Abstract
Purpose
High flow nasal cannula (HFNC) is a relatively recent respiratory support technique which delivers high flow, heated and humidified controlled concentration of oxygen via the nasal route. Recently, its use has increased for a variety of clinical indications. To guide clinical practice, we developed evidence-based recommendations regarding use of HFNC in various clinical settings.
Methods
We formed a guideline panel composed of clinicians, methodologists and experts in respiratory medicine. Using GRADE, the panel developed recommendations for four actionable questions.
Results
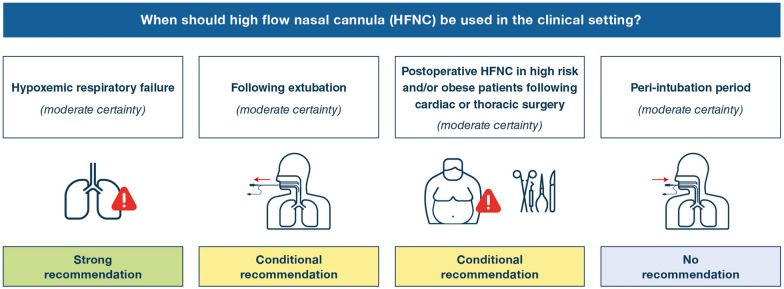
The guideline panel made a strong recommendation for HFNC in hypoxemic respiratory failure compared to conventional oxygen therapy (COT) (moderate certainty), a conditional recommendation for HFNC following extubation (moderate certainty), no recommendation regarding HFNC in the peri-intubation period (moderate certainty), and a conditional recommendation for postoperative HFNC in high risk and/or obese patients following cardiac or thoracic surgery (moderate certainty).
Conclusions
This clinical practice guideline synthesizes current best-evidence into four recommendations for HFNC use in patients with hypoxemic respiratory failure, following extubation, in the peri-intubation period, and postoperatively for bedside clinicians.
Electronic supplementary material
The online version of this article (10.1007/s00134-020-06312-y) contains supplementary material, which is available to authorized users.
Keywords: High flow nasal cannula, Respiratory failure, Extubation, Peri-intubation, Postoperative, Mortality
Take-home message
| The guideline panel made a strong recommendation for HFNC in hypoxemic respiratory failure (moderate certainty), a conditional recommendation for HFNC following extubation (moderate certainty), no recommendation regarding HFNC in the peri-intubation period (moderate certainty), and a conditional recommendation for postoperative HFNC in high risk and/or obese patients following cardiac or thoracic surgery (moderate certainty) |
Introduction
Clinicians use various non-invasive modalities to deliver oxygen to patients. Each modality is associated with specific advantages and disadvantages. Two of the most commonly used oxygen-delivery modalities, traditional nasal cannula and regular or Venturi masks, typically accommodate flow rates of around 15 L (L) per minute (although Venturi can provide total gas flow > 60L/min) and therefore have limited ability to meet the inspiratory demands of patients, especially patients with dyspnea [1]. The high flow nasal cannula (HFNC) has recently garnered interest as a system that is capable of delivering high flow of 30–60L/min of heated and humidified gas at a controlled concentration of oxygen via the nasal route [2]. Despite these potential benefits, use of HFNC for various clinical scenarios is variable and clinicians lack evidence-based guidance. We developed a clinical practice guideline to help clinicians regarding HFNC use for four specific clinical indications.
Methods
Scope and panel composition
The PLUG (https://www.plugwgroup.org/), a working group of the European Society of Intensive Care Medicine (ESICM) nominated a joint panel of experts to develop guidelines for respiratory support using HFNC. The panel includes intensive care physicians, respirologists and five clinician-methodologists (BR, SE, KB, DC, YH) with experience in guideline development using Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology [3]. The executive group for the guideline included a smaller subset of experts and methodologists (BR, TM, JM, KB, SE, LB). ESCIM provided videoconference software and meeting space for face-to-face panel meetings. We did not receive financial support to develop this clinical practice guideline.
Following initial discussions, panel members identified four actionable PICO (patients, intervention, comparator, outcomes) questions. We prioritized questions of importance to stakeholders and in areas where practice variation was expected to exist based on widespread HFNC use for selected clinical conditions [4, 5].
Conflict of interest (COI) policy
All panel members were required to disclose all potential financial conflicts of interested prior to guideline initiation. An ad-hoc COI management committee was chaired by one member of the guideline executive (JM) and two other guideline panel members (RDS and ASS). Panel members judged to have important financial COI ($5,000 or higher) were able to participate in discussion around the evidence but were excluded from voting (where required) and formulating recommendations.
Literature search
Panel members rated outcomes based on perceived importance to patients for clinical decision-making on a scale of 1 (not important) to 9 (critically important). Working with a medical librarian, methodologists conducted systematic reviews of the literature to seek studies examining the use of the HFNC for four indications: hypoxic respiratory failure, peri-intubation, post extubation and post-operative usage. We searched MEDLINE, EMBASE and Web of Science from January 1st 2007 through November 1st 2019 as HFNC was not widely used in adults prior to 2007. We included randomised controlled studies (RCTs) conducted in adults that compared HFNC use to either conventional oxygen therapy (COT), continuous positive airway pressure (CPAP) or NIPPV and reported one or more outcomes of interest. We limited our search to trials published in English. We considered prior meta-analyses that met acceptable quality standards. We also reviewed the reference lists of eligible trials, reviews, and meta-analyses and inquired with panel experts to ensure that no trials were missed. To respond to the actionable PICO questions, we focused on RCTs. To address the non-actionable narrative questions, we also identified observational studies. Although we did not conduct a formal systematic review for patient values and preferences, we retained any relevant information found addressing these from the literature search. We searched clinical trial registries (clinicaltrials.gov, controlled-trials.com, anzctr.org.au, and who.int/ictrp) to identify trials currently in progress. Further details on our search and methodology can be found in standalone published meta-analyses performed to support this guideline [6, 7].
Data collection and analysis
Two methodologists (SE, YH) screened titles and abstracts and subsequently full-text manuscripts independently and in duplicate. Similarly, multiple methodologists (DC, BR, YH, SE) performed data extraction and risk of bias assessment independently and in duplicate for each included trial. We assessed risk of bias using the modified Cochrane Risk of Bias tool [8] which assesses random sequence generation (selection bias), allocation concealment (selection bias), blinding of outcome assessors (performance and detection bias), incomplete outcome data, intention-to-treat (attrition bias) and selective reporting. Trials were assigned a risk of bias corresponding to the highest rating for any of these domains.
