contrast to many of the other cancers examined in this systematic review, NK cells were not significantly associated with improved survival in either of these studies. Neither study reported a HR and therefore these could not be included in the meta-analysis. Although further study is warranted, the available evidence suggests that NK cells are not associated with improved prognosis in renal cell carcinoma, a finding consistent with reports that T cell infiltration is also not beneficial in this cancer type108.
Sarcoma (1 study, n = 249 patients)
Soft tissue sarcoma, a rare group of cancers, develop in the mesenchyme, a portion of the embryo that establishes the connective and skeletal tissues109. One study examined the prognostic effect of infiltrating NK cells in soft tissue sarcoma. Sorbye et al. (2012) evaluated 249 patients by staining for NK cells using CD57. High numbers of CD57+ NK cells in the peritumoral capsule non-significantly trended towards better OS of soft tissue sarcoma patients (p = 0.797) (Table 1, Fig. 2B)82. The median survival for high NK cell infiltration in this tumor compartment (29 patients, 36%) was 138 months, compared to 47 months for low expression (50 patients, 63%)82. There was no trend observed between CD57+ NK cell infiltration into the tumor and OS82. Overall, the potential association between better prognosis and NK cell infiltration warrants further studies.
Melanoma (1 study, n = 147 patients)
Melanoma is the most aggressive form of skin cancer and the global incidence is currently 160,000 new cases per year and steadily increasing110,111. Melanoma is notable for its resistance to classical therapies and susceptibility to immunotherapy112. Only one study examined infiltration of NK cells into melanoma, using the CD56 marker (Table 1)83. No significant association between NK cell infiltration and OS was reported, but further studies are needed in order to understand the role of NK infiltration in melanoma.
Periampullary adenocarcinoma (1 study, n = 175 patients)
Periampullary cancer arises from tissue surrounding the ampulla of Vater, the area where the common bile duct and pancreatic duct come together and open into the duodenum113. This cancer type is a heterogenous group of malignancies which arise from tissue from the distal bile duct, pancreatic duct and pancreas itself. Lundgren et al. (2016) was the only study we identified that explored immune infiltration in periampullary adenocarcinomas and stratified into intestinal and pancreatobiliary subtypes (Table 1)84. Those with high NK cell infiltration had significantly longer OS (p = 0.002) compared to those without84. Risk of dying in the NK cell infiltrated population was lower in the intestinal (HR=0.23, 95% CI: 0.07–0.78, p<0.05) but not pancreatobiliary (HR=0.59, 95% CI: 0.34–1.02, ns) subtype (Table 2, Fig. 3B)84. This study suggests NK cells are associated with improved survival in periampullary adenocarcinoma but may be more protective in intestinal type periampullary carcinoma; additional studies will help to validate this association.
Gallbladder (1 study, n = 45 patients)
We identified one study that evaluated the prognostic impact of NK cell infiltration in gallbladder cancer, specifically gallbladder adenocarcinoma85. Nakakubo et al. (2003) evaluated 45 tissue samples using CD57 to identify NK cells85. While significance was not reached, a trend toward improved survival in the high NK cell infiltrated group emerged (Table 1, Table 2)85. With growing interest in immunotherapeutic options for gallbladder patients, additional studies evaluating the prognostic value of infiltrating NK cells are warranted.
Glioblastoma (1 study, n = 25 patients)
Glioblastoma is the most common primary central nervous system neoplasm and is most often located in the brain114. Vaquero et al. (1989) evaluated 25 patients with glioblastoma, and found no change in survival with respect to the frequency of infiltrating NK cells86. More research into the prognostic value of NK infiltration in glioblastoma is required to confirm these findings.
Discussion
We report the first systematic review describing the prognostic value of NK cell infiltration into solid tumors. Thirty two of the 54 (59.3%) reported positive associations between OS and NK cell infiltration; twenty (38.9%) reported no impact and just one (1.9%) found a negative association between NK cell infiltration and OS. NK infiltration was more common with earlier stage and higher-grade tumours. When considering localization, the reviewed studies revealed that NK cells infiltrating intraepithelial regions impacted survival more than NK cells infiltrating the adjacent stroma. These findings define NK cell infiltration in solid tumors as a positive prognostic factor and prompt further research to understand and maximize NK cell function in solid tumors.
The total number of NK cells infiltrating solid tumors – including those considered “highly” infiltrated – was relatively low compared with other immune populations, and several studies asserted that the number of NK cells was too low to pursue prognostication[115], [116], [117], [118]. Notwithstanding, the presence of a single NK cell within a high powered microscopic field was associated with significantly improved OS and DFS in colorectal cancer58, HER2+ breast cancer48 and hepatocellular carcinoma73. Although the exact role(s) of infiltrating NK cells remains to be determined, that NK cells are competent “serial killers”119,120, capable of polarizing the TME, recruiting and activating additional effector cells suggests that their low frequency should not be interpreted as a lack of power or importance121.
