Abstract
The ability of cells to migrate is a fundamental physiological process involved in embryonic development, tissue homeostasis, immune surveillance, and wound healing. Therefore, the mechanisms governing cellular locomotion have been under intense scrutiny over the last 50 years. One of the main tools of this scrutiny is live-cell quantitative imaging, where researchers image cells over time to study their migration and quantitatively analyze their dynamics by tracking them using the recorded images. Despite the availability of computational tools, manual tracking remains widely used among researchers due to the difficulty setting up robust automated cell tracking and large-scale analysis. Here we provide a detailed analysis pipeline illustrating how the deep learning network StarDist can be combined with the popular tracking software TrackMate to perform 2D automated cell tracking and provide fully quantitative readouts. Our proposed protocol is compatible with both fluorescent and widefield images. It only requires freely available and open-source software (ZeroCostDL4Mic and Fiji), and does not require any coding knowledge from the users, making it a versatile and powerful tool for the field. We demonstrate this pipeline's usability by automatically tracking cancer cells and T cells using fluorescent and brightfield images. Importantly, we provide, as supplementary information, a detailed step-by-step protocol to allow researchers to implement it with their images.
Keywords: Cell migration, Image analysis, StarDist, TrackMate, Deep-learning, Automated tracking
Introduction
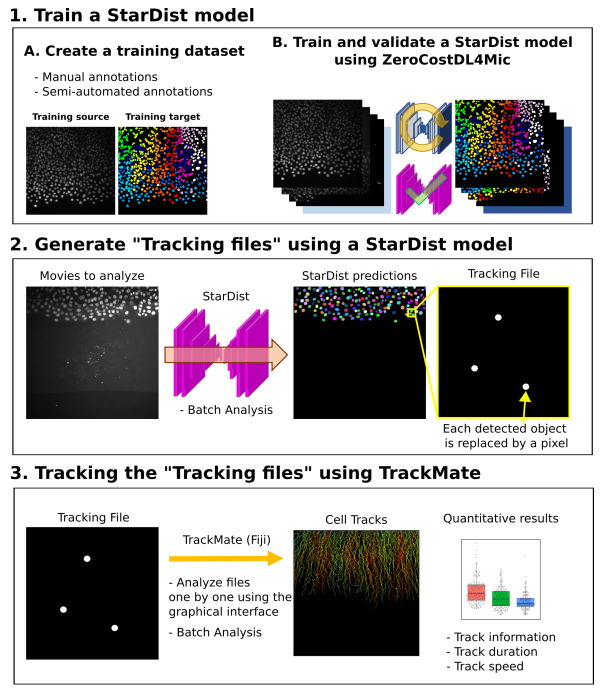
The study of cell motility typically involves recording cell behavior, using live-cell imaging, and tracking their movement over time 1, 2. To enable the analysis of such data, various software solutions have been developed 3– 9. However, despite the availability of these computational tools, manual tracking remains widely used among researchers due to the difficulty in setting up fully automated cell tracking analysis pipelines. Automated tracking pipelines share a typical workflow that starts with a segmentation strategy that identifies the objects to track in each image. Tracking algorithms are then used to link these objects between frames. One challenging aspect of an automated tracking pipeline is often achieving an accurate segmentation of the objects to track. One option to facilitate cell segmentation is to label their nuclei, using fluorescent dyes or protein markers. Nuclei can then be automatically segmented using intensity-based thresholding. However, this approach tends to become inaccurate when images are noisy or when the cells to track are very crowded 10. Deep-Learning approaches have demonstrated their robustness against these two issues 11. In this work, we present a new analysis workflow that builds upon a Deep-Learning segmentation tool and a cell tracking tool to achieve robust cell tracking in cell migration assays. We combine StarDist, a powerful deep learning-based segmentation tool, and TrackMate, a user-friendly tracking tool, into a tracking pipeline that can be used without requiring expertise in or specialized hardware for computing ( Figure 1) 12– 15.
Figure 1. Workflow depicting how StarDist and TrackMate can be combined to track cells automatically.
Methods
Pipeline
The use of deep learning networks, such as StarDist, often requires the user to train or retrain a model using their images. While high-quality StarDist pre-trained models are readily available, they are likely to underperform when used on different data with, e.g., different staining, noise, and microscope type 15. To train StarDist models, we took advantage of the ZeroCostDL4Mic platform, allowing researchers to train (and retrain), validate, and use deep learning networks 15. Importantly, the ZeroCostDL4Mic StarDist 2D notebook can directly output a file containing all the nuclei's geometric center coordinates (named tracking files), that can be used as input for TrackMate ( Figure 1). Therefore, our proposed pipeline can be divided into three parts ( Figure 1; Extended data 16). 1) First, a StarDist model is trained using the ZeroCostDL4Mic platform. This part needs to be performed only once for each type of data. 2) Second, the trained StarDist model is used to segment the object to track and generate Tracking files. 3) Finally, the tracking files can be used in TrackMate to track the identified objects.