Evidence summaries
We used Revman v.5.3 software for pooled analysis using inverse variance weighting and random effects models. We generated an evidence profile for each of the PICO questions [9]. Following GRADE methodology, certainty in each outcome was rated as high, moderate, low or very low [10]. Data from RCTs started as high certainty and data from observational studies started as low certainty evidence. We subsequently downgraded certainty by one or two level for concerns related to individual study risk of bias (RoB), inconsistency, indirectness, imprecision or publication bias.
Formulation of recommendations
The panel developed recommendations using the GRADE Evidence-to-Decision framework which considers the certainty in the evidence, the balance between desirable and undesirable effects (positive effects and negative effects), patient values and preferences, resource use, health equity, acceptability and feasibility [11]. We designated recommendations as strong (using the phrasing “we recommend”) or conditional (using the phrasing “we suggest”) [12]. Table 1 describes the implications of the strength of a recommendation. We finalized the recommendations at an in-person meeting in Brussels on November 4, 2019. The final wording of each recommendation was reviewed, approved, and voted on by panel members without COI. Figure 1 summarises our recommendations.
Table 1.
Interpretation of strong and conditional recommendations for stakeholders (patients, clinicians and policymakers)
| Strong recommendation | Conditional recommendation | |
|---|---|---|
| For patients | Most individuals in this situation would want the recommended course of action and only a small proportion would not | The majority of individuals in this situation would want the suggested course of action, but many would not |
| For clinicians | Most individuals should receive the recommended course of action. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences | Different choices are likely to be appropriate for different patients and therapy should be tailored to the individual patient’s circumstances. Those circumstances may include the patient or family’s values and preferences |
| For policy makers | The recommendation can be adapted as policy in most situations including for the use as performance indicators | Policy making will require substantial debates and involvement of many stakeholders. Policies are also more likely to vary between regions. Performance indicators would have to focus on the fact that adequate deliberation about the management options has taken place |
Fig. 1.
Scheme of recommendations
For non-actionable PICO questions, we drafted narratives using studies identified as part of the literature search. These drafts were circulated among panel members for review and approval.
Manuscript preparation
After generating the recommendations, the panel divided into writing groups focusing on each of the eight questions. Editing and feedback were coordinated by the panel executive and accomplished through electronic communication.
As new practice changing RCTs are published, guideline members will plan to update the guideline accordingly.
Actionable PICO Questions:
PICO 1: Acute hypoxemic respiratory failure
Recommendation
We recommend using HFNC compared to COT for patients with hypoxemic respiratory failure (strong recommendation, moderate certainty evidence).
Evidence summary
Nine trials compared HFNC to COT in this population [13–21]. Five trials were conducted in the ICU setting [13, 15, 17, 19, 21] and four trials were conducted in the Emergency Department (ED) [14, 16, 18, 20]. One trial [18] only included patients with acute pulmonary edema and two trials [10, 14] examined exclusively immune compromised patients. All included trials were unblinded. Four trials [14, 16, 17, 20] were judged to be at high risk of bias due to issues with features related to trial quality. The meta-analysis supporting this recommendation has been published elsewhere [6]. See the supplementary material to review the evidence profiles (Suppl material 1) and for a discussion of physiologic mechanisms (Suppl material 2).
Compared to COT, HFNC use decreased the need for intubation relative risk (RR) 0.85 (95% confidence interval [CI] 0.74–0.99; 7 trials, n = 1647, moderate certainty) and escalation of respiratory support RR 0.71 (0.51–0.98; 8 trials, n = 1703, moderate certainty). We lowered the certainty of both outcomes due to imprecision. We did not find evidence of an effect of HFNC compared to COT on mortality (moderate certainty), ICU length of stay (low certainty), hospital length of stay (moderate certainty), patient reported dyspnea (low certainty) and comfort (very low certainty). Complications of therapy were variably reported across the included trials which did not permit quantitative pooling; however, qualitative assessment did not suggest an increased risk of complications for HFNC-treated patients.
Although we pre-planned subgroup analyses based on several factors (hypoxemic versus hypercapnic respiratory failure, pulmonary edema versus other causes, immune compromised versus immunocompetent, mild versus severe hypoxia, bilateral infiltrates versus no bilateral infiltrates), there were insufficient data to perform these analyses.
Rationale
For patients with hypoxemic respiratory failure, the anticipated desirable effects of HFNC when compared to COT include a reduction in the rates of intubation (moderate certainty) and escalation of respiratory support (moderate certainty). HFNC may have a small effect on comfort and dyspnea; however, the effect of HFNC on mortality and length of stay was much less certain. The panel judged the overall desirable effects to be moderate—understanding that any decrease in intubation was almost certainly important to patients.
The panel judged the anticipated undesirable effects of HFNC compared to COT and NIPPV to be minimal. Adverse events directly related to HFNC are uncommon and have been mostly reported in children and infants [22–26]. The use of HFNC may delay intubation, however the due to the heterogeneity of study designs and risk of confounding we were not able to pool data evaluating this outcome. A large retrospective study evaluated the effect on patient outcome of the timing of intubation after failure of HFNC [27]. Patients intubated within 48 h had lower ICU mortality (39 vs. 67%; P = 0.001) than those intubated later. A similar association was observed with extubation success, ventilator weaning and ventilator-free days. Noticeably, patients in the early intubation group were intubated a mean of 10 h after initiating HFNC, while those in the late intubation group were intubated at a mean of 126 h, more than 5 days, after starting HFNC. Hence, this study actually compares early intubation to “very late” rather than late intubation. Post-hoc analysis in of the FLORALI trial did not demonstrate this potential harm of HFNC in delaying intubation as no association between timing of intubation and risk of mortality was demonstrated [28]. These studies evaluating the risk of delayed intubation are conflicting however the panel was reassured that there was no increase in mortality or duration of ICU/hospital length of stay with HFNC, as would be expected there was an important delay in intubation.
Although not reported in the included trials, nasal bleeding related to HFNC use was a concern and may limit treatment with HFNC. Importantly, patients randomized to HFNC had reduced rates of intubation and similar survival and length of stay compared to COT. Overall, the panel judged the balance of desirable and undesirable effects to favor use of HFNC over COT in patients with acute hypoxemic respiratory failure.