Historically, IHC was limited both by the number of chromogens available and the relatively few species in which antibodies can be raised, but modern multiplex IHC technology now allows for simultaneous identification of up to nine markers on a single slide, regardless of the species in which the antibodies are raised, and machine learning to identify cellular infiltration, sub-tumor localization and sociology134,135. With this new technology, we look forward to better understanding the features that impact NK cells’ relationship with solid tumor control, including the function of NK cells within tumors.
In addition to infiltration, the solid tumor microenvironment restricts NK cell reactivity. In tumors infiltrated with NK cells that are suppressed, interventions to support NK cell reactivity and overcome immunosuppression, such as checkpoint blockade or local cytokine production may prove efficacious[122], [123], [124], [125]. NK cells isolated from patients with solid tumors including head and neck 126,127, gallbladder128, and ovarian129 cancers exhibit increased expression of inhibitory receptors and decreased expression of activating receptors. In the TME, high levels of TGF-β directly suppress NK cell proliferation and cytotoxicity, leading to impaired anti-tumor activity in vivo130,131. Hypoxia and adenosine signaling through the high-affinity A2A receptor can interfere with NK cell development and cytotoxicity132. Immunosuppression, particularly that driven by myeloid-derived suppressor cells, increases with tumor stage; this has been observed in bladder 133, pancreatic 133, hepatocellular 134,135, gastric [134], [135], [136], non-small cell lung 137, and head and neck squamous cell138 cancers. Our meta-analysis reveals that these higher stage tumours are more often infiltrated by NK cells; in them, highlighting that NK cells may have an important role in these hard-to-treat tumors.
Within sub-tumor regions, NK cell phenotypes and infiltration differ. The best survival outcomes were observed specifically with high NK infiltration to the intraepithelial region45. In hepatocellular carcinoma, NK cells in the peritumoral (stromal) region expressed high levels of CD69 (a marker of activation), while intraepithelial NK cells from the same patients did not71. Although intraepithelial NK cells expressed CD107a, a marker associated with degranulation, they also exhibited low perforin, TRAIL, and granzyme B, suggesting that NK cells within this region may be exhausted71. In tongue cancer, intraepithelial NK cells were more likely to express the NKG2A inhibitory receptor than their stromal counterparts6. Together, these results suggest that like T cells139, NK cells can be activated for antitumor activity, but become suppressed as they enter further into the TME and closer to the tumor cells.
The phenotype of NK cells can be highly informative of their reactive capacity or suppression. Germline-encoded receptors for activation and inhibition are differentially expressed and co-expressed, and modified through NK cell “education” for missing self reactivity22,140,141. NK cytotoxicity occurs through several mechanisms including perforin and granzyme release and the death receptor pathways (i.e. Fas, TRAIL). Other markers often expressed or upregulated by activated NK cells include CD69, NKG2D, NKp46, or DNAM-1 (all associated with activation), immune checkpoints (i.e. TIGIT, PD1/PD-L1, NKG2A and TIM-3) and inhibitory receptors for HLA (i.e. killer immunoglobulin-like receptors, NKG2A, LIR-1)142. Whether the ligands for these receptors are present within solid tumors, or may be attenuated (inhibitory)125,143 or triggered (activating)144,145 are active areas of research in the field of NK-based immunotherapy. An overabundance of inhibitory ligands or infiltration of cells with immunosuppressive activity, including the ILC1 subset which has several overlapping features with conventional NK cells, would be expected to be associated with poor prognosis91. With novel multiplexing technologies and improved understanding of the distinction between NK cells and ILC subsets, it is now possible to distinguish conventional NK subsets from other populations that share markers with NK cells, including ILC1s146,147. Understanding the roles and distribution of ligands for NK cells in solid tumors will be important toward refining and precisely delivering NK-based immunotherapies.
Of the 53 studies evaluated in this review, only five used more than one NK cell marker to quantitate NK cell infiltration; the rest used CD56, CD57, or NKp46 exclusively. Compared with NKp46, definitions of NK cells using CD56 or CD57 were more consistently associated with improved survival. NKp46 is an activating receptor that is more dynamic in its expression than CD56 and CD57, and whose expression varies between tissues92 and may be diminished in patients with solid tumors93,94 . While informative as an activation marker, the definition of NK cells using NKp46 may be problematic because it is expressed by innate-like lymphocyte populations, including subsets associated with immunosuppression90,91. In fact, the only study in our meta-analysis to report a significantly negative association between NK cell infiltration and OS used NKp46 to define NK cells and reported a substantially higher number of infiltrating cells compared with other studies50. Noteworthy, CD56 and CD57 expression also varies on human NK cells, and CD56 can be expressed on cells of neural origin, so studies aiming to identify NK cells in vivo should include exclusion staining and redundancy in NK cell markers.
Cancer therapy – especially immunotherapy – is a rapidly evolving field, and the roles and benefits of NK cells likely intersect with treatment. Our analysis included data from treatment-naïve samples but reported survival of patients who may have undergone treatment. Patient diagnoses and therapy occurred over a period of three decades, during which treatment, cancer detection and the understanding of NK cell biology have improved. Studies did not report systematically on the impact of patient/tumor genetics, driver mutations, mutational burdens, virus infections and patient characteristics such as environmental or workplace exposures, smoking, obesity and alcohol use histories; these features may all impact the immunologic landscape of a tumor and the resulting necessary immune responses to control its growth.