Training a StarDist model requires a set of images and their corresponding masks ( Figure 1 and Figure 2). Generating a training dataset is by far the most time-consuming part of the analysis pipeline presented here as it requires the manual annotations of the images to analyze ( Extended data: Supplementary protocol 16). For instance, to generate the training datasets presented in Figure 2, each cell/nuclei contour was drawn manually using the freehands selection tool in Fiji. The creation of a high-quality training dataset is a critical part of the process as it will impact the specificity and performance of the StarDist model. However, the generation of a training dataset is only required once per dataset type. If a StarDist model already exists for similar images it can be used to significantly accelerate the creation of the training dataset via semi-automated annotation (see Extended data: Supplementary protocol 16).
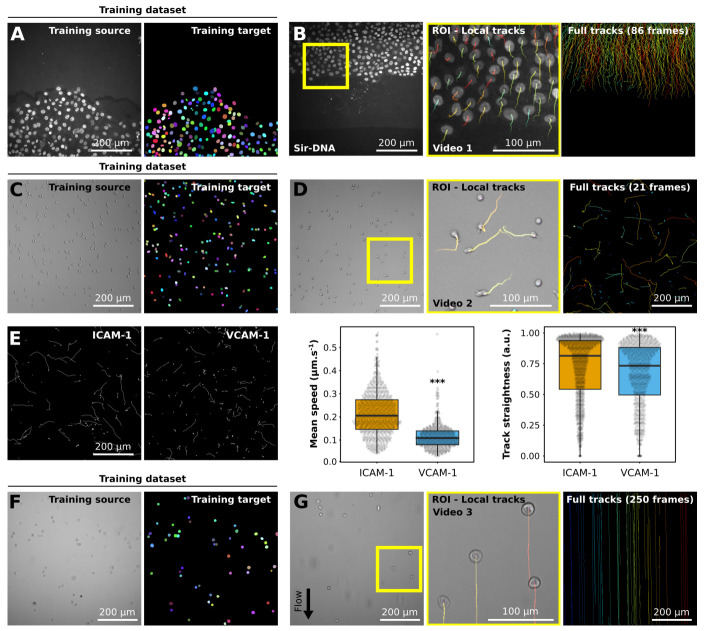
Figure 2. Example of datasets analyzed using StarDist and TrackMate.
( A, B) Migration of MCF10DCIS.com, labeled with Sir-DNA, recorded using a spinning disk confocal microscope and automatically tracked. Examples of images used to train StarDist ( A), and an example of results obtained using automated tracking are displayed ( B, Video 1). The yellow square indicates a magnified ROI, where the local track of each nucleus is displayed. The full cell tracks are displayed on the left. Tracks are color-coded as a function of their maximum instantaneous velocity (blue slow, red fast tracks). ( C– E) Migration of activated T cell plated on VCAM-1 or ICAM-1, recorded using a brightfield microscope and automatically tracked. Examples of images used to train StarDist ( C) and an example of results obtained using automated tracking are displayed ( D, Video 2). ( E) Comparison of the migration of activated T cells on VCAM-1 or ICAM-1. Track mean speed and track straightness were quantified. Data are displayed as boxplots. *** p-value = <0.001, p-values were determined using a randomization test. ( F, G) Cancer cells flowing in a microfluidic chamber, recorded live using a brightfield microscope and automatically tracked (Video 3). Examples of images used to train StarDist ( F), and an example of results obtained using automated tracking are displayed ( G). The full tracks shown here were color-coded as a function of their x coordinate.
One of our analysis pipeline's key features is that, once a StarDist model has been satisfactorily trained, movies of migrating cells can efficiently be processed in batch. Indeed, while individual tracking files can be analyzed one by one using the TrackMate graphical interface, we also provide a Fiji macro to analyze a folder containing multiple tracking files. Our batch processing macro will provide basic quantitative information for each track, including median and maximal speeds. If more information is needed, the tracking results generated by our script are directly compatible with the Motility lab website, where they can be further processed 17.
Implementation and operation
The described image analysis pipeline is composed of a Jupiter notebook optimized to run in Google Colab (ZeroCostDL4Mic framework 15) and a Python script that can run in Fiji 14. A step-by-step protocol describing how to use our analysis pipeline is provided as Extended data 16.