The panel did not identify important considerations with regard to patient’s values and preferences (how different patients may place different levels of importance on each outcome of interest) as most patients value avoiding intubation. Cost-effectiveness data suggests a net cost savings with HFNC compared to COT in the range of 500–1000 British Pounds per patient (600–1200 US Dollars or Euros [equivalent currency]) [29]. This cost-effectiveness considers both the cost of the equipment but also the cost savings in intubations avoided. Consequently, HFNC was judged by the panel to be associated with at least moderate cost savings. We did not find any cost data evaluating the comparison of HFNC with NIPPV. The panel judged HFNC use (as compared to COT or NIPPV) to be both acceptable and feasible to implement. However, for of all the reasons mentioned above, constant monitoring of the patients and an experienced assessment of their response to treatment are critical and may require appropriate human resources. HFNC should therefore be initiated in an environment that has sufficient staff to closely monitor the patient’s clinical course and that is well trained to recognize the early signs of failure.
The panel debated between a conditional or strong recommendation regarding the use of HFNC for acute respiratory failure. This recommendation was the only recommendation that required a formal vote amongst the panel; however, the majority of panel members favored making a strong recommendation. Those who favored a conditional recommendation recognized the persistent uncertainty regarding the risks associated with delayed intubation and specific populations that may not benefit from HFNC. At this time, patients with acute hypoxemic respiratory failure requiring high HFNC settings (flow and/or FiO2) should be managed in a monitored setting as they have a high likelihood of decompensating and requiring invasive ventilation.
Research priorities
Despite the number of RCTs studying HFNC in patients with hypoxemic respiratory failure, further data are required to improve precision in point estimates. Future research should focus on specific patient populations to determine which patients derive the greatest benefit from HFNC support (e.g. congestive heart failure, COPD, septic shock, those with moderate to severe hypoxemia (PF ratio < 200 mmHg). Also, we require more data examining HFNC as compared to NIPPV in many of these populations. Additional research is needed to inform implementation considerations including the setting in which patients are managed (ICU, wards, step-up or step-down units). There also remain several areas of uncertainty (optimal settings, early detection of patients who are likely to fail, protocols, methods to escalate and de-escalate support, and weaning strategies) to guide HFNC use in patients with acute hypoxemic respiratory failure. Cost-effectiveness data are needed to clarify the impact of widespread HFNC use for hypoxemic patients in different healthcare contexts. Data are needed regarded the effects of HFNC in low income countries given differing etiologies for respiratory failure and co-interventions in this setting. Finally, recent studies have examined co-administration of HFNC and NIPPV so as to combine the benefits of both interventions. Further research is needed to explore the role of multimodal support strategies.
PICO 2: Post-extubation respiratory failure
Recommendation
We suggest HFNC as compared to COT following extubation for patients who are intubated more than 24 h and have any high-risk feature (conditional recommendation, moderate certainty evidence).
For patients who clinicians would normally extubate to NIPPV, we suggest continued use of NIPPV as opposed to HFNC (conditional recommendation, low certainty evidence).
Evidence summary
Five trials [30–34] compared @@HFNC vs COT and three trials [35–37] compared HFNC to NIPPV (CPAP or bi-level NIPPV). Of the eight trials, seven were judged to be low risk of bias and one trial had insufficient information to assess risk of bias [36]. Although the definition for high risk was variably defined amongst included trials, the largest defined it as at least 1 of: age over 65, congestive heart failure, moderate-severe COPD, APACHE II score > 12, BMI > 30, airway patency or secretion problems, difficulty weaning, two or more comorbidities or duration of mechanical ventilation over 7 days [35]. See the supplementary material to review the evidence profiles (Suppl material 1) and for a discussion of physiologic mechanisms (Suppl material 2).
Compared to COT, HFNC reduced reintubation (RR 0.46 [0.30–0.70; 4 trials, n = 847, moderate certainty]) with no important inconsistency and reduced post-extubation respiratory failure (RR 0.52 [0.30–0.91; 3 trials, n = 787, very low certainty]). The certainty in post-extubation respiratory failure was lowered for important inconsistency, indirectness and imprecision.
Compared to NIPPV, HFNC had no effect on the rates of reintubation (low certainty) with no inconsistency or post-extubation respiratory failure (very low certainty). We lowered certainty based on considerations related to risk of bias, indirectness, and imprecision. Only one trial [37] examined comfort which favored HFNC (moderate certainty).
We did not identify effects of HFNC vs COT or NIV on mortality (moderate certainty), need for escalation to NIV (moderate certainty, COT comparison only), or ICU (moderate certainty) and hospital LOS (moderate certainty). Also, we did not identify credible subgroup effects for any outcome when comparing high vs low risk populations, obese versus non-obese patients or based on risk of bias.
Rationale
We identified desirable effects of HFNC vs. COT in reducing rates of reintubation (moderate certainty) and post-extubation respiratory failure (very low certainty) recognizing that trial authors variably defined the latter outcome. Conversely, we found that compared to NIPPV, HFNC had no effect on rates of reintubation (low certainty) and post-extubation respiratory failure (very low certainty). The desirable effects of HFNC compared to COT were larger than when HFNC was compared with NIPPV, where treatment effects with HFNC were more variable and uncertain.
The undesirable effects of HFNC compared to both COT and NIPPV were small. For a more detailed discussion on the risks of HFNC, please see PICO 1. On balance, the evidence supported use of HFNC compared to COT but neither favored nor disfavored use of HFNC over NIPPV, especially when patients were already being treated with NIPPV.
We did not identify important considerations related to patient values and preferences. We did not have sufficient information to assess resource and health equity considerations and anticipated that cost-effectiveness of HFNC (vs. COT) would vary based on jurisdiction. We judged HFNC use (compared to COT and NIPPV) to be both acceptable and feasible. The panel identified the fact that patients that are intubated for very short periods of time (e.g., < 24 h) and have no high-risk features may not need HFNC. A number of important practical questions remain including how long to use HFNC and how to de-escalate HFNC use in this population (see research priorities).
Research priorities
Further research is needed to identify the subgroups (e.g. medical versus surgical) of patients most likely to benefit from use of the HFNC among those at risk for extubation failure. As all of the trials were conducted in high income countries, it is unclear if the results and recommendation are generalizable to low income settings. Most trials examined prophylactic application of HFNC after extubation. Consequently, data are needed to assess the role of HFNC, if any, once post-extubation respiratory failure has developed [38].