Our meta-analysis reveals that NK cells are prognostically beneficial across an array of solid tumor types. Consideration of NK cells as therapy or therapeutic collaborators will be important for successful immunotherapy of solid tumors. For example, strategies to preserve or engineer NK cell expression of chemokine receptors could enhance their efficacy for solid tumor therapy148. Clinical approaches to expand and activate patients’ NK cells ex vivo may result in loss of CXCR2 expression, and genetic modification of NK cells to express CXCR2 increases migration to renal cell carcinoma in vitro149. Across an array of cancer types, infiltration of NK cells is associated with improved response to immunotherapy121. In sum, either alone or with an extra “boost” from therapeutic interventions, NK cells are highly promising effectors in solid tumor therapy. Optimizing NK cell anti-cancer efficacy will require supporting their infiltration, activation and resilience against immunosuppression in the TME.
CRediT authorship contribution statement
Sarah Nersesian: Conceptualization, Formal analysis, Data curation, Writing - review & editing, Investigation, Methodology, Supervision, Visualization. Sarah L. Schwartz: Data curation, Writing - review & editing, Investigation. Stephanie R. Grantham: Data curation, Writing - review & editing, Investigation. Leah K. MacLean: Data curation, Writing - review & editing, Investigation. Stacey N. Lee: Data curation, Writing - review & editing, Investigation. Morgan Pugh-Toole: Data curation, Writing - review & editing, Investigation. Jeanette E. Boudreau: Conceptualization, Formal analysis, Writing - review & editing, Investigation, Methodology, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors gratefully acknowledge Sam Cutler for assistance with meta-analysis code development and Kara Matheson from the Research Methods Unit at the Nova Scotia Health Authority for validation of our statistical analysis.
Funding
We gratefully acknowledge funding from the Canadian Cancer Research Institute and the Terry Fox New Investigator program to J.E.B within the Pan-Canadian Immunotherapeutic Network (iTNT). S.N. is supported by a Killam Scholarship and President's Award through Dalhousie University. S.L.S. is supported by scholarships from the Dalhousie Medicine Research Foundation (CIBC Graduate) and Research Nova Scotia. S.N., S.L.S., and S.N.L are supported by Nova Scotia Graduate Scholarships. S.N., L.K.M and S.N.L. are trainees in the Cancer Research Training Program of the Beatrice Hunter Cancer Research Institute. S.R.G. is supported by the Beatrice Hunter Cancer Research Institute through the IWK Foundation Jeremy Ingham Summer Studentship. S.N.L. is supported by a Canadian Institutes of Health Research Canadian Graduate Scholarship.
Footnotes
One sentence summary: NK cell infiltration into solid tumors is a positive prognostic factor for overall survival.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100930.
Appendix. Supplementary materials
References
- 1.Havel J.J., Chowell D., Chan T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer. 2019;19:133–150. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binnewies M. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fridman W.H., Pages F., Sautes-Fridman C., Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 4.Finotello F., Rieder D., Hackl H., Trajanoski Z. Next-generation computational tools for interrogating cancer immunity. Nat. Rev. Genet. 2019;20:724–746. doi: 10.1038/s41576-019-0166-7. [DOI] [PubMed] [Google Scholar]
- 5.Connolly James L., M., Schnitt Stuart J., MD, Wang Helen H., MD, Longtine Janina A., MD, Dvorak Ann, MD, Dvorak Harold F., MD . 6th edition. BC Decker; 2003. Holland-Frei Cancer Medicine. [Google Scholar]
- 6.Katou F. Differing phenotypes between intraepithelial and stromal lymphocytes in early-stage tongue cancer. Cancer Res. 2007;67:11195–11201. doi: 10.1158/0008-5472.CAN-07-2637. [DOI] [PubMed] [Google Scholar]
- 7.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Idos G.E. The prognostic implications of tumor infiltrating lymphocytes in colorectal cancer: a systematic review and meta-analysis. Sci. Rep. 2020;10:3360. doi: 10.1038/s41598-020-60255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang W.T., Adams S.F., Tahirovic E., Hagemann I.S., Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol. Oncol. 2012;124:192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu Q. Prognostic value of tumor-infiltrating lymphocytes in melanoma: a systematic review and meta-analysis. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2019.1593806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadler-Olsen E., Wirsing A.M. Tissue-infiltrating immune cells as prognostic markers in oral squamous cell carcinoma: a systematic review and meta-analysis. Br. J. Cancer. 2019;120:714–727. doi: 10.1038/s41416-019-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuminello S. Prognostic value of immune cells in the tumor microenvironment of early-stage lung cancer: a meta-analysis. Oncotarget. 2019;10:7142–7155. doi: 10.18632/oncotarget.27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen M., Wang J., Ren X. New insights into tumor-infiltrating b lymphocytes in breast cancer: clinical impacts and regulatory mechanisms. Front. Immunol. 2018;9:470. doi: 10.3389/fimmu.2018.