Use case
To illustrate our analysis pipeline's functionality and flexibility, we first trained a StarDist model to analyze the behavior of breast cancer cells migrating collectively ( Figure 2A; Extended data: Video 1 16). The cancer cell's nuclei were fluorescently labeled, and the cells imaged using fluorescence-based microscopy. The creation of the training dataset used in this example was greatly facilitated by the availability of a StarDist model, released by the StarDist creators, capable of segmenting fluorescent nuclei. In this case, the StarDist Fiji plugin was used to segment the location of nuclei in the training images, and all miss-annotations were manually corrected ( Extended data: Supplementary protocol 16).
Video 1: Automated tracking of breast cancer cell migrating collectively. MCF10DCIS.com cells, labeled with Sir-DNA, were recorded using a spinning disk confocal microscope and automatically tracked using StarDist and TrackMate. Local tracks are displayed.
Copyright: © 2020 Fazeli E et al.
To highlight that our pipeline can also be used to analyze brightfield images, we generated a StarDist model to track T cells migrating on ICAM-1 or VCAM-1 ( Figure 2C–E; Extended data: Video 2 16). Importantly, automated analysis of these data could reproduce the results obtained via manual tracking 19.
Video 2: Automated tracking of T cell migrating on ICAM-1. Activated T cell plated ICAM-1 were recorded using a brightfield microscope and automatically tracked using StarDist and TrackMate. Local tracks are displayed.
Copyright: © 2020 Fazeli E et al.
Finally, we used our pipeline to automatically track non-adherent cancer cells flowing in a microfluidic chamber ( Figure 2F and G; Extended data: Video 3 16). In this case, automated tracking is especially useful due to the very high number of frames to analyze. For the last two examples, no suitable pre-trained StarDist models were available. Therefore, to generate the training datasets, we manually annotated 20 images and trained a first StarDist model. This model was then used to accelerate the annotation of the rest of the training images.
Video 3: Automated tracking of cancer cells flowing in a microfluidic chamber. AsPC1 pancreatic cancer cells flowing in a microfluidic chamber were recorded live using a brightfield microscope and automatically tracked using StarDist and TrackMate. Local tracks are displayed.
Copyright: © 2020 Fazeli E et al.
Use case dataset creation
Breast cancer cell dataset. MCF10DCIS.com cells were described previously 15, 22. DCIS.COM lifeact-RFP cells were incubated for 2h with 0.5 µM SiR-DNA (SiR-Hoechst, Tetu-bio, Cat Number: SC007) before being imaged live for 14 h using a spinning-disk confocal microscope (1 picture every 10 min). The spinning-disk confocal microscope used was a Marianas spinning disk imaging system with a Yokogawa CSU-W1 scanning unit on an inverted Zeiss Axio Observer Z1 microscope (Intelligent Imaging Innovations, Inc.) equipped with a 20x (NA 0.8) air, Plan Apochromat objective (Zeiss).
T cell dataset. Lab-Tek 8 chamber slides (ThermoFisher) were coated with 2 μg/mL ICAM-1 or VCAM-1 overnight at 4°C 19. Activated primary mouse CD4+ T cells were washed and resuspended in L-15 media containing 2 mg/mL D-glucose. T cells were then added to the chambers, incubated 20 min, gently washed to remove all unbound cells, and imaged. Imaging was done using a 10x phase contrast objective at 37°C on a Zeiss Axiovert 200M microscope equipped with an automated X-Y stage and a Roper EMCCD camera. Time-lapse images were collected every 30 sec for 10 min using SlideBook 6 software (Intelligent Imaging Innovations).
Flow chamber dataset. Cancer cells (500,000 cells/ml in PBS) were perfused at a speed of 300 µm/sec using a peristaltic pump (ISMATEC MS12/4 analogic) and a homemade tubing system (Ismatek 3-Stop tubes and Ibidi ® tubings and connectors) in a microchannel (Ibidi ® µ-slides400 LUER). Images were acquired with a brightfield microscope (Zeiss Laser-TIRF 3 Imaging System, Carl Zeiss) and a 10X objective.
Data display and statistical analyses
Box plots were generated using PlotsOfData 23. Randomization tests were performed using the online tool PlotsOfDifferences 24.
Conclusions
Here we show that StarDist and TrackMate can be integrated seamlessly and robustly to automate cell tracking in fluorescence and brightfield images. We envision that this pipeline can also be applied to any circular or oval-shaped objects. However, we acknowledge that using brightfield images may not always work directly with our pipeline, especially if cells display complex and interchanging shapes, since StarDist is mostly designed to detect round or compact shapes. In this case, other tools, such as Usiigaci, could also be considered 8. Still, brightfield images could also be artificially labeled using deep learning, transforming the brightfield dataset into a pseudo-fluorescence one, as can be done with ZeroCostDL4Mic already 15. The pipeline described here is currently limited to the tracking of objects in 2D. However, a similar workflow can be applied to 3D datasets as both StarDist and TrackMate can accommodate 3D images 12, 13, 25.