For patients who are treated with HFNC after extubation, we have minimal data to guide treatment duration and de-escalation. More data regarding the potential role for combination treatment (HFNC combined with NIPPV) are needed. Finally, cost-effectiveness data are needed to inform policy regarding HFNC use after extubation.
PICO 3: Peri-intubation
Recommendation
We make no recommendation regarding use of HFNC in the peri-intubation period. For patients who are already receiving HFNC, we suggest continuing HFNC during intubation (conditional recommendation, moderate certainty).
Evidence summary
Ten trials [39–48] compared HFNC to COT (face mask and/or bag mask ventilation) or NIPPV in the peri-intubation period. Half of these trials enrolled non-hypoxemic patients undergoing intubation during induction of general anaesthesia before surgery and the remaining trials examined critically ill hypoxemic patients who required intubation [39−41, 43, 45]. Of the peri-operative trials, two trials included patients undergoing emergency surgery [42, 47], one trial each included patients undergoing bariatric surgery [35], any surgery, [48] and neurosurgery [46]. See the supplementary material to review the evidence profiles (Suppl material 1) and for a discussion of physiologic mechanisms (Suppl material 2). The results of the meta-analysis have been published elsewhere [7].
Six of the ten trials [39, 41–43, 46, 47] compared HFNC to COT, two [40, 48] compared HFNC to NIPPV only, and one trial compared HFNC with NIPPV to NIPPV only [45]. One trial included three arms that compared HFNC to NIPPV and facemask with bag mask [44]. With the exception of blinding, all trials except one [48] were judged to be at low or probably low risk of bias. Only one trial was judged to be at high risk of bias due to inadequate concealment.
When compared to COT and NIPPV, HFNC had no effect on the incidence of peri-intubation hypoxemia, defined as SpO2 < 80% (moderate certainty), 28-day mortality (moderate certainty); serious complications (low certainty), or ICU LOS (moderate certainty). Authors characterized serious complications as a composite of severe hypoxia (SpO2 < 80%), significant hypotension, use of vasopressors and cardiac arrest. Seven trials contributed data to the pooled analyses for hypoxemia and serious complications, whereas only four trials contributed data to the pooled analysis for ICU LOS and 28-day mortality. We lowered the certainty for all outcomes due to imprecision related to wide confidence intervals that failed to exclude serious benefit or harm. We further lowered certainty for the serious complications (various outcomes, different levels of patient importance) due to indirectness.
HFNC use had no effect on apneic time (low certainty), PaO2 measured after preoxygenation (moderate certainty), PaO2 measured after intubation (moderate certainty) or PaCO2 measured after intubation (low certainty) when compared to COT or NIPPV.
Preplanned subgroup analysis based on patient type (ICU vs. preoperative patients) demonstrated a larger increase in PaO2 levels after preoxygenation in the operative subgroup of patients who received HFNC; however, this increase is of questionable clinical significance as both subgroups had very high PaO2 levels after undergoing pre-oxygenation. Otherwise, we did not identify credible subgroup effects for any outcomes of interest by patient type, the comparator used (NIPPV vs. COT), or risk of bias (high vs. low). Sensitivity analysis excluding a single trial [43] that did not include patients with severe hypoxia did not change the strength or direction of the summary estimates.
Rationale
The desirable effects of HFNC compared to COT and NIPPV include a possible increase in PaO2 in preoperative patients (moderate certainty) but no effect on ICU LOS, peri-intubation complications, apneic time or oxygenation. Although, a single trial that compared HFNC plus NIPPV to NIPPV alone found a reduction in severe hypoxia (SpO2 < 80%) and an increase in PaO2, the panel agreed that any anticipated desirable effects of HFNC were likely small.
Conversely, the anticipated undesirable effects of HFNC use in the peri-intubation period were judged to be trivial. Although HFNC reportedly increases the risk for nasal bleeding this complication was not reported in the included trials. Overall, the panel judged the balance between desirable and undesirable effects of HFNC use in the peri-intubation period to be at least equal, favoring neither conventional therapy, NIPPV, or HFNC. Some panel members felt that the potential desirable effects outweighed the trivial harms associated with HFNC use. This viewpoint considered the trend towards improved oxygenation in pooled analysis and data suggesting that HFNC use may occasionally prevent catastrophic harm (hypoxic arrest, anoxic brain injury) due to hypoxia and apnea.
We did not identify important considerations regarding individual patient’s values or preferences. Although the use of HFNC would be associated with equipment costs, we did not have sufficient data to assess the cost-effectiveness of HFNC for this indication. The panel judged peri-intubation HFNC implementation (as compared to COT or NIPPV) to be acceptable and feasible.
Research priorities
Trials examining peri-intubation HFNC included a heterogeneous group of patients with various levels of hypoxia and undergoing intubation for diverse reasons. The effect of HFNC use in specific patient populations (severe hypoxia (P/F < 100); obese; different surgeries) is unknown. None of the trials examined HFNC use in patients who were considered to be difficult to intubate or at high risk for desaturation. Additionally, only three trials [33, 38, 41] compared HFNC use to NIPPV and one trial combined NIPPV with HFNC for intubation [38]. Data regarding intubator level of training, patient positioning, ease of intubation and patient experience were rarely or never reported. Data related to the long-term effects of HFNC use in the peri-intubation period were absent. This is particularly important as the costs and negative outcomes associated with severe hypoxemia or hypoxemic arrest are not seen in the short term [49]. It is unclear what effect prolonging safe apnea time may have on patient outcomes [50].
PICO 4: Post-operative
Recommendation
In high risk and/or obese patients undergoing cardiac or thoracic surgery, we suggest using HFNC compared to COT to prevent respiratory failure in the immediate postoperative period (conditional recommendation, moderate certainty evidence). We suggest against prophylactic HFNC use to prevent respiratory failure in other postoperative patients (conditional recommendation, very low certainty evidence).
Evidence summary
Ten trials [51–60] compared prophylactic HFNC to COT and only one trial compared HFNC to NIPPV [61]. Of these, six trials were conducted in patients following cardiac surgery [53, 55, 57, 58], four trials were conducted following thoracic surgery [51, 52, 56], and one trial was performed in patients after major combined thoracic and abdominal surgery [54]. With the exception of blinding, all trials, except one [52], were judged to be at low or probably low risk of bias. One trial was judged to be at high risk of bias as it did not adhere to an intention-to-treat analysis. See the supplementary material to review the evidence profiles (Suppl material 1) and for a discussion of physiologic mechanisms (Suppl material 2).