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei J. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: a systemic review and meta-analysis. Oncotarget. 2016;7:34217–34228. doi: 10.18632/oncotarget.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan X. Prognostic significance of tumor-associated macrophages in ovarian cancer: a meta-analysis. Gynecol. Oncol. 2017;147:181–187. doi: 10.1016/j.ygyno.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Kannan G.S., Aquino-Lopez A., Lee D.A. Natural killer cells in malignant hematology: a primer for the non-immunologist. Blood Rev. 2017;31:1–10. doi: 10.1016/j.blre.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Gasser S., Raulet D. The DNA damage response, immunity and cancer. Semin. Cancer Biol. 2006;16:344–347. doi: 10.1016/j.semcancer.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Cheent K., Khakoo S.I. Natural killer cells: integrating diversity with function. ImmunologyImmunology. 2009;126:449–457. doi: 10.1111/j.1365-2567.2009.03045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raulet D.H., Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat. Rev. Immunol. 2009;9:568–580. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S. Licensing of natural killer cells by host major histocompatibility complex class I molecules. NatureNature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen C.M., White M.J., Goodier M.R., Riley E.M. Functional significance of CD57 expression on human NK cells and relevance to disease. Front. Immunol. 2013;4:422. doi: 10.3389/fimmu.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horowitz A. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3006702. 208ra145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lunemann A., Lunemann J.D., Munz C. Regulatory NK-cell functions in inflammation and autoimmunity. Mol. Med. 2009;15:352–358. doi: 10.2119/molmed.2009.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnould L. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism. Br. J. Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smyth M.J., Hayakawa Y., Takeda K., Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Verges S. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. BloodBlood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angelo L.S. Practical NK cell phenotyping and variability in healthy adults. Immunol. Res. 2015;62:341–356. doi: 10.1007/s12026-015-8664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srivastava R.M. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin. Cancer Res. 2013;19:1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggeri L. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. BloodBlood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu K.C. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee D.A. Cellular therapy: adoptive immunotherapy with expanded natural killer cells. Immunol. Rev. 2019;290:85–99. doi: 10.1111/imr.12793. [DOI] [PubMed] [Google Scholar]
- 32.Liu E. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020;382:545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melero I., Rouzaut A., Motz G.T., Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho Y. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res. 2003;63:1555–1559. [PubMed] [Google Scholar]
- 35.Fang J. Prognostic significance of tumor infiltrating immune cells in oral squamous cell carcinoma. BMC Cancer. 2017;17:375. doi: 10.1186/s12885-017-3317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsia J.Y. Prognostic significance of intratumoral natural killer cells in primary resected esophageal squamous cell carcinoma. Chang Gung Med. J. 2005;28:335–340. [PubMed] [Google Scholar]
- 37.Lazaris A.C., Segas J.V., Nikolopoulos T.P., Patsouris E.S. Tissue detection of natural killer cells in laryngeal carcinoma. NeoplasmaNeoplasma. 2007;54:379–382. [PubMed] [Google Scholar]
- 38.Lu J. Detailed analysis of inflammatory cell infiltration and the prognostic impact on nasopharyngeal carcinoma. Head Neck. 2018;40:1245–1253. doi: 10.1002/hed.25104. [DOI] [PubMed] [Google Scholar]
- 39.Lv L. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS ONE. 2011;6:e18219. doi: 10.1371/journal.pone.0018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoenfeld J.D. Evaluating the PD-1 axis and immune effector cell infiltration in oropharyngeal squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2018;102:137–145. doi: 10.1016/j.ijrobp.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Svensson M.C. The integrative clinical impact of tumor-infiltrating T lymphocytes and NK cells in relation to B lymphocyte and plasma cell density in esophageal and gastric adenocarcinoma. Oncotarget. 2017;8:72108–72126. doi: 10.18632/oncotarget.19437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taghavi N., Bagheri S., Akbarzadeh A. Prognostic implication of CD57, CD16, and TGF-beta expression in oral squamous cell carcinoma. J. Oral Pathol. Med. 2016;45:58–62. doi: 10.1111/jop.12320. [DOI] [PubMed] [Google Scholar]
- 43.Tsuchikawa T. Association of CD8+ T cell infiltration in oesophageal carcinoma lesions with human leucocyte antigen (HLA) class I antigen expression and survival. Clin. Exp. Immunol. 2011;164:50–56. doi: 10.1111/j.1365-2249.2010.04311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner S. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int. J. Cancer. 2016;138:2263–2273. doi: 10.1002/ijc.29962. [DOI] [PubMed] [Google Scholar]