Data availability
Underlying data
Zenodo: Combining StarDist and TrackMate example 1 - Breast cancer cell dataset, http://doi.org/10.5281/zenodo.4034976 26
Zenodo: Combining StarDist and TrackMate example 2 - T cell dataset, http://doi.org/10.5281/zenodo.4034929 27
Zenodo: Combining StarDist and TrackMate example 3 - Flow chamber dataset, http://doi.org/10.5281/zenodo.4034939 28
Extended data
Zenodo: Combining StarDist and TrackMate - Extended data, http://doi.org/10.5281/zenodo.4091467 16.
This project contains the following extended data:
Supplementary protocol
Video 1: Automated tracking of breast cancer cell migrating collectively. MCF10DCIS.com cells, labeled with Sir-DNA, were recorded using a spinning disk confocal microscope and automatically tracked using StarDist and TrackMate. Local tracks are displayed.
Video 2: Automated tracking of T cell migrating on ICAM-1. Activated T cell plated ICAM-1 were recorded using a brightfield microscope and automatically tracked using StarDist and TrackMate. Local tracks are displayed.
Video 3: Automated tracking of cancer cells flowing in a microfluidic chamber. AsPC1 pancreatic cancer cells flowing in a microfluidic chamber were recorded live using a brightfield microscope and automatically tracked using StarDist and TrackMate. Local tracks are displayed.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Software availability
Source code available from: https://github.com/HenriquesLab/ZeroCostDL4Mic
Archived source code at time of publication: http://doi.org/10.5281/zenodo.4091474 26
License: MIT license.
Acknowledgments
The Cell Imaging and Cytometry Core facility (Turku Bioscience, University of Turku, Åbo Akademi University, and Biocenter Finland), the Finnish Euro-Bioimaging Node, and Turku Bioimaging are acknowledged for services, instrumentation and expertise.
This publication was supported by COST Action NEUBIAS (CA15124), funded by COST (European Cooperation in Science and Technology).
Funding Statement
This work was supported by grants awarded by the Academy of Finland (G.J.), the Sigrid Juselius Foundation (G.J.), Åbo Akademi University Research Foundation (CoE CellMech; G.J.) and by Drug Discovery and Diagnostics strategic funding to Åbo Akademi University (G.J.). G.F. was supported by National Cancer Center Finland (FICAN). NHR acknowledges support from the Cancer Research Institute and NIH T32 AR007442. R.F.L. acknowledges the support of the MRC Skills development fellowship (MR/T027924/1).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved
References
- 1. Jacquemet G, Baghirov H, Georgiadou M, et al. : L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat Commun. 2016;7:13297. 10.1038/ncomms13297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacquemet G, Morgan MR, Byron A, et al. : Rac1 is deactivated at integrin activation sites through an IQGAP1-filamin-A-RacGAP1 pathway. J Cell Sci. 2013;126(Pt 18):4121–4135. 10.1242/jcs.121988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DuChez BJ: Automated Tracking of Cell Migration with Rapid Data Analysis. Curr Protoc Cell Biol. 2017;76:12.12.1–12.12.16. 10.1002/cpcb.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cordelières FP, Petit V, Kumasaka M, et al. : Automated Cell Tracking and Analysis in Phase-Contrast Videos (iTrack4U): Development of Java Software Based on Combined Mean-Shift Processes. PLoS One. 2013;8(11):e81266. 10.1371/journal.pone.0081266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McQuin C, Goodman A, Chernyshev V, et al. : CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018;16(7):e2005970. 10.1371/journal.pbio.2005970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piccinini F, Kiss A, Horvath P: CellTracker (not only) for dummies. Bioinformatics. 2016;32(6):955–957. 10.1093/bioinformatics/btv686 [DOI] [PubMed] [Google Scholar]
- 7. Barry DJ, Durkin CH, Abella JV, et al. : Open source software for quantification of cell migration, protrusions, and fluorescence intensities. J Cell Biol. 2015;209(1):163–180. 10.1083/jcb.201501081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsai HF, Gajda J, Sloan TFW, et al. : Usiigaci: Instance-aware cell tracking in stain-free phase contrast microscopy enabled by machine learning. SoftwareX. 2019;9:230–237. 10.1016/j.softx.2019.02.007 [DOI] [Google Scholar]