Compared to COT, post-operative HFNC use was associated with a significantly lower reintubation rate (RR 0.32 [0.12–0.88; 6 trials, n = 900, moderate certainty]) and decreased need to escalate respiratory support (RR 0.54 [0.31–0.94; 7 trials, n = 1120, very low certainty]). We lowered certainty of evidence for the rate of reintubation due to imprecision related to a very low event rate. We also lowered certainty of evidence regarding the need to escalate respiratory support due to inconsistency, indirectness, and imprecision.
When compared to COT, HFNC had no effect on mortality (low certainty), ICU length of stay (high certainty), hospital length of stay (moderate certainty) or the incidence of postoperative hypoxia (low certainty).
Pre-planned subgroup analyses based on type of surgery, risk of postoperative respiratory complications, and obesity did not demonstrate credible subgroup effects. However, a post-hoc subgroup analysis, comparing high risk (combination of obese patients and those at high risk of postoperative respiratory complication) vs. non high-risk patients showed increased benefit of HFNC for high risk patients. Only two trials [55, 56] examined non high-risk patients. We performed two post-hoc sensitivity analyses excluding, (1) two trials that excluded obese patients [54, 56] and (2) a single trial that evaluated patients following thoracoabdominal surgery [54]. Neither sensitivity analysis changed the overall results or conclusions.
When compared to NIPPV, HFNC had no effect on reintubation rate (low certainty), the need for respiratory support (low certainty), or ICU length of stay (low certainty). Compared to HFNC use, skin breakdown was more common with NIPPV use (low certainty).
Rationale
The desirable effects of HFNC compared to COT included a reduction in the rates of reintubation (moderate certainty) and escalation of respiratory support (very low certainty). Although, the effect sizes for these outcomes were large (RR 0.32), the absolute effects were small (absolute risk reduction 2.9%). These effects were driven by obese patients and patients at high risk of postoperative respiratory complications. High-risk was variably defined in the included trials; two used an ARISCAT score of 26 or greater, while two others considered obesity or history of cardiac or respiratory disease as high risk. Similar benefits were not seen when HFNC was compared to NIPPV or in low-risk patients. We noted an increase in skin breakdown with NIPPV use compared to HFNC use.
The anticipated undesirable effects of HFNC compared to COT and NIPPV were minimal. The trials included in this dataset did not report nasal bleeding with HFNC. For a more detailed discussion on the risks of HFNC (see PICO 1). Overall, the panel judged the balance of desirable and undesirable effects to favor the use of prophylactic HFNC over COT for high risk or obese patients after major cardiac and thoracic surgery, and neither favored or disfavored HFNC use compared to NIPPV. Since only one trial examined non-cardiac or thoracic surgery patients and did not show benefit associated with HFNC use, the panel judged the evidence insufficient to recommend prophylactic HFNC use in other post-operative patients.
The panel did not identify any important considerations regarding individual patient’s values or preferences. There were insufficient data to consider the implications of HFNC use based on resources and health equity concerns. Nonetheless, NIPPV use typically requires admission to a monitored setting (e.g., ICU) which may not be true for HFNC use [61]. The panel judged HFNC implementation (as compared to COT or NIPPV) to be both acceptable and feasible.
Research priorities
The included trials examined patients after cardiac, thoracic, and major abdominal surgery. The effect of HFNC use postoperatively in other surgical populations at risk of respiratory failure (neurosurgery, otolaryngology surgery or major vascular surgery) remains unknown. Given that HFNC is likely most beneficial in high-risk surgical populations, HFNC use in these other populations should be investigated. HFNC use in patients after thoracic and abdominal surgery also requires further evaluation in order to increase precision of findings [54]. Additional trials are needed to assess HFNC alone vs. HFNC co-administered in combination with NIPPV in postoperative patients [62].
We did not identify any long term or cost-effectiveness data examining postoperative HFNC use. All trials investigated prophylactic HFNC use to prevent respiratory failure consequently, the role for HFNC as a treatment for acute respiratory failure in postoperative patients is unknown. Finally, there were insufficient data on the effectiveness of HFNC in high risk subgroups (elderly, poor lung reserve). Trials focused on these vulnerable populations are needed.
Discussion
Our guideline has several strengths. To address our PICO questions, we conducted a comprehensive systematic review of the literature, including risk of bias assessment of individual RCTs, assessment of certainty of evidence for each outcome, and adhered to GRADE methodology. The guideline process was accompanied by transparent reporting of COIs. The panel included both content and methods experts. We used the Evidence-to-Decision framework to ensure incorporation of all relevant considerations into the recommendations. We prioritized outcomes that were perceived to be important to patients and highlighted areas for future investigation. Our guideline also has limitations. Although we did not include patient representatives on the panel, we included speculated patient perspectives (in regards to the importance of outcomes and how patients may value the balance of specific benefits and harms). Because HFNC is a relatively new technology, imprecision and inconsistency in pooled estimates limited certainty assessments for several recommendations. As trials examining HFNC use continue to be conducted, we anticipate that recommendations will need to be updated.
Conclusion
We make four recommendations to guide HFNC use in practice including a strong recommendation for HFNC in hypoxemic respiratory failure (moderate certainty), conditional recommendation for HFNC following extubation (moderate certainty), no recommendation regarding HFNC in the peri-intubation period (moderate certainty), and a conditional recommendation for postoperative HFNC in high risk and/or obese patients following cardiac or thoracic surgery (moderate certainty).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The pleural pressure working group (PLUG) is supported by the European Society of Intensive Care medicine. We would also like to acknowledge David Granton and Dominic Wang for their help with the systematic reviews and Iris Arad for her assistance with conducting literature searches.
Abbreviations
- CI
Confidence interval
- COT
Conventional oxygen therapy. Defined as low-flow oxygen (≤ 15 l/min) delivered either by nasal cannula or face mask.
- CPAP
Continuous positive airway pressure
- ESICM
European society of intensive care medicine
- GRADE
Grading of recommendations, assessment, development, and evaluation
- HFNC
High flow nasal cannula
- ICU
Intensive care unit
- NIPPV
Non-invasive positive pressure ventilation
- OR
Odds ratio
- PaO2FiO2 ratios
Ratio between arterial oxygen pressure and inspired fraction of oxygen
- PEEP
Positive end-expiratory pressure
- PICO
Population/intervention/comparison/outcome
- RCT
Randomised clinical trial
- RR
Relative risk
- SpO2
Arterial oxygen saturation
Funding
The European Society of Intensive Care Medicine provided logistical support and endorsed the guideline.