- 45.Xu B. Prognostic value of tumor infiltrating NK cells and macrophages in stage II+III esophageal cancer patients. Oncotarget. 2016;7:74904–74916. doi: 10.18632/oncotarget.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zancope E. Differential infiltration of CD8+ and NK cells in lip and oral cavity squamous cell carcinoma. J. Oral Pathol. Med. 2010;39:162–167. doi: 10.1111/j.1600-0714.2009.00792.x. [DOI] [PubMed] [Google Scholar]
- 47.Honkanen T.J. Prognostic and predictive role of spatially positioned tumour infiltrating lymphocytes in metastatic HER2 positive breast cancer treated with trastuzumab. Sci. Rep. 2017;7:18027. doi: 10.1038/s41598-017-18266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muntasell A. NK cell infiltrates and HLA class I expression in primary HER2(+) breast cancer predict and uncouple pathological response and disease-free survival. Clin. Cancer Res. 2019;25:1535–1545. doi: 10.1158/1078-0432.CCR-18-2365. [DOI] [PubMed] [Google Scholar]
- 49.Park M.H., Lee J.S., Yoon J.H. High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J. Surg. Oncol. 2012;106:386–392. doi: 10.1002/jso.23095. [DOI] [PubMed] [Google Scholar]
- 50.Rathore A.S., Goel M.M., Makker A., Kumar S., Srivastava A.N. Is the tumor infiltrating natural killer cell (NK-TILs) count in infiltrating ductal carcinoma of breast prognostically significant? Asian Pac. J. Cancer Prev. 2014;15:3757–3761. doi: 10.7314/apjcp.2014.15.8.3757. [DOI] [PubMed] [Google Scholar]
- 51.Tian W. A prognostic risk model for patients with triple negative breast cancer based on stromal natural killer cells, tumor-associated macrophages and growth-arrest specific protein 6. Cancer Sci. 2016;107:882–889. doi: 10.1111/cas.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Triki H. CD155 expression in human breast cancer: clinical significance and relevance to natural killer cell infiltration. Life Sci. 2019;231 doi: 10.1016/j.lfs.2019.116543. [DOI] [PubMed] [Google Scholar]
- 53.Vgenopoulou S. Immunohistochemical evaluation of immune response in invasive ductal breast cancer of not-otherwise-specified type. Breast. 2003;12:172–178. doi: 10.1016/s0960-9776(03)00004-3. [DOI] [PubMed] [Google Scholar]
- 54.Wang B. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 2014;74:5746–5757. doi: 10.1158/0008-5472.CAN-13-2563. [DOI] [PubMed] [Google Scholar]
- 55.Alderdice M. Natural killer-like signature observed post therapy in locally advanced rectal cancer is a determinant of pathological response and improved survival. Mod. Pathol. 2017;30:1287–1298. doi: 10.1038/modpathol.2017.47. [DOI] [PubMed] [Google Scholar]
- 56.Coca S. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. CancerCancer. 1997;79:2320–2328. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 57.Lim S.H. Effect of neoadjuvant chemoradiation on tumor-infiltrating/associated lymphocytes in locally advanced rectal cancers. Anticancer Res. 2014;34:6505–6513. [PubMed] [Google Scholar]
- 58.Liska V. Infiltration of colorectal carcinoma by S100+ dendritic cells and CD57+ lymphocytes as independent prognostic factors after radical surgical treatment. Anticancer Res. 2012;32:2129–2132. [PubMed] [Google Scholar]
- 59.Menon A.G. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab. Invest. 2004;84:493–501. doi: 10.1038/labinvest.3700055. [DOI] [PubMed] [Google Scholar]
- 60.Sconocchia G. Tumor infiltration by FcgammaRIII (CD16)+ myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int. J. Cancer. 2011;128:2663–2672. doi: 10.1002/ijc.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sconocchia G. NK cells and T cells cooperate during the clinical course of colorectal cancer. Oncoimmunology. 2014;3 doi: 10.4161/21624011.2014.952197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amoueian S., Attaranzadeh A., Montazer M. Intratumoral CD68-, CD117-, CD56-, and CD1a-positive immune cells and the survival of Iranian patients with non-metastatic intestinal-type gastric carcinoma. Pathol. Res. Pract. 2015;211:326–331. doi: 10.1016/j.prp.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 63.Ishigami S. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer-Am. Cancer Soc. 2000;88:577–583. [PubMed] [Google Scholar]
- 64.Pernot S. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer. 2020;23:73–81. doi: 10.1007/s10120-019-00983-3. [DOI] [PubMed] [Google Scholar]
- 65.Rusakiewicz S. Immune infiltrates are prognostic factors in localized gastrointestinal stromal tumors. Cancer Res. 2013;73:3499–3510. doi: 10.1158/0008-5472.CAN-13-0371. [DOI] [PubMed] [Google Scholar]
- 66.Platonova S. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–5422. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 67.Takanami I., Takeuchi K., Giga M. The prognostic value of natural killer cell infiltration in resected pulmonary adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2001;121:1058–1063. doi: 10.1067/mtc.2001.113026. [DOI] [PubMed] [Google Scholar]