- 9. Pijuan J, Barceló C, Moreno DF, et al. : In vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front Cell Dev Biol. 2019;7:107. 10.3389/fcell.2019.00107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chenouard N, Smal I, de Chaumont F, et al. : Objective comparison of particle tracking methods. Nat Methods. 2014;11(3):281–289. 10.1038/nmeth.2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caicedo JC, Roth J, Goodman A, et al. : Evaluation of Deep Learning Strategies for Nucleus Segmentation in Fluorescence Images. Cytometry A. 2019;95(9):952–965. 10.1002/cyto.a.23863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tinevez JY, Perry N, Schindelin J, et al. : TrackMate: An open and extensible platform for single-particle tracking. Methods. 2017;115:80–90. 10.1016/j.ymeth.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 13. Schmidt U, Weigert M, Broaddus C, et al. : Cell Detection with Star-Convex Polygons.In: Medical Image Computing and Computer Assisted Intervention – MICCAI 2018(eds. Frangi, A. F., Schnabel, J. A., Davatzikos, C., Alberola-López, C. & Fichtinger, G.) (Springer International Publishing, 2018).11071:265–273. 10.1007/978-3-030-00934-2_30 [DOI] [Google Scholar]
- 14. Schindelin J, Arganda-Carreras I, Frise E, et al. : Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chamier LV, Laine RF, Jukkala J, et al. : ZeroCostDL4Mic: an open platform to use Deep-Learning in Microscopy. bioRxiv. 2020;2020.03.20.000133 10.1101/2020.03.20.000133 [DOI] [Google Scholar]
- 16. Jacquemet G, Roy NH, Follain G, et al. : Combining StarDist and TrackMate - Extended data. Zenodo. 2020. 10.5281/zenodo.4091467 [DOI] [Google Scholar]
- 17. Wortel IMN, Dannenberg K, Berry JC, et al. : CelltrackR: an R package for fast and flexible analysis of immune cell migration data.2019. 10.1101/670505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacquemet G: Video 1: Automated tracking of breast cancer cell migrating collectively.f1000research.com. Media. 2020. 10.6084/m9.figshare.13122635.v1 [DOI] [Google Scholar]
- 19. Roy NH, Kim SHJ, Buffone A, Jr, et al. : LFA-1 signals to promote actin polymerization and upstream migration in T cells. J Cell Sci. 2020;133(17):jcs248328. 10.1242/jcs.248328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacquemet G: Video 2: Automated tracking of T cell migrating on ICAM-1.f1000research.com. Media. 2020. 10.6084/m9.figshare.13122755.v1 [DOI] [Google Scholar]
- 21. Jacquemet G: Video 3: Automated tracking of cancer cells flowing in a microfluidic chamber.f1000research.com. Media. 2020. 10.6084/m9.figshare.13122764.v1 [DOI] [Google Scholar]
- 22. Jacquemet G, Paatero I, Carisey AF, et al. : FiloQuant reveals increased filopodia density during breast cancer progression. J Cell Biol. 2017;216(10):3387–3403. 10.1083/jcb.201704045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Postma M, Goedhart J: PlotsOfData—A web app for visualizing data together with their summaries. PLoS Biol. 2019;17(3):e3000202. 10.1371/journal.pbio.3000202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goedhart J: PlotsOfDifferences – a web app for the quantitative comparison of unpaired data. bioRxiv. 2019;578575 10.1101/578575 [DOI] [Google Scholar]
- 25. Weigert M, Schmidt U, Haase R, et al. : Star-convex Polyhedra for 3D Object Detection and Segmentation in Microscopy.2020;8 10.1109/WACV45572.2020.9093435 [DOI] [Google Scholar]
- 26. Jacquemet G: Combining StarDist and TrackMate example 1 - Breast cancer cell dataset (Version 1) [Data set]. Zenodo. 2020. 10.5281/zenodo.4034976 [DOI] [Google Scholar]
- 27. Roy NH, Jacquemet G: Combining StarDist and TrackMate example 2 - T cell dataset (Version 1) [Data set]. Zenodo. 2020. 10.5281/zenodo.4034929 [DOI] [Google Scholar]
- 28. Follain G, Jacquemet G: Combining StarDist and TrackMate example 3 - Flow chamber dataset (Version 1) [Data set]. Zenodo. 2020. 10.5281/zenodo.4034939 [DOI] [Google Scholar]
- 29. Jacquemet G, Fazeli E, Laine RF, et al. : Combining StarDist and TrackMate - Archived source code (Version v1). Zenodo. 2020. 10.5281/zenodo.4091474 [DOI] [Google Scholar]