Compliance with ethical standards
Conflicts of interest
SE received funding for travel, given lectures, owns patents with and/or performed consultancy work for Zoll, Medtronic and Diasorin and has participated in multicentre trials run by Artisanpharma, Eisai and Astra Zeneca. JM received personal fees from Faron Pharmaceuticals, Medtronic and Janssen; IMT Medical provided travel and hotel expenses to attend a meeting; he is a coinvestigator on a PAV + multinational trial funded by the CIHR in partnership with Covidien (Medtronic); more than 3 years ago Fisher-Paykel, General Electric and A-Lung provided grant funding to conduct clinical research. TM received lecture fees from Drager, Fisher and Paykel, Mindray and BBraun. EG supported by an Early Career Investigator award from the Canadian Institutes of Health Research. He receives financial and nonfinancial support from Getinge and Timpel outside the submitted work. SJ received Consulting fees from Drager, Xenios, and Fisher & Paykel. J-DR received travel expenses and accommodation coverage from Fisher&Paykel Healthcare to attend scientific meetings. Fisher&Paykel Healthcare provided support for the ongoing High Flow ACRF trial (NCT03406572) but took no part in design or conduct of the study. NR received travel expenses and lecture fees from Fisher&Paykel. OR’s institution received fees for consultation from Hamilton Medical and received lecture fees from Air Liquide. MA’s institution received unrestricted research grant from Fisher & Paykel and GE Healthcare, he received consulting fees from Getinge and Intersurgical. SMM received consulting fees from Draeger Medical and General Electric Healthcare; his Institution received a research grant from Fisher and Paykel Healthcare (the RINO trial, clinicaltrials.gov NCT02107183). AD reports research grants from Philips, Respinor, Lungpacer, French Ministry of Health, Fisher and Paykel Healthcare (the RINO trial, clinicaltrials.gov NCT02107183), financial and nonfinancial support from Fisher&Paykel (including payment for lectures and registration to one scientific meeting), personal fees from Baxter (board meeting), Hamilton (lecture), Getinge (lecture), Lowenstein (advisory board). CH was supported by a Heart Foundation Fellowship. AM received personal fees from Faron Pharmaceuticals, Air Liquid Medical Systems, Pfizer, Resmed and Draeger and grants and personal fees from Fisher and Paykel and Covidien. EA has received fees for lectures from Gilead, Pfizer, Baxter, and Alexion. His research group has been supported by Ablynx, Fisher & Paykel, Jazz Pharma, and MSD. GC received consulting fees from Maquet. AD-M reports research grants from Fisher Paykel, Baxter, Philips, Ferring and GSK; participation to advisory board for Air Liquide, Baxter, and Amomed, lectures for Getinge and Addmedica. JF received grant funding and travel support to meetings from Fisher Paykel ( no role in research from FP) and Xenios. His research group has received research support from CSL, Timpel, Draeger. J-PF received travel expenses and lectures fees from Fisher&Paykel and SOS oxygene. GG received travel expenses and lecture fees from Getinge, Draeger Medical, MSD, Fisher&Paykel, Thermofisher and Biotest. GH received personal fees and travel expenses from Fisher and Paykel Healthcare. AT received financial and nonfinancial support from Fisher&Paykel (including payment for lectures and travel expense coverage to attend scientific meetings), and received financial support from Covidien, Maquet-Getinge, and GE Healthcare outside the submitted work. LB conducts an investigator-initiated trial on PAV + funded by the Canadian Institute for Health Research in partnership with Medtronic Covidien. His laboratory also received grants and non-financial support from Fisher & Paykel, non-financial support from Air Liquide, non-financial support from Philips, non-financial support from Sentec, other from General Electric.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61(4):529–541. doi: 10.4187/respcare.04577. [DOI] [PubMed] [Google Scholar]
- 2.Papazian L, Corley A, Hess D, et al. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. 2016;42(9):1336–1349. doi: 10.1007/s00134-016-4277-8. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clin Res Ed) 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito J, Nagata K, Sato S, et al. The clinical practice of high-flow nasal cannula oxygen therapy in adults: a Japanese cross-sectional multicenter survey. Respir Investig. 2018;56(3):249–257. doi: 10.1016/j.resinv.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Besnier E, Hobeika S, Nseir S, et al. High-flow nasal cannula therapy: clinical practice in intensive care units. Ann Intensive Care. 2019;9(1):98. doi: 10.1186/s13613-019-0569-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rochwerg B, Granton D, Wang DX, et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhuri D, Granton D, Wang DX, et al. Moderate certainty evidence suggests the use of high-flow nasal cannula does not decrease hypoxia when compared with conventional oxygen therapy in the peri-intubation period: results of a systematic review and meta-analysis. Critical Care Med. 2020 doi: 10.1097/CCM.0000000000004217. [DOI] [PubMed] [Google Scholar]
- 8.RIsk of Bias in Systematic Reviews. Evidence Partners. https://www.evidencepartners.com/resources/methodological-resources/risk-of-bias-commentary/. Published 2018. Accessed September 30, 2018, 2018.