- 68.Villegas F.R. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 69.Yamada N. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol. Immunother. 2010;59:1543–1549. doi: 10.1007/s00262-010-0881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chew V. Toll-like receptor 3 expressing tumor parenchyma and infiltrating natural killer cells in hepatocellular carcinoma patients. J. Natl. Cancer Inst. 2012;104:1796–1807. doi: 10.1093/jnci/djs436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Y. Monocyte/macrophage-elicited natural killer cell dysfunction in hepatocellular carcinoma is mediated by CD48/2B4 interactions. Hepatology. 2013;57:1107–1116. doi: 10.1002/hep.26192. [DOI] [PubMed] [Google Scholar]
- 72.Zhao J.J. Interleukin-37 mediates the antitumor activity in hepatocellular carcinoma: role for CD57+ NK cells. Sci. Rep. 2014;4:5177. doi: 10.1038/srep05177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu L.Y., Zhou J., Liu Y.Z., Pan W.D. [Prognostic significance of natural killer cell infiltration in hepatocellular carcinoma] Ai Zheng. 2009;28:1198–1202. doi: 10.5732/cjc.009.10284. [DOI] [PubMed] [Google Scholar]
- 74.Henriksen J.R. Favorable prognostic impact of Natural Killer cells and T cells in high-grade serous ovarian carcinoma. Acta Oncol. 2020;59:652–659. doi: 10.1080/0284186X.2019.1711173. [DOI] [PubMed] [Google Scholar]
- 75.Li K. Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol. Immunother. 2009;58:641–652. doi: 10.1007/s00262-008-0585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ino K. Inverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin. Cancer Res. 2008;14:2310–2317. doi: 10.1158/1078-0432.CCR-07-4144. [DOI] [PubMed] [Google Scholar]
- 77.Versluis M.A.C. The prognostic benefit of tumour-infiltrating Natural Killer cells in endometrial cancer is dependent on concurrent overexpression of Human Leucocyte Antigen-E in the tumour microenvironment. Eur. J. Cancer. 2017;86:285–295. doi: 10.1016/j.ejca.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Zinovkin D., Pranjol M.Z. Tumor-infiltrated lymphocytes, macrophages, and dendritic cells in endometrioid adenocarcinoma of corpus uteri as potential prognostic factors: an immunohistochemical study. Int. J. Gynecol. Cancer. 2016;26:1207–1212. doi: 10.1097/IGC.0000000000000758. [DOI] [PubMed] [Google Scholar]
- 79.Sznurkowski J.J., Zawrocki A., Biernat W. Subtypes of cytotoxic lymphocytes and natural killer cells infiltrating cancer nests correlate with prognosis in patients with vulvar squamous cell carcinoma. Cancer Immunol. Immunother. 2014;63:297–303. doi: 10.1007/s00262-013-1511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jasinski-Bergner S. Clinical relevance of miR-mediated HLA-G regulation and the associated immune cell infiltration in renal cell carcinoma. Oncoimmunology. 2015;4 doi: 10.1080/2162402X.2015.1008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jensen H.K. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J. Clin. Oncol. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 82.Sorbye S.W. Prognostic impact of CD57, CD68, M-CSF, CSF-1R, Ki67 and TGF-beta in soft tissue sarcomas. BMC Clin. Pathol. 2012;12:7. doi: 10.1186/1472-6890-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erdag G. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lundgren S. The prognostic impact of NK/NKT cell density in periampullary adenocarcinoma differs by morphological type and adjuvant treatment. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0156497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakakubo Y. Clinical significance of immune cell infiltration within gallbladder cancer. Br. J. Cancer. 2003;89:1736–1742. doi: 10.1038/sj.bjc.6601331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaquero J. Presence and significance of NK cells in glioblastomas. J. Neurosurg. 1989;70:728–731. doi: 10.3171/jns.1989.70.5.0728. [DOI] [PubMed] [Google Scholar]
- 87.Rosen R.D., Sapra A. 2020. StatPearls. [Google Scholar]
- 88.Sznurkowski J.J., Zawrocki A., Emerich J., Biernat W. Prognostic significance of CD4+ and CD8+ T cell infiltration within cancer cell nests in vulvar squamous cell carcinoma. Int. J. Gynecol. Cancer. 2011;21:717–721. doi: 10.1097/IGC.0b013e3182131f36. [DOI] [PubMed] [Google Scholar]
- 89.Mamessier E. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J. Immunol. 2013;190:2424–2436. doi: 10.4049/jimmunol.1200140. [DOI] [PubMed] [Google Scholar]
- 90.Nagasawa M. KLRG1 and NKp46 discriminate subpopulations of human CD117(+)CRTH2(-) ILCs biased toward ILC2 or ILC3. J. Exp. Med. 2019;216:1762–1776. doi: 10.1084/jem.20190490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crome S.Q. A distinct innate lymphoid cell population regulates tumor-associated T cells. Nat. Med. 2017;23:368–375. doi: 10.1038/nm.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tomasello E. Mapping of NKp46(+) cells in healthy human lymphoid and non-lymphoid tissues. Front. Immunol. 2012;3:344. doi: 10.3389/fimmu.2012.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pasero C. Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Cancer Res. 2016;76:2153–2165. doi: 10.1158/0008-5472.CAN-15-1965. [DOI] [PubMed] [Google Scholar]
- 94.Krijgsman D. Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer Immunol. Immunother. 2019;68:1011–1024. doi: 10.1007/s00262-019-02343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schantz S.P., Shillitoe E.J., Brown B., Campbell B. Natural killer cell activity and head and neck cancer: a clinical assessment. J. Natl. Cancer Inst. 1986;77:869–875. [PubMed] [Google Scholar]
- 96.Bisheshar S.K., De Ruiter E.J., Devriese L.A., Willems S.M. The prognostic role of NK cells and their ligands in squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Oncoimmunology. 2020;9 doi: 10.1080/2162402X.2020.1747345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dekker E., Tanis P.J., Vleugels J.L.A., Kasi P.M., Wallace M.B. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 98.Ho S.W.T., Tan P. Dissection of gastric cancer heterogeneity for precision oncology. Cancer Sci. 2019;110:3405–3414. doi: 10.1111/cas.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ishigami S. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett. 2000;159:103–108. doi: 10.1016/s0304-3835(00)00542-5. [DOI] [PubMed] [Google Scholar]
- 100.Barta J.A., Powell C.A., Wisnivesky J.P. Global epidemiology of lung cancer. Ann. Glob. Health. 2019;85 doi: 10.5334/aogh.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yuan F., Lu L., Zou Q. Analysis of gene expression profiles of lung cancer subtypes with machine learning algorithms. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165822. [DOI] [PubMed] [Google Scholar]
- 102.Liu Z. The trends in incidence of primary liver cancer caused by specific etiologies: results from the global burden of disease study 2016 and implications for liver cancer prevention. J. Hepatol. 2019;70:674–683. doi: 10.1016/j.jhep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 103.Liu P., Chen L., Zhang H. Natural killer cells in liver disease and hepatocellular carcinoma and the NK cell-based immunotherapy. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/1206737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Labidi-Galy S.I. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 2017;8:1093. doi: 10.1038/s41467-017-00962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosen D.G. Ovarian cancer: pathology, biology, and disease models. Front. Biosci. (Landmark Ed.) 2009;14:2089–2102. doi: 10.2741/3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodriguez A.C., Blanchard Z., Maurer K.A., Gertz J. Estrogen signaling in endometrial cancer: a key oncogenic pathway with several open questions. Horm. Cancer. 2019;10:51–63. doi: 10.1007/s12672-019-0358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jonasch E., Gao J., Rathmell W.K. Renal cell carcinoma. BMJ. 2014;349:g4797. doi: 10.1136/bmj.g4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bromwich E.J. The relationship between T-lymphocyte infiltration, stage, tumour grade and survival in patients undergoing curative surgery for renal cell cancer. Br. J. Cancer. 2003;89:1906–1908. doi: 10.1038/sj.bjc.6601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Linch M., Miah A.B., Thway K., Judson I.R., Benson C. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat. Rev. Clin. Oncol. 2014;11:187–202. doi: 10.1038/nrclinonc.2014.26. [DOI] [PubMed] [Google Scholar]
- 110.Domingues B., Lopes J.M., Soares P., Populo H. Melanoma treatment in review. Immunotargets Ther. 2018;7:35–49. doi: 10.2147/ITT.S134842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matthews N.H., Li W.Q., Qureshi A.A., Weinstock M.A., Cho E. In: Ward W.H., Farma J.M., editors. 2017. [Google Scholar]
- 112.McDermott D. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat. Rev. 2014;40:1056–1064. doi: 10.1016/j.ctrv.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 113.Beger H.G., Mayer B., Rau B.M. Parenchyma-sparing, limited pancreatic head resection for benign tumors and low-risk periampullary cancer-a systematic review. J. Gastrointest. Surg. 2016;20:206–217. doi: 10.1007/s11605-015-2981-2. [DOI] [PubMed] [Google Scholar]
- 114.Davis M.E. Glioblastoma: overview of disease and treatment. Clin. J. Oncol. Nurs. 2016;20:S2–S8. doi: 10.1188/16.CJON.S1.2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Balatoni T. Tumor-infiltrating immune cells as potential biomarkers predicting response to treatment and survival in patients with metastatic melanoma receiving ipilimumab therapy. Cancer Immunol. Immunother. 2018;67:141–151. doi: 10.1007/s00262-017-2072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aguilar-Cazares D. Relationship of dendritic cell density, HMGB1 expression, and tumor-infiltrating lymphocytes in non-small cell lung carcinomas. Appl. Immunohistochem. Mol. Morphol. 2014;22:105–113. doi: 10.1097/PAI.0b013e3182849808. [DOI] [PubMed] [Google Scholar]
- 117.Webb J.R., Milne K., Watson P., Deleeuw R.J., Nelson B.H. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res. 2014;20:434–444. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- 118.Wang B. CD103+ tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J. Urol. 2015;194:556–562. doi: 10.1016/j.juro.2015.02.2941. [DOI] [PubMed] [Google Scholar]
- 119.Bhat R., Watzl C. Serial killing of tumor cells by human natural killer cells-enhancement by therapeutic antibodies. PLoS ONE. 2007;2:e326. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Srpan K. Shedding of CD16 disassembles the NK cell immune synapse and boosts serial engagement of target cells. J. Cell Biol. 2018;217:3267–3283. doi: 10.1083/jcb.201712085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huntington N.D., Cursons J., Rautela J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer. 2020 doi: 10.1038/s41568-020-0272-z. [DOI] [PubMed] [Google Scholar]