- 9.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Li SA, Alexander PE, Reljic T, et al. Evidence to decision framework provides a structured "roadmap" for making grade guidelines recommendations. J Clin Epidemiol. 2018;104:103–112. doi: 10.1016/j.jclinepi.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ (Clin Res Ed) 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azoulay E, Lemiale V, Mokart D, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018;320(20):2099–2107. doi: 10.1001/jama.2018.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell N, Hutchinson CL, Green TC, Rogan E, Bein KJ, Dinh MM. Randomised control trial of humidified high flow nasal cannulae versus standard oxygen in the emergency department. Emerg Med Austr EMA. 2015;27(6):537–541. doi: 10.1111/1742-6723.12490. [DOI] [PubMed] [Google Scholar]
- 15.Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 16.Jones PG, Kamona S, Doran O, Sawtell F, Wilsher M. Randomized controlled trial of humidified high-flow nasal oxygen for acute respiratory distress in the emergency department: the HOT-ER study. Respir Care. 2016;61(3):291–299. doi: 10.4187/respcare.04252. [DOI] [PubMed] [Google Scholar]
- 17.Lemiale V, Mokart D, Mayaux J, et al. The effects of a 2-h trial of high-flow oxygen by nasal cannula versus Venturi mask in immunocompromised patients with hypoxemic acute respiratory failure: a multicenter randomized trial. Crit Care (Lond Engl) 2015;19:380. doi: 10.1186/s13054-015-1097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makdee O, Monsomboon A, Surabenjawong U, et al. High-flow nasal cannula versus conventional oxygen therapy in emergency department patients with cardiogenic pulmonary edema: a randomized controlled trial. Ann Emerg Med. 2017;70(4):465–472 e462. doi: 10.1016/j.annemergmed.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Parke RL, McGuinness SP, Eccleston ML. A preliminary randomized controlled trial to assess effectiveness of nasal high-flow oxygen in intensive care patients. Respir Care. 2011;56(3):265–270. doi: 10.4187/respcare.00801. [DOI] [PubMed] [Google Scholar]
- 20.Rittayamai N, Tscheikuna J, Praphruetkit N, Kijpinyochai S. Use of high-flow nasal cannula for acute dyspnea and hypoxemia in the emergency department. Respir Care. 2015;60(10):1377–1382. doi: 10.4187/respcare.03837. [DOI] [PubMed] [Google Scholar]
- 21.Schwabbauer N, Berg B, Blumenstock G, Haap M, Hetzel J, Riessen R. Nasal high-flow oxygen therapy in patients with hypoxic respiratory failure: effect on functional and subjective respiratory parameters compared to conventional oxygen therapy and non-invasive ventilation (NIV) BMC Anesthesiol. 2014;14:66. doi: 10.1186/1471-2253-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hegde S, Prodhan P. Serious air leak syndrome complicating high-flow nasal cannula therapy: a report of 3 cases. Pediatrics. 2013;131(3):e939–944. doi: 10.1542/peds.2011-3767. [DOI] [PubMed] [Google Scholar]
- 23.Piastra M, Morena TC, Antonelli M, Conti G. Uncommon barotrauma while on high-flow nasal cannula. Intensive Care Med. 2018;44(12):2288–2289. doi: 10.1007/s00134-018-5279-5. [DOI] [PubMed] [Google Scholar]
- 24.Baudin F, Gagnon S, Crulli B, Proulx F, Jouvet P, Emeriaud G. Modalities and complications associated with the use of high-flow nasal cannula: experience in a pediatric ICU. Respir Care. 2016;61(10):1305–1310. doi: 10.4187/respcare.04452. [DOI] [PubMed] [Google Scholar]
- 25.Iglesias-Deus A, Pérez-Muñuzuri A, López-Suárez O, Crespo P, Couce ML. Tension pneumocephalus induced by high-flow nasal cannula ventilation in a neonate. Arch Dis Child Fetal Neonatal Ed. 2017;102(2):F173–f175. doi: 10.1136/archdischild-2015-309777. [DOI] [PubMed] [Google Scholar]
- 26.Inoue S, Tamaki Y, Sonobe S, Egawa J, Kawaguchi M. A pediatric case developing critical abdominal distension caused by a combination of humidified high-flow nasal cannula oxygen therapy and nasal airway. JA Clin Rep. 2018;4(1):4. doi: 10.1186/s40981-017-0143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang BJ, Koh Y, Lim CM, et al. Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Intensive Care Med. 2015;41(4):623–632. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- 28.Frat JP, Ragot S, Coudroy R, et al. Predictors of intubation in patients with acute hypoxemic respiratory failure treated with a noninvasive oxygenation strategy. Crit Care Med. 2018;46(2):208–215. doi: 10.1097/CCM.0000000000002818. [DOI] [PubMed] [Google Scholar]
- 29.Eaton Turner E, Jenks M. Cost-effectiveness analysis of the use of high-flow oxygen through nasal cannula in intensive care units in NHS England. Expert Rev Pharmacoecon Outcomes Res. 2018;18(3):331–337. doi: 10.1080/14737167.2018.1411804. [DOI] [PubMed] [Google Scholar]
- 30.Maggiore SM, Idone FA, Vaschetto R, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190(3):282–288. doi: 10.1164/rccm.201402-0364OC. [DOI] [PubMed] [Google Scholar]
- 31.Rittayamai N, Tscheikuna J, Rujiwit P. High-flow nasal cannula versus conventional oxygen therapy after endotracheal extubation: a randomized crossover physiologic study. Respir Care. 2014;59(4):485–490. doi: 10.4187/respcare.02397. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez G, Vaquero C, Gonzalez P, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315(13):1354–1361. doi: 10.1001/jama.2016.2711. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez R, Subira C, Frutos-Vivar F, et al. High-flow nasal cannula to prevent postextubation respiratory failure in high-risk non-hypercapnic patients: a randomized multicenter trial. Ann Intensive Care. 2017;7(1):47. doi: 10.1186/s13613-017-0270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song HZ, Gu JX, Xiu HQ, Cui W, Zhang GS. The value of high-flow nasal cannula oxygen therapy after extubation in patients with acute respiratory failure. Clin (Sao Paulo) 2017;72(9):562–567. doi: 10.6061/clinics/2017(09)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez G, Vaquero C, Colinas L, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316(15):1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 36.Theerawit PN, Sutherasan Y. The efficacy of the Whispherflow CPAP system versus high flow nasal cannula in patients at high risk for postextubation failure. Intensive Care Med Exp. 2017;5:206. doi: 10.1016/j.jcrc.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 37.Jing G, Li J, Hao D, et al. Comparison of high flow nasal cannula with noninvasive ventilation in chronic obstructive pulmonary disease patients with hypercapnia in preventing postextubation respiratory failure: a pilot randomized controlled trial. Res Nurs Health. 2019;42(3):217–225. doi: 10.1002/nur.21942. [DOI] [PubMed] [Google Scholar]