- 122.Solocinski K. Overcoming hypoxia-induced functional suppression of NK cells. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2019-000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Trefny M.P. PD-1(+) natural killer cells in human non-small cell lung cancer can be activated by PD-1/PD-L1 blockade. Cancer Immunol. Immunother. 2020 doi: 10.1007/s00262-020-02558-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hsu J. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest. 2018;128:4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Andre P. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. CellCell. 2018;175:1731–1743.e1713. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bottcher A. Gene expression profiling of circulating natural killer cells in head and neck squamous cell carcinoma. Cancer Genomics Proteomics. 2013;10:197–207. [PubMed] [Google Scholar]
- 127.Weil S. Natural killer group 2D ligand depletion reconstitutes natural killer cell immunosurveillance of head and neck squamous cell carcinoma. Front. Immunol. 2017;8:387. doi: 10.3389/fimmu.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu G., Ren H., Sun X.J., Shi J.S. Distribution of natural killer cells and T-lymphocyte subsets in peripheral blood, gallbladder cancer and surrounding tissue. Hepatobiliary Pancreat. Dis. Int. 2007;6:81–86. [PubMed] [Google Scholar]
- 129.Nersesian S., Glazebrook H., Toulany J., Grantham S.R., Boudreau J.E. Naturally killing the silent killer: NK cell-based immunotherapy for ovarian cancer. Front. Immunol. 2019;10:1782. doi: 10.3389/fimmu.2019.01782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Viel S. TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 2016;9:ra19. doi: 10.1126/scisignal.aad1884. [DOI] [PubMed] [Google Scholar]
- 131.Cortez V.S. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat. Immunol. 2017;18:995–1003. doi: 10.1038/ni.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Young A. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. 2018;78:1003–1016. doi: 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 133.Porembka M.R. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol. Immunother. 2012;61:1373–1385. doi: 10.1007/s00262-011-1178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shen P., Wang A., He M., Wang Q., Zheng S. Increased circulating Lin(-/low) CD33(+) HLA-DR(-) myeloid-derived suppressor cells in hepatocellular carcinoma patients. Hepatol. Res. 2014;44:639–650. doi: 10.1111/hepr.12167. [DOI] [PubMed] [Google Scholar]
- 135.Arihara F. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol. Immunother. 2013;62:1421–1430. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang L. Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J. Immunol. 2013;190:794–804. doi: 10.4049/jimmunol.1202088. [DOI] [PubMed] [Google Scholar]
- 137.Huang A. Increased CD14(+)HLA-DR (-/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol. Immunother. 2013;62:1439–1451. doi: 10.1007/s00262-013-1450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vasquez-Dunddel D. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J. Clin. Invest. 2013;123:1580–1589. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Friese M.A. RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 2004;64:7596–7603. doi: 10.1158/0008-5472.CAN-04-1627. [DOI] [PubMed] [Google Scholar]
- 140.Boudreau J.E., Hsu K.C. Natural killer cell education in human health and disease. Curr. Opin. Immunol. 2018;50:102–111. doi: 10.1016/j.coi.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Boudreau J.E., Hsu K.C. Natural killer cell education and the response to infection and cancer therapy: stay tuned. Trends Immunol. 2018;39:222–239. doi: 10.1016/j.it.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khan M., Arooj S., Wang H. NK cell-based immune checkpoint inhibition. Front. Immunol. 2020;11:167. doi: 10.3389/fimmu.2020.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vey N. A phase 1 study of lirilumab (antibody against killer immunoglobulin-like receptor antibody KIR2D; IPH2102) in patients with solid tumors and hematologic malignancies. Oncotarget. 2018;9:17675–17688. doi: 10.18632/oncotarget.24832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Davis Z.B., Vallera D.A., Miller J.S., Felices M. Natural killer cells unleashed: checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin. Immunol. 2017;31:64–75. doi: 10.1016/j.smim.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ben-Shmuel A., Biber G., Barda-Saad M. Unleashing natural killer cells in the tumor microenvironment-the next generation of immunotherapy? Front. Immunol. 2020;11:275. doi: 10.3389/fimmu.2020.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Seillet C., Belz G.T., Huntington N.D. Development, homeostasis, and heterogeneity of NK cells and ILC1. Curr. Top. Microbiol. Immunol. 2016;395:37–61. doi: 10.1007/82_2015_474. [DOI] [PubMed] [Google Scholar]
- 147.Taube J.M. The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2019-000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wennerberg E., Kremer V., Childs R., Lundqvist A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol. Immunother. 2015;64:225–235. doi: 10.1007/s00262-014-1629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kremer V. Genetic engineering of human NK cells to express CXCR2 improves migration to renal cell carcinoma. J. Immunother. Cancer. 2017;5:73. doi: 10.1186/s40425-017-0275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.