- 38.Esteban A, Frutos-Vivar F, Ferguson ND, et al. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350(24):2452–2460. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- 39.Simon M, Wachs C, Braune S, de Heer G, Frings D, Kluge S. High-flow nasal cannula versus bag-valve-mask for preoxygenation before intubation in subjects with hypoxemic respiratory failure. Respir Care. 2016;61(9):1160–1167. doi: 10.4187/respcare.04413. [DOI] [PubMed] [Google Scholar]
- 40.Frat JP, Ricard JD, Quenot JP, et al. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomised, multicentre, open-label trial. Lancet Respir Med. 2019;7(4):303–312. doi: 10.1016/S2213-2600(19)30048-7. [DOI] [PubMed] [Google Scholar]
- 41.Vourc'h M, Asfar P, Volteau C, et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015;41(9):1538–1548. doi: 10.1007/s00134-015-3796-z. [DOI] [PubMed] [Google Scholar]
- 42.Mir F, Patel A, Iqbal R, Cecconi M, Nouraei SA. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre-oxygenation with facemask pre-oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia. 2017;72(4):439–443. doi: 10.1111/anae.13799. [DOI] [PubMed] [Google Scholar]
- 43.Guitton C, Ehrmann S, Volteau C, et al. Nasal high-flow preoxygenation for endotracheal intubation in the critically ill patient: a randomized clinical trial. Intensive Care Med. 2019;45(4):447–458. doi: 10.1007/s00134-019-05529-w. [DOI] [PubMed] [Google Scholar]
- 44.Sebastian HTH, Benedikt S, et al. Benefits of heated and humidified high flow nasal oxygen for preoxygenation in morbidly obese patients undergoing bariatric surgery: a randomized controlled study. J Obes Bariatrics. 2014;1:1–7. [Google Scholar]
- 45.Jaber S, Monnin M, Girard M, et al. Apnoeic oxygenation via high-flow nasal cannula oxygen combined with non-invasive ventilation preoxygenation for intubation in hypoxaemic patients in the intensive care unit: the single-centre, blinded, randomised controlled OPTINIV trial. Intensive Care Med. 2016;42(12):1877–1887. doi: 10.1007/s00134-016-4588-9. [DOI] [PubMed] [Google Scholar]
- 46.Ng I, Krieser R, Mezzavia P, et al. The use of transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) for pre-oxygenation in neurosurgical patients: a randomised controlled trial. Anaesth Intensive Care. 2018;46(4):360–367. doi: 10.1177/0310057X1804600403. [DOI] [PubMed] [Google Scholar]
- 47.Lodenius A, Piehl J, Ostlund A, Ullman J, Jonsson FM. Transnasal humidified rapid-insufflation ventilatory exchange (THRIVE) vs facemask breathing pre-oxygenation for rapid sequence induction in adults: a prospective randomised non-blinded clinical trial. Anaesthesia. 2018;73(5):564–571. doi: 10.1111/anae.14215. [DOI] [PubMed] [Google Scholar]
- 48.Vourc'h M, Baud G, Feuillet F, et al. High-flow nasal cannulae versus non-invasive ventilation for preoxygenation of obese patients: the PREOPTIPOP randomized trial. EClinicalMedicine. 2019;13:112–119. doi: 10.1016/j.eclinm.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khot S, Tirschwell DL. Long-term neurological complications after hypoxic-ischemic encephalopathy. Semin Neurol. 2006;26(4):422–431. doi: 10.1055/s-2006-948323. [DOI] [PubMed] [Google Scholar]
- 50.Chua MT, Khan FA, Ng WM, et al. Pre- and Apnoeic high flow oxygenation for RApid sequence intubation in The Emergency department (Pre-AeRATE): study protocol for a multicentre, randomised controlled trial. Trials. 2019;20(1):195. doi: 10.1186/s13063-019-3305-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ansari BM, Hogan MP, Collier TJ, et al. A randomized controlled trial of high-flow nasal oxygen (Optiflow) as part of an enhanced recovery program after lung resection surgery. Ann Thorac Surg. 2016;101(2):459–464. doi: 10.1016/j.athoracsur.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 52.Brainard J, Scott BK, Sullivan BL, et al. Heated humidified high-flow nasal cannula oxygen after thoracic surgery—a randomized prospective clinical pilot trial. J Crit Care. 2017;40:225–228. doi: 10.1016/j.jcrc.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corley A, Bull T, Spooner AJ, Barnett AG, Fraser JF. Direct extubation onto high-flow nasal cannulae post-cardiac surgery versus standard treatment in patients with a BMI >/=30: a randomised controlled trial. Intensive Care Med. 2015;41(5):887–894. doi: 10.1007/s00134-015-3765-6. [DOI] [PubMed] [Google Scholar]
- 54.Futier E, Paugam-Burtz C, Godet T, et al. Effect of early postextubation high-flow nasal cannula vs conventional oxygen therapy on hypoxaemia in patients after major abdominal surgery: a French multicentre randomised controlled trial (OPERA) Intensive Care Med. 2016;42(12):1888–1898. doi: 10.1007/s00134-016-4594-y. [DOI] [PubMed] [Google Scholar]
- 55.Parke R, McGuinness S, Dixon R, Jull A. Open-label, phase II study of routine high-flow nasal oxygen therapy in cardiac surgical patients. Br J Anaesth. 2013;111(6):925–931. doi: 10.1093/bja/aet262. [DOI] [PubMed] [Google Scholar]
- 56.Pennisi MA, Bello G, Congedo MT, et al. Early nasal high-flow versus Venturi mask oxygen therapy after lung resection: a randomized trial. Crit Care (Lond Engl) 2019;23(1):68. doi: 10.1186/s13054-019-2361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahin M, El H, Akkoc I. Comparison of mask oxygen therapy and high-flow oxygen therapy after cardiopulmonary bypass in obese patients. Can Respir J. 2018;2018:1039635. doi: 10.1155/2018/1039635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tatsuishi W, Sato T, Kataoka G, Sato A, Asano R, Nakano K. High-Flow nasal cannula therapy with early extubation for subjects undergoing off-pump coronary artery bypass graft surgery. Respir Care. 2020;65(2):183–190. doi: 10.4187/respcare.06382. [DOI] [PubMed] [Google Scholar]
- 59.Yu Y, Qian X, Liu C, Zhu C. Effect of high-flow nasal cannula versus conventional oxygen therapy for patients with thoracoscopic lobectomy after extubation. Can Respir J. 2017;2017:7894631. doi: 10.1155/2017/7894631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zochios V, Collier T, Blaudszun G, et al. The effect of high-flow nasal oxygen on hospital length of stay in cardiac surgical patients at high risk for respiratory complications: a randomised controlled trial. Anaesthesia. 2018;73(12):1478–1488. doi: 10.1111/anae.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stephan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313(23):2331–2339. doi: 10.1001/jama.2015.5213. [DOI] [PubMed] [Google Scholar]
- 62.Brueckmann B, Villa-Uribe JL, Bateman BT, et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology. 2013;118(6):1276–1285. doi: 10.1097/ALN.0b013e318293065c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.